Abstract
Background
Most gastric cancers are diagnosed at an advanced or metastatic stage with poor prognosis and survival rate. Fatty acid 2-hydroxylase (FA2H) with high expression in stomach generates chiral (R)-2-hydroxy FAs ((R)-2-OHFAs) and regulates glucose utilization which is important for cell proliferation and invasiveness. We hypothesized that FA2H impacts gastric tumor growth and could represent a novel target to improve gastric cancer therapy.
Methods
FA2H level in 117 human gastric tumors and its association with tumor growth, metastasis and overall survival were examined. Its roles and potential mechanisms in regulating tumor growth were studied by genetic and pharmacological manipulation of gastric cancer cells in vitro and in vivo.
Findings
FA2H level was lower in gastric tumor tissues as compared to surrounding tissues and associated with clinicopathologic status of patients, which were confirmed by analyses of multiple published datasets. FA2H depletion decreased tumor chemosensitivity, partially due to inhibition of AMPK and activation of the mTOR/S6K1/Gli1 pathway. Conversely, FA2H overexpression or treatment with (R)-2-OHFAs had the opposite effects. In line with these in vitro observations, FA2H knockdown promoted tumor growth with increased level of tumor Gli1 in vivo. Moreover, (R)-2-OHFA treatment significantly decreased Gli1 level in gastric tumors and enhanced tumor chemosensitivity to cisplatin, while alleviating the chemotherapy-induced weight loss in mice.
Interpretation
Our results demonstrate that FA2H plays an important role in regulating Hh signaling and gastric tumor growth and suggest that (R)-2-OHFAs could be effective as nontoxic wide-spectrum drugs to promote chemosensitivity.
Fund
Grants of NSF, NIH, and PAPD.
Keywords: Fatty acid 2-hydroxylation, Gastric cancer, Lipid metabolism, mTOR, Chemotherapy, Hedgehog pathway
Research in context.
Evidence before this study
Cancer cells acquire characteristic changes in FA metabolism to support cell proliferation and metastasis and altered lipid composition is increasingly recognized as a signature of cancer. Palmitic acid impairs carcinoma development by modulating membrane fluidity. Fatty acid 2-hydroxylase (FA2H) specifically introduces a chiral (R)-hydroxyl group at the second carbon and is highly expressed in stomach. However, potential involvement of this structural modification in gastric tumor growth is not clear.
Added value of this study
We describe here for the first time the aberrant suppression of a specific fatty acid hydroxylation pathway in advanced gastric cancers. We demonstrate its association with tumor growth, metastasis and overall survival of the patients, which is verified by analyses of multiple public data sets. This FA2H-mediated pathway plays an important role in regulating Hedgehog signaling and gastric tumor growth both in vitro and in vivo.
Implications of all the available evidence
Our results not only demonstrate the effect of FA2H on the Hedgehog signaling and growth of gastric cancer, but also emphasize the potential of its enzymatic product (R)-2-hydroxy fatty acids could be effective as nontoxic wide-spectrum drugs to improve chemosensitivity. Given that gastric cancers are usually diagnosed at an advanced or metastatic stage with resistance to chemotherapy, our results provide a promising approach to improve the clinical management and the outcomes of the patients.
Alt-text: Unlabelled Box
1. Introduction
Gastric cancer is one of the leading causes of cancer-related death since most gastric cancers are diagnosed at advanced or metastatic stages when the tumor is usually considered unresectable, and prognosis is poor, resulting in low survival rate [1]. Most patients presenting advanced gastric cancer are treated upfront with chemotherapy which has been shown to improve survival and quality of life [2]. However, cancer chemotherapy resistance often develops, limiting treatment efficiency [3]. Therefore, it is urgent to identify novel therapeutic targets for better treatments of gastric cancer and to establish new biomarkers useful for its early detection in high-risk populations.
Cancer cells commonly have characteristic changes in glucose and fatty acid (FA) metabolism to support cell proliferation and metastasis [4] and altered lipid composition is increasingly recognized as a signature of cancer [[5], [6], [7]]. Hydroxylated FAs (OHFAs) are FA derivatives that are naturally occurring in mammalian cell lipids. The OHFAs in mammalian cells can be provided by microorganisms or food intake [8], and are also generated by endogenous hydroxylases [9]. FA 2-hydroxylase (FA2H) specifically introduces a chiral (R)-hydroxyl group at the second carbon of long chain FAs [10]. During the course of our studies on alterations in FA modifications in regulating tumor growth, we identified the FA2H enzyme as novel regulator of gastric tumorigenesis. FA2H is highly expressed in brain, skin, colon and stomach [11,12], and is essential for the normal functioning of multiple organ systems [13]. In addition, it impacts differentiation of various cell types and has been shown to regulate membrane trafficking of nutrient transporters [13,14]. There is evidence to demonstrate that FA2H and racemic 2-OHFA improve sensitivity to Elisidepsin (PM02734), a synthetic plasma membrane-disrupting cyclodepsipeptide drug in cancer treatment in vitro and in vivo, presumably by regulating membrane drug interaction [15,16]. The resulting (R)-2-hydroxy FAs ((R)-2-OHFAs) are incorporated into sphingolipids that induce apoptosis at significantly lower concentrations as compared to non-OH counterparts in C6 glioma cells, suggesting that FA2H and (R)-2-OHFAs induce specific proapoptotic signaling although this remains little studied [17]. Moreover, FA2H silencing facilitates cell growth and inhibits cAMP-induced cell cycle exit and growth arrest in D6P2T Schwannoma cells [18], indicating diverse functions of FA2H in regulating signaling pathways as related to cell proliferation.
FA2H is highly expressed in stomach [12], but whether it plays any role in the development of gastric tumors remains unexplored. Hedgehog (Hh) signaling is critical in the development and homeostasis of many organs and tissues. Constitutive activation of the Hh pathway is intimately involved in the genesis and maintenance of gastric cancer and is associated with poor prognosis in gastrointestinal cancer patients [19]. Regulation of Hh signaling is complex in cancer cells and its molecular mechanisms have not been completely understood. The classical Hh signaling pathway involves translocation of a G protein-coupled receptor-like protein Smoothened (SMO) to the primary cilium, phosphorylation of suppressor of fused (SUFU) and translocation of the Gli zinc-finger transcription factors to the nucleus, where they induce expression of Hh target genes that control cell growth, survival, and differentiation [20]. Moreover, Gli activation by multiple non-canonical pathways including mTOR/S6K1 and MAPK has also been identified [21], which explains the disappointing results of SMO inhibitors in cancer treatment.
Involvement of the energy sensing mTOR in Hh signaling suggests that specific changes in nutrient utilization as regulated by FA2H may play a role in the development of gastric cancer. Herein, we examined the potential involvement of FA 2-hydroxylation in the documented carcinogenic Hh signaling pathway and its associated resistance to chemotherapy [22]. Our results demonstrated an important role of a specific FA modification pathway in gastric cancer development and revealed its potential usefulness in disease diagnosis and treatment.
2. Materials and methods
2.1. Collection of human tissue samples
Paired 117 human gastric cancer tissues and adjacent normal tissues were collected immediately after surgical resection in the Department of General Surgery of the First Affiliated Hospital of Soochow University (Suzhou, China) from 2008 to 2012. Twenty-three patients were treated with radical gastrectomy (RG), 66 with RG/XELOX, 21 with RG/TP and 7 with palliative surgery/TP. Written informed consent was obtained from all patients in this study, which was approved by the Biomedical Research Ethics Committee of Soochow University.
2.2. Gene expression data analysis
The raw data of the Affymetrix Human Genome U133 Plus 2 and U133A microarray in CEL format were downloaded from Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/). Three datasets with normal and cancer samples were accessed through GSE13911 [23], GSE29272 [24] and GSE79973 [25]. One genechip dataset for cancer samples with corresponding survival data was obtained from GSE62254 [26]. Self-developed R (version 3·4·1, http://cran.r-project.org/) scripts were used for the bioinformatics analysis. The “affy” package (version 1·50·0) in Bioconductor (https://bioconductor.org) was applied to pre-process Affymetrix arrays [27]. The Microarray Suite 5·0 (MAS5) algorithm was taken for the probe set expression summaries [28]. The implementations of background correction, between-array normalization, and probeset summarization were undertaken with the “mas5” function in the “aff” package. The “limma” package (version 3·28·21) in Bioconductor was utilized to determine significant changes in gene expression between cancer and normal samples [29,30]. Empirical Bayes methods for assessing differentially expressed genes were implemented by the “eBayes” function in the “limma” package. P values adjusted by the Benjamini-Hochberg correction were used to evaluate the significance level for differentially expressed genes. P value < .05 was considered as the significance threshold.
2.3. Survival analysis
R programs were developed to perform the sample classification and prognostic analysis. Patients were stratified into two subgroups according to the media value of the mRNA expression level or immunohistochemistry definition. The Kaplan-Meier survival plots were yielded, using the “survminer” package (version: 0·4·0, https://cran.r-project.org/web/packages/survminer/index.html). The P value generated from the log-rank test was applied to indicate the statistical significance of survival difference between different subgroups. P value < 0·05 was considered as the significance threshold.
2.4. Immunohistochemistry
Tissues were fixed with formalin, embedded in paraffin, cut into sections of 5 μm in thickness and stained by IHC as previously described [31]. Briefly, sections were affixed to slides and incubated with the polyclonal antibody recognizing human FA2H or human Gli1 at 1:200 dilution at room temperature for 2 or 3 h. The proteins were visualized using a tissue staining kit (Zhongshan Biotechnology, Beijing, China) and staining scores were evaluated using two blinded researchers. Five random regions were analyzed based on the percentage of cells stained positively per section with scoring criteria as follows: 0, 0–5%; 1, 6–25%; 2, 26–50%; 3, 51–75%; 4, >75%. The staining intensity was scored as: 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). The final score was calculated by the multiple of the intensity and extent score. A final score of 0 was considered as −; 1–4 as +; 5–8 as ++; 9–12 as +++. In our study, ++ or +++ was considered as positive expression, and – or + as negative. Antibodies used in this study are listed in supplementary Table 4.
2.5. Cell culture
Human gastric cancer cell lines MKN45, SGC7901, HGC27, MGC803, AGS and normal gastric cell line GES1 were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and grown in RPMI Medium 1640 (Hyclone) containing 10% FBS (Gibco), 100 units/ml penicillin G sodium, and 100 μg/ml streptomycin sulfate (Gibco) and cultured at 37 °C under 5% CO2.
2.6. Preparation of FA/BSA complexes
FFAs were dissolved in ethanol and precipitated with the addition of half molar equivalence of 1 M NaOH. Ethanol was then evaporated under nitrogen gas and reconstituted in water at 60 °C for 30 min to yield a final concentration of 21 mM. The FA emulsion was added dropwise to 2 volumes of 30% BSA in PBS pre-warmed at 37 °C. The FA/BSA (2:1) solution was gently mixed on a shaker for 1 h at room temperature and stored in multiple aliquots at −70 °C prior to experiments.
2.7. Plasmid and siRNA transfection
Transfection of a pcDNA-FLAG-h FA2H plasmid (provided by Dr. Hama Hiroko) was performed using Lipofectamine™ 2000 (Invitrogen). Transfection of siRNAs targeting human Gli1 or FA2H used Lipofectamine™ RNAiMax (Invitrogen) at a final concentration of 20 nM as described. The sequences specific for human Gli1 (5′-CUCCACAGGCAUACAGGAU-3′) and human FA2H (5′-GGCTAAAGAGAAGCAGTTT-3′) were selected based on their potency to inhibit the target gene expression. A scrambled siRNA was used as a negative control. Most experiments were performed at 3 days after transfection.
2.8. Protein extraction and Western blotting
Whole cell lysates were prepared with RIPA lysis buffer containing cocktails of protease and phosphatase inhibitors (Sigma). Proteins separated by SDS-PAGE were transferred to nitrocellulose membranes, and the membranes were blocked with 5% non-fat milk and probed with the indicated primary antibodies (1:500–1000 dilution). After incubation with horseradish peroxidase-conjugated secondary antibodies (1:5000 dilution), the proteins were visualized by chemiluminescence and signals were quantified by ImageJ software (version: 1·4·3) as previously described [32].
2.9. Cell viability assay
Cell viability was determined using an MTT assay kit (Amresco, USA). After treatments cells seeded in 96-well plateswere incubated with MTT solution-containing culture medium for 4 h and formation of the formazan product was measured at 490 nm in a microplate reader.
2.10. Cell wound healing assay
Confluent cells grown in six-well plates were scratched with sterile tips, washed with PBS and cultured in growing media. Cells were photographed at 0, 24, 48, 72 h and wound closure (%) was evaluated by the TScratch software (version: 1·0).
2.11. Cell migration assay
The ability of cell migration was evaluated with 24-well transwell plates (Corning Incorporated, USA). Briefly, cells were seeded into the upper chamber in serum-free RPMI 1640 and the lower chamber was filled with RPMI 1640 containing 10% FBS. 24 h later, cells that had migrated through the membrane were stained with 0·5% crystal violet and counted. Migration levels were quantified by counting the invaded cells in five random regions per sample.
2.12. Subcutaneous xenograft
SPF grade BALB/c nude mice (16-18 g, 3–5 weeks old, male) were purchased from Shanghai SLRC laboratory Animal Co., Ltd. (Shanghai, China). Nude mice were injected with 5 × 106 gastric cancer cells subcutaneously into the left and right dorsal flank, respectively. Seven days after injection, mice were randomly separated into different groups (n = 5 per group) on day 0. Then, the mice received cisplatin (3 mg/kg) on days 1, 8 and 15. 2-OHPA enantiomers (15 μmol/kg) were injected intraperitoneally on days 1, 4, 8, 11, 15 and 18. Body weight and tumor size were measured twice a week. At the endpoint, tumors were harvested, weighted and stored for further analysis. Quantity analysis of protein expression in xenograft tumors was conducted by Image-Pro Plus (version: 6·0). All animal experimental procedures were approved by the Animal Ethics Committee of Soochow University (Suzhou, China).
2.13. Statistical analysis
All experiments presented were repeated for at least three times and n indicate the number of biological replicates per treatment condition for in vitro experiments or the number of mice per treatment group for in vivo experiments. Results are expressed as means ±SEM and the student's t-test or ANOVA was used to compare means between two groups. Analysis of IHC results was performed by Chi-squared test or Fisher's exact test. A two-tailed P value < 0·05 was considered statistically significant.
3. Results
3.1. FA2H expression is lower in gastric cancer tissues than in surrounding normal tissues and is inversely associated with Gli1 expression
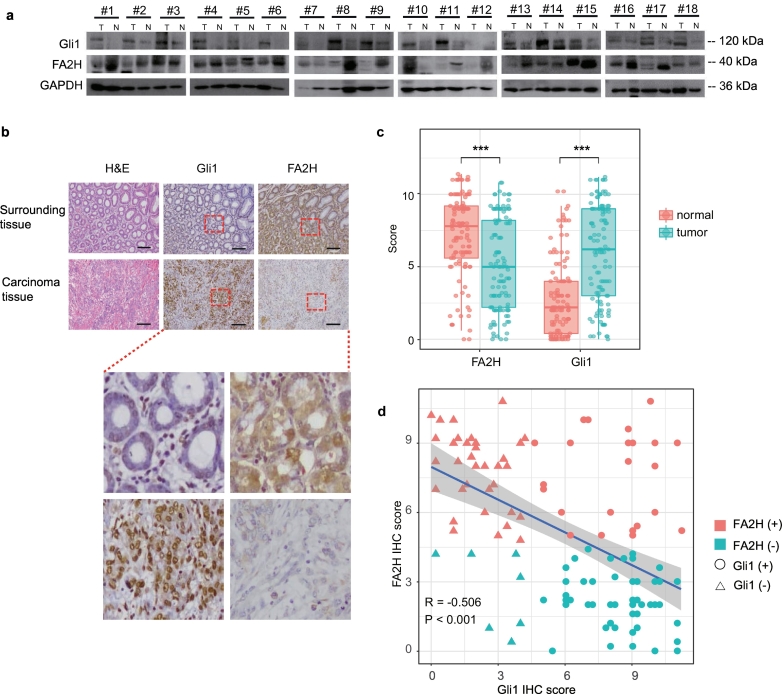
To investigate potential role of FA2H in the process of gastric carcinogenesis, we first compared the level of the FA2H and Gli1 protein in primary human gastric cancer and surrounding normal tissues. Consistent with previous reports [12] and the pilot immunohistochemistry (IHC) staining experiments in mouse stomachs (data not shown), our results demonstrated that FA2H protein was abundantly expressed in normal human gastric tissues, and its level was significantly lower in primary gastric cancer tumors (Fig. 1a, Supplementary Fig.1a, two sample t-test P = 0·0479). Conversely, level of the Hh signaling effecter Gli1 showed the opposite regulation that its overall expression level in cancer tissues was significantly higher (Fig. 1a, Supplementary Fig.1b, two sample t-test P = 0·0011). Moreover, IHC staining confirmed the low or absent expression of FA2H and high abundance of Gli1 (Fig. 1b). These differences were strengthened by statistical significance from the comparison between the 117 gastric cancer tissues and corresponding adjacent noncancerous tissues (Fig. 1c, Supplementary Table 1). These gastric cancer tissues showed significantly lower FA2H expression (one-way ANOVA P < 0·001) and higher Gli1 level (one-way ANOVA P < 0·001). These results imply not only a potential counterbalance with Gli1 expression, but also a probably involvement of FA2H in gastric carcinogenesis.
Fig. 1.
FA2H expression is significantly reduced in gastric cancer tissues.
a) Western blotting for Gli1 and FA2H proteins in gastric cancer tumors. Eighteen randomly selected pairs of gastric cancer tumors (T) and matched surrounding normal tissues (N) are presented.
b) IHC staining of Gli1 and FA2H in representative carcinoma and the surrounding tissue of gastric cancer (scale bar, 100 μm).
c) Column scatter plots showing the difference of Gli1 and FA2H expression between 117 human gastric cancer tumors and matched surrounding normal tissues. Statistical significance are indicated with asterisks, where ***, P < 0·001.
d) Scatter plot diagram showing a trend of the negative relationship between Gli1 and FA2H expression levels in 117 gastric cancer tissue samples.
To explore its potential involvement of prognostic significance, we examined the FA2H expression level in different pathological subtypes. Subgroup analysis for the 117 cancer tissues by LNM status (with or without LNM) evidenced a superior expression level of FA2H and an inferior level of Gli1 (Supplementary Fig. 1c-d, one-way ANOVA P < 0·001) in samples without LNM. When stratifying according to TNM stage (I-II or III-IV), the advanced stage (III-IV) tumors showed significantly lessening FA2H level and remarkably elevated Gli1 expression (Supplementary Fig. 1e-f, one-way ANOVA P < 0·001). Further molecular subtype classification of involved gastric tumors based on gene expression revealed that Gli1 was positively expressed in 24 out of 61 FA2H positive tumors, versus in 49 out of 56 FA2H negative tumors, indicating an apparently opposite trend in the expression of these two genes. The statistical significance revealed by the correlation analysis further validated this inverse association [Pearson correlation coefficient (R) = −0·506, P < .001, Fig. 1d]. Association analysis uncovered that having smaller tumor size (≤5 cm), shallower tumor invasion (T1–2), negative LNM status, negative venous invasion, or earlier TNM stage conferred a higher possibility of positive FA2H expression and negative Gli1 expression. Additionally, well-differentiated gastric cancers also conferred greater likelihood of positive FA2H expression (Supplementary Table 2), suggesting the prognostic potential of FA2H for gastric cancers.
3.2. Low FA2H expression is associated with a poor survival in gastric cancer
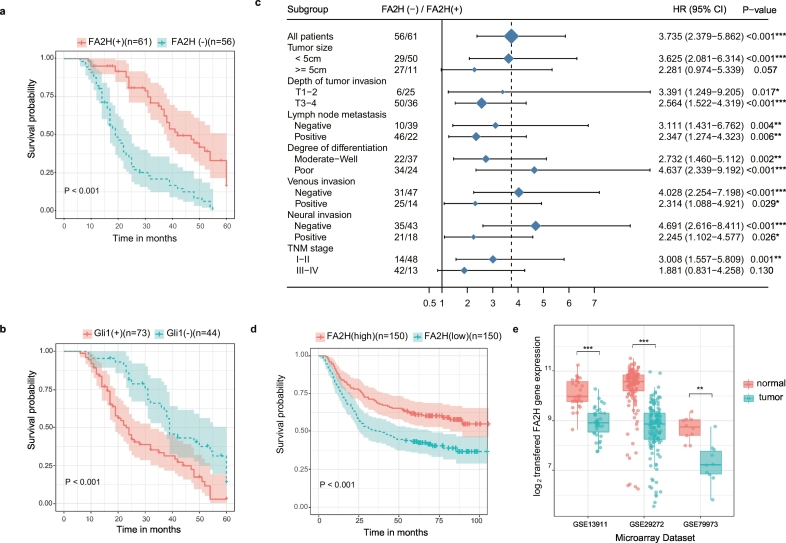
Given the observed significant difference of FA2H gene expression in comparison between different pathological conditions, we hypothesized the prognostic value of FA2H gene in gastric cancer. To test this, we assessed the prognostic impact of FA2H and Gli1 expression on overall survival (OS) in our gastric cancer cohort. Kaplan-Meier estimator showed low FA2H expression (Fig. 2a, log rank test P < 0·001) and high Gli1 level (Fig. 2b, log rank test P < 0·001) were associated with poor survival, which also coincides with the observed inverse association between FA2H and Gli1 expression (Fig. 1d). Moreover, univariate and multivariate Cox proportional hazards model analyses were also undertaken to screen prognostic factors with significant impact on OS of gastric cancers. Besides clinical factors including tumor size, depth of tumor invasion, lymph node metastasis, degree of differentiation, venous invasion, neural invasion, and TNM stage, univariate analysis also suggested that higher FA2H expression and lower Gli1 expression were both significant predictors for better OS of the 117 involved gastric cancers (P < 0·05, Supplementary Table 3). Further Cox's proportional hazard model analysis demonstrated that only the depth of tumor invasion, LNM status and FA2H expression were verified to be independent prognostic factors for the overall survival of involved gastric cancers (P < 0·05, Supplementary Table 3).
Fig. 2.
High expression of FA2H correlates with better survival of gastric cancer patients.
a, b) Kaplan-Meier curves for overall survival of 117 gastric cancer samples according to the expression of FA2H (a) or GLI1 (b). Cohort was stratified by the immunohistochemistry definition (− indicates negative, + indicates positive). The color-shaded areas around the estimated survival curves represent the 95% confidence bands.
c) Forest Plot of Hazard Ratios (HR) between low and high expression of FA2H by patient subgroups. Estimates with 95% confidence intervals (95% CI) for overall survival (OS) of 117 cases of gastric cancer were stratified according to different clinical factors. The area of the blue squares centred on the estimated HR for overall population and individual subgroups are proportional to their corresponding percentage weights. The vertical dashed line represents the HR for overall population, while the vertical solid line represents no effect (HR = 1).
d) Kaplan-Meier curve for overall survival of 300 gastric cancer samples from the public microarray dataset GSE62254 [26] according to the expression of FA2H. Cohort was stratified by the median value of FA2H relative expression (− indicates below median expression of FA2H, + above median). The color-shaded areas around the estimated survival curves represent the 95% confidence bands.
e) Column scatter plots validating the difference of FA2H expression between human gastric cancer tumors and normal tissues based on three public microarray datasets [GSE13911 [23], GSE29272 [24] and GSE79973 [25]].
*, P < 0·05; **, P < 0·01; ***, P < 0·001. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
To further define the prognostic effects of FA2H for OS of gastric cancer, the overall cohort was subgrouped to reassess the hazard and survival rates. The univariate HRs for OS according to the presence of FA2H in various subgroups showed statistical significance (Fig. 2c). FA2H expression status had significant prognostic relevance in patients in TNM earlier stage (P = 0·001) and those with smaller tumor size (P < 0·001). However, FA2H expression status did not significantly predict OS in the subgroup of gastric cancer patients in TNM III-IV stage (P = 0·130) or with larger size tumor (P = 0·057). Patients with negative FA2H expression had a significantly poorer survival than did those patients with positive expression, no matter LNM status (Supplementary Fig. 2a, without LNM: log rank test P = 0·002; Supplementary Fig. 2b, with LNM: log rank test P = 0·004). However, subgroup analysis by TNM stage revealed the prognostic value of FA2H expression level in TNM I-II stage (Supplementary Fig. 2c, log rank test P < 0·001), but not in TNM III-IV stage (Supplementary Fig. 2d, log rank test P = 0·110). Interestingly, significant inverse association between FA2H and Gli1 expression could be observed in subgroups without LNM (Supplementary Fig. 2e, P = 0·016), with LNM (Supplementary Fig. 2f, P = 0·006), and in TNM I-II stage (Supplementary Fig. 2 g, P = 0·005), but not in cohort in TNM III-IV stage (Supplementary Fig. 2 h, P = 0·078). These results evidenced the prognostic value of the FA2H gene for gastric cancer, especially for those in TNM early stage.
To support the hypothesis proposed above, public data sets were integrated for verification. The prognostic value of FA2H was validated by one published gene expression data set (GSE62254) with 300 gastric cancer samples (Fig. 2d, log rank test P < 0·001). On the other hand, the transcriptional expression levels of FA2H gene were also measured in three public gene expression profile data sets (Fig. 2e) of clinical gastric tumor specimens and noncancerous tissues. Consistent decrease of FA2H expression in gastric cancer samples showed statistical significance (GSE13911: P = 2·82e-10; GSE29272: P = 2·06e-29; GSE79973: P = 4·38e-3). Together, these data strengthened the evidence for the role of the FA2H gene as a prognostic signature in gastric cancer.
3.3. mTOR/S6K1 activates Gli1 expression in gastric cancer cells
Given the observed counterbalance between FA2H and Gli1 expression in clinical specimens, we measured the relative protein levels of Gli1 and FA2H in several human gastric cancer cell lines. The highest FA2H expression was detected in MKN45 cells, while the highest Gli1 expression was observed in SGC7901 (Supplementary Fig. 3 a-b). GES-1, a normal gastric cell line, had modest expression of FA2H and lower expression of Gli1 than gastric cancer cell lines. No significant correlation between FA2H and Gli1 levels was observed presumably due to low sample size (Pearson Correlation Test P = 0·272). Aberrant activation of Gli1 plays an important role in both gastrointestinal tract carcinogenesis and chemotherapy resistance [33,34]. To evaluate the carcinogenic effect of Gli1 gene, Gli1 gene was knockdown in SGC7901 cells with high Gli1 level (Supplementary Fig. 3c). Significantly deceased cell proliferation (Supplementary Fig. 3d) and inhibited migration (Supplementary Fig. 3 e-h) led by Gli knockdown were observed. Moreover, diminished Gli1 expression also enhanced cellular sensitivity to cisplatin treatment (Supplementary Fig. 3i). These data highlighted the significance of the Gli1 gene in both gastric carcinogenesis and chemosensitivity.
We then examined the crosstalk of the Akt/mTOR/S6K1 and Hh signaling pathways implicated in tumor development in MKN45 and SGC7901 cells. Our results showed that inhibition of Akt by A6730 (Supplementary Fig. 4 a-b) or mTOR by rapamycin (Supplementary Fig. 4 c-d) significantly decreases Gli1 level with no effect on SMO level in both cell lines, suggesting an important role of SMO-independent pathway in Gli1 activation in gastric cancer cells. In agreement with Gli1 contribution to cellular resistance to cisplatin, we observed that both the mTOR inhibitor rapamycin and the Gli1 inhibitor GANT61 increased cellular sensitivity to cisplatin and the combination of rapamycin and GANT61 had additive effect (Supplementary Fig. 4e).
3.4. FA2H and (R)-2-OHFA regulate the mTOR/S6K1/Gli1 pathway and cellular chemosensitivity
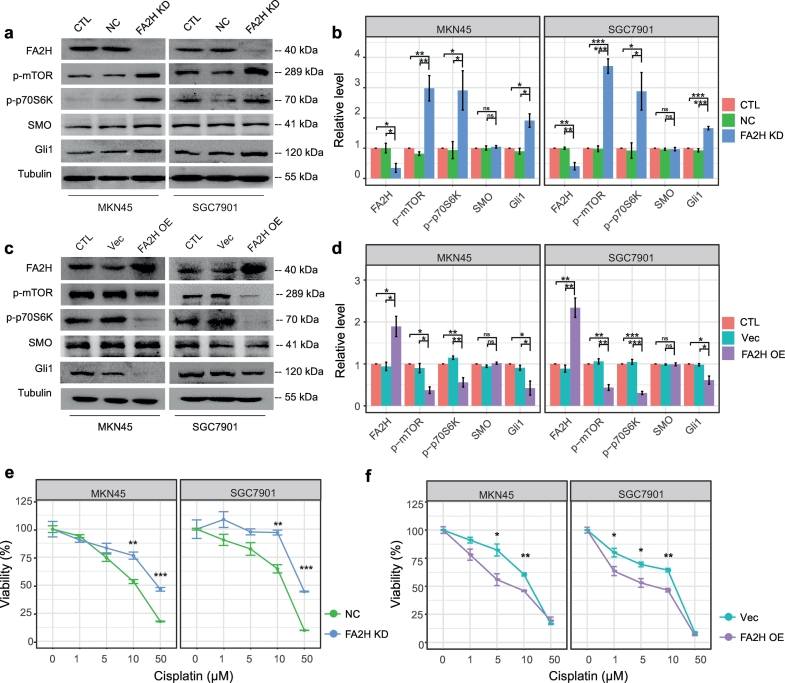
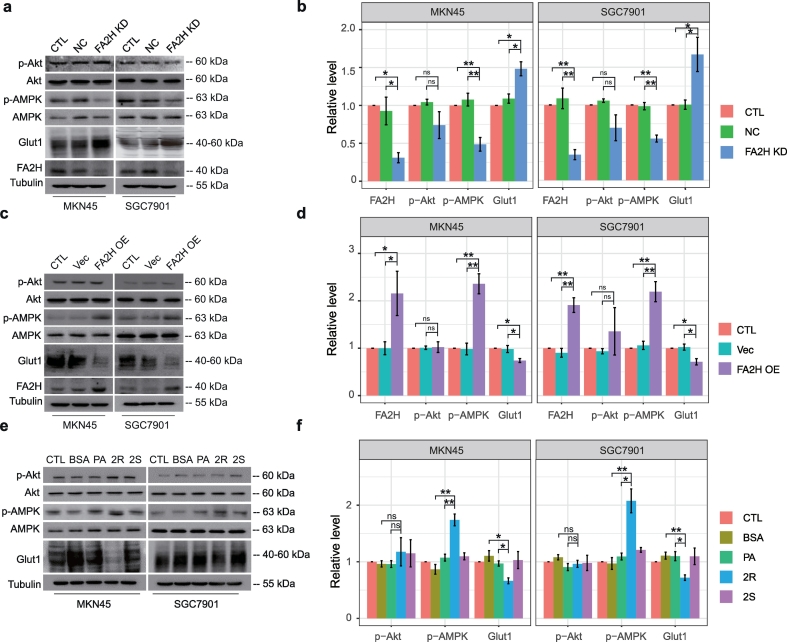
Given the observed counterbalance between FA2H and Gli1 expression, we hypothesized that FA2H may modulate cisplatin sensitivity through its regulation in the mTOR/S6K1/Gli1 pathway. FA2H knockdown in SGC7901 and MKN45 cells significantly enhanced the activation of mTOR/S6K1 and increased Gli1 level (Fig. 3 a-b) while FA2H over-expression suppressed mTOR/S6K1 signaling and Gli1 level (Fig. 3 c-d). Moreover, FA2H depletion decreased, while its over-expression enhanced cellular sensitivity to cisplatin (Fig. 3 e-f). These observations suggested that FA2H overexpression could increase chemosensitivity potentially through its inhibition of the mTOR/S6K1/Gli1 pathway.
Fig. 3.
FA 2-hydroxylation regulates Gli1 level and sensitivity to cisplatin.
a, b) MKN45 and SGC7901 cells were untreated (CTL) or transfected with negative control (NC) siRNA or siRNA against FA2H (FA2H KD). The whole cell lysates were prepared and subjected to Western blot analysis with antibodies directed against each specific protein as indicated (a). (b)The bands were quantified and presented as the mean ± SEM (n = 3).
c, d) MKN45 and SGC7901 cells were untreated or transfected with empty vector (Vec) or plasmid encoding hFA2H (FA2H OE). The whole cell lysates were prepared and subjected to Western blot analysis with antibodies directed against each specific protein as indicated (c). (d) The bands were quantified and presented as the mean ± SEM (n = 3).
e, f) MTT assay of SGC7901 and MKN45 cells in response to cisplatin. (e) Cells were transfected with negative control (NC) siRNA or siRNA against FA2H (FA2H KD). (f) Cells were transfected with empty vector (Vec) or plasmid encoding hFA2H (FA2H OE). Results presented as mean ± SEM (n = 3). *, P < 0·05; **, P < 0·01; ***, P < 0·001.
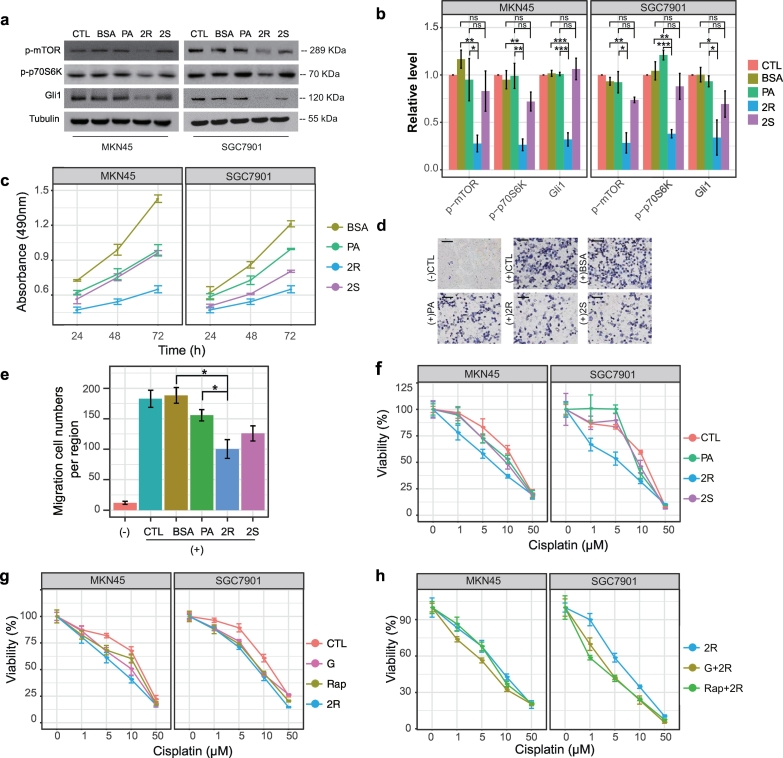
We next examined whether generation of (R)-2-OHFA mediates FA2H regulation of Gli1 level and chemosensitivity. Pre-treatment of SGC7901 and MKN45 cells with 50 μM (R)-2-OHPA for 24 h leads to decreased phosphorylation of mTOR and p70S6K and suppressed the expression of Gli1 protein (Fig. 4a-b). Treatment with palmitic acid (PA) which is not hydroxylated at the same concentration has no significant effect and the enantiomeric (S)-2-OHPA is much less or not effective. (R)-2-OHPA also effectively inhibited cell growth (Fig. 4c) and migration (Fig. 4d-e), and enhanced cellular sensitivity to cisplatin (Fig. 4f). Interestingly, the efficiency of (R)-2-OHPA is similar to the effect of GANT61 or rapamycin (Fig. 4g). Moreover, addition of (R)-2-OHPA further enhanced sensitivity to cisplatin in presence of GANT61 or rapamycin (Fig. 4h). These results showed that (R)-2-OHPA may be used as endogenous lipid surrogate for Hh signaling inhibitors to alleviate cellular resistance to chemotherapy.
Fig. 4.
(R)-2-OHPA increases sensitivity of gastric cancer cell lines to cisplatin.
a, b) SGC7901 cells were untreated (CTL) or treated with BSA, 50 μM PA, (R)-2-OHPA (2R), or (S)-2-OHPA (2S) for 24 h. (a) The whole cell lysates were prepared and subjected to Western blot analysis with antibodies directed against each specific protein as indicated in MKN45 and SGC7901cells. (b) The bands were quantified and presented as the mean ± SEM (n = 3).
c) MTT assay of MKN45 and SGC7901 cells treated with BSA, 50 μM PA, (R)-2-OHPA, or (S)-2-OHPA after incubation for 24 h, 48 h and 72 h, presented as mean ± SEM (n = 3). Statistical significance for MKN45 cell: BSA versus all other treatments showed P < 0·05 at least from 24 h onwards; 2R versus PA showed P < 0·05 at least from 24 h onwards; 2S versus PA showed no statistical difference from 24 h onwards. Statistical significance for SGC7901 cell: BSA versus all other treatments showed P < 0·05 at least from 48 h onwards; 2R versus PA showed P < 0·05 at least from 24 h onwards; 2S versus PA showed P < 0·05 at least from 48 h or 72 h, respectively.
d, e) Migration assay of SGC7901 cells treated with BSA, 50 μM PA, (R)-2-OHPA, or (S)-2-OHPA for 24 h. (d) Representative photographs are presented (Scale bar, 100 μm) and (e) the relative number of migratory cells were counted and presented as mean ± SEM (n = 5). DMEM and growing media were used as negative and positive controls, respectively.
f) MTT assay of MKN45 and SGC7901 cells pre-treated with 50 μM PA, (R)-2-OHPA (2R), or (S)-2-OHPA (2S) in response to cisplatin, presented as mean ± SEM (n = 3). Statistical significance for MKN45 cell: PA treatment or 2S treatment versus control showed no statistical difference from 1 μM onwards; 2R versus control showed P < 0·05 at least from 5 μM or 10 μM, respectively. Statistical significance for SGC7901 cell: PA treatment versus control showed P < 0·05 at least from 5 μM or 10 μM, respectively; 2R treatment versus control showed P < 0·05 at least from 1 μM to 10 μM; 2S treatment versus control showed no statistical difference from 1 μM onwards.
g) MTT assay of MKN45 and SGC7901 cells untreated (CTL) or pre-treated with 10 μm Gant61 (G), 1 μm Rapamycin (Rap), 50 μM (R)-2-OHPA (2R) in response to cisplatin, presented as mean ± SEM (n = 3). Statistical significance for MKN45 cell: G treatment versus control showed P < 0·05 at least from 5 μM; Rap treatment versus control showed P < 0·05 at least from 5 μM; 2R treatment versus control showed P < 0·05 at least from 5 μM onwards. Statistical significance for SGC7901 cell: G treatment versus control showed P < 0·05 at least from 5 μM or 10 μM, respectively; Rap treatment versus control showed P < 0·05 at least from 5 μM onwards; 2R treatment versus control showed P < 0·05 at least from 1 μM onwards.
h) MTT assay of MKN45 and SGC7901 cells pre-treated with 50 μM (R)-2-OHPA (2R), 50 μM (R)-2-OHPA in combination with 10 μM Gant61 (G + 2R) or 1 μM Rapamycin (Rap+2R) in response to cisplatin, presented as mean ± SEM (n = 3). Statistical significance for both MKN45 and SGC7901 cells: G + 2R treatment or Rap+2R treatment versus 2R treatment showed no statistical difference from 1 μM onwards.
*, P < 0·05; **, P < 0·01; ***, P < 0·001.
3.5. FA2H regulates AMPK activation in gastric cancer cell lines
We next explored potential mechanisms underlying FA2H regulation of mTOR/S6K1/Gli1 pathway. AMPK and Akt are important upstream regulators in mTOR activation in cancer [35]. We examined FA2H regulation of AMPK and Akt in gastric cancer cells. FA2H knockdown reduced AMPK phosphorylation in both SGC7901 and MKN45 cells (Fig. 5a-b), while FA2H overexpression enhanced it (Fig. 5c-d), suggesting FA2H induces AMPK activation, which leads to mTOR inhibition. Moreover, treatment of SGC7901 and MKN45 cells with (R)-2-OHPA has similar effect with FA2H overexpression in upregulating AMPK phosphorylation, while PA or (S)-2-OHPA had no significant effects (Fig. 5e-f). In contrast, Akt phosphorylation was not affected by either FA2H manipulation or (R)-2-OHPA treatment (Fig. 5a-f). FA2H or (R)-2-OHPA significantly reduces membrane fluidity [14], which suppresses expression of glucose transporters in Hep3B cells [5]. As anticipated, FA2H overexpression or treatment with (R)-2-OHPA in SGC7901 and MKN45 cells decreased GLUT1 level (Fig. 5c-e), while FA2H KD has the opposite effect (Fig. 5a). Collectively, our results indicate that FA2H regulates GLUT1 level and AMPK activation, mediating mTOR/S6K1/Gli1 pathway.
Fig. 5.
FA 2-hydroxylation regulates AMPK phosphorylation.
a, b) SGC7901 and MKN45 cells were untreated (CTL) or transfected with negative control (NC) siRNA or siRNA against FA2H (FA2H KD). (a) The whole cell lysates were prepared and subjected to Western blot analysis with antibodies directed against each specific protein as indicated. (b) The bands were quantified and presented as the mean ± SEM (n = 3).
c, d) SGC7901 and MKN45 cells were untreated (CTL) or transfected with empty vector (Vec) or plasmid encoding hFA2H (FA2H OE). (c) The whole cell lysates were prepared and subjected to Western blot analysis with antibodies directed against each specific protein as indicated. (d) The bands were quantified and presented as the mean ± SEM (n = 3).
e, f) SGC7901 and MKN45 cells were untreated (CTL) or pre-treated with BSA, 50 μM PA, (R)-2-OHPA (2R), or (S)-2-OHPA (2S). (e) The whole cell lysates were prepared and subjected to Western blot analysis with antibodies directed against each specific protein as indicated. (f) The bands were quantified and presented as the mean ± SEM (n = 3).
*, P < 0·05; **, P < 0·01, ns, not significant.
3.6. FA2H and (R)-2-OHPA inhibit gastric tumorigenesis in vivo
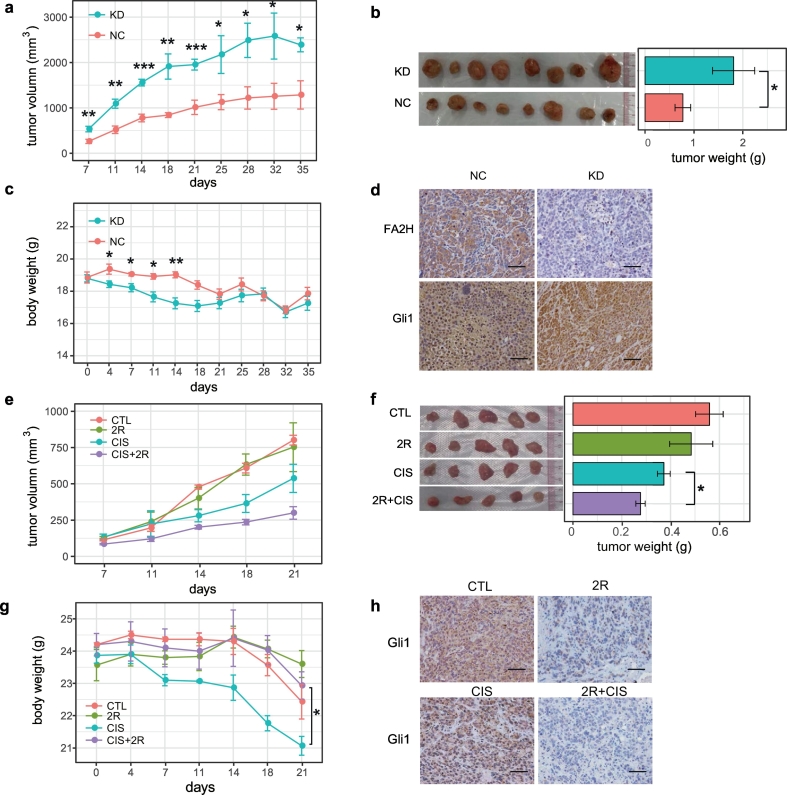
We then evaluated the role of FA2H protein and 2-OHFA in gastric cancer cell growth in an in vivo mice model with MKN45 and SGC7901 gastric cancer cells implanted onto the subcutaneous sites of nude mice. Depletion of FA2H in the implanted cancer cells greatly promoted tumor growth size and weight of tumors derived from FA2H knockdown cells were markedly increased as compared to control cells (Fig. 6a-b and Supplementary Fig. 5a-b). Nude mice implanted with FA2H depleted gastric cancer cells also had lower body weights as they grew larger tumors (Fig. 6c). Efficiency of FA2H knockdown was confirmed by IHC analysis in the tumors and Gli1 level was higher in tumors derived from the knockdown cells (Fig. 6d and Supplementary Fig. 5c). Since FA2H suppressed gastric tumor growth, we examined whether its enzymatic product, (R)-2-OHFA, can enhance the efficiency of cisplatin in inhibiting tumor growth and found that 5 mg/kg cisplatin was sufficient in inhibiting the size (Fig. 6e and Supplementary Fig. 5d) and the weight of the tumor (Fig. 6f and Supplementary Fig. 5e). A combinatory treatment using 15 μmol/kg (R)-2-OHPA substantiated the inhibitory effects of 5 mg/kg cisplatin. Importantly, treatment with 15 μmol/kg (R)-2-OHPA also alleviated cisplatin-induced body weight loss (Fig. 6g). IHC results revealed decreased Gli1 level in tumors treated with 15 μmol/kg (R)-2-OHPA (Fig. 6h and Supplementary Fig. 5f), suggesting involvement of Gli1 in tumor growth regulated by FA2H and (R)-2-OHPA.
Fig. 6.
FA2H knockdown promotes while (R)-2-OHPA inhibited in vivo tumorigenesis of MKN45 cells.
a-d) MKN45 cells stably expressing FA2H shRNA (KD) or control shRNA (NC) were transplanted into nude mice (n = 8). (a) The volumes of the tumors were measured twice a week during the indicated period. (b)The average tumor mass of each group was also presented. (c) The body weights were measured twice a week during the indicated period. (d) IHC staining of Gli1 and FA2H in representative tumors (Scale bar, 100 μm).
e-h) MKN45 cells were transplanted into nude mice (n = 5) which untreated (CTL), or received 15 μmol/kg (R)-2-OHPA, 5 mg/kg cisplatin (CIS), cisplatin combination with (R)-2-OHPA (CIS + 2R). (e) The volumes of the tumors were measured twice a week during the indicated period. (f) The average tumor mass of each group. (g) The body weights were measured twice a week during the indicated period. (h) IHC staining of Gli1 in representative tumors (Scale bar, 100 μm).
*P < 0·05, **P < 0·01, ***P < 0·001.
4. Discussion
Cancer cells are characterized with reprogrammed metabolism to support tumor growth and metabolic manipulation represents a promising therapeutic approach in cancer treatment [4]. In the current study, we identified the FA2H enzyme which is abundantly expressed in the stomach and its chiral specific enzymatic product (R)-2-OHPA as novel regulators of gastric tumor growth. FA2H levels are significantly lower in primary gastric cancer tumors as compared to surrounding normal tissues, which are confirmed in three public genechip data sets. Moreover, patients with high FA2H expression have better OS and the prognostic value of FA2H was validated by one published microarray data set (GSE62254) with 300 gastric cancer samples.
We demonstrated FA2H functions in cell culture as well as in vivo models by genetic manipulation and by pharmaceutical supplementation of (R)-2-OHPA. Our results suggest a crosstalk of Hh and a FA hydroxylation pathway in regulating sensitivity of gastric cancer cells to the chemotherapy agent cisplatin. Inhibition of Gli1 by FA 2-hydroxylation was evidenced by several observations. Overexpression of FA2H or treatment with (R)-2-OHPA diminished Gli1 in cultured gastric cancer cells and in tumors developed in nude mice. Moreover, a reverse association between the levels of Gli1 and FA2H was observed in human gastric tumors. The suppression of Gli1 by FA 2-hydroxylation is unlikely due to canonical Hh signaling since SMO level was not affected by FA2H or (R)-2-OHPA (Fig. 3). In contrast, SMO-independent Gli1 activation by mTOR/S6K1 pathway was inhibited. AMPK can phosphorylate TSC2 on S1387 or Raptor on S722 and S792, thereby promoting its inhibition of mTORC1 [36]. Activation of AMPK, presumably by increasing ADP level as previously described [37], was observed with FA2H overexpression or treatment of its product which might explain at least some of the observed inhibition of mTOR. Moreover, phosphorylation of Akt was not affected by FA2H or 2-OHPA, supporting that AMPK activation may be the major contributor to the observed mTOR inhibition. Fructose-1,6-bisphosphate and aldolase mediate glucose sensing by AMPK [38] and FA2H may regulate AMPK activation by diminishing GLUT1-mediated glucose utilization in cancer cells. We previously established that FA2H or (R)-2-OHPA significantly decreased membrane fluidity [14], which has been shown to limit glucose uptake and utilization in Hep3B cells [5]. In our current study, we showed that GLUT1 levels were decreased by FA2H overexpression or treatment of (R)-2-OHPA in gastric cancer cells, most likely due to decreased membrane fluidity [5]. Although the exact mechanism underlying FA2H activation of AMPK in gastric cancer cells as we observed is not clear and warrants future investigation, our results demonstrated that FA 2-hydroxylation regulates non-canonical Gli1 activation and may provide a rationale for its inclusion in combination therapy for gastric cancer.
The efficiency of (R)-2-OHPA treatment in enhancing cellular chemosensitivity to cisplatin is similar to that of Gli1 inhibitor GANT61 (Fig. 4g), suggesting that FA2H increased chemosensitivity partially through its inhibition of the mTOR/S6K1/Gli1 pathway. However, since (R)-2-OHPA and GANT61 have addictive effect, we cannot exclude the possibility that FA2H/(R)-2-OHPA may also regulate chemosensitivity via Gli1-independent pathway. A recent study demonstrated crucial roles of malic enzyme 1-mediated production of NADPH to promote gastric cancer growth and metastasis via suppression of ROS-induced cell apoptosis [39]. FA2H-regulated suppression of GLUT1 in gastric cancer cells may induce oxidative stress [40], which contributes to the observed inhibition of tumor growth. Alternatively, incorporation of 2-OHFAs into ceramides may alter ceramide regulation of electron transport chain and ROS generation [41,42]. However, whether this potential regulation of free radicals and the resulting lipid peroxides is involved in the (R)-2-OHFA-induced apoptosis requires extensive investigation in the future.
Our in vitro experiments showed similar effects of (R)-2-OHPA treatment and FA2H overexpression in regulating cell growth and migration (Fig. 3, Fig. 4). However, the effect of (R)-2-OHPA administration alone in tumor suppression or tumor regression in vivo was minimal, while depletion of FA2H significantly promoted tumor growth (Fig. 6 and S5). Although the mechanism for the observed difference is still not clear, these results suggested that the endogenous and exogenous (R)-2-OHPA may have distinct biological effects. (R)-2-OHPA generated by FA2H on the ER may have easier access to metabolic pathway which generates downstream lipid effectors with higher potency in inhibiting tumor growth. The metabolic fate of (R)-2-OHPA is still not completely understood, especially under in vivo conditions. Future studies on 2-OHFA metabolism would help identify more potent intermediate(s) in limiting tumor growth. Nevertheless, our in vivo study with two separate cell lines demonstrated that the (R)-2-OHPA was able to improve inhibitory effects of cisplatin on tumor growth.
Activation of Hh signaling pathway and the Gli1 expression contribute to cellular resistance to cisplatin through inhibition of platinum-DNA adduct repair and altered cellular accumulation of the drug [33,34]. Our present study also confirmed that Gli1 depletion improved cellular sensitivity to cisplatin in gastric cancer cells. FA2H overexpression or (R)-2-OHPA treatment inhibited Gli1 expression, resulting in enhanced cellular sensitivity to cisplatin, while FA2H knockdown has the opposite effects. Interestingly, inhibition of Gli1 by GANT61, mTOR by rapamycin and treatment with (R)-2-OHPA has similar efficiency in improving chemosensitivity with no effect on SMO level, suggesting that FA 2-hydroxylation may play an important role in cellular chemosensitivity via mTOR-regulated Hh signaling and Gli1 expression. Current ongoing clinical trials evaluating various Hh inhibitors for different cancers report many common adverse events including muscle spasms, alopecia, fatigue and weight loss [43], and rapamycin use causes significant metabolic impairments [44]. In contrast, treatment with (R)-2-OHPA improved cisplatin effectiveness at the same time that it alleviated cisplatin-induced body weight loss (Fig. 6f). Previous studies demonstrated that FA 2-hydroxylation in sebaceous glands is important for keratinocyte differentiation and fur development [45], suggesting that addition of (R)-2-OHFAs could alleviate notable chemotherapy associated side effects that were not tested here such as hair loss. Collectively, our results suggested that (R)-2-OHFA could be used as non toxic endogenous lipid surrogates for inhibiting mTOR and Hh signaling in combination therapy of gastric cancer.
2-OHFAs are constituents of sphingolipids and membrane lipids in plants [46,47] and animals and are abundant in microorganisms [48], animal wool waxes, skin lipids and some specialized tissues (e.g. brain) [13]. 2-OHFAs were also identified in food (e.g. dairy products and vegetable oils) with the (R) enantiomers being the predominant form in most samples [8]. All these biological sources for 2-OHFAs could be used to improve therapies treating gastric cancers. It is possible that consumption of food with high concentration of (R)-2-OHFAs could exert protection against gastric cancer risk. Interestingly, a meta-analysis of epidemiological studies showed significant inverse associations between total diary food consumption and gastric cancer risk in European and US cohorts, although not in the Asian population [49]. Prospective studies are required to confirm the association between the consumption of food enriched in (R)-2-OHFAs and the risk of cancers including gastric cancer. Recent study showed alteration of microbiota in gastric cancer with increased quantity of bacteria, diversified microbial communities, and enrichment of bacteria with potential cancer-promoting activities [50,51]. It would be interesting to investigate whether microorganism-originated 2-OHFAs in patients of gastric cancer may contribute to tumor growth and metastasis.
In summary, our results not only demonstrate the effect of FA 2-hydroxylation on the Hh signaling and growth of gastric cancer, but also emphasize the potential of (R)-2-OHFAs as a wide-spectrum anti-cancer drug. Moreover, foods enriched in 2-OHFAs may be protective against gastric cancer risk. Future clinical studies are needed to validate effectiveness of hydroxylated FA in treatment of gastric cancer and other cancer types, especially those where FA 2-hydroxylation activity is high.
Acknowledgments
Acknowledgements
This work was supported, in whole or in part, by Project of Nature Science Foundation (NSF) of China (31570806, 31620103906 and 81672348); National Science Foundation (NSF) of Jiangsu Province, China (BK20150006 and BK2016255); project funded by Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and by National Institutes of Health (NIH) Grants DK097608 and DK60022.
The sponsors of this study had no role in gathering, analyzing, interpreting the data, writing the manuscript, or decision to submit the manuscript for publication.
Declarations of interests
The authors declare that they have no competing interests.
Author contributions
Y.Y. and X.Y. conducted the research, analyzed the data, and wrote the manuscript. L.S., S.S., X.H., D.Z., T.L. and W.Z. contributed to data collection and analysis. N.A. contributed to data analysis and edited the manuscript. X.Z. and S.H. designed the study and edited the manuscript. X.S. designed the study and wrote the manuscript. Y.Y., X.Y. and X.S. are guarantors of this work and as such, had full access to all data and take responsibility for data integrity and accuracy of analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.01.066.
Contributor Information
Xinguo Zhu, Email: zxg45@hotmail.com.
Songbing He, Email: hesongbing1979@suda.edu.cn.
Xiong Su, Email: xsu@suda.edu.cn.
Appendix A. Supplementary data
Supplementary material
References
- 1.Van Cutsem E., Sagaert X., Topal B., Haustermans K., Prenen H. Gastric cancer. Lancet. 2016;388(10060):2654–2664. doi: 10.1016/S0140-6736(16)30354-3. [DOI] [PubMed] [Google Scholar]
- 2.Lordick F., Shitara K., Janjigian Y.Y. New agents on the horizon in gastric cancer. Ann. Oncol. 2017;28(8):1767–1775. doi: 10.1093/annonc/mdx051. [DOI] [PubMed] [Google Scholar]
- 3.Shi W.J., Gao J.B. Molecular mechanisms of chemoresistance in gastric cancer. World J. Gastroint. Oncol. 2016;8(9):673–681. doi: 10.4251/wjgo.v8.i9.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavlova N.N., Thompson C.B. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23(1):27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin L., Ding Y., Wang Y., Wang Z., Yin X., Yan G. Functional lipidomics: Palmitic acid impairs hepatocellular carcinoma development by modulating membrane fluidity and glucose metabolism. Hepatology. 2017;66(2):432–448. doi: 10.1002/hep.29033. [DOI] [PubMed] [Google Scholar]
- 6.Harayama T., Riezman H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018;19(5):281–296. doi: 10.1038/nrm.2017.138. [DOI] [PubMed] [Google Scholar]
- 7.Li J.J., Condello S., Thomes-Pepin J., Ma X.X., Xia Y., Hurley T.D. Lipid desaturation is a metabolic marker and therapeutic target of ovarian cancer stem cells. Cell Stem Cell. 2017;20(3):303–314. doi: 10.1016/j.stem.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenske R., Vetter W. Enantioselective analysis of 2-and 3-hydroxy fatty acids in food samples. J. Agric. Food Chem. 2008;56(24):11578–11583. doi: 10.1021/jf802772a. [DOI] [PubMed] [Google Scholar]
- 9.Hama H. Fatty acid 2-Hydroxylation in mammalian sphingolipid biology. Biochim. Biophys. Acta. 2010;1801(4):405–414. doi: 10.1016/j.bbalip.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo L., Zhang X., Zhou D., Okunade A.L., Su X. Stereospecificity of fatty acid 2-hydroxylase and differential functions of 2-hydroxy fatty acid enantiomers. J. Lipid Res. 2012;53(7):1327–1335. doi: 10.1194/jlr.M025742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alderson N.L., Rembiesa B.M., Walla M.D., Bielawska A., Bielawski J., Hama H. The human FA2H gene encodes a fatty acid 2-hydroxylase. J. Biol. Chem. 2004;279(47):48562–48568. doi: 10.1074/jbc.M406649200. [DOI] [PubMed] [Google Scholar]
- 12.Eckhardt M., Yaghootfam A., Fewou S.N., Zoller I., Gieselmann V. A mammalian fatty acid hydroxylase responsible for the formation of alpha-hydroxylated galactosylceramide in myelin. Biochem. J. 2005;388(Pt 1):245–254. doi: 10.1042/BJ20041451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kota V., Hama H. 2'-Hydroxy ceramide in membrane homeostasis and cell signaling. Adv. Biol. Regulat. 2014;54:223–230. doi: 10.1016/j.jbior.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo L., Zhou D., Pryse K.M., Okunade A.L., Su X. Fatty acid 2-hydroxylase mediates diffusional mobility of Raft-associated lipids, GLUT4 level, and lipogenesis in 3T3-L1 adipocytes. J. Biol. Chem. 2010;285(33):25438–25447. doi: 10.1074/jbc.M110.119933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrero A.B., Astudillo A.M., Balboa M.A., Cuevas C., Balsinde J., Moreno S. Levels of SCS7/FA2H-mediated fatty acid 2-hydroxylation determine the sensitivity of cells to antitumor PM02734. Cancer Res. 2008;68(23):9779–9787. doi: 10.1158/0008-5472.CAN-08-1981. [DOI] [PubMed] [Google Scholar]
- 16.Kiraly A., Varadi T., Hajdu T., Ruhl R., Galmarini C.M., Szollosi J. Hypoxia reduces the efficiency of elisidepsin by inhibiting hydroxylation and altering the structure of lipid rafts. Mar Drugs. 2013;11(12):4858–4875. doi: 10.3390/md11124858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kota V., Dhople V.M., Fullbright G., Smythe N.M., Szulc Z.M., Bielawska A. 2′-hydroxy C16-ceramide induces apoptosis-associated proteomic changes in C6 glioma cells. J. Proteome Res. 2013;12(10):4366–4375. doi: 10.1021/pr4003432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alderson N.L., Hama H. Fatty acid 2-hydroxylase regulates cAMP-induced cell cycle exit in D6P2T schwannoma cells. J. Lipid Res. 2009;50(6):1203–1208. doi: 10.1194/jlr.M800666-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akyala A.I., Peppelenbosch M.P. Gastric cancer and Hedgehog signaling pathway: emerging new paradigms. Genes Cancer. 2018;9(1–2):1–10. doi: 10.18632/genesandcancer.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng J.M., Curran T. The Hedgehog's tale: developing strategies for targeting cancer. Nat. Rev. Cancer. 2011;11(7):493–501. doi: 10.1038/nrc3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y., Ding Q., Yen C.J., Xia W., Izzo J.G., Lang J.Y. The crosstalk of mTOR/S6K1 and Hedgehog pathways. Cancer Cell. 2012;21(3):374–387. doi: 10.1016/j.ccr.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoon C., Park D.J., Schmidt B., Thomas N.J., Lee H.J., Kim T.S. CD44 expression denotes a subpopulation of gastric cancer cells in which hedgehog signaling promotes chemotherapy resistance. Clin. Cancer Res. 2014;20(15):3974–3988. doi: 10.1158/1078-0432.CCR-14-0011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.D'Errico M., de Rinaldis E., Blasi M.F., Viti V., Falchetti M., Calcagnile A. Genome-wide expression profile of sporadic gastric cancers with microsatellite instability. Eur. J. Cancer (Oxford, England : 1990) 2009;45(3):461–469. doi: 10.1016/j.ejca.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 24.Wang G., Hu N., Yang H.H., Wang L., Su H., Wang C. Comparison of global gene expression of gastric cardia and noncardia cancers from a high-risk population in China. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0063826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He J., Jin Y., Chen Y., Yao H.B., Xia Y.J., Ma Y.Y. Downregulation of ALDOB is associated with poor prognosis of patients with gastric cancer. OncoTargets Ther. 2016;9:6099–6109. doi: 10.2147/OTT.S110203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cristescu R., Lee J., Nebozhyn M., Kim K.M., Ting J.C., Wong S.S. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat. Med. 2015;21(5):449–456. doi: 10.1038/nm.3850. [DOI] [PubMed] [Google Scholar]
- 27.Gautier L., Cope L., Bolstad B.M., Irizarry R.A. Affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20(3):307–315. doi: 10.1093/bioinformatics/btg405. [DOI] [PubMed] [Google Scholar]
- 28.Hubbell E., Liu W.-M., Mei R. Robust estimators for expression analysis. Bioinformatics. 2002;18(12):1585–1592. doi: 10.1093/bioinformatics/18.12.1585. [DOI] [PubMed] [Google Scholar]
- 29.Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7) doi: 10.1093/nar/gkv007. (e47-e) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smyth G.K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 2004;3 doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 31.Thomas J.D., Zhang Y.J., Wei Y.H., Cho J.H., Morris L.E., Wang H.Y. Rab1A is an mTORC1 activator and a colorectal oncogene. Cancer Cell. 2014;26(5):754–769. doi: 10.1016/j.ccell.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y., Zhou D., Abumrad N.A., Su X. ADP-ribosylation factor 6 modulates adrenergic stimulated lipolysis in adipocytes. Am. J. Phys. Cell Phys. 2010;298(4):C921–C928. doi: 10.1152/ajpcell.00541.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kudo K., Gavin E., Das S., Amable L., Shevde L.A., Reed E. Inhibition of Gli1 results in altered c-Jun activation, inhibition of cisplatin-induced upregulation of ERCC1, XPD and XRCC1, and inhibition of platinum-DNA adduct repair. Oncogene. 2012;31(44):4718–4724. doi: 10.1038/onc.2011.610. [DOI] [PubMed] [Google Scholar]
- 34.Amable L., Fain J., Gavin E., Reed E. Gli1 contributes to cellular resistance to cisplatin through altered cellular accumulation of the drug. Oncol. Rep. 2014;32(2):469–474. doi: 10.3892/or.2014.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimobayashi M., Hall M.N. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat. Rev. Mol. Cell Biol. 2014;15(3):155–162. doi: 10.1038/nrm3757. [DOI] [PubMed] [Google Scholar]
- 36.Howell J.J., Hellberg K., Turner M., Talbott G., Kolar M.J., Ross D.S. Metformin inhibits hepatic mTORC1 signaling via dose-dependent mechanisms involving AMPK and the TSC complex. Cell Metab. 2017;25(2):463–471. doi: 10.1016/j.cmet.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei J.W., Shimazu J., Makinistoglu M.P., Maurizi A., Kajimura D., Zong H.H. Glucose uptake and Runx2 synergize to orchestrate osteoblast differentiation and bone formation. Cell. 2015;161(7):1576–1591. doi: 10.1016/j.cell.2015.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang C.S., Hawley S.A., Zong Y., Li M., Wang Z., Gray A. Fructose-1,6-bisphosphate and aldolase mediate glucose sensing by AMPK. Nature. 2017;548(7665):112–116. doi: 10.1038/nature23275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu Y.X., Ju H.Q., Liu Z.X., Chen D.L., Wang Y., Zhao Q. ME1 regulates NADPH homeostasis to promote gastric cancer growth and metastasis. Cancer Res. 2018;78(8):1972–1985. doi: 10.1158/0008-5472.CAN-17-3155. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez-Menendez P., Hevia D., Alonso-Arias R., Alvarez-Artime A., Rodriguez-Garcia A., Kinet S. GLUT1 protects prostate cancer cells from glucose deprivation-induced oxidative stress. Redox Biol. 2018;17:112–127. doi: 10.1016/j.redox.2018.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fucho R., Casals N., Serra D., Herrero L. Ceramides and mitochondrial fatty acid oxidation in obesity. FASEB J. 2017;31(4):1263–1272. doi: 10.1096/fj.201601156R. [DOI] [PubMed] [Google Scholar]
- 42.Kota V., Dhople V.M., Fullbright G., Smythe N.M., Szulc Z.M., Bielawska A. 2 '-Hydroxy C16-ceramide induces apoptosis-associated proteomic changes in C6 glioma cells. J. Proteome Res. 2013;12(10):4366–4375. doi: 10.1021/pr4003432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandhiya S., Melvin G., Kumar S.S., Dkhar S.A. The dawn of hedgehog inhibitors: Vismodegib. J. Pharmacol. Pharmacother. 2013;4(1):4–7. doi: 10.4103/0976-500X.107628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salmon A.B. About-face on the metabolic side effects of rapamycin. Oncotarget. 2015;6(5):2585–2586. doi: 10.18632/oncotarget.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maier H., Meixner M., Hartmann D., Sandhoff R., Wang-Eckhardt L., Zoller I. Normal fur development and sebum production depends on fatty acid 2-hydroxylase expression in sebaceous glands. J. Biol. Chem. 2011;286(29):25922–25934. doi: 10.1074/jbc.M111.231977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hitchcock C., Rose A. Stereochemistry of alpha-oxidation of fatty acids in plants - configuration of biosynthetic long-chain 2-hydroxy acids. Biochem. J. 1971;125(4):1155–1156. doi: 10.1042/bj1251155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bohn M., Heinz E., Luthje S. Lipid composition and fluidity of plasma membranes isolated from corn (Zea mays L.) roots. Arch. Biochem. Biophys. 2001;387(1):35–40. doi: 10.1006/abbi.2000.2224. [DOI] [PubMed] [Google Scholar]
- 48.Nurminen T., Suomalainen H. Occurrence of long-chain fatty acids and glycolipids in cell envelope fractions of bakers yeast. Biochem. J. 1971;125(4):963–969. doi: 10.1042/bj1250963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo Y.J., Shan Z.L., Ren H.Y., Chen W.H. Dairy consumption and gastric cancer risk: a meta-analysis of epidemiological studies. Nutr. Cancer. 2015;67(4):555–568. doi: 10.1080/01635581.2015.1019634. [DOI] [PubMed] [Google Scholar]
- 50.Yu G., Torres J., Hu N., Medrano-Guzman R., Herrera-Goepfert R., Humphrys M.S. Molecular characterization of the human stomach microbiota in gastric cancer patients. Front. Cell. Infect. Microbiol. 2017;7:302. doi: 10.3389/fcimb.2017.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang L., Zhou J., Xin Y., Geng C., Tian Z., Yu X. Bacterial overgrowth and diversification of microbiota in gastric cancer. Eur. J. Gastroenterol. Hepatol. 2016;28(3):261–266. doi: 10.1097/MEG.0000000000000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material