Abstract
Background
We and others have shown that dipeptidyl peptidase-IV (DPP4) expression is increased in obesity/atherosclerosis and is positively correlated with atherosclerotic burden. However, the mechanism by which DPP4 expression is regulated in obesity remains unclear. In this study, we investigated the pathways regulating the expression of DPP4 on macrophages.
Methods
Flowsight® Imaging Flow Cytometry was employed for the detection of DPP4 and immunophenotyping. DPP4 enzymatic activity was measured by a DPPIV-Glo™ Protease Assay kit.
Findings
Human monocytes expressed a moderate level of membrane-bound DPP4. Obese patients with body mass index (BMI) ≥ 30 had a higher level of monocyte DPP4 expression, in parallel with higher levels of HOMA-IR, blood glucose, triglycerides, and non-HDL cholesterol, compared to those in the non-obese (BMI < 30) patients. Oxidized low-density lipoprotein (oxLDL), but not native LDL, up-regulated DPP4 expression on macrophages with a preferential increase in CD36+ cells. OxLDL mediated DPP4 up-regulation was considerably diminished by Toll-like receptor-4 (TLR4) knockdown and CD36 deficiency. TRIF deficiency, but not MyD88 deficiency, attenuated oxLDL-induced DPP4 increase.
Interpretation
Our study suggests a key role for oxLDL and downstream CD36/TLR4/TRIF in regulating DPP4 expression. Increased DPP4 in response to oxidized lipids may represent an integrated mechanism linking post-prandial glucose metabolism to lipoprotein abnormality-potentiated atherosclerosis.
Keywords: Dipeptidyl peptidase IV, Inflammation, Obesity, Atherosclerosis, Oxidized LDL
Research in context.
Evidence before this study
The expression of DPP4 has been linked to a number of disease conditions including diabetes and cardiovascular disease. We and others have reported that DPP4 expression were increased in patients with obesity and atherosclerosis. The levels of DPP4 are associated with degree of insulin resistance and plasma lipids. However, the regulation of DPP4 expression is not well understood.
Added value of this study
In the current study, we identified that CD36/TLR4/TRIF inflammatory pathways are responsible for the upregulation of monocyte/macrophage DPP4 in obesity/atherosclerosis. These findings demonstrated an important role for oxidized lipids in immune cell DPP4 regulation and may provide an integrated mechanism linking abnormal post-prandial glucose metabolism with oxidized lipids and inflammation.
Implications of all the available evidence
In this study, we also found that catalytic inhibition of DPP4 increases DPP4 expression on macrophages. Given the pro-inflammatory role of DPP4, this may provide an explanation for the neutral effect of DPP4 inhibition on cardiovascular outcomes seen in clinical trials. Modulation of DPP4 expression by targeting its regulatory pathways may represent a novel strategy for reducing DPP4-induced inflammation.
Alt-text: Unlabelled Box
1. Introduction
Dipeptidyl peptidase-IV (DPP4) is a single-pass type II transmembrane glycoprotein that is best known for its role as a catalytic inactivator of the incretin hormones glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP) [1,2]. In addition to its catalytic function, DPP4 interacts with a number of ligands such as adenosine deaminase (ADA), caveolin-1, Middle East respiratory syndrome coronavirus (MERS-CoV) spike protein, fibronectin, and is thought to play an important role as a mediator of inflammation [[3], [4], [5], [6]].
In addition to its cell surface membrane-bound form, DPP4 also exists as a cleaved catalytically active extracellular domain circulating in plasma [7]. DPP4 is highly expressed on T cells, monocytes and antigen-presenting cells in adipose tissue and associated with T cell-mediated inflammation [8,9]. In addition, DPP4 is increased in obese patients with expression positively correlating to the degree of insulin resistance [8,9]. Membrane bound DPP4 on hematopoietic and adipocytes may contribute to a substantial proportion of plasma DPP4 [9,10], implicating that DPP4 on these cell types acts as an important regulator of incretin action. Studies by our team and other groups have suggested an important role for immune cell-expressing DPP4 in diabetes and cardiovascular disease [8,[11], [12], [13]]. However, the mechanisms and mediators of DPP4 expression on these cells are poorly understood. Given the central role of monocyte/macrophage in atherosclerosis, we recently reported that the expression of DPP4 on monocytes and plasma DPP4 activity were increased in patients with atherosclerosis and positively correlated with atherosclerotic plaque volume [14]. In addition, the DPP4 expression level on monocytes was also associated with plasma levels of non-HDL cholesterol and triglycerides [14]. However, the mechanisms by which DPP4 is up-regulated in cardiometabolic disease remain elucidated. In this study, we found that treatment of oxidized low-density lipoprotein (oxLDL), but not native LDL, elevated DPP4 expression on macrophages, which was attenuated by the inhibition of Toll-like receptor 4 (TLR4) or downstream Toll/IL-1R domain-containing adaptor-inducing IFN-β (TRIF). DPP4 expression almost exclusively occurred on CD36+ monocytes and CD36 deficient macrophages showed an attenuated up-regulation of DPP4 in response to oxLDL. For the first time to our knowledge, these findings demonstrated an important role for oxidized lipids in immune cell DPP4 regulation and may provide an integrated mechanism linking abnormal post-prandial glucose metabolism with oxidized lipids and inflammation.
2. Materials and methods
2.1. Participants and ethical approval
All procedures of this study were approved by the Institutional Review Board (IRB) at The Ohio State University and Case Western Reserve University. A written informed consent was obtained from all the participants before the study. A total of 27 patients with previously documented cardiovascular disease enrolled in the ALPINE trial were included in this study. The ALPINE, a phase 4 clinical trial (ClinicalTrials.gov Identifier: NCT01417104), has been described elsewhere [15]. Patients with established cardiovascular disease were recruited in Columbus, Ohio from 2009 to 2011 and randomized to receive either placebo or aliskiren treatment. Cardiovascular disease was defined as at least 1 of the following: myocardial infarction, cerebrovascular accident, previous coronary artery bypass graft surgery, and/or percutaneous coronary intervention or peripheral arterial disease (ankle-brachial index <0.9 and/or prior peripheral intervention or surgery). HOMA-IR was calculated as follows: HOMA-IR = Fasting Serum Glucose (mg/dL) × Fasting Plasma insulin (μU/mL) / 405. Patients with one or more of the following conditions were excluded from the study: history of malignancy, diagnosis of type 1 diabetes or use of hypoglycemic drugs, uncontrolled hypertension (>145/90 mmHg); renal insufficiency defined as glomerular filtration rate < 40 mL/min (derived with the Modified Diet in Renal Disease equation); unstable cardiac syndromes. Data from atherosclerotic patients within the placebo group were re-analyzed. Fourteen age- and race-matched healthy volunteers without cardiovascular disease as defined above were served as controls.
Biological markers including fasting blood glucose, fasting insulin glycosylated hemoglobin (HbA1c), leptin, adiponectin, total cholesterol, HDL cholesterol (HDL—C), LDL cholesterol (LDL-C), triglycerides, systolic blood pressure (SBP), diastolic blood pressure (SBP), standing heart rate (HR), body mass index (BMI), blood urea nitrogen, creatinine, creatinine kinase, uric acid, albumin, total protein, alkaline phosphatase, sodium, potassium, and chloride were examined in clinical laboratories.
2.2. Induction of human monocyte-derived macrophages (MDMs)
Peripheral blood mononuclear cells (PBMCs) were isolated from human peripheral blood through density gradient centrifugation using Ficoll. In brief, blood collected into EDTA-coated tubes was diluted 1:1 with sterile PBS and layered on Ficoll-Paque Plus (GE Healthcare, Piscataway, NJ), followed by centrifugation for 30 min at 500g without applying a brake. The PBMCs in the interface were carefully removed and washed twice with PBS. PBMCs were then placed in a 6-well plate for 2 h, and then adherent cells (monocytes) were cultured in RPMI-1640 medium supplemented with 10% FBS and 10 ng/mL recombinant human macrophage colony-stimulating factor (R&D, Minneapolis, MN) for 4 days. Media were replaced once at day 2.
2.3. DPP4 enzymatic activity measurement
Human plasma was isolated from EDTA anticoagulated peripheral blood by centrifugation at 1500 g for 15 min. The enzymatic activity of DPP4 in the plasma was measured using a DPPIV-Glo™ Protease Assay kit from Promega (Madison, WI) following the manufacturer's instruction.
2.4. Animals and reagents
C57BL/6, MyD88−/−, TRIF−/−, and CD36−/− mice were purchased from Jackson Laboratory. All procedures were approved by the IACUC committee at the Case Western Reserve University.
The antibodies used for flow cytometry were purchased from the following companies: anti-human DPP4 (clone # 2A6 [PE-labeled], purchased from eBioscience, San Diego, CA; Clone # BA5b [APC-labeled], purchased from Biolegend, San Diego, CA), PE-labeled anti-mouse DPP4 (Clone # 155202, R&D system, Minneapolis, MN), anti-human CD36 (clone # 5–271 [PE- or APC-labeled], Biolegend, San Diego, CA), APC-labeled anti-mouse CD36 (clone # 72–1, eBioscience, San Diego, CA), PE/Cy5-labeled anti-human/mouse CD11b (Clone # M1/70, Biolegend, San Diego, CA), FITC-labeled anti-human CD3 (Clone # OKT3, eBioscience, San Diego, CA), anti-human CD45 (Clone # Hi30, eBioscience, San Diego, CA), and anti-human CD4 (Clone # OKT4, eBioscience, San Diego, CA). Oxidized LDL was purchased from Thermo Fisher Scientific (Cat. # AAJ652618PL, Thermo Fisher Scientific, Waltham, MA). DPP4 enzymatic inhibitor (DPP4i) Linagliptin was a kind gift from Boehringer Ingelheim (Ingelheim am Rhein, Germany).
2.5. Induction of bone marrow-derived macrophages (BMMs)
To obtain bone marrow-derived macrophages (BMMs), bone marrow cells isolated from mouse tibia and femur were cultured in RPMI-1640 with 10% FBS and 10 ng/mL recombinant mouse M-CSF (R&D Systems, Minneapolis, MN) for 5 days. Media was replaced every 2 days. Adherent BMMs were used for experiments at day 5.
2.6. Flow cytometry
All antibodies used in imaging flow cytometry were purchased from BioLegend (San Diego, CA), BD (San Jose, CA), or R&D Systems (Minneapolis, MN). Cells were stained with the indicated antibodies as described elsewhere [8] and then analyzed on either a FlowSight® imaging cytometer (Amnis, Seattle, WA) or a LSR-II flow cytometer (BD, San Jose, CA).
2.7. Statistical analysis
All data in this study is presented as mean ± standard error of the mean (SE). A P value of <0.05 was considered statistically significant. GraphPad Prism 5 was used for statistical analysis using student's t-test, or ANOVA analysis with Boneferroni's post hoc test, or linear regression where appropriate. Patients with missing data were excluded from analysis.
3. Results
3.1. DPP4 expression on immune cells and the potential contribution of immune cells to systemic DPP4 activity
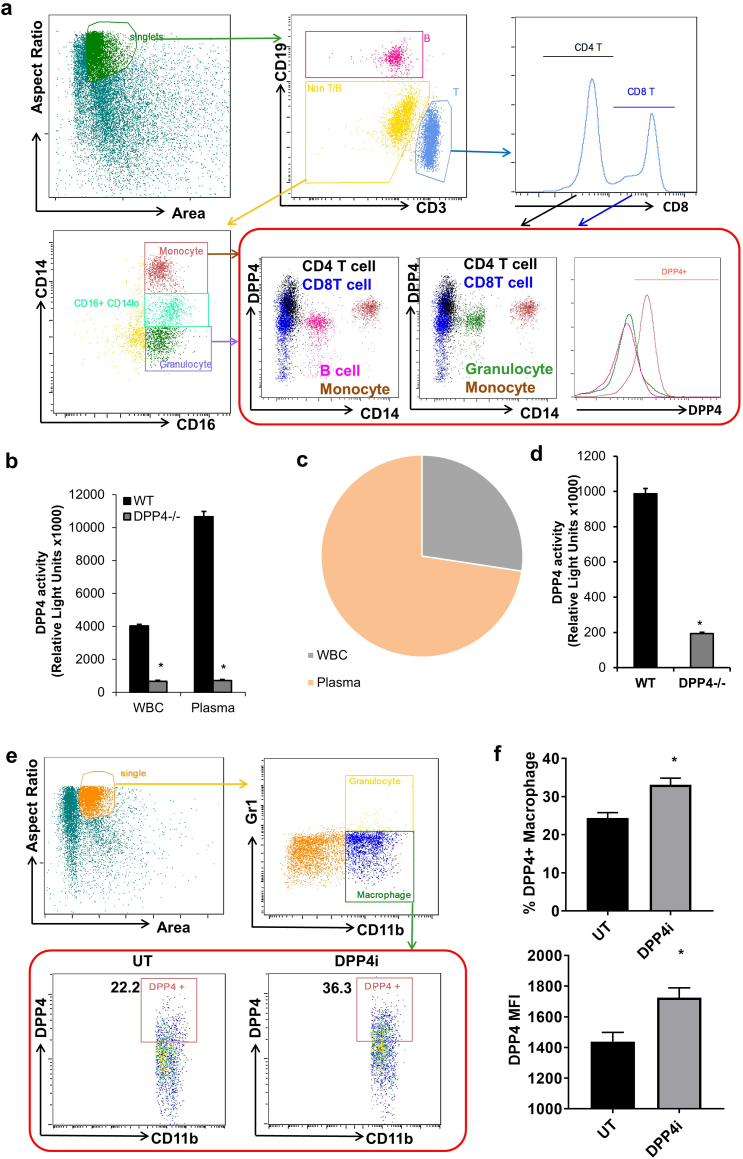
DPP4, an important regulator of the incretin-insulin axis, is expressed by a number of immune cells [2]. While the role of global DPP4 in diabetes and cardiovascular disease has been well studied, the function of immune cell-derived DPP4 in cardiometabolic disease remains elucidated. To determine the role of immune cell-expressing DPP4 in cardiovascular disease, we first examined the expression of DPP4 on circulating immune cells using Flowsight® imaging flow cytometry. Results indicate that monocytes and T cells are the major populations expressing DPP4 in the human peripheral blood, while the majority of granulocytes and B cells did not express DPP4 (Fig. 1a). In addition to membrane-bound DPP4, DPP4 can also be cleaved from the cell membrane and present as a soluble form in the plasma [2]. We next detected the enzymatic activity of membrane-bound DPP4 on the white blood cells and soluble DPP4 in the plasma. As a result, leukocytes contributed considerably to the enzymatic activity of DPP4 in the circulation (Figs. 1b & 1c). To examine if immune cells also contribute to the generation of soluble DPP4 in the plasma, DPP4 knockout mice were irradiated and transplanted with wild-type (WT) or DPP4−/− bone marrow. Plasma was isolated for the detection of DPP4 activity after 12 weeks. As depicted in Fig. 1d, mice with WT bone marrow showed a significantly higher level of plasma DPP4 activity compared to those with DPP4−/− bone marrow, suggesting that immune cells contribute to the generation of soluble DPP4. In addition, enzymatic inhibition of DPP4 inhibitor (DPP4i) for 4 weeks increased the expression of DPP4 on macrophages (Figs. 1e & 1f), suggesting that there may be a negative feedback loop to control DPP4 expression and enzymatic activity.
Fig. 1.
DPP4 expression and enzymatic activity contributed by immune cells.
A, DPP4 expression on circulating immune cells. Peripheral blood mononuclear cells (PBMCs) were isolated from healthy volunteers and CD14 + CD16+ monocyte, CD16 + CD14-granulocyte, CD19+ B lymphocyte, CD3 + CD4+ T lymphocyte and CD3 + CD4- T lymphocyte were gated for the detection of DPP4 using an imaging flow cytometer. B & C, White blood cells (WBC) and plasma were isolated from wild-type (WT) and DPP4−/− mice for the detection of DPP4 enzymatic activity. WBC and plasma DPP4 activity (B) and blood DPP4 activity composition in the WT mouse (C) are shown. D, Irradiated DPP4−/− mice were transplanted with WT or DPP4−/− bone marrow cells. Plasma were then isolated for the detection of DPP4 enzymatic activity after 12 weeks. E & F, WT C57BL/6 mice (n = 5–6/group) were treated with 5 mg/kg body weight (BW)/day linagliptin (DPP4i) in drinking water for 4 weeks and then subjected to flow cytometric detection of DPP4 expression. Representative dot plot (E) and statistical analyses (F; upper panel: % of DPP4+ macrophages; lower panel: DPP4mean fluorescence intensity) are shown. *, p < .05.
3.2. DPP4 increased in patients with atherosclerosis
Monocytes and macrophages are central effectors of innate immunity and are now recognized as key pathophysiologic players in the development of various chronic conditions such as atherosclerosis [16,17]. Our recent study indicates that DPP4 expression on circulating monocytes was increased in atherosclerosis and was associated with atherosclerotic burden [14]. Since DPP4 up-regulation has been associated with obesity [8,9,11], we divided patients into two groups: obese (BMI ≥ 30) and non-obese (BMI < 30). The biological characteristics and markers are shown in Table 1. There were no significant differences in age, SBP, DBP, HR, and biochemical parameters such as aspartate transaminase (AST), alanine transaminase (ALT), creatinine, creatine kinase, alkaline phosphatase, albumin, sodium, potassium, chloride, HDL—C, blood urea nitrogen, and total protein. The levels of HOMA-IR, non-HDL cholesterol, uric acid, triglycerides, fasting blood glucose, fasting insulin, and leptin were significantly increased, while adiponectin levels were lower in obese patients with atherosclerosis (Table 1).
Table 1.
Biological characteristics of obese and non-obese atherosclerotic patients.
| Characteristics | Non-obese (N = 15) |
Obese (N = 12) |
P | ||
|---|---|---|---|---|---|
| Mean | SE | Mean | SE | ||
| BMI (kg/m2) | 26.8 | 0.5 | 34.8 | 0.8 | 6.2949E-09 |
| Age (years) | 63.0 | 2.6 | 60.4 | 1.8 | 0.1826 |
| HOMA-IR | 3.4 | 0.3 | 14.4 | 4.2 | 0.0058 |
| Blood urea nitrogen (mg/dL) | 14.0 | 1.0 | 14.2 | 3.0 | 0.9543 |
| Creatinine (mg/dL) | 1.546 | 0.536 | 0.993 | 0.078 | 0.3699 |
| ALT (Unit/L) | 29.27 | 2.98 | 25.75 | 2.80 | 0.4076 |
| AST (Unit/L) | 28.47 | 2.04 | 26.25 | 2.05 | 0.4553 |
| Total cholesterol (mg/dL) | 151.7 | 6.6 | 171.8 | 7.6 | 0.0571 |
| non-HDL cholesterol (mg/dL) | 104. 2 | 8.2 | 130.7 | 5.9 | 0.0271 |
| HDL cholesterol (mg/dL) | 44.3 | 3. 2 | 38.9 | 2.3 | 0.2000 |
| Creatine Kinase (Unit/L) | 167.4 | 24.9 | 145.2 | 20.2 | 0.5091 |
| Alkaline phosphatase (IU/L) | 66.6 | 4.9 | 65.3 | 5.1 | 0.8600 |
| Sodium (mEq/L) | 139.0 | 0.5 | 138.9 | 0.4 | 0.9045 |
| Potassium (mEq/L) | 4.45 | 0.08 | 4.21 | 0.12 | 0.0952 |
| Chloride (mEq/L) | 104.2 | 0.7 | 104.3 | 0.7 | 0.8957 |
| Total protein (g/dL) | 6.83 | 0.07 | 6.98 | 0.12 | 0.2659 |
| Albumin (g/dL) | 4.06 | 0.07 | 3.93 | 0.07 | 0.2217 |
| Uric acid (mg/dL) | 6.11 | 0.34 | 7.58 | 0.56 | 0.0261 |
| Triglycerides (mg/dL) | 95.5 | 11.1 | 165.6 | 25.1 | 0.0110 |
| Glucose (mg/dL) | 99.9 | 3.0 | 120. 1 | 9.3 | 0.0285 |
| Insulin (μU/mL) | 15.5 | 1.4 | 47. 5 | 12.5 | 0.0039 |
| Leptin (ng/mL) | 9.38 | 1.98 | 34.16 | 8.70 | 0.0067 |
| Adiponectin (ng/mL) | 5990.4 | 884.1 | 3198.9 | 348.8 | 0.0162 |
| Standing HR (beats/min) | 63.9 | 2.5 | 68.2 | 2.9 | 0.2631 |
| SBP (mmHg) | 128.3 | 3.5 | 124. 7 | 4.4 | 0.5216 |
| DBP (mmHg) | 79.9 | 1.7 | 81.3 | 2.6 | 0.6321 |
| Monocyte DPP4 MFI | 4836.5 | 223.4 | 5564.7 | 263.1 | 0.0318 |
| Plasma DPP4 activity (relative light unit) | 207,872 | 2558 | 220,505 | 4938 | 0.0229 |
Bold numbers in P value column indicate statistical significance (p < .05).
3.3. Oxidized low-density lipoprotein (oxLDL) increased DPP4 expression
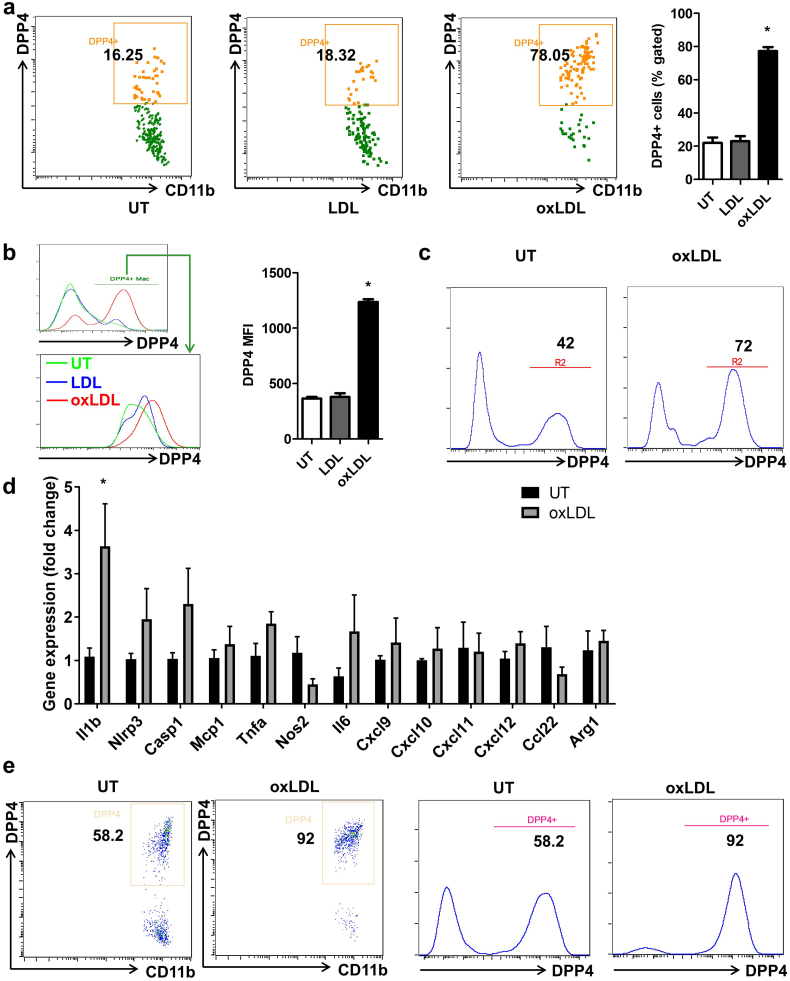
Given that oxidatively modified cholesterol is fundamental to the pathogenesis of atherosclerosis and is widely believed to represent a plausible mechanism of accelerated atherosclerosis in insulin resistance/type 2 diabetes, we tested the effect of LDL (native or oxidized) on the regulation of DPP4 expression. After 24-h treatment with 25 μg/mL lipoprotein of either native LDL or oxLDL, DPP4 expression on bone marrow derived macrophages (BMMs) from C57BL/6 mice was detected by flow cytometry. Results showed that 24-h treatment with native LDL did not affect DPP4 expression on macrophages. In contrast, oxLDL significantly increased the frequency of DPP4+ macrophages (22.1 ± 3.2 vs. 23.0 ± 3.0 vs. 77.3 ± 2.4 for untreated vs. native LDL vs. oxLDL respectively, Fig. 2a). In addition to the percentage change, DPP4 expression increased in DPP4+ macrophages as evidenced by the fact that the MFI of DPP4+ cells was higher in oxLDL-treated group (364.2 ± 15.6 vs. 379.3 ± 34.3 vs. 1236.9 ± 23.2 for untreated vs. native LDL vs. oxLDL respectively, Fig. 2b). Dose response experiments revealed that oxLDL dose-dependently enhanced DPP4 expression on macrophages (Supplemental Fig. 1). Similar findings were observed in peritoneal macrophages from C57BL/6 mice (Fig. 2c). Furthermore, the upregulation of DPP4 following oxLDL treatment was accompanied by an increased expression of inflammatory cytokine IL-1β (Fig. 2d). There was also a trend of increase in the expressions of NLRP3 and Caspase-1, two important components of NLRP3 inflammasome complex which is responsible for the maturation of IL-1β (Fig. 2d). To further confirm this result in humans, we prepared monocyte-derived macrophages (MDMs) from human peripheral blood monocytes and treated these cells with oxLDL. oxLDL increased DPP4 expression on human macrophages by nearly two fold (Fig. 2e). We next examined if 27-hydroxycholesterol (27OH-Ch), an oxidized cholesterol and important component of oxLDL, could induce DPP4 upregulation. Interestingly, DPP4 was not significantly upregulated after 27OH-Ch treatment, suggesting that the upregulation of DPP4 may not be mediated by free form of oxidized cholesterol (Supplemental Fig. 2).
Fig. 2.
Oxidized low-density lipoprotein (oxLDL) increased DPP4 expression.
A, Bone marrow cells isolated from C57BL/6 mice were cultured in the presence of 10 ng/mL M-CSF for 5 days and treated with 25 μg/mL native LDL or oxLDL for 24 h. Cells were then collected for the flow cytometric detection of DPP4 expression, DPP4 expression on bone marrow derived macrophages (BMMs) was detected by flow cytometry. Representative dot plots and statistical bar graph are shown. B, The DPP4+ macrophages were next gated for the analysis of DPP4 MFI. Histograms and bar graph show the DPP4 MFI on DPP4+ macrophages under different treatment conditions. C, Peritoneal macrophages were isolated from C57BL/6 mice and treated with 25 μg/mL oxLDL or vehicle for 24 h. Representative histograms show the expression of DPP4 on macrophages. D, Bone marrow-derived macrophages from C57BL/6 mice were treated with 25 μg/mL oxLDL for 24 h and the expression inflammatory genes was examined using quantitative real-time PCR. E, Monocytes were isolated from human peripheral blood and cultured in the presence of 10 ng/mL M-CSF for 5 days. Cells were then treated with 25 μg/mL oxLDL or vehicle for 24 h. Representative dot plots and histograms are shown. *, p < .05. MFI, mean fluorescence intensity; UT, untreated.
3.4. TLR4/TRIF signaling is partially responsible for oxLDL-induced DPP4 up-regulation
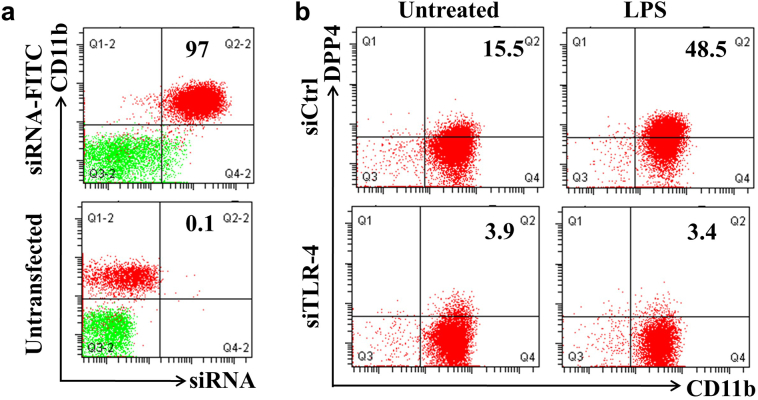
Activation of TLR4 may occur through binding of atherogenic lipids on receptors such as CD36, providing the proximal signaling event required for proinflammatory mediators, chemokines, inflammasome activation and ER stress [[18], [19], [20]]. We therefore initially examined the dependence of TLR4 as a proximal trigger for the induction of DPP4. We induced TLR4 activation with lipopolysaccharide (LPS) stimulation and examined expression of DPP4. LPS enhanced the expression of DPP4 on human monocytes, while siRNA-mediated knockdown of TLR4 substantially abrogated LPS-induced DPP4 up-regulation (Figs. 3a & 3b).
Fig. 3.
TLR4 activation induces DPP4 up-regulation.
A, Human monocyte derived macrophages (MDMs) were transfected with FITC-labeled scramble siRNA. After 24 h, both transfected and untransfected MDMs were used for the flow cytometric detection of transfection efficiency. B, Human MDMs were transfected with either TLR4-specific siRNA (siTLR4) or control siRNA (siCtrl). After 24 h, cells were then treated with 1 μg/mL lipopolysaccharide (LPS) overnight and collected for the detection of DPP4.
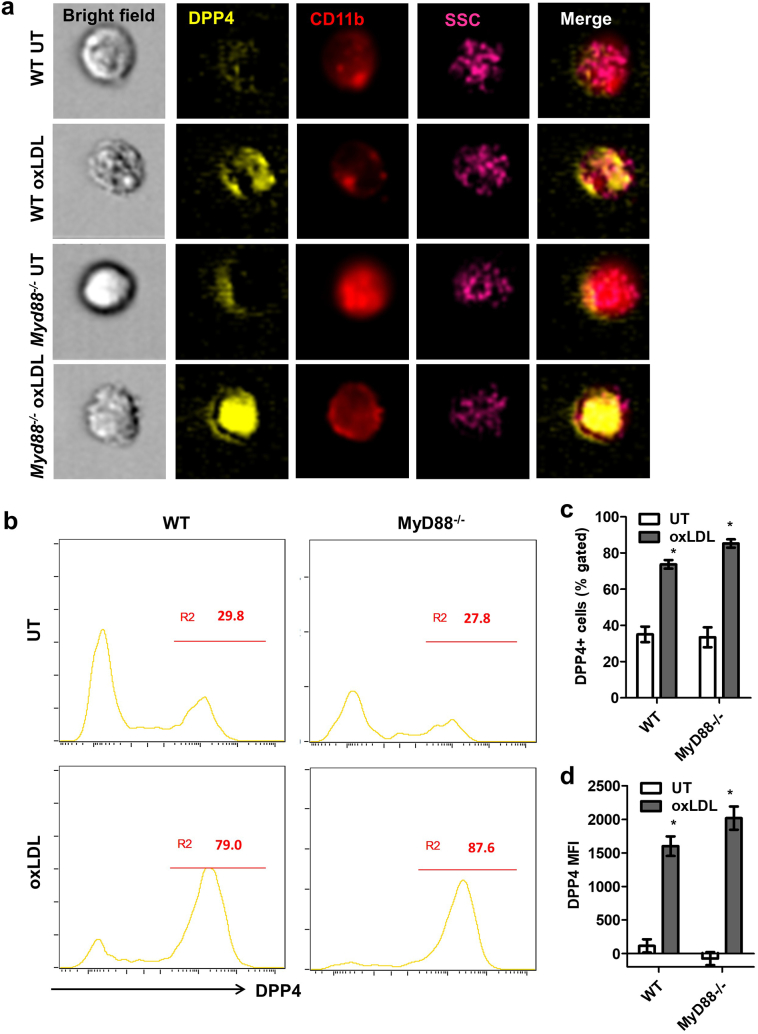
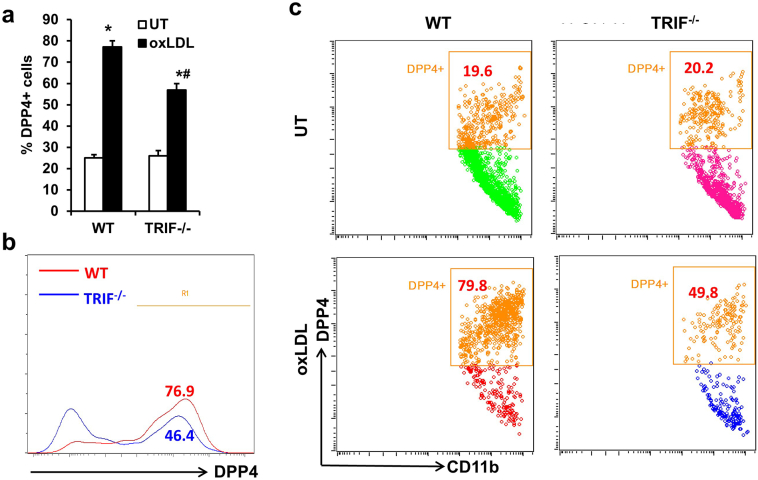
MyD88 and TRIF are two main downstream molecules mediating TLR4 signaling. We then tested if TLR4-mediated DPP4 up-regulation is dependent on MyD88, a major adaptor molecule for TLR4 signaling. Bone marrow cells isolated from wild-type (WT) or Myd88−/− mice were used for the induction of BMMs. The expressions of DPP4 on both WT and Myd88−/− BMMs increased after treatment with 25 μg/mL oxLDL. However, deficiency of MyD88 did not diminish the upregulation of DPP4. In contrast, there was even a slight increase of DPP4 expression after oxLDL treatment in Myd88−/− BMMs (Figs. 4a-4d). We then used Trif−/− mice to examine the involvement of MyD88-independent pathways of TLR4 mediated DPP4 expression. Compared to WT BMMs, Trif−/− BMMs showed impaired up-regulation of DPP4 following oxLDL treatment although it did not completely abolished oxLDL-induced DPP4 up-regulation (Figs. 5a-5c).
Fig. 4.
MyD88 signaling is not responsible for oxLDL-induced DPP4 up-regulation.
Bone marrows isolated from wild-type (WT) or Myd88−/− mice were used for the induction of BMMs. The expressions of DPP4 on both WT and Myd88−/− BMMs were detected by imaging flow cytometry after 24-h treatment of 25 μg/mL oxLDL. Representative images (A), histograms (B) and statistical analysis of DPP4+ macrophage frequency (C) or DPP4 MFI (D) are shown. *, p < .05 compared to UT controls.
Fig. 5.
TRIF signaling partially mediates oxLDL-induced DPP4 up-regulation.
WT or Trif−/− BMMs were treated with 25 μg/mL oxLDL for 24 h and DPP4 expression was then examined. Statistical analysis (A), representative histogram (B) and dot plots (C) are shown. *, p < .05 compared with UT; #, p < .05 compared with WT.
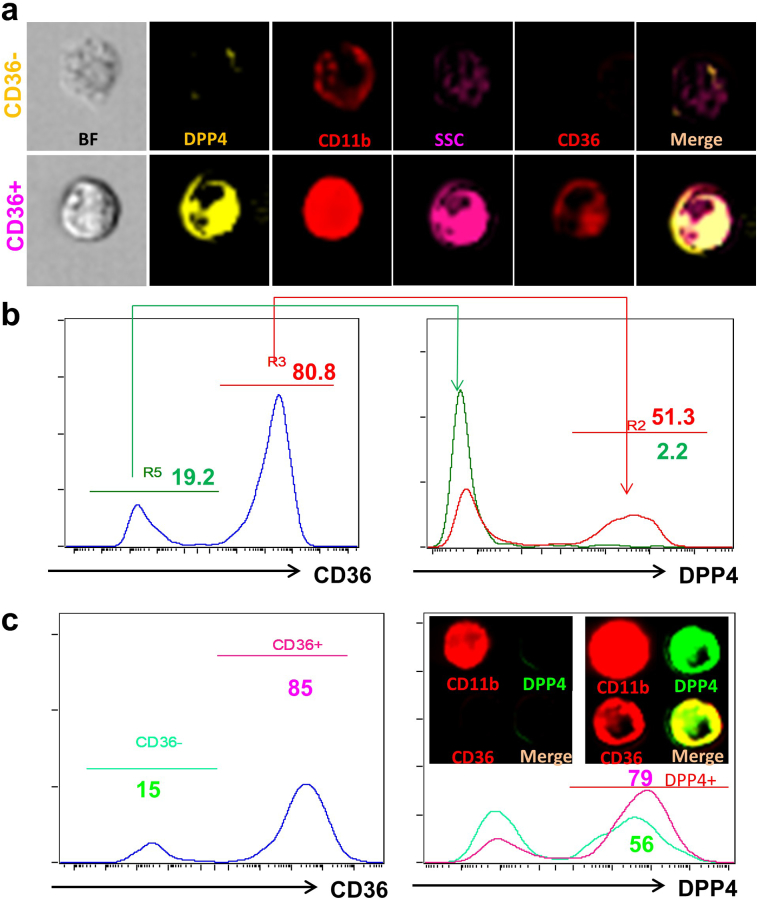
3.5. CD36 is required for oxLDL-induced DPP4 up-regulation
Since TLRs may mediate an oxLDL-induced inflammatory response in cooperation with CD36 [18], we next tested the involvement of CD36. Mouse macrophages were co-stained with CD36 and DPP4. Approximately 80% of the peritoneal macrophages expressed CD36. CD36+ and CD36− populations were gated for the analysis of DPP4 expression. Most CD36− macrophages did not express DPP4, while about 51.3% CD36+ macrophages expressed high levels of DPP4 (Figs. 6a & 6b). After oxLDL treatment, the frequency of CD36+ macrophages increased to 97.6% with DPP4 expression also coordinately increasing within CD36+ cells (Supplemental Fig. 3). Similar findings were also observed in human MDMs (Fig. 6c).
Fig. 6.
CD36 is associated with DPP4 expression on macrophages.
A&B, Peritoneal macrophages were co-stained with CD36 and DPP4 and then examined by imaging flow cytometry. CD36+ and CD36− macrophages were gated for the analysis of DPP4 expression. Representative images (A) and histograms (B) showing the expression of DPP4 on CD36+ and CD36− macrophages. C, Human MDMs were co-stained with CD36 and DPP4 and DPP4 expression on CD36+ or CD36− MDMs are shown.
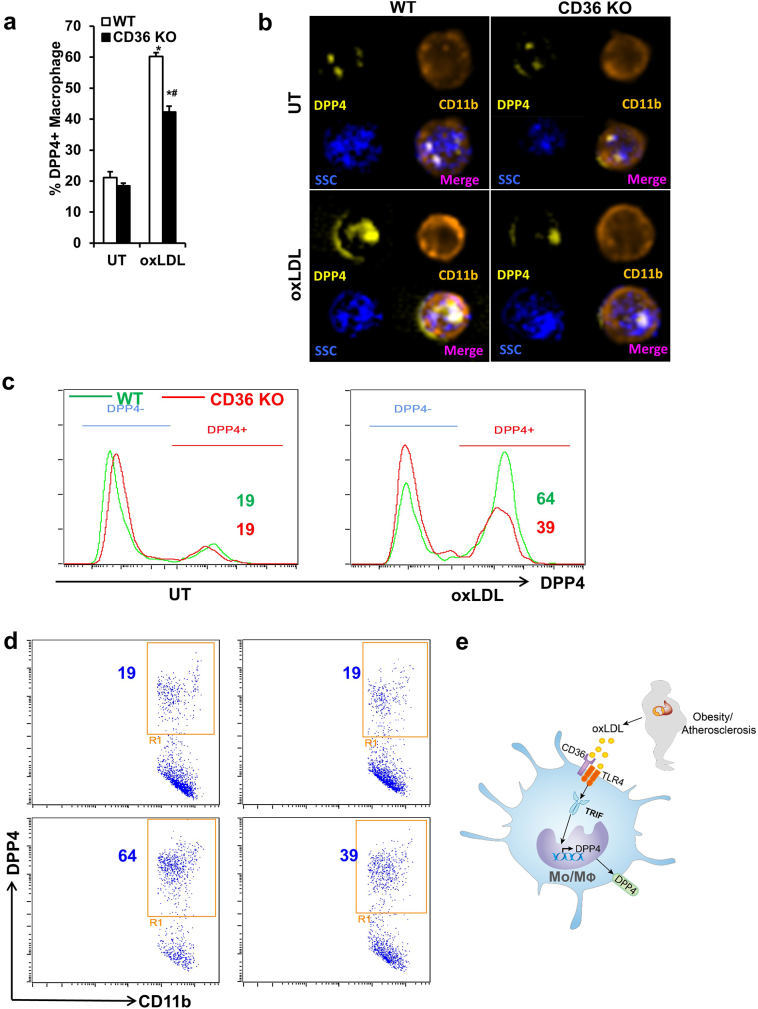
To further confirm the dependence of DPP4 up-regulation on CD36, WT or Cd36−/− BMMs were treated with 25 μg/mL oxLDL and DPP4 expression was measured. As expected, Cd36−/− BMMs had decreased DPP4 expression after treatment of oxLDL as compared to WT BMMs (Figs. 7a-7d), suggesting that CD36 is required for oxLDL-induced DPP4 up-regulation. Taken together, oxLDL upregulates DPP4 expression on monocyte and macrophage via TLR4/TRIF and CD36 pathways (Fig. 7e).
Fig. 7.
CD36 is implicated in oxLDL-induced DPP4 up-regulation.
Bone marrows isolated from wild-type (WT) or Cd36−/− mice were used for the induction of BMMs. The expressions of DPP4 on both WT and Cd36−/− BMMs were detected by imaging flow cytometry after 24-h treatment with 25 μg/mL oxLDL. Statistical analysis (A), representative images (B), histograms (C), and dot plots (D) are shown. *, p < .05 compared with UT; #, p < .05 compared with WT. E, Lipid dysregulation in obesity and atherosclerosis induces DPP4 up-regulation through oxLDL and CD36/TLR4/TRIF pathway. DPP4, dipeptidyl peptidase 4; oxLDL, oxidized low density lipoprotein; TLR4, Toll-like receptor 4; TRIF, Toll/IL-1R domain-containing adaptor-inducing IFN-β.
4. Discussions
In this paper, we demonstrate an important role of TLR4/TRIF and CD36 pathways in the regulation of DPP4 on monocyte/macrophage lineage. DPP4 expression on macrophages demonstrated heterogeneity of expression with high levels seen in CD36+ cells. OxLDL markedly up-regulated DPP4 expression via TLR4/TRIF- and CD36-dependent pathways. Our results suggest an important role for oxidized lipids and inflammatory signaling in DPP4 regulation which may provide an integrated mechanism linking abnormal post-prandial glucose metabolism with oxidized lipids and inflammation.
Our prior study has shown that DPP4 expression is markedly increased on the surface of antigen presenting cells (APCs) such as macrophages and dendritic cells in the visceral adipose of obese humans, with the levels of DPP4 expression on APC's positively correlating with the degree of insulin resistance [8]. In line with these findings, Sell et al. also confirmed the up-regulation of adipose tissue DPP4 in obese insulin resistant subjects and adipose tissue DPP4 was correlated with measures of insulin resistance and inflammation [9]. These findings clearly implicate DPP4 as a marker/mediator of inflammation in adipose tissue.
Experimental studies have strongly demonstrated a role for DPP4 in the progression of atherosclerosis with an increased expression in pro-inflammatory monocytes/macrophages in plaque [12]. However, given the relative neutrality of recent trials with DPP4 catalytic inhibitors [21,22], the role of DPP4 has been questioned. One potential reason for the lack of efficacy in these studies may relate to a divergent role/function of the catalytic versus non-catalytic function of DPP4 particularly in cell types such as monocytes and T cells. Thus our investigation was focused on the expression of membrane bound DPP4 in atherosclerosis. We have previously shown that DPP4 on macrophages and dendritic cells may enhance adipose tissue inflammation via non-catalytic pathways [8]. A recent study by Ghorpade et al. also reported that DPP4 acts with plasma factor Xa to inflame adipose tissue macrophage. Silencing expression of DPP4 in hepatocytes, but not enzymatic inhibition of DPP4 by sitagliptin, reduced adipose tissue inflammation and insulin resistance [23]. In the current study, we observed that catalytic inhibition of DPP4 increased the expression of DPP4 on macrophages. These results indicate that the compensatory upregulation of DPP4 by DPP4 catalytic inhibition may promote inflammation, which could be a possible explanation for the neutral effects of DPP4 inhibitors on cardiovascular outcome.
In a large cross-sectional study in China, plasma DPP4 activity was shown to correlate with carotid intima media thickness. DPP4 activity in this study associated positively with the degree of insulin resistance (HOMA-IR), oxidized LDL and other measures of oxidative stress including nitrotyrosine and 8-isoPGF2a [24]. Interestingly, our recent study indicates there is no correlation between plasma DPP4 activity and atherosclerosis, suggesting that catalytic activity of circulating/soluble DPP4 alone may not be sufficient to serve as a mediator of atherosclerosis [14]. Additionally, one could conclude that monocytes may not be an important source of circulating DPP4. Indeed, adipocytes, endothelial cells and bone marrow derived cells (other than monocytes) have been confirmed as important sources of circulating DPP4 [10,25]. We have previously shown that monocyte DPP4 expression is positively associated with non-HDL cholesterol and triglycerides, but not with fasting blood glucose or insulin levels, suggesting that the increase of monocyte DPP4 in obese patients might be related to dysregulated lipid metabolism in insulin resistance [14]. Since both LDL and its oxidatively modified forms are highly prevalent in atherosclerosis and may link diabetes with accelerated atherosclerosis [18,[26], [27], [28], [29], [30], [31], [32]], we examined the effect of both native LDL and oxLDL on DPP4 expression. Interestingly, only oxLDL, but not native LDL and free form of oxidized cholesterol (27OH-Ch), enhanced the expression of DPP4 on macrophages. In addition to DPP4 upregulation, IL-1β expression was also upregulated following oxLDL treatment, accompanied by a trend of increase in inflammasome components NLRP3 and caspase-1. This result is consistent with previous reports [33,34]. Recent studies have demonstrated that CD36 may coordinate with TLRs to enhance oxLDL-induced NLRP3 inflammasome activation and atherosclerosis [[35], [36], [37]]. However, the causal relationship between DPP4 upregulation and IL-1β production was not examined in this study and requires further investigation. Activation of TLRs and downstream inflammatory signaling could be activated by excessive free fatty acids, very low density lipoprotein, apolipoprotein CIII, and lipoprotein oxidation [18,[38], [39], [40], [41], [42], [43]]. In addition, the expression and activation of TLRs were also reported to increase in patients with metabolic syndrome [18,38,[43], [44], [45]]. In line with previous findings, we found that TLR4 knockdown or deficiency of downstream molecule TRIF abolished oxLDL-mediated up-regulation of DPP4 on macrophages. This suggests that oxLDL-induced TLR4/TRIF signaling may be responsible for elevated monocyte DPP4 in obesity. MyD88 and TRIF are the two major downstream adaptor proteins for TLRs. In the present study, we found TRIF rather than MyD88 is required for oxLDL-induced DPP4 up-regulation. TRIF is responsible for mediating the activation of NF-κB and IFN-β production mediated by TLR3 and TLR4 [46,47]. In our study, oxLDL-induced DPP4 upregulation was even slightly enhanced in MyD88 deficient macrophages. This could be a result of the compensatory enhancement of TRIF signaling in MyD88 knockout macrophages [48]. The role of TRIF signaling in atherosclerosis is complex. Depending on the cellular context and upstream pathways such as TLR4/3, TRIF could be proatherogenic or atheroprotective. Loss-of-function mutation of TRIF improved atherosclerosis in Ldlr−/− mice, while TLR3 deficiency in Ldlr−/− mice enhanced atherosclerosis development [49]. Since TRIF signaling is primarily induced by TLR3 and TLR4, these data suggest TLR4-mediated TRIF signaling promotes atherosclerosis, while the TLR3-dependent TRIF responses may be atheroprotective [49]. However, another study reported TLR3 and TLR4 signaling in bone marrow cells had proatherogenic effects in Ldlr−/− mice [50]. Studies by Ohnuma suggest that DPP4 may induce NFκB activation and monocyte maturation by interacting with caveolin-1 [4,51,52]. Therefore, up-regulation of DPP4 by oxLDL-induced innate signaling may in turn activate inflammatory signaling, resulting in a feed-forward loop.
Stewart et al. previously reported that TLRs cooperate with CD36 to mediate oxLDL-induced inflammatory response [18]. CD36 is well-known as a scavenger receptor responsible for oxLDL uptake and foam cell formation [53,54]. We found in this study that CD36 is also involved in oxLDL-induced DPP4 up-regulation. Almost all DPP4+ cells also expressed CD36. Treatment of oxLDL increased the expression of CD36, which is consistent with previously reported findings [55]. In addition, deficiency of CD36 at least partially abolished oxLDL-induced DPP4 up-regulation. These findings suggest an important role for CD36 in oxLDL-induced DPP4 up-regulation.
We acknowledge a number of important limitations in this study. Importantly, we have not demonstrated a link between mediators of triglycerides such as post-prandial remnants. Remnant lipoproteins are markedly increased in diabetic dyslipidemia and may represent a fraction that could potentially participate in linking disordered lipid metabolism in the post-prandial state with abnormalities in glucose metabolism. In addition, we acknowledged that the DPP4 expression in human monocytes was not examined in a large sample of patients. However, one critical finding in our human study that DPP4 is increased in obesity and atherosclerosis is supported by other reports with a larger population size [9,24]. We have also not provided additional mechanisms that link changes in TLR4 expression with downstream activation of TRIF and further kinase pathways that may function upstream of DPP4. In conclusion we demonstrate a role for oxidized lipids mediated up-regulation of DPP4 in atherosclerosis via TLR4/TRIF and CD36 dependent pathways. Our data provides a link between disordered lipid metabolism in atherosclerosis and impaired glucose intolerance through upregulation of DPP4.
Acknowledgments
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (81670431, 31870906, 81370942, and Y2110580), National Institutes of Health (K01DK105108, R03DK119680, and K99ES026241), American Diabetes Association (1-19-JDF-117), National Science and Technology Major Project (2016YFC1305803), American Heart Association (17GRNT33670485), and Hubei Regenerative Medicine Research Center. The funding sources had no role in the study design and execution, data analysis and interpretation, or decision to submit results.
Declaration of interests
The authors declare that there is no conflict of interest regarding the publication of this article.
Author contribution
X.R., L.D., Y.W., and J.Z., researched data. X.R. and J.Z. wrote the manuscript. Z.B., M.R., A.C.T., S.R., and J.Z. reviewed and edited the manuscript. S.Z. and H.M. contributed to discussion of experimental design.
Fund
This work was supported by grants from National Natural Science Foundation of China (81670431, 31870906, 81370942, and Y2110580), National Institutes of Health (K01DK105108, R03DK119680, and K99ES026241), American Diabetes Association (1-19-JDF-117), National Science and Technology Major Project (2016YFC1305803), American Heart Association (17GRNT33670485), and Hubei Regenerative Medicine Research Center.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.01.065.
Contributor Information
Shi Zhao, Email: zhaoshiwuhan@126.com.
Jixin Zhong, Email: jixin.zhong@case.edu.
Appendix A. Supplementary data
Supplementary material
References
- 1.Mulvihill E.E., Drucker D.J. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev. 2014;35(6):992–1019. doi: 10.1210/er.2014-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhong J., Maiseyeu A., Davis S.N., Rajagopalan S. DPP4 in cardiometabolic disease: recent insights from the laboratory and clinical trials of DPP4 inhibition. Circ Res. 2015;116(8):1491–1504. doi: 10.1161/CIRCRESAHA.116.305665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu G., Hu Y., Wang Q. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature. 2013;500(7461):227–231. doi: 10.1038/nature12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohnuma K., Yamochi T., Uchiyama M. CD26 up-regulates expression of CD86 on antigen-presenting cells by means of caveolin-1. Proc Natl Acad Sci U S A. 2004;101(39):14186–14191. doi: 10.1073/pnas.0405266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richard E., Arredondo-Vega F.X., Santisteban I., Kelly S.J., Patel D.D., Hershfield M.S. The binding site of human adenosine deaminase for CD26/Dipeptidyl peptidase IV: the Arg142Gln mutation impairs binding to cd26 but does not cause immune deficiency. J Exp Med. 2000;192(9):1223–1236. doi: 10.1084/jem.192.9.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng H.C., Abdel-Ghany M., Pauli B.U. A novel consensus motif in fibronectin mediates dipeptidyl peptidase IV adhesion and metastasis. J Biol Chem. 2003;278(27):24600–24607. doi: 10.1074/jbc.M303424200. [DOI] [PubMed] [Google Scholar]
- 7.Zhong J., Rajagopalan S. Dipeptidyl peptidase-4 regulation of SDF-1/CXCR4 axis: implications for cardiovascular disease. Front Immunol. 2015;6:477. doi: 10.3389/fimmu.2015.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong J., Rao X., Deiuliis J. A potential role for dendritic cell/macrophage-expressing DPP4 in obesity-induced visceral inflammation. Diabetes. 2013;62(1):149–157. doi: 10.2337/db12-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sell H., Bluher M., Kloting N. Adipose dipeptidyl peptidase-4 and obesity: correlation with insulin resistance and depot-specific release from adipose tissue in vivo and in vitro. Diabetes Care. 2013;36(12):4083–4090. doi: 10.2337/dc13-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z., Grigo C., Steinbeck J., von Horsten S., Amann K., Daniel C. Soluble DPP4 originates in part from bone marrow cells and not from the kidney. Peptides. 2014;57:109–117. doi: 10.1016/j.peptides.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Lee S.A., Kim Y.R., Yang E.J. CD26/DPP4 levels in peripheral blood and T cells in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2013;98(6):2553–2561. doi: 10.1210/jc.2012-4288. [DOI] [PubMed] [Google Scholar]
- 12.Shah Z., Kampfrath T., Deiuliis J.A. Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation. 2011;124(21):2338–2349. doi: 10.1161/CIRCULATIONAHA.111.041418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brenner C., Franz W.M., Kuhlenthal S. DPP-4 inhibition ameliorates atherosclerosis by priming monocytes into M2 macrophages. Int J Cardiol. 2015;199:163–169. doi: 10.1016/j.ijcard.2015.07.044. [DOI] [PubMed] [Google Scholar]
- 14.Rao X., Deiuliis J.A., Mihai G. Monocyte DPP4 expression in human atherosclerosis is associated with obesity and dyslipidemia. Diabetes Care. 2018;41(1):e1–e3. doi: 10.2337/dc17-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mihai G., Varghese J., Kampfrath T. Aliskiren effect on plaque progression in established atherosclerosis using high resolution 3D MRI (ALPINE): a double-blind placebo-controlled trial. J Am Heart Assoc. 2013;2(3) doi: 10.1161/JAHA.112.004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore K.J., Sheedy F.J., Fisher E.A. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13(10):709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore K.J., Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145(3):341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart C.R., Stuart L.M., Wilkinson K. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11(2):155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seimon T.A., Nadolski M.J., Liao X. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010;12(5):467–482. doi: 10.1016/j.cmet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bae Y.S., Lee J.H., Choi S.H. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ Res. 2009;104(2):210–218. doi: 10.1161/CIRCRESAHA.108.181040. 21p following 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scirica B.M., Bhatt D.L., Braunwald E. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 22.White W.B., Cannon C.P., Heller S.R. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 23.Ghorpade D.S., Ozcan L., Zheng Z. Hepatocyte-secreted DPP4 in obesity promotes adipose inflammation and insulin resistance. Nature. 2018;555(7698):673–677. doi: 10.1038/nature26138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng T.P., Liu Y.H., Yang L.X., Qin S.H., Liu H.B. Increased plasma dipeptidyl peptidase-4 activities are associated with high prevalence of subclinical atherosclerosis in Chinese patients with newly diagnosed type 2 diabetes: a cross-sectional study. Atherosclerosis. 2015;242(2):580–588. doi: 10.1016/j.atherosclerosis.2015.07.042. [DOI] [PubMed] [Google Scholar]
- 25.Lamers D., Famulla S., Wronkowitz N. Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome. Diabetes. 2011;60(7):1917–1925. doi: 10.2337/db10-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kesaniemi Y.A., Grundy S.M. Increased low density lipoprotein production associated with obesity. Arteriosclerosis. 1983;3(2):170–177. doi: 10.1161/01.atv.3.2.170. [DOI] [PubMed] [Google Scholar]
- 27.Klop B., Elte J.W., Cabezas M.C. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013;5(4):1218–1240. doi: 10.3390/nu5041218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Njajou O.T., Kanaya A.M., Holvoet P. Association between oxidized LDL, obesity and type 2 diabetes in a population-based cohort, the health, aging and body composition study. Diabetes Metab Res Rev. 2009;25(8):733–739. doi: 10.1002/dmrr.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norris A.L., Steinberger J., Steffen L.M., Metzig A.M., Schwarzenberg S.J., Kelly A.S. Circulating oxidized LDL and inflammation in extreme pediatric obesity. Obesity (Silver Spring) 2011;19(7):1415–1419. doi: 10.1038/oby.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kato R., Mori C., Kitazato K. Transient increase in plasma oxidized LDL during the progression of atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2009;29(1):33–39. doi: 10.1161/ATVBAHA.108.164723. [DOI] [PubMed] [Google Scholar]
- 31.Steinberg D., Witztum J.L. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30(12):2311–2316. doi: 10.1161/ATVBAHA.108.179697. [DOI] [PubMed] [Google Scholar]
- 32.Boullier A., Bird D.A., Chang M.K. Scavenger receptors, oxidized LDL, and atherosclerosis. Ann N Y Acad Sci. 2001;947:214–222. doi: 10.1111/j.1749-6632.2001.tb03943.x. [discussion 22-3] [DOI] [PubMed] [Google Scholar]
- 33.Peng K., Liu L., Wei D. P2X7R is involved in the progression of atherosclerosis by promoting NLRP3 inflammasome activation. Int J Mol Med. 2015;35(5):1179–1188. doi: 10.3892/ijmm.2015.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang S., Xie X., Lei T. Statins attenuate activation of the NLRP3 inflammasome by oxidized LDL or TNFalpha in vascular endothelial cells through a PXR-dependent mechanism. Mol Pharmacol. 2017;92(3):256–264. doi: 10.1124/mol.116.108100. [DOI] [PubMed] [Google Scholar]
- 35.Sheedy F.J., Grebe A., Rayner K.J. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;14(8):812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu W., Yin Y., Zhou Z., He M., Dai Y. OxLDL-induced IL-1 beta secretion promoting foam cells formation was mainly via CD36 mediated ROS production leading to NLRP3 inflammasome activation. Inflamm Res. 2014;63(1):33–43. doi: 10.1007/s00011-013-0667-3. [DOI] [PubMed] [Google Scholar]
- 37.Rao X., Zhong J., Maiseyeu A. CD36-dependent 7-ketocholesterol accumulation in macrophages mediates progression of atherosclerosis in response to chronic air pollution exposure. Circ Res. 2014;115(9):770–780. doi: 10.1161/CIRCRESAHA.115.304666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi H., Kokoeva M.V., Inouye K., Tzameli I., Yin H., Flier J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116(11):3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pal D., Dasgupta S., Kundu R. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012;18(8) doi: 10.1038/nm.2851. [DOI] [PubMed] [Google Scholar]
- 40.den Hartigh L.J., Altman R., Hutchinson R. Postprandial apoE isoform and conformational changes associated with VLDL lipolysis products modulate monocyte inflammation. PloS one. 2012;7(11) doi: 10.1371/journal.pone.0050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawakami A., Osaka M., Aikawa M. Toll-like receptor 2 mediates apolipoprotein CIII-induced monocyte activation. Circ Res. 2008;103(12):1402–1409. doi: 10.1161/CIRCRESAHA.108.178426. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Dichtl W., Nilsson L., Goncalves I. Very low-density lipoprotein activates nuclear factor-kappaB in endothelial cells. Circ Res. 1999;84(9):1085–1094. doi: 10.1161/01.res.84.9.1085. [DOI] [PubMed] [Google Scholar]
- 43.Howell K.W., Meng X., Fullerton D.A., Jin C., Reece T.B., Cleveland J.C., Jr. Toll-like receptor 4 mediates oxidized LDL-induced macrophage differentiation to foam cells. J Surg Res. 2011;171(1):e27–e31. doi: 10.1016/j.jss.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 44.Jialal I., Huet B.A., Kaur H., Chien A., Devaraj S. Increased toll-like receptor activity in patients with metabolic syndrome. Diabetes Care. 2012;35(4):900–904. doi: 10.2337/dc11-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reyna S.M., Ghosh S., Tantiwong P. Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes. 2008;57(10):2595–2602. doi: 10.2337/db08-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamamoto M., Sato S., Hemmi H. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301(5633):640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 47.Oshiumi H., Matsumoto M., Funami K., Akazawa T., Seya T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol. 2003;4(2):161–167. doi: 10.1038/ni886. [DOI] [PubMed] [Google Scholar]
- 48.Shaik-Dasthagirisaheb Y.B., Huang N., Weinberg E.O., Shen S.S., Genco C.A., Gibson F.C., 3rd. Aging and contribution of MyD88 and TRIF to expression of TLR pathway-associated genes following stimulation with Porphyromonas gingivalis. J Periodontal Res. 2015;50(1):89–102. doi: 10.1111/jre.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richards M.R., Black A.S., Bonnet D.J. The LPS2 mutation in TRIF is atheroprotective in hyperlipidemic low density lipoprotein receptor knockout mice. Innate Immun. 2013;19(1):20–29. doi: 10.1177/1753425912447130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lundberg A.M., Ketelhuth D.F., Johansson M.E. Toll-like receptor 3 and 4 signalling through the TRIF and TRAM adaptors in haematopoietic cells promotes atherosclerosis. Cardiovasc Res. 2013;99(2):364–373. doi: 10.1093/cvr/cvt033. [DOI] [PubMed] [Google Scholar]
- 51.Ohnuma K., Yamochi T., Uchiyama M. CD26 mediates dissociation of Tollip and IRAK-1 from caveolin-1 and induces upregulation of CD86 on antigen-presenting cells. Mol Cell Biol. 2005;25(17):7743–7757. doi: 10.1128/MCB.25.17.7743-7757.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohnuma K., Uchiyama M., Yamochi T. Caveolin-1 triggers T-cell activation via CD26 in association with CARMA1. J Biol Chem. 2007;282(13):10117–10131. doi: 10.1074/jbc.M609157200. [DOI] [PubMed] [Google Scholar]
- 53.Febbraio M., Podrez E.A., Smith J.D. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest. 2000;105(8):1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silverstein R.L., Febbraio M. CD36 and atherosclerosis. Curr Opin Lipidol. 2000;11(5):483–491. doi: 10.1097/00041433-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Feng J., Han J., Pearce S.F. Induction of CD36 expression by oxidized LDL and IL-4 by a common signaling pathway dependent on protein kinase C and PPAR-gamma. J Lipid Res. 2000;41(5):688–696. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material