Abstract
Objectives
To evaluate, by applying pharmacokinetic/pharmacodynamic (PK/PD) analysis, if the change in antibiotic susceptibility after the introduction of the 7-valent pneumococcal conjugate vaccine (PCV7) in Spain had any influence on the usefulness of the antimicrobials more frequently used as empirical treatment of pediatric acute otitis media (AOM).
Material and methods
PK parameters and susceptibility of Streptococcus pneumoniae and Haemophilus influenzae were obtained from bibliography. Monte Carlo simulation was used to estimate the cumulative fraction of response (CFR), understood as the expected probability of therapy success. For amoxicillin and amoxicillin/clavulanate, the target was free antibiotic concentration remaining above the minimum inhibitory concentration (MIC) for ≥50% of the dosing interval (fT>MIC≥50%), whereas for cefuroxime axetil and cefotaxime, the target was fT>MIC≥60%. CFR values ≥90% were considered successful.
Results
When all serotypes of S. pneumoniae are considered, amoxicillin and cefotaxime turned out to reach a high probability of success, and difference before and after vaccination was scarce. For H. influenzae, CFR values were higher with amoxicillin/clavulanate than with amoxicillin. For both microorganisms, cefuroxime axetil resulted in low probability of success in the two periods of study.
Conclusions
We have shown that the introduction of the PCV7 vaccination did not lead to changes in the probability of success of the current empiric treatments of the AOM. Integrated PK/PD analysis has demonstrated to be a useful tool to identify changes in antimicrobial activity after the implantation of a vaccination program, providing complementary information to the simple assessment of MIC values.
Keywords: acute otitis media, pharmacokinetics/pharmacodynamics analysis, 7-valent pneumococcal conjugate vaccine
Abstract
Objetivo
Evaluar mediante análisis farmacocinético/farmadocinámico (PK/PD) si el cambio en la sensibilidad antimicrobiana tras la introducción en España de la vacuna antineumocócica heptavalente (VNC7) ha implicado cambios en la adecuación del tratamiento antibiótico de la otitis media aguda (OMA) en niños.
Materiales y métodos
Los parámetros PK y datos de sensibilidad de Streptococcus pneumoniae y Haemophilus influenzae fueron obtenidos de la bibliografía. Mediante simulación de Montecarlo, calculamos la probabilidad de éxito del tratamiento antibiótico, expresada como fracción de respuesta acumulada (CFR). Para amoxicilina y amoxicilina/ácido clavulánico, el objetivo farmacodinámico considerado fue el tiempo durante el cual las concentraciones libres en sangre permanecen por encima de la concentración mínima inhibitoria (CMI), expresado como porcentaje del intervalo de dosificación (fT>CMI≥50%). Para cefuroxima axetilo y cefotaxima, el objetivo fue fT>CMI≥60%. Valores de CFR≥90% se consideraron indicativos de éxito.
Resultados
Si se tienen en cuenta todos los serotipos de S. pneumoniae, amoxicilina y cefotaxima proporcionaron una alta probabilidad de éxito, sin apenas diferencia entre ambos periodos. En el caso de H. influenzae, los valores de CFR fueron más altos con amoxicilina/ácido clavulánico que con amoxicilina. Para ambos microorganismos, las probabilidades de éxito de cefuroxima axetilo fueron bajas en ambos periodos de estudio.
Conclusiones
La introducción de la vacuna PCV7 no ha implicado cambios en la probabilidad de éxito del tratamiento antibiótico empírico de la OMA. Hemos demostrado la utilidad del análisis PK/PD para detectar cambios en la adecuación del tratamiento antibiótico tras la implantación de una vacuna, proporcionando información complementaria al seguimiento de los valores de CMI.
Palabras clave: otitis media aguda, análisis farmacocinético/farmacodinámico, vacuna antineumocócica heptavalente
INTRODUCTION
Acute otitis media (AOM) is one of the most frequent illnesses in children and the most commonly cited indication for antimicrobial treatment [1]. Since it is treated mainly empirically, the antimicrobial agents must target the most frequently isolated pathogens. Streptococcus pneumoniae has been the predominant pathogen related to AOM. The 7-valent pneumococcal conjugate vaccine (PCV7) has decreased AOM in children <2 years, as demonstrated by a ≥28% reduction in recurrent AOM [2,3] and a ≥43% reduction in AOM outpatient visits or prescriptions [4]. Since the introduction of the PCV7 for the prevention of invasive pneumococcal disease, many studies have shown a decrease in AOM cases in vaccinated as well as in non-vaccinated children due to herd protection [5]. In Spain, the PCV7 was introduced for child immunization in June 2001, and, as expected, it has induced a continuous decline in the prevalence of PCV7 serotypes. A recent study carried out in the north of Spain over a 12-year period [6] revealed that the most frequent serotypes of pneumococci causing AOM under the influence of the PCV7 were 19A (27.8%) and 3 (11.2%), serotypes not included in the vaccine, and 19F (9%). Specifically, the proportion of serotype 19A increased from 17.9% to 37.9%, and that of serotype 3 increased to 5.1% to 15.0%. However, the rate of serotypes included in the PCV7 sharply decreased from 62.4% in 1999-2001 to 2.2% in the 2008-2010. In another study carried out in Spain, a similar decrease in the proportion of PVC7 serotypes was shown: from 70.7% in 1999-2000 to 10.6% in 2009 [7]. On the other hand, in Spain the introduction of PCV7 has been associated to an increase in the proportion of AOM caused by Haemophilus influenzae [6]. In fact, the association of H. influenzae and S. pneumoniae in AOM has been largely demonstrated [8], especially in complex AOM [2,9,10], and several authors have suggested the possibility of an increased rate of H. influenzae AOM after the introduction of the PCV7 [11].
Pharmacokinetic/pharmacodynamic (PK/PD) analysis integrates information about the required concentration of antibiotic that reaches the infection site and produces the desirable effect, and information about the susceptibility of the pathogen against the antibiotic, expressed as minimum inhibitory concentration (MIC). PK/PD analysis with Monte Carlo simulation allows the researcher or clinician to select the optimal antibiotic and dosing regimen for each infectious process and patient in order to enhance the effect of the antibiotic, minimizing the side effect incidence and the emergence of resistance [12]. It can also be applied in drug development to scale from animal studies, establish the optimal dosing regimens in clinical trials or describe the kinetic and dynamic relation for new drugs, as required by regulatory agencies. Moreover, PK/PD analysis has also been proved to be useful to assess changing antimicrobial activity against clinical isolates, as complementary to the simply assessment of MIC values [13].
The goal of the current study was to elucidate, by means of PK/PD analysis, if the change in antibiotic susceptibility after the implementation of the PCV7 in Spain had any influence on the adequacy of the antimicrobials more frequently used as empirical treatment of pediatric AOM: amoxicillin, amoxicillin/clavulanate, cefuroxime axetil, and when oral antibiotics are not indicated, cefotaxime.
MATERIALS AND METHODS
The methodology included the following steps: (i) dosing regimen selection and acquisition of pharmacokinetic data; (ii) microbiological data acquisition; and (iii) Monte Carlo simulation of the antibiotics studied in children. Monte Carlo simulation allowed us to estimate the probability of target attainment (PTA), defined as the probability that at least a specific value of a PK/PD index is achieved at a certain MIC, and to calculate the cumulative fraction of response (CFR), defined as the expected population PTA for a specific drug dose and a specific population of microorganisms [14].
Dosing regimen selection and acquisition of pharmacokinetic data. Oral amoxicillin alone and associated with clavulanate, oral cefuroxime axetil, and intravenous cefotaxime were chosen based on their use for the treatment of AOM in children in Spain. The following drug regimens were evaluated: 1) amoxicillin and amoxicillin/clavulanate: 20 mg/kg, 40 mg/kg, 45 mg/kg and 50 mg/kg every 12h (q12h) and 13 mg/kg, 27 mg/kg, 30 mg/kg and 33 mg/kg every 8h (q8h); the dose of amoxicillin/clavulanate is expressed as amoxicillin and is administered as an oral suspension of 100/12.5 mg, 2) cefuroxime axetil: 10 mg/kg and 15 mg/kg q12h, and 3) cefotaxime: 33 mg/kg, 50 mg/kg and 66 mg/kg q8h, as a 0.5 h infusion. Pharmacokinetic parameters were obtained from published pharmacokinetic studies in pediatric populations [15-20]. All parameters were expressed as means and standard deviation (table 1). In the case of cefuroxime axetil and cefotaxime, published pharmacokinetic parameters were available only as mean values, without variability. In order to carry out the PK/PD analysis we assumed a variability (expressed as variation coefficient) of 20% for the volume of distribution (V) and elimination rate constant (K), and 25% for absorption rate constant (Ka). Unbound fraction was included as a fix value [21].
Table 1.
Pharmacokinetic parameters for each antimicrobial agent from published studies carried out in children (mean±standard deviation).
| Amoxicillin | Cefuroxime axetil | Cefotaxime | |
|---|---|---|---|
| V/F (L/Kg) | 1.44 ± 0.37 | 0.72 ± 0.14 | 0.295 ± 0.059 |
| K (h-1) | 0.276 ± 0.137 | 0.5 ± 0.1 | 0.75 ± 0.15 |
| Ka (h-1) | 1.77 ± 0.99 | 0.43 ± 0.11 | - |
| fu | 0.8 | 0.6 | 0.6 |
| Reference | [14,15] | [16] | [17-19] |
V/F: volume of distribution/drug bioavailability, K: elimination constant rate, KA: absorption constant rate, fu: unbound fraction
Acquisition of microbiological data. Susceptibility data of clinical isolates to each antibiotic before and after the implementation of the PCV7 were obtained from recently published studies (tables 2 and 3). Pre- (2000-2001) and post- (2010-2011) vaccination bacterial population MIC distribution of S. pneumoniae isolates for each antibiotic was provided by Fenoll et al. [7] (table 2). The proportion of the non-vaccine serotypes varied from 44.5% (85/191) in the pre-PCV7 period to 92.1% (128/139) in the post-PCV7 period. The most frequent serotypes in the post-vaccination period were 19A (47.5%, 66/139) and 3 (10.8%, 15/139). Pre-vaccination data of H. influenzae (1998-1999) were provided by the Medical Department of GlaxoSmithKline [22], and post-vaccination data of H. influenzae (2011) were obtained from a study performed by García-Cobos et al. [23] (table 3). The proportion of β-lactamase-producing H. influenzae strains varied from 20.6% in the pre-PCV7 period to 12.5% in the post-PCV7 period, and β-lactamase-nonproducing amoxicillin-resistant (BLNAR) strains varied from 25.3% to 22.9%.
Table 2.
Activity of the antibiotic studied against S. pneumoniae isolates from AOM in children on pre-vaccination period (May 2000-May 2001), and post-vaccination period (May 2010-May 2011). Pre-vaccination period: all isolates: 191, serotype 3: 18 isolates, serotype 19A: 18 isolates; post-vaccination period: all isolates: 139, serotype 3: 15 isolates, serotype 19A: 66 isolates.
| % of strains inhibited at MIC (mg/L) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All isolates | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | |
| Amoxicillin | Pre-PCV7 | 40.8 | 3.7 | 4.7 | 9.9 | 12 | 18.3 | 3.7 | 6.3 | 0.5 | ||||
| Post-PCV7 | 41.0 | 2.9 | 4.3 | 10.1 | 10.1 | 7.9 | 18.7 | 5.0 | ||||||
| Cefuroxime axetil | Pre-PCV7 | 4.2 | 20.9 | 5.2 | 6.3 | 4.2 | 6.3 | 6.8 | 7.3 | 26.2 | 12.6 | |||
| Post-PCV7 | 6.5 | 29.5 | 2.2 | 2.9 | 5 | 2.9 | 7.9 | 10.1 | 24.5 | 4.3 | 4.3 | |||
| Cefotaxime | Pre-PCV7 | 16.8 | 17.3 | 8.4 | 4.2 | 10.5 | 13.1 | 26.2 | 3.7 | |||||
| Post-PCV7 | 34.5 | 1.4 | 3.6 | 3.6 | 2.9 | 8.6 | 23.7 | 17.3 | 4.3 | |||||
| % of strains inhibited at MIC (mg/L) | ||||||||||||||
| Serotype 19A | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | |
| Amoxicillin | Pre-PCV7 | 66.7 | 5.6 | 5.6 | 16.7 | 5.6 | ||||||||
| Post-PCV7 | 7.6 | 1.5 | 4.5 | 16.7 | 12.1 | 12.1 | 37.9 | 7.6 | ||||||
| Cefuroxime axetil | Pre-PCV7 | 44.4 | 16.7 | 5.6 | 16.7 | 16.7 | ||||||||
| Post-PCV7 | 1.5 | 4.5 | 1.5 | 3 | 9.1 | 15.2 | 48.5 | 9.1 | 7.6 | |||||
| Cefotaxime | Pre-PCV7 | 22.2 | 38.9 | 5.6 | 5.6 | 11.1 | 11.1 | 5.6 | ||||||
| Post-PCV7 | 6.1 | 3 | 1.5 | 9.1 | 36.4 | 36.4 | 7.6 | |||||||
| % of strains inhibited at MIC (mg/L) | ||||||||||||||
| Serotype 3 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | |
| Amoxicillin | Pre-PCV7 | 100 | ||||||||||||
| Post-PCV7 | 100 | |||||||||||||
| Cefuroxime axetil | Pre-PCV7 | 27.7 | 55.5 | 11.1 | 5.5 | |||||||||
| Post-PCV7 | 26.7 | 73.3 | ||||||||||||
| Cefotaxime | Pre-PCV7 | 77.0 | 22.0 | |||||||||||
| Post-PCV7 | 100 | |||||||||||||
Table 3.
Activity of the antibiotic studied against H. influenzae isolates from AOM in children in the pre-vaccination period (1998-1999, n=146 isolates), and in the post-vaccination period (2011, n=48 isolates).
| % of strains inhibited at MIC (mg/L) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | |
| Amoxicillin | Pre-PCV7 | 54.1 | 18.5 | 6.8 | 1.4 | 19.2 | ||||||||
| Post-PCV7 | 62.5 | 18.7 | 6.3 | 4.2 | 8.3 | |||||||||
| Amoxicillin/clavulanate | Pre-PCV7 | 0.7 | 0.7 | 0.7 | 5.5 | 43.1 | 28.7 | 15.1 | 5.5 | |||||
| Post-PCV7 | 4.2 | 60.4 | 27.1 | 8.3 | ||||||||||
| Cefuroxime axetil | Pre-PCV7 | 7.5 | 11.0 | 47.3 | 22.6 | 10.3 | 1.4 | |||||||
| Post-PCV7 | 2.1 | 25.0 | 47.9 | 25 | ||||||||||
| Cefotaxime | Pre-PCV7 | 99.2 | 0.3 | 0.3 | 0.3 | |||||||||
| Post-PCV7 | 81.3 | 18.8 | ||||||||||||
The susceptibility (expressed as minimum inhibitory concentration, MIC) to amoxicillin, amoxicillin/clavulanate, cefuroxime axetil and cefotaxime was studied considering the Clinical and Laboratory Standards Institute (CLSI) breakpoints [24]. H. influenzae strains were classified as amoxicillin susceptible (MIC ≤ 1 mg/L), or resistant (MIC > 1 mg/L). BLNAR was determined according to the CLSI breakpoints.
Estimation of probability of target attainment (PTA). A 10,000 subject Monte Carlo simulation was conducted for each antibiotic agent using Oracle® Crystal Ball Fusion Edition v.11.1.1.1.00 (Oracle USA Inc., Redwood City, CA). As β-lactam antibiotics show time-dependent antimicrobial activity, the PK/PD parameter related to its activity is the percentage of time that free drug concentration remains over de MIC (fT>MIC). The target was the unbound antibiotic concentration remaining above the MIC for ≥50% of the dosing interval for penicillins (fT>MIC ≥50%) and ≥ 60% for cephalosporins (fT>MIC ≥60%) [16, 25]. The fraction of time (expressed as percentage of the dosing interval) that the drug concentration remains above the MIC (fT> MIC) was calculated for over an MIC range of serial twofold dilutions from 0.015 mg/L to 64 mg/L. We assumed a one-compartment pharmacokinetic model and, according statistical criteria, a log-normal distribution for the pharmacokinetic parameters was used.
For cefotaxime (intravenous infusion), the following equation was used to calculate fT>MIC [25]:
| Eq. 1 |
where tinf (h) is the infusion time, t1 (h) corresponds to the time at which the drug concentration reaches de MIC during the infusion phase, t2 (h) corresponds to the post-infusion time at which the serum concentration equals the MIC and t is the dosing interval. Assuming that cefotaxime shows linear pharmacokinetics, t1 and t2 were calculated as follows:
| Eq. 2 |
| Eq. 3 |
where fCmin,ss and fCmax,ss are the minimum and maximum unbound serum concentrations (mg/L) at steady state, respectively.
Total body clearance (CL), volume of distribution (V), and unbound fraction (fu) were used to estimate fCmin,ss and fCmax,ss according to the following equations:
| Eq. 4 |
| Eq. 5 |
For amoxicillin, amoxicillin/clavulanate and cefuroxime axetil, which are administered by oral route, the following equation was used:
| Eq. 6 |
where F is the drug bioavailability, Ka is the absorption rate constant, K is the elimination rate constant, and n is the number of administered doses that ensures that the steady state is reached (10 doses was always selected).
Using Oracle® Crystal Ball, the values of time at which concentration equals the MIC values were calculated and used to estimate ƒT>MIC (%) as follows:
| Eq. 7 |
where t1 and t2 corresponds to the time at which the drug concentration reaches the MIC in the ascendant and in the elimination phase of the plasma concentration-time curve, respectively.
The PTA (probability that ƒT>MIC (%) reaches the PK/PD target: 50% for amoxicillin, and 60% for cefuroxime axetil and cefotaxime), were estimated for every dosing regimen. The treatment was considered successful if the PTA was ≥90 % [26].
Estimation of cumulative fraction of response (CFR). The CFR, understood as the expected probability of success of a dosing regimen against bacteria in the absence of the specific value of MIC, was also calculated. It results from the total sum of the products of the PTA at a certain MIC times the frequency of isolates of microorganism exhibiting that MIC over the range of susceptibility, according to the following equation:
| Eq. 8 |
where i indicates the MIC category, PTAi is the PTA of each MIC category, and Fi is the fraction of microorganisms population in each MIC category. As for PTA, the dosing regimen was considered successful if the CFR value was equal to 90 % or higher [26].
RESULTS
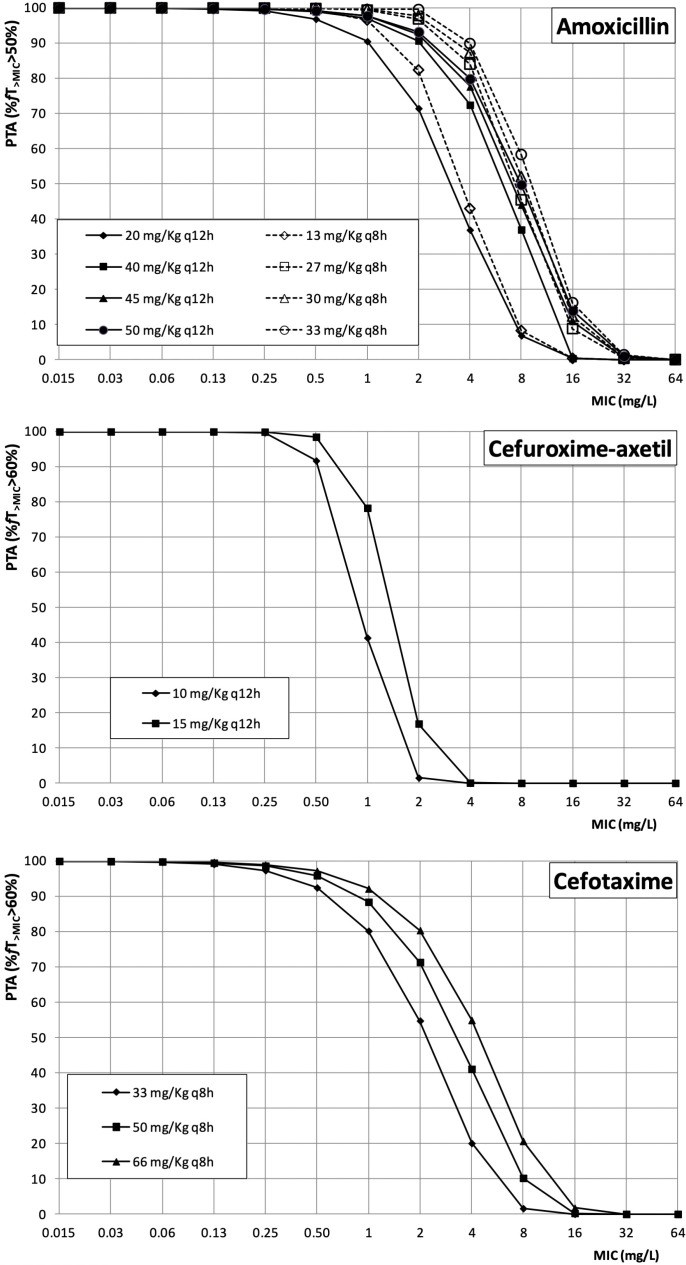
Figure 1 features the PTA values of amoxicillin, cefuroxime axetil and cefotaxime for all the dosing regimens studied. As expected, for each target (fT>MIC>50% for amoxicillin, and fT>MIC>60% for cefuroxime axetil and cefotaxime), the highest PTA values were achieved with the highest doses. Regarding amoxicillin, if the infection is caused by microorganisms with an MIC≤1 mg/L, a high probability of therapy success (PTA≥90%) was achieved even with the lowest dose. For an MIC value of 2 mg/L, all dosing regimens except 20 mg/kg q12h and 13 mg/kg q8h provided PTA≥90%, and PTA was higher than 90% when the MIC is 4 mg/L only with the dose of 33 mg/kg q8h). Both cephalosporins, cefuroxime axetil and cefotaxime, cover infections caused by microorganisms with an MIC≤0.5 mg/L, but for a MIC value of 1 mg/L, only cefotaxime 66 mg/kg q8h ensured a probability of therapy success higher than 90%.
Figure 1.
Probability of target attainment (PTA) of amoxicillin, cefuroxime-axetil, and cefotaxime in simulated pediatric patients. MIC: minimum inhibitory concentration.
The proportion of S. pneumoniae isolates amoxicillin-susceptible and cefotaxime-susceptible (MICs ≤ 2 mg/L and ≤ 1 mg/L, respectively) decreased 15% and 19% from pre-PCV7 to the post-PCV7 period, respectively. Table 4 shows CFR values of each antibiotic against S. pneumoniae taking into account the MIC distribution data in the pre- and post- vaccination periods (pre- and post-PCV7). When all serotypes of S. pneumoniae are considered, amoxicillin (except 20 mg/Kg q12h and 13 mg/Kg q8h) and cefotaxime turned out to reach a high probability of success (CFR≥90%), and difference before and after vaccination was scarce. However, for serotype 19A, CFR values decreased in the post-vaccination period, and the probability of success was ≥90% only with the highest doses of amoxicillin. As can be seen in table 4, serotype 3 is fully susceptible to all antimicrobial agents, and when this serotype is responsible for the infection, no difference in the probability of success of the antibiotic therapy between the pre- and post-vaccination period was detected.
Table 4.
CFR values for S. pneumoniae pre- and post-PCV7.
| All serotypes |
Serotype 19A |
Serotype 3 |
||||
|---|---|---|---|---|---|---|
| Amoxicillin | Pre-PCV7 | Post-PCV7 | Pre-PCV7 | Post-PCV7 | Pre-PCV7 | Post-PCV7 |
| 20 mg/kg q12h | 85 | 80 | 96 | 63 | 100 | 100 |
| 40 mg/kg q12h | 93 | 90 | 98 | 83 | 100 | 100 |
| 45 mg/kg q12h | 94 | 92 | 98 | 86 | 100 | 100 |
| 50 mg/kg q12h | 94 | 93 | 99 | 88 | 100 | 100 |
| 13 mg/kg q8h | 88 | 83 | 97 | 69 | 100 | 100 |
| 27 mg/kg q8h | 95 | 94 | 96 | 89 | 100 | 100 |
| 30 mg/kg q8h | 96 | 95 | 99 | 91 | 100 | 100 |
| 33 mg/kg q8h | 96 | 96 | 99 | 93 | 100 | 100 |
| Cefuroxime axetil | Pre-PCV7 | Post-PCV7 | Pre-PCV7 | Post-PCV7 | Pre-PCV7 | Post-PCV7 |
| 10 mg/kg q12h | 49 | 47 | 73 | 11 | 100 | 100 |
| 15 mg/kg q12h | 54 | 49 | 80 | 12 | 100 | 100 |
| Cefotaxime | Pre-PCV7 | Post-PCV7 | Post-PCV7 | Post-PCV7 | Pre-PCV7 | Post-PCV7 |
| 33 mg/kg q8h | 92 | 83 | 98 | 70 | 100 | 100 |
| 50 mg/kg q8h | 95 | 90 | 99 | 81 | 100 | 100 |
| 66 mg/kg q8h | 97 | 93 | 99 | 86 | 100 | 100 |
Numbers in bold indicates CFR≥90%.
Table 5 shows the CFR values obtained for H. influenzae. As expected, CFR values were higher with amoxicillin/clavulanate than with amoxicillin, and cefuroxime axetil resulted in a very low probability of success. In the two periods of study, cefotaxime led to a high probability of success (≥97%).
Table 5.
CFR values for H. influenzae in the pre- and post-PCV7.
| Amoxicillin | Pre-PCV7 | Post-PCV7 |
|---|---|---|
| 20 mg/kg q12h | 77 | 82 |
| 40 mg/kg q12h | 86 | 86 |
| 45 mg/kg q12h | 87 | 86 |
| 50 mg/kg q12h | 88 | 86 |
| 13 mg/kg q8h | 79 | 85 |
| 27 mg/kg q8h | 89 | 87 |
| 30 mg/kg q8h | 90 | 87 |
| 33 mg/kg q8h | 91 | 87 |
| Amoxicillin/clavulanate | Pre-PCV7 | Post-PCV7 |
| 20 mg/kg q12h | 89 | 93 |
| 40 mg/kg q12h | 96 | 98 |
| 45 mg/kg q12h | 96 | 98 |
| 50 mg/kg q12h | 97 | 98 |
| 13 mg/kg q8h | 93 | 97 |
| 27 mg/kg q8h | 98 | 99 |
| 30 mg/kg q8h | 99 | 100 |
| 33 mg/kg q8h | 99 | 100 |
| Cefuroxime axetil | Pre-PCV7 | Post-PCV7 |
| 10 mg/kg q12h | 38 | 46 |
| 15 mg/kg q12h | 59 | 67 |
| Cefotaxime | Pre-PCV7 | Post-PCV7 |
| 33 mg/kg q8h | 97 | 100 |
| 50 mg/kg q8h | 98 | 100 |
| 66 mg/kg q8h | 99 | 100 |
Numbers in bold indicates CFR≥90%.
DISCUSSION
In the present work, we have studied the antimicrobial activity of the antibiotics used for the treatment of AOM in children against clinical isolates of S. pneumoniae and H. influenzae before and after the introduction of the PCV7 in Spain, by using integrated PK/PD analysis.
Since the availability of PCV7, there have been changes in the overall serotype distribution of S. pneumoniae; in particular, an increase in serotype 19A has been observed globally [27]. In Spain, the rate of non-PCV7 serotypes increased after the vaccine was introduced, including serotypes 19A and 3 [6].
The efficacy of an antimicrobial drug depends on the relationship between the MIC of the microorganism and the exposure of the microorganism to the agent in the patient. For b-lactams, the time for which free drug levels exceed the MIC, expressed as the percentage of the dosing interval (fT>MIC), correlates best with bacterial eradication [28]. Accordingly, we have estimated the probability of treatment success as the probability of this index to reach the target value (50% for penicillins and 60% for cephalosporins), expressed as PTA. Current AOM management guidelines recommend high-dose amoxicillin (80-90 mg/kg/day) as the first-line drug of choice in children [29] and, according to our results, these dose levels would be effective against organisms with MICs up to 2 mg/L (figure 1). Taking into account the susceptibility patterns of S. pneumoniae (table 2), 89.5% (pre-PCV7) and 76.3% (post-PCV7) of all isolates have a MIC≤2 mg/L, although for the serotype 19A the rate of isolates with MIC≤2 mg/L has decreased from 94.4% to 54.5% after the implementation of the PCV7. In the case of H. influenzae, data are even more favorable, since most isolates present MIC≤2 mg/L for amoxicillin/clavulanate (table 3). Regarding cephalosporins, every dosing level is enough to treat infections due to microorganisms with MIC≤0.5 mg/L, but if MIC is 1 mg/L, only the highest dose of cefotaxime (66 mg/kg q8h) seems to be adequate.
Considering that AOM is typically treated empirically, the treatment of choice should target the most frequently isolated pathogens. As previously mentioned, in the post-vaccination period it was not only a serotype replacement, but also an increase of non-susceptibility rate of some serotypes not included in the vaccine against b-lactams, as serotype 19A [7]. Therefore we have calculated the probability of empirical treatment successful (CFR) in the pre- and post-vaccination period taking into account the MIC values. If we consider all serotypes of S. pneumoniae, only a slight decrease in the probability of success in the post-vaccination period was observed in comparison to the pre-vaccination period. Therefore, when the serotype is not identified, amoxicillin and cefotaxime may be good options for the treatment of AOM, although the probability of success slightly depends on the dose.
Although H. influenzae is not involved in the PCV7, mixed infections are common, and the association of this microorganism with S. pneumoniae has been widely demonstrated [9,10]. Moreover, an increase in the proportion of AOM cases caused by H. influenzae has been shown after the introduction of pneumococcal vaccines [2,3]. This is the reason why we have also studied the probability of treatment success before and after the introduction of the PCV7 when H. influenzae is responsible for the infection. According to the CFR values obtained and, as expected, the implantation of the vaccine hardly led to relevant changes in the activity of the antibiotics studied. In spite that in the post-PCV7 period, the rate of β-lactamase-producing H. influenzae strains was lower than before the introduction of the vaccine, and that the rate of BLNAR isolates hardly changed, only small differences in the CFR values of amoxicillin and amoxicillin/clavulanate were found between both periods. Amoxicillin/clavulanate provided slightly probabilities of treatment success than amoxicillin. Regarding cephalosporins, cefotaxime provided very high probability of therapy success both before and after the introduction of the PCV7. On the contrary, cefuroxime axetil resulted in a very low success probability in both periods.
National vaccination recommendations outside routine infant immunization programs differ among EU countries. Some countries have age-based vaccination programs, while others have risk-based programs, and some countries have regional variations with respect to recommendations [27]. Previous studies have shown that PK/PD analysis is a useful tool to identify differences in the antibiotic treatment success due to different susceptibility patterns [21]. Our study reveals that this methodology is also useful for the surveillance programs to evaluate the effect of a vaccine.
The change of serotype epidemiology due to the PCV7 has led to the development and introduction of higher-valent pneumococcal conjugate vaccines, including PCV13, which includes serotype 19A, to provide improved serotype coverage against pneumococcal diseases. In Spain, PCV13 has been available since June 2010 and vaccination is recommended for pediatric patients. However, before 2016 in most provinces of Spain, the vaccine was not financed by the public health insurance and it had to be paid by parents, leading to non-universal coverage. Although after the introduction of the PCV13, a reduction in the frequency of infections due to vaccine serotypes, mainly 19A and 1, was observed [30,31], the lack of available data does not allow to include the post-PCV13 period in the present study.
In conclusion, this study demonstrates the value of integrated PK/PD analysis to identify changes in antimicrobial activity after the implantation of a vaccination program, providing complementary information to the simply assessing of MIC values. We have shown that the introduction of the PCV7 vaccination did not lead to changes in the probability of success of the current empiric treatments of the AOM.
ACKNOWLEDGMENTS
The authors would like to Asunción Fenoll, Lorenzo Aguilar, Silvia García Cobos, and José Campos, for providing specific MIC distributions of S. pneumoniae and H. influenzae.
FUNDING
This study was supported by the University of the Basque Country UPV/EHU (PPG17/65, GIU17/032).
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Hoberman A, Paradise JL, Rockette HE, Kearney DH, Bhatnagar S, Shope TR et al. Shortened antimicrobial treatment for acute otitis media in young children. N Engl J Med. 2016;375(25):2446-56. DOI: 10.1056/NEJMoa1606043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben-Shimol S, Givon-Lavi N, Leibovitz E, Raiz S, Greenberg D, Dagan R. Impact of widespread introduction of pneumococcal conjugate vaccines on pneumococcal and nonpneumococcal otitis media. Clin Infect Dis. 2016;63(5):611-8. DOI: 10.1093/cid/ciw347. [DOI] [PubMed] [Google Scholar]
- 3.Dagan R, Pelton S, Bakaletz L, Cohen R. Prevention of early episodes of otitis media by pneumococcal vaccines might reduce progression to complex disease. Lancet Infect Dis. 2016;16(4):480-92. DOI: 10.1016/S1473-3099(15)00549-6. [DOI] [PubMed] [Google Scholar]
- 4.Zhou F, Shefer A, Kong Y, Nuorti JP. Trends in acute otitis media-related health care utilization by privately insured young children in the United States, 1997–2004. Pediatrics. 2008;121(2):253-60. DOI: 10.1542/peds.2007-0619. [DOI] [PubMed] [Google Scholar]
- 5.Block SL, Hedrick J, Harrison CJ, Tyler R, Smith A, Findlay R et al. Community-wide vaccination with the heptavalent pneumococcal conjugate significantly alters the microbiology of acute otitis media. Pediatr Infect Dis J. 2004;23(9):829-33. [DOI] [PubMed] [Google Scholar]
- 6.Alonso M, Marimon JM, Ercibengoa M, Pérez-Yarza EG, Pérez-Trallero E. Dynamics of Streptococcus pneumoniae serotypes causing acute otitis media isolated from children with spontaneous middle-ear drainage over a 12-year period (1999-2010) in a region of northern Spain. PLoS One. 2013;8(1):e54333 DOI: 10.1371/journal.pone.0054333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenoll A, Aguilar L, Vicioso MD, Gimenez MJ, Robledo O, Granizo JJ. Increase in serotype 19A prevalence and amoxicillin non-susceptibility among paediatric Streptococcus pneumoniae isolates from middle ear fluid in a passive laboratory-based surveillance in Spain, 1997-2009. BMC Infect Dis. 2011;11:239 DOI: 10.1186/1471-2334-11-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewnard JA, Huppert A, Givon-Lavi N, Pettigrew MM, Regev-Yochay G, Dagan R et al. Density, serotype diversity, and fitness of Streptococcus pneumoniae in upper respiratory tract co-colonization with nontypeable Haemophilus influenzae. J Infect Dis. 2016; 214(9):1411-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leibovitz E, Serebro M, Givon-Lavi N, Greenberg D, Broides A, Leiberman A et al. Epidemiologic and microbiologic characteristics of culture-positive spontaneous otorrhea in children with acute otitis media. Pediatr Infect Dis J. 2009;28(5):381-4. doi: 10.1097/INF.0b013e318194e783. [DOI] [PubMed] [Google Scholar]
- 10.Hausdorff WP, Yothers G, Dagan R, Kilpi T, Pelton SI, Cohen R et al. Multinational study of pneumococcal serotypes causing acute otitis media in children. Pediatr Infect Dis J. 2002;21(11):1008-16. PMID: DOI: 10.1097/01.inf.0000035588.98856.05. [DOI] [PubMed] [Google Scholar]
- 11.Leibovitz E, Jacobs MR, Dagan R (2004) Haemophilus influenzae: a significant pathogen in acute otitis media. Pediatr Infect Dis J. 2004;23(12):1142-52. [PubMed] [Google Scholar]
- 12.Asín-Prieto E, Rodríguez-Gascón A, Isla A. Applications of the pharmacokinetic/pharmacodynamic (PK/PD) analysis of antimicrobial agents. J Infect Chemother. 2015;21(5):319-29. PMID: DOI: 10.1016/j.jiac.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Zelenitsky SA, Rubinstein E, Ariano RE, Zhanel GG. Integrating pharmacokinetics, pharmacodynamics and MIC distributions to assess changing antimicrobial activity against clinical isolates of Pseudomonas aeruginosa causing infections in Canadian hospitals (CANWARD). J Antimicrob Chemother. 2013;68:i67-72. DOI: 10.1093/jac/dkt028 [DOI] [PubMed] [Google Scholar]
- 14.Mouton JW, Dudley MN, Cars O, Derendorf H, Drusano GL. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs: an update. J Antimicrob Chemother. 2005;55(5):601-7. DOI: 10.1093/jac/dki079 [DOI] [PubMed] [Google Scholar]
- 15.Canafax DM, Yuan Z, Chonmaitree T, Deka K, Russlie HQ, Gienbink GS. Amoxicillin middle ear fluid penetration and pharmacokinetics in children with acute otitis media. Pediatr Infect Dis J. 1998;17(2):149-56. [DOI] [PubMed] [Google Scholar]
- 16.Isla A, Trocóniz IF, Canut A, Labora A, Martín-Herrero JE, Pedraz JL et al. Pharmacokinetic/pharmacodynamic evaluation of amoxicillin, amoxicillin/clavulanate and ceftriaxone in the treatment of paediatric acute otitis media in Spain. Enferm Infecc Microbiol Clin. 2011;29(3):167-73. DOI: 10.1016/j.eimc.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Scott LJ, Ormrod D, Goa KL. Cefuroxime axetil: an updated review of its use in the management of bacterial infections. Drugs. 2001;61(10):1455-500. [DOI] [PubMed] [Google Scholar]
- 18.Patel KB, Nicolau DP, Nightingale CH, Quintiliani R. Pharmacokinetics of cefotaxime in healthy volunteers and patients. Diagn Microbiol Infect Dis. 1995;22(1-2):49-55. [DOI] [PubMed] [Google Scholar]
- 19.Harding SM, Monro AJ, Thornton JE, Ayrton J, Hogg MI. The comparative pharmacokinetics of ceftazidime and cefotaxime in healthy volunteers. J Antimicrob Chemother. 1981;8:263-72. [DOI] [PubMed] [Google Scholar]
- 20.Kearns GL, Young RA, Jacobs RF.. Cefotaxime dosage in infants and children. Pharmacokinetic and clinical rationale for an extended dosage interval. Clin Pharmacokinet. 1992;22(4):284-97. DOI: 10.2165/00003088-199222040-00004. [DOI] [PubMed] [Google Scholar]
- 21.Canut A, Isla A, Betriu C, Gascón AR. Pharmacokinetic-pharmacodynamic evaluation of daptomycin, tigecycline, and linezolid versus vancomycin for the treatment of MRSA infections in four western European countries. Eur J Clin Microbiol Infect Dis. 2012;31(9): 2227-35. DOI: 10.1007/s10096-012-1560-7. [DOI] [PubMed] [Google Scholar]
- 22.Susceptibility to antimicrobials agents used in the community in Spain (SAUCE 3 Project). GlaxoSmithKline, S.A: 2004. (Data on file). [Google Scholar]
- 23.García-Cobos S, Moscoso M, Pumarola F, Arroyo M, Lara N, Pérez-Vázquez M et al. Frequent carriage of resistance mechanisms to b-lactams and biofilm formation in Haemophilus influenzae causing treatment failure and recurrent otitis media in young children. J Antimicrob Chemother. 2014;69(9):2394–9. DOI: 10.1093/jac/dku158. [DOI] [PubMed] [Google Scholar]
- 24.Clinical and Laboratory Standards Institute (CLSI) Performance standards for antimicrobial susceptibility testing. 28th ed. CLSI supplement M100 Wayne, PA,USA: CLSI; 2018. [Google Scholar]
- 25.Canut A, Isla A, Rodríguez-Gascón A. Pharmacokinetic/pharmacodynamic analysis to evaluate ceftaroline fosamil dosing regimens for the treatment of community-acquired bacterial pneumonia and complicated skin and skinstructure infections in patients with normal and impaired renal function. Int J Antimicrob Agents. 2015;45(4):399–405. doi: 10.1016/j.ijantimicag.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 26.Bradley JS, Dudley MN, Drusano GL. Predicting efficacy of antiinfectives with pharmacodynamics and Monte Carlo simulation. Pediatr Infect Dis J. 2003;22(11):982-92. DOI: 10.1097/01.inf.0000094940.81959.14. [DOI] [PubMed] [Google Scholar]
- 27.Torres A, Bonanni P, Hryniewicz W, Moutschen M, Reinert RR, Welte T. Pneumococcal vaccination: what have we learnt so far and what can we expect in the future? Eur J Clin Microbiol Infect Dis. 2015;34(1):19-31. DOI: 10.1007/s10096-014-2208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lieberthal AS, Carroll AE, Chonmaitree T, Ganiats TG, Hoberman A, Jackson MA et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131(3):e964-99. DOI: 10.1542/peds.2012-3488. [DOI] [PubMed] [Google Scholar]
- 29.Ozawa D, Yano H, Endo S, Hidaka H, Kakuta R, Okitsu N et al. Impact of the seven-valent pneumococcal conjugate vaccine on acute otitis media in Japanese children: Emergence of serotype 15A multidrug-resistant Streptococcus pneumoniae in middle ear fluid isolates. Pediatr Infect Dis J. 2015;34(9):e217-21. DOI: 10.1097/INF.0000000000000776. [DOI] [PubMed] [Google Scholar]
- 30.Payeras A, Villoslada A, Garau M, Salvador MN, Gallegos MC. Evolution of pneumococcal infections in adult patients during a four-year period after vaccination of a pediatric population with 13-valent pneumococcal conjugate vaccine. Int J Infect Dis. 2015;33:22-7. DOI: 10.1016/j.ijid.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 31.Lewnard JA, Givon-Lavi N, Weinberger DM, Lipsitch M, Dagan R. Pan-serotype reduction in progression of Streptococcus pneumoniae to otitis media after rollout of pneumococcal conjugate vaccines. Clin Infect Dis. 2017;65(11):1853-1861. DOI: 10.1093/cid/cix673. [DOI] [PMC free article] [PubMed] [Google Scholar]