Abstract
A man in his early 60s with myotonic dystrophy type 1 (DM1) and an extensive history of non-melanoma skin cancer presented with multiple pearly, erythematous papules on his face, head, trunk and extremities, clinically consistent with basal cell carcinoma (BCC). Due to the numerous BCC and history of multiple and early-onset BCC, examination was concerning for a hereditary BCC syndrome. Subsequent histopathology confirmed BCC. Genetic testing was negative for basal cell nevus syndrome and clinical findings were inconsistent with other known hereditary BCC syndromes. There have been reports of an association between DM1 and BCC, however, it is not well known among clinicians. We hope to raise awareness among clinicians about this association.
Keywords: dermatology, genetics, muscle disease
Background
Myotonic dystrophy type 1 (DM1), or Steinert Disease, is an autosomal dominant disease caused by a cytosine–thymine–guanine (CTG) trinucleotide repeat mutation in the myotonic dystrophy protein kinase (DMPK) gene on chromosome 19, locus 19q13.3.1 It is the most common muscular dystrophy in adults, characterised by myotonia, progressive muscle wasting and weakness, cardiac conduction abnormalities, cataracts and testicular atrophy.1 Known associated skin findings include androgenic alopecia and pilomatrixomas.1 An association with basal cell carcinoma (BCC) has been previously reported, but this is not well recognised by clinicians (table 1).2–9
Table 1.
Summary of published case reports of BCCs associated with myotonic dystrophy type 1
| Age | Sex | BCC features (number of tumours, anatomic site) | References |
| 34 | Male | Three tumours; nose, neck and back | 2 |
| 42 | Female | Four tumours, nodular subtype; left posterior thigh x2, right upper back and right submammary | 3 |
| 50 | Female | Three tumours; scalp | 4 |
| 41 | Male | Multiple tumours; left upper eyelid x1, left cheek x1, and thorax (multiple, number not specified) | 5 |
| 42 | Female | Multiple tumours, number not specified, nodular subtype; face | 6 |
| 62 | Female | Four tumours, nodular and superficial subtype; face | 7 |
| 29 | Male | Multiple tumours, number not specified; chest | 8 |
| 18 | Female | Three tumours; right parasternal, left breast and abdomen | 9 |
BCC, basal cell carcinoma.
Case presentation
A 60-year-old man with DM1 and a history of numerous BCCs presented for skin cancer evaluation and management. Prior skin cancer history was notable for 28 primary BCCs and four squamous cell carcinomas, with his first skin cancer diagnosed in his early 40s. He denied any systemic or other cutaneous complaints. He reported no family history of skin cancer. He is currently retired and describes his previous occupation as office-based without significant occupation-related sun exposure. He reports a substantial history of recreational sun exposure with multiple sunburns in his youth but denies a history of blistering sunburns or indoor tanning.
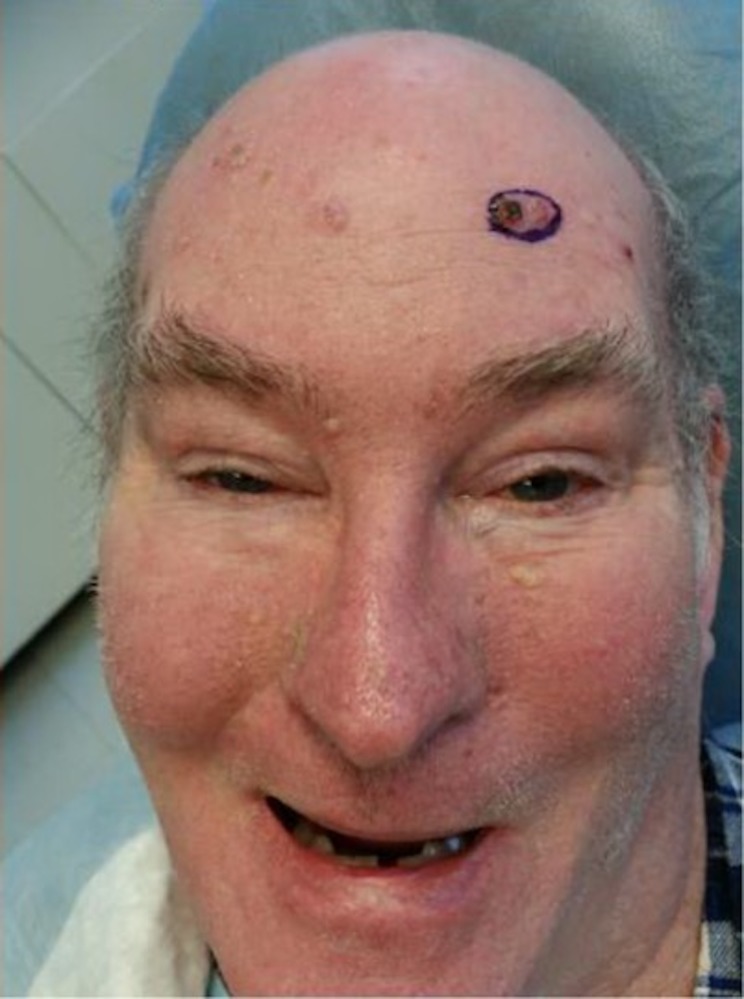
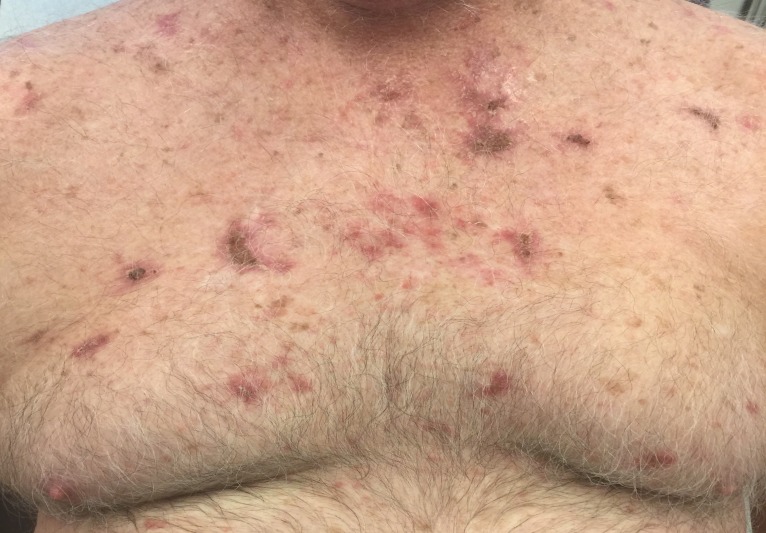
Skin exam was notable for Fitzpatrick type I skin, androgenic pattern alopecia (figure 1), and multiple (>20) pearly erythematous papules and plaques distributed on sun-exposed areas of the head and neck, trunk and extremities, all clinically consistent with BCC (figure 2). Sun-protected areas of his buttocks and upper inner arms were spared. There was no frontal bossing, palmar pits or atrophoderma.
Figure 1.
The patient presents with the characteristic androgenic alopecia of myotonic dystrophy type 1 as well as multiple pearly papules on the face and forehead clinically concerning for basal cell carcinoma.
Figure 2.
Numerous well-demarcated, erythematous, pearly crusted-papules and plaques on the patient’s chest, clinically concerning for basal cell carcinoma.
Investigations
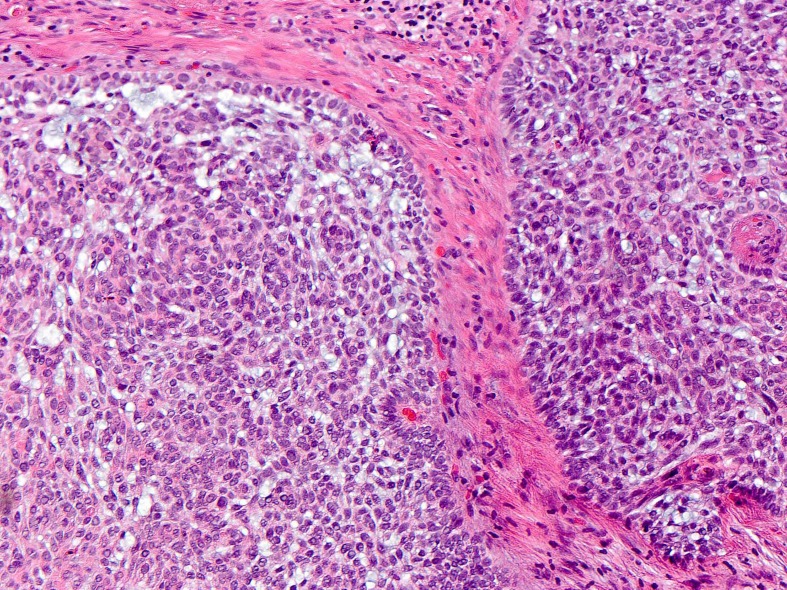
Biopsy of multiple tumours showed superficial, nodular and infiltrative type BCC (figure 3). Formal genetic evaluation was negative for known hereditary BCC-associated syndromes and there were no mutations or copy number variants in PTCH1, PTCH2 and SUFU.
Figure 3.
A biopsy of the patient’s right anterior shoulder showing basal cell carcinoma, nodular subtype.
Differential diagnosis
This 60-year-old patient with DM1 presented with numerous and early onset BCCs predominantly on sun-exposed skin, raising clinical suspicion for an underlying hereditary BCC syndrome, such as basal cell nevus syndrome, Rombo syndrome and Bazex-Dupré-Christol syndrome. However, clinical exam and genetic testing was negative for basal cell nevus syndrome. Clinical exam was not consistent with other hereditary BCC syndromes, including Rhombo and Bazex-Dupré-Christol, as the patient lacked the characteristic physical exam findings of atrophoderma and milia.
Treatment
The patient was treated with multiple modalities including field therapy using fluorouracil 5% cream and photodynamic therapy, electrodessication and curettage, excision and Mohs surgery. Systemic treatment with nicotinamide was initiated. Acitretin and SUBA-itraconazole were discussed and declined by the patient.
Outcome and follow-up
The patient had an excellent response to field therapy. He is closely followed with complete skin examinations and referrals for excisions and Mohs surgery as needed.
Discussion
To our knowledge, this is the ninth reported case of an association between DM1 and multiple, early-onset BCCs (table 1).2–9 A population-based nested case-control study found a significantly higher BCC risk among DM1 patients than age- and sex-matched controls (HR=5.78, 95% CI 3.36 to 9.92, p<0.0001).10 Another study found that, BCCs developed at a significantly younger age in patients with DM1 as compared with the general population (51 years vs 66 years, p=0.035).11 There may also be an association between DM1 other forms of skin cancer. A recent study found higher numbers of nevi, dysplastic nevi and cutaneous melanoma in DM1 patients when compared with controls, despite a greater proportion of the control subjects reporting a history sunburn.12
The mechanism of action for increased BCC risk may involve the mutated DMPK gene, which creates abnormally spliced mRNA that may interfere with protein production, DNA repair (including ultraviolet-induced damage) and cell death.1 7 Defects in the 19q chromosomal locus near the DMPK gene are associated with an increased risk of BCC.13 Mutations to this region may affect the nuclear genome or disrupt the normal function of tumour suppressors or DNA repair mechanisms. Further evidence of the role of the DMPK gene in BCC carcinogenesis comes from analysis of tumour-normal pairs where BCCs from a DM1 patient contained a larger trinucleotide repeat expansion than the patient’s healthy skin.9 Additional case reports of patients with DM1 have found markedly longer CTG expansions in tumours (endometroid carcinoma, insulinoma, gastric adenocarcinoma and adenocarcinoma of the colon) as compared with healthy tissue of the same organ.14 15 However, it has also been reported that the trinucleotide expansion in the oligodendroglioma of a patient with DM1 was shorter than surrounding healthy brain tissue.16 Thus, alterations (consisting of both expansions and contractions) in CTG repeat length appear to be implicated in tumourigenesis in DM1 patients.
Multiple BCCs in DM1 patients may suggest a link between the DMPK gene and keratinocyte carcinomas, possibly highlighting a novel pathway for BCC carcinogenesis.
Learning points.
There have been multiple reports of numerous and early basal cell carcinomas (BCCs) in patients with myotonic dystrophy type 1 (DM1).
Future research is needed to elucidate the exact molecular mechanism underlying the increased BCC risk in DM1.
Sun protection and skin cancer surveillance should be emphasised with DM1 patients
Dermatologists should be involved in the interdisciplinary approach for the care of patients with DM1.
Acknowledgments
The authors would like to acknowledge Dr Eduardo De Flammineis for his assistance with this case report.
Footnotes
Contributors: JF and MA were responsible for the conceptualisation of the project. JF, AL and MA collected the data, analysed and interpreted the data. JF was the primary author of the manuscript. All authors (JF, AL, DAS and MA) were involved in the drafting and critical revision of the article. All authors approved the final version of the article.
Funding: The study was funded by National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant number: K24 AR069760).
Competing interests: MA receives grant funding from Pfizer Inc. and Valeant Pharmaceuticals for unrelated studies on health outcomes in psoriasis and eczema. D.A.S. was supported by the Glenn Foundation for Medical Research and grants from the NIH (R37 AG028730, R01 AG019719 and R01 DK100263). D.A.S. is a founder, equity owner, board member, advisor to, director of, consultant to, investor in and/or inventor on patents licensed to Vium, Jupiter Orphan Therapeutics, Cohbar, Galilei Biosciences, GlaxoSmithKline, OvaScience, EMD Millipore, Wellomics, Inside Tracker, Caudalie, Bayer Crop Science, Longwood Fund, Zymo Research, EdenRoc Sciences (and affiliates Arc-Bio, Dovetail Genomics, Claret Bioscience, Revere Biosensors, UpRNA and MetroBiotech (an NAD booster company), Liberty Biosecurity), Life Biosciences (and affiliates Selphagy, Senolytic Therapeutics, Spotlight Biosciences, Animal Biosciences, Iduna, Immetas, Prana, Continuum Biosciences, Jumpstart Fertility (an NAD booster company), and Lua Communications). D.A.S. sits on the board of directors of both companies. D.A.S. is an inventor on a patent application filed by Mayo Clinic and Harvard Medical School that has been licensed to Elysium Health; his personal royalty share is directed to the Sinclair lab. For more information see https://genetics.med.harvard.edu/sinclair-test/people/sinclair-other.php.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Turner C, Hilton-Jones D. Myotonic dystrophy: diagnosis, management and new therapies. Curr Opin Neurol 2014;27:599–606. 10.1097/WCO.0000000000000128 [DOI] [PubMed] [Google Scholar]
- 2. Stieler W, Plewig G. [Multiple basaliomas in Curschmann-Steinert myotonia atrophica]. Hautarzt 1986;37:226–9. [PubMed] [Google Scholar]
- 3. Azurdia RM, Verbov JL. Myotonic dystrophy and basal cell carcinoma-a true association? Br J Dermatol 1999;141:941–2. 10.1046/j.1365-2133.1999.03184.x [DOI] [PubMed] [Google Scholar]
- 4. Itin PH, Laeng RH. [Multiple pigmented basalioma of the scalp in a patient with Curschmann-Steinert myotonia dystrophica. Confirmation of a rare symptom constellation]. Hautarzt 2001;52:244–6. [DOI] [PubMed] [Google Scholar]
- 5. Bañuls J, Botella R, Palau F, et al. Tissue and tumor mosaicism of the myotonin protein kinase gene trinucleotide repeat in a patient with multiple basal cell carcinomas associated with myotonic dystrophy. J Am Acad Dermatol 2004;50:1–3. 10.1016/S0190-9622(03)00125-7 [DOI] [PubMed] [Google Scholar]
- 6. Saponaro AE, Marini MA, Rossi GC, et al. Multiple basal cell carcinomas in a patient with myotonic dystrophy type 1. Int J Dermatol 2006;45:87–8. 10.1111/j.1365-4632.2004.02583.x [DOI] [PubMed] [Google Scholar]
- 7. Goolamali SI, Edmonds EV, Francis N, et al. Myotonic dystrophy and basal cell carcinomas: coincidence or true association? Clin Exp Dermatol 2009;34:e370 10.1111/j.1365-2230.2009.03321.x [DOI] [PubMed] [Google Scholar]
- 8. Zemtsov A. Association between basal, squamous cell carcinomas, dysplastic nevi and myotonic muscular dystrophy indicates an important role of RNA-binding proteins in development of human skin cancer. Arch Dermatol Res 2010;302:169–70. 10.1007/s00403-009-0997-8 [DOI] [PubMed] [Google Scholar]
- 9. Miraglia E, Cantisani C, Giustini S, et al. Basal cell carcinomas in a young woman with Steinert’s disease. Dermatol Online J 2014;20. [PubMed] [Google Scholar]
- 10. Wang Y, Pfeiffer RM, Alsaggaf R, et al. Risk of skin cancer among patients with myotonic dystrophy type 1 based on primary care physician data from the U.K. Clinical Practice Research Datalink. Int J Cancer 2018;142:1174–81. 10.1002/ijc.31143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marcoval J, Olivé M, Bonfill-Ortí M, et al. Cutaneous Neoplasms in Myotonic Dystrophy Type 1. Dermatology 2016;232:700–3. 10.1159/000456074 [DOI] [PubMed] [Google Scholar]
- 12. Zampetti A, Silvestri G, Manco S, et al. Dysplastic nevi, cutaneous melanoma, and other skin neoplasms in patients with myotonic dystrophy type 1: a cross-sectional study. J Am Acad Dermatol 2015;72:85–91. 10.1016/j.jaad.2014.09.038 [DOI] [PubMed] [Google Scholar]
- 13. Yin J, Rockenbauer E, Hedayati M, et al. Multiple single nucleotide polymorphisms on human chromosome 19q13.2-3 associate with risk of Basal cell carcinoma. Cancer Epidemiol Biomarkers Prev 2002;11:1449–53. [PubMed] [Google Scholar]
- 14. Jinnai K, Sugio T, Mitani M, et al. Elongation of (CTG)n repeats in myotonic dystrophy protein kinase gene in tumors associated with myotonic dystrophy patients. Muscle Nerve 1999;22:1271–4. [DOI] [PubMed] [Google Scholar]
- 15. Kinoshita M, Igarashi A, Komori T, et al. Differences in CTG triplet repeat expansions in an ovarian cancer and cyst from a patient with myotonic dystrophy. Muscle Nerve 1997;20:622–4. [DOI] [PubMed] [Google Scholar]
- 16. Rakocevic-Stojanovic V, Peric S, Ralic V, et al. Shorter CTG repeats length in an oligodendroglioma from a myotonic dystrophy type 1 patient. Acta Neurol Belg 2015;115:505–7. 10.1007/s13760-014-0336-5 [DOI] [PubMed] [Google Scholar]