Abstract
Whole-person care is a new paradigm for serious illness, but few programs have been robustly studied. We sought to test the effect of LifeCourse (LC), a person-centered program for patients living with serious illness, on health-care utilization, care experience, and quality of life, employing a quasi-experimental design with a Usual Care (UC) comparison group. The study was conducted 2012 to 2017 at an upper-Midwest not-for-profit health-care system with outcomes measured every 3 months until the end of life. Enrolled patients (N = 903) were estimated to be within 3 years of end of life and diagnosed with 1+ serious illness. Exclusion criteria included hospice enrollment at time of screening or active dying. Community health workers (CHWs) delivered standardized monthly 1-hour home visits based on palliative care guidelines and motivational interviewing to promote patients’ physical, psychosocial, and financial well-being. Primary outcomes included health-care utilization and patient- and caregiver-experience and quality of life. Patients were elderly (LC 74, UC 78 years) and primarily non-Hispanic, white, living at home with cardiovascular disease as the primary diagnosis (LC 69%, UC 57%). A higher proportion of LC patients completed advance directives (N = 173, 38%) than UC patients (N = 66, 15%; P < .001). LifeCourse patients who died spent more days in hospice (88 ± 191 days) compared to UC patients (44 ± 71 days; P = .018). LifeCourse patients reported greater improvements than UC in communication as part of the care experience (P = .016). Implementation of person-centered programs delivered by CHWs is feasible; inexpensive upstream expansion of palliative care models can yield benefits for patients and caregivers. Trial Registration: Trial NCT01746446 was registered on November 27, 2012 at ClinicalTrials.gov.
Keywords: person-centered program, palliative care, community health worker, whole-person care, care navigator, late life
Introduction
The suffering attendant to cancer, heart failure, and other advanced disease is manifest in pain, breathlessness, and progressive weakness. Just as great is the existential struggle with loss of function, social isolation, and impending mortality.1,2 When patients look for support with serious illness, they look beyond medical care, seeking face to face relationships with the health system, assurance of proactive care as they leave the hospital, help with practical tasks, in-home visits for companionship or prayer, supportive social networks, a sense of autonomy, and enhanced meaning in life as its end approaches.3–7
Over the course of chronic illness, family members serve as the default caregivers. They provide unpaid care, often for years preceding death, in an enterprise that rivals Medicare and Medicaid in economic scope and hours of care.8–11 Caregivers are both providers and receivers of care; they desire acknowledgment of their important role, respect for their value systems, and practical support for caregiving.12–14
Whole-person care has been proposed as a paradigm for the care of serious illness that acknowledges the nonmedical aspects of suffering and the importance of family support.15–18 Palliative care teams that provide this approach are largely focused on clinical services in hospitals and ambulatory settings, where licensed clinicians deliver expert services to a limited population.19,20 The need for more accessible whole-person care, inclusive of family, and delivered earlier in the disease, prompted this study. The success of clinic-based navigators for chronic illness led to consideration of a community health worker (CHW) model.21 Defined by the American Public Health Association as “a frontline public health worker who is a trusted member of and/or has an unusually close understanding of the community served. This trusting relationship enables the worker to serve as a liaison/link/intermediary between health/social services and the community to facilitate access to services and improve the quality and cultural competence of service delivery. A community health worker also builds individual and community capacity by increasing health knowledge and self-sufficiency through a range of activities such as outreach, community education, informal counseling, social support, and advocacy.”22
Community health workers contribute to better care in chronic conditions such as hypertension, diabetes, asthma, obesity, smoking, cognitive impairment, tuberculosis treatment, and morbidity that increases risk of readmission.4,7,21,23–31 Community health workers have proved acceptable to medical teams.32,33 In cancer care, CHWs have been employed in 2 large multisite trials. In the National Cancer Institute-funded Patient Navigation Research Program, time from screening to diagnosis was reduced, but without change in stage at diagnosis.34,35 The Patient Care Connect intervention utilized 8 community and academically based cancer-care teams. A lay navigator followed patients from onset of symptoms to death.36 Navigation was accompanied by reductions in emergency visits, inpatient utilization, and total cost.37 Activities associated with the CHW role in these studies included motivational interviewing, familiarity with social supports, advance care planning, and goal-setting.31,33,38–40
Systematic reviews of CHW outcomes are limited by statistical power, formal economic analysis, and difficulty quantifying CHW tasks. Generally positive trends are found for cardiovascular and cancer screening, particularly with underserved populations.38 Studies reporting utilization show 20% to 50% reduction in emergency visits, urgent care, or hospitalization.41 The cost savings from CHW interventions are less often reported, ranging in annual savings from US$368-3124.21,35,37
This study was initiated to understand the effectiveness of a program using trained lay members of the health-care team working in the home setting with patients experiencing serious illness. This program, named LifeCourse by end users, involved monthly in-person contacts and a consistent structure for engaging patients and families in all domains of palliative care, based on national consensus guidelines.42 The primary aim of this quasi-experimental trial was to assess the effect of the LifeCourse (LC) program on health-care utilization with a secondary aim to understand the effect of LC on patients’ and caregivers’ care experience and quality of life (QOL).
Methods
Study Setting, Population, and Study Design
Study participants were recruited from a not-for-profit health system in the upper Midwest that includes 12 hospitals, 90 clinics, and specialty medical services. Patients were recruited from 7 hospitals and care centers in the Minneapolis and Saint Paul metro areas. Eligible patients were identified via electronic health record (EHR) review to (1) be over age 18, (2) have a current health system primary care provider, (3) reside within a 35-mile radius of the primary hospital, (4) be diagnosed with at least one complex illness (including cancer, dementia, Parkinson’s disease, heart failure, chronic obstructive pulmonary disease, cardiovascular disease, diabetes, or liver disease), and (5) be within 3 years or less from the end of life as determined by an algorithm and nurse chart review. Patients were excluded if they were (1) eligible for or enrolled in hospice at time of screening, (2) actively dying or (3) had major mental health or chemical dependency issues, (4) unable to speak and write English, or (5) refused or were unable to provide written informed consent. Patients who screened eligible were contacted by study staff via phone. Interested patients scheduled a home visit to complete informed consent.
This trial was a quasi-experimental intervention study with a Usual Care (UC) comparison group. Patients assigned to the LifeCourse intervention group were recruited from 6 area hospitals or care centers and were enrolled in the study between October 2012 and September 2015. Patients declining the intervention were offered participation in UC group. Patients assigned to UC, with a 1:1 allocation ratio, were primarily recruited from one separate hospital and were enrolled between April 2014 and July 2016.
Intervention Description (Following the NIH Treatment Fidelity Framework)
The LC intervention was informed by the Clinical Practice Guidelines for Quality Palliative Care and used motivational interviewing principles.43,44 LifeCourse was structured to promote person-centered care through discussion of patients’ physical, psychosocial, and financial-legal concerns. Intervention content was delivered via standardized assessments and question sets during hour-long, monthly in-home visits (see addendum for question sets and standardized assessments used in LC). Caregivers were invited to participate in visits as patients desired. Visits continued through end of life or hospice enrollment. Each visit was structured to set intentions for the visit, discuss goals, complete question sets, and conduct guided assessments. Visits were documented in the EHR. LifeCourse enabled patients to articulate what matters most to them and their goals for living with illness through the use of these standardized question sets answered and revisited over the course of several visits. LifeCourse helped patients seek care that aligned with their goals through the inclusion of patient goals in the electronic health record, coupled with behavioral changes elicited through motivational interviewing.
LifeCourse visits were delivered by a CHW, called a care guide, who completed a standard 2 week training focused on palliative care domains, relationship skills, and visit protocols. Care guides were trained to document each visit in the EHR and to enhance communication between patients and their care teams. Care guides had bachelor’s degrees or higher and were supervised by a social worker or nurse manager. Throughout the intervention, patients continued to receive standard medical care, including palliative, care management, home care, and/or hospice care services.
Treatment fidelity
The number of delivered home visits was collected via the EHR. Patients and caregivers were asked to report their satisfaction with the LC program.
Usual Care Description
Patients assigned to usual care did not receive the LC intervention but continued to receive standard medical care which could include palliative, care management, home care, and/or hospice care services.
Data Collection and Measures
After enrollment, study participants completed the baseline survey. Follow-up surveys were completed every 3 months through the mail, via phone, or in-person with a trained data collection team member. To ensure surveys were returned by mail, the Dillman method was employed.45 Care guides were not permitted access to survey responses. Data collection team members were not blinded to treatment groups. Additional information was collected from patients’ EHRs.
Patient demographic variables
Collected via survey and EHR, demographic variables collected included age, sex, race, ethnicity, highest educational level, and marital status.
Patient health-related variables
Health-related variables were collected from the EHR, including primary diagnosis, comorbidity score, and date of death.
Patient healthcare utilization
Hospice length of stay, number of palliative care visits, completion of advance care plans, emergency department (ED) visits, intensive care unit (ICU) stays, and inpatient days were extracted from patient EHRs.
Patient QOL
Patient QOL was measured using the Functional Assessment of Chronic Illness Therapy-Palliative v4 (FACIT-Pal) survey, a general measure of health-related QOL in 4 domains: physical, social, emotional, and functional, plus a measure of end-of-life experiences.46 Items were reverse scored according to scoring guidelines and domain scores were calculated via prorated scores when there were <50% missing items for a given domain and <20% missing domains for a total score.
Patient care experience
Patient care experience was collected via a previously validated survey tool focused on the patient’s experience with their care team in the last 30 days.47 Three domains were scored: care team, goals, and communication with prorated scores when there were <50% missing items for a given domain and <20% missing domains for a total score.
Caregiver care experience
Caregiver care experience was measured by a developed tool, addressing various aspects of care experience. An overall score was calculated by summing answers to all items. Scores were also calculated in 3 domains: care team, communication, and support.
Caregiver QOL
Caregiver QOL was assessed via the patient-reported outcomes measurement information system (PROMIS) which asks caregivers to report their QOL in 8 domains.48 The PROMIS-29 scores were calculated by summing answers to all items within each domain.
Statistical Analysis
Descriptive statistics (means, standard deviations, and frequencies) were used to describe the study sample at baseline. Two-tailed independent sample t tests and χ2 were used to compare baseline characteristics across treatment groups. To test the association between treatment group and health-care utilization variables (ED visits, ICU stays, and inpatient days), negative binomial regression models were used. Models were adjusted for utilization 12 months prior to enrollment, demographics, and health-related variables. The χ2 was used to test differences in hospice enrollment across groups and Wilcoxon rank-sum was used to test between-group differences in length of hospice stay. The association between intervention and changes in QOL and care experience (both patient and caregiver) was assessed using random coefficients regression allowing for a random intercept and random slope. Models were adjusted for patient age, sex, race, ethnicity, education, marital status, primary diagnosis, baseline location, and comorbidity score. Models testing caregiver outcomes were also adjusted for caregiver age, sex, and relationship to patient. All tests are 2-tailed with α set at .05. Analyses were conducted in Stata version 14.
Institutional Review Board Approval
This study was approved by Quorum Review institutional review board prior to data collection and all study participants provided informed consent.
Results
Recruitment and Participant Flow
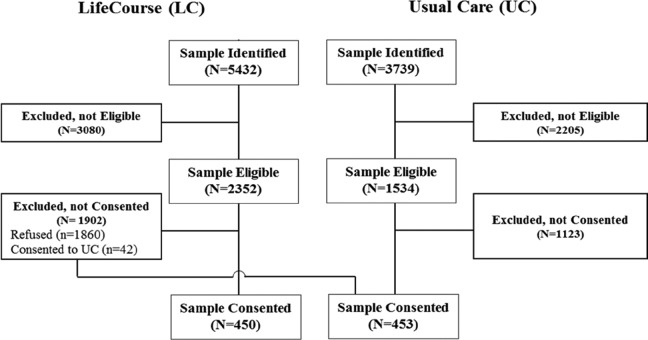
Figure 1 depicts how patients were enrolled in the study. Nine thousand one hundred seventy one patients were identified as potentially eligible through EHR screening, (59%, N = 5432 in LC and 41%, N = 3739 in UC.) The relative percent of the identified sample deemed ineligible was similar across groups (58% in LC and 59% in UC). The percent of eligibles not consenting was 35% in LC (N = 1902) and 30% in UC (N = 1123). Forty-two patients identified as eligible for the intervention refused, but consented to UC. A total of 903 patients consented to participate in the study, 10% of the identified sample.
Figure 1.
Modified CONSORT diagram for the LifeCourse study.
Information on the number of patients retained throughout the study is presented in Table 1. By 12 months, 18% (n = 176) of enrolled patients died, 16% (n = 140) dropped out of the study (n = 140), 14% (n = 125) were active in the study but did not complete the 12-month survey, and 51% (n = 462) completed the 12-month survey. The survey return rate, excluding those who died prior to the round, was 64% at 12 months (462 returned survey/[587 participated in the round + 140 dropped out prior to round]). The survey return rate was 65% in the UC group and 62% in the LC group at 12 months. Of the 301 LC patients who participated in the study through 30 months, 81% returned the 30-month survey (data not shown).
Table 1.
Response Patterns and Retention of Patients in the LifeCourse Study, (N = 903).
| 0M | 3M | 6M | 9M | 12M | 15M | 18M | 21M | 24M | 27M | 30M | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Died prior to round | 0 | 44 | 90 | 139 | 176 | 209 | 233 | 252 | 271 | 296 | 309 |
| Dropped out prior to roundA | 1 | 56 | 85 | 109 | 140 | 168 | 199 | 232 | 264 | 270 | 293 |
| Participated in roundB | 902 | 803 | 728 | 655 | 587 | 526 | 471 | 419 | 368 | 337 | 301 |
| Unable to reach | 17 | 42 | 39 | 61 | 106 | 136 | 154 | 160 | 181 | 188 | 203 |
| Unable to complete | 8 | 8 | 7 | 8 | 2 | 1 | 1 | 1 | 0 | 1 | 1 |
| Refused | 5 | 15 | 18 | 18 | 17 | 15 | 17 | 11 | 9 | 9 | 6 |
| Returned surveyC | 872 | 738 | 664 | 568 | 462 | 374 | 299 | 247 | 178 | 139 | 91 |
| Survey Return Rate a | 97% | 86% | 82% | 74% | 64% | 54% | 45% | 38% | 28% | 23% | 15% |
| UC Survey Rate | 95% | 90% | 91% | 82% | 65% | 49% | 38% | 34% | 26% | 20% | 10% |
| LC Survey Rate | 87% | 77% | 73% | 66% | 62% | 59% | 52% | 43% | 31% | 26% | 21% |
Abbreviations: LC, LifeCourse; UC, Usual Care.
a The survey return rate is calculated as the percent of patients who returned surveys out of those who were eligible to participate in the round, C/(A + B) × 100.
Baseline Descriptive Characteristics
Descriptive statistics for UC and LC patients at baseline are presented in Table 2. The 2 groups were similar in terms of sex, race, ethnicity, and education. Approximately half of the patients were female, the majority were non-Hispanic and white, and about two-thirds had some college education. There were statistically significant differences in terms of age (UC patients were younger, P < .0001) and marital status (UC patients were more likely to be married, P = .0200). At baseline, more UC patients still lived at home (91%) compared to LC patients (73%) and their primary diagnosis was more likely to be cardiovascular and less likely to be dementia when compared to UC (P < .0001). However, the comorbidity score of those in the UC and LC groups were similar (P = .2757). Health-care utilization in the 12 months prior to study enrollment was also similar across groups, as was baseline QOL, with the exception of the palliative domain. Patients in the UC group reported slightly better palliative QOL compared to the LC group at baseline (P = .0348). Patients in the UC group also reported better care experience, particularly in the Care Team domain (P = .0002) when compared to LC at baseline.
Table 2.
Baseline Demographic and Outcome Characteristics for the Overall Population, When Available, and Enrolled Study Patients by Treatment Group (Usual Care or Intervention).
| Usual Care, (N = 453), M (SD) or N (%) | Intervention, (N = 450), M (SD) or N (%) | P Valuea | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 74.3 (12.5) | 78.1 (12.0) | <.001 |
| Sex (% female) | 234 (52%) | 228 (51%) | .766 |
| Race | |||
| American Indian or Alaska native | 1 (<1%) | 0 (0%) | .694 |
| Asian | 3 (1%) | 2 (<1%) | |
| Black or African American | 19 (4%) | 20 (4%) | |
| Patient declined | 0 (0%) | 1 (<1%) | |
| White | 430 (95%) | 427 (95%) | |
| Ethnicity (% non-Hispanic) | 451 (>99%) | 442 (98%) | .238 |
| Marital status | |||
| Single | 26 (6%) | 40 (9%) | .020 |
| Married, domestic partnership | 224 (50%) | 202 (45%) | |
| Unmarried partnership | 11 (2%) | 4 (1%) | |
| Divorce, separated | 72 (16%) | 58 (13%) | |
| Widowed | 120 (26%) | 146 (32%) | |
| Highest level of education | |||
| 8th grade or less | 11 (3%) | 11 (3%) | .445 |
| Some high school | 18 (4%) | 23 (5%) | |
| High school graduate | 129 (30%) | 100 (23%) | |
| Some college or 2-year degree | 119 (27%) | 125 (29%) | |
| 4-year college degree | 77 (18%) | 81 (19%) | |
| Graduate or professional degree | 81 (19%) | 88 (21%) | |
| Health-related variables | |||
| Baseline location | |||
| Home | 405 (91%) | 319 (73%) | <.001 |
| Assisted living | 4 (1%) | 53 (12%) | |
| Nursing home | 36 (8%) | 65 (15%) | |
| Unknown | |||
| Primary diagnosis | |||
| Cardiovascular | 311 (69%) | 258 (57%) | <.001 |
| Dementia | 64 (14%) | 121 (27%) | |
| Cancer | 78 (17%) | 71 (16%) | |
| Comorbidity score | 4.6 (1.9) | 4.5 (2.2) | .311 |
| Preenrollment utilization | |||
| Advance directives in place (% yes) | 189 (42%) | 163 (36%) | .090 |
| ED visits in previous 12 months | 1.5 (2.3) | 1.7 (2.0) | .137 |
| Inpatient days in previous 12 months | 4.1 (5.9) | 4.3 (6.2) | .532 |
| ICU stays in previous 12 months | 0.4 (2.8) | 0.6 (2.9) | .445 |
| Quality of life | |||
| Total (range 0-184) | 134.3 (28.1) | 132.6 (27.2) | .381 |
| Domain | |||
| Physical | 19.6 (6.2) | 20.2 (5.6) | .177 |
| Emotional | 17.5 (5.0) | 17.7 (4.9) | .621 |
| Social | 22.0 (4.9) | 21.5 (5.2) | .110 |
| Functional | 17.0 (6.2) | 16.5 (6.2) | .221 |
| Palliative | 54.8 (11.8) | 53.1 (11.7) | .035 |
| Care experience | |||
| Total | 72.3 (8.8) | 69.7 (9.6) | .001 |
| Domain | |||
| Care team | 44.8 (6.3) | 42.9 (7.3) | <.001 |
| Communication | 17.3 (2.7) | 16.9 (2.8) | .076 |
| Goals | 10.0 (1.6) | 9.9 (1.7) | .188 |
Abbreviations: ED, emergency department; ICU, intensive care unit.
a P-values from t tests or χ2 comparing usual care and intervention group patients.
Treatment Fidelity
The average LC patients attended 11.2 (standard deviation [SD] 10.9) home visits and 4.1 (SD 4.2) phone calls with a care guide and were enrolled in the LC program for 510 (SD 369) days. After 3 months, 95% of LC patients and 89% of LC caregivers said they would probably or definitely recommend the program. This remained high throughout the study, with 97% of patients and 94% of caregivers stating they would probably or definitely recommend the LC program after 30 months.
Aim 1: Utilization
A significantly higher proportion of LC patients completed an advance directive since enrolling in the study (n = 173, 38%) than did the UC patients (n = 66, 15%; Pearson Χ2 = 72.43, P < .001). Of patients who died during the course of the study, the proportion of LC patients who utilized hospice (n = 105, 47%) was higher but not significantly different than the proportion of UC patient who utilized hospice (n = 65, 41%; Χ2 = 1.23, P = .267). However, LC patients who died spent significantly more days in hospice (M = 88 days, SD = 191 days) compared to UC patients (M = 44 days, SD = 71 days; rank sum z = 2.364, P = .018). There was no significant difference in number of days spent in the ED, hospital, or ICU between groups after controlling for baseline levels of utilization, demographics, and health-related variables.
Aim 2: Patient Experience and Quality of Life
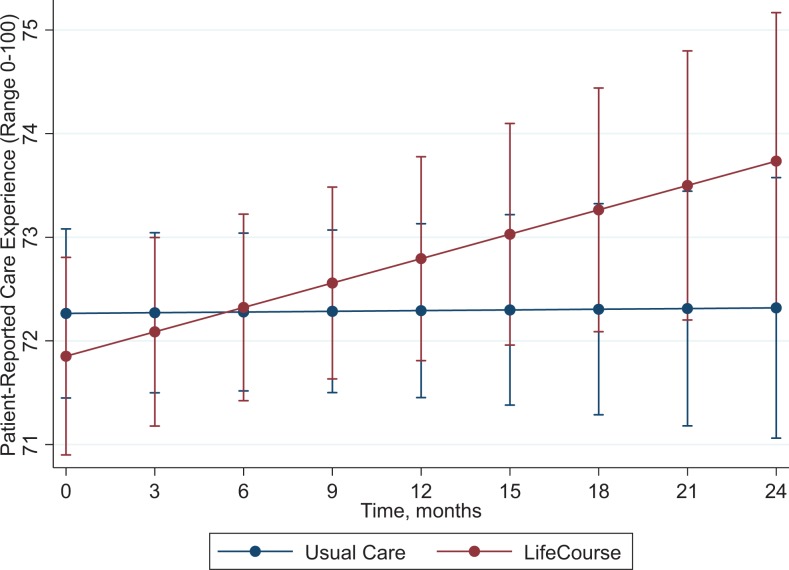
Table 3 provides the results from random coefficient regression models exploring the association between treatment groups and changes in QOL or care experience over the course of the study. Each of these models was adjusted for patient demographic and health-related variables. Patients in the LC group reported greater improvement in the communication domain of care experience throughout the study than did patients in the UC group (P = .016, see Figure 2). There was no other statistically significant treatment by time effects on the patient care experience or QOL domains. Previous analyses revealed early positive effects on patient quality of life.29
Table 3.
Regression Coefficients Representing the Treatment by Time Effect on Each of the Listed Outcome Variables.a
| B | P Value | 95% CI | ||
|---|---|---|---|---|
| Patient care experience | ||||
| Overall | .046 | .667 | −1.53 | 0.981 |
| Care team | .031 | .233 | −.020 | .083 |
| Communication | .022 | .032 | .002 | .042 |
| Goals | −.010 | .080 | −.022 | .001 |
| Patient Quality of Life | ||||
| Overall | .042 | .649 | −.138 | .221 |
| Physical | −.004 | .707 | −.043 | .033 |
| Social | .011 | .521 | −.022 | .044 |
| Emotional | .006 | .773 | −.026 | .037 |
| Functional | −.005 | .788 | −.048 | .036 |
| Palliative | −004 | .927 | −.082 | .075 |
| Caregiver care experience | ||||
| Overall | .033 | .573 | −.083 | .145 |
| Care team | .037 | .383 | −.046 | .119 |
| Communication | .010 | .519 | −.021 | .041 |
| Support | .020 | .084 | −.003 | .043 |
| Caregiver quality of life | ||||
| Physical Functioning | .045 | .236 | −.029 | .118 |
| Anxiety | −.098 | .038 | −.190 | −.005 |
| Depression | −.098 | .014 | −.176 | −.020 |
| Fatigue | −.060 | .235 | −.160 | .039 |
| Sleep | −.065 | .066 | −.134 | .004 |
| Social | .036 | .443 | −.056 | .127 |
| Pain | .010 | .847 | −.088 | .108 |
Abbreviation: CI, confidence interval.
a All models adjusted for caregiver demographics, patient demographic, and patient health-related variables.
Figure 2.
Random coefficients regression model showing the relationship between treatment group and the communication domain of care experience over time.
Aim 3: Caregiver Experience and Quality of Life
The results from random coefficient regression models exploring the association between treatment group and changes in caregiver QOL or care experience over the course of the study are presented in Table 3. Each of these models were adjusted for caregiver demographics, patient demographic, and patient health-related variables. There were no associations between treatment group and changes over time in caregivers’ care experience. When examining changes in caregiver’s QOL over the course of the study, the UC group caregivers had greater increases in the anxiety and depression domains throughout the study compared to LC group caregivers, whose levels remained stable (treatment by time B = −.098, P = .038, and B = −.098, P = .014, respectively). There was no statistically significant treatment by time effects on the other QOL domains.
Discussion
In an effort to concurrently address the challenges of an aging demographic, increasing longevity despite serious illness, and a shortage of palliative practitioners, we developed and studied a late life supportive care intervention delivered via CHWs employed by a health system. We found that development and implementation of such a model was possible, with high fidelity to expected home-based visit frequency, accompanied by high patient and caregiver satisfactions. Substantial enrollment of patients and their caregivers in the trial was achieved through chart review and development of a selection algorithm, demonstrating that it is reasonable to systematically identify eligible patients and to engage their close friends and family members. Use of CHWs allowed current care teams to include nontraditional service providers with whom patients and families can develop a different type of relationship compared to licensed clinicians. Community health workers in the LC program were allowed to build deep relationships with patients and families, could build liaison bridges between health-care team members and other community and social service supports, and could meet patients where they were at in their understanding, experience of, and decision-making related to late life. Because CHWs were the only team members delivering the LC intervention, we are unable to determine which component had specific effects.
A quasi-experimental approach was selected as the study design; this choice was appropriate because this was the first major test of such an approach and because offering a longer-term intervention through a randomized trial could be troubling ethically. Additionally, a comparison between group of patients and caregivers was successfully enrolled and the research team achieved a reasonable response rate among vulnerable patients over the duration of the study. This enabled long-term understanding of effectiveness—somewhat unique in care delivery research.
The research questions for this trial were designed to help understand what type of effect is possible for a palliative care informed intervention deployed upstream in the disease trajectory for patients and their families. The LC approach yielded a significant increase in the completion of advance directives. It also was associated with doubling the number of days in hospice—a service that improves outcomes at the end of life.30 While we saw no change in the QOL of LC patients as compared to those in UC, we did find an improvement in care experience, especially around communication. Further, while caregivers did not experience an improvement in experience, we noted that UC caregivers were more likely to see an increase in anxiety and depression over time compared to caregivers in LC. LifeCourse caregivers maintained stable levels of anxiety and depression over the course of the study. These results, taken in sum, suggest that efficient and inexpensive expansions of the palliative care model for individuals upstream in their disease process can yield benefits for patients and caregivers alike. Discernment of whether the LC model can be deployed by other team members or whether CHWs are able to yield positive outcomes in late life patients without the structure of the LC model are research questions that remain to be explored.
While this study was restricted to a single geographic location and healthcare system and homogenous patients and caregivers, we believe the model has promise. Faced with the question of how we are going to take care of those with long-term serious illness, we must strive to consider approaches that transform care to align health and social resources with the needs of the entire population of patients living with serious illness. The challenge undertaken here was to develop and study a novel care delivery approach rapidly enough to yield knowledge at a time when additional supports for the most vulnerable are desperately needed. The LC model is one step toward that challenge, one that we encourage other leaders across healthcare to continue to tackle.
Supplemental Material
Supplemental Material, LifeCourse_Visit_Framework_and_Questions_for_AJHPM for Quasi-Experimental Evaluation of LifeCourse on Utilization and Patient and Caregiver Quality of Life and Experience by Heather R. Britt, Meghan M. JaKa, Karl M. Fernstrom, Paige E. Bingham, Anne E. Betzner, Jessica R. Taghon, Nathan D. Shippee, Tetyana P. Shippee, Sandra E. Schellinger, and Eric W. Anderson in American Journal of Hospice and Palliative Medicine®
Footnotes
Authors’ Note: Meghan M. JaKa is now affiliated with Health Partners Institute, Bloomington, MN, USA. H.R.B. outlined the article and was a major contributor in writing. K.M.F., A.E.B., and M.M.J. performed analysis of data, created the figures and tables, and were major contributors in writing. T.P.S. and N.D.S. were partners outlining the manuscript and major editors through the development of the manuscript. P.E.B. and S.E.S. were major contributors in writing. J.R.T. was a contributor in writing and a major editor of the final manuscript. E.W.A. was a partner outlining the manuscript and was a major writer of the introduction and discussion sections. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The design, methods, subject recruitment, data collections, analysis, and preparation of the article were all supported by the Robina Foundation.
ORCID iD: Jessica R. Taghon  https://orcid.org/0000-0001-5985-7461
https://orcid.org/0000-0001-5985-7461
Eric W. Anderson  https://orcid.org/0000-0002-9203-7980
https://orcid.org/0000-0002-9203-7980
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Murray SA, Boyd K, Kendall M, Worth A, Benton TF, Clausen H. Dying of lung cancer or cardiac failure: prospective qualitative interview study of patients and their carers in the community. BMJ. 2002;325(7370):929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hegarty MM, Abernethy AP, Olver I, Currow DC. Former palliative caregivers who identify that additional spiritual support would have been helpful in a population survey. Palliat Med. 2011;25(3):266–277. [DOI] [PubMed] [Google Scholar]
- 3. Hanson LC, Armstrong TD, Green MA, et al. Circles of care: development and initial evaluation of a peer support model for African Americans with advanced cancer. Health Edu & Behav. 2013;40(5):536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hartgerink JM, Cramm JM, Bakker TJ, Mackenbach JP, Nieboer AP. The importance of older patients’ experiences with care delivery for their quality of life after hospitalization. BMC Health Serv. 2015;15:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kastbom L, Milberg A, Karlsson M. A good death from the perspective of palliative cancer patients. Support Care Cancer. 2017;25(3):933–939. [DOI] [PubMed] [Google Scholar]
- 6. Schneider H, Lehmann U. Lay health workers and HIV programmes: implications for health systems. AIDS Care. 2010;22:60–67. [DOI] [PubMed] [Google Scholar]
- 7. Kangovi S, Mitra N, Grande D, et al. Patient-centered community health worker intervention to improve posthospital outcomes: a randomized clinical trial. JAMA Intern Med. 2014;174(4):535–543. [DOI] [PubMed] [Google Scholar]
- 8. Wittenberg E, Prosser LA. Health as a family affair. N Engl J Med. 2016;374(19):1804–1806. [DOI] [PubMed] [Google Scholar]
- 9. Arno PS, Levine C, Memmott MM. The economic value of informal caregiving. Health Aff. 1999;18(2):182–188. [DOI] [PubMed] [Google Scholar]
- 10. Chari AV, Engberg J, Ray KN, Mehrotra A. The opportunity costs of informal elder-care in the United States: new estimates from the American Time Use Survey. Health Serv Res. 2015;50(3):871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Alliance for Caregiving and AARP Public Policy Institute. Caregiving in the U.S. 2015. https://www.aarp.org/content/dam/aarp/ppi/2015/caregiving-in-the-united-states-2015-report-revised.pdf. Accessed November 30, 2018.
- 12. Farquhar M, Penfold C, Walter FM, Kuhn I, Benson J. What are the key elements of educational interventions for lay carers of patients with advanced disease? A systematic literature search and narrative review of structural components, processes and modes of delivery. J Pain Symptom Manage. 2016;52(1):117–130.e127. [DOI] [PubMed] [Google Scholar]
- 13. Grande G, Stajduhar K, Aoun S, et al. Supporting lay carers in end of life care: current gaps and future priorities. Palliat Med. 2009;23(4):339–344. [DOI] [PubMed] [Google Scholar]
- 14. Anderson EW, White KM. “This is what family does”: the family experience of caring for serious illness. Am J Hosp Palliat Care. 2018;35(2):348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mount B, Kearney M. Healing and palliative care: charting our way forward. Palliat Med. 2003;17(8):657–658. [DOI] [PubMed] [Google Scholar]
- 16. Kearney M. A Place of Healing: Working with Nature and Soul at the End of Life. New Orleans: Spring Journal; 2009. [Google Scholar]
- 17. Hutchinson TA, Hutchinson N, Arnaert A. Whole person care: encompassing the two faces of medicine. CMAJ. 2009;180(8):845–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cassel C, Katherine F. Principles for Care of Patients at the End of Life: An Emerging Consensus Among the Specialties of Medicine. New York: Millbank Memorial Fund; 1999. [Google Scholar]
- 19. Spetz J, Dudley N, Trupin L, Rogers M, Meier DE, Dumanovsky T. Few hospital palliative care programs meet national staffing recommendations. Health Aff. 2016;35(9):1690–1697. [DOI] [PubMed] [Google Scholar]
- 20. Overview for policymakers: Growing demand, limited supply. Center to Advance Palliative Care; 2016. https://www.capc.org/policymakers/overview/. Accessed November 30, 2018. [Google Scholar]
- 21. Adair R, Wholey DR, Christianson J, White KM, Britt H, Lee S. Improving chronic disease care by adding laypersons to the primary care team: a parallel randomized trial. Ann Intern Med. 2013;159(3):176–184. [DOI] [PubMed] [Google Scholar]
- 22. American Public Health Association. Community Health Workers. 2018. https://www.apha.org/apha-communities/member-sections/community-health-workers. Accessed November 30, 2018. [DOI] [PubMed]
- 23. Lewin S, Babigumira S, Bosch-Capblanch X, et al. Lay Health Workers in Primary and Community Health Care: A Systematic Review of Trials. Geneva: WHO; 2006. [DOI] [PubMed] [Google Scholar]
- 24. Thom DH, Ghorob A, Hessler D, De Vore D, Chen E, Bodenheimer TA. Impact of peer health coaching on glycemic control in low-income patients with diabetes: a randomized controlled trial. Ann Fam Med. 2013;11(2):137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Viswanathan M, Kraschnewski J, Nishikawa B, et al. Outcomes of community health worker interventions. Evid Rep Technol Assess (Full Report). June 2009;(181):1–44. [PMC free article] [PubMed] [Google Scholar]
- 26. Galbraith AA, Meyers DJ, Ross-Degnan D, et al. Long-term impact of a postdischarge community health worker intervention on health care costs in a safety-net system. Health Serv Res. 2017;52(6):2061–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Herber OR, Johnston BM. The role of healthcare support workers in providing palliative and end-of-life care in the community: a systematic literature review. Health Soc Care Community. 2013;21(3):225–235. [DOI] [PubMed] [Google Scholar]
- 28. Lanese BS, Dey A, Srivastava P, Figler R. Introducing the health coach at a primary care practice: a pilot study (part 2). Hosp Top. 2011;89(2):37–42. [DOI] [PubMed] [Google Scholar]
- 29. Lanese BS, Dey A, Srivastava P, Figler R. Introducing the health coach at a primary care practice: impact on quality and cost (Part 1). Hosp Top. 2011;89(1):16–22. [DOI] [PubMed] [Google Scholar]
- 30. Leahey TM, Wing RR. A randomized controlled pilot study testing three types of health coaches for obesity treatment: professional, peer, and mentor. Obesity. 2013;21(5):928–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Litzelman DK, Inui TS, Griffin WJ, et al. Impact of community health workers on elderly patients’ advance care planning and health care utilization: moving the dial. Med Care. 2017;55(4):319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Adair R, Christianson J, Wholey DR, et al. Care guides: employing nonclinical laypersons to help primary care teams manage chronic disease. J Ambul Care Manage. 2012;35(1):27–37. [DOI] [PubMed] [Google Scholar]
- 33. Adelman AM, Graybill M. Integrating a health coach into primary care: reflections from the Penn State Ambulatory Research Network. Ann Fam Med. 2005;3(suppl 2):S33–S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hendren S, Griggs JJ, Epstein RM, et al. Study protocol: a randomized controlled trial of patient navigation-activation to reduce cancer health disparities. BMC Cancer. 2010;10:551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bensink ME, Ramsey SD, Battaglia T, et al. Costs and outcomes evaluation of patient navigation after abnormal cancer screening: evidence from the Patient Navigation Research Program. Cancer. 2014;120(4):570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rocque GB, Partridge EE, Pisu M, et al. The patient care connect program: transforming health care through lay navigation. J Oncol Pract. 2016;12(6):e633–e642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rocque GB, Pisu M, Jackson BE, et al. Resource use and Medicare costs during lay navigation for geriatric patients with cancer. JAMA Oncol. 2017;3(6):817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim K, Choi JS, Choi E, et al. Effects of community-based health worker interventions to improve chronic disease management and care among vulnerable populations: a systematic review. Am J Public Health. 2016;106(4):e3–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kangovi S, Mitra N, Grande D, Huo H, Smith RA, Long JA. Community health worker support for disadvantaged patients with multiple chronic diseases: a randomized clinical trial. Am J Public Health. 2017;107(10):1660–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Calhoun EA, Whitley EM, Esparza A, et al. A national patient navigator training program. Health Promo Practice. 2010;11(2):205–215. [DOI] [PubMed] [Google Scholar]
- 41. Jack HE, Arabadjis SD, Sun L, Sullivan EE, Phillips RS. Impact of community health workers on use of healthcare services in the United States: a systematic review. J Gen Intern Med. 2017;32(3):325–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. National Consensus Project for Quality Palliative Care. Clinical Practice Guidelines for Quality Palliative Care, 3rd ed Pittsburgh, PA: National consensus project for quality palliative care; 2013. [Google Scholar]
- 43. National Consensus Project for Quality Palliative Care. Clinical practice guidelines for quality palliative care. Kans Nurse. 2004;79(9):16. [PubMed] [Google Scholar]
- 44. Rollnick S, Miller WR, Butler CC, Aloia MS. Motivational interviewing in health care: helping patients change behavior. New York, NY: The Guilford Press; 2008. [Google Scholar]
- 45. Dillman DA. Mail and Internet Surveys; The Tailored Design Method, 2nd ed New York, NY: John Wiley & Sons, Inc; 2000: 167–170. [Google Scholar]
- 46. Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fernstrom KM, Shippee ND, Jones AL, Britt HR. Development and validation of a new patient experience tool in patients with serious illness. BMC Palliat Care. 2016;15(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, LifeCourse_Visit_Framework_and_Questions_for_AJHPM for Quasi-Experimental Evaluation of LifeCourse on Utilization and Patient and Caregiver Quality of Life and Experience by Heather R. Britt, Meghan M. JaKa, Karl M. Fernstrom, Paige E. Bingham, Anne E. Betzner, Jessica R. Taghon, Nathan D. Shippee, Tetyana P. Shippee, Sandra E. Schellinger, and Eric W. Anderson in American Journal of Hospice and Palliative Medicine®