ABSTRACT
Background:
Formation of struvite stones is associated with urinary tract infection by urease-producing bacteria. Biogenic crystal growth in natural and synthetic materials is regulated by the action of inhibitors, ranging from small ions, molecules to large macromolecules.
Materials and Methods:
We report the dynamics of in vitro crystallization of struvite in presence of vitamin C in synthetic urine using single diffusion gel growth technique. Sodium metasilicate gel of specific gravity 1.05 and the aqueous solution of ammonium dihydrogen phosphate were used as the medium for growing the struvite crystals. The crystallization process was induced by a urease positive struvite stone associated Pseudomonas aeruginosa to mimic the infection leading to stone formation. The grown crystals were characterized by ATR-FTIR and powder XRD. The surface morphology was analysed through FE-SEM for comparison between treatments.
Results:
We observed decrease in number, dimension, and growth rate of struvite crystals with the increasing concentrations of vitamin C. Crystals displayed well-defined faces and dendritic morphology of struvite in both control and biogenic systems.
Conclusion:
The results strongly suggest that, vitamin C can modulate the formation of struvite crystals in the presence of uropathogenic bacteria.
Keywords: Struvite, Pseudomonas aeruginosa, Ascorbic Acid
INTRODUCTION
Struvite or magnesium ammonium phosphate stone is associated with infections by urease-producing bacteria and accounts for about 10-15% of all kidney stones (1). The enzyme urease produced by the bacteria can cause super-saturation and crystallization of Mg2+ and PO4 3– as carbonate apatite (Ca10 (PO4)6.CO3) and struvite (MgNH4PO4.6H2O), respectively (2, 3). Struvite crystals can aggregate and form large crystals in the branches of the collecting system, to form large aggregates called staghorns. If untreated, they can cause significant kidney damage, and can sometimes also be life-threatening due to loss of kidney function (4).
Previous study from our group reported the high diversity of mixed stones having two or more than two types of mineral compositions among kidney stone patients (5). Struvite stones can be present as pure types or along with other compositions such as calcium oxalate and hydroxyapatite crystals. Struvite crystallization is mediated by the urease producing bacteria such as: Staphylococcus (Gram-positive), Proteus, Pseudomonas, Providencia and Klebsiella (Gram-negative). In addition, certain species of Serratia, Corynebacterium and Morganella also produce the enzyme urease which can lead to stone formation. Treatment of struvite stones involves stone removal followed by antibiotic therapy to eliminate bacteria from the urinary tract (6). Crystal growth in biogenic, natural and synthetic medium is regulated by the action of various inhibitors. In vitro crystallization of struvite has been increasingly investigated over the last years (7-9). However, bacterially induced struvite crystallization and its inhibition studies have higher implications in healthcare (6, 8, 10).
Compounds such as curcumin and vanillic acid can inhibit the growth of struvite in bacteria--induced crystallization in vitro (6, 11). Ascorbic acid, or vitamin C, is an essential micronutrient required for the normal metabolic functions and acts as an electron donor or reducing agent in biochemical reactions (12). Studies on vitamin C in kidney stone disease have shown mixed results with respect to oxalate metabolism and excretion (13, 14). It was initially reported that vitamin C can reduce the urinary pH (15, 16), however, others have found it as an ineffective urinary acidifier (17, 18).
Here we report the role of vitamin C on crystallization and pathogenesis of struvite crystal caused by P. aeruginosa isolated from the infectious kidney stone. To best of our knowledge there are no reports on uropathogenic P. aeruginosa induced struvite crystallization and inhibition of the same by vitamin C.
MATERIALS AND METHODS
Chemicals and Reagents
Ammonium di-hydrogenphosphate (NH4H2PO4), sodium metasilicate (Na2SiO3), magnesium acetate solution (C4H6MgO4) and vitamin C were of analytical grade and purchased from commercial sources.
Bacterial Strain and Culture Conditions
All the procedures performed in studies involving human participants were approved by the Institutional Scientific Review Board (YRCSRB/034/17) and Institutional Ethics Committee (YUEC.No.2016/022) of the Yenepoya University. Pseudomonas aeruginosa strain YU22S, previously isolated from the stone culture of a patient with struvite stone was used. P. aeruginosa was tested for urease activity using urea agar and phenol hypochlorite assay (secondary screening). The bacteria were cultured on tryptic soy broth (TSB) for 18h at 37°C and cells were harvested by centrifugation (6000rpm, 8 min). Density of the suspension was determined using McFarland standards and spectrophotometrically (OD 550nm). The cells were suspended in synthetic urine (artificially prepared aqueous solution with mineral compositions for simulating the urine) to an appropriate concentration (105cells/mL).
Preparation of Synthetic Urine
The synthetic urine used for the crystallization was prepared according to Griffith et al., (19) (g/L): CaCl2 2H2O, (0.651); MgCl2 6H2O, (0.651); NaCl, (4.6); Na2SO4, (2.3); KH2PO4, (2.8); KCl, (1.6); NH4Cl, (1.0); sodium citrate, (0.65); sodium oxalate, (0.02); urea, (25.0); creatine, (1.1); and TSB, (10). The content of the mineral components in the synthetic urine corresponds to mean concentration found in 24h period in normal human urine.
Experimental Setup
The single diffusion gel growth technique was used to study the growth and inhibition of struvite crystals as described elsewhere (8, 20). Briefly, sodium metasilicate (SMS) solution of specific gravity 1.05 was used to prepare the gel. An aqueous solution of ammonium di-hydrogenphosphate (0.5M) was mixed with the SMS solution in appropriate amount, so that the gel pH was set at 7.0 in test tubes (140mm length and 25mm diameter). To this, 20mL supernatant solutions of 0.5M magnesium acetate in synthetic urine along with different concentrations of vitamin C were gently poured on the gels without disturbing it. Bacteria suspended in synthetic urine at a density of 5×105 CFU/mL was used for the groups having bacterial addition. All the procedures were done aseptically. Bacterial growth in the synthetic urine was assessed every 24h by plating on trypticase soy agar (TSA) to monitor the viability. All the experiments were performed in triplicates at 37±0.5°C. During the experiment, pH of the samples was measured using a digital pH meter. Additionally, samples from different stages of the crystallization process were observed under a light microscope. Macro-morphology of grown crystals was recorded using stereomicroscope (Carl Zeiss, Göttingen, Germany).
Characterization of Struvite Crystals
Samples from different stages of the crystallization process were collected and used for investigations. The grown crystals were harvested from the test tube set up and characterized by Field Emission Scanning Electron Microscope (FE-SEM), Attenuated Total Reflectance-Fourier transform infrared spectroscopy (ATR-FTIR), and X-ray diffraction (XRD). ATR-FTIR of the amorphous crystals was directly recorded using the instrument (Shimadzu IR Prestige-21) and compositions were determined by the FTIR spectra at mid frequency range (4000-400cm−1) at 4cm−1 resolution. The XRD patterns were recorded with a Rigaku MiniFlex 600 laboratory diffractometer using a Cu-Κα radiation (λ=1.5406Å). Diffraction patterns were registered within the 2° angle range from 10 to 80° and the phase identification was calculated from the diffractograms. The microstructure and morphology were observed using FE-SEM (Carl Zeiss, Germany).
Statistical analysis
All the experiments were performed in triplicates. Continuous variables are reported as means ± standard deviation. Pearson's correlation was used to correlate the amount of struvite crystals formed in control and test groups. The value of p<0.05 was considered statistically significant. Statistical analysis was performed using SPSS, Version 22.0. (IBM Corp).
RESULTS
Inhibitory Activity of Vitamin C during Bacteria-induced Struvite Crystallization.
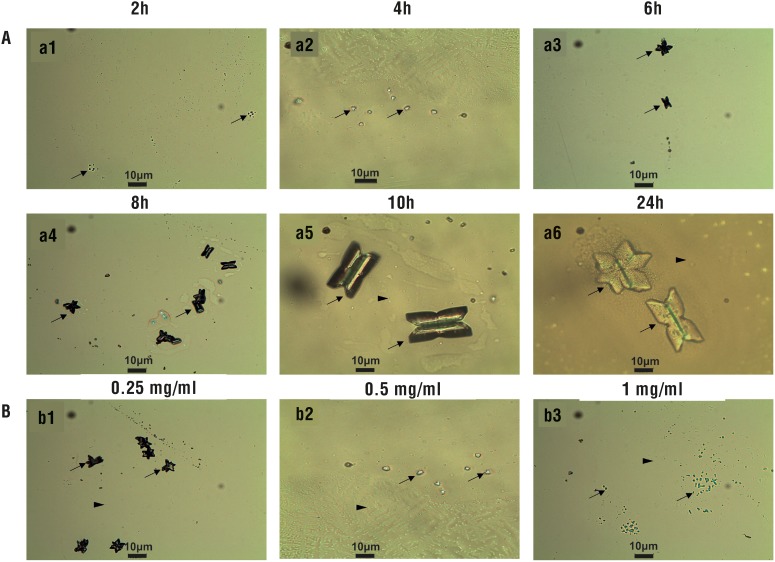
The early stages (2-24h) of struvite crystallization are given in Figure-1. During this interval, struvite exhibited typical hemimorphic morphology of coffin-lid shape along with bacterial cell. In the presence of different concentrations of vitamin C, crystal formation was delayed compared to control. In both control and vitamin C treated groups, dendritic and X-shaped crystals were formed. However, in the presence of vitamin C, the crystals formed were comparatively smaller and lesser in numbers.
Figure 1. Struvite crystals grown in artificial urine infected with Pseudomonas aeruginosa (a). Temporal growth pattern in control (a1-a6) (b). Inhibition of struvite crystals due to vitamin C treatment 24 h (b1:0.25 mg/mL, b2:0.5 mg/mL and b3:1 mg/mL). “Arrow” shows crystals and “arrowhead” shows bacteria.
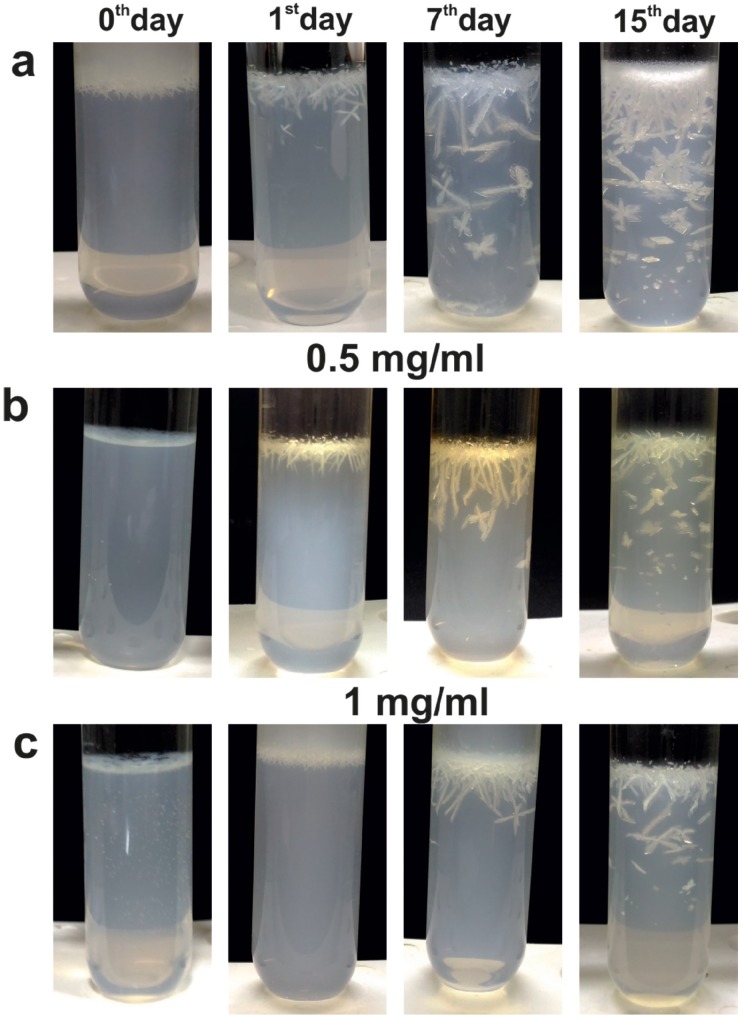
Due to the urease activity, at higher pH, the crystals frequently formed twins, and large dendritic branches (Figure-1A, image a6). The time-resolved experiments in the gel media showed the growth of small dendritic type crystals at the end of the first day in the gel at the gel-liquid interface. The initial pinpoint crystals tend to nucleate randomly throughout the test tube and in its surfaces (Figure-2). With the increase in time, the amount of crystals and size gradually increased. However, vitamin C concentrations and the weight of the struvite crystals showed significant inverse correlation (r= 0.962, p<0.05).
Figure 2. Photograph of the struvite crystals grown in gel medium in artificial urine infected with Pseudomonas aeruginosa and treated with different concentrations of vitamin C at different time points (0th, 1st, 7th and 15th day). (a) In the absence of vitamin C (b & c) In the presence of 0.5 mg/mL and 1 mg/mL vitamin C respectively.
Effect of pH, Bacterial Viability and Dimensions of Struvite during Crystallization.
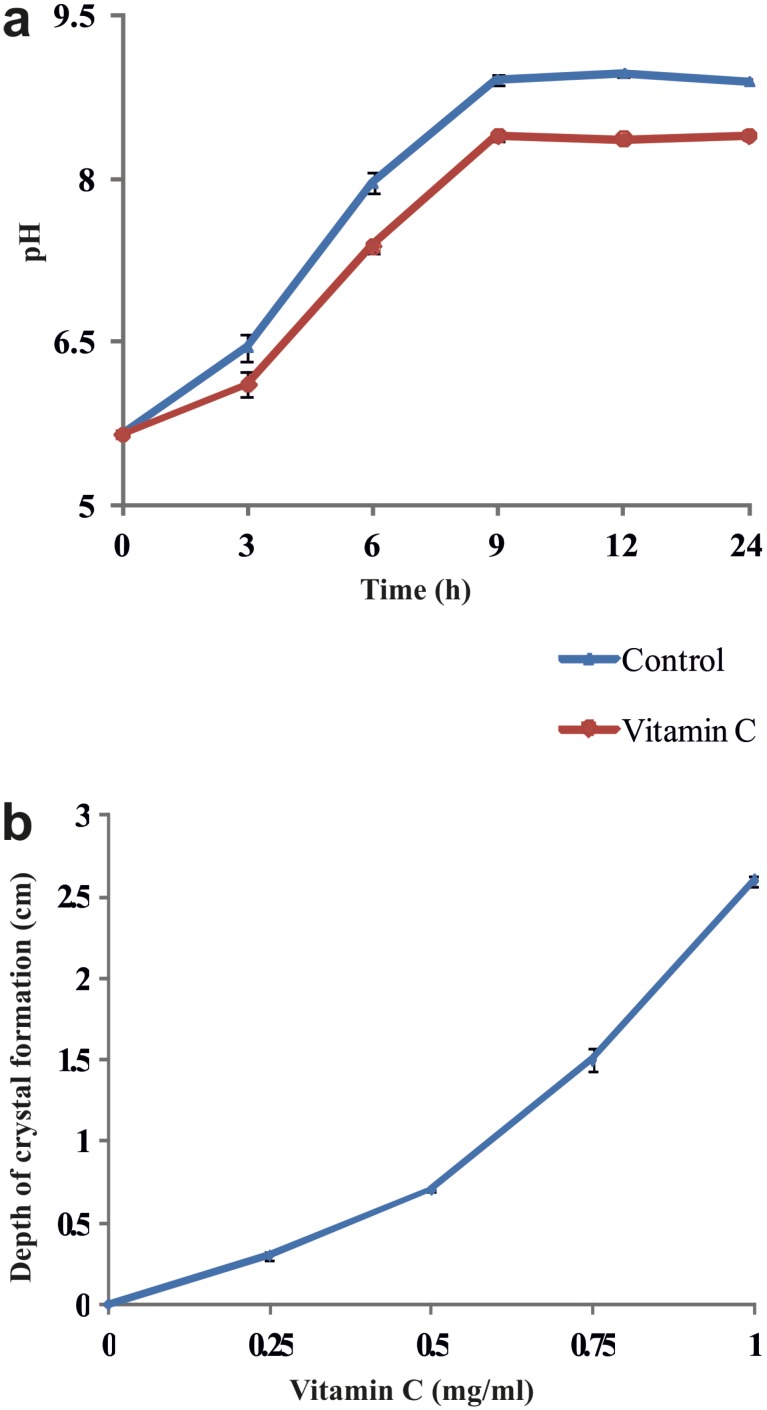
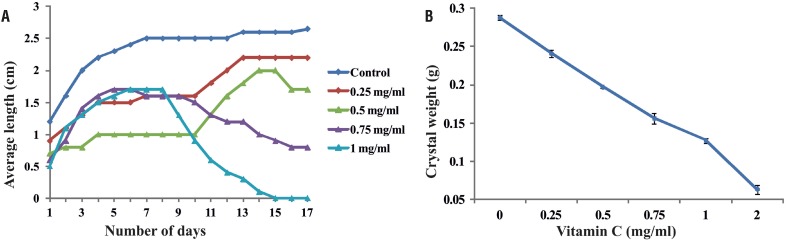
In the P. aeruginosa infected synthetic urine, a progressive change in the pH was observed during the initial 24h, from pH 5.65 to 8.9. In particular, in the presence of vitamin C, urine pH increased slower compared with the control (Figure-3A). Interestingly, crystal formation presence of vitamin C in the gel was in the higher depth compared to control. This may be possibly due to the penetration of vitamin C into the upper region of the gel. The depth at which crystals formed increased with the concentration of vitamin C (Figure-3B). The size and weight of the harvested crystals showed concentration dependent changes with vitamin C treatment (Figure-4).
Figure 3. a) Kinetics of pH of the artificial urine infected with Pseudomonas aeruginosa without (control) and with vitamin C. b) Depth of struvite crystals formed in the gel media in different concentration of vitamin C.
Figure 4. Relationship between vitamin C concentrations and (a) length and (b) weight of struvite crystals obtained in gel growth technique.
Structural and Morphological Characterization of Struvite Crystals
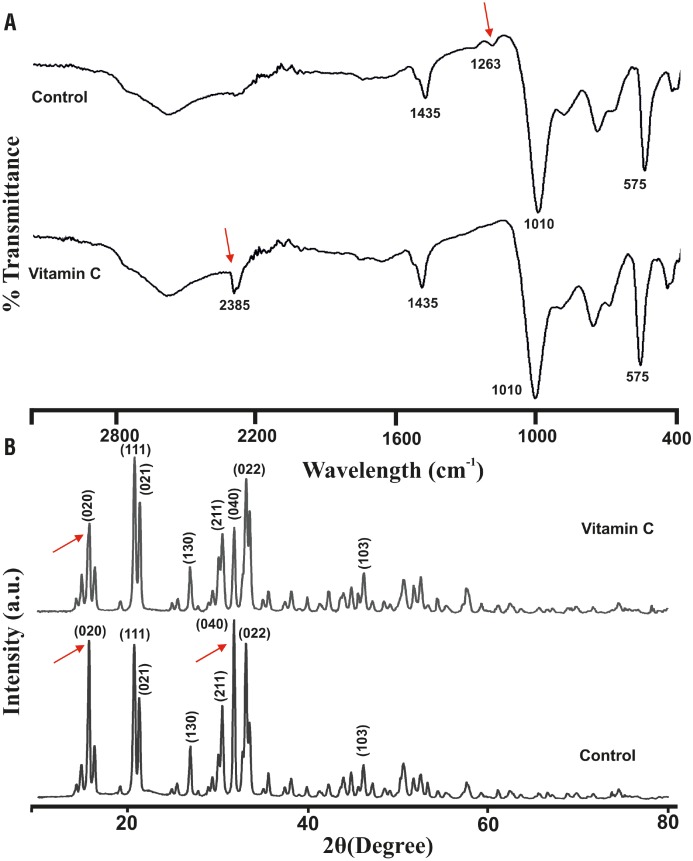
Struvite crystals showed characteristic IR spectrum at 1010cm−1 due to the absorption of PO4 3– (v3). Peaks at 1469, 1435 and 1400 cm−1 attributed to (v4) NH4+ bending and the peaks at 892 and 761cm−1 correspond to the ammonium-water H bonding and water-water H bonding respectively. The P-O bend (v4) and the PO4 3– (v2) modes were represented by peaks at 572 and 462cm−1 represent. In the presence of vitamin C, the band at 1263cm−1 was absent and a new peak at 2385cm−1 was found (Figure-5A). XRD patterns for the crystals obtained with and without vitamin C treatment are shown in Figure-5B. The struvite crystallizes were in the orthorhombic Pmn21 space group (cell parameters a=6.955 Å, b=11.2 Å, c=6.142 Å). Vitamin C induced struvite exhibited increased peak intensity corresponding to (021) and decreased intensity of (020) and (040) planes as compared to the control. It shows that vitamin C interfere in the crystal growth and the preferred growth being at (111) plane. This indicates preferential adsorption and binding of vitamin C onto these faces and results in the prominent development in (111) face.
Figure 5. Representative (a) FTIR and (b) XRD patterns of the of the grown struvite crystal. Arrow indicates differences in the peak.
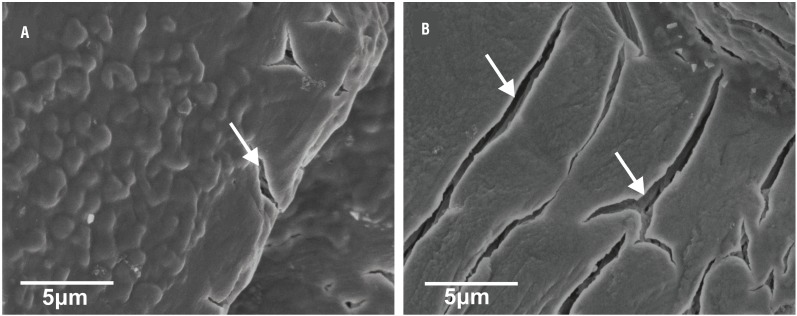
The struvite crystals exhibited porous nature with characteristic tubular pores in FE-SEM and the single struvite crystals had well-defined crystalline faces and multi-layered depositions. In addition, vitamin C induced struvite crystals had highly porous appearance compared to control (Figure-6). Moreover, the presence of Mg, N, P and O using X-ray spectroscopy confirmed the major elements present in struvite crystal.
Figure 6. FE-SEM micrographs of struvite crystals grown in artificial urine infected with Pseudomonas aeruginosa revealing the porous structure and mesoscopic arrangement (a) control (b) in presence of vitamin C (1 mg/mL). Arrow indicates porous morphology.
DISCUSSION
The effect of vitamin C on P. aeruginosa-induced struvite crystallization was analysed in detail using microscopy, changes in pH, and bacterial viability during 24h incubation in synthetic urine. Struvite exhibited typical hemimorphic morphology in the presence of uropathogenic bacteria. These characteristic hemimorphic habit and morphology were reported previously in detail (9). And similar observations were also made for struvite crystallized in the presence of Proteus mirabilis (8). In the presence of vitamin C, crystal formation was delayed compared to control and X-shaped crystals were observed. These characteristic X-shapes have been reported in bacteria--induced struvite crystallization (8, 21).
The induction time for crystallization in the presence of vitamin C was delayed. Similar observation was also found with other inhibitory compounds during bacteria induced struvite crystallization in artificial urine (11). Moreover, the size and weight of the harvested crystals showed concentration dependent changes with vitamin C treatment. Such inhibitory reduction was observed in struvite crystallization under polyaspartic acid treatment of different concentrations (9). In FTIR spectra, shift in the bands to lower frequencies were seen, which may be due to the vibrations related to stretching Mg-O modes. Such changes in the structure of the struvite crystals were also reported earlier (22, 23). Vitamin C interferes in the crystal growth and the preferred growth being at (111) plane and the XRD pattern of struvite crystals grown in the gel medium are identical with the reported literature (7, 24). The struvite crystals exhibited porous nature and the multi-layered depositions. These multi-layered depositions are commonly observed during the formation of struvite crystals in vitro in the presence of natural compounds (24). The internal porous nature with characteristic tubular pores of struvite crystal during P. mirabilis induced crystallization was well explained (8).
Possible Mode of Action for the Struvite Inhibition by Vitamin C
The results obtained with respect to in vitro inhibition of struvite showed a decrease of crystal size and weight in the presence of vitamin C in a concentration dependent manner. pH of supernatant solution was acidic in nature, indicating that vitamin C can constantly bring down the pH thereby preventing the crystallization in the synthetic urine. Vitamin C can lower the pH and also inhibit the urease activity due the altered pH. In addition, these characteristics delay the nucleation and appearance of struvite crystals in the presence of vitamin C. Citrus fruits containing vitamin C had similar interaction with struvite minerals and inhibited crystal growth in vitro (20).
Blood levels of vitamin C can have a significant role in preventing struvite stone formation. Vitamin C has been shown to play a significant role in preventing infection progression through increased reactive oxygen species production against bacteria (25). Current treatment of infection stones remains challenging, and management of the struvite calculi requires a comprehensive approach. Dietary manipulation, antibiotic therapy along with acidification therapy with vitamin C may improve the clinical outcome of the patients. Furthermore, understanding the mechanism of vitamin C interaction with urinary tract bacteria can be studied to understand its role in the prevention of bacteria-induced struvite. The effect of vitamin C on struvite stone formation needs to be evaluated with other urease-producing bacteria. The effect of vitamin C on struvite stone formation needs to be further evaluated using in vivo models to establish the findings of the present study to support vitamin C as a potential choice for prevention of struvite stones.
CONCLUSIONS
Struvite crystals displayed well-defined faces and dendritic morphology in biogenic systems. Vitamin C has inhibitory activity on bacterially induced struvite crystal formation.
ACKNOWLEDGEMENTS
M.A.P.M. acknowledge Yenepoya University research fellowship (YU-SRF). Authors acknowledge Dr. K.K. Achary and Mrs. Megha Nair for their assistance in data analysis.
Surya Ram Duwal contributed similarly as first author.
ABBREVIATIONS
- ADP
= Ammonium di-hydrogenphosphate
- ATR-FTIR
= Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy
- FE-SEM
= Field Emission Scanning Electron Microscope
- SMS
= Sodium metasilicate
- TSA
= Trypticase soy agar
- TSB
= Tryptic soy broth
- XRD
= X-ray diffraction
Footnotes
Compliance with Ethical Standards
Disclosure of potential conflicts of interest: All authors have nothing to declare and no competing financial interests in relation to the work described.
Research involving Human Participants and/or Animals: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Not applicable
REFERENCES
- 1.Flannigan R, Choy WH, Chew B, Lange D. Renal struvite stones-pathogenesis, microbiology, and management strategies. Nat Rev Urol. 2014;11:333–341. doi: 10.1038/nrurol.2014.99. Review. Erratum in: Nat Rev Urol. 2014;11:page after 341. [DOI] [PubMed] [Google Scholar]
- 2.Rodman JS. Struvite stones. Nephron. 1999;81(Suppl 1):50–59. doi: 10.1159/000046299. [DOI] [PubMed] [Google Scholar]
- 3.Prywer J, Sadowski RR, Torzewska A. Aggregation of struvite, carbonate apatite, and Proteus mirabilis as a key factor of infectious urinary stone formation. Cryst. Growth Des. 2015;15:1446–1451. [Google Scholar]
- 4.Thomas B, Tolley D. Concurrent urinary tract infection and stone disease: pathogenesis, diagnosis and management. Nat Clin Pract Urol. 2008;5:668–675. doi: 10.1038/ncpuro1254. [DOI] [PubMed] [Google Scholar]
- 5.Manzoor MA, Mujeeburahiman M, Rekha PD. Association of serum biochemical panel with mineralogical composition of kidney stone in India. Acta Med Int. 2017;4:26–30. [Google Scholar]
- 6.Torzewska A, Rozalski A. Inhibition of crystallization caused by Proteus mirabilis during the development of infectious urolithiasis by various phenolic substances. Microbiol Res. 2014;169:579–584. doi: 10.1016/j.micres.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Chethan K.C., Joseph K. C., Bharat, B. P., Joshi M. J. Growth and Characterization of struvite crystals. Indian Journal of pure and applied Physics. 2008;46:507–512. [Google Scholar]
- 8.Prywer J, Torzewska A, Płociński T. Unique surface and internal structure of struvite crystals formed by Proteus mirabilis. Urol Res. 2012;40:699–707. doi: 10.1007/s00240-012-0501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Yao QZ, Wang YY, Li YL, Zhou GT. Biomimetic synthesis of struvite with biogenic morphology and implication for pathological biomineralization. Sci Rep. 2015;5:7718–7718. doi: 10.1038/srep07718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clapham L, McLean RJ, Nickel JC, Downey J, Costerton JW. The influence of bacteria on struvite crystal habit and its importance in urinary stone formation. J. Cryst. Growth. 1990;104:475–484. [Google Scholar]
- 11.Prywer J, Torzewska A. Effect of Curcumin Against Proteus mirabilis During Crystallization of Struvite from Artificial Urine. Evid Based Complement Alternat Med. 2012;2012 doi: 10.1155/2012/862794. 862794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA. 1999;281:1415–1423. doi: 10.1001/jama.281.15.1415. [DOI] [PubMed] [Google Scholar]
- 13.Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Intake of vitamins B6 and C and the risk of kidney stones in women. J Am Soc Nephrol. 1999;10:840–845. doi: 10.1681/ASN.V104840. [DOI] [PubMed] [Google Scholar]
- 14.Baxmann AC, De O G Mendonça C, Heilberg IP. Effect of vitamin C supplements on urinary oxalate and pH in calcium stoneforming patients. Kidney Int. 2003;63:1066–1071. doi: 10.1046/j.1523-1755.2003.00815.x. [DOI] [PubMed] [Google Scholar]
- 15.Takasaki E. An observation on the composition and recurrence of urinary calculi. Urol Int. 1975;30:228–236. doi: 10.1159/000279983. [DOI] [PubMed] [Google Scholar]
- 16.Coe F. Treatment and prevention of renal stones. Consultant. 1978;18:47–50. [Google Scholar]
- 17.Wall I, Tiselius HG. Long-term acidification of urine in patients treated for infected renal stones. Urol Int. 1990;45:336–341. doi: 10.1159/000281732. [DOI] [PubMed] [Google Scholar]
- 18.Hetey SK, Kleinberg ML, Parker WD, Johnson EW. Effect of ascorbic acid on urine pH in patients with injured spinal cords. Am J Hosp Pharm. 1980;37:235–237. [PubMed] [Google Scholar]
- 19.Griffith DP, Musher DM, Itin C. Urease. The primary cause of infection-induced urinary stones. Invest Urol. 1976;13:346–350. [PubMed] [Google Scholar]
- 20.Chauhan CK, Joshi MJ. Growth inhibition of Struvite crystals in the presence of juice of Citrus medica Linn. Urol Res. 2008;36:265–273. doi: 10.1007/s00240-008-0154-4. [DOI] [PubMed] [Google Scholar]
- 21.Prywer J, Torzewska A. Biomineralization of struvite crystals by Proteus mirabilis from artificial urine and their mesoscopic structure. Cryst. Res. Technol. 2010;45:1283–1289. [Google Scholar]
- 22.Stefov V, Šoptrajanov B, Spirovski F, Kuzmanovski I, Lutz HD, Engelen B. Infrared and Raman spectra of magnesium ammonium phosphate hexahydrate (struvite) and its isomorphous analogues. I. Spectra of protiated and partially deuterated magnesium potassium phosphate hexahydrate. J Mol Struct. 2004;689:1–10. [Google Scholar]
- 23.Cahil A., Najdoski M., Stefov V. Infrared and Raman spectra of magnesium ammonium phosphate hexahydrate (struvite) and its isomorphous analogues. IV. FTIR spectra of protiated and partially deuterated nickel ammonium phosphate hexahydrate and nickel potassium phosphate hexahydrate. J Mol Struct. 2007;834:408–413. [PubMed] [Google Scholar]
- 24.Bindhu B, Swetha AS, Veluraja K. Studies on the effect of phyllanthus emblica extract on the growth of urinary type struvite crystals invitro. Clin Phytoscience. 2015;1:3–3. [Google Scholar]
- 25.Hemilä H. Vitamin C and Infections. Nutrients. 2017;9:339–339. doi: 10.3390/nu9040339. [DOI] [PMC free article] [PubMed] [Google Scholar]