Abstract
Background: The incidence of postoperative acute kidney injury (AKI) is predominantly determined by renal hemodynamics. Beside arterial blood pressure, the role of factors causing a deterioration of venous congestion (intraabdominal pressure, central venous pressure, mechanical ventilation) has emerged. The value of combined hemodynamic, respiratory and intra-abdominal pressure (IAP) monitoring in predicting postoperative acute kidney injury has received only limited exploration to date.
Methods: Data were collected for adult patients admitted after major abdominal surgery at nine Hungarian ICUs. Hemodynamic parameters were compared in AKI vs. no-AKI patients at the time of admission and 48 h thereafter. Regarding ventilatory support, we tested mean airway pressures (Pmean). Effective renal perfusion pressure (RPP) was calculated as MAP−(IAP + CVP + Pmean). The Mann–Whitney U and the chi-square tests were carried out for statistical analysis with forward stepwise logistic regression for AKI as a dependent outcome.
Results: A total of 84 patients (34 ventilated) were enrolled in our multicenter observational study. The median values of MAP were above 70 mmHg, IAP not higher than 12 mmHg and CVP not higher than 8 mmHg at all time-points. When we combined those parameters, even those belonging to the ‘normal’ range with Pmean, we found significant differences between no-AKI and AKI groups only at 12 h after ICU admission (median and IQR: 57 (42–64) vs. 40 (36–52); p < .05). Below it’s median (40.7 mmHg) on admission, AKI developed in all patients. If above 40.7 mmHg on admission, they were protected against AKI, but only if it did not decrease within the first 12 h.
Conclusions: Calculated effective RPP with the novel formula MAP−(IAP + CVP + Pmean) may predict the onset of AKI in the surgical ICU with a great sensitivity and specificity. Maintaining effective RPP appears important not only at ICU admission but during the next 12 h, as well. Additional, larger studies are needed to explore therapeutic interventions targeting this parameter.
Keywords: Postoperative acute kidney injury, central venous pressure, intraabdominal pressure, mean airway pressure
Introduction
Acute kidney injury (AKI) associates with poor prognosis in critically ill patients [1–3]. There are well-known risk factors for postoperative AKI: (1) sepsis especially due to intraabdominal infections; (2) deterioration of renal blood supply; (3) type of surgery (intraabdominal, cardiac, emergency interventions); (4) mechanical ventilation; (5) vasopressor therapy; (6) preexisting kidney disease [1,4–9]. The deterioration of arterial blood supply as indicated by a mean arterial blood pressure (MAP) less than 65 mmHg clearly leads to worsening of kidney function, but until now only limited animal and human studies have been conducted for highlighting the role of elevated renal venous pressures [10–12].
Abdominal perfusion pressure (APP), recommended by the WSACS – the Abdominal Compartment Society for estimating the adequacy of renal perfusion, is computed by MAP – intraabdominal pressure (IAP) [13]. Several studies have been published on this method [14–18]. Elevated IAP with or without a decreased APP has been shown to be present in connection with a higher incidence of AKI [13]. On the other hand, other possible contributors to impair venous return and net renal perfusion pressures, such as central venous pressure (CVP) or transmitted airway pressures induced by mechanical ventilation have not been sufficiently considered to date. Several other intensive care investigators use the term ‘transrenal pressure’, defined as MAP – CVP as a predictor of AKI, albeit without considering the impact of IAP as a contributing factor [10–12]. To date, only one study has been conducted to investigate the relationship between elevated CVP and the rise of IAP; however, this study found poor correlation between elevated CVP and IAP in decompensated heart failure patients [19]. The WSACS – the Abdominal Compartment Society proposed the filtration gradient (FG = MAP − 2 × IAP) for computing the renal perfusion pressure in the Society’s former guidelines, but this recommendation was subsequently withdrawn in 2013 [13,20].
In daily practice, mechanical ventilation has a well-known association with a higher risk of AKI, but none of the individual respiratory parameters, such as positive end-expiratory pressure (PEEP), tidal volume, mean airway pressure (Pmean), either alone or combined with any circulatory pressure parameters has proven predictive with regard to AKI until now [21].
Proper hemodynamic monitoring improves outcomes after major abdominal surgery [22]. For this reason, the continuous measurement of MAP and IAP is generally accepted and widely practiced [13]. The majority of postoperative AKI cases are attributable to the impairment of the effective vascular perfusion to the kidney; therefore, the optimization of macro-hemodynamics remains critically important [9].
Most recently, we reported [MAP−(IAP + CVP + Pmean)], a novel formula for predicting renal perfusion pressure (RPP) and calculated on admission to the ICU after major abdominal surgery, appears to be the most precise way to predict AKI in critically ill postoperative patients from the twelve investigated formulas [23]. The aim of our study was to test the hypothesis whether the formula, we termed ‘effective RPP’ that includes MAP, IAP, CVP and Pmean are appropriate for predicting the onset of AKI in surgical ICU patients after major abdominal surgery.
Materials and methods
Patients
Data were collected prospectively in adult patients admitted after major abdominal surgery in nine Hungarian Intensive Care Units (ICU) (4 universities, 4 regional and 1 city hospitals) in our multicenter observational between 5 January 2015 and 6 October in 2015 [23]. Ethical approval for this study was provided by the Hungarian Health Registration and Training Center, the national ethical committee (Ethical Committee N° 039320/2014/OTIG). Written informed consent was obtained from all study participants or granted by their legal guardians or next-of-kin. The ethical standards of the experiments were in accordance with the guidelines provided by the World Medical Association Declaration of Helsinki on Ethical Principles for Medical Research Involving Humans for Studies.
Patients were enrolled after major abdominal surgery requiring a minimum of 48-h ICU postoperative therapy. Patients under 18 years of age, those with an end-stage kidney disease, those having surgery with suprarenal cross-clamping or undergoing kidney or urinary bladder operations were excluded from the study. Patients enrolled in other clinical studies were also excluded. To collect data, we used an Excel-based data collection file and, included demographic and perioperative information, severity of illness on admission (Simplified Acute Physiology Score version II (SAPS II), Sepsis-related Organ Failure Assessment Score (SOFA)) and the outcome data (presence of AKI at 48 h) [24,25]. The internationally accepted Acute Kidney Injury Network (AKIN) criteria (both creatinine and urine output) were used for defining AKI [26]. We considered a preoperative serum creatinine level at baseline [23].
Measurements
MAP, CVP and IAP were recorded by commercially available patient monitors at the time of admission and at 6, 12, 24 and 48 h thereafter. The measurement of IAP was performed by nursing staff via indwelling urinary catheters and according to the WSACS – the Abdominal Compartment Society guidelines. RPP was calculated at the given time points according to the novel formula of MAP−(IAP + CVP + Pmean) and the onset of AKI was detected at 48 h after ICU admission. The measurement of CVP was performed at the end of expiration. All pressures were given in mmHg (1 mmHg = 1.36 cmH2O).
Statistical analysis
All values were presented as median with interquartile range (IQR). The median values of the different groups were compared using the Mann–Whitney U-test and the occurrence rates using the chi-square test. To test the sensitivity and specificity of changes of RPP between 6 and 12 h, a Receiver Operating Curve (ROC) analysis was performed for parameters discriminating AKI and no-AKI groups [Group 1: patients without AKI; Group 0: patients with AKI (grade 1, 2 or 3)]. Confidence intervals (95%) were calculated according to the formula described by Hosmer and Lemeshow. While CVP and airway pressures can influence IAP, their independency has been analyzed by linear regression analysis, as shown in our previous work [23]. Data were dichotomized regarding effective RPP, which demonstrated the best predictivity with reference to its previously published median of 40.7 mmHg (measured on admission) when comparing no-AKI and AKI groups at later time points.
To explore the relationship between abdominal perfusion pressure and the incidence of AKI, forward stepwise logistic regression was performed. All the basic hemodynamic parameters, Pmean and the effective RPP on admission, 6 h and 12 h, as well as all the changes between these time points were included. All the pressures (MAP, CVP, IAP, Pmean and RPP) were considered as independent predictors measured at the same time or interval, and the presence of AKI as the outcome parameter.
Our study had an 80% statistical power of effective RPP 12 h after admission between the AKI and no-AKI groups at a significance level of 0.05. For estimating the effect size of our study, we calculated Hedges’ g modified for small samples. For MAP−(IAP + CVP + Pmean) Hedges’ g values were 0.74 on admission, 1.02 at 12 h after admission, and 0.93 at the change between 6 and 12 h. Hedges’ g is considered to be high above 0.8. All differences were deemed to be significant if p ≤ .05. All analyses were performed by the SPSS statistical software package 23.0 (IBM SPSS, Armonk, NY, US).
Results
A total of 84 patients were admitted to the ICU during the study period (type of surgery and number of cases – gastric: 9; bowel: 28; rectal: 19; hepatic: 3; pancreatic: 4; aortic: 13; trauma: 5 and miscellaneous others: 3). Between the AKI and no-AKI groups, there were no significant differences in age, gender and serum albumin levels. A higher incidence of sepsis, as well as higher serum lactate levels and severity-of-disease scores were found in the AKI group (Table 1).
Table 1.
Demographics of patients at admission (median and IQR; case numbers and percentage).
| no-AKI (n = 45) |
AKI (n = 39) |
p | |
|---|---|---|---|
| Male/female |
30/15 |
19/20 |
NS |
| Age (years) | 65.0 (60.0–76.5) |
68.0 (59.0–79.0) |
NS |
| SAPS II | 33.0 (22.0–40.0) |
49.5 (32.8–65.3) |
<.01 |
| SOFA | 5.0 (2.0–10.0) |
10.0 (5.8–11.8) |
<.001 |
| Non-renal SOFA | 3.0 (1.0–7.0) |
7.0 (4.0–9.0) |
<.01 |
| Albumin (g/L) |
29.4 (24.0–35.5) |
26.9 (22.1–32.1) |
NS |
| Lactate (mmol/L) |
1.5 (1.1–2.4) |
2.4 (1.8–3.5) |
<.01 |
| BUN (mmol/L) | |||
| Preop | 7.0 (4.5–9.0) |
8.7 (5.1–14.8) |
NS |
| Admission | 5.1 (3.9–7.3) |
10.0 (5.5–15.9) |
<.01 |
| Creatinine (micromol/L) | |||
| Preop | 85 (70–104) |
96 (73–137) |
NS |
| Admission | 73 (56–90) |
127 (72–213) |
<.001 |
| Sepsis | 3 (6.7%) | 16 (41.0%) | <.001 |
| Need of ventilation | 15 (33.3%) |
25 (64.1%) |
<.05 |
| ICU mortality | 2 (4.4%) |
9 (24%) |
<.01 |
| Hospital mortality | 5 (11.1%) |
13 (33.3%) |
<.05 |
| ICU length of stay (days) | 2.0 (2.0–4.0) |
5.0 (2.0–11.5) |
<.001 |
| Hospital length of stay (days) | 11.0 (9.0–14.0) |
13.0 (8.0–18.5) |
NS |
AKI: Acute Kidney Injury; BUN: Blood Urea Nitrogen; SAPS II: Simplified Acute Physiology Score version II; SOFA: Sepsis-related Organ Failure Assessment Score.
The MAP and the calculated effective RPP values were significantly lower in the AKI group (Table 2) on admission. No differences were found in airway pressures between the two groups. The change of RPP between time points is presented in Table 3.
Table 2.
The values of parameters (mmHg, median (IQR)).
| Parameters | n | No AKI | n | AKI | p | |
|---|---|---|---|---|---|---|
| MAP | Admission | 45 | 89 (77–100) | 39 | 82 (65–91) | NS |
| 6 h | 45 | 75 (66–85) | 39 | 72 (67–85) | NS | |
| 12 h | 45 | 76 (68–87) | 39 | 72 (69–82) | NS | |
| 24 h | 42 | 81 (71–91) | 37 | 80 (70–88) | NS | |
| 48 h | 32 | 81 (75–101) | 37 | 82 (70–90) | NS | |
| IAP | Admission | 45 | 10 (6–13) | 39 | 11 (7–16) | NS |
| 6 h | 45 | 10 (6–12) | 39 | 9 (7–14) | NS | |
| 12 h | 45 | 11 (7–13) | 39 | 11 (7–15) | NS | |
| 24 h | 39 | 9 (7–13) | 36 | 12 (8–16) | < .05 | |
| 48 h | 29 | 10 (7–14) | 37 | 12 (8–17) | NS | |
| CVP | Admission | 45 | 6 (2–10) | 39 | 6 (4–8) | NS |
| 6 h | 45 | 5 (1–8) | 39 | 7 (4–8) | NS | |
| 12 h | 45 | 5 (2–7) | 39 | 6 (4–9) | NS | |
| 24 h | 42 | 6 (4–7) | 37 | 8 (3–11) | NS | |
| 48 h | 31 | 7 (4–9) | 37 | 7 (5–11) | NS | |
| Pmean | Admission | 12 | 9 (7–10) | 22 | 9 (8–11) | NS |
| 6 h | 7 | 8 (7–10) | 19 | 10 (6–11) | NS | |
| 12 h | 6 | 7 (7–10) | 17 | 9 (7–12) | NS | |
| 24 h | 4 | 7 (6–9) | 18 | 10 (7–12) | NS | |
| 48 h | 3 | 6 (6–7) | 16 | 8 (7–14) | NS | |
| MAP-IAP | Admission | 45 | 88 (69–93) | 39 | 65 (49–85) | < .005 |
| 6 h | 45 | 66 (56–74) | 39 | 62 (55–77) | NS | |
| 12 h | 45 | 66 (57–77) | 39 | 61 (53–76) | NS | |
| 24 h | 41 | 72 (60–84) | 37 | 64 (57–77) | NS | |
| 48 h | 32 | 72 (63–91) | 37 | 69 (57–82) | NS | |
| MAP-(IAP + CVP + Pmean) | Admission | 12 | 55 (50–59) | 22 | 41 (30–52) | < .005 |
| 6 h | 7 | 49 (39–55) | 19 | 44 (40–60) | NS | |
| 12 h | 6 | 57 (42–64) | 17 | 40 (36–52) | < .05 | |
| 24 h | 4 | 53 (45–58) | 18 | 31 (18–46) | NS | |
| 48 h | 3 | 55 (52–59) | 16 | 36 (3–50) | NS |
AKI: Acute Kidney Injury; CVP: Central Venous Pressure; IAP: Intraabdominal Pressure; MAP: Mean Arterial Pressure.
Table 3.
The change of RPP in the first 12 h after admission to ICU (mmHg, median (IQR)).
| Parameter | n | No AKI | n | AKI | p | |
|---|---|---|---|---|---|---|
| Δ[MAP-(IAP + CVP + Pmean)] | 0–6 h | 8 | −5 (−41 to –6) | 23 | 6 (−9 to –18) | NS |
| 6–12 h | 7 | 9 (2–13) | 21 | −4 (−10 to –1) | <.05 | |
| 0–12 h | 7 | 3 (−28 to –14) | 21 | −6 (−14 to –11) | NS |
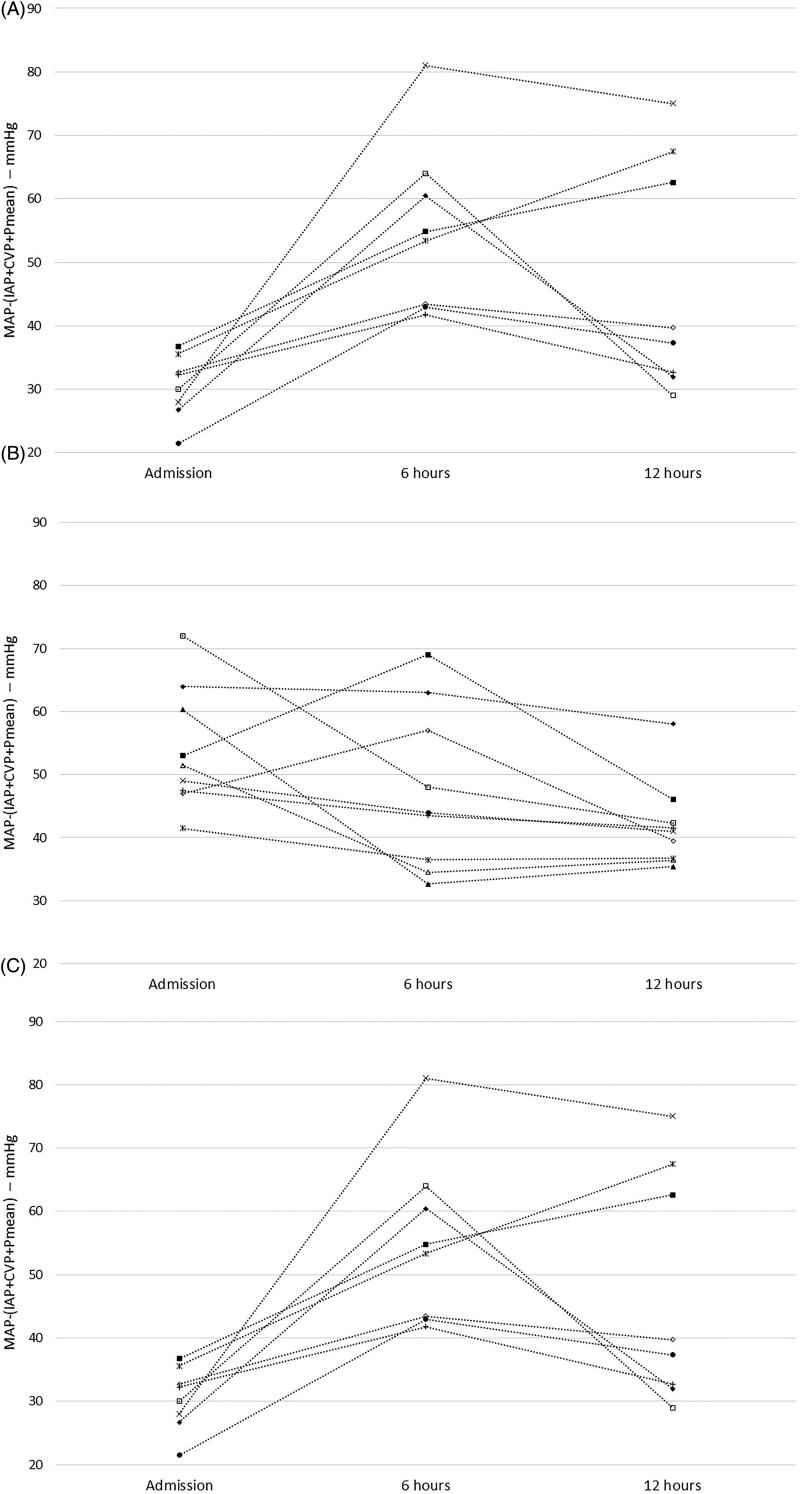
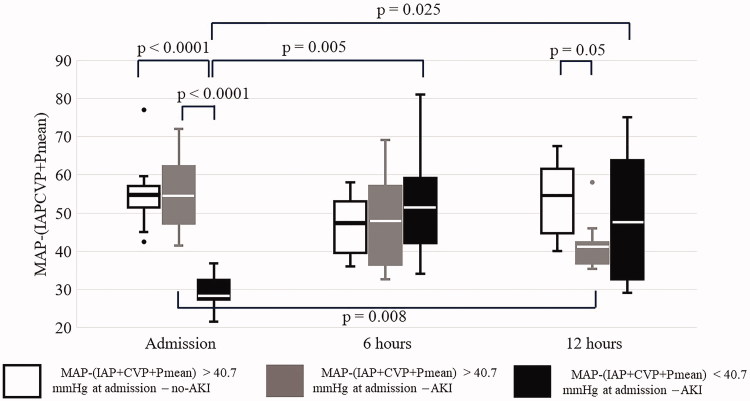
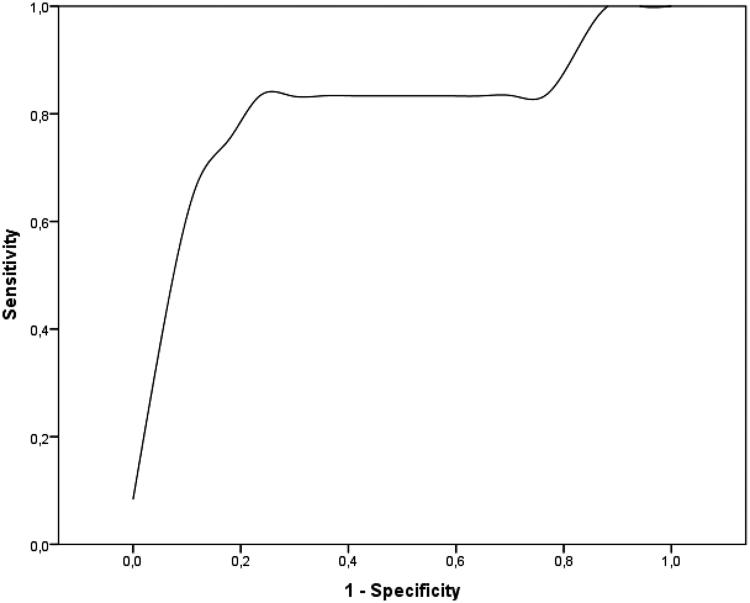
Below the median of this parameter AKI developed in all cases. If effective RPP was above the median on admission and increased in the first 12 h in the ICU, none of the study participants developed AKI, whereas most patients with decreasing effective RPP did (Figure 1). In most AKI patients, effective RPP decreased between 6 and 12 h. Even though perfusion improved both at 6 and 12 h in the poor RPP group, AKI remained unavoidable (Figure 2). ROC analysis of changes of RPP between 6 and 12 h revealed moderate probability of the RPP for AKI according to both positive and negative likelihood ratios (LR) (1-AUC: 0.804, sensitivity: 86%, specificity: 81%, LR +: 4.50, LR −: 0.18) (Figure 3).
Figure 1.
The patients' data with (A) below 40.7 mmHg of MAP−(IAP + CVP + Pmean) and above it (B) with or (C) without AKI in the first 12 h.
Figure 2.
The course of MAP−(IAP + CVP + Pmean) in the first 12 h in ICU after major abdominal surgery. The p values within or between groups not shown were not statistically significant.
Figure 3.
ROC analysis of the changes of RPP between 6 and 12 h after admission.
After logistic regression analysis, the change of effective RPP between 6 and 12 h was the only parameter that remained in the equation and that showed an almost significant result (B: −0.091, SE: 0.048, Wald: 3.641, Sig: 0.056, Exp (B): 0.913, 95% CI for Exp (B): 0.813–1.002). The logistic regression analysis with the parameters at other time points or intervals has not revealed significant relationship between basic pressures or RPP and the presence of AKI at 48 h.
Discussion
The role of arterial blood supply is clear and well-studied, but venous congestion is underestimated in different guidelines, and currently, only the measurement of IAP is emphasized [13,27]. In this paper, having analyzed parameters recorded within 12 h after admission in our study, we are reporting the value of the formula of MAP−(IAP + CVP + Pmean) in predicting AKI beyond ICU admission, incorporating elements of hemodynamic, as well as ventilator-derived and intra-abdominal pressures.
Human studies for investigating the optimal MAP were conducted mostly in patients with septic shock. The generally accepted target is 65 mmHg, a number extrapolated from intraoperative observations and from clinical experience derived from monitoring septic patients’ hemodynamics. Keeping MAP above this value is protective against AKI [28]. From the parameters affecting renal congestion, the elevated IAP (>12 mmHg) is associated with a higher risk of AKI [13]. The CVP (but not the cardiac index) has been shown to be a risk factor of renal failure in cardiac patients and is proved to be independent of IAP [10]. In septic ICU patients, higher CVP and lower diastolic pressure have been associated with AKI [11]. In another study, mean perfusion pressure deficit (defined as MAP − CVP) and diastolic perfusion pressure deficit have been shown to be predictors of worsening kidney function [12]. Against these results, in our prospective study, the median values of MAP were above 70 mmHg, IAP not higher than 12 mmHg and CVP not higher than 8 mmHg at all time-points. To reach these hemodynamic parameters, many patients needed vasopressor therapy and various amounts of intravenous fluids. The aim of our study was to find the simplest, continuously recordable and proper parameter for determining the adequacy of renal perfusion pressure in patients requiring ICU admission after major abdominal surgery.
Mechanical ventilation can increase the risk of AKI, but in our study, we found no differences in the Pmean values between the groups [23]. Because no unique and simple parameter is suitable for monitoring and selecting patients who are at risk of AKI after major abdominal surgery, we tried to find the best combination. In our previous paper, we reported that the MAP−(IAP + CVP + Pmean) formula was the best predictor of AKI on admission to ICU [23]. Moreover, when we combined those parameters even with parameters of ‘normal’ range, we found significant differences between groups in effective RPP at 12 h. We should emphasize that this effect was not due to a change of arterial blood supply as MAP remained unchanged. The factors affecting the venous congestion of the kidney (IAP, CVP, Pmean) have been proved to be independent of each other [23].
The physiological basis of computing these equations was the previously mentioned data concerning venous congestion [10,19]. Medians of all parameters were in so-called ‘normal’ range, and also the CVP was there. There were no significant differences in medians of AKI and of no-AKI groups. The IQRs of MAP are the same across groups at 6 and 12 h, as you can see in Table 2. The IQR of IAP is lower in no-AKI group at 6 h, and this difference disappears at 12 h. The medians and IQRs of CVP are higher in AKI group at both 6 and 12 h, although this difference is not significant statistically. The medians of Pmean are higher in AKI group at both 6 and 12 h (difference is not significant). The net driving pressure of filtration in the glomeruli is about 10 mmHg under physiological condition. The medians and IQRs of the parameters mentioned above are the same range. Therefore, the cumulation of seemingly small changes can result in AKI. CVP represents not only extracapsular, but also the intracapsular pressure of kidney, which can differ because of the tight fibrous capsule of it.
The predictivity of effective RPP was found to be suitable not only on admission but at later time points as well: our prediction model has a sensitivity of 86% and specificity of 81%. When this pressure fell below 40.7 mmHg on admission to the ICU, AKI developed in every case, regardless of any further improvement in perfusion. On the other hand, keeping the effective RPP above its median (40.7 mmHg) on admission and the subsequent 12 h in patients having undergone major abdominal surgery protected against AKI. The individual course of RPP above its median measured on admission to ICU is an important tool in prediction of postoperative AKI and can be a part of the patient's personalized care of the patient. Moreover, monitoring this parameter is simpler and may be much easier than using the points of different scoring systems or models.
Our study has several limitations: first, we had only a relatively small-sized cohort with a low number of subjects recruited from the individual centers. The proportion of patients receiving mechanical ventilation was even smaller and likely received heterogeneous and somewhat different from center-to-center care. Details regarding preexisting or total body fluid excess, vasopressors types and doses or intraoperative blood pressures were not available. In our study, only moderately elevated IAP and a normal range of CVP were found, representing both relative strength and weakness of the study, and our conclusions need to be verified with even sicker patients with higher IAP, Pmean or massive volume overload. The value of effective RPP during renal replacement therapy in the course of AKI remains fully unexplored and likely a fertile territory for future investigations.
Conclusions
Continuous measurement of MAP, IAP and CVP can be useful in patients with shock and potential intrabdominal hypertension. We were able to demonstrate that computing the effective RPP as [MAP−(IAP + CVP + Pmean)] and combining values even 'near-to-normal’ values of hemodynamic and respiratory parameters can be a predictor of AKI in the early postoperative period. Accordingly, this formula may represent a valuable addition to the care of ICU patients at-risk for AKI. Even if there is a modest elevation of venous pressures then MAP should be kept correspondingly higher to keep RPP above 40.7 mmHg. Additionally, larger studies are needed to explore therapeutic interventions targeting this parameter.
We sincerely appreciated the assistance of Mr. Attila Lénárt-Muszka (Debrecen, Hungary) and of Richard Mann during editing and grammar review.
Acknowledgements
List of investigators: Anita Zsirmik, Domonkos Trásy MD, Ildikó László MD, Zsolt Molnár (Department of Anesthesiology and Intensive Therapy, Faculty of Medicine, University of Szeged); Gabriella Kecskés, Béla Gartner (Petz Aladár Hospital, Győr); Zoltán Petró, Zoltan Fülep (Bács-Kiskun Provincial Hospital, Kecskemét); István Laszló, Béla Fülesdi (Department of Anesthesiology and Intensive Therapy, Faculty of Medicine, University of Debrecen); Miklós Siptár, Barbara Benkovics, Lajos Bogár (Institute of Anesthesiology and Intensive Therapy, Faculty of Medicine, University of Pécs); Judit Lőrincz, László Nagy (Central Military Hospital, Budapest); Emese Helbocsányi, Gábor Bencsik (Hetényi Géza Hospital, Szolnok); Gábor Szigligeti, Csaba Antek (Jósa András Hospital, Nyíregyháza). We sincerely appreciated the assistance of Mr. Richard Mann (Budapest, Hungary) and of Mr. Attila Lénárt-Muszka (Debrecen, Hungary) during editing and grammar review.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability
The data that support the findings of this study are available from the corresponding author, CsK, upon reasonable request.
References
- 1.Medve L, Antek C, Paloczi B, et al. Epidemiology of acute kidney injury in Hungarian intensive care units: a multicenter, prospective, observational study. BMC Nephrol. 2011;12:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu HC, Wang WJ, Chen YW, et al. The association between the duration of postoperative acute kidney injury and in-hospital mortality in critically ill patients after non-cardiac surgery: an observational cohort study. Renal Failure. 2015;37:985–993. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, Xue FS, Liu GP, et al. Assessing association between duration of postoperative acute kidney injury and in-hospital mortality after noncardiac surgery. Renal Failure. 2016;38:342–343. [DOI] [PubMed] [Google Scholar]
- 4.Fülöp T, Pathak MB, Schmidt DW, et al. Volume-related weight gain and subsequent mortality in acute renal failure patients treated with continuous renal replacement therapy. Asaio J. 2010;56:333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brienza N, Giglio MT, Marucci M, et al. Does perioperative hemodynamic optimization protect renal function in surgical patients? A meta-analytic study. Crit Care Med. 2009;37:2079–2090. [DOI] [PubMed] [Google Scholar]
- 6.Medve L, Gondos T. Epidemiology of postoperative acute kidney injury in Hungarian intensive care units: an exploratory analysis. Renal Failure. 2012;34:1074–1078. [DOI] [PubMed] [Google Scholar]
- 7.Moore EM, Bellomo R, Nichol AD. The meaning of acute kidney injury and its relevance to intensive care and anaesthesia. Anaesth Intensive Care. 2012;40:929–948. [DOI] [PubMed] [Google Scholar]
- 8.Makris K, Spanou L. Acute kidney injury: definition, pathophysiology and clinical phenotypes. Clin Biochem Rev. 2016;37:85–98. [PMC free article] [PubMed] [Google Scholar]
- 9.Romagnoli S, Ricci Z, Ronco C. Perioperative acute kidney injury: prevention, early recognition, and supportive measures. Nephron. 2018;140:105–110. [DOI] [PubMed] [Google Scholar]
- 10.Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Legrand M, Dupuis C, Simon C, et al. Association between systemic hemodynamics and septic acute kidney injury in critically ill patients: a retrospective observational study. Crit Care. 2013;17:R278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito S, Uchino S, Takinami M, et al. Postoperative blood pressure deficit and acute kidney injury progression in vasopressor dependent cardiovascular surgery patients. Crit Care. 2016;20:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirkpatrick AW, Roberts DJ, De Waele J, Pediatric Guidelines Sub-Committee for the World Society of the Abdominal Compartment Syndrome, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39:1190–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirkpatrick AW, Brenneman FD, McLean RF, et al. Is clinical examination an accurate indicator of raised intraabdominal pressure in critically injured patients. Can J Surg. 2000;43:207–211. [PMC free article] [PubMed] [Google Scholar]
- 15.Cheatham ML, White MW, Sagraves SG, et al. Abdominal perfusion pressure: a superior parameter in the assessment of intra-abdominal hypertension. J Trauma. 2000;49:621–627. [DOI] [PubMed] [Google Scholar]
- 16.Cheatham ML. Nonoperative management of intraabdominal hypertension and abdominal compartment syndrome. World J Surg. 2009;33:1116–1122. [DOI] [PubMed] [Google Scholar]
- 17.Ouellet JF, Leppaniemi A, Ball CG, et al. Alternatives to formal abdominal decompression. Am Surg. 2011;77:S51–S57. [PubMed] [Google Scholar]
- 18.De Keulenaer BL, De Waele JJ, Malbrain ML. Nonoperative management of intra-abdominal hypertension and abdominal compartment syndrome: evolving concepts. Am Surg. 2011;77:S34–S41. [PubMed] [Google Scholar]
- 19.Mullens W, Abrahams Z, Skouri HN, et al. Elevated intra-abdominal pressure in acute decompensated heart failure: a potential contributor to worsening renal function? J Am Coll Cardiol. 2008;51:300–306. [DOI] [PubMed] [Google Scholar]
- 20.Cheatham ML. Abdominal Compartment Syndrome: pathophysiology and definitions. Scand J Trauma Resusc Emerg Med. 2009;17:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van den Akker JP, Egal M, Groeneveld AB. Invasive mechanical ventilation as a risk factor for acute kidney injury in the critically ill: a systematic review and meta-analysis. Crit Care. 2013;17:R98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salzwedel C, Puig J, Carstens A, et al. Perioperative goal-directed hemodynamic therapy based on radial arterial pulse pressure variation and continuous cardiac index trending reduces postoperative complications after major abdominal surgery: a multi-center. prospective. randomized study. Crit Care. 2013;17:R191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kopitko C, Medve L, Gondos T. Renoprotective postoperative monitoring: what is the best method for computing renal perfusion pressure? – an observational, prospective, multicentre study. Nephron. 2018;139:228–236. [DOI] [PubMed] [Google Scholar]
- 24.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–2963. [DOI] [PubMed] [Google Scholar]
- 25.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. [DOI] [PubMed] [Google Scholar]
- 26.Mehta RL, Kellum JA, Shah SV, the Acute Kidney Injury Network, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 28.Leone M, Asfar P, Radermacher P, et al. Optimizing mean arterial pressure in septic shock: a critical reappraisal of the literature. Crit Care. 2015;19:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, CsK, upon reasonable request.