Abstract
There are numerous abnormalities that present with similar signs and symptoms to arthritis. In this article some of these conditions that can masquerade as arthritis are discussed. Synovial osteochondromatosis is an uncommon benign disorder marked by the metaplastic proliferation of multiple cartilaginous nodules in the synovial membrane of the joints, bursae, or tendon sheaths. Pigmented villonodular synovitis, also known as diffuse-type tenosynovial giant cell tumor, is a locally destructive fibrohistiocytic proliferation, characterized by many villous and nodular synovial protrusions which affects the joints. Synovial hemangioma is a rare benign lesion whose pathogenesis is still unclear. It commonly affects the knee joint, although the other articulations, such as elbow, wrist, and ankle may also be involved. Lipoma arborescens, also known as villous lipomatous proliferation of the synovial membrane, is a rare intra-articular disorder characterized by a non-neoplastic lipomatous proliferation of the synovium. The term “arborescens” refers to the characteristic tree-like morphology of the lesion, which resembles a frond-like mass.
Keywords: arthritis, synovial osteochondromatosis, pigmented villonodular synovitis, synovial hemangioma, lipoma arborescens
Joint symptoms, such as pain and swelling, are most commonly caused by the various forms of arthritis, therefore clinicians will often put arthritis high on the list of differential diagnoses when faced with patients exhibiting joint-based symptoms and signs. The correct diagnosis will usually only become clear once laboratory data and imaging studies are available(1). In this article we highlight a number of conditions that present with similar signs and symptoms to arthritis and may be initially diagnosed as such.
Synovial osteochondromatosis
Definition
Synovial osteochondromatosis is also known as synovial chondromatosis or synovial chondrometaplasia. In this article we are addressing the primary form of the disease, which is a benign disorder marked by the metaplastic proliferation of multiple cartilaginous nodules in the synovial membrane of the joints, bursae, or tendon sheaths(2). The cartilaginous nodules will frequently ossify at which point synovial chondromatosis becomes known as synovial osteochondromatosis(3). The term secondary osteochondromatosis is occasionally used to describe the development of multiple osteochondral bodies within the joint, usually the knee or hip, affected by advanced osteoarthritis. This is most commonly seen in older patients(4).
Epidemiology
This is an uncommon condition, mostly seen in adults in the third to fifth decade. Male-to-female ratio is 2:1.
Site of involvement
It is almost invariably a monoarticular disorder; rarely, multiple joints may be affected. The knee is a preferential site of involvement, with the hip, shoulder, and elbow accounting for most of the remaining cases.
Clinical findings
Patients usually report pain and swelling, and occasionally locking of the joint. Joint effusion, tenderness, and restricted movement of the joint are common findings. Sometimes the condition may present as a painless soft-tissue mass adjacent to a joint. Three phases of articular disease have been identified: an initial phase, characterized by the metaplastic formation of cartilaginous nodules in the synovium; a transitional phase, characterized by detachment of those nodules and formation of free intraarticular bodies; and an inactive phase, in which synovial proliferation has resolved but the loose bodies, which frequently become ossified (synovial osteochondromatosis), remain in the joint, usually with variable amounts of joint fluid.
Imaging findings
The imaging findings depend on the degree of calcification within the cartilaginous bodies, ranging from mere joint effusion to visualization of multiple radiopaque osteochondral bodies, which are usually small and uniform in size (Fig. 1 and Fig. 2). The best proof that the bodies are indeed intraarticular is achieved by arthrography or computed tomography (CT) (Fig. 3). These modalities can visualize even noncalcified bodies(5–7). Ultrasound is hampered by its frequent inability to access all aspects of the joint. Nevertheless, it will readily identify both calcified and non-calcified bodies within the joint (Fig. 4 and Fig. 5). Magnetic resonance imaging (MRI) may also be helpful, although MRI appearance is variable and depends on the relative preponderance of synovial proliferation, loose bodies formation, and extent of calcification or ossification. Unmineralized hyperplastic synovial masses exhibit high signal intensity on T2-weighted images, whereas calcifications can be seen as signal void against the high-signal-intensity fluid (Fig. 6 and Fig. 7). In addition to revealing loose bodies in the joint, CT and MRI may demonstrate bone erosion (Fig. 8).
Fig. 1.
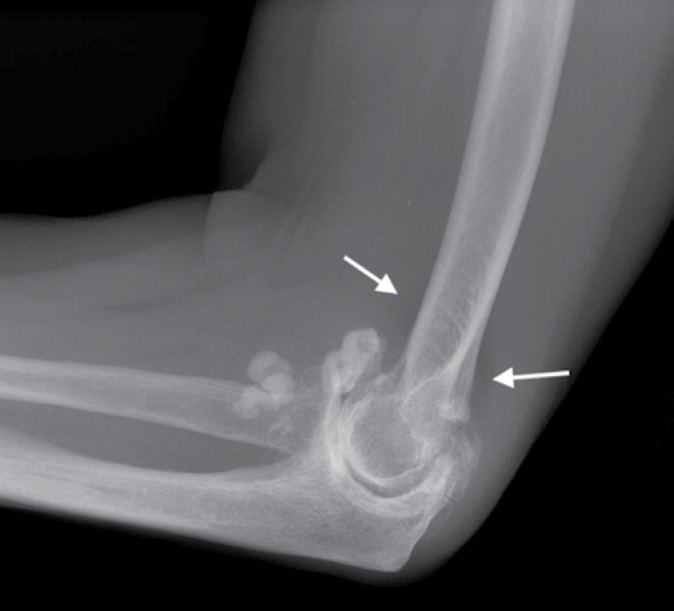
Lateral radiograph of the elbow of a 37-year-old man with synovial osteochondromatosis shows, in addition to multiple osteochondral bodies, displaced anterior and posterior fat pads (arrows) caused by the associated joint effusion. Note the absence of features of osteoarthritis such as joint space narrowing or osteophytosis
Fig. 2.
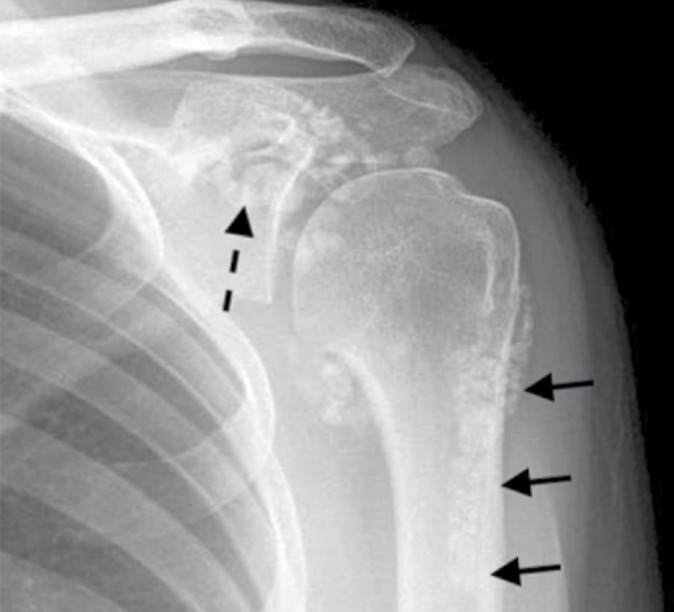
Radiograph of the shoulder of a 34-year-old woman with synovial osteochondromatosis. Multiple osteochondral bodies spread throughout the joint and its recesses including the subscapular recess (dashed arrow) and the biceps tendon sheath (arrows). There is no evidence of osteoarthritic changes
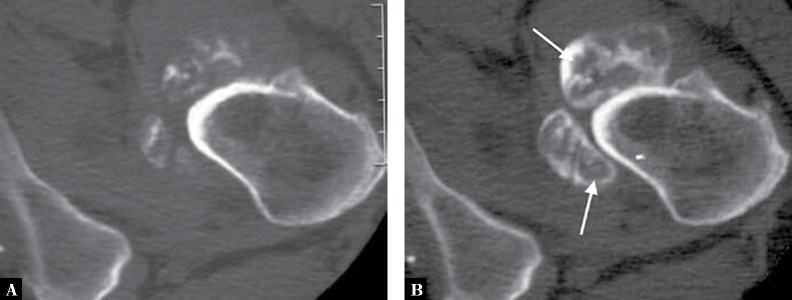
Fig. 3.
A. Conventional axial CT of the inferior aspect of the left hip joint of a 27-year-old man with synovial osteochondromatosis shows various degrees of calcification in osteochondral bodies. B. In addition, axial CT-arthrogram reveals the presence of non-calcified chondral bodies (arrows)
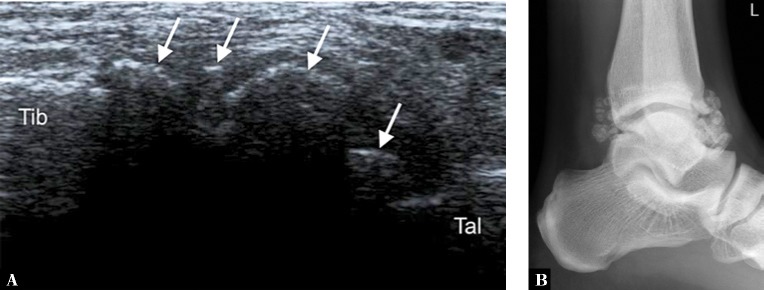
Fig. 4.
A. Longitudinal ultrasound through the anterior aspect of the ankle joint (Tib – anterior tibia, Tal – dorsal talus) of a 31-year-old man with synovial osteochondromatosis reveals multiple calcified loose bodies (arrows), corresponding to ossified osteochondral bodies seen on the lateral radiograph (B)
Fig. 5.
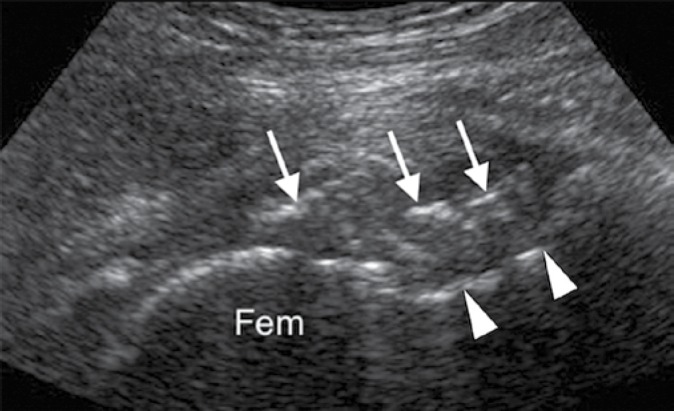
Ultrasound of the hip (Fem – femoral head, arrowheads indicate anterior cortex of femoral neck) of the same patient as depicted in Fig. 3 shows calcified osteochondral bodies in the anterior joint line (arrows)
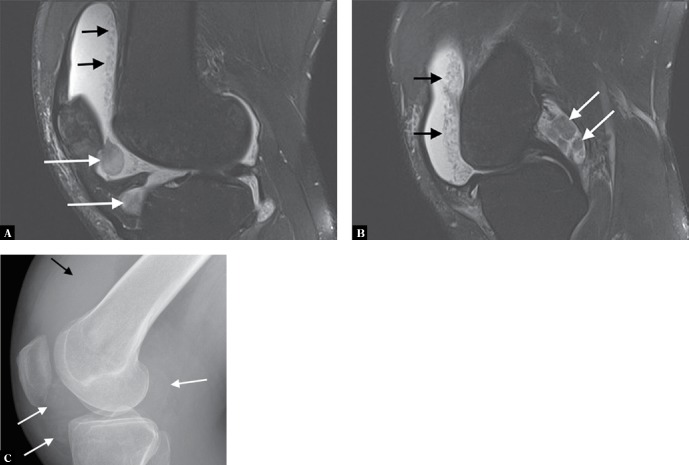
Fig. 6.
A, B. Two sagittal proton-density fat-suppressed MR images of the knee of a 37-year-old man with synovial osteochondromatosis show joint effusion with several large chondral bodies in the joint (white arrows). In addition, note also numerous tiny chondral bodies layering in the supra-patellar recess (black arrows). These resemble rice bodies that can be seen in rheumatoid arthritis and tuberculous arthritis, two conditions to be considered in the differential diagnosis. C. Lateral radiograph of the knee shows the larger chondral bodies exhibiting very early calcification (white arrows). The joint effusion is apparent in the suprapatellar recess (black arrows), but the uncalcified small bodies seen on MRI are not evident
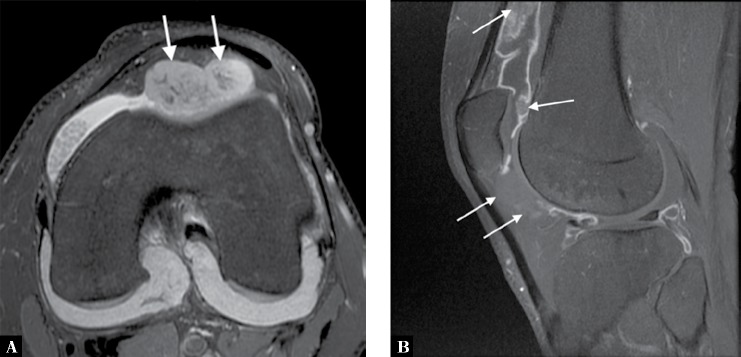
Fig. 7.
A. Axial proton-density fat-suppressed MRI of the knee of a 28-year-old man with synovial osteochondromatosis demonstrates joint effusion along with large high T2-signal bodies in the anterior joint space (arrows). Low-signal areas within these bodies represent areas of calcification. Note also multiple small bodies, similar to those depicted in Fig. 6, in the medial joint compartment. B. Sagittal T1-weighted fat-suppressed MRI obtained after intravenous administration of gadolinium shows isointense bodies within adjacent fluid and suppressed fat. High-signal-enhanced synovium surrounds some of the bodies in the suprapatellar recess
Fig. 8.

Coronal reformatted CT image of the left shoulder of a 36-year-old man with synovial osteochondromatosis shows numerous calcified bodies within the glenohumeral joint and in the subacromial bursa. Note erosion of the humeral head (arrow)
Pathologic findings
Gross pathologic findings consist of multiple, small blue/white ovoid nodules within synovial tissue. By microscopy, these nodules are covered by fibrous tissue with synovial lining, are highly cellular, and the cells themselves may exhibit a moderate pleomorphism, with occasional plump and double nuclei. The cartilaginous nodules, which often undergo calcification and endochondral ossification, may detach and become loose bodies. The loose bodies continue to be viable and may increase in size as they receive nourishment from the synovial fluid.
Genetics
Most cases show near-diploid karyotypes with some cases exhibiting only numerical changes (-X, -Y, and +5, respectively). Recently ERK and NOG (Noggin) gene association has been postulated(8).
Differential diagnosis
Synovial osteochondromatosis should be differentiated from the secondary osteochondromatosis caused by osteoarthritis, particularly in the knee and hip joints. Distinguishing primary from secondary osteochondromatosis usually presents no problems. In the latter condition, there is invariably radiographic evidence of osteoarthritis with all of its typical features, such as narrowing of the radiographic joint space, subchondral sclerosis, osteophyte formation, and, occasionally, periarticular cysts or cyst-like lesions (Fig. 9). The loose bodies are fewer, larger, and invariably of different sizes (Fig. 10). Conversely, in primary synovial osteochondromatosis the joint is not affected by degenerative changes. In some cases, however, the bone may show erosions secondary to pressure of the calcified bodies on the outer aspects of the cortex. The intraarticular bodies are numerous, small, and usually of uniform size.
Fig. 9.
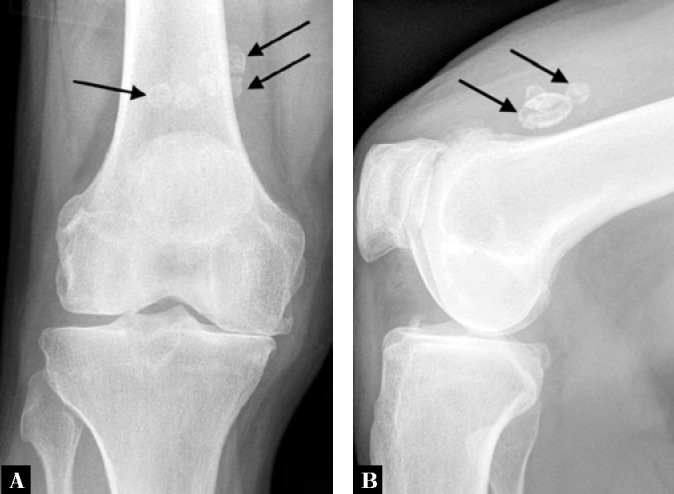
A. Anteroposterior and B. lateral weight-bearing radiographs of the knee of a 73-year-old man with osteoarthritis and secondary osteochondromatosis shows joint space narrowing of the medial and patellofemoral compartments associated with the patellofemoral osteophytosis. Several osteochondral bodies of varying sizes are seen within the suprapatellar pouch (arrows)
Fig. 10.
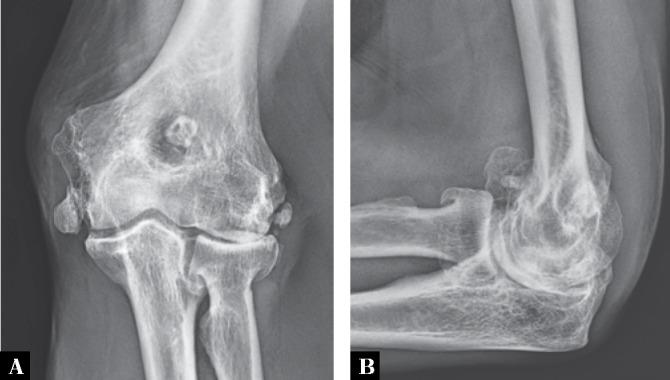
A. Anteroposterior and B. lateral radiographs of the left elbow of a 57-year-old man with a history of several prior elbow dislocations show advanced osteoarthritis complicated by the presence of a few large but different in size intraarticular osteochondral bodies
The other conditions that can radiologically mimic synovial chondromatosis include pigmented villonodular synovitis (PVNS), synovial hemangioma, and lipoma arborescens (discussed in detail later). In pigmented villonodular synovitis, the filling defects in the joint are more confluent and less distinct. MRI may show foci of decreased intensity of the synovium in all sequences because of the paramagnetic effects caused by deposition of hemosiderin (see Fig. 15). Synovial hemangioma usually presents as a single soft-tissue mass. On MRI, T1-weighted images show that the lesion is either isointense or slightly higher (brighter) in signal intensity than surrounding muscles, but much lower in intensity than subcutaneous fat. On T2-weighted images, the mass is invariably much brighter than fat (see Fig. 18). Phleboliths and fibrofatty septa in the mass are common findings that show low-signal characteristics. Lipoma arborescens is a villous lipomatous proliferation of the synovial membrane. This rare condition usually affects the knee joint but has occasionally been reported in other joints, including the wrist and ankle. The disease has been variously reported to have a developmental, traumatic, inflammatory, or neoplastic origin, but its true cause is still unknown. The clinical findings include slowly increasing but painless synovial thickening as well as joint effusion with sporadic exacerbation. Imaging studies reveal a joint effusion, and frond-like masses arising from the synovium, occasionally accompanied by various degrees of osteoarthritis (see Fig. 20 and Fig. 21).
Fig. 15.
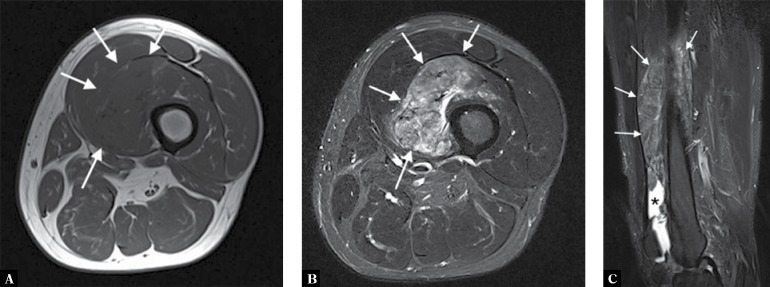
A 57-year-old man presented with a thigh mass that on the biopsy was diagnosed as PVNS. A. Axial T1-weighted, B. axial T2-weighted fat-suppressed, and C. sagittal T2-weighed fat-suppressed MR images show the mass to be arising from the suprapatellar recess and showing intermediate T1 signal. The mass shows typical signal characteristics on the T2-weighted images, appearing intermediate-to-low signal with foci of high signal representing fluid and synovium (arrows). The sagittal image also reveals joint effusion (*)
Fig. 18.
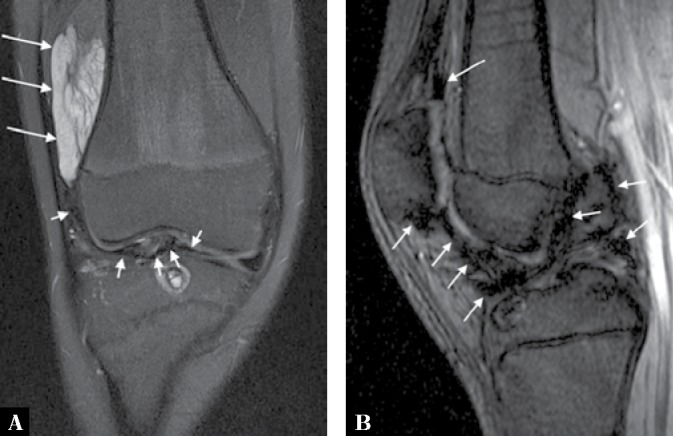
A. Coronal proton-density fat-suppressed MRI of the knee of the same patient depicted in Fig. 16 shows the mass exhibiting high signal intensity with some internal low-signal separation and striation (long arrows). Note also a low signal change in the joint (short arrows) due to hemosiderinladen synovium from recurrent hemarthroses. B. Sagittal gradient-echo scout image shows a typical blooming artefact from the joint due to a susceptibility artefact from the hemosiderin demonstrated on the T2* image
Fig. 20.
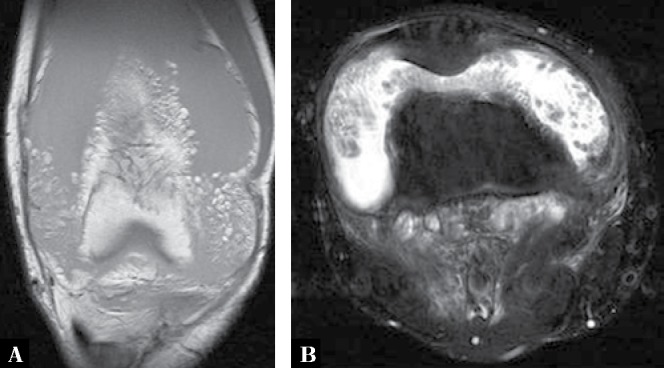
A. Coronal T1-weighted MR image of the right knee of a 42-year-old woman who presented with painless swelling of the knee shows large joint effusion with high signal intensity frond-like synovitis due to fat deposition within the synovium. B. Axial proton-density fat-suppressed MRI shows the fatty synovial proliferation as dark (due to the fat suppressed sequence) against the high-signal fluid within the joint; findings characteristic of lipoma arborescens
Fig. 21.
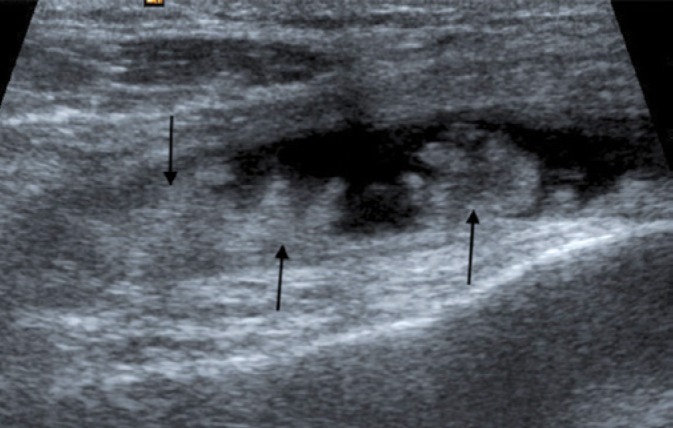
Longitudinal ultrasound through the suprapatellar recess of the right knee of the patient depicted in Fig. 20, shows hyperechoic fronds of lipid-laden synovium (arrows) surrounded by the anechoic joint fluid
Treatment and prognosis
Treatment of synovial chondromatosis usually consists of removal of the intraarticular bodies and synovectomy, but local recurrence is not uncommon. Rare cases of chondrosarcoma arising in synovial chondromatosis have been described(9,10).
Pigmented villonodular synovitis
Definition
Pigmented villonodular synovitis (PVNS) is a locally destructive fibrohistiocytic proliferation, characterized by multiple villous and nodular synovial protrusions, composed of synovial-like mononuclear cells admixed with multinucleate giant cells, foam cells, siderophages, and inflammatory cells. It affects joints, bursae, and tendon sheaths, and the condition can be diffuse or localized. When the entire synovium of the joint is affected, and when there is a major villous component, the condition is referred to as diffuse pigmented villonodular synovitis. When a discrete intraarticular mass is present, the condition is called localized pigmented villonodular synovitis. When the process affects the tendon sheaths, it is called localized giant cell tumor of the tendon sheaths. In the new revised classification of soft tissue tumors, the World Health Organization (WHO) classifies localized intraarticular and extraarticular lesions as giant cell tumor of tendon sheath, whereas the diffuse intraarticular and extraarticular forms are categorized as diffuse-type giant cell tumor (keeping PVNS as a synonym)(11–13).
Epidemiology
Although patients range in age from 4 to 60 years, the young and middle-aged individuals are most commonly affected, with a peak incidence in the third and fourth decades. Male-to-female ratio is 1:2(14).
Site of involvement
Both the diffuse and the localized forms of villonodular synovitis usually occur as a single lesion. The most common site is the knee joint (75%), less frequently affected are the hip (15%), ankle, wrist, elbow, and shoulder joints(15,16).
Clinical findings
PVNS is a slowly progressing process, with duration of symptoms ranging from 6 months to as long as 25 years. Patients usually present with mild joint pain, joint swelling, and limitation of movement in the affected joint, mimicking arthritis. Occasionally, increased skin temperature is noted over the affected joint. About 66% of patients present with bloody joint effusion. In fact, the presence of a serosanguinous synovial fluid in the absence of a history of recent trauma should strongly suggest the diagnosis of PVNS.
Imaging findings
Radiography reveals a soft-tissue density in the affected joint, frequently interpreted as joint effusion. However, the density is greater than that of simple effusion, and it reflects not only a hemorrhagic fluid but also lobulated synovial masses. A marginal, well-defined erosion of subchondral bone with a sclerotic margin may be present (incidence reported from 15% to 50%), usually on both sides of the affected articulation (Fig. 11). Narrowing of the joint space has also been reported. In the hip, multiple cyst-like or erosive areas involving non–weight-bearing regions of the acetabulum, as well as the femoral head and neck, are characteristic (Fig. 12). Calcifications are encountered only in exceptional cases(17).
Fig. 11.
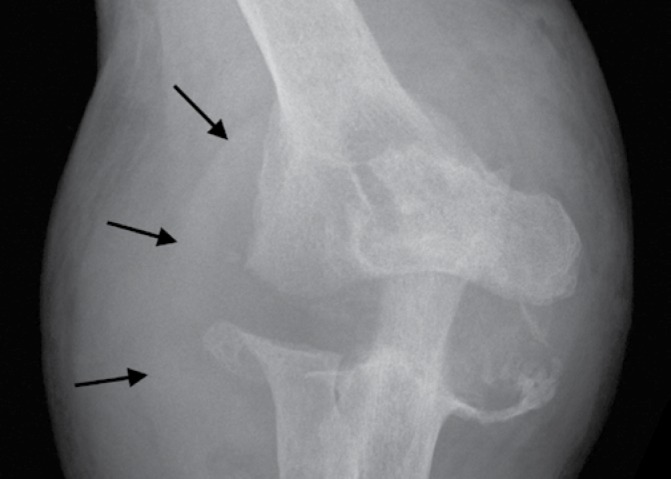
Anteroposterior radiograph of the right elbow of a 35-year-old woman with PVNS shows dense soft tissue shadowing representing hemorrhagic joint effusion and synovitis (arrows) along with extensive well-defined subchondral erosions on both sides of the joint
Fig. 12.
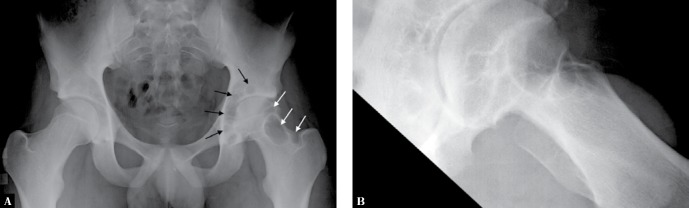
A. Anteroposterior and B. lateral views of the left hip of a 17-year-old boy with PVNS demonstrate cyst-like erosions of the acetabulum and femoral head and neck (arrows). Note also joint space narrowing relative to the right hip
Arthrography reveals multiple lobulated masses with villous projections, which appear as filling defects in the contrast-filled joint (Fig. 13). CT effectively demonstrates the extent of the disease. The increase in iron content of the synovial fluid results in high Hounsfield values, a feature that can help in the differential diagnosis. Ultrasound can demonstrate an intraarticular synovial mass, or show diffuse involvement in the joint (Fig. 14). It usefully distinguishes effusion from a solid mass. However, it can be difficult to access parts of some joints for assessment. Furthermore, on ultrasound the appearances are non-specific because hypoechoic areas are also common in other causes of hypertrophic synovium, including inflammatory arthritis. MRI has advantages in showing that the intraarticular mass has characteristic signal changes which effectively make the diagnosis. This technique is extremely useful because on T2-weighted images the intraarticular masses demonstrate a combination of high-signal-intensity areas, representing fluid and congested synovium, interspersed with areas of intermediate to low signal intensity, secondary to random distribution of hemosiderin in the synovium (Fig. 15). In general, MRI shows a low signal on T1- and T2-weighted images because of hemosiderin deposition and thick fibrous tissue. In addition, within the mass, signals consistent with fat can be noted, which are caused by clumps of lipid-laden macrophages. Other MRI findings include hyperplastic synovium and occasionally bone erosions. Administration of gadolinium in the form of Gd-DTPA leads to a notable increase in overall heterogeneity, which tends toward an overall increase in signal intensity of the capsule and septa. This enhancement of the synovium allows it to be differentiated from the fluid invariably present, which does not enhance. Apart from its diagnostic effectiveness, MRI is also useful in defining the extent of the disease(18,19).
Fig. 13.
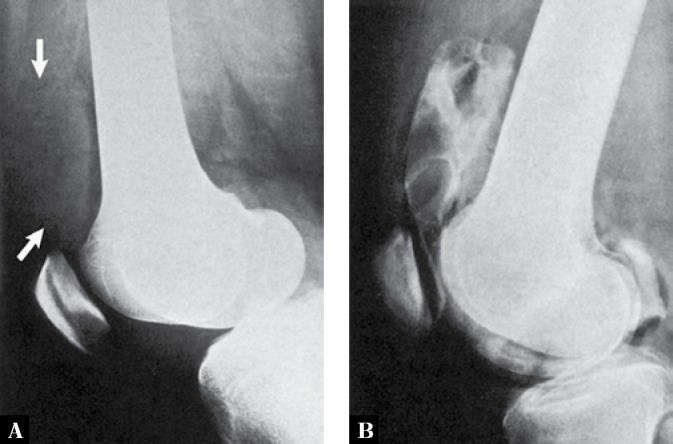
A. Lateral radiograph of the knee of a 25-year-old man with PVNS shows what appears to be suprapatellar joint effusion (arrows). However, the density of the fluid is increased, and there is some lobulation present. B Contrast arthrogram of the knee shows lobulated filling defects in the suprapatellar bursa, representing lumpy synovial masses
Fig. 14.
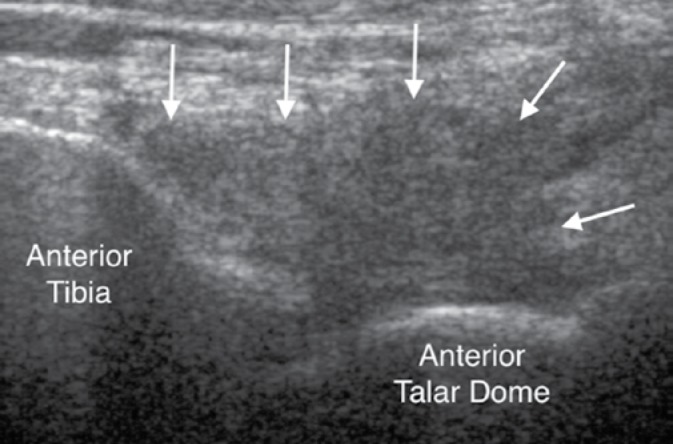
Longitudinal ultrasound image of the ankle of a 26-year-old woman with PVNS demonstrates an intraarticular soft tissue mass within the anterior aspect of the joint showing low reflectivity (arrows)
Pathologic findings
Jaffe, Lichtenstein, and Sutro first described PVNS in 1941, and used this name to identify the lesion because of its yellow–brown, villous, and nodular appearance(20). The yellow–brown pigmentation is caused by excessive deposits of lipids and hemosiderin. One of the most characteristic findings in PVNS is the ability of the hyperplastic synovium to invade the subchondral bone, producing cysts and erosions. Although the cause is unknown and is often controversial, some investigators have suggested an autoimmune pathogenesis(21). Some investigators contended that PVNS represents a true benign neoplasm. Although the latter theory was presumed to be supported by pathologic studies indicating that the histiocytes present in PVNS may function as facultative fibroblasts and that foam cells may derive from histiocytes, thus relating PVNS to a benign neoplasm of a fibrohistiocytic origin, these findings do not constitute a definite proof that PVNS is a true neoplasm. They are rather indicative of a special form of a chronic proliferative inflammation process(20).
Gross pathologic examination shows a tan-colored or reddish-brown synovial mass, firm or sponge-like, with hypertrophic villi. On histopathologic examination, PVNS reveals tumor-like proliferation of the synovial tissue. A dense infiltration of mononuclear histiocytes is observed, accompanied by plasma cells, xanthoma cells, lymphocytes, and variable numbers of giant cells. Mitotic figures are not uncommon. Iron deposits and aggregates of foam cells may be present usually at the periphery of the lesion. Long-standing lesions show fibrosis and hyalinization(22–24).
Immunohistochemistry
Positivity for CD68, CD163, CD45, and in some cases for CD34 is observed.
Genetics
The majority of cases show t(1;2)(p11;q37) translocation with COL66A3-CSF1 gene fusion. Rearrangements of the 1p11-13 regions are also encountered(25).
Differential diagnosis
The most common diagnostic possibilities include hemophilic arthropathy, synovial chondromatosis, synovial hemangioma, and synovial sarcoma. MRI is very effective in distinguishing these entities because it can reveal hemosiderin deposition in PVNS. Although this feature may also be present in hemophilic arthropathy, detection of diffuse hemosiderin clumps, synovial irregularity and thickening, and distention of the synovial sac favors the diagnosis of PVNS. In addition, hemophilia, unlike PVNS, commonly affects multiple joints and is associated with growth disturbance at the articular ends of the affected bones. It should be noted that synovial hemangioma may also lead to recurrent hemarthrosis, which may result in hemosiderin deposition within the joint, but this condition is commonly associated with the formation of phleboliths. Synovial chondromatosis may manifest with pressure erosions of the bone similar to those of PVNS (see Fig. 8). However, it can be distinguished by the presence of multiple joint bodies, calcified or uncalcified. Synovial sarcoma tends to have a shorter T1 and longer T2 on MRI compared with PVNS, and when calcifications are present the latter diagnosis can be excluded.
Treatment and prognosis
Treatment usually consists of surgical open or arthroscopic synovectomy. Occasionally, intraarticular radiation synovectomy is used when the abnormal synovial tissue is less than 5 mm thick. Recently, reports appeared of postsynovectomy adjuvant treatment with external beam radiation therapy or intraarticular injection of radioactive material, such as yttrium-90 (90Y)(26). Local recurrence is not uncommon and is reported in approximately 50% of cases.
Synovial hemangioma
Definition
Synovial hemangioma is a rare lesion, consisting of benign proliferation of blood vessels arising in a synovium-lined surface, including the joints, bursae, and tendon sheaths.
Epidemiology
The lesion most commonly occurs in children and adolescents, with predilection for males.
Sites of involvement
Most commonly the knee joint is affected, usually its anterior compartment.
Clinical findings
Almost all patients with synovial hemangioma are symptomatic, frequently presenting with a swollen knee or with mild pain or limitation of movement in the joint. Sometimes patients report a history of recurrent episodes of joint swelling and various degrees of pain of several years’ duration. Recurrent hemarthrosis may be also encountered. Synovial hemangioma is often associated with an adjacent cutaneous or deep soft-tissue hemangioma. For this reason, some investigators classify knee joint lesions as intraarticular, juxtaarticular, or intermediate, depending on the extent of involvement. Synovial hemangioma is frequently misdiagnosed. According to one estimate, a correct preoperative diagnosis is made in only 22% of cases(27,28).
Imaging findings
Until recently, synovial hemangiomas were evaluated by a combination of conventional radiography, arthrography, angiography, and contrast-enhanced CT. Although radiographs appear normal in at least half of the patients, they may reveal soft-tissue swelling, a mass around the joint, joint effusion, or erosions. Phleboliths, periosteal thickening, advanced maturation of the epiphysis, and arthritic changes are also occasionally noted on conventional radiographs(29). Arthrography usually shows nonspecific filling defects with a villous configuration. They can often reveal a vascular lesion and can demonstrate pathognomonic features of hemangioma. Ultrasound has limitations in assessing some joint recesses as it may be challenging to find a sonographic window. However, when the hemangioma is identified, it typically appears as a vascular mass of heterogenous hypoechogenicity. Phleboliths, where present, will be seen as brightly echogenic foci within the lesion. Vascular channels may be identified, depending on their size. Doppler examination may demonstrate flow within the vessels, which may be high or slow flow depending on the nature of the lesion (Fig. 16). Angiograms yield much more specific information. They can often reveal a vascular lesion and can demonstrate pathognomonic features of hemangioma (Fig. 17). Contrast-enhanced CT of the joint typically reveals a heterogeneous-appearing soft-tissue mass that displays tissue attenuation approximating that of skeletal muscle and containing areas of decreased attenuation, some approaching that of fat. CT is effective for demonstrating phleboliths and revealing patchy enhancement around them, as well as enhancement of tubular areas and contrast pooling within the lesion. In some cases, CT reveals enlarged vessels feeding and draining the mass, as well as enlarged adjacent subcutaneous veins. At present, MRI has become the modality of choice for the evaluation of hemangiomas because, with this modality, a presumptive diagnosis can be made. The soft-tissue mass typically exhibits an intermediate signal intensity on T1-weighted sequences, appearing isointense with or slightly brighter than muscle but much less bright than fat. The mass is usually much brighter than subcutaneous fat on T2-weighted images and on fat suppression sequences and shows thin, often serpentine, low-intensity septa within it (Fig. 18). In general, the signal intensity characteristics of hemangiomas appear to be related to a number of factors, including slow flow, thrombosis, vessel occlusion, and stagnant blood that pools in larger vessels and dilated sinuses, as well as to the variable amounts of adipose tissue in the lesion(30). After intravenous injection of gadolinium, there is evidence of enhancement of the hemangioma. In patients with a cavernous hemangioma of the knee, fluid–fluid levels are also observed (Fig. 19), a finding recently reported also in soft-tissue hemangiomas of this type. MRI may also demonstrate low signal synovium as a result of hemosiderin deposition, if there have been recurrent hemarthroses (see Fig. 18).
Fig. 16.
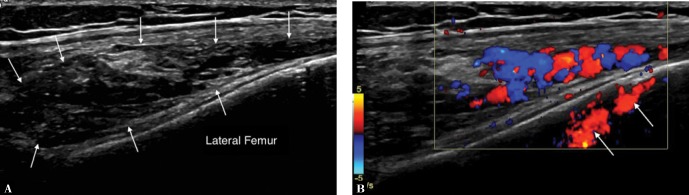
A Longitudinal ultrasound of the knee of a 17-year-old girl who presented with a history of recurrent hemarthrosis and palpable mass over anterolateral aspect of the knee shows a heterogeneous predominantly hyporeflective mass alongside the lateral femoral metaphysis (arrows). B. Color Doppler examination demonstrates flow in the tortuous vessels within the lesson. The apparent “flow” within the femur (arrows) is caused by reverberation artefact
Fig. 17.
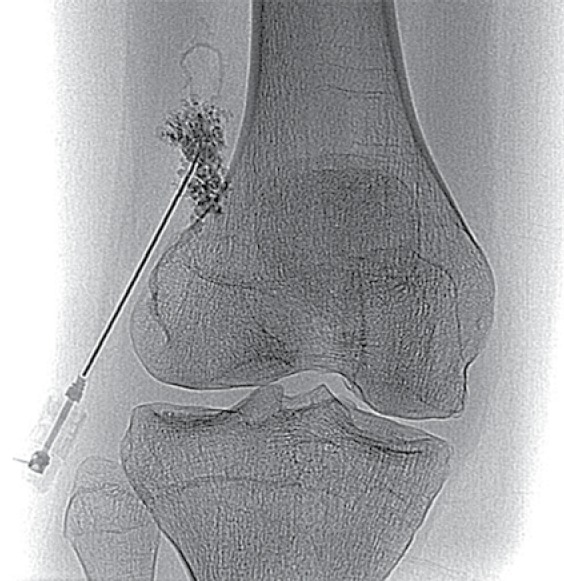
Angiogram performed in the patient depicted in Fig. 16 at the time of direct puncture of the lesion and injection of contrast during sclerotherapy demonstrates disorganized vessels within the hemangioma
Fig. 19.
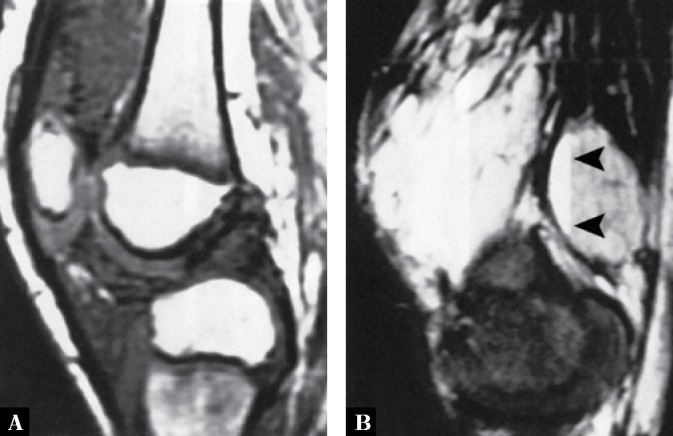
A. Sagittal T1-weighted MR image of the knee of a 9-year-old boy with synovial hemangioma shows isointense with muscle masses within the suprapatellar bursa and infrapatellar Hoffa fat pad. B. Sagittal T2-weighted fat-suppressed MR image shows the masses becoming very bright. The fluid-fluid levels seen in the popliteal region (arrowheads) are typical for the cavernous type of the lesion
Pathologic findings
Grossly, the tumor is a lobulated soft, brown, doughy mass with overlying villous synovium that is often stained mahogany brown by hemosiderin. When the lesion is completely intraarticular, it is usually well circumscribed and apparently encapsulated, attached to the synovial membrane by a pedicle of variable size, and adherent to the synovium on one or more surfaces by separable adhesions. On microscopic examination, the lesion exhibits arborizing vascular channels of different sizes and hyperplastic overlying synovium, which may show abundant iron deposition in chronic cases with repeated hemarthrosis. Originating in the subsynovial layer mesenchyme of the synovial membrane, synovial hemangioma is a vascular lesion that contains variable amounts of adipose, fibrous, and muscle tissue, as well as thrombi in the vessels.
Differential diagnosis
The differential diagnosis of synovial hemangioma includes PVNS and synovial chondromatosis. All proliferative chronic inflammatory processes, such as rheumatoid arthritis, tuberculous arthritis, and hemophilic arthropathy, should also be considered in the differential diagnosis, but these conditions, when involving the knee, can usually be distinguished clinically. Because it is extremely uncommon, lipoma arborescens is rarely included in the differential diagnosis. MRI is diagnostic for the latter condition, showing typical frond-like projections of the lesion and fat characteristics (bright on T1- and intermediate on T2-weighted images). In PVNS, radiography commonly reveals findings similar to those of synovial hemangioma, such as joint effusion and a mass in the suprapatellar bursa or popliteal fossa region. Radiographs may also demonstrate bone erosions on both sides of the joint. MRI, however, is usually diagnostic for PVNS, demonstrating that the synovium exhibits nodular thickening and masses of heterogeneous signal intensity (see previous discussion). Synovial osteochondromatosis can be distinguished from synovial hemangioma if radiography shows calcified bodies. Intraarticular osteochondral fragments of uniform size are almost pathognomonic for this condition. CT may be helpful in demonstrating faint calcifications not otherwise seen.
Treatment and prognosis
Small lesions can be removed completely without risk of local recurrence.
Lipoma arborescens
Definition
Lipoma arborescens, also known as villous lipomatous proliferation of the synovial membrane, is a rare intraarticular disorder characterized by non-neoplastic lipomatous proliferation of the synovial membrane. The term “arborescens” (from the Latin word arbor, meaning tree) describes the characteristic tree-like morphology of the hypertrophied synovium, which exhibits a frond-like appearance(31–33). The term “lipoma” is a misnomer, because there is no focal mass. It has been suggested that a more appropriate term for this condition would be synovial lipomatosis. The cause of this disorder remains uncertain, although an association with rheumatoid arthritis, psoriasis, and diabetes mellitus has been postulated. It has been suggested that there may be an association between lipoma arborescens and premature onset of osteoarthritis(34).
Epidemiology
It is a rare condition, consisting of less than 1% of all lipomatous lesions, most commonly seen in patients between the fourth and seventh decades, and more prevalent in males.
Sites of involvement
Lipoma arborescens may be monoarticular or polyarticular(35). This lesion most commonly affects the knee joint(36,37), although involvement of other joints, such as the shoulder, hip, wrist, elbow, and ankle, has been sporadically reported by various authors(38). Occasionally this condition may affect multiple joints. There have been also sporadic reports of bursae and tendon sheaths involvement(39).
Clinical findings
The patients present with slowly increasing but painless joint effusion accompanied by synovial thickening. Occasionally limited motion within the joint is encountered.
Imaging findings
Imaging studies, particularly MRI, are very characteristic and allow definite diagnosis of this condition(40). Joint effusion is invariably present, associated with frond-like masses arising from the synovium that have the signal intensity of fat on all imaging sequences (Fig. 20). Occasionally, a chemical shift artifact is present at the fat–fluid interface. Ultrasound typically shows lipoma arborescens as a synovial based hyperechoic frond-like mass, associated with joint effusion (Fig. 21). While the lesion has a characteristic appearance on ultrasound, MRI remains the imaging modality of choice when this diagnosis is suspected.
Pathologic findings
Gross pathology shows yellowish-white mass with villous proliferation. Histopathologically, lipoma arborescens is characterized by hyperplasia of subsynovial fat, formation of mature fat cells, and the presence of proliferative villous projections. Osseous and chondroid metaplasia can occur.
Differential diagnosis
Differential diagnosis should include PVNS, synovial chondromatosis, synovial hemangioma, hemophilic arthropathy, and a variety of intraarticular inflammatory conditions.
Treatment and prognosis
The lesion is cured by synovectomy; recurrences are uncommon.
Conclusions
It is important that clinicians and radiologists are aware of the range of articular abnormalities that can masquerade as arthritis. This is particularly important when faced with a patient presenting with a monoarticular disease. As has been shown, imaging has an important responsibility, not only in suggesting that the diagnosis is not arthritis, but in many cases making the specific diagnosis. While radiographs, ultrasound, and CT have roles to play in the imaging work-up, MRI is particularly valuable in making the final diagnosis with its ability to define a lesion based on its signal characteristics. For example, the presence of low T2 signal is invaluable in identifying blood products associated with PVNS, and high T1 signal indicating fat in lipoma arborescens. Synovial osteochondromatosis can be readily diagnosed on radiographs, ultrasound, and CT, when calcification exists in the chondral bodies, but MRI is able to demonstrate the chondral bodies before they calcify. Imaging also has an important role in identifying damage to the joint associated with these conditions.
Footnotes
Conflict of interest
Authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
References
- 1.Greenspan A, Gershwin ME: Imaging in Arthritis: A Clinical Approach. Wolters Kluwer, Philadelphia: 2018: 377–419. [Google Scholar]
- 2.Hermann G, Abdelwahab IF, Klein M, Kenan S, Lewis M: Synovial chondromatosis. Skeletal Radiol 1995; 24: 298–300. [DOI] [PubMed] [Google Scholar]
- 3.Crotty JM, Monu JU, Pope TL Jr: Synovial osteochondromatosis. Radiol Clin North Am 1996; 34: 327–342. [PubMed] [Google Scholar]
- 4.Jacobson JA, Girish G, Jiang Y, Sabb BJ: Radiographic evaluation of arthritis: degenerative joint disease and variations. Radiology 2008; 248: 737–747. [DOI] [PubMed] [Google Scholar]
- 5.Blacksin MF, Ghelman B, Freiberger RH, Salvati E: Synovial chondromatosis of the hip. Evaluation with air computed arthrotomography. Clin Imaging 1990; 14: 315–318. [DOI] [PubMed] [Google Scholar]
- 6.Murphey MD, Vidal JA, Fanburg-Smith JC, Gajewski DA: Imaging of synovial chondromatosis with radiologic-pathologic correlation. Radiographics 2007; 27: 1465–1488. [DOI] [PubMed] [Google Scholar]
- 7.Sheldon PJ, Forrester DM, Learch TJ: Imaging of intraarticular masses. Radiographics 2005; 25: 105–119. [DOI] [PubMed] [Google Scholar]
- 8.Hopyan S, Nadesan P, Yu C, Wunder J, Alman BA: Dysregulation of hedgehog signaling predisposes to synovial chondromatosis. J Pathol 2005; 206: 143–150. [DOI] [PubMed] [Google Scholar]
- 9.Evans S, Boffano M, Chaudhry S, Jeys L, Grimer R: Synovial chondrosarcoma arising in synovial chondromatosis. Sarcoma 2014. DOI: 10.1155/2014/647939. [DOI] [PMC free article] [PubMed]
- 10.Ontell F, Greenspan A: Chondrosarcoma complicating synovial chondromatosis: findings with magnetic resonance imaging. Can Assoc Radiol J 1994; 45: 318–323. [PubMed] [Google Scholar]
- 11.De St. Aubain Sommerhausen N, Dal Cin P: Giant cell tumour of tendon sheath In: Fletcher CDM, Unni KK, Mertens F (eds.): World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. IARC Press, Lyon: 2002: 110–111. [Google Scholar]
- 12.De St. Aubain Sommerhausen N, Dal Cin P: Diffuse-type giant cell tumour In: Fletcher CDM, Unni KK, Mertens F (eds.): World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. IARC Press, Lyon: 2002: 112–114. [Google Scholar]
- 13.Fletcher CDM, Bridge J, Hogendoorn P, Mertens F (eds.): WHO Classification of Tumors of Soft Tissue and Bone. IARC Press, Lyon: 2013. [Google Scholar]
- 14.Greenspan A, Borys D: Radiology and Pathology Correlation of Bone Tumors. A Quick Reference and Review. Wolters Kluwer, Philadelphia: 2016: 353–380. [Google Scholar]
- 15.Bravo SM, Winalski CS, Weissman BN: Pigmented villonodular synovitis. Radiol Clin North Am 1996; 34: 311–326. [PubMed] [Google Scholar]
- 16.Cotten A, Flipo RM, Chastanet P, Desvigne-Noulet MC, Duquesnoy B, Delcambre B: Pigmented villonodular synovitis of the hip: review of radiographic features in 58 patients. Skeletal Radiol 1995; 24: 1–6. [DOI] [PubMed] [Google Scholar]
- 17.Lin J, Jacobson JA, Jamadar DA, Ellis JH: Pigmented villonodular synovitis and related lesions: the spectrum of imaging findings. AJR Am J Roentgenol 1999; 172: 191–197. [DOI] [PubMed] [Google Scholar]
- 18.Eustace SE, Harrison M, Srinivasen U, Stack J: Magnetic resonance imaging in pigmented villonodular synovitis. Can Assoc Radiol J 1994; 45: 283–286. [PubMed] [Google Scholar]
- 19.Besette PR, Cooley PA, Johnson RP, Czarnecki DJ: Gadolinium-enhanced MRI of pigmented villonodular synovitis of the knee. J Comput Assist Tomogr 1992; 16: 992–994. [DOI] [PubMed] [Google Scholar]
- 20.Jaffe HL, Lichtenstein L, Sutro CJ: Pigmented villonodular synovitis, bursitis and tenosynovitis. Arch Pathol Lab Med 1941; 31: 731–765. [Google Scholar]
- 21.Stout AP, Lattes R: Atlas of Tumor Pathology. Tumors of the Soft Tissues (2nd Series Fascicle 1). Armed Forces Institute Pathology, Washington DC: 1967: 38–52. [Google Scholar]
- 22.Dorwart RH, Genant HK, Johnston WH, Morris JM: Pigmented villonodular synovitis of synovial joints: clinical, pathologic, and radiologic features. AJR Am J Roentgenol 1984; 143: 877–885. [DOI] [PubMed] [Google Scholar]
- 23.Llauger J, Palmer J, Rosón N, Cremades R, Bagué S: Pigmented villonodular synovitis and giant cell tumors of the tendon sheath: radiologic and pathologic features. AJR Am J Roentgenol 1999; 172: 1087–1091. [DOI] [PubMed] [Google Scholar]
- 24.Rubin BP: Tenosynovial giant cell tumor and pigmented villonodular synovitis: a proposal for unification of these clinically distinct but histologically and genetically identical lesions. Skeletal Radiol 2007; 36: 267–268. [DOI] [PubMed] [Google Scholar]
- 25.Mendenhall WM, Mendenhall CM, Reith JD, Scarborough MT, Gibbs CP, Mendenhall NP: Pigmented villonodular synovitis. Curr Opin Oncology 2011; 23: 361–366. [Google Scholar]
- 26.Chen DY, Lan JL, Chou SJ: Treatment of pigmented villonodular synovitis with yttrium-90: changes in immunologic features. Tc-99m uptake measurements, and MR imaging of one case. Clin Rheumatol 1992; 11: 280–285. [DOI] [PubMed] [Google Scholar]
- 27.Brodsky AE: Synovial hemangioma of the knee joint. Bull Hosp Joint Dis 1956; 17: 58–69. [PubMed] [Google Scholar]
- 28.Cotten A, Flipo RM, Herbaux B, Gougeon F, Lecomte-Houcke M, Chastanet P: Synovial haemangioma of the knee: a frequently misdiagnosed lesion. Skeletal Radiol 1995; 24: 257–261. [DOI] [PubMed] [Google Scholar]
- 29.Devaney K, Vinh TN, Sweet DE: Synovial hemangioma: report of 20 cases with differential diagnostic considerations. Hum Pathol 1993; 24: 737–745. [DOI] [PubMed] [Google Scholar]
- 30.Greenspan A, Azouz EM, Matthews J, Décarie JC: Synovial hemangioma: imaging features in eight histologically proven cases, review of the literature, and differential diagnosis. Skeletal Radiol 1995; 24: 583–590. [DOI] [PubMed] [Google Scholar]
- 31.Armstrong SJ, Watt I: Lipoma arborescens of the knee. Br J Radiol 1989; 62: 178–180. [DOI] [PubMed] [Google Scholar]
- 32.Grieten M, Buckwalter KA, Cardinal E, Rougraff B: Case report 873. Lipoma arborescens (villous lipomatous proliferation of the synovial membrane). Skeletal Radiol 1994; 23: 652–655. [DOI] [PubMed] [Google Scholar]
- 33.Hallel T, Lew S, Bansal M: Villous lipomatous proliferation of the synovial membrane (lipoma arborescens). J Bone Joint Surg Am 1988; 70: 264–270. [PubMed] [Google Scholar]
- 34.Al-Ismail K, Torreggiani WC, Al-Sheikh F, Keogh C, Munk PL: Bilateral lipoma arborescens associated with early osteoarthritis. Eur Radiol 2002; 12: 2799–2802. [DOI] [PubMed] [Google Scholar]
- 35.Bejia I, Younes M, Moussa A, Said M, Touzi M, Bergaoui N: Lipoma arborescens affecting multiple joints. Skeletal Radiol 2005; 34: 536–538. [DOI] [PubMed] [Google Scholar]
- 36.Kloen P, Keel SB, Chandler HP, Geiger RH, Zarins B, Rosenberg AE: Lipoma arborescens of the knee. J Bone Joint Surg Br 1998; 80: 298–301. [DOI] [PubMed] [Google Scholar]
- 37.Ryu KN, Jaovisidha S, Schweitzer M, Motta AO, Resnick D: MR imaging of lipoma aborescens of the knee joint. AJR Am J Roentgenol 1996; 167: 1229–1232. [DOI] [PubMed] [Google Scholar]
- 38.Laorr A, Peterfy CG, Tirman PF, Rabassa AE: Lipoma arborescens of the shoulder: magnetic resonance imaging findings. Can Assoc Radiol J 1995; 46: 311–313. [PubMed] [Google Scholar]
- 39.White EA, Omid R, Matcuk GR, Domzalski JT, Fedenko AN, Gottsegen CJ. et al. : Lipoma arborescens of the biceps tendon sheath. Skeletal Radiol 2013; 42: 1461–1464. [DOI] [PubMed] [Google Scholar]
- 40.Parsonage S, Mehr A, Davies AM: Lipoma arborescens: a definitive MR imaging diagnosis. Osteol Közlem 2001; 9: 80–82. [Google Scholar]