Abstract
We present a report on ultrasound findings in extragenital endometriosis and a literature review accompanied by illustrations. Intestinal endometriosis should be considered in female patients of reproductive age who present with constipation, gastrointestinal bleeding, nausea, vomiting, cramp-like abdominal pain, diarrhoea and pelvic pain. Although definitive preoperative diagnosis of endometriosis is difficult, clinical suspicion and appropriate imaging might prevent extensive surgical procedures with higher morbidity. Contrast-enhanced ultrasound is an efficient non-invasive imaging method without any radiation exposure that supports the early diagnosis of intestinal endometriosis and may help assess the vascularization of endometriotic lesions within the distinct layers of the intestinal wall.
Keywords: guideline, inflammatory bowel disease, Crohn disease, colitis, diarrhea
Introduction
We present a report on ultrasound findings in extragenital endometriosis and a literature review accompanied by illustrations. Clinically, intestinal endometriosis is rare and may present a major diagnostic challenge for all imaging methods(1–6). The introduction of contrast enhanced ultrasound (CEUS) using contrast agents has raised the same questions as for computed tomography (CT) three decades ago. Today, CT without contrast agents is not “state of the art” any more, but contrast injection was controversially discussed at the beginning of the CT era as it is currently for CEUS. CEUS is an efficient non-invasive imaging method with no exposure to radiation and with much less side effects compared to CT contrast agents. CEUS is promising to characterize the enhancement features (vascularization) of endometriotic lesions and might be superior compared to CT due to its strict intravascular distribution. This modified review has been published on the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) website (www.efusmb.org) and might serve as an illustration and comment paper of the most recent guidelines and recommendations of the EFSUMB and the guidelines and position papers of the World Federation for Ultrasound in Medicine and Biology (WFUMB) to better link efforts between the federation of societies. EFSUMB and WFUMB guidelines have been published for a variety of educational purposes which can be freely downloaded from the EFSUMB website(7).
Illustrations
Illustration 1
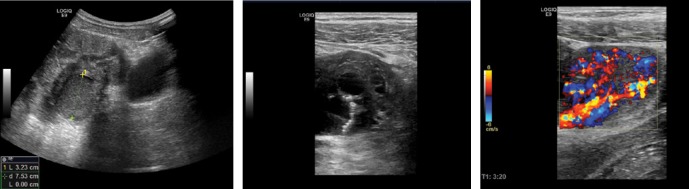
The following figures show transmural endometriosis infiltrating the sigmoid colon and present endoscopy, ultrasound, CEUS and endoscopic ultrasound findings. A 24-year old woman complained of recurrent lower abdominal pain for 6 months. She was referred for colonoscopy. There was no palpable mass on digital rectal examination. Blood chemistry, full blood count, coagulation profile, alfa-fetoprotein and carcinoembryonic antigen were within normal limits. Colonoscopy revealed a semi-circular polypoid lesion in the sigmoid colon suggesting malignancy (Fig. 1). The biopsy taken at colonoscopy was diagnostic for endometriosis and surgical treatment was planned. Other imaging modalities were arranged to stage the local disease and to exclude further manifestations. Transabdominal B-mode ultrasound (BMUS) confirmed a 40 mm sized heterogeneous hypoechoic transmural lesion infiltrating the sigmoid colon (Fig. 2). Contrast enhanced ultrasound showed a rapidly and heterogeneously enhancing lesion during the arterial and venous phases (Fig. 3). Contrast enhanced colour Doppler ultrasound confirmed the finding. Endorectal endoscopic ultrasound of the sigmoid colon revealed transmural extension of the mass and confirmed the transcutaneous finding (Fig. 4). The lesion was well vascularized (Fig. 2 and Fig. 4). Magnetic resonance imaging did not add any additional information. Subsequently, the patient underwent laparoscopic sigmoid resection. Pathological examination revealed extragenital endometriosis. Microscopic examination disclosed endometrial stroma and gland islands located between muscular fibres, subserosa and serosa. The postoperative period was uneventful.
Fig. 1.
Colonoscopy showed a semicircular polypoid lesion in the sigmoid colon suggesting neoplasia (A). The lesion was partially resected by endoscopic mucosal resection for tissue sampling (B)
Fig. 2.
Transabdominal B-mode ultrasound (BMUS) confirmed a 40 mm sized heterogeneous hypoechoic and hypervascular lesion infiltrating the sigmoid colon (A–C) using the conventional abdominal probe (A), high frequency transducer for more details (B) and color Doppler imaging (C)
Fig. 3.
After administering contrast agents, the lesion showed rapid heterogeneous hyperenhancement during early arterial phase (A) and late phase (B). After CEUS, blood flow signals in the lesion were increased (C)
Fig. 4.
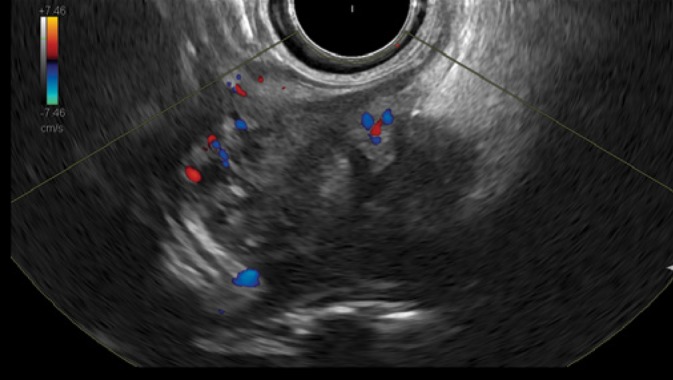
Endorectal endoscopic ultrasound of the sigmoid colon revealed transmural extension of the mass confirming the transcutaneous finding. The lesion was vascularized
Illustration 2
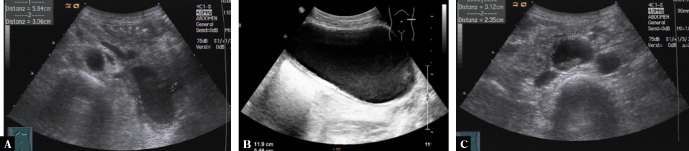
Pancreatic and retroperitoneal endometriosis mimicking pancreatic pseudocyst is presented by the second image sequence. A 40-year old woman presented in March 2015 with diffuse abdominal pain, nausea and weight loss. Medical history included active alcohol abuse and uterine rupture during pregnancy five years before. Ultrasound (Fig. 5) and computed tomography (Fig. 6) revealed multiple confluent cystic lesions with a thick cystic wall in the left abdomen with contact to the pancreatic tail suggestive of pancreatic pseudocysts. However, neither ultrasound nor CT revealed abnormalities of the pancreatic parenchyma or the pancreatic duct. A further cystic lesion was detected in the retroperitoneum between the abdominal aorta and the inferior caval vein. Percutaneous ultrasound guided aspiration of the cystic fluid and biopsy of the cystic wall were performed to rule out malignancy. Analysis of the chocolate-like fluid revealed a markedly increased lipase concentration (13.697 U/L), supporting the diagnosis of pancreatic pseudocysts. Histological examination, however, resulted in the diagnosis of pancreatic endometriosis. This diagnosis was confirmed after surgical resection and pathological analysis of the surgical specimen.
Fig. 5.
Transabdominal ultrasound of a pancreatic and retroperitoneal endometriosis mimicking pancreatic pseudocysts. Left upper abdominal oblique scan showing a large cystic lesion with slightly echogenic content, thick cystic wall and contact to the pancreatic tail (A). Left flank scan demonstrating a large cystic lesion with echogenic sediment and a thick cystic wall with several layers (B). Transverse abdominal scan revealing a mixed cystic solid lesion with a thick wall between the abdominal aorta and the inferior caval vein (C)
Fig. 6.
Contrast enhanced computed tomography (portal venous phase) of a pancreatic and retroperitoneal endometriosis mimicking pancreatic pseudocysts. Transverse scan showing two cystic lesions inside the pancreatic tail and in the neighborhood of the pancreatic tail with contrast enhancement of the cystic wall (A). Transverse scan of several cystic lesions in the neighborhood of the pancreatic tail with contrast enhancement of the cystic wall (B). Transverse scan of the lower abdomen showing multiple retroperitoneal cystic lesions in the neighborhood of the left paracolic gutter and the psoas muscle. Note contrast-enhancement of the cystic wall (C)
Illustration 3
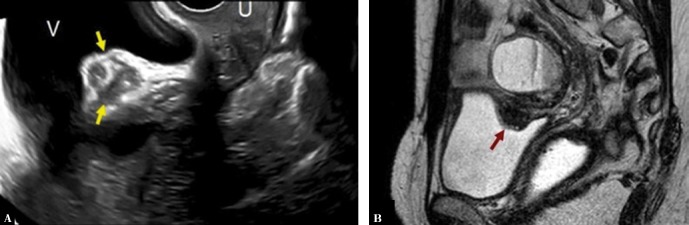
The following figures show transmural endometriosis infiltrating the posterior bladder wall and present ultrasound and MRI findings. Bladder wall is an uncommon location; it is typically located in the posterior bladder wall and it is usually associated with endometriosis elsewhere in the pelvis. A 38-year-old woman complained of intense dysmenorrhea and dysuria. Transvaginal ultrasound revealed a hypoechogenic nodule attached to the posterior bladder wall infiltrating the muscle layer (Fig. 7). Magnetic resonance imaging showed hypointense nodular lesion with characteristic intralesional 1–4-mm high-signal intensity foci that represent ectopic endometrial glands (Fig. 7). MRI showed an associated ovarian endometrioma. Laparoscopy showed a fibrous nodule in the wall of the bladder adhered to the anterior uterine surface. An additional endometrioma in the pouch of Douglas was found. The bladder nodule was dissected after adhesiolysis and the integrity of the bladder mucosa was verified. The diagnosis of extragenital endometriosis was confirmed by pathological analysis of the surgical specimen.
Fig. 7.
Bladder wall deep endometriosis (A) Transvaginal ultrasound image with endometriosis implant inside the upper bladder wall (arrows) V: bladder, U: uterus. (B) Sagittal single-shot fast spin-echo T2-weighted image shows a hypointense nodular lesion with characteristic hyperintense foci (arrow). Note a hyperintense rounded lesion located cranial to the implant with a fluid-liquid level that proved to be an ovarian endometrioma
Discussion
Definition, etiology and pathogenesis
Endometriosis is a common condition defined as the presence of endometrial glands and stroma outside the uterine cavity, most often involving the pelvis, including the ovaries. Extragenital endometriosis, however, is rare and can affect all organs, most often the gastrointestinal (GI) and urinary tract. It occurs in about 8–12% of women with endometriosis(8). Endometriosis is detected more frequently in the genital organs and pelvic peritoneum, rarely in the gastrointestinal tract, bladder, greater omentum, surgical scars, pancreas, kidneys, umbilical, thorax, abdominal wall and even nasal cavity.
The most common location of extragenital endometriosis is the gastrointestinal tract(4). Clinical suspicion is of importance for achieving the diagnosis. The most common site affected within the gastrointestinal tract is the rectosigmoid junction, followed by the ileum and the appendix(9,10). Endometriosis involving the mucosa of the intestine is very rare and may lead to diagnostic pitfalls and subsequent mismanagement. Deep endometriosis is defined as endometriosis involving the muscularis layer. Deep infiltrating endometriosis is associated with reactive inflammation of the surrounding area, including proliferation of smooth muscle cells, fibrosis and adhesions. For optimal management of patients with endometriosis involving the sigmoid and/or rectum, it is important to understand the clinical context and pre-operative imaging characteristics. Most importantly we refer on how to learn gastrointestinal ultrasound, which has been published in detail(11–14).
The first laparoscopic approach to intestinal endometriosis has been reported in 1980(15). The assumed pathomechanism for extragenital endometriosis is retrograde spread as proposed by Sampson, which refers to propagation of endometrial cells into the peritoneal cavity through the fallopian tubes during menstruation followed by dissemination to other areas(16). Recently, Nezhat et al. summarized the current knowledge regarding diagnosis and management of bowel endometriosis(4).
Endometriosis is also a risk factor for development of extrauterine endometrial stromal sarcoma (EESS) and history and/or histological evidence for endometriosis is usually present. Hormone replacement therapy (HRT) is an acknowledged risk factor as well. The condition can mimic a chronic or acute abdominal pathology, and laparoscopic core biopsy is the best way to achieve the diagnosis and management(17).
The endometrial inclusions in the abdominal wall scar are iatrogenic ‘implants’, created at the same time with the surgical operation performed on patients with genital endometriosis. Symptoms included cyclical pain and palpable subcutaneous masses fixed to the surrounding tissues(18). The only curable treatment is the surgical removal of all the pathological tissue through a large excision. Hormonal therapy is adjuvant(19).
Endometriosis lesions may also relatively often involve the urinary tract. Renal endometriosis is extremely rare. It might initially be misinterpreted as complicated hemorrhagic cysts(20). Treatment involves hormonal manipulation or hysterectomy with bilateral salpingo-oophorectomy. Whether nephrectomy is indicated also depends on renal function. Early diagnosis may prevent unnecessary nephrectomy in cases of uncomplicated renal endometriosis(21).
Symptoms
Endometrial tissue implanted into the gastrointestinal tract can cause gastrointestinal symptoms including abdominal pain, rectal bleeding and dyschezia. Symptoms can be similar to irritable bowel syndrome and may even mimic colonic adenocarcinoma(22). Compared with peritoneal and ovarian endometriosis, intestinal endometriosis is more frequently associated with dysmenorrhea, dyspareunia, noncyclic pelvic pain and infertility, as well as specific bowel symptoms, including cyclic bowel alterations, dyschezia and rectal bleeding(9), constipation and bowel cramping. Women with deeply infiltrating endometriosis with implants in the Douglas pouch and rectovaginal septum, typically present with dyspareunia and painful defecation.
A prospective study performed by Roman et al.(23) demonstrated that women presenting with rectal endometriosis were more likely to present with various digestive complaints such as cyclic pain during defecation and cyclic constipation. If left untreated, progressive endometriosis may result in partial or complete bowel obstruction requiring surgical resection.
The degree of symptoms may not correlate to the size of the lesions and painful symptoms are not indicative of surgical intervention. Some patients with extensive endometriosis affecting the rectosigmoid can be almost asymptomatic, while others with small lesions can present with severe symptoms. This makes it more difficult to determine the need for intervention, especially radical surgery. An evaluation of patients with endometriosis in the rectosigmoid showed that 48% and 84% of them also had ovarian endometriosis and retrocervical lesions, respectively(24).
Diagnosis
The diagnosis of intestinal endometriosis is often difficult and delayed since the clinical presentation may be confused with other diseases including inflammatory bowel disease (IBD), diverticulitis or neoplasia (adenocarcinoma, lymphoma). Endometriosis may appear as a cystic, solid, or combined solid-cystic lesion and usually involves the serosa or subserosal layer, although it can sometimes involve all layers of the colon. As the infiltration of the intestinal wall by endometriosis rarely involves the mucosa, conventional endoscopic investigations are of little help in diagnosing intestinal involvement and will often fail to detect the disease(25). When endometriosis involves the intestinal mucosa, it may cause diagnostic difficulties, especially in endoscopic biopsies. The findings may vary depending on the day of the menstrual cycle, the ratio of stromal and glandular elements, and the amount of bleeding and inflammatory response in the surrounding tissue(4).
The majority of patients with intestinal endometriosis are diagnosed at laparoscopy or laparotomy. Diagnosing intestinal endometriosis in the bowel wall involving the serosa, muscularis propria and submucosa is usually in resected bowel specimen. There are no pathognomonic laboratory findings for intestinal endometriosis. Serum cancer antigen 125 (CA-125) levels may be elevated in women with endometriosis.
Preoperative evaluation
Clinical examination and a history of cycle-related symptoms can only raise suspicion of endometriosis. There is still ongoing debate which imaging technique is the most appropriate method for pre-surgical assessment. While definitive diagnosis requires tissue biopsy and histologic confirmation, the combination of symptoms, signs, and imaging findings can be used to make a presumptive, nonsurgical diagnosis of endometriosis. According to Sampson’s theory on the pathomechanism leading to endometriosis, endometriotic lesions affect the rectosigmoid starting from the serosa, invade towards the lumen of the bowel and finally infiltrate the entire wall. The fibrotic component represents around 80% of the lesions in intestinal endometriosis rendering surgical management more difficult(8,26). The number and size of the lesions, depth of infiltration, percentage of the intestinal wall circumference infiltrated, and lymph node involvement need to be considered when planning surgery. In a literature review, Meuleman et al.(27) reported that 95% of patients undergoing bowel resection had bowel serosa involvement; 95% had lesions infiltrating the muscularis propria while 38% had lesions infiltrating the submucosa and 6% had lesions infiltrating the mucosa.
Ultrasound findings
Little is known about the transcutaneous ultrasound findings of intestinal endometriosis, which represents the most common site of extragenital endometriosis (about 5%). With respect to the examination technique we refer to the published papers on how to learn and how to perform gastrointestinal ultrasound(11–14,28). Most commonly the rectum, rectosigmoid and sigmoid colon are involved (about 75% of intestinal endometriosis), followed by the terminal ileum, cecum and appendix. All these regions are easily accessible by transabdominal ultrasound. However, currently, transvaginal ultrasound (TVUS) is the most commonly used approach.
There are only few reports on transrectal and transabdominal ultrasound to assess intestinal endometriosis(24,29). Ultrasound findings in intestinal endometriosis have been described as hypoechoic masses with irregular and sometimes hyperechoic margins presenting in the mucosa, submucosa, muscular wall layer, serosa or other surrounding structures in close attachment to the intestinal wall. Endometriosis is not typically cystic. A fibrotic (retractive) and often painful intestinal segment may show a characteristic C-shape appearance with convergence of both edges. The uterus appears stiff and with lost mobility due to adhesions. In the colon, the muscularis propria layer is most often involved with a longitudinal, fusiform and sometimes spiculated (comet-tail) appearance and distribution on both sides. The overlying mucosa and submucosa may be intact but can also show a similar picture as seen in colorectal carcinoma(30). The urinary bladder is a less common location; if the urinary bladder is affected, endometriosis is most likely to be found in the posterior wall. Other rare locations include the kidney, retroperitoneum and mesenterium. The vascularity of endometriosis may be scarce or moderate. To our knowledge, little is known about contrast enhanced ultrasound and endometriosis.
Other imaging findings
It is remarkably difficult to diagnose intestinal endometriosis by conventional imaging methods. Endoscopic and imaging findings may mimic other diseases including any forms of colitis, acute and chronic inflammatory bowel disease, solitary rectal ulcer syndrome, diverticulitis, colorectal adenoma, colorectal cancer and many other diseases. Diagnosing intestinal endometriosis remains a challenge (“chameleon”)(31).
Ultrasound, computed tomography (CT), magnetic resonance imaging (MRI) and colonoscopy can be helpful in localising the pathology. Meuleman et al.(27) described that in 59% of the studies analyzed, the pre-operative assessment of bowel endometriosis included barium enema (26%), CT (31%) and/or MRI (28%). Advances in imaging technology and adequate training in image analysis have made it possible to pre-operatively identify characteristics of endometriosis nodules(8). Detailed imaging findings allow to define and to plan the optimal surgical procedure. This enables proper patient counselling and consenting. It facilitates appropriate selection of a multidisciplinary surgical team aiming at the best patient outcome. A sensitivity of 84% and specificity of 99% was reported in 60 patients with intestinal involvement using MRI for the diagnosis of intestinal endometriosis(32). There are scarce reports on the use of contrast enhanced ultrasound in the assessment of endometriosis. However, there are descriptions of lesion enhancement on MRI. Enhancement may or may not occur after contrast injection, depending on the proportions of inflammatory reaction, glandular tissue, and fibrosis that are present(33).
Treatment
The best treatment approach for patients with asymptomatic bowel endometriosis is still controversial. Asymptomatic patients whose lesions were diagnosed incidentally on radiologic imaging do not generally require surgery. However, large lesions involving the lumen of the rectosigmoid, causing severe haemorrhage, or progressive disease should be considered for surgery. Laparoscopy is preferred to laparotomy due to lower morbidity, less post-operative discomfort, shorter hospital stay, cosmetic reasons and faster wound healing. There is also a debate whether full thickness resection of the bowel wall is always required as in some cases shaving of the endometriotic lesions from the intestinal wall might be sufficient.
For asymptomatic patients, the indications for surgery are limited to the risk of bowel obstruction and, possibly, improvement of fertility after previous IVF failures. For patients who are not trying to conceive, medical treatment should be the first option. Although most patients respond to medical treatment, the recurrence rate is very high after cessation of therapy. Therefore, surgery should be considered the treatment of first choice, especially in young patients and those with severe symptoms. The recurrence rate after total excision is very low. Surgical treatment provides excellent results, with >85% of women showing complete improvement of symptoms and recurrence rates lower than 5%(34).
Prognosis
A review evaluating the effects of conservative surgery for rectovaginal and rectosigmoid endometriosis on reproductive function demonstrated that the mean pregnancy rate after surgery in all patients planning pregnancy, regardless of pre-operative fertility status and IVF performance, was 39%, but the spontaneous pregnancy rate was 24% only(35). It remains unclear whether surgery for deep infiltrating endometriosis might improve fertility. The completeness of surgical excision seems to determine the rate of recurrence. This was shown when clinical and histological characteristics were examined as possible predictive factors for bowel endometriosis recurrence after laparoscopic segmental bowel resection. Three independent predictor factors, positive bowel resection margins, age <31 years and body mass index ≥23 kg/m2, were also significantly associated with recurrence which was observed in 16% of all patients. The complete excision of bowel endometriosis appears most effective for avoiding disease recurrence.
Endometriosis is also a risk factor for the development of extrauterine endometrial stromal sarcoma. History and/or histological evidence of endometriosis is usually present in this disease. The condition can mimic a chronic or acute abdominal pathology and laparoscopic core biopsy is the best way to achieve a diagnosis and plan therapeutic management(17).
Conclusion
Extragenital endometriosis is a so-called diagnostic “chameleon” and should be considered in atypical clinical presentations in female patients of reproductive age. Intestinal endometriosis should be considered in patients who present with a variety of gastrointestinal symptoms, including gastrointestinal bleeding, and pelvic pain. Although definitive diagnosis of extragenital endometriosis is sometimes difficult preoperatively, clinical suspicion, appropriate imaging and biopsy with histological examination might prevent extensive surgical procedures with higher mortality. The diagnosis cannot be achieved by imaging alone since the differential diagnosis most importantly includes malignant diseases. Contrast enhanced ultrasound is an efficient non-invasive imaging method without any radiation exposure that supports the early diagnosis of intestinal endometriosis and can assess the vascularisation of endometriotic lesions within the distinct layers of the intestinal wall.
Footnotes
Conflict of interest
Authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
References
- 1.Dietrich CF, Lembcke B, Jenssen C, Hocke M, Ignee A, Hollerweger A: Intestinal ultrasound in rare gastrointestinal diseases, update, part 1. Ultraschall Med 2014; 35: 400–421. [DOI] [PubMed] [Google Scholar]
- 2.Dietrich CF, Lembcke B, Jenssen C, Hocke M, Ignee A, Hollerweger A: Intestinal ultrasound in rare gastrointestinal diseases, update, part 2. Ultraschall Med 2015; 36: 428–456. [DOI] [PubMed] [Google Scholar]
- 3.Dietrich CF, Brunner V, Lembcke B: [Intestinal ultrasound in rare small and large intestinal diseases]. Z Gastroenterol 1998; 36: 955–970. [PubMed] [Google Scholar]
- 4.Nezhat C, Li A, Falik R, Copeland D, Razavi G, Shakib A. et al. : Bowel endometriosis: diagnosis and management. Am J Obstet Gynecol 2017; 218: 549–562. [DOI] [PubMed] [Google Scholar]
- 5.Nezhat C, Hajhosseini B, King LP: Laparoscopic management of bowel endometriosis: Predictors of severe disease and recurrence. JSLS 2011; 15: 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis LA, Nezhat C: Laparoscopic treatment of bowel endometriosis. Surg Technol Int 2007; 16: 137–141. [PubMed] [Google Scholar]
- 7.Dietrich CF, Rudd L, Saftiou A, Gilja OH: The EFSUMB website, a great source for ultrasound information and education. Med Ultrason 2017; 19: 102–110. [DOI] [PubMed] [Google Scholar]
- 8.Abrão MS, Petraglia F, Falcone T, Keckstein J, Osuga Y, Chapron C: Deep endometriosis infiltrating the recto-sigmoid: Critical factors to consider before management. Hum Reprod Update 2015; 21: 329–339. [DOI] [PubMed] [Google Scholar]
- 9.Remorgida V, Ferrero S, Fulcheri E, Ragni N, Martin DC: Bowel endometriosis: presentation, diagnosis, and treatment. Obstet Gynecol Surv 2007; 62: 461–470. [DOI] [PubMed] [Google Scholar]
- 10.Rana R, Sharma S, Narula H, Madhok B: A case of recto-sigmoid endometriosis mimicking carcinoma. Springerplus 2016; 5: 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atkinson NS, Bryant RV, Dong Y, Maaser C, Kucharzik T, Maconi G. et al. : How to perform gastrointestinal ultrasound: Anatomy and normal findings. World J Gastroenterol 2017; 23: 6931–6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atkinson NS, Bryant RV, Dong Y, Maaser C, Kucharzik T, Maconi G. et al. : WFUMB position paper. Learning gastrointestinal ultrasound: Theory and practice. Ultrasound Med Biol 2016; 42: 2732–2742. [DOI] [PubMed] [Google Scholar]
- 13.Maconi G, Nylund K, Ripolles T, Calabrese E, Dirks K, Dietrich CF. et al. : EFSUMB Recommendations and Clinical Guidelines for Intestinal Ultrasound (GIUS) in inflammatory bowel diseases. Ultraschall Med 2018; 39: 304–317. [DOI] [PubMed] [Google Scholar]
- 14.Nylund K, Maconi G, Hollerweger A, Ripolles T, Pallotta N, Higginson A. et al. : EFSUMB Recommendations and Guidelines for Gastrointestinal Ultrasound. Ultraschall Med 2017; 38: e1–e15. [DOI] [PubMed] [Google Scholar]
- 15.Nezhat C, Hajhosseini B, King LP: Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS 2011; 15: 387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Acar T, Acar N, Çelik SC, Ekinci N, Tarcan E, Çapkınoğlu E: Endometriosis within the sigmoid colon/extragenital endometriosis. Ulus Cerrahi Derg 2015; 31: 250–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchholz V, Kiroff G, Trochsler M, Kanhere H: An unexpected diagnosis of primary omental endometrial stromal sarcoma in a patient with acute right abdominal pain: A case report and review of literature. Int J Surg Case Rep 2017; 36: 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marinis A, Vassiliou J, Kannas D, Theodosopoulos TK, Kondi-Pafiti A, Kairi E. et al. : Endometriosis mimicking soft tissue tumors: Diagnosis and treatment. Eur J Gynaecol Oncol 2006; 27: 168–170. [PubMed] [Google Scholar]
- 19.Paşalega M, Mirea C, Vîlcea ID, Vasile I, Pleşea IE, Calotă F. et al. : Parietal abdominal endometriosis following Cesarean section. Rom J Morphol Embryol 2011; 52 (Suppl.): 503–508. [PubMed] [Google Scholar]
- 20.Giambelluca D, Albano D, Giambelluca E, Bruno A, Panzuto F, Agrusa A. et al. : Renal endometriosis mimicking complicated cysts of kidney: Report of two cases. G Chir 2017; 38: 250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng CH, Kuo HC, Su B: Endometriosis in a kidney with focal xanthogranulomatous pyelonephritis and a perinephric abscess. BMC Res Notes 2015; 8: 591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haggag H, Solomayer E, Juhasz-Böss I: The treatment of rectal endometriosis and the role of laparoscopic surgery. Curr Opin Obstet Gynecol 2011; 23: 278–282. [DOI] [PubMed] [Google Scholar]
- 23.Roman H, Ness J, Suciu N, Bridoux V, Gourcerol G, Leroi AM. et al. : Are digestive symptoms in women presenting with pelvic endometriosis specific to lesion localizations? A preliminary prospective study. Hum Reprod 2012; 27: 3440–3449. [DOI] [PubMed] [Google Scholar]
- 24.Goncalves MO, Podgaec S, Dias JA Jr, Gonzalez M, Abrao MS: Transvaginal ultrasonography with bowel preparation is able to predict the number of lesions and rectosigmoid layers affected in cases of deep endometriosis, defining surgical strategy. Hum Reprod 2010; 25: 665–671. [DOI] [PubMed] [Google Scholar]
- 25.Bergamini V, Ghezzi F, Scarperi S, Raffaelli R, Cromi A, Franchi M: Preoperative assessment of intestinal endometriosis: A comparison of transvaginal sonography with water-contrast in the rectum, transrectal sonography, and barium enema. Abdom Imaging 2010; 35: 732–736. [DOI] [PubMed] [Google Scholar]
- 26.Sampson JA: Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol 1927; 3: 93.43–110.43. [PMC free article] [PubMed] [Google Scholar]
- 27.Meuleman C, Tomassetti C, D’Hoore A, Van Cleynenbreugel B, Penninckx F, Vergote I. et al. : Surgical treatment of deeply infiltrating endometriosis with colorectal involvement. Hum Reprod Update 2011;17: 311–326. [DOI] [PubMed] [Google Scholar]
- 28.Dietrich CF, Averkiou M, Nielsen MB, Barr RG, Burns PN, Calliada F. et al. : How to perform Contrast-Enhanced Ultrasound (CEUS). Ultrasound Int Open 2018; 4: E2–E15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hudelist G, Tuttlies F, Rauter G, Pucher S, Keckstein J: Can transvaginal sonography predict infiltration depth in patients with deep infiltrating endometriosis of the rectum? Hum Reprod 2009; 24: 1012–1017. [DOI] [PubMed] [Google Scholar]
- 30.Chamié LP, Pereira RM, Zanatta A, Serafini PC: Transvaginal US after bowel preparation for deeply infiltrating endometriosis: protocol, imaging appearances, and laparoscopic correlation. Radiographics 2010; 30: 1235–1249. [DOI] [PubMed] [Google Scholar]
- 31.Nasim H, Sikafi D, Nasr A: Sigmoid endometriosis and a diagnostic dilemma: A case report and literature review. Int J Surg Case Rep 2011; 2: 181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bazot M, Darai E, Hourani R, Thomassin I, Cortez A, Uzan S. et al. : Deep pelvic endometriosis: MR imaging for diagnosis and prediction of extension of disease. Radiology 2004; 232: 379–389. [DOI] [PubMed] [Google Scholar]
- 33.Coutinho A Jr, Bittencourt LK, Pires CE, Junqueira F, Lima CM, Coutinho E. et al. : MR imaging in deep pelvic endometriosis: A pictorial essay. Radiographics 2011; 31: 549–567. [DOI] [PubMed] [Google Scholar]
- 34.Koninckx PR, Ussia A, Adamyan L, Wattiez A, Donnez J: Deep endometriosis: definition, diagnosis, and treatment. Fertil Steril 2012; 98: 564–571. [DOI] [PubMed] [Google Scholar]
- 35.Vercellini P, Barbara G, Buggio L, Frattaruolo MP, Somigliana E, Fedele L: Effect of patient selection on estimate of reproductive success after surgery for rectovaginal endometriosis: Literature review. Reprod Biomed Online 2012; 24: 389–395. [DOI] [PubMed] [Google Scholar]