To the Editor:
Although antidepressants reduce anxiety and depressive symptoms in youth (Strawn et al. 2015; Locher et al. 2017), side effects—including weight gain—may complicate antidepressant treatment. Moreover, clinical and demographic factors potentially influence the emergence of side effects. In adults, escitalopram or citalopram (es/citalopram) is associated with increases in body weight (Uguz et al. 2015); although in adolescents with major depressive disorder, weight gain did not differ between escitalopram-treated adolescents and those receiving placebo during the 8-week double-blind placebo-controlled registration trial (Emslie et al. 2009). Currently, the time course of es/citalopram-related weight gain in youth and the factors that influence its emergence have not been systematically evaluated.
We retrospectively examined clinician-documented weight gain in youth <19 years of age (N = 248, mean age: 14.4 ± 2.2 years, range: 6.4–18.8 years) who initiated es/citalopram during an inpatient psychiatric hospitalization and received subsequent outpatient treatment within a tertiary care pediatric medical center from September 2013 to May 2017. After IRB approval, data were abstracted from electronic medical records (EMRs) during the total es/citalopram treatment period. Using an adaptive natural language processing algorithm that was refined by manual chart review to achieve a false-positive rate <10%, we examined the presence of weight gain-related terms in >32,000 outpatient notes. Patients were excluded by diagnoses of traumatic brain injury, substance use disorders, intellectual disability, congenital brain abnormality, and/or bipolar disorder. Patients must have been prescribed es/citalopram for ≥1 day and have ≥1 outpatient follow-up visit. Time to first documented weight gain was the length in days between the prescription start date and the earliest date when a weight gain-related term appeared in the EMR during es/citalopram treatment. The maximum dose of es/citalopram was extracted from the EMR, and citalopram doses were normalized to escitalopram doses by dividing by 2. Time to clinician-documented weight gain was analyzed with the log-rank test using Prism 7 for Windows (GraphPad, La Jolla, CA).
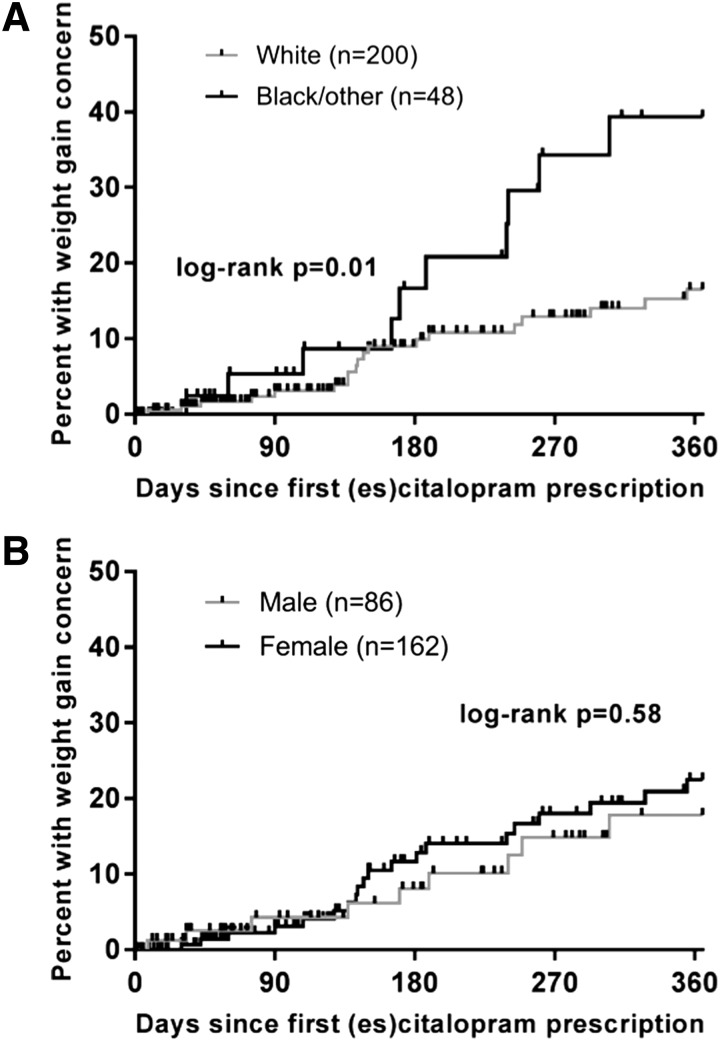
The majority of patients (62%, n = 153) had DSM-IV/5 anxiety and depressive disorders, 12% had only anxiety disorders (n = 30), and 26% (n = 65) had only depressive disorders. The mean maximum dose of es/citalopram was 16.0 ± 7.8 mg/day (range: 2.5–40 mg). The time course of documented weight gain was significantly associated with race (p = 0.01, log-rank test, Fig. 1A) but not by gender (p = 0.58, Fig. 1B). Individuals in the black/other racial category included African and African American patients in addition to mixed-race patients, Asians, and Latinos. No significant differences in concomitant medications were observed between white patients and patients classified as “black/other” who gained weight (total number of concomitant medications p = 0.841; second generation antipsychotics p = 0.50; antihistamines/anxiolytics: p = 0.906).
FIG. 1.
Time to first weight gain concern during treatment with escitalopram/citalopram is associated with race (p = 0.01, log-rank test for trend, A), but not gender (p = 0.58, B). (A) White patients are shown in gray and black or other races are shown in black. (B) Males are shown in gray and females are shown in black.
Although weight gain could represent improvement (e.g., normalization of appetite and food intake) or a side effect, our observation that clinician-documented weight gain in es/citalopram-treated youth with anxiety and depressive disorders differs between white and nonwhite patients suggests that clinicians should consider population-specific monitoring strategies. These findings highlight (1) the utility of using EMR-derived data to examine variability in side effects and (2) problems with “one size fits all” treatment monitoring strategies in youth and argue for precision medicine monitoring approaches. In the spirit of precision medicine, myriad patient-specific factors (e.g., age, gender, primary disorder, and pharmacogenomic profile) may inform treatment selection and monitoring.
Disclosures
S.L.A. is a paid contractor of Facial Dysmorphology Novel Analysis (FDNA). J.R.S. has received research support from the National Institute of Mental Health and the National Institute of Environmental and Health Science, Allergan, Edgemont Pharmaceuticals, Lundbeck, Neuronetics, and Shire. He has received material support from Genesight/Assurex and has provided consultation to Genesight/Assurex. He is an associate editor for Current Psychiatry and the Journal of Child & Adolescent Psychopharmacology and receives royalties from UptoDate and Springer and honoraria from CMEology. The other authors report no conflicts of interest.
References
- Emslie GJ, Ventura D, Korotzer A, Tourkodimitris S: Escitalopram in the treatment of adolescent depression: A randomized placebo-controlled multisite trial. J Am Acad Child Adolesc Psychiatry 48:721–729, 2009 [DOI] [PubMed] [Google Scholar]
- Locher C, Koechlin H, Zion SR, Werner C, Pine DS, Kirsch I, Kessler RC, Kossowsky J: Efficacy and safety of selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and placebo for common psychiatric disorders among children and adolescents: A systematic review and meta-analysis. JAMA Psychiatry 74:1011–1020, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Welge JA, Wehry AM, Keeshin B, Rynn MA: Efficacy and tolerability of antidepressants in pediatric anxiety disorders: A systematic review and meta-analysis. Depress Anxiety 32:149–157, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uguz F, Sahingoz M, Gungor B, Aksoy F, Askin R: Weight gain and associated factors in patients using newer antidepressant drugs. Gen Hosp Psychiatry 37:46–48, 2015 [DOI] [PubMed] [Google Scholar]