Abstract
Objectives: Psychotherapy is an effective, recommended treatment for pediatric anxiety disorders. Nevertheless, individuals with mental health conditions often do not receive psychotherapy, with variation across provider types. This study sought to examine psychotherapy claims surrounding medication initiation in U.S. children with diagnosed anxiety disorders.
Methods: The study cohort included privately insured children (3–17 years) with a diagnosed anxiety disorder initiating a medication to treat anxiety from 2004 to 2014. We examined psychotherapy claims in the 3 months before and 3 months after medication initiation and described children with multiple (2+) psychotherapy claims per 3-month period.
Results: Of the 75,024 children initiating a medication for anxiety (median age = 14 years, 58% female), 35% had multiple psychotherapy claims before medication initiation, with variation by age, anxiety disorder, and psychiatric comorbidity and with little change across time. Psychotherapy claims after medication initiation varied by whether the child had prior psychotherapy: 80% in children with prior psychotherapy and 30% in children without prior psychotherapy claims (44% of children diagnosed by a psychiatrist, 21% of children diagnosed by a pediatrician).
Conclusion: Many privately insured children do not have claims for psychotherapy before or after pharmacotherapy initiation for anxiety. Findings can inform future research and efforts to ultimately increase appropriate psychotherapy utilization in children with anxiety disorders.
Keywords: child, adolescent, anxiety disorder, psychotherapy, pharmacotherapy
Introduction
In the United States, an estimated 4 million children (4.6% between 0–19 years) have an anxiety disorder (Global Burden of Disease Pediatrics Collaboration 2016). Psychotherapy, particularly cognitive behavioral therapy (CBT), is an effective treatment for pediatric anxiety disorders (Connolly et al. 2007; James et al. 2015; Wang et al. 2017). The American Academy of Child and Adolescent Psychiatry (AACAP) recommends psychotherapy as part of the treatment for anxiety disorders in children and as the initial treatment for anxiety disorders of mild severity (Connolly et al. 2007; Cohen et al. 2010; Geller et al. 2012). Reasons for combining pharmacotherapy with psychotherapy, or starting pharmacotherapy, in the treatment of pediatric anxiety disorders include the need for acute symptom reduction, moderate-to-severe anxiety disorder symptoms, impairment that limits participation in psychotherapy, comorbid conditions, partial response to psychotherapy, and potential for improved outcomes (Connolly et al. 2007; Cohen et al. 2010; Geller et al. 2012).
Individuals with anxiety disorders often do not receive adequate treatment, including underutilization of psychotherapy and of follow-up care (Whiteside et al. 2016; Gunter and Whittal 2010; Soria-Saucedo et al. 2018); a third of adults diagnosed with anxiety received psychotherapy, lower than other mental health conditions (Harpaz-Rotem et al. 2012). Prior research described low utilization of psychosocial services surrounding antipsychotic initiation in children (Finnerty et al. 2016) and before psychotropic polypharmacy (Hincapie-Castillo et al. 2017), with variation in utilization by age, sex, race/ethnicity, urban community, and psychiatric diagnosis (Harris et al. 2012; Finnerty et al. 2016). However, to our knowledge, it is unknown how many children with anxiety disorders receive psychotherapy in the months before and after initiating a pharmacotherapy for anxiety and whether there is variation across time and across child characteristics.
Children often receive care for mental health conditions from primary care providers (Anderson et al. 2015). In children with depression, provider specialty was a key determinant in treatment selection, with mental health specialists more likely to choose combination therapy or psychotherapy alone than primary care providers (Soria-Saucedo et al. 2016). Describing psychotherapy claims in children diagnosed by mental health and nonmental health specialists can identify variations in treatment and targets for future research in pediatric anxiety disorders.
The purpose of this study is to describe psychotherapy claims surrounding medication initiation for anxiety in children and adolescents diagnosed with anxiety disorders and to assess whether psychotherapy claims varied over time and by child characteristics, including diagnosing provider type.
Methods
Datasource and study population
We used the MarketScan Commercial Claims and Encounters database (Truven Health Analytics, Inc. 2011), which includes individuals and their dependents covered by employer health plans across the United States. We utilized records of outpatient and inpatient diagnoses and procedures and outpatient dispensed prescriptions. The cohort included children (3–17 years) with an anxiety diagnosis who filled a new prescription for a medication used to treat anxiety from 2004 to 2014. Children were required to have at least one anxiety disorder diagnosis corresponding to anxiety disorders in the DSM-5 (American Psychiatric Association 2013), obsessive–compulsive disorder (OCD) diagnosis, or posttraumatic stress disorder (PTSD) diagnosis (ICD-9-CM = 293.84, 300.0x, 300.2x, 300.3x, 309.21, 309.81, 313.23) ≤30 days before medication initiation. To define new use, children were required to have no anxiety medication prescriptions in the year before medication initiation along with continuous insurance enrollment with mental health services coverage. Prescription medications for anxiety, referring broadly to medications prescribed for anxiety disorders, included selective serotonin reuptake inhibitors (SSRIs), the recommended first-line pharmacotherapy (Connolly et al. 2007), other antidepressants (ex. serotonin norepinephrine reuptake inhibitors, bupropion, tricyclic antidepressants), benzodiazepines (anxiolytic), beta-blockers, buspirone, hydroxyzine, clonidine, guanfacine, selected anticonvulsants, selected atypical antipsychotics, and other therapies (<0.1%). This cohort was previously used to describe the initial pharmacotherapy children receive for anxiety, 70% initiated with an SSRI (Bushnell et al. 2018). We applied an additional inclusion criteria of 3 months insurance coverage following medication initiation to evaluate psychotherapy claims.
Psychotherapy claims
We examined inpatient and outpatient psychotherapy claims (Current Procedural Terminology, CPT, codes = 90804–90819, 90821–90824, 90826–90829, 90847, 90849, 90853, and 90857, and additional 2013 codes: 90832–90834, 90836–90839) billed through insurance in the 3 months before and 3 months after a child began pharmacotherapy for anxiety. We were specifically interested in children with at least two recorded psychotherapy claims per 3-month period, as a minimal requirement of consistent psychotherapy use and helping ensure the child actually received psychotherapy in that window. Psychotherapy claims on the date of medication initiation (first prescription fill) were grouped with psychotherapy before medication start.
Statistical analyses
We estimated the proportion and 95% confidence interval of children with at least two recorded psychotherapy claims per 3-month period, overall and stratified by year, sex, age, anxiety disorder, psychiatric comorbidities, geographical division of the primary beneficiary, and initial anxiety pharmacotherapy. Among children without multiple psychotherapy claims before medication initiation, we estimated how many had psychotherapy claims after medication initiation; this was stratified by provider specialty recording the anxiety disorder diagnosis most before pharmacotherapy initiation. Results for provider specialty were stratified by year of medication initiation and internally standardized by age group, sex, and geographical region.
In a sensitivity analysis, we evaluated psychotherapy claims surrounding pharmacotherapy initiation in children without PTSD or OCD, conditions no longer classified under “anxiety disorders” in the DSM-5. To increase the likelihood medication was initiated for an anxiety disorder rather than a comorbidity, we conducted a sensitivity analysis examining the proportion of children with multiple psychotherapy claims before and after medication initiation in selected subsets: (1) children with an anxiety disorder diagnosis on the same date as the initial prescription, (2) children without a baseline psychiatric comorbidity diagnosis (ICD-9-CM: 290–319, excluding anxiety ICD-9-CM codes used to define the cohort), (3) children who initiated pharmacotherapy with an SSRI (the recommended first-line medication), and (4) children who initiated pharmacotherapy with an SSRI and had no baseline psychiatric comorbidity diagnosis. Analyses were completed with SAS version 9.4, Cary, NC. The University of North Carolina Institutional Review Board approved this study.
Results
Of the 75,024 children initiating a medication for anxiety, 58% were female and the median age was 14 years (Table 1). Half of children had an unspecified anxiety disorder diagnosis before medication initiation, 24% generalized anxiety disorder, 8% OCD, 5% panic disorder, and 4% PTSD. The most common provider types associated with the anxiety disorder diagnosis before medication initiation were psychiatrist (25%), pediatrician (16%), family practitioner (13%), and psychologist/therapist (12%).
Table 1.
Psychotherapy Claims Surrounding Initiation of Pharmacotherapy for Anxiety by Child Characteristic
| Characteristica | Total | Psychotherapy claims priorb | Psychotherapy claims afterc |
|---|---|---|---|
| N (%) | Row % (95% CI) | Row % (95% CI) | |
| Full cohort | 75,024 | 35 (34–35) | 47 (48–48) |
| Full cohort, excluding OCD and PTSD | 65,522 (87) | 33 (33–34) | 45 (45–46) |
| Female | 43,601 (58) | 35 (34–35) | 48 (48–48) |
| Male | 31,423 (42) | 35 (34–35) | 47 (46–47) |
| Age at pharmacotherapy initiation, median (IQR) | 14 (11–16) | ||
| 3–9 Years | 12,450 (17) | 41 (40–42) | 51 (50–51) |
| 10–13 Years | 19,605 (26) | 39 (38–39) | 52 (51–52) |
| 14–17 Years | 42,969 (57) | 31 (31–32) | 45 (44–45) |
| Anxiety disorder | |||
| Unspecified | 37,702 (50) | 28 (28–29) | 40 (39–40) |
| GAD | 18,212 (24) | 44 (43–44) | 56 (55–56) |
| OCD | 5647 (8) | 41 (40–42) | 58 (57–59) |
| Panic disorder | 3834 (5) | 24 (23–25) | 41 (39–42) |
| PTSD | 3211 (4) | 51 (50–53) | 66 (64–67) |
| Social phobia | 1722 (2) | 46 (44–49) | 61 (58–63) |
| Other, multiple | 4696 (6) | 38 (36–39) | 51 (50–52) |
| No psychiatric comorbidity diagnosis | 32,309 (43) | 20 (20–21) | 35 (34–35) |
| Any psychiatric comorbidity diagnosis | 42,715 (57) | 46 (45–46) | 57 (56–57) |
| Depression | 19,172 (26) | 48 (47–49) | 63 (63–64) |
| ADHD | 13,090 (17) | 42 (41–43) | 50 (49–51) |
| Adjustment disorder | 11,467 (15) | 63 (62–64) | 69 (68–69) |
| Conduct disorder | 4694 (6) | 51 (49–52) | 58 (57–60) |
| Other episodic mood disorder | 3520 (5) | 50 (49–52) | 65 (64–67) |
| Sleep problems | 3386 (5) | 35 (33–36) | 46 (44–47) |
| Substance use disorder | 2445 (3) | 37 (35–39) | 53 (51–55) |
| Initial anxiety pharmacotherapy | |||
| SSRI alone | 46,982 (63) | 37 (36–37) | 50 (49–50) |
| SSRI+another anxiety pharmacotherapy | 5159 (7) | 32 (30–33) | 52 (51–54) |
| Non-SSRI anxiety pharmacotherapyd | 22,883 (31) | 31 (31–32) | 41 (41–42) |
| Benzodiazepine | 6479 (9) | 19 (18–20) | 34 (32–35) |
| Hydroxyzine | 2873 (4) | 20 (19–22) | 28 (26–30) |
| Non-SSRI antidepressant | 5492 (7) | 40 (39–42) | 47 (46–49) |
| Other initial pharmacotherapye | 8039 (11) | 40 (39–41) | 48 (47–49) |
| Geographical divisionf | |||
| New England | 5570 (7) | 49 (48–50) | 62 (60–63) |
| Middle Atlantic | 7334 (10) | 43 (42–44) | 57 (56–58) |
| South Atlantic | 12,843 (17) | 28 (28–29) | 42 (42–43) |
| East North Central | 18,187 (24) | 37 (37–38) | 51 (50–52) |
| East South Central | 5066 (7) | 22 (21–24) | 34 (33–35) |
| West North Central | 4848 (6) | 29 (28–30) | 42 (40–43) |
| West South Central | 6329 (8) | 23 (22–24) | 35 (34–36) |
| Mountain | 4235 (6) | 28 (26–29) | 40 (38–41) |
| Pacific | 9914 (13) | 44 (43–45) | 53 (52–54) |
Measures were defined in the year up to and at pharmacotherapy initiation; therefore, some measures could have been affected by prior psychotherapy.
Psychotherapy claims (2+) in the 3 months before pharmacotherapy initiation.
Psychotherapy claims (2+) in the 3 months after pharmacotherapy initiation.
Does not include children initiating with an SSRI and another medication for anxiety.
Other initial pharmacotherapy included: 3% selected atypical antipsychotics, 1% buspirone, 1% beta-blocker, 1% selected anticonvulsants, 3% other medication (primarily guanfacine or clonidine), 1% two non-SSRI medication classes.
U.S. states included in each division (of primary beneficiary): New England (CT, ME, MA, NH, RI, VT); Middle Atlantic (NJ, NY, PA); South Atlantic (DE, MD, FL, GA, NC, SC, VA, WV); East North Central (IL, IN, MI, OH, WI); East South Central (AL, KY, MS, TN); West North Central (IA, KS, MN, MO, NE, SD, ND); West South Central (AR, LA, OK, TX); Mountain (AZ, CO, ID, MT, NV, NM, UT, WY); Pacific (AK, CA, HI, OR, WA); 1% unknown division.
ADHD, attention-deficit/hyperactivity disorder; CI, confidence interval; IQR, interquartile range; GAD, generalized anxiety disorder; OCD, obsessive–compulsive disorder; PTSD, posttraumatic stress disorder; SSRI, selective serotonin reuptake inhibitor.
Half of children (50%, n = 37,480) had at least one psychotherapy claim in the year before medication initiation. A third (35%, n = 26,085) of children had multiple (2+) psychotherapy claims in the 3 months before initiating a medication for anxiety, with a median of 4 psychotherapy claims (interquartile range, IQR = 3–7) during those 3 months (Table 1). The proportion of children with psychotherapy claims before medication initiation remained relatively stable across the study period (2004–2014, range: 31%–37%). After medication initiation, 47% of children had psychotherapy claims. The proportion of children with psychotherapy claims before or after medication initiation was similar by sex and was lowest in the oldest children, children diagnosed with panic disorder or unspecified anxiety, children without psychiatric comorbidities, and children initiating on a benzodiazepine or hydroxyzine, with variation by geographical division (Table 1). Overall 54% of children had multiple psychotherapy claims before or after medication initiation.
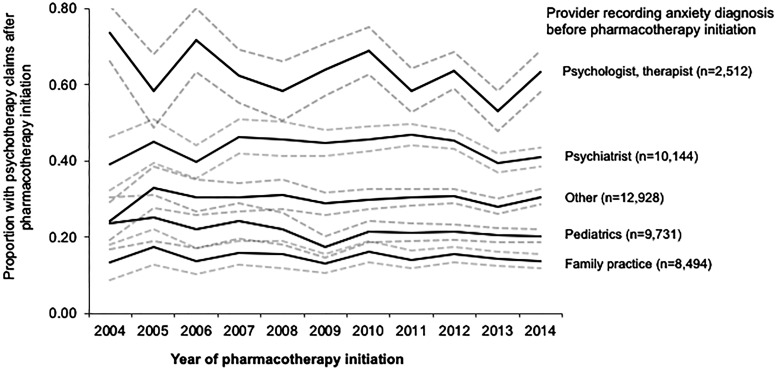
Psychotherapy use after medication initiation varied by whether the child had prior psychotherapy. The majority of children with psychotherapy claims before medication initiation also had multiple psychotherapy claims in the 3 months after medication initiation (80%, n = 20,881/26,085) (Table 2). Among children without psychotherapy claims in the 3 months before medication initiation, 30% (n = 14,688/48,939) had psychotherapy claims after medication initiation (median number of psychotherapy claims = 4, IQR = 3–6). When excluding children with PTSD or OCD (n = 65,522), results were consistent (Table 2 footnote). In children without psychotherapy claims before medication initiation and diagnosed with anxiety by a psychiatrist, 44% had psychotherapy claims after medication initiation and 21% of children diagnosed by a pediatrician had psychotherapy claims after medication initiation (Fig. 1); within each provider, there was no increase in the proportion of children with psychotherapy claims after medication initiation from 2004 to 2014.
Table 2.
Proportion of Children with Psychotherapy Claims Surrounding Pharmacotherapy Initiation in Restricted Subsets to Increase the Likelihood Pharmacotherapy was Initiated for Anxiety
| Primary analysis: cohort of children initiating anxiety medicationa | Sensitivity analysis: selected subsets | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Children with anxiety disorder diagnosis on date of medication start | Children with no comorbid psychiatric diagnosisb | Children initiating pharmacotherapy with an SSRI | Children initiating pharmacotherapy with an SSRI and with no comorbid psychiatric diagnosisb | |||||||
| Total No. | 75,024 | 42,318 | 32,309 | 52,141 | 22,217 | |||||
| Psychotherapy claims before medication (% of total)c | 26,085 | 35% (34–35)d | 11,915 | 28% (28–29) | 6562 | 20% (20–21) | 18,879 | 36% (36–37) | 4897 | 22% (22–23) |
| Psychotherapy claims after medication (% of prior users) | 20,881 | 80% (80–81) | 9634 | 81% (80–82) | 5165 | 79% (78–80) | 15,365 | 81% (81–82) | 3926 | 80% (79–81) |
| No psychotherapy claims before medication (% of total)c | 48,939 | 65% (65–66) | 30,403 | 72% (71–72) | 25,747 | 80% (79–80) | 33,262 | 64% (63–64) | 17,320 | 78% (77–78) |
| Psychotherapy claims after medication initiation (% of prior non-users) | 14,688 | 30% (30–30)e | 8484 | 28% (27–28) | 6082 | 24% (23–24) | 10,766 | 32% (32–33) | 4462 | 26% (25–26) |
Of the 65,522 children with non-PSTD and non-OCD anxiety, 33% (n = 21,819) had prior psychotherapy claims (80%, n = 17,379 of those children had psychotherapy claims after medication start), 67% (n = 43,703) did not have prior psychotherapy claims (28%, n = 12,417 of those children had psychotherapy claims after medication start).
No comorbid psychiatric diagnosis in year before initiating a medication for anxiety.
Psychotherapy claims defined as 2+ psychotherapy claims during 3-month period.
Additional 10% had one psychotherapy claim in the 3 months before medication initiation.
Additional 9% had one psychotherapy claim in the 3 months after medication initiation.
OCD, obsessive–compulsive disorder; SSRI, selective serotonin reuptake inhibitor.
FIG. 1.
Proportion of children with psychotherapy claims after medication initiation who did not have psychotherapy claims before medication initiation by provider specialty. Dotted lines represent 95% confidence intervals. Figure is restricted to children without multiple psychotherapy claims before medication initiation (<2 claims in 3 months); psychotherapy claims after medication initiation defined as 2+ claims in the following 3 months; the majority of children with an anxiety diagnosis from a psychologist/therapist had psychotherapy claims before medication initiation and therefore were excluded from this figure. Standardized internally (reference year = 2012) by sex, age group (3–9, 10–13, 14–17 years), and region to account for shifts in the population covered across the study period; children with an unknown region or provider specialty and with prior psychotherapy claims were excluded (included n = 43,809). Primary values under “other” provider specialty include acute care hospital, medical doctor (not elsewhere classified), multispecialty physician group, internal medicine (not elsewhere classified), nurse practitioner, and mental health facilities; the remaining include 80+ different values.
In the sensitivity analysis, the proportion of children with multiple psychotherapy claims surrounding pharmacotherapy initiation remained fairly consistent in the subsets of children with an increased likelihood that anxiety was the indication (Table 2). Overall, the proportion with psychotherapy claims was lower in children with anxiety and no baseline psychiatric comorbidity diagnosis.
Discussion
Our findings, based on recorded psychotherapy claims, suggest that psychotherapy utilization needs to be improved surrounding initiation of anxiety medication in privately insured children and adolescents with anxiety disorders. Only a third of children had psychotherapy claims in the months before starting pharmacotherapy and around half in the months after, with no notable changes from 2004 to 2014. This low rate of psychosocial service utilization has been observed in Medicaid enrolled children during 3 months before antipsychotic initiation (49%) (Finnerty et al. 2016) and in the 6 months before psychotropic polypharmacy (40% in 2005–2007) (Hincapie-Castillo et al. 2017). In children who initiated pharmacotherapy after a new PTSD diagnosis, less than half also received psychotherapy (Soria-Saucedo et al. 2018). Taken together, psychotherapy and behavioral interventions are not consistently used before, or after, psychotropic initiation in children; perhaps indicative of an overreliance on medications (Hincapie-Castillo et al. 2017). In fact, from 1998 to 2007 a higher proportion of individuals with anxiety disorders were treated with medication alone and a lower proportion with psychotherapy alone (Olfson and Marcus 2010).
The low use of psychotherapy surrounding pharmacotherapy initiation is despite pediatric anxiety guidelines recommending, in many situations, psychotherapy before or with pharmacotherapy (Connolly et al. 2007) and benefits of treating pediatric anxiety disorders with an SSRI and CBT compared with an SSRI alone (Walkup et al. 2008). While we focused on children with at least two psychotherapy claims per 3-month period, more sessions are recommended with trials and systematic reviews in pediatric anxiety requiring at least 9–14 sessions of CBT over a similar period of time (Compton et al. 2010; James et al. 2015). We cannot know whether, or how often, pharmacotherapy could have been precluded or delayed if psychotherapy was received first, but this could potentially reduce exposure to pharmacological side effects.
The differences in psychotherapy utilization across initial pharmacotherapies may represent varying treatment approaches. Benzodiazepines have been used to reduce anxiety symptoms before psychotherapy is initiated (Connolly et al. 2007), possibly explaining a lower proportion with psychotherapy before benzodiazepine initiation (19% of initiators) than SSRI initiation (37% of initiators). Furthermore, benzodiazepines and hydroxyzine are often prescribed for short-term or as-needed treatment, with only 5% of benzodiazepine and 3% of hydroxyzine initiators continuing treatment for 6 months compared with 55% of SSRI initiators (Bushnell et al. 2018). Prior psychotherapy use differed by psychiatric diagnoses in children initiating antipsychotics (Finnerty et al. 2016), similarly, we observed variation in psychotherapy use by psychiatric comorbidity and by specific anxiety disorder, ranging from 24% of children with panic disorder to 51% of children with PTSD having psychotherapy claims before pharmacotherapy. Variations in psychotherapy use by initial pharmacotherapy and psychiatric diagnoses may be appropriate and represent varying treatment approaches based on the clinical situation, including disorder severity, evidence of effectiveness of psychosocial and pharmacological interventions for the specific disorder and comorbidities, and expected need for long-term treatment. Further research is needed to understand the complexity of how patient factors interact to influence psychotherapy utilization in children (Finnerty et al. 2016).
Primary care providers face a variety of barriers when treating pediatric mental health conditions (O'Brien et al. 2016). The receipt of psychotherapy after medication initiation was higher in children diagnosed by a mental health provider than primary care providers. Relatedly, a higher proportion of children with anxiety or mood disorders seeing primary care providers were prescribed psychotropic medication than children seeing psychiatrists (Anderson et al. 2015). However, children seeing mental health providers have access and perhaps a greater proclivity for psychosocial interventions. In parts of the United States, lack of qualified practitioners to deliver quality, evidence-based psychotherapy, often makes medication the default treatment (Gunter and Whittal 2010; Finnerty et al. 2016). Access could be one factor explaining our observed differences in psychotherapy claims by geographical division. The number of U.S. psychiatrists per 100,000 residents has declined from 2003 to 2013, likely affecting access to mental health care (Bishop et al. 2016). However, in children seen by primary care providers, availability of community psychiatrists only partially explained the variation in mental health diagnoses and psychotropic prescribing (Mayne et al. 2016).
Efforts to ensure providers and families are aware of all treatment options and have access to adequate psychosocial treatment are important (Harris et al. 2012). The advent of online and electronic sources of therapy (Ebert et al. 2015; Jones et al. 2015; O'Dea et al. 2015) may increase psychotherapy access for children unable to access traditional office-based face-to-face therapy. Moreover, therapy delivered in primary care settings, such as brief behavioral therapy for pediatric anxiety disorders and depression, may provide another avenue to increase access (Walkup et al. 2017; Weersing et al. 2017). Families may also have access to psychotherapy through school, employee, or community programs, sources that may go unrecorded in claims data. Research on how alternative sources of therapy can be effectively utilized and integrated into care may be vital to increase psychotherapy use in pediatric anxiety disorders.
Our study has some important limitations. First, we are only able to evaluate psychotherapy billed through insurance; therefore, we likely underestimate psychotherapy use. We may be more likely to underestimate psychotherapy in children diagnosed with anxiety outside mental health providers. In 1999, a third of outpatient psychotherapy visits for anxiety disorders were reported to be self-paid (Olfson et al. 2004). In 2005–2006, 72% of psychiatrists accepted private noncapitated insurance, decreasing to 55% in 2009–2010 (Bishop et al. 2014). However, it is unclear how often psychotherapy is not billed through insurance in privately insured children with anxiety disorders; furthermore, psychotherapy may be available through community, school, or employee programs outside of what we observe. Additionally, we do not have specific information on type of psychotherapy provided, which is particularly relevant given variation in effectiveness of psychotherapy types across disorders. We describe psychotherapy utilization by provider type and did not account for differences in condition severity, which likely influence provider type seen and psychotherapy use. As medications used to treat anxiety disorders have multiple indications, we cannot be certain the medication was initiated for anxiety, but sensitivity analyses increase assurance that results would be consistent. Finally, findings are not generalizable to all U.S. children; we evaluated privately insured children who received an anxiety disorder diagnosis and initiated a medication used to treat anxiety, only a subset of all children with anxiety disorders.
Conclusion
In conclusion, a third of privately insured children had psychotherapy claims in the months before initiating a medication for anxiety and less than half in the 3 months after initiation, providing important insight into existing treatment practices. Future research is needed to understand the amount and type of psychotherapy children receive outside insurance. However, these findings reinforce the necessity of efforts to increase psychotherapy utilization in children with anxiety disorders, particularly in children seen outside mental health specialists.
Clinical Significance
Despite recommendations, psychotherapy in children with anxiety was underutilized surrounding pharmacotherapy initiation. Psychotherapy use could preclude exposure to pharmacotherapy and initiating pharmacotherapy with psychotherapy could result in greater symptom improvement. Findings can inform future research and efforts to increase psychotherapy utilization in children most likely to benefit from psychotherapy, including children treated for anxiety disorders by primary care providers.
Disclaimer
The funding source had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. This content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Source of the data: Copyright ©2016 Truven Health Analytics, Inc. All Rights Reserved.
Disclosures
Authors Dr. Bradley Gaynes and Dr. Stacie Dusetzina report no financial interests or potential conflicts of interest. Dr. Bushnell received support from the National Institute of Mental Health as a Ruth L. Kirschstein National Research Service Award (NRSA) Individual Predoctoral Fellow (F31MH107085) and currently under award number T32MH013043. Dr. Bushnell previously held a graduate research assistantship with GlaxoSmithKline and was the Merck fellow for the Center for Pharmacoepidemiology (both ended December 2015). Dr. Brookhart receives investigator-initiated research funding from the National Institutes of Health (NIH) and through contracts with the Agency for Healthcare Research and Quality's DEcIDE program and the Patient-Centered Outcomes Research Institute. Within the past 3 years, he has received research support from Amgen and AstraZeneca and has served as a scientific advisor for Amgen, Merck, and GlaxoSmithKline (honoraria/payment received by the institution). He has received consulting fees from RxAnte, Inc. and World Health Information Consultants. Dr. Stürmer receives investigator-initiated research funding and support as Principal Investigator (R01 AG056479) from the National Institute on Aging (NIA), and as Coinvestigator (R01 CA174453; R01 HL118255, R21-HD080214), NIH. He also receives salary support as Director of Comparative Effectiveness Research (CER), NC TraCS Institute, UNC Clinical and Translational Science Award (UL1TR002489), the Center for Pharmacoepidemiology (current members: GlaxoSmithKline, UCB BioSciences, Merck, Shire), and from pharmaceutical companies (Amgen, AstraZeneca, Novo Nordisk) to the Department of Epidemiology, University of North Carolina at Chapel Hill. Dr. Stürmer does not accept personal compensation of any kind from any pharmaceutical company. He owns stock in Novartis, Roche, BASF, AstraZeneca, and Novo Nordisk. Dr. Compton receives research support from the National Institute of Mental Health, NC GlaxoSmithKline Foundation, Mursion, Inc. and has been a consultant for Shire, received honoraria from the Journal of Consulting and Clinical Psychology, Nordic Long-Term OCD Treatment Study Research Group, and The Center for Child and Adolescent Mental Health, Eastern and Southern Norway, and given expert testimony for Duke University.
References
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013 [Google Scholar]
- Anderson LE, Chen ML, Perrin JM, Van Cleave J: Outpatient visits and medication prescribing for US children with mental health conditions. Pediatrics 136:e1178–e1185, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop TF, Press MJ, Keyhani S, Pincus HA: Acceptance of insurance by psychiatrists and the implications for access to mental health care. JAMA Psychiatry 71:176–181, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop TF, Seirup JK, Pincus HA, Ross JS: Population of US practicing psychiatrists declined, 2003–13, which may help explain poor access to mental health care. Health Aff (Millwood) 35:1271–1277, 2016 [DOI] [PubMed] [Google Scholar]
- Bushnell GA, Compton SN, Dusetzina SB, Gaynes BN, Brookhart MA, Walkup JT, Rynn MA, Sturmer T: Treating pediatric anxiety: Initial use of SSRIs and other antianxiety prescription medications. J Clin Psychiatry 79:e1–e9, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JA, Bukstein O, Walter H, Benson SR, Chrisman A, Farchione TR, Hamilton J, Keable H, Kinlan J, Schoettle U, Siegel M, Stock S, Medicus J; AACAP Work Group on Quality Issues: Practice parameter for the assessment and treatment of children and adolescents with posttraumatic stress disorder. J Am Acad Child Adolesc Psychiatry 49:414–430, 2010 [PubMed] [Google Scholar]
- Compton SN, Walkup JT, Albano AM, Piacentini JC, Birmaher B, Sherrill JT, Ginsburg GS, Rynn MA, McCracken JT, Waslick BD, Iyengar S, Kendall PC, March JS: Child/Adolescent Anxiety Multimodal Study (CAMS): Rationale, design, and methods. Child Adolesc Psychiatry Ment Health 4:1–15, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly SD, Bernstein GA; Work Group on Quality Issues: Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders J Am Acad Child Adolesc Psychiatry 46:267–283, 2007 [DOI] [PubMed] [Google Scholar]
- Ebert DD, Zarski AC, Christensen H, Stikkelbroek Y, Cuijpers P, Berking M, Riper H: Internet and computer-based cognitive behavioral therapy for anxiety and depression in youth: A meta-analysis of randomized controlled outcome trials. PLoS One 10:e0119895, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerty M, Neese-Todd S, Pritam R, Leckman-Westin E, Bilder S, Byron SC, Hudson Scholle S, Crystal S, Olfson M: Access to psychosocial services prior to starting antipsychotic treatment among Medicaid-insured youth. J Am Acad Child Adolesc Psychiatry 55:69–76, 2016 [DOI] [PubMed] [Google Scholar]
- Geller DA, March J; AACAP Committee on Quality Issues: Practice parameter for the assessment and treatment of children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry 51:98–113, 2012 [DOI] [PubMed] [Google Scholar]
- Global Burden of Disease Pediatrics Collaboration: Global and national burden of diseases and injuries among children and adolescents between 1990 and 2013: Findings from the Global Burden of Disease 2013 Study. JAMA Pediatr 170:267–287, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter RW, Whittal ML: Dissemination of cognitive-behavioral treatments for anxiety disorders: Overcoming barriers and improving patient access. Clin Psychol Rev 30:194–202, 2010 [DOI] [PubMed] [Google Scholar]
- Harpaz-Rotem I, Libby D, Rosenheck RA: Psychotherapy use in a privately insured population of patients diagnosed with a mental disorder. Soc Psychiatry Psychiatr Epidemiol 47:1837–1844, 2012 [DOI] [PubMed] [Google Scholar]
- Harris E, Sorbero M, Kogan JN, Schuster J, Stein BD: Concurrent mental health therapy among Medicaid-enrolled youths starting antipsychotic medications. Psychiatr Serv 63:351–356, 2012 [DOI] [PubMed] [Google Scholar]
- Hincapie-Castillo JM, Liu X, Bussing R, Winterstein AG: Prevalence of psychotherapy surrounding initiation of psychotropic polypharmacy in the Medicaid-insured population, 1999–2010. Psychiatr Serv 68:1120–1126, 2017 [DOI] [PubMed] [Google Scholar]
- James AC, James G, Cowdrey FA, Soler A, Choke A: Cognitive behavioural therapy for anxiety disorders in children and adolescents. Cochrane Database Syst Rev 2:CD004690, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E, Manassis K, Arnold P, Ickowicz A, Mendlowitz S, Nowrouzi B, Wilansky-Traynor P, Bennett K, Schmidt F: Translating cognitive behavioral therapy for anxious youth to rural-community settings via tele-psychiatry. Community Ment Health J 51:852–856, 2015 [DOI] [PubMed] [Google Scholar]
- Mayne SL, Ross ME, Song L, McCarn B, Steffes J, Liu W, Margolis B, Azuine R, Gotlieb E, Grundmeier RW, Leslie LK, Localio R, Wasserman R, Fiks AG: Variations in mental health diagnosis and prescribing across pediatric primary care practices. Pediatrics 137:1–10, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien D, Harvey K, Howse J, Reardon T, Creswell C: Barriers to managing child and adolescent mental health problems: A systematic review of primary care practitioners' perceptions. Br J Gen Pract 66:e693–e707, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dea B, Calear AL, Perry Y: Is e-health the answer to gaps in adolescent mental health service provision? Curr Opin Psychiatry 28:336–342, 2015 [DOI] [PubMed] [Google Scholar]
- Olfson M, Marcus SC: National trends in outpatient psychotherapy. Am J Psychiatry 167:1456–1463, 2010 [DOI] [PubMed] [Google Scholar]
- Olfson M, Marcus SC, Wan GJ, Geissler EC: National trends in the outpatient treatment of anxiety disorders. J Clin Psychiatry 65:1166–1173, 2004 [DOI] [PubMed] [Google Scholar]
- Soria-Saucedo R, Chung JH, Walter H, Soley-Bori M, Kazis LE: Factors that predict the use of psychotropics among children and adolescents with PTSD: Evidence from private insurance claims. Psychiatr Serv 69:1007–1014, 2018 [DOI] [PubMed] [Google Scholar]
- Soria-Saucedo R, Walter HJ, Cabral H, England MJ, Kazis LE: Receipt of evidence-based pharmacotherapy and psychotherapy among children and adolescents with new diagnoses of depression. Psychiatr Serv 67:316–323, 2016 [DOI] [PubMed] [Google Scholar]
- Truven Health Analytics, Inc.: 2011 Truven Health Marketscan® commercial claims and encounters Medicare supplemental and coordination of benefits data dictionary. Alexandria, VA: American Academy of Otolaryngology, 2011 [Google Scholar]
- Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, Ginsburg GS, Rynn MA, McCracken J, Waslick B, Iyengar S, March JS, Kendall PC: Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med 359:2753–2766, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkup JT, Mathews T, Green CM: Transdiagnostic behavioral therapies in pediatric primary care: Looking ahead. JAMA Psychiatry 74:557–558, 2017 [DOI] [PubMed] [Google Scholar]
- Wang Z, Whiteside SPH, Sim L, Farah W, Morrow AS, Alsawas M, Barrionuevo P, Tello M, Asi N, Beuschel B, Daraz L, Almasri J, Zaiem F, Larrea-Mantilla L, Ponce OJ, LeBlanc A, Prokop LJ, Murad MH: Comparative effectiveness and safety of cognitive behavioral therapy and pharmacotherapy for childhood anxiety disorders: A systematic review and meta-analysis. JAMA Pediatr 171:1049–1056, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weersing VR, Brent DA, Rozenman MS, Gonzalez A, Jeffreys M, Dickerson JF, Lynch FL, Porta G, Iyengar S: Brief behavioral therapy for pediatric anxiety and depression in primary care: A randomized clinical trial. JAMA Psychiatry 74:571–578, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside SP, Ale CM, Young B, Olsen MW, Biggs BK, Gregg MS, Geske JR, Homan K: The length of child anxiety treatment in a regional health system. Child Psychiatry Hum Dev 47:985–992, 2016 [DOI] [PubMed] [Google Scholar]