Abstract
The present study aimed to study the sequence of developing the oviduct of the Alexandria chicken during the embryonic and posthatching period by using the light and scanning electron microscopic (SEM) examination. The Mullerian duct began to appear as left and right urogenital ridges composed of stratified cuboidal epithelium at the ventrolateral aspect of the mesonephros at the 5-day-old embryo. At the 6-day-old embryo, the left urogenital ridge canalized and the tubal wall surrounded a circular lumen composed of three cellular components; inner simple columnar epithelium, multilayers of mesenchymal cells, and outer stratified cuboidal epithelium. At the 8-day-old embryo, the inner tubal layer became composed of simple-to-pseudostratified ciliated columnar epithelium, the density of the mesenchymal cells increased, and the outer layer became simple squamous epithelium at the medial aspect of the duct and stratified epithelium at the lateral aspect of the duct. The left oviduct of the 1-day-old chick resembled the oviduct of 8-day-old embryo except the SEM observations of the tunica mucosa of the 1-day-old chick which showed extensive mucosal folds with many straight cilia. At the 1-week-old chick, the left oviduct showed a folded lumen surrounded by simple columnar ciliated epithelial layer followed by a layer of mesenchymal cells, many layers of smooth muscles surrounded the mesenchymal cells layer and outer simple squamous epithelium layer. At the 1-month-old chick, the left oviduct wall was composed of five layers surrounded by a star-shaped lumen.
Keywords: Chicken, light microscopy, oviduct, scanning electron microscopy
INTRODUCTION
Alexandria strain of chicken is a tetra hybrid cross mating of the local Egyptian breed between Fayoumi and Barred Plymouth Rock, then with Rhode Island Red and with White Leg Horn.[1,2] The fertility of Alexandria chicken reaches 88.17% and the hatchability is 76.82%. It reaches the sexual maturity at 172 days posthatching and it is characterized by high egg yield which reaches about 47.4 and highest egg mass about 1987 g.[2]
The female genital tract has an obvious importance to the continuation of the species, the developmental differences between species reflect on the reproduction of the adult.
The avian oviduct is a complex biological organ that undergoes a series of cellular changes during the prenatal and postnatal development. The avian oviduct is of special interest to the commercial egg industry. Any change in the development and function of the oviduct of a laying hen affects directly on the egg production and egg quality. The avian oviduct appears as a groove on the dorsolateral surface of the nephrogenic ridge and rolls up into a tube that sinks beneath the surface of the genital ridge on the day 4 of development.[3] In 1-day-old chick, the oviduct is present as a tiny tube just visible to naked eye and it gradually increases in size until puberty.[4]
The present study aimed to tracking the development of the oviduct of the Alexandria native breed during the embryonic and posthatching period.
MATERIALS AND METHODS
Egg incubation
One hundred fertilized eggs of Alexandria chicken were obtained from Abees farm belonging to the Faculty of Agriculture, Alexandria University, Egypt. These eggs incubated horizontally at 37.5°C ± 2°C with 52% relative humidity until embryos reached the proper stages using the chick embryos staging guide.[5]
Collection of embryos
The whole embryo was examined at HH26 (day 5), HH29 (day 6), and HH34 (day 8). Immediately before the procedures were carried out, eggs were sprayed with 70% ethyl alcohol. The cracked eggs and their content were transferred to sterile, clean, and dry Petri dish. The embryo and the overlying vitelline membrane were very gently peeled away from the yolk and the older embryos were obtained after cutting the main umbilical blood vessels. The embryos were transferred to a Petri dish containing sterile phosphate-buffered saline (PBS) and then the embryos were washed, removed by small spatula, and transferred to 2 ml sterile Eppendorf tube. A mid-ventral incision was made, the abdominal organs were removed, and then the female gonads were dissected, separated from the embryos, washed with PBS, and transferred into 2 ml Eppendorf tube.
Samples from posthatching chicks
After hatching, the female left oviduct from 1-day-old chicks, 1-week-old chicks, and 1-month-old chicks were separated and obtained by mid-ventral incision of the chick abdomen, evisceration of the abdominal cavity from the abdominal organs then the oviduct dissected and separated from the body and transferred into a Petri dish containing sterile PBS.
Light microscopic examination
The samples were fixed in 10% buffered neutral formalin for 48 h, and then dehydrated in ascending grades of ethyl alcohol.[6] They were cleared in xylene and embedded in three changes of paraffin. The paraffin blocks were cut at 4 μm thickness and stained by Harris hematoxylin and eosin for general studies, Periodic acid–Schiff technique (PAS).
Scanning electron microscopic examination
The specimens were immersed in a fixative (2% formaldehyde, 1.25% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.2) at 4°C. Once fixed, the samples were washed in 0.1 M sodium cacodylate containing 5% sucrose, processed through tannic acid, and finally dehydrated in graded ethanol series for 15 min in each (50%, 70%, 80%, 90%, 95%, and 100%). The samples were critical point dried in carbon dioxide, attached to stubs with colloidal carbon, and coated with gold palladium in a sputtering device. Specimens were examined and photographed with JEOL (Gute Änger, Freising Germany) 5300 JSM SEM operating at 25 kV at the Faculty of Science, Alexandria University.
RESULTS
At HH26 (5-day-old embryo)
The female Mullerian duct appeared as a groove at the thickened peritoneal epithelium known as a tubal ridge that attached to the ventrolateral border of the mesonephros alongside to the lateral body wall and grew at an anteroposterior direction [Figure 1]. The tubal ridge composed of stratified cuboidal epithelium [Figure 2].
Figure 1.
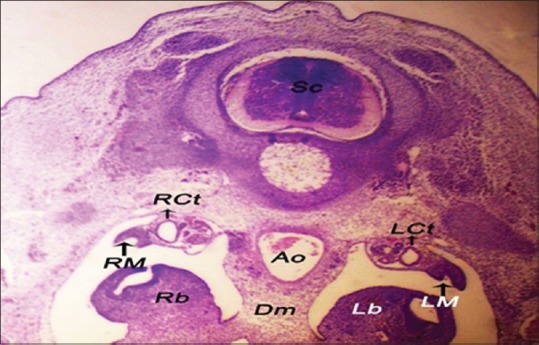
The left tubal ridge and the right tubal ridge ventrolateral to the mesonephroi of 5-day-old embryo. Sc: Spinal cord, LCt: Left collecting tubule, RCt: Right collecting tubule, AO: Aorta, Dm: Dorsal mesentery, Lb: Left lung bud, Rb: Right lung bud (H and E, ×10)
Figure 2.
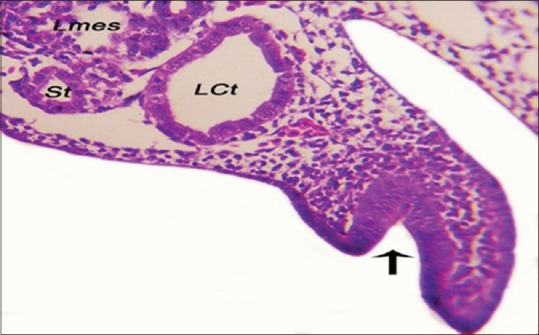
The epithelial lining of the left tubal ridge of 5-day-old embryo (black arrow). LCt: Left collecting tubule, Lmes: Left mesonephros, St: Secreting tubule (H and E, ×40)
At HH29 (6-day-old embryo)
The development of the Mullerian duct completed when the canalization of the tubal ridge appeared and became a female Mullerian duct that run caudolateral to the Wolffian duct [Figure 3]. The Mullerian duct was composed of three cellular components: epithelial cells forming the inner tube that called the Mullerian duct epithelium (MDE). This epithelial layer composed of simple vacuolated columnar epithelium. This layer showed a weak positive PAS reaction because of the decrease thickness of the basal lamina of the Mullerian duct. The mesenchymal cells were multilayers surrounding the MDE called Mullerian duct mesenchyme (MDM). The coelomic epithelium defined the external borders of the Mullerian duct by a layer called Mullerian coelomic epithelium (MCE) that were composed of stratified vacuolated cuboidal epithelium. The three layers of the Mullerian duct enclosed a circular lumen [Figure 4].
Figure 3.
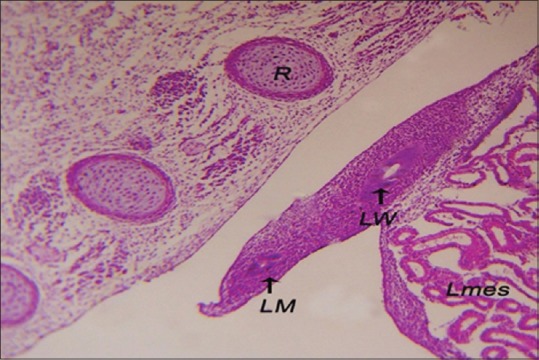
The left Mullerian duct caudolateral to the left Wolffian duct of 6-day-old embryo. Lmes: Left mesonephros, R: Rib (H and E, ×40)
Figure 4.
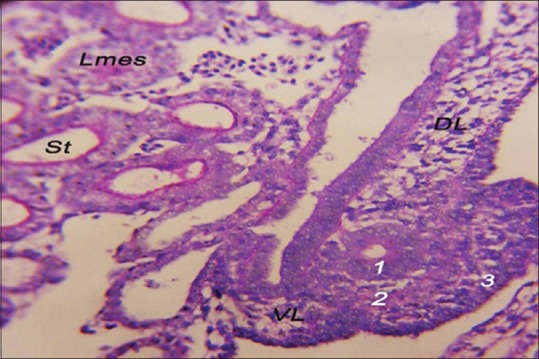
The structure of the left Mullerian duct of 6-day-old embryo. Mullerian duct epithelium (1), Mullerian duct mesenchyme (2), Mullerian coelomic epithelium (3), DL: Dorsal ligament of oviduct, VL: Ventral ligament of oviduct, Lmes: Left mesonephros, and St: Secreting tubules (periodic acid–Schiff, ×40)
At HH34 (8-day-old embryo)
The MDE was composed of simple-to-pseudostratified ciliated columnar epithelium, and the density of the mesenchymal cells at the layer of MDM increased. The MCE showed a layer of simple squamous epithelium at the medial aspect of the Mullerian duct; however, the lateral aspect showed a layer of stratified epithelium [Figure 5]. The three layers of the Mullerian duct were still enclosing a circular lumen.
Figure 5.
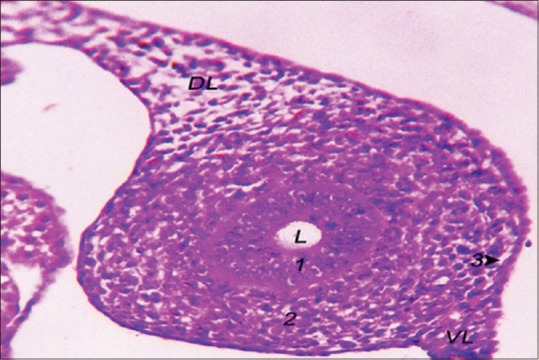
The structure of the left Mullerian duct of 8-day-old embryo. Mullerian duct epithelium (1), Mullerian duct mesenchyme (2), Mullerian coelomic epithelium (3), DL: Dorsal ligament of oviduct, VL: Ventral ligament of oviduct, L: Lumen (H and E, ×40)
One-day-old chick
The left oviduct of the 1-day-old chick resembles the oviduct of 8-day-old chicken embryo as it was composed of epithelial layer of simple-to-pseudostratified ciliated columnar epithelium that surrounds a circular lumen. The mesenchymal layer showed a large density of mesenchymal cells. The tunica serosa of the left oviduct was composed of a single thin layer of squamous epithelium [Figure 6].
Figure 6.
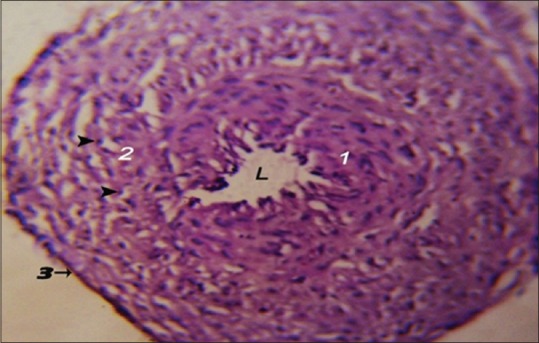
Photomicrograph showing the histological structure of the 1-day-old chick left oviduct. Inner epithelial layer (1), mesenchymal cell layer (2) with peripheral nucleus (arrowhead), tunica serosa (3), L: Lumen (H and E, ×10)
The SEM observations of the tunica mucosa of the left oviduct of the 1-day-old chick showed extensive mucosal folds arranged as a rugae with many straight cilia which were variable in length (about 0.5–1.5 mm in length) [Figure 7].
Figure 7.
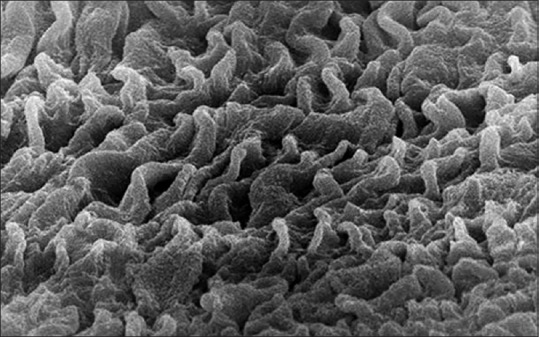
Scanning electron micrograph showing the tunica mucosa of the 1-day-old chick left oviduct with extensive mucosal folds and many straight cilia (×10,000)
One-week old chick
The left oviduct of the 1-week old chick showed a folded lumen surrounded by simple columnar ciliated epithelial layer followed by a layer of mesenchymal cells with a peripheral compressed flattened nucleus and either clear or vesicular cytoplasm. These features of the mesenchymal cells showed a lipid storage of these cells. Many layers of smooth muscles occurred around the mesenchymal cell layer. The outermost layer was a layer of tunica serosa that was composed of simple squamous epithelium [Figure 8].
Figure 8.
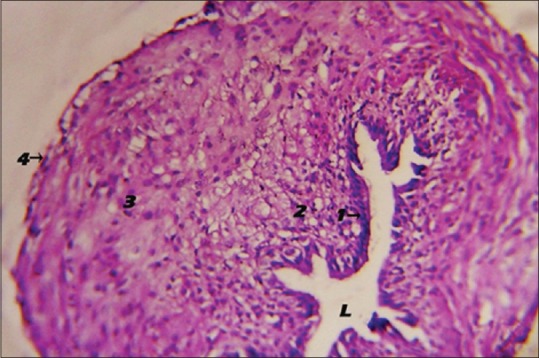
Photomicrograph showing the histological structure of the 1-week-old chick left oviduct. Inner epithelial layer (1), mesenchymal cell layer (2), circular smooth muscle layer (3), tunica serosa (4), folded lumen (H and E, ×40). L: Lumen
The SEM observations of the tunica mucosa of the left oviduct of the 1-week-old chick were similar to the tunica mucosa of the left oviduct of the 1-day-old chick as it contained extensive mucosal folds with numerous cilia of variable length but the cilia were more corrugated [Figure 9].
Figure 9.
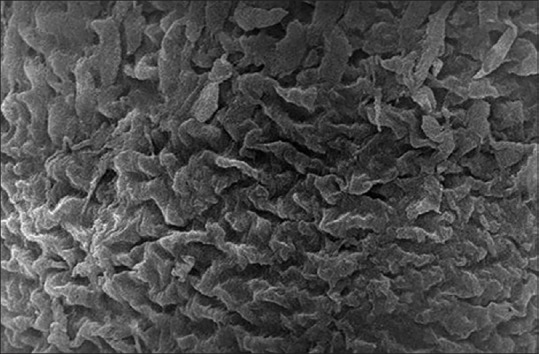
Scanning electron micrograph showing the tunica mucosa of the 1-week-old chick left oviduct with extensive mucosal folds and many corrugated cilia (×5000)
One-month-old chick
The left oviduct of the one-month old chick showed 5 layers surrounded a star shape lumen that were an inner epithelial lining that composed of simple tall columnar ciliated epithelial cells, a lamina propria of connective tissue supporting the inner epithelial layer, a layer of connective tissue, a layer of circular smooth muscle, an outer tunica serosa of simple squamous epithelial cells [Figure 10].
Figure 10.
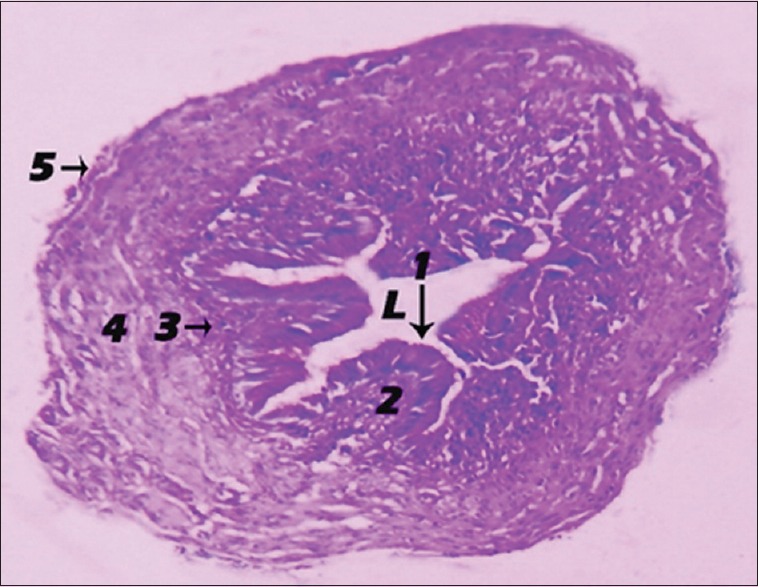
Photomicrograph showing the histological structure of the 1-month-old chick left oviduct. Inner epithelial layer (1), lamina propria (2), connective tissue layer (3), circular smooth muscle layer (4), tunica serosa (5), star-shaped lumen (periodic acid–Schiff, ×3.2). L: Lumen
The SEM observations of the tunica mucosa of the left oviduct of the 1-month-old chick showed long large longitudinal mucosal folds [Figure 11]. The accidently detached cilia were long as they estimated around 6–9 mm length and had the shape of the matches [Figures 12 and 13].
Figure 11.
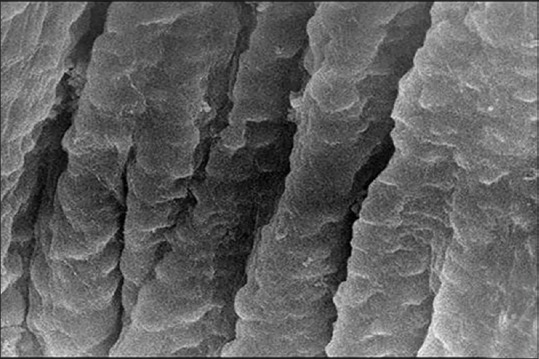
Scanning electron micrograph showing the tunica mucosa of the 1-month-old chick left oviduct with longitudinal mucosal folds (×5000)
Figure 12.
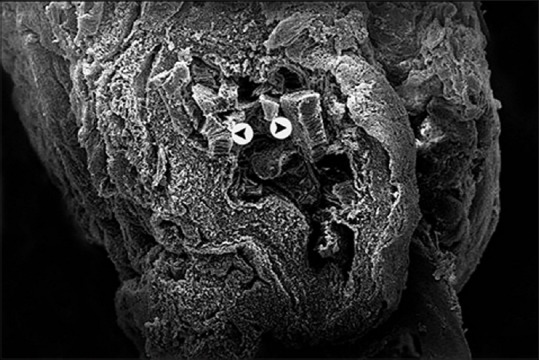
Scanning electron micrograph showing the 1-month-old chick left oviduct with star-shaped lumen and mucosal cilia (arrow head) (×500)
Figure 13.
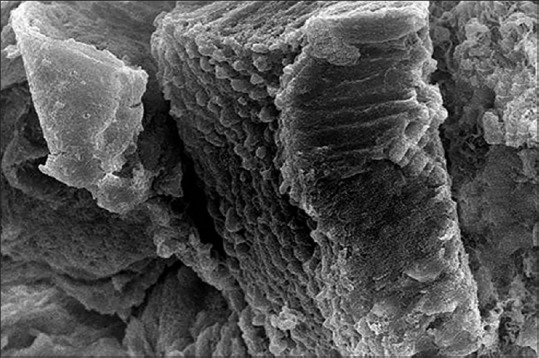
Scanning electron micrograph showing the mucosal cilia of the 1-month-old chick left oviduct (×3500)
DISCUSSION
In this article, we study the way in which the Mullerian duct forms. We track the genital ridge from day 5, where the left and right tubal ridges appeared as thickening ventrolateral to the genital ridge and were different to traditional concept that the Mullerian duct develops as a groove dorsolateral to nephrogenic ridge and then sinks beneath the genital ridge as reported previously by Jacob et al.[7,8] While Bellairs and Osmond[3] and Romanoff[9] stated that the Mullerian duct firstly appeared in the chicken embryo at the 4-day old embryo. In addition, Al-Saffar and Ab. Abood[10] observed that the Mullerian duct firstly appeared as ventrolateral thickening of the mesonephros in the Mallard duck embryo at 8-day-old embryo.
At the 6-day-old embryo, the left Mullerian duct enlarged and canalized to become the left oviduct and run caudolateral to the mesonephric duct, while the right Mullerian duct began to degenerate, similar to recorded by Bellairs and Osmond.[3,11]
The present work claimed that the smooth muscle layer firstly observed at the left oviduct of the 1-week-old chick and this result come in contrast to those of Nickel et al.[4] who noted this layer at the left oviduct of 1-day-old chick.
It is obvious in the present study at the left oviduct of 1-month-old chick that it composed of five layers that are inner epithelial layer, lamina propria that empty from any glands, connective tissue layer, circular smooth muscle layer, and outer peritoneal coat. The lumen became folded due to the presence of the layer of the circular smooth muscle. This results disagree with the results of King[12] on the definitive left oviduct who claimed that the left oviduct of the laying hen is composed of seven layers that include the inner epithelial layer, lamina propria, inner layer of connective tissue, inner layer of circular smooth muscle, outer layer of connective tissue, outer layer of longitudinal smooth muscle, and outer peritoneal coat.
Financial support and sponsorship
Funding was kindly provided by Alexandria University.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kosba M. Analysis of an Experiment on Selection for Economic Traits in Chickens. M. Sc. Thesis, Faculty of Agriculture Alexandria University Egypt. 1966 [Google Scholar]
- 2.Kosba M, El-Halim HA. Evaluation of the egyption local strains of chickens. Egypt Poult Sci. 2008;28:1239–51. [Google Scholar]
- 3.Bellairs R, Osmond M. Elsevier: Academic Press, UK; 2005. Atlas of chick development. [Google Scholar]
- 4.Nickel R, Schummer A, Seiferle E. Anatomy of the Domestic Birds. Verlag Paul Parey. 1977 [Google Scholar]
- 5.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo 1951. Dev Dyn. 1992;195:231–72. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- 6.Bancroft JD, Gamble M. Theory and Practice of Histological Techniques. Elsevier Health Sciences. 2008 [Google Scholar]
- 7.Jacob M, Konrad K, Jacob HJ. Early development of the müllerian duct in avian embryos with reference to the human. An ultrastructural and immunohistochemical study. Cells Tissues Organs. 1999;164:63–81. doi: 10.1159/000016644. [DOI] [PubMed] [Google Scholar]
- 8.Guioli S, Sekido R, Lovell-Badge R. The origin of the Mullerian duct in chick and mouse. Dev Biol. 2007;302:389–98. doi: 10.1016/j.ydbio.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 9.Romanoff AL. The avian embryo Structural and functional development. New York and London: The Macmillan Company; 1960. The avian embryo. Structural and functional development. [Google Scholar]
- 10.Al-Saffar FJ, Ab.Abood D. Histomorphological study of the pre hatching development of the female genital system in the indigenous mallard duck (Anas platyrhynchos) Int J Adv Res. 2014;2:248–63. [Google Scholar]
- 11.Hyttel P, Sinowatz F, Vejlsted M. Edinburgh, London, New York, Oxford, Philadelphia, St. Louis: Sydney Toronto, Saunders Elsevier; 2009. Essentials of Domestic Animal Embryology E-Book. [Google Scholar]
- 12.King A. Sisson and Grossman's the Anatomy of the Domestic Animals. 5th ed. Philadelphia: Saunders; 1975. Aves Urogenital System; pp. 1919–64. [Google Scholar]


