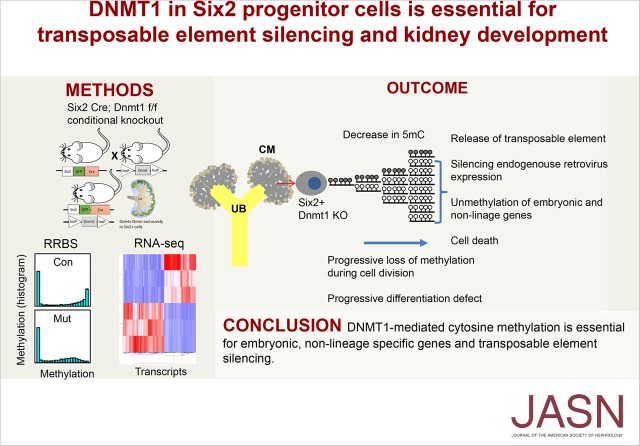
Significance Statement
Although cytosine methylation plays a key role in regulating gene expression, including expression of transposable elements such as endogenous retroviruses, its role in kidney development is unknown. Using genome-wide methylation analysis in a mouse model, the authors showed dynamic changes in methylation of gene promoters and enhancers in developing kidneys. Genetic deletion of de novo methyltransferases in nephron progenitor cells did not lead to developmental alterations, whereas deletion of Dnmt1, which encodes a maintenance hemimethylase, caused a severe kidney developmental defect. Dnmt1 deletion resulted in a marked loss of methylation of transposable elements, accumulation of endogenous retroviral transcript, and activation of viral sensing pathways and cell death. These findings indicate that DNMT1-mediated methylation to silence embryonic nonrenal lineage genes and transposable elements is essential for kidney development.
Keywords: DNA methylation, DNMT, podocyte, kidney development, Transposable element
Visual Abstract
Abstract
Background
Cytosine methylation of regulatory regions, such as promoters and enhancers, plays a key role in regulating gene expression, however, its role in kidney development has not been analyzed.
Methods
To identify functionally important epigenome-modifying enzymes and genome regions where methylation modifications are functionally important for kidney development, we performed genome-wide methylation analysis, expression profiling, and systematic genetic targeting of DNA methyltransferases (Dnmt1, Dnmt3a, and Dnmt3b) and Ten-eleven translocation methylcytosine hydroxylases (Tet2) in nephron progenitor cells (Six2Cre) in mice.
Results
Genome-wide methylome analysis indicated dynamic changes on promoters and enhancers during development. Six2CreDnmt3af/f, Six2CreDnmt3bf/f, and Six2CreTet2f/f mice showed no significant structural or functional renal abnormalities. In contrast, Six2CreDnmt1f/f mice died within 24 hours of birth, from a severe kidney developmental defect. Genome-wide methylation analysis indicated a marked loss of methylation of transposable elements. RNA sequencing detected endogenous retroviral transcripts. Expression of intracellular viral sensing pathways (RIG-I), early embryonic, nonrenal lineage genes and increased cell death contributed to the phenotype development. In podocytes, loss of Dnmt1, Dnmt3a, Dnmt3b, or Tet2 did not lead to functional or structural differences at baseline or after toxic injury.
Conclusions
Genome-wide cytosine methylation and gene expression profiling showed that by silencing embryonic, nonrenal lineage genes and transposable elements, DNMT1-mediated cytosine methylation is essential for kidney development.
DNA cytosine methylation (5mC) is largely erased and then reestablished between generations in mammals. At the blastocyst stage, most cytosines are unmethylated because of active and passive demethylation. During cell type diversification and differentiation, CpG methylation increases.1 DNA methylation is accomplished by the de novo DNA methyltransferases DNMT3A and DNMT3B.2,3 DNMT3A and DNMT3B have been proposed to be critical for establishing methylation of enhancers that are important for cell type–specific transcription factor binding and cell differentiation. DNMT1 is the key hemimethylase responsible for methylating the newly synthesized DNA strand during replication.2,4 The combination of cytosine methylation, histone modification, and chromatin structure defines genome accessibility and allows the differentiation of multiple cell types from a single fertilized egg.5 De novo cytosine methylation of promoters and enhancers plays a key role in establishing gene expression therefore it is key for cellular differentiation. Although this model of gene expression and differentiation is widely accepted, the direct evidence linking promoter and enhancer methylation to gene expression in vivo is limited because of technical difficulties in manipulating cytosine methylation in a temporal and site-specific manner.
Animal models with genetic deletion of Dnmts in different cells and organs have been used to understand the role of methylation in regulating gene expression and cellular differentiation. In embryonic stem cells, pancreatic, gut epithelium, and skin, Dnmt1 depletion leads to dramatic organ development failure.6–9 The mechanism of Dnmt1 loss-induced organ defect has been attributed to differential methylation of cell type–specific genes leading to cell cycle arrest, premature differentiation, and a failure of tissue self-renewal.10,11 Deletion of de novo methyltransferases (Dnmt3a or Dnmt3b) in different compartments have been associated with milder phenotypes.12
Transposable elements (TEs) control is one of the most ancient and fundamental function of cytosine methylation. TEs are generally fully methylated in all cells.13 Transposons are mobile genetic elements found in every eukaryotic genome sequenced to date and account for at least half of the mammalian genome.14 In most mammals, retrotransposons are the predominant TEs. These can be divided into endogenous retroviruses (ERVs), long interspersed elements (LINEs), and short interspersed elements (SINEs).13,15 Because of the low methylation state of the blastomere, some ERVs are highly expressed at this stage. Although some believe ERV expression is a simple byproduct of epigenetic reprogramming, studies indicate ERVs could contribute to multipotency. Recent studies also found significant ERV expression in some cancer cells due to epigenetic misregulation. Release of TE silencing in cancer cells induces the cytosolic double-stranded RNA (dsRNA) sensing pathway that triggers a type 1 IFN response.16,17
Nephron number in humans is highly variable. Six2-expressing cap mesenchymal cells represent a multipotent nephron progenitor population, which undergo self-renewal and give rise to the functioning nephron epithelium.18 Nephron number at birth shows strong association with hypertension and kidney disease development later in life. Studies have shown that intrauterine nutritional and environmental alterations are key determinants of nephron endowment. Epigenome-modifying enzymes use products of intermediate metabolism (methyl or acetyl groups) as their substrates. Variations in substrate availability can lead to changes in the epigenome. Therefore, epigenetic changes during development have been proposed to be the mediators of fetal programming that eventually lead to kidney disease development.19–22
This study aimed to identify functionally important epigenome-modifying enzymes and regions in the genome where methylation modifications are functionally important for kidney development by genome-wide methylation and expression analysis.
Methods
Mice
Dnmt1f/f mice were obtained from Mutant Mouse Regional Resource Center (MMRRC_014114-UCD). Dnmt3af/f and Dnmt3bf/f mice were obtained from the Jackson Laboratory.23,24 Tet2f/f mice were purchased from the Jackson Laboratory (stock number 017573). Dnmts and Tet2 mice were crossed to transgenic mice carrying the PodCre 25 (Jackson Laboratory stock number 008205) or Six2Cre (Jackson Laboratory stock number 009606). Double transgenic mice and animal gender were identified by genomic PCR analysis.26 Podocyte injury was induced in 6-week-old male mice by doxorubicin (20 mg/kg, intravenous; Pfizer) or streptozotocin injection (50 mg/kg administered intraperitoneally, for 5 days; Sigma-Aldrich).
Histologic Procedures and Staining
Renal histologic changes were examined by PAS stained sections. Immunostaining were performed using the following primary antibodies: DNMT1 (ab188453; Abcam), SIX2 (11562–1-AP; Proteintech), p-53 (#2526; CST), fluorescein labeled Peanut agglutinin (PNA), Lotus tetragonolobus lectin (LTL), and Dolichos biflorus agglutinin (DBA) (FL-1071, FL-1321, FL-1031; Vector) after heat-induced antigen retrieval by Tris-EDTA buffer (pH 9.0 or 6.0).
Isolation of Six2-Positive Cells
Kidneys from Six2Cre control and Six2Cre Dnmt1f/f mice were dissected under a stereo microscope and placed in RPMI, briefly dissociated using 18G and 21G needles. Kidneys were incubated in collagenase for 10 minutes at 37°C. After being passed through a 40 μm cell strainer, 10% serum was added to neutralize collagenase. After red blood cell lysis, single-cell suspension was collected by centrifugation and suspended in 500 μl of PBS containing 2% serum. FACS analysis was performed using BD FACSAria II.
Reduced Representation Bisulfite Sequencing
Genome-wide DNA methylome analysis was performed using the Premium Reduced Representation Bisulfite Sequencing (RRBS) Kit (Diagenode) following manufacturer’s instruction. In brief, genomic DNA was extracted from whole kidneys using Puregene Core Kit A (Qiagen), 100 ng genomic DNA was then digested using the methylation-insensitive enzyme MspI. Fragment ends were filled in, and adapters were ligated. After size selection, fragments were subjected for bisulfite conversion. Libraries were sequenced on Illumina Hiseq 50 bp SE configuration. The adapter sequences and low-quality base calls (<30) were trimmed from the 3′ end using Trim_Galore program with–rrbs option. The bisulfite converted sequencing reads were then aligned to the mouse reference genome (mm10) using Bismark program (default parameters). Differentially methylated regions (DMRs) were determined using methylKit in R package with definition number of CpG ≥3 and DMC ≥1 and q <0.05. Chromatin states of DMRs were annotated using mouse kidney specific ChromHMM maps.27,28 Transcription factor motif were identified in the DMRs using Homer program.
RNA Sequencing
RNA was isolated using RNeasy mini kit (Qiagen). First, 200 ng total RNA was used to isolate poly A purified mRNA using the Illumina TruSeq RNA Preparation Kit. Sequencing was performed in Illumina by paired-end 150 bp, and the annotated RNA counts (fastq) were calculated by Illumina’s CASAVA 1.8.2. Sequence quality was surveyed with FastQC. Adaptor and lower-quality bases were trimmed with Trim-galore. Reads were aligned to the Gencode mouse genome (GRCm38) using STAR-2.4.1d. The aligned reads were mapped to the genes (GRCm38; version 7 Ensembl 82) using HTSeq-0.6.1. DESeq2 was used to test for differential gene expression between control and knockout groups.
To analyze the TEs, the sequencing reads aligned to the mouse genes were firstly removed. First, the aligned reads were mapped to the genes (GRCm38; version 7 Ensembl 82) using HTSeq-0.6.1 with intersection-nonempty parameter for the mode option. Then, the reads which were mapped to the Ensembl genes were filtered out from the sam files. Finally, the rest of the sequencing reads (marked as __no_feature) were aligned to the Repeatmasker which was downloaded from the UCSC genome browser. In regarding to qRT-PCR, 1 µg RNA was reverse transcribed using the cDNA Archive kit (Life Technology), and qRT-PCR was run in the ViiA7 System (Life Technology) machine using SYBR Green Master Mix and gene-specific primers (Supplemental Table 1). The data were normalized and analyzed using the ΔΔCt method.
Statistical Analyses
Statistical analyses were performed using GraphPad Prism software (GraphPad Software Inc., La Jolla, CA). All values are expressed as mean and SD. Two-tailed t test was used to compare two groups. One-way ANOVA with post hoc Tukey test was used to compare multiple groups. A P value <0.05 was considered statistically significant.
Study Approval
All experiments in animals were reviewed and approved by the Institutional Animal Care and Use Committee of University of Pennsylvania and were performed in accordance with the institutional guidelines.
Data Access
All sequencing data (RRBS and RNA sequencing) have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO accession number GSE110481.
Results
Genome-Wide Methylation During Kidney Development
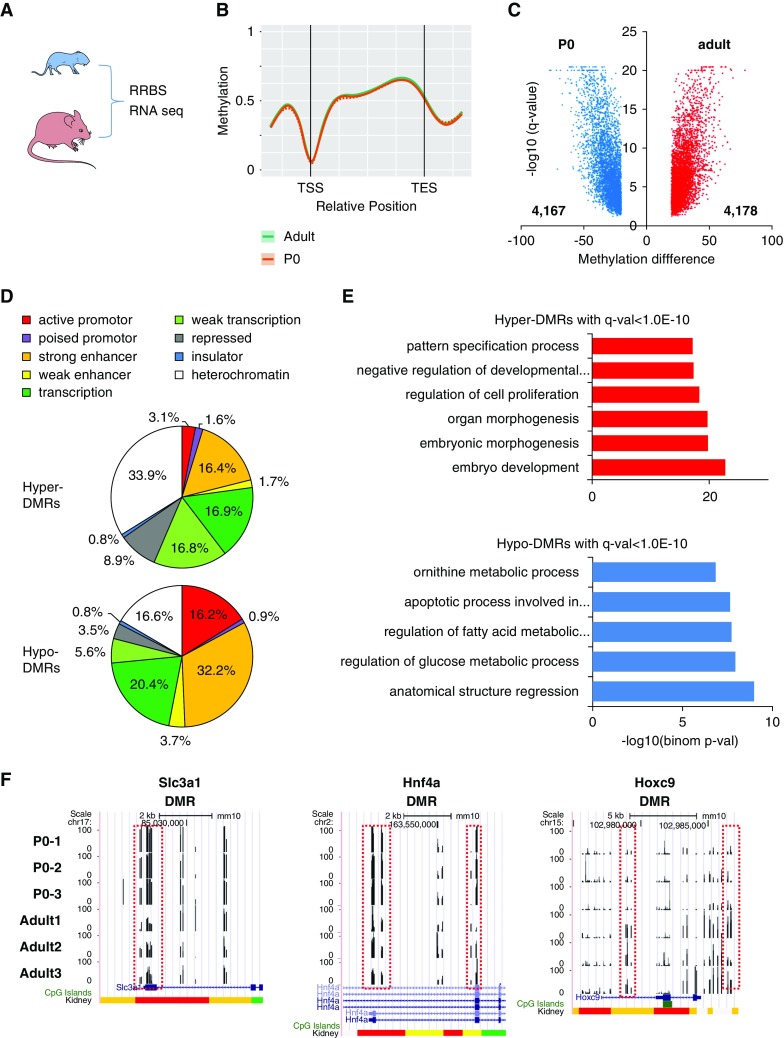
We first analyzed methylation changes in developing (newborn) and differentiated (adult) kidneys (Figure 1A) by genome-wide RRBS. 5mC levels were lower in adult kidneys when compared with newborn kidneys that are still undergoing nephrogenesis. Upon mapping 5mC to RefSeq-based regulatory annotation, we found low 5mC levels around transcription start sites (Figure 1B). We identified 4178 DMRs with higher methylation and 4167 regions with lower methylation in adult kidneys (Figure 1C).
Figure 1.
Methylation changes during kidney development. (A) RRBS and RNA sequencing of P0 and adult mice. (B) Methylation profiles near and within all mouse genes. TES, transcription end site; TSS, transcription start site. (C) Volcano plot showing the DMRs between kidneys of control P0 and adult mice. (D) Annotation of DMRs by kidney-specific chromatin states. (E) Functional annotation analysis for the DMRs between P0 and adult mice. (F) UCSC genome browser screenshot showing the representative DMRs. DMRs are marked by red boxes. y-axis represents methylation level from 0% to 100%. Chromatin states are shown at the bottom (color scheme as above; red promoter, yellow enhancer).
Next, we mapped DMRs onto kidney specific functional regulatory regions, defined by combination of histone modifications and ChromHMM maps.27,28 Promoters and enhancers were significantly enriched in regions showing lower methylation levels in adult samples (Figure 1D), whereas 5mC levels were increased in heterochromatin regions. Regions with increased methylation levels were enriched for developmental transcription factors, such as LHX binding sites (Supplemental Figure 1). On the other hand, methylation levels were lower at HNF binding motifs (Supplemental Figure 1). Gene ontology analysis for DMRs with increased methylation indicated an enrichment around pathways involved in embryonic morphogenesis and development, and a decrease in developmental and metabolic processes (Figure 1E). We showcase some of the DMRs at Slc3a1, Hnf4a, and Hoxc9 gene loci (Figure 1F). In summary, our results show dynamic methylation changes of regulatory regions (promoter and enhancer) in the developing kidney.
Changes in Dnmt and Tet Expression in Developing Kidneys
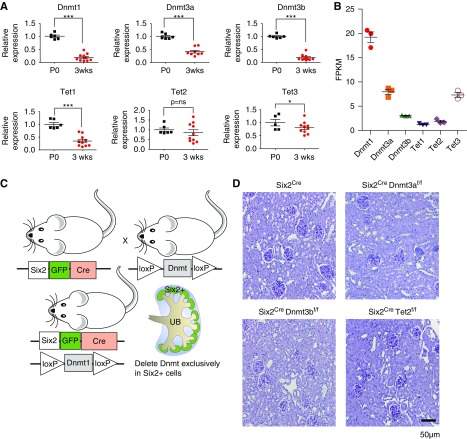
Next, we investigated the temporal course and relative expression of methylome editing enzymes, Dnmt and Tet, in mouse kidneys. Gene expression analysis (by qRT-PCR and RNA sequencing) indicated the expression of Dnmt1, Dnmt3a, Dnmt3b, Tet1, and Tet3 were higher at birth (P0) when compared with adults, whereas Tet2 expression did not change (Figure 2, A and B).
Figure 2.
Differences in expression of methylome editing enzymes in developing mouse kidneys. Expression of Dnmt1, Dnmt3a, Dnmt3b, Tet1, Tet2, and Tet3 in newborn (P0) and 3-week-old mouse kidneys analyzed by (A) qRT-PCR and (B) in P0 mouse kidneys analyzed by RNA sequencing. *P<0.05 and ***P<0.001. (C) Breeding scheme of generating Six2Cre Dnmt1f/f mice. (D) Representative PAS-stained sections of control, Six2Cre Dnmt3af/f, Six2Cre Dnmt3bf/f, and Six2Cre Tet2 f/f kidneys in 3-week-old mice. FPKM, fragments per kilobase of transcript per million mapped reads.
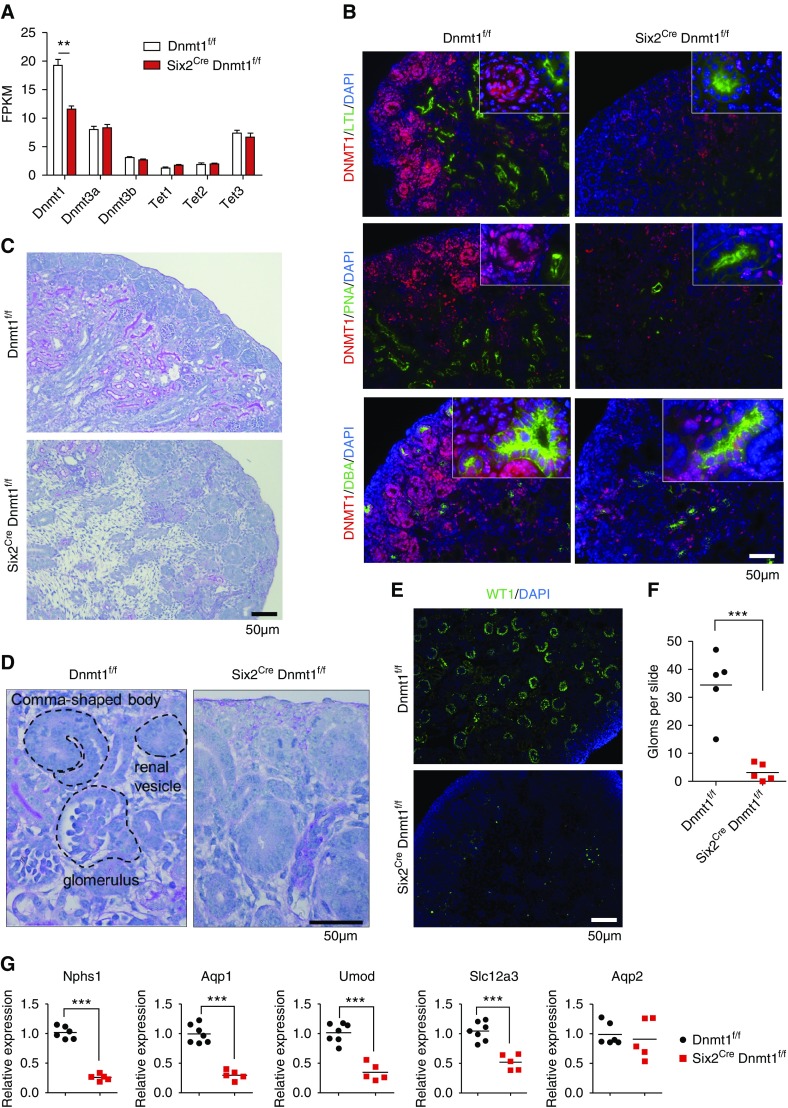
Immunofluorescence staining indicated that in the P0 control mouse kidney cortex, DNMT1 was highly expressed in the cap mesenchyme and nephron precursors such as renal vesicle and S-shaped body (Figure 3B). Although we observed some regional differences in DNMT1 expression, the double-immunofluorescence staining of DNMT1 with differentiated kidney segment markers LTL (proximal tubule), PNA (loop of Henle and distal tubule), and DBA (collecting duct) showed that the expression of DNMT1 was much lower in differentiated epithelial cell (Figure 3B).
Figure 3.
Dnmt1 is critical for kidney development. (A) Expression of Dnmt1, Dnmt3a, Dnmt3b, Tet1, Tet2, and Tet3 in Dnmt1f/f and Six2Cre Dnmt1f/f mouse kidneys (P0) as analyzed by RNA sequencing. Asterisks represent FDR significant differences calculated by DESeq2. **P<0.01. (B) Immunofluorescence staining of DNTM1 (red) and kidney segment markers LTL, PNA, and DBA (green) in P0 kidneys from Dnmt1f/f and Six2Cre Dnmt1f/f mice. (C and D) Representative PAS-stained kidney sections from control (Dnmt1f/f) and Six2Cre Dnmt1f/f kidneys (P0). Specific kidney structures are highlighted. (E) Immunofluorescence staining of Dnmt1f/f and Six2Cre Dnmt1f/f kidneys with WT1 (podocyte marker). (F) Total number of glomeruli per longitudinal kidney sections in Dnmt1f/f and Six2Cre Dnmt1f/f kidney sections at P0 (n=5 per group). (G) Expression of differentiated kidney segment markers in Dnmt1f/f and Six2Cre Dnmt1f/f kidneys by qRT-PCR. FPKM, fragments per kilobase of transcript per million mapped reads. ***P<0.001.
Dnmt3a, Dnmt3b, and Tet2 Are Dispensable for Kidney Development
In mice, the progeny of Six2 positive cells give rise to the functional nephron epithelium from the glomerulus to distal tubule, but not to the last tubule segment, the collecting duct. To determine the functionally significant methylome editing enzymes, we crossed Dnmt1, Dnmt3a, Dnmt3b, and Tet2 conditional mice with Six2-EGFPCre mouse 18 (Figure 2C). Six2CreDnmt3af/f, Six2CreDnmt3bf/f, and Six2CreTet2f/f mice showed no clear structural and functional renal abnormalities (Figure 2D), indicating that Dnmt3a, Dnmt3b, and Tet2 are dispensable for kidney development.
Loss of Dnmt1 in Six2-positive Cells Results in Severe Kidney Developmental Defect
To study the role of Dnmt1 in kidney development, we created Six2CreDnmt1f/f mice. Dnmt1 transcript level was lower in Six2CreDnmt1f/f kidneys compared with controls, whereas no transcription change of Dnmt3a and Dnmt3b was detected (Figure 3A). Immunofluorescence staining of Six2CreDnmt1f/f samples confirmed the lower expression of DNMT1 in Six2-positive cell lineages such as LTL- and PNA-positive tubule segment, but not in ureteric bud lineage (DBA-positive segment) (Figure 3B).
Six2CreDnmt1f/f mice were born at expected Mendelian ratio, but they died within 24 hours of birth. Animals had visibly smaller kidneys and their bladders were void of urine (Supplemental Figure 2). Structural analysis indicated significant defect in nephron differentiation. In control P0 mouse kidneys, we observed multiple layers of nascent nephrons as they formed in the nephrogenic zone, followed by a thick layer of renal cortex (Figure 3C). However, Six2CreDnmt1f/f kidneys showed lack of tubule structures, expanded interstitium, and very few comma and S-shape bodies, or fully differentiated nephrons (Figure 3, C and D). The expression of Ccnd1 (marker of renal vesicle) was slightly increased in Dnmt1 knockout kidneys, which is consistent with a differentiation defect (Supplemental Figure 3). Expression of WT1, a podocyte marker, was decreased (Figure 3E) and very few LTL-positive and PNA-positive segments were found in knockout kidneys (Figure 3B), which confirmed that Six2CreDnmt1f/f mice had a broad differentiation defect (Figure 3F). On the other hand, DBA-positive collecting duct cells, which are differentiated from ureteric bud, appeared to be unaffected (Figure 3B). Gene expression analysis showed lower levels of Nphs1 (podocyte), Aqp1, Slc34a1, Hnf4a (proximal tubule), Umod (loop of Henle), and Slc12a3 (distal convoluted tubule) in Six2CreDnmt1f/f mice,29 but conserved Aqp2 level (Figure 3G, Supplemental Figure 4). In summary, our studies indicate that very few, if any fully differentiated nephrons are formed in the absence of Dnmt1 from Six2-positive progenitor cells.
Dnmt1 Depletion Did Not Reduce Six2-Positive Cell Population
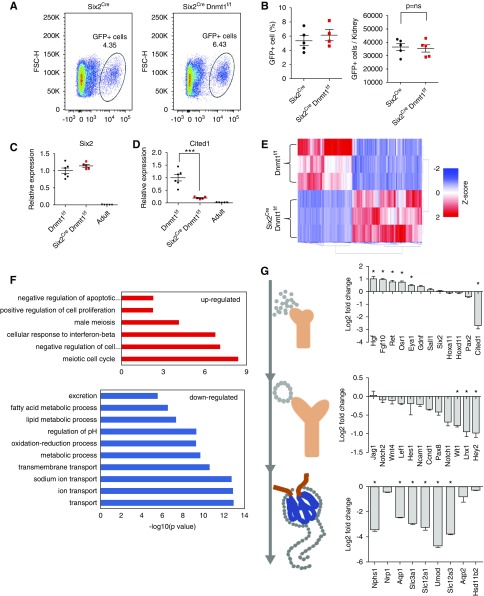
To understand the mechanism of Dnmt1 depletion-induced defect in nephrogenesis, we first quantified the number of Six2-positive cell population. Through fluorescence-activated cell sorting, we found no significant differences in GFP-positive (Six2-positive) cell number between control P0 kidneys and Six2CreDnmt1f/f samples (Figure 4, A and B). Immunofluorescence staining with SIX2 antibody showed that Six2CreDnmt1f/f mice had slightly, but not significantly higher SIX2-positive staining (Supplemental Figure 5). We also found no change in Six2 transcript levels (Figure 4, C and D), although Cited1 level was decreased.30
Figure 4.
Dnmt1 is indispensable for renal epithelial cell differentiation. (A) Representative flow cytometry images of GFP-positive cells in Six2Cre and Six2Cre Dnmt1f/f kidneys. (B) Quantification of GFP-positive cells. (C and D) Expression of Six2 and Cited1 in Dnmt1f/f P0, Six2Cre Dnmt1f/f P0, and wild-type adult kidneys, as analyzed by qRT-PCR. (E) Heatmap of the DEGs between Dnmt1f/f and Six2Cre Dnmt1f/f mice. Color scheme uses z-score distribution. (F) Functional annotation analysis (DAVID) of the differentially expressed (up- and downregulated) genes. (G) Log2 fold change of expression genes involved in kidney development. Asterisks represent FDR significant differences calculated by DESeq2. *P<0.05 and ***P<0.001.
Next, we performed comprehensive gene expression analysis by RNA sequencing. We identified 1829 differentially expressed genes (DEGs) when we compared Dnmt1 knockout P0 kidneys to controls (FDR<0.05) (Figure 4E, Supplemental Table 2). Genes that showed significantly lower expression in knockout kidneys were related to ion transport and renin-angiotensin pathways (Figure 4F, Supplemental Figure 6), indicating the lack of renal tubule epithelia in the knockout mice. Although Six2 expression was unaffected, we found that level of Hgf, Fgf10, and Ret were increased in Dnmt1 null kidneys (Figure 4G). These genes are usually expressed before Six2, as they are critically involved in the ureteric bud and metanephric mesenchyme interaction before nephron progenitors establishment. Genes that play a role in elongation and segmentation of the renal epithelial vesicles or markers of differentiated glomerular and proximal tubule cells were almost uniformly decreased in Dnmt1 knockout mice (Figure 4G). Markers of collecting duct cell fate, such as Aqp2 and Hsd11b2, were not changed in Six2CreDnmt1f/f mice, as this segment develops from a ureteric bud and not from a Six2-positive population. These results indicate an absence of differentiation of Six2 progenitor cells and reexpression of early nephric duct markers.
We found male primordial germ cell–specific genes, such as Dazl and Sohlh2, showed one of the highest increases in Dnmt1 null kidneys (Supplemental Figure 7, A and B). There was an enrichment for genes with function in homologous recombination and meiosis in absence of Dnmt1 (Figure 4F, Supplemental Figure 6). We observed the expression of even earlier markers such as Zscan4b and Zscan4d that are normally only expressed in 2–4 cell-stage embryos.31 The full list of DEGs can be found in Supplemental Table 2. In summary, genome-wide expression analysis not only indicated the lack of differentiated epithelial cell markers but also the expression of nonlineage and early embryonic markers in absence of Dnmt1.
Dnmt1 Loss Results in Broad Cytosine Methylation Changes
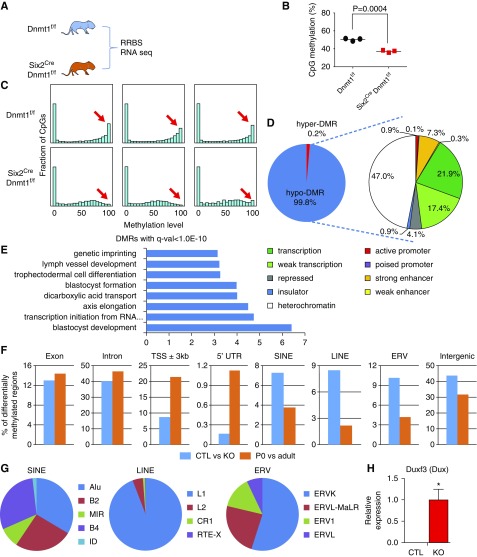
Next, we analyzed the DNA methylomes of control and knockout kidneys (Figure 5A). Genome-wide RRBS analysis revealed a significant decrease in overall CpG methylation (Figure 5B). A bimodal distribution pattern of CpG methylation was observed in controls. There was a marked decrease in the number of fully methylated loci in knockout kidneys (Figure 5C). Most of the DMRs were hypo-DMRs (45,026 hypo versus 88 hyper) (Figure 5D). Next, we mapped DMRs onto kidney-specific regulatory regions, defined by histone modifications and ChromHMM maps. Surprisingly, only a few promoters or enhancers showed differential methylation in Dnmt1 null kidneys (Figure 5D). Transcription factor motifs analysis of the DMRs revealed enrichment for ELK4, MEF2C, and GATA2 binding motifs (Supplemental Figure 8). Lower methylated regions in Dnmt1 knockout mice were enriched at genes with blastocyst development and formation function (Figure 5E).
Figure 5.
Dnmt1 loss results in broad cytosine methylation changes. (A) RRBS and RNA sequencing analysis of kidneys of Dnmt1f/f and Six2Cre Dnmt1f/f mice. (B) Global CpG methylation in Dnmt1f/f and Six2Cre Dnmt1f/f mouse kidneys. (C) Methylation distributions of CpGs in Dnmt1f/f and Six2Cre Dnmt1f/f mouse kidneys. Histogram plots showing counts by CpG methylation level (x-axis: 0%–100%) distributed across 20 bins of 5% intervals. (D) Annotation of DMRs by kidney-specific chromatin states. (E) Gene ontology annotation analysis for the DMRs between Dnmt1f/f and Six2Cre Dnmt1f/f mouse kidneys. (F) Distribution of DMRs in different genomic elements. Blue bar represents DMRs detected between Dnmt1f/f and Six2Cre Dnmt1f/f mice. Orange bar represents DMRs detected between Dnmt1f/f P0 and wild-type adult mice. (G) Distribution of DMRs in different transposable elements. (H) Relative expression level of Duxf3 (Dux). y-axis is normalized expression level determined by RNA sequencing. Asterisks represent significant differences calculated by DESeq2. *P<0.05.
More than 50% of the observed DMRs in the Dnmt1 knockout mice were on regions annotated as repressed region or heterochromatin (Figure 5D). These regions are normally fully methylated. In absence of Dnmt1, methylation differences of intergenic regions, including LINE, SINE, and ERV were the most prominent (Figure 5F). LTR retrotransposons include ERVs derived from infectious retroviruses that integrated in the germline. Most of DMRs detected in ERV were hypo-DMRs and more than half of them were observed in ERVK family (Figure 5G). In contrast, transcription start sites and 5′ UTR showed differential methylation during development (Figure 5F). Furthermore, in Dnmt1 knockout mice, we detected a significant increase in the expression level of the Dux transcription factor, which is a known activator of ERV elements (Figure 5H).32,33 In summary, loss of Dnmt1 was associated with significantly lower methylation levels in the kidney, which specifically affected the normally fully methylated heterochromatin regions (LINE, SINE, and ERV).
Dnmt1 Maintains Silencing of TEs in Six2-Positive Progenitor Cells
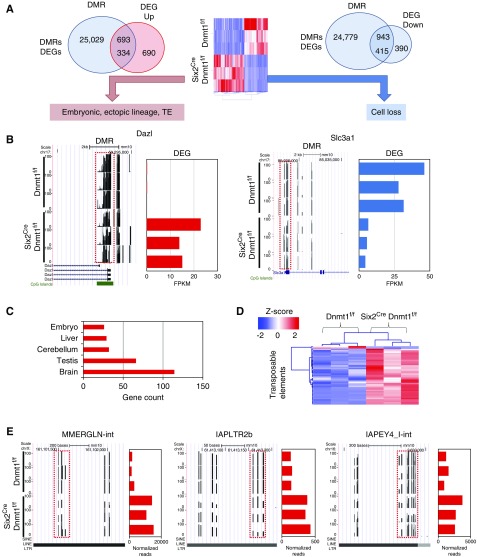
Next, we integrated methylation changes with transcriptome differences. In Six2CreDnmt1f/f kidneys, 1829 DEGs and 25,722 DMRs within promoter or gene body regions were identified (Figure 6A). Large numbers of DEGs (749 out of 1829) were in the vicinity of DMRs. A total of 415 genes showed a decrease both in methylation and gene expression levels. Genes in this group mostly included renal tubule epithelial–specific genes such as Slc3a1, Slc23a1, and Umod. Expression of these genes were lower despite the low methylation in their regulatory regions. These results are consistent with dropout of differentiated epithelial cells in Six2CreDnmt1f/f kidneys (Figure 6B, Supplemental Figures 9 and 10A). A limitation of our work is the lack of lineage enriched transcriptome analysis, as whole tissue analysis identified not only cell-specific but also cell composition changes (dropout). A small fraction (18%) of all DEGs (334) followed a classic pattern of lower methylation and increase in gene expression. Gene ontology analysis showed this cluster of genes are enriched for meiotic cell cycle, and spermatogenesis. (Supplemental Figure 10B). Dnmt1 knockout kidneys expressed high levels of primordial germ cell–specific genes, including Dazl,34,35 Sohlh2,36,37 Tex19.1,38 Pet2,39 and also genes exclusively expressed in 2–4 cell-stage embryos, Zscan4b and Zscan4d.31 Such genes were undetectable in control P0 kidneys (Figure 6B, Supplemental Figure 7). Tissue enrichment analysis of the DEGs indicated enrichment for brain, testis, and embryo genes (Figure 6C), highlighting the expression nonlineage specific genes and a broad dysregulation in cell identity in Dnmt1 null kidneys.
Figure 6.
Methylation changes in Six2Cre Dnmt1f/f mice are associated with embryonic, ectopic lineage and transposable element expression. (A) The number of identified DMR and DEGs and their consistency and directionality in control and Six2Cre Dnmt1f/f mice. (B) Representative DMRs and their target gene expression changes. DMRs are marked by red boxes. Left panel shows a UCSC genome browser screenshot with DNA methylation patterns and y-axis represents methylation level from 0% to 100%. Right panel shows gene expression level and x-axis represents normalized sequencing reads. (C) Tissue enrichment analysis of the target genes (DAVID). (D) Heatmap of the differentially expressed TEs between Dnmt1f/f and Six2Cre Dnmt1f/f mice. Color scheme uses z-score distribution. (E) Representative DMRs and their target TE expression changes. DMRs are marked by red boxes. Left panel shows a UCSC genome browser screenshot with DNA methylation patterns and y-axis represents methylation level from 0% to 100%. Right panel shows TE expression level and x-axis represents normalized sequencing reads.
Next, we wanted to understand whether reduced methylation on TE was associated with increased ERV transcription. Among 619 LINE, SINE, and ERV families, we have identified 43 differentially expressed TEs, of which 38 ERV families were increased and only two ERV families were decreased in knockout kidneys (Figure 6D, Supplemental Table 3). These increased ERV families included all three ERV classes. On the other hand, three LINE families (L1Md_Gf, L1Md_T, and L1Md_F) were increased in knockout kidneys. Figure 6E illustrates that Dnmt1 depletion–induced hypomethylation was associated with increase in RNA levels of three different ERVs, MMERGLN, IAPLTR2b, and IAPEY4.
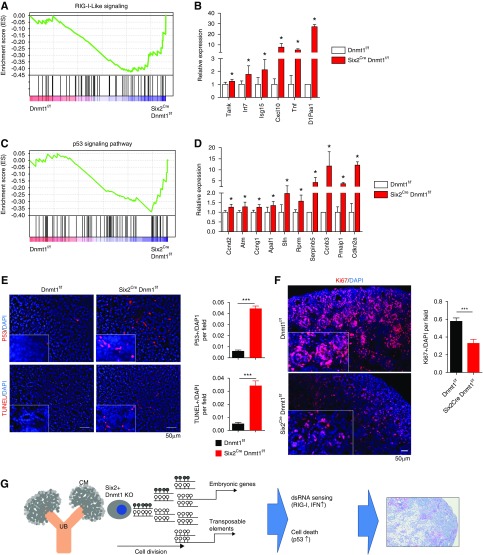
An increase in ERV in cancer cells triggers cytosolic viral sensors, IFN release, and immune activation. Increased expression of IFN and retinoid acid inducible gene I (RIG-I) signaling was observed in Dnmt1 null kidneys (Figure 7, A and B, Supplemental Figure 11). RIG-I-like receptor is a dsRNA helicase that functions as a pattern recognition receptor and viral sensor. In addition, increased p53 signaling was observed in Dnmt1 knockout mice (Figure 7, C and D, Supplemental Figure 11). We quantitatively confirmed the increase in protein expression of P53 in Dnmt1 knockout mice (Figure 7E). Furthermore, the cell death rate was higher in knockout animals (Figure 7E), whereas cell proliferation rate was lower (Figure 7F). Altogether, our data indicates that Dnmt1 deletion in the progeny of Six2-positive cells leads to release of TEs, silencing ERVs transcription, and subsequent RIG-I and P53 activation, contributing to the severe kidney developmental defect, but the contribution of other factors such as increased senescence cannot be excluded (Figure 7G).
Figure 7.
RIG-I-Like signaling and apoptosis are increased in kidneys of Six2Cre Dnmt1f/f mice. (A) The GSEA enrichment plots of RNA sequencing data showing enrichment for RIG-I–like signaling in Six2Cre Dnmt1f/f mice. (B) Relative expression level of genes involved in RIG-I–like signaling. y-axis is normalized expression level determined by RNA sequencing. Asterisks represent significant differences calculated by DESeq2. (C) The GSEA enrichment plots of RNA sequencing data of P53 signaling in Six2Cre Dnmt1f/f mice. (D) Relative expression level of genes involved in p53 signaling. y-axis is normalized expression level determined by RNA sequencing. Asterisks represent significant differences calculated by DESeq2. (E) Left panel: immunofluorescence staining of P53 (red) and TUNEL assay (red) in P0 Dnmt1f/f and Six2Cre Dnmt1f/f mice kidneys; right panel: quantification of P53- and TUNEL-positive signaling according to the staining. (F) Left panel: immunofluorescence staining of Ki67 (red) in P0 Dnmt1f/f and Six2Cre Dnmt1f/f mice kidneys; right panel: quantification of Ki67-positive cells. (G) Schematic of our model which Dnmt1 deletion in progeny of Six2-positive cells affects kidney development. *P<0.05 and ***P<0.001.
Dnmts and Tets Are Dispensable in Podocytes
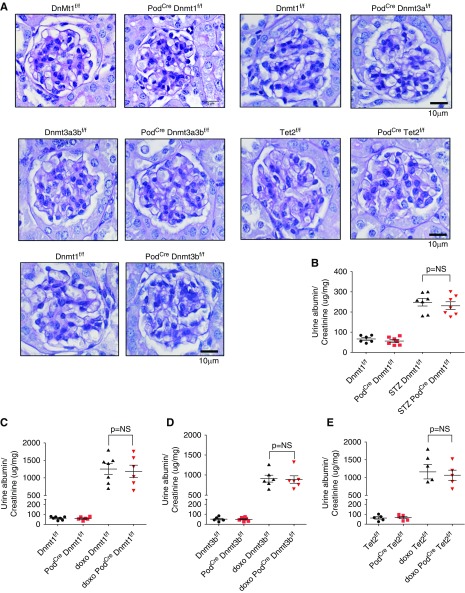
To study the role of 5mC in differentiated podocytes, we crossed Dnmt1 f/f, Dnmt3a f/f, Dnmt3b f/f, and Tet2 f/f animals with the Podcre mice 25. Mice were born at expected Mendelian frequency and we did not observe significant functional or structural renal abnormalities even at 20 weeks of age (Figure 8A). Next, we tested their response to podocyte injury. To induce diabetes we injected podocyte-specific knockout mice and control littermates with streptozotocin. Phenotype analysis performed 24 weeks after the initiation of diabetes showed no differences in albuminuria level (Figure 8B). Doxorubicin is known to be toxic to podocytes and can induce FSGS. Even after careful characterization of control and Dnmt1, Dnmt3b, and Tet2 knockout animals, we failed to observe differences in albuminuria level in knockout animals (Figure 8, C–E). These finding indicated that Dnmt1, Dnmt3b, and Tet2 proteins are dispensable in podocytes, even under injury conditions.
Figure 8.
Epigenetic editing enzymes are dispensable in mature podocytes. (A) Representative PAS-stained mouse kidney sections of control (Dnmt1f/f) and mice with podocyte-specific deletion of Dnmt1, Dnmt3a, Dnmt3b, Dnmt3a/3b, and Tet. (B) Albuminuria (urine albumin-to-creatinine, micrograms per milligram) of Dnmt1f/f and PodCre Dnmt1f/f mice at baseline and 20 weeks after streptozotocin-induced diabetes. (C–E) Albuminuria (urine albumin-to-creatinine, micrograms per milligram) of control, PodCre Dnmt1f/f, PodCre Dnmt3bf/f, and PodCre Tet2f/f mice at baseline and 7 days after doxorubicin-induced glomerular injury.
Discussion
Overall, this is the first study to define the methylome at base-pair resolution in the developing and adult mouse kidneys. To define functionally critical methylation changes, we have complemented the methylome atlas by deleting most epigenome editing enzymes. Methylome analysis of the wild-type developing mouse kidney indicated dynamic changes of methylation levels enriched in gene promoters and enhancers, which is consistent with the consensus model of cell differentiation where loss of methylation was associated with expression of cell type–specific genes and nonlinage gene silencing was associated with increased methylation of cis-regulatory elements. It was, however, to our surprise that genetic deletion of de novo methyltransferases Dnmt3a or Dnmt3b, which are supposed to be responsible for regulatory region methylation, did not result in observable phenotype development. It is possible that no further de novo methylation is needed for kidney development at this stage; however, this is not consistent with our methylome data. Further studies are needed to determine base resolution methylation and gene expression levels in Dnmt3a/3b animals.
Once differentiation completed, such as in terminally differentiated (nondividing) podocytes, deletion of Dnmt1, Dnmt3a, Dnmt3b, and Tet2 did not lead to phenotypic alterations at baseline and even after injury. These results indicate that either methylation changes play only a minor role in podocyte diseases or only minimal remodeling of the podocyte methylome occurs in disease states.
Dnmt1 plays a critical and nonredundant role in the differentiation of the Six2-positive progenitor population by maintaining methylation. Dnmt1 loss was associated with marked decrease in global methylation levels and resulted in very severe kidney developmental defect with barely any fully differentiated nephrons. On the other hand, in contrast to the developing mice, more than 50% of the observed DMRs in the Dnmt1 knockout mice were on regions annotated as repressed region or heterochromatin, in addition to promoters and enhancers. The broad loss of methylation leads to expression of early embryonic genes and nonrenal linage genes (brain, testes, and early embryonic genes). This effect was marked and progressive in the progeny of the Six2-positive cells and resulted in a broad and severe differentiation defect that was not specific for a developmental stage, whereas the Six2-positive cell number was not seemed to be altered. This is consistent with Dnmt1’s function, hemimethylating the newly synthesized DNA strand during cell division. While our work was under consideration, a study by Wanner et al.40 was published also reporting on the critical role of Dnmt1 in progenitor cell renewal and differentiation of the kidney. This paper reported statistically significant differences in Six2-positive cell number at embryonic day 19.5 (P=0.03, one-tailed t test) by immunostaining studies. Using gene expression analysis and sensitive FACS-based cell counting, we could not confirm a defect in the Six2-positive cell number.
Our data suggest that the most profound effect on phenotype development in the Six2CreDnmt1f/f mice was caused by lowering the methylation of TEs, regions that are fully methylated in the genome. Methylation is essential for TE silencing, and the lower methylation was associated in an increase in transcriptional activity of these regions, such as ERV expression.11,41,42 Although ERV activation is important in pluripotent stem cells,43 it seems that Six2-positive progenitor cells are unable to tolerate cellular dsRNA. The presence of cellular dsRNA induces expression of viral sensing pathways, such as an IFN response,44 which was observed in the Six2CreDnmt1f/f mice. In our review of the literature, this is the first study describing ERV activation precipitated by loss of Dnmt1 in tissue progenitor cells. Release of TEs has recently been identified as major mechanism of the antitumor effect of azacytidine in various cancer models.16,17 Ultimately, ERV activation is associated with reduction in cell proliferation and increased cell death, such as DNA damage related-Trp53 activation, in the progeny of Six2-positive cells. These downstream pathways are consistent with previous descriptions in other organ systems.45 Haploinsufficiency of p53 has partially protected from phenotype development in the Dnmt1-depleted pancreas.8
In summary, to understand the role of 5mC in kidney development, we performed genome-wide methylation and gene expression analysis coupled with comprehensive genetic deletion of methylome editing enzymes. We observed dynamic methylation changes on promoters and enhancers in kidney development and show that DNMT1-mediated DNA methylation is essential for kidney development by silencing embryonic, nonlineage specific genes and TEs.
Disclosures
The Susztak laboratory receives research support from Biogen, Boehringer Ingelheim, GSK, Merck, Regeneron, Gilead, and ONO Pharma for work not related to this manuscript.
Supplementary Material
Acknowledgments
S.-Y.L., K.C., and Y.G. created transgenic mouse models, S.-Y.L. performed animal experiments. J.P. analyzed the RRBS and RNA sequencing data. R.S. and Y.G. performed FACS analysis and immunostaining. M.B.P. performed histopathological analysis. S.-Y.L. and K.S. conceptualized the work and wrote the manuscript.
S.-Y.L. received research support from Taipei Veterans General Hospital–National Yang-Ming University Excellent Physician Scientists Cultivation Program (grant 104-V-A-003) and Ministry of Science and Technology (grants 107-2314-B-075-037-MY3 and 105-2314-B-075 -050 -MY3). J.P. is supported by American Diabetes Association training grant 1-17-PDF-036. Work in the Susztak laboratory is supported by the National Institutes of Health grants R01 DK076077, DK087635, and DP3 DK108220.
The work was presented at the annual meeting of the American Society of Nephrology on November 4, 2017 in New Orleans, LA and October 26, 2018 in San Diego, CA and deposited in BioRx on June 29, 2018 (doi: https://doi.org/10.1101/359448).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018070687/-/DCSupplemental.
Supplemental Figure 1. Transcription factor motif enrichment analysis of DMRs.
Supplemental Figure 2. Gross morphology of Dnmt1f/f and Six2Cre Dnmt1f/f mice.
Supplemental Figure 3. Immunofluorescence staining of CCND1 in Dnmt1f/f and Six2Cre Dnmt1f/f kidneys.
Supplemental Figure 4. Expression of proximal tubule markers in Dnmt1f/f and Six2Cre Dnmt1f/f kidneys.
Supplemental Figure 5. Immunofluorescence staining of SIX2 in Dnmt1f/f and Six2Cre Dnmt1f/f kidneys.
Supplemental Figure 6. Heatmap of representative target genes showing gene expression differences.
Supplemental Figure 7. Gene expression patterns in Dnmt1f/f and Six2Cre Dnmt1f/f mice.
Supplemental Figure 8. Transcription factor motif enrichment analysis of DMRs.
Supplemental Figure 9. Representative DMRs and their target gene expression changes.
Supplemental Figure 10. Functional annotation analysis (DAVID).
Supplemental Figure 11. KEGG pathways for RIG-I–like receptor signaling pathway and P53 signaling pathway.
Supplemental Table 1. Primer sequences used in this study.
Supplemental Table 2. List of DEGs between P0 kidneys of Dnmt1 knockout mice and controls.
Supplemental Table 3. List of differentially expressed transposable elements between P0 kidneys of Dnmt1 knockout mice and controls.
References
- 1.Weber M, Hellmann I, Stadler MB, Ramos L, Pääbo S, Rebhan M, et al.: Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat Genet 39: 457–466, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Okano M, Bell DW, Haber DA, Li E: DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 99: 247–257, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Jeltsch A: Molecular enzymology of mammalian DNA methyltransferases. Curr Top Microbiol Immunol 301: 203–225, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Li E, Bestor TH, Jaenisch R: Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell 69: 915–926, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Palacios D, Summerbell D, Rigby PW, Boyes J: Interplay between DNA methylation and transcription factor availability: Implications for developmental activation of the mouse Myogenin gene. Mol Cell Biol 30: 3805–3815, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott EN, Sheaffer KL, Schug J, Stappenbeck TS, Kaestner KH: Dnmt1 is essential to maintain progenitors in the perinatal intestinal epithelium. Development 142: 2163–2172, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sen GL, Reuter JA, Webster DE, Zhu L, Khavari PA: DNMT1 maintains progenitor function in self-renewing somatic tissue. Nature 463: 563–567, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Georgia S, Kanji M, Bhushan A: DNMT1 represses p53 to maintain progenitor cell survival during pancreatic organogenesis. Genes Dev 27: 372–377, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao J, Karnik R, Gu H, Ziller MJ, Clement K, Tsankov AM, et al.: Targeted disruption of DNMT1, DNMT3A and DNMT3B in human embryonic stem cells. Nat Genet 47: 469–478, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheaffer KL, Kim R, Aoki R, Elliott EN, Schug J, Burger L, et al.: DNA methylation is required for the control of stem cell differentiation in the small intestine. Genes Dev 28: 652–664, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karimi MM, Goyal P, Maksakova IA, Bilenky M, Leung D, Tang JX, et al.: DNA methylation and SETDB1/H3K9me3 regulate predominantly distinct sets of genes, retroelements, and chimeric transcripts in mESCs. Cell Stem Cell 8: 676–687, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sproul D, Nestor C, Culley J, Dickson JH, Dixon JM, Harrison DJ, et al.: Transcriptionally repressed genes become aberrantly methylated and distinguish tumors of different lineages in breast cancer. Proc Natl Acad Sci U S A 108: 4364–4369, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gifford WD, Pfaff SL, Macfarlan TS: Transposable elements as genetic regulatory substrates in early development. Trends Cell Biol 23: 218–226, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, et al.: International Human Genome Sequencing Consortium : Initial sequencing and analysis of the human genome. Nature 409: 860–921, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Singer MF: SINEs and LINEs: Highly repeated short and long interspersed sequences in mammalian genomes. Cell 28: 433–434, 1982 [DOI] [PubMed] [Google Scholar]
- 16.Chiappinelli KB, Strissel PL, Desrichard A, Li H, Henke C, Akman B, et al.: Inhibiting DNA methylation causes an interferon response in cancer via dsRNA including endogenous retroviruses. Cell 169: 361, 2017 [DOI] [PubMed] [Google Scholar]
- 17.Roulois D, Loo Yau H, Singhania R, Wang Y, Danesh A, Shen SY, et al.: DNA-demethylating agents target colorectal cancer cells by inducing viral Mimicry by endogenous transcripts. Cell 162: 961–973, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, et al.: Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3: 169–181, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu AY, Tin A, Schlosser P, Ko YA, Qiu C, Yao C, et al.: Epigenome-wide association studies identify DNA methylation associated with kidney function. Nat Commun 8: 1286, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Susztak K: Understanding the epigenetic syntax for the genetic alphabet in the kidney. J Am Soc Nephrol 25: 10–17, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beckerman P, Ko YA, Susztak K: Epigenetics: A new way to look at kidney diseases. Nephrol Dial Transplant 29: 1821–1827, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ko YA, Mohtat D, Suzuki M, Park AS, Izquierdo MC, Han SY, et al.: Cytosine methylation changes in enhancer regions of core pro-fibrotic genes characterize kidney fibrosis development. Genome Biol 14: R108, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaneda M, Okano M, Hata K, Sado T, Tsujimoto N, Li E, et al.: Essential role for de novo DNA methyltransferase Dnmt3a in paternal and maternal imprinting. Nature 429: 900–903, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Lin H, Yamada Y, Nguyen S, Linhart H, Jackson-Grusby L, Meissner A, et al.: Suppression of intestinal neoplasia by deletion of Dnmt3b. Mol Cell Biol 26: 2976–2983, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sweetwyne MT, Gruenwald A, Niranjan T, Nishinakamura R, Strobl LJ, Susztak K: Notch1 and Notch2 in podocytes play differential roles during diabetic nephropathy development. Diabetes 64: 4099–4111, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McFarlane L, Truong V, Palmer JS, Wilhelm D: Novel PCR assay for determining the genetic sex of mice. Sex Dev 7: 207–211, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Bogu GK, Vizán P, Stanton LW, Beato M, Di Croce L, Marti-Renom MA: Chromatin and RNA maps reveal regulatory long noncoding RNAs in mouse. Mol Cell Biol 36: 809–819, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ernst J, Kellis M: ChromHMM: Automating chromatin-state discovery and characterization. Nat Methods 9: 215–216, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luyckx VA, Bertram JF, Brenner BM, Fall C, Hoy WE, Ozanne SE, et al.: Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet 382: 273–283, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Little M, Georgas K, Pennisi D, Wilkinson L: Kidney development: Two tales of tubulogenesis. Curr Top Dev Biol 90: 193–229, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Nakai-Futatsugi Y, Niwa H: Zscan4 is activated after telomere shortening in mouse embryonic stem cells. Stem Cell Reports 6: 483–495, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young JM, Whiddon JL, Yao Z, Kasinathan B, Snider L, Geng LN, et al.: DUX4 binding to retroelements creates promoters that are active in FSHD muscle and testis. PLoS Genet 9: e1003947, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geng LN, Yao Z, Snider L, Fong AP, Cech JN, Young JM, et al.: DUX4 activates germline genes, retroelements, and immune mediators: Implications for facioscapulohumeral dystrophy. Dev Cell 22: 38–51, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsui S, Dai T, Roettger S, Schempp W, Salido EC, Yen PH: Identification of two novel proteins that interact with germ-cell-specific RNA-binding proteins DAZ and DAZL1. Genomics 65: 266–273, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Chen HH, Welling M, Bloch DB, Muñoz J, Mientjes E, Chen X, et al.: DAZL limits pluripotency, differentiation, and apoptosis in developing primordial germ cells. Stem Cell Reports 3: 892–904, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin YH, Ren Y, Suzuki H, Golnoski KJ, Ahn HW, Mico V, et al.: Transcription factors SOHLH1 and SOHLH2 coordinate oocyte differentiation without affecting meiosis I. J Clin Invest 127: 2106–2117, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ballow DJ, Xin Y, Choi Y, Pangas SA, Rajkovic A: Sohlh2 is a germ cell-specific bHLH transcription factor. Gene Expr Patterns 6: 1014–1018, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Crichton JH, Playfoot CJ, MacLennan M, Read D, Cooke HJ, Adams IR: Tex19.1 promotes Spo11-dependent meiotic recombination in mouse spermatocytes. PLoS Genet 13: e1006904, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bergsagel PL, Timblin CR, Eckhardt L, Laskov R, Kuehl WM: Sequence and expression of a murine cDNA encoding PC326, a novel gene expressed in plasmacytomas but not normal plasma cells. Oncogene 7: 2059–2064, 1992 [PubMed] [Google Scholar]
- 40.Wanner N, Vornweg J, Combes A, Wilson S, Plappert J, Rafflenbeul G, et al.: DNA methyltransferase 1 controls nephron progenitor cell renewal and differentiation. J Am Soc Nephrol 30: 63–78, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hackett JA, Reddington JP, Nestor CE, Dunican DS, Branco MR, Reichmann J, et al.: Promoter DNA methylation couples genome-defence mechanisms to epigenetic reprogramming in the mouse germline. Development 139: 3623–3632, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walter M, Teissandier A, Pérez-Palacios R, Bourc’his D: An epigenetic switch ensures transposon repression upon dynamic loss of DNA methylation in embryonic stem cells. ELife 5, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robbez-Masson L, Rowe HM: Retrotransposons shape species-specific embryonic stem cell gene expression. Retrovirology 12: 45, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chuong EB, Elde NC, Feschotte C: Regulatory evolution of innate immunity through co-option of endogenous retroviruses. Science 351: 1083–1087, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jackson-Grusby L, Beard C, Possemato R, Tudor M, Fambrough D, Csankovszki G, et al.: Loss of genomic methylation causes p53-dependent apoptosis and epigenetic deregulation. Nat Genet 27: 31–39, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.