Significance Statement
Differential diagnosis of transplant glomerulopathy, a common lesion observed after kidney transplant that is associated with poor prognosis, remains challenging because its morphologic pattern (double contour of the glomerular basement membrane) is found in several disease processes. The authors used archetype analysis, a probabilistic data-driven unsupervised statistical approach, to identify distinct groups of patients (archetypes) with this condition. By applying this approach to a large, comprehensively phenotyped multicenter cohort from patients diagnosed with transplant glomerulopathy on the basis of post-transplant biopsies, the authors identified five archetypes with distinct clinical, histologic, and immunologic features, as well as different outcomes (kidney allograft survival rates). The findings suggest that an archetype-based characterization of this condition may improve risk stratification for individual patients undergoing kidney transplant and those included in clinical trials.
Keywords: transplant glomerulopathy, Archetype, antibody-mediated rejection, transplant outcomes, kidney transplantation, donor-specific anti-HLA antibody
Visual Abstract
Abstract
Background
Transplant glomerulopathy, a common glomerular lesion observed after kidney transplant that is associated with poor prognosis, is not a specific entity but rather the end stage of overlapping disease pathways. Its heterogeneity has not been precisely characterized to date.
Methods
Our study included consecutive kidney transplant recipients from three centers in France and one in Canada who presented with a diagnosis of transplant glomerulopathy (Banff cg score ≥1 by light microscopy), on the basis of biopsies performed from January of 2004 through December of 2014. We used an unsupervised archetype analysis of comprehensive pathology findings and clinical, immunologic, and outcome data to identify distinct groups of patients.
Results
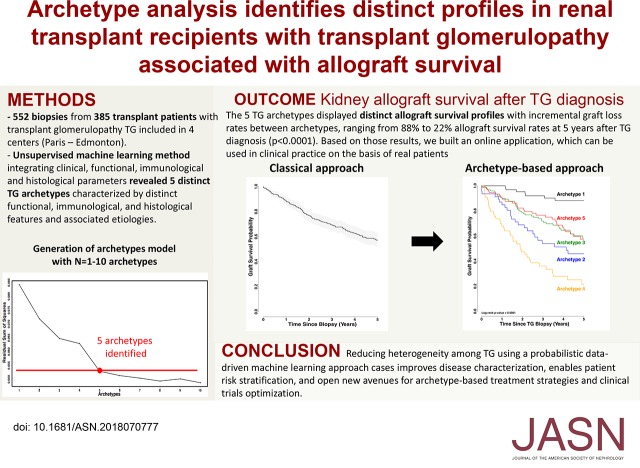
Among the 8207 post-transplant allograft biopsies performed during the inclusion period, we identified 552 biopsy samples (from 385 patients) with transplant glomerulopathy (incidence of 6.7%). The median time from transplant to transplant glomerulopathy diagnosis was 33.18 months. Kidney allograft survival rates at 3, 5, 7, and 10 years after diagnosis were 69.4%, 57.1%, 43.3%, and 25.5%, respectively. An unsupervised learning method integrating clinical, functional, immunologic, and histologic parameters revealed five transplant glomerulopathy archetypes characterized by distinct functional, immunologic, and histologic features and associated causes and distinct allograft survival profiles. These archetypes showed significant differences in allograft outcomes, with allograft survival rates 5 years after diagnosis ranging from 88% to 22%. Based on those results, we built an online application, which can be used in clinical practice on the basis of real patients.
Conclusions
A probabilistic data-driven archetype analysis approach applied in a large, well defined multicenter cohort refines the diagnostic and prognostic features associated with cases of transplant glomerulopathy. Reducing heterogeneity among such cases can improve disease characterization, enable patient-specific risk stratification, and open new avenues for archetype-based treatment strategies and clinical trials optimization.
Double contour of the glomerular basement membrane is a pattern of glomerular injury found in the repair/remodeling phase after several disease processes, including immune complex– or complement-mediated membranoproliferative glomerulonephritis (MPGN) and thrombotic microangiopathy (TMA).1 In the transplant setting, transplant glomerulopathy (TG) represents a glomerular lesion common in long-standing kidney allografts, first described by Porter et al.2 in 1967 and characterized by duplication of the glomerular basement membrane in the absence of immune complex deposits.3–5 Patients with TG frequently present with nephrotic range proteinuria, hypertension, or kidney graft function deterioration; TG is also associated with poor kidney graft outcome.6,7 In recent years, the strong association between TG and the presence of circulating anti-HLA donor-specific antibodies (anti-HLA DSAs) has made TG a key pathology lesion of antibody-mediated rejection (ABMR).6–8 However, the epidemiology and heterogeneity of the disease processes associated with TG, including risk stratification, remain largely unknown. These represent major limitations for improving clinical management and therapeutic options for patients presenting with TG and potentially contribute to the failure of clinical trials aiming in an unselective fashion at ameliorating TG progression to expand survival of allografts with TG.
From an epidemiologic perspective, the prevalence of TG has been mainly assessed in small series of patients8 to range from 5% to 20% at 5 years after kidney transplantation in centers performing protocol biopsies. Disease processes that are known to cause glomerular double contours (i.e., TG) include chronic ABMR, immune complex– (hepatitis C, lupus) or complement-mediated MPGN, TMA, and T cell–mediated rejection.4,8–11 Because the assessment of TG relies on a semiquantitative score (Banff cg score) identified by light and/or electron microscopy (EM), it oversimplifies a rather complex phenotype. Only one retrospective study including 25 patients has studied in detail the associated diagnostic entities of TG including ABMR, HCV infection, and TMA,8 suggesting overlapping pathways of TG and underlying disease heterogenicity. Currently, the differential diagnoses of TG are primarily supported by performing immunofluorescence for IgG, IgA, IgM, C1q, C3, κ, and λ; therefore, potential overlap between diagnoses may be overlooked.4,11–14 Most studies have consistently shown a strong association of TG with poor allograft outcomes; however, no previous report has addressed, in a large and appropriately annotated and followed multicenter population, potential distinct survival profiles of patients with TG with different underlying pathogeneses.8,15–17
In this study, we applied a probabilistic data-driven unsupervised approach to a comprehensively phenotyped multicenter cohort. We hypothesized that by deconstructing data matrixes that include pathologic data and serologic and clinical information correlated with meaningful clinical and outcome features, new insights in TG can be gained.
Methods
Participants
Among 8207 biopsy specimens collected between January of 2004 and January of 2014 from three French centers (Necker Hospital, Saint-Louis Hospital, and Foch Hospital) and one North American center (University of Alberta Hospital, Edmonton, Canada), we identified 552 biopsy specimens showing TG (Banff cg score ≥1 by light microscopy), corresponding to a total of 385 patients. In patients with multiple biopsy samples, only the first biopsy sample showing TG was included in the survival analyses. All transplants were ABO compatible. This study was approved by local institutional review boards with patients providing informed consent where indicated. Clinical data on donors and recipients for the French cohorts were obtained from the Paris Transplant Group database. Anonymized data were prospectively entered into the registry at specific time points for each patient (on day 0 and 6 months and 1 year after transplantation) and updated annually thereafter (see the Methods section in the Supplemental Appendix for the clinical and biologic parameters assessed). Clinical data for the University of Alberta Hospital were obtained retrospectively from the medical records.
Kidney Allograft Phenotypes
All biopsy specimens were scored according to the Banff criteria for histologic lesions: glomerulitis (g), tubulitis (t), interstitial inflammation (i), endarteritis (v), peritubular capillary inflammation (ptc), TG (cg), interstitial fibrosis (ci), tubular atrophy (ct), arterial fibrous intimal thickening (cv), and arteriolar hyaline thickening (ah).18–20 Microvascular inflammation was defined as the sum of the g and ptc scores. C4d staining was performed by immunohistochemistry on paraffin sections using the human C4d polyclonal antibody for the French biopsy specimens and by immunofluorescence on frozen sections for the Edmonton cases. Immunofluorescence including IgA, IgG, IgM, C3, and C1q was performed in biopsy specimens with suspicion for recurrent disease, “unknown” cause of original disease, history of autoimmune disease, history of hepatitis C infection, TMA (biologic or histologic glomerular or arteriolar), glomerular disease (i.e., MPGN, lupus nephropathy, membranous nephropathy), and/or isolated TG with a microvascular inflammation score ≤1. EM was performed in all biopsy samples from Edmonton and for Paris biopsy samples if light microscopy and immunofluorescence were not sufficient to classify a TG case. In such cases, EM was performed from paraffin-embedded material. In biopsy samples with available EM, sequential peritubular capillaries were examined, and the number of circumferential layers in multiple capillaries was averaged for each case. Peritubular basement membrane multilayering (PTCML) was graded as absent (one layer), mild (2–4 layers), moderate (5–6 layers), or severe (>6 layers). To take into account the low reproducibility and intrarater variability in Banff scoring, experienced pathologists were involved (J.-P.D.V.H., M. Rabant, S.H., M.M., B.S.). Five pathologists reviewed the biopsy samples with granular scoring and diagnoses analyzed together and individually. EM was also performed in the case of isolated TG in order to avoid misclassification in the case of ABMR, MPGN, or immunologic lesions that would have been missed by histology.
Detection and Characterization of Circulating Donor-Specific Anti-HLA Antibodies
The presence of circulating donor-specific anti–HLA-A, -B, -Cw, -DR, -DQ, and -DP antibodies was analyzed using single-antigen bead assays (One Lambda, Inc., Canoga Park, CA) on a Luminex platform on serum samples collected at the time of transplantation and at the time of biopsy. For each patient, we recorded the number, class, specificities, and mean fluorescence intensity (MFI) of all donor-specific HLA antibodies. The maximum MFI for the DSA (DSA MFImax) was defined as the highest ranked donor-specific bead. HLA typing of donors and recipients was performed using DNA typing. Donor HLA typing for HLA-Cw and HLA-DP was only performed if the recipients had circulating anti–HLA-Cw and/or anti–HLA-DP antibodies.
Exploration of Complement Dysregulation
Exploration of complement dysregulation was performed in patients with a TMA and/or an MPGN pattern compatible with C3 glomerulopathy. This exploration included serum C3 and C4. Plasma C5b-9 and C3NeF were measured in serum and plasma from the patients. Exon sequencing of CFH, CD46, CFI, CFB, C3, and THBD was performed by direct sequencing analysis as previously described.21
Statistical Analyses
Continuous variables were described using means and SDs or medians and interquartile ranges. We compared means and proportions between groups using t test, ANOVA (Mann–Whitney test for MFI), or chi-squared test (or Fisher exact test if appropriate). Venn diagrams of the histologic diagnosis were created using the R package Vennerable. The kidney survival analysis was performed from the time of transplantation until a maximum follow-up of 10 years with kidney graft loss as the event of interest, defined as the patient’s return to dialysis. For the 36 (9.4%) patients who died with a functioning graft, graft survival was censored at the time of death.22 Kidney allograft survival was plotted using Kaplan–Meier curves and compared using the log-rank test.
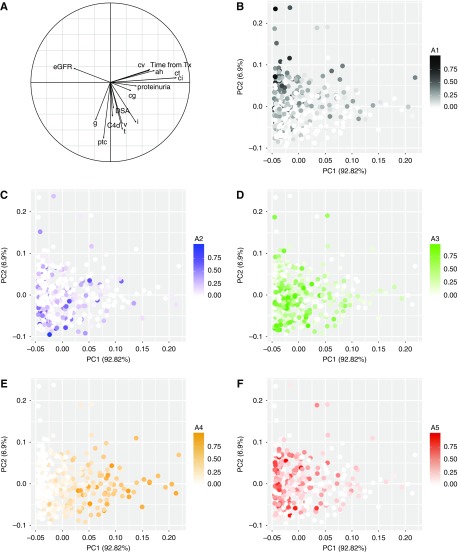
For the archetypal analysis, we first developed models with different numbers of archetypes and chose which to use as the final model according to the residual sum of squares using the “elbow” method.24 The final five-archetype model assigns five scores to each patient using the time from transplant to the biopsy and the functional (eGFR, proteinuria), immunologic (presence of a DSA at the time of TG biopsy), and histologic Banff scores, one for each archetype, with the scores summing to 1.0. Each parameter was also assigned to a single archetype cluster on the basis of its highest archetype score. The individual scores provide more detail than the cluster assignments regarding each functional, immunologic, and histologic parameter. However, the cluster assignments are convenient for summarizing results and were therefore used in the presentation of some of our results. We used a principal component analysis to visualize the data matrix used as the input for the archetypal analysis.
We used STATA (version 14, Data Analysis and Statistical Software) and R (version 3.2.1, R Foundation for Statistical Computing) for the descriptive and survival analyses. Archetypes were assigned using the “archetypes” package in R. All statistical tests were two-sided, and probability values <0.05 were considered to be significant.
Results
Baseline Characteristics of the Kidney Transplant Recipients
The baseline and immunologic characteristics of the 385 patients with TG included, overall and according to participating center, are presented in Table 1 and Supplemental Table 1, respectively. The most common primary renal disease was GN (n=162, 42.08%), including IgA nephropathy (n=56, 34.57%), MPGN (n=27, 16.67%), and FSGS (n=20, 12.35%). Thirty-five recipients were hepatitis C positive (9.09%) and 27 were hepatitis B positive (7.40%). At the time of transplantation, 64 patients (16.62%) presented with circulating anti-HLA DSA with a median MFI of the immuno-dominant anti-HLA DSA of 2625 (interquartile range [IQR], 868–10,155). The mean donor age was 47±17 years, and 283 were deceased donors (73.51%). The median time from transplantation to TG diagnosis was 33.18 months (IQR, 12.12–78.72 months; Supplemental Figure 1). At the time of TG diagnosis, the mean eGFR was 38.39±18.87 ml/min per 1.73 m2, the mean proteinuria was 1.57±2.14 g/creatinine, and 235 (61.04%) patients presented with circulating anti-HLA DSA, including 179 (76.17%) with de novo DSA and 56 (23.83%) with preexisting/recurrent DSA.
Table 1.
Recipient-, donor-, and transplant-associated characteristics of patients with a diagnosis of TG
| Characteristic | Patients with TG (n=385) | |
|---|---|---|
| n | ||
| Recipient characteristics | ||
| Age (yr), mean (SD) | 385 | 43.95 (15.69) |
| Male, n (%) | 385 | 226 (58.70) |
| ESRD causes, n (%) | 385 | |
| GN | 162 (42.08) | |
| Diabetes | 38 (9.87) | |
| Vascular | 20 (5.19) | |
| Tubulo-interstitial | 84 (21.82) | |
| Other | 9 (2.34) | |
| Unknown | 72 (18.70) | |
| GN ESRD causes, n (%) | 162 | |
| MPGN | 27 (16.67) | |
| TMA | 12 (7.41) | |
| IgA nephropathy | 56 (34.57) | |
| Lupus | 13 (8.02) | |
| Membranous nephropathy | 7 (4.32) | |
| Alport | 8 (4.94) | |
| Amyloidosis | 1 (0.62) | |
| FSGS | 20 (12.35) | |
| Crescent glomerulopathy | 9 (5.56) | |
| Unspecified | 9 (5.56) | |
| Positive serology at the time of transplant, n (%) | ||
| HCV | 385 | 35 (9.09) |
| HBV | 365 | 27 (7.40) |
| HIV | 385 | 2 (0.52) |
| CMV | 381 | 238 (62.47) |
| Donor characteristics | ||
| Age (yr), mean (SD) | 368 | 47.40 (16.97) |
| Male, n (%) | 376 | 189 (50.27) |
| Creatinine (μmol/L), mean (SD) | 357 | 81.04 (40.87) |
| Deceased donor, n (%) | 385 | 283 (73.51) |
| Double transplantation, n (%) | 385 | 19 (4.94) |
| Positive serology, n (%) | ||
| Hepatitis C virus | 353 | 4 (1.13) |
| Hepatitis B virus | 355 | 10 (2.82) |
| HIV | 385 | 0 |
| CMV | 379 | 210 (55.41) |
| Transplant baseline characteristics | ||
| Prior kidney transplant, n (%) | 385 | 78 (20.26) |
| Cold ischemia time (h), mean (SD) | 381 | 15.54 (10.16) |
| Delayed graft function, n (%)a | 385 | 107 (27.79) |
| HLA A/B/DR mismatch, mean (SD), number | 385 | 3.47 (1.46) |
| Donor-specific anti-HLA antibodies on d 0, n (%) | 385 | 64 (16.62) |
| Circulating anti-HLA DSA MFI, median (IQR) | 2625 (868–10,155) | |
HCV, hepatitis C virus; HBV, hepatitis B virus; CMV, Cytomegalovirus; HLA, human leukocyte antigen; DSA, donor-specific antibodies.
Delayed graft function was defined as the use of dialysis in the first postoperative wk.
Diagnoses Overlap in Biopsy Samples Presenting cg Lesions
Among the 552 biopsy samples included, 488 (88.41%) were from for-cause biopsies whereas 64 (11.59%) were from protocol biopsies, 21 (32.81%) of which were performed at 3 months and 43 (67.19%) of which were performed at 1 year post-transplantation. Supplemental Table 2 depicts the Banff lesion scores for all 552 TG biopsy samples (Supplemental Table 3 depicts the Banff lesion scores at the time of the first biopsy with TG diagnosis). The Banff cg score distribution was cg=1 in 237 biopsies (42.9%), cg=2 in 160 biopsies (29.0%), and cg=3 in 155 biopsies (28.1%). The mean g, ptc, and microcirculation inflammation (g+ptc) scores were 1.36±1.06, 1.44±1.04, and 2.94±1.75, respectively. A total of 167 biopsy samples (30.25%) showed positive c4d staining. The mean i and t Banff scores were 0.63±0.90 and 0.48±0.82, respectively. The mean tubular atrophy and interstitial fibrosis score was 1.59±0.97. The mean cv, ah, and v scores were 1.54±0.96, 1.67±1.06, and 0.12±0.43, respectively.
Three predominant, overlapping causes accounted for 466 (84.4%) cases. A total of 417 biopsy samples (75.5%) showed chronic active ABMR, 90 biopsy samples (16.3%) showed TMA lesions, 65 (11.8%) showed an MPGN pathology pattern, and 86 (15.6%) remained equivocal with no specific causes identified. Among the biopsy specimens without specific causes identified, EM revealed no immune complex deposit, which confirmed the absence of GN. In those biopsy specimens, the mean PTCML score was 3.83±1.98. Twelve percent did not show PTCML, whereas 47%, 34%, and 7% presented with mild, moderate, and severe PTCML, respectively. The final associated diagnoses reached by pathologists regarding MPGN or TMA patterns are presented in Supplemental Table 4 and Supplemental Table 5 for the first biopsy with TG.
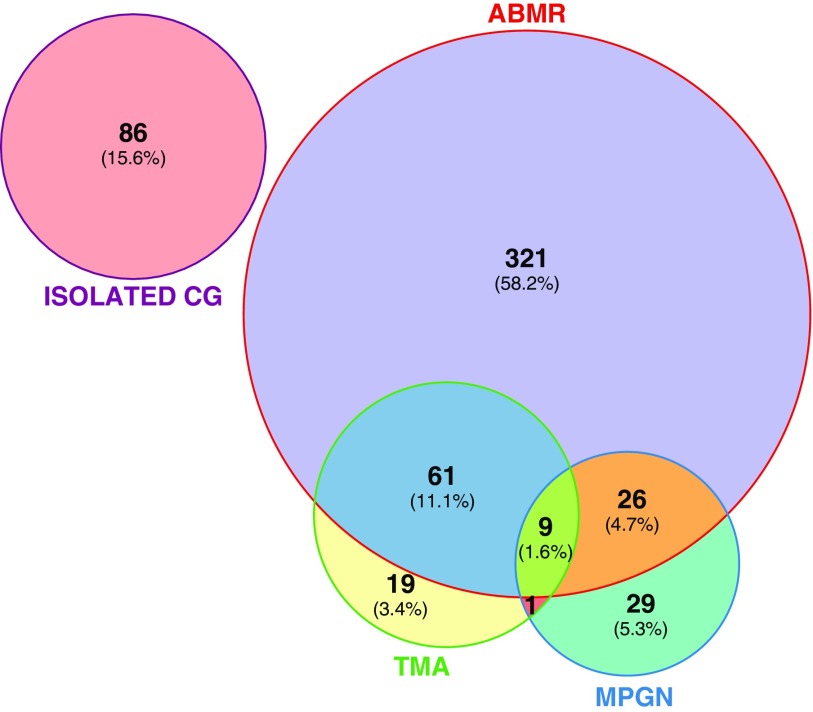
We identified 97 (17.6%) biopsy samples that showed overlapping causes (Figure 1). A total of 61 (11.1%) biopsy samples showed cg lesions associated with ABMR and TMA (fibrin thrombi, endotheliosis, and mesangiolysis in glomeruli); 26 (4.7%) biopsy samples showed ABMR and MPGN; one biopsy sample showed TMA and MPGN; and nine (1.6%) biopsy samples showed ABMR, TMA, and MPGN features (Figure 1).
Figure 1.
Overlapping causes in biopsy samples presenting cg lesions. Data are on the basis of 552 kidney allograft biopsy specimens with TG. Each circle corresponds to one cause. Circle sizes are correlated to the number of biopsy specimens in each TG-related cause category. cg, Banff cg lesion.
Identifying TG Archetypes
On the basis of the overlapping diagnoses and heterogeneity in cg lesions, we used an unsupervised archetype analysis to identify distinct groups of patients with cg lesions. After integrating clinical, histologic, and immunologic features at the time of TG diagnosis, five distinct archetypes were identified (Figure 2).
Figure 2.
Generation of TG archetypes using clinical, histologic, and immunologic data. (A) We generated ten archetype models using clinical, histologic, and immunologic data in the dataset. (B) The residual sum of squares decreases with increasing numbers of archetypes. We selected five archetypes as the final archetype model. All patients are assigned a score for each of the five archetypes, and cluster assignments are made on the highest score within that patient. (C) The table shows what typical data look like with real patients.
Figure 3 presents the projection of patients in a principal component analysis and the corresponding correlation circle according to the five archetypes identified. Baseline recipient, donor, and transplant characteristics according to archetypes are presented in Table 2. Clinical, immunologic, and Banff lesion scores according to archetypes are presented in Figure 4, A and B.
Figure 3.
TG archetypes are associated with distinct phenotypes. (A) The correlation circle. (B–F) Each figure corresponds to one archetype and its PCA. The final five-archetype model assigns five scores to each patient using the time from transplant to the biopsy, and the functional (eGFR, proteinuria), immunologic (presence of a DSA at the time of TG biopsy), and histologic Banff scores, one for each archetype, with the scores summing to 1.0. Each dot corresponds to a patient, and its intensity corresponds to the score for a given archetype (score from 0 to 1). (B) Corresponds to the PCA of archetype 1. (C) Corresponds to the PCA of archetype 2. (D) Corresponds to the PCA of archetype 3. (E) Corresponds to the PCA of archetype 4. (F) Corresponds to the PCA of archetype 5. A, archetype; cpt, peritubular capilary inflammation; PCA, principal component analysis; PC1, Principal component 1; PC2, Principal component 2; Tx, transplantation.
Table 2.
Baseline characteristics of patients with a diagnosis of TG according to their archetype
| Characteristic | Archetype 1 n=68 | Archetype 2 n=47 | Archetype 3 n=122 | Archetype 4 n=56 | Archetype 5 n=92 | P Value |
|---|---|---|---|---|---|---|
| Recipient characteristics | ||||||
| Age (yr), mean (SD) | 43.45 (15.25) | 38.44 (15.67) | 46.45 (14.99) | 37.46 (16.19) | 47.76 (14.88) | <0.001 |
| Male, n (%) | 37 (54.41) | 33 (70.21) | 68 (55.74) | 35 (62.50) | 53 (57.61) | 0.42 |
| ESRD causes, n (%) | ||||||
| GN | 30 (44.12) | 19 (40.43) | 41 (33.61) | 30 (53.57) | 42 (45.65) | 0.12 |
| Diabetes | 8 (11.76) | 6 (12.77) | 16 (13.11) | 4 (7.14) | 4 (4.35) | 0.18 |
| Vascular | 4 (5.88) | 3 (6.38) | 5 (4.10) | 2 (3.57) | 6 (6.52) | 0.89 |
| Tubulo-interstitial | 11 (16.18) | 8 (17.02) | 31 (25.41) | 10 (17.86) | 24 (26.09) | 0.39 |
| Other | 3 (4.41) | 0 | 2 (1.64) | 3 (5.36) | 1 (1.09) | 0.23 |
| Unknown | 12 (17.65) | 11 (23.40) | 27 (22.13) | 7 (12.50) | 15 (16.30) | 0.49 |
| GN ESRD causes, n (%) | ||||||
| MPGN | 6 (20.00) | 6 (31.58) | 7 (17.07) | 4 (13.33) | 4 (9.52) | 0.39 |
| TMA | 4 (13.33) | 0 | 3 (7.32) | 2 (6.67) | 3 (7.14) | 0.52 |
| IgA nephropathy | 12 (40.00) | 5 (26.32) | 14 (34.15) | 11 (36.67) | 14 (33.33) | 0.51 |
| Lupus | 2 (6.67) | 2 (10.53) | 3 (7.32) | 2 (6.67) | 4 (9.52) | 0.91 |
| Membranous nephropathy | 0 | 0 | 3 (7.32) | 0 | 4 (9.52) | 0.22 |
| Alport | 1 (3.33) | 2 (10.53) | 1 (2.44) | 1 (3.33) | 3 (7.14) | 0.52 |
| Amyloidosis | 0 | 0 | 0 | 0 | 1 (2.38) | 0.68 |
| FSGS | 3 (10.00) | 3 (15.79) | 6 (14.63) | 3 (10.00) | 5 (11.90) | 0.99 |
| Crescent glomerulopathy | 2 (6.67) | 1 (5.26) | 1 (2.44) | 3 (10.00) | 2 (4.76) | 0.39 |
| Unspecified | 0 | 0 | 3 (7.32) | 4 (13.33) | 2 (4.76) | 0.11 |
| Positive serology at the time of transplant, n (%) | ||||||
| HCV | 7 (10.29) | 4 (8.51) | 10 (8.20) | 3 (5.36) | 11 (11.96) | 0.74 |
| HBV | 3 (4.48) | 2 (4.35) | 15 (12.71) | 2 (4.76) | 5 (5.43) | 0.20 |
| HIV | 0 | 0 | 0 | 1 (1.79) | 1 (1.09) | 0.38 |
| CMV | 41 (60.29) | 29 (63.04) | 80 (65.57) | 27 (50.94) | 61 (66.30) | 0.38 |
| Donor characteristics | ||||||
| Age (yr), mean (SD) | 43.52 (16.84) | 41.70 (18.40) | 53.08 (16.55) | 40.88 (16.41) | 48.79 (14.59) | <0.001 |
| Male, n (%) | 34 (50.75) | 20 (42.55) | 57 (47.50) | 30 (58.82) | 48 (52.75) | 0.53 |
| Creatinine (μmol/L), mean (SD) | 74.22 (30.50) | 76.11 (30.59) | 85.98 (52.96) | 83.50 (33.83) | 80.84 (36.24) | 0.47 |
| Deceased donor, n (%) | 42 (61.76) | 32 (68.09) | 99 (81.15) | 37 (66.07) | 73 (79.35) | 0.01 |
| Double transplantation, n (%) | 3 (4.41) | 2 (4.26) | 9 (7.38) | 1 (1.79) | 4 (4.35) | 0.66 |
| Positive serology, n (%) | ||||||
| Hepatitis C virus | 1 (1.59) | 1 (2.27) | 1 (0.85) | 0 | 1 (1.11) | 0.89 |
| Hepatitis B virus | 2 (3.12) | 1 (2.27) | 4 (3.39) | 0 | 3 (3.33) | 0.96 |
| HIV | 0 | 0 | 0 | 0 | 0 | >0.99 |
| CMV | 39 (57.35) | 22 (46.81) | 64 (52.46) | 34 (66.67) | 51 (56.04) | 0.34 |
| Transplant baseline characteristics | ||||||
| Prior kidney transplant, n (%) | 10 (14.71) | 5 (10.64) | 29 (23.77) | 6 (10.71) | 28 (30.43) | <0.01 |
| Cold ischemia time (h), mean (SD) | 12.88 (10.16) | 13.53 (8.84) | 16.91 (9.79) | 15.93 (12.04) | 16.49 (9.78) | 0.05 |
| Delayed graft function, n (%)a | 9 (13.24) | 11 (23.40) | 47 (38.52) | 12 (21.43) | 28 (30.43) | 0.003 |
| HLA A/B/DR mismatch, mean (SD), number | 3.50 (1.52) | 3.91 (1.43) | 3.41 (1.52) | 3.29 (1.33) | 3.41 (1.42) | 0.13 |
| Donor specific anti-HLA antibodies on d 0, n (%) | 10 (14.71) | 5 (10.64) | 22 (18.03) | 2 (3.57) | 25 (27.17) | 0.002 |
| Circulating anti-HLA DSA MFI, median (IQR) | 3396 (1129–9441) | 945 (868–1400) | 2479 (889–8736) | 2625 (2625–2625) | 3679 (858–15,106) | 0.52 |
| Treatments, n (%) | ||||||
| Steroids | 9 (14.06) | 18 (39.13) | 19 (16.96) | 6 (10.91) | 22 (25.00) | 0.004 |
| Anti-CD20 | 7 (10.94) | 12 (26.09) | 10 (8.93) | 5 (9.09) | 21 (24.14) | <0.01 |
| Plasma exchange | 6 (9.38) | 10 (22.22) | 12 (10.71) | 0 | 21 (23.86) | <0.001 |
| IVIG | 9 (14.06) | 18 (39.13) | 22 (19.47) | 10 (18.18) | 46 (52.17) | <0.001 |
| Eculizumab | 0 | 0 | 3 (2.68) | 0 | 4 (4.60) | 0.21 |
| Bortezomib | 0 | 0 | 0 | 0 | 4 | 0.02 |
| Centers | ||||||
| Edmonton | 34 (50.00) | 26 (55.32) | 26 (21.31) | 47 (83.93) | 29 (31.52) | |
| Paris | 34 (50.00) | 21 (44.68) | 96 (78.69) | 9 (16.07) | 63 (68.48) | <0.001 |
Fisher exact tests were conducted to compare proportions, and unpaired tests were conducted to compare continuous variables. HCV, hepatitis C virus; HBV, hepatitis B virus; CMV, Cytomegalovirus; HLA, human leukocyte antigen; DSA, donor-specific antibodies; IVIG, intravenous immunoglobulin.
Delayed graft function was defined as the use of dialysis in the first postoperative wk.
Figure 4.
TG archetypes are associated with distinct functional, immunologic, and histologic phenotypes. (A) Functional parameters, anti-HLA DSA at the time of TG, and time of onset TG diagnosis according to archetypes. (B) Histologic parameters at the time of TG diagnosis according to archetypes. A, archetype; DSA, donor-specific antibodies; IFTA, Interstitial fibrosis and tubular atrophy; HLA, human leukocyte antigen.
Archetype 1 (n=68, 17.7%) was characterized by the highest mean eGFR (52.81±24.68 ml/min per 1.73 m2) and low-grade proteinuria (0.97±1.44 g/g). Histologically, archetype 1 presented with the lowest cg Banff score (1.57±0.72), the lowest microcirculation inflammation and tubulointerstitial inflammation Banff scores, and the lowest IFTA Banff score compared with other archetypes. Archetype 1 also included the highest proportion of patients with isolated cg lesions (28 of 68, 41.2%). Isolated cg was defined as the presence of cg without concomitant MPGN, TMA patterns, or microvascular inflammation. At the time of TG diagnosis, circulating anti-HLA DSAs were found in 30 of 68 (44.1%) patients belonging to archetype 1.
Archetype 2 (n=47, 12.2%) was characterized by the highest interstitial inflammation/tubulitis and v lesion scores, with a high microcirculation inflammation and c4d deposition score (21 of 47, 44.7%). Circulating anti-HLA DSAs were present at the time of TG diagnosis in 33 of 47 (70.2%) patients from this archetype.
Archetype 3 (n=122, 31.7%) included the oldest donors, with the highest proportion of delayed graft function (38.5%). Patients from archetype 3 presented with higher allograft arteriosclerosis and IFTA lesions and low levels of interstitial inflammation, tubulitis, and C4d capillary deposition. In total, 62 of 122 (50.8%) patients from archetype 3 presented with circulating anti-HLA DSAs at the time of TG diagnosis. The mean microcirculation inflammation score (g+ptc Banff score) was 2.23±1.74.
Archetype 4 (n=56, 14.5%) was characterized by similar graft function at the time of TG diagnosis to archetypes 2, 3, and 5 (mean eGFR of 32.34±13.93 ml/min per 1.73 m2), but with the highest proteinuria level (mean 3.66±3.43 g/g); the highest mean cg score (2.11±0.73); high IFTA, cv, and arterial hyalinosis lesions; but low microcirculation inflammation and interstitial inflammation/tubulitis. Archetype 4 also showed the longest delay between transplantation and TG diagnosis (Figure 4).
Archetype 5 (n=92, 23.9%) revealed similarities to archetype 2 for several functional and histologic features (e.g., eGFR, time of TG diagnosis post-transplant, and microvascular inflammation at the time of TG diagnosis). Moreover, archetype 5 presented with lower scores of chronic lesions (IFTA), interstitial inflammation, tubulitis, and vasculitis compared with archetype 2. No isolated cg case was observed in archetype 5.
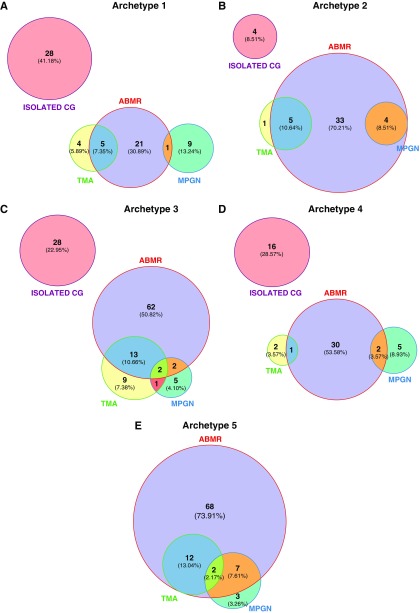
The overlapping diagnoses at the time of TG according to the five archetypes are represented in Figure 5.
Figure 5.
TG archetypes are associated with distinct overlapping causes. Data are on the basis of the 385 patients with TG. Each circle corresponds to one cause. Circle sizes are correlated to the number of biopsy specimens in each TG-related cause category. (A) Archetype 1. (B) Archetype 2. (C) Archetype 3. (D) Archetype 4. (E) Archetype 5.
TG Archetypes and Allograft Survival Patterns
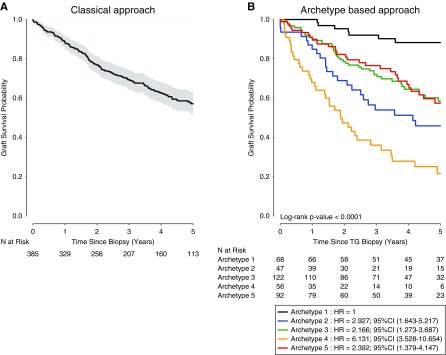
Figure 6A shows the overall death-censored allograft survival after TG diagnosis (classic approach). Kidney allograft survival rates after TG diagnosis were 69.4%, 57.1%, 43.3%, and 25.5% at 3, 5, 7, and 10 years, respectively. Figure 6B shows the archetype-based allograft survival approach, demonstrating different allograft survival profiles. When using archetype 1 as the reference (allograft survival at 3 and 5 years post–TG biopsy: 92% and 88%), a gradually decreasing allograft survival rate was observed at 3 and 5 years post–TG biopsy in patients from archetype 3 (72% and 58%; hazard ratio [HR], 2.17; 95% confidence interval [95% CI], 1.27 to 3.69; P=0.004), archetype 5 (77% and 57%; HR, 2.39; 95% CI, 1.38 to 4.15; P=0.002), archetype 2 (54% and 46%; HR, 2.93; 95% CI, 1.64 to 5.22; P<0.001), and archetype 4 (36% and 22%; HR, 6.13; 95% CI, 3.53 to 10.65; P<0.001). We compared allograft survival according to the archetypes and the treatment of TG lesions (plasmaphereses/IVIg ± steroids). Statistical difference was found for archetype 2, with 27.9% allograft survival at 5 years for the untreated patients versus 64.6% for the treated patients (P value=0.04) (Supplemental Table 6).
Figure 6.
TG archetypes are associated with distinct outcomes. (A) Allograft survival probability (Kaplan–Meier) since the diagnosis of TG. (B) Probability of allograft survival after the TG biopsy, on the basis of the five archetypes. The overall difference between the five archetypes was significant (P value <0.001).
Application Online for the Clinicians
We built an application online that can be used by the clinicians to assess the archetype of an individual patient and the corresponding kidney allograft survival.
To develop this application, we:
Used the Paris Transplant Group reference set with all of the variables included in the archetype analysis;
Extracted the archetype coefficients;
Used the Shiny application package from RStudio (https://shiny.rstudio.com/).
The application allows clinicians to enter the clinical, histologic, and immunologic parameters of a given patient to get:
The corresponding probabilities of belonging to each archetype.
The corresponding long-term allograft survival.
The application is available online: https://dyshinyapps.shinyapps.io/Archetype_Shiny/.
Examples of how this application can be used in clinical practice on the basis of real patients are provided in Supplemental Figure 2.
Sensitivity Analysis
Center Effect and Distribution of TG-Related Diagnoses
We compared the overlapping pathways associated with TG lesions between the French and Canadian centers (Supplemental Figure 3). Among patients with double contour lesions, 268 (80.5%) had a definitive diagnosis of chronic active ABMR in the Paris Transplant Group cohort versus 149 (69.3%) patients from the University of Alberta Hospital, Edmonton. Regarding TMA, 53 (15.9%) and eight (3.7%) had a concomitant diagnosis of chronic active ABMR in the Paris Transplant Group and the Edmonton cohorts, respectively. MPGN was found in 15 (4.5%) patients with a chronic active ABMR and was found as an isolated diagnosis among nine (2.7%) patients with double contours in patients from the Paris Transplant Group cohort. In the Edmonton cohort, 20 (9.1%) patients were found in each subgroup (chronic active ABMR + MPGN and MPGN alone).
Center Effect, Time Frame of TG Occurrence, and Long-Term Allograft Loss Post-Transplantation
When the time to occurrence of TG post-transplant was compared between the French and Canadian recruiting centers, the median time to presentation was earlier among patients from the Paris cohort (median time, 16.82 months; IQR, 7.69–37.98) compared with patients from the Edmonton cohort (median time, 77.24; IQR, 34.96–142.03) (Supplemental Figure 4A). However, there was no significant difference in terms of allograft survival after TG diagnosis between the two centers (P=0.11) (Supplemental Figure 4B).
Discussion
By integrating functional, immunologic, and histologic data in an unsupervised probabilistic archetypal approach, we identified five archetypes of TG revealing distinct clinical, functional, immunologic, and histologic features associated with different allograft outcomes.
Previous TG studies were limited by small numbers of patients, limited phenotype details, a lack of immunologic assessment, and the absence of unsupervised analysis to address the heterogeneity within TG.8,15 In this study, we characterized and phenotyped a large population of patients with TG with a detailed assessment of clinical, biologic, immunologic, and histologic features including long-term follow-up, allowing us to address TG heterogeneity and subset-specific prognostication.
To effectively address the TG heterogeneity, we applied machine learning–derived methods to improve disease characterization and risk stratification. There is growing evidence that traditional statistical models may not be optimal to address heterogeneity and risk stratification, particularly in datasets that encompass multiple entries that likely bias conventional models by overfitting and over-adjustments. To address these challenges, new methods such as machine learning, random forest analysis, archetype analysis, and artificial neural networks derived from artificial intelligence have shown great potential in high-dimensional data23 and have been recently used in the field of nephrology and in transplantation using gene expression assessed in transplant biopsy samples.25–27 The aim of the archetype analysis, introduced by Cutler and Breimer in 1993,24 is to address the heterogeneity of a study population by defining a limited set of extreme or pure phenotypes within a dataset. This method describes each patient as a composite of the underlying archetypes, which allows precise probabilistic assessment while retaining the uniqueness of each patient.
Applying the archetype approach to the present well annotated TG cohort permitted us to reduce patient heterogeneity and formed meaningful groups in terms of morphologic patterns, disease activity/progression, and risk of failure. Indeed, archetype analyses are performed in the case of a heterogeneous population to better identify distinct clusters with similar and homogeneous patients within clusters. We found that cg lesions were not equal in terms of the associated overall histologic and clinical phenotype and allograft survival. Archetype 1 presented with the highest proportion of patients with isolated cg (i.e., double contours without concomitant glomerulitis or GN), the highest eGFR, the lowest range of proteinuria, and the lowest inflammation-associated Banff scores (MVI, i+t scores), suggesting an “isolated cg archetype” that carries the best long-term outcome. Interestingly, the archetype analysis also identified a substantial number of patients with TG (archetype 2) presenting with features of active “mixed” rejection at the time of TG diagnosis. These cases presented with i and t lesions, microcirculation inflammation, and vasculitis lesions that could be referred to a “mixed-rejection” TG archetype. A causative role for T cell–mediated rejection, alone or associated with ABMR in mixed rejection, has been previously suggested in the development of TG.4 Five-year death-censored allograft survival was low for archetype 2, in line with the identification of v lesions associated with ABMR as a risk factor of allograft loss.28 In this archetype, 28 of 47 patients (60%) developed de novo DSA. This archetype would correspond to patients who are potentially noncompliant. Two other archetypes predominantly associated with chronic lesions were also identified. Archetype 3 presented with high IFTA and cv scores and low inflammation. We labeled this archetype the “chronic inactive ABMR archetype.” Conversely, archetype 4 presented much later after kidney transplantation than all other archetypes, with a high level of proteinuria; high scores for IFTA, cv, and ah; but low inflammation scores (MVI, i, and t); it was associated with the worst long-term allograft outcomes, suggesting a “late-terminal cg” archetype. Archetype 5 was characterized by a high microcirculation inflammation burden, the highest proportion of C4d positivity, and patients with circulating DSA at the time of TG. No cases of isolated cg were found in this archetype; accordingly, we labeled this archetype the “chronic active ABMR archetype.”
Strengths and Limitations
This study responds to a current challenge in transplant medicine, highlighted by regulatory agencies and international societies, to establish and refine diagnostic standards and improve risk stratification in renal transplantation. Accordingly, the Banff process has recently evolved from being a primarily pathology-driven approach to a more comprehensive and multidisciplinary approach including the integration of multidimensional data combining clinical, biologic, histologic, and immunologic parameters to define realistic and feasible end points and approaches for next-generation clinical trials.3 To achieve this goal, and as illustrated in the most recent Banff meeting report,3 new statistical methods such as machine learning and unsupervised approaches are required. The strength of this analysis is that it addresses overlaps and archetypes without preconceived hypotheses by using unsupervised analyses to obtain distinct phenotypes not only on the basis of clinician belief or classification but identified directly from the data. In fact, this combines a most comprehensive TG cohort data matrix and unsupervised analysis without being constrained by existing histologic classes, i.e., letting the data itself show the patterns of variation defining the five TG archetypes described above. This new approach might be useful for clinical trials that are limited by the heterogeneity of the TG population. Using the archetypes would make two groups of patients comparable regarding clinical, functional, immunologic, and histologic parameters and reduce the bias and negative results due to the comparison of different populations with different prognoses at the time of inclusion.
A limitation is that the biologic disease processes, distinct mechanisms, and pathways causing TG in organ transplants are still not fully deciphered in all cases that we included. This would require comprehensive molecular studies. Another limitation is that no therapeutic implications of the five archetypes could be assessed because no standard treatment protocols for TG exist, and our patients historically have been treated at the discretion of the most responsible physician. However, without precise diagnostic classification, no standardized treatment can be developed. Whether an archetype-based management of patients with TG would ultimately offer any benefit for the patients requires further prospective validation.
In conclusion, a probabilistic data-driven archetypal approach applied to a large, well annotated, multicentric cohort of kidney biopsy specimens with TG refines the diagnostic and prognostic features associated with TG and reduces heterogeneity, thereby improving TG characterization and risk stratification in the individual patient.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors would like to thank La Fondation Bettencourt Schueller and INSERM–Action thématique incitative sur programme Avenir (ATIP-Avenir) for their financial support. This project received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No. 754995.
O.A., A.L., S.H., M.M., and J.-P.D.V.H. designed the study; O.A., S.H., N.S., and P.C. gathered the data; S.H., M.R., B.S., and J.-P.D.V.H. reviewed the slides; O.A., S.H., Y.B., and A.L. analyzed the data; O.A. made the figures; and O.A., S.H., Y.B., D.V., D.G., C. Legendre, C. Lefaucheur, X.J., J.-P.E. and A.L. drafted and revised the paper. All authors approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018070777/-/DCSupplemental.
Supplemental Table 1. Baseline patient characteristics according to the centers.
Supplemental Table 2. Histological and allograft function parameters for all transplant glomerulopathy (TG) biopsies.
Supplemental Table 3. Histological and allograft function parameters at the time of the first transplant glomerulopathy (TG) diagnosis.
Supplemental Table 4. Histological diagnosis of biopsies with double contour associating arguments for antibody mediated rejection (ABMR) and thrombotic microangiopathy (TMA) or ABMR and a membranoproliferative pattern (MPGN).
Supplemental Table 5. Histological diagnosis of the first biopsies with double contour associating arguments for antibody mediated rejection (ABMR) and thrombotic microangiopathy (TMA) or ABMR and a membranoproliferative pattern (MPGN).
Supplemental Table 6. 5-year kidney allograft survival for each Archetype according to the treatment.
Supplemental Figure 1. Cumulative incidence of biopsy with a cg score ≥1 since transplantation and allograft survival.
Supplemental Figure 2. Archetype practical application for clinicians: Ready-to-use interface for clinicians. Real-life patients for whom we used the Archetypes to predict allograft survival.
Supplemental Figure 3. Venn Diagram according to the centers.
Supplemental Figure 4. Cumulative incidence of biopsy with transplant glomerulopathy (TG) since transplantation and Kaplan-Meier curve of allograft survival after TG biopsy according to the center.
References
- 1.Sethi S, Haas M, Markowitz GS, D’Agati VD, Rennke HG, Jennette JC, et al.: Mayo clinic/renal pathology society consensus report on pathologic classification, diagnosis, and reporting of GN. J Am Soc Nephrol 27: 1278–1287, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porter KA, Dossetor JB, Marchioro TL, Peart WS, Rendall JM, Starzl TE, et al.: Human renal transplants. I. Glomerular changes. Lab Invest 16: 153–181, 1967 [PubMed] [Google Scholar]
- 3.Haas M, Loupy A, Lefaucheur C, Roufosse C, Glotz D, Seron D, et al.: The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant 18: 293–307, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filippone EJ, McCue PA, Farber JL: Transplant glomerulopathy. Mod Pathol 31: 235–252, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, et al.: Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant 8: 753–760, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Loupy A, Hill GS, Jordan SC: The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol 8: 348–357, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Adam BA, Smith RN, Rosales IA, Matsunami M, Afzali B, Oura T, et al.: Chronic antibody-mediated rejection in nonhuman primate renal allografts: Validation of human histological and molecular phenotypes. Am J Transplant 17: 2841–2850, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baid-Agrawal S, Farris AB 3rd, Pascual M, Mauiyyedi S, Farrell ML, Tolkoff-Rubin N, et al.: Overlapping pathways to transplant glomerulopathy: Chronic humoral rejection, hepatitis C infection, and thrombotic microangiopathy. Kidney Int 80: 879–885, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Gloor JM, Sethi S, Stegall MD, Park WD, Moore SB, DeGoey S, et al.: Transplant glomerulopathy: Subclinical incidence and association with alloantibody. Am J Transplant 7: 2124–2132, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Husain S, Sis B: Advances in the understanding of transplant glomerulopathy. Am J Kidney Dis 62: 352–363, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Cosio FG, Gloor JM, Sethi S, Stegall MD: Transplant glomerulopathy. Am J Transplant 8: 492–496, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Sellarés J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, et al.: Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 12: 388–399, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Suri DL, Tomlanovich SJ, Olson JL, Meyer TW: Transplant glomerulopathy as a cause of late graft loss. Am J Kidney Dis 35: 674–680, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Kieran N, Wang X, Perkins J, Davis C, Kendrick E, Bakthavatsalam R, et al.: Combination of peritubular c4d and transplant glomerulopathy predicts late renal allograft failure. J Am Soc Nephrol 20: 2260–2268, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patri P, Seshan SV, Matignon M, Desvaux D, Lee JR, Lee J, et al.: Development and validation of a prognostic index for allograft outcome in kidney recipients with transplant glomerulopathy. Kidney Int 89: 450–458, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malheiro J, Santos S, Tafulo S, Dias L, Martins S, Fonseca I, et al.: Correlations between donor-specific antibodies and non-adherence with chronic active antibody-mediated rejection phenotypes and their impact on kidney graft survival. Hum Immunol 79: 413–423, 2018 [DOI] [PubMed] [Google Scholar]
- 17.Kamal L, Broin PO, Bao Y, Ajaimy M, Lubetzky M, Gupta A, et al.: Clinical, histological, and molecular markers associated with allograft loss in transplant glomerulopathy patients. Transplantation 99: 1912–1918, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Sis B, Mengel M, Haas M, Colvin RB, Halloran PF, Racusen LC, et al.: Banff ’09 meeting report: Antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant 10: 464–471, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Mengel M, Sis B, Haas M, Colvin RB, Halloran PF, Racusen LC, et al.: Banff Meeting Report Writing Committee : Banff 2011 meeting report: New concepts in antibody-mediated rejection. Am J Transplant 12: 563–570, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, et al. ; Banff Meeting Report Writing Committee : Banff 2013 meeting report: Inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 14: 272–283, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Marinozzi MC, Roumenina LT, Chauvet S, Hertig A, Bertrand D, Olagne J, et al.: Anti-factor B and anti-C3b autoantibodies in C3 glomerulopathy and ig-associated membranoproliferative GN. J Am Soc Nephrol 28: 1603–1613, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamb KE, Lodhi S, Meier-Kriesche HU: Long-term renal allograft survival in the United States: A critical reappraisal. Am J Transplant 11: 450–462, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Hart Y, Sheftel H, Hausser J, Szekely P, Ben-Moshe NB, Korem Y, et al. : Inferring biological tasks using Pareto analysis of high-dimensional data. Nat Methods 12: 233–235, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Cutler A, Breiman L: Archetypal analysis. Technometrics 36: 338–347, 1994 [Google Scholar]
- 25.Yoo KD, Noh J, Lee H, Kim DK, Lim CS, Kim YH, et al.: A machine learning approach using survival statistics to predict graft survival in kidney transplant recipients: A multicenter cohort study. Sci Rep 7: 8904, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reeve J, Böhmig GA, Eskandary F, Einecke G, Lefaucheur C, Loupy A, et al.: MMDx-Kidney Study Group : Assessing rejection-related disease in kidney transplant biopsies based on archetypal analysis of molecular phenotypes. JCI Insight 2: 12, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iatropoulos P, Daina E, Curreri M, Piras R, Valoti E, Mele C, et al.: Registry of Membranoproliferative Glomerulonephritis/C3 Glomerulopathy; Nastasi : Cluster Analysis identifies distinct pathogenetic patterns in C3 glomerulopathies/immune complex-mediated membranoproliferative GN. J Am Soc Nephrol 29: 283–294, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lefaucheur C, Loupy A, Vernerey D, Duong-Van-Huyen JP, Suberbielle C, Anglicheau D, et al.: Antibody-mediated vascular rejection of kidney allografts: A population-based study. Lancet 381: 313–319, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.