Significance Statement
Phosphate binders are currently the only medications available to reduce elevated serum phosphate in patients with ESRD receiving hemodialysis. Tenapanor, a minimally absorbed inhibitor of gastrointestinal sodium/hydrogen exchanger 3 (NHE3), acts via a non–phosphate-binding mechanism, reducing paracellular phosphate transport in the intestine. The authors found that tenapanor significantly lowered elevated serum phosphate in patients receiving hemodialysis, resulting in a mean reduction of 1.0–1.2 mg/dl over 8 weeks. Tenapanor also showed a significant benefit over placebo in patients rerandomized to either continue tenapanor treatment or receive a placebo for 4 weeks. Adverse effects were largely limited to softening of stool and more frequent bowel movements. By targeting paracellular phosphate transport’s substantial contribution to net phosphate absorption in the gut, tenapanor has the potential to improve management of mineral bone disorder in CKD.
Keywords: tenapanor, hyperphosphatemia, NHE3, hemodialysis, phosphate
Visual Abstract
Abstract
Background
Guidelines recommend reducing elevated serum phosphate in patients with CKD. Tenapanor, a minimally absorbed inhibitor of gastrointestinal sodium/hydrogen exchanger 3 (NHE3), reduces paracellular phosphate transport.
Methods
In this phase 3 randomized, double-blind trial, we randomly assigned patients with hyperphosphatemia receiving maintenance hemodialysis to receive twice-daily oral tenapanor (3, 10, or 30 mg [the latter down-titrated, if needed]) for 8 weeks. Patients were then rerandomized 1:1 to receive either their previously assigned dose or placebo for a 4-week ‘withdrawal’ period. We measured serum phosphate levels over the course of the trial. The primary end point was mean change in serum phosphate over the 4-week withdrawal period for the tenapanor group (using pooled data) versus the placebo group.
Results
Of 219 patients randomized, 152 completed both study phases. During the initial 8-week treatment period, all three treatment groups experienced significant decreases in mean serum phosphate (reductions of 1.00, 1.02, and 1.19 mg/dl, corresponding to the 3, 10, and 30 mg [down-titrated] dose groups, respectively). Tenapanor also showed a significant benefit over placebo during the withdrawal period, with a mean increase of 0.85 mg/dl in the placebo group versus a mean increase of 0.02 mg/dl in the pooled tenapanor group. Adverse events were largely limited to softened stool and a modest increase in bowel movement frequency, resulting from increased stool sodium and water content, stemming from tenapanor’s mechanism of action.
Conclusions
Tenapanor significantly reduced elevated serum phosphate in patients with hyperphosphatemia receiving maintenance hemodialysis. Adverse effects were limited to those induced by its known mechanism of action, which increases stool sodium and water content.
Reducing elevated serum phosphate in patients with ESRD receiving maintenance dialysis has been a therapeutic goal for nearly three decades.1 Initial clinical concerns were focused on the contribution of serum phosphate to the development of secondary hyperparathyroidism and uremic pruritus; subsequently, these concerns were overshadowed by consistent observations demonstrating a monotonic relationship between serum phosphate concentration and cardiovascular risk in patients spanning the entire spectrum of kidney function.2–5 Today, most nephrologists attempt to reduce serum phosphate as part of a comprehensive cardiovascular risk-reduction strategy.6 Therapeutic interventions designed to accomplish this goal remain limited to phosphate binders and reducing dietary phosphate intake. Frequent hemodialysis improves phosphate control, yet logistic and regulatory hurdles preclude adoption of this intervention at a population level.7
Surprisingly little is known about the physiology of phosphate homeostasis in patients with CKD or in healthy persons. Serum phosphate concentration is known to be influenced by circadian rhythm as well as by phosphate ingestion, absorption, and excretion.8–11 Absolute intestinal phosphate absorption is dependent on both active transport and passive, paracellular transport. Active phosphate transport is thought to be saturated at relatively low intraluminal phosphate concentrations; thus, the bulk of net phosphate transport is thought to be driven by the concentration-dependent, nonsaturable, paracellular pathway.12
Tenapanor is a minimally systemically absorbed inhibitor of intestinal sodium/hydrogen exchanger 3 (NHE3) that reduces phosphate absorption in healthy persons and lowers elevated serum phosphate concentrations in patients receiving maintenance hemodialysis.13–15 The effect of tenapanor on phosphate absorption is mediated by transiently increasing the intracellular proton concentration in cells lining the gastrointestinal lumen, a result of NHE3 inhibition, which induces a conformational change in tight junction proteins, thereby decreasing permeability to paracellular phosphate transport; this action has no apparent effect on the absorption of other ions (except sodium) or nutrients.12 A consequence of intestinal NHE3 inhibition is that stool sodium and water content are increased, loosening stool consistency and increasing bowel movement frequency.15–17 We conducted this phase 3 placebo-controlled, randomized clinical trial in patients receiving maintenance hemodialysis with hyperphosphatemia to test the safety and efficacy of tenapanor.
Methods
Study Design
The trial (Clinicaltrials.gov identifier NCT02675998) was conducted at 41 sites in the United States between January 20, 2016 and January 6, 2017 in accordance with the Declaration of Helsinki, International Conference on Harmonization, and Good Clinical Practice guidelines. The protocol and all amendments were approved by an independent ethics committee or institutional review board. All participants provided written informed consent.
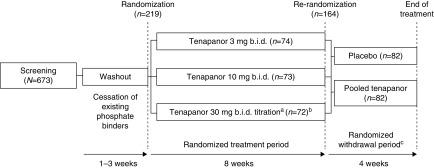
The trial was originally designed as a double-blind, dose-ranging phase 2 study with the primary end point being the change in serum phosphate from baseline to the end of the 8-week randomized treatment period (RTP). After trial initiation, the US Food and Drug Administration (FDA) informed the sponsor that a previous phase 2 study13 was sufficient for dose range finding and proposed conversion to a phase 3 trial incorporating a 4-week, double-blind, placebo-controlled, randomized withdrawal period (RWP); the protocol was amended on March 3, 2016, when 22 patients were enrolled (Figure 1). The primary end point was amended to the between-groups (pooled tenapanor versus placebo) difference in the mean change in serum phosphate from the end of the RTP to the end of the RWP (or the end point visit for this period) in a protocol amendment dated May 27, 2016, when 94 patients were enrolled. A responder analysis of serum phosphate change in the RWP was also requested by the FDA, which was performed among patients who experienced at least a 1.2-mg/dl decrease in serum phosphate during the RTP. All protocol amendments were made before any efficacy data had been analyzed. Details of key protocol changes are provided in the Supplemental Material.
Figure 1.
Study design. aPatients initially receiving tenapanor 30 mg twice a day were allowed to down-titrate weekly (stepwise 30 → 20 → 15 → 10 → 3 mg twice a day) during the first 4 weeks of the RTP, on the basis of gastrointestinal tolerability. Mean final dose of tenapanor in this group at the end of the RTP was 24.4 mg twice a day for the safety and ITT analysis sets, and 22.8 mg twice a day for the secondary efficacy analysis set. bOne patient did not receive any dose of study drug and was excluded from analyses. cThe study was initiated on January 20, 2016 as an 8-week randomized dose range–finding study; the RWP was added to the protocol in an amendment dated March 3, 2016, when 22 patients were enrolled. Patients randomized to tenapanor in the RWP remained on their previous dose from the RTP. Mean exposure during the RTP was 48 days in all three treatment groups, and during the RWP was 26 days for patients receiving placebo and 27 days for patients receiving tenapanor. bid, twice daily.
Adults (aged 18–80 years) with ESRD who had been on maintenance hemodialysis for at least 3 months, who were receiving at least three doses of phosphate-binding medication per day, and who had serum phosphate concentrations of 4.0–7.0 mg/dl (inclusive) were eligible. Vitamin D and/or calcimimetic therapy were required to have been stable for at least 4 weeks before screening. After the 1–3-week washout period, patients must have had an increase in serum phosphate of at least 1.5 mg/dl, with an absolute value from 6.0 mg/dl to <10.0 mg/dl, to be eligible for randomization. Exclusion criteria included parathyroid hormone >1200 pg/ml, serum phosphate >10.0 mg/dl at any time in the previous 3 months, serum bicarbonate <18 mmol/L on two consecutive measurements, diarrhea/loose stool (≥3 bowel movements/day on two or more days or any stool of Bristol Stool Form Scale [see Supplemental Material]18 ≥6 during the week before randomization), and life expectancy <6 months.
On day 1, eligible patients were randomly assigned to one of three parallel tenapanor regimens—3- or 10-mg fixed dose twice a day, or 30 mg twice a day which could be down-titrated during the first 4 weeks of the RTP in a step-wise fashion to 20, 15, 10, or 3 mg twice a day on the basis of gastrointestinal tolerability—in a 1:1:1 ratio using a computer-generated randomization schedule and a block size of 3. Study site staff and patients were blinded to treatment assignment. To preserve blinding, all patients were asked about tolerability at each study visit. Tenapanor was formulated as round, 9-mm diameter, plain, white, film-coated tablets irrespective of dose. Patients took two tablets in the morning before breakfast and two tablets in the evening before dinner; patients did not take tablets at the meal before dialysis and instead took them before another meal on that day (see Supplemental Table 1 for dosing regimens).
All randomized patients entered the 8-week RTP. At the end of the RTP, patients were rerandomized 1:1 to either remain on their previously assigned dose of tenapanor or to receive matching placebo and entered a 4-week RWP. We pooled data from all three tenapanor groups in the RWP.
Study Assessments
Study visits included a screening visit, 1–3 postwashout visits, a randomization visit, weekly visits at weeks 1–4, and every other week thereafter. All study assessments occurred after a short dialysis interval and predialysis. Serum parathyroid hormone and fibroblast growth factor 23 (FGF23) were assessed using intact assays (Roche Diagnostics, Indianapolis, IN and Kainos Laboratories, Tokyo, Japan, respectively). To assess tolerability, all patients used an electronic diary (phone) to record daily bowel habits (frequency and stool form, using the Bristol Stool Form Scale18) for the entire study. Safety assessments included physical examination, vital signs, laboratory tests, 12-lead electrocardiograms, and adverse event recording. We assessed adherence to study medication by pill count. Full details of study assessments are provided in the Supplemental Material.
Study Outcomes and Statistical Analyses
Separate safety analysis sets were analyzed in the RTP and RWP, each of which included all patients who received at least one dose of study treatment in the respective period and was used for the analysis of all safety outcomes. The intention-to-treat (ITT) analysis set for efficacy assessments included all patients who received at least one dose of study treatment and had at least one postbaseline serum phosphate assessment during the RTP. Key efficacy outcomes were the change in serum phosphate from baseline to the end of the 8-week RTP for each tenapanor group (the original primary end point) and the change in serum phosphate from the end of the RTP to the end of the RWP (or the end point visit for this period) for the pooled tenapanor and placebo groups (the revised primary end point). The proportion of patients with serum phosphate <5.5 mg/dl at each visit during the RTP was a secondary efficacy outcome. The secondary analysis requested by the FDA, change in serum phosphate from the end of the RTP to the end of the RWP among responders, was assessed using an efficacy analysis set composed of all patients who completed the RTP and achieved at least a 1.2-mg/dl reduction in serum phosphate from baseline to the end of the RTP.
Continuous efficacy variables were assessed using an analysis of covariance model with investigator site and treatment group as fixed factors and baseline value as a covariate, with change from baseline to each assessment as the dependent variable. We log-transformed FGF23 data before inference testing owing to the highly skewed distribution and, as such, we report ratios of the geometric means to aid interpretability.
Further details of the study outcome measures and statistical analyses are provided in the Supplemental Material.
Results
Patient Disposition and Baseline Characteristics
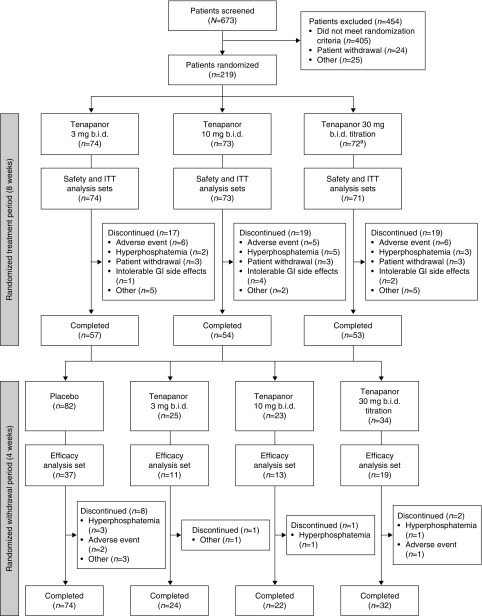
Of 673 patients screened, 219 patients were randomized to one of three tenapanor treatment groups (Figure 2). A total of 164 patients (75%) completed the RTP, of whom 152 (93%) completed the 4-week RWP. The proportions of patients not completing the RTP were approximately equal in all three tenapanor dose groups (23%, 26%, and 26% in the 3, 10, and 30 mg [down-titration] twice a day groups, respectively). During the RWP, 10% of patients randomized to placebo and 4%–6% of patients randomized to tenapanor withdrew before completion. The ITT and safety analysis sets for the RTP included 218 patients, because one patient withdrew before receiving study drug, and for the RWP included 164 patients (82 pooled tenapanor, 82 placebo). The secondary (FDA-requested) efficacy analysis set included 80 patients (37 placebo, 43 pooled tenapanor).
Figure 2.
Patient flow. aOne patient discontinued before receiving a dose of study drug and was excluded from the analyses. bid, twice daily; GI, gastrointestinal.
Baseline characteristics were similar in all randomized groups (Table 1). Mean adherence to study drug was >92% in all three treatment groups during the RTP and >95% during the RWP. Mean final dose of tenapanor in the 30 mg twice a day down-titration group at the end of the RTP was 24.4 mg twice a day for the safety and ITT analysis sets.
Table 1.
Baseline characteristics for patients entering the RTP and RWP
| Characteristic | RTP | RWP | |||
|---|---|---|---|---|---|
| Tenapanor | Placebo, n=82 | Pooled Tenapanor, n=82 | |||
| 3 mg Twice Daily, n=74 | 10 mg Twice Daily, n=73 | 30 mg Twice Daily Titration, n=71 | |||
| Age, yr | 55.7±11.5 | 57.4±10.8 | 54.2±10.9 | 55.8±11.8 | 55.2±10.4 |
| Men, n (%) | 46 (62.2) | 34 (46.6) | 48 (67.6) | 44 (53.7) | 52 (63.4) |
| Race, n (%) | |||||
| White | 30 (40.5) | 25 (34.2) | 30 (42.3) | 26 (31.7) | 29 (35.4) |
| Black | 40 (54.1) | 45 (61.6) | 40 (56.3) | 51 (62.2) | 51 (62.2) |
| Asian | 2 (2.7) | 0 (0.0) | 0 (0.0) | 2 (2.4) | 0 (0.0) |
| Native American or Alaskan native | 1 (1.4) | 2 (2.7) | 1 (1.4) | 2 (2.4) | 2 (2.4) |
| Other | 1 (1.4) | 1 (1.4) | 0 (0.0) | 1 (1.2) | 0 (0.0) |
| Ethnicity, n (%) | |||||
| Hispanic or Latino | 13 (17.6) | 8 (11.0) | 18 (25.4) | 12 (14.6) | 16 (19.5) |
| Baseline BMI, kg/m2 | 32.5±8.5 | 33.6±8.5 | 33.4±8.1 | 33.0±7.9 | 34.3±8.2 |
| Duration since first hemodialysis, mo | 58.1±63.1 | 62.0±53.1 | 57.1±57.1 | 55.7±52.2 | 57.9±51.7 |
| Kt/V valuea | NA | 1.62±0.38 | 1.61±0.28 | 1.63±0.32 | 1.61±0.34 |
| Serum phosphate, mg/dlb | 7.40±1.57 | 7.46±1.69 | 7.62±1.43 | NA | NA |
| PTH value before study entry, pg/ml | 471±268 | 393±237 | 433±213 | 405±206 | 443±241 |
Data are mean±SD unless otherwise stated. BMI, body mass index; Kt/V, a marker of dialysis adequacy, where K is dialyzer clearance of urea, t is dialysis time, and V is volume distribution of urea (approximately equal to the participant’s total body water); NA, not applicable/available; PTH, parathyroid hormone.
Data for 3 mg twice a day group not included due to a recording error.
On day 1, i.e., postwashout of phosphate binders.
Efficacy
Serum Phosphate
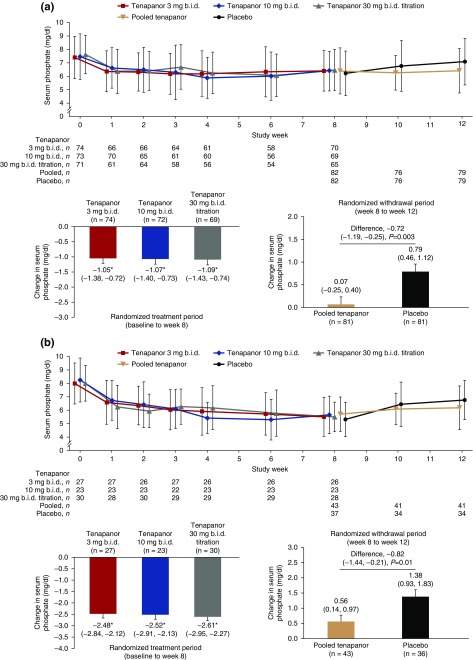
In the RTP, there were significant decreases in serum phosphate in all three tenapanor groups; mean±SD serum phosphate in the ITT set decreased by 1.00±1.73, 1.02±1.66, and 1.19±1.82 mg/dl in patients assigned to tenapanor 3, 10, and 30 mg twice a day down-titration, respectively, from postwashout baseline to week 8 (Figure 3A). There was no clear dose-response relationship during the RTP. The proportion of patients with serum phosphate <5.5 mg/dl at each visit during the RTP was 28.8%–37.7%, 24.6%–41.1%, and 25.0%–40.7% for the tenapanor 3, 10, and 30 mg twice a day down-titration groups, respectively (Supplemental Table 2).
Figure 3.
Tenapanor significantly decreased serum phosphate levels in patients with hyperphosphatemia receiving maintenance hemodialysis. Data presented are for the change in serum phosphate during the RTP and the RWP for (A) the ITT analysis set and (B) the efficacy (responder) analysis set. Line graph data are mean±SD. Bar chart data are LSM change (95% CI) in serum phosphate concentration and error bars show SEM, from an analysis of covariance with treatment and pooled investigator sites as factors and baseline (left) or end of 8-week RTP (right) serum phosphate concentration as a covariate. Data in (B) are shown for the responder population, defined as all patients with a reduction in serum phosphate concentration of at least 1.2 mg/dl during the RTP. The analyses used a patient’s last study center visit as the end point visit; there may be apparent discrepancies in patient numbers between figure panels if patients did not visit the study center after the first visit of each period (i.e., had no end point visit for the RTP/RWP). *P<0.001 versus baseline. bid, twice daily; 95% CI, 95% confidence interval; LSM, least squares mean.
In the RWP, the difference in serum phosphate change between the pooled tenapanor group and the placebo group was significant (mean±SD increase of 0.85±1.68 mg/dl with placebo versus 0.02±1.63 mg/dl with tenapanor; least squares mean difference, −0.72 mg/dl; 95% confidence interval, −1.19 to −0.25 mg/dl; P=0.003; Figure 3A).
Eighty of 164 patients in the RTP were deemed responders (mean±SD serum phosphate reduction, 2.56±1.10 mg/dl) after 8 weeks’ treatment. In the RWP, the difference in serum phosphate change between pooled tenapanor and placebo among responders was statistically significant (Figure 3A).
Other Biochemical End Points
Mean changes from baseline to the end of the RTP in mean serum parathyroid hormone concentration were small in magnitude (least squares mean change, +1.0, +7.3, and −24.6 pmol/L in the 3, 10, and 30 mg twice a day down-titration groups, respectively) and none were statistically significant.
Mean FGF23 was reduced from baseline to the end of the RTP in all three treatment groups, with a significant reduction observed in the 3 and 30 mg twice a day down-titration groups (Supplemental Table 3).
Safety and Tolerability
Stool Form and Frequency
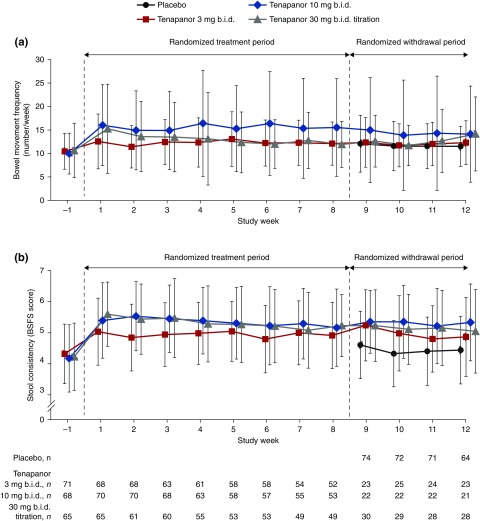
Mean bowel movement frequency remained in the normal range for healthy individuals19 in all groups throughout the study (Figure 4). At the end of the RTP, mean stool frequency increased by 2.8/wk (equivalent to 0.4/d or one incremental movement every 2.5 days) from baseline. During the RWP, the mean bowel movement frequency was 0.8−2.7 movements per week higher in patients receiving tenapanor versus those receiving placebo. The mean Bristol Stool Form Scale score increased by 0.8 from baseline during the RTP and was 0.4–0.9 points higher in tenapanor- versus placebo-treated patients during the RWP.
Figure 4.
Mean bowel movement frequency increased with tenapanor treatment, but remained within the normal range for healthy individuals. Data presented are for (A) bowel movement frequency and (B) stool consistency during the RTP and the RWP. Data are mean±SD. BSFS scores are weekly averages. bid, twice daily; BSFS, Bristol Stool Form Scale.
Adverse Events and Other Assessments
The most common adverse events were gastrointestinal in nature and were largely confined to diarrhea, as defined by any change in stool form or frequency (Tables 2 and 3, Supplemental Tables 4 and 5). During the RTP, diarrhea prompted drug discontinuation in 18 patients (8.3%). No patients discontinued treatment owing to diarrhea during the RWP. Very few serious adverse events occurred during the RTP and no patients receiving tenapanor had a serious adverse event during the RWP. One patient receiving tenapanor 3 mg twice a day died from sudden cardiac death, a finding assessed as unrelated to study treatment. Hyperphosphatemia was reported in 12 patients during the RTP. No clinically meaningful changes from baseline were observed in other laboratory parameters (Supplemental Table 6), including serum bicarbonate, or in vital signs, 12-lead electrocardiograms, or physical examination findings.
Table 2.
Summary of AEs
| AE category | Tenapanor | Placebo, n=82 | Tenapanor | ||||
|---|---|---|---|---|---|---|---|
| 3 mg Twice Daily, n=74 | 10 mg Twice Daily, n=73 | 30 mg Twice Daily Titration, n=71 | 3 mg Twice Daily, n=25 | 10 mg Twice Daily, n=23 | 30 mg Twice Daily Titration, n=34 | ||
| RTP | |||||||
| Any AE | 39 (52.7) | 51 (69.9) | 49 (69.0) | ||||
| Treatment-related AE | 24 (32.4) | 38 (52.1) | 33 (46.5) | ||||
| AE leading to study discontinuation | 8 (10.8) | 16 (21.9) | 11 (15.5) | ||||
| AE leading to death | 1 (1.4) | 0 (0.0) | 0 (0.0) | ||||
| SAE | 11 (14.9) | 5 (6.8) | 5 (7.0) | ||||
| AEs by system organ classa | |||||||
| Gastrointestinal disorders | 24 (32.4) | 35 (47.9) | 40 (56.3) | ||||
| Infections and infestations | 11 (14.9) | 5 (6.8) | 8 (11.3) | ||||
| Metabolism and nutrition disorders | 4 (5.4) | 10 (13.7) | 9 (12.7) | ||||
| Injury, poisoning, and procedural complications | 5 (6.8) | 11 (15.1) | 5 (7.0) | ||||
| General disorders and administration site conditions | 7 (9.5) | 5 (6.8) | 3 (4.2) | ||||
| Respiratory, thoracic, and mediastinal disorders | 3 (4.1) | 3 (4.1) | 5 (7.0) | ||||
| Skin and subcutaneous tissue disorders | 3 (4.1) | 4 (5.5) | 2 (2.8) | ||||
| Cardiac disorders | 3 (4.1) | 2 (2.7) | 3 (4.2) | ||||
| Vascular disorders | 0 (0.0) | 4 (5.5) | 3 (4.2) | ||||
| Investigations | 3 (4.1) | 2 (2.7) | 1 (1.4) | ||||
| Nervous system disorders | 4 (5.4) | 1 (1.4) | 1 (1.4) | ||||
| Musculoskeletal and connective tissue disorders | 2 (2.7) | 1 (1.4) | 2 (2.8) | ||||
| Blood and lymphatic system disorders | 2 (2.7) | 2 (2.7) | 0 (0.0) | ||||
| Renal and urinary disorders | 2 (2.7) | 1 (1.4) | 1 (1.4) | ||||
| RWP | |||||||
| Any AE | 21 (25.6) | 4 (16.0) | 7 (30.4) | 12 (35.3) | |||
| Treatment-related AE | 5 (6.1) | 0 (0.0) | 1 (4.3) | 0 (0.0) | |||
| AE leading to study discontinuation | 5 (6.1) | 0 (0.0) | 1 (4.3) | 1 (2.9) | |||
| AE leading to death | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| SAE | 4 (4.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| AEs by system organ classa | |||||||
| Metabolism and nutrition disorders | 7 (8.5) | 0 (0.0) | 1 (4.3) | 3 (8.8) | |||
| Injury, poisoning, and procedural complications | 4 (4.9) | 2 (8.0) | 0 (0.0) | 2 (5.9) | |||
| Infections and infestations | 2 (2.4) | 2 (8.0) | 1 (4.3) | 2 (5.9) | |||
| Gastrointestinal disorders | 4 (4.9) | 0 (0.0) | 0 (0.0) | 2 (5.9) | |||
| Investigations | 2 (2.4) | 1 (4.0) | 0 (0.0) | 2 (5.9) | |||
| Respiratory, thoracic, and mediastinal disorders | 1 (1.2) | 1 (4.0) | 3 (13.0) | 0 (0.0) | |||
| General disorders and administration site conditions | 1 (1.2) | 0 (0.0) | 0 (0.0) | 2 (5.9) | |||
| Skin and subcutaneous tissue disorders | 2 (2.4) | 0 (0.0) | 1 (4.3) | 0 (0.0) | |||
| Cardiac disorders | 2 (2.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
Data are number of patients experiencing AE (%). AE, adverse event; SAE, serious adverse event.
Data shown for system organ classes for which two or more patients in any group experienced an AE.
Table 3.
Gastrointestinal AEs
| AE category | Tenapanor | Placebo, n=82 | Tenapanor | ||||
|---|---|---|---|---|---|---|---|
| 3 mg Twice Daily, n=74 | 10 mg Twice Daily, n=73 | 30 mg Twice Daily Titration, n=71 | 3 mg Twice Daily, n=25 | 10 mg Twice Daily, n=23 | 30 mg Twice Daily Titration, n=34 | ||
| RTP | |||||||
| Gastrointestinal disorders | 24 (32.4) | 35 (47.9) | 40 (56.3) | ||||
| Gastrointestinal disorders by preferred terma | |||||||
| Diarrhea | 22 (29.7) | 30 (41.1) | 34 (47.9) | ||||
| Mild | 9 (12.2) | 11 (15.1) | 14 (19.7) | ||||
| Moderate | 12 (16.2) | 16 (21.9) | 17 (23.9) | ||||
| Severe | 1 (1.4) | 3 (4.1) | 3 (4.2) | ||||
| Vomiting | 2 (2.7) | 3 (4.1) | 3 (4.2) | ||||
| Flatulence | 2 (2.7) | 3 (4.1) | 2 (2.8) | ||||
| Abdominal discomfort | 1 (1.4) | 4 (5.5) | 1 (1.4) | ||||
| Abdominal distension | 0 (0.0) | 1 (1.4) | 2 (2.8) | ||||
| Abdominal pain | 0 (0.0) | 3 (4.1) | 0 (0.0) | ||||
| Abdominal pain upper | 2 (2.7) | 1 (1.4) | 0 (0.0) | ||||
| Frequent bowel movements | 0 (0.0) | 3 (4.1) | 0 (0.0) | ||||
| Nausea | 2 (2.7) | 1 (1.4) | 0 (0.0) | ||||
| Defecation urgency | 0 (0.0) | 2 (2.7) | 0 (0.0) | ||||
| RWP | |||||||
| Gastrointestinal disorders | 4 (4.9) | 0 (0.0) | 0 (0.0) | 2 (5.9) | |||
| Gastrointestinal disorders by preferred terma | |||||||
| Diarrhea | 2 (2.4) | 0 (0.0) | 0 (0.0) | 1 (2.9) | |||
| Mild | 1 (1.2) | 0 (0.0) | 0 (0.0) | 1 (2.9) | |||
| Moderate | 1 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Abdominal pain | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.9) | |||
| Food poisoning | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.9) | |||
Data are number of patients experiencing AE (%). AE, adverse event.
Data shown for AEs that were experienced by >2% of patients in any group.
Discussion
In this phase 3 placebo-controlled trial with an RWP, treatment with tenapanor, a minimally absorbed specific inhibitor of NHE3 that neither binds phosphate nor inhibits active phosphate transport, resulted in a significant reduction in serum phosphate among patients receiving maintenance hemodialysis with elevated serum phosphate concentrations. Adverse effects were uncommon; there was an expected modest increase in the frequency of bowel movements (on average one additional movement every 2.5 days) and a detectable, modest softening of the stool by a conventional criterion (the Bristol Stool Form Scale).
Tenapanor is a minimally absorbed compound with detectable levels in serum only rarely being described,15–17 a potential advantage given the continued concern with inadvertent systemic accumulation of cations from phosphate binders including those containing aluminum, lanthanum, iron, and calcium. Indeed, on the basis of several rigorous calcium balance studies in patients with CKD and from observational data and randomized clinical trials demonstrating higher mortality related to the use of calcium-based phosphate binders,20–23 the 2017 Kidney Disease Improving Global Outcomes guidelines update recommends limiting calcium exposure from phosphate binders in all patients with CKD.24 Also relevant is the form and method of administration of tenapanor. One 9-mm-diameter tablet taken twice daily represents a dramatic reduction in pill burden when compared with commonly used doses of phosphate binders (often 9–12 or more tablets or capsules per day). However, the design of this trial precludes any accurate prediction of the proportion of individuals that might be treated successfully with tenapanor monotherapy.
Contrary to conventional belief, serum phosphate concentration is not simply the net result of phosphate ingestion, absorption, and excretion. Elegant work has demonstrated that the nicotinamide phosphoribosyl transferase (Nampt)/(NAD+) intracellular pathway plays a fundamentally important role in the expression of renal and intestinal phosphate transporters (NaPi-2a, NaPi-2b, and NaPi-2c), and likely plays an additional role in the transcellular shifts from other organs that occur independent of oral phosphate ingestion, and which determine diurnal variation in serum phosphate concentration.25–27 Even our understanding of what constitutes a “phosphate-restricted diet” is now known to be on the basis of basic misunderstandings about the relative contribution of animal- versus plant- versus additive-based phosphate exposure.9,28 Our findings here indicate a clear and substantial contribution of paracellular phosphate transport to net phosphate absorption. One conclusion from this body of work is the erroneous and pejorative labeling of patients as “noncompliant” when their serum phosphate fails to conform to clinical expectations despite prescribed phosphate-restricted dietary limits and phosphate binders. The nearly universal finding that phosphate binders provide a maximal serum phosphate reduction of approximately 2.0 mg/dl at their highest dose suggests that novel mechanisms will be required to achieve further improvements in population phosphate control.29–31 Other interventions that target renal, intestinal, or cell membrane active phosphate transport (expression or function), or alternatively tight junction protein function, might provide additional novel pathways.
Tenapanor, which increases stool sodium and water content through its effects on NHE3, resulted in softer stool and increased frequency of bowel movements, as we expected. Diarrhea was experienced by approximately 40% of patients receiving tenapanor during the RTP, although by only one patient receiving tenapanor and two patients receiving placebo during the RWP. Interestingly, although we chose to classify any increase in stool softness or frequency as “diarrhea,” patients themselves only rarely discontinued study drug as a result—only 18 patients after 8 weeks of exposure in the RTP. It is plausible to speculate that the acute effect on stool sodium and water content may become the “new normal,” with patients becoming accustomed to the effect. Alternatively, the constipating effect of most phosphate binders may allow for some individuals to prefer more frequent and slightly softer, yet still formed, stool.
We did not observe a dose-response relationship in the RTP of our study. We had previously conducted a dose-ranging study13 and chose two doses that were deemed efficacious, along with a down-titration arm, recognizing that the effects of tenapanor on stool frequency and consistency were not necessarily dose related. The trial design precluded any ability to titrate the dose of tenapanor to a particular phosphate target or to match tenapanor dose to those requiring the highest amount of phosphate binder at baseline. An ongoing phase 3 trial of tenapanor includes a 26-week, open-label treatment period followed by a 12-week, placebo-controlled RWP, and includes the capacity to titrate tenapanor dose to patient response32; this design should be more capable of robustly estimating the dose response over the longer term. The mean 2.56 mg/dl reduction in serum phosphate observed in the responder population suggests the possibility of improving phosphate control relative to historical or current standard of care. Studies using tenapanor in combination with intestinal phosphate binders would also be informative.
This trial has two key limitations. First, the protocol was modified after the trial was launched, at the request of the FDA. Thus, the primary outcome was changed from the original (change in serum phosphate relative to baseline at completion of the RTP) to the revised (change in serum phosphate relative to end of RTP at completion of the RWP) version. The FDA also requested a secondary analysis examining the change in serum phosphate during the RWP among “responders,” defined as patients with a reduction in serum phosphate during the RTP of at least 1.2 mg/dl (the efficacy analysis set). For transparency, we present all three results, although we consider the analysis in responders as secondary; because only responders were included, any conclusions drawn from this analysis have limited generalizability. Second, the primary efficacy end point—the mean change in serum phosphate—is a surrogate. Ideally, a long-term, randomized, placebo-controlled trial would be conducted examining the effects of tenapanor on mortality, cardiovascular events, and fractures. However, many experts are concerned about the risks of untreated hyperphosphatemia for periods longer than 4–6 weeks. Alternatively, a trial could be conducted comparing the effects of tenapanor with those of phosphate binders, or a placebo-controlled trial of tenapanor in patients who remain hyperphosphatemic on fixed doses of phosphate binders. Blinding of such trials would be difficult, given the disparate effects of tenapanor and phosphate binders on the gastrointestinal tract.
Our trial has several strengths as well. The population was diverse in terms of age, sex, race/ethnicity, and primary cause of kidney disease. On the basis of experience from an earlier phase 2 trial, we carefully evaluated the effects of tenapanor on stool form and the frequency of bowel movements, and patients who started on the highest dose of tenapanor (30 mg twice a day) could be titrated down if there were untoward gastrointestinal effects. Adherence to tenapanor dosing was excellent.
In conclusion, we demonstrate the relative safety and efficacy of tenapanor in lowering serum phosphate through the inhibition of paracellular phosphate transport. Adverse effects were limited to those expected by its mechanism of action, which increases stool sodium and water content.
Disclosures
G.A.B. has received consulting fees from and has ownership interest in Ardelyx. He was affiliated with Denver Nephrology at the time this study was conducted and has since become an employee of Reata Pharmaceuticals. D.P.R. and A.Y. are employees of and have ownership interest in Ardelyx. G.M.C. is a consultant to and has equity ownership interest in Ardelyx.
Supplementary Material
Acknowledgments
This study was funded by Ardelyx. Medical writing support was provided by Richard Claes, of Oxford PharmaGenesis, UK, funded by Ardelyx.
Individual deidentified participant data and additional study-related documents will not be available.
G.A.B., conception, design, and conduct of the study; interpretation of data; drafting and revision of the manuscript for important intellectual content; and approval of the final version for submission. D.P.R., conception, design, and conduct of the study; analysis and interpretation of data; revision of the manuscript for important intellectual content; and approval of the final version for submission. A.Y., design and conduct of statistical analyses and interpretation of data, revision of the manuscript for important intellectual content, and approval of the final version for submission. G.M.C., conception and design of study, interpretation of data, drafting and revision of the manuscript for important intellectual content, and approval of the final version for submission.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018080832/-/DCSupplemental.
Key Protocol Amendments
Study Outcome Measures
Study Assessments
Study drug exposure and adherence
Serum intact FGF23 assay
Serum intact PTH assay
Stool frequency and consistency
Adverse event (AE) recording
Statistical Analyses
Sample size calculation
Other
Supplemental Table 1. Tenapanor dosing regimens.
Supplemental Table 2. Proportion of patients with serum phosphate below 5.5 mg/dL during the randomized treatment period (RTP) (intention-to-treat analysis set).
Supplemental Table 3. Change in serum FGF23 from baseline to the end of the randomized treatment period (RTP) (intention-to-treat analysis set).
Supplemental Table 4. AEs occurring in at least 2% of patients in any treatment group.
Supplemental Table 5. Treatment-related AEs occurring in at least 2% of patients in any treatment group.
Supplemental Table 6. Serum chemistry and hematology values.
References
- 1.Block GA, Hulbert-Shearon TE, Levin NW, Port FK: Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis 31: 607–617, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Chue CD, Edwards NC, Moody WE, Steeds RP, Townend JN, Ferro CJ: Serum phosphate is associated with left ventricular mass in patients with chronic kidney disease: A cardiac magnetic resonance study. Heart 98: 219–224, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Eddington H, Hoefield R, Sinha S, Chrysochou C, Lane B, Foley RN, et al.: Serum phosphate and mortality in patients with chronic kidney disease. Clin J Am Soc Nephrol 5: 2251–2257, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGovern AP, de Lusignan S, van Vlymen J, Liyanage H, Tomson CR, Gallagher H, et al.: Serum phosphate as a risk factor for cardiovascular events in people with and without chronic kidney disease: A large community based cohort study. PLoS One 8: e74996, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G; Cholesterol And Recurrent Events Trial Investigators : Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 112: 2627–2633, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group : KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl (113): S1–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, et al.: FHN Trial Group : In-center hemodialysis six times per week versus three times per week. N Engl J Med 363: 2287–2300, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galassi A, Cupisti A, Santoro A, Cozzolino M: Phosphate balance in ESRD: Diet, dialysis and binders against the low evident masked pool. J Nephrol 28: 415–429, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Moe SM, Zidehsarai MP, Chambers MA, Jackman LA, Radcliffe JS, Trevino LL, et al.: Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol 6: 257–264, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Portale AA, Halloran BP, Morris RC Jr.: Dietary intake of phosphorus modulates the circadian rhythm in serum concentration of phosphorus. Implications for the renal production of 1,25-dihydroxyvitamin D. J Clin Invest 80: 1147–1154, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trivedi H, Szabo A, Zhao S, Cantor T, Raff H: Circadian variation of mineral and bone parameters in end-stage renal disease. J Nephrol 28: 351–359, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King AJ, Siegel M, He Y, Nie B, Wang J, Koo-McCoy S, et al.: Inhibition of sodium/hydrogen exchanger 3 in the gastrointestinal tract by tenapanor reduces paracellular phosphate permeability. Sci Transl Med 10: pii eaam6474, 2018. doi: 10.1126/scitranslmed.aam6474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Block GA, Rosenbaum DP, Leonsson-Zachrisson M, Åstrand M, Johansson S, Knutsson M, et al.: Effect of tenapanor on serum phosphate in patients receiving hemodialysis. J Am Soc Nephrol 28: 1933–1942, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansson S, Leonsson-Zachrisson M, Knutsson M, Spencer AG, Labonté ED, Deshpande D, et al.: Preclinical and healthy volunteer studies of potential drug–drug interactions between tenapanor and phosphate binders. Clin Pharmacol Drug Dev 6: 448–456, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansson S, Rosenbaum DP, Knutsson M, Leonsson-Zachrisson M: A phase 1 study of the safety, tolerability, pharmacodynamics, and pharmacokinetics of tenapanor in healthy Japanese volunteers. Clin Exp Nephrol 21: 407–416, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spencer AG, Labonte ED, Rosenbaum DP, Plato CF, Carreras CW, Leadbetter MR, et al.: Intestinal inhibition of the Na+/H+ exchanger 3 prevents cardiorenal damage in rats and inhibits Na+ uptake in humans. Sci Transl Med 6: 227ra36, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Rosenbaum DP, Yan A, Jacobs JW: Pharmacodynamics, safety, and tolerability of the NHE3 inhibitor tenapanor: Two trials in healthy volunteers. Clin Drug Investig 38: 341–351, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis SJ, Heaton KW: Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 32: 920–924, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Walter SA, Kjellström L, Nyhlin H, Talley NJ, Agréus L: Assessment of normal bowel habits in the general adult population: The popcol study. Scand J Gastroenterol 45: 556–566, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Jamal SA, Vandermeer B, Raggi P, Mendelssohn DC, Chatterley T, Dorgan M, et al.: Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: An updated systematic review and meta-analysis. Lancet 382: 1268–1277, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Hill KM, Martin BR, Wastney ME, McCabe GP, Moe SM, Weaver CM, et al.: Oral calcium carbonate affects calcium but not phosphorus balance in stage 3-4 chronic kidney disease. Kidney Int 83: 959–966, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spiegel DM, Brady K: Calcium balance in normal individuals and in patients with chronic kidney disease on low- and high-calcium diets. Kidney Int 81: 1116–1122, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel L, Bernard LM, Elder GJ: Sevelamer versus calcium-based binders for treatment of hyperphosphatemia in CKD: A meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol 11: 232–244, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ketteler M, Block GA, Evenepoel P, Fukagawa M, Herzog CA, McCann L, et al.: Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) guideline update: What’s changed and why it matters. Kidney Int 92: 26–36, 2017 [DOI] [PubMed] [Google Scholar]
- 25.Miyagawa A, Tatsumi S, Takahama W, Fujii O, Nagamoto K, Kinoshita E, et al.: The sodium phosphate cotransporter family and nicotinamide phosphoribosyltransferase contribute to the daily oscillation of plasma inorganic phosphate concentration. Kidney Int 93: 1073–1085, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Zheng J, Glezerman IG, Sadot E, McNeil A, Zarama C, Gonen M, et al. : Hypophosphatemia after hepatectomy or pancreatectomy: Role of the nicotinamide phosphoribosyltransferase. J Am Coll Surg 225: 488–497 e482, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nomura K, Tatsumi S, Miyagawa A, Shiozaki Y, Sasaki S, Kaneko I, et al.: Hepatectomy-related hypophosphatemia: A novel phosphaturic factor in the liver-kidney axis. J Am Soc Nephrol 25: 761–772, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karp HJ, Kemi VE, Lamberg-Allardt CJ, Kärkkäinen MU: Mono- and polyphosphates have similar effects on calcium and phosphorus metabolism in healthy young women. Eur J Nutr 52: 991–996, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Renagel (sevelamer hydrochloride) tablet for oral use. Prescribing information (revised 2016). Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021179s032lbl.pdf. Accessed May 23, 2018
- 30.Fosrenol (lanthanum carbonate) chewable tablets/oral powder for oral use. Prescribing information (revised 2016). Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021468s020,204734s001lbl.pdf. Accessed May 23, 2018
- 31.Auryxia (ferric citrate) for oral use. Prescribing information (revised 2015). Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/205874s004lbl.pdf. Accessed May 23, 2018
- 32.A phase 3 study of tenapanor to treat hyperphosphatemia in ESRD patients on dialysis. ClinicalTrials.gov identifier: NCT03427125. 2018. Available at: https://clinicaltrials.gov/ct2/show/NCT03427125. Accessed March 15, 2018
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.