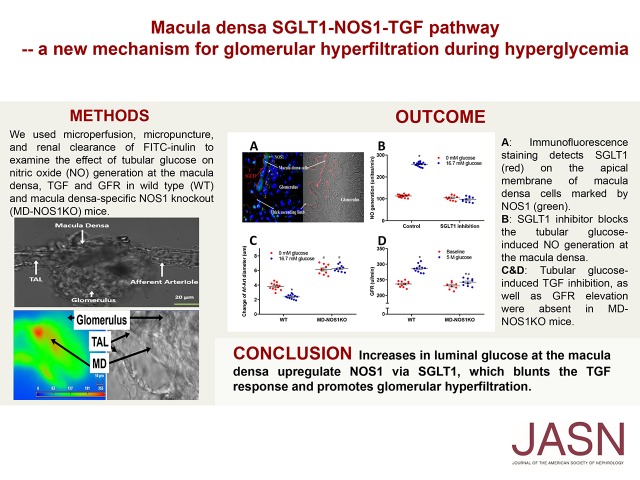
Significance Statement
Although glomerular hyperfiltration is common in early diabetes and considered a risk factor for later diabetic nephropathy, the mechanisms underlying glomerular hyperfiltration have not been fully clarified. The authors identified a novel mechanism of acute hyperglycemia–induced hyperfiltration in which increases in luminal glucose at the macula densa upregulate the expression and activity of neuronal nitric oxide synthase 1 (NOS1) via sodium-glucose cotransporter 1 (SGLT1); this blunts the tubuloglomerular feedback (TGF) response and promotes glomerular hyperfiltration. This novel SGLT1-NOS1-TGF pathway mediates the glomerular hyperfiltration observed in response to acute hyperglycemia. These findings establish a critical role of macula densa NOS1 and SGLT1 as key determinants of, and potential therapeutic targets for, acute hyperglycemia–associated glomerular hyperfiltration, and possibly for diabetes as well.
Keywords: glomerular hyperfiltration, hyperglycemia, SGLT1, NOS1, tubuloglomerular feedback
Visual Abstract
Abstract
Background
Glomerular hyperfiltration is common in early diabetes and is considered a risk factor for later diabetic nephropathy. We propose that sodium-glucose cotransporter 1 (SGLT1) senses increases in luminal glucose at the macula densa, enhancing generation of neuronal nitric oxide synthase 1 (NOS1)–dependent nitric oxide (NO) in the macula densa and blunting the tubuloglomerular feedback (TGF) response, thereby promoting the rise in GFR.
Methods
We used microperfusion, micropuncture, and renal clearance of FITC–inulin to examine the effects of tubular glucose on NO generation at the macula densa, TGF, and GFR in wild-type and macula densa–specific NOS1 knockout mice.
Results
Acute intravenous injection of glucose induced hyperglycemia and glucosuria with increased GFR in mice. We found that tubular glucose blunts the TGF response in vivo and in vitro and stimulates NO generation at the macula densa. We also showed that SGLT1 is expressed at the macula densa; in the presence of tubular glucose, SGLT1 inhibits TGF and NO generation, but this action is blocked when the SGLT1 inhibitor KGA-2727 is present. In addition, we demonstrated that glucose increases NOS1 expression and NOS1 phosphorylation at Ser1417 in mouse renal cortex and cultured human kidney tissue. In macula densa–specific NOS1 knockout mice, glucose had no effect on NO generation, TGF, and GFR.
Conclusions
We identified a novel mechanism of acute hyperglycemia–induced hyperfiltration wherein increases in luminal glucose at the macula densa upregulate the expression and activity of NOS1 via SGLT1, blunting the TGF response and promoting glomerular hyperfiltration.
More than 30 million Americans have diabetes. Diabetic nephropathy is a major complication of diabetes mellitus1–3 and the leading cause of ESRD. An increase in GFR or glomerular hyperfiltration has been observed in about 70% of patients with type 1 diabetes4,5 and 50% of patients with type 2 diabetes,5–8 and is associated with an increased risk for diabetic nephropathy and worse prognosis.4,7–9 The pathogenesis of glomerular hyperfiltration in diabetes has not been fully elucidated. Several mechanisms have been implicated, primarily including vascular and tubular theories. According to the vascular mechanism, glomerular hyperfiltration results from an imbalance between vasoconstrictive factors and vasodilatory factors.4,5,10 The tubular theory proposes that tubular growth and the upregulated sodium-glucose cotransporter 2 (SGLT2) enhance proximal tubular reabsorption, which reduces sodium chloride (NaCl) delivery to the macula densa and increases GFR via the tubuloglomerular feedback (TGF) response (SGLT2-NaCl pathway).11–13
The TGF response describes a mechanism by which an increase in NaCl delivery to the macula densa promotes the release and formation of ATP and/or adenosine,14–17 which then constricts the afferent arteriole (Af-Art) and induces a tonic inhibition of single-nephron GFR.18–20 neuronal nitric oxide synthase (NOS1) is the predominant nitric oxide synthase (NOS) isoform expressed in macula densa cells,21,22 and nitric oxide (NO) generated by the macula densa blunts TGF response.23–26 Recently, several studies from our laboratory demonstrated the decisive role of macula densa NOS1 in the TGF response and the long-term control of GFR, sodium excretion, and BP.27–29 Mice with deletion of NOS1 from the macula densa exhibit enhanced TGF responsiveness and develop salt-sensitive hypertension.28 Although many previous studies have assessed the TGF response and renal NO production in diabetes,11,30–33 whether macula densa NOS1 is a causal factor for diabetic hyperfiltration remains elusive.
More than 99% of filtered glucose in the kidney is reabsorbed by sodium-glucose cotransporter 1 (SGLT1) and SGLT2 in the proximal tubules. SGLT2 is present in the S1 and S2 segments of proximal tubules and mediates approximately 97% of glucose resorption, whereas SGLT1 is present in the S3 segment of proximal tubules and accounts for the remaining 2%–3% of the filtered glucose.34 The luminal glucose concentration at the macula densa is usually negligible under normoglycemic conditions. Luminal glucose concentration at the macula densa rises, however, when the amount of filtered glucose exceeds the maximal capacity of reabsorption by proximal tubules in hyperglycemic states. Moreover, SGLT1 has recently been detected on the apical membrane of macula densa cells in mouse35 and rat kidneys36 with a custom-made antibody. However, the role of macula densa SGLT1 in the control of TGF and GFR in a hyperglycemic setting is not known and has not been investigated.
In this study, we tested a novel hypothesis that the increase in luminal glucose concentration at the macula densa enhances NOS1-dependent NO formation via SGLT1, thereby inhibiting TGF responsiveness and promoting glomerular hyperfiltration in hyperglycemia (SGLT1-NOS1 pathway).
Methods
Animal
C57BL/6 mice (male, 13–15 weeks old) were purchased from Jackson Laboratory. The macula densa–specific NOS1 knockout (MD-NOS1KO) mice (NKCC2cre/NOS1flox/flox) were generated by crossing an NKCC2-Cre line with NOS1-floxed mice as described previously.28 All protocols were approved by the Institutional Animal Care and Use Committee at the University of South Florida, College of Medicine (IS00003816 and IS00004119). All chemicals were purchased from Sigma (St. Louis, MO), except as indicated.
Induction of Acute Hyperglycemia
To determine the intravenous dose of glucose that is able to increase blood glucose levels over the renal threshold of glucose (corresponding to blood glucose concentration of approximately >180 mg/dl in humans and >250 mg/dl in rodents37,38), we applied a bolus injection of 50 µl of glucose (2.5 or 5 M in saline) in lightly anesthetized mice via the retro-orbital venous sinus, and the blood glucose levels were measured at 3, 10, 20, 40, 60, and 80 minutes after glucose injection, by Precision Xtra Glucose Meter K (Fisher Scientific, Waltham, MA). Mice injected with 50 µl saline (0 M glucose) served as controls.
Measurement of Glucose Concentration in Urine and Early Distal Tubule
To determine the effect of hyperglycemia on glucose concentration at macula densa, the glucose concentration in urine and early distal tubule fluid were measured. Micropuncture preparation was performed as previously described.28,39,40 Briefly, mice were anesthetized with inactin (80 mg/ml, intraperitoneal injection) and ketamine (50 mg/ml, intramuscular injection). A tracheostoma was placed to facilitate breathing and femoral artery was catheterized for BP measurement. The femoral vein was catheterized for infusion of saline with 1% BSA at the rate of 1 ml/h per 100 g body wt throughout the experiment. After an abdominal incision, the left ureter was catheterized for the collection of urine and the left kidney was exposed and immobilized in a kidney holder cup. The kidney orientation was positioned so that superficial tubules could be clearly visualized under the microscope (SZX16; Olympus, Tokyo, Japan). The segment of early distal tubule was identified by the movement of green dye along the same nephron, after a bolus injection of artificial tubular fluid (ATF) with 5% fast green into a random proximal segment. After 30 minutes of equilibration, urine was collected through a catheterized ureter for 20 minutes, and the fluid in early distal tubule was collected by oil-filled pipette for 3–5 minutes after a blockade of oil droplet as basal. Then, 50 µl glucose solution (5 M in saline) was intravenously injected via retro-orbital venous sinus. Urine was collected during 0–20, 20–40, 40–60, and 60–80 minutes after the injection of glucose solution, and the fluid in early distal tubule was collected at 20, 40, 60, and 80 minutes. The glucose concentration in the urine and early distal tubular fluid samples was measured with mouse glucose assay kit (Crystal Chem, Elk Grove Village, IL).
Measurement of GFR in Conscious Mice
GFR was measured by the clearance of plasma FITC–inulin with a single bolus injection in conscious mice as previously described.28,41,42 Mice were lightly anesthetized with isoflurane and injected with FITC–inulin solution (3.74 µl/g body wt) through the retro-orbital venous sinus. Blood (10 µl) was collected into heparinized capillary tubes through the retro-orbital venous sinus at 3, 7, 10, 15, 35, 55, and 75 minutes after inulin injection. The blood samples were centrifuged at 8000 rpm for 5 minutes at 4°C. Plasma (1 µl) was collected from each sample. FITC–inulin concentration of the plasma was measured using a plate reader (Cytation3; BioTek, Winooski, VT) with 485 nm excitation and 538 nm emission. GFR were calculated with GraphPad Prism 6 (GraphPad Software, San Diego, CA).
To determine the effect of hyperglycemia on GFR, we first measured the basal GFR in mice, and then 7 days later, the mice were intravenously injected with 50 µl glucose solution (5 M in saline) together with FITC–inulin (3.74 µl/g body wt) through the retro-orbital venous sinus for repeated measurement of GFR. To determine whether osmolality affects GFR, we repeated the experiments by replacing D-glucose with L-glucose (50 µl, 5 M in saline) or mannitol (50 µl, 5 M in saline).
Measurement of TGF in vivo with Micropuncture
The preparation of micropuncture in mouse kidney was performed as described above. Tubular flow of a selected proximal tubule with multiple visible loops was obstructed with a grease block. Stop-flow pressure (Psf) in proximal tubule upstream of the grease block was measured with the servo-nulling method (Model 900A; World Precision Instruments, Sarasota, FL). The micropipette for pressure measurement with a tip diameter of 2–3 µm was filled with 2 M KCl colored with 1% fast green. The proximal tubule segment distal to the grease block was perfused with ATF (containing in mM: 4 NaHCO3, 5 KCl, 2 CaCl2,7 urea, 2 MgCl2, 128 NaCl, and 1% fast green; pH 7.4; pH 7.4). Psf was measured when tubular perfusion rate was set at 0 nl/min and 40 nl/min, each for 3–5 minutes. The change of Psf (ΔPsf) was used as an index of the TGF response. To determine the effect of glucose on TGF response in vivo, ΔPsf was measured twice consecutively in the same nephron: first ΔPsf was measured with glucose-free ATF, and second ΔPsf was measured using ATF with 16.7 mM glucose. Second ΔPsf measurements using glucose-free ATF, ATF with 16.7 mM L-glucose, or ATF with 16.7 mM mannitol served as control groups. In addition, because twice consecutive measurements of ΔPsf in the same nephron may introduce a systematic error, a single measurement of ΔPsf was also performed directly under high-glucose conditions, using ATF with 16.7 mM glucose. Measurements with glucose-free ATF, ATF containing 16.7 mM L-glucose, or ATF containing 16.7 mM mannitol served as control groups.
Measurement of TGF in vitro with Microperfusion
Mice were anesthetized with isoflurane and kidneys were removed and sliced. Kidney slices were placed in ice-cold DMEM containing 5% BSA. A single, superficial Af-Art and its intact glomerulus were microdissected together with adherent tubular segments consisting of the thick ascending limb (TAL), macula densa, and early distal tubule under a stereomicroscope (SMZ1500; Nikon, Yuko, Japan), as described previously.28,39 The microdissection was completed within 60 minutes and the dissected sample was transferred to a temperature-regulated chamber mounted on an inverted microscope (Axiovert 100TV; ZEISS). The bath solution (containing 5.5 mM glucose) in the chamber (total volume of 1.5 ml) was exchanged continuously at a rate of 1 ml/min and the temperature of the bath was maintained at 37°C throughout the experiment. The Af-Art was cannulated with one glass pipette and perfused with DMEM, and intraluminal pressure of Af-Art was maintained at 60 mm Hg throughout the experiment. The TAL was cannulated with another glass pipette and perfused with glucose-free macula densa solution containing, in mM: 10 HEPES, 1.0 CaCO3, 0.5 K2HPO4, 4.0 KHCO3, 1.2 MgSO4, 0.5 Na acetate, 0.5 Na lactate, and either 10 NaCl or 80 NaCl; pH 7.4. The imaging system consisted of a microscope (Eclipse Ti; Nikon), a digital charge–coupled device camera (CoolSnap; Photometrics, Tucson, AZ), xenon light (LB-LS/30; Shutter Instruments), and optical filter changer (Lambda 10–3; Shutter Instruments). Images were displayed and analyzed with NIS-Elements imaging software (Nikon).
After a 20-minute equilibration period, macula densa perfusate was switched from 10 to 80 mM NaCl, and luminal diameter of the Af-Art was observed for at least 5 minutes. The TGF response was determined by the average change in the luminal diameter of the Af-Art. To determine the effect of luminal glucose on TGF response in vitro, the TGF response was measured twice consecutively in the same isolated juxtaglomerular apparatus (JGA). First TGF response was measured by switching macula densa perfusate from 10 to 80 mM NaCl in the absence of glucose. In the second TGF response measurement, macula densa was perfused with 10 mM NaCl macula densa solution plus 16.7 mM glucose for 15 minutes, followed by switching macula densa perfusate from 10 to 80 mM NaCl while maintaining the same glucose level.
To determine whether the osmolality has any effect on TGF response in vitro, we repeated the above experiments, replacing D-glucose with L-glucose (16.7 mM) or mannitol (16.7 mM).
To determine the effect of glucose in the basolateral side of macula densa on TGF response in vitro, we changed the glucose concentration in the bath solution while maintaining the glucose-free tubular perfusate. The first TGF response was measured with normal bath solution (containing 5.5 mM glucose), and the second TGF response was measured with glucose concentration in the bath solution increased to 16.7 mM.
To determine the effect of luminal glucose on the TGF response under constant and low tubular NaCl at the macula densa, as observed in diabetic rats, the diameter of the Af-Art was assessed when tubular glucose concentration was increased from 0 to 16.7 mM while the NaCl concentration was maintained at 15 mM.12
In separate experiments, to determine the significance of SGLT1 in the effect of glucose on the TGF response, the macula densa of isolated JGA from mouse kidney was treated with the selective SGLT1 inhibitor KGA-2727 (10−6 M) (Kissei Pharmaceutical Co., Ltd., Nagano, Japan)43 via the tubular lumen perfusate for 30 minutes before the TGF response was measured by the above protocol.
Measurement of NO Generation at the Macula Densa in Isolated Perfused Juxtaglomerular Apparatus
The preparation of the mouse Af-Art and attached macula densa was performed as described for the measurement of TGF in vitro with microperfusion. After a 30-minute equilibration period, the macula densa was loaded with fluorescent NO probe 4-amino-5-methylamino-2', 7'-difluorofluorescein diacetate (DAF-2 DA; 10 μM plus 0.1% pluronic acid) from the tubular lumen for 30 minutes, then washed for 10 minutes with macula densa perfusate. DAF-2 DA was excited at 490 nm with a xenon light, and the emitted fluorescence was recorded at wavelengths of 510–550 nm. The rate of increase in fluorescence intensity of DAF-2 DA was used to determine NO generation by the macula densa.28,44 To determine the effect of luminal glucose on the NO generation in the macula densa, basal NO generation was measured with glucose-free macula densa perfusate for 5 minutes, followed by measurement of NO generation in the macula densa as the glucose concentration in the tubular perfusate was increased to 16.7 mM. In addition, we repeated the above experiments, replacing D-glucose with L-glucose (16.7 mM) or mannitol (16.7 mM) as the osmotic controls to determine whether the osmolality has any effect on NO generation in the macula densa.
To determine the effect of glucose at the basolateral side of the macula densa on NO generation, we measured the NO generation in the macula densa when the glucose concentration in the bath solution was increased from 5.5 to 16.7 mM when the tubular perfusate was maintained glucose-free.
In separate experiments, to determine the significance of SGLT1 in the effect of glucose on the NO generation at the macula densa, the macula densa of isolated JGA from mouse kidney was treated with the selective SGLT1 inhibitor KGA-2727 via the tubular lumen perfusate for 30 minutes before the NO generation was measured by the above protocol.
Analysis of the Single-Cell RNA-Sequencing Profile
We analyzed the single-cell gene expression profiles of kidney samples (normal 1, normal 2, normal 3, and normal 4) from C57BL/6 mice that were recently reported by Park et al.45 The datasets were accessed in National Center for Biotechnology Information Gene Expression Omnibus (GEO) database (accession no. GSE107585). We identified and selected the macula densa cells from 12,090 kidney cells, using the transcriptional copies of both marker genes “Na/K/2Cl cotransporter (Slc12a1)” and “nitric oxide synthase 1 (Nos1)” >0 as cut-off (Table 1). Then, we compared the mRNA expression levels of glucose transporters (Slc2a1, Slc2a2, Slc2a3, Slc2a4, Slc2a5, Slc2a6, Slc2a8, Slc2a9, Slc2a12, Slc2a13, Slc5a1, and Slc5a2) in these selected macula densa cells. All statistical analyses were performed using R (version 3.4.3).
Table 1.
Identification and selection of macula densa cells from single kidney cells
| Condition | Normal 1 | Normal 2 | Normal 3 | Normal 4 | Total |
|---|---|---|---|---|---|
| Subtotal | 2943 | 5060 | 1383 | 2704 | 12,090 |
| Slc12a1>0 | 268 | 268 | 165 | 279 | 980 |
| Nos1>0 | 7 | 6 | 8 | 19 | 40 |
| Slc12a1>0&Nos1>0 | 6 | 5 | 8 | 19 | 38 |
Immunofluorescence
Double-immunofluorescence staining of SGLT1 as well as NOS1 to localize the macula densa in human kidney was performed as previously reported.28,39 The human kidney biopsy samples were collected from cadaveric kidney donors in Tampa General Hospital, Florida. The samples were fixed overnight with 4% paraformaldehyde and then embedded in paraffin. After deparaffinization and antigen unmasking, 2 µm sections were blocked with 10% normal goat serum and 0.1% Tween-20 in PBS for 30 minutes. For the detection of SGLT1, the slices were incubated overnight with a SGLT1 antibody (rabbit polyclonal IgG; 1:500).35,36 After washing, the sections were incubated for 1 hour with a fluorescent secondary antibody (Alexa Fluor 594 goat anti-rabbit, 1:1000; Abcam, Cambridge, MA). For the detection of NOS1, the slices were incubated overnight with a NOS1 antibody (A-11; mouse monoclonal IgG, 1:500; Santa Cruz Biotechnology, Santa Cruz, CA) followed by the fluorescent secondary antibody (Alexa Fluor 488 goat anti-mouse; 1:1000). Negative controls included sections incubated without primary and/or secondary antibodies. All slices were mounted with VECTASHIELD antifade medium with DAPI (Vector Laboratories, Burlingame, CA), and images were captured with Nikon Eclipse E600FN Confocal Microscope equipped with a Cascade 131 512F digital camera (Photometrics, Tucson, AZ).
Human Kidney Tissue Culture
The human kidney biopsy samples were cultured with the hanging drop technique, as recently described.46 The culture medium contained DMEM (Invitrogen, Carlsbad, CA); 0.1 mM MEM nonessential amino acids (Invitrogen); 2 mM sodium pyruvate; 2 nM L-glutamine; 0.01 mg/ml insulin, 5.5 μg/ml transferrin, and 5 μg/ml selenium supplement; 100 U/ml penicillin; 100 mg/ml streptomycin; and 10% FBS. A drop of medium (approximately 30 μl) was prepared on the lid of a petri dish. A piece of human kidney biopsy tissue (1 mm3) was placed into the drop, and the dish was carefully inverted to keep the drop intact with the tissue suspended. PBS was added to the bottom of the dish to prevent dehydration. The kidney biopsy tissue pieces were cultured at 37°C in a humidified atmosphere of 5% CO2 and 95% room air. Because most of the NOS1 in the renal cortex comes from macula densa cells, to determine whether glucose alters macula densa NOS1 expression or phosphorylation, we cultured human renal cortex tissue in hanging drop medium with either 5.5 mM glucose or 16.7 mM glucose for 30 minutes, and then measured NOS1 expression and phosphorylation by Western blotting.
Western Blotting
An intravenous injection of 50 µl of 5 M glucose was applied in mice to induce hyperglycemia as described above, and after 60 minutes,47 we harvested the kidneys and measured NOS1 expression and phosphorylation in the renal cortex where most of the NOS1 comes from macula densa cells. Kidney tissue protein extracts (50 μg per lane) were separated on a 7.5% SDS-PAGE gel as described previously.28,41 After blocking for 1 hour at room temperature with 5% skim milk, the membranes were incubated overnight at 4°C with a C-terminal NOS1 antibody28,48 (mouse polyclonal IgG; 1:3000; BD Biosciences, San Jose, CA) or an antibody of NOS1 phosphorylated at Ser141749–51 (P-NOS1) (rabbit polyclonal IgG; 1:500; Abcam), respectively. The membranes were then incubated with horseradish peroxidase–conjugated secondary antibody (goat anti-mouse, IgG; 1:300,000; Bio-Rad, Hercules, CA; or goat anti-rabbit, IgG; 1:300,000). The immunoreactive bands were revealed by enhanced chemiluminescence detection on Hyperfilm (Amersham Pharmacia Biotech, Piscataway, NJ).
Statistical Analyses
Statistical analyses were performed using SPSS 13.0 (IBM, Armonk, NY). The effects of interest were tested using t test, or ANOVA, followed by multiple comparisons post hoc test when appropriate. Data are presented as mean±SEM, and P<0.05 was considered statistically significant.
Results
A Bolus Intravenous Injection of Glucose Induced Hyperglycemia and Glucosuria with Increased GFR
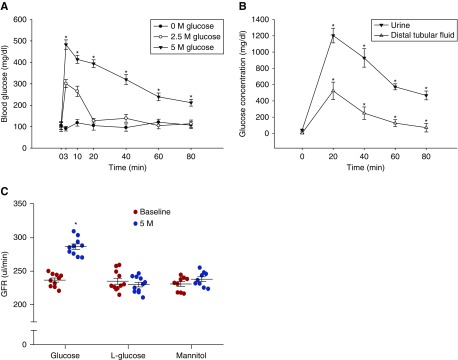
A bolus intravenous injection of 50 µl of 2.5 M glucose increased blood glucose immediately after injection, but the blood glucose quickly returned to normal levels at 20 minutes (Figure 1A). An intravenous injection of 50 µl of 5 M glucose raised blood glucose over 200 mg/dl and induced significant glucosuria as well as increased glucose concentration in the early distal tubule for 80 minutes (Figure 1B).
Figure 1.
Intravenous injection of glucose induces hyperglycemia and glucosuria with increased GFR. (A) The blood glucose concentration in response to different doses of intravenous glucose injection; n=10–12; *P<0.01 versus baseline. (B) The glucose concentration in urine and early distal tubular fluid in response to an intravenous injection of 50 µl of 5 M glucose; n=5–7; *P<0.01 versus baseline. (C) An intravenous injection of 50 µl of 5 M glucose increased GFR by 21.2±5.3%; n=10; *P<0.01 versus baseline. Intravenous injection of L-glucose or mannitol had no significant effect on the GFR; n=9–11. Statistical difference was calculated by two-way ANOVA followed by multiple comparisons post hoc test.
Using the latter glucose dose, we found that an intravenous injection of glucose significantly increased GFR by 21.2±5.3%. However, neither L-glucose nor mannitol had any effect on GFR. The data demonstrates that an increase in blood glucose over the renal threshold raises GFR (Figure 1C).
Tubular Glucose Blunts TGF Response in vivo and in vitro
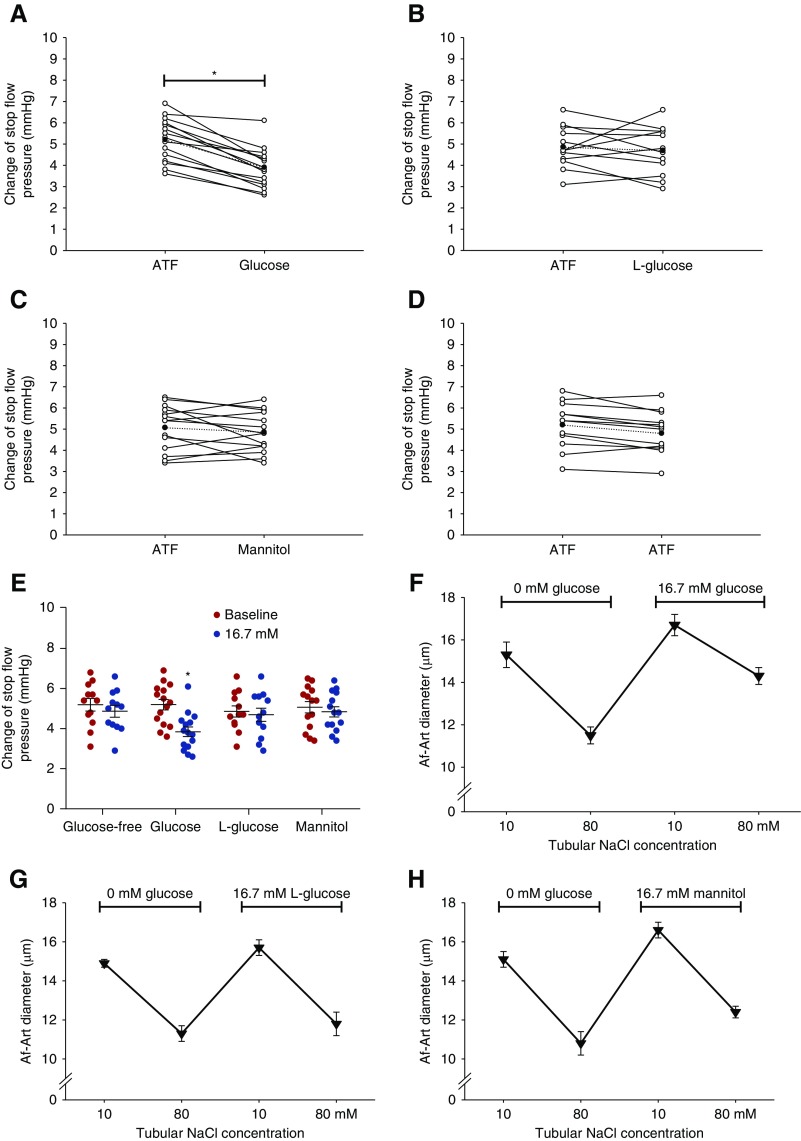
TGF responses in vivo were measured twice consecutively in each nephron using ATF without and with glucose. In the first measurement, when tubular perfusion rate of ATF without glucose was increased from 0 to 40 nl/min, Psf decreased from 38.4±1.9 to 33.2±2.5 mm Hg (ΔPsf=5.2±1.0 mm Hg), whereas the mean arterial pressure was 88.9±3.9 mm Hg during measurement. In the second measurement with ATF containing 16.7 mM glucose, Psf decreased from 38.2±1.6–34.4±1.8 mm Hg (ΔPsf =3.8±0.9 mm Hg), whereas the mean arterial pressure was 87.2±4.2 mm Hg (Figure 2A). When we used glucose-free ATF, ATF with L-glucose or ATF with mannitol in the second measurement, there were no significant differences between the first and second TGF response measurements (Figure 2, B–D). These data demonstrate that tubular glucose inhibits the TGF response in vivo (Figure 2E).
Figure 2.
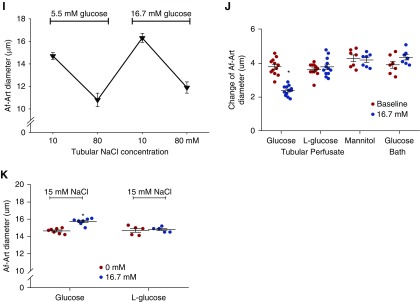
Tubular glucose blunts TGF response in vivo and in vitro. The TGF responses in vivo were measured twice consecutively in each nephron with micropuncture using ATF without and with glucose. TGF response was indicated by the maximum ΔPsf when the tubular perfusion rate was increased from 0 to 40 nl/min. (A) In the presence of glucose, ΔPsf was reduced from 5.2±1.0 to 3.8±0.9 mm Hg; n=5 mice per 15 tubules; *P<0.05 versus baseline. (B–E) None of ATF with L-glucose, ATF with mannitol, or glucose-free ATF significantly changed ΔPsf; n=3–5 mice per 12–14 tubules; *P<0.05 versus baseline. (F–J) TGF response in vitro was determined by the change of Af-Art diameter when switching the tubular perfusate from 10 to 80 mmol/L of NaCl, and in the presence of 16.7 mM glucose in the tubular perfusate, TGF response was reduced from 3.8±0.2 to 2.4±0.2 µm. Neither L-glucose nor mannitol significantly altered TGF response in vitro. When the glucose concentration in the bath solution was increased from 5.5 to 16.7 mM while the tubule was perfused with a glucose-free solution, the TGF response in vitro was not significantly changed; n=7–12; *P<0.01 versus 0 mM glucose. (K) Under conditions of constant low tubular NaCl of 15 mM, the diameter of Af-Art increased from 14.6±0.3 to 15.7±0.4 μm when glucose concentration in tubular perfusate was increased from 0 to 16.7 mM; n=5–7; *P<0.05 versus 0 mM glucose. Statistical difference in (A–D) was calculated by paired t test. Statistical difference in (E, J, and K) was calculated by two-way ANOVA followed by multiple comparisons post hoc test. Statistical difference in (F–I) was calculated by one-way ANOVA.
We also measured the TGF response in vitro in isolated and double-perfused JGAs. In the absence of glucose in the tubular perfusate, the TGF response indicated by the average change of Af-Art diameter was 3.8±0.2 μm. In the presence of glucose in the tubular perfusate, the TGF response was reduced to 2.4±0.2 µm (Figure 2F). When D-glucose was replaced by L-glucose or mannitol, no significant differences were observed between the first and second TGF response measurements (Figure 2, G and H). These data demonstrate that tubular glucose inhibits TGF response in vitro.
In contrast, the TGF response was not significantly changed when the glucose concentration in the bath solution was increased from 5.5 to 16.7 mM while the tubule was perfused with a glucose-free solution (Figure 2I). These data demonstrate that it is the glucose in the lumen rather than at the basolateral side of the macula densa that blunts the TGF response (Figure 2J).
In addition, under conditions of constant, low tubular NaCl of 15 mM, the diameter of Af-Art increased from 14.6±0.3 to 15.7±0.4 μm when D-glucose concentration in tubular perfusate was increased from 0 to 16.7 mM, whereas the Af-Art diameter was not significantly changed when D-glucose in the tubular perfusate was replaced by L-glucose (Figure 2K).
Tubular Glucose Inhibits TGF Response via SGLT1
To explore the expression of glucose transporters in the macula densa, we identified 38 macula densa cells that express both Slc12a1 and Nos1 out of 12,090 kidney cells from C57BL/6 mice, and compared the mRNA expression levels of glucose transporters in these selected macula densa cells. Our analysis showed the highest transcriptional level of Slc5a1 among the glucose transporters, suggesting that SGLT1 might be the primary glucose transporter in the macula densa cells (Table 2).
Table 2.
The transcriptional copy of glucose transporters in the macula densa
| Gene | Transcriptional Level |
|---|---|
| Slc5a1 | 0.55263±0.175681 |
| Slc2a1 | 0.13158±0.055572 |
| Slc2a4 | 0.10526±0.050452 |
| Slc2a8 | 0.07895±0.044332 |
| Slc2a2 | 0.05263±0.036709 |
| Slc5a2 | 0.05263±0.036709 |
| Slc2a13 | 0.02632±0.026316 |
| Slc2a3 | 0±0 |
| Slc2a5 | 0±0 |
| Slc2a6 | 0±0 |
| Slc2a9 | 0±0 |
| Slc2a12 | 0±0 |
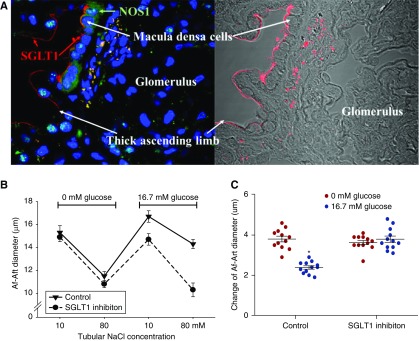
To confirm the expression of SGLT1 at the macula densa in the human kidney, we performed double-immunofluorescence staining of SGLT1 and NOS1 in slices of human kidney biopsy specimens. We found that SGLT1 (red) was clearly detected on the apical membrane of macula densa cells marked with an NOS1 antibody (green) using confocal microscopy (Figure 3A).
Figure 3.
Tubular glucose-induced TGF inhibition is mediated via macula densa SGLT1. SGLT1 on macula densa cells was identified by immunofluorescence staining in human kidney biopsy specimens. (A) Positive staining of SGLT1 (red) was observed in macula densa cells marked with NOS1 antibody (green). (B and C) SGLT1 inhibitor KGA-2727 blocked the inhibitory effect of luminal glucose on the TGF response in vitro in isolated perfused JGA from mouse kidney; n=10–12; *P<0.01 versus baseline. Statistical difference was calculated by two-way ANOVA followed by multiple comparisons post hoc test.
In the presence of the selective SGLT1 inhibitor, the TGF response in vitro was not significantly attenuated by increasing the luminal glucose concentration (Figure 3B). These data demonstrate that the SGLT1 inhibitor blocks the inhibitory effect of luminal glucose on the TGF response (Figure 3C).
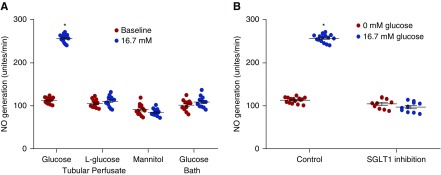
Tubular Glucose Stimulates NO Generation in the Macula Densa
Adding 16.7 mM glucose to the tubular perfusate increased NO generation in the macula densa of isolated JGAs by 117.2±13.4%. In contrast, macula densa NO generation in the macula densa was not significantly changed by luminal L-glucose, luminal mannitol, or glucose in the bath solution (Figure 4A). These data indicated that it is the glucose in the lumen rather than bath that stimulates macula densa NO generation.
Figure 4.
Tubular glucose stimulates NO generation in the macula densa. The macula densa NO generation was measured in isolated perfused JGA with DAF-2 DA. In the presence of glucose in tubular perfusate, NO generation in the macula densa was increased from 118.3±7.7 to 257±9.2 U/min. NO generation was not significantly altered by luminal L-glucose or mannitol. (A) Increased glucose concentration in the bath had no significant effect on NO generation in the macula densa; n=13–15; *P<0.01 versus baseline. (B) SGLT1 inhibitor KGA-2727 blocked the glucose-induced NO generation at the macula densa in isolated perfused JGA from mouse kidney; n=10–15; *P<0.01 versus baseline. Statistical difference was calculated by two-way ANOVA followed by multiple comparisons post hoc test.
Additionally, in the presence of the SGLT1 inhibitor, NO generation in the macula densa was not significantly changed after adding 16.7 mM glucose in the tubular perfusate (Figure 4B), indicating that luminal glucose induces NO generation in the macula densa via SGLT1.
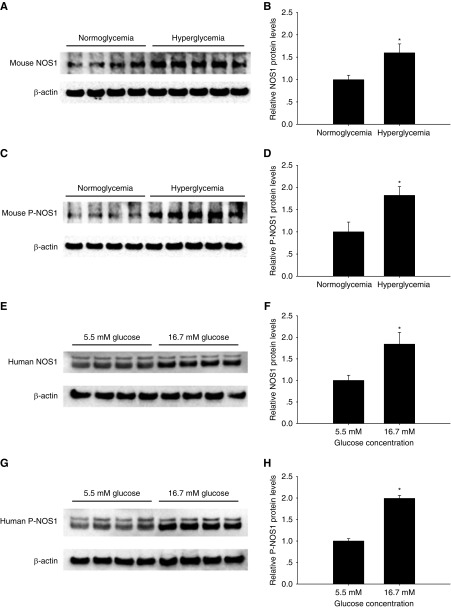
Glucose Increases Macula Densa NOS1 Expression and NOS1 Phosphorylation at Ser1417
Hyperglycemia resulted in a 1.6±0.2-fold increase in protein level of NOS1 (Figure 5, A and B) and a 1.8±0.2-fold increase in protein level of P-NOS1 (Figure 5, C and D) in mouse renal cortex.
Figure 5.
Glucose upregulates macula densa NOS1 expression and phosphorylation. The protein levels of (A and B) NOS1and (C and D) P-NOS1 in the renal cortex. Hyperglycemia was induced by an intravenous injection of 50 µl of 5 M glucose in mice; n=4–5; *P<0.05 versus normoglycemia. The protein levels of (E and F) NOS1 and (G and H) P-NOS1 in human renal cortex tissue cultured with different glucose concentrations; n=4; *P<0.05 versus 5.5 mM glucose group. Statistical difference was calculated by t test.
There was a 1.8±0.3-fold increase in the protein level of NOS1 (Figure 5, E and F) and a 1.9±0.1-fold increase in the protein level of P-NOS1 in human renal cortex tissue cultured with 16.7 mM glucose compared with 5.5 mM glucose (Figure 5, G and H).
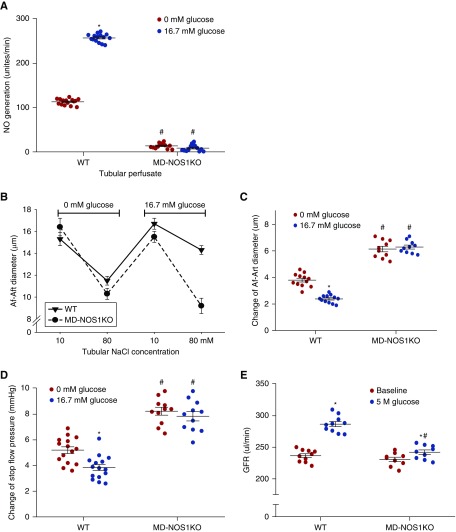
Glucose-Induced Hyperfiltration Is Mediated by Macula Densa NOS1
To determine the significance of macula densa NOS1 in glucose-induced hyperfiltration, we repeated the experiments above in MD-NOS1KO mice. Adding glucose to the macula densa perfusate had no effect on the NO generation in MD-NOS1KO mice (Figure 6A). The TGF in vitro (Figure 6, B and C) or TGF in vivo (Figure 6D) was not significantly changed in MD-NOS1KO mice after adding glucose to tubular perfusate. Furthermore, acute hyperglycemia–induced elevation in GFR was significantly attenuated in MD-NOS1KO mice compared with wild-type mice (Figure 6E). These results indicated that macula densa NOS1 mediates the glucose-induced hyperfiltration.
Figure 6.
Glucose-induced hyperfiltration is mediated by macula densa NOS1. (A) The effects of tubular glucose on NO generation in the macula densa (n=13–15), (B and C) TGF response in vitro (n=10–12), d an(D) TGF response in vivo (n=3–5 mice per 11–15 tubules) were measured in MD-NOS1KO mice and compared with wild-type (WT) mice. *P<0.05 versus baseline; #P<0.05 versus WT. (E) The change of GFR in response to an intravenous injection of 50 µl of 5 M glucose was measured in MD-NOS1KO mice and compared with WT mice; n=9–10; *P<0.05 versus baseline; #P<0.05 versus WT. The data of WT were the same data presented in Figure 1C, Figure 2, A and F, Figure 3C, and Figure 4A. Statistical difference was calculated by two-way ANOVA followed by multiple comparisons post hoc test.
Discussion
This study provides evidence for a novel mechanism of hyperglycemia-induced glomerular hyperfiltration in nondiabetic mice by showing that (1) luminal glucose at the macula densa is sensed via SGLT1 and thereby increases the activity and expression of NOS1, (2) luminal glucose at the macula densa blunts the TGF vasoconstrictor response, (3) this effect is dependent on intact SGLT1 and NOS1 in the macula densa, and (4) macula densa NOS1 mediates the acute hyperglycemia–induced increase in GFR.
The significance of blood glucose levels in diabetic hyperfiltration has been recognized in studies that reported a lowering of glomerular hyperfiltration in patients with diabetes after effective insulin therapy, but further increases with worsening of plasma glucose control.4,52 In accordance, acute intravenous infusion of glucose can increase GFR in both individuals with and without diabetes,53–55 as well as in experimental animals.56,57 The renal threshold for glucose reabsorption corresponds to blood glucose concentrations of approximately 180 mg/dl.58–60 In this study, we determined that an intravenous dose of glucose is able to induce a sustained increase in blood glucose levels over the renal threshold, along with significant glucosuria and increased glucose concentration in early distal tubular fluid in C57BL/6 mice. Because there is only fluid reabsorption but no more glucose reabsorption from the beginning of distal tubule to the end of nephron, the glucose concentration in the urine was much higher than that in distal tubule. In addition, consistent with the acute effect of hyperglycemia on GFR, this hyperglycemia model demonstrated that an intravenous injection of D-glucose, but not L-glucose or mannitol, significantly increased GFR in nondiabetic mice.
Previous studies have suggested a critical role of the TGF mechanism in glomerular hyperfiltration in response to moderate levels of hyperglycemia.13 The glucose infusion induced GFR elevation in nondiabetic dogs was absent in animals with nonfiltering kidneys, in which the TGF response was interrupted.57 In addition, the TGF response has been extensively studied in db/db mice and STZ-induced diabetic rats via measurement of proximal tubular Psf, proximal-distal differences in single-nephron GFR, or freeflow perturbation analysis of TGF efficiency at the natural operating point. In both type 1 and type 2 diabetic animal models, the TGF response was found to be inhibited or reset.11,12,33,61,62 However, it remained unclear whether glucose per se has a significant effect on the TGF response. Our study demonstrated that an increase in the concentration of D-glucose, but not L-glucose or mannitol, in the tubular perfusate attenuated the TGF response. Moreover, there was no significant effect when the glucose was applied in the bath, indicating it is the glucose at the apical rather than basolateral sides of the macula densa that inhibits the TGF response.
NO generated by NOS1 in the macula densa is a major modulator of the TGF response, which buffers or attenuates the vasoconstrictor TGF tone.21–26 However, the effect of glucose on macula densa NO generation remained unclear. In this study, we found an increase in luminal concentration of D-glucose rather than L-glucose or osmolarity enhanced NO generation in the macula densa. Moreover, the basolateral increase of glucose concentration in the macula densa had no significant effect on NO production, which suggests that it is the luminal increase in glucose concentration at the macula densa that enhances NO generation.
To explore the expression of glucose transporters in macula densa cells, we analyzed the single-cell RNA-sequencing profile of mouse kidneys that was deposited in the GEO database in a recently reported study.45 We defined macula densa cells by the expression of the two marker genes NOS1 and NKCC2, and compared the mRNA expression levels of established glucose transporters in these macula densa cells. We found that the glucose transporter with highest transcriptional level was SGLT1. SGLT1 mediates most of the sodium-dependent glucose uptake across apical cell membranes in the small intestine and the reabsorption of approximately 3% of the filtered glucose in the kidney, predominantly in the S2 and S3 segment of the proximal tubule.34,63 More recently, the expression of SGLT1 was also detected on the apical domain of the TAL of the loop of Henle and the macula densa in both mouse35 and rat kidneys36, but it was not detected in human macula densa cells with a specific antibody made by H.K.64 Because the localization and identification of the macula densa cells in the human kidney tissue in the latter study appeared ambiguous, we requested and used the same antibody to perform double-immunofluorescence staining of SGLT1 as well as NOS1 to localize the macula densa in human kidney biopsy specimens that had been immediately fixed after harvest. Our results confirmed the expression of SGLT1 in human macula densa, which provides clinical relevance and significance to the SGLT1 aspect of our study. Moreover, when the selective SGLT1 inhibitor KGA-2727 was added to the tubular perfusate, the glucose-induced macula densa NO generation and TGF inhibition were blocked. These results indicate that an increase in glucose concentration at the macula densa blunts the TGF response and enhances NO generation via SGLT1. Besides SGLT1, the second most highly expressed glucose transporter in the macula densa cells was GLUT1. For mammalian cells, GLUT1 is the most prominent transporter for glucose influx with a manner of passive and facilitative diffusion after concentration gradient. In the proximal tubule epithelial cells, GLUT1 also mediates the efflux of intracellular glucose in the basolateral membrane because the influx of luminal glucose via SGLTs elevates intracellular glucose concentration above the interstitium. Accordingly, we speculated that under normoglycemic conditions, GLUT1 in the basolateral membrane may mediate the influx of interstitial glucose as an energy source because of the minimal luminal glucose at the macula densa; however, under hyperglycemic conditions, GLUT1 may facilitate the efflux of intracellular glucose to interstitium through basolateral membrane because of the active glucose influx via luminal SGLT1.
This study also demonstrated that acute hyperglycemia was associated with the upregulation of protein levels of NOS1, along with increased phosphorylation of NOS1 at Ser1417 in mouse renal cortex, where most of the NOS1 comes from macula densa cells. Moreover, in cultured human renal cortex tissue, hanging drop medium with a high concentration of glucose increased NOS1 expression and stimulated the NOS1 phosphorylation at Ser1417. Regarding potential mechanisms, on the basis of the related literature, we speculate that glucose upregulates NOS1 expression and phosphorylates NOS1 via the PI3K/Akt65–69 and/or cAMP/PKA70–74 pathways.
Recently, our laboratory developed the MD-NOS1KO mouse strain by crossing NKCC2-Cre mice with NOS1-floxed mice, in which all of the splice variants of NOS1 were deleted from macula densa cells.28 Because the expression of NOS1 is negligible in TAL of loop of Henle compared with that in the macula densa, this NKCC2cre/NOS1flox/flox mouse strain could be considered as a macula densa–selective NOS1 knockout. Thus, in this study, the MD-NOS1KO mice were utilized to determine the significance of macula densa NOS1 in the effects of glucose on macula densa NO generation, TGF response, and GFR. We found that all of the glucose-induced effects that were observed in wild-type mice were absent in MD-NOS1KO mice, which suggests that macula densa NOS1 mediates the acute luminal glucose–induced increase in NO generation in the macula densa and TGF inhibition, as well as the acute hyperglycemia-induced glomerular hyperfiltration.
The major limitation of our study is that no functional TGF measurements are available in humans to show the clinical relevance of the proposed macula densa SGLT1-NOS1-TGF pathway. Although the SGLT1-NOS1 signaling pathway is the focus of this study, we expect that the effects of the SGLT1-NOS1 and SGLT2-NaCl pathways on TGF and GFR are separate mechanisms operating in the diabetic kidney. We assume that hyperglycemia increases glucose filtration and enhances SGLT2-mediated sodium-glucose reabsorption, which decreases NaCl delivery to the macula densa and inhibits the vasoconstrictor TGF tone.12,34,75 At the same time, the increased luminal glucose at the macula densa is sensed by SGLT1, which upregulates NOS1 expression and activity, and further reduces the vasoconstrictor TGF tone. Thus, these two signaling pathways additively promote the glomerular hyperfiltration in diabetes. Beyond these two TGF-related mechanisms, high glucose-induced vasodilation of Af-Art should be a possible vascular mechanism that cannot be discounted for diabetic hyperfiltration. We recently demonstrated that an increase in luminal glucose concentration dilates the mouse Af-Art by stimulation of the endothelium-derived NO production via GLUT1.76 Another study by Toma et al.77 also showed that high glucose in vascular perfusate rather than bath solution dilates the rabbit Af-Art, which involves the local accumulation of succinate and activation of G protein–coupled metabolic receptor GPR91. Additionally, acute hyperglycemia stimulates β-cells to produce and secret insulin. It has been demonstrated that insulin per se could induce an increase in GFR,78,79 which is associated with the direct renal vasodilation80,81 and sodium retention by enhanced reabsorption in distal tubule.80,82–84 Therefore, the elevation of insulin level might be another potential mechanism for the increases of GFR in response to acute hyperglycemia.
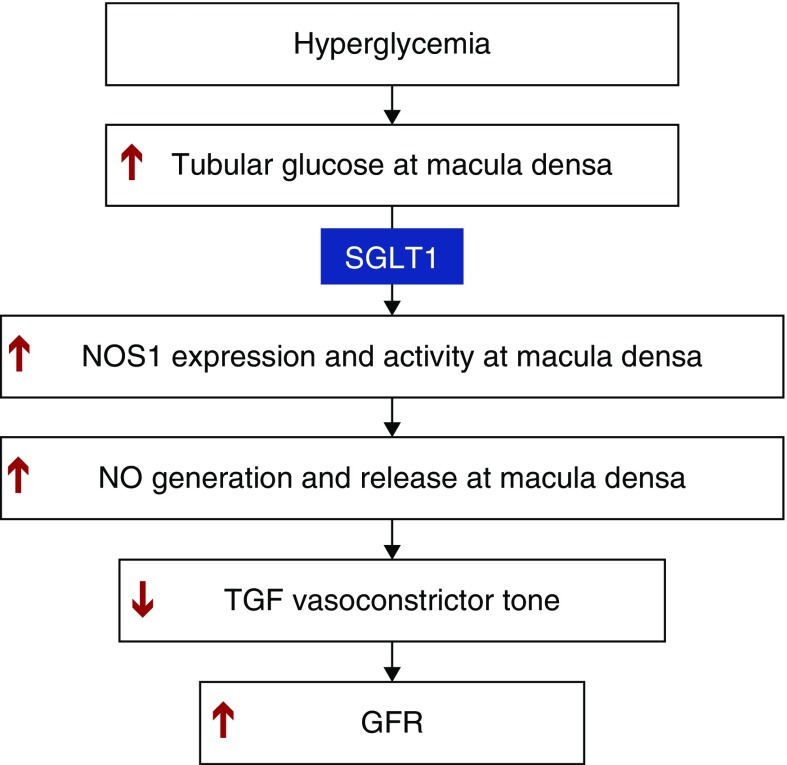
In conclusion, our study indicates that an acute increase in glucose concentration at the luminal side of the macula densa enhances the expression and activity of NOS1 via SGLT1, which blunts the TGF response and promotes the rise in GFR (Figure 7). This novel SGLT1-NOS1-TGF pathway mediates the glomerular hyperfiltration observed in response to acute hyperglycemia. These findings establish a critical role of macula densa NOS1 and SGLT1 as key determinants and potential novel therapeutic targets for glomerular hyperfiltration associated with acute hyperglycemia and thus, potentially, diabetes.
Figure 7.
Macula densa SGLT1-NOS1-TGF pathway mediates the glomerular hyperfiltration during hyperglycemia. An increase in glucose concentration at the luminal side of the macula densa upregulates the expression and activity of NOS1 via SGLT1, which blunts the TGF response and thereby promotes the rise in GFR.
Disclosures
Over the past 36 months, V.V. has served as a consultant and received honoraria from Bayer, Boehringer Ingelheim, Eli Lilly, Janssen Pharmaceutical, and Merck, and received grant support for investigator-initiated research from Astra-Zeneca, Bayer, Boehringer Ingelheim, Fresenius, and Janssen Pharmaceutical.
Supplementary Material
Acknowledgments
J.Z., J.W., and R.L. designed the study. J.Z., J.W., and S.J. performed the experiments. J.Z., J.W., L.X., and F.C. analyzed the data. J.Z. and J.W. drafted the manuscript. L.W., J.B., H.K., V.V., and R.L. revised the manuscript. All authors approved the final version of the manuscript.
This work was supported by American Society of Nephrology Ben J. Lipps Research Fellowship Awards (to J.Z. and J.W.), an American Heart Association Career Development Award 18CDA34110441 (to L.W.), and the National Institutes of Health (grants DK112042 and DK106102 to V.V.; and grants DK099276, HL142814, and HL137987 to R.L.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “The Other Glucose Transporter, SGLT1 – Also a Potential Trouble Maker in Diabetes?,” on pages 519–521.
Supplemental Materials
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018080844/-/DCSupplemental.
Supplemental Figure 1. To avoid any systematic error introduced by consecutive measurements of ΔPsf in the same nephron, TGF response in vivo was also assessed directly under high-glucose conditions. The ΔPsf measured with ATF containing 16.7 mM glucose was significantly lower than the control measurements with glucose-free ATF, ATF containing 16.7 mM L-glucose, or ATF containing 16.7 mM mannitol.
Supplemental Figure 2. The complete gels for the Western blot of NOS1 and P-NOS1 in the (A and B) mouse renal cortex and (C and D) human kidney biopsy tissue.
References
- 1.Selby JV, FitzSimmons SC, Newman JM, Katz PP, Sepe S, Showstack J: The natural history and epidemiology of diabetic nephropathy. Implications for prevention and control. JAMA 263: 1954–1960, 1990 [PubMed] [Google Scholar]
- 2.Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T: Diabetic nephropathy: Diagnosis, prevention, and treatment. Diabetes Care 28: 164–176, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Tervaert TW, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, et al.: Renal Pathology Society : Pathologic classification of diabetic nephropathy. J Am Soc Nephrol 21: 556–563, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Bank N: Mechanisms of diabetic hyperfiltration. Kidney Int 40: 792–807, 1991 [DOI] [PubMed] [Google Scholar]
- 5.Levine DZ: Can rodent models of diabetic kidney disease clarify the significance of early hyperfiltration?: Recognizing clinical and experimental uncertainties. Clin Sci (Lond) 114: 109–118, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Vora JP, Dolben J, Dean JD, Thomas D, Williams JD, Owens DR, et al.: Renal hemodynamics in newly presenting non-insulin dependent diabetes mellitus. Kidney Int 41: 829–835, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Nelson RG, Bennett PH, Beck GJ, Tan M, Knowler WC, Mitch WE, et al.: Diabetic Renal Disease Study Group : Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. N Engl J Med 335: 1636–1642, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Keller CK, Bergis KH, Fliser D, Ritz E: Renal findings in patients with short-term type 2 diabetes. J Am Soc Nephrol 7: 2627–2635, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Helal I, Fick-Brosnahan GM, Reed-Gitomer B, Schrier RW: Glomerular hyperfiltration: Definitions, mechanisms and clinical implications. Nat Rev Nephrol 8: 293–300, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Anderson S, Vora JP: Current concepts of renal hemodynamics in diabetes. J Diabetes Complications 9: 304–307, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Thomson SC, Deng A, Bao D, Satriano J, Blantz RC, Vallon V: Ornithine decarboxylase, kidney size, and the tubular hypothesis of glomerular hyperfiltration in experimental diabetes. J Clin Invest 107: 217–224, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vallon V, Richter K, Blantz RC, Thomson S, Osswald H: Glomerular hyperfiltration in experimental diabetes mellitus: Potential role of tubular reabsorption. J Am Soc Nephrol 10: 2569–2576, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Vallon V, Thomson SC: Renal function in diabetic disease models: The tubular system in the pathophysiology of the diabetic kidney. Annu Rev Physiol 74: 351–375, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, et al.: Mediation of tubuloglomerular feedback by adenosine: Evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci U S A 98: 9983–9988, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren Y, Arima S, Carretero OA, Ito S: Possible role of adenosine in macula densa control of glomerular hemodynamics. Kidney Int 61: 169–176, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Ren Y, Garvin JL, Liu R, Carretero OA: Role of macula densa adenosine triphosphate (ATP) in tubuloglomerular feedback. Kidney Int 66: 1479–1485, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Thomson S, Bao D, Deng A, Vallon V: Adenosine formed by 5′-nucleotidase mediates tubuloglomerular feedback. J Clin Invest 106: 289–298, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ollerstam A, Pittner J, Persson AE, Thorup C: Increased blood pressure in rats after long-term inhibition of the neuronal isoform of nitric oxide synthase. J Clin Invest 99: 2212–2218, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welch WJ, Tojo A, Wilcox CS: Roles of NO and oxygen radicals in tubuloglomerular feedback in SHR. Am J Physiol Renal Physiol 278: F769–F776, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Ren YL, Garvin JL, Carretero OA: Role of macula densa nitric oxide and cGMP in the regulation of tubuloglomerular feedback. Kidney Int 58: 2053–2060, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Wilcox CS, Welch WJ, Murad F, Gross SS, Taylor G, Levi R, et al.: Nitric oxide synthase in macula densa regulates glomerular capillary pressure. Proc Natl Acad Sci U S A 89: 11993–11997, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mundel P, Bachmann S, Bader M, Fischer A, Kummer W, Mayer B, et al.: Expression of nitric oxide synthase in kidney macula densa cells. Kidney Int 42: 1017–1019, 1992 [DOI] [PubMed] [Google Scholar]
- 23.Liu R, Ren Y, Garvin JL, Carretero OA: Superoxide enhances tubuloglomerular feedback by constricting the afferent arteriole. Kidney Int 66: 268–274, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Welch WJ, Wilcox CS: Role of nitric oxide in tubuloglomerular feedback: Effects of dietary salt. Clin Exp Pharmacol Physiol 24: 582–586, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Liu R, Carretero OA, Ren Y, Garvin JL: Increased intracellular pH at the macula densa activates nNOS during tubuloglomerular feedback. Kidney Int 67: 1837–1843, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Vallon V, Traynor T, Barajas L, Huang YG, Briggs JP, Schnermann J: Feedback control of glomerular vascular tone in neuronal nitric oxide synthase knockout mice. J Am Soc Nephrol 12: 1599–1606, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Lu D, Fu Y, Lopez-Ruiz A, Zhang R, Juncos R, Liu H, et al.: Salt-sensitive splice variant of nNOS expressed in the macula densa cells. Am J Physiol Renal Physiol 298: F1465–F1471, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu Y, Wei J, Stec DE, Roman RJ, Ge Y, Cheng L, et al.: Macula densa nitric oxide synthase 1β protects against salt-sensitive hypertension. J Am Soc Nephrol 27: 2346–2356, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Chandrashekar K, Wang L, Lai EY, Wei J, Zhang G, et al.: Inhibition of nitric oxide synthase 1 induces salt-sensitive hypertension in nitric oxide synthase 1α knockout and wild-type mice. Hypertension 67: 792–799, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tolins JP, Shultz PJ, Raij L, Brown DM, Mauer SM: Abnormal renal hemodynamic response to reduced renal perfusion pressure in diabetic rats: Role of NO. Am J Physiol 265: F886–F895, 1993 [DOI] [PubMed] [Google Scholar]
- 31.Mattar AL, Fujihara CK, Ribeiro MO, de Nucci G, Zatz R: Renal effects of acute and chronic nitric oxide inhibition in experimental diabetes. Nephron 74: 136–143, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Thomson SC, Vallon V, Blantz RC: Kidney function in early diabetes: The tubular hypothesis of glomerular filtration. Am J Physiol Renal Physiol 286: F8–F15, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Vallon V, Blantz RC, Thomson S: Homeostatic efficiency of tubuloglomerular feedback is reduced in established diabetes mellitus in rats. Am J Physiol 269: F876–F883, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Vallon V, Thomson SC: Targeting renal glucose reabsorption to treat hyperglycaemia: The pleiotropic effects of SGLT2 inhibition. Diabetologia 60: 215–225, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madunić IV, Breljak D, Karaica D, Koepsell H, Sabolić I: Expression profiling and immunolocalization of Na+-D-glucose-cotransporter 1 in mice employing knockout mice as specificity control indicate novel locations and differences between mice and rats. Pflugers Arch 469: 1545–1565, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balen D, Ljubojevic M, Breljak D, Brzica H, Zlender V, Koepsell H, et al.: Revised immunolocalization of the Na+-D-glucose cotransporter SGLT1 in rat organs with an improved antibody. Am J Physiol Cell Physiol 295: C475–C489, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Mühlbauer RC, Fleisch H: Abnormal renal glucose handling in X-linked hypophosphataemic mice. Clin Sci (Lond) 80: 71–76, 1991 [DOI] [PubMed] [Google Scholar]
- 38.Liang Y, Arakawa K, Ueta K, Matsushita Y, Kuriyama C, Martin T, et al.: Effect of canagliflozin on renal threshold for glucose, glycemia, and body weight in normal and diabetic animal models. PLoS One 7: e30555, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu Y, Hall JE, Lu D, Lin L, Manning RD Jr., Cheng L, et al.: Aldosterone blunts tubuloglomerular feedback by activating macula densa mineralocorticoid receptors. Hypertension 59: 599–606, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu Y, Lu Y, Liu EY, Zhu X, Mahajan GJ, Lu D, et al.: Testosterone enhances tubuloglomerular feedback by increasing superoxide production in the macula densa. Am J Physiol Regul Integr Comp Physiol 304: R726–R733, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei J, Zhang J, Wang L, Cha BJ, Jiang S, Liu R: A new low-nephron CKD model with hypertension, progressive decline of renal function, and enhanced inflammation in C57BL/6 mice. Am J Physiol Renal Physiol 314: F1008–F1019, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L, Wei J, Jiang S, Li HH, Fu L, Zhang J, et al.: Effects of different storage solutions on renal ischemia tolerance after kidney transplantation in mice. Am J Physiol Renal Physiol 314: F381–F387, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shibazaki T, Tomae M, Ishikawa-Takemura Y, Fushimi N, Itoh F, Yamada M, et al.: KGA-2727, a novel selective inhibitor of a high-affinity sodium glucose cotransporter (SGLT1), exhibits antidiabetic efficacy in rodent models. J Pharmacol Exp Ther 342: 288–296, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Wang L, Shen C, Liu H, Wang S, Chen X, Roman RJ, et al.: Shear stress blunts tubuloglomerular feedback partially mediated by primary cilia and nitric oxide at the macula densa. Am J Physiol Regul Integr Comp Physiol 309: R757–R766, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park J, Shrestha R, Qiu C, Kondo A, Huang S, Werth M, et al.: Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science 360: 758–763, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang S, Wang X, Boone J, Wie J, Yip KP, Zhang J, et al.: Application of hanging drop technique for kidney tissue culture. Kidney Blood Press Res 42: 220–231, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baig MS, Zaichick SV, Mao M, de Abreu AL, Bakhshi FR, Hart PC, et al.: NOS1-derived nitric oxide promotes NF-κB transcriptional activity through inhibition of suppressor of cytokine signaling-1. J Exp Med 212: 1725–1738, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu Q, Shao R, Qian HS, George SE, Rockey DC: Gene transfer of the neuronal NO synthase isoform to cirrhotic rat liver ameliorates portal hypertension. J Clin Invest 105: 741–748, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Z, Ma J, Miyoshi C, Li Y, Sato M, Ogawa Y, et al.: Quantitative phosphoproteomic analysis of the molecular substrates of sleep need. Nature 558: 435–439, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sastre E, Caracuel L, Prieto I, Llévenes P, Aller MA, Arias J, et al.: Decompensated liver cirrhosis and neural regulation of mesenteric vascular tone in rats: Role of sympathetic, nitrergic and sensory innervations. Sci Rep 6: 31076, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han JY, Kang MJ, Kim KH, Han PL, Kim HS, Ha JY, et al.: Nitric oxide induction of parkin translocation in PTEN-induced putative kinase 1 (PINK1) deficiency: Functional role of neuronal nitric oxide synthase during mitophagy. J Biol Chem 290: 10325–10335, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG: Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia 52: 691–697, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Christiansen JS, Frandsen M, Parving HH: Effect of intravenous glucose infusion on renal function in normal man and in insulin-dependent diabetics. Diabetologia 21: 368–373, 1981 [DOI] [PubMed] [Google Scholar]
- 54.Bounous G, Shumacker HB Jr.: Influence of blood sugar levels upon renal blood flow. Ann Surg 151: 441–452, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fox M, Thier S, Rosenberg L, Segal S: Impaired renal tubular function induced by sugar infusion in man. J Clin Endocrinol Metab 24: 1318–1327, 1964 [DOI] [PubMed] [Google Scholar]
- 56.Noonan WT, Shapiro VM, Banks RO: Renal glucose reabsorption during hypertonic glucose infusion in female streptozotocin-induced diabetic rats. Life Sci 68: 2967–2977, 2001 [DOI] [PubMed] [Google Scholar]
- 57.Woods LL, Mizelle HL, Hall JE: Control of renal hemodynamics in hyperglycemia: Possible role of tubuloglomerular feedback. Am J Physiol 252: F65–F73, 1987 [DOI] [PubMed] [Google Scholar]
- 58.Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J: Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes 54: 3427–3434, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Lawrence RD: Renal threshold for glucose: Normal and in diabetics. BMJ 1: 766–768, 1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DeFronzo RA, Hompesch M, Kasichayanula S, Liu X, Hong Y, Pfister M, et al.: Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care 36: 3169–3176, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levine DZ, Iacovitti M, Robertson SJ, Mokhtar GA: Modulation of single-nephron GFR in the db/db mouse model of type 2 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol 290: R975–R981, 2006 [DOI] [PubMed] [Google Scholar]
- 62.Levine DZ, Iacovitti M, Robertson SJ: Modulation of single-nephron GFR in the db/db mouse model of type 2 diabetes mellitus. II. Effects of renal mass reduction. Am J Physiol Regul Integr Comp Physiol 294: R1840–R1846, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Gorboulev V, Schürmann A, Vallon V, Kipp H, Jaschke A, Klessen D, et al.: Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 61: 187–196, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vrhovac I, Balen Eror D, Klessen D, Burger C, Breljak D, Kraus O, et al.: Localizations of Na(+)-D-glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine, liver, lung, and heart. Pflugers Arch 467: 1881–1898, 2015 [DOI] [PubMed] [Google Scholar]
- 65.Hinchee-Rodriguez K, Garg N, Venkatakrishnan P, Roman MG, Adamo ML, Masters BS, et al.: Neuronal nitric oxide synthase is phosphorylated in response to insulin stimulation in skeletal muscle. Biochem Biophys Res Commun 435: 501–505, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qin D, Zhang GM, Xu X, Wang LY: The PI3K/Akt signaling pathway mediates the high glucose-induced expression of extracellular matrix molecules in human retinal pigment epithelial cells. J Diabetes Res 2015: 920280, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lonze BE, Ginty DD: Function and regulation of CREB family transcription factors in the nervous system. Neuron 35: 605–623, 2002 [DOI] [PubMed] [Google Scholar]
- 68.Sasaki M, Gonzalez-Zulueta M, Huang H, Herring WJ, Ahn S, Ginty DD, et al.: Dynamic regulation of neuronal NO synthase transcription by calcium influx through a CREB family transcription factor-dependent mechanism. Proc Natl Acad Sci U S A 97: 8617–8622, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tanaka S, Hosogi S, Sawabe Y, Shimamoto C, Matsumura H, Inui T, et al.: PPARα induced NOS1 phosphorylation via PI3K/Akt in Guinea pig antral mucous cells: NO-enhancement in Ca(2+)-regulated exocytosis. Biomed Res 37: 167–178, 2016 [DOI] [PubMed] [Google Scholar]
- 70.Gourdon L, Lou DQ, Raymondjean M, Vasseur-Cognet M, Kahn A: Negative cyclic AMP response elements in the promoter of the L-type pyruvate kinase gene. FEBS Lett 459: 9–14, 1999 [DOI] [PubMed] [Google Scholar]
- 71.Viollet B, Kahn A, Raymondjean M: Protein kinase A-dependent phosphorylation modulates DNA-binding activity of hepatocyte nuclear factor 4. Mol Cell Biol 17: 4208–4219, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boissel JP, Bros M, Schröck A, Gödtel-Armbrust U, Förstermann U: Cyclic AMP-mediated upregulation of the expression of neuronal NO synthase in human A673 neuroepithelioma cells results in a decrease in the level of bioactive NO production: Analysis of the signaling mechanisms that are involved. Biochemistry 43: 7197–7206, 2004 [DOI] [PubMed] [Google Scholar]
- 73.Yen DH, Chen LC, Shen YC, Chiu YC, Ho IC, Lou YJ, et al.: Protein kinase A-dependent neuronal nitric oxide synthase activation mediates the enhancement of baroreflex response by adrenomedullin in the nucleus tractus solitarii of rats. J Biomed Sci 18: 32, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hurt KJ, Sezen SF, Lagoda GF, Musicki B, Rameau GA, Snyder SH, et al.: Cyclic AMP-dependent phosphorylation of neuronal nitric oxide synthase mediates penile erection. Proc Natl Acad Sci U S A 109: 16624–16629, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thomson SC, Rieg T, Miracle C, Mansoury H, Whaley J, Vallon V, et al.: Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol 302: R75–R83, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang J, Jiang S, Wei J, Yip KP, Wang L, Lai EY, et al.: Glucose dilates renal afferent arterioles via glucose transporter-1. Am J Physiol Renal Physiol 315: F123–F129, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Toma I, Kang JJ, Sipos A, Vargas S, Bansal E, Hanner F, et al.: Succinate receptor GPR91 provides a direct link between high glucose levels and renin release in murine and rabbit kidney. J Clin Invest 118: 2526–2534, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hall JE, Coleman TG, Mizelle HL, Smith MJ Jr.: Chronic hyperinsulinemia and blood pressure regulation. Am J Physiol 258: F722–F731, 1990 [DOI] [PubMed] [Google Scholar]
- 79.Cohen AJ, McCarthy DM, Stoff JS: Direct hemodynamic effect of insulin in the isolated perfused kidney. Am J Physiol 257: F580–F585, 1989 [DOI] [PubMed] [Google Scholar]
- 80.Stenvinkel P, Bolinder J, Alvestrand A: Effects of insulin on renal haemodynamics and the proximal and distal tubular sodium handling in healthy subjects. Diabetologia 35: 1042–1048, 1992 [DOI] [PubMed] [Google Scholar]
- 81.Stenvinkel P, Ottosson-Seeberger A, Alvestrand A: Renal hemodynamics and sodium handling in moderate renal insufficiency: The role of insulin resistance and dyslipidemia. J Am Soc Nephrol 5: 1751–1760, 1995 [DOI] [PubMed] [Google Scholar]
- 82.Skøtt P, Hother-Nielsen O, Bruun NE, Giese J, Nielsen MD, Beck-Nielsen H, et al.: Effects of insulin on kidney function and sodium excretion in healthy subjects. Diabetologia 32: 694–699, 1989 [DOI] [PubMed] [Google Scholar]
- 83.Friedberg CE, van Buren M, Bijlsma JA, Koomans HA: Insulin increases sodium reabsorption in diluting segment in humans: Evidence for indirect mediation through hypokalemia. Kidney Int 40: 251–256, 1991 [DOI] [PubMed] [Google Scholar]
- 84.Ter Maaten JC, Voorburg A, Heine RJ, Ter Wee PM, Donker AJ, Gans RO: Renal handling of urate and sodium during acute physiological hyperinsulinaemia in healthy subjects. Clin Sci (Lond) 92: 51–58, 1997 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.