The cellular response of Lactococcus lactis MG1363 to infection by the virulent phage p2 was characterized through label-free quantitative proteomics. Combined with a targeted approach, we identified the highest proteome coverage for phage p2, a model for the most predominant group of lactococcal phages in the dairy industry worldwide. Four bacterial gene candidates possibly involved in phage infection were disrupted through specific knockouts to investigate their importance. Deletion of gene llmg_0219 (unknown function) resulted in a resistance to phage p2.
Keywords: Mass Spectrometry, Label-free quantification, Bacteria, Viruses, Proteogenomics, Genome editing, Lactococcus lactis, Phage infection
Graphical Abstract
Highlights
The proteomes of L. lactis MG1363 and phage p2 at different stages of infection were characterized.
16% (226/1412) of the bacterial proteins detected were unique to infected cultures.
A targeted approach using synthetic peptides improved the coverage of phage p2 proteome.
By means of proteogenomics, we uncovered a conserved phage protein coded by a previously unannotated gene.
Deletion of the bacterial gene llmg_0219 (unknown function) impedes phage p2 infection.
Abstract
Phages are viruses that specifically infect and eventually kill their bacterial hosts. Bacterial fermentation and biotechnology industries see them as enemies, however, they are also investigated as antibacterial agents for the treatment or prevention of bacterial infections in various sectors. They also play key ecological roles in all ecosystems. Despite decades of research some aspects of phage biology are still poorly understood. In this study, we used label-free quantitative proteomics to reveal the proteotypes of Lactococcus lactis MG1363 during infection by the virulent phage p2, a model for studying the biology of phages infecting Gram-positive bacteria. Our approach resulted in the high-confidence detection and quantification of 59% of the theoretical bacterial proteome, including 226 bacterial proteins detected only during phage infection and 6 proteins unique to uninfected bacteria. We also identified many bacterial proteins of differing abundance during the infection. Using this high-throughput proteomic datasets, we selected specific bacterial genes for inactivation using CRISPR-Cas9 to investigate their involvement in phage replication. One knockout mutant lacking gene llmg_0219 showed resistance to phage p2 because of a deficiency in phage adsorption. Furthermore, we detected and quantified 78% of the theoretical phage proteome and identified many proteins of phage p2 that had not been previously detected. Among others, we uncovered a conserved small phage protein (pORFN1) coded by an unannotated gene. We also applied a targeted approach to achieve greater sensitivity and identify undetected phage proteins that were expected to be present. This allowed us to follow the fate of pORF46, a small phage protein of low abundance. In summary, this work offers a unique view of the virulent phages' takeover of bacterial cells and provides novel information on phage-host interactions.
Phages are ubiquitous, abundant, genetically diverse and can produce a large burst of progeny virions in a short period of time following the infection of their bacterial host. Over the last century, the study of phage biology led to the rise of molecular biology and biotechnologies (1). Phage studies were key to the discovery of powerful research tools such as restriction enzymes and CRISPR-Cas systems (2). Despite their importance, some aspects of phage biology are still unknown. For example, except for a few classical models, technical limitations have left much of the phage proteome unresolved. However, recent instrumental improvements and the development of highly sensitive analytical methods contributed to advances in viral proteomics (3), with most studies focusing on the biology of eukaryotic viruses.
Phage p2 belongs to the Siphoviridae family of the Caudovirales order (4) and is a member of the Sk1virus genus (5, 6). This highly virulent phage infects Lactococcus lactis subsp. cremoris MG1363, a laboratory strain used in basic research on Gram-positive bacteria. Strains of L. lactis as well as other lactic acid bacteria (LAB) are used to ferment milk during the manufacture of several cheeses. Phages of the Sk1virus genus are by far the most prevalent in cheese factories worldwide and are responsible for milk fermentation defects. A wealth of data has been generated on phage p2, making it a model system for studying the biology of phages infecting LAB and Gram-positive bacteria. For example, its structural proteins have been analyzed in detail, which led to solving its complete structure using single-particle electron microscopy (7). The genome of the reference siphophage p2 has 49 annotated ORFs organized in three large gene clusters, chronologically transcribed upon infection. Although the late-expressed gene module mainly encodes proteins found in the virion structure, the early- and middle-expressed gene modules encode non-structural proteins responsible for hijacking the cellular machinery.
The transcriptional response of only a few phage-host pairs has been analyzed to identify viral and bacterial genes differentially expressed during the infection. For instance, the infection of the Gram-positive bacterium Bacillus subtilis by the virulent phage φ29 (Podoviridae family) was studied through deep RNA-sequencing (8) whereas the infection of L. lactis subsp. lactis IL1403 by the lytic siphophage c2 was studied with whole-genome microarrays (9). The effects of phage infection on gene expression were also studied through transcriptomic and metabolomic analysis of Pseudomonas aeruginosa cells infected with the lytic phage PaP1 (Myoviridae family) (10). Those reports provided insight into the control of gene expression during phage infection. However, the expression of genes can be regulated both at the post-transcriptional and translational levels. This implies that proteome analysis is essential to fully understand the cellular response to specific conditions and to follow the fate of each single protein.
An in-depth analysis of P. aeruginosa infection by the virulent podophage Luz19 characterized the cellular response at the DNA, RNA and protein levels, combining quantitative PCR, microarray, RNA-Seq, and two-dimensional gel electrophoresis (2D-GE) (11). It came as some surprise that only subtle changes in the bacterial proteome could be observed. Although 2D-GE can be used for protein fractionation prior to MS analysis, separation by one-dimensional (1D) SDS-PAGE (GeLC-MS/MS) or gel-free techniques can significantly increase proteome coverage for global proteomic studies (12). The entire genome of L. lactis MG1363 (GenBank AM406671) has been previously (re)sequenced and found to encode 2383 deduced proteins (13, 14). A few studies revealed the proteomic signatures, or proteotypes, of this strain in response to different stresses, such as acidic (15) and anaerobic conditions (16).
Here, we used label-free quantitative proteomics to reveal the proteotypes of L. lactis MG1363 during infection by the virulent phage p2. The bacterial and viral proteomes were characterized at different stages of infection. Several bacterial proteins were only detected during phage infection. Using the CRISPR-Cas9 genome editing tool, the genes coding for some of these proteins were inactivated. The inactivation of one of them led to a phage-resistant L. lactis derivative. Moreover, we report an increased proteome coverage for phage p2, including identification of many non-structural proteins. This high-throughput and discovery-based research provides a comprehensive view of phage infection at the proteome level and paves the way for future studies on phage-host interactions.
EXPERIMENTAL PROCEDURES
Phage Propagation
All bacterial strains, phages and plasmids used in this study are listed in supplemental Table S1. Phage p2 and its bacterial host L. lactis MG1363 were obtained from the Félix d'Hérelle Reference Center for Bacterial Viruses (www.phage.ulaval.ca). L. lactis MG1363 and its derivatives were grown statically at 30 °C in M17 broth (Oxoid, Ontario, Canada) supplemented with 0.5% (w/v) glucose monohydrate (GM17). For phage infection, growth media were supplemented with 10 mm CaCl2 (GM17+Ca). For phage amplification, a scraping of phage lysate glycerol stock preserved at −80 °C was co-inoculated with L. lactis MG1363 and incubated until lysis. The resulting phage lysate was filtered (0.45 μm PES filter) and used for a second round of amplification. In the latter instance, L. lactis MG1363 was grown to an optical density at 600 nm (OD600) of 0.35 and 100 μl of the first phage amplification lysate was added. The infected culture was incubated until lysis, filtered and stored at 4 °C until use. To obtain concentrated and purified samples of phage p2, one liter of a second amplification lysate was purified on a discontinuous cesium chloride gradient (17). For phage titration, double layer plaque assays (18) were performed with plates containing a bottom layer of GM17+Ca supplemented with 1.0% (w/v) agar and a top layer of the same medium supplemented with 0.75% (w/v) agar.
For transformation, L. lactis MG1363 electrocompetent cells were prepared as described previously (19). To maintain cloning plasmid vectors, chloramphenicol and/or erythromycin were added to the growth media to a final concentration of 5 μg/ml, each. Chemically competent E. coli NEB5α were purchased from New England Biolabs and transformed according to the manufacturer's instructions. E. coli transformants were grown in BHI medium supplemented with 150 μg/ml erythromycin (Em150) and incubated at 37 °C with agitation. For solid media, 1.5% (w/v) agar was added to BHI Em150 broth.
Time-course Infection and Protein Extraction
L. lactis MG1363 cells were incubated at 30 °C and grown until the OD600 reached 0.5. The culture was then concentrated 5-fold by centrifugation and resuspended in fresh medium. A sample of uninfected bacteria (T0) was collected just prior to adding the purified phage p2 at a multiplicity of infection (MOI) of 5. The infected culture was kept at 30 °C and samples T10, T20 and T40 were collected 10, 20- and 40-min post-infection, respectively. All samples were centrifuged immediately, and cell pellets frozen in liquid nitrogen for storage at −80 °C. Time-course infections were done in biological triplicates. For protein extraction, samples were rapidly thawed and lysed in 50 mm tris(hydroxymethyl)aminomethane (Tris)-HCl pH 8.0 containing 5% (w/v) sodium dodecyl sulfate (SDS), 10 mm ethylenediaminetetraacetic acid (EDTA)1 pH 8.0, 100 mm dithiothreitol, 10% (v/v) glycerol and bromphenol blue. Cells were disrupted with 0.1 mm glass beads and a homogenizer (5 cycles of 30 s at 6.5 m/s). Between each cycle, samples were put on ice for 5 min. Beads were removed by centrifugation and pipetting of the homogenates.
GeLC-MS/MS
Protein extracts were separated by 1D SDS-PAGE (12% polyacrylamide) and the gels were stained with Coomassie Brilliant Blue as previously described (20). For each sample, ten gel bands were excised, destained and digested with trypsin as reported elsewhere (20). Trypsin cleaves peptide bonds C-terminal to lysine and arginine residues. To recover the peptides, gel pieces were covered with ultra-pure water and incubated 15 min in an ultrasonic water bath. Just before MS analysis, the indexed retention time (iRT) standard kit (Biognosys, Schlieren, Switzerland) was prepared according to manufacturer's instructions and was added to each sample in a 1:100 ratio. Tryptic peptides were separated by liquid chromatography (LC) using an EASY-nLC II system and measured online by ESI-mass spectrometry on an Orbitrap Velos instrument. Peptides were loaded on a self-made analytical column (Aeris PEPTIDE 3.6 μm XB - C18 (phenomenex), OD 360 μm, ID 100 μm, length 20 cm) and eluted by a binary nonlinear gradient of 5–55% acetonitrile in 0.1% acetic acid over 100 min with a flow rate of 300 nl/min. For MS analysis, a full scan in the Orbitrap (m/z 300–1700) with a resolution of 60,000 was followed by CID MS/MS experiments of the twenty most abundant precursor ions acquired in the linear ion trap.
Targeted MS
In this study, a Tier 2 assay has been developed and applied, which allows to quantify the proteins of interest (pORF27, pORF46 and pORF47) repeatedly within samples thereby using isotopically labeled peptide standards for confident detection and precise quantification of those proteins. For the development of the SRM assay and for protein quantification applying this assay, a time-course phage infection was performed as described above, except that samples were taken at time 0 (uninfected culture), 5, 12, 15, 20, 30, and 40 min postinfection. Cell pellets were resuspended in TE-buffer (20 mm Tris-HCl pH 8.0, 1 mm EDTA) and disrupted similarly to GeLC-MS/MS samples (homogenization with glass beads). Protein concentration was determined for every sample using the Bradford assay (21). For discovery and quantification, 20 μg protein of three biological replicates was prepared for in-solution digestion. For digestion with trypsin, samples were prepared as previously described (22). Briefly, proteins were diluted in 50 mm TEAB-Buffer, pH 8.0 containing 0.1% (w/v) RapiGest (Waters) to a final concentration of 1 μg/μl. After protein reduction and alkylation, trypsin (Promega) was added. After 5 h at 37 °C, digestion was terminated by adding concentrated HCl. Hydrolyzed RapiGest was removed by centrifugation. For digestion with AspN, an endoproteinase that cleaves peptide bonds N-terminal to aspartic acid residues, protein concentration was adjusted to 1 μg/μl using 50 mm triethylammonium bicarbonate. For digestion with GluC, protein concentration was adjusted to the same concentration using 0.1% (w/v) RapiGest in 100 mm ammonium bicarbonate (pH 8.0). In this buffer, GluC specifically cleaves peptide bonds C-terminal to glutamic acid residues. All samples were reduced and alkylated as described in (22). Digestion was performed with either 0.5 μg AspN at 37 °C overnight (16 h) or with 0.4 μg GluC at 27 °C for 8 h. All digested samples were desalted via C18 columns (StageTip, Thermo) and mixed with iRT peptides (Biognosys) for retention time alignment according to the manufacturer's protocol. Peptides were separated by LC as described for the shotgun proteomics approach before being subjected to selected reaction monitoring (SRM) analysis using a TSQ Vantage mass spectrometer. The selectivity for both Q1 and Q3 were set to 0.7 Da (FWHM). The instrument was operated in SRM mode applying a collision gas pressure of 1.2 mTorr in Q2.
During discovery phase trypsin, AspN, and GluC were possible proteases for the generation of suitable peptides. All peptides suitable for targeted analysis were selected based on the following criteria: unique peptides against background proteome L. lactis MG1363 and phage p2, peptide length 7–25 amino acids, no histidine, no glycosylation motif (NxT, NxS), no possible ragged end (KP/RP). Furthermore, the derived peptides were categorized according to their expected quantification accuracy: Category A) no miss cleavages, no cysteine, no methionine; Category B) up to 2 miss cleavages, no cysteine, no methionine; Category C) up to 2 miss cleavages, cysteine (including possible carbamidomethylation), no methionine; Category D) up to 2 miss cleavages, cysteine (including possible carbamidomethylation), methionine (including possible oxidation). Transitions were selected to include all doubly and triply charged precursor ions within the analytical window of the mass spectrometer and all fragment ions ranging from ion 4 to the last ion. Transition lists were exported from Skyline (most recent version on February 7th, 2017) with default collision energies for Thermo mass spectrometers. The resulting raw files were loaded into skyline and peptides were evaluated for their chromatographic behavior (peak shape, interference, intensity, signal-to-noise-ratio) and their expected expression profile (early, middle, late expression during phage infection) to select up to three peptides per protein to be ordered in their isotopically labeled isoform.
These synthetic peptides (Innovative Peptide Solutions, Berlin, Germany) (supplemental Table S2) were used to optimize the collision energy (CE) by ramping the CE in 10 steps from −5 eV to +5 eV from the predicted CE calculated by the default starting linear equation for Thermo instruments in Skyline (most recent version on February 7th, 2017). For quantification based on heavy standard peptides, the samples were spiked with the corresponding synthetic peptides before being digested with either AspN or GluC. Sequences of quantified peptides and their optimized acquisition parameters as well as the transitions monitored for each analyte are provided in the Supporting Information (supplemental Table S3).
Bacterial Gene Knockout Using CRISPR-Cas9
The protocols used to clone new spacers in the vector pL2Cas9 (Addgene plasmid # 98841) and to construct repair templates for homologous recombination are detailed elsewhere (19). To edit the genome of L. lactis MG1363, the repair template containing the bacterial gene to modify was flanked with two homologous arms of ∼1 kb to allow efficient recombination. Because all spacers were targeting a sequence found in the genome of L. lactis MG1363, pL2Cas9 constructs were first cloned into the cloning host E. coli NEB5α. The resulting plasmids were purified from overnight E. coli cultures using the QIAprep Spin Miniprep kit (Qiagen) and were electroporated into L. lactis MG1363 already containing a compatible plasmid with the appropriate repair template. The presence of the correct spacers in the crRNA of pL2Cas9 and the sequences of the repair templates were confirmed by sequencing PCR products with an ABI 3730xl analyzer at the Plateforme de séquençage et de génotypage des génomes of the CRCHUL (Quebec City, Quebec, Canada). Sequences were analyzed using the Geneious software (version 7.1.4). All primers and oligonucleotides used in this study are listed in supplemental Table S4.
Analysis of L. lactis MG1363 Recombinants
After genome editing, isolated colonies of L. lactis MG1363 were analyzed by migration of PCR products on a 2% (w/v) agarose gel. Subsequent sequencing confirmed the expected genomic deletions. To check for phage resistance, double layer plaque assays were performed as described above. L. lactis MG1363 recombinants and the phage-sensitive L. lactis MG1363 harboring the corresponding repair template and a nontargeting pL2Cas9 were infected with phage p2. The efficiency of plaquing (EOP) was calculated by dividing the phage titer obtained on the resistant strain by the phage titer obtained on the sensitive strain.
To investigate the effect of the deletions on bacterial growth and on phage p2 infection in broth, 2 × 10 ml GM17-Ca were inoculated with overnight cultures (1% (v/v)) of each mutant and corresponding controls (phage sensitive strain with the repair template and a non-targeting pL2Cas9). Cultures were incubated statically at 30 °C and the OD600 was followed until it reached 0.3. Then, phage p2 was added at a MOI of 0.06 in half of the cultures and the OD600 was followed until clear lysis of the infected cultures. Cultures were kept at 30 °C for the whole experiment.
Phage adsorption assays were performed as previously described (23). Briefly, 100 μl (1.5 × 104 pfu/ml) from a diluted phage lysate were mixed with 900 μl of bacteria (OD600 of 0.6 to 0.8) or 900 μl of media (negative control). After incubation at 30 °C for 10 min, mixtures were centrifuged at 14,000 rpm for 1 min and the phages remaining in the supernatants were titered on the sensitive strain. The percentage of adsorption was calculated using the formula: 100 × (phage titer in negative control - phage titer in supernatant after incubation with bacteria)/phage titer in negative control). Adsorption assays were performed in technical and biological triplicates.
Data Processing
For GeLC-MS/MS, relative protein quantification was achieved using the MaxQuant software (version 1.6.0.1) (24, 25) and the Andromeda plug-in (26). The *.raw files were searched against a L. lactis MG1363 and a phage p2 database (downloaded from Uniprot (27) on June 23rd, 2015) including one entry for iRT peptides (2,433 entries in total). For proteogenomics, phage p2 genome (GenBank GQ979703) was translated to amino acid sequences using all 6 reading frames with the Geneious software (version 7.1.4). Peptide fragments with at least 33 amino acids were retained (343 entries in total). MaxQuant's generic contamination list was included during search. Database search was performed with following parameters: digestion mode, trypsin/P with up to 2 missed cleavages; peptide tolerance 20 ppm; fragment ion tolerance 0.5 Da, variable modification, methionine oxidation (15.99 Da), and maximal number of 5 modifications per peptide; activated LFQ option with minimal ratio count of 2 and “match-between-runs” feature. The false discovery rates of peptide spectrum match and protein were set to 0.01. Only unique peptides were used for protein quantification. The data from MaxQuant output files were filtered for contaminants, only identified by site and reverse hits with the Perseus software (version 1.6.0.2) (28), log2-tranformed, and used for statistical analysis.
For targeted MS, raw data of three biological replicates analyzed with three technical replicates each were imported into Skyline (most recent version on February 7th, 2017) using the implemented algorithm for peak integration. All peak areas were inspected manually to avoid possible interference in the mass spectra. Only if the dot-product was >0.7 the calculated ratio of native to standard peptide was used for protein quantification. Precision of measurements was determined by calculating the coefficient of variation (CV) for every peptide in every sample by following a random effect model as recently described (29) (supplemental Table S5).
Experimental Design and Statistical Rationale
In shotgun-proteomics experiments, nine samples (biological triplicates of three time points) of L. lactis MG1363 infected by phage p2 were analyzed by GeLC-MS/MS. The time points (10, 20, and 40 min postinfection) were chosen considering the lytic cycle of phage p2 in our laboratory conditions. Three samples of the bacterial cultures right before the phage infection served as the negative control. Proteins were accepted if at least two unique peptides could be identified in at least two of the three biological replicates (filtered in Perseus, version 1.6.0.2.) (supplemental Tables S6 and S7) (28). Statistical analysis (ANOVA) was performed using TM4 (30) by applying p values of 0.01 (supplemental Table S8). For classification and visualization purposes, all L. lactis MG1363 proteins were assigned a TIGRFAM functional role (TIGRRole) (31). Voronoi tree maps were built to compare protein expression between samples (32).
For targeted MS, eighteen samples (biological triplicates of six time points) of L. lactis MG1363 infected by phage p2 were analyzed. Infected bacterial cultures were sampled 5, 12, 15, 20, 30, and 40 min postinfection. Once again, three uninfected bacterial cultures served as the negative control. Technical replicates of each samples were analyzed using Skyline (33). Statistical analysis (ANOVA) was performed using TM4 (30) by applying p values of 0.05 (supplemental Table S5).
The MS proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository (34) with the dataset identifier PXD011263.
RESULTS
Sensitivity of the Method
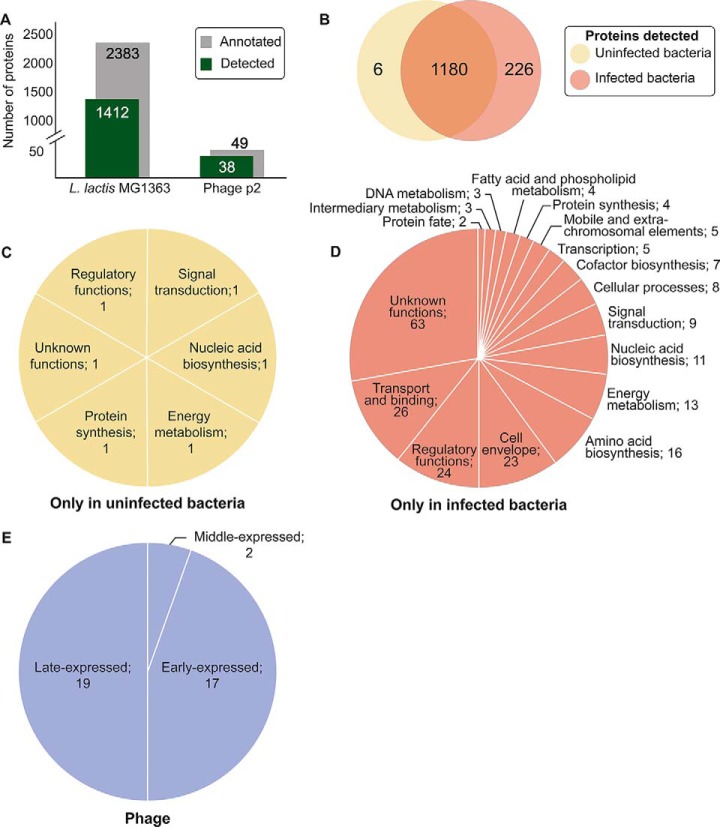
Our MS approach resulted in the high-confidence detection and quantification of 1412 bacterial proteins, representing 59% (1412/2383) of the theoretical bacterial proteome (supplemental Table S8) as well as 78% (38/49) of the theoretical phage proteome (Fig. 1A, supplemental Table S9). Among these datasets, 226 bacterial proteins were detected only during phage infection (supplemental Table S10) whereas 6 other bacterial proteins were found only in uninfected bacterial cells (supplemental Table S11, Fig. 1B). Although no pathway was overrepresented among the proteins detected only in uninfected bacteria (Fig. 1C), proteins involved in transport and binding, regulatory and cell envelope functions represented almost a third of the proteins detected only in infected cultures (Fig. 1D). We identified many non-structural proteins of phage p2, mainly encoded by early- and middle-expressed genes, that had not previously been detected (Figs. 1E and 2, supplemental Tables S9 and S10).
Fig. 1.
Proteins detected in this study. A, Bar graph comparing the number of proteins detected with the theoretical annotated proteome for both L. lactis MG1363 and phage p2. B, Venn diagram depicting the overlaps of identified bacterial proteins among samples. C, Pie chart illustrating the proportion and the classification according to metabolic pathways of bacterial proteins detected only in uninfected cultures or D, detected strictly in cultures infected with phage p2. E, The blue pie chart illustrates the proportion and the classification according to the expression modules of phage proteins detected. In (C), (D) and (E), the number of proteins are indicated after their respective classification.
Fig. 2.
Viral proteins detected during time-course infections. The genome of phage p2 now contains 50 annotated genes represented by arrows and numbers (top). Early-expressed genes are in green, middle-expressed in yellow and late-expressed in dark red. Colored boxes (bottom) indicate that the corresponding phage proteins were detected at the time-points indicated on the y axis. The newly annotated orfN1 is located between orf22 and orf23. The data for the orf46-encoded protein (boxes colored in lighter yellow) was obtained separately, by SRM (see Fig. 3 for more details). MCP: Major capsid protein, MTP: Major tail protein, TMP: Tail tape measure protein, Dit: Distal tail protein, Tal: Tail associated lysozyme, RBP: Receptor binding protein, Hol: Holin, Lys: Lysin, SaV: Sensitivity to AbiV protein, SSB: Single stranded binding protein.
Detection of a New Phage Protein
Our GeLC-MS/MS approach also detected a phage protein that was not expected to be present. Mass spectrometry spectra can be screened against a database containing all possible protein sequences in the six reading frames, thereby avoiding bias toward annotated proteomes. Phage p2 genome has previously been shown to encode 49 ORFs (supplemental Table S12). Using proteogenomics, we uncovered a conserved phage protein (pORFN1) of 59 amino acids coded by a previously unannotated gene located in the early module of the genome (Fig. 2, supplemental Tables S12 and S13). Therefore, we have detected 39/50 of the phage p2 proteome by GeLC-MS/MS.
Detection of Missing Proteins
To try to improve the coverage of phage p2 proteins (Fig. 1A), we investigated if we could use targeted MS for phage proteome analysis. We selected three small phage proteins (pORF27, pORF46 and pORF47) that were not detected for further analysis. First, peptides from these proteins were synthesized with an isotopically labeled amino acid (supplemental Table S2). To determine the quantity of peptides from these phage proteins, we programmed a triple quadrupole mass analyzer to analyze only their characteristic fragmentation reactions. In combination with standard isotope dilution, this SRM method allowed us to achieve greater sensitivity compared with our GeLC-MS/MS approach.
The synthetic peptide from pORF27 could not be detected during MS analysis under any conditions tested, so no quantification of the native peptide could be performed. Three peptides from pORF47 were synthesized but peptide (ELYIELSSDL) could not be quantified because of quality scores not reaching the thresholds. Indeed, the DotProduct, which scores the similarity between SRM intensities of native and synthetic peptide, was too low in every sample. The other two peptides (DIGEITKELYIELSS and DIGEITKELYIELSSDL) were quantified but their abundance during the time-course phage infection was not significantly different (ANOVA, p = 0.05). On the other hand, all three pORF46 peptides were quantified. The abundance of one peptide (MTEEQLLFK) was not significantly different during the time-course of the infection and it produced an expression profile different from the other two peptides. This is likely because of the methionine residue, which is prone to oxidation during sample preparation, leading to fractions of the peptide (both synthetic and native) that are undetectable with the approach used. The other two peptides (DTALIFKGE and QLLFKQE) could be quantified and their abundance differed significantly during the time-course of infection (Fig. 3).
Fig. 3.
Expression profile of the phage p2 protein encoded by orf46. The orf46-encoded protein was first detected 10 min post-infection and its abundance peaked 20 to 30 min post-infection.
Visualization of Host Protein Abundances During Infection
One of the challenges in quantitative proteomic studies is to display the large amount of data obtained in an interpretable form. Voronoi tree maps were built to visualize major metabolic pathways perturbed or induced by phage p2. At 10 mins postinfection (T10 versus T0), host protein changes were already observed in all functional categories, with larger increases for proteins involved in pyrimidine ribonucleotide biosynthesis, signal transduction and cell envelope functions (Fig. 4A, supplemental Fig. 1). At 20 min postinfection (T20 versus T0), a decrease in protein concentrations was observed in pathways associated with restriction-modification and degradation of DNA. On the other hand, a subsection of proteins involved in protein synthesis (ribosomal proteins synthesis and modification) were more abundant (Fig. 4B, supplemental Fig. 2). At 40 min postinfection (T40 versus T0) the most readily detectable changes occurred in pathways associated with protein synthesis where a global decrease was observed, whereas the concentration of proteins associated with transport and binding and energy metabolism increased (Fig. 4C, supplemental Fig. 3).
Fig. 4.
Voronoi treemaps depicting the proteotypes of L. lactis cells during phage p2 infection. Each cell represents a single gene locus grouped with other functionally related elements. Metabolic pathways assigned to gene locus are based on TIGR roles (left panel) and TIGR subroles (right panel). Protein abundances were determined at 10 (T10), 20 (T20) and 40 (T40) minutes post-infection and compared with uninfected cultures (T0). For high-resolution images, see supplemental Figs. S1, S2, and S3.
Many proteins were only detected at a specific stage of the infection (T10, T20, or T40) whereas others were only detected in the uninfected cultures (T0) prior to phage infection. These proteins are depicted as dark red and dark blue cells, respectively. There are 99, 189, and 124 dark red cells in T10 versus T0 (Fig. 4A), T20 versus T0 (Fig. 4B) and T40 versus T0 (Fig. 4C), respectively. These proteins are prevailing in metabolic pathways associated with regulatory functions (subrole DNA interactions), cell envelope (subrole other) and proteins of unknown function (subrole general). On the other hand, 24, 13, and 21 proteins are detected in the uninfected cultures, but not 10, 20, or 40 min post-infection, respectively. These proteins are scattered across various metabolic pathways, illustrating that phage p2 infection suppresses the expression of genes that are not functionally related.
Genome Editing with CRISPR-Cas9
The detection of some of the host proteins strictly during p2 infection (226/1412, 16%) suggests that they are important for phage replication and their gene inactivation may lead to a phage resistance phenotype. To investigate this hypothesis, we generated specific knockout mutants using our CRISPR-Cas9 tool adapted for L. lactis MG1363 (35). Five of the 226 genes (supplemental Table S10) were selected at random. Knockout of genes llmg_0219, llmg_2214, dltC, and nth were generated individually through deletions, ensuring no residual gene function (Table I). One of the genes (tadA) could not be inactivated with our CRISPR-Cas9 tool, suggesting that it is essential for the viability of L. lactis MG1363 cells under our laboratory conditions.
Table I. L. lactis MG1363 genes selected for inactivation with CRISPR-Cas9.
| Gene | Protein IDs | TIGR roles | TIGR subroles | Number of amino acids remaining after deletion |
|---|---|---|---|---|
| llmg_0219 | A2RHT8 | Cell envelope | Other | 29/118 |
| llmg_2214 | A2RN93 | Cell envelope | Other | 40/310 |
| dltC | A2RKK0 | Cell envelope | Biosynthesis and degradation of murein sacculus and peptidoglycan | 6/80 |
| nth | A2RLB2 | DNA metabolism | DNA replication, recombination, and repair | 29/219 |
| tadA | A2RM87 | Protein synthesis | tRNA and rRNA base modification | NA |
NA, not applicable as we could not generate the deletion, gene likely essential.
Then, we measured the level of phage p2 resistance by EOP and plaque morphology. One of the four knockout strains had a phage resistance phenotype, namely L. lactis MGΔ0219, lacking gene llmg_0219 (unknown function). This strain had a difference in EOP of 0.11 ± 0.01 and phage plaques were significantly smaller than the plaques on the phage sensitive control strain (supplemental Fig. S4). L. lactis MGΔ0219 also showed resistance to phage p2 in liquid broth (Fig. 5). Because llmg_0219 is localized within a large gene cluster involved in the production of a thin polysaccharide pellicle at the cell surface, which was previously associated with phage adsorption (7), we performed phage adsorption assays. Phage p2 adsorbed significantly less to L. lactis MGΔ0219 (2.7 ± 2.6%) as compared with the phage-sensitive control (90.6 ± 1.8%), indicating that the phage resistance phenotype is because of a deficiency in adsorption.
Fig. 5.
Growth curves of phage-sensitive L. lactis pKO0219 pL2Cas9 (control) and derivative L. lactis MGΔ0219 in presence or absence of phage p2. The knockout strain (orange and gray) takes longer to grow than the control strain (yellow and blue) and it is resistant to phage p2 infection (gray).
Of note, the growth of the knockout mutant L. lactis MGΔdltC was slower than the control, revealing its importance for growth under laboratory conditions. However, no difference in bacterial lysis was observed after phage infection (supplemental Figs. S5). The two other mutants (L. lactis MGΔ2214 and L. lactis MGΔnth) grew and lysed comparably to their control.
DISCUSSION
Uncovering Phage p2 Proteome
Phage structural proteins, typically encoded by late-expressed genes, are the target of most phage proteomic studies because their analyses are often done on purified virions. Only a few studies identified nonstructural phage proteins (36–38), notable exceptions being the endolysins among a few others. Here, we made use of GeLC-MS/MS to search for both structural and nonstructural proteins of phage p2 during infection of L. lactis MG1363. We detected 17/24 proteins encoded by early-expressed genes, 2/5 proteins encoded by middle-expressed genes and 19/20 proteins encoded by late-expressed genes (Fig. 1C, Fig. 2 and supplemental Fig. S5). We also generated a database containing the products of all possible six-frame translations of the phage p2 genome to search for novel proteins and refine the annotation (39, 40). This proteogenomic approach led to the detection of a small 59-amino acid protein coded by a previously unannotated phage p2 gene (supplemental Tables S12 and S13). This early-expressed gene, dubbed orfN1 (pORFN1), is present in 73 other publicly available lactococcal phage genomes but it is often not annotated, demonstrating the relevance of proteogenomics.
We also applied a targeted proteomic approach using SRM and synthetic peptides to detect and quantify low-abundance small phage p2 proteins during L. lactis infection. We obtained an expression profile for pORF46, a small 42-amino acid phage protein not previously detected. This currently uncharacterized phage protein was first detected 10 min post-infection and its abundance peaked 10 to 20 min later (Fig. 3), which is consistent with a middle-expressed gene. Targeted proteomics can complement global proteome analysis, but the technique is not suitable for all proteins as it did not allow us to quantify two other candidates, pORF27 and pORF47.
Investigating Metabolic Pathways Triggered by Phage p2
This study also investigated the impact of phage infection on bacterial physiology. A substantial proportion of the L. lactis MG1363 theoretical proteome was detected (Fig. 1A). We detected many bacterial proteins unique to infected (226 proteins) or uninfected (6 proteins) lactococcal cells (supplemental Table S10 and S11), which corresponds to more than 16% of the bacterial proteins detected in this study. Clearly, a significant host response occurred during phage p2 infection (Fig. 1B). Moreover, almost all bacterial proteins detected differed in abundance throughout the phage infection (Fig. 4, supplemental Table S8). In comparison, transcriptomic analysis of B. subtilis cells infected with phage φ29 revealed significant changes in less than 2% of the host genes (8). Approximately 7% of P. aeruginosa genes were differentially expressed in phage-infected cells when compared with uninfected cells, most being downregulated in the late infection phase (10). In a culture of L. lactis IL1403 infected by phage c2, whole-genome microarrays detected 61 differentially expressed genes, corresponding to less than 3% of the annotated genes (9).
Ten minutes post-infection, changes in the bacterial proteome were observable (Fig. 4A, supplemental Fig. S1). The increase in abundance of proteins associated with pyrimidine ribonucleotide biosynthesis is consistent with phages using the host machinery to rapidly replicate their genomic DNA. Notable changes also occurred in proteins involved in signal transduction and cell envelope functions. Under stress conditions, bacteria exploit various sensors to monitor the intracellular and extracellular environments and to regulate cell physiology. Changes are also triggered at the bacterial cell surface to maintain the integrity of this physical barrier and promote cell survival (41). Our results suggest that phage infection is sensed as a significant stress threatening membrane integrity. Twenty minutes post-infection, a reduction of proteins involved in restriction-modification and degradation of DNA can be observed. It is likely that phages trigger the repression of these metabolic pathways to protect the newly replicated viral DNA. A massive build-up of proteins involved in ribosomal proteins synthesis and modification suggests that phages have hijacked the host machinery to support viral protein production (Fig. 4B, supplemental Fig. S2). Forty minutes post-infection, we observed a decrease in proteins involved in protein synthesis (Fig. 4C, supplemental Fig. S3). This is consistent with phage p2 taking ∼35 min to complete its lytic cycle and kill L. lactis MG1363 under laboratory conditions. The increase in proteins associated with transport and binding and energy metabolism is consistent with phages hijacking the bacteria energy resources for virion assembly. It is also possible that more membrane proteins are detected 40 min post-infection because the bacteria are lysed and the cell wall is degraded.
It was previously reported that the lactococcal phage c2 downregulated many host genes involved in amino acid and energy metabolism pathways, suggesting a global repression of energy-consuming functions (9). Similarly, the lytic phage PaP1 suppressed 85% of the differentially expressed P. aeruginosa genes, with most genes belonging to amino acid metabolism pathways (10). In B. subtilis, phage infection triggered the repression of genes involved in utilization of specific carbon sources (8). We did not see this trend in the proteome of L. lactis MG1363 infected by phage p2. Bacterial cells infected by phages are under stress, but the response is more difficult to predict. To enhance survival under stress conditions, cells typically lower their metabolic activities, decreasing energy production and reducing growth (41). Once the viral genome is inside the bacterial cell, phages will redirect the host machinery to their own benefit. They act as both positive and negative regulators of gene expression. Phage proteins will also create their own unique interaction network, thereby creating a more complex bacterial response than the response to an inorganic stressor.
The proteotypes of L. lactis MG1363 during infection by phage p2 strongly differ from the changes in transcriptome profiles observed in L. lactis IL1403 infected by phage c2 (9). The only shared findings were the increase of SpxB and ArgC. In L. lactis, SpxB is involved in the response to cell envelope stress induced by peptidoglycan hydrolysis with lysozyme (3). Gene argC is involved in the arginine biosynthesis pathway. Like phage p2, the phage c2 is a member of the Caudovirales order and the Siphoviridae family. However, it belongs to the C2virus genus (instead of Sk1virus for p2) and has a smaller genome encoding 39 ORFs. Also, in this previous study, phage c2 was added at a much higher MOI (160-fold) than the one used for our p2 infection, making it difficult to compare our proteomic data with their transcriptomic results.
Exploiting Proteomics to Design Phage-resistant Bacterial Strains
Because phages rely on host machinery for replication, some bacterial genes are critical for an efficient lytic cycle. By comparing the proteomes of infected and uninfected bacteria, we identified host gene candidates possibly involved in phage infection. We hypothesized that the upregulation of some genes during infection was the consequence of phages repurposing host cellular processes for viral progeny production. We investigated the importance of five bacterial genes for phage multiplication by generating specific knockout mutants. Genes were selected if their resultant protein was detected in infected bacterial cultures but not in the uninfected control (supplemental Table S8). Proteins involved in different metabolic pathways were targeted (Table I). Notably, one of our knockout mutants lacking gene llmg_0219 showed resistance to phage p2 because of a deficiency in phage adsorption. Gene llmg_0219 is in the genomic region encoding proteins involved in the biosynthesis of the cell wall polysaccharide and p2 has previously been shown to bind to this structure to infect L. lactis MG1363 (7).
Although the other mutations did not provide a phage resistance phenotype, these results highlight the complexity of phage-host interactions. Deleting single genes from the bacterial genome might not be enough to generate phage-resistant bacterial strains as more than one protein may need to be perturbed. Additionally, genes/proteins that are essential for bacterial growth and fitness cannot be investigated for their importance to the lytic cycle using this approach. Our datasets highlighted many genes and proteins for their possible involvement in phage-host interactions, but additional work is required to investigate their specific roles.
Proteomic analyses are important to understand the response of L. lactis to different stress conditions, including phage infection. These analyses may even contribute to the development of strategies to increase the biotechnological potential of this species. In a recent study, the L. lactis core proteome was characterized, using a two-dimensional reversed phase nano ultra-performance LC-MS approach, in four L. lactis strains including MG1363 (42). Overall, 1108 proteins were detected in at least two of the three biological replicates for the four strains. This result is similar to the 1186 proteins detected in our negative control (uninfected L. lactis MG1363 cultures). The five proteins that were found to be the most abundant in this previous study were the DNA topoisomerase 4 subunit A (parC, A2RLE3), the 50S ribosomal protein L29 (rpmC, A2RNP7), the alanine-tRNA ligase (alaS, A2RME6), the elongation factor Tu (tuf, A2RMT1), and the thioredoxin H-type (trxH, A2RIB5). We also detected those five proteins in our negative control and at all time-points during the infection. However, they were not the most abundant proteins in our study (supplemental Table S8). This may be explained by the different quantitative approach used as well as the growing conditions.
CONCLUSION
In the last decade, the emergence of high-resolution quantitative proteomics has reinvigorated interest in bacterial proteomics (43). Quantitative analysis of proteomes from multiple systems can be done concurrently, for example, a bacterial host and its virulent phage. One advantage of investigating viral proteomics is that uninfected cultures provide a robust negative control. Here, we established a comprehensive and quantitative proteomic framework to study L. lactis MG1363 cells as they are infected by phage p2 and investigated pathways critical for phage biology. A number of host and phage proteins were detected, and their relative abundance measured during phage infection. It remains to be seen if the abundance of these host proteins will follow the same trend when the strain is infected by other phages from the same phage genus. We believe this approach will be instrumental in defining lactococcal phage-host interactions so the dairy industry can respond to phage problems from a foundation of better information to develop or optimize rational solutions. For example, it may lead to a better selection of phage-resistant bacterial strains. In other systems, knowledge of pathways inhibited by phages may lead to the discovery of novel drug targets or to a better selection of phages for therapy purposes.
DATA AVAILABILITY
The raw MS data were deposited on PRIDE. Project Name: Investigating Lactococcus lactis MG1363 response to phage p2 infection at the proteome level; Project accession: PXD011263.
Supplementary Material
Acknowledgments
We thank Barbara-Ann Conway (Medical Writer & Editor) for editorial assistance.
Footnotes
* This work was funded by grants from the Fonds de Recherche du Québec - Nature et Technologies (FRQNT), Novalait, PROTEO and the Natural Sciences and Engineering Research Council of Canada (NSERC). M-LL was supported by scholarships from NSERC, FRQNT, Novalait, and Op+Lait. SM holds a Tier 1 Canada Research Chair in Bacteriophages.
 This article contains supplemental Figures and Tables.
This article contains supplemental Figures and Tables.
1 The abbreviations used are:
- EDTA
- ethylenediaminetetraacetic acid
- EOP
- efficiency of plaquing
- Cas
- CRISPR associated proteins
- CRISPR
- clustered regularly interspaced short palindromic repeats
- LAB
- lactic acid bacteria
- LC
- liquid chromatography
- MOI
- multiplicity of infection
- MS
- mass spectrometry
- ORF
- open reading frame
- PAGE
- polyacrylamide gel electrophoresis
- PAM
- protospacer adjacent motif
- PFU
- plaque forming units
- SDS
- sodium dodecyl sulfate
- SRM
- selected reaction monitoring
- Tris
- tris(hydroxymethyl)aminomethane.
REFERENCES
- 1. Santos S. B., Costa A. R., Carvalho C., Nóbrega F. L., and Azeredo J. (2018) Exploiting bacteriophage proteomes: the hidden biotechnological potential. Trends Biotechnol. 36, 966–984 [DOI] [PubMed] [Google Scholar]
- 2. Ofir G., and Sorek R. (2018) Contemporary phage biology: from classic models to new insights. Cell 172, 1260–1270 [DOI] [PubMed] [Google Scholar]
- 3. McBride A. A. (2017) The promise of proteomics in the study of oncogenic viruses. Mol. Cell. Proteomics 16, S65–S74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ackermann H. W. (2007) 5500 phages examined in the electron microscope. Arch. Virol. 152, 227–243 [DOI] [PubMed] [Google Scholar]
- 5. Deveau H., Labrie S. J., Chopin M. C., and Moineau S. (2006) Biodiversity and classification of lactococcal phages. Appl. Environ. Microbiol. 72, 4338–4346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mahony J., Murphy J., and van Sinderen D (2012) Lactococcal 936-type phages and dairy fermentation problems: from detection to evolution and prevention. Front Microbiol 3, 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bebeacua C., Tremblay D., Farenc C., Chapot-Chartier M.P., Sadovskaya I., van Heel M., Veesler D., Moineau S., and Cambillau C. (2013) Structure, adsorption to host, and infection mechanism of virulent lactococcal phage p2. J. Virol. 87, 12302–12312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mojardin L., and Salas M. (2016) Global transcriptional analysis of virus-host interactions between phage φ29 and Bacillus subtilis. J. Virol. 90, 9293–9304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fallico V., Ross R. P., Fitzgerald G. F., and McAuliffe O. (2011) Genetic response to bacteriophage infection in Lactococcus lactis reveals a four-strand approach involving induction of membrane stress proteins, D-alanylation of the cell wall, maintenance of proton motive force, and energy conservation. J. Virol. 85, 12032–12042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao X., Shen M., Jiang X., Shen W., Zhong Q., Yang Y., Tan Y., Agnello M., He X., Hu F., and Le S. (2017) Transcriptomic and metabolomics profiling of phage-host interactions between phage PaP1 and Pseudomonas aeruginosa. Front. Microbiol. 8, 548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lavigne R., Lecoutere E., Wagemans J., Cenens W., Aertsen A., Schoofs L., Landuyt B., Paeshuyse J., Scheer M., Schobert M., and Ceyssens P. J. (2013) A multifaceted study of Pseudomonas aeruginosa shutdown by virulent podovirus LUZ19. mBio 4, e00061–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dzieciatkowska M., Hill R., and Hansen K. C. (2014) GeLC-MS/MS analysis of complex protein mixtures. Methods Mol. Biol. 1156, 53–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wegmann U., O'Connell-Motherway M., Zomer A., Buist G., Shearman C., Canchaya C., Ventura M., Goesmann A., Gasson M. J., Kuipers O. P., van Sinderen D., and Kok J. (2007) Complete genome sequence of the prototype lactic acid bacterium Lactococcus lactis subsp. cremoris MG1363. J. Bacteriol. 189, 3256–3270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Linares D., Kok J., and Poolman B. (2010) Genome sequences of Lactococcus lactis MG1363 (revised) and NZ9000 and comparative physiological studies. J Bacteriol 192, 5806–5812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Budin-Verneuil A., Pichereau V., Auffray Y., Ehrlich D. S., and Maguin E. (2005) Proteomic characterization of the acid tolerance response in Lactococcus lactis MG1363. Proteomics 5, 4794–4807 [DOI] [PubMed] [Google Scholar]
- 16. Akyol I. (2013) Proteomics analysis of the Flp regulon in Lactococcus lactis subsp. cremoris. Electrophoresis 34, 2218–2228 [DOI] [PubMed] [Google Scholar]
- 17. Sambrook J., and Russell W. D. (2001) Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Lab Press, Cold Spring Harbor, NY [Google Scholar]
- 18. Kropinski A. M., Mazzocco A., Waddell T. E., Lingohr E., and Johnson R. P. (2009) Enumeration of bacteriophages by double agar overlay plaque assay. Methods Mol. Biol. 501, 69–76 [DOI] [PubMed] [Google Scholar]
- 19. Lemay M.-L., Renaud A. C., Rousseau G. M., and Moineau S. (2018) Targeted genome editing of virulent phages using CRISPR-Cas9. Bio-protocol 8, e2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bonn F., Bartel J., Buttner K., Hecker M., Otto A., and Becher D. (2014) Picking vanished proteins from the void: how to collect and ship/share extremely dilute proteins in a reproducible and highly efficient manner. Anal. Chem. 86, 7421–7427 [DOI] [PubMed] [Google Scholar]
- 21. Bradford M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 22. Maass S., Sievers S., Zühlke D., Kuzinski J., Sappa P. K., Muntel J., Hessling B., Bernhardt J., Sietmann R., Völker U., Hecker M., and Becher D. (2011) Efficient, global-scale quantification of absolute protein amounts by integration of targeted mass spectrometry and two-dimensional gel-based proteomics. Anal. Chem. 83, 2677–2684 [DOI] [PubMed] [Google Scholar]
- 23. Sanders M. E., and Klaenhammer T. R. (1980) Restriction and modification in group N streptococci: effect of heat on development of modified lytic bacteriophage. Appl. Environ. Microbiol. 40, 500–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cox J., and Mann M. (2008) MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 [DOI] [PubMed] [Google Scholar]
- 25. Cox J., Hein M. Y., Luber C. A., Paron I., Nagaraj N., and Mann M. (2014) Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell. Proteomics 13, 2513–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cox J., Neuhauser N., Michalski A., Scheltema R. A., Olsen J. V., and Mann M. (2011) Andromeda: a peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 10, 1794–1805 [DOI] [PubMed] [Google Scholar]
- 27. UniProt Consortium (2017) UniProt: the universal protein knowledgebase. Nucleic Acids Res. 45, D158–D169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tyanova S., Temu T., Sinitcyn P., Carlson A., Hein M. Y., Geiger T., Mann M., and Cox J. (2016) The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 13, 731–740 [DOI] [PubMed] [Google Scholar]
- 29. Maass S. (2018) Absolute protein quantification using AQUA-calibrated 2D-PAGE. Methods Mol. Biol. 1841, 141–162 [DOI] [PubMed] [Google Scholar]
- 30. Saeed A. I., Sharov V., White J., Li J., Liang W., Bhagabati N., Braisted J., Klapa M., Currier T., Thiagarajan M., Sturn A., Snuffin M., Rezantsev A., Popov D., Ryltsov A., Kostukovich E., Borisovsky I., Liu Z., Vinsavich A., Trush V., and Quackenbush J. (2003) TM4: a free, open-source system for microarray data management and analysis. BioTechniques 34, 374–378 [DOI] [PubMed] [Google Scholar]
- 31. Haft D. H., Selengut J. D., Richter R. A., Harkins D., Basu M. K., and Beck E. (2013) TIGRFAMs and genome properties in 2013. Nucleic Acids Res. 41, D387–D95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liebermeister W., Noor E., Flamholz A., Davidi D., Bernhardt J., and Milo R. (2014) Visual account of protein investment in cellular functions. Proc. Natl. Acad. Sci. U.S.A. 111, 8488–8493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. MacLean B., Tomazela D. M., Shulman N., Chambers M., Finney G. L., Frewen B., Kern R., Tabb D. L., Liebler D. C., and MacCoss M. J. (2010) Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 26, 966–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vizcaino J. A., Cote R. G., Csordas A., Dianes J. A., Fabregat A., Foster J. M., Griss J., Alpi E., Birim M., Contell J., O'Kelly G., Schoenegger A., Ovelleiro D., Pérez-Riverol Y., Reisinger F., Ríos D., Wang R., and Hermjakob H. (2013) The PRoteomics IDEntifications (PRIDE) database and associated tools: status in 2013. Nucleic Acids Res. 41, D1063–D1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lemay M. L., Tremblay D. M., and Moineau S. (2017) Genome engineering of virulent lactococcal phages using CRISPR-Cas9. ACS Synth. Biol. 6, 1351–1358 [DOI] [PubMed] [Google Scholar]
- 36. Van den Bossche A., Ceyssens P. J., De Smet J., Hendrix H., Bellon H., Leimer N., Wagemans J., Delattre A. S., Cenens W., Aertsen A., Landuyt B., Minakhin L., Severinov K., Noben J. P., and Lavigne R. (2014) Systematic identification of hypothetical bacteriophage proteins targeting key protein complexes of Pseudomonas aeruginosa. J. Proteome Res. 13, 4446–4456 [DOI] [PubMed] [Google Scholar]
- 37. Pope W. H., Jacobs-Sera D., Russell D. A., Rubin D.H., Kajee A., Msibi Z. N., Larsen M. H., Jacobs W. R. Jr, Lawrence J. G., Hendrix R. W., and Hatfull G. F. (2014) Genomics and proteomics of mycobacteriophage patience, an accidental tourist in the Mycobacterium neighborhood. mBio 5, e02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Skowron P. M., Kropinski A. M., Zebrowska J., Janus L., Szemiako K., Czajkowska E., Maciejewska N., Skowron M., £oœ J., £oœ M., and Zylicz-Stachula A. (2018) Sequence, genome organization, annotation and proteomics of the thermophilic, 47.7-kb Geobacillus stearothermophilus bacteriophage TP-84 and its classification in the new Tp84virus genus. PLoS ONE 13, e0195449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Venter E., Smith R. D., and Payne S. H. (2011) Proteogenomic analysis of bacteria and archaea: a 46 organism case study. PLoS ONE 6, e27587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Armengaud J., Trapp J., Pible O., Geffard O., Chaumot A., and Hartmann E. M. (2014) Non-model organisms, a species endangered by proteogenomics. J. Proteomics 105, 5–18 [DOI] [PubMed] [Google Scholar]
- 41. Papadimitriou K., Alegria A., Bron P. A., de Angelis M., Gobbetti M., Kleerebezem M., Lemos J. A., Linares D. M., Ross P., Stanton C., Turroni F., van Sinderen D., Varmanen P., Ventura M., Zúñiga M., Tsakalidou E., and Kok J. (2016) Stress physiology of lactic acid bacteria. Microbiol. Mol. Biol. Rev. 80, 837–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Silva W. M., Sousa C. S., Oliveira L. C., Soares S. C., Souza G. F. M. H., Tavares G. C., Resende C. P., Folador E. L., Pereira F. L., Figueiredo H., and Azevedo V. (2018) Comparative proteomic analysis of four biotechnological strains Lactococcus lactis through label-free quantitative proteomics. Microb. Biotechnol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Park A. J., Krieger J. R., and Khursigara C. M. (2016) Survival proteomes: the emerging proteotype of antimicrobial resistance. FEMS Microbiol. Rev. 40, 323–342 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw MS data were deposited on PRIDE. Project Name: Investigating Lactococcus lactis MG1363 response to phage p2 infection at the proteome level; Project accession: PXD011263.