Abstract
Despite tremendous advances in traditional imaging technology over the past few decades, the intraoperative identification of lesions is still based on naked eye observation or pre-operative image evaluation. However, these two-dimensional image data cannot objectively reflect the complex anatomical structure of the liver and the detailed morphological features of the lesion, which directly limits the clinical application value of these imaging data in surgery in that it cannot improve the curative efficacy of surgery and the prognosis of the patient. This traditional mode of diagnosis and treatment has been changed by digital medical imaging technology in the new era with its significant function of accurate and efficient diagnosis of diseases, selection of reasonable treatment schemes, improvement of radical resection rate and reduction of surgical risk. In this paper, we reviewed the latest application of digital intelligent diagnosis and treatment technology related to liver surgery in the hope that it may help to achieve accurate treatment of liver surgery diseases.
Keywords: Digital medicine, Liver surgery, 3D visualization, 3D printing, Virtual reality
1. Introduction
In 1989, the National Library of Medicine (NLM) filed a “visualization plan”. Afterwards, a male and female VHP dataset were successfully collected in 1994 and 1996, respectively in order to establish a database of human body structure images, which has created a new era of visual human research [1,2]. Since then, countries including South Korea, Japan, and China have started digital virtual human research; meanwhile, virtual human research has extended to virtual physiological human and physical human [3,4]. The study of human internal anatomy by visualizing the three-dimensional reconstruction of human body data indicates the development of surgery from the era of traditional anatomy into the era of digital anatomy, and from “digital virtual human” to “digital medicine” for clinical diagnosis and treatment.
Since CT was applied to clinic in 1972, its equipment and technology have continued to evolve [5]. From conventional CT and spiral CT to multi-slice spiral CT, CT scanning speed and resolution have been significantly improved. Meanwhile, medical digital image and communication (DICM) workstations have been used to process a large amount of image data for three-dimensional reconstruction and angiography imaging, cardiac imaging, cerebral perfusion imaging, etc. [6,7]. From the three-dimensional reconstruction of traditional two-dimensional CT images, virtual simulation surgery and three-dimensional physical printing to virtual reality technology and real-time navigation of fluorescent images, all of these are the innovative development of modern digital medical imaging technology, which have changed the common use of palpation and vision in liver surgery to determine the boundaries between normal tissue and cancer tissue [8]. The Cleveland Medical Center released “2019 Top 10 Medical Innovations” at its 2018 Medical Innovation Summit, which includes artificial intelligence, 3D -printing, and virtual and mixed reality in digital medicine. The development of liver surgery is based on the anatomical study of the hepatic vascular system (hepatic artery, hepatic vein, portal vein and biliary system). Its breakthrough point is to grasp the complexity and variability of the internal structure of the organ. Digital medical intelligent diagnosis and treatment technology has exerted tremendous impetus on the development of liver surgery, promoting liver surgery into the era of “intelligence”.
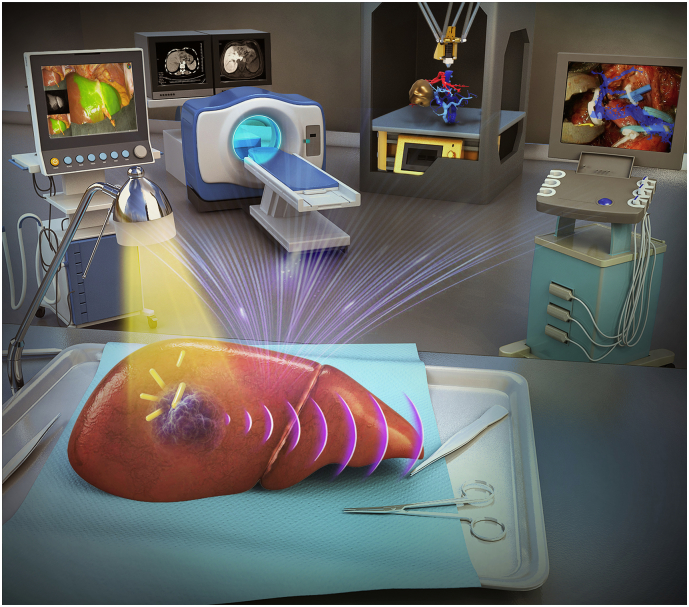
Therefore, it is important for surgeons to accumulate the basic knowledge of intelligent diagnosis and treatment technology of digital medicine (Fig. 1) and to learn how to apply it into clinical practice. This paper introduces seven aspects of digital medicine intelligent diagnosis and treatment technology: (1) 3D visualization and virtual simulation surgery; (2) 3D printing; (3) virtual reality; (4) molecular fluorescence imaging technology; (5) artificial intelligence-imaging omics; (6) navigation technology for abdominal surgery; (7) new tumour imaging technology-photoacoustic imaging. Meanwhile, their limitations and future impact on surgeons are also introduced.
Fig. 1.
Intelligent diagnosis and treatment technology of digital medicine.
2. Digital imaging technology
2.1. Three-dimensional visualization and virtual simulation surgery
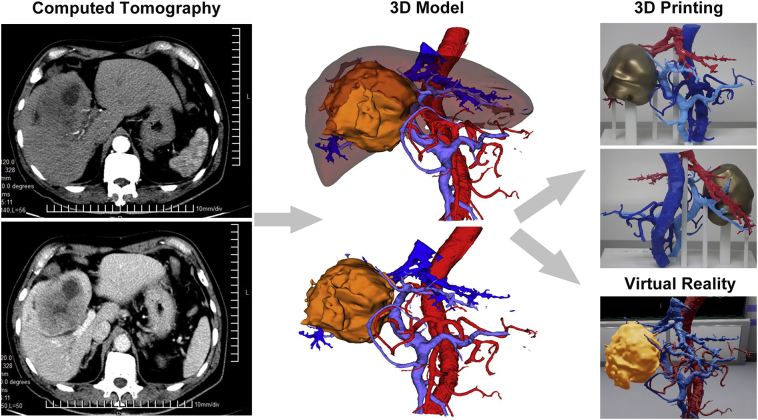
In 1998, Professor Marescaux [9] pointed out that three-dimensional visualization of organs helps us to understand the complex anatomy of the liver. The use of virtual reality concept for surgical planning, surgical simulation as well as medical training and education will revolutionize the development of liver surgery in the 21st century. Though the development of modern imaging technology has promoted that of liver surgery, surgeons still have to rely on two-dimensional images provided by B-ultrasound, CT, MRI, etc., as well as on their experience and abstract thinking to form a three-dimensional cognition of the spatial structure of diseases. However, this cognition is sometimes inaccurate and vague, and even affects the selection of surgical method. Through three-dimensional reconstruction and analysis of the CT data, surgeons can stereoscopically and comprehensively observe the location, shape and size of lesions to better preoperative planning [10,11]. It helps to observe the spatial relationship between tumour and intrahepatic ducts stereoscopically and multi-angle, identify the anatomy and variation of vascular system accurately, perform simulated surgery with surgical simulated software preoperatively, adjust the liver pre-cut surface and calculate the residual liver volume so as to determine the optimal surgical plan. Meanwhile, three-dimensional reconstruction images can comprehensively reflect the pathological changes of the lesion to guide surgeons to identify lesions intraoperatively, which can shorten the operation time, reduce the resection scope and reduce the amount of bleeding [[12], [13], [14]]. There is controversy about the selection of surgical methods for central liver cancer. Based on the location of tumour, Fang et al. proposed a three-dimensional visual classification of central liver cancer, and then constructed a platform for three-dimensional visual diagnosis and treatment of it. The three-dimensional visualization and individualized simulation surgery provide a safe and effective treatment method for central liver cancer [15]. A case of patient with centrally located hepatocellular carcinoma is presented in Fig. 2. Initially, this patient was considered inoperable according to the findings of two dimension CT images; however, 3D images re-evaluated the patient's remnant liver volume and found it was enough to operate. Based on 3D printing model and virtual reality, we observed the spatial position relationship between the huge tumour and blood vessels in the liver in a more stereoscopic and intuitive way, and found that the tumour did not invade the main blood vessels. Finally, we decided to perform mesohepatectomy. Hepatectomy guided by three-dimensional visualization is a safe and effective method for the treatment of intrahepatic cholelithiasis. Three-dimensional reconstruction images can clearly show the stereoscopic morphology, hypertrophy, and atrophy of the patient's liver, dilatation or stenosis of the intrahepatic bile duct tree; so it is beneficial for the selection of optimal surgical path, thus improving the success rate of operation and reducing the residual rate and recurrence rate of calculus [[16], [17], [18], [19]]. The special anatomical structure and infiltrating growth pattern of hilar cholangiocarcinoma are important factors influencing surgical decision-making. Three-dimensional visualization technology helps to clearly understand the complex anatomy of the hilar and quickly identify important anatomical variations, objectively display suspicious sites of tumour invasion of blood vessels, and prepare for intraoperative revascularization. On the basis of radical resection, functional liver volume is preserved to prevent postoperative liver failure [20,21]. The limit point of portal vein separation under normal conditions refers to the extreme site in which the bile duct can be separated from the parallel portal vein and hepatic artery during hepatectomy [22]. At this limit point, the upper bile duct cannot be separated and cut off alone. In the case of portal vein variation, the portal vein separation limit point will move forward or downward. Identification of variations in intrahepatic vascular structure is particularly important for determining the location of the hepatic resection limit point [23]. A new non-invasive method for portal vein pressure measurement may appear when the portal vein three-dimensional model is combined with hydrodynamic theory [[24], [25], [26]]. Moreover, virtual hepatectomy provides an opportunity for radical hepatectomy, even for liver cancer patients with impaired liver function and colorectal cancer liver metastases with advanced tumors. In living liver transplantation, virtual hepatectomy can improve the success rate of operation by optimizing donor selection and venous reconstruction, as well as balancing the relationship between the recipient and the donor [27].
Fig. 2.
A case of centrally located hepatocellular carcinoma. Thin-layer CT image is segmented, reconstructed and optimized to generate 3D model; 3D model data are imported into 3D printer, and 3D physical model of hepatic vascular and liver tumour is printed layer by layer and then reconstructed; 3D model data are imported into and displayed in virtual reality.
At present, three-dimensional reconstruction and simulation surgery software with independent intellectual property rights have been developed. Virtual hepatectomy are performed with three-dimensional reconstructed models and the volume of hepatectomy is calculated. These preoperative planning data are used for intraoperative real-time guidance, which makes liver surgery safer and more standardized [[15], [16],[28], [29], [30]]. 3D reconstruction and virtual simulation surgery have played an important role in reducing the right hepatectomy, retaining more residual liver volume, and expanding indications of liver surgery in some patients who may not have considered surgery [31,32]. Because of the retrospective nature and heterogeneity of patients, it is vital to design high-quality multicenter randomized controlled trials to evaluate its effect on liver surgery [33]. More randomized controlled trials help strengthen the evidence base and assist in the selection of suitable cases. In addition, 3D image assisted surgery is not only used in liver surgery, but also widely used in other surgical fields (Table 1).
Table 1.
3D reconstruction in other surgical fields.
| Fields of surgical application | Practical examples |
|---|---|
| Pancreatic surgery [[34], [35], [36]] | Assess the resectability of pancreatic tumors, in addition to schedule optimal surgery planning for vasculature reconstruction. |
| Vascular surgery [[37], [38], [39]] | 1. Constructing the abdominal aortic aneurysm simulating surgery training platform for junior doctors not only improve their essential surgery skills but also shorten their learning curve. |
| 2. Evaluate the feasibility and efficacy of trans-jugular intrahepatic portosystem shunt (TIPS). | |
| Breast surgery [40] | Establish and optimize the surgery schedule for breast cancer, meanwhile, providing the plannings for three-dimensional breast reconstruction. |
| Orthopedics [[41], [42], [43]] | 1. The 3D printing physical models improve the feasibility and efficacy of surgical treatment for complex tibial plateau fractures (ORIF). |
| 2. 3D printing technique instruct plate assisted hollow screw fixation for unstable pelvic fractures. | |
| 3. Application in the treatment of ankle fracture, segmental bone defect, orthosis, orthopedic and reconstruction. | |
| Plastic surgery [44,45] | 1. Aiding the surgery planning for silicone implants that aim to correct the thoracic deformities caused by Polish syndrome. |
| 2. Providing a novel surgical planning approach for surgeons to operate reconstruction surgery after maxillary or mandibular resection. |
2.2. 3D printing
Three-dimensional reconstruction images can well display the three-dimensional relationship among important blood vessels, biliary tract structures and liver parenchyma, but the three-dimensional images on a two-dimensional computer screen still lack authenticity. Due to the lack of reliable liver surface markers, the intraoperative use of three-dimensional images to guide surgery is still difficult. Meanwhile, morphological changes caused by liver traction and breathing movement have been the main limitations for the use of three-dimensional images during surgery and the use of high-precision 3D printed model is expected to overcome these difficulties. In 2013, Zein et al. [46] first reported the use of 3D printing technology to print out a translucent liver three-dimensional model. Three living liver transplantations have been successfully implemented. The intraoperative adjustment of 3D printing model in real-time is helpful for the anatomical location of the key site, the vascular structure treatment of the liver section and the reduction of the operation time and postoperative complications. High-precision 3D printed models can truthfully reflect the spatial relationship of important blood vessels and biliary system with liver [47]. For infant living liver transplantation, 3D printing technology can further improve the accuracy and safety of surgery and expand its indications [48]. During liver tumour resection, 3D printed model is brought into the operating room, and then adjusted in the optimal anatomical position. By the comparison with the intraoperative real-time surgery, intuitive real-time navigation for key surgical steps can be provided. The model can also be placed in a sterilized transparent bag and placed in the operating area for comparison with the liver. 3D printing technique can be used to guide the resection of middle lobe tumors and small hepatocellular carcinoma lesions that cannot be detected by intraoperative ultrasound [[49], [50], [51]]. It has improved the surgeon's understanding of liver anatomy and is beneficial to the improvement of hepatectomy techniques.
3D printing technology has realized a leap-forward transition from three-dimensional images to three-dimensional physical models, which may bring more in-depth information and spatial realism of anatomical structure. However, the material, time, cost and ethical issues of 3D printing have limited the clinical application of 3D printing technology to a certain extent [52]. With the development of biomaterials, the cost of 3D printing materials will continue to decrease, which will promote the widespread use of 3D printing technology [53]. At the same time, the functional model of biological 3D printing can construct artificial tissues with physiological functions. Hopefully, it can solve the problem of donor shortage in transplant surgery, which is worthy of attention and expectation [54,55].
2.3. Virtual reality
Advances in three-dimensional reconstruction image processing technology have transformed liver two-dimensional image data into three-dimensional stereoscopic models, and three-dimensional reconstructed image can be restored to real physical model by 3D printing technology. However, it is still very difficult to connect three-dimensional reconstruction images or three-dimensional physical models to the actual operation process. There is still a separation of space and time at this stage, which leads to insufficient synchronization, resulting in hand-eye disharmony [56]. As a method of displaying complex images, virtual reality (VR) technology simulates the real scene in a virtual environment by a computer. Surgeons immersed in the virtual environment can look around the operating room as usual and perform visual operation and interaction by browsing virtual menu and switching image data. Moreover, other clinical data can also be visualized [57]. In an immersive virtual reality environment, virtual anatomical information interaction is interacted to provide learners with a realistic three-dimensional learning environment that enables surgeons to overcome the difficulties of mastering complex anatomical structures and to clearly understand the spatial relationships among portal veins, hepatic veins, hepatic arteries and hepatic segments, all of which are the basis for surgical treatments of the liver diseases [58,59]. A randomized controlled trial shows that virtual reality simulated surgery training can help beginners master the basic skills of laparoscopic cholecystectomy, shorten the learning curve and surgery time [60]. As the further development of virtual reality technology, augmented reality technology and mixed reality technology have been widely used in medical field. In liver surgery, the augmented reality technique is to reconstruct the three-dimensional images of liver and intrahepatic vascular system by using computed tomography and magnetic resonance imaging data. These superimposed virtual images can help surgeons to concretize and visualize the intrahepatic structure, thus achieving accurate operation and improving the surgical effect [61]. For laparoscopic hepatectomy, visual and tactile feedback is essential for accurate evaluation of complex intrahepatic anatomical structures. Augmented reality can project three-dimensional visual liver models into the surgical area and achieve accurate registration in the coverage area. These superimposed virtual images can help to solve the problem of hand-eye disharmony in laparoscopic surgery [[62], [63], [64]]. The mixed reality presents the information of the virtual scene in the real environment, and sets up an interactive feedback information loop between the real world, the virtual world and the user, in order to enhance the users' experience of reality. The application of mixed reality in abdominal surgery is still in its infancy. Introducing this new method to display three-dimensional anatomical models near the site of surgery can reduce the deviation between operation space and visualization [56].
Three-dimensional visualization is the first step in creating a virtual immersion. By automatically blending different images, surgeons can provide a coherent and multi-modal virtual view of the changing surgical scene [65]. The application of virtual reality, augmented reality and mixed reality provides a new solution to reduce the gap between the three-dimensional reconstruction model and the actual operating space. It has obvious advantages and broad prospects in many aspects, such as preoperative planning, intraoperative navigation, surgical education and doctor-patient communication, etc. [66].
2.4. Indocyanine green molecular fluorescence imaging technique
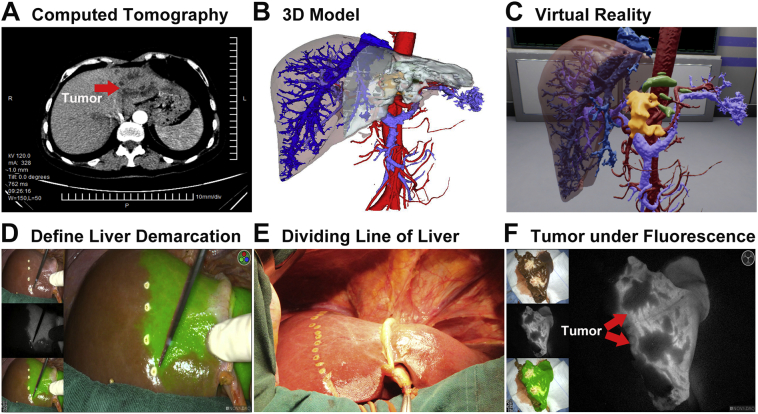
How can we precisely demarcation the tumour boundary? This is a significant scientific issue. What is more,how to achieve radical resection? This is a universal clinical need. Indocyanine Green-mediated near-infrared light detection is widely used in surgical navigation [67]. Based on the near-infrared fluorescence characteristics of ICG molecules, ICG molecular fluorescence imaging has obvious advantages in liver tumour boundary demarcation, minimal lesion detection, intraoperative real-time navigation and portal vein drainage area identification [68]. Ishizawa et al. [69] applied ICG molecular fluorescence imaging in liver cancer resection to identify small lesions or lesions that are indistinguishable to the naked eyes in real time with high sensitivity, thus achieving radical resection of the tumour. A case of left hemi-hepatectomy in a patient with left hepatic cholangiocarcinoma is shown in Fig. 3. Although two-dimension CT cannot display the location of tumour and dilated intrahepatic duct, 3D images and virtual reality could do that and provide surgeons clearer information. Besides, in contrast with just using the middle hepatic vein or ultrasound as the guide for the transection plane, ICG fluorescent dye can stay in the liver for a long time, and the staining will not be removed during the process of liver parenchyma dissection. This dynamic observation can be carried out throughout the operation, and the direction of liver resection can also be adjusted and corrected according to the fluorescence boundary of liver parenchyma. According to the fluorescence morphology of the specimen, the degree of tumour differentiation can be preliminarily distinguished. The low-differentiated liver cancer tissues or metastatic cancer tissues show circular fluorescence around the cancer tissue; while the moderately differentiated liver cancer tissues show partial fluorescence signal; the well-differentiated liver cancer tissues show full fluorescence signal [70]. Satou et al. [71] first used ICG molecular fluorescence imaging technique to detect the extrahepatic metastases of primary liver cancer in order to identify and locate the extrahepatic metastases. The technique can also be used to guide the resection of hepatoblastoma [72] and to navigate the location of hepatoblastoma lung metastasis lesions, so as to accurately remove the tumour-transferred lung lobe and achieve no residual lesions [73]. Abo, et al. [74] showed that ICG molecular fluorescence imaging has more advantages than traditional imaging techniques in the detection of liver surface tumors and small lesions. However, this technique has two main disadvantages: (1) when there is ICG excretion disorder caused by obstructive jaundice, liver cirrhosis and liver fibrosis, some interference may occur. It is necessary to judge by combining the comprehensive results of intraoperative pathology to determine specific operation methods. (2) Infrared rays have limited ability to penetrate in human tissues (about 10 mm). In the face of deeper tumors, it is necessary to combine intraoperative ultrasound to improve the detection rate of liver cancer.
Fig. 3.
A case of left hemihepatectomy in a patient with left hepatic cholangiocarcinoma. A: CT showing the location of tumour in the left extrahepatic lobe; B: 3D model based on CT; C: 3D model displayed on Virtual Reality; D: hepatic tangent marked by fluorescent signals; E: left and right hepatic demarcation line; F: tumour not developed under fluorescent.
ICG molecular fluorescence imaging reflects the pathological state of cell molecular level in vivo, and initially realizes the boundary of cell function. During the operation, dynamic observation can be carried out, and the plane of hepatectomy can be adjusted and corrected according to the fluorescence boundary of liver parenchyma, so as to achieve real-time intraoperative navigation. The portal vein staining method produces a fluorescent signal in the target liver lobe or segment, and effectively distinguishes adjacent liver segments and determines the extent of hepatectomy through the fluorescence imaging range to achieve anatomical, functional, and radical hepatectomy [68,75,76]. In addition, living donor liver transplantation can provide long-term survival opportunities for more patients, but postoperative complications are more likely to occur than traditional cadaveric donor livers. ICG molecular fluorescence imaging technology has great potential in guiding biliary reconstruction, preventing biliary stricture, and preventing liver failure during liver transplantation [77].
ICG molecular fluorescence imaging can provide surgeons with real-time feedback. With the further development of clinical practice and technological innovation, ICG molecular fluorescence imaging technology will continue to improve. It shows a good application prospect for accurate diagnosis and treatment of liver diseases [78].
2.5. Artificial intelligence-radiomics
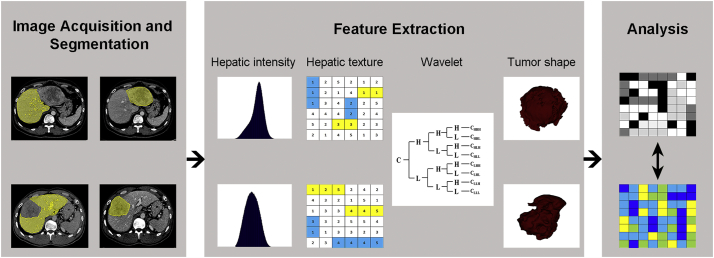
Deep learning based on artificial neural network expands the scope of artificial intelligence in medical field. The research of Radiomics of deep learning is one of the most popular research fields at present. The concept of Radiomics was put forward by Dutch scholar Lambin et al. [79] in 2012.The connotation of Radiomics is to realize tumour segmentation, feature extraction and model establishment through means of high-throughput machine learning, automatic quantitative analysis of image data such as CT, MRI and PET, and then carry out deeper mining, prediction and analysis to transform image information into high-dimensional data to improve medical decision-making. The flow of image processing is summarized in Fig. 4. Simpson et al. [80] used the conventional CT image to extract the features of radiomics to analyse the texture of preoperative CT images of patients with liver metastases from colorectal cancer undergoing hepatectomy. It was found that the texture analysis results of pixel changes in CT images were related to the occurrence of liver dysfunction after hepatectomy. In CHESS1701 trial, a new measurement method of radiomics-based hepatic venous pressure gradient (rHVPG) based on imaging features was developed to provide an accurate and non-invasive method for the measurement of hepatic venous pressure in cirrhotic patients with clinically significant portal hypertension (CSPH) [81]. A prospective multi-centre study has shown that in-depth learning imaging with ultrasound elastography can significantly improve the diagnosis of hepatic fibrosis in chronic hepatitis B, and has a certain practical value for non-invasive accurate diagnosis of hepatic fibrosis staging [82]. In primary liver cancer, the characteristics of Radiomics constructed after extracting features from liver CT images are included in routine clinical factors to predict the early recurrence of the tumour after hepatectomy, which is important for early postoperative intervention [83]. Radiography is an important direction for digital medicine to play a role in preoperative prediction, which needs to be verified by further laboratory and multi-centre research results. Although radiology analysis is expected to improve the accuracy of diagnosis, prognosis evaluation and therapeutic effect prediction, as an emerging subject, it still faces a great challenge in clinical application [84].
Fig. 4.
Flow chart of image acquisition and segmentation, feature extraction and quantitative analysis.
2.6. Abdominal surgery navigation technology
With the development of computer technology and imaging technology, “navigation” has been extended from the global positioning system (GPS) to the field of surgery or sub-surgery, which is called surgical navigation technology. The collection of multi-disciplinary image-guided surgery techniques has evolved along with the development of various related disciplines. Accurate surgical navigation can better guide surgeons to perform surgery and improve the safety of surgery [85]. Surgical navigation technology means that doctors can visualize patient's pre-operative multi-modal image data by using medical imaging equipment and computer image processing methods before operation. The rapid registration procedure is used to accurately match the anatomical structure of the patients during the operation, and the position of the surgical instruments in the space is acquired and displayed in real time by using the three-dimensional positioning system. By observing the relative position between the surgical instruments and the diseased sites in the 3D model, surgeons conducted accurate surgical navigation accurately corresponds to the anatomy of the intraoperative patient through a rapid registration procedure [86]. Unlike orthopedics and neurosurgical navigation, surgical navigation for abdominal surgery belongs to non-rigid registration. Surgical procedures, liver deformation, and respiratory movements can change image quality.
How can we simulate the deformation of anatomical structure during operation? How can we achieve real-time simulation while ensuring accuracy? Respiratory movement and soft tissue deformation are important problems in navigation for abdominal surgery. ICG fluorescence imaging technology provides real-time anatomical images for hepatobiliary surgery. Combined with image projection technology, the three-dimensional image of preoperative planning can be projected onto the liver surface to accurately identify anatomical markers, which can achieve real-time three-dimensional navigation. With the renewal of instruments and equipment and the progress of technology, laparoscopic hepatectomy has been popularized and developed rapidly. It has great potential to integrate ICG images with laparoscopic system in the near future [87]. The addition of augmented reality and mixed reality technology makes the anatomy of the surgical area clearer and provides another solution for intraoperative real-time navigation [88].
2.7. New tumour imaging-photoacoustic imaging
Most of the traditional tumour imaging devices can only describe the shape of the tumour, but cannot achieve precise boundary definition of the tumour in vivo. How can we precisely define the living boundary of tumour? This is a key scientific issue and a hot topic of current research. Photoacoustic imaging (PAI), as a new non-invasive imaging method, combines the characteristics of high contrast and spectral recognition of optical imaging, as well as the characteristics of ultrasound imaging with high resolution at large penetration depth. The tumour boundary is defined from the molecular, cellular and microvascular levels to achieve high-precision and cross-scale photoacoustic microscopy imaging to guide the accurate diagnosis of tumors [89]. Photoacoustic imaging technology has shown great potential in clinical and biomedical research. Multi-spectral photoacoustic tomography (MSOT) is a non-invasive and quantitative assessment of intestinal hemoglobin levels, with the potential to distinguish between active Crohn's disease patients and remission patients, avoiding more invasive procedures [90]. Compared with ultrasound and X-ray molybdenum target imaging, MSOT can find the characteristic of peripheral blood vessel abundance in breast cancer tissue with high resolution [91]. Aguirre et al. [92] obtained the hyperfine structure of skin by using raster scanning photoacoustic imaging (RSOM) and comparatively analysed the structural difference between normal skin and psoriasis skin, and visualized the morphological and vascular morphology of the dermis and dermis of patients with psoriasis. Thus, inflammation and other biomarkers of psoriasis can be quantified. At present, the application of photoacoustic imaging technology in the definition of hepatocellular carcinoma is mainly focused on specific molecular probes [93]. The combination of photoacoustic-fluorescence dual-mode probe can realize preoperative photoacoustic diagnosis and intraoperative fluorescence real-time navigation [[94], [95], [96]]. It is a great challenge to realize the transformation of basic research into clinical applications.
3. Prospects
Digital medicine is a new interdisciplinary subject that combines modern medicine with digital and intelligent high-tech. It covers many fields, such as medicine, computer science, mathematics, informatics, mechanical engineering. Like many new technologies, intelligent diagnosis and treatment of digital medicine still has much to be continuously improved and developed. The limitations of 3D visualization technology cannot be ignored: 3D model images cannot replace the original two-dimensional image data, and for some complex diseases, the production process depends on manual segmentation. If the operator's understanding of the anatomical structure or the determination of the extent of the lesion is insufficient, then the situation reflected on the model image is significantly different from the reality. Therefore, to achieve a comprehensive evaluation of the disease, on the one hand, the 3D model maker needs rich clinical experience; on the other hand, it needs to combine the original 2D image when using it. Three-dimensional and two-dimensional comparisons are needed repeatedly to avoid being misled by the deviation of the model [97].
In order to standardize the use of 3D visualization and 3D printing technology, the Digital Medical Society of the Chinese Medical Association has reached a consensus on the use of 3D visualization techniques for accurate surgery for complicated liver cancer, hilar cholangiocarcinoma, and hepatolithiasis [98,99]. However, based on the consensus of experts, the strength of evidence-based medical evidence needs to be improved. As the standard of clinical guidelines, randomized controlled trials are relatively rare in the field of digital medicine, mainly because the costs of such trials are far higher than the risks associated with the application of digital technology. On the other hand, both the continuous improvement of surgical instruments and the improvement of surgical operation level are the key factors limiting clinical trials. Designing high-quality multi-centre randomized controlled trial data to assess the impact of digital medicine on surgery. In addition, Digital Liver Surgery, Digital Pancreas Surgery and Digital Biliary Surgery were published to guide the clinical diagnosis and treatment of hepatopancreatic diseases. Meanwhile, further scientific verification is needed in the following popularization.
With the rapid development of modern digital information technology in the 21st century, digital medicine has broken through the traditional diagnosis and treatment model. Digital imaging technology is expected to significantly improve the effect of liver surgery in the next decade. However, the uncertainties of digital medicine, clinical efficacy and cost-effectiveness issues have also aroused heated discussion. Even though some digital technology has more diagnostic and therapeutic value than traditional technology, the application of digital medical technology in clinical practice may lead to related ethical disputes, and the advancement of technology has also brought many concerns. The potential impact of these technologies on medicine deserves our attention.
4. Conclusion
Preoperative evaluation of liver lesions by 3D reconstruction based on imaging images, virtual simulation surgery and 3D printing is directly related to the decision of the operation plan. The prediction of postoperative complications and recurrence by imaging is helpful to improve the controllability of the operation. ICG molecular fluorescence imaging and abdominal surgery navigation technology have solved the problem that the recognition of lesions in traditional surgery is still based on the direct observation by naked eye or evaluation of preoperative imaging images. Thus, the uncertainty of operation, the complicated decision-making and prolonged surgery are to be solved appropriately. Although the intelligent diagnosis and treatment technology of digital medicine integrates multidisciplinary technology to serve the diagnosis and treatment of clinical diseases, there are still insufficient areas that need to be perfected in continuous practice. In the future, in-depth study of imaging omics, surgical navigation and new photoacoustic imaging technologies will bring new opportunities for the further development of surgery.
4.1. Outstanding questions
Although facing opportunities and challenges, digital intelligent technologies have been widely used in clinical practice like many other emerging technologies. It is vital to design high-quality multicentre randomized controlled trials to assess the effects of 3D image technologies in liver surgery. The outstanding issues includes how to use artificial intelligence-radiomics to demonstrate the individualized accurate diagnosis and treatment giving full play to the risk prediction ability, and how to solve the registration error caused by real-time simulation and organ deformation while ensuring accuracy in the navigation of liver surgery.
4.2. Search strategy and selection criteria
Data for this review were identified by searches of PubMed and Web of Science using MeSH and search terms that relate to the topic. The articles published in either English or Chinese between 1998 and 2018 were included.
Declaration of interests
No author has any potential conflict to disclose (financial, professional or personal) relevant to the manuscript.
Acknowledgement
This work was supported by the grants from the National Key R&D Program (No. 2016YFC0106500), National Natural Science Foundation of China (81600510), the NSFC-GD Union Foundation (No. U1401254), the Major Instrument Project of National Natural Science Fund (No. 81627805), Guangdong Science Fund for Distinguished Young Scholars (2018B030306019), Guangzhou Industry-Academia-Research Collaborative Innovation Major Project (201704020015) and National High TechnologyResearch and Development Program of China (863 program) (2006AA02Z346, 2012AA021105).
Contributor Information
Chihua Fang, Email: fangch_dr@163.com.
Xiaolong Qi, Email: qixiaolong@vip.163.com.
References
- 1.Spitzer V.M., Whitlock D.G. The visible human dataset: the anatomical platform for human simulation. Anat Rec. 1998;253:49–57. doi: 10.1002/(SICI)1097-0185(199804)253:2<49::AID-AR8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 2.Hohne K.H., Pflesser B., Pommert A., Riemer M., Schubert R., Schiemann T. A realistic model of human structure from the visible human data. Methods Inf Med. 2001;40:83–89. [PubMed] [Google Scholar]
- 3.Chung M.S., Kim S.Y. Three-dimensional image and virtual dissection program of the brain made of Korean cadaver. Yonsei Med J. 2000;41:299–303. doi: 10.3349/ymj.2000.41.3.299. [DOI] [PubMed] [Google Scholar]
- 4.Yuan Y., Qi L., Luo S. The reconstruction and application of virtual Chinese human female. Comput Methods Programs Biomed. 2008;92:249–256. doi: 10.1016/j.cmpb.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs T., Kachelriess M., Kalender W.A. Technical advances in multi-slice spiral CT. Eur J Radiol. 2000;36:69–73. doi: 10.1016/s0720-048x(00)00269-2. [DOI] [PubMed] [Google Scholar]
- 6.Vaquerizo B., Spaziano M., Alali J., Mylote D., Theriault-Lauzier P., Alfagih R. Three-dimensional echocardiography vs. computed tomography for transcatheter aortic valve replacement sizing. Eur Heart J Cardiovasc Imaging. 2016;17:15–23. doi: 10.1093/ehjci/jev238. [DOI] [PubMed] [Google Scholar]
- 7.Rydberg J., Buckwalter K.A., Caldemeyer K.S., Phillips M.D., Conces D.J., Aisen A.M. Multisection CT: scanning techniques and clinical applications. Radiographics. 2000;20:1787–1806. doi: 10.1148/radiographics.20.6.g00nv071787. [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal E.L., Warram J.M., Bland K.I., Zinn K.R. The status of contemporary image-guided modalities in oncologic surgery. Ann Surg. 2015;261:46–55. doi: 10.1097/SLA.0000000000000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marescaux J., Clement J.M., Tassetti V., Koehl C., Cotin S., Russier Y. Virtual reality applied to hepatic surgery simulation: the next revolution. Ann Surg. 1998;228:627–634. doi: 10.1097/00000658-199811000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamade W., Glombitza G., Fischer L., Chiu P., Cardenas C.S., Thorn M. The impact of 3-dimensional reconstructions on operation planning in liver surgery. Arch Surg. 2000;135:1256–1261. doi: 10.1001/archsurg.135.11.1256. [DOI] [PubMed] [Google Scholar]
- 11.Fang C.H., LauWan Y.Y., Cai W. The present status and future prospects of application of digital medical technology in general surgery in China. Zhonghua Wai Ke Za Zhi. 2017;55:11–14. doi: 10.3760/cma.j.issn.0529-5815.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Uchida M. Recent advances in 3D computed tomography techniques for simulation and navigation in hepatobiliary pancreatic surgery. J Hepatobiliary Pancreat Sci. 2014;21:239–245. doi: 10.1002/jhbp.82. [DOI] [PubMed] [Google Scholar]
- 13.Cai W., Fan Y., Hu H., Xiang N., Fang C., Jia F. Postoperative liver volume was accurately predicted by a medical image three dimensional visualization system in hepatectomy for liver cancer. Surg Oncol. 2017;26:188–194. doi: 10.1016/j.suronc.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Mutter D., Dallemagne B., Bailey C., Soler L., Marescaux J. 3D virtual reality and selective vascular control for laparoscopic left hepatic lobectomy. Surg Endosc. 2009;23:432–435. doi: 10.1007/s00464-008-9931-y. [DOI] [PubMed] [Google Scholar]
- 15.Fang C., Tao H., Yang J., Fang Z., Cai W., Liu J. Impact of three-dimensional reconstruction technique in the operation planning of centrally located hepatocellular carcinoma. J Am Coll Surg. 2015;220:28–37. doi: 10.1016/j.jamcollsurg.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 16.Fang C., Liu J., Fan Y., Yang J., Xiang N., Zeng N. Outcomes of hepatectomy for hepatolithiasis based on 3-dimensional reconstruction technique. J Am Coll Surg. 2013;217:280–288. doi: 10.1016/j.jamcollsurg.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Xiang N., Fang C. Application of hepatic segment resection combined with rigid choledochoscope in the treatment of complex hepatolithiasis guided by three-dimensional visualization technology. Zhonghua Wai Ke Za Zhi. 2015;53:335. [PubMed] [Google Scholar]
- 18.Xie A., Fang C., Huang Y., Fan Y., Pan J., Peng F. Application of three-dimensional reconstruction and visible simulation technique in reoperation of hepatolithiasis. J Gastroenterol Hepatol. 2013;28:248–254. doi: 10.1111/jgh.12066. [DOI] [PubMed] [Google Scholar]
- 19.Li G., Fang C.H., Fan Y.F., Wu T.C., Zhong S.Z. A comparative study of the diagnostic accuracy of the medical image three-dimensional visualization system, MRCP, CT and US in hepatolithiasis. Hepatogastroenterology. 2014;61:1901–1907. [PubMed] [Google Scholar]
- 20.Zhang J., Qiao Q.L., Guo X.C., Zhao J.X. Application of three-dimensional visualization technique in preoperative planning of progressive hilar cholangiocarcinoma. Am J Transl Res. 2018;10:1730–1735. [PMC free article] [PubMed] [Google Scholar]
- 21.Okuda Y., Taura K., Seo S., Yasuchika K., Nitta T., Ogawa K. Usefulness of operative planning based on 3-dimensional CT cholangiography for biliary malignancies. Surgery. 2015;158:1261–1271. doi: 10.1016/j.surg.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 22.Hirano S., Tanaka E., Shichinohe T., Suzuki O., Hazama K., Kitagami H. Treatment strategy for hilar cholangiocarcinoma, with special reference to the limits of ductal resection in right-sided hepatectomies. J Hepatobiliary Pancreat Surg. 2007;14:429–433. doi: 10.1007/s00534-006-1190-5. [DOI] [PubMed] [Google Scholar]
- 23.Zeng N., Tao H., Fang C., Fan Y., Xiang N., Yang J. Individualized preoperative planning using three-dimensional modeling for Bismuth and Corlette type III hilar cholangiocarcinoma. World J Surg Oncol. 2016;14:44. doi: 10.1186/s12957-016-0794-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi X., Li Z., Huang J., Zhu Y., Liu H., Zhou F. Virtual portal pressure gradient from anatomic CT angiography. Gut. 2015;64:1004–1005. doi: 10.1136/gutjnl-2014-308543. [DOI] [PubMed] [Google Scholar]
- 25.Chengxi L., Rentao L., Wei Z. Progress in non-invasive detection of liver fibrosis. Cancer Biol Med. 2018;15:124. doi: 10.20892/j.issn.2095-3941.2018.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qi X., Berzigotti A., Cardenas A., Sarin S.K. Emerging non-invasive approaches for diagnosis and monitoring of portal hypertension. Lancet Gastroenterol Hepatol. 2018;3:708–719. doi: 10.1016/S2468-1253(18)30232-2. [DOI] [PubMed] [Google Scholar]
- 27.Mise Y., Hasegawa K., Satou S., Shindoh J., Miki K., Akamatsu N. How has virtual hepatectomy changed the practice of liver surgery? Ann Surg. 2018;1 doi: 10.1097/SLA.0000000000002213. [DOI] [PubMed] [Google Scholar]
- 28.Oshiro Y., Ohkohchi N. Three-dimensional liver surgery simulation: computer-assisted surgical planning with three-dimensional simulation software and three-dimensional printing. Tissue Eng Part A. 2017;23:474–480. doi: 10.1089/ten.TEA.2016.0528. [DOI] [PubMed] [Google Scholar]
- 29.Mise Y., Tani K., Aoki T., Sakamoto Y., Hasegawa K., Sugawara Y. Virtual liver resection: computer-assisted operation planning using a three-dimensional liver representation. J Hepato-Biliary-Pancreat Sci. 2013;20:157–164. doi: 10.1007/s00534-012-0574-y. [DOI] [PubMed] [Google Scholar]
- 30.Nakayama K., Oshiro Y., Miyamoto R., Kohno K., Fukunaga K., Ohkohchi N. The effect of three-dimensional preoperative simulation on liver surgery. World J Surg. 2017;41:1840–1847. doi: 10.1007/s00268-017-3933-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu M., Hu H., Cai W., Mo Z., Xiang N., Yang J. The safety and feasibility of three-dimensional visualization technology assisted right posterior lobe allied with Part of V and VIII sectionectomy for right hepatic malignancy therapy. J Laparoendosc Adv Surg Tech A. 2018;28:586–594. doi: 10.1089/lap.2017.0479. [DOI] [PubMed] [Google Scholar]
- 32.Yang J., Tao H., Cai W., Zhu W., Zhao D., Hu H. Accuracy of actual resected liver volume in anatomical liver resections guided by 3-dimensional parenchymal staining using fusion indocyanine green fluorescence imaging. J Surg Oncol. 2018;118:1081–1087. doi: 10.1002/jso.25258. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X., Wei X., Chen Z., Cheng S. The virtual hepatectomy changed the practice of liver surgery. Ann Surg. 2018;1 doi: 10.1097/SLA.0000000000003007. [DOI] [PubMed] [Google Scholar]
- 34.Fang C.H., Kong D., Wang X., Wang H., Xiang N., Fan Y. Three-dimensional reconstruction of the peripancreatic vascular system based on computed tomographic angiography images and its clinical application in the surgical management of pancreatic tumors. Pancreas. 2014;43:389–395. doi: 10.1097/MPA.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 35.Fang C., Zhu W., Wang H., Xiang N., Fan Y., Yang J. A new approach for evaluating the resectability of pancreatic and periampullary neoplasms. Pancreatology. 2012;12:364–371. doi: 10.1016/j.pan.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Miyamoto R., Oshiro Y., Nakayama K., Kohno K., Hashimoto S., Fukunaga K. Three-dimensional simulation of pancreatic surgery showing the size and location of the main pancreatic duct. Surg Today. 2017;47:357–364. doi: 10.1007/s00595-016-1377-6. [DOI] [PubMed] [Google Scholar]
- 37.Torres I.O., De Luccia N. A simulator for training in endovascular aneurysm repair: the use of three dimensional printers. Eur J Vasc Endovasc Surg. 2017;54:247–253. doi: 10.1016/j.ejvs.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 38.Luo X., Wang X., Zhao Y., Ma H., Ye L., Yang L. Real-Time 3D CT image guidance for transjugular intrahepatic portosystemic shunt creation using preoperative CT: a prospective feasibility study of 20 patients. Am J Roentgenol. 2017;208:W11–W16. doi: 10.2214/AJR.15.15210. [DOI] [PubMed] [Google Scholar]
- 39.Liu X., Yang J., Li X.X., Xiang N., Zeng N., Fan Y.F. The anatomy features and variations of the point where right gastroepiploic VEIN flows into superior mesenteric vein/portal vein: anatomical study of catheterization of portal vein infusion chemotherapy. J Laparoendosc Adv Surg Tech A. 2018;28:794–798. doi: 10.1089/lap.2017.0655. [DOI] [PubMed] [Google Scholar]
- 40.Zhang P.S., Luo Y.F., Yu J.L., Fang C.H., Shi F.J., Deng J.W. Application of digital 3D technique combined with nanocarbon-aided navigation in endoscopic sentinel lymph node biopsy for breast cancer. Nan Fang Yi Ke Da Xue Xue Bao. 2016;36:1129–1133. [PubMed] [Google Scholar]
- 41.Xie L., Chen C., Zhang Y., Zheng W., Chen H., Cai L. Three-dimensional printing assisted ORIF versus conventional ORIF for tibial plateau fractures: a systematic review and meta-analysis. Int J Surg. 2018;57:35–44. doi: 10.1016/j.ijsu.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 42.Yang H., Lei Q., Cai L., Liu F., Zhou W., Chen S. Treatment of unstable pelvic fractures by cannulated screw internal fixation with the assistance of three-dimensional printing insertion template. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018;32:145–151. doi: 10.7507/1002-1892.201708059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei D., Li C., Xu Y. Research progress of three-dimensional printing technique in foot and ankle surgery. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2017;31:880–884. doi: 10.7507/1002-1892.201611044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chavoin J.P., Taizou M., Moreno B., Leyx P., Grolleau J.L., Chaput B. Correcting poland syndrome with a custom-made silicone implant: contribution of three-dimensional computer-aided design reconstruction. Plast Reconstr Surg. 2018;142:109e–119e. doi: 10.1097/PRS.0000000000004605. [DOI] [PubMed] [Google Scholar]
- 45.Shenaq D.S., Matros E. Virtual planning and navigational technology in reconstructive surgery. J Surg Oncol. 2018;118:845–852. doi: 10.1002/jso.25255. [DOI] [PubMed] [Google Scholar]
- 46.Zein N.N., Hanouneh I.A., Bishop P.D., Samaan M., Eghtesad B., Quintini C. Three-dimensional print of a liver for preoperative planning in living donor liver transplantation. Liver Transpl. 2013;19:1304–1310. doi: 10.1002/lt.23729. [DOI] [PubMed] [Google Scholar]
- 47.Fang C., Fang Z., Fan Y., Li J., Xiang F., Tao H. Application of 3D visualization, 3D printing and 3D laparoscopy in the diagnosis and surgical treatment of hepatic tumors. Nan Fang Yi Ke Da Xue Xue Bao. 2015;35:639–645. [PubMed] [Google Scholar]
- 48.Soejima Y., Taguchi T., Sugimoto M., Hayashida M., Yoshizumi T., Ikegami T. Three-dimensional printing and biotexture modeling for preoperative simulation in living donor liver transplantation for small infants. Liver Transpl. 2016;22:1610–1614. doi: 10.1002/lt.24516. [DOI] [PubMed] [Google Scholar]
- 49.Igami T., Nakamura Y., Hirose T., Ebata T., Yokoyama Y., Sugawara G. Application of a three-dimensional print of a liver in hepatectomy for small tumors invisible by intraoperative ultrasonography: preliminary experience. World J Surg. 2014;38:3163–3166. doi: 10.1007/s00268-014-2740-7. [DOI] [PubMed] [Google Scholar]
- 50.Igami T., Nakamura Y., Oda M., Tanaka H., Nojiri M., Ebata T. Application of three-dimensional print in minor hepatectomy following liver partition between anterior and posterior sectors. ANZ J Surg. 2018;88:882–885. doi: 10.1111/ans.14331. [DOI] [PubMed] [Google Scholar]
- 51.Xiang N., Fang C., Fan Y., Yang J., Zeng N., Liu J. Application of liver three-dimensional printing in hepatectomy for complex massive hepatocarcinoma with rare variations of portal vein: preliminary experience. Int J Clin Exp Med. 2015;8:18873–18878. [PMC free article] [PubMed] [Google Scholar]
- 52.Ligon S.C., Liska R., Stampfl J., Gurr M., Mülhaupt R. Polymers for 3D printing and customized additive manufacturing. Chem Rev. 2017;117:10212–10290. doi: 10.1021/acs.chemrev.7b00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Y. Oshiro, J. Mitani, T. Okada, N. Ohkohchi. A novel three-dimensional print of liver vessels and tumors in hepatectomy. Surg Today; 47:521–524. [DOI] [PubMed]
- 54.Dzobo K., Thomford N.E., Senthebane D.A., Shipanga H., Rowe A., Dandara C. Advances in regenerative medicine and tissue engineering: innovation and transformation of medicine. Stem Cells Int. 2018:2495848. doi: 10.1155/2018/2495848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazzocchi A., Devarasetty M., Huntwork R.C., Soker S., Skardal A. Optimization of collagen type I-hyaluronan hybrid bioink for 3D bioprinted liver microenvironments. Biofabrication. 2018;11 doi: 10.1088/1758-5090/aae543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sauer I.M., Queisner M., Tang P., Moosburner S., Hoepfner O., Horner R. Mixed reality in visceral surgery: development of a suitable workflow and evaluation of intraoperative use-cases. Ann Surg. 2017;266:706–712. doi: 10.1097/SLA.0000000000002448. [DOI] [PubMed] [Google Scholar]
- 57.Draelos M., Keller B., Viehland C., Carrasco-Zevallos O.M., Kuo A., Izatt J. Real-time visualization and interaction with static and live optical coherence tomography volumes in immersive virtual reality. Biomed Opt Express. 2018;9:2825. doi: 10.1364/BOE.9.002825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Silverstein J.C., Dech F., Edison M., Jurek P., Helton W.S., Espat N.J. Virtual reality: immersive hepatic surgery educational environment. Surgery. 2002;132:274–277. doi: 10.1067/msy.2002.125723. [DOI] [PubMed] [Google Scholar]
- 59.Moro C., Stromberga Z., Raikos A., Stirling A. The effectiveness of virtual and augmented reality in health sciences and medical anatomy. Anat Sci Educ. 2017;10:549–559. doi: 10.1002/ase.1696. [DOI] [PubMed] [Google Scholar]
- 60.Kowalewski K.F., Garrow C.R., Proctor T., Preukschas A.A., Friedrich M., Muller P.C. LapTrain: multi-modality training curriculum for laparoscopic cholecystectomy-results of a randomized controlled trial. Surg Endosc. 2018;32:3830–3838. doi: 10.1007/s00464-018-6110-7. [DOI] [PubMed] [Google Scholar]
- 61.Tang R., Ma L.F., Rong Z.X., Li M.D., Zeng J.P., Wang X.D. Augmented reality technology for preoperative planning and intraoperative navigation during hepatobiliary surgery: a review of current methods. Hepatobiliary Pancreat Dis Int. 2018;17:101–112. doi: 10.1016/j.hbpd.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 62.Hallet J., Soler L., Diana M., Mutter D., Baumert T.F., Habersetzer F. Trans-thoracic minimally invasive liver resection guided by augmented reality. J Am Coll Surg. 2015;220:e55–e60. doi: 10.1016/j.jamcollsurg.2014.12.053. [DOI] [PubMed] [Google Scholar]
- 63.Phutane P., Buc E., Poirot K., Ozgur E., Pezet D., Bartoli A. Preliminary trial of augmented reality performed on a laparoscopic left hepatectomy. Surg Endosc. 2018;32:514–515. doi: 10.1007/s00464-017-5733-4. [DOI] [PubMed] [Google Scholar]
- 64.Bichlmeier C., Heining S.M., Feuerstein M., Navab N. The virtual mirror: a new interaction paradigm for augmented reality environments. IEEE Trans Med Imaging. 2009;28:1498–1510. doi: 10.1109/TMI.2009.2018622. [DOI] [PubMed] [Google Scholar]
- 65.Conrad C., Fusaglia M., Peterhans M., Lu H., Weber S., Gayet B. Augmented reality navigation surgery facilitates laparoscopic rescue of failed portal vein embolization. J Am Coll Surg. 2016;223:e31–e34. doi: 10.1016/j.jamcollsurg.2016.06.392. [DOI] [PubMed] [Google Scholar]
- 66.Li L., Yu F., Shi D., Shi J., Tian Z., Yang J. Application of virtual reality technology in clinical medicine. Am J Transl Res. 2017;9:3867–3880. [PMC free article] [PubMed] [Google Scholar]
- 67.Zelken J.A., Tufaro A.P. Current trends and emerging future of indocyanine green usage in surgery and oncology: an update. Ann Surg Oncol. 2015;22:1271–1283. doi: 10.1245/s10434-015-4743-5. [DOI] [PubMed] [Google Scholar]
- 68.Fang C.H., Liang H.B., Chi C.W., Tao H.S., Fang C., Zhu W. Application of indocyanine green-fluorescent imaging technique in planning resection line and real-time surgical navigation in small hepatocellular carcinoma. Zhonghua Wai Ke Za Zhi. 2016;54:444–450. doi: 10.3760/cma.j.issn.0529-5815.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 69.Ishizawa T., Fukushima N., Shibahara J., Masuda K., Tamura S., Aoki T. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer-Am Cancer Soc. 2009;115:2491–2504. doi: 10.1002/cncr.24291. [DOI] [PubMed] [Google Scholar]
- 70.Lim C., Vibert E., Azoulay D., Salloum C., Ishizawa T., Yoshioka R. Indocyanine green fluorescence imaging in the surgical management of liver cancers: current facts and future implications. J Visc Surg. 2014;151:117–124. doi: 10.1016/j.jviscsurg.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 71.Satou S., Ishizawa T., Masuda K., Kaneko J., Aoki T., Sakamoto Y. Indocyanine green fluorescent imaging for detecting extrahepatic metastasis of hepatocellular carcinoma. J Gastroenterol. 2013;48:1136–1143. doi: 10.1007/s00535-012-0709-6. [DOI] [PubMed] [Google Scholar]
- 72.Yamamichi T., Oue T., Yonekura T., Owari M., Nakahata K., Umeda S. Clinical application of indocyanine green (ICG) fluorescent imaging of hepatoblastoma. J Pediatr Surg. 2015;50:833–836. doi: 10.1016/j.jpedsurg.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 73.Kitagawa N., Shinkai M., Mochizuki K., Usui H., Miyagi H., Nakamura K. Navigation using indocyanine green fluorescence imaging for hepatoblastoma pulmonary metastases surgery. Pediatr Surg Int. 2015;31:407–411. doi: 10.1007/s00383-015-3679-y. [DOI] [PubMed] [Google Scholar]
- 74.Abo T., Nanashima A., Tobinaga S., Hidaka S., Taura N., Takagi K. Usefulness of intraoperative diagnosis of hepatic tumors located at the liver surface and hepatic segmental visualization using indocyanine green-photodynamic eye imaging. Eur J Surg Oncol. 2015;41:257–264. doi: 10.1016/j.ejso.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 75.Inoue Y., Arita J., Sakamoto T., Ono Y., Takahashi M., Takahashi Y. Anatomical liver resections guided by 3-dimensional parenchymal staining using fusion indocyanine green fluorescence imaging. Ann Surg. 2015;262:105–111. doi: 10.1097/SLA.0000000000000775. [DOI] [PubMed] [Google Scholar]
- 76.Kobayashi Y., Kawaguchi Y., Kobayashi K., Mori K., Arita J., Sakamoto Y. Portal vein territory identification using indocyanine green fluorescence imaging: technical details and short-term outcomes. J Surg Oncol. 2017;116:921–931. doi: 10.1002/jso.24752. [DOI] [PubMed] [Google Scholar]
- 77.Mizuno S., Inoue H., Tanemura A., Murata Y., Kuriyama N., Azumi Y. Biliary complications in 108 consecutive recipients with duct-to-duct biliary reconstruction in living-donor liver transplantation. Transplant Proc. 2014;46:850–855. doi: 10.1016/j.transproceed.2013.11.035. [DOI] [PubMed] [Google Scholar]
- 78.Koch M., Ntziachristos V. Advancing Surgical Vision with Fluorescence Imaging. Annu Rev Med. 2016;67:153–164. doi: 10.1146/annurev-med-051914-022043. [DOI] [PubMed] [Google Scholar]
- 79.Lambin P., Riosvelazquez E., Leijenaar R., Carvalho S., van Stiphout R.G., Granton P. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2007;43:441–446. doi: 10.1016/j.ejca.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Simpson A.L., Adams L.B., Allen P.J., Angelica M.I.D., DeMatteo R.P., Fong Y. Texture analysis of preoperative ct images for prediction of postoperative hepatic insufficiency: a preliminary study. J Am Coll Surg. 2015;220:339–346. doi: 10.1016/j.jamcollsurg.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu F., Ning Z., Liu Y., Liu D., Tian J., Luo H. Development and validation of a radiomics signature for clinically significant portal hypertension in cirrhosis (CHESS1701): a prospective multicenter study. EBioMedicine. 2018;36:151–158. doi: 10.1016/j.ebiom.2018.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang K., Lu X., Zhou H., Gao Y., Zheng J., Tong M. Deep learning Radiomics of shear wave elastography significantly improved diagnostic performance for assessing liver fibrosis in chronic hepatitis B: a prospective multicentre study. Gut. 2018;0:1–13. doi: 10.1136/gutjnl-2018-316204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou Y., He L., Huang Y., Chen S., Wu P., Ye W. CT-based radiomics signature: a potential biomarker for preoperative prediction of early recurrence in hepatocellular carcinoma. Abdom Radiol. 2017;42:1695–1704. doi: 10.1007/s00261-017-1072-0. [DOI] [PubMed] [Google Scholar]
- 84.Gillies R.J., Kinahan P.E., Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278:563–577. doi: 10.1148/radiol.2015151169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dilley J., Hughes-Hallett A., Pratt P.J., Pucher P.H., Camara M., Darzi A.W., Mayer E.K. Perfect Registration Leads to Imperfect Performance: A Randomized Trial of Multimodal Intraoperative Image Guidance. Ann Surg. 2019;269:236–242. doi: 10.1097/SLA.0000000000002793. [DOI] [PubMed] [Google Scholar]
- 86.Peterhans M., Vom B.A., Dagon B., Inderbitzin D., Baur C., Candinas D. A navigation system for open liver surgery: design, workflow and first clinical applications. Int J Med Robot Comp Assist Surg. 2011;7:7–16. doi: 10.1002/rcs.360. [DOI] [PubMed] [Google Scholar]
- 87.Nishino H., Hatano E., Seo S., Nitta T., Saito T., Nakamura M. Real-time navigation for liver surgery using projection mapping with indocyanine green fluorescence. Ann Surg. 2018;267:1134–1140. doi: 10.1097/SLA.0000000000002172. [DOI] [PubMed] [Google Scholar]
- 88.Fernandes A.T., Apisarnthanarax S., Yin L., Zou W., Rosen M., Plastaras J.P. Comparative assessment of liver tumor motion using cine-magnetic resonance imaging versus 4-dimensional computed tomography. Int J Radiat Oncol Biol Phys. 2015;91:1034–1040. doi: 10.1016/j.ijrobp.2014.12.048. [DOI] [PubMed] [Google Scholar]
- 89.Ntziachristos V., Pleitez M.A., Aime S., Brindle K.M. Emerging technologies to image tissue metabolism. Cell Metab. 2018 doi: 10.1016/j.cmet.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 90.Knieling F., Neufert C., Hartmann A., Claussen J., Urich A., Egger C. Multispectral optoacoustic tomography for assessment of crohn's disease activity. N Engl J Med. 2017;376:1292–1294. doi: 10.1056/NEJMc1612455. [DOI] [PubMed] [Google Scholar]
- 91.Diot G., Metz S., Noske A., Liapis E., Schroeder B., Ovsepian S.V. Multispectral Optoacoustic Tomography (MSOT) of human breast cancer. Clin Cancer Res. 2017;23:6912–6922. doi: 10.1158/1078-0432.CCR-16-3200. [DOI] [PubMed] [Google Scholar]
- 92.Aguirre J., Schwarz M., Garzorz N., Omar M., Buehler A., Eyerich K. Precision assessment of label-free psoriasis biomarkers with ultra-broadband optoacoustic mesoscopy. Nat Biomed Eng. 2017;1:0068. [Google Scholar]
- 93.Zeng C., Shang W., Liang X., Liang X., Chen Q., chi C. Cancer diagnosis and imaging-guided photothermal therapy using a dual-modality nanoparticle. ACS Appl Mater Interfaces. 2016;8:29232–29241. doi: 10.1021/acsami.6b06883. [DOI] [PubMed] [Google Scholar]
- 94.Ai T., Shang W., Yan H., Zeng C., Wang K., Gao Y. Near infrared-emitting persistent luminescent nanoparticles for hepatocellular carcinoma imaging and luminescence-guided surgery. Biomaterials. 2018;167:216–225. doi: 10.1016/j.biomaterials.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 95.Guan T., Shang W., Li H., Yang X., Fang C., Tian J. From detection to resection: photoacoustic tomography and surgery Guidance with indocyanine green loaded gold Nanorod@liposome core–shell nanoparticles in liver Cancer. Bioconjug Chem. 2017;28:1221–1228. doi: 10.1021/acs.bioconjchem.7b00065. [DOI] [PubMed] [Google Scholar]
- 96.Song X., Shang W., Peng L., Jiang H., Wang K., Fang C. Novel GPC3-binding WS2-Ga(3+)-PEG-peptide nanosheets for in vivo bimodal imaging-guided photothermal therapy. Nanomedicine. 2018 doi: 10.2217/nnm-2017-0367. [DOI] [PubMed] [Google Scholar]
- 97.Fang C.H., Lau Y.Y., Zhou W.P., Cai W. Ten years retrospective review of the application of digital medical technology in general surgery in China. Zhonghua Wai Ke Za Zhi. 2017;55:887–890. doi: 10.3760/cma.j.issn.0529-5815.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 98.Chinese Society of Digital Medicine, Chinese Research Hospital Association of Digital Surgery Committee Expert consensus of precise diagnosis and treatment for pancreatic head cancer using three-dimensional visualization technology. Zhonghua Wai Ke Za Zhi. 2017;55:881–886. doi: 10.3760/cma.j.issn.0529-5815.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 99.Dong J., Qi X. Liver imaging in precision medicine. EBioMedicine. 2018;32:1–2. doi: 10.1016/j.ebiom.2018.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]