Abstract
Animal models of chronic kidney disease (CKD) are critical for understanding its pathophysiology and for therapeutic development. The cardiovascular and renal anatomy and physiology of the pig are virtually identical to humans. This study aimed to develop a novel translational model of CKD that mimics the pathological features of CKD in humans. CKD was induced in seven domestic pigs by bilateral renal artery stenosis and diet-induced dyslipidemia. Animals were observed for a total of 14 wk. Renal hemodynamics and function were quantified in vivo using multi-detector CT after 6, 10, and 14 wk of CKD. Urine and blood were collected at each time-point, and blood pressure was continuously measured (telemetry). After completion of in vivo studies, pigs were euthanized, kidneys were removed, and microvascular (MV) architecture (μCT), markers of renal injury, inflammation, and fibrosis were evaluated ex vivo. Additional pigs were used as controls (n = 7). Renal blood flow and glomerular filtration were reduced by 50% in CKD, accompanied by hypertension and elevated plasma creatinine, albumin-to-creatinine ratio and increased urinary KIM-1 and NGAL, suggesting renal injury. Furthermore, 14 wk of CKD resulted in cortical and medullary MV remodeling and loss, inflammation, glomerulosclerosis, tubular atrophy, and tubule-interstitial fibrosis compared with controls. The current study characterizes a novel model of CKD that mimics several of the pathological features observed in human CKD, irrespective of the etiology. Current approaches only slow rather than halt CKD progression, and this novel model may offer a suitable platform for the development of new treatments in a translational fashion.
Keywords: chronic renal disease, fibrosis, inflammation, microcirculation, renal hemodynamics
INTRODUCTION
Chronic kidney disease (CKD) is a progressive disorder affecting almost 14% of the general population and its prevalence has continuously grown over the past 2 decades (51). CKD is an independent risk factor for cardiovascular morbidity and mortality, as patients with diagnosed cardiovascular disease show a staggering 40.8% prevalence of CKD, representing a doubling of CKD in less than 20 yr (51). Over 90% of patients with CKD have hypertension (39), which also contributes to the higher rates of hospitalization, greater mortality, shorter life expectancy, and healthcare costs, which are up to 5 times more expensive than non-CKD patients and represent an enormous burden to the healthcare budget. Furthermore, the progressive nature of CKD and the lack of reliable clinical biomarkers to define the best time for interventions reduce opportunities to recover kidney function or to delay development of end-stage renal disease.
Animal models are critical for understanding the pathophysiology of CKD and for development of new therapies. We have learned a lot from rodent models of CKD, which are often preferred over large animals for a variety of reasons, such as lower maintenance costs and the possibility of genetic manipulations or specific interventions (4). However, pathophysiology in rodents is often not replicated in larger animals, and differences in cardio-renal anatomy (e.g., unilobular/unipapillary), physiology, and pathophysiology in rodents (e.g., sensitivity to develop fibrosis) compared with human kidneys stress some limitations for clinical translation. Therefore, animal models that could address these limitations and complement the mechanistic knowledge from current models could improve our understanding of CKD, facilitate development of new treatments, and ultimately help to move the field forward.
Previous studies from our laboratory using a swine model of unilateral renovascular disease (RVD; induced by renal artery stenosis) and hypertension demonstrated that renal microvascular (MV) dysfunction, damage, and even loss develop in the stenotic kidney, are progressive in cortex and medulla, and correlate with loss of renal function, hypertension, and severity of renal damage (6, 7, 13, 25). Chronic RVD affects 9–11% of the general population but is observed in up to 30–60% of older patients or in those with atherosclerotic coronary and/or peripheral vascular disease (42, 49, 50), increasing their cardiovascular death and risk for developing CKD by 25% (28).
Renal MV rarefaction is a hallmark and a universal pathological feature of CKD irrespective of the cause (35, 53). The renal microvessels are not only highly susceptible to rarefaction, but also display limited regenerative capacity (34). Furthermore, major cardiovascular factors and causes of CKD like hypertension and diabetes associate with intrarenal MV rarefaction, which may develop even before deterioration of renal function (36), supporting a potential cause-effect relationship and suggesting a pathophysiological role of MV damage in the development and progression of renal dysfunction and injury. We have also shown that superimposing dyslipidemia on RVD synergistically exacerbates pathological aspects of CKD in this model, but only in the stenotic kidney (8, 9), whereas the contralateral kidney is relatively preserved (25, 43). Therefore, by inducing bilateral RVD and dyslipidemia in the swine, the aim of this study was to develop a translational model of CKD that mimics major pathological features observed in patients with this disease.
METHODS
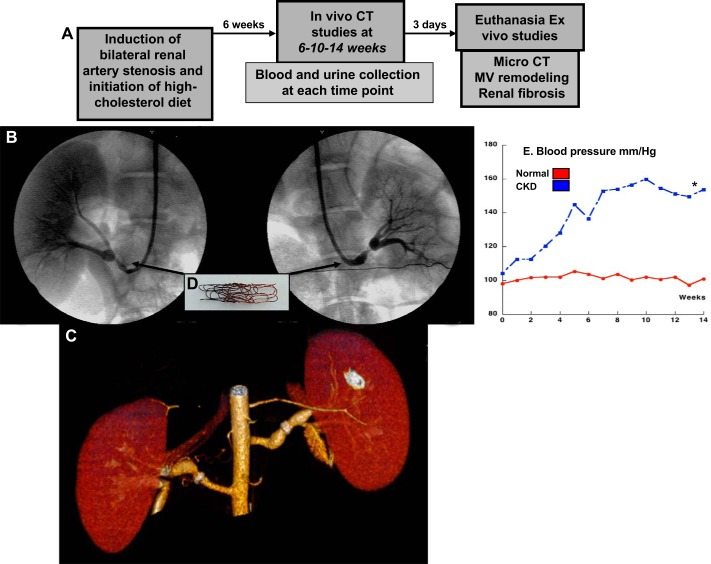
The Institutional Animal Care and Use Committee at the University of Mississippi Medical Center approved all the studies. Fourteen prejuvenile (not sexually mature) female domestic pigs (Sus scrofa domesticus) were used for the study, which lasted a total of 14 wk. In seven pigs, CKD was induced by bilateral renal artery stenosis (8, 9, 11) combined with a 15% lard-2% high-cholesterol atherogenic diet (Tables 1 and 2) (8, 9) initiated on the same day of induction of renal artery stenosis and maintained for 14 wk (Fig. 1A). Briefly, under sterile conditions, a vascular cutdown was performed in the right or left femoral artery, with a 9F vascular sheath introduced, and then under fluoroscopic guidance a 7F arterial guide was advanced from the femoral artery to the aorta. A 7-mm PTCA balloon containing a copper wire coil was deployed and engaged in the proximal-middle section of the left renal artery over a 0.014-in. PTCA guide wire, inflated once to 14 atm for coil expansion, and then deflated and removed, leaving the coil in place. Using the same vascular access, the process was repeated to engage a new coper coil in the proximal-middle section of the right renal artery. We have shown that the coil leads to a progressive narrowing of the arterial lumen on average 70% and an increase in blood pressure within 10 days (8, 32, 33). Blood pressure was continuously measured by telemetry, as previously described (8, 9, 11). The other seven pigs were used as normal controls.
Table 1.
Composition of the high-cholesterol diet (15% lard, 2% cholesterol)
| Formula | Amount, g/kg |
|---|---|
| Corn | 276.6 |
| Wheat middlings | 250 |
| Soybean meal (44%) | 223.9 |
| Alfalfa meal (17%), dehydrated | 30 |
| Lard | 150 |
| Cholesterol | 20 |
| Dicalcium phosphate, FG (18.5% P, 21% Ca) | 14 |
| Calcium carbonate, FG (38%) | 11.2 |
| Mineral mix, swine (84246) | 5 |
| dl-methionine, FG (99%) | 1.6 |
| l-lysine HCl, FG (78%) | 0.6 |
| Vitamin mix, AIN-76A (40077) | 15 |
| Choline chloride, FG (60%) | 2 |
| Vitamin B12 (0.1% in mannitol) | 0.01 |
| Vitamin B12 (MSB complex) | 0.06 |
AIN, American Institute of Nutrition; FG, feed grade; MSB, menadione sodium bisulfate.
Table 2.
Nutritional information of normal and HC diets
| Normal diet, %kcal from | HC diet, %kcal from | |
|---|---|---|
| Protein | 21 | 19 |
| Carbohydrates | 68 | 37.2 |
| Fat | 11 | 43.8 |
HC, high cholesterol.
Fig. 1.
A: design of the study. B and C: conventional renal angiography (arm; B and C) and CT angiography [multidetector computer tomography (MDCT); C] after 14 wk of chronic kidney disease (CKD). D and E: copper coil deployed inside each main renal artery on day 0 to induce renal artery stenosis (D), which resulted in a significant increase in blood pressure (measured by telemetry; E). *P < 0.05 vs. normal.
Six weeks after induction of CKD, all animals (CKD and normal controls) were anesthetized (induction with intramuscular 2 mg/kg ketamine + 5 mg/kg of telazol; maintenance with intravenous cocktail of 20 mg/kg ketamine + 2 mg/kg of xylazine), intubated, and mechanically ventilated. Under sterile conditions, a vascular cutdown was performed and vascular access gained to place 9F vascular sheaths in the carotid artery and external jugular vein, respectively. Under fluoroscopic guidance, a 7F arterial guide was advanced to the renal arteries and degree of renal artery stenosis quantified in all pigs by renal angiography as shown (8, 9, 11). The arterial guide was then placed and left at the level of the suprarenal aorta and used for collection of renal arterial blood, direct blood pressure recording, and infusion of acetylcholine (Ach) or sodium nitroprusside (SNP) needed for the in vivo studies. In addition, a 7F catheter was advanced through a jugular vein to the renal veins for collection of blood and then replaced with a 5F pigtail catheter through the venous sheath and placed in the right atrium for administration of a commercially available and clinically used nonionic low-osmolar contrast medium (20-ml bolus in 2 s) using a power injector. The bolus served for the in vivo helical multidetector computer tomography (MDCT) flow studies, which were performed for quantification of RBF, regional perfusion, and GFR, as previously shown and validated (8, 9, 11, 16). MDCT-derived quantifications of renal hemodynamics were repeated during suprarenal infusion of Ach and SNP to test endothelium-dependent and -independent renal MV endothelial function responses as shown and validated (8, 9, 11, 16).
Pigs were observed for 8 additional wk and MDCT in vivo studies repeated at 10 and 14 wk to assess progression of renal deterioration. Blood and urine samples were collected at each in vivo study (6, 10, and 14 wk; Fig. 1A) to measure plasma creatinine, blood urea nitrogen (BUN), albuminuria (spot urine, single collection) and albumin-to-creatinine ratio (spot urine, single collection, fluorometry, cat. no. K551-100; BioVision, Milpitas, CA), and urinary measurements of kidney-injury molecule (KIM)-1 and neutrophil gelatinase-associated lipocalin (NGAL) by ELISA (cat. no. DKM100 and cat. no. KIT 036; R & D Systems, Minneapolis, MN, and BioPorto Diagnostics, Hellerup, Denmark, respectively) (17), following vendors’ instructions. After completion of all of the in vivo studies, the pigs were euthanized with pentobarbital sodium (100 mg/kg iv), the kidneys were removed, and ex vivo studies were performed to quantify MV density by μCT reconstructions, renal MV remodeling, renal inflammation, and morphometric analysis.
High-resolution CT imaging.
MDCT analysis was used to calculate single-kidney RBF (ml/min), GFR (ml/min), and renal perfusion (ml·min−1·ml tissue−1), using previously validated methods (7, 11, 12, 16, 30).
μCT reconstruction and quantification of renal MV density and distribution in cortex and medulla was performed as extensively described (7, 10–13).
Western blotting.
Standard blotting protocols were followed as described (9, 11) to determine renal expression of vascular endothelial growth factor (VEGF), the specific receptor Flk-1, proinflammatory nuclear factor (NF)-κB and mediator IκB, profibrotic transforming growth factor (TGF)-β, and connective tissue growth factor (CTGF), and tissue remodeling factors matrix metalloproteinases (MMP)-2 and -9 and their inhibitor tissue inhibitors of metalloproteinases (TIMP-1) (Santa Cruz Biotechnology, CA for all) were also measured.
Renal inflammation.
Immunoreactivity against CD68, indolamine 2,3-dioxygenase (IDO), and manose receptor C type 1 (MRC1) were performed in paraffin-embedded renal sections following established costaining protocols (18) to identify M1 and M2 macrophage infiltration in the kidney. IDO and MRC1 equally identify M1 and M2 phenotypes in human and swine (22, 29, 45). Briefly, 5-µm paraffin-embedded kidney cross-sections were stained with mouse anti-CD68, rabbit anti-MRC1, and goat anti-IDO1, followed by Alexa Fluor 488 conjugated donkey anti-mouse IgG, Alexa Fluor 546 conjugated donkey anti-rabbit IgG, and Alexa Fluor Cy5 conjugated donkey anti-goat IgG. Sections from CKD and normal controls pigs were evaluated using a Leica TCS SP8 confocal microscope and quantified using ImageJ 1.45s (National Institutes of Health) (12). Positive cells were quantified as number of cells positive for either CD68 + IDO (M1) or CD68 + MRC1 (M2) and expressed as number of dual-positive cells per high power (×63) field in 15 randomly selected fields per slide.
Renal morphology.
Mid-hilar 5-µm cross- sections of each kidney (1 per animal) stained with trichrome were examined to quantify (using ImageJ 1.45s, National Institutes of Health) tubule/interstitial fibrosis, glomerulosclerosis, and media-to-lumen ratio, as shown (11, 12).
Statistical analysis.
Results are expressed as means ± SD or SE as indicated. Comparisons within groups were performed using the paired Student’s t-test, and among groups using one-way ANOVA, with Bonferroni correction for multiple comparisons. Statistical significance was defined as P ≤ 0.05.
RESULTS
General characteristics.
Body weights of the animals were similar in normal and CKD. After 14 wk of observation the pigs were very close to the size of an average adult human (68.8 ± 2.9 kg). The degree of renal artery stenosis was similar in both renal arteries after 6 wk of CKD and remained unchanged at 10 and 14 wk, as were plasma cholesterol and hypertension (Table 3 and Fig. 1B), which were all significantly higher compared with controls.
Table 3.
Body weight, degree of stenosis, MAP, plasma cholesterol (total), plasma creatinine, BUN, albuminuria (spotted urine), and renal volume in normal animals and animals with CKD
| Parameter | Normal | CKD |
|---|---|---|
| Body weight, kg | 59.2 ± 8.8 | 68.8 ± 2.9 |
| Degree of stenosis, % | 0.0 ± 0.0 | 71.8 ± 10.2* |
| MAP, mm/Hg | 92.4 ± 4.6 | 156.4 ± 8.7* |
| Cholesterol, mg/dl | 74.9 ± 5.6 | 831.9 ± 34.3* |
| Plasma creatinine, μmol/l | 112.3 ± 5.8 | 203.2 ± 13.2* |
| BUN, mg/dl | 4.8 ± 0.4 | 6.6 ± 0.6* |
| Albuminuria, mg/dl | 1.68 ± 0.04 | 2.45 ± 0.3* |
| Renal volume, ml | 198.1 ± 13.3 | 132.6 ± 12.8* |
Values are means ± SE; n = 7/group. BUN, blood urea nitrogen; CKD, chronic kidney disease; MAP, mean arterial pressure. Parameters were obtained after 14 wk of observation.
P < 0.05 vs. normal.
CT-derived renal hemodynamics and function.
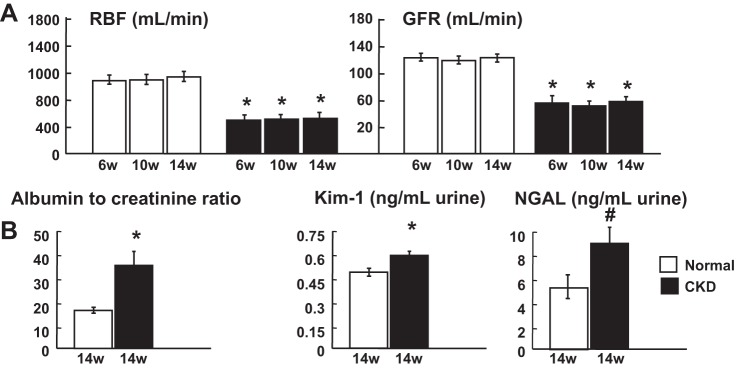
Compared with normal controls, animals with CKD showed significant reductions in total RBF (−41.3% vs. normal, P < 0.05), GFR (−48.5% vs. normal, P < 0.05; Fig. 2A), and renal volume (−33.1% vs. normal, P < 0.05), with a relatively preserved renal tissue perfusion [−8% vs. normal, P = not significant (NS)], which were all sustained throughout 14 wk of observation. The reduction in basal renal hemodynamics was accompanied by blunted responses to Ach and SNP at 6 (RBF increased between 10 and 16%, and GFR increased between 10 and 16%, respectively; P = NS vs. baseline), 10 (RBF increased between 0 and 10%, and GFR increased between 6 and 10%, respectively, P = NS vs. baseline), and at 14 wk (RBF increased between 0 and 4%, and GFR increased between 0 and 4%, respectively, P = NS vs. baseline), suggesting prolonged renal MV endothelial dysfunction (normal controls; RBF and GFR increases in response to Ach and SNP were between 38 and 49% vs. baseline, P < 0.05 vs. baseline throughout the study). In addition, these changes were associated with progressive increases in plasma creatinine, BUN, microalbuminuria (Tables 1 and 2), elevated albumin-to-creatinine ratio, and increased urinary concentrations of KIM-1 and NGAL, suggesting significant renal parenchymal injury (Fig. 2B).
Fig. 2.
Experimental chronic kidney disease (CKD) resulted in a significant reduction of bilateral renal hemodynamics and function (compatible with CKD stage 2) and development of renal injury. A: renal blood flow (RBF) and glomerular filtration rate (GFR) after 6, 10, and 14 wk of CKD. B: albumin-to-creatinine ratio, urinary kidney injury molecule-1 (KIM-1), and neutrophil gelatinase associated lipocalin (NGAL) after 14 wk of CKD. *P < 0.05 vs. normal; #P = 0.07 vs. normal.
Micro CT-derived MV analysis.
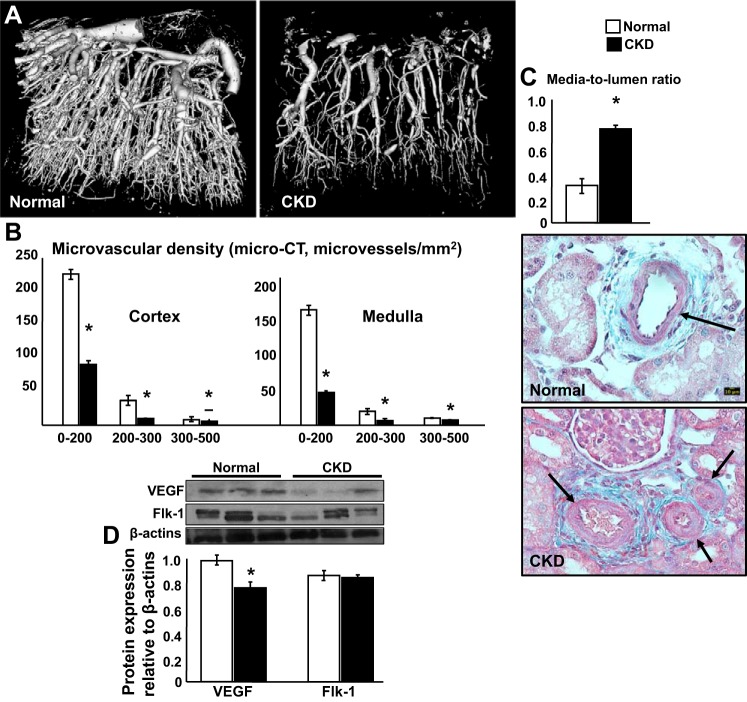
Cortical and medullary MV rarefaction was significant after 14 wk of CKD, with greater reduction in MV density of those microvessels with diameters <200 μm (Fig. 3, A and B). In addition, media-to-lumen ratio, a marker of MV remodeling and damage, was also increased (Fig. 3C) and accompanied by blunted renal expression of VEGF (Flk-1 was not significantly reduced), suggesting altered signaling for MV proliferation and repair (Fig. 3D).
Fig. 3.
Experimental chronic kidney disease (CKD) induced significant renal microvascular rarefaction and remodeling in both kidneys. A and B: representative pictures of renal microvascular (MV) density (3-dimensional μCT reconstruction) and quantification (MV density of microvessels of diameter between 0 and 200, 200 and 300, and 300 and 500 μm. C: quantification of MV media to lumen ratio (both kidneys). D: representative protein expression (3 bands/group) of VEGF and Flk-1 receptor in normal pigs and pigs after 14 wk of CKD. *P < 0.05 vs. normal.
Renal protein expression/inflammation.
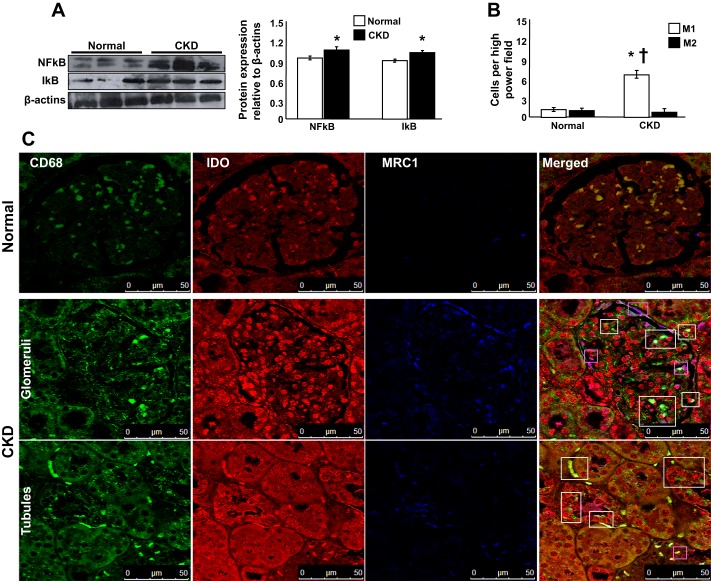
CKD pigs showed increased NF-κB/IκB expression, suggesting proinflammatory activity (Fig. 4A). In addition, confocal microscopy of costained renal cross-sections revealed a substantial number of total (CD68+) macrophages in both cortex and medulla shown by differential staining an abundance of M1 (CD68+/IDO+) over M2 (CD68+/MRC1+) macrophages (Fig. 4B), indicating renal inflammation.
Fig. 4.
Experimental kidney disease (CKD) resulted in substantial inflammation in both kidneys. A: representative protein expression (3 bands/group) of proinflammatory NF-κB and IκB in normal pigs and pigs after 14 wk of CKD. B and C: representative quantification and pictures of renal sections costained with CD68 (total macrophages), indolamine 2,3-dioxygenase (IDO; M1 macrophages), manose receptor C type 1 (MRC1; M2 macrophages), and merged CD68/IDO/MRC-1 (×63) shown as examples to illustrate renal inflammatory infiltrates and identify M1 and M2 macrophages after 14 wk of CKD. The renal expression of NF-κB/IκB was increased in CKD compared with controls, whereas the presence of proinflammatory M1 macrophages (white boxes) was significantly higher than M2 (magenta boxes) in pigs after 14 wk of CKD. *P < 0.05 vs. normal; †P < 0.01 vs. M2.
Renal morphology.
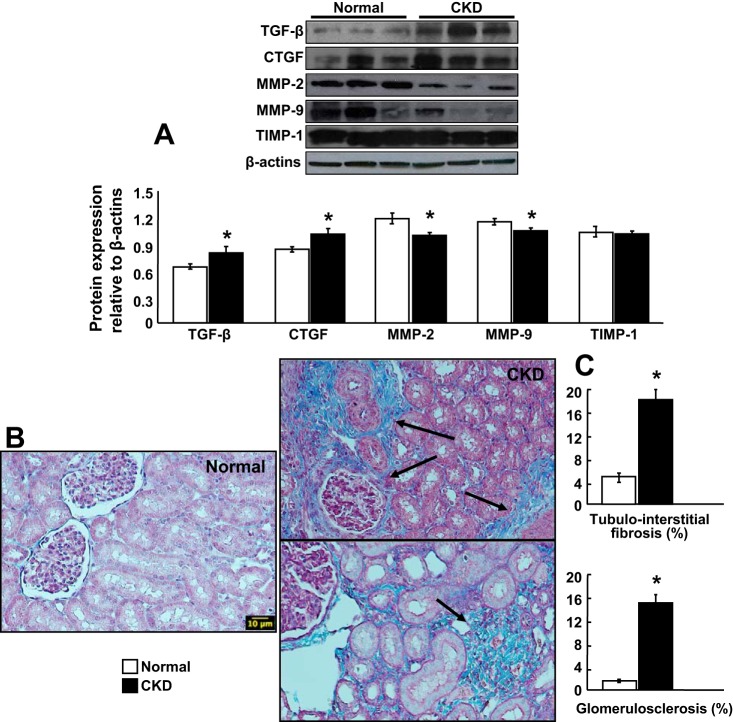
Fourteen weeks of CKD resulted in a significantly increased expression of TGFβ, CTGF, and blunted MMP-2 and -9 (TIMP-1 was unchanged), suggesting altered signaling for extracellular matrix turnover (Fig. 5A). This was accompanied by development of significant renal fibrosis in tubulointerstitial, perivascular, and glomerular compartments. The presence of fibrosis was similar in both kidneys and more evident at the tubulointerstitial level (Fig. 5, B and C).
Fig. 5.
Experimental kidney disease (CKD) induced significant glomerulosclerosis and tubule/interstitial fibrosis in both kidneys. A: representative protein expression (3 bands/group) of profibrotic transforming growth factor-β (TGFβ), connective tissue growth factor (CTGF), and tissue remodeling factors matrix metalloproteinase (MMP)-2, MMP-9, and tissue inhibitors of metalloproteinases (TIMP-1). Representative renal cross-sections stained with trichrome (×20), shown as examples to illustrate (B) and quantify (C) the degree of renal fibrosis after 14 wk of CKD. *P < 0.05 vs. normal.
DISCUSSION
The current study aimed to develop and characterize a new model of CKD. Bilateral renal ischemia combined with dyslipidemia resulted in a significant decrease in total RBF and GFR (compatible with CKD stage 2) that remained decreased throughout the 14 wk of observation and was associated with increased plasma creatinine, BUN, urine albumin-to-creatinine ratio, and urinary markers of tubular injury. The deterioration of renal function was accompanied by progressive MV rarefaction and remodeling in cortex and medulla, reduction in kidney size, renal inflammation, fibrosis, and hypertension. These data show that our approach successfully led to CKD in the swine and reproduced major pathological features (loss of renal function, microalbuminuria, tubular injury, glomerular injury, inflammation, tubule-interstitial fibrosis, glomerulosclerosis, MV rarefaction and remodeling, and hypertension) that are observed in human CKD irrespective of the etiology (48, 55).
In general, research using large animals like dogs, pigs, or monkeys has become less frequent when compared with studies using small animal models of disease. Numerous factors contributing to this trend include practical reasons such as cost, easier manipulation and maintenance, and the advantage of a large availability of genetically modified rodent models of disease. Undoubtedly, genetically modified models allow in-depth studies of pathways involved in health and disease in a mechanistic fashion. Genetically modified large animals are also available but much less frequent (Table 4).
Table 4.
Representative table summarizing differences, similarities, advantages, and disadvantages of rodent and swine (Sus scrofa domesticus) models of renal disease to humans
| Parameter | Rodents | Pigs |
|---|---|---|
| Anatomy and organ distribution | Similar to H | Similar to H |
| Renal anatomy | Unipapillary | Multipapillary as in H |
| Organ and body size | Distant from H | Closer to H |
| Metabolic rate | Distant from H | Closer to H |
| Cardiac parameters | Distant from H | Closer to H |
| Renal parameters | Distant from H | Closer to H |
| Genetic similarities | Distant from H | Closer to H |
| Renal pathophysiology | Often not replicated in H | Usually replicated in H |
| Tissue availability | Low | High |
| Inducible genetic modifications | Available and abundant | Limited |
| Combined etiologies of CKD | Possible | Possible |
| Cost | Low | High |
CKD, chronic kidney disease; H, humans.
Large animal models of disease offer several advantages related to their size and similarities in anatomy, physiology, and pathophysiology of clinically prevalent cardiovascular risk factors and target organ injury (Table 4). The pig shows cardiovascular and metabolic parameters comparable with humans and displays predictable responses to dietary, pharmacological, or instrumental interventions (8, 33, 38). The pig’s size, anatomy, and organ distribution are virtually identical to humans and allow performance of clinically available interventions and techniques used in patients (6, 10). Furthermore, recent work demonstrated that similarities between pigs and humans are deeply expressed at the genetic level and that some genetic mutations in swine (Sus scrofa domesticus) and humans display a similar pathological phenotype (21), underscoring the high potential of the swine as a suitable model for studying mechanisms of human disease (Table 4).
A new platform in a large animal with numerous genetically, anatomic, and physiological similarities compared with humans (1, 21) may extend and complement the current knowledge on CKD. In addition, the characteristics of the swine model may also serve as a novel tool to test potential treatments for CKD in a highly translational fashion. The current study developed a model of CKD using prejuvenile female Sus scrofa domesticus. Sexual maturation in this strain of swine is not reached until 8–10 mo of age and/or weights of 90 kg or higher (37) and thus mitigates any potential effect of changes in sex hormones on the pathophysiology of CKD in the model.
The induction of bilateral RVD led to hypertension in this model, which was progressive until 6–8 wk and then stabilized for the rest of the observation. Hypertension relates to CKD as both a cause and a consequence and increases cardiovascular risk and risk for progression to end-stage renal disease proportionally (26). In general, hypertension in CKD is a highly prevalent feature that ranges from 23% in CKD stage 1 to 84% in CKD stages 4–5 and is even higher when renal artery stenosis or diabetes is present (>90%) (48). In a recent study on 3,612 adults with CKD (majority at moderate stage), the prevalence of hypertension was 86% compared with ∼30% observed in the general population (31). Hypertension in this model is likely initiated by a significant stimulation of the renin-angiotensin and endothelin systems driven by induction of renal ischemia and is sustained by expansion of extracellular volume, increased systemic and renal oxidative stress, reduced bioavailability of vasodilators, and endothelial dysfunction (32, 40, 44). Progressive damage of the renal parenchyma in CKD plays an important role in development and maintenance of hypertension, as hypertension propels the progression of renal injury in CKD (20), and reduction in renal damage may attenuate hypertension (12).
Remodeling, altered vascular tone, increased permeability, and even loss of the renal microvasculature are pathological features of experimental and clinical CKD of different etiologies (3). The progressive damage of the renal microvasculature possibly plays a prominent role in the evolving nature of CKD since renal vessels provide nutrition to the organ and participate in whole body homeostasis. The swine model of CKD develops a significant and progressive functional and structural MV rarefaction that initiates in the cortex [as we showed in unilateral RVD after 6 wk (25)] but expands to the medulla as disease evolves (7, 11). We also showed that combined dyslipidemia with renal ischemia synergistically reduces the renal bioavailability of nitric oxide and results in a vasoconstrictive milieu that alters renal hemodynamics and function (8). These are likely the major underlying mechanisms of the blunted RBF and GFR (reduced by ∼50% compared with normal controls) and deficient responses to endothelium-dependent and -independent challenges throughout the study, suggesting prolonged MV endothelial dysfunction.
The abnormal MV function in the CKD model likely plays a prominent role in blunted renal hemodynamics, but prolonged renal ischemia can also disrupt the integrity of the MV endothelium as well. Although a decrease in oxygen supply initially upregulates expression of hypoxia-inducible factor (HIF)-1α and VEGF in renal cells, sustained hypoxia may destabilize or depress the HIF-1α/VEGF axis and interfere with renal MV and tissue repair (7, 25). Our model of CKD displays a significant decrease in renal VEGF. Thus, chronic hypoxia may both stimulate and restrict compensatory mechanisms of MV repair and proliferation in the kidney. These actions may explain the impaired vascular reactivity, angiogenic, proliferative and likely migratory capacity of the cells, and contribute to erode protective properties of the MV endothelial barrier. A disruption of renal MV integrity in our model may also be suggested by the increased albuminuria and albumin-to-creatinine ratio (52). Furthermore, structural MV rarefaction in CKD may also be favored by unopposed pro-fibrotic stimuli in the kidney such as upregulation of TGF-β and CTGF paired with altered activity of MMPs/TIMPs. These data suggest a multifold deleterious imbalance in matrix turnover toward fibrosis together with reduced renal VEGF and blunted MV repair in the CKD model. Notably, we recently showed that recovery of the renal MV architecture and function is feasible and functionally consequential (7, 11, 12, 47). Therefore, MV protection may represent an attractive therapeutic target to slow, halt, or even reverse progression of renal injury in CKD.
Another important pathological footprint of CKD is inflammation. Renal MV endothelium damage and oxidative stress are powerful stimuli for development of systemic and renal inflammation in CKD (46). Persistent and often progressive inflammation in CKD plays a role in the increased cardiovascular and all-cause mortality of the disease (2). We showed that coexistence of lipid abnormalities with renal ischemia in the swine model of RVD exacerbates development of renal inflammation, which is provoked by upregulation of proinflammatory pathways such as the NF-κB/IκB(5, 9, 10), a master transcription path that activates transcription of other proinflammatory cytokines, including TNF-α and interleukins (54). Both kidneys in the CKD model display increased renal expression of NF-κB accompanied by the abundant presence of macrophages in the glomeruli and tubule/interstitium. Differential staining showed a significant imbalance between proinflammatory M1 macrophages related to anti-inflammatory M2, which was likely driven by the upregulation of NF-κB and TNFα (41). Indeed, the M1/M2 balance reflects the renal microenvironment, which defines the differentiation of the macrophages to M1 or M2 phenotype and could influence the progression of CKD (22). Thus, chronic inflammation is a major pathological component of this model of CKD that likely contributes to the development of renal dysfunction and injury. Importantly, compared with mice, the immune system of the swine has been shown to more closely resemble humans in >80% of parameters studied (19). We used M1/M2 markers (IDO and MRC1, respectively [22, 29, 45)] that are expressed in both humans and swine, which underscored the translational potential of these findings. Increased inflammation was also accompanied by increased urinary KIM-1 and NGAL, which are accepted markers of renal injury that also play a role in determining macrophage phenotype (27, 56). Thus, all these changes indicate that chronic renal inflammation develops and likely plays a pathological role in the swine model of CKD, as observed in patients, which may offer a translational platform to test potential therapeutic interventions targeting inflammation in CKD.
Limitations and opportunities.
There are certain limitations that need to be addressed. Although the weight of the model is similar to the average weight of an adult human, the animals were prejuvenile. CKD is a disease more prevalent in middle-aged and older patients. Therefore, future studies may need to address whether age plays a role in the pathophysiology or, eventually, responses to treatments in the swine model and whether older animals will still present comparable pathological features as the most prevalent population of patients suffering CKD. However, the natural growth pattern of these animals may also impose a limitation for very long-term studies since they can reach >300 kg at adult age. Similarly, we will consider future studies in prejuvenile males as well as in sexually mature male and female pigs to address whether sex may act as a biological variable in this model. Another limitation of the model and study is the lack of frequently observed comorbidities and factors that contribute to the development and progression of CKD, such as diabetes, obesity, or smoking, to name a few. However, the model offers an opportunity to induce additional insults such as metabolic syndrome, obesity, or diabetes (18, 23, 57, 58) that may exacerbate renal injury and the progression of CKD into more advanced stages. Furthermore, a common clinical scenario is acute kidney injury that occurs in the context of CKD. AKI and CKD being interconnected as one is a risk factor for the other, and both are risk factors for cardiovascular disease (15, 24). AKI is a significant risk factor for progression of preexisting CKD (14), but underlying mechanisms are not fully understood. Thus, a translational platform like the swine model with so many pathological features observed in human patients also offers an opportunity for elucidation of such mechanisms that determine the chances of renal recovery and the testing of therapeutic interventions. Finally, this model may also offer a new experimental setting for future studies to investigate cardiac disease in CKD in a translational fashion.
In summary, the current study characterized a novel and highly translational model of CKD that, independent of the initial cause, reproduces many pathological features of human disease. The model allows for additional insults that may accelerate the progression of the disease, offering a platform to test new treatments and new targets and identify windows of opportunity to halt progression of CKD, recover the kidney, or pinpoint traits of irreversible damage that could define progression toward end-stage and the need of renal replacement therapies.
GRANTS
This work was supported by Grant No. 18490005 from the American Heart Association, NIH Grants HL-51971 and GM-104357, and an Intramural Research Support Program-University of Mississippi Medical Center grant.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.R.C. conceived and designed research; A.R.C., M.L.W., J.E.E., E.G., and T.W.H. performed experiments; A.R.C. and M.L.W. analyzed data; A.R.C. and M.L.W. interpreted results of experiments; A.R.C., J.E.E., and E.G. prepared figures; A.R.C. drafted manuscript; A.R.C., M.L.W., J.E.E., E.G., and T.W.H. edited and revised manuscript; A.R.C., M.L.W., J.E.E., E.G., and T.W.H. approved final version of manuscript.
REFERENCES
- 1.Abbott A. Pig geneticists go the whole hog. Nature 491: 315–316, 2012. doi: 10.1038/491315a. [DOI] [PubMed] [Google Scholar]
- 2.Akchurin OM, Kaskel F. Update on inflammation in chronic kidney disease. Blood Purif 39: 84–92, 2015. doi: 10.1159/000368940. [DOI] [PubMed] [Google Scholar]
- 3.Bábíčková J, Klinkhammer BM, Buhl EM, Djudjaj S, Hoss M, Heymann F, Tacke F, Floege J, Becker JU, Boor P. Regardless of etiology, progressive renal disease causes ultrastructural and functional alterations of peritubular capillaries. Kidney Int 91: 70–85, 2017. doi: 10.1016/j.kint.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 4.Becker GJ, Hewitson TD. Animal models of chronic kidney disease: useful but not perfect. Nephrol Dial Transplant 28: 2432–2438, 2013. doi: 10.1093/ndt/gft071. [DOI] [PubMed] [Google Scholar]
- 5.Chade AR, Best PJ, Rodriguez-Porcel M, Herrmann J, Zhu X, Sawamura T, Napoli C, Lerman A, Lerman LO. Endothelin-1 receptor blockade prevents renal injury in experimental hypercholesterolemia. Kidney Int 64: 962–969, 2003. doi: 10.1046/j.1523-1755.2003.00170.x. [DOI] [PubMed] [Google Scholar]
- 6.Chade AR, Kelsen S. Renal microvascular disease determines the responses to revascularization in experimental renovascular disease. Circ Cardiovasc Interv 3: 376–383, 2010. doi: 10.1161/CIRCINTERVENTIONS.110.951277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chade AR, Kelsen S. Reversal of renal dysfunction by targeted administration of VEGF into the stenotic kidney: a novel potential therapeutic approach. Am J Physiol Renal Physiol 302: F1342–F1350, 2012. doi: 10.1152/ajprenal.00674.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chade AR, Rodriguez-Porcel M, Grande JP, Krier JD, Lerman A, Romero JC, Napoli C, Lerman LO. Distinct renal injury in early atherosclerosis and renovascular disease. Circulation 106: 1165–1171, 2002. doi: 10.1161/01.CIR.0000027105.02327.48. [DOI] [PubMed] [Google Scholar]
- 9.Chade AR, Rodriguez-Porcel M, Grande JP, Zhu X, Sica V, Napoli C, Sawamura T, Textor SC, Lerman A, Lerman LO. Mechanisms of renal structural alterations in combined hypercholesterolemia and renal artery stenosis. Arterioscler Thromb Vasc Biol 23: 1295–1301, 2003. doi: 10.1161/01.ATV.0000077477.40824.52. [DOI] [PubMed] [Google Scholar]
- 10.Chade AR, Tullos N, Stewart NJ, Surles B. Endothelin-a receptor antagonism after renal angioplasty enhances renal recovery in renovascular disease. J Am Soc Nephrol 26: 1071–1080, 2015. doi: 10.1681/ASN.2014040323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chade AR, Tullos NA, Harvey TW, Mahdi F, Bidwell GL III. Renal therapeutic angiogenesis using a bioengineered polymer-stabilized vascular endothelial growth factor construct. J Am Soc Nephrol 27: 1741–1752, 2016. doi: 10.1681/ASN.2015040346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chade AR, Williams ML, Guise E, Vincent LJ, Harvey TW, Kuna M, Mahdi F, Bidwell GL III. Systemic biopolymer-delivered vascular endothelial growth factor promotes therapeutic angiogenesis in experimental renovascular disease. Kidney Int 93: 842–854, 2018. doi: 10.1016/j.kint.2017.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chade AR, Zhu X, Lavi R, Krier JD, Pislaru S, Simari RD, Napoli C, Lerman A, Lerman LO. Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation 119: 547–557, 2009. doi: 10.1161/CIRCULATIONAHA.108.788653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, Bittleman D, Cruz D, Endre Z, Fitzgerald RL, Forni L, Kane-Gill SL, Hoste E, Koyner J, Liu KD, Macedo E, Mehta R, Murray P, Nadim M, Ostermann M, Palevsky PM, Pannu N, Rosner M, Wald R, Zarbock A, Ronco C, Kellum JA; Acute Disease Quality Initiative Workgroup 16. . Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 13: 241–257, 2017. doi: 10.1038/nrneph.2017.2. [DOI] [PubMed] [Google Scholar]
- 15.Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 371: 58–66, 2014. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daghini E, Primak AN, Chade AR, Krier JD, Zhu XY, Ritman EL, McCollough CH, Lerman LO. Assessment of renal hemodynamics and function in pigs with 64-section multidetector CT: comparison with electron-beam CT. Radiology 243: 405–412, 2007. doi: 10.1148/radiol.2432060655. [DOI] [PubMed] [Google Scholar]
- 17.Eirin A, Saad A, Tang H, Herrmann SM, Woollard JR, Lerman A, Textor SC, Lerman LO. Urinary Mitochondrial DNA Copy Number Identifies Chronic Renal Injury in Hypertensive Patients. Hypertension 68: 401–410, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eirin A, Zhu XY, Li Z, Ebrahimi B, Zhang X, Tang H, Korsmo MJ, Chade AR, Grande JP, Ward CJ, Simari RD, Lerman A, Textor SC, Lerman LO. Endothelial outgrowth cells shift macrophage phenotype and improve kidney viability in swine renal artery stenosis. Arterioscler Thromb Vasc Biol 33: 1006–1013, 2013. doi: 10.1161/ATVBAHA.113.301164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fairbairn L, Kapetanovic R, Sester DP, Hume DA. The mononuclear phagocyte system of the pig as a model for understanding human innate immunity and disease. J Leukoc Biol 89: 855–871, 2011. doi: 10.1189/jlb.1110607. [DOI] [PubMed] [Google Scholar]
- 20.Griffin KA. Hypertensive kidney injury and the progression of chronic kidney disease. Hypertension 70: 687–694, 2017. doi: 10.1161/HYPERTENSIONAHA.117.08314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groenen MA, Archibald AL, Uenishi H, Tuggle CK, Takeuchi Y, Rothschild MF, Rogel-Gaillard C, Park C, Milan D, Megens HJ, Li S, Larkin DM, Kim H, Frantz LA, Caccamo M, Ahn H, Aken BL, Anselmo A, Anthon C, Auvil L, Badaoui B, Beattie CW, Bendixen C, Berman D, Blecha F, Blomberg J, Bolund L, Bosse M, Botti S, Bujie Z, Bystrom M, Capitanu B, Carvalho-Silva D, Chardon P, Chen C, Cheng R, Choi SH, Chow W, Clark RC, Clee C, Crooijmans RP, Dawson HD, Dehais P, De Sapio F, Dibbits B, Drou N, Du ZQ, Eversole K, Fadista J, Fairley S, Faraut T, Faulkner GJ, Fowler KE, Fredholm M, Fritz E, Gilbert JG, Giuffra E, Gorodkin J, Griffin DK, Harrow JL, Hayward A, Howe K, Hu ZL, Humphray SJ, Hunt T, Hornshøj H, Jeon JT, Jern P, Jones M, Jurka J, Kanamori H, Kapetanovic R, Kim J, Kim JH, Kim KW, Kim TH, Larson G, Lee K, Lee KT, Leggett R, Lewin HA, Li Y, Liu W, Loveland JE, Lu Y, Lunney JK, Ma J, Madsen O, Mann K, Matthews L, McLaren S, Morozumi T, Murtaugh MP, Narayan J, Nguyen DT, Ni P, Oh SJ, Onteru S, Panitz F, Park EW, Park HS, Pascal G, Paudel Y, Perez-Enciso M, Ramirez-Gonzalez R, Reecy JM, Rodriguez-Zas S, Rohrer GA, Rund L, Sang Y, Schachtschneider K, Schraiber JG, Schwartz J, Scobie L, Scott C, Searle S, Servin B, Southey BR, Sperber G, Stadler P, Sweedler JV, Tafer H, Thomsen B, Wali R, Wang J, Wang J, White S, Xu X, Yerle M, Zhang G, Zhang J, Zhang J, Zhao S, Rogers J, Churcher C, Schook LB. Analyses of pig genomes provide insight into porcine demography and evolution. Nature 491: 393–398, 2012. doi: 10.1038/nature11622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guiteras R, Flaquer M, Cruzado JM. Macrophage in chronic kidney disease. Clin Kidney J 9: 765–771, 2016. doi: 10.1093/ckj/sfw096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hara H, Lin YJ, Zhu X, Tai HC, Ezzelarab M, Balamurugan AN, Bottino R, Houser SL, Cooper DK. Safe induction of diabetes by high-dose streptozotocin in pigs. Pancreas 36: 31–38, 2008. doi: 10.1097/mpa.0b013e3181452886. [DOI] [PubMed] [Google Scholar]
- 24.Heung M, Chawla LS. Acute kidney injury: gateway to chronic kidney disease. Nephron Clin Pract 127: 30–34, 2014. doi: 10.1159/000363675. [DOI] [PubMed] [Google Scholar]
- 25.Iliescu R, Fernandez SR, Kelsen S, Maric C, Chade AR. Role of renal microcirculation in experimental renovascular disease. Nephrol Dial Transplant 25: 1079–1087, 2010. doi: 10.1093/ndt/gfp605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Judd E, Calhoun DA. Management of hypertension in CKD: beyond the guidelines. Adv Chronic Kidney Dis 22: 116–122, 2015. doi: 10.1053/j.ackd.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung M, Brüne B, Hotter G, Sola A. Macrophage-derived Lipocalin-2 contributes to ischemic resistance mechanisms by protecting from renal injury. Sci Rep 6: 21950, 2016. doi: 10.1038/srep21950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalra PA, Guo H, Kausz AT, Gilbertson DT, Liu J, Chen SC, Ishani A, Collins AJ, Foley RN. Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int 68: 293–301, 2005. doi: 10.1111/j.1523-1755.2005.00406.x. [DOI] [PubMed] [Google Scholar]
- 29.Kapetanovic R, Fairbairn L, Beraldi D, Sester DP, Archibald AL, Tuggle CK, Hume DA. Pig bone marrow-derived macrophages resemble human macrophages in their response to bacterial lipopolysaccharide. J Immunol 188: 3382–3394, 2012. doi: 10.4049/jimmunol.1102649. [DOI] [PubMed] [Google Scholar]
- 30.Krier JD, Ritman EL, Bajzer Z, Romero JC, Lerman A, Lerman LO. Noninvasive measurement of concurrent single-kidney perfusion, glomerular filtration, and tubular function. Am J Physiol Renal Physiol 281: F630–F638, 2001. doi: 10.1152/ajprenal.2001.281.4.F630. [DOI] [PubMed] [Google Scholar]
- 31.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI; Chronic Renal Insufficiency Cohort (CRIC) Study Group . Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 4: 1302–1311, 2009. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lerman LO, Nath KA, Rodriguez-Porcel M, Krier JD, Schwartz RS, Napoli C, Romero JC. Increased oxidative stress in experimental renovascular hypertension. Hypertension 37: 541–546, 2001. doi: 10.1161/01.HYP.37.2.541. [DOI] [PubMed] [Google Scholar]
- 33.Lerman LO, Schwartz RS, Grande JP, Sheedy PF, Romero JC. Noninvasive evaluation of a novel swine model of renal artery stenosis. J Am Soc Nephrol 10: 1455–1465, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Ligresti G, Nagao RJ, Xue J, . et al. A novel three-dimensional human peritubular microvascular system. J Am Soc Nephrol 27: 2370–2381, 2016. doi: 10.1681/ASN.2015070747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mack M, Yanagita M. Origin of myofibroblasts and cellular events triggering fibrosis. Kidney Int 87: 297–307, 2015. doi: 10.1038/ki.2014.287. [DOI] [PubMed] [Google Scholar]
- 36.Maric-Bilkan C, Flynn ER, Chade AR. Microvascular disease precedes the decline in renal function in the streptozotocin-induced diabetic rat. Am J Physiol Renal Physiol 302: F308–F315, 2012. doi: 10.1152/ajprenal.00421.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nowak R. Walker’s Mammals of the World. Baltimore, Maryland: Johns Hopkins University Press, 1991. [Google Scholar]
- 38.Pawar AS, Zhu XY, Eirin A, Tang H, Jordan KL, Woollard JR, Lerman A, Lerman LO. Adipose tissue remodeling in a novel domestic porcine model of diet-induced obesity. Obesity (Silver Spring) 23: 399–407, 2015. doi: 10.1002/oby.20971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao MV, Qiu Y, Wang C, Bakris G. Hypertension and CKD: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES), 1999-2004. Am J Kidney Dis 51, Suppl 2: S30–S37, 2008. doi: 10.1053/j.ajkd.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 40.Reckelhoff JF, Romero JC. Role of oxidative stress in angiotensin-induced hypertension. Am J Physiol Regul Integr Comp Physiol 284: R893–R912, 2003. doi: 10.1152/ajpregu.00491.2002. [DOI] [PubMed] [Google Scholar]
- 41.Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest 118: 3522–3530, 2008. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ritchie J, Green D, Chrysochou C, Chalmers N, Foley RN, Kalra PA. High-risk clinical presentations in atherosclerotic renovascular disease: prognosis and response to renal artery revascularization. Am J Kidney Dis 63: 186–197, 2014. doi: 10.1053/j.ajkd.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez-Porcel M, Krier JD, Lerman A, Sheedy PF II, Romero JC, Napoli C, Lerman LO. Combination of hypercholesterolemia and hypertension augments renal function abnormalities. Hypertension 37: 774–780, 2001. doi: 10.1161/01.HYP.37.2.774. [DOI] [PubMed] [Google Scholar]
- 44.Romero JC, Reckelhoff JF. Oxidative stress may explain how hypertension is maintained by normal levels of angiotensin II. Braz J Med Biol Res 33: 653–660, 2000. doi: 10.1590/S0100-879X2000000600006. [DOI] [PubMed] [Google Scholar]
- 45.Schroder K, Irvine KM, Taylor MS, Bokil NJ, Le Cao KA, Masterman KA, Labzin LI, Semple CA, Kapetanovic R, Fairbairn L, Akalin A, Faulkner GJ, Baillie JK, Gongora M, Daub CO, Kawaji H, McLachlan GJ, Goldman N, Grimmond SM, Carninci P, Suzuki H, Hayashizaki Y, Lenhard B, Hume DA, Sweet MJ. Conservation and divergence in Toll-like receptor 4-regulated gene expression in primary human versus mouse macrophages. Proc Natl Acad Sci USA 109: E944–E953, 2012. doi: 10.1073/pnas.1110156109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stenvinkel P, Alvestrand A. Inflammation in end-stage renal disease: sources, consequences, and therapy. Semin Dial 15: 329–337, 2002. doi: 10.1046/j.1525-139X.2002.00083.x. [DOI] [PubMed] [Google Scholar]
- 47.Stewart N, Chade AR. Renoprotective effects of hepatocyte growth factor in the stenotic kidney. Am J Physiol Renal Physiol 304: F625–F633, 2013. doi: 10.1152/ajprenal.00504.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tedla FM, Brar A, Browne R, Brown C. Hypertension in chronic kidney disease: navigating the evidence. Int J Hypertens 2011: 132405, 2011. doi: 10.4061/2011/132405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Textor SC, Lerman LO. Reality and renovascular disease: when does renal artery stenosis warrant revascularization? Am J Kidney Dis 63: 175–177, 2014. doi: 10.1053/j.ajkd.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 50.Textor SC, Misra S, Oderich GS. Percutaneous revascularization for ischemic nephropathy: the past, present, and future. Kidney Int 83: 28–40, 2013. doi: 10.1038/ki.2012.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.U.S. Renal Data System, USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: NIH, National Institute of Diabetes and Digestive and Kidney Diseases, 2012. [Google Scholar]
- 52.Viazzi F, Leoncini G, Ratto E, Vaccaro V, Tomolillo C, Falqui V, Parodi A, Conti N, Deferrari G, Pontremoli R. Microalbuminuria, blood pressure load, and systemic vascular permeability in primary hypertension. Am J Hypertens 19: 1183–1189, 2006. doi: 10.1016/j.amjhyper.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 53.Xavier S, Vasko R, Matsumoto K, Zullo JA, Chen R, Maizel J, Chander PN, Goligorsky MS. Curtailing endothelial TGF-β signaling is sufficient to reduce endothelial-mesenchymal transition and fibrosis in CKD. J Am Soc Nephrol 26: 817–829, 2015. doi: 10.1681/ASN.2013101137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiao L, Liu Y, Wang N. New paradigms in inflammatory signaling in vascular endothelial cells. Am J Physiol Heart Circ Physiol 306: H317–H325, 2014. doi: 10.1152/ajpheart.00182.2013. [DOI] [PubMed] [Google Scholar]
- 55.Yamaguchi J, Tanaka T, Nangaku M. Recent advances in understanding of chronic kidney disease. F1000Res 4: 4, 2015. doi: 10.12688/f1000research.6970.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang L, Brooks CR, Xiao S, Sabbisetti V, Yeung MY, Hsiao LL, Ichimura T, Kuchroo V, Bonventre JV. KIM-1-mediated phagocytosis reduces acute injury to the kidney. J Clin Invest 125: 1620–1636, 2015. doi: 10.1172/JCI75417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang X, Li ZL, Eirin A, Ebrahimi B, Pawar AS, Zhu XY, Lerman A, Lerman LO. Cardiac metabolic alterations in hypertensive obese pigs. Hypertension 66: 430–436, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang X, Li ZL, Woollard JR, Eirin A, Ebrahimi B, Crane JA, Zhu XY, Pawar AS, Krier JD, Jordan KL, Tang H, Textor SC, Lerman A, Lerman LO. Obesity-metabolic derangement preserves hemodynamics but promotes intrarenal adiposity and macrophage infiltration in swine renovascular disease. Am J Physiol Renal Physiol 305: F265–F276, 2013. doi: 10.1152/ajprenal.00043.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]