Abstract
Major renal functions such as renal blood flow, glomerular filtration rate, and urinary excretion are known to exhibit circadian oscillations. However, the underlying mechanisms that govern these variations have yet to be fully elucidated. To better understand the impact of the circadian clock on renal solute and water transport, we have developed a computational model of the renal circadian clock and coupled that model to an epithelial transport model of the proximal convoluted cell of the rat kidney. The activity of the Na+-H+ exchanger 3 (NHE3) is assumed to be regulated by changes in transcription of the NHE3 mRNA due to regulation by circadian clock proteins. The model predicts the rhythmic oscillations in NHE3 activity, which gives rise to significant daily fluctuations in Na+ and water transport of the proximal tubule cell. Additionally, the model predicts that 1) mutation in period 2 (Per2) or cryptochrome 1 (Cry1) preserves the circadian rhythm and modestly raises Na+ reabsorption; 2) mutation in Bmal1 or CLOCK eliminates the circadian rhythm and modestly lowers Na+ reabsorption; 3) mutation in Rev-Erb or ROR-related orphan receptor (Ror) has minimal impact on the circadian oscillations. The model represents the first step in building a tool set aimed at increasing our understanding of how the molecular clock affects renal ion transport and renal function, which likely has important implications for kidney disease.
Keywords: NHE3, mRNA, rhythm, transcription
INTRODUCTION
Circadian clocks are cell-autonomous time-keeping mechanisms that organize physiological functions in a 24-h periodicity. In mammals, the circadian system comprises a hierarchy of oscillators that function at the cellular, tissue, and systems levels. The central circadian clock, which is located in the suprachiasmatic nucleus (SCN) of the hypothalamus, acts as a central pacemaker for the organism that contributes to rhythms in behavior, body temperature, and hormones (23). The circadian clock mechanism is present in most peripheral organs, and cells and these peripheral clocks can oscillate independently of the SCN (48). Whereas light is the dominant zeitgeber, or synchronization cue, for the SCN, food intake appears to be the most potent zeitgeber for peripheral clocks including the liver and kidney (36). Exactly how each peripheral clock receives signals from and responds to the SCN is currently an area of active investigation.
In both animals and humans, plasma Na+ concentration and renal Na+ excretion have been observed to exhibit significant diurnal fluctuations (8, 27). While the molecular mechanisms that drive these diurnal variations have yet to be completely elucidated, a number of genes that play key roles in these processes have been identified. Here, we focus on the rhythmic regulation of the Na+/H+ exchanger (NHE3). NHE3 is expressed on the apical membrane of the proximal tubule cell and catalyzes the electroneutral exchange of one extracellular Na+ for one intracellular H+ (44). The expression of NHE3 has been shown to exhibit diurnal variations at the level of mRNA, protein, and localization and is regulated directly by CLOCK:BMAL1 heterodimers of the peripheral circadian clock of the kidney (33).
In the rat, the proximal tubule is responsible for reabsorbing approximately two-thirds of the filtered Na+ and volume, with a large fraction of that reabsorption mediated by NHE3. Proximal tubule specific knockout of NHE3 resulted in substantial reduction in bicarbonate and volume reabsorption in mutant mice, although no significant change in systolic blood pressure was observed (21). Thus, as the expression of NHE3 fluctuates diurnally, the transport of water and sodium by the proximal tubule is expected to fluctuate correspondingly, likely resulting in variations in water and salt excretion. To assess the extent to which proximal tubule cellular transport is modulated by the clock gene circadian rhythm, we have developed the very first computational model of the renal circadian clock and connected the clock model to a published model of the proximal convoluted tubule epithelial cell model of the rat. The resulting model was applied to simulate changes in cellular transport in wild type and mutants.
MODEL FORMULATION
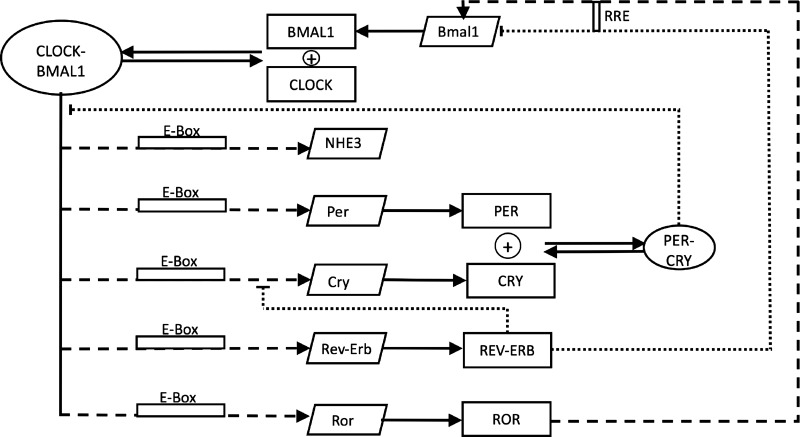
To simulate the modulation of NHE3 activity by the circadian clock, we first developed a mathematical model of the core clock in the rat kidney. The clock model comprises a number of transcription factors that regulate gene expression: period (Per), cryptochromes (Cry), Rev-Erb and RAR-related orphan receptor retinoic acid receptor-related orphan receptor (Ror), brain and muscle ARNT-Like 1 (Bmal1), and circadian locomotor output cycles kaput (CLOCK). A schematic diagram of the clock network is shown in Fig. 1. The model represents the direct regulation of NHE3 expression level by Bmal1 and CLOCK via a transcriptional mechanism (33). Equations for the clock model are given in the appendix. Model parameters were obtained by fitting predicted profiles for mRNA expression levels of Per, Cry, Rev-Erb, Ror, Bmal1, and NHE3 with their corresponding experimentally measured profiles reported in Ref 49 and database (http://circadb.hogeneschlab.org), obtained for the dark-dark cycles.
Fig. 1.
Schematic network of our mathematical model, which describes the interactions of core clock components with Na+/H+ exchanger (NHE3) gene. mRNAs are denoted by slanted boxes, proteins by squares, and protein complexes by ovals. Dashed arrows represent the transactivation and dotted arrows represent inhibition.
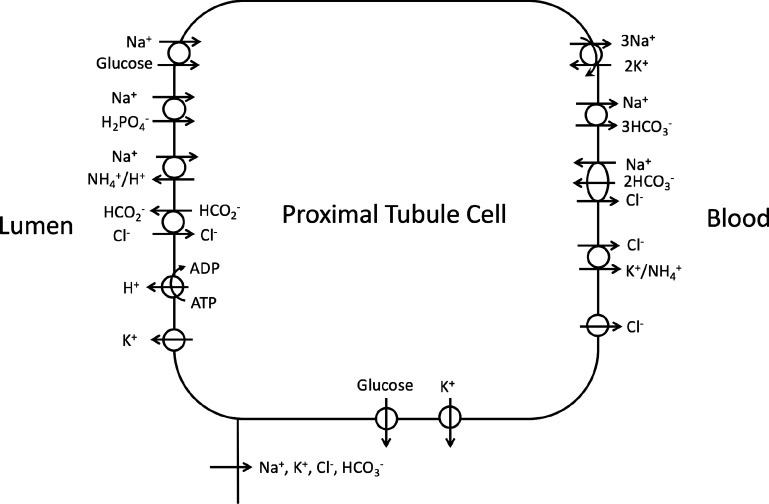
The predicted NHE3 activity was then incorporated into a detailed mathematical model of the proximal convoluted tubule cell of a rat kidney (15, 16, 18). The model was formulated for the time-dependent state (7) and represents 15 major solutes (Na+, K+, Cl−, , H2CO3, CO2, , , urea, NH3, , H+, , H2CO2, and glucose). The proximal tubule cell is represented as a well-stirred, compliant cellular compartment surrounded by luminal and peritubular (bath) solutions, the compositions of which are assumed known a priori (Table 1), as well as a lateral, paracellular space. Transporters and channels are represented on the apical and basolateral membranes, some of which are shown in Fig. 2. Model equations are based on mass conservation and electroneutrality constraints. Model equations and parameters are described in the appendix. With this configuration, the proximal tubule cell model predicts solute concentrations of the cellular and lateral innerspace compartments, membrane potential, and solute and water fluxes across the apical membrane, basolateral membrane, and paracellular pathway, as functions of time.
Table 1.
Luminal and bath concentrations
| Solute | Concentration (mM) |
|---|---|
| Na+ | 144.0 |
| K+ | 4.9 |
| Cl− | 117.0 |
| 25.0 | |
| 4.41 × 10−3 | |
| CO2 | 1.50 |
| + | 3.9 |
| urea | 5.0 |
| NH3 + † | 1.0 |
| + H2CO2 | 1.0 |
| Glucose | 5.0 |
| Protein | 2.0 |
| pH | 7.323 |
Fig. 2.
Schematic diagram of the proximal convoluted tubule cell model. The model accounts for 15 major solutes (see text). The diagram displays only the major Na+, K+, and Cl− transporters.
MODEL RESULTS
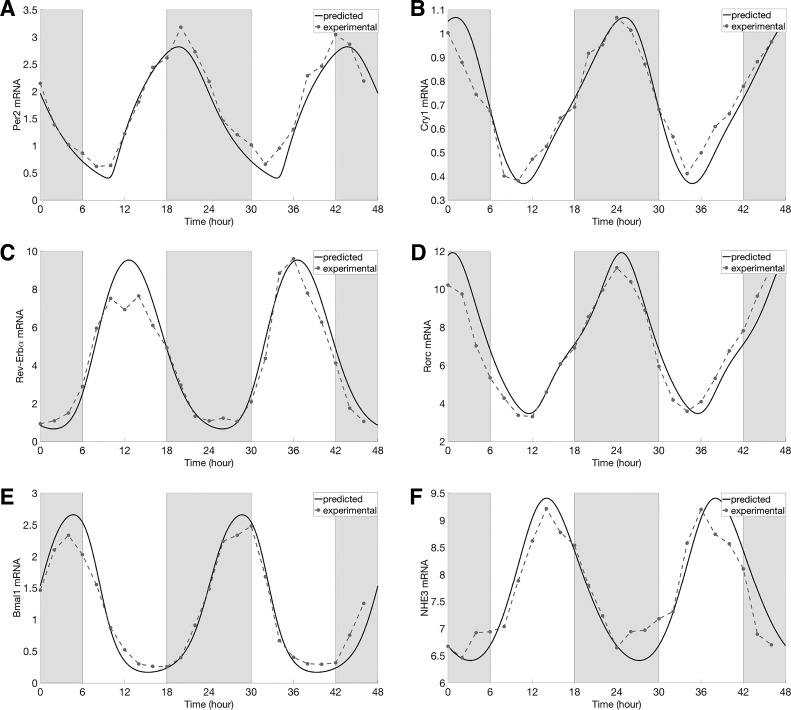
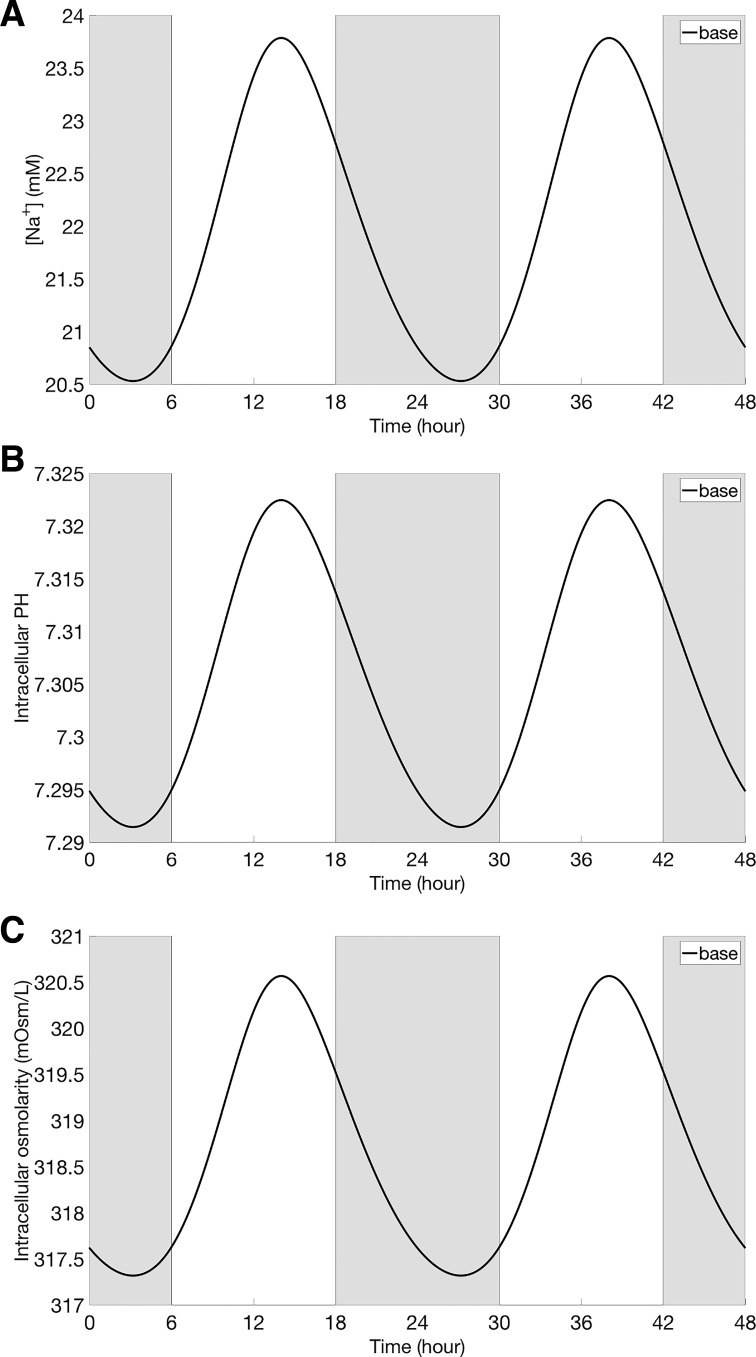
Using the baseline model parameters (see appendix), the circadian clock model predicts that the expression levels of all clock components exhibit rhythmic oscillations (i.e., limit-cycle oscillations) with a period of 24 h. Time-profiles of core clock components and the expression level of NHE3 are shown in Fig. 3, together with the experimental data (49) and database http://circadb.hogeneschlab.org. Model parameters were chosen to ensure good agreement with data. Oscillations in NHE3 activity gave rise to in-phase oscillations in NHE3-mediated Na+ fluxes and consequently cellular [Na+] (Figs. 4, A and B, and 5A). Similarly, cellular pH exhibited in-phase oscillation with NHE3 expression (Fig. 5B). Changes in NHE3-mediated Na+ flux are proportionally smaller than changes in NHE3 expression: amplitude of the NHE3 expression and Na+ flux oscillation is 39% and 15% of the corresponding mean, respectively. That difference can be attributed to the changes in intracellular [Na+], a rise in which, taken in isolation, would attenuate the NHE3-mediated Na+ flux.
Fig. 3.
Comparison of predicted [solid lines, period (Per; A), cryptochrome (Cry; B), Rev-Erb (C), Ror (D), Bmal1 (E), and NHE3 (F)] with experimental (dashed lines with dots, Per2, Cry1, Rev-Erbα, Rorc, Bmal1, and NHE3 mRNA expression levels obtained in constant darkness. Gray shading and white regions correspond to activity and rest cycles, respectively.
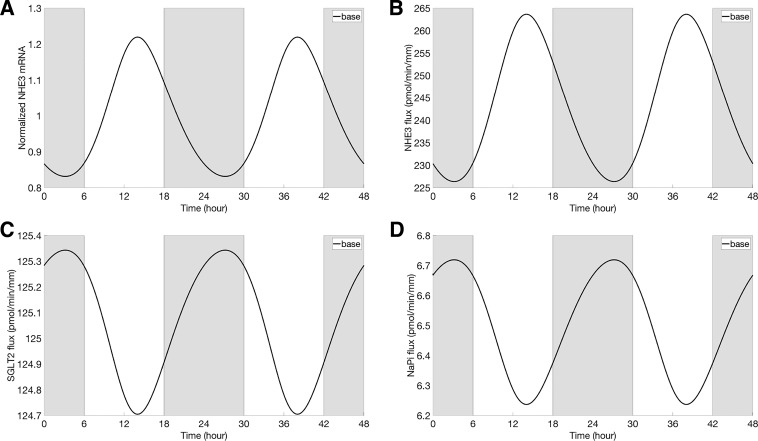
Fig. 4.
Normalized NHE3 mRNA (with respect to the average of the baseline; A), NHE3-mediated Na+ flux (B), Na+-dependent glucose cotransporter 2 (SGLT2)-mediated Na+ flux (C), and Na+-phosphate cotransporter (NaPi)-mediated Na+ flux (D), obtained under baseline conditions. Gray shading and white regions correspond to activity and rest cycles, respectively.
Fig. 5.
Intracellular Na+ concentration (A), intracellular pH value (B), and intracellular osmolarity (C), obtained under baseline conditions. Gray shading and white regions correspond to te activity and rest cycles, respectively.
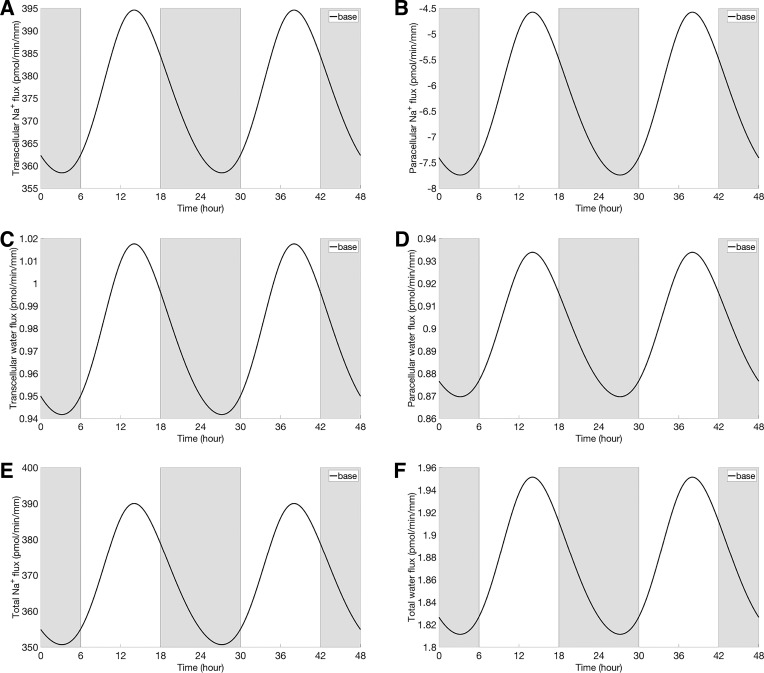
Oscillations in cellular [Na+] also induced oscillations in Na+ fluxes that are mediated by other apical Na+ transporters (Na+-glucose cotransporter SGLT2, and the Na+-phosphate cotransporter NaPi2); these oscillations are out-of-phase with those of the NHE3 activity and cellular [Na+] (Fig. 4, C and D). Together, the changes in NHE3-, SGLT2-, and NaPi2-mediated Na+ fluxes gave rise to oscillation in transcellular Na+ flux, with an amplitude that is 10% of its mean (Fig. 6A). As cellular [Na+] and other solute concentrations fluctuated, cellular fluid osmolarity fluctuated also (Fig. 5C). As a result, transcellular water flux exhibited oscillations that were in-phase with NHE3 expression (Fig. 6C).
Fig. 6.
Transcellular Na+ flux (A), paracellular Na+ flux (B), transcellular water flux (C), paracellular water flux (D), total Na+ flux (E), and total water flux (F) at baseline. Gray shading and white regions correspond to activity and rest cycles, respectively.
Furthermore, the model predicted rhythmic oscillations in intracellular concentrations of all solutes. In particular, intracellular [Cl−] and [] exhibit oscillations that are in phase with NHE3, whereas [K+] exhibits out-of-phase oscillations with NHE3 (results not shown). These oscillations in turn induced rhythmic changes in all transmembrane fluxes.
Rhythmic oscillations in basolateral Na+/K+-ATPase-mediated [Na+] (result not shown) yielded fluctuations in fluid osmolarity in the lateral innerspace. These oscillations, which are in phase with the NHE3 oscillations, generated in-phase oscillations in paracellular water flux and subsequently in paracellular Na+ flux (Fig. 6, B and D). Taken together, oscillations in NHE3 expression yielded in-phase oscillations in total (transcellular and paracellular) Na+ and water reabsorptive fluxes (Fig. 6, E and F), with amplitudes that are 11% and 8% of their respective means.
Below, we describe model simulations that predict the impact of eliminating individual clock genes on the circadian rhythm and on solute and water transport of the proximal tubule cell.
Per2 mutation.
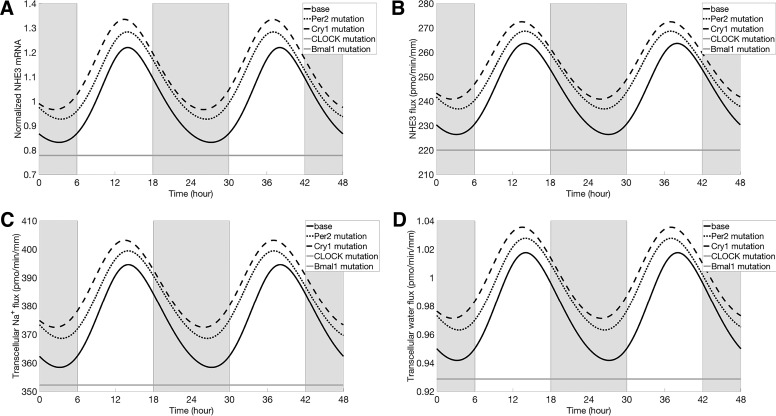
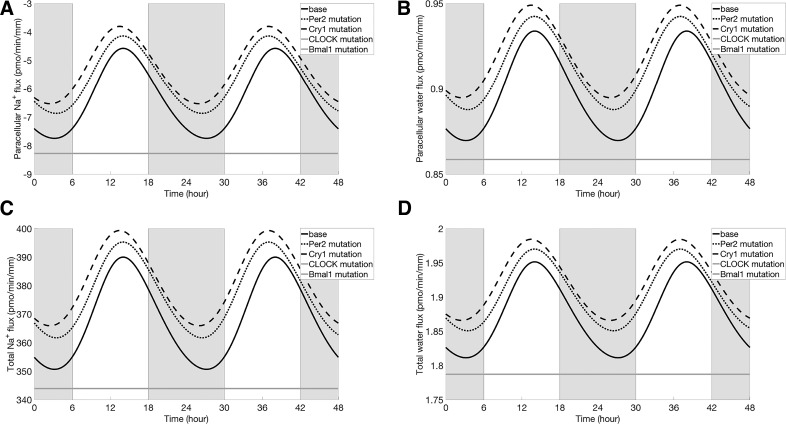
In mammals, there are three known Per isoforms: Per1, Per2, and Per3. In the kidney and liver, the abundance of Per3 is low relative to Per1 and Per2. The present clock model ignores Per3, and, because the specific actions of Per1 and Per2 have yet to be sufficiently well characterized, for simplicity the model does not distinguish between Per1 and Per2. Thus, to simulate the effect of Per2 mutation on proximal tubular Na+ and water transport, we reduced the maximal transcription rate of Per ( in Eq. A1) by 50%. The model predicted that, following Per2 mutation, the rhythmic oscillations in clock gene expression persisted. PER protein decreased by 30% (in mean relative to baseline), whereas CRY protein is increased by 2% (in mean). Recall that these two proteins combine in a reversible reaction to form the PER-CRY complex (see Fig. 1). Their competing changes resulted in a decrease in PER-CRY complex abundance (27% in mean), in an increase in NHE3 expression (9% in mean), and, consequently, in elevated proximal tubular Na+ fluxes (transcellular and paracellular; see Figs. 7 and 8). The higher transcellular Na+ transport raised intracellular [Na+] and augmented transcellular water reabsorption. Changes in transcellular fluxes of other solutes (e.g., K+, Cl−, etc.) were minimal, whereas mean flux increased significantly, by 7%. The circadian period was predicted to be shortened by an hour (i.e., the period became 23 h), due to the attenuated inhibition from PER-CRY complex.
Fig. 7.
Normalized NHE3 mRNA (with respect to the average of the baseline; A), NHE3-mediated Na+ flux (B), transcellular Na+ flux (C), and transcellular water flux (D). Results were obtained for baseline conditions (black solid line), Per2 mutation (black dotted line), Cry1 mutation (black dashed line), CLOCK mutation (gray solid line), and Bmal1 mutation (gray solid line). Profiles corresponding to CLOCK mutation and Bmal1 mutation are indistinguishable. Gray shading and white regions correspond to activity and rest cycles, respectively.
Fig. 8.
Paracellular Na+ flux (A), paracellular water flux (B), total Na+ flux (C), and total water flux (D). Results were obtained for baseline conditions (black solid line), Per2 mutation (black dotted line Cry1 mutation (black dashed line), CLOCK mutation (gray solid line), and Bmal1 mutation (gray solid line). Profiles corresponding to CLOCK mutation and Bmal1 mutation are indistinguishable. Gray shading and white regions correspond to activity and rest cycles, respectively.
Cry1 mutation.
The clock model does not distinguish between the two Cry isoforms Cry1 and Cry2. Due to their similar abundance in the kidney and liver (25, 26, 52), we simulated a selective mutation of Cry1 by reducing the associate rate-maximal transcription rate of Cry ( in Eq. A2) by 50%. The model predicted that, when Cry1 was eliminated, the rhythmic oscillations in clock gene expression would persist. Specifically, Cry1 mutation increased PER protein abundance by 55% (in mean relative to baseline) and decreased CRY protein abundance by 60% (in mean). Taken together, these competing changes yielded a 36% reduction in PER-CRY complex. The resulting attenuated inhibition (from PER-CRY complex) sped up the system. We thus observed a reduction in the circadian period of 30 min. The attenuated inhibition also elevated mean NHE3 expression (Fig. 7A) by 13% relative to baseline.
As shown in Figs. 7 and 8, transcellular and paracellular Na+ and water fluxes of a proximal tubule cell in a Cry1 mutant oscillated in phase with the NHE3 expression (black dashed line). Mean NHE3-mediated Na+ flux (Fig. 7B, black dashed line) increased by 5% relative to baseline. That increase is substantially smaller than the increase in NHE3 expression level (13%) due to the higher cellular Na+ concentration, which limited the driving force of Na+/H+ antiporter. The mean value of total (transcellular plus paracellular) Na+ and water fluxes are increased by 4% and 3%, respectively.
In another simulation, we considered the Cry1/Cry2 double mutant by reducing the maximal transcription rate of Cry () to zero. The model predicted that the concentration of PER-CRY protein complex decreased to zero. The disappearance of the inhibitor of the circadian system broke the feedback loop; consequently, core clock and its target genes ceased to oscillate (results not shown). Without the inhibiting effect of PER-CRY, the NHE3 expression level increased by 157% above baseline, resulting in a 24% increase in total Na+ flux, a 9% increase in water flux, a 16% decrease in total K+ flux, an 11% decrease in total Cl− flux, and a 141% increase in flux.
Rev-Erbα mutation.
The current model does not distinguish between Rev-Erbα and Rev-Erbβ. Assuming similar abundance of these two isoforms, we reduced the maximal transcription rate of Rev-Erb ( in Eq. A3) by 50% to simulate the selective mutation of Rev-Erbα. The model predicted that the circadian rhythms of clock component would persist. Specifically, Rev-Erbα mutation decreased REV-ERB protein by 48% (in mean), thus leading to an increase in BMAL1 protein by 6% (in mean). That was followed by an increase in CLOCK-BMAL1 protein complex (by 6% in mean). The resulting stronger activation sped up the circadian system, modestly lowering its period by 30 min. Another consequence of the enhanced activation was the elevated mean NHE3 expression (by 5%) above baseline (results not shown).
In the proximal tubule cell of a Rev-Erbα mutant, transcellular and paracellular and water fluxes oscillated in phase with NHE3 activity. The elevations in the mean NHE3-mediated Na+ flux, transcellular and paracellular Na+ flux, and water fluxes were minimal relative to the corresponding baseline fluxes. Changes in other solute (K+, Cl−, and ) fluxes were similarly small.
In another simulation, we considered a Rev-Erbα/Rev-Erbβ double mutant by reducing the maximal transcription rate of Rev-Erb () to zero. The model predicted that core clock and its target genes ceased to oscillate (results not shown), following disappearance of the inhibitor of the Bmal1, which broke the feedback loop.
Rorc mutation.
The model does not distinguish among the three Ror isoforms Rora, Rorb, and Rorc. To simulate the selective mutation of Rorc, we reduced the maximal transcription rate of Ror ( in Eq. A4) by 30, 50, and 75%. The model predicted that, in all cases following Rorc mutation, the rhythmic oscillation in clock gene expression persisted. Rorc mutation (30, 50, and 75%) yielded a decrease in mean ROR protein by 7, 13, and 28%, respectively; this led to minor reductions in BMAL1 protein and CLOCK-BMAL1 complex (by 3, 5, and 12%, respectively). Subsequently, REV-ERB protein mean abundance decreased by 2, 5, and 16%, respectively. Because REV-ERB protein inhibits the formation of Bmal1, the lower REV-ERB protein level attenuated the reduction in Bmal1. Attenuated activation of target genes resulting from a decrease in CLOCK-BMAL1 complex slowed down the circadian system. We thus observed an increase in the period of oscillation by 0.9, 1.7, and 2.8 h, respectively.
With a 75% reduction in , the decrease in the activator CLOCK-BMAL1 abundance lowered NHE3 expression by 7% (in mean, relative to baseline). Following Ror mutation, the proximal tubule cell Na+ and water fluxes oscillated in phase with NHE3 expression. The mean NHE3-mediated Na+ and fluxes decreased by 3 and 6%, respectively. The reductions in the mean total Na+ and water fluxes were minimal. Changes in other solute fluxes (e.g., K+, Cl−, etc.) were similarly small.
Bmal1 mutation.
Next, we simulated Bmal1 mutation by setting the maximal transcription rate of Bmal1 ( in Eq. A5) to zero. The model predicted that the concentration of Bmal1 mRNA would decrease to zero, as would the concentrations of BMAL1 protein and the activator CLOCK-BMAL complex. The feedback loop of the gene clock network was thus broken, and the concentrations of other target genes ceased to oscillate. Similarly, NHE3 expression level no longer oscillated but instead settled at a level below the mean (by 22%) baseline level (Fig. 7A). That reduction can be attributed to the disappearance of the transcriptional activator CLOCK-BMAL1 complex (i.e., attenuated activation). The lower NHE3 level decreased NHE3-mediated and overall transcellular Na+ flux by 10% and 6%, respectively; decreased transcellular water flux by 5% (Fig. 7). The mean total flux decreased by 19% relative to the baseline. The changes on total K+ and Cl− fluxes were minimal. Model predictions are consistent with experimental observations showing that immediate and complete loss of circadian rhythmicity are seen in SCN and liver of BMAL1 mutant mice in constant darkness (3).
CLOCK mutation.
Elimination of the CLOCK protein caused the concentration of CLOCK-BMAL complex to decrease almost to zero. As in Bmal1 mutation, the rhythmic oscillations disappeared, and the model predicted a NHE3 expression level that was essentially the same as the Bmal1 mutation case (22% below baseline, Fig. 7). Model predictions are consistent with experimental reports that mice carrying CLOCK mutation exhibit abnormalities of circadian behavior in SCN, including loss of rhythmicity (43).
DISCUSSION
The principle goal of this study was to predict the extent to which Na+ and water transport by a proximal convoluted tubule cell of the rat kidney is modulated by the circadian rhythm. To accomplish that goal, we have developed the first computational model of the renal circadian clock. Most published modeling studies of proximal tubule epithelial transport focus on steady-state results (15, 16, 18, 45), an exception being Ref 7, where cellular response to abrupt changes in luminal or bath composition was simulated. None of the published modeling studies have considered sustained oscillations in the expression level of NHE3 or other transporters. Given that the regulation of NHE3 by clock gene has been well established (33), the impact of circadian rhythms on cellular transport of salt and water, in wild-type and various clock gene mutants, is worthy of investigation.
To simulate the regulation of NHE3 activity by the circadian clock, we combined the new computational model of the core clock with a model of epithelial transport of the proximal convoluted tubule cell of the rat kidney. The clock model simulates the interactions among transcription factors Per, Cry, Rev-Erb, Ror, Bmal1, and CLOCK; it also represents the direct regulation of NHE3 expression level by Bmal1 and CLOCK (see Fig. 1). A set of model parameters was identified that yielded mRNA profiles that are consistent with the experimental data (49). With these parameters, the model predicts circadian rhythms in clock gene expression levels, which drive NHE3 activity to fluctuate with an amplitude that is 39% of its mean baseline value (see Fig. 3).
The predicted NHE3 activity is incorporated into a computational model of proximal tubule epithelial transport to predict intracellular solute concentration, membrane potential, luminal solute and water fluxes, basolateral solute and water fluxes, and paracellular solute and water fluxes (see Fig. 2). The model predicts that fluctuations in NHE3 activity result in fluctuations in NHE3-mediated Na+ flux and consequently transcellular and paracellular Na+ flux and water flux (Figs. 4–6). However, the amplitudes of the flux fluctuations are significantly smaller than that of the NHE3 activity due to fluctuations in intracellular [Na+] that are out of phase with NHE3 activity and thus have the opposite effect on Na+ transport.
Comparison with experimental observations.
Model simulations predicted that mutation of Bmal1 or CLOCK would sufficiently eliminate the activator CLOCK-BMAL complex to break the feedback loop, leading to the disappearance of the circadian rhythms. These predictions are consistent with experimental observations in BMAL1 and CLOCK mutant mice (3, 43).
Model simulation of Per2 mutation predicted an increase in NHE3 expression level, consistent with findings in Ref 33, and a 1-h reduction in circadian period (Fig. 7A). In comparison, findings in the SCN of mPer2 mutant mice in constant darkness revealed clock gene mRNA rhythms that exhibit an even shorter (by 1.5 h) circadian period followed by a loss of circadian rhythmicity (1, 51). The differences in rhythmicity may have resulted from organ-dependent clock genes.
Simulation results of Cry1 mutation are qualitatively similar to those of Per2 mutation: a significant rise in NHE3 expression level, together with a 0.5-h reduction in circadian period (Fig. 7A). These results are consistent with findings in the liver nuclei of Rev-Erbα-deficient mice, where clock gene rhythms have been reported to exhibit a period reduction of 0.5 h (28).
Model limitations and potential extensions.
The mammalian Per gene exhibits three isoforms: Per1, Per2, and Per3. Due to the lack of data regarding Per3 action in the kidney, the present clock model ignores Per3. Furthermore, for simplicity, the model does not distinguish between Per1 and Per2. Recent experimental studies have shed light into the distinct functions of Per1 and Per2 (31, 33). Findings in Ref 31. suggest that Per1 potentially activates renal sodium transport through a Cry2-CLOCK/Bmal1-dependent mechanism, in which Per1 transcriptionally represses Cry2, preventing its repressions of CLOCK/Bmal1. In contrast, Per2 has been shown to inhibit Na+ reabsorption (33). In future modeling studies of the renal circadian clock, separate differential equations can be used to represent and reveal the distinct roles of Per1 and Per2 in renal Na+ handling.
Again for simplicity, the present model does not distinguish between the two Cry isoforms Cry1 and Cry2. It has been observed in the brain that Cry1 and Cry2 mutations have opposite effects on the circadian period (~1-h increase/decrease, respectively) (42), although the underlying mechanism has not been elucidated. Furthermore, Cry2 is suppressed by Per1, but Cry1 is not (31). Thus, a more detailed computational model that separates Cry1 and Cry2 would better capture their different roles in the renal circadian system.
Compensation is common in knockout animals; that is, when one clock gene is deleted, other isoforms may upregulate. For example, Cry2 mRNA and protein expression are increased in the liver and kidney of Per1 KO mice (31). On the other hand, kidney-specific KO of Bmal1 did not appear to result in increased expression of CLOCK mRNA (25). Because the issue of compensation is not fully understood in the kidney, we did not consider compensation in our knockout simulations. However, we consider this a benefit of computational modeling: in silico studies can simulate “clean” knockouts and clearly demonstrate the effects of deleting a given gene component without the influence of compensation.
An additional limitation of the present model is that it does not consider the role of the circadian clock in other cellular functions that may affect NHE3 activity. For example, paracellular transport pathways may be subject to circadian regulation. Indeed, claudin-1 mRNA levels oscillate in the kidney (CircaDB database), and this finding has been replicated by independent studies (25). Related to adherens and tight junctions, E-cadherin and claudin-4, respectively, exhibit rhythmic changes in mRNA and protein levels in the rat kidney (47). NHE3 activity is also likely to be affected by changes in dietary sodium which has recently been shown to affect clock gene expression in the rat kidney (38). Based on the model presented here, future studies will be designed to add additional important elements, such as clock-regulation of other Na+-coupled transporters, e.g., SGLT1 (26). Such investigations should increase our overall understanding of how the circadian clock in the kidney contributes to the regulation of fluid and electrolyte homeostasis, acid-base balance, and the role of general proximal tubule dysfunction in case of core-clock disruption.
The present model represents a stand-alone proximal convoluted cell, with luminal and peritubular fluid composition assumed to be known a priori. Clearly, variations in epithelial transport of a given cell would affect luminal fluid composition and thus the solute and water transport downstream. To investigate the effect of circadian rhythm on overall proximal tubule Na+ transport, we may extend the present epithelial model to simulate a proximal tubule, by connecting a series of cells following a standard approach (15, 16, 18). The resulting proximal tubule model can be used to answer questions such as: 1) to what extent does the circadian rhythm affect solute and water transport along the proximal tubule? 2) how does segmental transport change when a key clock gene is eliminated? and 3) is there any significant impact on glomerulotubular balance?
The impact of the renal circadian clock reaches far beyond the proximal tubule. In addition to NHE3 (33), clock genes have also be shown to regulate SGLT1 of the proximal straight tubule cell (26), Na+-K+-Cl− cotransporter isoform 2 (NKCC2) and estrogen-related receptor-β (ERRβ) of the thick ascending limb cell (12, 26), Na+-Cl− cotransporter (NCC), and with-no-lysine kinase (WNK) of the distal convoluted tubule cell (32, 40), α-subunit of epithelial sodium channel (αENaC) (10, 52), and key regulators of sodium transport of the renal collecting duct cell (39, 52). The circadian rhythm of these transporters can be simulated in a computational model of the nephron (14, 16, 18) using the approach we have used for NHE3. The resulting model can be used to predict the interactions of the clock-controlled transporters and their effects on renal tubular transport and urinary excretion.
Clinical perspectives.
It is becoming increasingly clear that circadian rhythms are directly relevant to human health. For instance, Montaigne et al. (24) recently demonstrated that afternoon surgery was protective against perioperative myocardial injury compared with morning surgery in patients undergoing aortic valve replacement. The hazard ratio was 0.50 for afternoon surgery (n = 298 per group, P < 0.01). This effect was linked to the transcriptional actions of the molecular clock. The dramatic results of this first-of-its-kind clinical study have important implications for human health and are likely to extend to the pathophysiology of other organ systems such as the kidney. Indeed, circadian blood pressure disorders are common in chronic kidney disease (11). The model presented here represents an important step in extending our understanding of the kidney clock to the level of cellular transport function. This model provides a critical hypothesis-generating tool that is necessary to increase our understanding of how the molecular clock affects renal ion transport and renal function, which is likely to have important implications for kidney disease.
GRANTS
This research was supported in part by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, via Grant DK-106102 and by the National Science Foundation via Grant DMS-1263995 to A. T. Layton.
DISCLOSURES
Conflict of interest statement: No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.W., M.L.G., and A.T.L. conceived and designed research; N.W. performed experiments; N.W. and A.T.L. analyzed data; N.W., M.L.G., and A.T.L. interpreted results of experiments; N.W. prepared figures; N.W. and A.T.L. drafted manuscript; N.W., M.L.G., and A.T.L. edited and revised manuscript; N.W., M.L.G., and A.T.L. approved final version of manuscript.
APPENDIX: CIRCADIAN CLOCK MODEL EQUATIONS
The circadian system is a network of interlocked transcriptional-translational feedback loops that drives the circadian oscillations of core clock components with a cycle length of 24 h (30). In the primary negative feedback loop, CLOCK and BMAL1 heterodimerize to initiate the transcription of target clock genes, including Per (with isoforms Per1, Per2, Per3) and Cry (with isoforms Cry1 and Cry2), by binding the E-box elements in the promoter region (3, 5, 50). PERs and CRYs then heterodimerize to inhibit their own transcription by acting on CLOCK-BMAL1 protein complex (6, 13, 35, 37). In the secondary feedback loop, activators of CLOCK and BMAL1 dimerize to initiate the transcription of ROR genes Rev-Erbα and Rorc (28, 34, 41), which compete to bind to ROR response elements (ROREs) present in Bmal1 promoter. REV-ERBs (with isoforms REV-ERBα, REV-ERBβ) and RORs (with isoforms RORa, RORb, and RORc) are shown to repress and activate Bmal1 transcription, respectively (4, 9). In addition, Rev-Erb also inhibits Cry transcription (22) to ensure the robust oscillations (2, 29). NHE3 is a transporter that is directly regulated by the circadian system (33). NHE3 is an output gene transactivated by the heterodimerizer CLOCK-BMAL1, whose activation is repressed by the primary negative feedback loop (33). The result of this molecular regulation is that NHE3 protein expression and localization change over the course of a 24-h period. Thus, the molecular clock directly regulates NHE3 expression at the level of mRNA, protein, and localization (33). Consistent with this regulation, it is interesting to note that the well-established regulator of NHE3 trafficking, Nherf1, also exhibits circadian expression at the level of mRNA (CircaDB, gene name Slc9a3r1). Indeed, Nherf1 mRNA expression showed a similar time-dependent change in expression in the kidney in an independent study by Firsov et al. (25).
For simplicity, we represent the two period homologs (Per1 and Per2) as a single Per gene and ignore Per3, and we represent the two cryptochromes (Cry1, Cry2) as a single Cry gene. Similarly, the two isoforms Rev-Erbα and Rev-Erbβ and three isoforms Rora, Rorb and Rorc are represented by single variables Rev-Erb and Ror, respectively. Expression of the Clock protein is constitutive. Nuclear entry of clock proteins like PER is regulated by phosphorylation and is assumed to be rapid relative to the 24-h period of the clock (20); therefore, posttranslational modification are not included in the model.
Our mathematical model, inspired by Ref 46, describes the time variations of mRNA and corresponding protein concentrations of clock genes Per, Cry, Rev-Erb, Ror, and Bmal1 as well as mRNA concentration for the output clock gene NHE3. The rates of change of the mRNA and protein concentrations are determined by Eqs. A1–A13. Parameters involved in the model are listed in Tables A1–A7. All parameters are unknown and were estimated from the mouse circadian kidney database (http://circadb.hogeneschlab.org), which was developed using data obtained in constant darkness. It is noteworthy that the present model is based on the rat, whereas the parameters were estimated using mouse data. Despite known species differences, key core clock gene expressions of mouse and rat kidneys exhibit many similarities (38, 52). Below we adopt the notation where names with a mix of upper and lower case letters (e.g., Per) denote mRNA, and names in all caps (e.g., PER) denote protein.
| (A1) |
| (A2) |
| (A3) |
| (A4) |
| (A5) |
| (A6) |
| (A7) |
| (A8) |
| (A9) |
| (A10) |
| (A11) |
| (A12) |
| (A13) |
In Eqs. A1–A6, the rates of change of [Per], [Cry], [Rev-Erb], [Ror], [Bmal1], and [NHE3] are given by the mRNA degradation and transcription. The transcription process of Per, Cry, Rev-Erb, and Ror are activated by CLOCK-BMAL1 protein complex binding at the promoter region of target genes, and the CLOCK-BMAL1-dependent transcription is inhibited by the PER-CRY protein complex. Equation A2 also considers the repression of Cry by Rev-Erb. The transcription rate is formulated based on thermodynamic equilibrium and the fractions of time spent by the gene in its different states (free, bound by CLOCK-BMAL1, bound by CLOCK-BMAL1 and PER-CRY). Equations A7–A13 show that the rates of change of [PER], [CRY], [REV-ERB], [ROR], [BMAL1], [CLOCK-BMAL1], and [PER-CRY] are mediated by protein degradation, mRNA translation, and the protein-protein interactions. Model parameters are given in Tables A1–A7.
Table A1.
mRNA and protein degradation rate constant (in h–1)
| Parameter | Value | Description |
|---|---|---|
| dm_per | 0.171401 | Per mRNA degradation rate constant |
| dm_cry | 0.353319 | Cry mRNA degradation rate constant |
| dm_rev | 0.507872 | Rev-Erb mRNA degradation rate constant |
| dm_ror | 0.168399 | Ror mRNA degradation rate constant |
| dm_bmal | 5.26234 | Bmal1 mRNA degradation rate constant |
| dm_NHE3 | 0.524274 | NHE3 mRNA degradation rate constant |
| dp_per | 0.406045 | PER protein degradation rate constant |
| dp_cry | 1.81463 | CRY protein degradation rate constant |
| dp_rev | 0.249662 | REV-ERB protein degradation rate constant |
| dp_ror | 0.188237 | ROR protein degradation rate constant |
| dp_bmal | 0.229965 | BMAL protein degradation rate constant |
| d_cb | 0.164691 | CLOCK-BMAL protein complex degradation rate constant |
| d_pc | 0.167848 | PER-CRY protein complex degradation rate constant |
Table A2.
Maximal transcription rates (in nmol⋅l−1⋅h–1)
| Parameter | Value | Description |
|---|---|---|
| 0.608252 | Per maximal transcription rate | |
| 0.506614 | Cry maximal transcription rate | |
| 0.067494 | Rev-Erb maximal transcription rate | |
| 20.5441 | Ror maximal transcription rate | |
| 0.602884 | Bmal1 maximal transcription rate | |
| 3.145644 | NHE3 maximal transcription rate |
Table A3.
Activation ratios (dimensionless)
| Parameter | Value | Description |
|---|---|---|
| fold_per | 2.60968 | Activation ratio of Per by CLOCK-BMAL |
| fold_cry | 9.3413 | Activation ratio of Cry by CLOCK-BMAL |
| fold_rev | 120.772 | Activation ratio of Rev-Erb by CLOCK-BMAL |
| fold_ror | 2.61103 | Activation ratio of Ror by CLOCK-BMAL |
| fold_bmal | 29.7513 | Activation ratio of Bmal1 by ROR |
| fold_NHE3 | 49.0476 | Activation ratio of NHE3 by CLOCK-BMAL |
Table A4.
Regulation thresholds (in nmol/l)
| Parameter | Value | Description |
|---|---|---|
| Ka_per_cb | 2.20956 | Regulation threshold of Per by CLOCK-BMAL |
| Ki_per_pc | 0.13775 | Regulation threshold of Per by PER-CRY |
| Ka_cry_cb | 1.70642 | Regulation threshold of Cry by CLOCK-BMAL |
| Ki_cry_pc | 0.00286886 | Regulation threshold of Cry by PER-CRY |
| Ki_cry_rev | 0.338714 | Regulation threshold of Cry by REV-ERB |
| Ka_rev_cb | 0.253161 | Regulation threshold of Rev-Erb by CLOCK-BMAL |
| Ki_rev_pc | 233.541 | Regulation threshold of Rev-Erb by PER-CRY |
| Ka_ror_cb | 0.622771 | Regulation threshold of ROR by CLOCK-BMAL |
| Ki_ror_pc | 0.0657504 | Regulation threshold of ROR by PER-CRY |
| Ka_bmal_ror | 0.116865 | Regulation threshold of bmal by ROR |
| Ki_bmal_rev | 0.000255891 | Regulation threshold of bmal by REV-ERB |
| Ka_NHE3_cb | 7.71202 | Regulation threshold of NHE3 by CLOCK-BMAL |
| Ki_NHE3_pc | 0.0698202 | Regulation threshold of NHE3 by PER-CRY |
Table A5.
Hill coefficients (dimensionless)
| Parameter | Value | Description |
|---|---|---|
| hill_per_cb | 21.633 | Hill coefficient regulation of Per by CLOCK-BMAL |
| hill_per_pc | 22.0113 | Hill coefficient regulation of Per by PER-CRY |
| hill_cry_cb | 5.11758 | Hill coefficient regulation of Cry by CLOCK-BMAL |
| hill_cry_pc | 1.77601 | Hill coefficient regulation of Cry by PER-CRY |
| hill_cry_rev | 52.6298 | Hill coefficient regulation of Cry by Rev-Erb |
| hill_rev_cb | 5.37045 | Hill coefficient regulation of Rev-Erb by CLOCK-BMAL |
| hill_rev_pc | 3.96668 | Hill coefficient regulation of Rev-Erb by PER-CRY |
| hill_ror_cb | 7.16985 | Hill coefficient regulation of ROR by CLOCK-BMAL |
| hill_ror_pc | 3.68283 | Hill coefficient regulation of ROR by PER-CRY |
| hill_bmal_ror | 3.07146 | Hil coefficient regulation of bmal by ROR |
| hill_bmal_rev | 1.46683 | Hill coefficient regulation of bmal by REV-ERB |
| hill_NHE3_cb | 1.16369 | Hill coefficient regulation of NHE3 by CLOCK-BMAL |
| hill_NHE3_pc | 1.0005 | Hill coefficient regulation of NHE3 by PER-CRY |
Table A6.
Translation rates (in molecules per hour per mRNA)
| Parameter | Value | Description |
|---|---|---|
| kp_per | 2.20832 | Per translation rate |
| kp_cry | 1.82108 | Cry translation rate |
| kp_rev | 0.000696149 | Rev-Erb translation rate |
| kp_ror | 0.013315 | Ror translation rate |
| kp_bmal | 1.0651 | Bmal1 translation rate |
Table A7.
Complexation kinetic rates
| Parameter | Value | Unit | Description |
|---|---|---|---|
| kass_cb | 0.0063849 | nmol−1⋅l⋅h–1 | CLOCK-BMAL association rate |
| kass_pc | 0.13296611 | nmol−1⋅l⋅h–1 | PER-CRY association rate |
| kdiss_cb | 0.00073878 | h–1 | CLOCK-BMAL dissociation rate |
| kdiss_pc | 0.195142 | h–1 | PER-CRY dissociation rate |
REFERENCES
- 1.Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron 30: 525–536, 2001. doi: 10.1016/S0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- 2.Bugge A, Feng D, Everett LJ, Briggs ER, Mullican SE, Wang F, Jager J, Lazar MA. Rev-erbα and Rev-erbβ coordinately protect the circadian clock and normal metabolic function. Genes Dev 26: 657–667, 2012. doi: 10.1101/gad.186858.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103: 1009–1017, 2000. doi: 10.1016/S0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, Chong LW, DiTacchio L, Atkins AR, Glass CK, Liddle C, Auwerx J, Downes M, Panda S, Evans RM. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature 485: 123–127, 2012. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol 72: 517–549, 2010. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 6.Duong HA, Robles MS, Knutti D, Weitz CJ. A molecular mechanism for circadian clock negative feedback. Science 332: 1436–1439, 2011. doi: 10.1126/science.1196766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards A, Layton AT. Cell volume regulation in the proximal tubule of rat kidney: proximal tubule cell volume regulation. Bull Math Biol 79: 2512–2533, 2017. doi: 10.1007/s11538-017-0338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang Z, Carlson SH, Peng N, Wyss JM. Circadian rhythm of plasma sodium is disrupted in spontaneously hypertensive rats fed a high-NaCl diet. Am J Physiol Regul Integr Comp Physiol 278: R1490–R1495, 2000. doi: 10.1152/ajpregu.2000.278.6.R1490. [DOI] [PubMed] [Google Scholar]
- 9.Guillaumond F, Dardente H, Giguère V, Cermakian N. Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J Biol Rhythms 20: 391–403, 2005. doi: 10.1177/0748730405277232. [DOI] [PubMed] [Google Scholar]
- 10.Gumz ML, Stow LR, Lynch IJ, Greenlee MM, Rudin A, Cain BD, Weaver DR, Wingo CS. The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J Clin Invest 119: 2423–2434, 2009. doi: 10.1172/JCI36908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Judd E, Calhoun DA. Management of hypertension in CKD: beyond the guidelines. Adv Chronic Kidney Dis 22: 116–122, 2015. doi: 10.1053/j.ackd.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krid H, Dorison A, Salhi A, Cheval L, Crambert G. Expression profile of nuclear receptors along male mouse nephron segments reveals a link between ERRβ and thick ascending limb function. PLoS One 7: e34223, 2012. doi: 10.1371/journal.pone.0034223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, Reppert SM. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell 98: 193–205, 1999. doi: 10.1016/S0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- 14.Layton AT, Laghmani K, Vallon V, Edwards A. Solute transport and oxygen consumption along the nephrons: effects of Na+ transport inhibitors. Am J Physiol Renal Physiol 311: F1217–F1229, 2016. doi: 10.1152/ajprenal.00294.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Layton AT, Vallon V, Edwards A. Modeling oxygen consumption in the proximal tubule: effects of NHE and SGLT2 inhibition. Am J Physiol Renal Physiol 308: F1343–F1357, 2015. doi: 10.1152/ajprenal.00007.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Layton AT, Vallon V, Edwards A. A computational model for simulating solute transport and oxygen consumption along the nephrons. Am J Physiol Renal Physiol 311: F1378–F1390, 2016. doi: 10.1152/ajprenal.00293.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Layton AT, Vallon V, Edwards A. Predicted consequences of diabetes and SGLT inhibition on transport and oxygen consumption along a rat nephron. Am J Physiol Renal Physiol 310: F1269–F1283, 2016. doi: 10.1152/ajprenal.00543.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee E, Kim EY. A role for timely nuclear translocation of clock repressor proteins in setting circadian clock speed. Exp Neurobiol 23: 191–199, 2014. doi: 10.5607/en.2014.23.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li HC, Du Z, Barone S, Rubera I, McDonough AA, Tauc M, Zahedi K, Wang T, Soleimani M. Proximal tubule specific knockout of the Na+/H+ exchanger NHE3: effects on bicarbonate absorption and ammonium excretion. J Mol Med (Berl) 91: 951–963, 2013. doi: 10.1007/s00109-013-1015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu AC, Tran HG, Zhang EE, Priest AA, Welsh DK, Kay SA. Redundant function of REV-ERBalpha and β and non-essential role for Bmal1 cycling in transcriptional regulation of intracellular circadian rhythms. PLoS Genet 4: e1000023, 2008. doi: 10.1371/journal.pgen.1000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci 35: 445–462, 2012. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montaigne D, Marechal X, Modine T, Coisne A, Mouton S, Fayad G, Ninni S, Klein C, Ortmans S, Seunes C, Potelle C, Berthier A, Gheeraert C, Piveteau C, Deprez R, Eeckhoute J, Duez H, Lacroix D, Deprez B, Jegou B, Koussa M, Edme JL, Lefebvre P, Staels B. Daytime variation of perioperative myocardial injury in cardiac surgery and its prevention by Rev-Erbα antagonism: a single-centre propensity-matched cohort study and a randomised study. Lancet 391: 59–69, 2018. doi: 10.1016/S0140-6736(17)32132-3. [DOI] [PubMed] [Google Scholar]
- 25.Nikolaeva S, Ansermet C, Centeno G, Pradervand S, Bize V, Mordasini D, Henry H, Koesters R, Maillard M, Bonny O, Tokonami N, Firsov D. Nephron-specific deletion of circadian clock gene bmal1 alters the plasma and renal metabolome and impairs drug disposition. J Am Soc Nephrol 27: 2997–3004, 2016. doi: 10.1681/ASN.2015091055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pizarro A, Hayer K, Lahens NF, Hogenesch JB. CircaDB: a database of mammalian circadian gene expression profiles. Nucleic Acids Res 41: D1009–D1013, 2013. doi: 10.1093/nar/gks1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poulis JA, Roelfsema F, van der Heide D. Circadian urinary excretion rhythms in adrenalectomized rats. Am J Physiol Regul Integr Comp Physiol 251: R441–R449, 1986. doi: 10.1152/ajpregu.1986.251.3.R441. [DOI] [PubMed] [Google Scholar]
- 28.Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110: 251–260, 2002. doi: 10.1016/S0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 29.Relógio A, Westermark PO, Wallach T, Schellenberg K, Kramer A, Herzel H. Tuning the mammalian circadian clock: robust synergy of two loops. PLOS Comput Biol 7: e1002309, 2011. doi: 10.1371/journal.pcbi.1002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature 418: 935–941, 2002. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 31.Richards J, All S, Skopis G, Cheng KY, Compton B, Srialluri N, Stow L, Jeffers LA, Gumz ML. Opposing actions of Per1 and Cry2 in the regulation of Per1 target gene expression in the liver and kidney. Am J Physiol Regul Integr Comp Physiol 305: R735–R747, 2013. doi: 10.1152/ajpregu.00195.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richards J, Ko B, All S, Cheng KY, Hoover RS, Gumz ML. A role for the circadian clock protein Per1 in the regulation of the NaCl co-transporter (NCC) and the with-no-lysine kinase (WNK) cascade in mouse distal convoluted tubule cells. J Biol Chem 289: 11791–11806, 2014. doi: 10.1074/jbc.M113.531095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saifur Rohman M, Emoto N, Nonaka H, Okura R, Nishimura M, Yagita K, van der Horst GT, Matsuo M, Okamura H, Yokoyama M. Circadian clock genes directly regulate expression of the Na(+)/H(+) exchanger NHE3 in the kidney. Kidney Int 67: 1410–1419, 2005. doi: 10.1111/j.1523-1755.2005.00218.x. [DOI] [PubMed] [Google Scholar]
- 34.Sato TK, Panda S, Miraglia LJ, Reyes TM, Rudic RD, McNamara P, Naik KA, FitzGerald GA, Kay SA, Hogenesch JB. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron 43: 527–537, 2004. doi: 10.1016/j.neuron.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 35.Sato TK, Yamada RG, Ukai H, Baggs JE, Miraglia LJ, Kobayashi TJ, Welsh DK, Kay SA, Ueda HR, Hogenesch JB. Feedback repression is required for mammalian circadian clock function. Nat Genet 38: 312–319, 2006. doi: 10.1038/ng1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schibler U, Gotic I, Saini C, Gos P, Curie T, Emmenegger Y, Sinturel F, Gosselin P, Gerber A, Fleury-Olela F, Rando G, Demarque M, Franken P. Clock-talk: interactions between central and peripheral circadian oscillators in mammals. Cold Spring Harb Symp Quant Biol 80: 223–232, 2015. doi: 10.1101/sqb.2015.80.027490. [DOI] [PubMed] [Google Scholar]
- 37.Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B, Kume K, Lee CC, van der Horst GT, Hastings MH, Reppert SM. Interacting molecular loops in the mammalian circadian clock. Science 288: 1013–1019, 2000. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- 38.Speed JS, Hyndman KA, Roth K, Heimlich JB, Kasztan M, Fox BM, Johnston JG, Becker BK, Jin C, Gamble KL, Young ME, Pollock JS, Pollock DM. High dietary sodium causes dyssynchrony of the renal molecular clock in rats. Am J Physiol Renal Physiol 314: F89–F98, 2018. doi: 10.1152/ajprenal.00028.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stow LR, Richards J, Cheng KY, Lynch IJ, Jeffers LA, Greenlee MM, Cain BD, Wingo CS, Gumz ML. The circadian protein period 1 contributes to blood pressure control and coordinately regulates renal sodium transport genes. Hypertension 59: 1151–1156, 2012. doi: 10.1161/HYPERTENSIONAHA.112.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Susa K, Sohara E, Isobe K, Chiga M, Rai T, Sasaki S, Uchida S. WNK-OSR1/SPAK-NCC signal cascade has circadian rhythm dependent on aldosterone. Biochem Biophys Res Commun 427: 743–747, 2012. doi: 10.1016/j.bbrc.2012.09.130. [DOI] [PubMed] [Google Scholar]
- 41.Triqueneaux G, Thenot S, Kakizawa T, Antoch MP, Safi R, Takahashi JS, Delaunay F, Laudet V. The orphan receptor Rev-Erbα gene is a target of the circadian clock pacemaker. J Mol Endocrinol 33: 585–608, 2004. doi: 10.1677/jme.1.01554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Horst GT, Muijtjens M, Kobayashi K, Takano R, Kanno S, Takao M, de Wit J, Verkerk A, Eker AP, van Leenen D, Buijs R, Bootsma D, Hoeijmakers JH, Yasui A. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 398: 627–630, 1999. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- 43.Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW, Takahashi JS. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 264: 719–725, 1994. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang T, Yang CL, Abbiati T, Schultheis PJ, Shull GE, Giebisch G, Aronson PS. Mechanism of proximal tubule bicarbonate absorption in NHE3 null mice. Am J Physiol Renal Physiol 277: F298–F302, 1999. doi: 10.1152/ajprenal.1999.277.2.F298. [DOI] [PubMed] [Google Scholar]
- 45.Weinstein AM. A mathematical model of the rat proximal tubule. Am J Physiol Renal Fluid Electrolyte Physiol 250: F860–F873, 1986. doi: 10.1152/ajprenal.1986.250.5.F860. [DOI] [PubMed] [Google Scholar]
- 46.Woller A, Duez H, Staels B, Lefranc M. A mathematical model of the liver circadian clock linking feeding and fasting cycles to clock function. Cell Reports 17: 1087–1097, 2016. doi: 10.1016/j.celrep.2016.09.060. [DOI] [PubMed] [Google Scholar]
- 47.Yamato M, Ito T, Iwatani H, Yamato M, Imai E, Rakugi H. E-cadherin and claudin-4 expression has circadian rhythm in adult rat kidney. J Nephrol 23: 102–110, 2010. [PubMed] [Google Scholar]
- 48.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2:LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA 101: 5339–5346, 2004. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci USA 111: 16219–16224, 2014. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, Li Q, Sun ZS, Eichele G, Bradley A, Lee CC. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell 105: 683–694, 2001. doi: 10.1016/S0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]
- 51.Zheng B, Larkin DW, Albrecht U, Sun ZS, Sage M, Eichele G, Lee CC, Bradley A. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature 400: 169–173, 1999. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]
- 52.Zuber AM, Centeno G, Pradervand S, Nikolaeva S, Maquelin L, Cardinaux L, Bonny O, Firsov D. Molecular clock is involved in predictive circadian adjustment of renal function. Proc Natl Acad Sci USA 106: 16523–16528, 2009. doi: 10.1073/pnas.0904890106. [DOI] [PMC free article] [PubMed] [Google Scholar]