Abstract
Introduction/Objectives:
Assess the impact of chronic lung diseases (CLD) on survival in rheumatoid arthritis (RA).
Method:
Among participants in the Veterans Affairs Rheumatoid Arthritis (VARA) Registry, a prospective cohort of U.S. Veterans with RA, we identified CLD and cardiovascular disease (CVD) using administrative and registry data. Demographics, smoking status, RA characteristics including Disease Activity Score in 28 joints (DAS28), and disease-modifying anti-rheumatic drug (DMARD) use were obtained from registry data, which was linked to the National Death Index to obtain vital status. We evaluated associations of CLD with survival using multivariable Cox regression models.
Results:
Among a large (n=2,053), male predominant (91%) RA cohort, 554 (27%) had CLD at enrollment. Mortality risk was increased 1.51-fold (95% CI 1.26–1.81) in RA patients with CLD after multivariable adjustment, a risk that was similar to that observed with CVD (HR CLD alone 1.46 [1.03–2.06]; CVD alone 1.62 [1.35–1.94]). Survival was significantly reduced in those with interstitial lung disease (ILD) as well as other forms of CLD. Mortality risk with methotrexate and biologic use was not different in those with CLD compared to those without (p interaction ≥ 0.15) using multiple exposure definitions and propensity score adjustment.
Conclusions:
Mortality risk is significantly increased in RA patients with CLD. This risk is attributable not only to ILD but also to other chronic lung conditions and does not appear to be substantially greater in those receiving methotrexate or biologic therapies. Comorbid lung disease should be targeted as a means of improving long-term outcomes in RA.
Keywords: rheumatoid arthritis, lung disease, mortality, methotrexate, biologics
INTRODUCTION
Several chronic lung diseases have been described in rheumatoid arthritis (RA) including interstitial lung disease (ILD), obstructive lung diseases (chronic obstructive pulmonary disease [COPD], bronchiectasis, bronchiolitis), pulmonary nodules, medication toxicities, and pleural diseases. Perhaps the most concerning of these pulmonary manifestations is ILD, which is clinically apparent in up to 10–15% of RA patients and carries a poor long-term prognosis [1–4]. In our prior work, we demonstrated that respiratory-related deaths were the most overrepresented cause of death in men with RA [5] and similar findings were reported in women with RA in the Nurses’ Health Study [6]. Notably, in both studies COPD, rather than ILD, was the leading cause of respiratory-related death in RA. Despite their frequency, the prognostic importance of lung diseases in RA beyond ILD is not well established. In a population-based incident RA cohort study, obstructive lung disease (defined as an obstructive defect on spirometry and a physician diagnosis of airway or parenchymal lung disease) was associated with a 2-fold higher risk of mortality [7]. Bronchiectasis and bronchiolitis have also been reported to increase the risk of mortality in RA patients in a few small studies [8–10].
In addition to RA itself, several disease-modifying anti-rheumatic drugs (DMARDs) have been implicated in chronic lung diseases (e.g. drug-induced pneumonitis) [11–14]. Moreover, increased adverse events were reported in COPD patients receiving abatacept in a randomized controlled trial [15]. Because of the potential for pulmonary toxicity with these agents, there is significant concern regarding optimal DMARD selection in patients with chronic lung disease, evidenced by epidemiologic channeling to leflunomide (away from methotrexate) in RA patients with ILD [16]. The long-term safety and best practices for the use of DMARDs in RA patients with chronic lung disease remains an important and unanswered question.
Our objective was to evaluate the risk of death among RA patients with chronic lung disease, including chronic lung diseases other than ILD. To illustrate its importance on RA outcomes, we contrasted this risk with cardiovascular disease (CVD), another overrepresented comorbid condition in RA patients that is well-established as a determinant of poor long-term outcomes [5, 17, 18]. Additionally, we investigated whether select DMARD use in RA patients with chronic lung disease was associated with differential impact on survival.
MATERIALS AND METHODS
Participants
We utilized the Veterans Affairs Rheumatoid Arthritis (VARA) Registry, a multicenter longitudinal observational cohort study of U.S. Veterans with RA fulfilling the 1987 American College of Rheumatology (ACR) classification criteria [19]. The VARA Registry has been well described previously [20]. Patients were followed from the time of enrollment, initiated in 2003, until death or censoring at the end of available vital status data (December 31, 2013). All patients provided written informed consent before enrollment, and each site received institutional review board approval. This study was approved by the VARA Scientific Ethics Advisory Committee.
Chronic lung disease assessment
Recognizing that chronic lung disease are often characterized by an insidious onset of pulmonary symptoms leading to diagnosis, we assessed prevalent chronic lung disease by using outpatient diagnostic codes within the VA Corporate Data Warehouse (CDW) collected over a 2-year period, 12 months prior to and 12 months following enrollment. Diagnostic codes were categorized using the Healthcare Cost and Utilization Project Clinical Classification Software (HCUP-CCS, https://www.hcup-us.ahrq.gov/). HCUP-CCS is a freely available software tool developed by the Agency for Healthcare Research and Quality (AHRQ) that categorizes International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes into 295 distinct categories. Chronic lung disease categories included were 127: chronic obstructive pulmonary disease and bronchiectasis, 128: asthma, 132: lung disease due to external agents; and 133: other lower respiratory disease. Respiratory codes representing acute lung conditions were not included (122: pneumonia; 123: influenza; 125: acute bronchitis; 126: other upper respiratory infections; 129: aspiration pneumonitis, food/vomitus; 130: Pleurisy, pneumothorax, pulmonary collapse, 131: respiratory failure, insufficiency, arrest; 134: other upper respiratory disease). Diagnostic codes (ICD-9-CM) corresponding to these HCUP-CCS categories are shown in Supplemental Table 1. Chronic lung disease categories were not mutually exclusive (i.e. patients could have multiple chronic lung diseases). To enhance the specificity of disease classification, we required at least two HCUP-CCS codes within this 24-month window and at least one of these codes to have occurred prior to VARA enrollment. CVD was assessed in the same manner, using HCUP-CCS categories 96–97, 100–101, 105–110, and 112–114 (Supplemental Table 2). Recognizing that ICD-9-CM codes commonly used for ILD are included within a HCUP-CCS category that also contains non-ILD codes (Supplemental Table 3), we used diagnostic codes specific to ILD (ICD-9-CM: 495, 515–517, 714.81) entered into the registry at enrollment by treating rheumatologists. An overall measure of comorbidity burden was assessed using the Rheumatic Disease Comorbidity Index (RDCI) [21].
Clinical variables and vital status
In conjunction with routine rheumatology care, ACR core measures were collected by treating rheumatologists, including erythrocyte sedimentation rate (ESR, mm/hr), 28-joint swollen joint count (SJC), 28-joint tender joint count (TJC), patient and provider global assessment (0–100mm visual analogue scale), multidimensional health-assessment questionnaire (MD-HAQ) [22] as well as calculation of the Disease Activity Score in 28 joints (DAS28) [23]. DMARDs, both biologic and non-biologic, and prednisone use were similarly collected within the registry. Additional variables collected at enrollment were sex, smoking status (current, former, never), education level, and self-reported race. HLA-DRB1 shared epitope (SE) alleles, C-reactive protein (CRP), anti-cyclic citrullinated peptide (anti-CCP, U/ml) antibody, and rheumatoid factor (RF, IU/ml) were measured using banked serum and genomic DNA collected at enrollment, as previously described [24, 25]. Vital status was determined by linkage with the National Death Index (NDI; Center of Excellence for Suicide Prevention, Joint Department of VA and Department of Defense Suicide Data Repository – NDI; http://vaww.virec.research.va.gov/Mortality/Overview.htm; extract through 2013) [5].
Statistical analysis
Baseline characteristics were compared between patients with and without chronic lung disease at the time of enrollment using chi-square and independent t-tests. Multivariable Cox proportional hazards models were used to assess the association of chronic lung disease with all-cause mortality. Covariates included in the Cox models were age, sex, race, smoking status, MD-HAQ, DAS28, baseline DMARDs, and baseline prednisone use. MD-HAQ and DAS28 were allowed to vary over time, while all other variables were fixed at enrollment values. To compare mortality risk between chronic lung disease and CVD, a combined categorization was created: neither comorbidity (referent), chronic lung disease alone, CVD alone, or both comorbidities occurring together.
Associations of DMARDs with mortality in RA patients with chronic lung disease was determined in stratified analyses (all patients, patients with chronic lung disease, and patients without lung disease) and interaction terms were tested using multivariable Cox regression models adjusting for age, sex, race, smoking status, MD-HAQ, DAS28, and baseline prednisone use and clustered by enrollment site. DMARDs were modeled as time-varying and baseline use separately. Because tumor necrosis factor inhibitors (TNFi) comprised 95% of biologic DMARD use at baseline, all biologic (b)DMARDs were modeled together. In sensitivity analyses, we assessed DMARDs using propensity score adjustment. We calculated propensity scores for receiving methotrexate, leflunomide, hydroxychloroquine, sulfasalazine, azathioprine, or bDMARDs at baseline using age, sex, race, education level, smoking status, chronic lung disease, RDCI score, RA disease duration, anti-CCP antibody positivity, MD-HAQ, DAS28, and prednisone use as predictors in logistic regression models. The resulting propensity scores (both as continuous values and propensity score quintiles) were entered as a covariate into Cox models with the DMARD of interest. Proportional hazards assumptions were tested in Cox models by Schoenfeld residuals, which were not significant. A p value <0.05 was considered significant in all analyses, which were completed using Stata v15 (StataCorp, College Station, TX).
RESULTS
Baseline characteristics
Baseline characteristics of the study population (N = 2,053) stratified by chronic lung disease status are shown in Table 1. Those with chronic lung disease were older (p value < 0.001), male predominant (p value = 0.005), less likely to have a high-school education (p value = 0.004), more likely to be current or former smokers (p value < 0.001), and had higher comorbidity scores (p value < 0.001). Anti-CCP antibody concentrations were higher in those with chronic lung disease (mean 286 vs. 230 U/mL, p value = 0.007), while frequency of anti-CCP and RF positivity and RF concentration did not differ between those with and without chronic lung disease. Similarly, race, disease duration, subcutaneous nodules, and SE positivity did not differ by chronic lung disease status.
Table 1.
Baseline characteristics of rheumatoid arthritis cohort by chronic lung disease status.
| Variable | Lung Disease (n=554) | No Lung Disease (n=1499) | P value |
|---|---|---|---|
| Demographics and comorbidities | |||
| Age, years | 65.7 (9.9) | 62.7 (11.2) | <0.001 |
| Male sex, % | 93.1 | 89.2 | 0.005 |
| White, % | 79.0 | 76.0 | 0.14 |
| High-school education, % | 83.3 | 88.1 | 0.004 |
| Smoking status, % | <0.001 | ||
| Current | 27.9 | 25.9 | |
| Former | 58.4 | 50.5 | |
| Never | 13.7 | 23.6 | |
| Body mass index, kg/m2 | 28.5 (5.6) | 28.4 (5.6) | 0.54 |
| RDCI score | 3.9 (1.2) | 1.7 (1.3) | <0.001 |
| RA disease status | |||
| RA duration, years | 11.5 (11.5) | 11.8 (11.4) | 0.60 |
| Shared epitope positive, % | 72.3 | 71.7 | 0.82 |
| Anti-CCP positive, % | 79.3 | 77.0 | 0.25 |
| Anti-CCP, U/mL | 286 (439) | 230 (389) | 0.007 |
| RF positive, % | 80.5 | 79.4 | 0.59 |
| RF, IU/mL | 370 (719) | 328 (707) | 0.25 |
| Nodules, % | 31.9 | 29.5 | 0.27 |
| MD-HAQ, 0–3 | 1.0 (0.6) | 0.9 (0.6) | 0.002 |
| ESR, mm/Hr | 31.7 (1.2) | 24.6 (0.6) | <0.001 |
| C-reactive protein, mg/dL | 1.4 (2.1) | 1.1 (1.9) | 0.003 |
| DAS28 | 4.1 (1.5) | 3.9 (1.6) | 0.003 |
| Medications | |||
| Methotrexate, % | 50.2 | 57.3 | 0.003 |
| Leflunomide, % | 15.4 | 9.7 | <0.001 |
| Hydroxychloroquine, % | 34.8 | 34.0 | 0.72 |
| Sulfasalazine, % | 14.4 | 14.6 | 0.93 |
| Biologic DMARD, % | 28.3 | 27.5 | 0.69 |
| Prednisone, % | 47.0 | 38.4 | <0.001 |
P value by independent t-test or Χ2.
Abbreviations: RDCI, Rheumatic Disease Comorbidity Index; RA, rheumatoid arthritis; CCP, cyclic citrullinated peptide; RF, rheumatoid factor; ACR, American College of Rheumatology; MD-HAQ, multidimensional Health Assessment Questionnaire; ESR, erythrocyte sedimentation rate; DAS28, 28-joint Disease Activity Score; CDAI, Clinical Disease Activity Index; DMARD, disease-modifying antirheumatic drug; NSAIDs, non-steroidal anti-inflammatory drugs
Frequency of chronic lung disease
Using HCUP-CCS categories, 27% of participants (n = 554) had chronic lung disease with other lower respiratory disease being the most common (16.1%) followed by COPD/bronchiectasis (14.7%) (Table 2). Using physician entered diagnostic codes, the prevalence of COPD (18.1%) was similar while ILD was documented in 5.2% of patients.
Table 2.
Frequency of specific chronic lung disease comorbidities (N = 2,053).
| Lung disease | N | % of Patients |
|---|---|---|
| Any HCUP-CCS chronic lung disease | 554 | 27.0 |
| HCUP-CCS, COPD and bronchiectasis | 301 | 14.7 |
| HCUP-CCS, asthma | 62 | 3.0 |
| HCUP-CCS, lung disease due to external agents | 2 | 0.1 |
| HCUP-CCS, other lower respiratory disease† | 330 | 16.1 |
| Physician entered, ILD‡ | 106 | 5.2 |
| Physician entered, COPD | 371 | 18.1 |
Abbreviations: HCUP-CCS, Healthcare Cost and Utilization Project-Clinical Classification Software; COPD, chronic obstructive pulmonary disease; ILD, interstitial lung disease
Most diagnostic codes for interstitial lung disease are included in this HCUP-CCS category
ICD-9-CM: 495, 515–517, 714.81
Chronic lung disease and survival
During a total observation period of 6682 patient-years, there were a total of 341 deaths; 139 of these occurred in patients with chronic lung disease (81.6 per 1000 person-years [PY], 95% confidence interval [CI] 69.1–96.0) and 202 occurred in patients without lung disease (40.6 per 1000PY, 95% CI 35.4–46.6). Deaths among those with chronic lung disease occurred on average 1.3 years earlier compared to those without (mean age at death of 73.3 ± 9.4 vs 74.6 ± 9.4 years). Respiratory-related deaths accounted for 22.3% of deaths among those with chronic lung disease, but only for 9.9% among those without chronic lung disease. Chronic lung disease (per HCUP-CCS codes) was associated with a significantly greater mortality risk in all models (Table 3). In fully adjusted models, chronic lung disease was associated with a 51% increased risk of mortality (hazard ratio [HR] 1.51, 95% CI 1.26–1.81). Except for asthma, each individual HCUP-CCS chronic lung disease category was associated with a greater mortality risk (HRs: COPD/bronchiectasis 1.61, 95% CI 1.39–1.86; other lower respiratory 1.32, 95% CI 1.27–1.36). Using physician entered diagnostic codes from the registry, COPD was associated with a 1.48-fold higher mortality risk (95% CI 1.16–1.90) and ILD was associated with a 1.90-fold higher mortality risk (95% CI 1.23–2.96).
Table 3.
Associations of chronic lung disease with all-cause mortality in RA.
| Age & Sex | Intermediate† | Fully adjusted‡ | |
|---|---|---|---|
| HCUP-CCS | |||
| Chronic lung disease | 1.80 (1.54, 2.11) | 1.72 (1.41, 2.10) | 1.51 (1.26, 1.81) |
| COPD & bronchiectasis | 2.02 (1.60, 2.56) | 1.89 (1.50, 2.38) | 1.61 (1.39, 1.86) |
| Asthma | 1.02 (0.47, 2.17) | 1.11 (0.49, 2.53) | 0.77 (0.23, 2.54) |
| Other lower respiratory | 1.52 (1.45, 1.59) | 1.47 (1.35, 1.60) | 1.32 (1.27, 1.36) |
| Physician entered | |||
| COPD | 1.82 (1.43, 2.30) | 1.70 (1.34, 2.16) | 1.48 (1.16, 1.90) |
| Interstitial lung disease | 1.99 (1.35, 2.94) | 1.90 (1.26, 2.86) | 1.90 (1.23, 2.96) |
Values are hazard ratios and 95% confidence intervals.
Intermediate model includes age, sex, race, smoking status.
Fully adjusted model includes covariates from intermediate model and multidimensional Health Assessment Questionnaire, 28-joint Disease Activity Score, baseline DMARDs, baseline prednisone use.
Abbreviations: HCUP-CCS, Health Care Cost and Utilization Project Clinical Classification Software; COPD, chronic obstructive pulmonary disease
We then contrasted the mortality risk between chronic lung disease and CVD using a combined lung and CVD classification. Using HCUP-CCS codes, 55.6% of participants had neither lung nor CVD comorbidity, 16.5% of participants had only chronic lung disease, 17.3% had only CVD, and 10.6% had both comorbidities. Both chronic lung disease and CVD were associated with a similar increased risk of mortality alone compared to individuals free of both conditions (Figure 1; CVD HR 1.62, 95% CI 1.33–1.94; chronic lung HR 1.46, 95% CI 1.03–2.06). Those simultaneously afflicted with both chronic lung disease and CVD had numerically higher mortality risk than those with lung or CVD comorbidity alone (HR 2.28, 95% CI 1.80–2.89). In sub-analyses, COPD by both HCUP-CCS (HR 1.76, 95% CI 1.33–2.32) and physician entered diagnostic codes (1.61, 95% CI 1.28–2.02) were associated with a similar risk of death as CVD comorbidity (Supplemental Table 4). Other lower respiratory comorbidities identified by HCUP-CCS had a numerically lower risk of death than CVD comorbidity (other lower respiratory HR 1.13, 95% CI 0.85–1.51; CVD HR 1.56, 95% CI 1.35–1.79) while ILD by physician entered codes had a numerically higher risk of death (ILD HR 2.18, 95% CI 1.08–4.41; CVD HR 1.73, 95% CI 1.58–1.90). The presence of both lung disease and CVD was associated with the highest risk of death across all sub-analyses.
Figure 1.
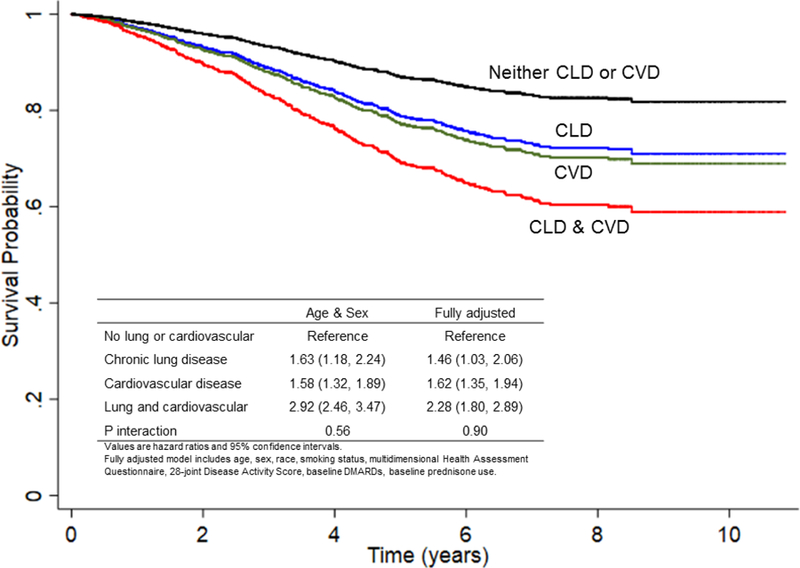
Comparison of all-cause mortality risk between chronic lung and cardiovascular disease comorbidity.
Probability of survival by chronic lung disease (CLD) and cardiovascular disease (CVD) status assessed during the 12 months prior to and following registry enrollment (neither CVD nor CLD [black], CVD only [green], CLD only [blue], both CVD and CLD [red]). The table contained within the figure shows hazard ratios for the association of CVD and CLD with all-cause mortality in multivariable Cox models as well as the null interaction between CVD and CLD
DMARDs and mortality risk
While individual DMARDs demonstrated associations with mortality in general, no DMARDs (baseline or time-varying use) were associated with an increased risk of mortality in RA patients with chronic lung disease (Table 4). Furthermore, there was no evidence of differential risk of mortality with baseline or time-varying methotrexate or bDMARD use in those with/without chronic lung disease (all p values for interaction ≥ 0.15). There was, however, evidence of a significant interaction between hydroxychloroquine use and chronic lung disease (P-values ≤ 0.04). In stratified analyses, hydroxychloroquine use was associated with a numerically more protective association with mortality in those with chronic lung disease compared RA patients without lung disease; however, associations were not statistically significant within each subgroup. Sub-analyses stratified by individual lung diseases (HCUP-CCS COPD/bronchiectasis, other lower respiratory disease; physician entered codes for COPD and ILD) were consistent with overall lung disease analyses (data not shown). In sensitivity analyses incorporating propensity scores for baseline medication use, again most DMARDs were not associated with increased risk of mortality in those with chronic lung disease, with the exception of baseline sulfasalazine use (continuous: HR 1.73, 95% CI 1.03–2.91; quintiles: HR 1.67, 95% CI 1.04–2.69; Supplementary Table 5).
Table 4.
Associations of disease-modifying anti-rheumatic drugs (DMARDs) with mortality in RA patients with and without comorbid chronic lung disease.
| DMARD | All subjects | Lung Disease | No lung Disease | P interaction |
|---|---|---|---|---|
| Time-varying | ||||
| Methotrexate | 0.60 (0.50, 0.71) | 0.56 (0.42, 0.75) | 0.62 (0.52, 0.74) | 0.59 |
| Leflunomide | 0.79 (0.60, 1.04) | 0.67 (0.40, 1.11) | 0.94 (0.63, 1.42) | 0.10 |
| Azathioprine | 1.30 (0.82, 2.06) | 0.81 (0.33, 1.96) | 1.72 (1.21, 2.44) | 0.09 |
| Hydroxychloroquine | 1.08 (0.88, 1.33) | 0.96 (0.71, 1.30) | 1.20 (0.97, 1.48) | 0.04 |
| Sulfasalazine | 0.81 (0.68, 0.96) | 0.90 (0.72, 1.13) | 0.74 (0.60, 0.91) | 0.33 |
| Biologic DMARD | 0.60 (0.48, 0.75) | 0.64 (0.46, 0.88) | 0.54 (0.41, 0.70) | 0.19 |
| Baseline | ||||
| Methotrexate | 0.79 (0.59, 1.05) | 0.80 (0.53, 1.19) | 0.76 (0.56, 1.04) | 0.80 |
| Leflunomide | 0.86 (0.59, 1.26) | 0.88 (0.45, 1.70) | 0.94 (0.63, 1.41) | 0.63 |
| Azathioprine | 0.96 (0.66, 1.39) | 0.71 (0.28, 1.79) | 1.36 (0.70, 2.63) | 0.25 |
| Hydroxychloroquine | 0.93 (0.77, 1.14) | 0.68 (0.44, 1.04) | 1.19 (0.97, 1.47) | 0.01 |
| Sulfasalazine | 1.00 (0.80, 1.24) | 1.27 (0.93, 1.74) | 0.82 (0.54, 1.23) | 0.14 |
| Biologic DMARD | 1.05 (0.88, 1.25) | 1.09 (0.87, 1.37) | 0.97 (0.74, 1.28) | 0.15 |
Values are hazard ratios and 95% confidence intervals.
Models adjusted for age, sex, race, smoking status, multidimensional Health Assessment Questionnaire, 28-joint Disease Activity Score, chronic lung comorbidity (all-subjects only), and baseline prednisone use.
DISCUSSION
Utilizing a cohort of U.S. Veterans with RA and adjustment for key confounders (e.g. smoking status), we have demonstrated that chronic lung disease is associated with reduced survival. These findings were not limited to ILD, but included the more common pulmonary manifestation of COPD. Among the most compelling findings, the effect of chronic lung disease on mortality risk was comparable to that of comorbid CVD. Our results emphasize the importance of targeting chronic lung diseases in RA patients with a similar urgency as has been proposed for CVD [26] in order to achieve optimal long-term patient outcomes.
Prior studies of long-term outcomes in RA patients with lung disease have primarily focused on ILD. There has been far less investigation into the long-term outcomes for RA patients with obstructive lung diseases, despite evidence that the risk of obstructive lung disease, such as COPD, is increased in RA patients [27, 28]. Only a few small studies have examined the survival of RA patients with non-ILD pulmonary manifestations, namely bronchiectasis and bronchiolitis. While the comparison group varied between these studies, findings were suggestive of an increased mortality risk [8–10]. Our study not only confirms the findings of increased mortality with non-ILD lung diseases, but expands on these observations. We studied over 2,000 RA patients of which 27% had chronic lung disease and found a 51% increase in mortality for those with any chronic lung disease relative to RA patients without. Those with chronic lung disease were also more likely to die from lung disease (22.3% in those with chronic lung disease vs. 9.9% in those without). While physician coding for ILD numerically carried the highest risk of death in our study (nearly 2-fold higher), COPD/bronchiectasis and other lower respiratory disease codes were associated with an increased risk of death by 32–61% in RA patients. These results clearly indicate that non-ILD pulmonary manifestations are a major determinant of mortality risk in RA. Furthermore, these results are in line with those from the general population where risk of death was increased 1.6-fold for moderately severe COPD and 1.7-fold for restrictive lung disease [29].
The heightened risk of CVD and CVD-related mortality in RA has been well established [5, 17, 18]. As a result, numerous efforts have been undertaken to enhance the identification of CVD and its risk factors as well as treating both RA and modifiable risk factors as a means of preventing premature CVD mortality [26]. In fact, recent reports suggest these efforts may be effectively narrowing the current gap in CVD incidence between RA patients and the general population [30]. Building on prior studies identifying respiratory-related mortality as the most overrepresented cause of death in RA [5, 6], we have illustrated that comorbid lung disease carries a similar mortality risk as comorbid CVD. Thus, our findings provide support for the concept that researchers and clinicians alike should aggressively target the identification and treatment of lung disease in RA as well as its risk factors, as has been previously done in CVD. It should be noted, however, that these efforts could render fewer gains than efforts focused on CVD because of the limited interventions that are currently available such as smoking cessation and oxygen supplementation. Unfortunately, data suggesting aggressive immunomodulatory therapy improves survival in RA patients with chronic lung disease is lacking, in contrast to some findings with CVD-related mortality in RA [31].
Selecting optimal DMARDs in RA patients with chronic lung disease is challenging. There is concern that select DMARDs could exacerbate pre-existing lung disease or cause pulmonary toxicity. Pre-existing lung disease is a risk factor for methotrexate pneumonitis [32] and, as suggested in the current study, individuals with/at-risk-for lung disease are often channeled to alternative therapies, such as leflunomide [16]. However, there is limited evidence to support or refute these safety concerns, making it critically important to evaluate the risk of poor long-term health outcomes with DMARDs in RA patients with chronic lung disease. Reassuringly, our analyses suggest that chronic lung disease does not appear to differentially impact the mortality risk attributable to most DMARDs, including methotrexate and bDMARDs. These findings of similar mortality risk in those with and without lung disease were robust to alternate definitions of DMARD use (baseline vs. time-varying) as well as statistical models (Cox models with multivariable adjustment vs. propensity score adjustment). As this is an observational study design in which treatment selection was informed rather than randomly selected, our observations should be interpreted as associations and not causal evidence. Moreover, we examined mortality risk stratified by a composite chronic lung disease measure. Therefore, future work will be needed to determine if there is a differential mortality risk with DMARDs based on specific sub-types of chronic lung disease in RA.
There are limitations to our study. Because our primary objective was to evaluate survival following lung disease, we ascertained lung disease at cohort inception. Left censoring may have occurred and lung disease occurring during follow-up was not included, but these would be anticipated to bias the risk of mortality towards the null. Furthermore, the insidious onset of lung disease makes it difficult to clearly define “incident” vs. “prevalent” lung disease and will need to be the subject of future studies. We assessed lung disease using diagnostic codes and categorized specific lung diseases using the HCUP-CCS. While this is a standardized, freely accessible classification software for diagnostic codes, it provides only limited classification of chronic lung diseases and has the potential to misclassify specific lung diseases. However, we also examined diagnoses of COPD and ILD annotated by the treating rheumatologist, with resulting COPD findings that were generally in agreement with results based on HCUP-CCS classification. Given the retrospective nature of these analyses, we were unable to apply American Thoracic Society/European Respiratory Society guidelines for ILD classification [33]. Our rates of chronic lung disease are higher than others have reported [7], which likely reflects the male predominance and frequent smoking history of our cohort and the VA population but may limit generalizability. Data regarding the severity of lung disease, imaging findings, and pulmonary function tests were not available. While we adjusted for smoking status at the time of enrollment, data was not available regarding duration, dose, or intensity of tobacco exposure as well as changes in tobacco use that may have occurred during follow-up. Due to limited use of non-TNF bDMARDs in those with chronic lung disease in our sample, we grouped all bDMARDs together. Thus, future studies are needed to adequately assess the mortality risk with individual bDMARDs.
Our study has numerous strengths including its cohort design and unique study population enriched with chronic lung disease. Moreover, these analyses leveraged robust data including longitudinal RA disease measures and functional status, autoantibodies, and enrollment smoking status that could be potential confounders and were adjusted for in multivariable analyses. We linked the clinical data within the VARA registry to the NDI to assess vital status and with administrative VA data to capture diagnostic codes related to usual care. Finally, we utilized the HCUP-CCS, readily available to other researchers for use in studying chronic lung disease in RA using administrative data.
In summary, we have demonstrated a greater risk of death for RA patients with chronic lung disease that is similar in magnitude to that of CVD and not limited to ILD. Reassuringly, methotrexate and bDMARD use, as occurring in regular care, were not observed to impart a higher risk of mortality in RA patients with chronic lung disease. While future studies should expand on our findings with further characterization of lung disease that includes imaging findings and pulmonary function testing, our study importantly brings to attention the poor prognosis that accompanies chronic lung disease in RA.
Supplementary Material
ACKNOWLEDGEMENTS
Work supported by Center of Excellence for Suicide Prevention, Joint Department of Veterans Affairs (VA) and Department of Defense (DoD) Suicide Data Repository – National Death Index (NDI)
Funding sources: Dr. England is supported by UNMC Internal Medicine Scientist Development Award, UNMC Physician-Scientist Training Program, and UNMC Mentored Scholars Program. Dr. Mikuls is supported by a VA Merit Award (CX000896) and grants from the National Institutes of Health: National Institute of General Medical Sciences (U54GM115458), National Institute on Alcohol Abuse and Alcoholism (R25AA020818), and National Institute of Arthritis and Musculoskeletal and Skin Diseases (2P50AR60772). Dr. Poole is supported by the National Institute of Environmental Health Sciences (R01ES019325). Dr. Caplan is supported by VA Merit Award IIR 14–048-3. Dr. Baker is supported by a VA Career Development Award from Clinical Science Research & Development (IK2 CX000955). Dr. Cannon is supported by Specialty Care Center of Innovation, Veterans Health Administration, Department of Veterans Affairs
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Veterans Affairs.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest
REFERENCES
- 1.Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum 2010;62(6):1583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw M, Collins BF, Ho LA, Raghu G. Rheumatoid arthritis-associated lung disease. Eur Respir Rev 2015;24(135):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olson AL, Swigris JJ, Sprunger DB, et al. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Respir Crit Care Med 2011;183(3):372–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koduri G, Norton S, Young A, et al. Interstitial lung disease has a poor prognosis in rheumatoid arthritis: results from an inception cohort. Rheumatology 2010;49(8):1483–9. [DOI] [PubMed] [Google Scholar]
- 5.England BR, Sayles H, Michaud K, et al. Cause-Specific Mortality in Male US Veterans With Rheumatoid Arthritis. Arthritis Care Res (Hoboken) 2016;68(1):36–45. [DOI] [PubMed] [Google Scholar]
- 6.Sparks JA, Chang SC, Liao KP, et al. Rheumatoid Arthritis and Mortality Among Women During 36 Years of Prospective Follow-Up: Results From the Nurses’ Health Study. Arthritis Care Res (Hoboken) 2016;68(6):753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nannini C, Medina-Velasquez YF, Achenbach SJ, et al. Incidence and mortality of obstructive lung disease in rheumatoid arthritis: a population-based study. Arthritis Care Res (Hoboken) 2013;65(8):1243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swinson DR, Symmons D, Suresh U, Jones M, Booth J. Decreased survival in patients with co-existent rheumatoid arthritis and bronchiectasis. Br J Rheumatol 1997;36(6):689–91. [DOI] [PubMed] [Google Scholar]
- 9.Tsuchiya Y, Takayanagi N, Sugiura H, et al. Lung diseases directly associated with rheumatoid arthritis and their relationship to outcome. Eur Respir J 2011;37(6):1411–7. [DOI] [PubMed] [Google Scholar]
- 10.Puechal X, Genin E, Bienvenu T, Le Jeunne C, Dusser DJ. Poor survival in rheumatoid arthritis associated with bronchiectasis: a family-based cohort study. PLoS One 2014;9(10):e110066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kremer JM, Alarcón GS, Weinblatt ME, et al. Clinical, laboratory, radiographic, and histopathologic features of methotrexate‐associated lung injury in patients with rheumatoid arthritis. A multicenter study with literature review. Arthritis & Rheumatology 1997;40(10):1829–37. [DOI] [PubMed] [Google Scholar]
- 12.Chikura B, Lane S, Dawson JK. Clinical expression of leflunomide-induced pneumonitis. Rheumatology 2009;48(9):1065–8. [DOI] [PubMed] [Google Scholar]
- 13.Khasnis AA, Calabrese LH. Tumor necrosis factor inhibitors and lung disease: a paradox of efficacy and risk. In: Seminars in arthritis and rheumatism Elsevier; Abstract 40. [DOI] [PubMed] [Google Scholar]
- 14.Lioté H, Lioté F, Séroussi B, Mayaud C, Cadranel J. Rituximab-induced lung disease: a systematic literature review. European Respiratory Journal 2010;35(3):681–7. [DOI] [PubMed] [Google Scholar]
- 15.Weinblatt M, Combe B, Covucci A, Aranda R, Becker J, Keystone E. Safety of the selective costimulation modulator abatacept in rheumatoid arthritis patients receiving background biologic and nonbiologic disease‐modifying antirheumatic drugs: a one‐year randomized, placebo‐controlled study. Arthritis & Rheumatology 2006;54(9):2807–16. [DOI] [PubMed] [Google Scholar]
- 16.Suissa S, Hudson M, Ernst P. Leflunomide use and the risk of interstitial lung disease in rheumatoid arthritis. Arthritis Rheum 2006;54(5):1435–9. [DOI] [PubMed] [Google Scholar]
- 17.Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis 2012;71(9):1524–9. [DOI] [PubMed] [Google Scholar]
- 18.England BR, Thiele GM, Anderson DR, Mikuls TR. Increased cardiovascular risk in rheumatoid arthritis: mechanisms and implications. BMJ 2018;361:k1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31(3):315–24. [DOI] [PubMed] [Google Scholar]
- 20.Mikuls TR, Reimold A, Kerr GS, Cannon GW. Insights and implications of the VA Rheumatoid Arthritis Registry. Fed Pract 2015;32:24–9. [PMC free article] [PubMed] [Google Scholar]
- 21.England BR, Sayles H, Mikuls TR, Johnson DS, Michaud K. Validation of the rheumatic disease comorbidity index. Arthritis Care Res (Hoboken) 2015;67(6):865–72. [DOI] [PubMed] [Google Scholar]
- 22.Pincus T, Sokka T, Kautiainen H. Further development of a physical function scale on a MDHAQ [corrected] for standard care of patients with rheumatic diseases. J Rheumatol 2005;32(8):1432–9. [PubMed] [Google Scholar]
- 23.van der Heijde DM, van ‘t Hof M, van Riel PL, van de Putte LB. Development of a disease activity score based on judgment in clinical practice by rheumatologists. J Rheumatol 1993;20(3):579–81. [PubMed] [Google Scholar]
- 24.Mikuls TR, Gould KA, Bynote KK, et al. Anticitrullinated protein antibody (ACPA) in rheumatoid arthritis: influence of an interaction between HLA-DRB1 shared epitope and a deletion polymorphism in glutathione S-transferase in a cross-sectional study. Arthritis Res Ther 2010;12(6):R213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miriovsky BJ, Michaud K, Thiele GM, et al. Anti-CCP antibody and rheumatoid factor concentrations predict greater disease activity in men with rheumatoid arthritis. Ann Rheum Dis 2010;69(7):1292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agca R, Heslinga SC, Rollefstad S, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis 2017;76(1):17–28. [DOI] [PubMed] [Google Scholar]
- 27.Sparks JA, Lin TC, Camargo CA Jr., et al. Rheumatoid arthritis and risk of chronic obstructive pulmonary disease or asthma among women: A marginal structural model analysis in the Nurses’ Health Study. Semin Arthritis Rheum 2017. [DOI] [PMC free article] [PubMed]
- 28.McGuire K, Avina-Zubieta JA, Esdaile JM, et al. Risk of Incident Chronic Obstructive Pulmonary Disease (COPD) in Rheumatoid Arthritis: A Population Based Cohort Study. Arthritis Care Res (Hoboken) 2017. [DOI] [PubMed]
- 29.Mannino DM, Buist AS, Petty TL, Enright PL, Redd SC. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax 2003;58(5):388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myasoedova E, Gabriel SE, Matteson EL, Davis JM 3rd, Therneau TM, Crowson CS. Decreased Cardiovascular Mortality in Patients with Incident Rheumatoid Arthritis (RA) in Recent Years: Dawn of a New Era in Cardiovascular Disease in RA? J Rheumatol 2017;44(6):732–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roubille C, Richer V, Starnino T, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis 2015;74(3):480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saravanan V, Kelly CA. Reducing the risk of methotrexate pneumonitis in rheumatoid arthritis. Rheumatology (Oxford) 2004;43(2):143–7. [DOI] [PubMed] [Google Scholar]
- 33.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188(6):733–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


