Abstract
Combination of Aconiti Lateralis Radix Praeparata (FZ) and Paeoniae Radix Alba (BS) shows a significant effect in rheumatoid arthritis (RA). This study aimed to investigate the efficacy enhancing and toxicity reducing mechanism of combination of them in adjuvant-induced arthritis (AIA) rats by metabolomics. Rats were randomly divided into seven groups, including A (healthy control), B (model control), C1 (therapy group), C2 (efficacy enhancing group), D1 (toxicity group), and D2 (toxicity reducing group), and dexamethasone group was used as positive control. The plasma biochemical indexes showed that therapeutic dose of lipid-soluble alkaloids of FZ could significantly inhibit the concentrations of IL-1β, TNF-α, and IFN-γ in AIA rats, and combination with total glucosides of peony could further reduce the concentration of IL-1β. Then, UPLC-LTQ/Orbitrap MS with untargeted metabolomics was performed to identify the possible metabolites and pathways. Through multivariate data analysis of therapeutic dose groups (A vs. B vs. C1 vs. C2) and multivariate data analysis of toxic dose groups (A vs. B vs. D1 vs. D2), 10 and 7 biomarkers were identified based on biomarker analysis, respectively. After inducing AIA model, the plasma contents of spermidine, vanillylmandelic acid, catechol, and linoleate were increased significantly, and the contents of citric acid, L-tyrosine, L-phenylalanine, leucine, L-tryptophan, and uridine 5'-monophosphate (UMP) were decreased significantly. High dose of lipid-soluble alkaloids of FZ could increase the plasma contents of L-lysine, L-arginine, and deoxycholic acid, while the plasma contents of UMP, carnitine, N-formylanthranilic acid, and adenosine were decreased significantly. The pathway analysis indicated that therapeutic dose of lipid-soluble alkaloids of FZ could regulate energy and amino acid metabolic disorders in AIA rats. However, toxic dose could cause bile acid, fat, amino acid, and energy metabolic disorders. And combination with total glucosides of peony could enhance the therapeutic effects and attenuate the toxicity induced by lipid-soluble alkaloids of FZ.
1. Introduction
Rheumatoid arthritis (RA) is an unknown etiology, chronic and inflammatory synovitis-based autoimmune systemic disease, which is characterized by invasive joint inflammation and symmetric lesions of the hands and foot facet joints, accompanied by positive serum anticyclic citrullinated peptide antibody (Anti-CCP) and rheumatoid factor (RF), even involving extra-articular organs and leading to joint deformity and functional injury [1, 2].
In recent years, drugs for the treatment of RA have been continuously put into clinical treatment, such as glucocorticoids, tumor necrosis factor-alpha (TNF-α) inhibitors, technetium [99Tc] methylene diphosphonate injection, nonsteroidal anti-inflammatory drugs (NSAIDs), biological targeting agents and disease-modifying antirheumatic drugs (DMARDs). However, they also bring many serious side effects during the treatment of RA, such as Cushing's syndrome, diabetes, hepatotoxicity, and even malignant tumors [3]. Therefore, it is urgently needed to find some drugs with effective and less side effects to solve clinical needs.
Aconiti Lateralis Radix Praeparata (Fuzi in Chinese, FZ), the daughter root of Aconitum carmichaelii Debx., has been used for more than a thousand years in clinical practice. In the documents of many classical Chinese medicine books, FZ can treat a variety of diseases, such as polyarthralgia, syncope, acute rheumatic fever, vomiting and diarrhea of asthenia cold, rheumatoid arthritis, heart failure, and inflammation [4–6]. Some studies reported that Aconitum alkaloids were the main bioactive components of FZ, which mainly consist of monoester-diterpenoid aconitines (MDAs) and diester-diterpenoid aconitines (DDAs). However, they were also reported to induce toxic reactions such as gastrointestinal toxicity, neurotoxicity, and fatal cardiac toxicity [7, 8]; therefore, excessive use of FZ in the treatment of RA might have serious consequences.
Paeoniae Radix Alba (Baishao in Chinese, BS), the dry root of Paeonia lactiflora Pall., has been widely used in the treatment of many diseases. According to the theory of traditional Chinese medicine, BS has good clinical efficacy in the treatment of menstrual irregularities, blood deficiency, sallow complexion, hypochondriac pain, spontaneous sweating, and headache [4]. In recent years, many studies showed that BS has obvious pharmacological effects, such as anti-inflammatory, antivirus, treating headache and vertigo, spasmolysis, pain relief, irregular menstruation, and liver protection [9, 10]. Total glucosides of peony were the main bioactive components of BS, which mainly consist of paeoniflorin, peony glucoside, hydroxy-paeoniflorin, benzoylpaeoniflorin, and albiflorin. Among them, paeoniflorin accounts for about 90% of total glucosides of peony [11].
Different from western medical system, traditional Chinese medicine focuses more on “system-to-system” treatment mode and pays more attention to pathological and physiological changes [12]. Combinatorial intervention is the main form of traditional Chinese medicine medication, which is summarized from long-term clinical experience. Through the appropriate combination of drugs, it can play an important role in enhancing efficacy and reducing toxicity in clinical treatment. Combinatorial intervention of FZ and BS is the most extensive used prescription in the treatment of RA; the commonly used combination proportions include 2:1, 1:1, and 1:2. Previous studies showed that after combination, the content of ester alkaloids decreased; therefore, the toxicity of Aconitum plants also decreased [13]. In the process of clinical treatment, although FZ is effective in the treatment of RA, excessive use of it will bring toxicity and side effects. Combination of FZ and BS can attenuate toxicity and increase efficiency; however, the mechanism is still unclear and needs to be studied.
Currently, metabolomics is a very popular research in the pharmaceutical field. It focuses on the study of the category, quantity, and variation of organism endogenous metabolites. Through a systematic and comprehensive research of the biochemical content change of cells, tissues or organism, metabolomics can detect and quantify the metabolic fingerprint differences in samples. Combined with pattern recognition method and other chemical analysis techniques, metabolomics can obtain the corresponding biomarkers. Metabolomics is capable of investigating the relationship between pathological and physiological changes by qualitative and quantitative analysis of potential biomarkers [14]. Metabolomics is suitable for the complex system study such as traditional Chinese medicine in order to explore its scientific connotation.
Through our study, the plasma metabolomics based on the UPLC-LTQ/Orbitrap MS technique combined with pattern recognition method, was applied to explore the endogenous metabolites changes caused by combination of lipid-soluble alkaloids of FZ and total glucosides of peony in adjuvant-induced arthritis (AIA) rats. Through the analysis of metabolite pathway changes, the efficacy enhancing and toxicity reducing mechanism of combination of two traditional Chinese medicines in the treatment of RA was investigated. This study can provide a scientific basis for rational use of the two Chinese herbal medicines.
2. Materials and Methods
2.1. Chemicals and Materials
Acetonitrile, methanol, and formic acid were HPLC grade and obtained from Merck Serono Co., Ltd. (Darmstadt, Germany), Shanghai URChem Co., Ltd. (Shanghai, China), and TCI development Co., Ltd. (Tokyo, Japan), respectively. Purified water was prepared by Arium mini ultrapure water system (Sartorius, Münster, Germany). Rat IL-1β, TNF-α, and IFN-γ ELLSA kits were obtained from Multi Sciences Co., Ltd. (Hangzhou, China). Complete Freund's adjuvant (CFA) was obtained from Sigma-Aldrich Co., Ltd. (St. Louis, MO, USA). Dexamethasone acetate tablets were purchased from Chongqing Kerui Pharmaceutical (group) Co., Ltd. (Chongqing, China). Aconiti Lateralis Radix Praeparata (FZ) was purchased from Sichuan Jiangyou Zhongba Fuzi Technology Development Co., Ltd. (Jiangyou, China). Paeoniae Radix Alba (BS) was purchased from Anhui Bozhou herbal medicine market (Bozhou, China). Professor Jin Pei of Chengdu University of Traditional Chinese Medicine identified the medicinal materials (Chengdu, China).
2.2. Drugs Preparation
The extracts of lipid-soluble alkaloids of FZ and total glucosides of peony were extracted from FZ and BS, respectively. 400 g FZ yielded 1 g lipid-soluble alkaloids of FZ, and 25 g BS yielded 1 g total glucosides of peony. The contents of aconitine, mesaconitine, and hypaconitine in lipid-soluble alkaloids of FZ were 0.264 % (2.64 mg/g), 8.24 % (82.4 mg/g), and 1.08 % (10.8 mg/g), respectively. The content of paeoniflorin in total glucosides of peony was 81.6 % (816 mg/g).
2.3. Animals
70 male SPF grade Sprague-Dawley rats (180 ± 20 g) were commercially purchased from Laboratory Animal Center of Sichuan Provincial Academy of Chinese Medical Sciences. The number of certification was SCXK 2013-19. All rats were acclimated for a week before the experiments began. They were placed in an appropriate environment, with 12 h light and dark cycle, average temperature: at 20 ± 2°C, and relative humidity: at 55 ± 5 %. The rats were provided with distilled water and rat food. The ethical review committee of Chengdu University of Traditional Chinese Medicine approved all experimental methods and procedures of this study.
2.4. AIA Rats Construction and Treatment
SD rats were randomly divided into seven groups, with 10 rats in each group (2 rats in D2 group died due to mistakes in the operation of the intragastric administration). Namely healthy control group (A), AIA model group (B), therapy group (C1), efficacy enhancing combination group (C2), toxicity group (D1), toxicity reducing combination group (D2) and dexamethasone group (DG). The rats in groups B, C1, C2, D1, D2, and DG were injected 0.1 mL CFA in the right hind foot pad, and the rats in group A were injected 0.1 mL 0.9 % sodium chloride injection [15].
19 days after the immunization, all groups began treatment through intragastric administration. Groups A and B were given distilled water as controls, the rats in C1 group received about one-third of the maximum tolerated dose of lipid-soluble alkaloids of FZ as a therapeutic dose (12.5 mg·kg−1·d−1), the rats in C2 group received lipid-soluble alkaloids of FZ (12.5 mg·kg−1·d−1) and total glucosides of peony (400 mg·kg−1·d−1), the rats in D1 group received a maximum tolerated dose of lipid-soluble alkaloids of FZ (35 mg·kg−1·d−1), the rats in D2 group received lipid-soluble alkaloids of FZ (35 mg·kg−1·d−1) and total glucosides of peony (1120 mg·kg−1·d−1), and the rats in DG group received dexamethasone (0.3 mg·kg−1·d−1).
2.5. Pharmacological Effects Evaluation
After the immunization, rats were weighed every 3 days. In order to record the development of arthritis erythema or edema in rat paws and observe the effects of the drugs, the paw volumes were measured every 4 days and the arthritis scores were evaluated simultaneously. The semiquantitative clinical score was used to assess the severity of arthritis: 0, normal joint condition; 1, slight swelling and erythema of the finger or ankle; 2, moderate swelling and erythema of the ankle; 3, swelling and erythema of the whole rat paw including fingers; and 4, severe arthritis of the limb and involvement of multiple joints. The recorded paw volumes were the average volumes of the two hind paws, and the recorded arthritis scores were the sum of the two hind paws [16].
2.6. Sample Collection
Drug administration lasted for 14 days. Two hours after last administration, all rats were anesthetized with 10 % chloral hydrate, 0.35 mL/100 g per rat. The blood samples were collected into EDTA tube from the rat abdominal aorta then immediately centrifuged at 1,600 g for 15 min at 4°C. The plasma samples were transferred into sterilized cryopreservation tube for metabolomics analysis and ELISA examinations. The plasma samples were stored at -80°C and thawed at 4°C before analysis.
2.7. ELISA Examinations
The levels of cytokines IL-1β, TNF-α, and IFN-γ in rat plasma were evaluated by ELISA kits.
2.8. Metabolomics Analysis Based on UPLC-LTQ/Orbitrap MS
UPLC-LTQ/Orbitrap MS analysis was based on Waters ACQUITY UPLC system combined with electrospray ionization (ESI) source and Thermo LTQ/Orbitrap XL mass spectrometry. The analysis equipment had an autosampler and a multisolvent delivery system. Analysis was carried out on an ACQUITY UPLC BEH C18 column (100×2.1 mm, 1.7 μm, Waters). The column and autosampler temperature were maintained at 40°C and 4°C, respectively. Solvent A (water contained 0.1% formic acid) was mixed with solvent B (acetonitrile contained 0.1% formic acid) in different proportions for gradient elution of analytes, and the flow rate was set at 0.25 mL/min. After equilibration, 5 μL supernatant plasma of each sample were injected into UPLC-LTQ/Orbitrap MS system for metabolomics analysis. Solvent B with an increasing linear gradient was used as follows: 0~1 min, 2% B; 1~9.5 min, 2%~50% B; 9.5~14 min, 50%~98% B; 14~15 min, 98% B; 15~15.5 min, 98%~2% B; 15.5~17 min, 2% B.
MS condition: the Thermo LTQ/Orbitrap XL mass spectrometer was applied to ESI-MS experiments. The spray voltages in positive and negative ion modes were 4.8 kV and 4.5 kV, respectively. Sheath and auxiliary gas were set at 45 and 15 arbitrary units, respectively. The normalized collision energy was set at 30 eV. The capillary temperature was maintained at 325°C. The capillary voltages in positive and negative modes were 35 V and -15 V, and the tube lens voltages in positive and negative modes were 50 V and -50 V.
2.9. Data Analysis
The Protein Wizard software was used to convert the analysis data of UPLC-LTQ/Orbitrap MS into CDF format. And then, XCMS (www.bioconductor.org/) was applied to preprocessing of the study. Peak areas of metabolites in each sample were normalized by sum method from metaboanalyst (www.metaboanalyst.ca). The data sets from all samples, including normalized peak area of metabolites and retention time, were used for data preprocessing by Simca-P software, version 13.0 (Umetrics AB, Umea, Sweden). The unit variance scaling and mean-centered method were applied to data processing. And then, principal component analysis (PCA) and partial least squares-discriminant analysis (PLS-DA) were used to pattern recognition analysis by multivariate statistical analysis tool. In PLS-DA model, variable importance in the projection (VIP) value higher than 1 was considered a potential variable. The SPSS software, version 21.0 (Chicago, IL, USA), was used for the analysis of potential variables by Student's t-test and one-way analysis of variance (ANOVA), and P < 0.05 was considered to be statistically different.
The potential metabolic biomarkers were identified by MS/MS fragmentation patterns and matching the exact molecular weight to the online database (the mass error was less than 20 ppm), mainly including HMDB database (http://www.hmdb.ca), Metlin database (https://metlin.scripps.edu), Massbank database (http://massbank.eu/MassBank), Lipid Maps database (http://www.lipidmaps.org), and Mzcloud database (https://www.mzcloud.org). Then KEGG database (http://www.kegg.jp) was applied to find out the correlation between candidate biomarkers and plot metabolomics pathways network diagram.
3. Results
3.1. Evaluation of AIA Model and Therapeutic Effects of Drugs
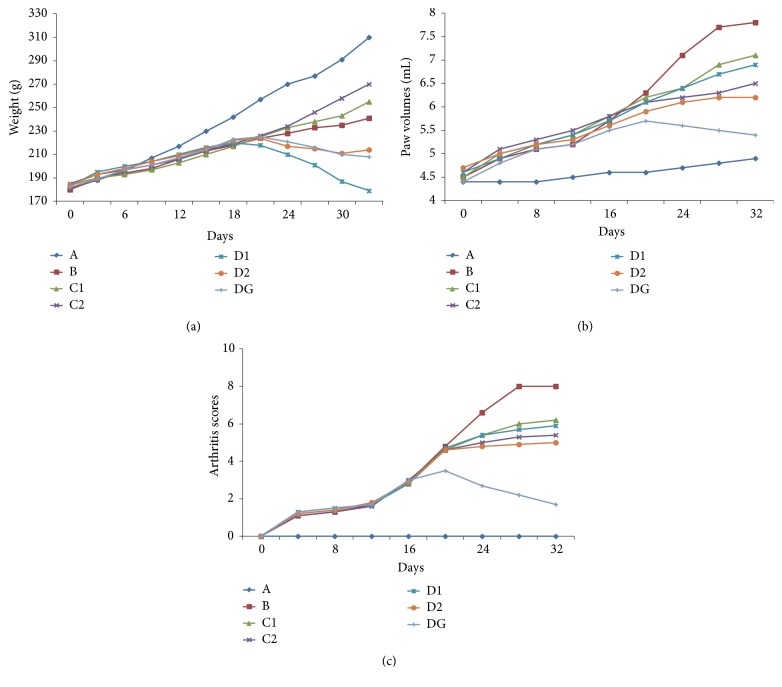
After immunization with CFA, swelling immediately appeared on the injected feet, on the 8th day after immunization, swelling and erythema gradually appeared on the left foot, and the model group all reached the peak of inflammation on the 28th day. As shown in Figure 1(a), C1, C2 groups had higher body weight than model group; however, the body weight of D1, D2, and dexamethasone groups were significantly reduced after administration. As shown in Figures 1(b) and 1(c), the paws swelling and arthritis scores in each group were significantly increased after immunization; however, in drug administration groups, the paws swelling and arthritis scores were significantly inhibited after administration (P < 0.05 vs. model group). The above results illustrated that dexamethasone had a strong anti-inflammatory effect, lipid-soluble alkaloids of FZ also had a good effect on AIA rats, and combination with total glucosides of peony could enhance this effect. However, excessive lipid-soluble alkaloids of FZ could cause toxic damage to rats.
Figure 1.
(a) The body weight of rats in each group after immunization, weighed every 3 days. (b) The change of paw volumes of rats in each group after immunization, measured every 4 days. (c) The clinical arthritis scores in each group after immunization, evaluated every 4 days. Healthy control group (A). AIA model group (B). Therapy group (C1). Efficacy enhancing combination group (C2). Toxicity group (D1). Toxicity reducing combination group (D2). Dexamethasone group (DG).
3.2. Plasma Biochemical Parameters
Inflammatory cytokines, such as IL-1β and TNF-α, played an important role in the inflammatory response of RA. They could act on synoviocytes, endothelial cells, bone-derived cells, and chondrocytes to produce collagenase and synthesize PGE2 in articular lesions. Especially, IL-1β was the main cause of bone and cartilage destruction [17]. IFN-γ was an inflammatory cytokine with the functions of immune system regulation and cell proliferation, and it was also considered to be an important marker of RA [18]. The ELISA results were shown in Table 1. The levels of IL-1β, TNF-α, and IFN-γ in model group were significantly higher than control group (P < 0.05). After administration, the levels of IL-1β, TNF-α, and IFN-γ were significantly reduced in dexamethasone group, the levels of IL-1β were significantly reduced in C1 group (P < 0.05), C2 group (P < 0.001), D1 group (P < 0.05), and D2 group (P < 0.001), the levels of TNF-α were also reduced in all administration groups, and the levels of IFN-γ were significantly reduced in C1 group (P < 0.01), C2 group (P < 0.01), D1 group (P < 0.05), and D2 group (P < 0.01). The results indicated that lipid-soluble alkaloids of FZ had anti-inflammatory effects on the inhibition of IL-1β, TNF-α, and IFN-γ, and combination with total glucosides of peony could enhance the regulation of IL-1β. However, beyond a certain range, the levels of IL-1β, TNF-α, and IFN-γ were not reduced continually when the dose of lipid-soluble alkaloids of FZ increased; instead, toxic reactions occurred.
Table 1.
Effect of combination of FZ and BS on IL-1β, TNF-α, and IFN-.
| Group | IL-1β/pg·mL−1 | TNF-α/pg·mL−1 | IFN-γ/pg·mL−1 |
|---|---|---|---|
| A group | 217.39±61.22 | 135.20±17.86 | 154.23±43.04 |
| B group | 731.26±156.34### | 164.24±18.82# | 506.03±191.55## |
| C1 group | 547.62±172.16∗ | 145.05±17.85 | 198.84±54.15∗∗ |
| C2 group | 262.96±191.44∗∗∗ | 143.20±18.85 | 182.98±88.86∗∗ |
| D1 group | 535.62±154.12∗ | 146.37±24.72 | 209.58±69.74∗ |
| D2 group | 375.62±143.54∗∗∗ | 148.54±26.84 | 186.47±71.19∗∗ |
| Dexamethasone group | 239.42±102.37∗∗∗ | 140.30±19.10∗ | 185.88±67.94∗∗ |
Significant differences were based on independent sample t-test.
# P < 0.05, compared with control group; ##P < 0.01, compared with control group; ###P < 0.001, compared with control group.
∗ P < 0.05, compared with model group; ∗∗P < 0.01, compared with model group; ∗∗∗P < 0.001, compared with model group
Healthy control group (A). AIA model group (B). Therapy group (C1). Efficacy enhancing combination group (C2). Toxicity group (D1). Toxicity reducing combination group (D2). Dexamethasone group (DG).
3.3. Multivariate Analysis of UPLC-LTQ/Orbitrap MS Data
Plasma chromatography in positive and negative ion modes was obtained by UPLC-LTQ/Orbitrap MS. The plasma metabolites of each sample were investigated by multivariate statistical analysis, to obtain the difference of metabolic components.
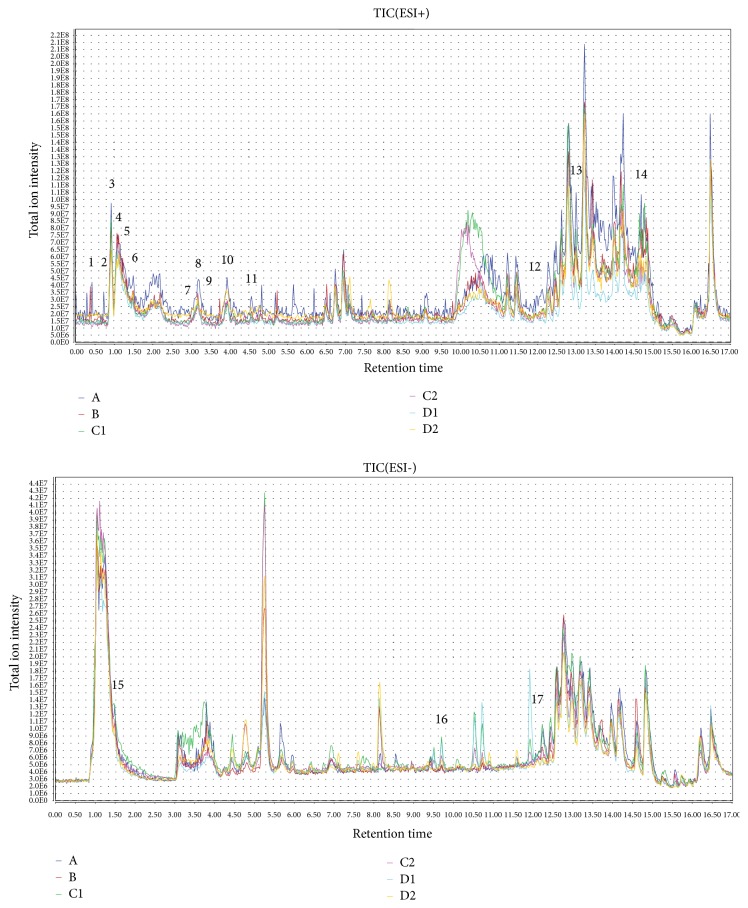
Through the conditions listed in Section 2.8, UPLC-LTQ/Orbitrap MS analysis could detected 1632 precursor molecules in positive ion mode and 3137 precursor molecules in negative ion mode, within 17 min. Typical total ion chromatograms (TICs) of UPLC-LTQ/Orbitrap MS in positive and negative modes were shown in Figure 2, some differences between each group could be observed by TICs. A total of 17 potential biomarkers associated with therapy and toxicity mechanism were marked in TICs. It could be observed that the screened potential biomarkers had obvious signal intensity differences in each group.
Figure 2.
UPLC-LTQ/Orbitrap MS TIC chromatograms of the plasma samples from six groups in positive and negative modes. 1. Uridine 5'-monophosphate (UMP); 2. Spermidine; 3. L-Lysine; 4. Carnitine; 5. L-Arginine; 6. L-Tyrosine; 7. N-Formylanthranilic acid; 8. L-Phenylalanine; 9. Leucine; 10. L-Tryptophan; 11. L-Isoleucine; 12. Vanillylmandelic acid; 13. Catechol; 14. Linoleate; 15. Citric acid; 16. Adenosine; 17. Deoxycholic acid. Healthy control group (A). AIA model group (B). Therapy group (C1). Efficacy enhancing combination group (C2). Toxicity group (D1). Toxicity reducing combination group (D2).
In order to clarify the relationship between therapeutic dose and toxic dose of lipid-soluble alkaloids of FZ, the efficacy enhancing and toxicity reducing mechanism after combination with total glucosides of paeony, multivariate data analysis was carried out in two comparison groups, namely, multivariate data analysis of therapeutic dose groups (A vs. B vs. C1 vs. C2) and multivariate data analysis of toxic dose groups (A vs. B vs. D1 vs. D2). After peak matching, all variables of each group were analyzed by PCA. In the clustering of the data, the abnormal samples were removed. The PCA score plots in positive and negative modes were shown in supplementary materials (Available here).
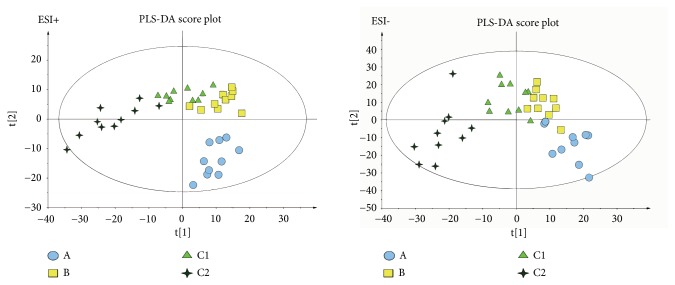
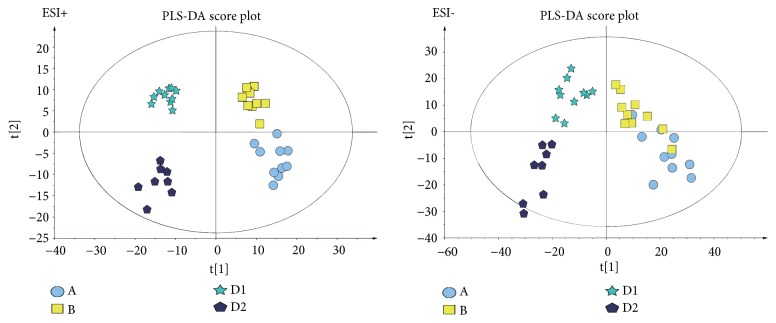
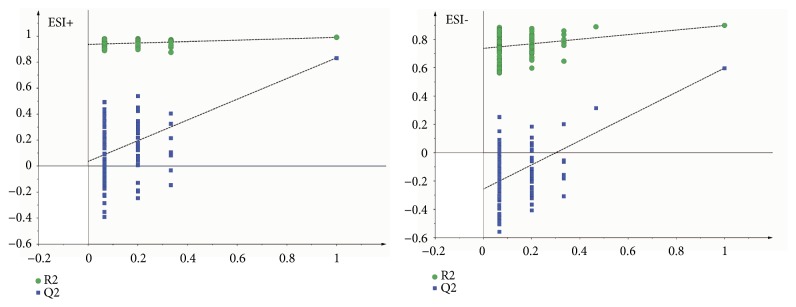
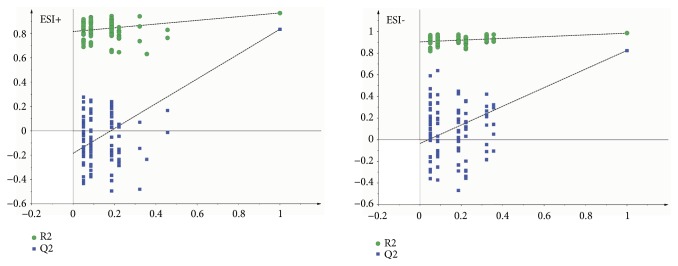
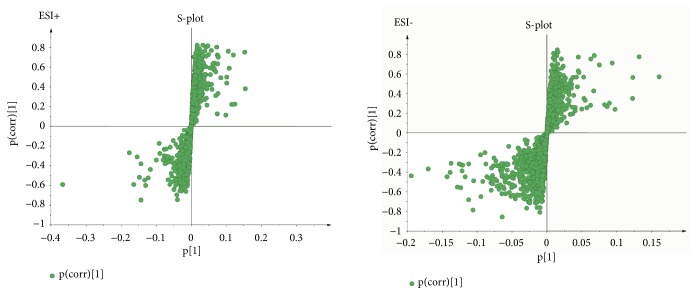
As the supervised analysis method of multivariate analysis, PLS-DA was applied to further distinguish the differences of analysis groups and confirm the potential biomarkers by VIP value. PLS-DA score plots in positive and negative modes were shown in Figures 3 and 4, the PLS-DA parameter of therapeutic dose groups (positive ion mode: R2X=0.463, R2Y=0.981, Q2=0.804; negative ion mode: R2X=0.421, R2Y=0.922, Q2=0.531) and toxic dose groups (positive ion mode: R2X=0.380, R2Y=0.978, Q2=0.850; negative ion mode: R2X=0.515, R2Y=0.986, Q2=0.850). The results of PLS-DA model showed an obvious separation trend among all groups; the samples of each group were separated from each other and clustered together individually. The model group and the control group were separated clearly, which indicated that the animal model was successful, and the occurrence of disease could cause significant changes in endogenous metabolites. Compared with the two low-dose groups, the two high-dose groups were further apart from the model group and the control group and clustered in another area individually, which suggested that the occurrence of toxicity might cause great changes in endogenous metabolites. Permutation tests in Figures 5 and 6 were applied 100 iterations to detect whether the PLS-DA models were overfitting; the results suggested that they were not overfitted. The S-plot was shown in Figure 7, which could visualize the relationship between correlation and covariance of PLS-DA. As shown in the picture, each spot corresponded to an endogenous substance, and the variables situated at the ends of the S-plot contributed highly to the differentiation.
Figure 3.
The PLS-DA score plot of therapeutic dose groups (A vs. B vs. C1 vs. C2) in ESI+ and ESI−. Healthy control group (A). AIA model group (B). Therapy group (C1). Efficacy enhancing combination group (C2).
Figure 4.
The PLS-DA score plot of toxic dose groups (A vs. B vs. D1 vs. D2) in ESI+ and ESI−. Healthy control group (A). AIA model group (B). Toxicity group (D1). Toxicity reducing combination group (D2).
Figure 5.
100-permutation test of PLS-DA model of therapeutic dose groups (A vs. B vs. C1 vs. C2) in ESI+ and ESI−.
Figure 6.
100-permutation test of PLS-DA model of toxic dose groups (A vs. B vs. D1 vs. D2) in ESI+ and ESI−.
Figure 7.
The PLS-DA S-plot in ESI+ and ESI−.
3.4. Identification and Analysis of Potential Biomarkers
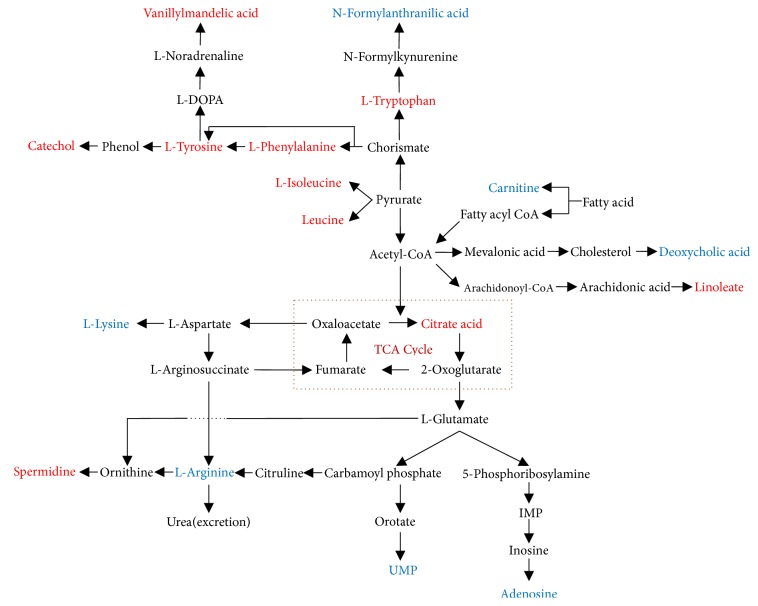
According to VIP values in PLS-DA model, the potential biomarkers with VIP > 1 were selected for further Student's t-test and ANOVA analysis, and P < 0.05 was considered to be statistically different. Following these steps and threshold above, differential metabolites related to therapeutic or toxic mechanism of drugs in AIA rats were screened out. Based on the retention time, exact mass and MS/MS fragmentation patterns information, the ions of metabolites were identified by standard sample spectra and online database information. The mass spectra of the identified compounds were shown in supplementary materials. As listed in Table 2, 10 metabolic biomarkers were considered as potential biomarkers related to therapy mechanism of lipid-soluble alkaloids of FZ and total glucosides of peony. As listed in Table 3, 7 metabolic biomarkers were considered as potential biomarkers related to toxicity mechanism of lipid-soluble alkaloids of FZ, and toxicity reducing mechanism of combination with total glucosides of peony. The identified metabolic biomarkers referenced to the KEGG database (http://www.kegg.jp) in order to investigate the interrelationship of the potential metabolic pathways involved in the development of RA, and the therapeutic or toxic mechanism of the two Chinese herbal medicines in the treatment of RA [19]. The metabolic pathway networks of potential biomarkers were shown in Figure 8.
Table 2.
List of the identification of potential biomarkers among groups.
| No. | Biomarker identification | m/z | ppm | ESI mode | Rt/min | Formula | B vs. A | C1 vs. B | C2 vs. B | Pathway |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Spermidine | 146.16467 | 6.64 | + | 0.91 | C7H19N3 | ↑## | ↓∗∗ | ↑ | Arginine and proline metabolism |
| 2 | Citric acid | 191.01813 | 9.03 | − | 1.52 | C6H8O7 | ↓# | ↑∗ | ↑∗∗ | Citrate cycle |
| 3 | L-Tyrosine | 182.08048 | 4.64 | + | 1.65 | C9H11NO3 | ↓# | ↓ | ↑∗∗∗ | Phenylalanine metabolism |
| 4 | L-Phenylalanine | 166.08602 | 1.49 | + | 3.15 | C9H11NO2 | ↓# | ↑ | ↑∗ | Phenylalanine metabolism |
| 5 | Leucine | 132.10130 | 2.60 | + | 3.23 | C6H13NO2 | ↓# | ↑ | ↑ | Valine, leucine and isoleucine biosynthesis |
| 6 | L-Tryptophan | 205.09676 | 1.79 | + | 3.92 | C11H12N2O2 | ↓# | ↑ | ↑∗∗∗ | Tryptophan metabolism |
| 7 | L-Isoleucine | 132.10129 | 2.41 | + | 4.63 | C6H13NO2 | ↓ | ↓ | ↑∗ | Valine, leucine and isoleucine biosynthesis |
| 8 | Vanillylmandelic acid | 199.16870 | 12.35 | + | 11.80 | C9H10O5 | ↑### | ↑ | ↓∗∗ | Tyrosine metabolism |
| 9 | Catechol | 111.01985 | 9.46 | + | 13.07 | C6H6O2 | ↑# | ↓∗ | ↓∗∗ | Tyrosine metabolism |
| 10 | Linoleate | 281.24722 | 5.86 | + | 14.84 | C18H32O2 | ↑## | ↓∗ | ↓ | Linoleic acid metabolism |
↑: the compound was upregulated; ↓: the compound was downregulated.
# P < 0.05, compared with control group; ##P < 0.01, compared with control group; ###P < 0.001, compared with control group.
∗ P < 0.05, compared with model group; ∗∗P < 0.01, compared with model group; ∗∗∗P < 0.001, compared with model group.
Table 3.
List of the identification of potential biomarkers among groups.
| No. | Biomarker identification | m/z | ppm | ESI mode | Rt/min | Formula | B vs. A | D1 vs.B | D2 vs. D1 | Pathway |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | UMP | 324.21578 | 7.38 | + | 0.78 | C9H13N2O9P | ↓# | ↓ | ↑ | Pyrimidine metabolism |
| 2 | L-Lysine | 147.11222 | 0.07 | + | 0.98 | C6H14N2O2 | ↑ | ↑∗ | ↓△ | Lysine biosynthesis |
| 3 | Carnitine | 162.11193 | 6.44 | + | 1.13 | C7H15NO3 | ↓ | ↓∗∗ | ↑△ | Bile secretion |
| 4 | L-Arginine | 175.11846 | 1.68 | + | 1.14 | C6H14N4O2 | ↑ | ↑∗ | ↓△ | Arginine and proline metabolism |
| 5 | N-Formylanthranilic acid | 166.04688 | 18.18 | + | 2.83 | C8H7NO3 | ↓ | ↓∗ | ↓ | Tryptophan metabolism |
| 6 | Adenosine | 265.94792 | 7.65 | − | 9.60 | C10H13N5O4 | ↓ | ↓∗ | ↑ | Purine metabolism |
| 7 | Deoxycholic acid | 391.28221 | 0.28 | − | 12.10 | C24H40O4 | ↑ | ↑∗∗ | ↑ | Bile secretion |
↑: the compound was upregulated; ↓: the compound was downregulated.
# P < 0.05, compared with control group; ##P < 0.01, compared with control group; ###P < 0.001, compared with control group.
∗ P < 0.05, compared with model group; ∗∗P < 0.01, compared with model group; ∗∗∗P < 0.001, compared with model group.
△ P < 0.05, compared with D1 group; △△P < 0.01, compared with D1 group; △△△P < 0.001, compared with D1 group.
Figure 8.
The metabolic pathway networks of potential biomarkers in response to therapeutic and toxic mechanism of combination of FZ and BS in AIA rats. The red marked metabolites were associated with the therapeutic mechanism. The blue marked metabolites were associated with the toxic mechanism.
4. Discussion
4.1. Analysis of Metabolites Associated with Therapeutic Effects
Nontargeted metabolomics was applied to analyze the therapeutic mechanism of lipid-soluble alkaloids of FZ and total glucosides of peony. The potential biomarkers were screened out by multivariate data analysis of therapeutic dose groups (A vs. B vs. C1 vs. C2), and the results showed that the therapeutic mechanism was related to the regulation of energy and amino acids metabolism.
4.1.1. Metabolites Alteration Related to Energy Metabolism
In the process of many diseases, with the occurrence and development of inflammation, the consumption of energy in the body would be reflected in the metabolites.
Citric acid was an important intermediate in mitochondria to participate tricarboxylic acid (TCA) cycle. TCA cycle was ubiquitous metabolic pathway in aerobic organisms and played an important role in energy metabolism. The levels of citric acid in urine and plasma of patients with bone diseases decreased significantly than those of normal people [20]. In our study, the level of citric acid was significantly decreased in model group, which suggested that the citric acid synthase in TCA cycle was impaired in AIA rats. Compared with model group, the levels of citric acid in C1 and C2 groups were significantly upregulated, especially in C2 group, which indicated that lipid-soluble alkaloids of FZ might regulate TCA cycle by increasing citric acid level, and total glucosides of peony could enhance this effect.
Linoleate was an essential polyunsaturated fatty acid, widely distributed in the nutrients needed by the human body. Linoleate was also a precursor of arachidonic acid and involved in the biosynthesis of many eicosanoid derivatives. Some studies clearly demonstrated that linoleate had strong proinflammatory properties and was directly associated with metabolic diseases [21]. As the epoxides of arachidonic acid and linoleate, epoxyeicosatrienoic acids (EETs) and epoxyoctadecenoic acids (EpOMEs) could be converted to dihydroxyeicosatrienoic acids (DHETEs) and dihydroxyoctadecenoic acids (DHOMEs), two compounds possessing mild anti-inflammatory and proinflammatory properties, respectively [22–24]. Furthermore, 9-HODE was another metabolite of linoleate, which could promote a strong proinflammatory reaction in wound-healing model rats [25]. In our study, the level of linoleate was significantly increased in model group, while the levels of linoleate in C1 and C2 groups were lower than that in model group, especially in C1 group, which suggested that lipid-soluble alkaloids of FZ might reduce the level of linoleate and thus lead to an anti-inflammatory response. However, the synergistic effect of combination with total glucosides of peony was not obvious.
4.1.2. Metabolites Alteration Related to Amino Acids Metabolism
Some studies demonstrated that amino acids were the main differential metabolites in plasma of RA patients. Amino acids played an essential role in the pathogenesis of RA. For example, inflammation and synovial hyperplasia were closely related to the alteration of amino acids metabolism in vivo [26–28].
L-tryptophan was an essential amino acid, which played an important role in physiological process of the body. Through the kynurenine metabolic pathway, tryptophan metabolism was considered to modulate the balance of immune system between tolerance to nonharmful antigens and responsiveness to pathogens. Interferon-gamma (IFN-γ), which is released by activated T-cells and leucocytes, was a proinflammatory cytokine in vivo. IFN-γ could induce catabolism of tryptophan during many different cell types of immune response [29]. Tryptophan depletion was closely related to the defense mechanism during inflammation by suppressing the proliferation of invading pathogens [16]. The decreased level of L-tryptophan in model group was closely related to immune response of RA, and C2 group could significantly increase the level of L-tryptophan. The above results suggested that combination of lipid-soluble alkaloids of FZ and total glucosides of peony had obvious regulation of tryptophan metabolism pathway.
L-phenylalanine, L-isoleucine, and leucine were all essential amino acids of the human body, and L-phenylalanine was also a precursor of L-tyrosine [30, 31]. The levels of tyrosine, phenylalanine, isoleucine, valine, alanine, and histidine had lower concentrations in patients with rheumatoid arthritis and systemic lupus erythematosus [32, 33]. Previous studies showed that AIA rats had more phenylalanine excretion, which suggested that phenylalanine metabolism could be partially blocked in inflammatory state and the protein metabolism might be disturbed [34, 35]. Leucine assumed a crucial role in the regulation of protein metabolism, the decreased level of leucine in plasma indicated a disorder in the regulation of protein degradation. Leucine could modulate proinflammatory cytokines and increase anti-inflammatory cytokines to maintain immune balance in the body [36]. As the results showed, L-phenylalanine, L-tyrosine, L-isoleucine, and leucine were significantly altered in model group. The changed levels of these four biomarkers indicated that leucine, isoleucine and valine biosynthesis pathway, tyrosine, tryptophan and phenylalanine biosynthesis pathway, and phenylalanine metabolism pathway had been disturbed in AIA rats. Compared with model group, C2 group could upregulate the levels of these amino acids in different degrees, and the effects were better than C1 group. The above results indicated that combination of lipid-soluble alkaloids of FZ and total glucosides of peony were superior to single dose of lipid-soluble alkaloids of FZ in the treatment of RA.
Spermidine was a polyamine with important physiological functions and played a crucial role in cell growth, aging, autophagy, and neurodegenerative diseases. Polyamines levels increased in many diseases, such as Alzheimer's disease, malignant tumor, Parkinson's disease, and inflammatory diseases. Many diseases were associated with inflammation, while polyamines were involved in inflammatory responses, the levels of polyamines increased with the development of inflammation [37]. Previous studies suggested that spermidine and its oxidative metabolites, mediated the anti-inflammatory activity in human rheumatoid synovial fluid, plasma of arthritic rats and inflammatory exudates [38]. Spermidine could protect macrophages from inflammatory injury by increasing the synthesis of anti-inflammatory factor IL-10 and inhibiting the expression of proinflammatory cytokines IL-12 and IFN-γ [39]. As the results showed that the level of spermidine was significantly increased in model group. Compared with model group, the level of spermidine decreased significantly in C1 group, but the therapeutic effect of C2 group was not obvious. The above results suggested that lipid-soluble alkaloids of FZ could reduce the level of spermidine to make an anti-inflammatory response. However, the synergistic effect of combination with total glucosides of peony was not significant.
The majority of catechols existed in body as derivatives of norepinephrine, adrenaline, and dopamine; all of them were collectively referred to catecholamines (CAs). And vanillylmandelic acid was the final metabolite of CAs. Catechol and vanillylmandelic acid were all involved in tyrosine metabolism pathway [40, 41]. In addition to regulating some visceral activities such as cardiovascular, respiratory, and digestive activities, CAs was also involved in regulating the immune function of the body [42]. Previous studies showed that CAs could inhibit the production of TNF-α, IL-1, and IFN-γ, while they inhibit TNF-α production by astrocytes, monocytes, and microglial cells and indirectly inhibit IL-1 production by inhibition of TNF-α and potentiation of IL-10 production [43]. Some studies also demonstrated the presence of catecholamine-producing cells in the synovial tissue during arthritis but not in normal tissue, which suggested that they were related to chronic inflammation [44]. In this study, the levels of catechol and vanillylmandelic acid were significantly increased in model group. After medication, the levels of catechol in C1 and C2 groups decreased significantly, and the level of vanillylmandelic acid in C2 group decreased significantly. The above results suggested that lipid-soluble alkaloids of FZ had anti-inflammatory effects, and total glucosides of peony could obviously enhance the anti-inflammatory effects in arthritis disease.
4.2. Analysis of Metabolites Associated with Toxicity Reduction
Nontargeted metabolomics was applied to analyze the toxicity mechanism of lipid-soluble alkaloids of FZ and the toxicity reducing mechanism of combination with total glucosides of peony. The potential biomarkers were screened out by multivariate data analysis of toxic dose groups (A vs. B vs. D1 vs. D2), and the results showed that the toxicity mechanism was related to the fat, bile acids, amino acids, and energy metabolic disorders.
4.2.1. Metabolites Alteration Related to Fatty Metabolism
As an essential nutrient of the organism, carnitine played an important role in promoting the oxidative decomposition of fatty acids. In the process of fatty metabolism, firstly, in the mitochondrial outer membrane and endoplasmic reticulum, long-chain fatty acid converted to fatty acyl CoA by ester of acyl CoA synthetase [45]. Then, the fatty acyl CoA combined with L-carnitine to form carnityl carnitine by carnitine acyltransferase I (CPT-I), carnityl carnitine transported into mitochondria, and release L-camitine by carnitine acyltransferase II (CPT-II). In mitochondria, fatty acyl CoA underwent β-oxidization to synthesize acetyl-CoA [46, 47]. In our study, the levels of carnitine were significantly decreased in D1 group and increased in D2 group. The above results suggested that toxic dose of lipid-soluble alkaloids of FZ could impair the activity of fatty acid esterase or CPT-I due to drug hepatotoxicity, and total glucosides of peony could reduce this toxicity by regulating the metabolic environments of fatty acids.
4.2.2. Metabolites Alteration Related to Bile Acids Metabolism
Deoxycholic acid was a free bile acid, derived from the loss of an oxygen atom of cholic acid which was synthesized by the liver. It served many indispensable physiological functions, such as absorption of vitamins and lipids, and the regulation of glucose and cholesterol [48]. The levels of bile acids were increased in hepatic and intestinal diseases, impaired renal tubules and hepatotoxicity injury, because of the synthesis and clearance of intrahepatic bile acids and their intestinal absorption were disturbed [49]. The damaged liver could lead to obstruction of bile acids enterohepatic circulation, resulting in the increased levels of blood bile acids. Bile acids were also considered as potential biomarkers of drug-induced liver injury [50]. In this study, the levels of deoxycholic acid were significantly increased in D1 and D2 groups, which suggesting that toxic dose of lipid-soluble alkaloids of FZ could cause liver injury, but total glucosides of peony could not prevent this damage.
4.2.3. Metabolites Alteration Related to Amino Acids Metabolism
Amino acids were mainly metabolized in the liver, and any liver injury might cause the disorder of amino acids metabolism. The results in our study showed that the metabolic environments and pathways of amino acids were seriously impacted by toxic dose of lipid-soluble alkaloids of FZ.
N-formylanthranilic acid was the metabolite of tryptophan by kynurenine pathway; then, N-formylanthranilic acid was further converted to quinolinic acid into tryptophan-NAD+ pathway or converted to acetyl-CoA to participate the TCA cycle [51]. The decreased level of N-formylanthranilic acid in D1 group suggested that toxic dose of lipid-soluble alkaloids of FZ might cause tryptophan and NAD metabolic disorders, energy metabolism blocked and hepatic toxicity occurred. However, the regulatory effects of total glucosides of peony on these disorders were not obvious.
L-arginine was usually considered to be a conditionally essential amino acid [52]. L-arginine occupied an important position because of its extensive biological functions; it was the critical intermediate in the urea cycle, the substrate for nitric oxide (NO) synthase, the source of ornithine for polyamine synthesis, and the source of amidino groups for creatine synthesis. In addition, it was also closely related to the metabolism of glutamine and pyrimidine [53, 54]. In liver, L-arginine was hydrolyzed by ornithine cycle, ornithine could subsequently be converted to proline and polyamines, and the production of urea was excreted by the kidney [55]. Other pathways of L-arginine catabolism were synthesis of agmatine, creatine, and NO [56, 57]. In vivo, pyrimidines synthesized through the formation of carbamoyl phosphate from glutamine, carbamoyl phosphate could also enter the urea cycle to participate the synthesis of L-arginine [58].
Previous studies showed that L-lysine and L-arginine were transported by the same carrier through intestinal mucosal transport, and they were also transported by the same carrier in renal tubule [59]. L-lysine could compete with L-arginine for intracellular transport, excess of L-lysine might indirectly affect L-arginine metabolism [60].
In our study, the levels of L-arginine and L-lysine were significantly increased in D1 group, which suggested that the metabolic disorder of L-arginine in the liver might be induced by lipid-soluble alkaloids of FZ, including suppressed pathways of urea, creatine, creatinine, and ornithine synthesis. Metabolic disorders of L-arginine and L-lysine also interacted with each other because of the same carrier was used by them. The level of uridine 5'-monophosphate (UMP) was downregulated in D1 group, which might also be indirectly caused by the metabolic disorder of L-arginine. The level of UMP was upregulated in D2 group, which indicated that total glucosides of peony could significantly regulate the metabolic disorders caused by hepatotoxicity and maintain the stability of the liver internal environments.
4.2.4. Metabolites Alteration Related to Energy Metabolism
Adenosine was an endogenous nucleoside distributed in human cells and directly involved in the energy metabolism of cells. Adenosine was the material basis for energy storage molecules, nucleic acids, neuromodulator of cellular activity and enzyme's substrate, etc. It was also an essential intermediate for the synthesis of adenylate, adenine and adenosine triphosphate (ATP) [61]. The level of adenosine was significantly decreased in D1 group, and this phenomenon suggested that lipid-soluble alkaloids of FZ could interfere with adenosine metabolism and directly affect the energy metabolism. The level of adenosine was upregulated in D2 group, which indicated that the metabolic disorder of adenosine could be regulated by total glucosides of peony.
5. Conclusions
Firstly, through the ELISA experiments, therapeutic dose of lipid-soluble alkaloids of FZ exhibited strong anti-inflammatory activity, it could significantly reduce the concentrations of IL-1β, TNF-α, and IFN-γ, and the combination with total glucosides of peony could further reduce the concentration of IL-1β. Then, through metabolomics study, the therapeutic mechanism of combination of lipid-soluble alkaloids of FZ and total glucosides of peony in AIA rats had been investigated. The therapeutic mechanism mainly involved in citrate cycle, phenylalanine, tyrosine, tryptophan metabolism and leucine, isoleucine biosynthesis. The effects of total glucosides of peony in the attenuation of toxicity induced by lipid-soluble alkaloids of FZ were also investigated. The bile secretion, purine, pyrimidine, lysine and arginine metabolic disorders caused by drug-induced liver injury could be regulated by total glucosides of peony.
In summary, this study showed that lipid-soluble alkaloids of FZ were both medicinal and toxic components, and different doses could affect different biological targets and produce different biological effects. Combination with total glucosides of peony could enhance the therapeutic effects and attenuate the toxicity induced by lipid-soluble alkaloids of FZ.
Acknowledgments
The study was supported by National Natural Science Foundation of China (81630101,81573583) and Sichuan Provincial Science and Technology Department of Youth and Technology Innovation Research Team Program (2017TD0001).
Abbreviations
- AIA:
Adjuvant-induced arthritis
- BS:
Paeoniae Radix Alba
- CFA:
Complete Freund's adjuvant
- FZ:
Aconiti Lateralis Radix Praeparata
- PCA:
Principal component analysis
- PLS-DA:
Partial least squares-discriminant analysis
- RA:
Rheumatoid arthritis
- TCA:
Tricarboxylic acid
- TIC:
Total ion chromatogram
- UMP:
Uridine 5'-monophosphate.
Contributor Information
Yun-xia Li, Email: lyxcdutcm@126.com.
Cheng Peng, Email: pengchengsub@126.com.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
All authors confirmed that there were no conflicts of interest associated with this study and publication.
Authors' Contributions
All authors participated and completed the experiments together. The manuscript was also reviewed and approved by them.
Supplementary Materials
Supplementary Table S1: the detailed information of each group. Supplementary Table S2: the nutrient composition of rat food (%). Supplementary Figure S1: the PCA score plot of therapeutic dose groups (A vs. B vs. C1 vs. C2) in ESI+ and ESI−. Supplementary Figure S2: the PCA score plot of toxic dose groups (A vs. B vs. D1 vs. D2) in ESI+ and ESI−. Supplementary Figure S3: the mass spectra of the compounds listed in the manuscript.
References
- 1.Zhang Q., Hu X., Yao W., et al. Pharmacologic effect of miao medicine Illicium simonsii maxim. on collagen-induced arthritis in rats. Evidence-Based Complementary and Alternative Medicine. 2018;2018:7. doi: 10.1155/2018/2398379.2398379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rantapää-Dahlqvist S., de Jong B. A. W., Berglin E., et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis & Rheumatology. 2003;48(10):2741–2749. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 3.Cheng B., Zheng H., Wu F., et al. Metabolomics analysis of Danggui Sini decoction on treatment of collagen-induced arthritis in rats. Journal of Chromatography B. 2017;1061-1062:282–291. doi: 10.1016/j.jchromb.2017.07.043. [DOI] [PubMed] [Google Scholar]
- 4.Pharmacopoeia Commission of the People’s Republic of China. The Pharmacopoeia of the People’s Republic of China. Beijing, China: China Medical Science Press; 2015. [Google Scholar]
- 5.Nyirimigabo E., Xu Y., Li Y., Wang Y., Agyemang K., Zhang Y. A review on phytochemistry, pharmacology and toxicology studies of Aconitum. Journal of Pharmacy and Pharmacology. 2015;67(1):1–19. doi: 10.1111/jphp.12310. [DOI] [PubMed] [Google Scholar]
- 6.Kim W., Lee W., Choi J. G., et al. Inhibitory effects of aconiti lateralis radix preparata on chronic intermittent cold-induced inflammation in the mouse hypothalamus. Journal of Ethnopharmacology. 2018;215:27–33. doi: 10.1016/j.jep.2017.12.042. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y.-B., Da J., Zhang J.-X., et al. A feasible, economical, and accurate analytical method for simultaneous determination of six alkaloid markers in Aconiti Lateralis Radix Praeparata from different manufacturing sources and processing ways. Chinese Journal of Natural Medicines. 2017;15(4):301–309. doi: 10.1016/S1875-5364(17)30048-1. [DOI] [PubMed] [Google Scholar]
- 8.Hu R., Zhao J., Qi L.-W., Li P., Jing S.-L., Li H.-J. Structural characterization and identification of C19- and C20-diterpenoid alkaloids in roots of Aconitum carmichaeli by rapid-resolution liquid chromatography coupled with time-of-flight mass spectrometry. Rapid Communications in Mass Spectrometry. 2009;23(11):1619–1635. doi: 10.1002/rcm.4038. [DOI] [PubMed] [Google Scholar]
- 9.Wang C.-H., Wang R., Cheng X.-M., et al. Comparative pharmacokinetic study of paeoniflorin after oral administration of decoction of Radix Paeoniae Rubra and Radix Paeoniae Alba in rats. Journal of Ethnopharmacology. 2008;117(3):467–472. doi: 10.1016/j.jep.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 10.Kong M., Liu H.-H., Wu J., et al. Effects of sulfur-fumigation on the pharmacokinetics, metabolites and analgesic activity of Radix Paeoniae Alba. Journal of Ethnopharmacology. 2018;212:95–105. doi: 10.1016/j.jep.2017.10.023. [DOI] [PubMed] [Google Scholar]
- 11.Wu D., Chen J., Zhu H., et al. UPLC-PDA determination of paeoniflorin in rat plasma following the oral administration of Radix Paeoniae Alba and its effects on rats with collagen-induced arthritis. Experimental and Therapeutic Medicine. 2014;7(1):209–217. doi: 10.3892/etm.2013.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu Y., Lu C., Zha Q., et al. Plasma metabonomics study of rheumatoid arthritis and its Chinese medicine subtypes by using liquid chromatography and gas chromatography coupled with mass spectrometry. Molecular BioSystems. 2012;8(5):1535–1543. doi: 10.1039/c2mb25022e. [DOI] [PubMed] [Google Scholar]
- 13.Yan Y., Zhang A., Dong H., et al. Toxicity and detoxification effects of herbal Caowu via ultra performance liquid chromatography/mass spectrometry metabolomics analyzed using pattern recognition method. Pharmacognosy Magazine. 2017;13(52):683–692. doi: 10.4103/pm.pm_475_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y., Gong X., Liu M., Peng C., Li P., Wang Y. Investigation of liver injury of Polygonum multiflorum Thunb in rats by metabolomics and traditional approaches. Frontiers in Pharmacology. 2017;8, article 791 doi: 10.3389/fphar.2017.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbosa C. P., Ritter A. M., Da Silva L. G., et al. Effects of simvastatin, ezetimibe, and their combination on histopathologic alterations caused by adjuvant-induced arthritis. Inflammation. 2014;37(4):1035–1043. doi: 10.1007/s10753-014-9826-0. [DOI] [PubMed] [Google Scholar]
- 16.Yue R., Zhao L., Hu Y., et al. Rapid-resolution liquid chromatography TOF-MS for urine metabolomic analysis of collagen-induced arthritis in rats and its applications. Journal of Ethnopharmacology. 2013;145(2):465–475. doi: 10.1016/j.jep.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Dayer J. The pivotal role of interleukin-1 in the clinical manifestations of rheumatoid arthritis. Rheumatology (Oxford) 2003;42(2):ii3–ii10. doi: 10.1093/rheumatology/keg326. [DOI] [PubMed] [Google Scholar]
- 18.Cardoso P. R. G., Matias K. A., Dantas A. T., et al. Losartan, but not enalapril and valsartan, inhibits the expression of IFN-γ, IL-6, IL-17F and IL-22 in PBMCs from rheumatoid arthritis patients. The Open Rheumatology Journal. 2018;12:160–170. doi: 10.2174/1874312901812010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yun X., Dong S., Hu Q., Dai Y., Xia Y. 1H NMR-based metabolomics approach to investigate the urine samples of collagen-induced arthritis rats and the intervention of tetrandrine. Journal of Pharmaceutical and Biomedical Analysis. 2018;154:302–311. doi: 10.1016/j.jpba.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 20.Renata C., Fabio V., Angela B., Sergio S. Citrate and mineral metabolism: kidney stones and bone disease. Frontiers in Bioscience. 2003;8:s1084–s1106. doi: 10.2741/1119. [DOI] [PubMed] [Google Scholar]
- 21.Choque B., Catheline D., Rioux V., Legrand P. Linoleic acid: between doubts and certainties. Biochimie. 2014;96(1):14–21. doi: 10.1016/j.biochi.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Newman J. W., Morisseau C., Hammock B. D. Epoxide hydrolases: their roles and interactions with lipid metabolism. Progress in Lipid Research. 2005;44(1):1–51. doi: 10.1016/j.plipres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Schmelzer K. R., Kubala L., Newman J. W., Kim I.-H., Eiserich J. P., Hammock B. D. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proceedings of the National Acadamy of Sciences of the United States of America. 2005;102(28):9772–9777. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmelzer K. R., Inceoglu B., Kubala L., et al. Enhancement of antinociception by coadministration of nonsteroidal anti-inflammatory drugs and soluble epoxide hydrolase inhibitors. Proceedings of the National Acadamy of Sciences of the United States of America. 2006;103(37):13646–13651. doi: 10.1073/pnas.0605908103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fritsche K. L. Too much linoleic acid promotes inflammation-doesn’t it? Prostaglandins, Leukotrienes and Essential Fatty Acids. 2008;79(3-5):173–175. doi: 10.1016/j.plefa.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi T., Okada M., Ito S., et al. Amino acid profiles in relation to chronic periodontitis and rheumatoid arthritis. Open Journal of Stomatology. 2014;4(2):49–55. doi: 10.4236/ojst.2014.42009. [DOI] [Google Scholar]
- 27.Smolenska Z., Smolenski R. T., Zdrojewski Z. Plasma concentrations of amino acid and nicotinamide metabolites in rheumatoid arthritis-potential biomarkers of disease activity and drug treatment. Biomarkers. 2016;21(3):218–224. doi: 10.3109/1354750X.2015.1130746. [DOI] [PubMed] [Google Scholar]
- 28.Ahn J. K., Kim S., Hwang J., Kim J., Kim K. H., Cha H.-S. GC/TOF-MS-based metabolomic profiling in cultured fibroblast-like synoviocytes from rheumatoid arthritis. Joint Bone Spine. 2016;83(6):707–713. doi: 10.1016/j.jbspin.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Moffett J. R., Namboodiri M. A. Tryptophan and the immune response. Immunology & Cell Biology. 2003;81(4):247–265. doi: 10.1046/j.1440-1711.2003.t01-1-01177.x. [DOI] [PubMed] [Google Scholar]
- 30.Neurauter G., Grahmann A. V., Klieber M., et al. Serum phenylalanine concentrations in patients with ovarian carcinoma correlate with concentrations of immune activation markers and of isoprostane-8. Cancer Letters. 2008;272(1):141–147. doi: 10.1016/j.canlet.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Kato H., Miura K., Nakano S., Suzuki K., Bannai M., Inoue Y. Leucine-enriched essential amino acids attenuate inflammation in rat muscle and enhance muscle repair after eccentric contraction. Amino Acids. 2016;48(9):2145–2155. doi: 10.1007/s00726-016-2240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ouyang X., Dai Y., Wen J. L., Wang L. X. 1H NMR-based metabolomic study of metabolic profiling for systemic lupus erythematosus. Lupus. 2011;20(13):1411–1420. doi: 10.1177/0961203311418707. [DOI] [PubMed] [Google Scholar]
- 33.Zabek A., Swierkot J., Malak A., et al. Application of (1)H NMR-based serum metabolomic studies for monitoring female patients with rheumatoid arthritis. Journal of Pharmaceutical and Biomedical Analysis. 2016;117:544–550. doi: 10.1016/j.jpba.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Qi Y., Li S. Z., Pi Z. F., et al. Metabonomic study of Wu-tou decoction in adjuvant-induced arthritis rat using ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences. 2014;953-954:11–19. doi: 10.1016/j.jchromb.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 35.Qi Y., Pi Z., Liu S., et al. A metabonomic study of adjuvant-induced arthritis in rats using ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. Molecular BioSystems. 2014;10(10):2617–2625. doi: 10.1039/c4mb00131a. [DOI] [PubMed] [Google Scholar]
- 36.Cruz B., Oliveira A., Gomes-Marcondes M. C. C. L-leucine dietary supplementation modulates muscle protein degradation and increases pro-inflammatory cytokines in tumour-bearing rats. Cytokine. 2017;96:253–260. doi: 10.1016/j.cyto.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 37.Minois N., Carmona-Gutierrez D., Madeo F. Polyamines in aging and disease. Aging (Albany NY) 2011;3(8):716–732. doi: 10.18632/aging.100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bjelaković G., Stojanović I., Stoimenov T. J., et al. Metabolic correlations of glucocorticoids and polyamines in inflammation and apoptosis. Amino Acids. 2010;39(1):29–43. doi: 10.1007/s00726-010-0489-3. [DOI] [PubMed] [Google Scholar]
- 39.Haskó G., Kuhel D. G., Marton A., Nemeth Z. H., Deitch E. A., Szabó C. Spermine differentially regulates the production of interleukin-12 p40 and interleukin-10 and suppresses the release of the t helper 1 cytokine interferon-γ. Shock. 2000;14(2):144–149. doi: 10.1097/00024382-200014020-00012. [DOI] [PubMed] [Google Scholar]
- 40.Kayama E., Yamada K., Aizawa Y. Urinary excretion of vanillylmandelic acid and effect of some anti-inflammatory drugs in cotton pellet inflammation. The Japanese Journal of Pharmacology. 1981;31(5):860–862. doi: 10.1254/jjp.31.860. [DOI] [PubMed] [Google Scholar]
- 41.Lee T., Yagi M., Kusunoki N., et al. Standard values for the urine HVA/VMA ratio in neonates as a screen for Menkes disease. Brain & Development. 2015;37(1):114–119. doi: 10.1016/j.braindev.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 42.Maestroni G. J. M. Sympathetic nervous system influence on the innate immune response. Annals of the New York Academy of Sciences. 2006;1069(1):195–207. doi: 10.1196/annals.1351.017. [DOI] [PubMed] [Google Scholar]
- 43.Calcagni E., Elenkov I. Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Annals of the New York Academy of Sciences. 2006;1069:62–76. doi: 10.1196/annals.1351.006. [DOI] [PubMed] [Google Scholar]
- 44.Capellino S., Cosentino M., Wolff C., Schmidt M., Grifka J., Straub R. H. Catecholamine-producing cells in the synovial tissue during arthritis: modulation of sympathetic neurotransmitters as new therapeutic target. Annals of the Rheumatic Diseases. 2010;69(10):1853–1860. doi: 10.1136/ard.2009.119701. [DOI] [PubMed] [Google Scholar]
- 45.Gao S., Casals N., Keung W., Moran T. H., Lopaschuk G. D. Differential effects of central ghrelin on fatty acid metabolism in hypothalamic ventral medial and arcuate nuclei. Physiology & Behavior. 2013;118:165–170. doi: 10.1016/j.physbeh.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 46.Fulgencio J.-P., Kohl C., Girard J., Pégorier J.-P. Troglitazone inhibits fatty acid oxidation and esterification, and gluconeogenesis in isolated hepatocytes from starved rats. Diabetes. 1996;45(11):1556–1562. doi: 10.2337/diab.45.11.1556. [DOI] [PubMed] [Google Scholar]
- 47.Skrede S., Sørensen H. N., Larsen L. N., et al. Thia fatty acids, metabolism and metabolic effects. Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism. 1997;1344(2):115–131. doi: 10.1016/S0005-2760(96)00138-5. [DOI] [PubMed] [Google Scholar]
- 48.Yu Y., Ma C., Bi K., et al. A metabonomic analysis of urine from rats treated with rhizoma alismatis using ultra-performance liquid chromatography/mass spectrometry. Rapid Communications in Mass Spectrometry. 2011;25(18):2633–2640. doi: 10.1002/rcm.5163. [DOI] [PubMed] [Google Scholar]
- 49.Palmeira C. M., Rolo A. P. Mitochondrially-mediated toxicity of bile acids. Toxicology. 2004;203(1-3):1–15. doi: 10.1016/j.tox.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 50.Luo L., Schomaker S., Houle C., Aubrecht J., Colangelo J. L. Evaluation of serum bile acid profiles as biomarkers of liver injury in rodents. Toxicological Sciences. 2014;137(1):12–25. doi: 10.1093/toxsci/kft221. [DOI] [PubMed] [Google Scholar]
- 51.Ji H., Liu Y., He F., An R., Du Z. LC–MS based urinary metabolomics study of the intervention effect of aloe-emodin on hyperlipidemia rats. Journal of Pharmaceutical and Biomedical Analysis. 2018;156:104–115. doi: 10.1016/j.jpba.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 52.Luiking Y. C., Poeze M., Dejong C. H., Ramsay G., Deutz N. E. Sepsis: an arginine deficiency state? Critical Care Medicine. 2004;32(10):2135–2145. doi: 10.1097/01.CCM.0000142939.81045.A0. [DOI] [PubMed] [Google Scholar]
- 53.Efron D., Barbul A. Role of arginine in immunonutrition. Journal of Gastroenterology. 2000;35(Suppl 12):20–23. doi: 10.1007/PL00009971. [DOI] [PubMed] [Google Scholar]
- 54.Brosnan M. E., Brosnan J. T. Orotic acid excretion and arginine metabolism. Journal of Nutrition. 2007;137(6) Suppl 2:1656S–1661S. doi: 10.1093/jn/137.6.1656S. [DOI] [PubMed] [Google Scholar]
- 55.Cynober L. Can arginine and ornithine support gut functions? Gut. 1994;35(Suppl 1):S42–S45. doi: 10.1136/gut.35.1_Suppl.S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu G., Morris S. M., Jr. Arginine metabolism: nitric oxide and beyond. Biochemical Journal. 1998;336(1):1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flynn N., Meininger C. J., Haynes T. E., Wu G. The metabolic basis of arginine nutrition and pharmacotherapy. Biomedicine & Pharmacotherapy. 2002;56(9):427–438. doi: 10.1016/s0753-3322(02)00273-1. [DOI] [PubMed] [Google Scholar]
- 58.Evans D. R., Guy H. I. Mammalian pyrimidine biosynthesis: fresh insights into an ancient pathway. The Journal of Biological Chemistry. 2004;279(32):33035–33038. doi: 10.1074/jbc.R400007200. [DOI] [PubMed] [Google Scholar]
- 59.Schwartz I. F., Iaina A., Benedict Y., et al. Augmented arginine uptake, through modulation of cationic amino acid transporter-1, increases GFR in diabetic rats. Kidney International. 2004;65(4):1311–1319. doi: 10.1111/j.1523-1755.2004.00508.x. [DOI] [PubMed] [Google Scholar]
- 60.Luiking Y. C., Deutz N. E. Biomarkers of arginine and lysine excess. Journal of Nutrition. 2007;137(6) Suppl 2:1662S–1668S. doi: 10.1093/jn/137.6.1662S. [DOI] [PubMed] [Google Scholar]
- 61.Porkka-Heiskanen T., Kalinchuk A. V. Adenosine, energy metabolism and sleep homeostasis. Sleep Medicine Reviews. 2011;15(2):123–135. doi: 10.1016/j.smrv.2010.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: the detailed information of each group. Supplementary Table S2: the nutrient composition of rat food (%). Supplementary Figure S1: the PCA score plot of therapeutic dose groups (A vs. B vs. C1 vs. C2) in ESI+ and ESI−. Supplementary Figure S2: the PCA score plot of toxic dose groups (A vs. B vs. D1 vs. D2) in ESI+ and ESI−. Supplementary Figure S3: the mass spectra of the compounds listed in the manuscript.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.