Abstract
Background
We investigated the effects of metformin on neurological function and oxidative stress in patients with type 2 diabetes mellitus with acute stroke.
Material/Methods
We randomly assigned 80 acute stroke patients to 2 groups: the metformin combined group and the insulin group. Each group had 40 patients and all were treated with standard stroke treatment. The indexes of nervous functional score and oxidative stress were measured before and 2 weeks after treatment. The primary fetal rat hippocampal neurons were gradually matured after 7 days of culture, and divided into the control group (Con), the oxygen-glucose deprivation model group (Mod), and the metformin group (Met). In the Met group, 10 mmol/L metformin was added, and the Con group and the Mod group received equal volumes of cell culture fluid. Cell viability, cell apoptosis rate, and the expression of Bax, Bcl-2, AMPK, pAMPK and mTOR were detected; MDA content and SOD activity were also detected.
Results
Before treatment, there was no difference in the metrical indexes between the 2 groups. After treatment, the treatment group was better than the control group in neurological function scores and multiple oxidative stress-related indicators. The experimental results of primary fetal rat hippocampal neuronal cells suggest that this mechanism of improvement is closely related to the AMPK/mTOR signaling pathway.
Conclusions
Metformin can improve the neurological function and oxidative stress status of acute stroke patients with type 2 diabetes, and its mechanism may be related to the AMPK/mTOR signaling pathway and oxidative stress.
MeSH Keywords: Cryopyrin-Associated Periodic Syndromes, Metformin, Stroke
Background
Stroke is a leading cause of death and disability globally, with 69% of all strokes experienced among people in low- and middle-income countries (LMIC). China is the most populous LMIC and acute stroke the leading cerebrovascular disease. Among stroke survivors, 70–80% are living with varying degrees of neurological impairment, and stroke is associated with high mortality and disability [1]. The incidence of acute stroke is increasing year by year, which brings a heavy burden to families and society. The occurrence of acute stroke has a close relationship with atherosclerosis, hemorheological changes, inflammatory state, oxidative stress, and many other factors. Neuronal cell death is the main injury caused by stroke, and its mechanism is by oxidative stress, inflammation, ischemia, hypoxia, calcium ion dysregulation, and apoptosis [2,3].
Metformin is a clinically common first-line agent for the treatment of type 2 diabetes. Researchers have shown that long-term metformin treatment can reduce the risk of dementia and stroke, and improve the mild cognitive impairment in type 2 diabetes, and metformin may be a potential therapeutic drug for stroke [4–7]. AMPK (adenosine monophosphate-activated protein kinase) is an important serine/threonine protein kinase, and is an upstream regulator of key enzymes in oxide synthesis and fat metabolism. Cell energy loss induces an increase in AMP/ATP ratio, which can directly activate AMPK. This process can transform cells from anabolic to catabolic mode, regulate energy metabolism by promoting glycolysis and fatty acid oxidation, and prevent gluconeogenesis [8,9]. The AMPK/mTOR (mammalian target of rapamycin) signaling pathway plays an important role in cell proliferation, survival, apoptosis, glucose metabolism, gene transcription, and cell migration [10,11]. Animal experiments have confirmed that metformin influences the formation of beta amyloid protein in dementia model rat neural cells, reduces apoptosis of neurons in rats, and improve memory function of dementia rats through the AMPK/mTOR signaling pathway. Metformin can reduce the area of cerebral infarction and reduce neuronal apoptosis in rats with cerebral ischemia, which is related to the AMPK signaling pathway and the decreased level of oxidative stress [12,13].
The aim of this study was to investigate the potential neuroprotective effects of metformin and explore the possible mechanisms involved in the primary fetal rat hippocampal neurons deprived of oxygen and glucose (in vitro model of stroke). We also investigated the effect of metformin on the functional level of patients following acute stroke combined with type 2 diabetes.
Clinical Data
Research objects
From January 2014 to December 2016, 80 cases of newly diagnosed acute stroke combined with type 2 diabetes mellitus in our hospital were enrolled in the study, including 46 males and 34 females, with an average age of 58.2±14.6 years. They were randomly divided into 2 groups – a metformin combined with insulin treatment group and an insulin treatment group – with 40 cases in each group. There was no significant difference in gender, age, time from onset to visiting, or number of coronary heart disease cases in the 2 groups before treatment (P>0.05).
Criteria for the admission of cases: (1) all patients were diagnosed with acute ischemic cerebral stroke according to the “China Guidelines for Cerebral Vascular Disease”; (2) patients with first acute stroke were diagnosed by head CT or MRI; (3) patients were diagnosed with type 2 diabetes, with fasting blood glucose ≥7 mmol/L or random blood glucose ≥11.1mmol/L; (4) patients were in stable condition, without serious complications such as liver or kidney dysfunction, myocardial infarction, heart failure, respiratory failure, or pulmonary infection; (5) no cerebral hemorrhage; and (6) the patient and family members volunteered to participate in this study, and the compliance was good. This study was approved by the Ethics Committee of our institute, and all patients signed informed consent before treatment.
Other exclusion factors included presence of: (1) severe liver, cardiovascular, hematopoietic system, urinary system diseases, or tumors; (2) acute or chronic infections; and (3) mental disorders and other incompatible patients.
Other exclusion factors included presence of acute or chronic infection, severe cardiovascular and cerebrovascular events, and other serious complications or adverse reactions during the study period. Subjects were free to withdraw from this study at any time.
Material and methods
All patients with acute stroke were immediately treatment with standard treatment after hospitalization, including antiplatelet, anticoagulation, thrombolysis, control of blood pressure, lipid, dehydration reducing intracranial pressure, protection of brain cells, and nerve nutrition, and dietary therapy was carried out according to the diabetes diet. The insulin group was treatment with insulin aspart (NovoRapid R, Novo Nordisk Pharmaceutical, China) subcutaneous injection before the meal, combined with Isophane Protamine Biosynthetic Human Insulin Injection before bedtime (Novolin N, Novo Nordisk Pharmaceutical, China). In the metformin combination treatment group, we added metformin on the basis of the treatment plan of the control group, at 500 mg 3 times a day (Gehua Zhi, Sino-US Shanghai Squibb Pharmaceutical Co.). The dosage of insulin and metformin was adjusted according to the levels of blood glucose. The duration of treatment was 2 weeks.
Assessments
The following indexes were measured at the end of the 2 weeks before and after the treatment: (1) the nerve function index: Comprehensive assessment was based on the Mini-Mental State Examination (MMSE), National Institutes of Health Stroke Scale (NIHSS), and daily life ability scale (Activities of Daily Living, ADL). (2) Oxidative stress related indicators: Microplate method for the detection of malondialdehyde (MDA), colorimetric method for measuring glutathione peroxidase (GSH-PX) and superoxide dismutase (SOD), the malondialdehyde test kit (A003), the glutathione peroxidase test kit (A005), and the superoxide dismutase kit (A001) were purchased from Nanjing Jiancheng Biotechnology Co. and used strictly in accordance with the instructions.
Basic Research
Materials and methods
In the animal experiment, SD rats pregnant for 17 days were purchased from Beijing Weitong Lihua Co.
Main reagents were: DMEM medium, Neurobasal medium, fetal bovine serum (FBS), horse serum, B27 medium additive, pancreatin, penicillin, and streptomycin were purchased from Gibco USA. 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT), poly-L-lysine, DMSO, bovine serum albumin, metformin and TUNEL kit were purchased from Sigma USA. B cell lymphoma/leukemia protein -2 gene (Bcl-2), Bcl-2-associated X protein gene protein (Bcl-2 X), AMPK, phosphorylation pAMPK, rapamycin (mammalian target of rapamycin, mTOR) and rabbit polyclonal antibody were purchased from the Abcam USA. Rat polyclonal antibody of β-actin, second antibody of goat anti-rabbit and goat anti-mouse were bought from Zhonghua Jinqiao Co., China. Caspase-3 activity assay kits and protein assay kits were purchased from Beijing Pulilai Company, China. ECL chemiluminescence kits were purchased from Millipore USA. The MDA and SOD kits were purchased from the Nanjing Jiancheng Bioengineering Institute in China.
Primary neuronal cell culture
Animal experiments were approved by the local animal ethics committee. The primary fetal rat hippocampal neurons were derived from the embryos of 17-days pregnant SD rats. An anatomic microscope was used to dissect the hippocampus of rats and put it into the Hank ‘s balanced salt solution. The primary fetal rat hippocampal tissue was digested with 0.25% trypsin at 37°C for 18 min, then oscillated 2~3 times. The digestion phase was stopped by DMEM culture solution containing 10% FBS. The cell suspension was then obtained by repeating aspirations through a Pasteur pipette following centrifugation at 1500×g for 5 min. The cells were cultured in plates coated with poly-L-lysine (0.1 g/L) with a density of 1×105/mL. The cultures were maintained in a humidified incubator with 5% CO2 at 37°C. Penicillin 100U/mL, streptomycin 100U/mL, 10% FBS, and 10% horse serum were added into the culture solution. After 24 h, the total amount of liquid was changed into Neurobasal+B27 serum-free culture system (sugar concentration 25 mmol/L), and this medium was subsequently half-changed every 3 days. The neuronal cells were used for experiment at 7 days.
The primary fetal rat hippocampal neurons, gradually matured after 7 days of culture, were used for cell experiments. The experimental group was divided into the control group (Con), the oxygen-glucose deprivation model group (Mod), and the metformin group (Met). On the seventh day of cell culture, metformin was diluted with culture medium, and for the metformin group we added metformin with the final concentration of 10 mmol/L, while in the control group and model group we added equal volumes of culture medium. The model group and the metformin group were then subjected to oxygen-glucose deprivation for 1 h, but the control group was not.
Oxygen-glucose deprivation treatment of primary fetal rat hippocampal neuron cells
The media in both model and metformin groups were carefully removed, washed with PBS 3 times, and transferred into glucose-free DMEM medium, then the cultured cells were transferred to a hypoxic incubator chamber containing 5% CO2 and 95% N2 at 37°C for 1 h. After the medium was removed, neurons were exposed to normal growth conditions at 37°C in a humidified incubator with 5% CO2 and 95% air for an additional 24 h.
Morphological study of hippocampal neurons
The cell growth was observed under a microscope (Leica, Germany).
MTT detection of survival rate
Neuronal cells activity was measured using the MTT method. We added 0.5 mg/mL MTT to each well and incubated for 4 h, then the liquid was discarded. 150μL DMSO was added to each well, and was shocked for 10 min. The enzyme absorbance (OD) was measured at 570 nm. The cell survival rate=(the average value of OD in the experimental group/the mean value of the OD in the control group)×100%.
TUNEL detection of apoptosis rate
The primary fetal rat hippocampal neuronal cells were placed 24-well clusters with a density of 5×104 cells/well, with coverslip coated by poly-L-lysine in the bottom for drug treatment and the treatment of oxygen-glucose deprivation. TUNEL and PI staining were carried out according to the TUNEL kit instructions. After PBS scrubbing, the morphology of the cells was observed by fluorescence microscope. Cells were counted in 20 fields of vision, selected randomly. We estimated the percentage of apoptotic cells using the following ratio: number of apoptotic cells/total cell number×100%.
Western blot assay of proteins related to apoptotic pathway (AMPK/pAMPK/mTOR/BAX/Bcl-2)
The primary fetal rat hippocampal neurons cells were placed a culture flask, and after the treatment with drugs and oxygen-glucose deprivation, cells were collected and trypsinized. The protein quantitative kit was used for protein quantification, and equivalent amounts of protein were separated on 12% SDS-polyacrylamide gels and transferred to PVDF membranes. Membranes were incubated with TBS containing 5% nonfat dry milk to block nonspecific binding overnight at 4°C, and were incubated with antibodies against BAX, BCL-2, AMPK, pAMPK, and mTOR at room temperature for 2 h. After being washed with TBST 3 times for 10 min each time, membranes were incubated with secondary antibodies at room temperature for 2 h. Membranes were washed with TBST 3 times for 15 min each time. The membranes were subsequently incubated for 2–3 min by adding ECL chemical luminescent working solution, and exposed.
Oxidative stress response markers (MDA, SOD) evaluation
The primary fetal rat hippocampal neurons cells were placed 6-well plates. After the treatment of drugs and oxygen-glucose deprivation, the cells were digested by trypsin. The cells were broken by ultrasound (300W, broken 25 s, and intermittent 25 s). After centrifugation and collection of supernatants, the MDA and SOD were detected by the kit provided by Nanjing Institute of Bioengineering using a blood gas analyzer (Analog Devices, Inc., USA), and the protein content was determined by Bradford method.
Statistical analysis
Data processing was performed using SPSS 18.0 statistical software. The statistical data with normal distribution are expressed as mean ±SD. Comparison before and after treatment and the comparison between the 2 groups were performed by t test. The normally distributed data were subjected to multiple comparisons by Tukey’s method in one-way ANOVA. P<0.05 was considered statistically significant (the confidence level was set up at 95%), and P<0.01 was statistically significant (the confidence level was set up at 99%).
Results
Neurological assessment of patients
We estimated the effect of metformin on neurological function in patients with acute stroke complicated with type 2 diabetes. As shown in Table 1, there was no significant difference between the 2 groups before treatment in MMSE, NIHSS, and ADL scores (P>0.05). After 2 weeks of treatment, the MMSE and ADL scores of the patients in the 2 groups increased significantly, while the NIHSS scores decreased significantly. Compared with those before treatment, the difference between the 2 groups was statistically significant (P<0.05). Moreover, when comparing the metformin combined treatment group with the insulin group, MMSE and ADL were increased and NIHSS was decreased significantly (P<0.05).
Table 1.
Effect of metformin on oxidative stress in patients with acute stroke complicated with type 2 diabetes (n=40).
| Group | MMSE score | NIHSS score | ADL score | |||
|---|---|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| Metformin combination treatment group | 22.18±1.93 | 25.16±2.16ab | 19.66±8.48 | 7.4±4.72ab | 26.45±9.19 | 63.86±12.20ab |
| Insulin group | 22.21±1.96 | 23.15±1.95a | 19.72±7.92 | 10.6±5.62a | 26.82±10.28 | 44.15±13.27a |
| t value | 0.687 | 8.116 | 0.839 | 6.525 | 0.718 | 8.382 |
| P value | 0.465 | <0.001 | 0.341 | <0.001 | 0.432 | <0.001 |
Compared with before treatment, P<0.05, the difference was statistically significant;
compared with the control group, P<0.05, the difference was statistically significant.
Oxidative stress index in patients
We estimated the effect of metformin on oxidative stress in patients with acute stroke complicated with type 2 diabetes. According to Table 2, there was no significant difference in malondialdehyde (MDA), glutathione peroxidase (GSH-PX) and superoxide dismutase (SOD) between the 2 groups before treatment (P>0.05). After 2 weeks of treatment in patients of the 2 groups, malondialdehyde (MDA) decreased significantly, glutathione peroxidase (GSH-PX) and superoxide dismutase (SOD) increased significantly, compared with before treatment (P<0.05). Compared to the insulin group, the levels of MDA, SOD, and GSH-PX significantly decreased in the metformin group.
Table 2.
Effect of metformin on oxidative stress in patients with acute cerebral stroke and type 2 diabetes (n=40).
| Group | MDA (μmol/L) | SOD (U/L) | GSH-PX (U/L) | |||
|---|---|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| Metformin combination treatment group | 4.95±0.96 | 2.23±0.74ab | 27.46±9.18 | 36.72±9.92ab | 155.4±24.7 | 188.2±20.1ab |
| Insulin group | 4.97±0.87 | 3.29±0.83a | 27.32±8.92 | 31.81±8.97a | 153.6±26.8 | 164.3±27.8a |
| t value | 1.122 | 5.721 | 0.542 | 7.246 | 0.361 | 7.324 |
| P value | 0.286 | <0.001 | 0.637 | <0.001 | 0. 794 | <0.001 |
Compared with before treatment, P<0.05, the difference was statistically significant;
compared with the control group, P<0.05, the difference was statistically significant.
The effect of metformin on hippocampal neurons morphology
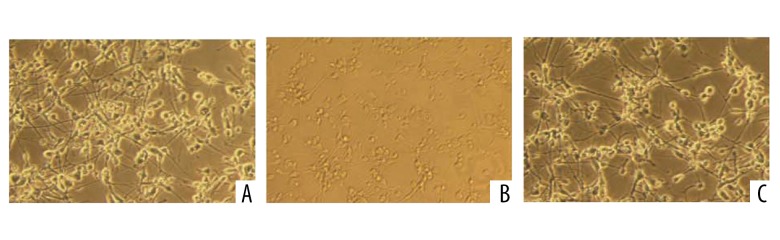
The effect of metformin on the morphology of the primary fetal rat hippocampal neurons in oxygen deprivation was observed. In the oxygen-glucose deprivation model group, many of the primary fetal rat hippocampal neurons showed apoptosis, necrosis, cell contraction, and limited growth. The metformin group had partial necrosis and apoptosis of the primary fetal rat hippocampal neuron cells, which was lower than in the oxygen-glucose deprivation model group (Figure 1).
Figure 1.
The morphological observation of the primary fetal rat hippocampal neurons in each group (×200). (A: Control group; B: Model group; C: Metformin group).
The effect of metformin on survival rate in the hippocampal cell culture
MTT assay was used to observe the effect of metformin on the survival rate of the primary fetal rat hippocampal neurons induced by oxygen-glucose deprivation. The survival rate of the primary fetal rat hippocampal neurons in the control group was 100%, while the survival rate of the primary fetal rat hippocampal neurons in the oxygen-glucose deprivation group was 38.7±5.24%, and the survival rate in the metformin group was 72.3±7.26%. The survival rate of the oxygen-glucose deprivation group was significantly lower than that of the control group (P<0.01). The survival rate of the metformin group was significantly higher than that of the oxygen-glucose deprivation group (P<0.01) (Figure 2).
Figure 2.

The effect of metformin on the survival rate of the primary fetal rat hippocampal neurons under oxygen-glucose deprivation. (** P<0.01 compared with the control group, there was a statistically significant difference; ## P<0.01 compared with the model group, the difference was statistically significant).
The effect of metformin on the apoptosis rate in the hippocampal cell culture
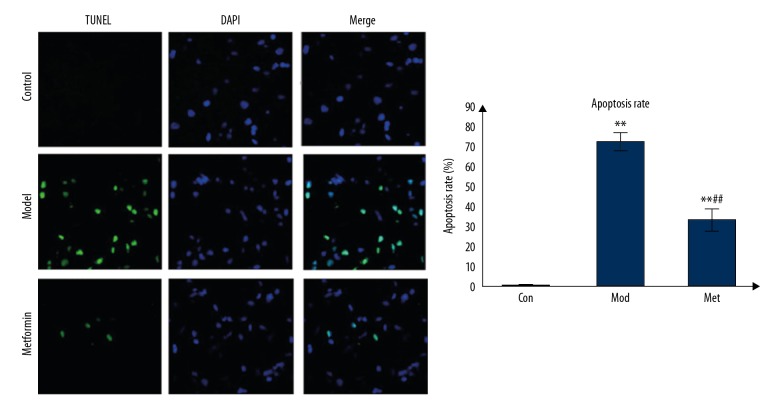
The effect of metformin on the apoptosis rate of the primary fetal rat hippocampal neurons in oxygen-glucose deprivation was observed by TUNEL staining. The TUNEL-positive cells were stained green, DAPI staining was blue, and the number of TUNEL-positive cells divided by the number of DAPI staining was the rate of apoptosis. The apoptosis rate of the control group was 1.1±0.3%, the rate of apoptosis in the model group was 72±4.8%, and the rate of apoptosis in the metformin group was 33±5.9%. The apoptotic rate in the model group was significantly higher than that in the control group (P<0.01), and the apoptosis rate in metformin group was significantly lower than that in the model group (P<0.01) (Figure 3).
Figure 3.
Effect of metformin on the apoptotic rate of the primary fetal rat hippocampal neurons under oxygen-glucose deprivation. (TUNEL-stained green cells showed apoptosis-positive cells, DAPI-stained blue as nucleated cells, Merge stained with TUNEL and DAPI. Apoptosis rate comparison: ** P<0.01 compared with control group, the difference was statistically significant; ## compared with the model group P<0.01, the difference was statistically significant).
The effect of metformin on apoptotic pathway in the hippocampal cell culture
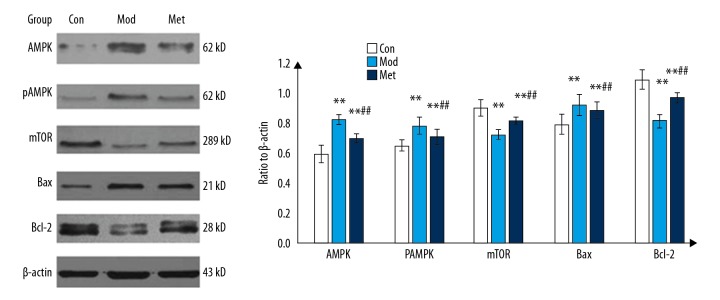
The effect of metformin on the AMPK/mTOR signaling pathway and apoptotic protein in the primary fetal rat hippocampal neurons induced by oxygen-glucose deprivation was observed. The expression of AMPK, pAMPK, and Bax in the model group increased significantly compared with the control group, and the expression of mTOR and Bcl-2 in the model group was significantly lower than that in the control group (P<0.05). The expression of AMPK, pAMPK, and Bax in metformin group was significantly lower than that in the model group, and the expression of mTOR and Bcl-2 in the metformin group was significantly higher than that in the model group (P<0.05) (Figure 4).
Figure 4.
Effect of metformin on the expression of protein in the primary fetal rat hippocampal neurons under oxygen-glucose deprivation. (** P<0.01 compared with the control group, the difference was statistically significant; ## compared with the model group P<0.01, the difference was statistically significant).
The effect of metformin on oxidative stress response in the hippocampal cell culture
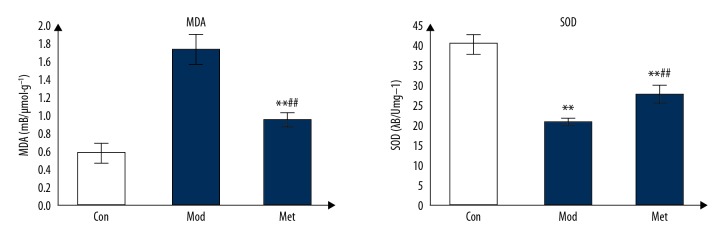
The MDA content in the model group was significantly higher than that in the control group (P<0.05). The MDA content in metformin group was significantly lower than that in the model group (P<0.05). SOD activity in the model group was significantly lower than that in the control group (P<0.05). The SOD activity in metformin group was significantly higher than that in the model group (P<0.05) (Figure 5).
Figure 5.
Effect of metformin on MDA and SOD in the primary fetal rat hippocampal neurons under oxygen-glucose deprivation. (** P<0.01 compared with the control group, the difference was statistically significant; ## compared with the model group P<0.01, the difference was statistically significant).
Discussion
Metformin is a clinically common first-line agent for the treatment of type 2 diabetes, which can reduce insulin resistance, reduce liver glycogen formation, promote the use of peripheral glucose, and then effectively control blood glucose levels. A number of epidemiological studies have shown that long-term metformin treatment in type 2 diabetes can reduce the risk and the severity of cerebrovascular disease [5,6,14–16]. A number of basic studies have shown that the use of metformin to pre-treat cerebral ischemia in rats can reduce the area of cerebral infarction, reduce neuronal apoptosis, and improve neurological deficits [14,17]. The use of metformin after cerebral ischemia in rats can reduce blood-brain barrier damage and improve cognitive function [12,13]. The present study shows that, compared with insulin alone, patients with type 2 diabetes treatment with metformin combined with insulin treatment had significantly higher MMSE and ADL scores, but NIHSS scores decreased significantly, which shows that metformin can improve the neurological deficit and improve cognitive function in patients with acute stroke with type 2 diabetes.
To demonstrate the mechanism underlying the neuroprotective effects of metformin, fetal rat hippocampal neuronal cell culture was used to study the molecular mechanism. Oxygen-glucose deprivation of the primary fetal rat hippocampal neurons can mimic the process of ischemic stroke at the cellular level [18], and our study showed that the apoptotic rate of the primary fetal rat hippocampal neurons in the metformin group was significantly lower than that in the oxygen-glucose deprivation model group, and the survival rate was significantly increased (P<0.01), indicating that metformin has neuroprotective effects on oxygen deprived primary fetal rat hippocampal neurons.
In patients with acute brain injury due to cerebral ischemia and hypoxia, the brain tissue exhibits a high level of oxidative stress, which induces the production of free radicals and inflammatory factors, further aggravating the damage of brain tissue and nerve cells [19,20]. MDA, SOD, and GSH-PX are all markers of oxidative stress, and MDA is the end-product of lipid peroxidation. SOD is a metalloenzyme that scavenges oxygen free radicals, GSH-PX is an antioxidant that blocks the cascade of oxidative stress systems and scavenges oxygen free radicals, MDA, and SOD, and GSH-PX reflects the level of oxidative stress in the body [2,21,22]. The clinical trials in this study showed that serum MDA levels were significantly decreased in patients with acute stroke treated with metformin combined with insulin, and SOD activity and GSH-PX levels were significantly increased; the basic experiment also reached the same conclusion. This indicates that metformin can improve neurological function by reducing the level of oxidative stress.
AMPK is a serine/threonine protein kinase that acts as an energy receptor and is involved in many physiological processes such as cell growth, metabolism, and apoptosis. Research shows that metformin can reduce brain tissue inflammation through the AMPK pathway, and reduce the production of inflammatory cytokines, thereby reducing damage to the blood-brain barrier and promoting nerve repair [14,23–25]. The present study shows that metformin can improve apoptosis of the primary fetal rat hippocampal neurons induced by oxygen-glucose deprivation. After metformin treatment, the expression of AMPK, pAMPK, and Bax was significantly decreased, and the expression of mTOR and Bcl-2 was significantly increased. This suggests that metformin inhibits apoptosis of the primary fetal rat hippocampal neurons through the AMPK/mTOR signaling pathway, and then plays a neuroprotective role. This study agrees with other studies [5] showing that metformin has neuroprotective effects on stroke in rats, and may be related to oxidative stress, endothelial nitric oxide synthase activation, angiogenesis, and autophagy [26–29].
Metformin may be able to promote angiogenesis and neuronal regeneration in the brain. Metformin was used to intervene in the model of cerebral ischemia in mice, and the density of neovascularization in the cerebral ischemic area was significantly increased, and the neuronal cells showed obvious regeneration, which actively promoted nerve repair [26–32]. The present study demonstrates that metformin may play a neuroprotective role through the AMPK/mTOR signaling pathway and oxidative stress. However, further studies on animal vascular regeneration, nerve cell regeneration, and other possible mechanisms are needed.
Conclusions
In summary, metformin can improve neurological function and oxidative stress in patients with acute stroke and type 2 diabetes, and its mechanism may be related to the AMPK/mTOR signaling pathway and oxidative stress.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Bettger JP, Li Z, Xian Y, et al. CNSR II investigators. Assessment and provision of rehabilitation among patients hospitalized with acute ischemic stroke in China: Findings from the China National Stroke Registry II. Int J Stroke. 2017;12(3):254–63. doi: 10.1177/1747493017701945. [DOI] [PubMed] [Google Scholar]
- 2.Narne P, Pandey V, Phanithi PB. Interplay between mitochondrial metabolism and oxidative stress in ischemic stroke: An epigenetic connection. Mol Cell Neurosci. 2017;82:176–94. doi: 10.1016/j.mcn.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Newton DF, Naiberg MR, Goldstein BI. Oxidative stress and cognition amongst adults without dementia or stroke: Implications for mechanistic and therapeutic research in psychiatric disorders. Psychiatry Res. 2015;227(2–3):127–34. doi: 10.1016/j.psychres.2015.03.038. [DOI] [PubMed] [Google Scholar]
- 4.Kaisar MA, Villalba H, Prasad S, et al. Offsetting the impact of smoking and e-cigarette vaping on the cerebrovascular system and stroke injury: Is Metformin a viable countermeasure? Redox Biol. 2017;13:353–62. doi: 10.1016/j.redox.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arbelaez-Quintero I, Palacios M. To use or not to use metformin in cerebral ischemia: A review of the application of metformin in stroke rodents. Stroke Res Treat. 2017;2017 doi: 10.1155/2017/9756429. 9756429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mima Y, Kuwashiro T, Yasaka M, et al. Impact of metformin on the severity and outcomes of acute ischemic stroke in patients with type 2 diabetes mellitus. J Stroke Cerebrovasc Dis. 2016;25(2):436–46. doi: 10.1016/j.jstrokecerebrovasdis.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Osei E, Fonville S, Zandbergen AA, et al. Metformin and sitAgliptin in patients with impAired glucose tolerance and a recent TIA or minor ischemic Stroke (MAAS): Study protocol for a randomized controlled trial. Trials. 2015;16:332. doi: 10.1186/s13063-015-0882-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams T, Courchet J, Viollet B, et al. AMP-activated protein kinase (AMPK) activity is not required for neuronal development but regulates axogenesis during metabolic stress. Proc Natl Acad Sci USA. 2011;108(14):5849–54. doi: 10.1073/pnas.1013660108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheng B, Liu J, Li GH. Metformin preconditioning protects Daphnia pulex from lethal hypoxic insult involving AMPK, HIF and mTOR signaling. Comp Biochem Physiol B Biochem Mol Biol. 2012;163(1):51–58. doi: 10.1016/j.cbpb.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Lv B, Li F, Han J, Fang J, et al. Hif-1alpha overexpression improves transplanted bone mesenchymal stem cells survival in rat MCAO stroke model. Front Mol Neurosci. 2017;10:80. doi: 10.3389/fnmol.2017.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu L, Huang L, Cao C, et al. Inhibition of AMP-activated protein kinase alleviates focal cerebral ischemia injury in mice: Interference with mTOR and autophagy. Brain Res. 2016;1650:103–11. doi: 10.1016/j.brainres.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 12.Venna VR, Li J, Hammond MD, et al. Chronic metformin treatment improves post-stroke angiogenesis and recovery after experimental stroke. Eur J Neurosci. 2014;39(12):2129–38. doi: 10.1111/ejn.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin Q, Cheng J, Liu Y, et al. Improvement of functional recovery by chronic metformin treatment is associated with enhanced alternative activation of microglia/macrophages and increased angiogenesis and neurogenesis following experimental stroke. Brain Behav Immun. 2014;40:131–42. doi: 10.1016/j.bbi.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Deng T, Zheng YR, Hou WW, et al. Pre-stroke metformin treatment is neuroprotective involving AMPK reduction. Neurochem Res. 2016;41(10):2719–27. doi: 10.1007/s11064-016-1988-8. [DOI] [PubMed] [Google Scholar]
- 15.Floyd JS, Wiggins KL, Sitlani CM, et al. Case-control study of second-line therapies for type 2 diabetes in combination with metformin and the comparative risks of myocardial infarction and stroke. Diabetes Obes Metab. 2015;17(12):1194–97. doi: 10.1111/dom.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.den Hertog HM, Vermeer SE, Zandbergen AA, et al. Safety and feasibiLIty of Metformin in patients with Impaired glucose Tolerance and a recent TIA or minor ischemic stroke (LIMIT) trial – a multicenter, randomized, open-label phase II trial. Int J Stroke. 2015;10(1):105–9. doi: 10.1111/ijs.12023. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Benashski SE, Venna VR, Mccullough LD. Effects of metformin in experimental stroke. Stroke. 2010;41(11):2645–52. doi: 10.1161/STROKEAHA.110.589697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meloni BP, Milani D, Edwards AB, et al. Neuroprotective peptides fused to arginine-rich cell penetrating peptides: Neuroprotective mechanism likely mediated by peptide endocytic properties. Pharmacol Ther. 2015;153:36–54. doi: 10.1016/j.pharmthera.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Zhang R, Xu M, Wang Y, et al. Nrf2-a promising therapeutic target for defensing against oxidative stress in stroke. Mol Neurobiol. 2017;54(8):6006–17. doi: 10.1007/s12035-016-0111-0. [DOI] [PubMed] [Google Scholar]
- 20.Weaver J, Liu KJ. Does normobaric hyperoxia increase oxidative stress in acute ischemic stroke? A critical review of the literature. Med Gas Res. 2015;5:11. doi: 10.1186/s13618-015-0032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodrigo R, Fernandez-Gajardo R, Gutierrez R, et al. Oxidative stress and pathophysiology of ischemic stroke: Novel therapeutic opportunities. CNS Neurol Disord Drug Targets. 2013;12(5):698–714. doi: 10.2174/1871527311312050015. [DOI] [PubMed] [Google Scholar]
- 22.Pradeep H, Diya JB, Shashikumar S, Rajanikant GK. Oxidative stress – assassin behind the ischemic stroke. Folia Neuropathol. 2012;50(3):219–30. doi: 10.5114/fn.2012.30522. [DOI] [PubMed] [Google Scholar]
- 23.Jia J, Cheng J, Ni J, Zhen X. Neuropharmacological actions of metformin in stroke. Curr Neuropharmacol. 2015;13(3):389–94. doi: 10.2174/1570159X13666150205143555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dadwal P, Mahmud N, Sinai L, et al. Activating endogenous neural precursor cells using metformin leads to neural repair and functional recovery in a model of childhood brain injury. Stem Cell Rep. 2015;5(2):166–73. doi: 10.1016/j.stemcr.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patil SP, Jain PD, Ghumatkar PJ, et al. Neuroprotective effect of metformin in MPTP-induced Parkinson’s disease in mice. Neuroscience. 2014;277:747–54. doi: 10.1016/j.neuroscience.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 26.Fidaleo M, Cavallucci V, Pani G. Nutrients, neurogenesis and brain ageing: From disease mechanisms to therapeutic opportunities. Biochem Pharmacol. 2017;141:63–76. doi: 10.1016/j.bcp.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 27.Smieszek A, Strek Z, Kornicka K, et al. Antioxidant and anti-senescence effect of metformin on mouse olfactory ensheathing cells (mOECs) may be associated with increased brain-derived neurotrophic factor levels-an ex vivo study. Int J Mol Sci. 2017;18(4) doi: 10.3390/ijms18040872. pii: E872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed S, Mahmood Z, Javed A, et al. Effect of metformin on adult hippocampal neurogenesis: Comparison with donepezil and links to cognition. J Mol Neurosci. 2017;62(1):88–98. doi: 10.1007/s12031-017-0915-z. [DOI] [PubMed] [Google Scholar]
- 29.Porceddu PF, Ishola IO, Contu L, Morelli M. Metformin prevented dopaminergic neurotoxicity induced by 3,4-methylenedioxymethamphetamine administration. Neurotox Res. 2016;30(1):101–9. doi: 10.1007/s12640-016-9633-5. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Tang G, Zhang Z, et al. Metformin promotes focal angiogenesis and neurogenesis in mice following middle cerebral artery occlusion. Neurosci Lett. 2014;579:46–51. doi: 10.1016/j.neulet.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Gallagher D, Devito LM, et al. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell. 2012;11(1):23–35. doi: 10.1016/j.stem.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 32.Potts MB, Lim DA. An old drug for new ideas: Metformin promotes adult neurogenesis and spatial memory formation. Cell Stem Cell. 2012;11(1):5–6. doi: 10.1016/j.stem.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]