Abstract
Background
Doxorubicin (DOX) is a potent chemotherapeutic agent used to treat colon cancer. Despite impressive initial clinical responses, drug resistance has dramatically compromised the effectiveness of DOX. However, the underlying mechanisms of chemotherapeutic resistance in colon cancer remain poorly understood.
Material/Methods
In this study, we compared the expression of miR-222-3p in DOX-resistant colon cancer cells (LoVo/ADR) with the corresponding DOX-sensitive parental cells (LoVo/S) using quantitative real-time PCR. In addition, miR-222-3p inhibitors were infected into LoVo/ADR cell lines and the effects of this treatment were assessed. The Cell Counting Kit 8 assay was employed to verify the sensitivity of colon cancer cell lines to DOX. EdU (5-ethynyl-2′-deoxyuridine) assay, flow cytometry, and in vivo subcutaneous tumorigenesis were used to assess cell proliferation and apoptosis. Transwell and wound healing assays were used to investigate cell migration after adding DOX. Additionally, the expression of forkhead box protein P2 (FOXP2), P-glycoprotein (P-gp) and caspase pathway-associated markers was assessed by western blotting.
Results
Our results showed that miR-222-3p was upregulated in LoVo/ADR compared with the expression in LoVo/S cells. Additionally, downregulation of miR-222-3p in LoVo/ADR cells increased their sensitivity to DOX, reduced P-gp expression, and activated the caspase pathway. However, the downregulation of FOXP2 could efficiently reverse the effect of miR-222-3p inhibitors on LoVo/ADR cells.
Conclusions
Taken together, our results showed that miR-222-3p induced DOX resistance via suppressing FOXP2, upregulating P-gp, and inhibiting the caspase pathway.
MeSH Keywords: Caspases, Colonic Neoplasms, Drug Resistance, MicroRNAs, P-Glycoprotein
Background
Colon cancer is a common malignant tumor, and the resultant mortality rate is the third highest of that of all cancers [1]. Despite continuous improvements in cancer-fighting strategies, chemotherapy remains the preferred treatment with prolonged patient survival [2]. However, tumor cells inevitably become resistant to chemotherapeutic drugs, due to the triggering of inner self-protection mechanism referred to as multidrug resistance (MDR) [3]. DOX has emerged as a vital chemotherapeutic agent in the treatment of colon cancer as it inhibits RNA and DNA synthesis in colon cancer [4]. MDR, whether inherent or acquired, has become the greatest challenge for effective systemic therapy in colon cancer [5,6]. When dealing with MDR, clinicians usually choose to increase the dose of drugs to increase topical drug concentration in the cancer region. However, this approach can lead to systemic toxicity [7]. Therefore, it is of great importance to improve the efficiency of drugs used to treat cancer by inhibiting mechanisms of MDR.
MicroRNAs (miRNAs) are endogenous non-coding single-strand RNAs that negatively regulate gene expression through binding to their 3′-untranslated regions (3′ UTRs) [8]. Key miRNAs involved in biological processes, such as proliferation, apoptosis, invasion, metastasis, and drug resistance are dysregulated in various cancers [9,10]. It is known that miR-222-3p plays a pivotal role in tumorigenesis in a diverse range of cancers including those of the pancreas, liver, stomach, and breast cancer [11–14]. Through regulating the expression of downstream DOX targets, such as PTEN, p27, BCL-2, Bax, and BIM, miR-222-3p could promote the progression of MDR [15,16].
However, little is known about the role of miR-222-3p in circumventing MDR in colon cancer. In this study, we investigated miR-222-3p expression in DOX-sensitive (LoVo/S) cells and DOX-resistant (LoVo/ADR) cells. We also explored the function of miR-222-3p in DOX resistance and potential causative mechanisms in vitro and in vivo.
Material and Methods
Cell culture
The LoVo/S human colon cancer cell line was obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). LoVo/S cells were exposed to DOX (Meilun, Dalian, China) at an initial concentration of 0.025 mg/L. Subsequently, surviving cells, which were tolerant to DOX, were selected and cultured in DOX at twice the previous concentration. This procedure was repeatedly applied to the cells until they could grow well in the presence of 1.0 mg/L DOX, at which time LoVo/ADR (resistant) cells were successfully established. The LoVo/ADR cells were continuously exposed to DOX for maintenance of resistance. The cells were cultured in high-sugar DMEM (HyClone, Logan, UT, USA) with 10% fetal bovine serum (FBS; Gibco, Carlsbad, CA, USA), 8 mg/mL penicillin, and 8000 U/mL streptomycin at 37°C in a humidified 5% CO2 incubator.
RNA infection
To establish cell lines with stable knockdown, lentiviruses expressing miR-222-3p inhibitors against miR-222-3p and the negative control (NC cells) sequence were constructed from Genechem Co., Ltd. (Shanghai, China). LoVo/ADR cells were infected with a mixture of the lentiviruses (multiplicity of infection, 40) and 5 μg/mL polybrene. After 72 hours, the infected cells (refer to as LoVo/ADR + miR-222-3p inhibitors cells) were used for further analysis. Meanwhile, lentiviruses expressing siRNAs (small interfering RNAs) against forkhead box protein P2 (FOXP2) (5′-AACTATGAATTTTATAAAAATGC-3′) were also utilized in LoVo/ADR + miR-222-3p inhibitors cells, to establish FOXP2-inhibited cells (referred to as LoVo/ADR + miR-222-3p inhibitors+si-FOXP2 cells).
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from cells using RNAiso reagent (Takara, Tokyo, Japan) and then subjected to reverse transcription reaction using the BuSuperScript RT Kit (Biouniquer Technology, Nanjing, China). Then quantitative real-time polymerase chain reaction (qRT-PCR) was performed using the SYBR Premix Ex Taq kit (Takara) following the manufacturer’s instructions. The specific primers for mature miR-222-3p were as follows: 5′-CAGTGCGTGTCGTGGAGT-3′. Relative mRNA expression was quantified as compared to internal control U6 using comparative CT method.
Western blotting
Total proteins from cells were lysed with RIPA buffer (Biouniquer Technology). Lysates were then separated by SDS-PAGE, and proteins were further transferred onto PVDF membranes (Sigma, Germany). After 5% non-fat dry milk blocking and overnight incubation with primary antibodies FOXP2, P-glycoprotein (P-gp), caspase-3, cleaved caspase-3, PARP, cleaved PARP, and GAPDH (1: 1000; Cell Signaling Technology). The blots were detected using appropriate secondary horseradish peroxidase-conjugated secondary antibodies (Sigma) and visualized by enhanced chemiluminescence detection (Millipore Corp., Billerica, MA, USA).
Cell Counting Kit 8 (CCK-8) assay
Cells were cultured in 96-well plates at a density of 3000 cells/well for 24 hours. Then, the cells were treated with different concentrations of DOX (64, 32, 16, 8, 4, 2, or 1 μg/mL). Cells were quantified after 48 hours using the Cell Counting Kit 8 (CCK-8) assay (Donjindo, Kumamoto, Japan) and a microplate reader at the absorbance of 450 nm.
5-ethynyl-2′-deoxyuridine (EdU) assay
Cells were seeded into 6-well plates at a density of 1×106 cells/well. After 24 hours, the cells were treated with 8 μg/mL DOX for 24 hours and cultured for another 24 hours, and subjected to the EdU (5-ethynyl-2′-deoxyuridine) assay (Ruibo, Guangzhou, China).
Wound closure assay
Cells were seeded into 6-well plates and cultured to 80–90% confluence. The cell monolayer was then scratched in a straight line with a 200-μL pipette tip to create a wound, and subsequently cultured with 8 μg/mL DOX. Images were taken 0 and 24 hours after the scratch.
Transwell migration assay
Cells were treated with 8 μg/mL DOX for 24 hours. Subsequently, cells were cultured in DMEM supplemented with 10% FBS for another 24 hours. Cells were harvested (5×104) in 200 μL of serum-free medium and added to the top Transwell chambers with 8 μm pores in 24-well plates (Corning Incorporated, Corning, NY, USA). Migration was induced by the 10% FBS medium in the bottom chambers. After 24 hours, the cells were fixed in 4% paraformaldehyde and stained with 0.5% crystal violet. The migrated cells were counted in 5 different fields using an inverted microscope (Nikon, Tokyo, Japan).
Flow cytometry
Cells were seeded into 6-well plates at a density of 1×106 cells per well and cultured for 24 hours. Cells were then treated with either fresh DMEM or 8 μg/mL DOX. After incubation for 24 hours, cells were stained with an Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis kit (Keygen, Nanjing, China). The staining of cells was assessed by flow cytometry.
In addition, to evaluate the interaction of miR-222-3p and FOXP2, the LoVo/ADR cells infected with miR-222-3p inhibitors were again treated with siRNA-FOXP2, before being analyzed by CCK8, EdU assay, and flow cytometry as described.
Dual-luciferase reporter assay
The candidate target genes of miR-222-3p was predicted using TargetScan software program. LoVo/ADR cells were transfected with wild-type or mutant FOXP2-3′UTR region plasmid along with miR-222-3p mimic. A commercial dual luciferase assay kit was used to detect luciferase activity following transfection for 24 hours according to the manufacturer’s instructions.
In vivo anti-tumor assay
Male nude mice were purchased from the Experimental Animal Centre of Southern Medical University and were randomly divided into 3 groups (LoVo/S, LoVo/ADR, and LoVo/ADR + miR-222-3p inhibitor, n=3). To develop the tumor model, cells were injected into the right flank of mice at density of 2×106 cells. Following the successful generation of tumor-bearing mice, DOX (5 mg/kg) was administered via tail vein injection every 2 days. To further test the relationship between FOXP2 and miR-222-3p, another 2 groups (miR-222-3p inhibitors and miR-222-3p inhibitors + si-FOXP2) were utilized. After 21 days, all treated mice were sacrificed with a pentobarbital overdose, and the tumor volume and weight recorded. All animal experiments were performed according to our institution’s guidelines for the use of laboratory animals and were approved by the Institutional Animal Care and Use Committee of Southern Medical University.
Immunohistochemistry
To investigate the expression of apoptosis protein in tumor tissue, a standard 2-step immunohistochemistry was performed. Primary antibodies against cleaved caspase-3 (1: 100) were incubated with sections overnight, and Mayer’s hematoxylin was used for nuclear counter staining.
Statistical analysis
Data were expressed as means ± standard deviation and analyzed by SPSS 22.0 software (SPSS, Chicago, IL, USA). All experiments were repeated at least 3 times with comparable results, unless indicated otherwise. Statistical evaluation of the data was performed using the unpaired Student’s t-test and one-way ANOVA. Differences were considered significant when P<0.05.
Results
miR-222-3p inhibitors partially alter the DOX/ADR-resistance of colon cancer cells
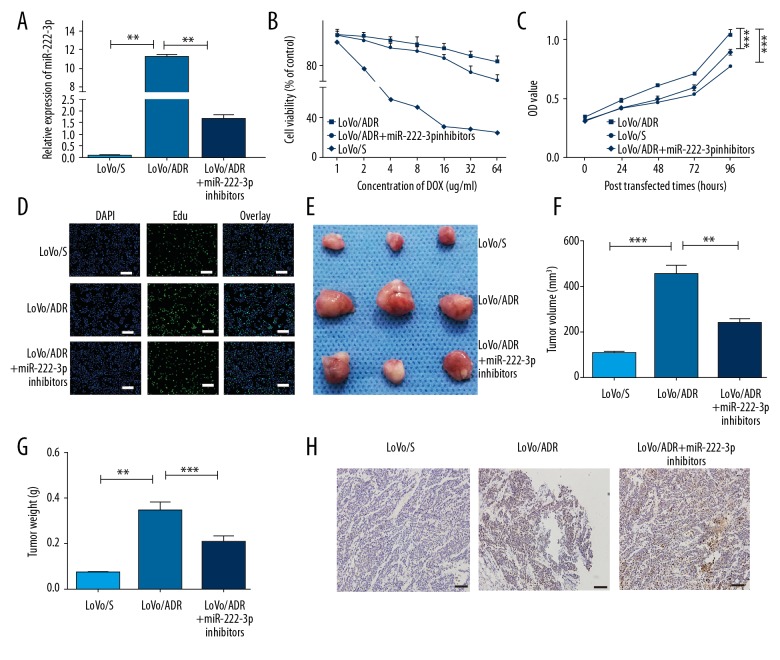
From qRT-PCR assay, we found that miR-222-3p was upregulated in the LoVo/ADR cells compared to the LoVo/S cells (P<0.01), while infection with miR-222-3p inhibitors significantly reduced miR-222-3p expressions (P<0.01) (Figure 1A). Compared to the LoVo/S cells, the LoVo/ADR cells showed enhanced tolerance to DOX, however, this resistance was inhibited after treatment with miR-222-3p inhibitors (Figure 1B, P<0.01). From the CCK8 assay, we found that the proliferative ability of the LoVo/ADR group was significantly higher than that of the LoVo/S group (P<0.001) (Figure 1C). Interestingly, LoVo/ADR cells infected with miR-222-3p inhibitors exhibited markedly reduced proliferative ability compared with those of the LoVo/ADR cells (P<0.001). EdU incorporation assays showed that the number of EdU positive cells were higher in the LoVo/ADR cells than in the LoVo/S cells, but again decreased with treatment of miR-222-3p inhibitors (Figure 1D). Xenografted LoVo/ADR cells showed larger tumor volume and higher tumor weight, than did xenografted LoVo/S cells (Figure 1E–1G). Moreover, the expression of caspase-3, a classic apoptosis marker, showed a similar tendency (Figure 1H). Consistently, proliferation abilities were suppressed by miR-222-3p inhibitors (Figure 1E–1H).
Figure 1.
MiR-222-3p inhibitors increase the sensitivity of LoVo/ADR cells to DOX. (A) The qRT-PCR analysis of miR-222-3p expression in LoVo/S (sensitive) cells, LoVo/ADR (resistant) cells, and LoVo/ADR + miR-222-3p inhibitors cells. (B) CCK8 assay of LoVo/S cells, LoVo/ADR cells, and LoVo/ADR + miR-222-3p inhibitors cells, in the presence of different concentration of DOX. Viability was assessed using the CCK8 assay. (C) OD values of LoVo/S cells, LoVo/ADR cells, and LoVo/ADR + miR-222-3p inhibitors cells. (D) EdU cell proliferation assays of LoVo/S cells, LoVo/ADR cells, and LoVo/ADR + miR-222-3p inhibitors cells. Scar bar, 50 um. (E) Images of tumors derived from the nude mice (LoVo/S, LoVo/ADR, and LoVo/ADR + miR-222-3p inhibitors, n=3). (F) Volume of tumors isolated from the mice. (G) Weight of tumors isolated from the mice. (H) Expression of cleaved caspase-3 in tumors isolated from the mice. Scar bar, 50 um. ** P<0.01, *** P<0.001. DOX – doxorubicin; qRT-PCR – quantitative real-time polymerase chain reaction; CCK8 – Cell Counting Kit 8; OD – optical density; EdU – 5-ethynyl-2′-deoxyuridine.
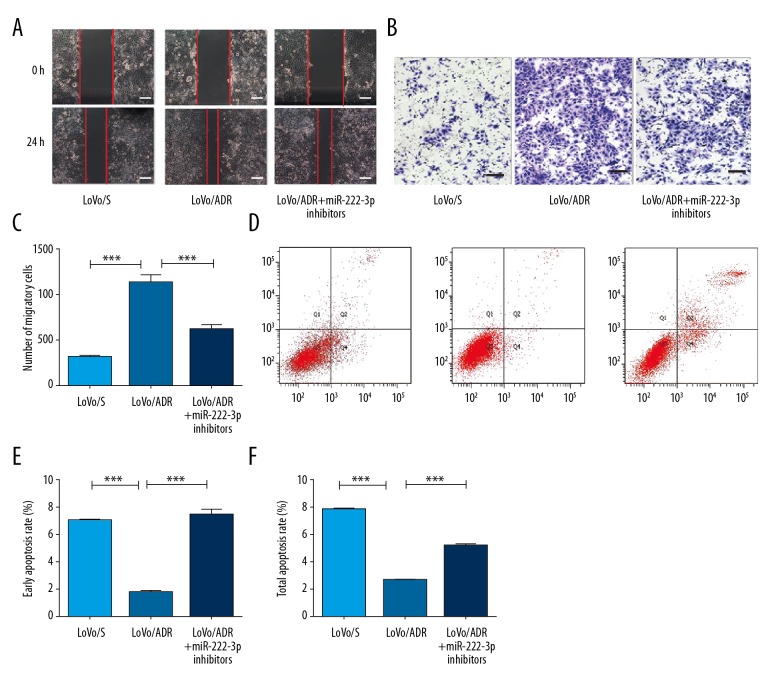
miR-222-3p inhibitors altered the migration and anti-apoptosis abilities of the LoVo/ADR cells
The migration ability of the LoVo/ADR cells was significantly greater than that of the LoVo/S cells (Figure 2A). Following depletion of miR-222-3p, decreased cell migration was observed in the LoVo/ADR + miR-222-3p inhibitor cells, compared to that in the LoVo/ADR cells. Consistently, Transwell migration assays showed a similar tendency (Figure 2B, 2C). The total and early apoptotic rates of the LoVo/ADR cells were significantly increased compared with those of the LoVo/S cells (P<0.001) (Figure 2D–2F), while miR-222-3p inhibitors again significantly decreased the apoptotic rates compared with those of the LoVo/ADR cells (P<0.001) (Figure 2D–2F).
Figure 2.
Depletion of miR-222-3p affects the migration and anti-apoptosis abilities of LoVo/ADR cells. (A, B) Wound healing and Transwell migration assays were performed for LoVo/S cells, LoVo/ADR cells, and LoVo/ADR + miR-222-3p inhibitors cells after treatment with DOX, Scar bar, 50 um. (C) A comparison of the average number of cells penetrating the artificial basement membrane in the migration assays described in B is shown. (D) Annexin V-FITC/PI-positive cells as determined by FlowJo software. (E, F) The total and early apoptotic rates of LoVo/S cells, LoVo/ADR cells, and LoVo/ADR + miR-222-3p inhibitors cells. ** P<0.01, *** P<0.001. DOX – doxorubicin.
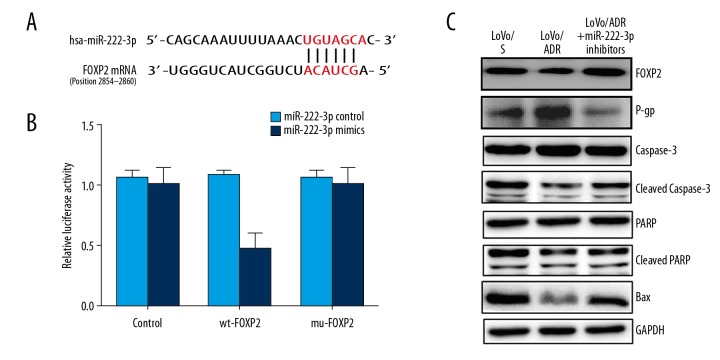
FOXP2 is a direct target of miR-222-3p
By searching TargetScan database, FOXP2 was found to be a potential target gene of miR-222-3p. The dual luciferase reporter showed a significant decrease in luciferase activity of the wild-type vector, whereas mutation in the putative binding site in the 3′UTR region of FOXP2 abrogated the suppressive ability of miR-222-3p, verifying that miR-222-3p regulates endogenous FOXP2 by directly binding to its 3′ UTR region (Figure 3A, 3B). To identify mechanisms of miR-222-3p action, the expression of FOXP2, P-gp, caspase-3, PARP, cleaved caspase-3, cleaved PARP, and Bax was analyzed by western blotting (Figure 3C). Compared to the LoVo/S group, the expression of FOXP2 was dramatically downregulated, while P-gp showed a relatively higher expression in the LoVo/ADR group. Furthermore, the expression of cleaved caspase-3, cleaved PARP, and Bax in the LoVo/ADR cells was significantly lower than that in the LoVo/S cells. Interestingly, the expression of FOXP2, P-gp, cleaved caspase-3, P-gp, and Bax was markedly reversed in the LoVo/ADR cells and the LoVo/ADR + miR-222-3p inhibitors cells.
Figure 3.
FOXP2 is a direct target of miR-222-3p. (A, B) Luciferase assay revealed that miR-222-3p directly binds to the 3′UTR region of FOXP2. (C) Western blot analysis of FXOP2, caspase-3, cleaved caspase-3, PARP, cleaved PARP, Bax, and P-gp proteins in LoVo/S cells, LoVo/ADR cells, and LoVo/ADR + miR-222-3p inhibitor cells. FOXP2 – forkhead box protein P2.
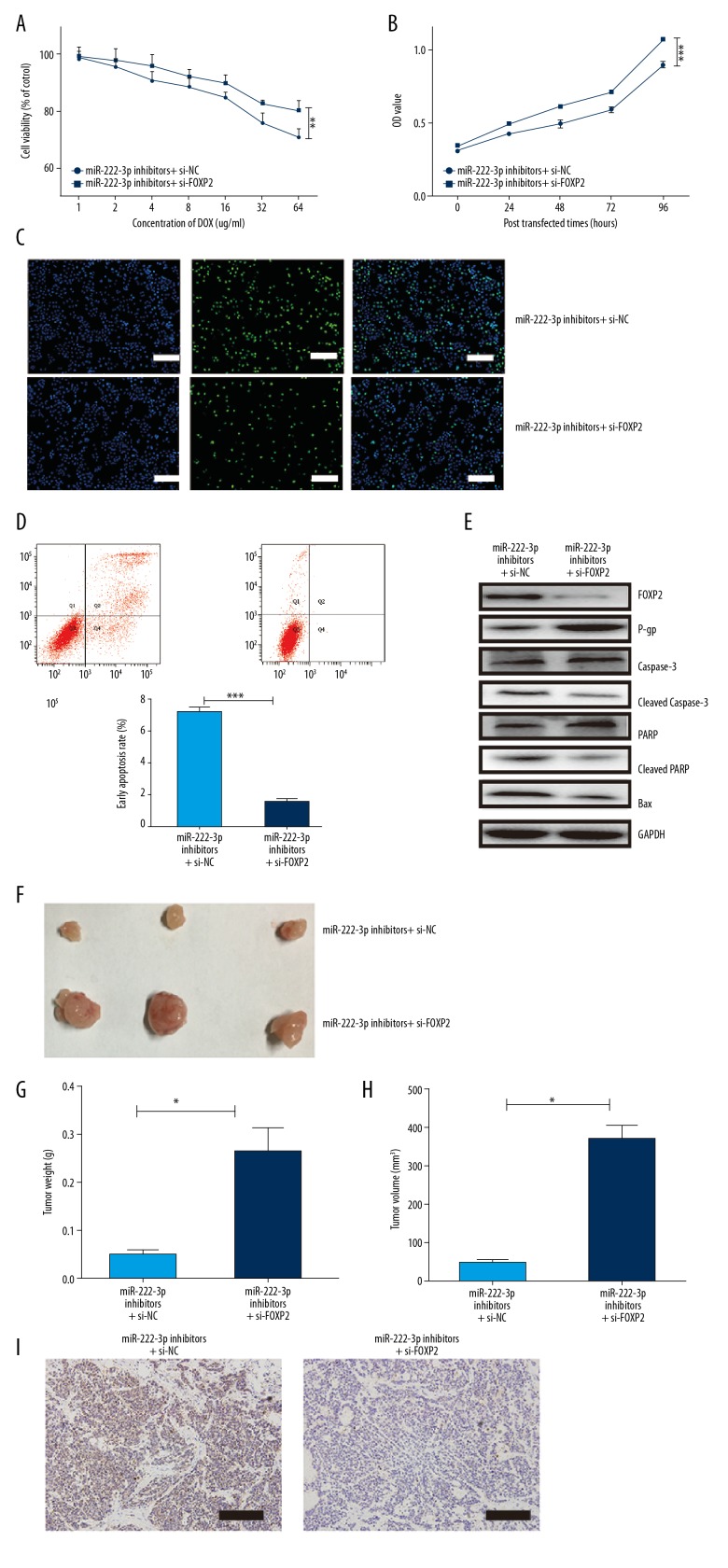
Downregulation of FOXP2 rescues the effect of miR-222-3p inhibitors
To assess the relation between miR-222-3p and FOXP2, we performed cell proliferation assays in the miR-222-3p inhibitor LoVo/ADR cells with the addition of si-FOXP2 or si-NC. Cell viability, cell proliferative ability, and the number of EdU positive cells in the si-FOXP2 group were significantly increased compared to that in the si-NC group (Figure 4A–4C), whereas the cell apoptosis rate was significantly reduced (Figure 4D). Furthermore, with the downregulation of FOXP2, the expression of cleaved caspase-3, cleaved PARP, and Bax in the LoVo/ADR cells were significantly depleted, with upregulation of P-gp (Figure 4E). From the in vivo assay, the si-FOXP2 group showed higher proliferation, resulting in larger volume and heavier weight (Figure 4F–4H). Moreover, the expression of caspase-3 showed a similar tendency (Figure 4I).
Figure 4.
Downregulation of FOXP2 rescues the effect of miR-222-3p inhibitors. (A) Cell viability of LoVo/ADR cells in the presence of different concentrations of DOX. Viability was assessed using the CCK8 assay. (B–D) OD values, EdU cell proliferation, cell apoptosis assays of LoVo/ADR cells after the infection of si-FOXP2 or si-NC. Scar bar, 50 um. (E) Western blot analysis of FOXP2, caspase-3, cleaved caspase-3, PARP, cleaved PARP, Bax, and P-gp proteins in LoVo/ADR cells after infection with si-FOXP2 or si-NC. (F) Images of tumors from the nude mice (miR-222-3p inhibitors + si-NC and miR-222-3p inhibitors + si-FOXP2, n=3). (G) Weight of tumors isolated from the mice. (H) Volume of tumors isolated from the mice. (I) Expression of cleaved caspase-3 in tumors isolated from the mice. Scar bar, 50 um. ** P<0.01, *** P<0.001. DOX – doxorubicin; CCK8 – Cell Counting Kit 8; OD – optical density; EdU – 5-ethynyl-2′-deoxyuridine; FOXP2 – forkhead box protein P2; NC – normal control.
Discussion
Many studies have suggested that aberrant miRNA expression is strongly associated with drug resistance [9,17,18]. Some studies have indicated that miR-222-3p is markedly upregulated in DOX/ADR-induced drug resistant breast cancer cells, while reduction of miR-222-3p inhibited this resistance ability [14–16]. Other studies have shown that miR-222-3p confers cisplatin, tamoxifen, and fulvestrant resistance in cancers [19,20]. In our study, miR-222-3p was selected as a candidate marker for DOX-resistance in colon cancer cells. It was shown that miR-222-3p expression was significantly higher in the LoVo/ADR cells than in the LoVo/S cells. Further investigation showed that the downregulation of miR-222-3p could alter the sensitivity of the LoVo cells to DOX. These findings indicated that miR-222-3p expression was associated with DOX-resistance in colon cancer. To determine the biological significance of miR-222-3p-induced drug resistance to DOX, we studied the effect of miR-222-3p on cell proliferation and metastasis in LoVo cells. Our in vitro experiments showed that DOX-resistance was closely correlated with increased proliferative capacity and metastasis in LoVo cells, and inhibition of miR-222-3p expression suppressed the vitality and migration of LoVo/ADR cells. The growth-suppression effect of miR-222-3p depletion was confirmed by in vivo tumor growth assays.
Cancer develops because of an imbalance between cell growth and death. Therefore, another important mechanism by which cancer cells develop resistance to therapeutic intervention is through apoptosis evasion [21,22]. To delineate the molecular basis of miR-222-3p-mediated drug resistance, we used FACS (fluorescence-activated cell sorting) analysis to detect the levels of apoptosis in the LoVo/S cells and the LoVo/ADR cells. Our results showed that the LoVo/ADR cells had considerably fewer Annexin V-positive cells than the LoVo/S cells. When miR-222-3p was knocked down, we observed an increase in the LoVo/ADR apoptotic rate. Consistently, expression levels of well-defined apoptosis protein markers, including Bax, cleaved PARP, and cleaved caspase 3, were markedly reduced in the LoVo/ADR cells, and increased upon silencing of miR-222-3p. Taken together, these results suggested that miR-222-3p may enhance drug resistance by eliciting apoptosis in LoVo cells.
The most common cause of drug resistance in cancer is acquired mutations or overexpression of transport proteins during cancer progression. P-gp is such a protein, as it can actively transport drugs out of cells, effectively reducing intracellular drug accumulation and concentration, leading to chemotherapy resistance [20]. Accumulating evidence suggests that aberrant P-gp expression is involved in MDR of cancer therapy [23,24]. In other words, MDR is reflected in the negative correlation between P-gp expression and chemosensitivity of cancers. In our study, upregulation of P-gp was detected in LoVo/ADR cells. Furthermore, miR-222-3p depletion reversed the increased expression of P-gp. These results suggested that miR-222-3p might promote the efflux of cytotoxic drugs by upregulating P-gp expression, and it should be considered as a novel P-gp inhibitor to reverse cancer drug-resistance.
To explore the mechanisms underlying drug resistance in colon cancer by miR-222-3p, we identified its potential target genes. With predicted programmer and experimental luciferase assays, miR-222-3p was found to directly bind to 3′-UTR of FOXP2 in vitro. FOXP2 transcription factor is a member of FOXP gene subfamily which all have a C-terminal winged helix forkhead DNA binding domain and it has been previously shown to regulate embryonic development and cell cycle via many neurogenesis signaling pathways such as Hedgehog, Wnt, and Notch pathways [25,26]. Prior investigations have shown that FOXP2 gene functions as a tumor suppressor in breast cancer, gastric cancer, bone cancer, and liver cancer [27–30]. It was also revealed that FOXP2 was negatively regulated by miR-190 in gastric cancer [28]. In this colon cancer study, we, for the first time, have shown that FOXP2 protein was downregulated in LoVo/ADR cells compared to LoVo/S cells. Furthermore, our experiment indicated that suppression of endogenous miR-222-3p in LoVo/ADR cells effectively promoted FOXP2 protein expression levels. This suggests that a potential interaction between miR-222-3p and FOXP2 might contribute to drug-resistance in colon cancer. To confirm this hypothesis, functional experiments were performed and showed that knockdown of FOXP2 in LoVo/ADR cells with low levels of miR-222-3p contributed to the enhancement of drug-resistance to DOX by inhibiting cell apoptosis and facilitating cell growth in vitro and in vivo. The present study might be the first to show miRNA-222-3p enhancing the drug resistance of colon cancer to DOX by downregulating FOXP2 expression.
Conclusions
We have shown that miR-222-3p was significantly upregulated in DOX-resistant cells. Moreover, knockdown of miR-222-3p expression constrained proliferation, metastasis, and caused cell death by apoptosis in DOX-resistant cells. Most importantly, our data revealed that miR-222-3p enhanced chemoresistance by suppressing FOXP2 expression in colon cancer cells. These results suggest that miR-222-3p could present a critical therapeutic strategy to enhance chemosensitivity for DOX treatment in colon cancer.
Footnotes
Source of support: This work was funded by Natural Science Foundation of Guagndong Province grant (2017A030310407) and Special Funds for the Cultivation of Guangdong College Students’ Scientific and Technological Innovation (pdjha0094)
Conflicts of interest
None.
References
- 1.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. Cancer J Clin. 2017;67:177–93. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 2.Gustavsson B, Carlsson G, Machover D, et al. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin Colorectal Cancer. 2015;14:1–10. doi: 10.1016/j.clcc.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Kathawala RJ, Gupta P, Ashby CR, Jr, Chen ZS. The modulation of ABC transporter-mediated multidrug resistance in cancer: A review of the past decade. Drug Resist Updat. 2015;18:1–17. doi: 10.1016/j.drup.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Lee CS, Kim H, Yu J, et al. Doxorubicin-loaded oligonucleotide conjugated gold nanoparticles: A promising in vivo drug delivery system for colorectal cancer therapy. Eur J Med Chem. 2017;142:416–23. doi: 10.1016/j.ejmech.2017.08.063. [DOI] [PubMed] [Google Scholar]
- 5.Lelong-Rebel I, Brisson C, Fabre M, et al. Effect of pO2 on antitumor drug cytotoxicity on MDR and non-MDR variants selected from the LoVo metastatic colon carcinoma cell line. Anticancer Res. 2008;28:55–68. [PubMed] [Google Scholar]
- 6.Boiocchi M, Tumiotto L, Giannini F, et al. P-glycoprotein but not topoisomerase II and glutathione-S-transferase-pi accounts for enhanced intracellular drug-resistance in LoVo MDR human cell lines. Tumori. 1992;78:159–66. doi: 10.1177/030089169207800303. [DOI] [PubMed] [Google Scholar]
- 7.Kibria G, Hatakeyama H, Harashima H. Cancer multidrug resistance: Mechanisms involved and strategies for circumvention using a drug delivery system. Arch Pharm Res. 2014;37:4–15. doi: 10.1007/s12272-013-0276-2. [DOI] [PubMed] [Google Scholar]
- 8.Wongfieng W, Jumnainsong A, Chamgramol Y, et al. 5′-UTR and 3′-UTR regulation of MICB expression in human cancer cells by novel microRNAs. Genes (Basel) 2017;8:9. doi: 10.3390/genes8090213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nijhuis A, Thompson H, Adam J, et al. Remodelling of microRNAs in colorectal cancer by hypoxia alters metabolism profiles and 5-fluorouracil resistance. Hum Mol Genet. 2017;26:1552–64. doi: 10.1093/hmg/ddx059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schetter AJ, Okayama H, Harris CC. The role of microRNAs in colorectal cancer. Cancer J. 2012;18:244–52. doi: 10.1097/PPO.0b013e318258b78f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee C, He H, Jiang Y, Di Y, et al. Elevated expression of tumor miR-222 in pancreatic cancer is associated with Ki67 and poor prognosis. Med Oncol. 2013;30:700. doi: 10.1007/s12032-013-0700-y. [DOI] [PubMed] [Google Scholar]
- 12.Yang YF, Wang F, Xiao JJ, et al. MiR-222 overexpression promotes proliferation of human hepatocellular carcinoma HepG2 cells by downregulating p27. Int J Clin Exp Med. 2014;7:893–902. [PMC free article] [PubMed] [Google Scholar]
- 13.Li N, Tang B, Zhu ED, et al. Increased miR-222 in H. pylori-associated gastric cancer correlated with tumor progression by promoting cancer cell proliferation and targeting RECK. FEBS Lett. 2012;586:722–28. doi: 10.1016/j.febslet.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 14.Shah MY, Calin GA. MicroRNAs miR-221 and miR-222: A new level of regulation in aggressive breast cancer. Genome Med. 2011;3:56. doi: 10.1186/gm272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang DD, Li J, Sha HH, et al. MiR-222 confers the resistance of breast cancer cells to Adriamycin through suppression of p27(kip1) expression. Gene. 2016;590:44–50. doi: 10.1016/j.gene.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Shen H, Wang D, Li L, et al. MiR-222 promotes drug-resistance of breast cancer cells to Adriamycin via modulation of PTEN/Akt/FOXO1 pathway. Gene. 2017;596:110–18. doi: 10.1016/j.gene.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Liang CQ, Fu YM, Liu ZY, et al. The effect of miR-224 downregulation on SW80 cell proliferation and apoptosis and weakening of ADM drug resistance. Eur Rev Med Pharmacol Sci. 2017;21:5008–16. [PubMed] [Google Scholar]
- 18.Li ZH, Weng X, Xiong QY, et al. MiR-34a expression in human breast cancer is associated with drug resistance. Oncotarget. 2017;8:106270–82. doi: 10.18632/oncotarget.22286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng LP, Hu ZM, Li K, Xia K. MiR-222 attenuates cisplatin-induced cell death by targeting the PPP2R2A/Akt/mTOR Axis in bladder cancer cells. J Cell Mol Med. 2016;20:559–67. doi: 10.1111/jcmm.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou S, Zhang S, Wang Y, et al. MiR-21 and miR-222 inhibit apoptosis of adult dorsal root ganglion neurons by repressing TIMP3 following sciatic nerve injury. Neurosci Lett. 2015;586:43–49. doi: 10.1016/j.neulet.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Mashima T, Tsuruo T. Defects of the apoptotic pathway as therapeutic target against cancer. Drug Resist Updat. 2005;8:339–43. doi: 10.1016/j.drup.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Indran IR, Tufo G, Pervaiz S, Brenner C. Recent advances in apoptosis, mitochondria and drug resistance in cancer cells. Biochim Biophys Acta. 2011;1807:735–45. doi: 10.1016/j.bbabio.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Hamada H, Tsuruo T. Characterization of the ATPase activity of the Mr 170,000 to 180,000 membrane glycoprotein (P-glycoprotein) associated with multidrug resistance in K562/ADM cells. Cancer Res. 1988;48:4926–32. [PubMed] [Google Scholar]
- 24.Weerasinghe P, Hallock S, Tang SC, et al. Sanguinarine overcomes P-glycoprotein-mediated multidrug-resistance via induction of apoptosis and oncosis in CEM-VLB 1000 cells. Exp Toxicol Pathol. 2006;58:21–30. doi: 10.1016/j.etp.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Spaeth JM, Hunter CS, Bonatakis L, et al. The FOXP1, FOXP2 and FOXP4 transcription factors are required for islet alpha cell proliferation and function in mice. Diabetologia. 2015;58:1836–44. doi: 10.1007/s00125-015-3635-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsui D, Vessey JP, Tomita H, et al. FoxP2 regulates neurogenesis during embryonic cortical development. J Neurosci. 2013;33:244–58. doi: 10.1523/JNEUROSCI.1665-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen MT, Sun HF, Li LD, et al. Downregulation of FOXP2 promotes breast cancer migration and invasion through TGFbeta/SMAD signaling pathway. Oncol Lett. 2018;15:8582–88. doi: 10.3892/ol.2018.8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia WZ, Yu T, An Q, et al. MicroRNA-190 regulates FOXP2 genes in human gastric cancer. Onco Targets Ther. 2016;9:3643–51. doi: 10.2147/OTT.S103682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gascoyne DM, Spearman H, Lyne L, et al. The forkhead transcription factor FOXP2 is required for regulation of p21WAF1/CIP1 in 143B osteosarcoma cell growth arrest. PLoS One. 2015;10:e0128513. doi: 10.1371/journal.pone.0128513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan X, Zhou H, Zhang T, et al. Downregulation of FOXP2 promoter human hepatocellular carcinoma cell invasion. Tumour Biol. 2015;36:9611–19. doi: 10.1007/s13277-015-3701-y. [DOI] [PubMed] [Google Scholar]