Abstract
Congenital pseudarthrosis of the tibia (CPT) is likely to be a primary periosteal disease and secondary bone disease. The primary goal of treatment is to obtain union, correct the diaphyseal deformity, correct any proximal fibular migration and prevent refracture. The pathobiology demonstrates increased osteoclasis by the surrounding fibrous hamartoma and reduced osteogenesis and bone morphogenic protein production by the bone. This leads to a loss of remodelling potential and gradual bowing and atrophy of the bone with eventual fracture of the tibia and or fibula. This recommends the synergistic use of bisphosphonates and bone morphogenic protein. The pathomechanics of CPT implicate the anterolateral bowing, narrow diameter of the atrophic bone ends and proximal fibular migration. These biomechanical factors can be addressed by means of straightening of the deformity, intramedullary support of both bones, stable fixation and reduction of proximal migration of the fibula. A summary of the literature on CPT shows that the mean probability of achieving primary union without refracture, by most treatments is 50% (12% to 80%). Two recent studies have shown a much higher success rate approaching 100%, by creating a cross-union between the tibia and fibula. The cross-union with intramedullary reinforcement of the bone makes refracture unlikely due to the cross-sectional area of union with its two-bar linkage. A new classification to guide such treatment is also proposed.
Level of Evidence
V – expert opinion
Keywords: congenital pseudarthrosis of the tibia, neurofibromatosis, cross-union
Introduction
Congenital pseudarthrosis of the tibia (CPT) is a rare condition that has defied and challenged orthopaedic surgeons for over 100 years. Its incidence is reported to be between 1:140,000 to 1:250,000 live births.1 It is commonly associated with neurofibromatosis and to a lesser extent with fibrous dysplasia or Campanacci’s osteofibrous dysplasia. It usually presents as a deformity of the tibia (anterolateral bowing) or as a fracture of the tibia and/or fibula. When it presents without fracture of the tibia, the standard of care is to prevent fracture by bracing. When it presents with a fracture of the tibia, surgery is indicated but there is no universal agreement as to what surgery offers the best union rate and the lowest refracture rate nor even what is the best age to perform surgery. Failure at obtaining union is accepted as part of the natural history of this condition. Failure at maintaining union, even when union is initially achieved, leads to repeated surgeries and secondary changes. These secondary changes include foot deformity, leg-length discrepancy (LLD), knee malalignment and even hip dysplasia due to valgus deformity of the proximal femur. These changes are all considered part of the natural history of CPT. Repeated surgeries due to failures of treatment or treatment for secondary changes lead to interruption of childhood, prolonged repeated disability and in some cases a recommendation for amputation either as a primary or secondary treatment.2,3
Until recently no single treatment has emerged that is based on an understanding of the pathobiology and pathomechanics of CPT. No single treatment has stood out as superior to all of the rest. To achieve this status, a treatment would have to demonstrate safety, reliability and reproducibility in both obtaining and maintaining union.
Pathology of CPT
Until recently little was understood about the pathology of CPT. Many theories have been put forward to explain the development of the pseudarthrosis. Codivilla4 was the first to recognize that the periosteum in CPT is diseased. McElvenny5 described a thickened, adherent periosteum that constricted the tibia and fibula causing atrophy and leading to fracture and pseudarthrosis. Boyd6 and Boyd and Sage,7 from a study of amputated specimens, postulated that CPT was caused by osteolytic fibromatosis. Blauth et al8 posited that the thickened periosteum was caused by myofibroblast overgrowth.9 Resection of hamartomatous fibrous tissue is part of many treatment protocols, but it does not ensure healing or prevent refracture. With the recognition that the periosteum was diseased, Codivilla4 recommended osteo-periosteal grafting more than 100 years ago. Cambras from Cuba (circa 1977, personal communication 1996) treated CPT with bone and periosteal grafting from the child’s mother, emphasizing the role of the periosteum to cure the disease. Paley proposed periosteal grafting as a treatment option in 1995 based on observations made about the lack of remodelling of pin sites (presented in: Paley D, Congenital pseudarthrosis: management. PanArabOrthopaediCongress, Muscat, Oman, Sept 11-14, 1995) and later published on this method in a doctoral thesis by El-Rosasy et al10 in Egypt in 2001 and then in two book chapters in 200711,13. Paley’s periosteal grafting method was also used and reported on by Franz Grill from Austria (presented at IPOS meeting 2006, Orlando, Florida). A two-centre study combining the experience with periosteal grafting from Paley and Kocaoglu was published in 2008 by Thabet et al.14 While union rate was high (100%), the refracture rate was high too (40%).
Hermanns-Sachweh et al15 2005, identified neural-like cells that surround the small periosteal vessels causing narrowing and obliteration of these vessels. They postulated that this leads to hypoxia of the subperiosteal bone with subsequent resorption, fracture and nonunion. Morphologically these cells were reminiscent of Schwann cells. This may tie the pathology to its strong association with neurofibromatosis.
Cho et al16 2008 showed that periosteal cells in the hamartoma of CPT have decreased osteoblastic responses to bone morphogenic protein-2 (BMP2). In contrast, the osteoclastic activity of the periosteum was significantly higher than that of controls. They concluded that failure of healing as well as resorption of bone graft is related to increased osteoclastic activity and decreased osteoblastic activity of the CPT periosteum compared with normal periosteum.
Schindeler et al17 2008, showed that neurofibromatosis-1(NF1) haploinsufficient (+/-) (NF positive) mouse cells had less osteogenic potential than NF1(+/+) (NF negative) cells (controls). There was much less bone formation in response to BMP in the NF positive cells compared with the NF negative cells. Co-treatment with zoledronic acid (ZA) a third-generation bisphosphonate, led to a synergistic increase in bone formation in both groups. They postulated that bisphosphonate-BMP combination therapy should be superior to BMP therapy alone.
To test this conclusion, these same authors18 looked at distal tibial fracture healing in NF (+/-) versus NF (+/+) mice, in which the periosteum was also stripped at the fracture site and the fracture site was treated with BMP2. The NF (+/-) mouse tibia remained unbridged in 1/15 (7%), while the NF (+/+) remained unbridged in 9/12(75%). When ZA infusion was added, the proportion remaining unbridged was halved in NF (+/-) 6/16 (37.5%). They concluded that anabolic treatment with local recombinant human (rh) BMP-2 and catabolic treatment with systemic ZA produced a higher rate of union than rhBMP-2 treatment alone.
Madhuri et al19 2016 studied stem cells harvested from hamartoma tissue in three CPT patients compared with healthy bone marrow stem cells in controls. They found that the mesenchymal stem cells in the CPT patients had a higher proliferation rate than in controls, but that these stem cells showed less differentiation potential and less osteogenic potential than control stem cells. Furthermore, bisphosphonate treatment alone did not increase the osteogenic potential of the hamartoma-derived stem cells. They concluded that the bisphosphonate must be combined with an additional stimulus to enhance bone formation.
Natural history of CPT
The natural history of CPT can be divided into primary and secondary. The anterolateral bowing (varus-procurvatum distal diaphyseal deformity associated with valgus recurvatum proximal metaphyseal deformity) is the primary deformity associated with CPT. As this bowing progresses, the tibia and/or fibula will eventually break. Fracture of the tibia leads to instability and loss of integrity of the primary weight-bearing bone of the lower leg. Fracture of the fibula leads to proximal migration of the distal fibula and valgus of the ankle joint. Therefore, the primary problems in CPT are: 1) anterolateral bowing; 2) non-healing fracture (pseudarthrosis); and 3) proximal migration of the fibula. As a consequence of these three primary conditions a myriad of secondary deformities develop. Most of the secondary conditions are due to the effect of the primary condition on the surrounding soft tissues and joints and the secondary effects on growth and development of the lower limb. The anterolateral bowing relaxes the posterior muscles leading to decreased tension on the Achilles tendon. This leads to atrophy and thinning of the calf muscles, and eventually to a calcaneo-cavus deformity of the foot with a pistol grip heel. The anterior bow of the tibia causes the foot to assume a dorsiflexed position. The anterior muscles and capsule are never stretched into equinus. This leads to a dorsiflexion contracture of the ankle (calcaneus deformity of the foot). The proximal migration of the fibula causes the talus to follow the fibula. This leads to lateral subluxation of the ankle joint, valgus wedging of the distal tibial epiphysis and instability of the ankle. The ankle valgus serves to compensate for the varus diaphyseal deformity (lateral bowing). Similarly, the proximal tibial physis grows into recurvatum and valgus to compensate for the procurvatum-varus diaphyseal deformity. The lack of loading on the tibia and the altered muscle forces as well as the proximity of the pseudarthrosis to the distal tibial physis leads to slowing of growth of the distal tibial and fibular physes and LLD. In response to the altered forces on the lower limb, the proximal femur responds by growing into coxa valga.20 The coxa valga may explain the overgrowth of the femur despite undergrowth of the tibia. CPT is one of the few conditions with growth inhibition in the tibia (LLD) that compensates by overgrowth in the femur. In some cases the coxa valga can be so extreme that it leads to hip dysplasia. The LLD, foot, ankle, knee, femur and hip deformities are all secondary problems associated with CPT. These secondary problems can be prevented by successfully treating the primary problems of union and angulation. Therefore, the primary objectives of CPT treatment are: 1) straighten the anterolateral bowing at the CPT site; 2) obtain and maintain union of the tibia at the CPT site; and 3) obtain union of the fibula and reduce/prevent proximal fibular migration. The secondary treatment objectives are correction of deformities of the ankle/foot/hip and LLD.
Treatment methods and results
The literature is replete with surgical techniques to treat CPT.2,3,21-25 Adjunctive treatment using electric stimulation has also been recommended.12,26 The primary four methods of treatment for CPT are: internal fixation with intramedullary rodding,22,27-35 external fixation (EF) (predominantly Ilizarov apparatus),10,36-40 combination treatment with an Ilizarov and rodding construct14,41-44 and vascularized fibula transfer.21,35,45,46 There have been many variations, primarily with the first three methods. These include resection of the hamartoma, bone grafting of the CPT site and pharmacologic treatment with BMP and bisphosphonates. For comparison, the union and refracture rate have been tabulated according to one of these four treatment groups (Table 1). We can define success as unequivocal radiographic union of the tibia with the index procedure, without subsequent refracture (union rate × (1−mean refracture rate)). Intramedullary (IM) rodding achieved this in 40% of cases. Ilizarov fixation achieved this in 57% of cases. Combined Ilizarov with rodding achieved primary union without refracture in 57% of cases. Free vascularized fibular grafting achieved this in 58% of cases. The mean union rate for all of these studies combined (Table 1) was 72% (21% to 100%). The mean refracture rate was 24% (0% to 68%). The average probability of achieving unequivocal union with the index procedure with no subsequent refracture was 50.7% (12% to 80%). A success rate of 50% is not very reassuring to a parent whose child has CPT.
Table 1.
Comparison of union, refracture and probability of union without refracture rates amongst different published studies for different methods of treatment
| Patients (n) | Primary union rate (%) | Refracture rate (%) | Success probability (%) | Other | |
|---|---|---|---|---|---|
| Rodding | |||||
| Birke et al 201027 | 8 | 75 | 0 | 75 | BMP |
| Das et al 201428 | 20 | 90 | 25 | 68 | BMP |
| Dobbs et al 200422 | 21 | 86 | 57 | 37 | |
| Johnston 200229 | 23 | 22 | 0 | 22 | |
| Joseph and Matthew 200030 | 14 | 21 | 5 | 20 | |
| Joseph et al 200331 | 26 | 73 | 15 | 62 | |
| Kim and Weinstein 200232 | 11 | 36 | 50 | 18 | |
| Liu et al 201833 | 42 | 95 | 26 | 67 | |
| Stephens Richards and Anderson 201834 | 21 | 76 | 31 | 24 | BMP |
| Vigouroux et al 201735 | 10 | 40 | 30 | 12 | |
| Total/Mean | 196 | 61 | 24 | 40 | |
| Ilizarov | |||||
| Boero et al 199736 | 21 | 81 | 19 | 66 | |
| Borzunov et al 201637 | 28 | 100 | 61 | 49 | |
| El-Rosasy et al 200110 | 17 | 100 | 68 | 32 | |
| Hissnauer et al 201738 | 7 | 86 | 50 | 43 | BMP |
| Ohnishi et al 200539 | 26 | 100 | 15 | 85 | |
| Paley et al 199240 | 16 | 94 | 31 | 65 | |
| Total/Mean | 115 | 93.5 | 41 | 57 | |
| Ilizarov + Rodding | |||||
| Agashe et al 201241 | 15 | 40 | 17 | 33 | |
| Shabtai et al 201542 | 10 | 80 | 0 | 80 | BMP |
| Thabet et al 200814 | 20 | 100 | 40 | 60 | |
| Yan et al 201743 | 51 | 51 | 0 | 51 | |
| Zhu et al 201644 | 56 | 89 | 26 | 66 | |
| Total/Mean | 152 | 72 | 17 | 58 | |
| Free vascularized fibula graft | |||||
| Grill et al 200059 | 31 | 61 | 16 | 51 | |
| Kalra and Agarwal 201245 | 26 | 92.6 | 15 | 79 | |
| Vigouroux et al 201735 | 8 | 38 | 0 | 38 | |
| Weiland et al 199046 | 19 | 74 | 14 | 64 | |
| Total/Mean | 84 | 66 | 11 | 58 | |
| Mean all groups | 72 | 24 | 50.7 |
Adjunctive pharmacological therapeutics used in the treatment of CPT, including BMP2, BMP7 and bisphosphonate therapy17,47,48 are now available. Lee et al47 reported five cases of CPT treated with BMP7 combined with corticocancellous allograft and IM rodding combined with EF. The authors concluded that the use of recombinant human BMP7 is not enough to overcome the poor healing environment associated with CPT. Hissnauer et al38 combined Ilizarov fixation with insertion of BMP2 and achieved 86% primary union but had a 50% refracture rate. All of these reports are retrospective studies of the use of BMP with CPT. Das et al28 have published the only prospective randomized study on the use of BMP. They randomized the use of BMP7 in 20 patients treated by resection of hamartoma and bone at the CPT site, autologous bone grafting from the other tibia and intramedullary fixation through the ankle joint. There were no statistically significant differences in healing time between the two groups: with BMP 14.5 months versus without BMP 17.1 months. Refracture occurred in 3/10 with BMP and 4/10 without BMP. This randomized study only adds to the controversy about whether BMP is effective or not. Carlier et al49 performed an ‘in silico’ clinical trial and concluded that BMP treatment reduced the severity of the CPT, but that the result was subject-specific. Birke et al27 used IM fixation, bone grafting and in some cases also EF together with BMP7 in surgery and zoledronic acid infusion after surgery. They reported primary union in 75% with no refractures.
Amputation is the final option in cases of CPT.2,3,50 Its incidence varies from series to series. McCarthy3 noted that foot condition, number of operations and severity of LLD are the factors that determine the need for amputation. Secondary procedures are often required even after amputation in up to 78% of cases.50 High functional level may be achieved if the patient has access to the appropriate prosthetic care.50 This is a major consideration in developing countries.
Recently there have been two multicentre or meta-analysis studies looking at large cohorts of patients with long follow-up in an effort to better understand the factors that increase success of achieving union and the actual refracture rate following union.51,52 Shah et al51 performed a long-term follow-up retrospective multicentre study to identify the factors that affect union and refracture of CPT. Patients were treated with a variety of methods including Williams rods, Ilizarov fixation, bone grafting and free vascularized fibula. Primary union was achieved after the index procedure in 102/119 (86%). Amputation was used in 11/17 that failed primary union. Data regarding refracture was available on 94 of the primary union cases. Forty of these sustained a refracture (42.6%). Therefore, the probability of union without refracture for this series was 49.4%. The mean age at the index surgery was five years (1 to 14) and the mean age at refracture was eight years (2.5 to 17.3). The refractured cases underwent 53 procedures. At skeletal maturity 82/119 were united (69%). Statistical analysis showed that a sound union of the tibia was associated with no surgery on the fibula, the use of cortical bone graft and either IM nailing or Ilizarov treatment. The combination of Ilizarov and IM nailing had a high rate of unsound union. The use of BMP was associated with a poorer outcome. Transfixation of the ankle was shown to improve the chance of obtaining union. Kesireddy et al52 published a meta-analysis of 33 studies encompassing 401 CPT cases. The mean age at treatment was 5.2 years and NF1 was present in 262 (65%). The mean follow-up was eight years. The mean rate of initial union was 75% and rate of refracture was 35%. The probability of union without refracture was 49%. Looking at the results from Table 1, Shah et al51 and Kesireddy et al,52 the probability of union without refracture was 50.7%, 49.5% and 49%, respectively. The probability of union without refracture for CPT treatment seems to be at an impasse (50%), which cannot be overcome irrespective of the method chosen.
While most studies focus on union and refracture, few studies have looked at the functional outcome for CPT. Karol et al53 showed that patients treated with IM rods across their ankles had 68% diminished ankle push off strength compared with only 36% in the group that did not have the rod inserted across the joint. Seo et al54 showed that the ankle function in the sagittal plan was well preserved after successful Ilizarov treatment of CPT. The clear lesson from these two studies is that the final ankle range of movement and push off strength is prognostic of a good functional result. Therefore, techniques that leave a rod across the ankle and subtalar joint are less desirable.
Classification of CPT
There have been several different published classifications of CPT.7,11,55,56 The Crawford classification, which considers the tibia as dysplastic progressing from intact anterolateral bowing to fractured and atrophic is currently the most commonly used classification.56 None of these classifications considers the status of the fibula. Choi et al57 classified the fibula in CPT with emphasis on its level of proximal migration. The goal of a classification is to guide treatment and to categorize cases for comparison, so that apples are compared with apples and not oranges. The Anderson, Boyd and Crawford classifications are all descriptive and emphasize the presence of sclerosis, cystic and atrophic changes of the tibia. These changes, however, do not necessarily impact treatment or prognosis. The El-Rosasy-Paley classification factored in the stability and width of the bone ends in the pseudarthrosis (mobile versus stiff, narrow atrophic versus wide hypertrophic) similar to what is done in adult nonunions.58 Mobile-atrophic-narrow bone ends requiring open treatment while stiff-hypertrophic-wide bone ends are amenable to gradual distraction. This is related to the anticipated tissue between the bone ends being dense fibro-cartilagenous for the stiff-hypertrophic-wide bone ends nonunion, making it amenable to distraction treatment. The mobile-narrow bone ends type was divided into two groups; without previous surgery versus with previous surgery (with the assumption that those with previous surgery had a bone defect or dead bone which after resection would produce a bone defect). The recommendation was that without a bone defect the CPT site would be treated by bone grafting, while cases with a bone defect would be treated by bone transport or acute shortening of the defect with relengthening of the tibia proximally11. The El-Rosasy-Paley classification was the first classification where the type was related to the treatment algorithm. The Choi et al57 classification of the fibula was the first to highlight the important consideration of proximal fibular migration. Although they documented the fibular migration, Choi et al57 did not recommend any treatment for this. Johnston29 (2002), concluded that treatment of the fibula to the CPT union rate.
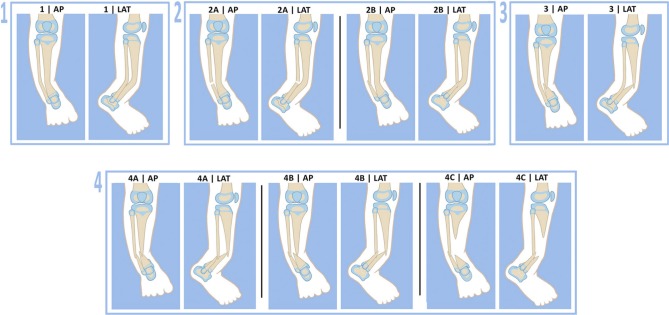
The author currently uses a different classification to guide treatment. The Paley Classification (Fig. 1) factors in: 1) the integrity of the tibia and fibula; 2) the presence or absence of proximal migration of the distal fibula; and 3) the presence of a significant bone defect. These three factors affect the treatment protocol. Other factors such as the presence or absence of NF or fibrous dysplasia, previous surgery, atrophic or hypertrophic bone ends and age are not considered in the classification.
Fig. 1.
Paley Classification of congenital pseudarthrosis of the tibia: type 1, no fractures; type 2, no fracture tibia, fracture fibula with fibula (a) at station (b) proximal migration; type 3, fracture tibia, no fracture fibula ; type 4, fracture tibia and fibula with fibula (a) at station (b) proximal migration (c) bone defect tibia with proximal migration fibula. Reproduced with permission by the Paley Foundation (AP, anteroposterior; LAT, lateral).
Prognostic factors
Factors reported to negatively affect union: 1) neurofibromatosis; 2) age at treatment less than three years; 3) previous failed surgery; and 4) years of follow-up after treatment. Neurofibromatosis is often touted to be a negative prognostic indicator. Several studies have now shown equivalent results in patients with or without NF1.29,37 Some studies have found higher nonunion rates in younger patients and have therefore recommended surgery between the ages of three and six years old36,59,60 leading to recommendations to delay the index treatment to an older age.61 Other studies suggest that surgery in younger patients (one to three years old) is safe and effective.31,33 Earlier definitive surgical intervention may allow for more normal development of the affected extremity with diminished LLD at skeletal maturity.31
Longer follow-up times clearly give a better idea of refracture rates. El-Rosasy reviewed the same group of patients that Paley had published on eight years earlier.10,11 The refracture rate of a group of patients treated with the Ilizarov method increased from 31% to 68%.
An ideal treatment would consider all of these factors and be based on the pathobiology and pathomechanics of CPT. An ideal treatment would include elements shown to be beneficial from previous treatment protocols and avoid factors that worsen the functional outcome (e.g. rodding across the ankle).
The pathobiological considerations are:
Fibrous hamartoma replaces the healthy periosteum.
Fibrous hamartoma leads to osteolysis and vascular constriction of the bone.
The bone is viable despite the osteolysis, atrophy and hamartomatous constriction.
Medullary canal obliterated due to sclerosis.
Osteocytes produce lower levels than normal of BMP.17
Increased osteoclasts and osteoclasis.15
The pathomechanical considerations are:
Diaphyseal angular deformity (antero-lateral bow).
Proximal migration of fibula.
Small cross-section of bone at fracture (CPT) site (atrophic bone ends).
Prior treatment lessons are:
Hamartoma resection should be comprehensive back to normal fat planes.
Medullary canal should be recanalized.
All bone and soft tissue at the CPT site should be alive.
Autogenous cancellous bone graft contains more stem cells than autogenous cortical or allograft bone and produces more bone for the same volume of graft.62
Bone graft is rapidly resorbed.
Intramedullary fixation helps prevent refracture.
Rigid fixation is critical to provide mechanical stability at the CPT site.
BMP may be helpful to boost decreased BMP production by the diseased tibia.
Zoledronic acid can help prevent osteoclasis at the CPT site.
Zoledronic acid given prior to bone graft harvest can protect the bone graft from resorption after implantation.
Angular correction at the CPT site is critical.
Periosteal grafting can help restore healthy periosteum at the CPT site.14
Larger cross-sectional area of union at the CPT site has a lower risk of fracture.10,11
Cases with accidental cross union between the tibia and fibula do not refracture.
The author used all of these considerations to construct a treatment protocol that is based on biology, biomechanics and more than a century of accumulated knowledge about CPT.
The cross-union concept: principles and preliminary results
Choi et al63 (2011) recommended creation of a cross-union between the tibia and fibula for CPT cases where the fibula was broken but minimally proximally migrated. They converged the two fibula bone ends towards the two tibia bone ends in what they called a ‘4-in-1 Osteosynthesis’. They used a cortico-cancellous sheet of the inner table of the ilium with or without its periosteum and when necessary additional cortical bone from the contralateral tibia combined with cancellous bone chips to achieve the cross-union. The cortical graft was placed posterior to the two bones and then cancellous chips between the bones and another layer of cortical bone anterior to the bones. They did not recommend this method when the fibula was intact or when the fibula was significantly proximally migrated. They reported eight cases treated at a mean age of 6.3 years (2.9 to 11.8). All eight united and developed a cross-union of the tibia to the fibula. There were no refractures at an average of 7.4 years (2.7 to 12.4). They compared this with a smaller group of five patients who had end-to-end repair of the tibia without cross-union. Four out of five refractured and required further treatment for the CPT. Choi et al63 attributed the large cross-section of the bone at the level of the cross-union as the reason for no refractures. To quantitate this, they measured what they called the relative cross-sectional area (rCSA = area at the CPT site after union divided by area at the upper tibial physis). The rCSA was significantly lower in the non-synostosis group that all went on to refracture (0.13) versus in the synostosis group that did not refracture (0.27).
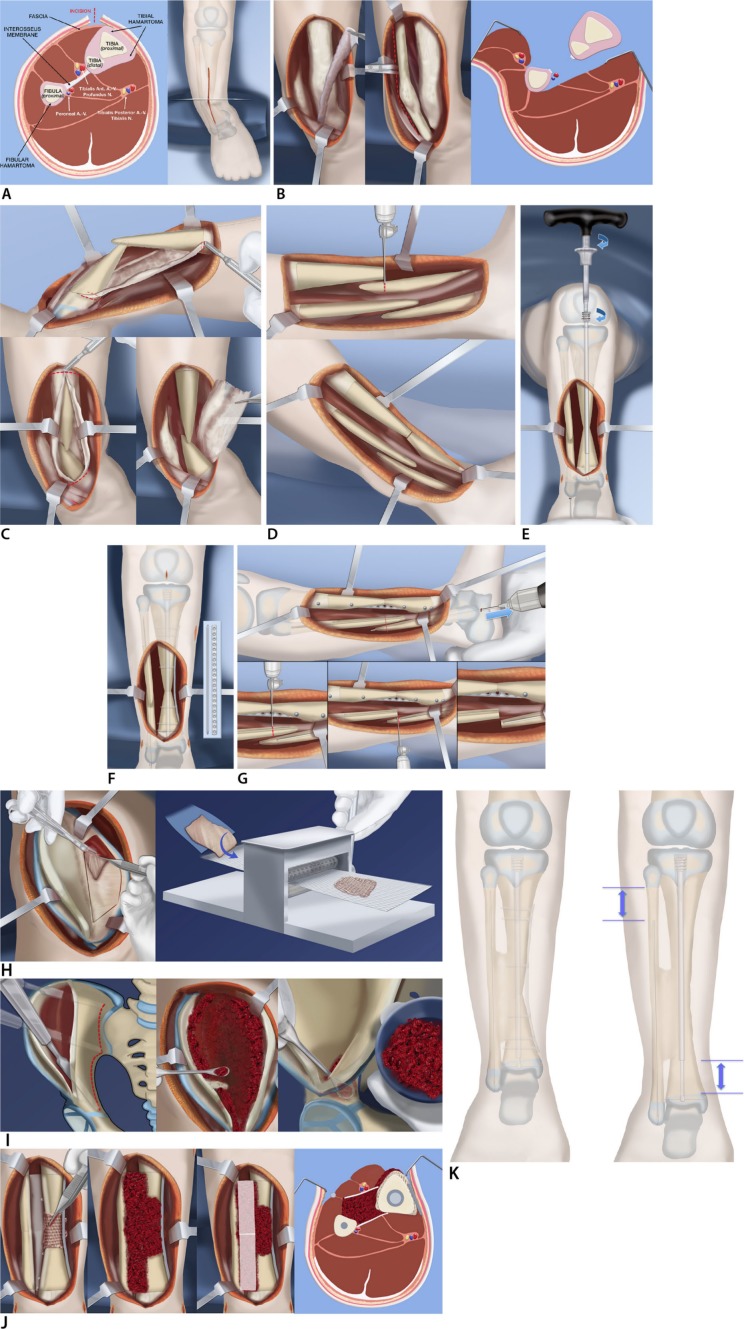
Paley64 (2012) reported preliminary results using combined pharmacological and surgical management with cross-union. The treatment protocol was (Fig. 2): presurgical infusion of zoledronic acid (ZA); hamartoma resection around tibia and fibula with resection of the interosseous membrane; tibial rodding with a telescopic growing rod and fibular rodding with a wire; decancellousization of the ilium to harvest a large cancellous bone graft (as much as 20 cc can be obtained in a 12-month-old child); harvest of periosteal graft from the underside of the iliacus muscle; application of a three-layer graft composed of 1) periosteum around the CPT, 2) cancellous bone between and around the tibia and fibula, and 3) BMP2 posterior and anterior to the bone graft covered by soft tissues. The last step was application of the Ilizarov apparatus to compress the CPT site and to give rotational stability. The smooth non-locking telescopic rod only gives angular support but does not prevent the bone ends pulling apart or rotating to each other. More recently in 2017, the author replaced the external fixator with an internal fixator (locking plate)65 (Fig. 2).
Fig. 2.
Reproduced with permission by the Paley Foundation: (a) anterior incision shown from front and cross section. Note hamartoma encircling tibia and fibula and the interosseous membrane between them; (b) anterior and deep posterior fasciotomy and muscle reflection to expose tibia, interosseous membrane, and fibula, allow resection of the membrane under direct vision without damage to the neurovascular bundles; (c) circumferential resection of the tibial fibrous hamartoma is carried out over the planned length of the cross-union. The same is done for the fibular hamartoma; (d) the tibial bowing is straightened and the bone ends overlapped and resected; (e) a customized Fassier-Duval telescopic nail is inserted and the male end locked with a wire into the distal epiphysis and the female end screwed into the proximal epiphysis; (f) a small diameter locking plate is fixed medially to the tibia with six screws; (g) the fibular ends can now be cut and the fibula fixed with a wire in its medullary canal; (h) a periosteal graft is harvested from the undersurface of the iliacus muscle. It is then expanded by passing it through the skin graft mesher; (i) decancellousization of the ilium is done by first splitting the two cortical tables of the ilium down to the roof of the acetabulum, triradiate cartilage, sciatic notch, posterior spines and sacro-iliac joint; (j) the periosteal graft is wrapped around the congenital pseudarthrosis site and bone morphogenic protein-2 (BMP2) collagen sponges are inserted overtop the posterior muscles behind the tibia and fibula (left). The cancellous bone is inserted between the tibia and fibula (left centre). The BMP2 sponges are placed overtop the bone graft (right centre). The anterior muscles lie over the BMP2. The interosseous space has a sandwich of cancellous bone between layers of BMP2 and its overlying soft tissues; (k) the cross-union forms between the bones by three months after surgery. The bone is well fixed with the telescopic rod in the tibia, the wire in the fibula and the plate on the tibia (left). Growth may occur despite the hardware leading to telescopic expansion of the male and female rods. The fibular wire descends with growth (left).
For more details of this technique please refer to the online supplemental material. (https://online.boneandjoint.org.uk/doi/suppl/10.1302/1863-2548.13.180147)
Since the 2012 manuscript64 was written and submitted before the publication of the Choi et al article in 201163, Paley was unaware of the Choi et al method, which predates the Paley method. Both publications recommend a cross-union. Although Paley reached this idea independently of Choi et al’s publication, Choi et al deserve credit for first recommending the intentional creation of a cross-union to reduce the risk of refracture. Choi et al began performing their version of the cross-union called, the ‘4-in-1 Osteosynthesis’ technique in 1999, while Paley did not start using his method intentionally until 2007 (Paley had observed since 1999, that when cross-union occurred unintentionally, those cases never had a refracture).
The first mention of cross-union as part of the treatment of CPT was by Johnston in 2002.29 He reported that 13 of the 23 cases were treated by a delayed posterolateral bone grafting between the tibia and fibula to create a cross-union. The delayed grafting occurred after the initial direct CPT grafting of the tibia (and in some cases fibula), had already been performed three to 12 months prior, at the discretion of the surgeon. Johnston’s study highlighted the importance of fixation and obtaining union of the fibula on the success of union and refracture of the CPT site. It did not highlight or specifically recognize the importance of the cross-union for CPT. The Johnston case series studied patients treated by seven surgeons between 1978 and 1992.
The Choi et al63 and Paley64,65,66 reports specifically recognized the importance of the cross-union in CPT treatment. There are, however, several notable differences. In the Paley cross-union technique, the tibia and fibula are both rodded straight, keeping the tibia and fibula apart by their normal interosseous distance. A telescopic growing rod is used in the tibia and the rods never cross the ankle or subtalar joints. In the Choi et al method the fibular ends are converged towards the tibia and the tibia is rodded but the fibula is not. In the Choi et al method rodding across the ankle and subtalar joint is often used initially. The Paley technique results in almost twice as large a cross-sectional area of healing as calculated using Choi et al’s relative cross-section area (0.46 versus 0.27). Choi et al also do not indicate their method for cases with intact tibia or fibula (Paley type 1 and 3) or with significant proximal migration of the fibula (Paley type 2b or 4b). Paley recommends the cross-union protocol for all types of CPT (types 1 to 4) (Figs 3, 4, 5 and 6). The two authors also use different techniques for autogenous bone graft harvesting. In the Paley technique, only cancellous bone is harvested by decancellousization of the ilium. In the 4-in-1 technique, a sheet of cortico-cancellous bone is harvested and used. Cancellous bone is known to produce ten-times as much bone as cortical bone.62 Paley also incorporates pharmacologic treatment with ZA and BMP to prevent resorption of the bone graft and the CPT sites and to stimulate osteogenesis respectively, both of which were not used by Choi et al. Obviously, creation of a cross-union can be achieved in more than one way.
Fig. 3.
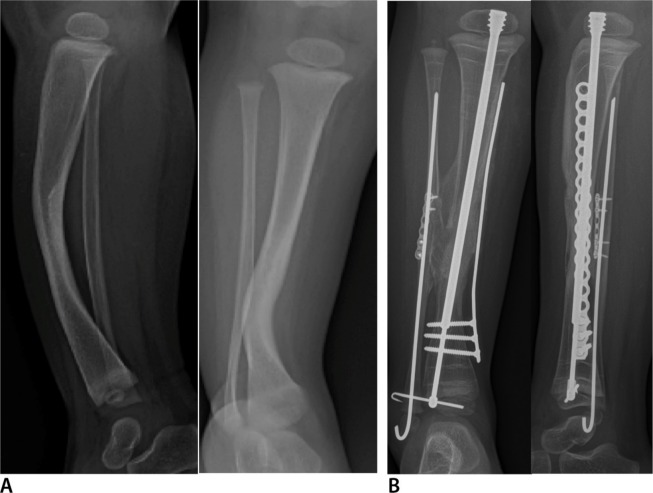
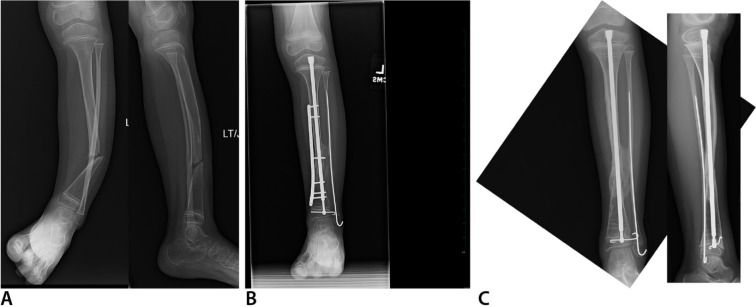
Anteroposterior (AP) (right) and lateral (left) radiographs (a) of right tibia and fibula with anterolateral bowing and neurofibromatosis. This is classified as Paley type 1. AP (left) and lateral (right) radiographs (b) one year after osteotomy of the tibia and fibula with cross-union protocol surgery at age two years. The tibia and fibula are straight fixated with the Fassier-Duval nail and an EVOS plate (Smith & Nephew Orthopedics, Memphis, Tennessee). The upper screws in the plate were removed after six months. There is a long tibio-fibular cross-union present.
Fig. 4.
Anteroposterior (AP) (left) and oblique (right) radiographs (a) of right tibia and fibula with anterolateral bowing and neurofibromatosis. The fibula has a pseudarthrosis with a bone defect and has proximal migration. This is classified as Paley type 2b. AP radiograph (b) of tibia and fibula immediately after cross-union surgery three years. Note the large amount of bone graft in the interosseous space. The rod and plate are stabilizing the leg well. The fibular bone defect is spanned by graft and an intramedullary fibular wire. The fibula was moved distally relative to the tibia and is fixed with one of the screws in the plate. AP radiograph (c) taken one year later showing a mature cross-union spanning the interosseous space and the fibular bone defect. The tibia is united end to end. Telescoping is seen in the Fassier-Duval nail indicating growth. The upper screws in the plate were removed after six months.
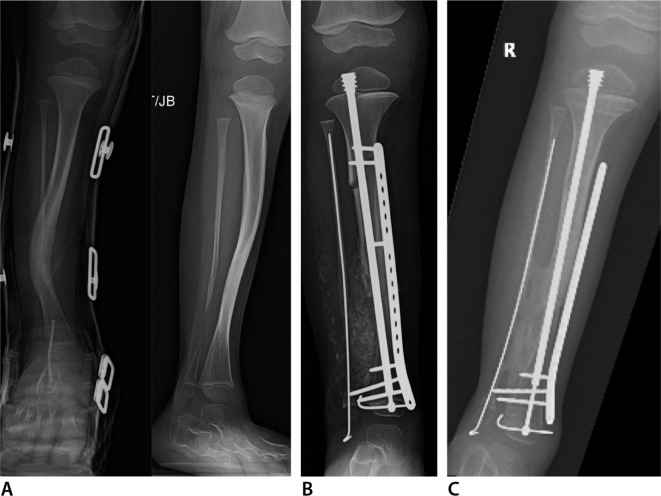
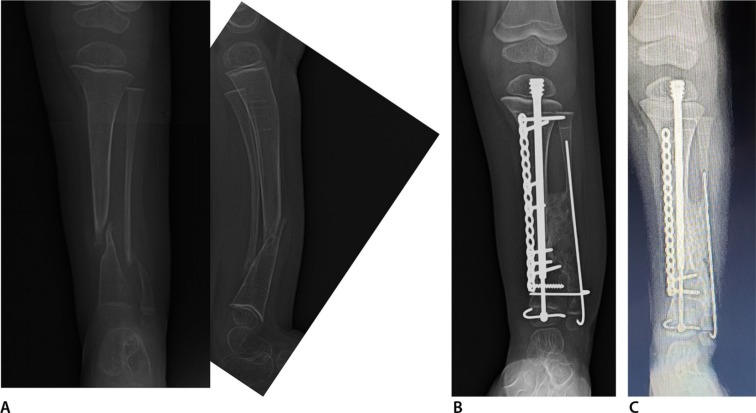
Fig. 5.
Anteroposterior (AP) (left) and lateral (right) radiographs (a) of left tibia and fibula with anterolateral bowing, neurofibromatosis and tibial pseudarthrosis with an intact fibula. This is classified as Paley type 3. AP radiograph (b) six months after cross-union surgery performed at age five years. AP radiograph (c) one and a half years after cross-union surgery. The plate has been removed. The rod was exchanged for a new Fassier-Duval rod.
Fig. 6.
Anteroposterior (AP) (left) and lateral (right) radiographs (a) of right tibia and fibula with anterolateral bowing and neurofibromatosis. This is classified as Paley type 4b since there is a pseudarthrosis of both bones and the distal fibula has proximal migration. The bones are very osteoporotic. He has been non-ambulatory for three years. AP tibial radiograph (b) six months after cross-union surgery which was performed at age three years. A well healed bony bridge is already seen. AP radiograph (c) one year after cross-union surgery after the upper screws in the plate were removed.
Paley et al65,66 recently reported the results of 17 CPTs treated using the Paley cross-union protocol using an external fixator. Preoperative ZA (0.02 mg/kg) was given in each case and a medium (four sponges) Infuse implant (BMP-2 from Medtronic, Memphis, Tennessee) were used in surgery (not weight-related). The average follow-up was 3.7 years (1 to 9). Primary union and cross-union with the index procedure were achieved in 17/17 (100%). The total EF time was an average of four months (3 to 5). The mean radiographic union time was four months (1.5 to 6). No refractures occurred in any of these patients. The calculated probability of union without refracture with this method is 100%, which is the same as in the Choi et al series63. Unpublished further follow-up of these 17 tibias has shown no deterioration or refracture with up to 11 years (mean 5.3 years, range 3 to 11) follow-up. Although no gait analysis was done, at last clinical follow-up, hip, knee, ankle and gait function were normal in all children. The rCSA was a mean of 0.46 ± 0.14. This rCSA is much higher than 0.27 reported by Choi et al.63 This is not surprising since the fibula and tibia in the Paley protocol are not converged as in the ‘4 in 1’ Choi et al technique. Dr. Raymond Liu from Cleveland, Ohio, also reviewed the centre-edge angle (CEA) and acetabular index (AI) after decancellousization of the ilium on the harvested versus unharvested side (unpublished data). There were no significant differences in CEA and AI between sides.
Dr. Anna Hell from Goettingen, Germany (study not published or presented yet, accepted for presentation at the POSNA meeting in May 2019) updated (on the first 17 cases) and expanded this retrospective review, to include the first 36 consecutive CPT cases, in a retrospective study of 34 children (36 tibias) all treated by the Paley cross-union protocol: 18 stabilized with EF and 18 with plate fixation (P). According to the Paley Classification there were EF/P: type 1 0/1; type 2a 2/0; type 2b 1/2; type 3 5/4; type 4a 5/4; and type 4b 5/7. In the six types 1 and 2 cases, the intact tibia was osteotomized while in the other 34 tibias there was a pseudarthrosis of the tibia. In the nine types 1 and 3 cases the fibula was osteotomized. The mean age at treatment was 5.6 years (1 to 13) EF and 3.5 years (1 to 5) P, and 4.6 years combined (C) group. The mean follow-up was: 5.3 years EF (3 to 11) and 1.7 years P (1 to 4) and 3.5 years C. Neurofibromatosis was present in 67% EF, 83% P and 75% C. Fibrous dysplasia was present in 11% EF, 6% P and 8% C. Failed previous CPT surgery occurred in 33% EF and 17% P.
Unequivocal radiographic CPT union and tibio-fibular cross-union was achieved in all 36 tibias. There were no refractures. Three very distal fibular pseudarthroses remained ununited but stable due to the cross-union of the proximal fibula to the tibia across the CPT site. The percent of cross-union length relative to total length of the tibia at seven, 28 and 70 months was 32%, 22% and 17%, respectively. The radiographic time to union was a mean of 16 weeks EF (6 to 24), 12 weeks P (6 to 18) and 14 weeks C. The telescopic rod pulled out of the epiphysis distally or proximally in six EF and 0 P cases; 17% C. There was an association of nail pull to cases where the proximal tibia grew into valgus. Valgus deformity at the knee was successfully treated with a medial proximal tibial hemiepiphysiodesis plate; five EF, 0 P. The rod pull-out was addressed at the time of planned rod exchange a few years later. There were two wound complications treated by debridement and closure; one EF, one P; three pin infections (EF) and two cellulitis (one EF, one P) treated with antibiotics. There were no iliac donor site complications. All complications were resolved without sequellae.
Identical results are seen using a plate combined with rodding as with using the external fixator combined with rodding. Both serve to compress the CPT site and to control rotation. Using the internal fixation eliminates the risk of pin infections and avoids wearing an external fixator for four months. A cast is used instead. This is more convenient to most families and surgeons. It is no surprise that the healing rate is the same for both methods. There is less concern with the internal plate since it is not removed for another several years until the planned rod exchange. It therefore acts as an internal brace for the tibia along with the telescopic nail and the fibular wire. The author has moved away from using the external fixator and now exclusively uses the plate, even in very distal CPT. The most common problem noted to date following cross-union with both EF and P fixation, has been telescopic nail pull-out of the proximal or distal epiphysis. This is posited to be due to stiction-friction on the telescopic mechanism due to the proximal tibia growing into valgus after union is already achieved. This problem can be remedied by insertion of a hemiepiphysiodesis plate and exchange rodding of the telescopic nail which is a secondary planned procedure for all young children treated with the Fassier-Duval nail. This most recent expanded cross-union study to include plate instead of external fixation, corroborates Paley and Choi’s previously published results, that the probability of achieving union without refracture remains 100%. In Choi’s cases the follow-up is up to 12 years, while in the Paley EF cross-union cases the follow-up is up to 11 years. In the Paley cases 4/17 have reached skeletal maturity. The rest show no deterioration in radiographic integrity or bone diameter. For this reason, the author remains cautiously optimistic that these results will not deteriorate with longer follow-up.
Combining BMP and bisphosphonate treatment in clinical practice is a useful adjunct as was shown in the animal model.67 To prevent bone graft resorption, ZA is given two weeks prior to surgery. This allows the zoledronic acid to be taken up by the cancellous bone of the ilium and should make the cancellous bone of the ilium less resorbable. Johnston and Birch68 advocated using BMP as an adjuvant treatment in all primary and recalcitrant CPT cases. In the United States, BMP is not Food and Drug Administration (FDA)-cleared for children. This is because the original FDA submission included limited indications for spine and adult fracture-nonunion surgery. It is not because testing showed increased complications in children. Its use in CPT is therefore considered off-label. In the United States, this means that physicians can choose to use it after informed consent explaining that BMP2 use in children has not been cleared by the FDA and that it has potential risks. Specifically, parents should be advised of the theoretical risk (no reported cases to date) of tumorigenesis because BMP stimulates the RAS pathway, which is also a tumour pathway.69 Patients with NF or fibrous dysplasia already have a propensity for both benign and malignant tumours.
Conclusions
Meta-analysis of the published results of various CPT treatments has shown that the probability of achieving unequivocal union without refracture with the index procedure is approximately 50%. Preliminary results with intentional tibio-fibular cross-union from Choi et al63 and Paley64,65,66 report a probability of primary union without refracture of 100%. Longer follow-up is needed to fully corroborate this strong statement which is based on preliminary experience from two centers extending back more than ten years. CPT is a primary periosteal and secondary bony disease associated with increased osteoclastic activity and decreased osteoblastic activity. As a consequence, the use of BMP2 to stimulate bone healing and ZA to decrease osteoclasis is a specific and synergistic therapy for CPT that should be combined with the cross-union technique. Creating a cross-union increases the cross-sectional diameter of the bone and makes refracture less likely. Intramedullary fixation is essential to help obtain and maintain union. Decancellousization of the ilium is a new useful bone graft harvest technique that allows the harvest of a sufficiently large volume of autogenous cancellous bone to span the interosseous space and create a cross-union even in very young children. The preliminary positive results of cross-union may also recommend early treatment for unfractured anterolateral bowing of the tibia by osteotomy and cross-union. Early treatment of both intact and fractured anterolateral bowing of the tibia and fibula is anticipated to reduce the secondary deformities of the ankle and foot and LLD.
Open access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (CC BY-NC 4.0) licence (https://creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed.
Acknowledgements
The author would like to thank Michelle Coleman, MD, PhD, for her editorial assistance with this manuscript. The author would also like to thank Pamela Boullier Ross who illustrated all of the figures in this manuscript and the Paley Foundation for funding the cost of making these illustrations and giving permission for their reproduction in the Journal of Children’s Orthopedics.
Compliance with ethical standards
Funding statement
The author has received or will receive benefits for personal or professional use from a commercial party related directly or indirectly to the subject of this article.
Ethical statement
Ethical approval: This article does not contain any studies with animals performed by the author. This review does include information on a retrospective study of congenital pseudarthrosis of the tibia patients the author conducted on humans with full institutional review board (IRB) approval.
Informed consent: Informed consent was not required by the IRB.
ICMJE Conflict of interest statement
The author receives royalties from Pega Medical (Montreal, Quebec, Canada) for the male component of the Fassier-Duval Nail that is discussed and used in the treatment of the congenital pseudarthrosis of the tibia patients discussed in this work.
References
- 1.Hefti F, Bollini G, Dungl P, et al. Congenital pseudarthrosis of the tibia: history, etiology, classification, and epidemiologic data. J Pediatr Orthop B 2000;9:11-15. [DOI] [PubMed] [Google Scholar]
- 2.Guille JT, Kumar SJ, Shah A. Spontaneous union of a congenital pseudarthrosis of the tibia after Syme amputation. Clin Orthop Relat Res 1998;351:180-185. [PubMed] [Google Scholar]
- 3.McCarthy RE. Amputation for congenital pseudarthrosis of the tibia. Indications and techniques. Clin Orthop Relat Res 1982;4:58-61. [PubMed] [Google Scholar]
- 4.Codivilla A. On the cure of the congenital pseudoarthrosis of the tibia by means of periosteal transplantation. J Bone Jt Surg [Am] 1906;S2-4-A:163-169. [Google Scholar]
- 5.McElvenny R. Congenital pseudarthrosis of the tibia: the findings in one case and a suggestion as to possible etiology and treatment. Q Bull Northwest Univ Med Sch 1949;23:413-423. [PMC free article] [PubMed] [Google Scholar]
- 6.Boyd HB. Pathology and natural history of congenital pseudarthrosis of the tibia. Clin Orthop Relat Res 1982;166:5-13. [PubMed] [Google Scholar]
- 7.Boyd HB, Sage FP. Congenital pseudarthrosis of the tibia. J Bone Joint Surg [Am] 1958;40-A:1245-1270. [PubMed] [Google Scholar]
- 8.Blauth M, Harms D, Schmidt D, Blauth W. Light- and electron-microscopic studies in congenital pseudarthrosis. Arch Orthop Trauma Surg 1984;103:269-277. [DOI] [PubMed] [Google Scholar]
- 9.Ippolito E, Corsi A, Grill F, Wientroub S, Bianco P. Pathology of bone lesions associated with congenital pseudarthrosis of the leg. J Pediatr Orthop B 2000;9:3-10. [DOI] [PubMed] [Google Scholar]
- 10.El-Rosasy M, Paley D, Herzenberg J. Ilizarov techniques for the management of congenital pseudarthrosis of the tibia. Tanta University; Tanta, Egypt, 2001. [Google Scholar]
- 11.El-Rosasy M, Paley D, Herzenberg J. Congenital pseudarthrosis of the tibia Rozbruch S, Ilizarov S, eds. Limb Lengthening and Reconstruction Surgery. New York: Informa Healthcare, 2007:485-493. [Google Scholar]
- 12.Paterson DC, Lewis GN, Cass CA. Treatment of congenital pseudarthrosis of the tibia with direct current stimulation. Clin Orthop Relat Res 1980;148:129-135. [PubMed] [Google Scholar]
- 13.Weber M. Congenital pseudarthrosis of the tibia redefined: congenital crural segemental dysplasia Rozbruch S, Ilizarov S, eds. Limb Lengthening and Reconstruction Surgery. New York: Informa Healthcare, 2007:495-509. [Google Scholar]
- 14.Thabet AM, Paley D, Kocaoglu M, et al. Periosteal grafting for congenital pseudarthrosis of the tibia: a preliminary report. Clin Orthop Relat Res 2008;466:2981-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hermanns-Sachweh B, Senderek J, Alfer J, et al. Vascular changes in the periosteum of congenital pseudarthrosis of the tibia. Pathol Res Pract 2005;201:305-312. [DOI] [PubMed] [Google Scholar]
- 16.Cho T-J, Seo J-B, Lee HR, et al. Biologic characteristics of fibrous hamartoma from congenital pseudarthrosis of the tibia associated with neurofibromatosis type 1. J Bone Joint Surg [Am] 2008;90-A:2735-2744. [DOI] [PubMed] [Google Scholar]
- 17.Schindeler A, Ramachandran M, Godfrey C, et al. Modeling bone morphogenetic protein and bisphosphonate combination therapy in wild-type andNf1 haploinsufficient mice. J Orthop Res 2008;26:65-74. [DOI] [PubMed] [Google Scholar]
- 18.Schindeler A, Birke O, Yu NYC, et al. Distal tibial fracture repair in a neurofibromatosis type 1-deficient mouse treated with recombinant bone morphogenetic protein and a bisphosphonate. Bone Joint J 2011;93-B:1134-1139. [DOI] [PubMed] [Google Scholar]
- 19.Madhuri V, Mathew SE, Rajagopal K, Ramesh S, Antonisamy B. Does pamidronate enhance the osteogenesis in mesenchymal stem cells derived from fibrous hamartoma in congenital pseudarthrosis of the tibia? Bone Rep 2016;5:292-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song MH, Park MS, Yoo WJ, Cho T-J, Choi IH. Femoral overgrowth in children with congenital pseudarthrosis of the tibia. BMC Musculoskelet Disord 2016;17:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grill F, Bollini G, Dungl P, et al. Treatment approaches for congenital pseudarthrosis of tibia: results of the EPOS multicenter study. European Paediatric Orthopaedic Society (EPOS). J Pediatr Orthop B 2000;9:75-89. [DOI] [PubMed] [Google Scholar]
- 22.Dobbs MB, Rich MM, Gordon JE, Szymanski DA, Schoenecker PL. Use of an intramedullary rod for treatment of congenital pseudarthrosis of the tibia. A long-term follow-up study. J Bone Joint Surg [Am] 2004;86-A:1186-1197. [DOI] [PubMed] [Google Scholar]
- 23.Dormans JP, Krajbich JI, Zuker R, Demuynk M. Congenital pseudarthrosis of the tibia: treatment with free vascularized fibular grafts. J Pediatr Orthop 1990;10:623-628. [DOI] [PubMed] [Google Scholar]
- 24.El-Gammal TA, El-Sayed A, Kotb MM. Telescoping vascularized fibular graft: a new method for treatment of congenital tibial pseudarthrosis with severe shortening. J Pediatr Orthop B 2004;13:48-56. [DOI] [PubMed] [Google Scholar]
- 25.Keret D, Bollini G, Dungl P, et al. The fibula in congenital pseudoarthrosis of the tibia: the EPOS multicenter study. European Paediatric Orthopaedic Society (EPOS). J Pediatr Orthop B 2000;9:69-74. [DOI] [PubMed] [Google Scholar]
- 26.Paterson DC, Simonis RB. Electrical stimulation in the treatment of congenital pseudarthrosis of the tibia. J Bone Joint Surg [Br] 1985;67-B:454-462. [DOI] [PubMed] [Google Scholar]
- 27.Birke O, Schindeler A, Ramachandran M, et al. Preliminary experience with the combined use of recombinant bone morphogenetic protein and bisphosphonates in the treatment of congenital pseudarthrosis of the tibia. J Child Orthop 2010;4:507-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Das SP, Ganesh S, Pradhan S, Singh D, Mohanty RN. Effectiveness of recombinant human bone morphogenetic protein-7 in the management of congenital pseudoarthrosis of the tibia: A randomised controlled trial. Int Orthop 2014;38:1987-1992. [DOI] [PubMed] [Google Scholar]
- 29.Johnston CE. Congenital pseudarthrosis of the tibia: results of technical variations in the charnley-williams procedure. J Bone Joint Surg [Am] 2002;84-A:1799-1810. [PubMed] [Google Scholar]
- 30.Joseph B, Mathew G. Management of congenital pseudarthrosis of the tibia by excision of the pseudarthrosis, onlay grafting, and intramedullary nailing. J Pediatr Orthop B 2000;9:16-23. [DOI] [PubMed] [Google Scholar]
- 31.Joseph B, Somaraju VVJ, Shetty SK. Management of congenital pseudarthrosis of the tibia in children under 3 years of age: effect of early surgery on union of the pseudarthrosis and growth of the limb. J Pediatr Orthop 2003;23:740-746. [DOI] [PubMed] [Google Scholar]
- 32.Kim HW, Weinstein SL. Intramedullary fixation and bone grafting for congenital pseudarthrosis of the tibia. Clin Orthop Relat Res 2002;405:250-257. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Mei H, Zhu G, et al. Congenital pseudarthrosis of the tibia in children: should we defer surgery until 3 years old? J Pediatr Orthop B 2018;27:17-25. [DOI] [PubMed] [Google Scholar]
- 34.Stephens Richards B, Anderson TD. RhBMP-2 and intramedullary fixation in congenital pseudarthrosis of the tibia. J Pediatr Orthop 2018;38:230-238. [DOI] [PubMed] [Google Scholar]
- 35.Vigouroux F, Mezzadri G, Parot R, et al. Vascularised fibula or induced membrane to treat congenital pseudarthrosis of the Tibia: A multicentre study of 18 patients with a mean 9.5-year follow-up. Orthop Traumatol Surg Res 2017;103:747-753. [DOI] [PubMed] [Google Scholar]
- 36.Boero S, Catagni M, Donzelli O, Facchini R, Frediani PV. Congenital pseudarthrosis of the tibia associated with neurofibromatosis-1: treatment with Ilizarov’s device. J Pediatr Orthop 1997;17:675-684. [DOI] [PubMed] [Google Scholar]
- 37.Borzunov DY, Chevardin AY, Mitrofanov AI. Management of congenital pseudarthrosis of the tibia with the Ilizarov method in a paediatric population: influence of aetiological factors. Int Orthop 2016;40:331-339. [DOI] [PubMed] [Google Scholar]
- 38.Hissnauer TN, Stiel N, Babin K, et al. Bone morphogenetic protein-2 for the treatment of congenital pseudarthrosis of the tibia or persistent tibial nonunion in children and adolescents: A retrospective study with a minimum 2-year follow-up. J Mater Sci Mater Med 2017;28:60. [DOI] [PubMed] [Google Scholar]
- 39.Ohnishi I, Sato W, Matsuyama J, et al. Treatment of congenital pseudarthrosis of the tibia: a multicenter study in Japan. J Pediatr Orthop 2005;25:219-224. [DOI] [PubMed] [Google Scholar]
- 40.Paley D, Catagni M, Argnani F, et al. Treatment of congenital pseudoarthrosis of the tibia using the Ilizarov technique. Clin Orthop Relat Res 1992;280:81-93. [PubMed] [Google Scholar]
- 41.Agashe MV, Song SH, Refai MA, Park KW, Song HR. Congenital pseudarthrosis of the tibia treated with a combination of Ilizarov’s technique and intramedullary rodding. Acta Orthop 2012;83:515-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shabtai L, Ezra E, Wientroub S, Segev E.. Congenital tibial pseudarthrosis, changes in treatment protocol. J Pediatr Orthop B 2015;24:444-449. [DOI] [PubMed] [Google Scholar]
- 43.Yan A, Mei HB, Liu K, et al. Wrapping grafting for congenital pseudarthrosis of the tibia. Medicine (Baltimore) 2017;96:e8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu GH, Mei HB, He RG, et al. Combination of intramedullary rod, wrapping bone grafting and Ilizarov’s fixator for the treatment of Crawford type IV congenital pseudarthrosis of the tibia: mid-term follow up of 56 cases. BMC Musculoskelet Disord 2016;17:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalra GD, Agarwal A. Experience with free fibula transfer with screw fixation as a primary modality of treatment for congenital pseudarthosis of tibia in children - Series of 26 cases. Indian J Plast Surg 2012;45:468-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiland AJ, Weiss AP, Moore JR, Tolo VT. Vascularized fibular grafts in the treatment of congenital pseudarthrosis of the tibia. J Bone Joint Surg [Am] 1990;72-A:654-662. [PubMed] [Google Scholar]
- 47.Lee FY-I, Sinicropi SM, Lee FS, et al. Treatment of congenital pseudarthrosis of the tibia with recombinant human bone morphogenetic protein-7 (rhBMP-7). A report of five cases. J Bone Joint Surg [Am] 2006;88-A:627-633. [DOI] [PubMed] [Google Scholar]
- 48.Högler W, Yap F, Little D, et al. Short-term safety assessment in the use of intravenous zoledronic acid in children. J Pediatr 2004;145:701-704. [DOI] [PubMed] [Google Scholar]
- 49.Carlier A, Vasilevich A, Marechal M, De Boer J, Geris L. In silico clinical trials for pediatric orphan diseases. Sci Rep 2018;8:2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Westberry DE, Carpenter AM, Tisch J, Wack LI. Amputation outcomes in congenital pseudarthrosis of the tibia. J Pediatr Orthop 2018;38:e475-e481. [DOI] [PubMed] [Google Scholar]
- 51.Shah H, Joseph B, Nair BVS, et al. What factors influence union and refracture of congenital pseudarthrosis of the tibia? A multicenter long-term study. J Pediatr Orthop 2018;38:e332-e337. [DOI] [PubMed] [Google Scholar]
- 52.Kesireddy N, Kheireldin RK, Lu A, et al. Current treatment of congenital pseudarthrosis of the tibia. J Pediatr Orthop B 2018;27:541-550. [DOI] [PubMed] [Google Scholar]
- 53.Karol LA, Haideri NF, Halliday SE, Smitherman TB, Johnston CE 2nd. Gait analysis and muscle strength in children with congenital pseudarthrosis of the tibia: the effect of treatment. J Pediatr Orthop 1998;18:381-386. [PubMed] [Google Scholar]
- 54.Seo SG, Lee DY, Kim YS, et al. Foot and ankle function at maturity after Ilizarov treatment for atrophic-type congenital pseudarthrosis of the tibia a comprehensive outcome comparison with normal controls. J Bone Joint Surg [Am] 2016;98:490-498. [DOI] [PubMed] [Google Scholar]
- 55.Andersen KS. Radiological classification of congenital pseudarthrosis of the tibia. Acta Orthop Scand 1973;44:719-727. [DOI] [PubMed] [Google Scholar]
- 56.Crawford AH, Bagamery N. Osseous manifestations of neurofibromatosis in childhood. J Pediatr Orthop 1986;6:72–88. [DOI] [PubMed] [Google Scholar]
- 57.Choi IH, Cho T-J, Moon HJ. Ilizarov treatment of congenital pseudarthrosis of the tibia: a multi-targeted approach using the Ilizarov technique. Clin Orthop Surg 2011;3:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paley D, Catagni MA, Argnani F, et al. Ilizarov treatment of tibial nonunions with bone loss. Clin Orthop Relat Res 1989;241:146-165. [PubMed] [Google Scholar]
- 59.Grill F, Ganger R, Petje G, Schmid R. Congenital pseudarthrosis of the tibia. Orthopade 2000;29:821-831. [DOI] [PubMed] [Google Scholar]
- 60.Wientroub S, Grill F. Congenital pseudarthrosis of the tibia: Part 1. European Pediatric Orthopaedic Society multicenter study of congenital pseudoarthrosis. J Pediatr Orthop B 2000;9:1-2. [DOI] [PubMed] [Google Scholar]
- 61.Morrissy RT. Congenital pseudarthrosis of the tibia. Factors that affect results. Clin Orthop Relat Res 1982;166:21-27. [PubMed] [Google Scholar]
- 62.Gray JC, Elves MW. Early osteogenesis in compact bone isografts: a quantitative study of contributions of the different graft cells. Calcif Tissue Int 1979;29:225-237. [DOI] [PubMed] [Google Scholar]
- 63.Choi IH, Lee SJ, Moon HJ, et al. ‘4-in-1 Osteosynthesis’ for atrophic-type congenital pseudarthrosis of the tibia. J Pediatr Orthop 2011;31:697-704. [DOI] [PubMed] [Google Scholar]
- 64.Paley D. Congenital pseudarthrosis of the tibia: combined pharmacologic and surgical treatment using biphosphonate intravenous infusion and bone morphogenic protein with periosteal and cancellous autogenous bone grafting, tibio-fibular cross union, intramedullary rodding and external fixation In Zorzi A. (ed.), 2012: 91-106; InTech, Available from: https://www.intechopen.com/books/bone-grafting/treatment-of-congenital-pseudarthrosis-with-periosteal-and-cancellous-bone-grafting-
- 65.Paley D. Congenital pseudarthrosis of the tibia Current Progress in Orthopaedics. Johari A and Waddell J. (eds) Mumbai: Tree Life Media; 2017:318-348. [Google Scholar]
- 66.Packer D, Robb J, Liu R, Robbins C, Paley D. Combined pharmacologic and biological treatment of congenital pseudoarthrosis of the tibia; 100 % union; no refractures! J Child Orthop 2016;10:S19-S20. [Google Scholar]
- 67.Schindeler A, Little DG. Recent insights into bone development, homeostasis, and repair in type 1 neurofibromatosis (NF1). Bone 2008;42:616-622. [DOI] [PubMed] [Google Scholar]
- 68.Johnston CE, Birch JG. A tale of two tibias: a review of treatment options for congenital pseudarthrosis of the tibia. J Child Orthop 2008;2:133-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stiel N, Hissnauer TN, Rupprecht M, et al. Evaluation of complications associated with off-label use of recombinant human bone morphogenetic protein-2 (rhBMP-2) in pediatric orthopaedics. J Mater Sci Mater Med 2016;27:184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.