Significance
Changes in climate and disturbance regimes may cause abrupt shifts in vegetation communities. Identifying climatic conditions that can limit tree regeneration is important for understanding when and where wildfires may catalyze such changes. This study quantified relationships between annual climate conditions and regeneration of Pinus ponderosa (ponderosa pine) and Pseudotsuga menziesii (Douglas-fir), two ecologically and economically important conifer species in low-elevation forests of western North America. We found that regeneration exhibited a threshold response to annual climate conditions and the forests we sampled crossed these climate thresholds in the past 20 years, resulting in fewer recruitment opportunities through time. In areas that have crossed climatic thresholds for regeneration, stand-replacing fires may result in abrupt ecosystem transitions to nonforest states.
Keywords: ecosystem transition, climate change, wildfire, ponderosa pine, Douglas-fir
Abstract
Climate change is increasing fire activity in the western United States, which has the potential to accelerate climate-induced shifts in vegetation communities. Wildfire can catalyze vegetation change by killing adult trees that could otherwise persist in climate conditions no longer suitable for seedling establishment and survival. Recently documented declines in postfire conifer recruitment in the western United States may be an example of this phenomenon. However, the role of annual climate variation and its interaction with long-term climate trends in driving these changes is poorly resolved. Here we examine the relationship between annual climate and postfire tree regeneration of two dominant, low-elevation conifers (ponderosa pine and Douglas-fir) using annually resolved establishment dates from 2,935 destructively sampled trees from 33 wildfires across four regions in the western United States. We show that regeneration had a nonlinear response to annual climate conditions, with distinct thresholds for recruitment based on vapor pressure deficit, soil moisture, and maximum surface temperature. At dry sites across our study region, seasonal to annual climate conditions over the past 20 years have crossed these thresholds, such that conditions have become increasingly unsuitable for regeneration. High fire severity and low seed availability further reduced the probability of postfire regeneration. Together, our results demonstrate that climate change combined with high severity fire is leading to increasingly fewer opportunities for seedlings to establish after wildfires and may lead to ecosystem transitions in low-elevation ponderosa pine and Douglas-fir forests across the western United States.
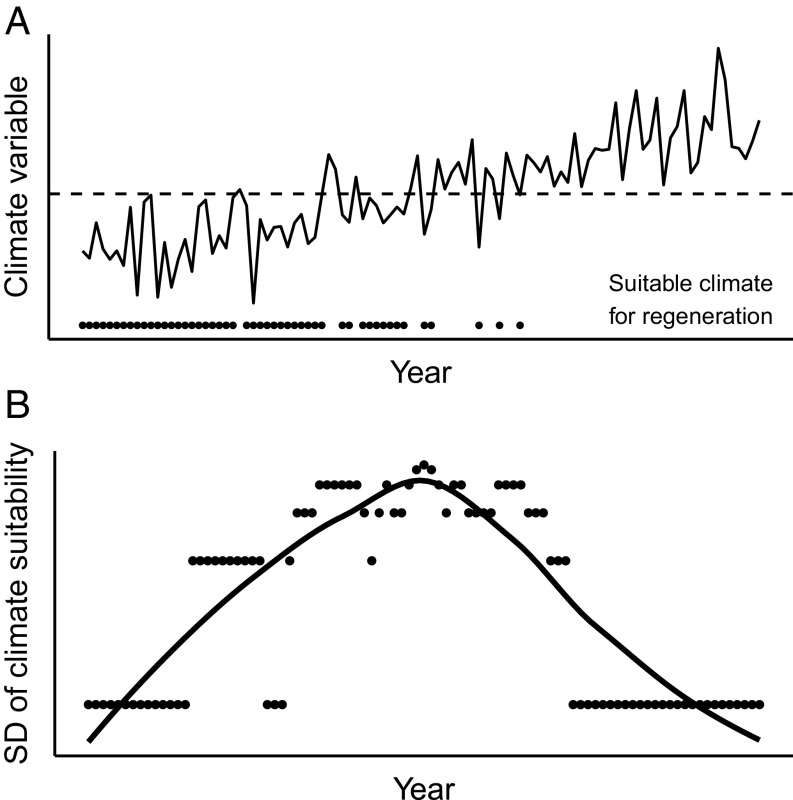
As climate and disturbance regimes change, abrupt transitions are increasingly recognized and predicted in ecological systems (1, 2). Abrupt transitions between different ecosystem states can occur when gradually changing environmental conditions cross critical thresholds beyond which small changes produce large ecosystem responses (1, 2). Before reaching a critical threshold or “tipping point,” gradual changes in environmental conditions may cause increased variance but do not necessarily lead to distinct changes in ecosystem states (1, 3, 4). In stochastic environments, systems may oscillate between two states before a transition (3, 5). For example, tree species that have climatic thresholds for regeneration may experience episodic recruitment as climate temporally varies between conditions that are suitable and unsuitable for regeneration (refs. 6 and 7, Fig. 1). Despite widespread interest in understanding ecological thresholds in an era of rapid global change (8–10), quantifying abrupt climate-induced vegetation shifts remains a substantial and important scientific challenge.
Fig. 1.
Conceptual diagram depicting a simulated hypothetical annual climate variable (e.g., mean summer temperature or vapor pressure deficit; solid line) and the corresponding expected changes to the frequency of years suitable for regeneration (dots), as mean climate conditions increase and cross a threshold for regeneration (dashed line) (A). When climate conditions are suitable for regeneration every year, other factors such as cone production may still cause episodic establishment. As climatic thresholds are approached, the SD of climate suitability for regeneration increases, shown here using a moving 10-y window and summarized with a locally weighted polynomial regression smoothing (LOESS) (B). Once a critical threshold is crossed, variability declines again, as fewer years are suitable for regeneration.
Stand-replacing fires can catalyze vegetation shifts during periods of directional climate change, accelerating changes that would otherwise take decades to centuries to play out (11–13). This is particularly true in forest ecosystems, where adult trees can live for centuries and tolerate a broader range of climate conditions than juveniles of the same species (6, 14, 15). Disturbance-catalyzed change at lower treeline, where trees grow at the warm, dry margin of their climatic tolerances, may be one of the first visible signs of forest ecosystems adjusting to new climate conditions. Recent evidence suggests that wildfires may already be catalyzing vegetation shifts in forests across the western United States (16), with limited tree regeneration following fires in recent decades (e.g., refs. 17–19). This is particularly acute in low-elevation forests (17, 20–23), implicating climate change as an important driver of regeneration failures. However, the annual climate conditions which limit tree regeneration are poorly resolved, and potential thresholds to regeneration have not been identified. Understanding if recent reductions in postfire tree regeneration signal an ecosystem transition (e.g., to a nonforested state) requires a quantitative understanding of how seasonal to interannual variations in climate impact tree seedling germination and establishment.
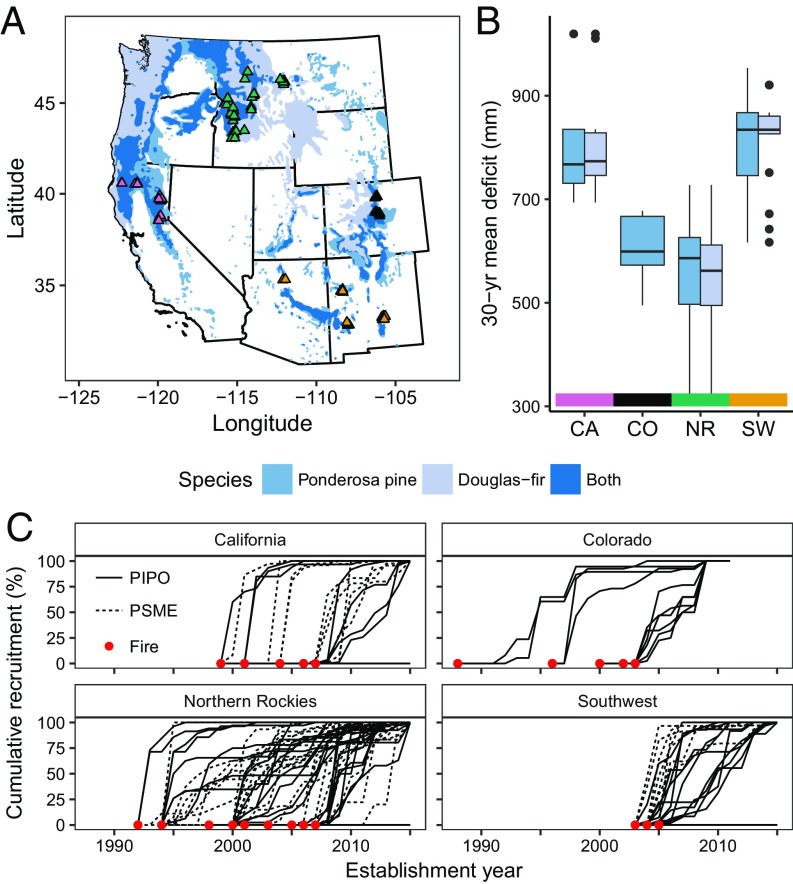
Here we demonstrate that dry low-elevation Pinus ponderosa (ponderosa pine) and Pseudotsuga menziesii (Douglas-fir) forests of the western United States have crossed a critical climate threshold for postfire tree regeneration. We focused on ponderosa pine and Douglas-fir because they are widespread ecologically and economically important conifers in low-elevation forests of western North America. We sampled across climate gradients in space and time (1988–2015) using annually resolved establishment dates from 2,935 trees that established after 33 wildfires in four regions across the western United States (Fig. 2) to (i) quantify the relationship between seasonal to annual climate and regeneration, (ii) identify critical climate thresholds for regeneration, and (iii) assess how climate suitability for postfire regeneration has changed over recent decades. To isolate the effect of annual climate, we accounted for the effect of other drivers of postfire regeneration, including fire severity and distance to seed source. Our results demonstrate threshold responses of annual tree recruitment to vapor pressure deficit (VPD), surface temperature, and soil moisture. Climate conditions in the low-elevation forests we sampled have repeatedly crossed these thresholds over the past 20 y, revealing a decline in the climate suitability for postfire tree regeneration across broad regions of the western United States. These findings imply that increased frequency of stand-replacing fires could initiate abrupt ecosystem transitions in low-elevation ponderosa pine and Douglas-fir forests.
Fig. 2.
Map of study site locations in the western United States (A), 30-y (1981–2010) mean climatic water deficit for sample sites within each region (B), and cumulative recruitment following fires for each site (C). Overall, 90 sites were sampled (see SI Appendix, Tables S1 and S2 for details). Ponderosa pine (PIPO) and Douglas-fir (PSME) ranges are shown in blue in A (72).
Results and Discussion
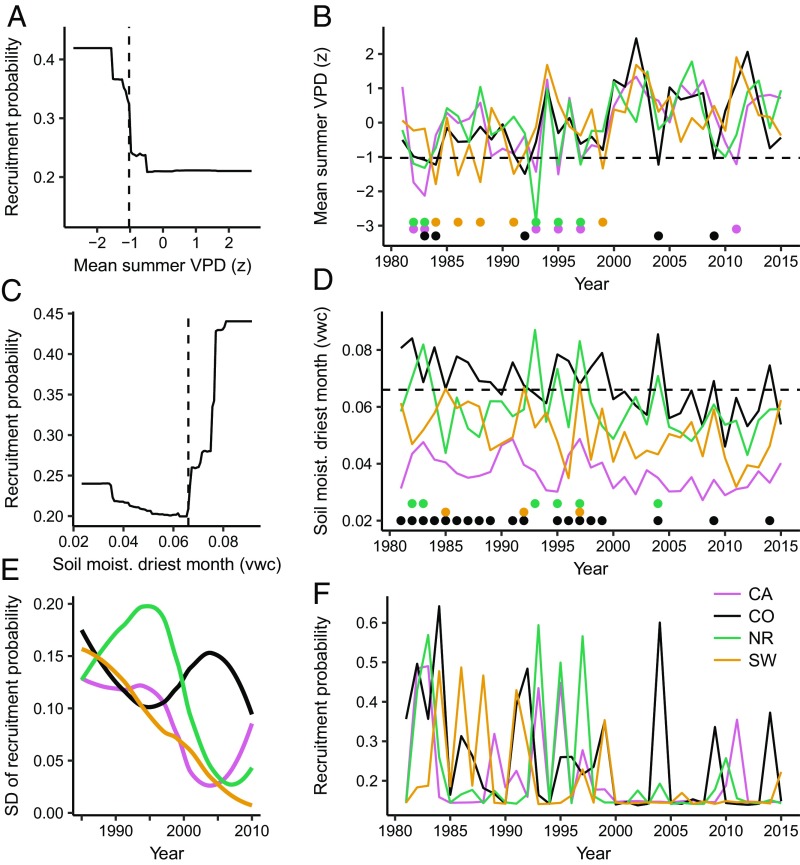
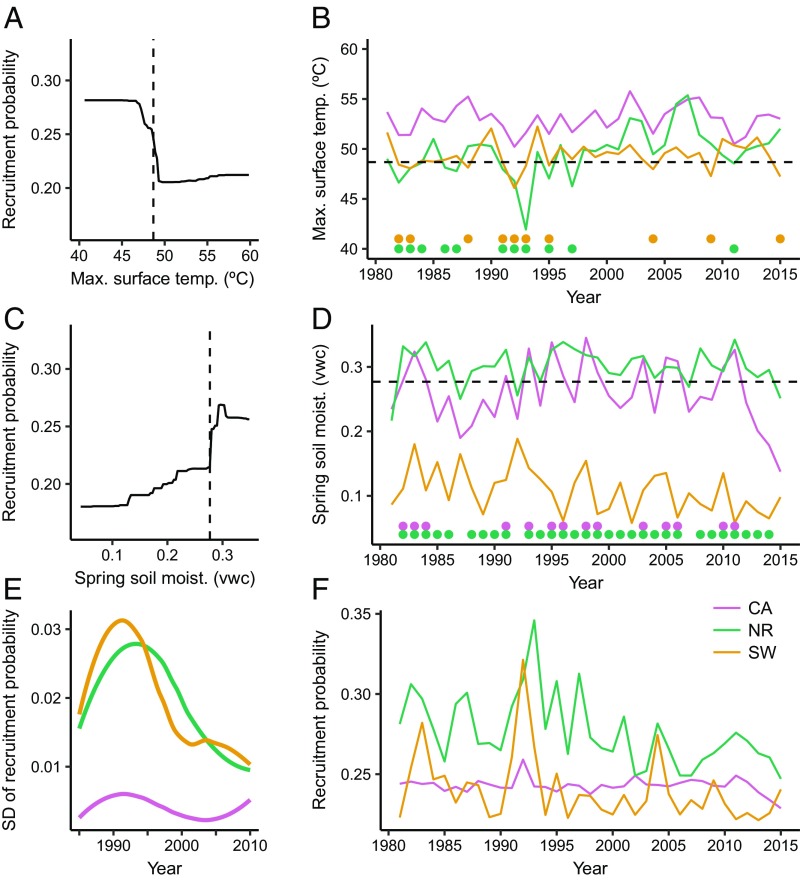
Annual rates of tree regeneration exhibited strongly nonlinear relationships with annual climate conditions, with distinct threshold responses to summer VPD, soil moisture, and maximum surface temperatures (Figs. 3 and 4). Across the study region, seasonal to annual climate conditions from the early 1990s through 2015 have crossed these climate thresholds at the majority of sites (SI Appendix, Fig. S8), indicating conditions that are increasingly unsuitable for tree regeneration, particularly for ponderosa pine. We assessed changes in the climate suitability for postfire regeneration by projecting our boosted regression tree models [mean area under the curve (AUC) 0.81; SI Appendix, Table S4] with climate time series for each site from 1980 to 2015, while holding nonclimatic factors constant (Figs. 3F and 4F). We found abrupt declines in modeled annual recruitment probability in the 1990s for both species and across all regions, with the exception of Douglas-fir in California and Colorado. The cumulative probability of postfire recruitment calculated on a 5- and 10-y basis also declined in the Southwest and Northern Rockies for both species, and in Colorado and California for ponderosa pine (SI Appendix, Figs. S9 and S10). Change-point detection analysis identified significant changes in modeled recruitment probability in the early to mid-1990s (SI Appendix, Table S5). These declines in annual and cumulative recruitment probability correspond directly to years when mean climate conditions approached threshold values for recruitment (Figs. 3 and 4), making it increasingly unlikely that future climate conditions will be favorable (i.e., a cool or wet year) for subsequent regeneration pulses.
Fig. 3.
Threshold response of recruitment to annual climate and modeled annual recruitment probability for ponderosa pine. Partial dependency plots from a boosted regression tree model show the marginal effect of the two most important climate variables on annual recruitment probability, after accounting for the average effects of all other variables in the model (A and C). Annual time series of climate variables at each site averaged by region (B and D). Climate thresholds are identified with vertical (A and C) and horizontal (B and D) dashed lines. Dots below the lines in B and D represent years when that specific climate variable was suitable for regeneration. The influence of both climate variables on regeneration are summarized by the modeled annual recruitment probability (F), while holding constant time since fire (1 y), distance to seed source (50 m), and fire severity (dNBR 412). Variability in annual recruitment probability (E) is shown as the SD of recruitment probability values from F, calculated in 10-y moving windows and plotted with a LOESS. The metric vwc indicates the ratio of water volume to soil volume.
Fig. 4.
Threshold response of recruitment to annual climate and modeled annual recruitment probability for Douglas-fir. Partial dependency plots from a boosted regression tree model show the marginal effect of the two most important climate variables on annual recruitment probability, after accounting for the average effects of all other variables in the model (A and C). Annual time series of climate variables at each site averaged by region (B and D). Climate thresholds are identified with vertical (A and C) and horizontal (B and D) dashed lines. Dots below the lines in B and D represent years when that specific climate variable was suitable for regeneration. The influence of both climate variables on regeneration are summarized by the modeled annual recruitment probability (F), while holding constant time since fire (1 y), distance to seed source (50 m), and fire severity (dNBR 412). Variability in annual recruitment probability (E) is shown as the SD of recruitment probability values from F, calculated in 10-y moving windows and plotted with a LOESS. The metric vwc indicates the ratio of water volume to soil volume.
Theory predicts that variance in state variables will increase as critical transitions are approached (3, 4), and then decrease once thresholds are crossed (ref. 2, Fig. 1). Consistent with this, in most regions we found that the variability in the modeled annual recruitment probability increased initially and then declined over time, once climate thresholds were crossed (Figs. 3E and 4E). There were two exceptions to this pattern, which highlight important complexities in how climate change may impact postfire tree regeneration. First, the annual recruitment probability of ponderosa pine in Colorado varied little over time (Fig. 3E), because as mean VPD increased, so too did interannual variability in VPD, such that some years were still suitable for recruitment (Fig. 3B). Increased variability in climate metrics was not seen in other regions. Second, the probability of Douglas-fir regeneration in California sites likewise varied little over time, reflecting little change in the mean and variability of the climate predictors of Douglas-fir recruitment (Fig. 4 B and D). Across our study regions, we also observed higher variability in observed recruitment at sites closer to the dry margins of tree distributions. By analyzing the annual patterns of observed recruitment across sites, we showed that sites with drier 30-y mean climate conditions exhibited more episodic recruitment than wetter sites (SI Appendix, Fig. S13; t = −2.9, df = 69, P = 0.005), highlighting the prevalence of episodic recruitment at sites near climatic thresholds (24, 25).
Differences between the sensitivity of ponderosa pine and Douglas-fir to annual climate have important implications for species-specific responses to climate change (26, 27). Both species were sensitive to soil moisture; first-year conifer seedlings have shallow root systems and frequently succumb to desiccation (28–31). However, Douglas-fir regeneration was more strongly related to spring soil moisture, which has not declined in recent decades, than to soil moisture of the driest month, which has recently crossed threshold values. This pattern explains the larger declines in annual recruitment probability for ponderosa pine relative to Douglas-fir (Figs. 3 and 4). Ponderosa pine was additionally sensitive to high summer VPD, which leads to increased transpiration rates and increased plant water stress. VPD, which has increased over the past several decades (ref. 32, Fig. 3), is recognized as an important factor determining tree mortality, growth rates, and seedling survival (31, 33–36). VPD is predicted to increase in the future (32). Consequently, the probability of ponderosa pine regeneration at low-elevation sites will likely decline. Douglas-fir recruitment was sensitive to high maximum surface temperatures, which kill seedlings by damaging vascular tissue (37–39). The threshold response we observed is consistent with previous experiments that show threshold mortality responses to soil surface temperatures at or near 55 °C (37, 40). This mechanism of mortality is especially important in disturbed areas where there is no remaining canopy cover to ameliorate high maximum temperatures near the soil surface (41, 42). Intraspecific differences in climatic tolerances are also likely to exist (43–45). For example, across the study area, ponderosa pine recruitment was more strongly related to site-specific VPD anomalies than raw VPD values, suggesting that ecotypic variation or local adaptation among populations in part influences how annual climate impacts germination and survival (36). However, in our models, geographic region had a negligible effect on annual recruitment probability and no strong interactions with annual climate variables. Thus, our results suggest that consistent threshold relationships with annual climate were identifiable across the entire range of both species.
While annual climate was an important driver of postfire regeneration, our findings also highlight that the nature of a fire event strongly influences postfire regeneration. For example, the combined relative influence of annual climate variables on tree recruitment in our boosted regression tree (BRT) models was 24% for ponderosa pine and 34% for Douglas-fir (SI Appendix, Fig. S3), while the relative influence of distance to seed source, which is largely determined by fire severity, was 32% for ponderosa pine and 21% for Douglas-fir (SI Appendix, Fig. S3). The importance of seed tree availability in determining postfire regeneration has been demonstrated across forest types in the western United States (e.g., refs. 17, 20, and 46), suggesting that increases in high severity burned patch sizes (e.g., ref. 47) will significantly reduce tree regeneration. Beyond affecting seed availability, fire severity can also affect regeneration by altering microclimate (41, 42, 48) or soil properties and biota (49). Furthermore, high severity fire is correlated with high postfire shrub dominance in some regions, which can limit ponderosa pine regeneration (19). Accordingly, we found that ponderosa pine regeneration was lower at sites that experienced higher fire severity (SI Appendix, Fig. S3). High shrub cover also corresponded with more episodic establishment of ponderosa pine (SI Appendix, Fig. S13; t = −3.2, df = 69, P = 0.002), with pronounced peaks of regeneration immediately following fire at sites with high shrub cover. Fire severity was much less influential for Douglas-fir regeneration (SI Appendix, Fig. S3). Overall, our results indicate that the impacts of annual climate conditions on tree regeneration are strongly mediated by multiple biotic and abiotic factors.
While our results reveal clear relationships between annual climate and the probability of tree regeneration, they are constrained by two limitations. First, our statistical models focus on climate conditions during the year of seedling germination. Theory and observations highlight that mortality during the year of germination is a key bottleneck affecting conifer demography (28, 30, 31, 39, 50). Our results are consistent with this perspective: climate during the year of germination was a significant predictor of tree demography at sites sampled years to decades after germination. However, annual climate conditions in the years following germination through the year of sampling, may also affect seedling survival. Second, our statistical models did not account for cone or seed production, an important determinant of tree regeneration that also varies with annual climate conditions (51, 52). As years with suitable conditions for regeneration become increasingly rare, the episodic nature of cone production will likely further limit regeneration, particularly if years with high cone production do not align with years with suitable climate for germination and survival.
Our results reveal an important pathway linking climate change and interannual climate variability to recently observed reductions in postfire tree regeneration. Our findings suggest that many low-elevation mixed conifer forests in the western United States have already crossed climatic thresholds beyond which the climate is unsuitable for regeneration. Once climate exceeds climatic thresholds for regeneration and fire results in adult mortality, sites that currently experience episodic recruitment may lose local tree populations (6). We sampled dry ponderosa pine and Douglas-fir sites within each region, but as climate continues to get warmer and drier (32, 53), more areas will cross climatic thresholds that limit conifer recruitment. Increasing frequency of extreme fire weather could lead to more high severity fire (54), while decreases in summer precipitation (55) coupled with changes in fuel aridity and timing of spring snowmelt, have led to more area burned across the western United States in the past several decades (56, 57). The combination of more area burned, potentially at high severity, with decreasing climate suitability for postfire regeneration could lead to rapid transitions from ponderosa pine and Douglas-fir forests to nonforest vegetation. Our results highlight the potential for ecological processes to exhibit rapid transitions, and we expect that fire will increasingly catalyze vegetation shifts at lower treeline in the future. The nonlinear relationships between annual climate and regeneration observed in this study are likely not unique to these two species (e.g., ref. 30). Thus, the combination of fire and climate change may lead to abrupt ecosystem changes where other tree species exhibit similar threshold responses to climate.
Materials and Methods
Our study was designed to understand the relationship between annual climate conditions and postfire tree regeneration in dry conifer forests dominated by P. ponderosa (ponderosa pine) and/or P. menziesii (Douglas-fir). We used dendrochronology to determine the germination year of trees established after 33 fires in 90 sites across four regions in the western United States: the Northern Rockies (NR), Colorado Front Range (CO), Southwest (SW), and Northern California (CA) (Fig. 2). Within each region, we sampled sites near the warm/dry limits of regional forest extent, to bracket the climatic conditions suitable for recruitment; further, these areas are where we would expect tree regeneration to be most sensitive to climate change. We constructed a statistical model predicting the annual recruitment probability at each site as a function of biophysical variables. The model results provide insights into the nature of climatic and nonclimatic controls of postfire tree regeneration, and through hindcasting, the model allowed us to assess how climate suitability for tree regeneration has varied over the past several decades across our study regions. The data and code used in this study are publicly available via the Dryad Data Repository: https://doi.org/10.5061/dryad.pc3f9d8 (58).
Field Sampling.
In each region, we selected sites that burned at moderate to high severity between 1988 and 2007 [based on Monitoring Trends in Burn Severity (MTBS) data (59) and later field verified], had no postfire planting and had 30-y (1980–2009) mean climatic water deficits within the top (i.e., driest) 50th percentile for each species within each region (SI Appendix, Supplemental Methods). From a random set of sites that fit these criteria, we sampled a total of 19 sites in CA (from six fires), 10 in CO (from five fires), 40 in NR (from 18 fires), and 21 in SW (from four fires) (SI Appendix, Tables S1 and S2). Historically, forests in these regions experienced mixed severity fire regimes, with SW and some CA sites characterized by low-severity surface fire regimes (60–62).
In NR, SW, and CA, sampling occurred in 60-m long belt transects with variable width (2–40 m), with the goal of destructively sampling ∼30 trees per transect. All trees that established following fire in each transect were sampled; as all sampled trees were less than 25 y old, we use the term “juvenile” hereafter to refer to sampled individuals. We destructively sampled juveniles to obtain precise germination years, because field-based methods for estimating tree ages are not accurate with annual precision (63). If no juveniles were present at initial randomly identified sample points, site data were recorded and the zero density was retained in the dataset; this occurred at 30% of sites. Where none of the preselected random sample points within a fire yielded at least 30 juveniles for sampling, new plots were located in areas with more regeneration. Thus, field sampling was designed to accurately reconstruct tree age structures (which inherently requires trees to be present), not the probability of juvenile presence/absence at each site.
To destructively sample juveniles, soil was excavated and a segment of the stem from at least 10 cm below and 10 cm above the root–shoot boundary was removed. At three points along each transect, shrub cover was estimated in 2 × 3 m plots. Distance to the nearest seed source (i.e., live reproductive tree) of each species was recorded from the center of the transect with a laser range finder. Data collection in CO followed similar protocols, although not enough Douglas-fir juveniles were sampled to be included in this analysis. Additionally, juveniles in CO were defined as trees <150 cm in height, which excluded three trees at two sites from sampling (25).
Dendrochronology.
To identify the germination year of juvenile trees with annual accuracy, sample stems were cut into 2.5-cm-long segments; the bottom of each segment was progressively sanded with finer-grit sandpaper (up to 600–1,500 grit; ref. 64) to reveal ring boundaries. Tree rings on each segment were counted at 10–40× with a Nikon SMZ stereomicroscope, and the segment with the most rings, which also corresponded with the first appearance of pith, was used to age the sample (24, 25, 65). We recorded visual marker years, but the young age of the trees precluded more formal cross-dating methods. To test the precision of our aging methods, 555 random samples were recounted by three technicians. The mean (SD) difference in ring-count-based ages among the technicians was 0.298 (0.461) y. If ring boundaries were indistinct or pith dates were otherwise ambiguous, then the sample was not included in the final dataset. In total, we used 2,935 aged juveniles in our analyses (SI Appendix, Table S1).
Climate and Biophysical Data.
We chose a suite of biophysical variables as potential predictors of tree recruitment, based on their direct effects on seed availability and plant–water relations impacting germination and survival. As potential predictors of seed availability and site conditions suitable for germination, we used the MTBS-calculated fire severity value for the 30 × 30 m pixel including each site [differenced normalized burn ratio (dNBR)] and the field-measured distance to seed source.
Bioclimatic variables included mean summer (June–August) VPD (in kilopascals), maximum surface temperature (in Celsius), mean spring (March–May) soil moisture [volumetric water content (vwc), ratio of water volume to soil volume], mean soil moisture of the driest month (vwc), and climatic water deficit (in millimeters). Bioclimatic variables were calculated from gridded climate data from 1979 to 2015 (55) with a resolution of 250 m at daily or subdaily timescales and then summarized to seasonal or annual values (SI Appendix, Supplemental Methods). Soil moisture and maximum land surface temperature (LST) were modeled using the ECH20 ecohydrology model (66) following methods described by Simeone et al. (31). Soil moisture was characterized at 0–10 cm, the depths reached by young conifer seedling roots.
Data Analysis.
We constructed BRT models for each species separately to predict annual recruitment as a function of annual climate during the year of germination and other biophysical variables (67). Annual recruitment was modeled as a binomial process, with “success” defined by annual recruitment rates (no. juveniles ha−1⋅yr−1) exceeding a region-specific threshold: the 25th percentile of annual recruitment rates from among all years with recruitment for a given species in a given region. This threshold accounts for varying forest density among regions (SI Appendix, Table S1), and we use this value to represent the annual recruitment rate needed for successful “regeneration” at each site. We also conducted our analysis using a 50th percentile threshold and juvenile presence/absence alone, which produced similar results to those reported in the main text (SI Appendix, Figs. S4–S7).
Predictors used for the BRT models included both static and dynamic, time-varying variables. Static variables, specific to each site, included distance to seed source and fire severity. Time-varying variables, specific to each year and site combination, included time since fire, mean summer VPD (calculated as a site-specific z score), maximum surface temperature, annual climatic water deficit (calculated as a site-specific z score), mean soil moisture of the driest month, and mean spring soil moisture. We developed an initial BRT model using all of the above predictor variables, and then used the relative influence of each bioclimatic variable to select the most influential moisture-related (i.e., deficit or soil moisture) and energy-related (i.e., VPD or maximum surface temperature) variables; this resulted in a final BRT model with five predictor variables: distance to seed source, fire severity, time since fire, a moisture-related annual bioclimatic variable, and an energy-related annual bioclimatic variable. We used R version 3.3.3 (68), the package “dismo” (69), and the function “gbm.step” to fit BRT models. To account for lack of spatial independence in our observations, each fold in the k-fold cross-validation used by gbm.step included data from one fire (70), resulting in 25 folds for the ponderosa pine model and 23 folds for the Douglas-fir model. We tested predictive performance by leaving out each site, fitting a BRT model with the same settings as above, predicting the holdout site, and then calculating accuracy (defined as the proportion of years with correct prediction) and the AUC statistic.
To examine how the climate suitability for recruitment has changed with shifts in annual climate over the past 35 y, we used the species-specific BRT models to hindcast annual recruitment probability at each site based on the site-specific annual climate time series (1981–2015; SI Appendix, Fig. S8) and a constant distance to seed source (50 m), time since fire (1 y), and dNBR value (412, the median across all sites). This median dNBR value loosely corresponds to the median observed fire-related tree mortality of 90%; such high mortality likely resulted from torching of individual trees, active crown fire, or smoldering at the base of trees, given the thick bark of both study species. A range of distance-to-seed-source and dNBR values were initially tested when hindcasting the models; while the mean annual recruitment probability differed based on these initial values, the temporal patterns were consistent among different initial values (SI Appendix, Figs. S11 and S12). Thus, because distance to seed source, time since fire, and fire severity were held constant, the modeled annual recruitment probability at each site is a measure of climate suitability for recruitment in the first year after a hypothetical fire. Modeled annual recruitment probabilities for sites within each region were averaged to create a single time series for each region and species combination (Figs. 3F and 4F). We characterized the temporal variability in the regional annual recruitment probability values by calculating the SD of recruitment probability in consecutive 10-y windows (Figs. 3E and 4E). We identified significant shifts in the regional time series of annual (year 1 postfire) and cumulative (years 1–5 or 1–10 postfire) (SI Appendix, Supplemental Methods) recruitment probabilities using a change-point detection algorithm [Sup(F)] in the “strucchange” package in R (9, 71).
Supplementary Material
Acknowledgments
We thank L. Hankin, S. Pracht, E. Berglund, and L. Crofutt for assistance with field and lab work; E. Burke and E. Heyerdahl for helpful insights about counting tree rings; K. Kemp for sharing data and information from sites in the Northern Rockies; and the many US Forest Service employees who assisted with obtaining permits and provided information about local fires and management history. K.T.D., S.Z.D., and P.E.H. were funded by the Joint Fire Science Program, project 16-1-01-15. K.T.D., S.Z.D., A.S., and M.P.M. were also funded by National Science Foundation Grant BCS-1461576. S.Z.D. received further support from the USDA National Institute of Food and Agriculture, McIntire Stennis program, project 1012438. Support for Z.A.H. was provided by National Aeronautics and Space Administration Applied Sciences Grant NNH11ZDA001N-FIRES. T.T.V. and M.T.R. were funded by National Science Foundation Grants BCS-1232997 and OISE-0966472.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. C.H. is a guest editor invited by the Editorial Board.
Data deposition: The data and code used in this study have been deposited in the Dryad Data Repository, https://doi.org/10.5061/dryad.pc3f9d8.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1815107116/-/DCSupplemental.
References
- 1.Scheffer M, Carpenter S, Foley JA, Folke C, Walker B. Catastrophic shifts in ecosystems. Nature. 2001;413:591–596. doi: 10.1038/35098000. [DOI] [PubMed] [Google Scholar]
- 2.Bestelmeyer BT, et al. Analysis of abrupt transitions in ecological systems. Ecosphere. 2011;2:1–26. [Google Scholar]
- 3.Scheffer M, et al. Early-warning signals for critical transitions. Nature. 2009;461:53–59. doi: 10.1038/nature08227. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter SR, Brock WA. Rising variance: A leading indicator of ecological transition. Ecol Lett. 2006;9:311–318. doi: 10.1111/j.1461-0248.2005.00877.x. [DOI] [PubMed] [Google Scholar]
- 5.Dakos V, van Nes EH, Scheffer M. Flickering as an early warning signal. Theor Ecol. 2013;6:309–317. [Google Scholar]
- 6.Jackson ST, Betancourt JL, Booth RK, Gray ST. Ecology and the ratchet of events: Climate variability, niche dimensions, and species distributions. Proc Natl Acad Sci USA. 2009;106:19685–19692. doi: 10.1073/pnas.0901644106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrus RA, Harvey BJ, Rodman KC, Hart SJ, Veblen TT. Moisture availability limits subalpine tree establishment. Ecology. 2018;99:567–575. doi: 10.1002/ecy.2134. [DOI] [PubMed] [Google Scholar]
- 8.Groffman P, et al. Ecological thresholds: The key to successful environmental management or an important concept with no practical application? Ecosystems (NY) 2006;9:1–13. [Google Scholar]
- 9.Andersen T, Carstensen J, Hernández-García E, Duarte CM. Ecological thresholds and regime shifts: Approaches to identification. Trends Ecol Evol. 2009;24:49–57. doi: 10.1016/j.tree.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Cavanaugh KC, et al. Poleward expansion of mangroves is a threshold response to decreased frequency of extreme cold events. Proc Natl Acad Sci USA. 2014;111:723–727. doi: 10.1073/pnas.1315800111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Svenning JC, Sandel B. Disequilibrium vegetation dynamics under future climate change. Am J Bot. 2013;100:1266–1286. doi: 10.3732/ajb.1200469. [DOI] [PubMed] [Google Scholar]
- 12.Crausbay SD, Higuera PE, Sprugel DG, Brubaker LB. Fire catalyzed rapid ecological change in lowland coniferous forests of the Pacific Northwest over the past 14,000 years. Ecology. 2017;98:2356–2369. doi: 10.1002/ecy.1897. [DOI] [PubMed] [Google Scholar]
- 13.Gavin D, Brubaker L, Greenwald D. Postglacial climate and fire-mediated vegetation change on the western Olympic Peninsula, Washington (USA) Ecol Monogr. 2013;83:471–489. [Google Scholar]
- 14.Bell DM, Bradford JB, Lauenroth WK. Early indicators of change: Divergent climate envelopes between tree life stages imply range shifts in the western United States. Glob Ecol Biogeogr. 2014;23:168–180. [Google Scholar]
- 15.Dobrowski SZ, et al. Forest structure and species traits mediate projected recruitment declines in western US tree species. Glob Ecol Biogeogr. 2015;24:917–927. [Google Scholar]
- 16.Davis KT, Higuera PE, Sala A. Anticipating fire-mediated impacts of climate change using a demographic framework. Funct Ecol. 2018;32:1729–1745. [Google Scholar]
- 17.Stevens-Rumann CS, et al. Evidence for declining forest resilience to wildfires under climate change. Ecol Lett. 2018;21:243–252. doi: 10.1111/ele.12889. [DOI] [PubMed] [Google Scholar]
- 18.Roccaforte JP, Fulé PZ, Chancellor WW, Laughlin DC. Woody debris and tree regeneration dynamics following severe wildfires in Arizona ponderosa pine forests. Can J For Res. 2012;42:593–604. [Google Scholar]
- 19.Welch KR, Safford HD, Young TP. Predicting conifer establishment post wildfire in mixed conifer forests of the North American Mediterranean-climate zone. Ecosphere. 2016;7:e01609. [Google Scholar]
- 20.Tepley AJ, Thompson JR, Epstein HE, Anderson-Teixeira KJ. Vulnerability to forest loss through altered postfire recovery dynamics in a warming climate in the Klamath Mountains. Glob Change Biol. 2017;23:4117–4132. doi: 10.1111/gcb.13704. [DOI] [PubMed] [Google Scholar]
- 21.Donato DC, Harvey BJ, Turner MG. Regeneration of montane forests 24 years after the 1988 yellowstone fires: A fire-catalyzed shift in lower treelines? Ecosphere. 2016;7:e01410. [Google Scholar]
- 22.Rother MT, Veblen TT. Limited conifer regeneration following wildfires in dry ponderosa pine forests of the Colorado Front Range. Ecosphere. 2016;7:e01594. [Google Scholar]
- 23.Kemp KB, Higuera PE, Morgan P, Abatzoglou JT. Climate will increasingly determine post-fire tree regeneration success in low-elevation forests, Northern Rockies, USA. Ecosphere. 2019;10:e02568. [Google Scholar]
- 24.League K, Veblen T. Climatic variability and episodic Pinus ponderosa establishment along the forest-grassland ecotones of Colorado. For Ecol Manage. 2006;228:98–107. [Google Scholar]
- 25.Rother MT, Veblen TT. Climate drives episodic conifer establishment after fire in dry ponderosa pine forests of the Colorado Front Range, USA. Forests. 2017;8:159. [Google Scholar]
- 26.Williams JW, Shuman BN, Webb T, Bartlein PJ, Leduc PL. Late-quaternary vegetation dynamics in North America: Scaling from taxa to biomes. Ecol Monogr. 2004;74:309–334. [Google Scholar]
- 27.Fisichelli N, et al. First-year seedlings and climate change: Species-specific responses of 15 North American tree species. Oikos. 2014;123:1331–1340. [Google Scholar]
- 28.Johnson DM, McCulloh KA, Reinhardt K. The earliest stages of tree growth: Development, physiology and impacts of microclimate. In: Meinzer FC, Lachenbruch B, Dawson TE, editors. Size- and Age-Related Changes in Tree Structure and Function, Tree Physiology. Vol 4. Springer; Dordrecht, The Netherlands: 2011. pp. 65–87. [Google Scholar]
- 29.Rother MT, Veblen TT, Furman LG. A field experiment informs expected patterns of conifer regeneration after disturbance under changing climate conditions. Can J For Res. 2015;45:1607–1616. [Google Scholar]
- 30.Reinhardt K, Germino MJ, Kueppers LM, Domec JC, Mitton J. Linking carbon and water relations to drought-induced mortality in Pinus flexilis seedlings. Tree Physiol. 2015;35:771–782. doi: 10.1093/treephys/tpv045. [DOI] [PubMed] [Google Scholar]
- 31.Simeone C, et al. Coupled ecohydrology and plant hydraulics modeling predicts ponderosa pine seedling mortality and lower treeline in the US Northern Rocky Mountains. New Phytol. September 27, 2018 doi: 10.1111/nph.15499. [DOI] [PubMed] [Google Scholar]
- 32.Ficklin DL, Novick KA. Historic and projected changes in vapor pressure deficit suggest a continental-scale drying of the United States atmosphere. J Geophys Res D Atmospheres. 2017;122:2061–2079. [Google Scholar]
- 33.Restaino CM, Peterson DL, Littell J. Increased water deficit decreases Douglas fir growth throughout western US forests. Proc Natl Acad Sci USA. 2016;113:9557–9562. doi: 10.1073/pnas.1602384113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eamus D, Boulain N, Cleverly J, Breshears DD. Global change-type drought-induced tree mortality: Vapor pressure deficit is more important than temperature per se in causing decline in tree health. Ecol Evol. 2013;3:2711–2729. doi: 10.1002/ece3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Will RE, Wilson SM, Zou CB, Hennessey TC. Increased vapor pressure deficit due to higher temperature leads to greater transpiration and faster mortality during drought for tree seedlings common to the forest-grassland ecotone. New Phytol. 2013;200:366–374. doi: 10.1111/nph.12321. [DOI] [PubMed] [Google Scholar]
- 36.McCullough IM, Davis FW, Williams AP. A range of possibilities: Assessing geographic variation in climate sensitivity of ponderosa pine using tree rings. For Ecol Manage. 2017;402:223–233. [Google Scholar]
- 37.Daubenmire RF. Soil temperature versus drought as a factor determining lower altitudinal limits of trees in the Rocky Mountains. Bot Gaz. 1943;105:1–13. [Google Scholar]
- 38.Kolb PF, Robberecht R. High temperature and drought stress effects on survival of Pinus ponderosa seedlings. Tree Physiol. 1996;16:665–672. doi: 10.1093/treephys/16.8.665. [DOI] [PubMed] [Google Scholar]
- 39.Hermann RK, Chilcote WW. Effect of Seedbeds on Germination and Survival of Douglas-Fir. Forest Research Laboratory, Oregon State University; Corvallis, OR: 1965. [Google Scholar]
- 40.Seidel KW. Tolerance of seedlings of ponderosa pine, Douglas-fir, grand fir, and Englemann spruce for high temperatures. Northwest Sci. 1986;60:1–7. [Google Scholar]
- 41.Davis KT, Dobrowski SZ, Holden ZA, Higuera PE, Abatzoglou JT. Microclimatic buffering in forests of the future: The role of local water balance. Ecography. 2018;41:1–11. [Google Scholar]
- 42.Feddema JJ, Mast JN, Savage M. Modeling high-severity fire, drought and climate change impacts on ponderosa pine regeneration. Ecol Modell. 2013;253:56–69. [Google Scholar]
- 43.St Clair JB, Mandel NL, Vance-Borland KW. Genecology of Douglas fir in western Oregon and Washington. Ann Bot. 2005;96:1199–1214. doi: 10.1093/aob/mci278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bansal S, Harrington CA, Gould PJ, St Clair JB. Climate-related genetic variation in drought-resistance of Douglas-fir (Pseudotsuga menziesii) Glob Change Biol. 2015;21:947–958. doi: 10.1111/gcb.12719. [DOI] [PubMed] [Google Scholar]
- 45.Shinneman DJ, Means RE, Potter KM, Hipkins VD. Exploring climate niches of ponderosa pine (Pinus ponderosa Douglas ex Lawson) haplotypes in the Western United States: Implications for evolutionary history and conservation. PLoS One. 2016;11:e0151811. doi: 10.1371/journal.pone.0151811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kemp KB, Higuera PE, Morgan P. Fire legacies impact conifer regeneration across environmental gradients in the U.S. Northern Rockies. Landsc Ecol. 2016;31:619–636. [Google Scholar]
- 47.Stevens JT, Collins BM, Miller JD, North MP, Stephens SL. Changing spatial patterns of stand-replacing fire in California conifer forests. For Ecol Manage. 2017;406:28–36. [Google Scholar]
- 48.Montes-Helu MC, et al. Persistent effects of fire-induced vegetation change on energy partitioning and evapotranspiration in ponderosa pine forests. Agric For Meteorol. 2009;149:491–500. [Google Scholar]
- 49.Certini G. Effects of fire on properties of forest soils: A review. Oecologia. 2005;143:1–10. doi: 10.1007/s00442-004-1788-8. [DOI] [PubMed] [Google Scholar]
- 50.Moyes AB, Castanha C, Germino MJ, Kueppers LM. Warming and the dependence of limber pine (Pinus flexilis) establishment on summer soil moisture within and above its current elevation range. Oecologia. 2013;171:271–282. doi: 10.1007/s00442-012-2410-0. [DOI] [PubMed] [Google Scholar]
- 51.Moreira X, Abdala-Roberts L, Linhart YB, Mooney KA. Effects of climate on reproductive investment in a masting species: Assessment of climatic predictors and underlying mechanisms. J Ecol. 2015;103:1317–1324. [Google Scholar]
- 52.Eis S. Cone production of Douglas fir and Grand fir and its climatic requirements. Can J For Res. 1973;3:61–70. [Google Scholar]
- 53.IPCC 2014. Climate change 2014: AR5 synthesis report. Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change, eds Pachauri RK, Meyer LA (IPCC, Geneva), p 151.
- 54.Parks SA, et al. High-severity fire: Evaluating its key drivers and mapping its probability across western US forests. Environ Res Lett. 2018;13:044037. [Google Scholar]
- 55.Holden ZA, et al. Decreasing fire season precipitation increased recent western US forest wildfire activity. Proc Natl Acad Sci USA. 2018;115:E8349–E8357. doi: 10.1073/pnas.1802316115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Westerling AL. Increasing western US forest wildfire activity: Sensitivity to changes in the timing of spring. Philos Trans R Soc Lond B Biol Sci. 2016;371:20150178, and erratum (2016) 371:20160373. doi: 10.1098/rstb.2015.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abatzoglou JT, Williams AP. Impact of anthropogenic climate change on wildfire across western US forests. Proc Natl Acad Sci USA. 2016;113:11770–11775. doi: 10.1073/pnas.1607171113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davis KT, et al. 2019 doi: 10.5061/dryad.pc3f9d8. Data from “Wildfires and climate change push low-elevation forests across a critical climate threshold for tree regeneration.” Dryad. Available at . . Deposited February 5, 2019. [DOI]
- 59.Eidenshink J, et al. A project for monitoring trends in burn severity. Fire Ecol. 2007;3:3–21. [Google Scholar]
- 60.Baker WL. Fire Ecology in Rocky Mountain Landscapes. Island Press; Washington, DC: 2009. p. 544. [Google Scholar]
- 61.Sugihara NG, Van Wagtendonk JW, Fites-Kaufman J, Shaffer KE, Thode AE. Fire in California’s Ecosystems. Univ California Press; Berkeley, CA: 2006. p. 596. [Google Scholar]
- 62.Agee JK. Fire Ecology of Pacific Northwest Forests. Island Press; Washington, DC: 1993. p. 493. [Google Scholar]
- 63.Hankin LE, Higuera PE, Davis KT, Dobrowski SZ. Accuracy of node and bud-scar counts for aging two dominant conifers in Western North America. For Ecol Manage. 2018;427:365–371. [Google Scholar]
- 64.Speer JH. Fundamentals of Tree-Ring Research. Univ. Arizona Press; Tucson, AZ: 2010. p. 333. [Google Scholar]
- 65.Telewski FW. Determining the germination date of woody plants: A proposed method for locating the root/shoot interface. Tree Ring Bull. 1993;53:13–16. [Google Scholar]
- 66.Maneta MP, Silverman NL. A spatially distributed model to simulate water, energy, and vegetation dynamics using information from regional climate models. Earth Interact. 2013;17:1–44. [Google Scholar]
- 67.Elith J, Leathwick JR, Hastie T. A working guide to boosted regression trees. J Anim Ecol. 2008;77:802–813. doi: 10.1111/j.1365-2656.2008.01390.x. [DOI] [PubMed] [Google Scholar]
- 68.R Core Team 2017. R: A Language and Environment for Statistical Computing Version 3.3.3 (R Foundation for Statistical Computing, Vienna)
- 69.Hijmans RJ, Phillips S, Leathwick J, Elith J. 2017 dismo: Species Distribution Modeling. R Package Version 1.1-4. Available at https://CRAN.R-project.org/package=dismo. Accessed February 26, 2019.
- 70.De’ath G. Boosted trees for ecological modeling and prediction. Ecology. 2007;88:243–251. doi: 10.1890/0012-9658(2007)88[243:btfema]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 71.Zeileis A, Leisch F, Hornik K, Kleiber C. strucchange: An R package for testing for structural change in linear regression models. J Stat Softw. 2002;7:1–38. [Google Scholar]
- 72.Little EL. Atlas of United States Trees. US Department of Agriculture, Forest Service; Washington, DC: 1971. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.