Midlife obesity has recently been identified as a global pandemic (1). Morbidities and mortalities attributable to excess adiposity include atherosclerosis, type 2 diabetes (T2D) (2), certain cancers (3), and dementia (4), each of which has reached epidemic proportions on its own. It is no exaggeration to state that T2D and dementia threaten the emotional and financial stabilities of most Western economies. Moreover, when T2D and dementia comorbidity is present, the severities and costs of management of each are dramatically increased (5). Thus, there are major imperatives that drive current inquiries into the borderland where cognition and metabolism intersect. New research in PNAS by Soto et al. (6) provides significant insight into this area by describing dramatic links to phenotypes relevant to cognition, behavior, and metabolism. A key set of principles drove this work: (i) insulin receptors (IRs) and insulin-like growth factor 1 (IGF-1) receptors (IGF1Rs), upon binding of their cognate ligand, trigger two closely related signaling pathways that promote both redundant and distinct intracellular effects (7), and (ii) this redundancy includes the formation of IR/IGF1R hybrids, and it has been estimated that at least half of the IRs and IGF1Rs in brain exist as heterodimers (8), which have greatest affinity for IGF-1 and IGF-2 (Fig. 1).
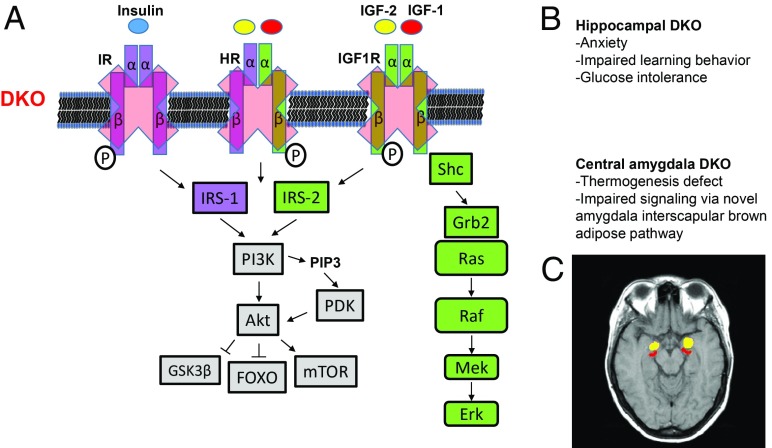
Fig. 1.
Focal forebrain IR/IGF1R DKO eliminates signaling by receptor homodimers and hybrids in hippocampus or central amygdala. (A) Focal forebrain IR/IGF1R DKO eliminates homodimers and IR/IGF1R heterodimers and precludes distinct and collateral insulin, IGF-1, and IGF-2 signaling via these receptors. HR, IR/IGF1R hybrid receptors; IRS, insulin receptor substrate. (B) Targeted DKO in forebrain causes dramatic region-specific phenotypes. Hippo-DKO mice show anxiety, learning behavior deficits, and glucose intolerance, while CeA-DKO mice show defective thermogenesis via a previously unknown pathway linking this brain region to interscapular brown adipose tissue function. (C) Axial human brain magnetic resonance image showing bilateral hippocampi (red) and amygdalae (yellow).
Although it is widely recognized that insulin and IGF-1 overlap extensively, most studies on insulin and brain aging have largely centered on the cognitive decline associated with T2D (3, 9–11), while studies on IGF-1 and brain aging largely focus on neurogenesis (12), cerebrovascular function (13), and/or proteostasis (14) Efforts at clarifying the role of brain insulin signaling in cognitive aging remain challenging (3, 10). Insulin is not required for glucose uptake by neurons, leading to the conclusions that brain neurons may be responding to insulin acting either as a growth factor during development or as a neurotransmitter peptide, or perhaps both (15, 16). Furthermore, controversy remains as to whether the brain “sees” less insulin in the setting of T2D or aging and/or insulin resistance manifests in the brain as is typically observed in metabolically relevant tissues of individuals with impaired insulin sensitivity (10).
The mainstay model of dysregulated brain insulin signaling has been the neuronal IR knockout (NIRKO) mouse, developed by Kahn and colleagues (17) about 20 y ago. Although brain IRs are widely dispersed, most studies in NIRKO mice by Kahn and others have focused on the role of brain insulin signaling in energy balance, glucose metabolism, and reproduction. NIRKO mice represent perhaps the most discrete and best defined model of brain insulin resistance. Neurobiological phenotypes of NIRKO mice appear to arise from the dysfunction of mitochondria and/or the dopaminergic system (18). These are important pieces of the puzzle but fail to explain completely what function(s) may be played by IRs localized to other brain regions, especially those in the forebrain.
This is where the new research comes in. Soto et al. (6) employ genetically modified adeno-associated viruses in a compound viral gene-knockout strategy that generates animals with targeted brain-regional combined deficiencies in both IRs and IGF1Rs. These animals display dramatic and unexpected region-specific phenotypes that provide insight into the nature and importance of brain insulin (and IGF-1) signaling.
The two brain regions that were each individually depleted of IRs and IGF1Rs (and by implication, depleted of IR/IGF1R hybrids) were the hippocampus and the central amygdala. The animals arising from these manipulations were designated hippocampus double knockout (Hippo-DKO) mice or central amygdala DKO (CeA-DKO) mice. Hippo-DKO mice displayed increased anxiety, impaired learning behavior, and glucose intolerance, while CeA-DKO mice were relatively normal in those parameters but displayed impaired cold-induced thermogenesis and brown adipose tissue activation.
When synaptosomes from basal and DKO brain regions were analyzed, NMDA receptor content was normal, but levels of AMPA receptor subtypes were decreased. This is consistent with what one might predict based on pharmacological effects of AMPA receptor subtypes (19). In one particular example (19), AMPA-modulated anxiety and learning behavior disorders were phenotypes associated with altered hippocampal neurogenesis. Further work will be required to determine whether the Hippo-DKO phenotype is associated with changes in hippocampal neurogenesis, but given the ability of these ligands to stimulate hippocampal neurogenesis, one could easily imagine bidirectional effects resulting from either increased or deficient IR/IGF1R pathway signaling on this process (15).
Quite strikingly, Hippo-DKO mice also displayed hyperglycemia and impaired glucose tolerance. Central control of insulin sensitivity is well described, but this regulatory function is dogmatically linked to insulin signaling in the mediobasal hypothalamus. Soto et al. (6) demonstrate that compound deficiency of IR/IGF1R (i.e., DKO) restricted to the hippocampus is sufficient to alter peripheral glucose homeostasis, a finding that further adds to the complexity of central regulation of peripheral metabolism.
In contrast, CeA-DKO mice displayed normal anxiety, normal learning behavior, and normal glucose tolerance. Prompted by the discovery that the CeA-DKO mice display altered thermogenesis, Soto et al. (6) subsequently discovered a previously unknown neuroanatomic pathway that physically connects the central amygdala with interscapular brown adipose tissue and thermogenesis.
Prompted by the discovery that the CeA-DKO mice display altered thermogenesis, Soto et al. subsequently discovered a previously unknown neuroanatomic pathway that physically connects the central amygdala with interscapular brown adipose tissue and thermogenesis.
The lessons here are twofold: (i) When studying systems with redundancy and collateral compensatory mechanisms, it is imperative that all possibility for redundancy be eliminated. Here it is worth emphasizing that homodimers of IRs, homodimers of IGF1Rs, and heterodimers of IR/IGF1R hybrids can all contribute in the intact system. (ii) When complex signaling systems are completely abolished such that no collateral signaling is possible, even focal dysfunction can cause systemic phenotypes. In this case, not only were the lesions focal and relatively small, but they also involved forebrain regions not typically associated with modulation of glucose or energy homeostasis. The conventional wisdom would suggest that these effects on metabolism would more likely be attributable to targeting these canonical receptors in defined hypothalamic regions, rather than in the central amygdala, as shown by Soto et al. (6).
Circling back to our opening thoughts regarding dementia associated with T2D, these data provide insight into how such metabolic complications might lead to cognitive decline. Although dementia associated with T2D is often equated with Alzheimer’s disease, recent neuropathological studies indicate that it is probably a variant of vascular cognitive impairment and dementia (VCID) syndrome (6, 9, 10). This formulation is consistent with evidence that damage to other end organs (kidney, retina, etc.) is vascular in nature. Focal VCID-induced hippocampal damage emerges as a potential specific pathogenesis for the dementia associated with T2D. Clinical metabolic-biomarker studies are underway (20) to determine whether the VCID prevalence observed at postmortem (6) can be validated during life (20). Moreover, maintaining adequate insulin and/or IGF-1 signaling in these discrete yet critical brain regions could prove vital to preserving cerebrovascular function and cognitive abilities in T2D and aging.
Acknowledgments
S.G. is supported by NIH Grants R01 AG061894, U01 AG046170, R01AG058469, U01OH011314, and P50AG005138 and by Department of Veterans Affairs Rehabilitation Research and Development Service Awards 1I01RX000684 and 1I01RX002333. Additional support to S.G. was provided by the Rudin Family Foundation, the Alzheimer’s Drug Discovery Foundation, the Werber Family Foundation, the Sara and Gideon Gartner Foundation, the Louis B. Mayer Foundation, the Jennifer and Scott Moskowitz Foundation, the Jane Martin and Stuart Katz Foundation, the Lady Va and Sir Deryck Maughan Foundation, the Georgianne and Dr. Reza Khatib Foundation, and the George B. Link Foundation. D.M.H. is supported by NIH Grants R01 AG057429, R21 AG055026, and P30 AG038072 and by the American Federation for Aging Research.
Footnotes
The authors declare no conflict of interest.
See companion article on page 6379.
References
- 1.Unnikrishnan R, Pradeepa R, Joshi SR, Mohan V. Type 2 diabetes: Demystifying the global epidemic. Diabetes. 2017;66:1432–1442. doi: 10.2337/db16-0766. [DOI] [PubMed] [Google Scholar]
- 2.Singh-Manoux A, et al. Obesity trajectories and risk of dementia: 28 years of follow-up in the Whitehall II Study. Alzheimers Dement. 2018;14:178–186. doi: 10.1016/j.jalz.2017.06.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 4.Stoeckel LE, et al. Complex mechanisms linking neurocognitive dysfunction to insulin resistance and other metabolic dysfunction. F1000 Res. 2016;5:353. doi: 10.12688/f1000research.8300.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cholerton B, Baker LD, Montine TJ, Craft S. Type 2 diabetes, cognition, and dementia in older adults: Toward a precision health approach. Diabetes Spectr. 2016;29:210–219. doi: 10.2337/ds16-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soto M, Cai W, Konishi M, Kahn CR. Insulin signaling in the hippocampus and amygdala regulates metabolism and neurobehavior. Proc Natl Acad Sci USA. 2019;116:6379–6384. doi: 10.1073/pnas.1817391116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai W, et al. Domain-dependent effects of insulin and IGF-1 receptors on signalling and gene expression. Nat Commun. 2017;8:14892. doi: 10.1038/ncomms14892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailyes EM, et al. Insulin receptor/IGF-I receptor hybrids are widely distributed in mammalian tissues: Quantification of individual receptor species by selective immunoprecipitation and immunoblotting. Biochem J. 1997;327:209–215. doi: 10.1042/bj3270209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abner EL, et al. Diabetes is associated with cerebrovascular but not Alzheimer’s disease neuropathology. Alzheimers Dement. 2016;12:882–889. doi: 10.1016/j.jalz.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnold SE, et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nat Rev Neurol. 2018;14:168–181. doi: 10.1038/nrneurol.2017.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talbot K, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122:1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen E, Dillin A. The insulin paradox: Aging, proteotoxicity and neurodegeneration. Nat Rev Neurosci. 2008;9:759–767. doi: 10.1038/nrn2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lichtenwalner RJ, et al. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- 14.Sonntag WE, et al. Insulin-like growth factor-1 in CNS and cerebrovascular aging. Front Aging Neurosci. 2013 doi: 10.3389/fnagi.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziegler AN, Levison SW, Wood TL. Insulin and IGF receptor signalling in neural-stem-cell homeostasis. Nat Rev Endocrinol. 2015;11:161–170. doi: 10.1038/nrendo.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Werner H, LeRoith D. Insulin and insulin-like growth factor receptors in the brain: Physiological and pathological aspects. Eur Neuropsychopharmacol. 2014;24:1947–1953. doi: 10.1016/j.euroneuro.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Brüning JC, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 18.Kleinridders A, et al. Insulin resistance in brain alters dopamine turnover and causes behavioral disorders. Proc Natl Acad Sci USA. 2015;112:3463–3468. doi: 10.1073/pnas.1500877112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez-Garcia G, et al. PTSD-related behavioral traits in a rat model of blast-induced mTBI are reversed by the mGluR2/3 receptor antagonist BCI-838. eNeuro. 2018 doi: 10.1523/ENEURO.0357-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg J, Lechea N, Pentang GN, Shah NJ. What magnetic resonance imaging reveals—A systematic review of the relationship between type II diabetes and associated brain distortions of structure and cognitive functioning. Front Neuroendocrinol. 2019;52:79–112. doi: 10.1016/j.yfrne.2018.10.001. [DOI] [PubMed] [Google Scholar]