Significance
Taurine transporter deficiency in mice results in hyperammonemia and reduced glutamine formation in hepatic scavenger cells due to an impaired ammonia transport at 3 months of age and a tyrosine nitration-dependent inactivation of glutamine synthetase in 12-month-old mice. The data suggest that down-regulation of the ammonia transporter RhBG can be rate limiting for the synthesis of glutamine in mouse liver.
Keywords: glutamine, hyperammonemia, taurine, scavenger cells, oxidative stress
Abstract
Hepatic ammonia handling was analyzed in taurine transporter (TauT) KO mice. Surprisingly, hyperammonemia was present at an age of 3 and 12 months despite normal tissue integrity. This was accompanied by cerebral RNA oxidation. As shown in liver perfusion experiments, glutamine production from ammonia was diminished in TauT KO mice, whereas urea production was not affected. In livers from 3-month-old TauT KO mice protein expression and activity of glutamine synthetase (GS) were unaffected, whereas the ammonia-transporting RhBG protein was down-regulated by about 50%. Double reciprocal plot analysis of glutamine synthesis versus perivenous ammonia concentration revealed that TauT KO had no effect on the capacity of glutamine formation in 3-month-old mice, but doubled the ammonia concentration required for half-maximal glutamine synthesis. Since hepatic RhBG expression is restricted to GS-expressing hepatocytes, the findings suggest that an impaired ammonia transport into these cells impairs glutamine synthesis. In livers from 12-, but not 3-month-old TauT KO mice, RhBG expression was not affected, surrogate markers for oxidative stress were strongly up-regulated, and GS activity was decreased by 40% due to an inactivating tyrosine nitration. This was also reflected by kinetic analyses in perfused liver, which showed a decreased glutamine synthesizing capacity by 43% and a largely unaffected ammonia concentration dependence. It is concluded that TauT deficiency triggers hyperammonemia through impaired hepatic glutamine synthesis due to an impaired ammonia transport via RhBG at 3 months and a tyrosine nitration-dependent inactivation of GS in 12-month-old TauT KO mice.
The liver plays a central role for ammonia homeostasis in the body. A sophisticated structural and functional organization of urea and glutamine synthesis as well as glutaminase has evolved, which not only allows for efficient ammonia detoxication by the liver, but also to adjust urea synthesis to the needs of systemic acid base homeostasis (1, 2). In this organization, glutaminase augments urea synthesis by amplifying the ammonia concentration in the periportal compartment, whereas glutamine synthetase (GS), which is restricted to a small perivenous cell population (3) acts as a high-affinity detoxification system for the ammonia, which escapes periportal urea synthesis (1, 2, 4). Due to this important function, the small perivenous subpopulation of hepatocytes has been termed “scavenger cells” (2). These cells exclusively express GS, the glutamate transporter 1 (Glt1) and the NH3/NH4+ transporter Rhesus type glycoprotein B (RhBG) as well as uptake systems for dicarboxylates (3, 5, 6). Pioneer work with the isolated perfused rat liver suggested already decades ago that intact scavenger cells are essential for maintenance of physiologically low ammonia concentrations in the blood (1, 2, 7), and liver-specific GS deletion results in systemic hyperammonemia in mice (8). This study also confirmed that chronic hyperammonemia in the absence of liver damage is sufficient to induce cerebral oxidative stress and to impair behavior and locomotion, which are hallmarks of hepatic encephalopathy (HE) (8).
The amino acid taurine is highly abundant in brain, liver, and other organs (9). Intracellular taurine concentrations are maintained at high levels through uptake by the taurine transporter TauT (10). Functions of taurine in the body are remarkably diverse: taurine acts as an osmolyte, an antioxidant, and a molecular chaperone, and promotes antiinflammatory effects and modulates neurotransmission (11–13). Studies from TauT KO mice showed that low taurine levels cause a variety of malfunctions in different organs in an age-dependent way (14–20). Within 1 y after birth TauT KO mice lose vision, suffer from auditory, olfactory, and muscle dysfunction, and show altered synaptic transmission in brain (for review see ref. 21). At higher age (beyond 15 mo), liver manifestations develop, such as fibrosis, unspecific hepatitis, and tumor formation (17, 21). However, the pathogenetic events that precede such structural liver dysfunction in aged TauT KO mice remained unclear until now.
In the present study, we analyzed effects of TauT deficiency on hepatic ammonia handling in 3- and 12-mo-old TauT KO mice. Our results suggest that TauT deficiency impairs ammonia detoxification by perivenous scavenger cells in the liver, thereby inducing systemic hyperammonemia. This is explained by down-regulation of the ammonium transporter RhBG at 3 mo of age and a tyrosine nitration-induced inactivation of GS at 12 mo of age due to oxidative/nitrosative stress. The data also suggest that RhBG expression can be a site of control of hepatic glutamine synthesis.
Results
Taurine Transporter Knockout Triggers Hyperammonemia Without Affecting Liver Integrity.
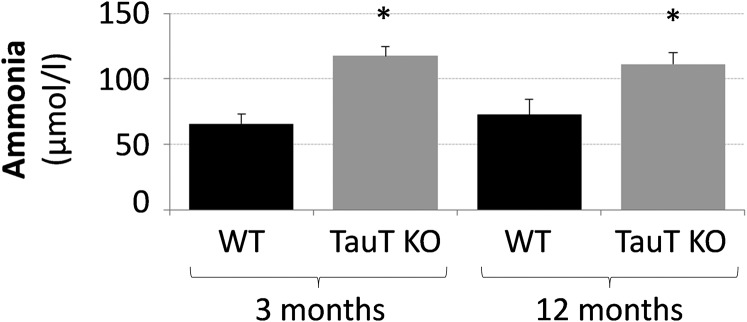
TauT deficiency was shown to trigger liver fibrosis in mice beyond an age of 15 mo (17). However, as shown by Sirius red staining (SI Appendix, Fig. S1A) and immunofluorescence analysis of collagens I and IV (SI Appendix, Fig. S1B), no signs of fibrosis were found in livers from 3- and 12-mo-old TauT KO and WT mice and expression of these surrogate markers of fibrosis was not different from respective wild-type controls. However, a strong Sirius red staining was noted in liver slices from bile duct ligated mice (21 d postsurgery), which served as a positive control (SI Appendix, Fig. S1A). These data suggest that taurine transporter knockout does not induce liver fibrosis or structural liver damage in TauT KO mice up to an age of 12 mo. However, unexpectedly, blood ammonia levels were significantly elevated to about 115 µmol/L in both, 3- and 12-mo-old TauT KO mice compared with WT mice, which presented blood ammonia levels of about 70 µmol/L (Fig. 1).
Fig. 1.
Blood ammonia levels in TauT KO and WT mice. Ammonia levels in blood from 3- and 12-mo-old WT and TauT KO mice were measured as described in Materials and Methods. *Statistically significantly different compared with WT. n = 3–8 animals for each condition.
Effects of TauT KO on Hepatic Glutamine and Urea Production from Ammonia in Perfused Mouse Liver.
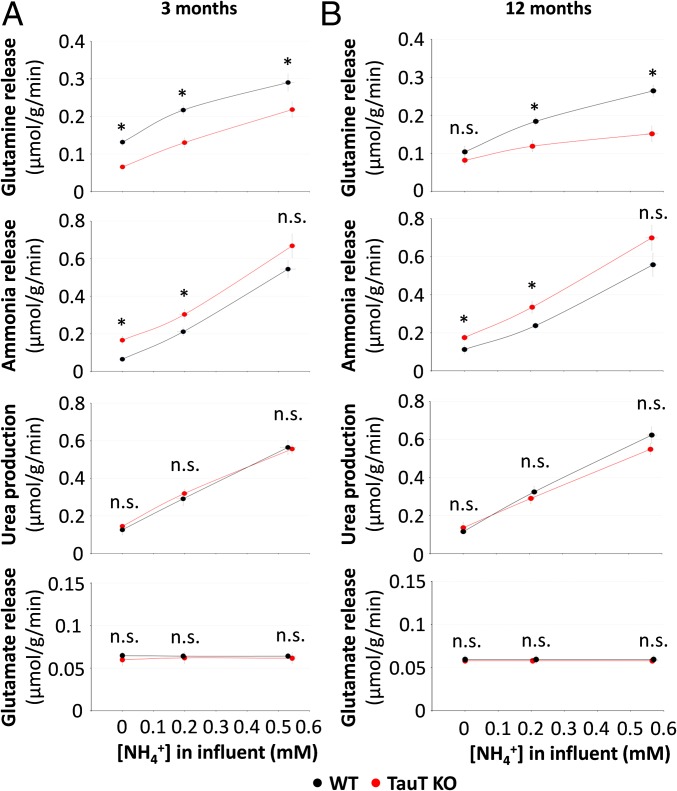
The effect of TauT deficiency on hepatic ammonia handling was analyzed in perfused livers from 3- and 12-mo-old WT and TauT KO mice. As shown in Fig. 2, ammonia clearance by livers from 3- (Fig. 2A) and 12-mo-old (Fig. 2B) TauT KO mice was significantly impaired compared with respective wild-type mice. This was due to an impaired glutamine synthesis, whereas urea production from ammonia and glutamate release were unaffected in TauT KO mice (Fig. 2). In line with this, mRNA and protein expression of carbamoyl-phosphate synthetase 1 (CPS1) and the mRNA levels of ornithine transcarbamylase (OTC) and argininosuccinate lyase (ASL) were not different in livers from 3- or 12-mo-old TauT KO mice compared with the respective wild-type mice (SI Appendix, Fig. S2 A and B). Likewise, neither liver type glutaminase (LGA) nor kidney type glutaminase (KGA) protein levels were altered in livers from TauT KO mice compared with WT mice at 3 or 12 mo of age, respectively (SI Appendix, Fig. S2B).
Fig. 2.
Urea, glutamine, ammonia, and glutamate release into effluent of perfused livers from 3- and 12-mo-old WT and TauT KO mice. Livers from 3 (A)- and 12 (B)-mo-old WT and TauT KO mice were perfused with 0, 0.2, or 0.5 mmol/L NH4Cl and concentrations of urea, glutamine, ammonia, and glutamate in the effluent were measured as described in Materials and Methods. Urea, glutamine, ammonia, and glutamate production is given as µmol/g/min. n.s., not statistically significantly different compared with WT. *Statistically significantly different compared with WT. n = 3–5 animals for each condition.
The data suggest that hyperammonemia in TauT-deficient mice involves an impairment of hepatic glutamine synthesis, but not of urea synthesis.
Effects of TauT Deficiency on Scavenger Cell Marker Expression.
In the liver acinus ammonia that escapes the urea cycle is subsequently detoxified by glutamine synthesis in a small perivenous hepatocyte population termed scavenger cells (1, 2). These scavenger cells not only exclusively express GS, but also the glutamate transporter Glt1 and the NH3/NH4+ transporter RhBG, which is under transcriptional control of T cell factor 4 (TCF4).
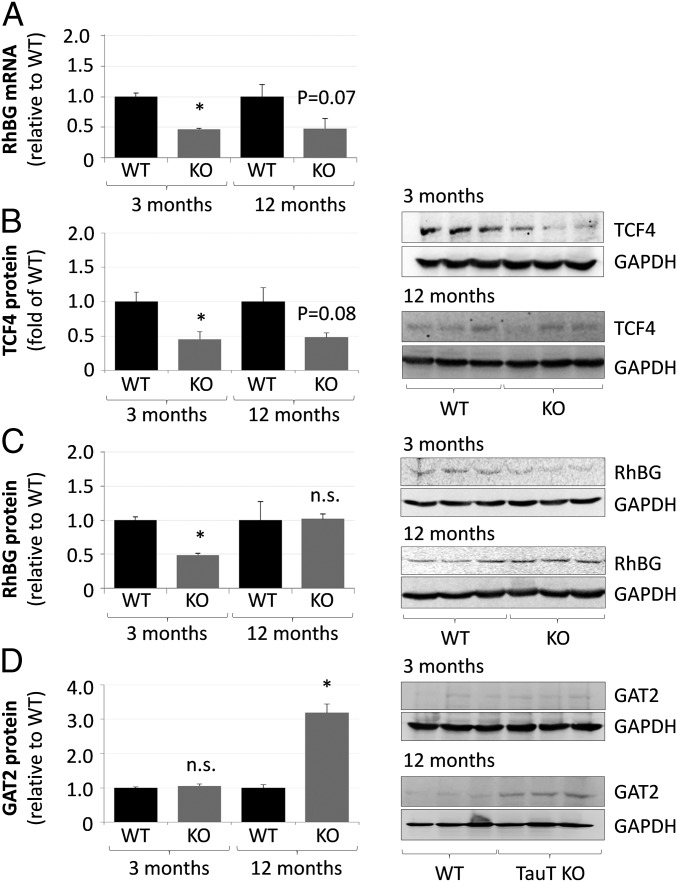
As shown by qPCR, mRNA levels of GS and Glt1 were not affected in both, 3- and 12-mo-old TauT KO mice (SI Appendix, Figs. S3A and S4A), whereas mRNA levels of RhBG were significantly down-regulated in livers from 3-mo-old TauT KO mice and a similar tendency was found in livers from 12-mo-old TauT KO mice (Fig. 3A). TCF4 (Fig. 3B) and RhBG (Fig. 3C and SI Appendix, Fig. S5) protein levels were significantly decreased by about 50% in livers from 3-mo-old TauT KO mice as shown by Western blot or immunofluorescence analysis. However, at an age of 12 mo RhBG (Fig. 3C and SI Appendix, Fig. S5) but not TCF4 (Fig. 3B) protein expression in liver had fully recovered and was no longer different from the wild-type control.
Fig. 3.
Expression levels of RhBG, TCF4, and GAT2 in livers from TauT KO and WT mice. mRNA and protein were isolated from livers of 3- or 12-mo-old TauT KO or WT mice and analyzed for RhBG expression by qPCR (A). Analysis of TCF4 (B), RhBG (C), or GAT2 (D) protein expression by Western blot. mRNA or protein levels in livers from TauT KO are given relative to WT mice. n.s., not statistically significantly different compared with WT. *Statistically significantly different compared with WT. (A and C) Three month, n = 3; 12 mo, n = 4; (B) three month, n = 3; 12 mo, n = 4 WT, and n = 8 KO; and (D) n = 3 animals for each condition.
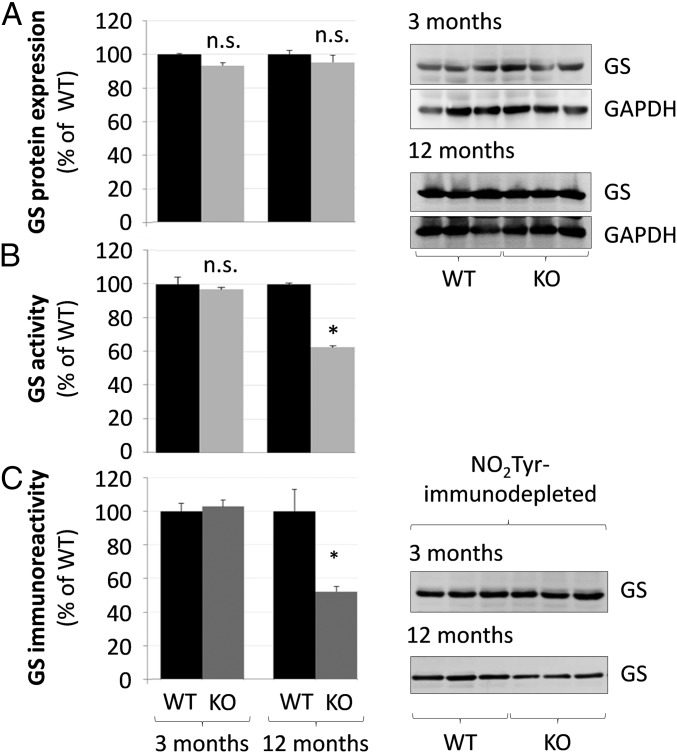
Neither GS mRNA (SI Appendix, Fig. S4A) and protein levels (Fig. 4A) nor the strict perivenous localization of GS in liver were altered in TauT KO mice at 3 and 12 mo of age (Fig. 4A and SI Appendix, Fig. S4B). GS activity was significantly reduced by about 40% in protein lysates of livers from 12- but not from 3-mo-old TauT KO mice compared with the respective wild-type mice (Fig. 4B).
Fig. 4.
Glutamine synthetase expression, activity, and tyrosine nitration in livers from TauT KO and WT mice. Protein was isolated from livers from 3- or 12-mo-old TauT KO or WT mice and analyzed for (A) GS protein expression by Western blot and (B) GS activity (n = 3). (C) GS immunoreactivity (Western blot) in protein lysates from TauT WT and KO mice after depletion of tyrosine-nitrated proteins as described in Materials and Methods (n = 4–6 animals). GS protein expression was quantified by densitometric analysis. GS expression in NO2Tyr-depleted TauT KO protein lysates is given relative to NO2Tyr-depleted WT liver samples. n.s., not statistically significantly different compared with WT. *Statistically significantly different compared with WT.
Glt1 protein levels were significantly increased by about 80% in livers from 3-mo-old and about fourfold in livers from 12-mo-old TauT KO mice as shown by Western blot and immunofluorescence analysis (SI Appendix, Fig. S3 B and C).
Mechanism of Impaired Glutamine Synthesis in Livers from 3-Mo-Old TauT KO Mice.
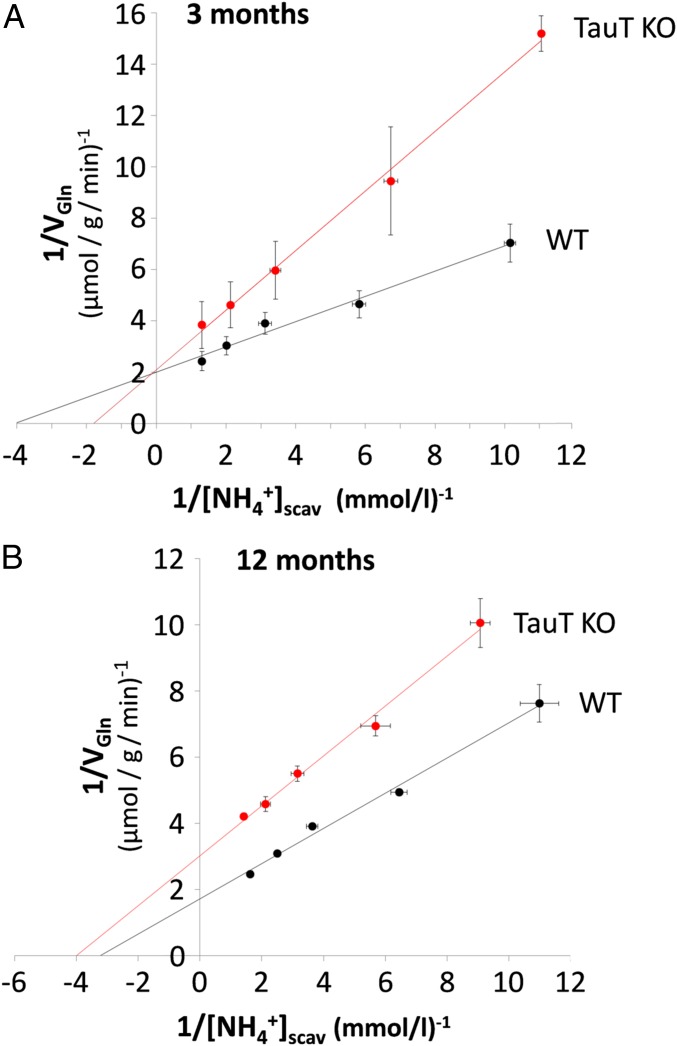
As shown above, in perfused livers from 3-mo-old TauT KO mice, glutamine synthesis from ammonia at concentrations up to 0.5 mmol/L is diminished (Fig. 2A), although GS expression (Fig. 4A) and activity (Fig. 4B) were not affected compared with 3-mo-old wild-type mice. We therefore analyzed the ammonia concentration dependence of glutamine production by the liver (VGln) using a double-reciprocal plot analysis in analogy to Lineweaver–Burk blotting. In this approach, the perivenous intrasinusoidal ammonia concentration available for GS-positive scavenger hepatocytes {[NH4+]scav} can be estimated as the sum of the ammonia concentration in effluent {[NH4+]effl} and the ammonia consumed by glutamine synthesis and being recovered in effluent perfusate {[Gln]effl)}: [NH4+]scav = [NH4+]effl + 2 [Gln]effl. As shown in Fig. 5A double reciprocal blotting of [NH4+]scav versus VGln showed that the maximal rates of hepatic glutamine production were 0.50 µmol/g/min and 0.48 µmol/g/min in 3-mo-old wild-type and TauT KO mice, respectively, indicating that the capacity for glutamine synthesis was not affected by TauT deficiency. On the other hand, the ammonia concentrations {[NH4+]scav} required for half-maximal glutamine production were 0.25 and 0.55 mmol/L in livers from wild-type and TauT KO mice, respectively (SI Appendix, Table S1). These observations are well compatible with an impaired ammonia import into scavenger cells due to a down-regulation of the RhBG ammonia transporter, which apparently becomes rate controlling for glutamine synthesis at near-physiological extracellular ammonia concentrations.
Fig. 5.
Double reciprocal blot analysis of glutamine synthesis versus perivenous ammonia concentration in perfused mouse livers from 3- and 12-mo-old WT and TauT KO mice. Livers were perfused with different NH4Cl concentrations (0–1 mmol/L) and glutamine release (VGln) and ammonia concentration in the effluent were measured as described in Materials and Methods. The perivenous intrasinusoidal ammonia concentration available for GS-positive scavenger hepatocytes {[NH4+]scav} was calculated as the sum of the ammonia concentration in effluent {[NH4+]effl} and the ammonia consumed by glutamine synthesis and being recovered in effluent perfusate {[Gln]effl}:[NH4+]scav = [NH4+]effl + 2 [Gln]effl. Linear regression analysis of the data shown in Fig. 5 yielded the following relationships with y = (VGln)−1 and x = [NH4+]scav−1. (A) Three-month-old animals: WT, y = 0.49x + 2.01; KO, y = 1.16x + 2.10. (B) Twelve-month-old mice: WT, y = 0.53x + 1.73; TauT KO, y = 0.76x + 3.02. Data are from n = 3–5 different livers for each condition.
Mechanism of Impaired Glutamine Synthesis in Livers from 12-Mo-Old TauT KO Mice.
A similar double reciprocal plot analysis in 12-mo-old wild-type and TauT KO mice (Fig. 5B) revealed that compared with 12-mo-old wild-type mice, glutamine synthesis capacity is decreased from 0.58 µmol/g/min to 0.33 µmol/g/min, i.e., by 43% in TauT KO mice. This value nicely corresponds to the 40% decrease of GS activity in the liver lysate (Fig. 4B). On the other hand, the ammonia concentrations {[NH4+]scav} required for half-maximal glutamine production were similar and calculated to be 0.31 and 0.25 mmol/L in livers from wild-type and TauT KO mice, respectively (SI Appendix, Table S1). This suggests that inhibition of glutamine synthesis in 12-mo-old TauT KO mice is primarily explained by a diminished GS activity.
As shown recently, GS is sensitive toward inactivation by tyrosine nitration (22, 23). Therefore, we investigated whether the decreased specific activity of GS (compare Fig. 4 A and B) in 12-mo-old TauT KO mice is due to tyrosine nitration. As shown in Fig. 4C, NO2Tyr immunodepletion lowered anti-GS immunoreactivity as analyzed by Western blot by about 50% in liver protein lysates from 12-mo, but not from 3-mo-old TauT KO mice. The data suggest that TauT KO inhibits GS activity through tyrosine nitration in 12-mo-old, but not in 3-mo-old TauT KO mice.
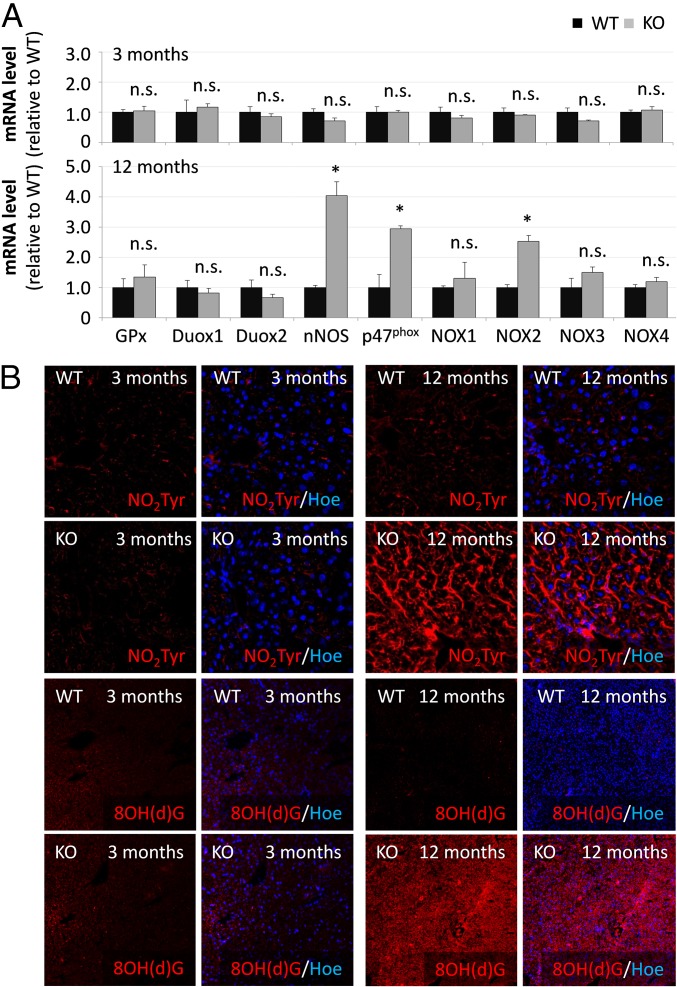
Taurine is considered to be an antioxidant (21) and taurine depletion may render cells and tissue sensitive to oxidative/nitrosative stress and trigger protein tyrosine nitration. As shown by qPCR, mRNA levels of glutathione peroxidase (GPx) as well as enzymes producing RNOS [NADPH oxidases (Nox), p47phox and neuronal nitric oxide synthase (nNOS)] in livers from 3-mo-old TauT KO mice did not differ from those found in wild type (Fig. 6A). However, mRNA levels of nNOS, Nox subunit p47phox and Nox2 were strongly up-regulated in livers from 12-mo-old TauT KO mice compared with WT mice (Fig. 6A). As shown by immunofluorescence analysis, levels of nNOS protein and serine-phosphorylated p47phox were strongly elevated in livers from 12- but not from 3-mo-old TauT KO mice compared with the respective WT mice (SI Appendix, Fig. S6). Immunoreactivities of the surrogate markers for oxidative/nitrosative stress, nitrotyrosine and 8OH(d)G (Fig. 6B) were strongly enhanced in livers from 12- but not from 3-mo-old TauT KO mice.
Fig. 6.
Oxidative/nitrosative stress markers in livers from TauT KO and WT mice. (A) mRNA was isolated from livers of 3- or 12-mo-old TauT KO or WT mice. mRNA expression levels of GPx, Nox isoforms, or nNOS were analyzed by qPCR. mRNA levels in livers from TauT KO mice are given relative to mRNA expression in TauT WT mice. n.s., not significantly different compared with WT; *Statistically significantly different compared with WT. n = 3–4 animals. (B) Immunofluorescence analysis of NO2Tyr and 8OH(d)G in livers from TauT KO and WT mice. Nuclei were counterstained using Hoechst. n = 3 animals for each condition.
The data show increased levels of nNOS, p47phox, and Nox2 mRNA, up-regulation of nNOS protein, enhanced serine phosphorylation of p47phox and up-regulation of oxidative/nitrosative stress surrogate markers NO2Tyr and 8OH(d)G in livers from 12- but not from 3-mo-old TauT KO mice.
Effects of Taurine Transporter Knockout on GAT2 Expression in the Liver.
Taurine levels in livers from 16- to 21-mo-old TauT KO mice are almost fourfold higher than in 2-mo-old TauT KO animals but are still decreased by more than 70% compared with respective WT controls (17). This may relate to a compensatory taurine uptake mechanism. Recently, the γ-aminobutyric acid transporter 2 (GAT2) was identified in mouse liver as a taurine transporter (24). As shown in Fig. 3D, GAT2 protein levels were by about threefold higher in livers from 12-mo-old, compared with 3-mo-old TauT KO mice. GAT2 was also strongly expressed in the plasma membrane of scavenger cells, whereas only weak immunostaining was found inside Kupffer cells (SI Appendix, Fig. S7). Up-regulation of GAT2 in the older animals may well explain why the taurine levels in liver tissue are almost fourfold higher than in young animals (17).
TauT Deficiency Triggers Cerebral RNA Oxidation and Protein Tyrosine Nitration.
As shown recently, systemic hyperammonemia triggers cerebral RNA oxidation in mouse brain where it was suggested to disturb cerebral neurotransmission (8). In line with this, systemic hyperammonemia in both 3- and 12-mo-old TauT KO mice was also associated with enhanced 8OH(d)G immunoreactivity in the brain (SI Appendix, Fig. S8). As shown by confocal laser-scanning microscopy, enhanced 8OH(d)G immunoreactivity was present in the somatosensory cortex, the hippocampus, the hypothalamus, and the cerebellum but not in the piriform cortex of 3-mo-old TauT KO mice (SI Appendix, Fig. S8). Anti-8OH(d)G immunoreactivity was absent in brain slices exposed to RNase (6 h, 37 °C, 100 µg/mL) indicative for enhanced oxidation of RNA but not of DNA in brain of TauT KO mice.
Discussion
The present study revealed an unexpected role of taurine for maintenance of systemic ammonia homeostasis in mice. Hyperammonemia was observed in 3-and 12-mo-old TauT KO mice and was not explained by altered expression of urea cycle enzymes or glutaminases, by alterations of zonal enzyme distribution or liver structure and there were no signs of liver fibrosis. As reported recently, fibrosis and unspecific hepatitis can develop in the TauT KO mice at an age beyond 15 mo (17, 21). Whereas urea production was not affected in livers from 3- or 12-mo-old TauT KO mice (Fig. 2), glutamine production was significantly reduced in livers from 3- and 12-mo-old TauT KO mice (Fig. 2). Therefore, the effects of TauT deficiency on hepatic scavenger cell function were analyzed in up to 12-mo-old TauT KO mice. These perivenous scavenger cells are the only liver cells expressing GS, which here acts as a high-affinity system for ammonia removal at the outflow of the sinusoidal bed. As shown previously these cells are decisive for the maintenance of systemic ammonia homeostasis (1, 2, 7, 8, 25).
As shown in the present study, GS localization and expression at the mRNA and protein levels were unchanged in livers from TauT KO mice up to 12 mo. However, the expression of the scavenger cell markers Glt1 and RhBG was affected in livers of TauT KO mice in an age-dependent way: whereas Glt1 was significantly up-regulated at 3 and 12 mo (SI Appendix, Fig. S3), RhBG protein expression was strongly down-regulated only in livers from 3-mo-old but not from 12-mo-old TauT KO mice (Fig. 3C and SI Appendix, Fig. S5). Together with the finding that GS activity in liver tissue remained unaffected in 3-mo-old TauT KO mice, this observation suggests that an impaired ammonia uptake by hepatic scavenger cells via RhBG may become rate controlling for glutamine synthesis in livers from 3-mo-old TauT KO mice. This view is substantiated by the kinetic analysis of glutamine formation in livers from 3-mo-old mice (Fig. 5A). This analysis shows that maximal rates of glutamine formation are not different between wild-type and TauT KO mice; however, the perivenous sinusoidal ammonia concentration {[NH4+]scav} required for half-maximal glutamine formation is about twice as high in TauT KO mice compared with wild-type mice. This suggests an impaired ammonia availability for GS in TauT KO mice, which can be overcome by increasing the ammonia concentration. These findings not only underline the importance of perivenous scavenger cells for maintenance of ammonia homeostasis, but also provide evidence that ammonia transport across the plasma membrane can be another site of regulation of hepatic glutamine synthesis.
Down-regulation of RhBG mRNA in livers from 3- and 12-mo-old TauT KO mice can be explained by decreased levels of TCF4 (Fig. 3B), which drives the transcription of RhBG (26), but not of GS (27), by interacting with β-catenin. However, decreased RhBG mRNA levels were not reflected at the protein level in livers from 12-mo-old TauT KO mice (Fig. 3C). This suggests that normal RhBG protein levels in livers from 12-mo-old TauT KO mice are maintained by posttranscriptional mechanisms that either enhance the translation of RhBG mRNA or stabilize the RhBG protein. Taurine is known to stabilize proteins (28) and to act as a chemical chaperone (29) and taurine levels in livers from TauT KO mice at an age of 16–21 mo are about fourfold higher than in 2-mo-old animals (17), probably due to a compensatory up-regulation of the also taurine-transporting (24) GAT2 which is also expressed in scavenger cells (Fig. 3D and SI Appendix, Fig. S7 A and B). In line with a role of taurine for stabilization of RhBG protein, siRNA-mediated knockdown of TauT in HuH-7.5 cells significantly down-regulated RhBG protein levels (SI Appendix, Fig. S9).
Interestingly, down-regulation of RhBG was accompanied by an up-regulation of the ammonia transporter RhCG in livers from 12-mo-old TauT KO mice (SI Appendix, Fig. S10). RhCG is solely expressed in epithelial cells of bile ducts and here RhCG may serve to secrete ammonia into the bile (5, 30). Thus, up-regulation of RhCG in livers from 12-mo-old TauT KO mice will not augment hepatic glutamine synthesis, but may represent a compensatory mechanism for ammonia removal in TauT deficiency. The mechanisms underlying the up-regulation of RhCG in the livers from 12-mo-old TauT KO mice remain to be determined. However, TNFα, whose plasma levels are elevated seven- to eightfold in 9- to 15-mo-old TauT KO animals (17), may trigger up-regulation of RhCG in mouse liver. In line with this, elevated TNFα was shown to up-regulate RhCG in vascular endothelial cells in mouse brain in acute liver failure (31). On the other hand, RhCG expression was not significantly increased in livers from 3-mo-old TauT KO mice (SI Appendix, Fig. S10) and in these animals the TNFα serum levels were not increased (SI Appendix, Fig. S11).
Ammonia is also transported by aquaporins (AQPs) 3, 7, 8, and 9 and in human liver AQPs 8 and 9 are coexpressed with Rhesus proteins (32). However, mRNA levels of aquaporins 3, 7, 8, or 9 were not significantly changed in livers from 3- and 12-mo-old TauT KO mice (SI Appendix, Fig. S12). This may indicate that decreased ammonia uptake due to RhBG down-regulation in livers from 3-mo-old TauT KO mice is probably not compensated by up-regulation of ammonia-transporting aquaporins.
At 12 mo of age, however, GS activity in liver tissue was strongly decreased in TauT KO mice, despite unchanged GS protein expression. This loss of GS activity by about 40% is also reflected in the kinetic analysis of hepatic glutamine formation in relation to [NH4+]scav (Fig. 5B), which indicates a 43% decrease of the capacity to produce glutamine without major effects on [NH4+]scav required for half-maximal glutamine formation. This decrease in GS capacity is due to a sizeable increase of tyrosine-nitrated GS, which was shown to inactivate mammalian GS (22, 23) and may trigger hyperammonemia in these mice. The situation resembles the pathomechanism for LPS-induced impairment of ammonia clearance in perfused rat liver, which also could be attributed to tyrosine nitration of GS (22). Interestingly, similar to findings in liver-specific GS knockout mice, the loss of GS activity in livers from 12-mo-old TauT KO mice led to hyperammonemia, which was not compensated by up-regulation of GS protein in skeletal muscle, kidney, or brain (SI Appendix, Fig. S13). These observations strengthen a role of taurine for maintenance of systemic ammonia homeostasis by the liver. It is unlikely that hyperammonemia inhibits taurine uptake and depletes taurine levels in the liver. This is suggested by unchanged TauT mRNA and protein levels in livers from liver-specific GS KO mice (SI Appendix, Fig. S14), which also present with hyperammonemia (8).
Down-regulation of RhBG at 3 mo and GS inactivation at 12 mo of age in livers from TauT KO mice were both associated with systemic hyperammonemia, suggesting that TauT deficiency disrupts ammonia homeostasis by impairing scavenger cell function. Up-regulation of Glt1 in livers from 3- and 12-mo-old TauT KO mice may represent an ineffective compensatory response to augment the impaired glutamine synthesis in the scavenger cells.
Tyrosine nitration of GS indicates the presence of oxidative/nitrosative stress and of peroxynitrite (ONOO−) which can recombine from NO and O2− (33). In line with this, up-regulation of nNOS, Nox2, and p47phox mRNA, nNOS protein and enhanced serine-phosphorylation of p47phox were found in livers from 12- (but not 3)-mo-old TauT KO mice. These changes may trigger NO and O2− synthesis and up-regulate the oxidative/nitrosative stress markers NO2Tyr and 8OH(d)G in livers from 12- but not from 3-mo-old TauT KO mice. The mechanisms underlying up-regulation of nNOS, Nox2, and p47phox and activation of p47phox in prolonged TauT deficiency have not yet been identified. However, activation of p47phox (34) and nNOS (35) may be triggered by TNFα which is significantly elevated in the blood of 9- to 15-mo-old (17) but not 3-mo-old TauT KO mice (SI Appendix, Fig. S11). In the liver, TNFα is mainly synthesized by Kupffer cells and TNFα synthesis is suppressed by taurine in liver injury and inflammation (36–38). Therefore, low taurine levels may trigger TNFα synthesis in Kupffer cells in 12-mo-old TauT KO mice and elevate systemic TNFα levels. Unchanged TNFα levels in 3-mo-old TauT KO mice may be explained by significantly elevated levels of IL10, which was shown to inhibit TNFα production in Kupffer cells (39). Levels of the proinflammatory cytokines IL1α/β and acute phase proteins were unchanged in the serum from TauT KO mice (SI Appendix, Fig. S11), suggesting that elevated IL6 levels in 3-mo-old TauT KO mice promote antiinflammatory effects.
Hyperammonemia was shown to trigger cerebral RNA oxidation not only in astrocytes, neurons, and brain slices in vitro, but also in mouse brain in vivo and to disturb cerebral neurotransmission (8). In line with this, systemic hyperammonemia in 3-mo-old TauT KO mice was also associated with enhanced 8OHG immunoreactivity in the somatosensory cortex, the hippocampus, the hypothalamus, and the cerebellum but not in the piriform cortex. This pattern of regional RNA oxidation in the brain resembled that observed in the liver-specific glutamine synthetase knockout mouse, which exhibits a similar degree of systemic hyperammonemia as the TauT KO mouse (8). These findings suggest that at least some of the neuropathologies reported in TauT KO mice such as impaired plasticity in corticostriatal neurotransmission (40) which are also found in liver-specific GS KO (41) mice may be attributed to systemic hyperammonemia. On the other hand, taurine was shown to diminish cerebral oxidative stress (42) and to counteract ammonia-induced edema in cerebrocortical brain slices (43). This clearly indicates that apart from effects on hepatic ammonia, handling other neuroprotective effects of taurine can come into play.
Taken together, the present study shows that taurine is essential for scavenger cell function in the liver and for the maintenance of systemic ammonia homeostasis.
TauT deficiency-induced hyperammonemia triggers cerebral oxidative stress, which provides another aspect of the role of taurine as neuroprotectant. Furthermore, prolonged TauT deficiency triggers oxidative/nitrosative stress in liver through induction of NADPH oxidase and nitric oxide synthases.
Serum taurine levels are frequently reduced in patients with liver cirrhosis (44), but it is currently unclear to what extent this will contribute to liver dysfunction. It remains also to be established, whether single nucleotide polymorphisms in the TauT gene exist that underlie an increased individual susceptibility for developing liver disease or its complications. Further research is required to identify the mechanisms underlying the beneficial actions of taurine in liver and brain.
Materials and Methods
Detailed information on genotyping, Sirius red staining, immunofluorescence, Western blot, qPCR analysis, and NO2-Tyr immunodepletion can be found in SI Appendix. GS activity was measured using a colorimetric transferase assay (SI Appendix), which is more sensitive compared with the synthetase assay. All experiments were approved by the Landesamt für Natur- und Verbraucherschutz, North Rhine-Westphalia.
Experiments were carried out with the indicated number of mice. Results are presented as arithmetric means ± SEM. Statistical testing was performed using two-sided unpaired Student’s t test (Excel, Microsoft) or one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test (Prism, GraphPad). A P value of ≤0.05 was considered significant.
Supplementary Material
Acknowledgments
The authors are grateful for expert technical assistance provided by Michaela Fastrich, Nicole Eichhorst, Vanessa Herbertz, and Torsten Janssen. This work was funded by the Deutsche Forschungsgemeinschaft Projektnummer 190586431–SFB 974 “Communication and Systems Relevance in Liver Injury and Regeneration” (Düsseldorf, Germany).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.J.L.C. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1813100116/-/DCSupplemental.
References
- 1.Häussinger D. Hepatocyte heterogeneity in glutamine and ammonia metabolism and the role of an intercellular glutamine cycle during ureogenesis in perfused rat liver. Eur J Biochem. 1983;133:269–275. doi: 10.1111/j.1432-1033.1983.tb07458.x. [DOI] [PubMed] [Google Scholar]
- 2.Häussinger D. Nitrogen metabolism in liver: Structural and functional organization and physiological relevance. Biochem J. 1990;267:281–290. doi: 10.1042/bj2670281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gebhardt R, Mecke D. Heterogeneous distribution of glutamine synthetase among rat liver parenchymal cells in situ and in primary culture. EMBO J. 1983;2:567–570. doi: 10.1002/j.1460-2075.1983.tb01464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper AJ, Nieves E, Coleman AE, Filc-DeRicco S, Gelbard AS. Short-term metabolic fate of [13N]ammonia in rat liver in vivo. J Biol Chem. 1987;262:1073–1080. [PubMed] [Google Scholar]
- 5.Weiner ID, Miller RT, Verlander JW. Localization of the ammonium transporters, Rh B glycoprotein and Rh C glycoprotein, in the mouse liver. Gastroenterology. 2003;124:1432–1440. doi: 10.1016/s0016-5085(03)00277-4. [DOI] [PubMed] [Google Scholar]
- 6.Stoll B, Häussinger D. Hepatocyte heterogeneity in uptake and metabolism of malate and related dicarboxylates in perfused rat liver. Eur J Biochem. 1991;195:121–129. doi: 10.1111/j.1432-1033.1991.tb15684.x. [DOI] [PubMed] [Google Scholar]
- 7.Häussinger D, Gerok W. Hepatocyte heterogeneity in ammonia metabolism: Impairment of glutamine synthesis in CCl4 induced liver cell necrosis with no effect on urea synthesis. Chem Biol Interact. 1984;48:191–194. doi: 10.1016/0009-2797(84)90120-0. [DOI] [PubMed] [Google Scholar]
- 8.Qvartskhava N, et al. Hyperammonemia in gene-targeted mice lacking functional hepatic glutamine synthetase. Proc Natl Acad Sci USA. 2015;112:5521–5526. doi: 10.1073/pnas.1423968112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- 10.Uchida S, et al. Molecular cloning of the cDNA for an MDCK cell Na(+)- and Cl(-)-dependent taurine transporter that is regulated by hypertonicity. Proc Natl Acad Sci USA. 1992;89:8230–8234. doi: 10.1073/pnas.89.17.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lambert IH, Kristensen DM, Holm JB, Mortensen OH. Physiological role of taurine–From organism to organelle. Acta Physiol (Oxf) 2015;213:191–212. doi: 10.1111/apha.12365. [DOI] [PubMed] [Google Scholar]
- 12.Bhattarai JP, Park SJ, Chun SW, Cho DH, Han SK. Activation of synaptic and extrasynaptic glycine receptors by taurine in preoptic hypothalamic neurons. Neurosci Lett. 2015;608:51–56. doi: 10.1016/j.neulet.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Kim C, Cha YN. Taurine chloramine produced from taurine under inflammation provides anti-inflammatory and cytoprotective effects. Amino Acids. 2014;46:89–100. doi: 10.1007/s00726-013-1545-6. [DOI] [PubMed] [Google Scholar]
- 14.Ito T, Yoshikawa N, Ito H, Schaffer SW. Impact of taurine depletion on glucose control and insulin secretion in mice. J Pharmacol Sci. 2015;129:59–64. doi: 10.1016/j.jphs.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Ito T, et al. Cardiac and skeletal muscle abnormality in taurine transporter-knockout mice. J Biomed Sci. 2010;17(Suppl 1):S20. doi: 10.1186/1423-0127-17-S1-S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang DY, et al. Impaired ability to increase water excretion in mice lacking the taurine transporter gene TAUT. Pflugers Arch. 2006;451:668–677. doi: 10.1007/s00424-005-1499-y. [DOI] [PubMed] [Google Scholar]
- 17.Warskulat U, et al. Chronic liver disease is triggered by taurine transporter knockout in the mouse. FASEB J. 2006;20:574–576. doi: 10.1096/fj.05-5016fje. [DOI] [PubMed] [Google Scholar]
- 18.Ito T, et al. Tissue depletion of taurine accelerates skeletal muscle senescence and leads to early death in mice. PLoS One. 2014;9:e107409. doi: 10.1371/journal.pone.0107409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito T, et al. Mass spectrometry-based metabolomics to identify taurine-modified metabolites in heart. Amino Acids. 2018;50:117–124. doi: 10.1007/s00726-017-2498-y. [DOI] [PubMed] [Google Scholar]
- 20.Jong CJ, Ito T, Prentice H, Wu JY, Schaffer SW. Role of mitochondria and endoplasmic reticulum in taurine-deficiency-mediated apoptosis. Nutrients. 2017;9:E795. doi: 10.3390/nu9080795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warskulat U, et al. Phenotype of the taurine transporter knockout mouse. Methods Enzymol. 2007;428:439–458. doi: 10.1016/S0076-6879(07)28025-5. [DOI] [PubMed] [Google Scholar]
- 22.Görg B, Wettstein M, Metzger S, Schliess F, Häussinger D. Lipopolysaccharide-induced tyrosine nitration and inactivation of hepatic glutamine synthetase in the rat. Hepatology. 2005;41:1065–1073. doi: 10.1002/hep.20662. [DOI] [PubMed] [Google Scholar]
- 23.Görg B, et al. Reversible inhibition of mammalian glutamine synthetase by tyrosine nitration. FEBS Lett. 2007;581:84–90. doi: 10.1016/j.febslet.2006.11.081. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Y, et al. Deletion of the γ-aminobutyric acid transporter 2 (GAT2 and SLC6A13) gene in mice leads to changes in liver and brain taurine contents. J Biol Chem. 2012;287:35733–35746. doi: 10.1074/jbc.M112.368175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Häussinger D, Schliess F. Glutamine metabolism and signaling in the liver. Front Biosci. 2007;12:371–391. doi: 10.2741/2070. [DOI] [PubMed] [Google Scholar]
- 26.Merhi A, De Mees C, Abdo R, Victoria Alberola J, Marini AM. Wnt/β-catenin signaling regulates the expression of the ammonium permease gene RHBG in human cancer cells. PLoS One. 2015;10:e0128683. doi: 10.1371/journal.pone.0128683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gebhardt R, Hovhannisyan A. Organ patterning in the adult stage: The role of Wnt/beta-catenin signaling in liver zonation and beyond. Dev Dyn. 2010;239:45–55. doi: 10.1002/dvdy.22041. [DOI] [PubMed] [Google Scholar]
- 28.Arakawa T, Timasheff SN. The stabilization of proteins by osmolytes. Biophys J. 1985;47:411–414. doi: 10.1016/S0006-3495(85)83932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yancey PH. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol. 2005;208:2819–2830. doi: 10.1242/jeb.01730. [DOI] [PubMed] [Google Scholar]
- 30.Weiner ID, Verlander JW. Renal and hepatic expression of the ammonium transporter proteins, Rh B glycoprotein and Rh C glycoprotein. Acta Physiol Scand. 2003;179:331–338. doi: 10.1046/j.0001-6772.2003.01210.x. [DOI] [PubMed] [Google Scholar]
- 31.Wang W, et al. Effect of tumor necrosis factor-α on the expression of the ammonia transporter Rhcg in the brain in mice with acute liver failure. J Neuroinflammation. 2018;15:234. doi: 10.1186/s12974-018-1264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Litman T, Søgaard R, Zeuthen T. Ammonia and urea permeability of mammalian aquaporins. Handb Exp Pharmacol. 2009:327–358. doi: 10.1007/978-3-540-79885-9_17. [DOI] [PubMed] [Google Scholar]
- 33.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 34.Dang PM, et al. Anti-inflammatory effect of interleukin-10 on human neutrophil respiratory burst involves inhibition of GM-CSF-induced p47PHOX phosphorylation through a decrease in ERK1/2 activity. FASEB J. 2006;20:1504–1506. doi: 10.1096/fj.05-5395fje. [DOI] [PubMed] [Google Scholar]
- 35.Stasko SA, Hardin BJ, Smith JD, Moylan JS, Reid MB. TNF signals via neuronal-type nitric oxide synthase and reactive oxygen species to depress specific force of skeletal muscle. J Appl Physiol. 2013;114:1629–1636. doi: 10.1152/japplphysiol.00871.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wettstein M, Häussinger D. Cytoprotection by the osmolytes betaine and taurine in ischemia-reoxygenation injury in the perfused rat liver. Hepatology. 1997;26:1560–1566. doi: 10.1053/jhep.1997.v26.pm0009397998. [DOI] [PubMed] [Google Scholar]
- 37.Seabra V, Stachlewitz RF, Thurman RG. Taurine blunts LPS-induced increases in intracellular calcium and TNF-alpha production by Kupffer cells. J Leukoc Biol. 1998;64:615–621. doi: 10.1002/jlb.64.5.615. [DOI] [PubMed] [Google Scholar]
- 38.Kincius M, et al. Taurine protects from liver injury after warm ischemia in rats: The role of kupffer cells. Eur Surg Res. 2007;39:275–283. doi: 10.1159/000102982. [DOI] [PubMed] [Google Scholar]
- 39.Grewe M, Gausling R, Gyufko K, Hoffmann R, Decker K. Regulation of the mRNA expression for tumor necrosis factor-alpha in rat liver macrophages. J Hepatol. 1994;20:811–818. doi: 10.1016/s0168-8278(05)80154-0. [DOI] [PubMed] [Google Scholar]
- 40.Sergeeva OA, et al. Taurine-induced long-lasting enhancement of synaptic transmission in mice: Role of transporters. J Physiol. 2003;550:911–919. doi: 10.1113/jphysiol.2003.045864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chepkova AN, et al. Impaired novelty acquisition and synaptic plasticity in congenital hyperammonemia caused by hepatic glutamine synthetase deficiency. Sci Rep. 2017;7:40190. doi: 10.1038/srep40190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aydın AF, et al. Carnosine and taurine treatments diminished brain oxidative stress and apoptosis in D-galactose aging model. Metab Brain Dis. 2016;31:337–345. doi: 10.1007/s11011-015-9755-0. [DOI] [PubMed] [Google Scholar]
- 43.Albrecht J, Wegrzynowicz M. Endogenous neuro-protectants in ammonia toxicity in the central nervous system: Facts and hypotheses. Metab Brain Dis. 2005;20:253–263. doi: 10.1007/s11011-005-7904-6. [DOI] [PubMed] [Google Scholar]
- 44.Agouza IE, Fouad R, Ahmed R, El-Sayed M, Menshawy A. Comparison between fibroscan and serum taurine for early diagnosis of liver fibrosis in Egyptian patients infected with HCV. Clin Med Biochem. 2017;3:127. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.