Significance
Despite available medications for depression, currently approved antidepressants take months to exert therapeutic effects, and ∼30% of patients remain treatment resistant. In contrast, a single subanesthetic dose of ketamine exerts rapid (within hours) and sustained antidepressant actions. Preclinical studies indicate that the ketamine metabolite (2R,6R)-hydroxynorketamine [(2R,6R)-HNK] is a rapid-acting antidepressant candidate with limited adverse effects compared with ketamine. Using behavioral, genetic, and pharmacological approaches and EEG measurements, we determined that the mechanism underlying antidepressant-relevant actions of (2R,6R)-HNK converges with metabotropic glutamate receptor subtype 2 (mGlu2) receptor signaling and identified high-frequency EEG oscillations as a marker associated with rapid antidepressant responses. Our data support the use of individually subtherapeutic doses of mGlu2 receptor inhibitors with ketamine or (2R,6R)-HNK in clinical trials for the treatment of depression.
Keywords: ketamine, hydroxynorketamine, antidepressant, mGlu2 receptor, cortical EEG
Abstract
Currently approved antidepressant drugs often take months to take full effect, and ∼30% of depressed patients remain treatment resistant. In contrast, ketamine, when administered as a single subanesthetic dose, exerts rapid and sustained antidepressant actions. Preclinical studies indicate that the ketamine metabolite (2R,6R)-hydroxynorketamine [(2R,6R)-HNK] is a rapid-acting antidepressant drug candidate with limited dissociation properties and abuse potential. We assessed the role of group II metabotropic glutamate receptor subtypes 2 (mGlu2) and 3 (mGlu3) in the antidepressant-relevant actions of (2R,6R)-HNK using behavioral, genetic, and pharmacological approaches as well as cortical quantitative EEG (qEEG) measurements in mice. Both ketamine and (2R,6R)-HNK prevented mGlu2/3 receptor agonist (LY379268)-induced body temperature increases in mice lacking the Grm3, but not Grm2, gene. This action was not replicated by NMDA receptor antagonists or a chemical variant of ketamine that limits metabolism to (2R,6R)-HNK. The antidepressant-relevant behavioral effects and 30- to 80-Hz qEEG oscillation (gamma-range) increases resultant from (2R,6R)-HNK administration were prevented by pretreatment with an mGlu2/3 receptor agonist and absent in mice lacking the Grm2, but not Grm3−/−, gene. Combined subeffective doses of the mGlu2/3 receptor antagonist LY341495 and (2R,6R)-HNK exerted synergistic increases on gamma oscillations and antidepressant-relevant behavioral actions. These findings highlight that (2R,6R)-HNK exerts antidepressant-relevant actions via a mechanism converging with mGlu2 receptor signaling and suggest enhanced cortical gamma oscillations as a marker of target engagement relevant to antidepressant efficacy. Moreover, these results support the use of (2R,6R)-HNK and inhibitors of mGlu2 receptor function in clinical trials for treatment-resistant depression either alone or in combination.
Although monoamine-targeting pharmacotherapies to treat depression, such as selective serotonin-reuptake inhibitors, are commonly used, more than 30% of severely depressed individuals remain treatment resistant (1). Moreover, even when effective, existing antidepressants often take months to exert their full therapeutic effects (2). Recent efforts to identify more effective antidepressant medications have focused on agents modulating glutamatergic neurotransmission (3). The relevance of targeting glutamatergic synapses is supported by numerous placebo-controlled trials that have provided strong evidence for the rapid (within 2 h) and sustained (∼7 d) antidepressant effects of subanesthetic doses of (R,S)-ketamine (ketamine) in depressed (4) and treatment-resistant depressed patients (5–9). Ketamine has been proposed to exert antidepressant actions via inhibition of glutamate NMDA receptor (NMDAR) function, which is also the mechanism attributed to its dissociative/anesthetic actions (10–13). However, no human clinical studies have replicated the full spectrum of robust, rapid, and sustained antidepressant actions observed with ketamine using alternative drugs that directly inhibit NMDAR function (14). Also, preclinical findings indicate NMDAR inhibition-independent mechanisms for the antidepressant-relevant actions of ketamine (15, 16). Thus, ketamine’s complete mechanism of action as a rapid-acting antidepressant remains controversial (10–13, 17).
In vivo, ketamine is rapidly metabolized to norketamine and thereafter, is hydroxylated at multiple locations to produce the hydroxynorketamines (HNKs). (2S,6S;2R,6R)-HNKs, which are produced by hydroxylation of the cyclohexyl ring at the C6 position, are the major HNK metabolites found in the plasma of humans, as well as plasma and brain of rodents after ketamine administration (18). Metabolism of ketamine to (2S,6S;2R,6R)-HNK was shown to be involved in ketamine’s sustained antidepressant-relevant actions in mice (15). The (2R,6R)-HNK stereoisomer has been identified as a potent, putative rapid-acting antidepressant drug in multiple animal behavioral tests (15, 19–22) and is reported to share ketamine’s antidepressant-relevant downstream signaling mechanisms (15, 16, 19, 22–25). Importantly, (2R,6R)-HNK does not share ketamine’s robust sensory dissociation or abuse potential properties in rodents (15, 20) and also, does not seem to inhibit NMDAR function at antidepressant-relevant concentrations (15, 18, 26–30). Thus, mechanistic studies with (2R,6R)-HNK enable the study of rapid and sustained antidepressant actions after a single administration that are independent of ketamine’s effect to inhibit NMDAR function.
Antidepressant-relevant actions of ketamine and (2R,6R)-HNK converge on a glutamate-associated enhancement of neuronal activity in mood-regulating synapses, which are proposed to both initiate and sustain their effects (13, 31, 32). Both ketamine (33–36) and (2R,6R)-HNK (15) enhance glutamatergic excitatory synaptic transmission in rodent brain slices. Such glutamate neurotransmission is regulated by the actions of group II metabotropic glutamate receptors subtypes 2 and 3 (mGlu2/3) (37). mGlu2 receptors are mainly expressed perisynaptically in close proximity to the presynaptic terminals, where they act as autoreceptors (38, 39) to decrease synaptic glutamate release when activated (40, 41). Indeed, activation of mGlu2/3 receptors reduces glutamate-dependent excitatory neurotransmission in rodent brain slices (42). In contrast, mGlu3 receptors are mainly expressed postsynaptically on neurons and glia (43–45).
Inhibitors of group II mGlu receptors have gained interest for their actions to exert rapid antidepressant-relevant effects, comparable with those observed with ketamine, in rodents (46–54). In addition to their similar antidepressant-relevant behavioral actions, downstream signaling pathways considered necessary for the antidepressant actions of ketamine are similarly involved in the actions of mGlu2/3 receptor antagonists (47, 48, 53, 55–58). Some evidence exists to suggest convergence in the mechanism of action of ketamine and mGlu2/3 receptor modulation. In particular, ketamine-induced enhancement of cortical extracellular glutamate levels was abolished by pretreatment with an mGlu2/3 receptor agonist in rats (59). Prevention of glutamate-dependent neurotransmission in the prefrontal cortex is expected to prevent hyperactivation of this brain region. Indeed, ketamine-induced cortical activation [as measured by [14C]2-deoxyglucose autoradiography, blood oxygenation-level dependence (BOLD) pharmacological magnetic resonance imaging, or [18F]fluorodeoxyglucose μ-positron emission tomography] was prevented by pretreatment with mGlu2/3 receptor agonists (60, 61) or selective mGlu2 receptor-positive allosteric modulators (62) in rodents. A similar effect was observed in humans using two different mGlu2/3 receptor agonist prodrugs while assessing BOLD pharmacological magnetic resonance imaging (63). In addition, pretreatment with mGlu2/3 agonists (64–67) or mGlu2 receptor-selective positive allosteric modulators (68, 69) prevented ketamine-induced enhancement of cortical quantitative EEG (qEEG) gamma oscillations, a marker of neuronal activation. These convergent processes have been hypothesized to involve ketamine-induced NMDAR inhibition, since mGlu2/3 activation also prevents cortical activation induced by other noncompetitive NMDAR open channel blockers, including MK-801 (68, 69), memantine (60, 62), and phencyclidine (70). However, whether there are convergent actions between mGlu2/3 receptor inhibition and (2R,6R)-HNK and if such effects are dependent on NMDAR inhibition are not known.
Here, we investigated the role of the mGlu2 and mGlu3 receptors in the antidepressant-relevant actions of (2R,6R)-HNK using pharmacological manipulations as well as mice in which mGlu2 (Grm2−/−) or mGlu3 (Grm3−/−) receptors are constitutively deleted. We also assessed the role of mGlu2 and mGlu3 receptors in the effects of (2R,6R)-HNK on cortical qEEG power. The results of our experiments reveal that (2R,6R)-HNK’s antidepressant-relevant actions converge with mGlu2, but not mGlu3, receptor signaling and highlight an NMDAR inhibition-independent mechanism underlying these effects. Cortical qEEG measurements implicate increases in high-frequency synchronized gamma oscillations as a putative mechanism contributing to rapid antidepressant efficacy and provide a target engagement marker with translational utility for human clinical trials.
Results
Effect of (2R,6R)-HNK on mGlu2/3 Receptor Agonist-Induced Hyperthermia.
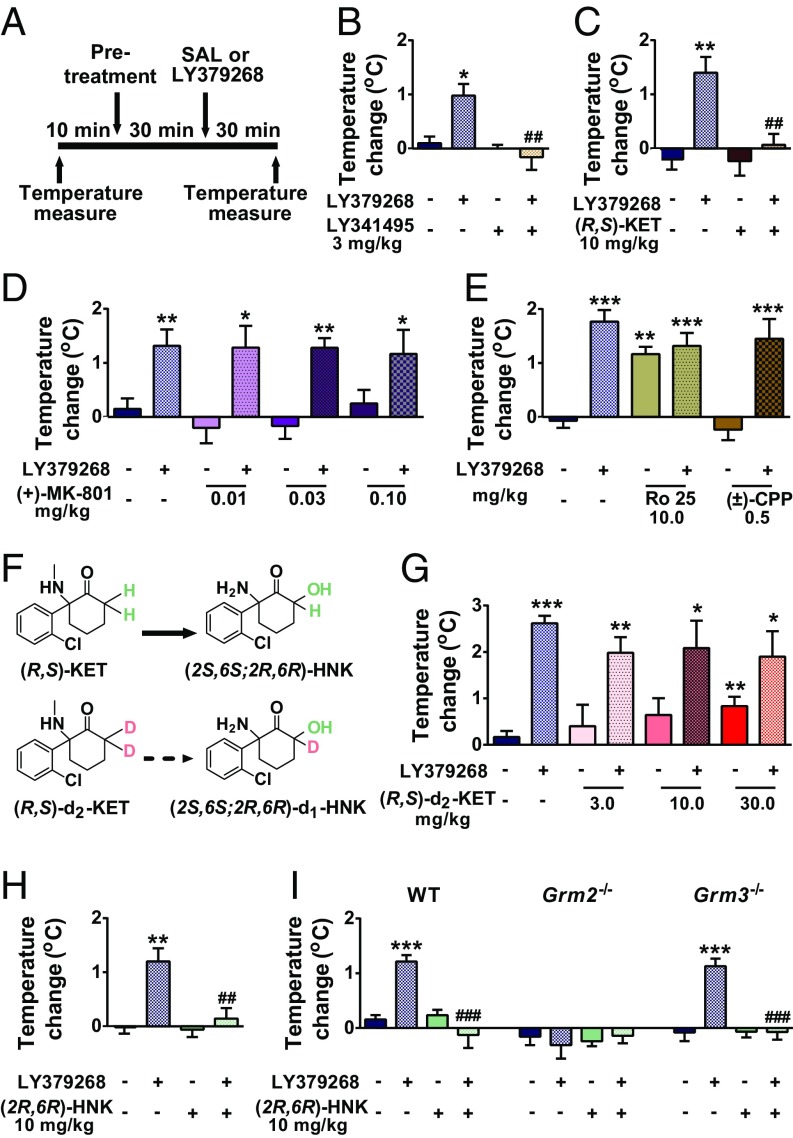
The mGlu2/3 receptor agonist-induced hyperthermia assay was previously established as a physiological readout sensitive to detect functional mGlu2/3 receptor antagonist activity of several orthosteric antagonists and negative allosteric modulators of mGlu2/3 receptors (71); Fig. 1A shows the experimental timeline. As previously published (71), we show that the mGlu2/3 receptor orthosteric antagonist LY341495 (3 mg/kg) prevented the increase in body temperature of mice induced by an mGlu2/3 receptor orthostatic agonist (LY379268; 3 mg/kg) (Fig. 1B). At the antidepressant-relevant dose of 10 mg/kg (15), ketamine also prevented LY379268-induced hyperthermia (Fig. 1C), indicating possible in vivo mGlu2/3 receptor antagonist activity of ketamine.
Fig. 1.
(2R,6R)-HNK prevents mGlu2/3 receptor agonist-induced hyperthermia in mice. (A) Timeline for the mGlu2/3 receptor agonist (LY379268)-induced hyperthermia assay. (B) The mGlu2/3 receptor antagonist LY341495 and (C) (R,S)-ketamine [(R,S)-KET] prevented LY379268-induced hyperthermia. In contrast, the NMDAR antagonists (D) (+)-MK-801, (E) Ro 25, and (±)-CPP and (F and G) the C6-deuterated analog of ketamine (R,S)-d2-KET did not prevent mGlu2/3 receptor agonist-induced hyperthermia. (H) Similar to ketamine, (2R,6R)-HNK prevented LY379268-induced hyperthermia, (I) an effect that was not present in mice lacking the Grm2 gene but was only present in WT and Grm3 knockout mice. Data are the mean ± SEM. SI Appendix, Table S1 has statistical analyses and n numbers. *P < 0.05 vs. control; **P < 0.01 vs. control; ***P < 0.001 vs. control; ##P < 0.01 vs. LY379268 group; ###P < 0.001 vs. LY379268 group.
To clarify whether ketamine’s action to prevent mGlu2/3 receptor agonist-induced hyperthermia is due to NMDAR inhibition, we assessed three distinct NMDAR antagonists: (i) (+)-MK-801, a noncompetitive NMDAR antagonist, which acts at the same phencyclidine (PCP)/ketamine site of the NMDAR; (ii) (±)-CPP, a competitive NMDAR antagonist; and (iii) Ro 25–6981, a GluN2B-specific NMDAR antagonist; we used doses known to exert putative antidepressant-relevant actions in rodents (15, 33, 72–76) and to induce NMDAR inhibition-mediated behaviors (75, 77–79). Administration of these NMDAR antagonists did not prevent mGlu2/3 receptor agonist-induced hyperthermia (Fig. 1 D and E), indicating that ketamine is unlikely to act through an NMDAR inhibition-dependent mechanism to prevent LY379268-induced hyperthermia.
To understand whether metabolism of ketamine to its 6-HNK metabolites is involved in its action to prevent mGlu2/3 receptor agonist-induced hyperthermia, we assessed the effects of a deuterated form of ketamine [deuterated at the C6 position; 6,6-dideuteroketamine; (R,S)-d2-ketamine (Fig. 1F)]. We previously showed that this compound retains the pharmacological properties of ketamine (i.e., NMDAR inhibition) and does not result in different levels of ketamine or norketamine in the brain but that it considerably decreases the levels of (2S,6S;2R,6R)-HNK after acute administration (15). In contrast to ketamine (10 mg/kg), (R,S)-d2-ketamine did not prevent mGlu2/3 receptor agonist-induced hyperthermia across a dose range of 3–30 mg/kg (Fig. 1G). These data suggest that metabolism of ketamine to its 6-HNK metabolites could be involved in its mGlu2/3 receptor antagonist-related actions. Indeed, (2R,6R)-HNK, similar to ketamine, prevented mGlu2/3 receptor agonist-induced hyperthermia (Fig. 1H).
To assess whether the effects of (2R,6R)-HNK are mGlu2 or mGlu3 receptor dependent, we examined the effects of (2R,6R)-HNK on mGlu2/3 receptor-induced hyperthermia in mice constitutively lacking either the Grm2 or Grm3 gene. As previously shown (71), LY379268 administration induced hyperthermia in WT and Grm3−/− mice but not in Grm2−/− mice (Fig. 1I). (2R,6R)-HNK prevented LY379268-induced hyperthermia in both WT and Grm3−/− mice, indicating that mGlu3 receptor is not involved in these effects (Fig. 1I). Notably, (2R,6R)-HNK was more potent in preventing the mGlu2/3 receptor agonist-induced hyperthermia than ketamine at the dose of 3 mg/kg (SI Appendix, Fig. S1). LY379268, (2R,6R)-HNK, or their combination did not have any effect on body temperature of Grm2−/− mice (Fig. 1I). We note that LY379268 has EC50 values at least 100 times lower for mGlu2 (EC50 ∼ 3 nM) and mGlu3 (EC50 ∼ 5 nM) receptors than for the other mGlu receptor subtypes (EC50 > 100 μM for mGlu1, mGlu5, mGlu7, and mGlu8; EC50 ∼ 400 nM and 20 μM for mGlu6 and mGlu4, respectively) (80, 81). As (2R,6R)-HNK prevents the hyperthermic effect of LY379268 in both WT and Grm3−/− mice, it is likely that the effect of (2R,6R)-HNK to prevent this physiological response of LY379268 specifically depends on the mGlu2 receptor.
(2R,6R)-HNK Interacts with the mGlu2/3 Receptor Antagonist LY341495 to Synergistically Exert Antidepressant-Relevant Behavioral Actions and Cortical qEEG Changes.
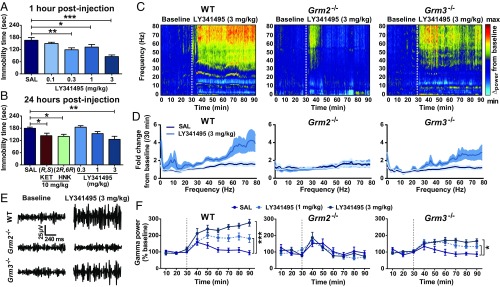
The results of the previous experiments provide evidence that the physiological actions of (2R,6R)-HNK converge with mGlu2 receptor signaling. We next examined whether these effects translate to rodent models predicting antidepressant efficacy. We first identified an optimal antidepressant-relevant dose of LY341495 by assessing different doses of the compound in the forced swim test (FST) 1 (Fig. 2A) and 24 h (Fig. 2B) postinjection. LY341495, similar to 10 mg/kg of ketamine and (2R,6R)-HNK, decreased immobility time in both tests at the dose of 3 mg/kg (Fig. 2 A and B).
Fig. 2.
mGlu2/3 receptor inhibition induces ketamine-like antidepressant-relevant actions and enhances cortical qEEG gamma power. (A) Administration of LY341495 at the doses of 0.3–3 mg/kg decreased immobility time in the FST 1 h after administration, (B) while only the dose of 3 mg/kg was effective in reducing immobility time in the FST 24 h after administration, similar to (R,S)-ketamine (KET) and (2R,6R)-HNK at the dose of 10 mg/kg. (C–F) LY341495 enhanced high-frequency (gamma) qEEG power in WT and Grm3−/−, but not Grm2−/−, knockout mice. (C) Average cortical qEEG spectrograms for a 30-min baseline period followed by an injection of LY341495 (3 mg/kg) in WT, Grm2−/−, and Grm3−/− knockout mice; power was normalized to mean values during the baseline. (D) Fold change in power spectra for a period of 30 min after injection normalized to the 30-min baseline values. (E) Representative gamma qEEG traces from WT, Grm2−/−, and Grm3−/− mice receiving LY341495 (3 mg/kg). (F) Gamma power changes (normalized to baseline) after administration of LY341495 at 1 and 3 mg/kg to WT, Grm2−/−, and Grm3−/− mice. Dashed lines denote injection times. Data are the mean ± SEM. SI Appendix, Table S1 has statistical analyses and n numbers. *P < 0.05; **P < 0.01; ***P < 0.001.
In agreement with convergent actions of mGlu2/3 receptor antagonists with (2R,6R)-HNK, we found that, similar to (2R,6R)-HNK (15), administration of LY341495 at antidepressant-relevant doses significantly increased cortical qEEG oscillations in WT mice within the 30- to 80-Hz (gamma oscillations) range (Fig. 2C). LY341495 also resulted in an increase of gamma power in Grm3−/−, although this effect was attenuated compared with the WT mice (Fig. 2 D–F). In contrast, the effect of LY341495 to increase gamma power was absent in Grm2−/− mice (Fig. 2 C–F). No significant effect of LY341495 was observed at lower-frequency qEEG oscillations at the antidepressant-relevant dose (SI Appendix, Fig. S2 C–E). The dose of 3 mg/kg was acutely associated with a hyperlocomotor effect in both WT and Grm2−/− mice (SI Appendix, Fig. S2 A and B), eliminating a role of hyperactivity in driving the differential qEEG effects observed. LY341495 administration at the dose of 1 mg/kg also induced an increase in the delta power range (1–3 Hz), which was not consistently shared by (2R,6R)-HNK administration. The nature of this increase in delta power is unclear, especially since there was no effect at 3 mg/kg (SI Appendix, Fig. S2C), which is the dose that we identified to exert antidepressant-like effects (Fig. 2 A and B).
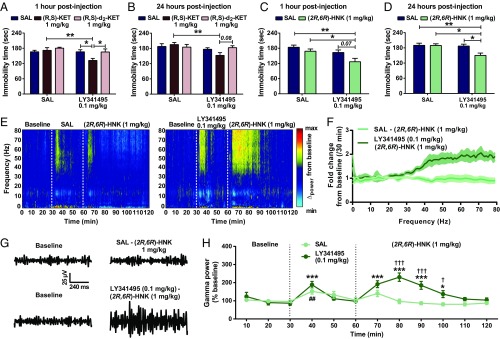
To assess for synergistic actions of mGlu2/3 receptor antagonists with ketamine, we coadministered subeffective doses of LY341495 (0.1 mg/kg) (no changes in immobility time compared with controls as determined in Fig. 2A) and ketamine (1 mg/kg; previously determined using the same behavioral test) [see Zanos et al. (15)] and assessed mice in the FST. To understand the role of ketamine’s conversion to its 6-HNK metabolites, we also compared the effects of ketamine with deuterated ketamine at the same dose. While subeffective doses of LY341495, ketamine, or deuterated ketamine did not individually induce any changes in the immobility time in the FST 1 or 24 h after administration compared with the controls (Fig. 3 A and B), combined administration of LY341495 and ketamine, but not deuterated ketamine, reduced immobility time in the FST 1 or 24 h after administration (Fig. 1 A and B). This finding indicates that conversion of ketamine to its 6-HNK metabolites is involved in its antidepressant efficacy-relevant synergism with mGlu2/3 receptor inhibition. Indeed, combined administration of subeffective doses of LY341495 and (2R,6R)-HNK [1 mg/kg; previously determined using the same behavioral test [see Zanos et al. (15)] reduced immobility time in the FST tested at 1 and 24 h after administration (Fig. 1 C and D). Subeffective doses of LY341495 and (2R,6R)-HNK also induced a synergistic enhancement of cortical qEEG gamma power (Fig. 3 E–H). No significant effects of combined administration of subeffective doses of LY341495 and (2R,6R)-HNK were observed at lower-frequency qEEG oscillations (SI Appendix, Fig. S3).
Fig. 3.
Subeffective doses of the mGlu2/3 receptor antagonist LY341495 and (2R,6R)-HNK induce synergistic antidepressant-relevant behavioral actions and enhance cortical qEEG gamma oscillations. Coadministration of subeffective doses of LY341495 (0.1 mg/kg) and (R,S)-ketamine [(R,S)-KET; 1 mg/kg] but not its C6-deuterated analog (R,S)-d2-KET induced a synergistic action to decrease immobility time in the FST (A) 1 and (B) 24 h postinjection. Similarly, subeffective doses of LY341495 and (2R,6R)-HNK (1 mg/kg) synergistically resulted in decreased immobility time in the FST (C) 1 and (D) 24 h postinjection. *P < 0.05; **P < 0.01. (E–H) Coadministration of subeffective doses of LY341495 and (2R,6R)-HNK induced a synergistic enhancement of high-frequency (gamma) qEEG power. (E) Average cortical qEEG spectrograms for a 30-min baseline period followed by an injection of SAL or LY341495 (0.1 mg/kg) and an injection of (2R,6R)-HNK 30 min later; power was normalized to the mean within the 30-min baseline time period. (F) Fold change in power spectra for a period of 30 min after injection normalized to the 30-min baseline values. (G) Representative gamma qEEG traces from mice receiving SAL or LY341495 and (2R,6R)-HNK. (H) Gamma power changes (normalized to baseline) showing effects of SAL or LY341495 and (2R,6R)-HNK administration. Dashed lines denote injection times. Data are the mean ± SEM. SI Appendix, Table S1 has statistical analyses and n numbers. *P < 0.05 vs. the 30-min baseline time point for the LY341495–(2R,6R)-HNK group; ***P < 0.001 vs. the 30-min baseline time point for the LY341495–(2R,6R)-HNK group; ##P > 0.01 vs. the 30-min baseline time point for the SAL–(2R,6R)-HNK group; †P < 0.05 for a comparison between the two groups; †††P < 0.001 for a comparison between the two groups.
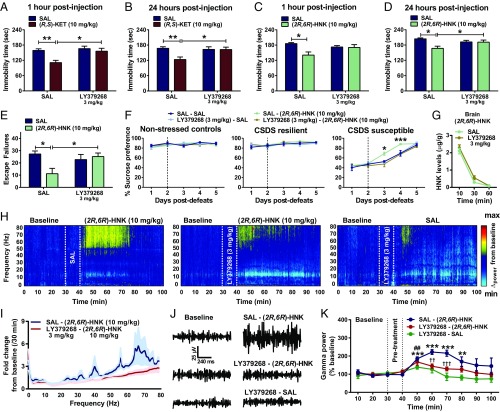
mGlu2/3 Receptor Activation Prevents the Antidepressant-Relevant Behavioral and Cortical qEEG Actions of (2R,6R)-HNK.
Since our data implicate an mGlu2/3 receptor antagonist-like functional action of (2R,6R)-HNK, we examined whether activation of mGlu2/3 receptors can prevent its antidepressant and cortical qEEG gamma power actions. We administered the mGlu2/3 receptor agonist LY379268 10 min before ketamine or (2R,6R)-HNK and tested mice in the FST 1 and 24 h later. Ketamine (Fig. 4 A and B) and (2R,6R)-HNK (Fig. 4 C and D) significantly decreased immobility time in the saline (SAL) pretreated animals at both 1 and 24 h postinjection. LY379268 pretreatment prevented the antidepressant behavioral actions of ketamine (at 1 and 24 h postinjection) (Fig. 4 A and B) and (2R,6R)-HNK (at 24 h postinjection) (Fig. 4 C and D) in the FST. In addition, LY379268 pretreatment prevented (2R,6R)-HNK’s ability to reverse inescapable shock-induced escape deficits (Fig. 4E) and chronic (10-d) social defeat-induced sucrose preference deficits in susceptible mice (Fig. 4F). There was no effect of LY379268 or (2R,6R)-HNK in nonstressed control or social defeat-resilient mice (Fig. 4F). Pretreatment with LY379268 did not affect (2R,6R)-HNK levels in the brain (Fig. 4G), eliminating altered pharmacokinetics as an explanation. Notably, when the mGlu2/3 receptor agonist LY379268 (3 mg/kg) was administered 4 h after (2R,6R)-HNK (10 mg/kg) administration, a time point when this metabolite is not detectable in the brain of mice (half-life = ∼24 min) (20), it did not affect the antidepressant actions of (2R,6R)-HNK in the FST 24 h after administration [SAL/SAL: 177.58 ± 7.30; (2R,6R)-HNK/SAL: 145.24 ± 12.17; SAL/LY379268: 181.08 ± 8.87; (2R,6R)-HNK/LY379268: 128.67 ± 11.07; treatment: F[1,28] = 0.42, P = 0.52; posttreatment: F[1,28] = 17.84, P < 0.001; interaction; F[1,28] = 1.00, P = 0.33].
Fig. 4.
mGlu2/3 receptor activation prevents (2R,6R)-HNK–induced antidepressant-relevant behavioral actions and cortical qEEG gamma enhancement. Administration of the mGlu2/3 receptor agonist LY379268 (3 mg/kg) 10 min before (R,S)-ketamine [(R,S)-KET; 10 mg/kg] prevented the decreased immobility time induced by (R,S)-KET in the FST (A) 1 and (B) 24 h postinjection. Similarly, administration of LY379268 10 min before (2R,6R)-HNK (10 mg/kg) prevented its antidepressant-relevant actions in the FST (C) 1 and (D) 24 h postinjection and (E) its actions to reverse inescapable shock-induced escape deficits 24 h postinjection. (F) LY379268 also prevented (2R,6R)-HNK’s reversal of sucrose preference deficits in susceptible mice after 10 d of chronic social defeat stress (CSDS). *P < 0.05; **P < 0.01; ***P < 0.001. (G) Administration of LY379268 (3 mg/kg) 10 min before (2R,6R)-HNK (10 mg/kg) did not change the distribution of (2R,6R)-HNK to the brain of mice. (H–K) Pretreatment with LY379268 prevents (2R,6R)-HNK–induced enhancement of high-frequency (gamma) qEEG power. (H) Average cortical qEEG spectrograms for a 30-min baseline period followed by an injection of SAL or LY379268 (3 mg/kg) and an injection of (2R,6R)-HNK or SAL 10 min later; power normalized to the mean baseline value. (I) Fold change in power spectra for a period of 30 min after injection normalized to the 30-min baseline. (J) Representative gamma qEEG traces from mice receiving SAL or LY379268 and (2R,6R)-HNK. (K) Normalized gamma power changes after administration of SAL or LY379268 and (2R,6R)-HNK. Dashed lines denote injection times. Data are the mean ± SEM. SI Appendix, Table S1 has statistical analyses and n numbers. **P < 0.01 vs. the 30-min baseline time point for the SAL–(2R,6R)-HNK group; ***P < 0.001 vs. the 30-min baseline time point for the SAL–(2R,6R)-HNK group; ##P < 0.01 vs. the 30-min baseline time point for the LY379268–(2R,6R)-HNK group; ††P < 0.01 for a comparison between the SAL–(2R,6R)-HNK and LY379268–(2R,6R)-HNK groups; †††P < 0.001 for a comparison between the SAL–(2R,6R)-HNK and LY379268–(2R,6R)-HNK groups.
To understand whether prevention of the antidepressant actions of (2R,6R)-HNK by activating the mGlu2/3 receptors also coincides with a prevention of enhanced cortical gamma qEEG power, we administered pretreatment injections of SAL or LY379268 (3 mg/kg) and 10 min later, administered (2R,6R)-HNK (10 mg/kg) while assessing mice for qEEG changes. Pretreatment with LY379268 significantly attenuated (2R,6R)-HNK–induced increases in gamma qEEG power (Fig. 4 H–K). Administration of LY379268 was also associated with a decrease in beta qEEG power, irrespective of (2R,6R)-HNK treatment (SI Appendix, Fig. S4), in line with previous findings using the same compound (64). No other effects were observed at lower-frequency qEEG oscillations (SI Appendix, Fig. S4).
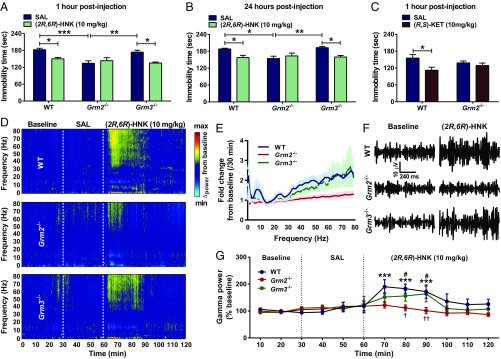
mGlu2, but Not mGlu3, Receptor Activity Is Required for the Antidepressant-Relevant and Cortical Gamma qEEG Actions of (2R,6R)-HNK.
To determine whether mGlu2 or mGlu3 receptor-dependent mechanisms converge with the actions of (2R,6R)-HNK, we assessed (2R,6R)-HNK’s actions in Grm2−/− and Grm3−/− mice. While (2R,6R)-HNK decreased immobility time in the FST in WT and Grm3−/− mice, it did not decrease immobility time in Grm2−/− male mice (Fig. 5 A and B) or female mice (SI Appendix, Fig. S5 A and B) 1 and 24 h postinjection. Similarly, ketamine failed to decrease immobility time in Grm2−/− mice (Fig. 5C). These data indicate that (2R,6R)-HNK acts via a mechanism that converges with mGlu2 receptor signaling to exert its antidepressant actions. We confirmed that this effect was not due to different levels of (2R,6R)-HNK in the brain of Grm2−/− mice compared with WT mice after 10-mg/kg dosing (SI Appendix, Fig. S5C). Also, this differential effect was not due to a shift in the dose–response, since at the dose of 30 or 90 mg/kg, (2R,6R)-HNK administration did not induce any decrease in the immobility time of Grm2−/− mice 1 h postinjection (SI Appendix, Fig. S5D), while both doses induced a decrease in immobility time in WT mice (SI Appendix, Fig. S5D). Lack of the Grm3 gene did not prevent (2R,6R)-HNK–induced reversal of escape deficits after inescapable shock stress (SI Appendix, Fig. S5E). Grm2−/− mice were found to be resilient in the development of escape deficits after inescapable shock stress; therefore, (2R,6R)-HNK was not assessed in Grm2−/− mice in this paradigm.
Fig. 5.
mGlu2 receptor is required for the antidepressant-relevant behavioral and cortical qEEG gamma power actions of (2R,6R)-HNK. Administration of (2R,6R)-HNK (10 mg/kg) decreased immobility time in WT and Grm3 knockout mice (Grm3−/−) but not Grm2 knockout mice (Grm2−/−; A) 1 and (B) 24 h postinjection. (C) (R,S)-ketamine [(R,S)-KET] administration did not result in a decrease in immobility time in the FST 1 h after administration in Grm2−/− mice. *P < 0.05; **P < 0.01; ***P < 0.001. (D–G) (2R,6R)-HNK enhanced high-frequency (gamma) qEEG power in WT and Grm3−/−, but not Grm2−/−, mice. (D) Average cortical qEEG spectrograms for a 30-min baseline period followed by an injection of SAL and an injection of (2R,6R)-HNK 30 min later in WT, Grm2−/−, and Grm3−/− mice; power normalized the mean values obtained during the baseline. (E) Fold change in power spectra for a period of 30 min after injection normalized to the 30-min baseline. (F) Representative gamma qEEG traces from WT, Grm2−/−, and Grm3−/− mice receiving (2R,6R)-HNK (10 mg/kg). (G) Normalized gamma power changes after administration of (2R,6R)-HNK to WT, Grm2−/−, and Grm3−/− mice. Data are the mean ± SEM. Dashed lines denote injection times. SI Appendix, Table S1 has statistical analyses and n numbers. ***P < 0.001 vs. the 30-min baseline time point for WT mice; #P < 0.05 vs. the 30-min baseline time point for Grm3−/− mice; †P < 0.05 for a comparison between the WT and Grm3−/− vs. Grm2−/− mice; ††P < 0.01 for a comparison between the WT and Grm3−/− vs. Grm2−/− mice.
Although ketamine did not induce any antidepressant-relevant behavioral actions in Grm2−/− mice, the drug exerted identical NMDAR inhibition-dependent (82) hyperlocomotor actions in both WT and Grm2−/− mice (SI Appendix, Fig. S5F), indicating that NMDAR inhibition-mediated effects are not altered. In addition, administration of the NMDAR antagonist (+)-MK-801 significantly reduced the immobility time of Grm2−/− mice in the FST 1 h postinjection (SI Appendix, Fig. S5G). Notably, Grm2−/− mice are not generally insensitive to showing antidepressant-relevant responses, since in addition to the behavioral actions of (+)-MK-801 that we observed here, an antidepressant-like effect of imipramine in the FST was previously reported in these mice (71). Consistent with a convergent mechanism of (2R,6R)-HNK antidepressant-relevant actions with mGlu2 receptor signaling, (2R,6R)-HNK increased qEEG gamma oscillations in WT and Grm3−/− mice but not Grm2−/− mice (Fig. 5 D–G). We note that there were no baseline differences in gamma oscillations between the WT, Grm2−/−, and Grm3−/− (WT: 26.58 ± 2.40 μV/30 min; Grm2−/−: 33.32 ± 3.92 μV/30 min; Grm3−/−: 29.96 ± 3.02 μV/30 min; one-way ANOVA: F[2,123] = 1.245, P = 0.29; post hoc comparisons: WT vs. Grm2−/−: P = 0.22; WT vs. Grm3−/−: P = 0.45). In addition, (2R,6R)-HNK administration increased alpha power only in WT mice (SI Appendix, Fig. S6A). Moreover, (2R,6R)-HNK administration increased delta qEEG power only in WT and Grm3−/− mice (SI Appendix, Fig. S6C). No effect of the drug was observed in the beta or theta qEEG power (SI Appendix, Fig. S6 B and D). In contrast to the effects of (2R,6R)-HNK on gamma qEEG power, (+)-MK-801 administration induced a significant increase in gamma qEEG power in Grm2−/− mice (SI Appendix, Fig. S5 F and G), highlighting that NMDAR inhibition does not mediate the lack of cortical qEEG actions of (2R,6R)-HNK in mice.
Discussion
Preclinical studies indicate that the ketamine metabolite (2R,6R)-HNK is a putative fast-acting antidepressant (15, 16, 19–25) devoid of ketamine’s adverse effects (15, 20). In particular, this metabolite was shown to be effective in rodent behavioral tests predictive of rapid antidepressant efficacy (15, 19–22) and to exert a long-lasting (at least 21-d) effect in rescuing chronic stress-induced behavioral despair and anhedonia in rats (19). In this study, using an in vivo measure predictive of mGlu2/3 receptor antagonist activity, we demonstrated that (2R,6R)-HNK, similar to the mGlu2/3 receptor antagonist LY341495, reverses mGlu2/3 receptor agonist-induced hyperthermia. Our experiments revealed this effect to be mGlu2, but not mGlu3, receptor mediated. We also show that subeffective doses of an mGlu2/3 receptor antagonist combined with (2R,6R)-HNK exert synergistic antidepressant-relevant behavioral actions and enhancement of cortical gamma qEEG power in mice. In addition, administration of the mGlu2/3 receptor agonist LY379268 before (2R,6R)-HNK prevented both the acute and sustained antidepressant-relevant actions of (2R,6R)-HNK. Importantly, the antidepressant-relevant behavioral effects of (2R,6R)-HNK were absent in mice lacking the Grm2, but not Grm3, gene. (2R,6R)-HNK’s action to increase gamma qEEG power was absent in mice lacking the Grm2, but not Grm3, gene, and it was abolished by pretreatment with an mGlu2/3 receptor agonist. We note that our findings do not reveal a direct interaction of (2R,6R)-HNK with the mGlu2 receptor, and we, therefore, do not exclude the possibility that this metabolite acts either upstream or downstream of mGlu2 receptors to exert convergent effects.
It was previously shown that peripheral administration of ketamine enhances extracellular glutamate levels in the prefrontal cortex of rats (59, 83) and that pretreatment with an mGlu2/3 receptor agonist blocked this effect (59), while the agonist itself had no effect on glutamate levels, suggesting that ketamine may act similarly to an mGlu2/3 receptor antagonist in vivo. Indeed, using the mGlu2/3 receptor agonist-induced hyperthermia assay, we show that both ketamine and (2R,6R)-HNK act similarly to mGlu2/3 receptor antagonists in vivo. This finding supports the hypothesis of a convergent mechanism of action between mGlu2/3 receptor inhibition and the effects of ketamine and (2R,6R)-HNK. In line with this hypothesis, in vitro experiments have previously shown that ketamine (33–36), (2R,6R)-HNK (15), and the mGlu2/3 receptor antagonist LY341495 (84, 85) similarly enhance excitatory glutamatergic synaptic transmission in rodent brain slices. Glutamate-dependent amplification of neuronal activity via activation of synaptic AMPARs has been broadly hypothesized to underlie the antidepressant actions of ketamine and other putative rapid-acting antidepressants, including (2R,6R)-HNK and mGlu2/3 receptor antagonists (52).
An in vivo marker of neuronal excitation, which is dependent on AMPAR throughput (15, 86), is the enhancement of high-frequency (gamma) qEEG oscillations (87). Results from human studies reveal that an antidepressant dose of ketamine induces an acute enhancement of the power (amplitude) of qEEG oscillations within the 30- to 80-Hz gamma range in the parietal (88), cingulate (88), and cerebral (89) as well as general cortical brain areas (90). Increases in cortical gamma power have been ascribed to a mechanism related to the psychotomimetic actions of ketamine (91–93), putatively via NMDAR inhibition-mediated modulation of interneuron activity (94). Similar to ketamine, (2R,6R)-HNK administration increases high-frequency cortical gamma qEEG oscillations in mice (15), but unlike ketamine, this metabolite does not exert behavioral changes in the prepulse inhibition task at doses up to 375 mg/kg, indicative of a lack of psychosis potential (15) and relevant NMDAR inhibition (26–30). Although ketamine’s overall effect on gamma power is likely influenced by its actions to inhibit the NMDAR expressed on GABAergic interneurons (83, 94, 95), the results here suggest an explanation whereby (2R,6R)-HNK exerts its effects on gamma oscillations via a mechanism that does not involve NMDAR inhibition.
High-frequency oscillations in vivo have parallels to many forms of activity-dependent plasticity, such as long-term potentiation, in which high-frequency stimuli induce sustained strengthening of excitatory synapses via an enhanced synchrony between limbic-connected brain regions, likely necessary for antidepressant behavioral actions (31, 96). The finding that (2R,6R)-HNK enhances cortical gamma qEEG oscillations indicates that this metabolite may engage endogenous processes that promote synaptic strengthening. Therefore, it is possible that enhancement of gamma qEEG oscillations is directly involved in the rapid antidepressant-relevant behavioral actions of (2R,6R)-HNK, especially considering our previous findings that in vivo pharmacological inhibition of AMPARs blocks both the behavioral actions and the increases in gamma power induced by (2R,6R)-HNK (15). These data highlight increases in gamma qEEG activity as a putative translational measure of antidepressant response. In agreement, ketamine-induced increases in gamma power have been recently associated with better antidepressant responses in patients treated for depression (97).
In this study, we demonstrated that administration of either (2R,6R)-HNK or an mGlu2/3 receptor antagonist at an antidepressant-relevant dose enhances cortical gamma qEEG oscillations and that these effects are mGlu2, but not mGlu3, receptor dependent. We also show that the antidepressant-relevant behavioral actions of (2R,6R)-HNK, similar to the effects previously shown for mGlu2/3 receptor antagonists (71), are also mGlu2, but not mGlu3, receptor dependent. These findings indicate that both mGlu2/3 receptor antagonists and (2R,6R)-HNK act in an mGlu2 receptor-dependent manner to enhance high-frequency neuronal activity and to exert antidepressant-relevant actions. In line with our findings, a previous study also showed increases in the gamma power range (30–49 Hz) 2–4 h (but not 1 h) after functional inhibition of the mGlu2/3 receptor in rats (98); however, that study restricted qEEG analyses to frequencies between 1 and 49 Hz, thus excluding gamma frequencies between 50 and 80 Hz. Moreover, the 10-mg/kg dose used for LY341495 to enhance gamma EEG oscillations in the aforementioned study is higher than the identified antidepressant-relevant dose (3 mg/kg) used in this study, where we observed increases of gamma power within 10 min after administration.
Important for understanding the mechanism of (2R,6R)-HNK action as an antidepressant is our finding that combined subeffective doses of an mGlu2/3 receptor antagonist and (2R,6R)-HNK synergistically exerted antidepressant-relevant actions and increased gamma qEEG oscillations. This finding suggests that a convergent mechanism in both the behavioral and physiological actions of these compounds exists. A similar synergistic antidepressant-relevant effect between ketamine and mGlu2/3 inhibition was previously demonstrated in the FST when rats were tested both acutely and 24 h after administration (99). This synergistic effect was reported to require AMPAR activity since pretreatment with an AMPAR antagonist prevented the effect (100). Our study extends these findings to (2R,6R)-HNK and implicates a non-NMDAR inhibition-mediated synergistic effect.
It was previously shown that activation of mGlu2/3 (64–67) or selective positive allosteric modulation of mGlu2 receptors (68, 69) can prevent ketamine-induced enhancement of gamma qEEG oscillations. This effect was thought to be due to the antipsychotic actions of mGlu2/3 receptor activation, since this pharmacological manipulation can prevent excessive glutamate levels in the prefrontal cortex of rodents after administration of NMDAR antagonists, which causes schizophrenia endophenotypes (59, 101, 102). Nevertheless, activation of mGlu2/3 receptors using the same doses as the ones used to prevent ketamine-induced gamma qEEG oscillations did not prevent ketamine-associated disruption in sensorimotor gating assessed via the prepulse inhibition task in rodents (66, 103, 104). These findings highlight that the actions of mGlu2/3 activation to reduce ketamine-induced increase in gamma power are unlikely to represent prevention of NMDAR inhibition-mediated psychotomimetic actions. Here, we show that gamma qEEG oscillation enhancement induced by (2R,6R)-HNK is also prevented by pretreatment with an mGlu2/3 receptor agonist and is absent in mice lacking the mGlu2 receptor. Therefore, mGlu2 receptor-dependent modulation of gamma frequency changes induced by (2R,6R)-HNK does not represent a mechanism whereby mGlu2/3 activation prevents NMDAR inhibition-induced excitation of cortical pyramidal neurons and thus, psychotomimetic effects, but rather, this could be important for (2R,6R)-HNK’s antidepressant mechanism of action. Indeed, pretreatment with an mGlu2/3 receptor agonist [similar to what was reported for ketamine (54)] or lack of mGlu2, but not mGlu3, receptors also prevented the antidepressant-relevant actions of (2R,6R)-HNK. This finding supports the hypothesis of a critical convergent mechanism between mGlu2 receptor signaling and (2R,6R)-HNK effects and suggests mGlu2 receptor inhibition as a promising target for rapid antidepressant effects. Although a clinical trial (n = 310) assessing the effects of an mGlu2/3 receptor-negative allosteric modulator (decoglurant) in patients suffering from depression failed to induce antidepressant actions compared with placebo (105), there was no measure of target engagement (such as gamma power) to ensure sufficient drug brain exposure.
Taken together, our findings highlight the presence of a convergent mechanism underlying the antidepressant-relevant actions of (2R,6R)-HNK and mGlu2/3 receptor antagonists and indicate that (2R,6R)-HNK acts in an mGlu2 receptor-dependent manner to exert these actions. Our data also support high-frequency gamma qEEG power as a marker relevant to the mechanism underlying rapid antidepressant efficacy. Moreover, our data support the use of drugs with mGlu2 receptor antagonist activity in experimental therapeutic trials either alone or in combination with low doses of (2R,6R)-HNK for treatment-resistant depression.
Materials and Methods
Detailed methods are described in SI Appendix.
Animals.
Male and female CD-1 mice (8–11 wk old at the start of testing; Charles River Laboratories) were housed in groups of four to five per cage with a 12-h light/dark cycle (lights on at 0700 h). Food and water were available ad libitum. For social defeat experiments, 8- to 9-wk-old male C57BL/6J mice (University of Maryland, Baltimore veterinary resources breeding colony) and retired male CD-1 breeders (Charles River Laboratories) were used. Grm2−/− and Grm3−/− mice (bred on a CD-1 background) as previously described by Linden et al. (106) and WT littermate controls were provided from an Eli Lilly Pharmaceuticals colony maintained at Taconic Biosciences. All experimental procedures were approved by the University of Maryland, Baltimore Animal Care and Use Committee and were conducted in full accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (107).
Behavioral Assays.
Mice were tested in the mGlu2/3 receptor agonist-induced hyperthermia assay as an in vivo measure of mGlu2/3 receptor antagonist activity (71). In addition, mice were assessed for behavioral despair in the FST 1 and/or 24 h postinjection (15), for escape deficits after inescapable shock (108), and for sucrose preference deficits after chronic social defeat stress (15). Details are in SI Appendix.
Cortical qEEG.
Surgeries, recordings, and data analysis for the qEEG studies were performed as previously described (15), with minor modifications.
Tissue Distribution and Clearance Measurements of (2R,6R)-HNK.
The concentrations of (2R,6R)-HNK in brain tissue were determined by achiral liquid chromatography–tandem mass spectrometry as previously described (15).
Statistical Analysis.
Statistical analyses were performed using GraphPad Prism software version 6. Holm–Šídák post hoc comparison was applied where ANOVAs reached statistical significance (i.e., P ≤ 0.05). The sample sizes, the specific statistical tests used, and the main effects of our statistical analyses for each experiment are reported in SI Appendix, Table S1. All post hoc comparison results are indicated in the figures. Raw data are provided in Dataset S1.
Supplementary Material
Acknowledgments
This work was supported by Brain & Behavior Research Foundation (NARSAD) Young Investigator Grant 26826 (to P.Z.), NIH Grant MH107615 (to T.D.G.), Veterans Affairs Merit Award 1I01BX004062 (to T.D.G.), and a Harrington Discovery Institute Scholar–Innovator grant (to T.D.G.). The laboratories of C.J.T., R.M., and C.A.Z. are supported by the NIH Intramural Research Program. The contents do not represent the views of the US Department of Veterans Affairs or the US Government.
Footnotes
Conflict of interest statement: P.Z., P.J.M., C.J.T., R.M., C.A.Z., and T.D.G. are listed as coauthors in patent applications related to the pharmacology and use of (2R,6R)-HNK in the treatment of depression, anxiety, anhedonia, suicidal ideation, and posttraumatic stress disorders. R.M. and C.A.Z. are listed as coinventors on a patent for the use of ketamine in major depression and suicidal ideation. T.D.G. has received research funding from Janssen, Allergan, and Roche Pharmaceuticals and was a consultant for FSV7 LLC during the preceding 3 years. All of the other authors report no conflict of interest.
This article is a PNAS Direct Submission.
See companion article on page 5160 in issue 11 of volume 116.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1819540116/-/DCSupplemental.
References
- 1.Rush AJ, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 2.Insel TR, Wang PS. The STAR*D trial: Revealing the need for better treatments. Psychiatr Serv. 2009;60:1466–1467. doi: 10.1176/ps.2009.60.11.1466. [DOI] [PubMed] [Google Scholar]
- 3.Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: An emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berman RM, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 5.Zarate CA, Jr, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 6.Lapidus KA, et al. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry. 2014;76:970–976. doi: 10.1016/j.biopsych.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fava M, et al. October 3, 2018. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry, 10.1038/s41380-018-0256-5erratum (2019) 10.1038/s41380-018-0311-2.
- 8.Singh JB, et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry. 2016;173:816–826. doi: 10.1176/appi.ajp.2016.16010037. [DOI] [PubMed] [Google Scholar]
- 9.Murrough JW, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: A two-site randomized controlled trial. Am J Psychiatry. 2013;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wohleb ES, Gerhard D, Thomas A, Duman RS. Molecular and cellular mechanisms of rapid-acting antidepressants ketamine and scopolamine. Curr Neuropharmacol. 2017;15:11–20. doi: 10.2174/1570159X14666160309114549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller OH, Moran JT, Hall BJ. Two cellular hypotheses explaining the initiation of ketamine’s antidepressant actions: Direct inhibition and disinhibition. Neuropharmacology. 2016;100:17–26. doi: 10.1016/j.neuropharm.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 12.Kavalali ET, Monteggia LM. Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am J Psychiatry. 2012;169:1150–1156. doi: 10.1176/appi.ajp.2012.12040531. [DOI] [PubMed] [Google Scholar]
- 13.Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23:801–811. doi: 10.1038/mp.2017.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newport DJ, et al. APA Council of Research Task Force on Novel Biomarkers and Treatments Ketamine and other NMDA antagonists: Early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172:950–966. doi: 10.1176/appi.ajp.2015.15040465. [DOI] [PubMed] [Google Scholar]
- 15.Zanos P, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wray NH, Schappi JM, Singh H, Senese NB, Rasenick MM. NMDAR-independent, cAMP-dependent antidepressant actions of ketamine. Mol Psychiatry. June 12, 2018 doi: 10.1038/s41380-41018-40083-41388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdallah CG. What’s the Buzz about hydroxynorketamine? Is it the history, the story, the debate, or the promise? Biol Psychiatry. 2017;81:e61–e63. doi: 10.1016/j.biopsych.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zanos P, et al. Ketamine and ketamine metabolite pharmacology: Insights into therapeutic mechanisms. Pharmacol Rev. 2018;70:621–660. doi: 10.1124/pr.117.015198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chou D, et al. (2R,6R)-HNK rescues chronic stress-induced depression-like behavior through its actions in the midbrain periaqueductal gray. Neuropharmacology. 2018;139:1–12. doi: 10.1016/j.neuropharm.2018.06.033. [DOI] [PubMed] [Google Scholar]
- 20.Highland JN, et al. Mouse, rat, and dog bioavailability and mouse oral antidepressant efficacy of ( 2R,6R)-HNK. J Psychopharmacol. November 29, 2018 doi: 10.1177/0269881118812095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pham TH, et al. Common neurotransmission recruited in (R,S)-ketamine and (2R,6R)-hydroxynorketamine-induced sustained antidepressant-like effects. Biol Psychiatry. 2018;84:e3–e6. doi: 10.1016/j.biopsych.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 22.Fukumoto K, et al. Activity-dependent brain-derived neurotrophic factor signaling is required for the antidepressant actions of (2R,6R)-HNK. Proc Natl Acad Sci USA. 2019;116:297–302. doi: 10.1073/pnas.1814709116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cavalleri L, et al. Ketamine enhances structural plasticity in mouse mesencephalic and human iPSC-derived dopaminergic neurons via AMPAR-driven BDNF and mTOR signaling. Mol Psychiatry. 2018;23:812–823. doi: 10.1038/mp.2017.241. [DOI] [PubMed] [Google Scholar]
- 24.Collo G, Cavalleri L, Chiamulera C, Merlo Pich E. (2R,6R)-hydroxynorketamine promotes dendrite outgrowth in human inducible pluripotent stem cell-derived neurons through AMPA receptor with timing and exposure compatible with ketamine infusion pharmacokinetics in humans. Neuroreport. 2018;29:1425–1430. doi: 10.1097/WNR.0000000000001131. [DOI] [PubMed] [Google Scholar]
- 25.Yao N, Skiteva O, Zhang X, Svenningsson P, Chergui K. Ketamine and its metabolite (2R,6R)-HNK induce lasting alterations in glutamatergic synaptic plasticity in the mesolimbic circuit. Mol Psychiatry. 2018;23:2066–2077. doi: 10.1038/mp.2017.239. [DOI] [PubMed] [Google Scholar]
- 26.Zanos P, et al. Zanos et al. reply. Nature. 2017;546:E4–E5. doi: 10.1038/nature22085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki K, Nosyreva E, Hunt KW, Kavalali ET, Monteggia LM. Effects of a ketamine metabolite on synaptic NMDAR function. Nature. 2017;546:E1–E3. doi: 10.1038/nature22084. [DOI] [PubMed] [Google Scholar]
- 28.Morris PJ, et al. Synthesis and N-methyl-d-aspartate (NMDA) receptor activity of ketamine metabolites. Org Lett. 2017;19:4572–4575. doi: 10.1021/acs.orglett.7b02177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moaddel R, et al. Sub-anesthetic concentrations of (R,S)-ketamine metabolites inhibit acetylcholine-evoked currents in α7 nicotinic acetylcholine receptors. Eur J Pharmacol. 2013;698:228–234. doi: 10.1016/j.ejphar.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lumsden E, et al. NMDA receptor function and the antidepressant effects of the ketamine metabolite (2R,6R)-HNK. Proc Natl Acad Sci USA. 2019;116:5160–5169. doi: 10.1073/pnas.1816071116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson SM, et al. An excitatory synapse hypothesis of depression. Trends Neurosci. 2015;38:279–294. doi: 10.1016/j.tins.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdallah CG, Sanacora G, Duman RS, Krystal JH. The neurobiology of depression, ketamine and rapid-acting antidepressants: Is it glutamate inhibition or activation? Pharmacol Ther. 2018;190:148–158. doi: 10.1016/j.pharmthera.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Autry AE, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang K, et al. Essential roles of AMPA receptor GluA1 phosphorylation and presynaptic HCN channels in fast-acting antidepressant responses of ketamine. Sci Signal. 2016;9:ra123. doi: 10.1126/scisignal.aai7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nosyreva E, et al. Acute suppression of spontaneous neurotransmission drives synaptic potentiation. J Neurosci. 2013;33:6990–7002. doi: 10.1523/JNEUROSCI.4998-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narimatsu E, Kawamata Y, Kawamata M, Fujimura N, Namiki A. NMDA receptor-mediated mechanism of ketamine-induced facilitation of glutamatergic excitatory synaptic transmission. Brain Res. 2002;953:272–275. doi: 10.1016/s0006-8993(02)03375-9. [DOI] [PubMed] [Google Scholar]
- 37.Niswender CM, Conn PJ. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience. 1996;71:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- 39.Shigemoto R, et al. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y-L, Huang C-C, Hsu K-S. Time-dependent reversal of long-term potentiation by low-frequency stimulation at the hippocampal mossy fiber-CA3 synapses. J Neurosci. 2001;21:3705–3714. doi: 10.1523/JNEUROSCI.21-11-03705.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tzounopoulos T, Janz R, Südhof TC, Nicoll RA, Malenka RC. A role for cAMP in long-term depression at hippocampal mossy fiber synapses. Neuron. 1998;21:837–845. doi: 10.1016/s0896-6273(00)80599-1. [DOI] [PubMed] [Google Scholar]
- 42.Anwyl R. Metabotropic glutamate receptors: Electrophysiological properties and role in plasticity. Brain Res Brain Res Rev. 1999;29:83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- 43.Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: An in situ hybridization study. J Comp Neurol. 1993;335:252–266. doi: 10.1002/cne.903350209. [DOI] [PubMed] [Google Scholar]
- 44.Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- 45.Joffe ME, Conn PJ. Antidepressant potential of metabotropic glutamate receptor mGlu2 and mGlu3 negative allosteric modulators. Neuropsychopharmacology. 2019;44:214–236. doi: 10.1038/s41386-018-0192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dwyer JM, Lepack AE, Duman RS. mTOR activation is required for the antidepressant effects of mGluR2/3 blockade. Int J Neuropsychopharmacol. 2012;15:429–434. doi: 10.1017/S1461145711001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koike H, Fukumoto K, Iijima M, Chaki S. Role of BDNF/TrkB signaling in antidepressant-like effects of a group II metabotropic glutamate receptor antagonist in animal models of depression. Behav Brain Res. 2013;238:48–52. doi: 10.1016/j.bbr.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 48.Fukumoto K, Iijima M, Chaki S. Serotonin-1A receptor stimulation mediates effects of a metabotropic glutamate 2/3 receptor antagonist, 2S-2-amino-2-(1S,2S-2-carboxycycloprop-1-yl)-3-(xanth-9-yl)propanoic acid (LY341495), and an N-methyl-D-aspartate receptor antagonist, ketamine, in the novelty-suppressed feeding test. Psychopharmacology (Berl) 2014;231:2291–2298. doi: 10.1007/s00213-013-3378-0. [DOI] [PubMed] [Google Scholar]
- 49.Dwyer JM, Lepack AE, Duman RS. mGluR2/3 blockade produces rapid and long-lasting reversal of anhedonia caused by chronic stress exposure. J Mol Psychiatry. 2013;1:15. doi: 10.1186/2049-9256-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ago Y, et al. Metabotropic glutamate 2/3 receptor antagonists improve behavioral and prefrontal dopaminergic alterations in the chronic corticosterone-induced depression model in mice. Neuropharmacology. 2013;65:29–38. doi: 10.1016/j.neuropharm.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 51.Dong C, et al. Rapid and sustained antidepressant action of the mGlu2/3 receptor antagonist MGS0039 in the social defeat stress model: Comparison with ketamine. Int J Neuropsychopharmacol. 2017;20:228–236. doi: 10.1093/ijnp/pyw089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zanos P, Thompson SM, Duman RS, Zarate CA, Jr, Gould TD. Convergent mechanisms underlying rapid antidepressant action. CNS Drugs. 2018;32:197–227. doi: 10.1007/s40263-018-0492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chaki S. mGlu2/3 receptor antagonists as novel antidepressants. Trends Pharmacol Sci. 2017;38:569–580. doi: 10.1016/j.tips.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 54.Witkin JM, et al. Comparative effects of LY3020371, a potent and selective metabotropic glutamate (mGlu) 2/3 receptor antagonist, and ketamine, a noncompetitive N-methyl-d-aspartate receptor antagonist in rodents: Evidence supporting the use of mGlu2/3 antagonists, for the treatment of depression. J Pharmacol Exp Ther. 2017;361:68–86. doi: 10.1124/jpet.116.238121. [DOI] [PubMed] [Google Scholar]
- 55.Koike H, Chaki S. Requirement of AMPA receptor stimulation for the sustained antidepressant activity of ketamine and LY341495 during the forced swim test in rats. Behav Brain Res. 2014;271:111–115. doi: 10.1016/j.bbr.2014.05.065. [DOI] [PubMed] [Google Scholar]
- 56.Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res. 2011;224:107–111. doi: 10.1016/j.bbr.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 57.Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol. 2014;18:pyu033. doi: 10.1093/ijnp/pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Witkin JM, et al. The rapidly acting antidepressant ketamine and the mGlu2/3 receptor antagonist LY341495 rapidly engage dopaminergic mood circuits. J Pharmacol Exp Ther. 2016;358:71–82. doi: 10.1124/jpet.116.233627. [DOI] [PubMed] [Google Scholar]
- 59.Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA. Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: Modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience. 2003;117:697–706. doi: 10.1016/s0306-4522(02)00652-8. [DOI] [PubMed] [Google Scholar]
- 60.Dedeurwaerdere S, Wintmolders C, Straetemans R, Pemberton D, Langlois X. Memantine-induced brain activation as a model for the rapid screening of potential novel antipsychotic compounds: Exemplified by activity of an mGlu2/3 receptor agonist. Psychopharmacology (Berl) 2011;214:505–514. doi: 10.1007/s00213-010-2052-z. [DOI] [PubMed] [Google Scholar]
- 61.Chin CL, et al. Awake rat pharmacological magnetic resonance imaging as a translational pharmacodynamic biomarker: Metabotropic glutamate 2/3 agonist modulation of ketamine-induced blood oxygenation level dependence signals. J Pharmacol Exp Ther. 2011;336:709–715. doi: 10.1124/jpet.110.173880. [DOI] [PubMed] [Google Scholar]
- 62.Wyckhuys T, et al. The [18F]FDG μPET readout of a brain activation model to evaluate the metabotropic glutamate receptor 2 positive allosteric modulator JNJ-42153605. J Pharmacol Exp Ther. 2014;350:375–386. doi: 10.1124/jpet.114.213959. [DOI] [PubMed] [Google Scholar]
- 63.Mehta MA, et al. Group II metabotropic glutamate receptor agonist prodrugs LY2979165 and LY2140023 attenuate the functional imaging response to ketamine in healthy subjects. Psychopharmacology (Berl) 2018;235:1875–1886. doi: 10.1007/s00213-018-4877-9. [DOI] [PubMed] [Google Scholar]
- 64.Jones NC, et al. Acute administration of typical and atypical antipsychotics reduces EEG γ power, but only the preclinical compound LY379268 reduces the ketamine-induced rise in γ power. Int J Neuropsychopharmacol. 2012;15:657–668. doi: 10.1017/S1461145711000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jones NC, et al. Effects of aberrant gamma frequency oscillations on prepulse inhibition. Int J Neuropsychopharmacol. 2014;17:1671–1681. doi: 10.1017/S1461145714000492. [DOI] [PubMed] [Google Scholar]
- 66.Fujáková M, et al. The effect of ((-)-2-oxa-4-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid (LY379268), an mGlu2/3 receptor agonist, on EEG power spectra and coherence in ketamine model of psychosis. Pharmacol Biochem Behav. 2014;122:212–221. doi: 10.1016/j.pbb.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 67.Hiyoshi T, Hikichi H, Karasawa J, Chaki S. Metabotropic glutamate receptors regulate cortical gamma hyperactivities elicited by ketamine in rats. Neurosci Lett. 2014;567:30–34. doi: 10.1016/j.neulet.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 68.Hiyoshi T, et al. Neurophysiologic and antipsychotic profiles of TASP0433864, a novel positive allosteric modulator of metabotropic glutamate 2 receptor. J Pharmacol Exp Ther. 2014;351:642–653. doi: 10.1124/jpet.114.218651. [DOI] [PubMed] [Google Scholar]
- 69.Hikichi H, et al. Antipsychotic profiles of TASP0443294, a novel and orally active positive allosteric modulator of metabotropic glutamate 2 receptor. J Pharmacol Sci. 2015;127:352–361. doi: 10.1016/j.jphs.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 70.Gozzi A, et al. Differential effects of antipsychotic and glutamatergic agents on the phMRI response to phencyclidine. Neuropsychopharmacology. 2008;33:1690–1703. doi: 10.1038/sj.npp.1301547. [DOI] [PubMed] [Google Scholar]
- 71.Gleason SD, et al. mGlu2/3 agonist-induced hyperthermia: An in vivo assay for detection of mGlu2/3 receptor antagonism and its relation to antidepressant-like efficacy in mice. CNS Neurol Disord Drug Targets. 2013;12:554–566. doi: 10.2174/18715273113129990079. [DOI] [PubMed] [Google Scholar]
- 72.Li N, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park MH, Choi M, Kim YS, Son H. The antidepressant action of 3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid is mediated by phosphorylation of histone deacetylase 5. Korean J Physiol Pharmacol. 2018;22:155–162. doi: 10.4196/kjpp.2018.22.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Talbot JN, et al. Rapid and sustained antidepressant properties of an NMDA antagonist/monoamine reuptake inhibitor identified via transporter-based virtual screening. Pharmacol Biochem Behav. 2016;150–151:22–30. doi: 10.1016/j.pbb.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Refsgaard LK, Pickering DS, Andreasen JT. Investigation of antidepressant-like and anxiolytic-like actions and cognitive and motor side effects of four N-methyl-D-aspartate receptor antagonists in mice. Behav Pharmacol. 2017;28:37–47. doi: 10.1097/FBP.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 76.Li SX, et al. Uncoupling DAPK1 from NMDA receptor GluN2B subunit exerts rapid antidepressant-like effects. Mol Psychiatry. 2018;23:597–608. doi: 10.1038/mp.2017.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mele A, Castellano C, Oliverio A. Chronic treatment with MK-801 affects the behavioral response to both D1 and D2 dopamine agonist in the one-trial inhibitory avoidance. Psychopharmacology (Berl) 1995;121:401–405. doi: 10.1007/BF02246081. [DOI] [PubMed] [Google Scholar]
- 78.Zhang L, Shirayama Y, Iyo M, Hashimoto K. Minocycline attenuates hyperlocomotion and prepulse inhibition deficits in mice after administration of the NMDA receptor antagonist dizocilpine. Neuropsychopharmacology. 2007;32:2004–2010. doi: 10.1038/sj.npp.1301313. [DOI] [PubMed] [Google Scholar]
- 79.Torrisi SA, et al. Buspirone counteracts MK-801-induced schizophrenia-like phenotypes through dopamine D3 receptor blockade. Front Pharmacol. 2017;8:710. doi: 10.3389/fphar.2017.00710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Monn JA, et al. Synthesis, pharmacological characterization, and molecular modeling of heterobicyclic amino acids related to (+)-2-aminobicyclo[3.1.0] hexane-2,6-dicarboxylic acid (LY354740): Identification of two new potent, selective, and systemically active agonists for group II metabotropic glutamate receptors. J Med Chem. 1999;42:1027–1040. doi: 10.1021/jm980616n. [DOI] [PubMed] [Google Scholar]
- 81.Collado I, et al. (2S,1′S,2′S,3′R)-2-(2′-carboxy-3′-methylcyclopropyl) glycine is a potent and selective metabotropic group 2 receptor agonist with anxiolytic properties. J Med Chem. 2002;45:3619–3629. doi: 10.1021/jm0110486. [DOI] [PubMed] [Google Scholar]
- 82.Irifune M, Shimizu T, Nomoto M, Fukuda T. Involvement of N-methyl-D-aspartate (NMDA) receptors in noncompetitive NMDA receptor antagonist-induced hyperlocomotion in mice. Pharmacol Biochem Behav. 1995;51:291–296. doi: 10.1016/0091-3057(94)00379-w. [DOI] [PubMed] [Google Scholar]
- 83.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: A novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thompson H, Neale SA, Salt TE. Activation of group II and group III metabotropic glutamate receptors by endogenous ligand(s) and the modulation of synaptic transmission in the superficial superior colliculus. Neuropharmacology. 2004;47:822–832. doi: 10.1016/j.neuropharm.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 85.Kew JN, Pflimlin MC, Kemp JA, Mutel V. Differential regulation of synaptic transmission by mGlu2 and mGlu3 at the perforant path inputs to the dentate gyrus and CA1 revealed in mGlu2-/- mice. Neuropharmacology. 2002;43:215–221. doi: 10.1016/s0028-3908(02)00084-9. [DOI] [PubMed] [Google Scholar]
- 86.Zanos P, et al. A negative allosteric modulator for α5 subunit-containing GABA receptors exerts a rapid and persistent antidepressant-like action without the side effects of the NMDA receptor antagonist ketamine in mice. eNeuro. 2017;4:ENEURO.0285-16.2017. doi: 10.1523/ENEURO.0285-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fitzgerald PJ, Watson BO. Gamma oscillations as a biomarker for major depression: An emerging topic. Transl Psychiatry. 2018;8:177. doi: 10.1038/s41398-018-0239-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Muthukumaraswamy SD, et al. Evidence that subanesthetic doses of ketamine cause sustained disruptions of NMDA and AMPA-mediated frontoparietal connectivity in humans. J Neurosci. 2015;35:11694–11706. doi: 10.1523/JNEUROSCI.0903-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shaw AD, et al. Ketamine amplifies induced gamma frequency oscillations in the human cerebral cortex. Eur Neuropsychopharmacol. 2015;25:1136–1146. doi: 10.1016/j.euroneuro.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 90.Sanacora G, et al. Lanicemine: A low-trapping NMDA channel blocker produces sustained antidepressant efficacy with minimal psychotomimetic adverse effects. Mol Psychiatry. 2014;19:978–985. doi: 10.1038/mp.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kocsis B, Brown RE, McCarley RW, Hajos M. Impact of ketamine on neuronal network dynamics: Translational modeling of schizophrenia-relevant deficits. CNS Neurosci Ther. 2013;19:437–447. doi: 10.1111/cns.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grent-’t-Jong T, et al. Acute ketamine dysregulates task-related gamma-band oscillations in thalamo-cortical circuits in schizophrenia. Brain. 2018;141:2511–2526. doi: 10.1093/brain/awy175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rivolta D, et al. Ketamine dysregulates the amplitude and connectivity of high-frequency oscillations in cortical-subcortical networks in humans: Evidence from resting-state magnetoencephalography-recordings. Schizophr Bull. 2015;41:1105–1114. doi: 10.1093/schbul/sbv051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Farber NB, Newcomer JW, Olney JW. The glutamate synapse in neuropsychiatric disorders. Focus on schizophrenia and Alzheimer’s disease. Prog Brain Res. 1998;116:421–437. doi: 10.1016/s0079-6123(08)60453-7. [DOI] [PubMed] [Google Scholar]
- 96.Gould TD, Zarate CA, Jr, Thompson SM. Molecular pharmacology and neurobiology of rapid-acting antidepressants. Annu Rev Pharmacol Toxicol. 2019;59:213–236. doi: 10.1146/annurev-pharmtox-010617-052811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nugent AC, et al. Ketamine has distinct electrophysiological and behavioral effects in depressed and healthy subjects. Mol Psychiatry. February 27, 2018 doi: 10.1038/s41380-018-0028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ahnaou A, Ver Donck L, Drinkenburg WH. Blockade of the metabotropic glutamate (mGluR2) modulates arousal through vigilance states transitions: Evidence from sleep-wake EEG in rodents. Behav Brain Res. 2014;270:56–67. doi: 10.1016/j.bbr.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 99.Podkowa K, Pochwat B, Brański P, Pilc A, Pałucha-Poniewiera A. Group II mGlu receptor antagonist LY341495 enhances the antidepressant-like effects of ketamine in the forced swim test in rats. Psychopharmacology (Berl) 2016;233:2901–2914. doi: 10.1007/s00213-016-4325-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Palucha-Poniewiera A, Podkowa K, Pilc A. Role of AMPA receptor stimulation and TrkB signaling in the antidepressant-like effect of ketamine co-administered with a group II mGlu receptor antagonist, LY341495, in the forced swim test in rats. Behav Pharmacol. February 1, 2019 doi: 10.1097/FBP.0000000000000471. [DOI] [PubMed] [Google Scholar]
- 101.Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- 102.Homayoun H, Jackson ME, Moghaddam B. Activation of metabotropic glutamate 2/3 receptors reverses the effects of NMDA receptor hypofunction on prefrontal cortex unit activity in awake rats. J Neurophysiol. 2005;93:1989–2001. doi: 10.1152/jn.00875.2004. [DOI] [PubMed] [Google Scholar]
- 103.Imre G, Fokkema DS, Ter Horst GJ. Subchronic administration of LY354740 does not modify ketamine-evoked behavior and neuronal activity in rats. Eur J Pharmacol. 2006;544:77–81. doi: 10.1016/j.ejphar.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 104.Imre G, et al. Effects of the mGluR2/3 agonist LY379268 on ketamine-evoked behaviours and neurochemical changes in the dentate gyrus of the rat. Pharmacol Biochem Behav. 2006;84:392–399. doi: 10.1016/j.pbb.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 105.Umbricht D, et al. P.2.f.021 results of a double-blind placebo-controlled study of the antidepressant effects of the mGLU2 negative allosteric modulator RG1578. Eur Neuropsychopharmacol. 2015;25:S447. [Google Scholar]
- 106.Linden AM, et al. Anxiolytic-like activity of the mGLU2/3 receptor agonist LY354740 in the elevated plus maze test is disrupted in metabotropic glutamate receptor 2 and 3 knock-out mice. Psychopharmacology (Berl) 2005;179:284–291. doi: 10.1007/s00213-004-2098-x. [DOI] [PubMed] [Google Scholar]
- 107.National Research Council . Guide for the Care and Use of Laboratory Animals. 8th Ed National Academies Press; Washington, DC: 2011. [Google Scholar]
- 108.Zanos P, et al. The prodrug 4-chlorokynurenine causes ketamine-like antidepressant effects, but not side effects, by NMDA/GlycineB-site inhibition. J Pharmacol Exp Ther. 2015;355:76–85. doi: 10.1124/jpet.115.225664. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.