Significance
Strong epidemiologic evidence indicates that aspirin is a powerful antitumorigenic agent. We now demonstrate that aspirin-triggered resolvins achieve the antitumor and chemopreventive activity of aspirin without toxicity, identifying a mechanism for aspirin’s anticancer activity. Our results suggest that differentiating between suppression and resolution of inflammation is highly relevant in cancer biology, revealing a class of endogenous antitumor mechanisms. These results have pivotal implications for cancer therapy and chemoprevention; unlike anti-inflammatory drugs, aspirin-triggered resolvins are active at nanogram doses and are not immunosuppressive. The antitumorigenic activity of aspirin-triggered resolvins may be harnessed to “mimic” aspirin without incurring aspirin-induced toxicity, such as bleeding, to contain minimal residual disease.
Keywords: metabolomics, eicosanoids, resolvins, inflammation, metastasis
Abstract
Inflammation in the tumor microenvironment is a strong promoter of tumor growth. Substantial epidemiologic evidence suggests that aspirin, which suppresses inflammation, reduces the risk of cancer. The mechanism by which aspirin inhibits cancer has remained unclear, and toxicity has limited its clinical use. Aspirin not only blocks the biosynthesis of prostaglandins, but also stimulates the endogenous production of anti-inflammatory and proresolving mediators termed aspirin-triggered specialized proresolving mediators (AT-SPMs), such as aspirin-triggered resolvins (AT-RvDs) and lipoxins (AT-LXs). Using genetic and pharmacologic manipulation of a proresolving receptor, we demonstrate that AT-RvDs mediate the antitumor activity of aspirin. Moreover, treatment of mice with AT-RvDs (e.g., AT-RvD1 and AT-RvD3) or AT-LXA4 inhibited primary tumor growth by enhancing macrophage phagocytosis of tumor cell debris and counter-regulating macrophage-secreted proinflammatory cytokines, including migration inhibitory factor, plasminogen activator inhibitor-1, and C-C motif chemokine ligand 2/monocyte chemoattractant protein 1. Thus, the pro-resolution activity of AT-resolvins and AT-lipoxins may explain some of aspirin’s broad anticancer activity. These AT-SPMs are active at considerably lower concentrations than aspirin, and thus may provide a nontoxic approach to harnessing aspirin’s anticancer activity.
More than 80 million aspirin tablets are consumed every year (1). Epidemiologic evidence suggests that the nonsteroidal anti-inflammatory drug (NSAID) aspirin reduces the risk and incidence of cancer and also prolongs survival when administered postdiagnosis (2). While initial studies have focused on colorectal cancers, low-dose aspirin has also demonstrated consistent antitumor activity in other cancers, including lung, breast, prostate, and metastatic cancers (3, 4). Studies have also identified survival and chemopreventive benefits of low-dose aspirin following cytotoxic therapy (e.g., radiation, chemotherapy) or surgical tumor resection (5, 6). Compelling evidence of aspirin’s anticancer activity stems from patients receiving low-dose aspirin for cardioprevention, in which a substantial fraction (20–30%) benefits from a decrease in cancer incidence (7). In contrast, several studies show that neither nonaspirin NSAIDs nor acetaminophen are associated with a reduced risk of cancer or chemopreventive activity (8, 9). While the known anti-inflammatory activity of aspirin offers a generic rationale, the unique antitumor mechanisms of aspirin compared with other NSAIDs remain poorly understood. Importantly, the use of low-dose aspirin in cancer patients is limited by adverse side effects, such as gastrointestinal bleeding and hemorrhagic stroke, that necessitate hospitalization (10).
The study of anti-inflammatory mechanisms in cancer has traditionally focused on the suppression of proinflammatory mediators, such as cytokines, eicosanoids, and enzymes (2). Cyclooxygenase (COX)-1 and COX-2 are key targets of aspirin and are involved in the biosynthesis of proinflammatory lipid autacoids, such as prostaglandins. Aspirin’s anticancer activity has previously been attributed to the irreversible acetylation of cyclooxygenases, which are overexpressed in colorectal and many other cancers (11, 12). Unlike other NSAIDs that reversibly block COX enzymes, aspirin has been shown to qualitatively alter the enzymatic substrate specificity and activity of COX. A unique activity of aspirin-acetylated COX is the production of aspirin-triggered (AT) specialized proresolving mediators (SPMs), including AT-lipoxin A4 (AT-LXA4) and AT-resolvins D1 (AT-RvD1) and D3 (AT-RvD3) (13–15). Other NSAIDs are not known to trigger endogenous SPM production (16). SPMs, such as resolvins and lipoxins, are immunoresolvent agonists that promote the resolution of inflammation by stimulating phagocytosis of cellular debris and counter-regulating proinflammatory cytokines without being immunosuppressive (16).
Aspirin-acetylated COX facilitates the biosynthesis of aspirin-triggered specialized proresolving mediators (AT-SPMs) from omega-3 polyunsaturated fatty acid substrates, including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (17). Human plasma resolvin levels are increased and detectable at 0.1–0.4 ng/mL in healthy individuals after dietary intake of EPA and aspirin (18). While AT-resolvins exhibit potent anti-inflammatory actions characteristic of native resolvins, the aspirin-triggered forms (R epimers) resist rapid inactivation by oxidoreductases and have longer half-lives (16). AT-RvD1 exhibits an approximate one log order increased potency in reducing total leukocyte infiltration in murine peritonitis compared with RvD1 (19). Intriguingly, AT-SPMs are increased in humans who respond to the anti-inflammatory activity of aspirin compared with those that do not respond to aspirin (20). Aspirin uses endogenous biosynthetic mechanisms to trigger transcellular biosynthesis of lipid mediators such as AT-lipoxins by human endothelial cell–leukocyte interactions or aspirin-stimulated neutrophils cocultured with human lung adenocarcinoma tumor cells (13, 14). AT-lipoxins also modulate tumor-associated macrophages and reduce bone cancer pain (21, 22). We recently demonstrated that SPMs, such as resolvins, enhance cytotoxic cancer therapy by promoting the clearance of therapy-generated tumor cell debris by macrophages (23). The antitumor mechanisms of the aspirin-triggered form of SPMs, such as AT-resolvins, remain to be addressed.
Here we provide evidence that AT-SPMs, including AT-resolvins and AT-lipoxins, are critical for the anticancer activity of low-dose aspirin by stimulating the resolution of tumor-promoting inflammation in mice. Both low-dose aspirin and AT-SPMs inhibit experimental primary tumor growth and metastasis by stimulating the clearance of therapy-generated tumor cell debris. Given that traditional cancer therapeutics (e.g., chemotherapy and radiation) induce inflammation and generate tumor cell debris (23–25), aspirin-triggered lipid autacoids that stimulate endogenous inflammation-clearing (resolution) mechanisms may offer a novel therapeutic approach to harness aspirin’s anti-cancer activity while avoiding the toxicity of aspirin.
Results
Antitumor Activity of Aspirin Is Resolvin-Receptor Dependent.
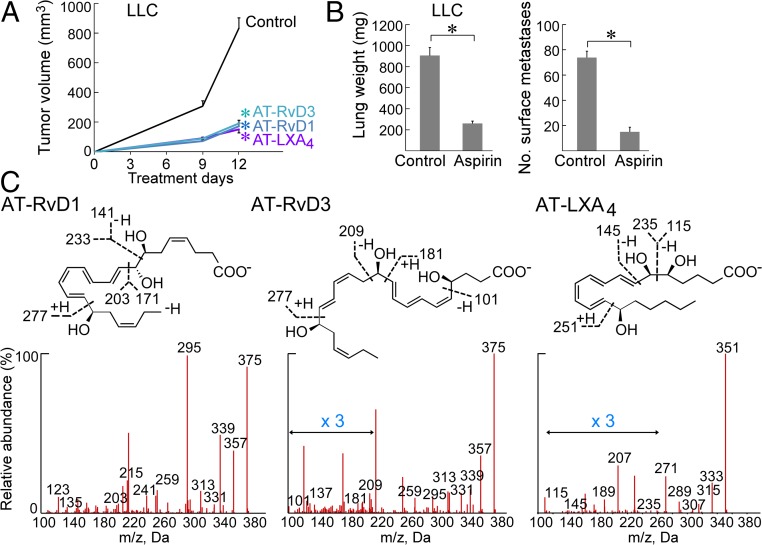
To evaluate the potential anticancer activity of AT-RvDs, we used AT-SPMs in an aggressive murine Lewis lung carcinoma (LLC) tumor model (26). Systemic treatment with AT-RvD1, AT-RvD3, or AT-LXA4 (0.6 μg/kg/d) inhibited LLC tumor growth (generated with an inoculum of 106 cells/mouse) for up to 12 treatment days compared with vehicle-treated mice (Fig. 1A). To confirm that the inhibition of primary tumor growth by AT-SPMs was not strain-, cell line-, or sex-specific, we next examined the tumor cell lines MC38 colon adenocarcinoma in male C57BL/6 mice and 4T1 mammary carcinoma in female BALB/c mice. AT-RvD1, AT-RvD3, or AT-LXA4 also inhibited MC38 and 4T1 tumor growth (106 MC38 or 4T1 cells/mouse) compared with control mice for up to 19 and 25 treatment days, respectively (SI Appendix, Fig. S1 A–C).
Fig. 1.
AT-SPMs or aspirin inhibits primary tumor growth. (A) AT-RvD1, AT-RvD3, or AT-LXA4 (0.6 μg/kg/d) with primary LLC tumor growth. Treatment was initiated on the day of tumor cell injection throughout. Values are represented as mean ± SEM; n = 5–10 mice/group. The two-tailed Student t test was used for final tumor measurements. *P < 0.05 vs. control. (B) Aspirin (30 mg/kg/d used throughout) with orthotopic LLC tumor growth. n = 4–5 mice/group. *P < 0.05 vs. control. Lungs were resected and weighed, and visible metastatic nodules were counted at 19 d after LLC injection. *P < 0.05 vs. control. (C) LC-MS/MS fragmentation spectra of AT-RvD1, AT-RvD3, and AT-LXA4 in LLC tumor tissue from mice systemically treated with aspirin (30 mg/kg/d) for 9 d.
We next evaluated whether aspirin has antitumor activity in these models and whether AT-SPMs can be detected in mice treated with aspirin. To do so, we treated orthotopic and spontaneous tumor models with low-dose aspirin (30 mg/kg/d) (27, 28). Aspirin suppressed lung (LLC) tumor growth, with a threefold reduction in tumor burden (lung weight) and a fivefold reduction in metastases, as well as orthotopic mammary carcinoma (4T1) growth (Fig. 1B and SI Appendix, Fig. S2 A and B). Moreover, aspirin inhibited spontaneous tumor growth in a genetically engineered mouse model [mouse mammary tumor virus (MMTV)-PyMT] (SI Appendix, Fig. S2 C and D). To determine whether aspirin induced AT-SPM production in our tumor models, we quantified AT-SPMs via LC-MS/MS profiling of tumor lysates and plasma isolated from LLC tumor-bearing mice following either 1 h or 9 d of systemic aspirin treatment. LC-MS/MS analysis identified significantly increased AT-SPMs in tumor tissues and plasma from mice given systemic aspirin for 9 d compared with control mice (Fig. 1C and SI Appendix, Tables S1 and S2). Specifically, AT-RvD1 in tumor tissue isolated from tumor-bearing mice increased from 4.4 pg/100 mg of tissue to 65.9 pg/100 mg of tissue following aspirin treatment compared with controls (SI Appendix, Table S1). Importantly, aspirin induced tumor cell apoptosis when administered systemically to mice injected with GFP-labeled LLC tumors (106 cells/mouse) (SI Appendix, Fig. S3A). In contrast, aspirin did not exhibit direct tumor cell cytotoxicity in cell cultures, suggesting a stroma-dependent cell-killing mechanism (SI Appendix, Fig. S3B).
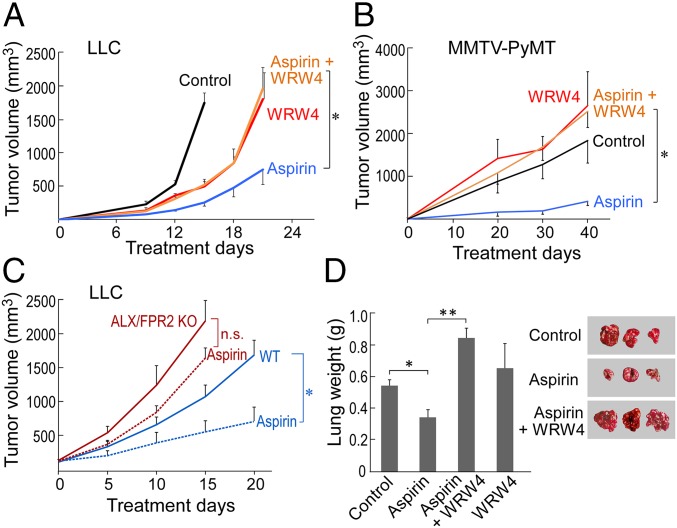
To further assess whether aspirin’s anticancer activity is mediated by AT-SPMs, we used WRW4, a pharmacologic antagonist of the AT-RvD1, RvD1, and the AT-LXA4 receptor ALX/FPR2 (29). The antitumor activity of aspirin in tumor-bearing mice (106 LLC cells/mouse) was neutralized by coadministration with the ALX/FPR2 antagonist (WRW4) (Fig. 2A). WRW4 further neutralized aspirin’s antitumor activity in orthotopic (4T1) and spontaneous (MMTV-PyMT) tumor models (Fig. 2B and SI Appendix, Fig. S4 A and B). To confirm that these results are not specific to WRW4, we used an additional blocking peptide to neutralize ALX/FPR2 function. Consistent with WRW4, the antitumor activity of aspirin was abrogated by coadministration with an anti-ALX/FPR2 blocking peptide (SI Appendix, Fig. S4C). We further characterized the role of ALX/FPR2 in aspirin’s anticancer activity by systemically treating established tumors (106 LLC cells/mouse) in genetically engineered ALX/FPR2 knockout (KO) or wild-type (WT) mice with low-dose aspirin or vehicle. Consistent with the antitumor activity of resolvins (23, 30–34), LLC tumor growth was accelerated in the ALX/FPR2 KO mice compared with WT mice (Fig. 2C). While systemic treatment with low-dose aspirin markedly inhibited LLC tumor growth in WT mice, it produced drastically reduced antitumor activity in ALX/FPR2 KO mice (Fig. 2C). The minimal residual antitumor activity of aspirin in ALX/FPR2 KO mice may be due to endogenous production of aspirin-triggered SPMs that act via receptors other than ALX/FPR2.
Fig. 2.
Antitumor activity of aspirin is resolvin-receptor dependent. WRW4 (1 mg/kg/d) and/or aspirin (30 mg/kg/d) with primary LLC (A) or spontaneous MMTV-PyMT (B) tumor growth. Values are mean ± SEM. For MMTV-PyMT mice, tumor volume represents the sum tumor volume of all visible tumors per mouse. n = 5–10 mice/group throughout. The two-tailed Student t test was used for final tumor measurements. *P < 0.05, aspirin vs. control; aspirin vs. aspirin + WRW4. (C) Aspirin (dashed lines) or control (solid lines) with LLC tumor growth in ALX/FPR2 KO mice (red lines) compared with WT mice (blue lines). Treatment was initiated once tumors reached 100–200 mm3. *P < 0.05 vs. WT control; n.s., not significant. (D) WRW4 and aspirin with primary LLC tumor resection and subsequent metastasis. Lung weights (g) and images are representative of lung metastases for each treatment group on day 14 after LLC tumor resection. *P < 0.05, aspirin vs. control; **P < 0.01, aspirin vs. aspirin + WRW4.
We next evaluated whether aspirin’s antitumor activity was limited to primary tumor sites by studying spontaneous lung metastasis following primary tumor resection. Using a well-established model of minimal residual disease in which resection of a primary tumor reproducibly stimulates the development of distant lung metastasis at 14–17 d after resection (26), we investigated whether aspirin exhibits antimetastatic activity mediated by resolvins after LLC resection. Aspirin alone inhibited spontaneous LLC metastatic growth triggered by primary tumor resection (Fig. 2D). In contrast, autopsies of moribund mice at 14 d after primary LLC tumor resection revealed a dramatic increase in lung weight and number of surface lung metastases in mice systemically administered the combination of low-dose aspirin and the pharmacologic resolvin receptor (ALX/FPR2) antagonist WRW4 compared with low-dose aspirin alone (Fig. 2D).
AT-SPMs Stimulate Macrophage Phagocytosis of Therapy-Generated Tumor Cell Debris.
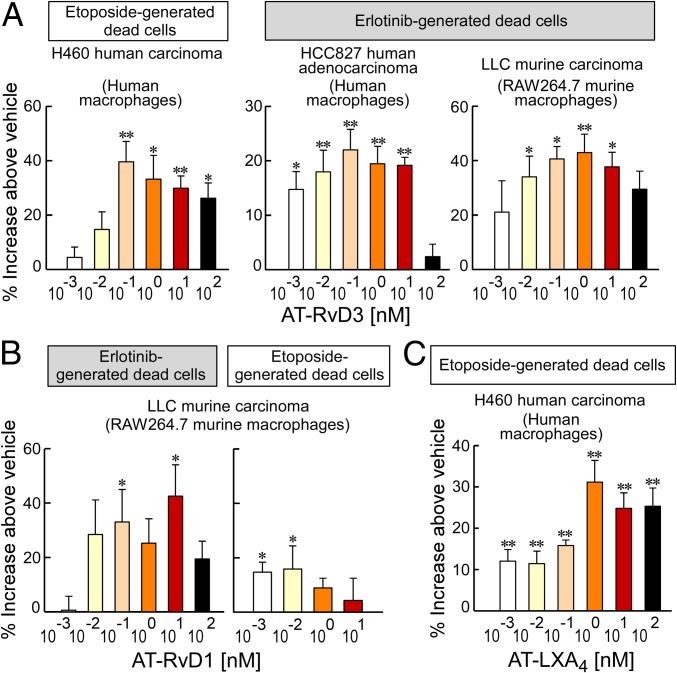
A critical function of resolvins and lipoxins is stimulation of nonphlogistic macrophage phagocytosis of debris (16). Therefore, we examined whether aspirin or AT-SPMs can stimulate the endogenous clearance of therapy-killed tumor cells, which, as we recently showed, promote tumor growth and recurrence (23). Using annexin V and propidium iodide staining, we confirmed the generation of apoptotic/necrotic tumor cells, hereinafter referred to as “tumor cell debris,” in cell cultures treated with chemotherapy (etoposide) or targeted therapy (erlotinib) (SI Appendix, Fig. S5 A–C). AT-RvD3 (100 pM–100 nM) stimulated human monocyte-derived macrophage phagocytosis of etoposide-generated human lung carcinoma (H460) debris or erlotinib-generated human lung carcinoma (HCC827) debris (Fig. 3A). Similarly, AT-RvD3 stimulated RAW264.7 murine macrophage phagocytosis of erlotinib-generated murine LLC tumor cell debris (Fig. 3A). Both AT-RvD1 and AT-LXA4 (100 pM–100 nM) also enhanced RAW264.7 murine macrophage or human monocyte-derived macrophage phagocytosis of therapy-generated LLC or H460 tumor cell debris, respectively, by 30–40% above vehicle (Fig. 3 B and C). Thus, aspirin-triggered lipoxins and resolvins stimulate phagocytosis of tumor cell debris in a dose-dependent and biphasic manner with activity diminishing at doses above 1 nM, a behavior characteristic of ligands that signal via G protein-coupled receptors (35).
Fig. 3.
AT-SPMs stimulate macrophage phagocytosis of therapy-generated tumor cell debris. Shown is human monocyte-derived macrophage or RAW264.7 murine macrophage phagocytosis of carboxyfluorescein diacetate-labeled tumor cell debris following AT-SPM treatment. Phagocytosis was quantified by relative fluorescent units (RFUs) normalized to percent increase above vehicle-treated macrophages. The two-tailed Student t test was used throughout; *P < 0.05; **P < 0.01 vs. vehicle. n = 6–12/group throughout. (A) AT-RvD3 with human or murine macrophage phagocytosis of etoposide- or erlotinib-generated tumor cell debris (H460, HCC827, and LLC). (B) AT-RvD1 with murine macrophage phagocytosis of erlotinib- or etoposide-generated LLC tumor cell debris. (C) AT-LXA4 with human macrophage phagocytosis of etoposide-generated H460 tumor cell debris.
Low-Dose Aspirin Triggers Macrophage Clearance of Therapy-Generated Tumor Cell Debris in a Receptor-Dependent Manner.
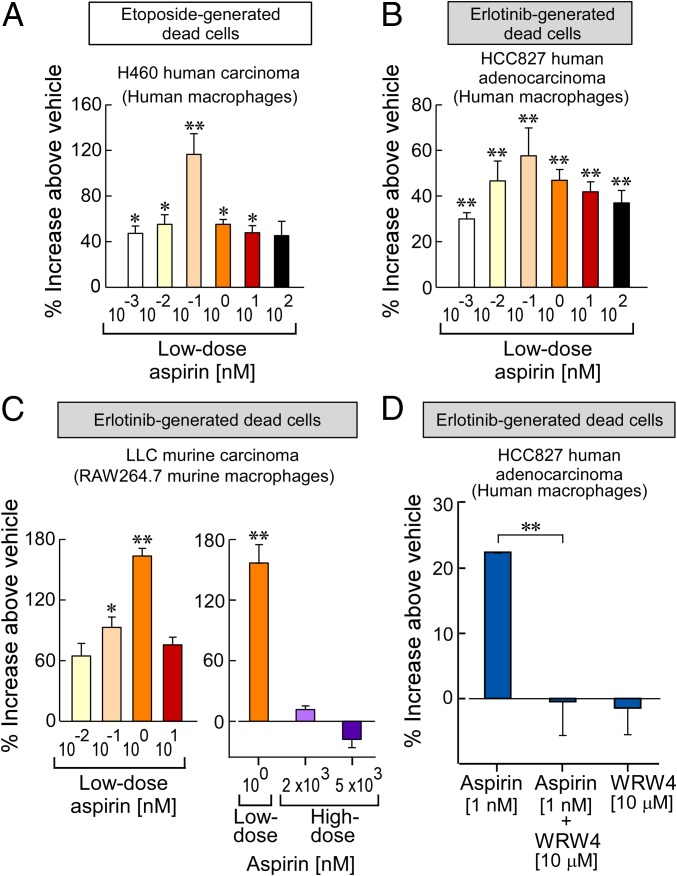
Macrophages express COX and LOX enzymes required for SPM biosynthesis, including resolvins (36, 37), and aspirin stimulates macrophage in vitro production of SPMs (16). Given that AT-SPMs stimulate the clearance of tumor cell debris, we also examined whether aspirin can stimulate macrophage phagocytosis of tumor cell debris. Consistent with AT-SPMs, aspirin significantly stimulated human monocyte-derived macrophage phagocytosis of tumor cell debris up to 116% above vehicle (Fig. 4 A and B). Similarly, aspirin stimulated RAW264.7 murine macrophage phagocytosis of erlotinib-generated LLC debris up to 163% above vehicle (Fig. 4C). Again, there was a biphasic response in which aspirin at high doses (2 or 5 μM) did not stimulate macrophage phagocytosis of tumor cell debris (Fig. 4C).
Fig. 4.
Low-dose aspirin stimulates macrophage phagocytosis of therapy-generated tumor cell debris. Shown is human monocyte-derived macrophage or RAW264.7 murine macrophage phagocytosis of carboxyfluorescein diacetate-labeled tumor cell debris following aspirin treatment. Phagocytosis was quantified by RFUs normalized to percent increase above vehicle-treated macrophages. The two-tailed Student t test was used throughout. *P < 0.05; **P < 0.01 vs. vehicle. n = 6–12/group throughout. (A and B) Low-dose aspirin (10−3−102 nM) with human macrophage phagocytosis of etoposide-generated H460 tumor cell debris (A) or erlotinib-generated HCC827 tumor cell debris (B). (C) Low- or high-dose aspirin (2,000 or 5,000 nM) with murine macrophage phagocytosis of erlotinib-generated LLC tumor cell debris. (D) WRW4 (10 μM) and aspirin (1 nM) with human macrophage phagocytosis of erlotinib-generated HCC827 tumor cell debris.
To determine whether the stimulation of macrophage phagocytosis by aspirin was mediated by AT-SPMs, including AT-LXA4 and AT-RvD1, macrophages were treated with the ALX/FPR2 antagonist WRW4 before treatment with aspirin and coincubation with tumor cell debris. While aspirin (1 nM) significantly stimulated human monocyte-derived macrophage phagocytosis, treatment with WRW4 neutralized aspirin-stimulated macrophage phagocytosis of debris (Fig. 4D). Macrophages treated with WRW4 alone did not exhibit increased phagocytosis of debris (Fig. 4D). To further characterize the role of the ALX/FPR2 receptor in aspirin-stimulated phagocytosis, peritoneal macrophages were isolated from WT or ALX/FPR2 KO mice and treated with low-dose aspirin. While aspirin stimulated phagocytosis of tumor cell debris by WT macrophages, ALX/FPR2 KO macrophages did not demonstrate increased phagocytosis in response to aspirin treatment (SI Appendix, Fig. S6 A and B). Moreover, AT-RvD1, a known ligand of the ALX/FPR2 receptor, also stimulated WT, but not ALX/FPR2 KO, macrophage phagocytosis of tumor cell debris (SI Appendix, Fig. S6 C and D).
AT-SPMs and Low-Dose Aspirin Suppress Macrophage Secretion of Proinflammatory Cytokines.
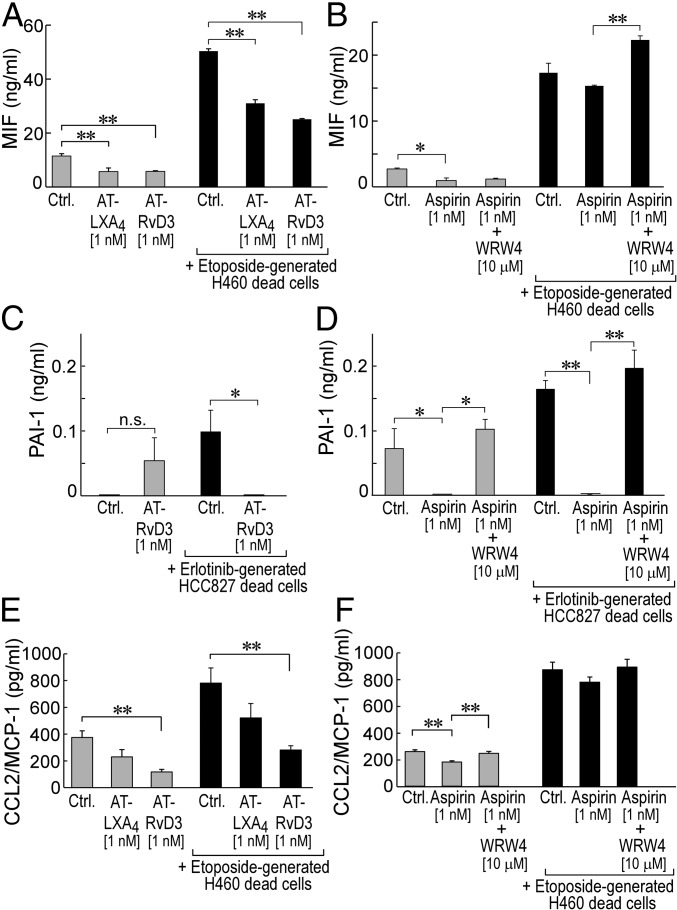
In addition to stimulating phagocytosis of cellular debris, SPMs and AT-SPMs (e.g., AT-LXA4, AT-RvD1, AT-RvD3) actively promote the resolution of inflammation by counter-regulating proinflammatory cytokines/chemokines (16). On screening for a broad panel of 36 proinflammatory cytokines, we identified several macrophage-produced cytokines whose secretion was suppressed by aspirin or AT-SPMs in the presence or absence of tumor cell debris. Both AT-LXA4 and AT-RvD3 (1 nM) inhibited baseline human monocyte-derived macrophage secretion of the proinflammatory cytokine macrophage migration inhibitory factor (MIF) (Fig. 5A, gray bars). Aspirin (1 nM) also significantly inhibited baseline macrophage secretion of MIF (Fig. 5B, gray bars). Coincubation of macrophages with etoposide-generated human H460 tumor cell debris drastically increased macrophage secretion of MIF by 4.5-fold, which was dampened by 40–50% by both AT-LXA4 and AT-RvD3 (Fig. 5A, black bars). Aspirin (1 nM) also exhibited a minimal, not statistically significant, reduction in MIF secretion by human monocyte-derived macrophages in the presence of etoposide-generated H460 tumor cell debris (Fig. 5B, black bars). Treatment of macrophages with the combination of aspirin and the ALX/FPR2 antagonist WRW4 did not suppress MIF secretion by debris-stimulated macrophages (Fig. 5B, black bars). AT-RvD3 treatment also inhibited plasminogen activator inhibitor-1 (PAI-1) secretion by human monocyte-derived macrophages in the presence of erlotinib-generated HCC827 debris (Fig. 5C, black bars). While aspirin also inhibited secretion of PAI-1 by macrophages in the absence or presence of erlotinib-generated HCC827 tumor cell debris, WRW4 again neutralized this aspirin-mediated inhibition (Fig. 5D). Both AT-LXA4 and AT-RvD3 also inhibited macrophage secretion of C-C motif chemokine ligand 2 (CCL2)/monocyte chemoattractant protein 1 (MCP-1) in the presence or absence of tumor cell debris (Fig. 5E). Consistent with MIF and PAI-1, aspirin inhibited macrophage secretion in the absence of tumor cell debris, whereas treatment of macrophages with both aspirin and WRW4 did not inhibit cytokine production (Fig. 5F). Thus, AT-SPMs and low-dose aspirin stimulate the resolution of inflammation by increasing macrophage phagocytosis of tumor cell debris and counter-regulating protumorigenic cytokines.
Fig. 5.
AT-SPMs and low-dose aspirin suppress macrophage secretion of proinflammatory cytokines. Shown is human monocyte-derived macrophage cytokine secretion following AT-SPM or aspirin (1 nM) treatment and/or coincubation with tumor cell debris. Values are represented as mean ± SEM throughout. The two-tailed Student t test was used throughout. *P < 0.05; **P < 0.01 vs. control (A–F) or aspirin alone (B, D, and F). n.s., not significant (C). n = 3–6/group throughout. (A and B) Baseline (gray bars) or H460 tumor cell debris-stimulated (black bars) macrophage secretion of macrophage MIF following treatment with AT-LXA4 (1 nM), AT-RvD3 (1 nM), aspirin (1 nM), or aspirin (1 nM) + WRW4 (10 μM). (C and D) Baseline (gray bars) or HCC827 tumor cell debris-stimulated (black bars) macrophage secretion of PAI-1 following treatment with AT-RvD3 (1 nM), aspirin (1 nM), or aspirin (1 nM) + WRW4 (10 μM). (E and F) Baseline (gray bars) or H460 tumor cell debris-stimulated (black bars) macrophage secretion of CCL2/MCP-1 following treatment with AT-LXA4 (1 nM), AT-RvD3 (1 nM), aspirin (1 nM), or aspirin (1 nM) + WRW4 (10 μM).
Discussion
Chronic inflammation has emerged as a critical factor in tumorigenesis and cancer progression (2). We recently demonstrated that tumor cell debris generated by cancer therapy stimulates tumor growth and metastasis, a process mediated by proinflammatory cytokines (23). Along with blocking the biosynthesis of prostaglandins from omega-6 fatty acid substrates, aspirin covalently interacts with COX-2 and alters COX-2 activity to trigger the production of anti-inflammatory and proresolving AT-SPMs (13, 19) from omega-3 fatty acid precursors. Here we demonstrate that both low-dose aspirin and AT-SPMs, including resolvins and lipoxins (AT-RvD1, AT-RvD3, and AT-LXA4), inhibit primary tumor growth and metastasis by enhancing endogenous macrophage clearance of tumor cell debris and quelling tumor-associated inflammation by counter-regulating protumorigenic cytokines. Importantly, we demonstrate via genetic and pharmacologic ablation that the antitumor activity of aspirin is resolvin receptor-dependent, thereby identifying a previously unknown mechanism for the unique chemopreventive activity of aspirin.
Genetic or pharmacologic ablation of ALX/FPR2 did not completely abrogate the antitumor activity of aspirin. This could potentially result from the diverse actions of SPMs. Aspirin-triggered SPMs encompass not only AT-RvD1, AT-RvD3, and AT-LXA4, each of which activates the ALX/FPR2 and GPR32 receptors (15, 38), but also AT-protectin D1 (AT-PD1), whose receptor remains to be identified, and the AT-resolvin E series, which act via the ChemR23 and BLT1 receptors (18, 39). Thus, aspirin-triggered PD1 and the resolvin E series may account for the residual antitumor activity of aspirin in the ALX/FPR2 KO mice or in the presence of the ALX/FPR2 antagonist WRW4. However, AT-SPMs (AT-RvD1, AT-RvD3, and AT-LXA4) that act via the receptor ALX/FPR2 collectively may be more potent than AT-PD1 or E series AT-resolvins, as pharmacologic and genetic ALX/FPR2 ablation almost completely neutralized both aspirin inhibition of tumor growth and aspirin stimulation of phagocytosis.
Aspirin reduces cancer risk and mortality by up to 30%; however, its use in chemoprevention of cancer is not recommended due to the increased risk of hemorrhagic events, such as gastrointestinal bleeding and stroke (3). Intriguingly, humans who respond to aspirin treatment of inflammatory lesions show elevated SPMs compared with those who do not respond to aspirin, suggesting that SPMs may play a critical role in the anti-inflammatory activity of aspirin (20). Dietary intake of EPA and DHA together with aspirin increases circulating resolvin levels in humans (17, 19). Moreover, low-dose aspirin administered to healthy volunteers for cardioprevention is also capable of producing bioactive levels of aspirin-triggered lipoxins (40). Our study demonstrates that aspirin-triggered SPMs are increased in tumor tissues and plasma following low-dose aspirin treatment, suggesting that SPMs may mediate aspirin’s broad anti-inflammatory and anti-cancer activities.
Our results demonstrate that systemic administration of aspirin or AT-SPMs inhibit the growth of primary tumors and metastasis in multiple murine tumor models, including orthotopic and genetically engineered models. The dose of aspirin used in this study (30 mg/kg/d) may be slightly higher than the equivalent dose of standard low-dose aspirin used in cardioprevention and may need to be adjusted to body size (41, 42). Importantly, the aspirin dose used in our in vivo tumor studies induced tumor cell apoptosis in mice compared with controls, in accordance with previous reports (43). However, aspirin treatment of cell cultures did not induce tumor cell apoptosis across a broad range of doses (100 –104 nM). Thus, aspirin did not exhibit direct tumor cell cytotoxicity; rather, aspirin induction of tumor cell death was determined to be tumor stroma-dependent.
In this study, AT-SPMs administered in our murine tumor models exhibited antitumor activity at a >1,000-fold lower dose than that of aspirin. This dose of AT-SPMs is also more than log orders of magnitude lower than the doses of their omega-3 fatty acid precursors (EPA and DHA) or NSAIDs required for tumor inhibition (44, 45). SPMs, including resolvins, are currently in clinical development. Given the risks associated with chronic low-dose aspirin intake, mediators such as aspirin-triggered resolvins and AT-SPMs may have more potent antitumor activity devoid of aspirin-related toxicity. Thus, aspirin-triggered resolvins may be optimal chemopreventive agents that represent a new treatment modality in cancer that remains to be evaluated in humans.
Materials and Methods
Methods used for the preparation of therapy-generated tumor cell debris (23, 46), flow cytometry (23, 46), LC-MS/MS profiling (47), isolation of human monocyte-derived macrophages and resident murine peritoneal macrophages (23, 46), and macrophage-conditioned medium for cytokine quantification (23, 46) have been described previously. ELISAs (R&D Systems) were performed according to provided recommended protocols. The protocols are described in detail in SI Appendix, Materials and Methods.
In Vivo Studies.
All animal studies were reviewed and approved by the Animal Care and Use Committee of Boston Children’s Hospital and Beth Israel Deaconess Medical Center. C57BL/6, BALB/c, and MMTV-PyMT mice were obtained from The Jackson Laboratory. ALX/FPR2 KO mice were generously provided by Mauro Perretti, Queen Mary University of London. The mice were systemically treated with 0.6 μg/kg/d of AT-SPMs [AT-LXA4, AT-RvD1 (Cayman Chemical), or AT-RvD3] via a mini osmotic pump (Alzet), low-dose aspirin (30 mg/kg/d; Sigma-Aldrich) via oral gavage, and/or WRW4 (1 mg/kg/d; EMD Millipore) or anti-ALX/FPR2 blocking peptide antibody (25 μg/kg/d; LifeSpan BioSciences) via i.p. injection. For metastasis studies, LLC tumors were surgically resected from the mid-dorsum of 6- to 8-wk-old C57BL/6 mice at 14 d postinjection.
Statistics.
For all animal and in vitro studies, comparisons of two groups were performed using the Student two-tailed unpaired t test. P values <0.05 were considered statistically significant. Data are represented as mean ± SEM.
Supplementary Material
Acknowledgments
We thank Steve Moskowitz (Advanced Medical Graphics) for preparing the figures and photographs. We also thank Romain Colas, Jaimie Chang, Haixia Yang, Suzan Lazo, John Daley, and Lucius Xuan for their excellent technical assistance. This work was supported by the National Institutes of Health (Grants R01 01CA170549, to D.P. and C.N.S., R0CA148633, to D.P., and 5P01 GM095467, to C.N.S.), the Credit Unions Kids at Heart Team (D.P.), the Stop and Shop Pediatric Brain Tumor Fund (M.W.K.), the C.J. Buckley Pediatric Brain Tumor Fund (M.W.K.), Alex’s Lemonade Stand (M.W.K.), Molly’s Magic Wand for Pediatric Brain Tumors (M.W.K.), the Markoff Foundation Art-In-Giving Foundation (M.W.K.), the Kamen Foundation (M.W.K.), Jared Branfman Sunflowers for Life (M.W.K.), and the Joe Andruzzi Foundation (M.W.K.).
Footnotes
Conflict of interest statement: M.W.K. is now an employee of Bristol-Myers Squibb. His position at Bristol-Myers Squibb is not related to this work.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1804000116/-/DCSupplemental.
References
- 1.Dubois RN. Will an aspirin a day keep the endoscope away? Gastroenterology. 2003;125:612–614. doi: 10.1016/s0016-5085(03)00962-4. [DOI] [PubMed] [Google Scholar]
- 2.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arber N, DuBois RN. Nonsteroidal anti-inflammatory drugs and prevention of colorectal cancer. Curr Gastroenterol Rep. 1999;1:441–448. doi: 10.1007/s11894-999-0027-1. [DOI] [PubMed] [Google Scholar]
- 4.Hudis CA, Subbaramaiah K, Morris PG, Dannenberg AJ. Breast cancer risk reduction: No pain, no gain? J Clin Oncol. 2012;30:3436–3438. doi: 10.1200/JCO.2012.44.8597. [DOI] [PubMed] [Google Scholar]
- 5.Restivo A, et al. Aspirin as a neoadjuvant agent during preoperative chemoradiation for rectal cancer. Br J Cancer. 2015;113:1133–1139. doi: 10.1038/bjc.2015.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fontaine E, et al. Aspirin and non-small cell lung cancer resections: Effect on long-term survival. Eur J Cardiothorac Surg. 2010;38:21–26. doi: 10.1016/j.ejcts.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Umar A, Steele VE, Menter DG, Hawk ET. Mechanisms of nonsteroidal anti-inflammatory drugs in cancer prevention. Semin Oncol. 2016;43:65–77. doi: 10.1053/j.seminoncol.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Salinas CA, et al. Use of aspirin and other nonsteroidal anti-inflammatory medications in relation to prostate cancer risk. Am J Epidemiol. 2010;172:578–590. doi: 10.1093/aje/kwq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bardia A, et al. Association of aspirin and nonaspirin nonsteroidal anti-inflammatory drugs with cancer incidence and mortality. J Natl Cancer Inst. 2007;99:881–889. doi: 10.1093/jnci/djk200. [DOI] [PubMed] [Google Scholar]
- 10.Rothwell PM, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741–1750. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 11.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 12.Kalgutkar AS, et al. Aspirin-like molecules that covalently inactivate cyclooxygenase-2. Science. 1998;280:1268–1270. doi: 10.1126/science.280.5367.1268. [DOI] [PubMed] [Google Scholar]
- 13.Clària J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci USA. 1995;92:9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clària J, Lee MH, Serhan CN. Aspirin-triggered lipoxins (15-epi-LX) are generated by the human lung adenocarcinoma cell line (A549)-neutrophil interactions and are potent inhibitors of cell proliferation. Mol Med. 1996;2:583–596. [PMC free article] [PubMed] [Google Scholar]
- 15.Dalli J, et al. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem Biol. 2013;20:188–201. doi: 10.1016/j.chembiol.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serhan CN, et al. Resolvins: A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arita M, et al. Stereochemical assignment, anti-inflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun YP, et al. Resolvin D1 and its aspirin-triggered 17R epimer: Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J Biol Chem. 2007;282:9323–9334. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- 20.Morris T, et al. Dichotomy in duration and severity of acute inflammatory responses in humans arising from differentially expressed pro-resolution pathways. Proc Natl Acad Sci USA. 2010;107:8842–8847. doi: 10.1073/pnas.1000373107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simões RL, et al. Lipoxin A4 selectively programs the profile of M2 tumor-associated macrophages which favour control of tumor progression. Int J Cancer. 2017;140:346–357. doi: 10.1002/ijc.30424. [DOI] [PubMed] [Google Scholar]
- 22.Hu S, et al. Lipoxins and aspirin-triggered lipoxin alleviate bone cancer pain in association with suppressing expression of spinal proinflammatory cytokines. J Neuroinflammation. 2012;9:278. doi: 10.1186/1742-2094-9-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sulciner ML, et al. Resolvins suppress tumor growth and enhance cancer therapy. J Exp Med. 2018;215:115–140. doi: 10.1084/jem.20170681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Revesz L. Effect of tumour cells killed by x-rays upon the growth of admixed viable cells. Nature. 1956;178:1391–1392. doi: 10.1038/1781391a0. [DOI] [PubMed] [Google Scholar]
- 25.Roca H, et al. Apoptosis-induced CXCL5 accelerates inflammation and growth of prostate tumor metastases in bone. J Clin Invest. 2018;128:248–266. doi: 10.1172/JCI92466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panigrahy D, et al. Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice. J Clin Invest. 2012;122:178–191. doi: 10.1172/JCI58128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlson LM, et al. Low-dose aspirin delays an inflammatory tumor progression in vivo in a transgenic mouse model of neuroblastoma. Carcinogenesis. 2013;34:1081–1088. doi: 10.1093/carcin/bgt009. [DOI] [PubMed] [Google Scholar]
- 28.Guillem-Llobat P, et al. Aspirin prevents colorectal cancer metastasis in mice by splitting the crosstalk between platelets and tumor cells. Oncotarget. 2016;7:32462–32477. doi: 10.18632/oncotarget.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dufton N, et al. Anti-inflammatory role of the murine formyl-peptide receptor 2: Ligand-specific effects on leukocyte responses and experimental inflammation. J Immunol. 2010;184:2611–2619. doi: 10.4049/jimmunol.0903526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuang H, Hua X, Zhou J, Yang R. Resolvin D1 and E1 alleviate the progress of hepatitis toward liver cancer in long-term concanavalin A-induced mice through inhibition of NF-κB activity. Oncol Rep. 2016;35:307–317. doi: 10.3892/or.2015.4389. [DOI] [PubMed] [Google Scholar]
- 31.Prevete N, et al. Formyl peptide receptor 1 suppresses gastric cancer angiogenesis and growth by exploiting inflammation resolution pathways. OncoImmunology. 2017;6:e1293213. doi: 10.1080/2162402X.2017.1293213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye Y, et al. Anti-cancer and analgesic effects of resolvin D2 in oral squamous cell carcinoma. Neuropharmacology. 2018;139:182–193. doi: 10.1016/j.neuropharm.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 33.Halder RC, et al. Curcuminoids and ω-3 fatty acids with anti-oxidants potentiate cytotoxicity of natural killer cells against pancreatic ductal adenocarcinoma cells and inhibit interferon γ production. Front Physiol. 2015;6:129. doi: 10.3389/fphys.2015.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee HJ, Park MK, Lee EJ, Lee CH. Resolvin D1 inhibits TGF-β1-induced epithelial mesenchymal transition of A549 lung cancer cells via lipoxin A4 receptor/formyl peptide receptor 2 and GPR32. Int J Biochem Cell Biol. 2013;45:2801–2807. doi: 10.1016/j.biocel.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 35.Chiang N, et al. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Werz O, et al. Human macrophages differentially produce specific resolvin or leukotriene signals that depend on bacterial pathogenicity. Nat Commun. 2018;9:59. doi: 10.1038/s41467-017-02538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halade GV, Norris PC, Kain V, Serhan CN, Ingle KA. Splenic leukocytes define the resolution of inflammation in heart failure. Sci Signal. 2018;11:eaao1818. doi: 10.1126/scisignal.aao1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arnardottir HH, et al. Resolvin D3 is dysregulated in arthritis and reduces arthritic inflammation. J Immunol. 2016;197:2362–2368. doi: 10.4049/jimmunol.1502268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arita M, et al. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 40.Chiang N, Bermudez EA, Ridker PM, Hurwitz S, Serhan CN. Aspirin triggers anti-inflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc Natl Acad Sci USA. 2004;101:15178–15183. doi: 10.1073/pnas.0405445101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ridker PM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 42.Rothwell PM, et al. Effects of aspirin on risks of vascular events and cancer according to body weight and dose: Analysis of individual patient data from randomised trials. Lancet. 2018;392:387–399. doi: 10.1016/S0140-6736(18)31133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stark LA, et al. Aspirin activates the NF-kappaB signalling pathway and induces apoptosis in intestinal neoplasia in two in vivo models of human colorectal cancer. Carcinogenesis. 2007;28:968–976. doi: 10.1093/carcin/bgl220. [DOI] [PubMed] [Google Scholar]
- 44.Grenon SM, et al. n-3 polyunsaturated fatty acids supplementation in peripheral artery disease: The OMEGA-PAD trial. Vasc Med. 2013;18:263–274. doi: 10.1177/1358863X13503695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: Mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002;94:252–266. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- 46.Chang J, et al. Chemotherapy-generated cell debris stimulates colon carcinoma tumor growth via osteopontin. FASEB J. 2019;33:114–125. doi: 10.1096/fj.201800019RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am J Physiol Cell Physiol. 2014;307:C39–C54. doi: 10.1152/ajpcell.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.