Significance
Adaptation to climate can promote the spread of nonnative species, but its wider impacts on native species have rarely been studied. Prickly lettuce (Lactuca serriola), an annual plant that has been introduced from Europe around the world, flowers earlier in arid climates such as those of southern California. By comparing L. serriola populations differing in flowering time within common garden experiments, we demonstrated that native California annual plants experienced stronger competition from the local early flowering L. serriola than later flowering plants from the ancestral European range, making their predicted persistence with L. serriola more difficult. Our study suggests that climate adaptation can have cascading effects on ecological communities, potentially increasing the impacts of biological invasions.
Keywords: biological invasions, coexistence, competition, ecoevolutionary dynamics, phenology
Abstract
Adaptation to climate is expected to increase the performance of invasive species and their community-level impacts. However, while the fitness gains from adaptation should, in general, promote invader competitive ability, empirical demonstrations of this prediction are scarce. Furthermore, climate adaptation, in the form of altered timing of life cycle transitions, should affect the phenological overlap between nonnative and native competitors, with potentially large, but poorly tested, impacts on native species persistence. We evaluated these predictions by growing native California grassland plants in competition with nonnative Lactuca serriola, a species that flowers earlier in parts of its nonnative range that are drier than its putative European source region. In common garden experiments in southern California with L. serriola populations differing in phenology, plants originating from arid climates bolted up to 48 d earlier than plants from more mesic climates, and selection favored early flowering, supporting an adaptive basis for the phenology cline. The per capita competitive effects of L. serriola from early flowering populations on five early flowering native species were greater than the effects of L. serriola from later flowering populations. Consequently, the ability of the native species to increase when rare in competition with L. serriola, as inferred from field-parameterized competition models, declined with earlier L. serriola phenology. Indeed, changes to L. serriola phenology affected whether or not one native species was predicted to persist in competition with L. serriola. Our results suggest that evolution in response to new climatic conditions can have important consequences for species interactions, and enhance the impacts of biological invasions on natural communities.
Rapid adaptive evolution in response to climate can enhance the spread of nonnative species (1, 2), and numerous nonnative plant species have evolved clines in traits related to size, resource allocation, and life history strategy as they spread along environmental gradients in their introduced range (2–4). Although rarely tested directly, the fitness benefit of climate adaptation can be substantial, sometimes exceeding the advantages that nonnative species gain from natural-enemy release (5), making rapid evolution an important contributor to the population dynamics of nonnative species (6, 7). At the same time, it is increasingly recognized that rapid evolution can affect how species interact with one another, with far-reaching impacts on community and ecosystem dynamics (8, 9). However, because invasion studies have thus far emphasized the demographic consequences of climate adaptation for the evolving invader, the wider community-level consequences of climate adaptation remain poorly studied.
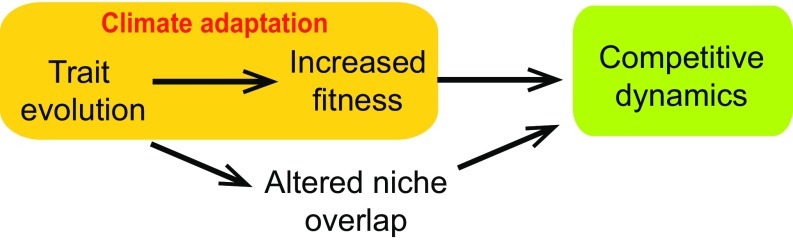
Climate adaptation in a nonnative species could impact the outcome of its interactions with native species through two distinct pathways (Fig. 1). Firstly, the fitness gains conferred by climate adaptation will, all else being equal, increase the invader’s population density, increasing its impact on native competitors (10). Indeed, modern coexistence theory shows how differences between species in their demographic potential are an important determinant of competitive outcomes (11, 12), and the evolution of more vigorously growing individuals plays a central role in several explanations for invader success. For example, ecologists have hypothesized that a relaxation of the selective pressures imposed by specialist enemies in the native range allows species to evolve greater competitive ability in their nonnative range (13), although empirical support is mixed (3, 14). Maybe more common are evolutionary responses to climate (2–4), and these should similarly promote the competitive ability of a nonnative species relative to its founder population.
Fig. 1.
Effects of climate adaptation on ecological dynamics. Trait evolution resulting in increased fitness through the process of adaption not only influences the population dynamics of the evolving species itself but will also increase that species’ population-level effect on competitors. However, trait evolution can also influence community dynamics by affecting the extent of niche overlap between competitors, which could either reinforce or weaken the competitive effects of the evolving species.
The second way in which climate adaptation in an invasive population can affect its competitive dynamics is through its effect on the extent of niche overlap between competitors (10), the other determinant of competitive outcomes in modern coexistence theory (12). More specifically, climate adaptation could enhance or weaken the ecological impacts of an invading species, depending on how the traits under selection by climate affect competitive niche overlap with native species (Fig. 1). This complex relationship between traits, climate, and competition is maybe most expected with the evolution of plant phenology, the timing of life history events.
Phenology is among the traits most commonly under selection by climate (e.g., refs. 5, 7, and 15), and differences between plant species in their phenology can strongly influence their niche overlap (16–18). Thus, while climate adaptation in phenology might affect invader competitive dynamics by increasing fitness, the first pathway in Fig. 1, it can also affect the niche overlap with native competitors, the second pathway. For example, in communities where selection generally favors species that flower early, the evolution of earlier flowering in an invading species will tend to increase niche overlap, increasing the probability that competitively inferior native species are excluded from the community (17). The consequences of climate adaptation in the invader for its impact on the native community will therefore depend on the combination of the demographic advantages conferred by adaptation, and the effect of trait evolution on niche overlap between competitors (Fig. 1 and refs. 17 and 19).
With biological invasions, we can test how trait changes associated with climate adaptation affect competitive outcomes, because information about the introduction history presents investigators with descendent populations that have evolved to the climate in the nonnative range, and ancestral populations that have not. By comparing the competitive effect of putative ancestor and descendent populations on native competitors in the nonnative range, one can reconstruct how competitive interactions have likely changed as a consequence of climate adaptation. Moreover, when this empirical information is used to inform models of competitive population dynamics, as we do here, one can predict how climate adaptation in the invader may affect the persistence of the native species.
Here, we evaluate the hypothesis that climate-related differences in the phenology of nonnative prickly lettuce [Lactuca serriola (Asteraceae)] leads to altered interactions with native annual plants in California. Nonnative L. serriola populations originate from Europe (possibly with limited admixture from Asia), representing a climatic and geographic subset of the native range, which also includes eastern and central Asia (20). L. serriola is an archaeophyte in Europe (i.e., introduced before AD 1500), has expanded its range in northern Europe in recent centuries (21), and has been widely introduced globally, including to North and South America, southern Africa, and Australasia. Following introduction outside of Europe, nonnative L. serriola has expanded its climatic envelope to occupy areas that are more arid than those it occupies in Europe but similar to some areas it occupies in Asia (20). At the same time, L. serriola from arid climates flowers more rapidly (20), which is presumably an adaptation to complete its life cycle before the onset of summer drought, and mirrors similar clines in phenology across the native range (20, 22). In the Mediterranean climate of the California grassland we study here, the native annual competitor species are generally earlier in phenology (17) than even the local L. serriola populations, and thus we hypothesized that an earlier phenology has increased L. serriola’s potential for negative effects on these native species.
To address this hypothesis, we established a field competition experiment in an arid part of L. serriola’s nonnative range in southern California, designed to parameterize a competitive population dynamics model (17, 19). We compared the competitive effect of a local Californian L. serriola population (expected to be adapted to the local, arid climate) on five native annual species with the competitive effect of L. serriola from the European source region (expected to be poorly adapted to the local climate). Because the precise origin of the nonnative genotypes is unknown, due to weak population structure within Europe (20, 23, 24), we included several L. serriola populations from across the species’ range to more generally address the potential effect of phenology evolution on competitive outcomes. We used these data to ask the following questions: (i) Do different L. serriola populations vary in their competitive effects on native species? (ii) Is this variation in competitive effects correlated with variation among L. serriola populations in their phenology? (iii) How does variation in L. serriola phenology relate to native Californian species persistence?
Results
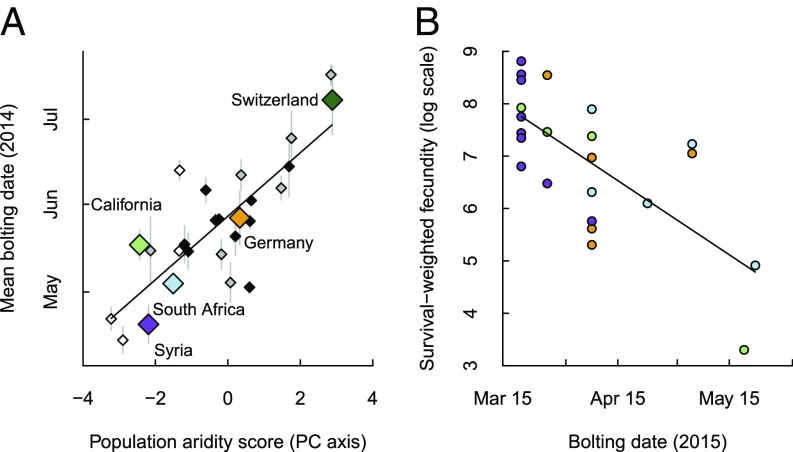
L. serriola originating from locations with arid climate bolted earlier than those from more mesic locations when growing in the southern California field site, and this conferred a selective advantage in terms of greater fecundity. L. serriola plants from 26 accessions from across the species’ range that were grown in the field in 2014 displayed clinal variation in phenology, with those originating from more arid climates bolting, on average, up to 48 d earlier than those from cooler and moister environments (F1,24 = 44.12, P < 0.001; Fig. 2A). The subset of five populations (from California, Syria, South Africa, Germany, and Switzerland) that were subsequently included in the competition experiment in 2015 also differed strongly in phenology, with a spread of 68 d between the average bolting dates of the earliest-bolting (from Syria) and latest-bolting (from Switzerland) populations. In the absence of competition, individuals from those populations that bolted early had significantly higher fecundity than those that bolted late (F1,21 = 20.09, P < 0.001; Fig. 2B). This indicates directional selection for early bolting in this environment, supporting an adaptive basis for the range-wide cline in bolting time (Fig. 2A and ref. 20).
Fig. 2.
Bolting phenology of L. serriola in a common garden in southern California. (A) Mean (±SE) bolting date in 2014 is related to the climate of origin (gradient from arid to mesic climate) of L. serriola populations (white, gray, and black diamonds are populations from Asia, Europe, and the nonnative range, respectively; large diamonds are populations included in the competition experiment in 2015). (B) The fecundity of L. serriola individuals growing without competition in 2015 is negatively related to the date at which they bolted (colors correspond to the populations indicated in A).
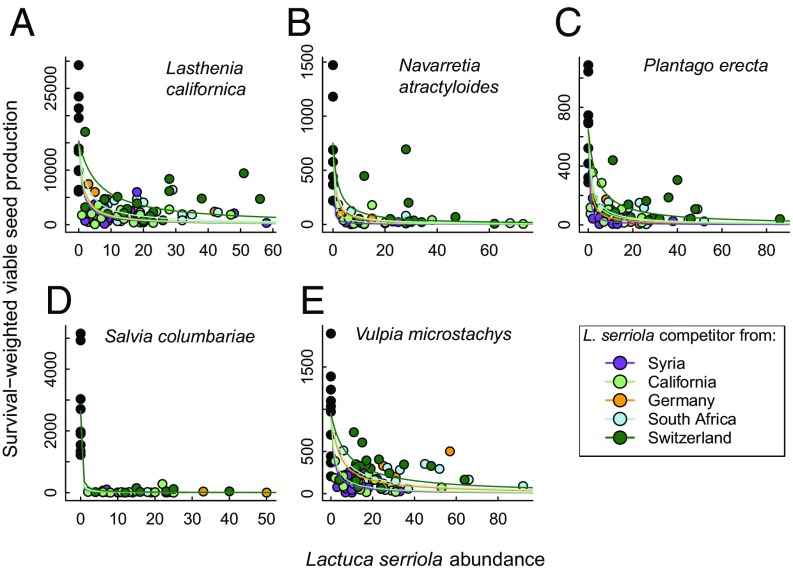
When we grew individuals of five native Californian species—Lasthenia californica, Navarretia atractyloides, Plantago erecta, Salvia columbariae, and Vulpia microstachys—in competition with L. serriola, we found that their survival-weighted fecundity declined as a function of L. serriola density. Importantly, the slope parameters of the fitted Beverton−Holt competition functions—the competition coefficients (α) describing the per capita competitive effects of L. serriola on native species fecundity—varied across the different L. serriola populations (Fig. 3). For all native species except S. columbariae, models including L. serriola population-specific competitive effects were more parsimonious than models including a common term across all L. serriola source populations (Table 1), while, for L. californica, the competition-free null model provided a marginally better fit to the data.
Fig. 3.
The fecundity of five native Californian annual species (A–E) as functions of the abundance of L. serriola competitors growing in an experimental garden in southern California, distinguishing effects of five L. serriola populations that differ in phenology. Fitted curves represent per capita competitive effects (α) of L. serriola on each focal species from maximum likelihood fits of the data to Beverton−Holt competition functions.
Table 1.
Comparison of three models fitted to describe per capita competitive effects (α) of L. serriola on five native Californian species
| Model | df | L. californica | N. atractyloides | P. erecta | S. columbariae | V. microstachys |
| No competition | 2 | 297.9 | 175.5 | 223.2 | 195.4 | 270.3 |
| Common L. serriola α | 2 | 309.9 | 159.3 | 205.6 | 146.5 | 274.1 |
| L. serriola population-specific α | 6 | 299.2 | 148.8 | 188.5 | 153.5 | 249.7 |
Data are values for AICc for each native species, with the most parsimonious model for each species indicated in bold.
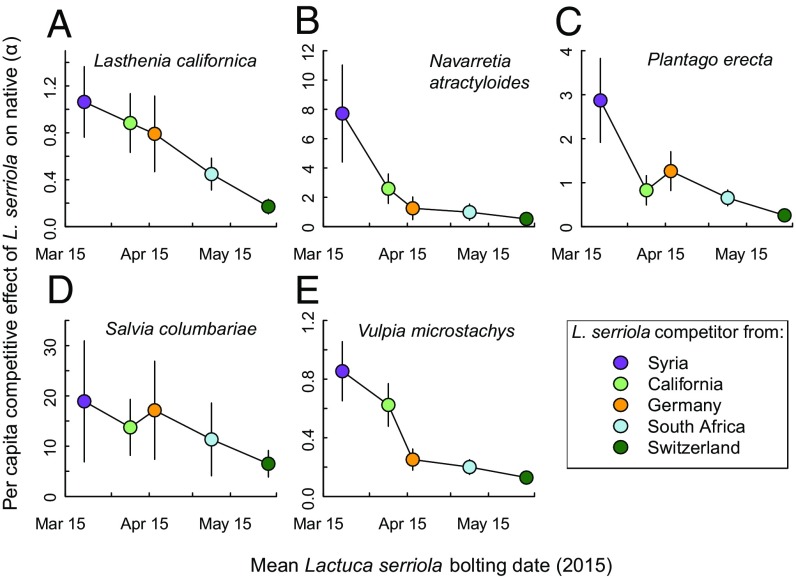
L. serriola phenology variation strongly predicted the population-level variation in the per capita competitive effects on native species (α) (main effect of L. serriola phenology on √α: F1,19 = 40.50, P < 0.001; the interaction of L. serriola phenology × native species identity was not significant; Fig. 4). All five native species experienced their strongest competitive suppression from the L. serriola population with the earliest bolting date (from Syria), and the weakest effects from the latest-bolting population (from Switzerland). On the whole, the weakening of competitive effects with delayed L. serriola bolting was monotonic.
Fig. 4.
Relationships between L. serriola phenology and the strength of the per capita competitive effect (α) of L. serriola on five different native Californian annual species (A–E). Estimates and SEs of α are from Beverton−Holt competition functions fitted by maximum likelihood.
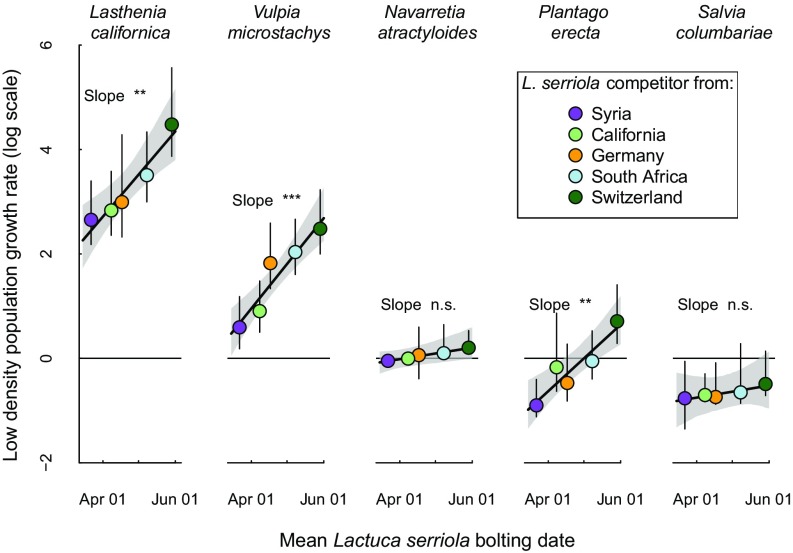
Finally, we asked how the strengthening of competitive effects associated with earlier L. serriola phenology affected the ability of the native species to persist with L. serriola. We did so by parameterizing, for each native species, the expression for its population growth rate when it is rare and its L. serriola competitor is at its single-species carrying capacity, following a common annual plant competition model (17), and regressing these growth rates on L. serriola bolting dates. The ability of all five native species to increase when rare (i.e., to persist) in competition with L. serriola declined with earlier L. serriola bolting, although these relationships were not statistically significant for N. atractyloides and S. columbariae (Fig. 5). The shift from late to early phenology affected whether or not one species, P. erecta, was predicted to persist with L. serriola. More generally, while all native species except S. columbariae were predicted to persist with the slowest-bolting L. serriola accession from Switzerland (Fig. 5), only L. californica and V. microstachys were predicted to persist with the fastest-bolting populations (Syria and California). However, there was large uncertainty in the low-density growth rate (LDGR) estimates, especially when propagating error in all eight parameters of the growth rate expression (SI Appendix, Table S1), and so these estimates should be interpreted with caution.
Fig. 5.
Predicted LDGR of five native species in competition with five L. serriola populations in an experimental garden in southern California, plotted against the mean bolting date of the L. serriola population. LDGR estimates are log-transformed, so that positive values predict that a focal species will persist with L. serriola, while negative values predict that they will be competitively excluded. Error bars and gray shading indicate the 95% confidence intervals around estimates of LDGR and the regression slopes, respectively, accounting for variance in estimates of each L. serriola population’s competitive effect on a native species (see Materials and Methods). ***P < 0.001; **P < 0.01; n.s. P > 0.05.
Discussion
The demographic benefits of adapting to local climatic conditions are well understood, but how climate adaptation impacts the community with which that species interacts is only beginning to be explored. Our study suggests that earlier flowering, potentially an adaptation to more arid climatic conditions, not only provides L. serriola with a fitness advantage in drier parts of its range (Fig. 1) but also influences its competitive effects on resident species. Specifically, earlier phenology increases the ability of L. serriola to competitively suppress its neighbors, in one case making the difference between whether or not the native species is predicted to persist with L. serriola. In nature, the effects of the other native and nonnative competitors simultaneously faced by these native species will modulate these results. Nonetheless, what we have presented here isolates the competitive consequences of changes to L. serriola phenology in the nonnative range.
The observed between-population variation in the strength of the competitive effects on native species supports the contention that rather than being a property of two species, the potential to coexist may be better understood as a property of two populations (10, 25, 26). More specifically, our results suggest that competitive outcomes and the potential for coexistence between species can depend on the genotypic composition of the interacting populations (10, 27, 28), insomuch as this affects the mean and variation of the traits that mediate interaction outcomes (29). This conclusion is not driven by the inclusion of the Swiss population, which possessed a relatively extreme phenology; after excluding this population, the regression of L. californica’s LDGR on L. serriola phenology was no longer significant, but, otherwise, our analyses of L. serriola phenology on αij and LDRG were qualitatively unaffected.
Variation among individuals in competitive traits may also arise with phenotypic plasticity, with similar implications for species coexistence (30). Plasticity in flowering phenology, which can be considerable for L. serriola (31), might explain why the rank mean bolting date of the South African L. serriola population differed between 2014 and 2015, years differing considerably in climate. For similar reasons, we might expect the impact of L. serriola phenology on competition with native species to change among years, especially in this Californian system with such a variable precipitation regime. For example, the relatively dry conditions of the 2015 study year might have led us to underestimate the “average year” relationship between phenology and competition, since drought compresses phenological differences between species. Alternatively, it may have enhanced the dominance of L. serriola populations with an early enough phenology to grow well in a dry year.
More generally, our study implicates phenology as an important trait mediating competitive interactions among the annual species in the California field site, consistent with other studies in this ecosystem (16, 17). Indeed, by explicitly considering variation in phenology within rather than between species, we partially control for variation in other (life history) traits that might be correlated with phenology and therefore cloud inferences about phenology per se. Still, we acknowledge that phenology is a complex trait, correlated with traits such as plant size or specific leaf area at any given calendar date (SI Appendix, Fig. S1); thus the extent to which our results are driven by phenology per se can be difficult to ascertain. Nonetheless, in our study, the biomass of plants at flowering did not differ between L. serriola populations (SI Appendix, Fig. S1A), indicating that phenology, as we measured it, captures the timing of life history events, and not other competition-relevant trait differences such as final plant size.
The strengthening of competitive effects caused by earlier phenology in L. serriola could be caused by a combination of increasing niche overlap with native species and a demographic advantage conferred by local adaptation (Fig. 1 and ref. 10). Due to high mortality of focal L. serriola individuals in the experiment, we do not have the data (separate estimates of intraspecific competition for each L. serriola population) needed to fully disentangle these mechanisms. Thus, when estimating native species’ LDGRs, we assumed that the intraspecific interaction coefficient and per germinant fecundity in the absence of any competition was constant across L. serriola populations (SI Appendix, Supplementary Materials and Methods). If we instead substituted L. serriola population-specific estimates (regardless of the statistical significance) of fecundity in the absence of competition (λj) and intraspecific competition (αjj) into this calculation, the native species’ growth rates when rare did not substantially change (SI Appendix, Fig. S2). This finding is surprising given the more than 10-fold difference in estimates of λj between populations from Syria and Switzerland, but can be explained by stronger intraspecific competition in those populations with a higher λj (SI Appendix, Table S2) (32, 33). Although more-detailed experiments are needed to validate this finding, it suggests that variation in competitive effects may be more strongly influenced by an effect of phenology evolution on niche overlap with native species than on a fitness benefit to L. serriola of adaptation (see also ref. 34).
Our finding that trait differences affect competition is bolstered by a large literature showing that traits of competing species can evolve in a way that affects coexistence (character displacement) (35–37). It is therefore conceivable that the invasion and evolution of L. serriola has altered selection regimes experienced by native species, as has been shown to be the effect of invasive Bromus tectorum in the western United States (38) and of several other nonnative species (39, 40). Native plant species in eastern North America, for example, have evolved to be stronger competitors with invasive Alliaria petiolata, leading to coevolutionary responses in the invader that promote coexistence (34, 39, 41). We might also expect the native species in our focal ecosystem to exert selection on L. serriola, with potential implications for L. serriola’s ability to adapt to the environment in the absence of competition, although we were not able to test this hypothesis with our data. Previous work has shown that species’ interactions can have both negative and positive effects on adaptation to changing environmental conditions (42–46), partly depending on whether biotic and abiotic selection pressures are aligned (47, 48). Nonetheless, selection by native species on L. serriola is likely minimal, since even the local L. serriola population in our experiment flowered later than all native species, and a previous study in this system found that late phenology species, including L. serriola, were competitively dominant in experimental arrays (16, 17). It is still possible that early season interactions with native species impose some selection on L. serriola phenology, but competitive selection pressures have apparently not been strong enough to mask the strong signal of climate on L. serriola’s phenology.
While previous work has focused on how biotic interactions can influence adaptation to the environment, our study is unusual in directly addressing possible impacts of climate adaptation on the outcome of biotic interactions (26). It demonstrates that, in principle, the outcome of species’ interactions could depend strongly on how well or poorly local populations are adapted to environmental conditions, and how traits under selection by the local environment also affect the intensity of biotic interactions. This has implications both for the impact of invasive species and for community dynamics following environmental change. It suggests that initial maladaptation in nonnative species might prevent or delay competitive suppression of native species. Adaptation during invasion will therefore not only promote invader spread and local abundance (5) but can also directly increase the impact that a nonnative species has on native communities. In our study, the local Californian population of L. serriola had neither the earliest phenology nor the highest fitness, and, consequently, did not have the strongest impact on native species. This suggests that L. serriola might have adapted to other local selection pressures favoring slightly later phenology but not eliciting a fitness advantage during our experiment, or that there is potential for continuing adaptation that would further enhance the impacts of this species. More generally, our results suggest that evolution in response to changing environmental conditions could affect the outcome of competition, contributing to the effects of environmental change on natural communities and ecosystems.
Materials and Methods
Field Site and Seed Material.
Our experiments were established in the Santa Ynez Valley, Santa Barbara County, California (34.7401°N, 120.0779°W) on the property of Midland School. The site is managed as a pasture, comprising a mixture of forbs and grasses (predominantly annual nonnative grasses). The area experiences a Mediterranean climate, with hot dry summers and most precipitation falling during winter (annual precipitation = 389 ± 164 mm, mean ± SD from 1909 to 2017 for a nearby weather station: Los Alamos Fire Station, www.countyofsb.org) (see ref. 49 for further details). During the period in which our experiments were conducted, the area was experiencing a prolonged drought, with total precipitation between September and August of 159 mm and 194 mm during the 2013–2014 and 2014–2015 seasons, respectively (www.countyofsb.org). Soils at the site were a well-drained, fine sandy loam in the Ballard series of alluvial soils derived from sedimentary rock (https://casoilresource.lawr.ucdavis.edu/gmap/). We fenced the site to exclude gophers, ground squirrels, and deer; tilled the experimental plots; and covered the ground between plots with landscape fabric to suppress weeds.
We selected an initial set of 26 L. serriola populations originating from across the species’ climatic and geographic range, including 12 populations from the nonnative range and 14 from the native range (5 from Asia and 9 from Europe) (SI Appendix, Table S3). We then selected a subset of five L. serriola populations that were used in the competition experiment: the local nonnative California population, a nonnative population from South Africa, and populations from the native range in Asia (Syria) and Europe (Germany, Switzerland), where L. serriola is an archaeophyte. These populations were chosen to cover the broad range of flowering phenology (20) observed in a common garden experiment (described in Common Garden Experiment; Fig. 2A), and to include replicate populations from both native and nonnative ranges, as well as putative source (Europe) and nonsource (Asia) regions of the native range. Seeds were obtained from plants that had grown for a generation in a previous experiment (20), with the exception of two populations from California and Switzerland that were field-collected (for the competition experiment, seeds of the Californian population were obtained from plants grown for a generation in a greenhouse in Zurich, Switzerland).
We propagated plants originating from these five populations to generate sufficient seed material for the competition experiment. Seeds from 3 to 15 (mean 7.6) maternal plants per population were sown onto compost in multipots in December 2013, vernalized in the dark at 4 °C for 3 wk, and allowed to germinate in the light, and then 30 individuals per population were potted up into 1-L pots and grown in a greenhouse in Zurich. At flowering, individual plants were bagged to capture seeds, and then harvested in May 2014 when plants had begun to senesce. Seeds belonging to the same population were pooled for use in the field experiment. Because the quantity of seed produced was insufficient, it was supplemented either with field-collected seed from the same population (for Switzerland and California) or with seed from greenhouse-grown plants that originated from nearby populations (from Netherlands, Syria, and South Africa). Seed from these replacement populations shared a similar phenology to the target population under common garden conditions (20) and, when used in the competition experiment, were never the focal individuals.
We selected five California native annual species to compete with the L. serriola populations: L. californica (Asteraceae), N. atractyloides (Polemoniaceae), P. erecta (Plantaginaceae), S. columbariae (Lamiaceae), and V. microstachys (Poaceae). These species were chosen because they exhibit a range of flowering phenologies (17), the earliest being L. californica and the latest being N. atractyloides, which released seeds in March and May, respectively, during the year of the experiment. Seeds of the five native species were collected from near the experimental field site (University of California Sedgwick Reserve) in 2013 and 2014, except for L. californica, which was sourced from a local commercial supplier (S&S Seeds, Inc.).
Common Garden Experiment.
We performed a common garden experiment to document variation in flowering phenology across the full set of 26 L. serriola populations. Seeds were sown into the field site in November 2013 into 36 circular 0.5-m2 plots, at five stations per plot separated by 29 cm. At each station, we sowed 30 seeds from a single population, with replication of 5 to 13 (mean 6.96) stations per population, and populations assigned at random to stations and plots. We thinned germinants to a single individual per station in March 2014, and monitored individuals to record the onset of bolting between April and September 2014. Focal plants were harvested after bolting and before setting seed. We fitted a linear model to analyze the mean date of bolting of a population as a function of the climate at the site of origin of the population. Climate was characterized as scores from the second axis of a principal component analysis (PC2) on eight bioclimatic variables capturing variability in temperature and precipitation (reported in ref. 20). PC2 reflects a gradient from warm locations with low and seasonal precipitation to cool locations with high and aseasonal precipitation (20).
Competition Experiment.
To estimate the per capita competitive effects (α) of different L. serriola populations on the five native species, we followed the performance of target native individuals sown into a density gradient of L. serriola competitors. Specifically, we grew focal individuals of the native species at different densities of L. serriola competitors in circular plots of 0.4 m2 that were established in early November 2014. For each L. serriola population, we sowed 10 plots with 2, 4, 6, 8, or 10 g/m2 of viable seed (2 plots per density; only 7 plots for the German population, due to limited seed availability). Within each plot, we marked 12 stations, arranged 17.5 cm apart, each receiving 30 or 50 (S. columbariae) viable seeds of one native species (n = 2 stations per species and plot), with two stations for focal individuals of L. serriola to estimate intraspecific competitive effects. To estimate fecundity in the absence of competition (λ), we also grew a single individual from each L. serriola population, or a single individual of each native species, spaced 26 cm apart in an additional 20 plots (10 plots for the L. serriola plants and 10 plots for the native species).
The plots were weeded of all nonplanted species in mid-December 2014 and again in mid-January 2015. In late January 2015, we counted the number of seedlings within a 7.5-cm radius around every focal individual in a competition plot. At this time, we also scored germination of the focal individuals, and thinned seedlings down to a single focal individual per station. Beginning on March 20, we made regular checks of L. serriola focal individuals’ flowering phenology (at least weekly until early May, then at least every 2 wk) until mid-July, by which time all L. serriola focal individuals had either flowered or senesced. We distinguished rosettes from plants that had bolted or begun to flower, and also collected additional functional trait data (SI Appendix, Fig. S1).
To estimate fecundity, we counted flower heads of all focal individuals in the field. Native species were sampled between early April and mid-May when focal individuals had completed flowering and senesced. For some species, we noted considerable variation in the size of flower heads, which we accounted for in our estimates of total plant fecundity (SI Appendix, Supplementary Materials and Methods). Fecundity was weighted by seed viability, as explained in Analysis of the Competition Experiment to give an estimate of viable seed production per individual. L. serriola plants (both focal individuals and background competitors) were harvested before their seeds had ripened, to prevent release of seed into the environment, but this procedure did not bias fecundity estimates for L. serriola (SI Appendix, Supplementary Materials and Methods). Finally, damage to L. serriola was caused by ground squirrels that entered the fence at the beginning of May, which we visually estimated as the percentage of biomass removed by squirrels on each individual. The extent of damage varied between populations (greatest on the Californian population and lowest on the Syrian population), but fecundity at the end of the experiment was unrelated to damage in a mixed-effects model that accounted for variation in fecundity across populations (main effect of squirrel damage: F1,129.25 = 1.644, P = 0.202; damage × population interaction: F4,122.8 = 0.553, P = 0.697).
Analysis of the Competition Experiment.
We estimated competitive effects of L. serriola on native species using our measurements of focal individual fecundity and neighborhood densities. To account for the fact that mortality occurred between germination and flowering, we weighted the per-germinant fecundity of species i (Fi) by the average survival rate of focal individuals of that species (significant density-dependent survival was only detected for one species, S. columbariae). We then used maximum likelihood methods to estimate competition coefficients (per capita competitive effects of species j on species i; αij) based on the survival-weighted per-germinant fecundity and neighborhood density estimates (number of germinants) of species j (Nj) using the function
We determined λi as the average fecundity of individuals growing without competition. Because this parameter is independent of the identity of competitors, it was fitted as a common value across different background competitors. Models were fitted using the “optim” function in R (version 3.4.2) using the Nelder and Mead method. For each native species, we fitted three models that differed in the way in which competition was modeled: a null model in which fecundity did not vary as a function of neighbor density, a model with a single competition coefficient for all L. serriola populations, and a full model with a separate coefficient for each L. serriola population. Models were compared based on Akaike Information Criteria (AICc) to test the hypothesis that L. serriola populations differ in their per capita competitive effects on native species.
To test the hypothesis that per capita competitive effects of L. serriola on native species vary as a function of L. serriola phenology, we fitted a linear model to the per capita effects of each L. serriola population on each native species (αij) as a function of the mean bolting date of all of the L. serriola focal individuals from that population, native species identity, and their interaction. The αij was square root-transformed to meet model assumptions. To test whether phenology is a trait potentially under selection in L. serriola at our field site, we fitted a linear model of log-transformed fecundity of plants growing without competition against the bolting date of each individual. Finally, we tested for differences in L. serriola population means of plant height, biomass, specific leaf area, and leaf dry matter content using mixed-effects models accounting for experimental plot as a random effect.
To test the hypothesis that differences in L. serriola phenology affect the ability of native species to persist with L. serriola, we parameterized a mathematical model describing how competition affects the population growth of annual plants (SI Appendix, Supplementary Materials and Methods). From this model, we derived the LDGR (also known as the invasion growth rate) of each native species in competition with L. serriola at its single-species equilibrium abundance. For each native species, we then fit linear regressions of the predicted log-transformed LDGR in the presence of each L. serriola population as a function of the mean bolting date of that population. We accounted for uncertainty in our estimates of LDGRs using Monte Carlo methods within the “propagate” function in R (50). Specifically, we generated probability distributions for each estimate of LDGR given the mean and variance of individual parameters in the expression for LDGR, based on 100,000 Monte Carlo simulations of these parameter values. We then used the probability distributions generated via this process to estimate an expected value and confidence intervals around our estimates of the LDGR. Given our focus on how phenology differences affect persistence, we only accounted for variation in L. serriola’s competitive effect on native species (αij), since none of the other parameters of the LDGR differed depending on the identity of the L. serriola population (SI Appendix, Supplementary Materials and Methods). To determine the statistical significance of the regressions between LDGR and L. serriola phenology, we generated confidence intervals for the regression slopes based on 10,000 draws of LDGR estimates from the Monte Carlo simulations. We also present confidence intervals for each estimate of LDGR after propagating variance in all of its eight parameters (assuming zero covariance among parameters) in SI Appendix, Table S1.
Data can be accessed from SI Appendix, Table S2 and Dataset S1.
Supplementary Material
Acknowledgments
This study would not have been possible without the help and logistic support of Carla D’Antonio during the field experiments. We also thank Dillon Polito, Lindsey Rice, Sara Giovanettina, Andrea Reid, and Tabea Kropf for assistance with field and laboratory work; Janneke HilleRisLambers for assistance with analyses; the Plant Ecology group at ETH and Carla D’Antonio for comments on an earlier draft of the manuscript; and Midland School for providing the field site. ETH Zurich funding to the Plant Ecology group supported the project.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1820569116/-/DCSupplemental.
References
- 1.Colautti RI, Lau JA. Contemporary evolution during invasion: Evidence for differentiation, natural selection, and local adaptation. Mol Ecol. 2015;24:1999–2017. doi: 10.1111/mec.13162. [DOI] [PubMed] [Google Scholar]
- 2.Oduor AMO, Leimu R, van Kleunen M. Invasive plant species are locally adapted just as frequently and at least as strongly as native plant species. J Ecol. 2016;104:957–968. [Google Scholar]
- 3.Colautti RI, Maron JL, Barrett SCH. Common garden comparisons of native and introduced plant populations: Latitudinal clines can obscure evolutionary inferences. Evol Appl. 2009;2:187–199. doi: 10.1111/j.1752-4571.2008.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Kleunen M, Fischer M. Adaptive rather than non-adaptive evolution of Mimulus guttatus in its invasive range. Basic Appl Ecol. 2008;9:213–223. [Google Scholar]
- 5.Colautti RI, Barrett SCH. Rapid adaptation to climate facilitates range expansion of an invasive plant. Science. 2013;342:364–366. doi: 10.1126/science.1242121. [DOI] [PubMed] [Google Scholar]
- 6.Suarez AV, Tsutsui ND. The evolutionary consequences of biological invasions. Mol Ecol. 2008;17:351–360. doi: 10.1111/j.1365-294X.2007.03456.x. [DOI] [PubMed] [Google Scholar]
- 7.Liao H, D’Antonio Carla M, Chen B, Huang Q, Peng S. How much do phenotypic plasticity and local genetic variation contribute to phenotypic divergences along environmental gradients in widespread invasive plants? A meta‐analysis. Oikos. 2016;125:905–917. [Google Scholar]
- 8.Turcotte MM, Reznick DN, Hare JD. The impact of rapid evolution on population dynamics in the wild: Experimental test of eco-evolutionary dynamics. Ecol Lett. 2011;14:1084–1092. doi: 10.1111/j.1461-0248.2011.01676.x. [DOI] [PubMed] [Google Scholar]
- 9.Ellner SP, Geber MA, Hairston NG., Jr Does rapid evolution matter? Measuring the rate of contemporary evolution and its impacts on ecological dynamics. Ecol Lett. 2011;14:603–614. doi: 10.1111/j.1461-0248.2011.01616.x. [DOI] [PubMed] [Google Scholar]
- 10.Lankau RA. Rapid evolutionary change and the coexistence of species. Annu Rev Ecol Evol Syst. 2011;42:335–354. [Google Scholar]
- 11.Hart SP, Freckleton RP, Levine JM. How to quantify competitive ability. J Ecol. 2018;106:1902–1909. [Google Scholar]
- 12.Chesson P. Mechanisms of maintenance of species diversity. Annu Rev Ecol Evol Syst. 2000;31:343–366. [Google Scholar]
- 13.Blossey B, Nötzold R. Evolution of increased competitive ability in invasive nonindigenous plants: A hypothesis. J Ecol. 1995;83:887–889. [Google Scholar]
- 14.Bossdorf O, et al. Phenotypic and genetic differentiation between native and introduced plant populations. Oecologia. 2005;144:1–11. doi: 10.1007/s00442-005-0070-z. [DOI] [PubMed] [Google Scholar]
- 15.Forrest J, Miller-Rushing AJ. Toward a synthetic understanding of the role of phenology in ecology and evolution. Philos Trans R Soc Lond B Biol Sci. 2010;365:3101–3112. doi: 10.1098/rstb.2010.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraft NJB, Godoy O, Levine JM. Plant functional traits and the multidimensional nature of species coexistence. Proc Natl Acad Sci USA. 2015;112:797–802. doi: 10.1073/pnas.1413650112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Godoy O, Levine JM. Phenology effects on invasion success: Insights from coupling field experiments to coexistence theory. Ecology. 2014;95:726–736. doi: 10.1890/13-1157.1. [DOI] [PubMed] [Google Scholar]
- 18.Wolkovich EM, Cleland EE. The phenology of plant invasions: A community ecology perspective. Front Ecol Environ. 2011;9:287–294. [Google Scholar]
- 19.Levine JM, HilleRisLambers J. The importance of niches for the maintenance of species diversity. Nature. 2009;461:254–257. doi: 10.1038/nature08251. [DOI] [PubMed] [Google Scholar]
- 20.Alexander JM. Evolution under changing climates: Climatic niche stasis despite rapid evolution in a non-native plant. Proc Biol Sci. 2013;280:20131446. doi: 10.1098/rspb.2013.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Andrea L, et al. Climate change, anthropogenic disturbance and the northward range expansion of Lactuca serriola (Asteraceae) J Biogeogr. 2009;36:1573–1587. [Google Scholar]
- 22.Prince SD. Vernalization and seed production in lettuce. In: Hebblethwaite PD, editor. Seed Production. Butterworths; London: 1980. pp. 485–499. [Google Scholar]
- 23.Lebeda A, et al. An insight into the genetic polymorphism among European populations of Lactuca serriola assessed by AFLP. Biochem Syst Ecol. 2009;37:597–608. [Google Scholar]
- 24.van de Wiel CCM, et al. Distribution of genetic diversity in wild European populations of prickly lettuce (Lactuca serriola): Implications for plant genetic resources management. Plant Genet Resour. 2010;8:171–181. [Google Scholar]
- 25.Aarssen LW. Ecological combining ability and competitive combining ability in plants: Toward a general evolutionary theory of coexistence in systems of competition. Am Nat. 1983;122:707–731. [Google Scholar]
- 26.Ehlers BK, Damgaard CF, Laroche F. Intraspecific genetic variation and species coexistence in plant communities. Biol Lett. 2016;12:20150853. doi: 10.1098/rsbl.2015.0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zerebecki RA, Crutsinger GM, Hughes AR. Spartina alterniflora genotypic identity affects plant and consumer responses in an experimental marsh community. J Ecol. 2017;105:661–673. [Google Scholar]
- 28.Bach LT, Lohbeck KT, Reusch TBH, Riebesell U. Rapid evolution of highly variable competitive abilities in a key phytoplankton species. Nat Ecol Evol. 2018;2:611–613. doi: 10.1038/s41559-018-0474-x. [DOI] [PubMed] [Google Scholar]
- 29.Hart SP, Schreiber SJ, Levine JM. How variation between individuals affects species coexistence. Ecol Lett. 2016;19:825–838. doi: 10.1111/ele.12618. [DOI] [PubMed] [Google Scholar]
- 30.Turcotte MM, Levine JM. Phenotypic plasticity and species coexistence. Trends Ecol Evol. 2016;31:803–813. doi: 10.1016/j.tree.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Alexander JM. Genetic differences in the elevational limits of native and introduced Lactuca serriola populations. J Biogeogr. 2010;37:1951–1961. [Google Scholar]
- 32.Watkinson AR, Freckleton RP. Quantifying the impact of arbuscular mycorrhiza on plant competition. J Ecol. 1997;85:541–545. [Google Scholar]
- 33.Siepielski AM, Nemirov A, Cattivera M, Nickerson A. Experimental evidence for an eco-evolutionary coupling between local adaptation and intraspecific competition. Am Nat. 2016;187:447–456. doi: 10.1086/685295. [DOI] [PubMed] [Google Scholar]
- 34.Huang F, Lankau R, Peng S. Coexistence via coevolution driven by reduced allelochemical effects and increased tolerance to competition between invasive and native plants. New Phytol. 2018;218:357–369. doi: 10.1111/nph.14937. [DOI] [PubMed] [Google Scholar]
- 35.Bassar RD, Simon T, Roberts W, Travis J, Reznick DN. The evolution of coexistence: Reciprocal adaptation promotes the assembly of a simple community. Evolution. 2017;71:373–385. doi: 10.1111/evo.13086. [DOI] [PubMed] [Google Scholar]
- 36.Germain RM, Williams JL, Schluter D, Angert AL. Moving character displacement beyond characters using contemporary coexistence theory. Trends Ecol Evol. 2018;33:74–84. doi: 10.1016/j.tree.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Kooyers NJ, James B, Blackman BK. Competition drives trait evolution and character displacement between Mimulus species along an environmental gradient. Evolution. 2017;71:1205–1221. doi: 10.1111/evo.13200. [DOI] [PubMed] [Google Scholar]
- 38.Leger EA, Goergen EM. Invasive Bromus tectorum alters natural selection in arid systems. J Ecol. 2017;105:1509–1520. [Google Scholar]
- 39.Lankau RA. Coevolution between invasive and native plants driven by chemical competition and soil biota. Proc Natl Acad Sci USA. 2012;109:11240–11245. doi: 10.1073/pnas.1201343109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lau JA. Beyond the ecological: Biological invasions alter natural selection on a native plant species. Ecology. 2008;89:1023–1031. doi: 10.1890/06-1999.1. [DOI] [PubMed] [Google Scholar]
- 41.Lankau RA, Nuzzo V, Spyreas G, Davis AS. Evolutionary limits ameliorate the negative impact of an invasive plant. Proc Natl Acad Sci USA. 2009;106:15362–15367. doi: 10.1073/pnas.0905446106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Den Elzen CL, Kleynhans EJ, Otto SP. Asymmetric competition impacts evolutionary rescue in a changing environment. Proc Biol Sci. 2017;284:20170374. doi: 10.1098/rspb.2017.0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Mazancourt C, Johnson E, Barraclough TG. Biodiversity inhibits species’ evolutionary responses to changing environments. Ecol Lett. 2008;11:380–388. doi: 10.1111/j.1461-0248.2008.01152.x. [DOI] [PubMed] [Google Scholar]
- 44.Lawrence D, et al. Species interactions alter evolutionary responses to a novel environment. PLoS Biol. 2012;10:e1001330. doi: 10.1371/journal.pbio.1001330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Northfield TD, Ives AR. Coevolution and the effects of climate change on interacting species. PLoS Biol. 2013;11:e1001685. doi: 10.1371/journal.pbio.1001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johansson J. Evolutionary responses to environmental changes: How does competition affect adaptation? Evolution. 2008;62:421–435. doi: 10.1111/j.1558-5646.2007.00301.x. [DOI] [PubMed] [Google Scholar]
- 47.Osmond MM, de Mazancourt C. How competition affects evolutionary rescue. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120085. doi: 10.1098/rstb.2012.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barraclough TG. How do species interactions affect evolutionary dynamics across whole communities? Annu Rev Ecol Evol Syst. 2015;46:25–48. [Google Scholar]
- 49.HilleRisLambers J, Yelenik SG, Colman BP, Levine JM. California annual grass invaders: The drivers or passengers of change? J Ecol. 2010;98:1147–1156. doi: 10.1111/j.1365-2745.2010.01706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spiess A-N. 2018 propagate: Propagation of Uncertainty. R Package Version 1.0-6. Available at https://cran.r-project.org/web/packages/propagate/index.html. Accessed October 16, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.