Abstract
Background
Increased vitamin B6 catabolism related to inflammation, as measured by the PAr index (the ratio of 4-pyridoxic acid over the sum of pyridoxal and pyridoxal-5'-phosphate), has been positively associated with lung cancer risk in two prospective European studies. However, the extent to which this association translates to more diverse populations is not known.
Materials and methods
For this study, we included 5323 incident lung cancer cases and 5323 controls individually matched by age, sex, and smoking status within each of 20 prospective cohorts from the Lung Cancer Cohort Consortium. Cohort-specific odds ratios (ORs) and 95% confidence intervals (CIs) for the association between PAr and lung cancer risk were calculated using conditional logistic regression and pooled using random-effects models.
Results
PAr was positively associated with lung cancer risk in a dose-response fashion. Comparing the fourth versus first quartiles of PAr resulted in an OR of 1.38 (95% CI: 1.19–1.59) for overall lung cancer risk. The association between PAr and lung cancer risk was most prominent in former smokers (OR: 1.69, 95% CI: 1.36–2.10), men (OR: 1.60, 95% CI: 1.28–2.00), and for cancers diagnosed within 3 years of blood draw (OR: 1.73, 95% CI: 1.34–2.23).
Conclusion
Based on pre-diagnostic data from 20 cohorts across 4 continents, this study confirms that increased vitamin B6 catabolism related to inflammation and immune activation is associated with a higher risk of developing lung cancer. Moreover, PAr may be a pre-diagnostic marker of lung cancer rather than a causal factor.
Keywords: PAr, vitamin B6, lung cancer, Lung Cancer Cohort Consortium, inflammation, nested case-control study
Key Message
Results from the Lung Cancer Cohort Consortium including 20 prospective cohorts across four continents confirm that increased vitamin B6 catabolism related to inflammation and immune activation is associated with a higher risk of developing lung cancer.
Introduction
Lung cancer remains the leading cause of cancer death in the United States [1] and worldwide [2], with less than one out of five cases surviving more than 5 years following diagnosis [3]. In addition to smoking, the primary risk factor for lung cancer, chronic inflammation is believed to play a critical role in cancer development [4] and may be involved in the tumor-promoting effect of smoking [4]. A recent randomized trial revealed a potential protective effect of anti-inflammatory therapy on lung cancer incidence and mortality [5].
Circulating pyridoxal-5'-phosphate (PLP), the widely used marker of vitamin B6 status, has been linked to risk of various cancers in epidemiological studies, including lung cancer [6]. However, the estimated associations of PLP with lung cancer risk vary considerably across studies [7–9], which may be due to the fact that circulating concentrations of PLP are influenced by several factors, including dietary or supplemental intake, inflammation, serum albumin, and alkaline phosphatase levels [10].
Considering the limitations of PLP as a biomarker, we have proposed the PAr index, defined as the ratio 4-pyridoxic acid (PA)/(pyridoxal + PLP) [11, 12]. Several inflammation-related processes involving PLP-catabolizing enzymes, oxidative stress, and kidney damage may contribute to a skewing of the concentrations of B6 vitamers in plasma toward more PA relative to pyridoxal+ PLP, resulting in an elevated PAr [13]. Therefore, PAr serves as a marker of increased vitamin B6 catabolism during inflammation and related cellular immune activation. We have previously reported findings from two studies, the Hordaland Health Study (HUSK) [14] and the European Prospective Investigation into Cancer and Nutrition (EPIC) [13], suggesting that PAr is associated with lung cancer risk. For instance, the EPIC study that included 892 cases and 1748 matched controls suggested that a doubling in PAr levels was associated with 52% higher lung cancer risk, and the risk increased most in former smokers and for squamous cell carcinoma (SCC) [13].
However, current evidence on PAr and lung cancer has been limited to European populations. Circulating levels of vitamins and their metabolites vary substantially across cohorts and continents due to many factors, including diet, lifestyle, vitamin supplementation, and food fortification [15]. Considering the large variations in PLP and PAr levels [15], it is not known if the reported positive association of PAr with lung cancer applies to populations with wide variance in the levels of this biomarker.
In order to comprehensively evaluate this question, we conducted a study of PAr within the Lung Cancer Cohort Consortium (LC3), the largest investigation to date assessing biomarkers of one-carbon metabolism in lung cancer, involving 20 prospective cohorts from around the world.
Methods
Study population and design
Details of the LC3 have been reported previously [8]. In brief, a total of 20 prospective cohort studies, which were members of the US National Cancer Institute (NCI) Cohort Consortium in 2009 and had cryopreserved plasma/serum samples available were included. The LC3 included 11 cohorts from the United States, 4 cohorts from Europe (Norway, Sweden, and Finland), 4 cohorts from Asia (China and Singapore), and 1 cohort from Australia, resulting in a combined cohort population of more than 2 000 000 participants [8]. Written informed consent was provided by all study participants, and the research was approved by the institutional review board of the International Agency for Research of Cancer and each participating cohort.
Cases ascertainment and control selection
Lung cancer cases were defined on the basis of the International Classification of Diseases for Oncology, Second Edition and included invasive cancers coded as C34.0-C34.9. From the 11 399 incident lung cancer cases with pre-diagnostic blood samples, 5545 cases were selected. Never and former smokers were oversampled to increase statistical power in analyses stratified by smoking. For each case, one control was selected by incidence density sampling and matched by cohort, sex, race (US cohorts only), date of birth (±1 year, relaxed to ±3 years), date of blood collection (±1 month, relaxed to ±3 months), and smoking status in five categories: never smokers, short- and long-term quitters among former smokers (<10 years, ≥10 years since quitting), and light and heavy smokers among current smokers (<15, ≥15 cigarettes per day). After various exclusions [8], 5364 lung cancer case–control pairs were included. We further excluded 41 case–control pairs with missing PA, pyridoxal or PLP measurements, yielding a final analytic sample of 5323 case–control pairs (10 646 participants).
Biochemical measurement
All blood samples were stored at ≤−80°C until shipment to the Bevital laboratory (www.bevital.no) for biochemical analyses. The time from blood draw to the measurement of PA, pyridoxal and PLP ranged from 2 to 38 years. Concentrations of PA, pyridoxal, PLP, cotinine (a marker of recent nicotine exposure) [16] and creatinine [17] were determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Cases and their matched controls were analyzed together within the same batches in random order, with laboratory staff blinded to the case-control status of the blood samples. The within-day coefficients of variation for the assays were 2.3%–4.6% and between-day coefficients of variation were 2.2%–12.3% [15, 16]. The estimated glomerular filtration rate (eGFR) was calculated on the basis of the chronic kidney disease-epidemiology creatinine equation [18].
Statistical analysis
Geometric mean [95% confidence interval (CI)] of PAr in each cohort was estimated by using generalized linear model adjusted for age, sex, and smoking (never, former, and current smokers) and eGFR (continuous). The correlation between PAr and eGFR was assessed using Spearman’s correlation coefficient, adjusted for age, sex, and cohort.
We used a two-stage modeling approach [19] to estimate the association between PAr and lung cancer risk. In the first stage, conditional logistic regression models were used to calculate cohort-specific odds ratios (ORs) with 95% CIs for lung cancer, conditioning on individual case sets. ORs were calculated for the fourth relative to the first quartile of PAr based on its distribution among the control subjects within each cohort, due to large differences in PAr levels across cohorts. The models were adjusted for pre-defined covariates including eGFR (continuous) and cotinine concentrations as quartiles defined from the distribution among current smokers. In sensitivity analysis, the models were additionally adjusted for body mass index (continuous). Also, we fitted models that were additionally adjusted for smoking duration or pack-years of smoking among ever smokers. In the second stage, study-specific ORs were pooled using random-effects meta-analysis, taking the possibility of between-study heterogeneity into account. Heterogeneity across subgroups was assessed by Cochrane’s Q test and the I2 index [20].
The primary analyses were conducted using all the study participants, and by region. We additionally generated risk estimates within strata by sex and smoking (never, former, and current smokers) using the same approach. Stratified risk analyses were also conducted by histology of lung cancer and by years from blood draw to diagnosis. Our secondary analysis included PAr as a continuous exposure, using log2-transformed PAr in conditional logistic regression models. Estimates from this model can be interpreted as the relative risk associated with a doubling in PAr levels.
All statistical analyses were carried out using SAS (version 9.4; SAS Institute, Inc., Cary, NC). Figures were produced using R (version 3.4.2, www.r-project.org). All tests were two sided and a P value <0.05 was considered statistically significant.
Results
Descriptive analyses
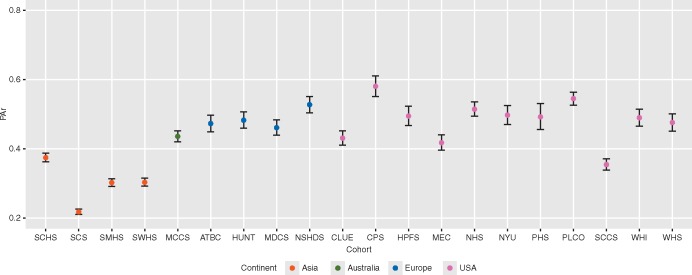
Characteristics of the 10 646 study participants at baseline are shown in Table 1. Of the individually matched cases and controls, 54% were men. Overall, the median age at blood draw was 62 years, and the median time from blood draw to diagnosis of lung cancer was 6.1 years. Nearly half of the participants (47%) were current smokers, 28% were former smokers, and 25% never smokers. In addition, the PAr level [median (5th–95th percentile)) among never, former, and current smokers was 0.36 (0.16–0.93), 0.50 (0.22–1.22), and 0.41 (0.14–0.96), respectively. PAr levels varied substantially across cohorts (Figure 1) and regions (Table 1). The adjusted geometric mean of PAr was highest (0.51) in the US cohorts and lowest (0.29) in the Asian cohorts. We observed an inverse relation between PAr and eGFR (Spearman's ρ = −0.19, P < 0.001).
Table 1.
Demographic and clinical characteristics of study participants at baseline by region, the Lung Cancer Cohort Consortium (LC3)a
| Asian cohorts |
Australian cohort |
European cohorts |
USA cohorts |
Pooled |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases (n = 1734) | Controls (n = 1734) | Cases (n = 354) | Controls (n = 354) | Cases (n = 835) | Controls (n = 835) | Cases (n = 2400) | Controls (n = 2400) | Cases (n = 5323) | Controls (n = 5323) | |
| Baseline characteristics | ||||||||||
| Age at blood draw (years) | 62 (46–74) | 62 (46–74) | 61 (46–68) | 61 (45–68) | 60 (44–71) | 60 (44–71) | 64 (48–78) | 64 (48–78) | 62 (47–76) | 62 (47–75) |
| Sex, men | 1188 (68.5) | 1188 (68.5) | 213 (60.2) | 213 (60.2) | 475 (56.9) | 475 (56.9) | 991 (41.3) | 991 (41.3) | 2867 (53.9) | 2867 (53.9) |
| Smoker | ||||||||||
| Never | 593 (34.2) | 593 (34.2) | 49 (13.8) | 49 (13.8) | 107 (12.8) | 107 (12.8) | 569 (23.7) | 569 (23.7) | 1318 (24.8) | 1318 (24.8) |
| Former | 175 (10.1) | 175 (10.1) | 145 (41.0) | 145 (41.0) | 190 (22.8) | 190 (22.8) | 1007 (42.0) | 1007 (42.0) | 1517 (28.5) | 1517 (28.5) |
| Current | 966 (55.7) | 966 (55.7) | 160 (45.2) | 160 (45.2) | 538 (64.4) | 538 (64.4) | 824 (34.3) | 824 (34.3) | 2488 (46.7) | 2488 (46.7) |
| eGFR (ml/min/1.73m²) | 89.8 (62.2–106.9) | 89.4 (62.7–106.7) | 92.7 (67.0–107.0) | 91.9 (65.1–108.5) | 91.3 (63.4–107.4) | 91.1 (64.1–106.6) | 83.7 (53.9–104.4) | 82.9 (53.4–103.2) | 87.9 (58.0–105.8) | 87.1 (57.4–105.4) |
| Biomarkers at baseline | ||||||||||
| Cotinine (nmol/l) | 494 (0.8–2159) | 218 (0.9–1772) | 23.3 (0.7–2324) | 9.2 (0.4–2084) | 959 (0–1866) | 792 (0–1698) | 4.0 (0–2142) | 3.1 (0–1964) | 98.4 (0–2103) | 12.6 (0–1864) |
| PLP (nmol/l) | 29.2 (11.0–114.9) | 31.3 (12.4–119.4) | 31.3 (14.2–212.1) | 31.3 (14.2–114.3) | 28.1 (12.4–104.9) | 30.9 (13.1–102.0) | 47.6 (15.2–266.2) | 49.9 (16.3–271.6) | 35.3 (12.5–205.4) | 37.3 (13.9–196.5) |
| Pyridoxal (nmol/l) | 11.3 (5.2–49.5) | 11.6 (5.3–48.6) | 15.2 (8.2–126.2) | 15.9 (9.1–65.5) | 13.3 (5.5–50.8) | 13.9 (5.9–52.9) | 18.9 (4.1–185.6) | 18.2 (4.5–184.7) | 14.3 (4.9–113.0) | 14.6 (5.2–112.8) |
| PA (nmol/l) | 12.2 (3.7–74.9) | 12.3 (4.5–65.0) | 19.5 (10.5–167.9) | 19.9 (11.0–74.6) | 19.9 (10.7–72.0) | 19.6 (10.9–81.3) | 32.9 (10.4–312.6) | 32.9 (10.2–323.9) | 20.6 (6.3–200.1) | 20.6 (6.7–187.6) |
| PArb | 0.30 (0.10–0.76) | 0.29 (0.11–0.71) | 0.41 (0.19–0.87) | 0.41 (0.22–0.82) | 0.50 (0.25–0.95) | 0.46 (0.23–0.95) | 0.51 (0.23–1.26) | 0.49 (0.22–1.20) | 0.43 (0.16–1.05) | 0.41 (0.16–1.02) |
| Clinical characteristics, cases only | ||||||||||
| Age at diagnosis (years) | 69 (52–80) | 70 (56–78) | 68 (53–82) | 70 (55–83) | 69.7 (53.6–82.0) | |||||
| Time from blood draw to diagnosis (years) | 6 (1–16) | 10 (2–17) | 10 (2–16) | 5 (1–16) | 6.1 (0–16.0) | |||||
| Histology | ||||||||||
| Large cell carcinoma | 16 (0.9) | 31 (8.8) | 15 (1.8) | 112 (4.7) | 174 (3.3) | |||||
| Small cell carcinoma | 97 (5.6) | 47 (13.3) | 103 (12.3) | 243 (10.1) | 490 (9.2) | |||||
| Squamous cell carcinoma | 311 (17.9) | 67 (18.9) | 162 (19.4) | 288 (12.0) | 828 (15.5) | |||||
| Adenocarcinoma | 608 (35.1) | 153 (43.2) | 260 (31.2) | 1028 (42.8) | 2049 (38.5) | |||||
| Missing /unknown | 726 (41) | 56 (15.8) | 295 (35.3) | 729 (30.4) | 1782 (33.5) | |||||
Characteristics are presented as n (%) for discrete variables and median (5th–95th percentile) for continuous variables.
PAr = PA/(pyridoxal + PLP).
eGFR, estimated glomerular filtration rate; PA, 4-pyridoxic acid; PLP, pyridoxal-5'-phosphate.
Figure 1.
Multivariable-adjusted geometric means of PAr in 20 cohorts. Error bars indicate 95% confidence intervals (CIs). Geometric mean (95% CI) of PAr in each cohort was estimated by using generalized linear model adjusted for age, sex and smoking (never, former, and current smokers) and estimated glomerular filtration rate (continuous). ATBC, The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; CLUE, The Campaign Against Cancer and Stroke (CLUE I) and the Campaign Against Cancer and Heart Disease (CLUE II); CPS-II, The American Cancer Society Cancer Prevention Study-II Nutrition Cohort; HPFS, Health Professionals Follow-up Study; HUNT, The Nord-Trøndelag Health Study; MCCS, The Melbourne Collaborative Cohort Study; MDCS, The Malmö Diet and Cancer Study; MEC, The Multiethnic Cohort; NHS, The Nurses’ Health Study; NSHDS, The Northern Sweden Health and Disease Study Cohort; NYU, The New York University Women’s Health Study; PHS, Physicians’ Health Study; PLCO, Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; SCCS, The Southern Community Cohort Study; SCHS, The Singapore Chinese Health Study; SCS, The Shanghai Cohort Study; SMHS, The Shanghai Men’s Health Study; SWHS, The Shanghai Women’s Health Study; WHI, The Women’s Health Initiative; WHS, Women’s Health Study.
Overall analysis of the association between PAr and lung cancer risk
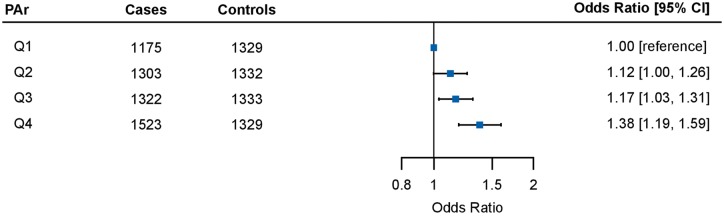
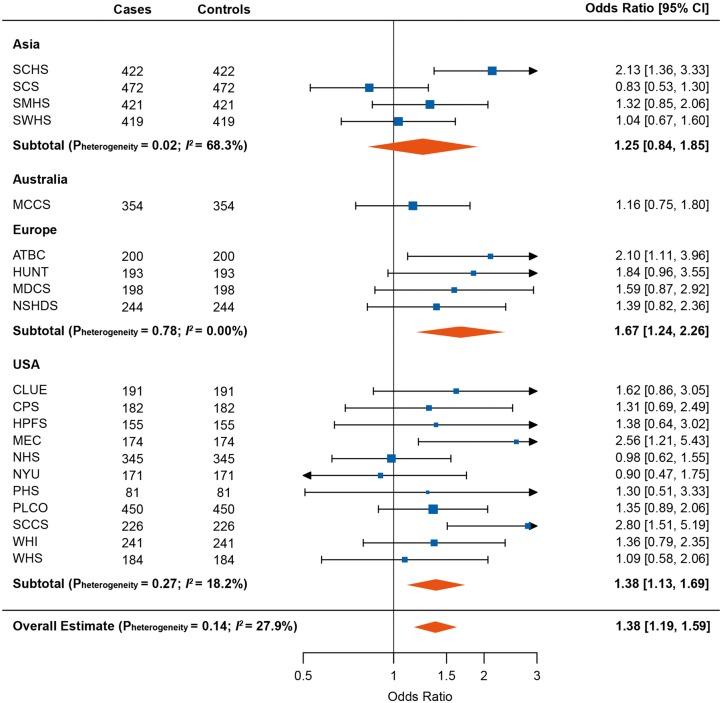
PAr was positively associated with lung cancer risk in a dose response fashion (Figure 2), with OR (95% CI) in the highest versus lowest quartile of 1.38 (1.19–1.59). When analyzing PAr as a continuous log2-transformed variable, a doubling in PAr was associated with 1.14-fold risk of lung cancer (OR for log2 PAr: 1.14, 95% CI: 1.05–1.25) (overall Pheterogeneity = 0.006; I2= 49.2%) (supplementary Figure S1, available at Annals of Oncology online). The strongest risk association was observed in Europe (OR: 1.67, 95% CI: 1.24–2.26), followed by the United States (OR: 1.38, 95% CI: 1.13–1.69), whereas no significant association was observed in Asia or Australia (overall Pheterogeneity = 0.14; I2= 27.9%) (Figure 3). The weakest associations were generally found in cohorts that only included women (supplementary Figure S1, available at Annals of Oncology online). Further adjustment for body mass index rendered the overall OR estimates slightly stronger (data not shown).
Figure 2.
Pooled odds ratios (OR) [95% confidence intervals (CIs)] for lung cancer risk across PAr quartiles. The first quartile of PAr was used as the reference. OR for each quartile was pooled using a random-effects model based on 20 cohorts. Cohort-specific estimates were calculated using conditional logistic regression adjusted for estimated glomerular filtration rate (continuous) and cotinine concentrations as quartiles defined from the distribution among current smokers.
Figure 3.
Forest plot showing odds ratios (ORs) [95% confidence intervals (CIs)] for lung cancer risk comparing the fourth to the first quartile of PAr. Cohort-specific ORs were calculated using conditional logistic regression adjusted for estimated glomerular filtration rate (continuous) and cotinine concentrations as quartiles defined from the distribution among current smokers. Results were combined using random effect models overall and for each region. ATBC, The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; CLUE, The Campaign Against Cancer and Stroke (CLUE I) and the Campaign Against Cancer and Heart Disease (CLUE II); CPS-II, The American Cancer Society Cancer Prevention Study-II Nutrition Cohort; HPFS, Health Professionals Follow-up Study; HUNT, The Nord-Trøndelag Health Study; MCCS, The Melbourne Collaborative Cohort Study; MDCS, The Malmö Diet and Cancer Study; MEC, The Multiethnic Cohort; NHS, The Nurses’ Health Study; NSHDS, The Northern Sweden Health and Disease Study Cohort; NYU, The New York University Women’s Health Study; PHS, Physicians’ Health Study; PLCO, Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; SCCS, The Southern Community Cohort Study; SCHS, The Singapore Chinese Health Study; SCS, The Shanghai Cohort Study; SMHS, The Shanghai Men’s Health Study; SWHS, The Shanghai Women’s Health Study; WHI, The Women’s Health Initiative; WHS, Women’s Health Study.
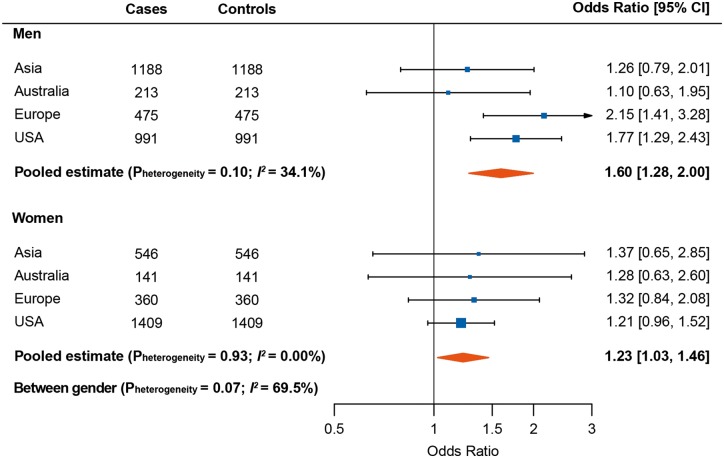
Stratified analysis by sex and smoking
As shown in Figure 4, the association between PAr and lung cancer appeared stronger in men than in women (Pheterogeneity = 0.07; I2= 69.5%), with a 60% increased risk when comparing the fourth versus first quartile in men. This association was mainly driven by men from the European and US cohorts. Effect modification was also present for smoking categories (Pheterogeneity = 0.006; I2= 79.6%), with the strongest association observed among former smokers (pooled OR: 1.69, 95% CI: 1.36–2.10 for the fourth versus first quartile of PAr) (supplementary Figure S2, available at Annals of Oncology online). After further adjustment by number of years of smoking or pack-years of smoking, the risk estimates did not change essentially among former smokers but were somewhat attenuated among current smokers (supplementary Table S1, available at Annals of Oncology online).
Figure 4.
Forest plot showing odds ratios (ORs) [95% confidence intervals (CIs)] for lung cancer risk comparing the fourth to the first quartile of PAr, stratified by sex. Cohort-specific ORs were calculated using conditional logistic regression adjusted for estimated glomerular filtration rate (continuous) and cotinine concentrations as quartiles defined from the distribution among current smokers. Results were combined using random effect models for each region among men and women.
Stratified analysis by histology and time to diagnosis
Stratified analysis by histology showed that the risk association of PAr appeared strongest for SCC (adjusted OR: 1.30, 95% CI: 0.95–1.78 for the fourth versus first quartile of PAr), followed by adenocarcinoma (adjusted OR: 1.26, 95% CI: 1.04–1.52), small-cell carcinoma (adjusted OR: 1.22, 95% CI: 0.84–1.78), and large cell carcinoma (adjusted OR: 0.91, 95% CI: 0.47–1.76). We also observed that the risk estimates were strongest for those who received their lung cancer diagnosis within 3 years of blood draw (adjusted OR: 1.73, 95% CI: 1.34–2.23), and gradually decreased by increasing time from blood draw to lung cancer diagnosis (supplementary Figure S3, available at Annals of Oncology online). In order to address an effect of potentially established cancer on PAr at baseline, we excluded 411 cases diagnosed within the first year after blood draw and their matched controls from the analysis, and observed consistent results. The risk estimates remained strongest for those who received their lung cancer diagnosis 1–3 years after blood draw (adjusted OR: 1.89, 95% CI: 1.38–2.59).
Discussion
Principal findings
In this study of pre-diagnostic individual level data from 20 nested case–control studies across Asia, Australia, Europe and the United States, we observed that study participants with increased vitamin B6 catabolism, as indicated by elevated PAr index, had an increased risk of developing lung cancer. This association was strongest in men, former smokers, and those who received a lung cancer diagnosis within the first 3 years after blood draw.
Comparison with previous studies
This study confirms our previously reported findings [13, 14] that PAr is positively associated with lung cancer risk, in particular among men who had ever smoked. Stratified analysis from the present large study showed that the risk association appeared to be strongest in men, former smokers and participants diagnosed with SCC, which is in agreement with results from the EPIC study including eight European countries [13]. Of note, the European cohorts in the LC3 generated a stronger risk estimate (OR: 1.31, 95% CI: 1.10–1.57 for log2 PAr) than cohorts from Asia, Australia or United States. However, the estimate was still lower than that in EPIC (OR: 1.52, 95% CI: 1.27–1.81 for log2 PAr), which is presumably attributable to differences in cohort recruitment and characteristics including levels of PLP and PAr. The European cohorts in the LC3 were exclusively from Finland, Norway, and Sweden, and had relatively low PLP concentrations and higher PAr levels, whereas the EPIC study additionally included cohorts from Central and Southern European regions, which had relatively higher plasma PLP and lower PAr levels compared with the Nordic countries.
Our findings in LC3 showed that the association between PAr and lung cancer risk was stronger for those who received their lung cancer diagnosis within the first 3 years after blood draw, which is also similar to previously reported findings for PLP [8] and functional vitamin B6 status [21] in LC3. This particular observation suggests preclinical metabolic changes, that is, increased vitamin B6 catabolism reflecting inflammation and immune activation in carcinogenesis before clinical lung cancer diagnosis. In other words, PAr may be a pre-diagnostic marker of lung cancer rather than a causal factor.
Possible mechanisms
The association between PAr and lung cancer risk among current smokers was attenuated after careful adjustment for smoking duration and intensity. This suggests that PAr may be related to inflammation and immune activation induced by smoking, which is one of the mechanisms through which smoking causes lung cancer [22]. More importantly, the strong association among former smokers remained essentially unchanged after such adjustment, indicating that inflammation and immune activation affecting lung cancer risk measured by PAr is beyond history of tobacco exposure. Current smokers had low levels of PLP in our study [8], and low circulating PLP may increase to levels observed in never smokers after smoking cessation [10], which is confirmed by our study. Nevertheless, the PAr among former smokers was even higher than current smokers in our study, largely due to a parallel increase in circulating PA and PLP. Therefore, focusing on increased vitamin B6 catabolism provides new insight into lung carcinogenesis beyond PLP.
Strengths and limitations
This study has several strengths. First, the large sample size of 5323 case–control pairs enabled well-powered subgroup analyses, and the inclusion of 20 prospective cohorts across four continents provided an unprecedented opportunity to evaluate the generalizability of the relation between PAr and lung cancer. Second, the centralized biochemical measurements with robust quality control further allowed for comparisons between individual cohorts and geographical regions. It has been shown that the components of the PAr (PA and PLP + pyridoxal) are stable during long-term storage at −80°C [23]. Lastly, we also controlled for current tobacco exposure using cotinine measurements, and the intentional oversampling of never and former smokers allowed for well-powered stratified analysis by smoking status. However, this study also has limitations. Some cohorts restricted the recruitment to certain subject categories, in particular, several cohorts recruitment was limited to a specific sex, thus complicating between-cohort comparisons. In addition, information on histological data was missing for 34% of the lung cancer cases, thus our finding regarding histological types should be interpreted with caution. As in all epidemiological studies based on measurements at a single time point, our estimates may have underestimated the real association between PAr and lung cancer due to regression dilution bias.
Conclusions
In this large analysis of 10 646 participants from 20 nested case–control studies, elevated PAr reflecting increased vitamin B6 catabolism was associated with an increased risk of lung cancer. This study robustly and comprehensively corroborates previous findings indicating that inflammation and immune activation as captured by increased PAr are associated with lung cancer.
Supplementary Material
Acknowledgements
The authors would like to thank the participants and staff of the Health Professionals Follow-up Study and Nurses’ Health Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data. Cancer incidence data for the Campaign Against Cancer and Stroke (CLUE I) and the Campaign Against Cancer and Heart Disease (CLUE II) cohorts were provided by the Maryland Cancer Registry, Center for Cancer Surveillance and Control, Department of Health 201 W. Preston Street, Room 400, Baltimore, MD 21201, https://phpa.health.maryland.gov/cancer/Pages/mcr_home.aspx, 410-767-4055. The CLUE authors would like to thank the State of Maryland, the Maryland Cigarette Restitution Fund, and the National Program of Cancer Registries of the Centers for Disease Control and Prevention for the funds that helped support the collection and availability of the cancer registry data. The CLUE authors would also like to thank the CLUE participants and staff at the George W. Comstock Center for Public Health Research and Prevention.
Funding
The Lung Cancer Cohort Consortium (LC3) was supported by National Institutes of Health/National Cancer Institute (1U01CA155340-01) and National Health and Medical Research Council, Australia (1050198). The Health Professionals Follow-up Study and Nurses’ Health Study were supported by a grant from the National Institutes of Health (NIH) grants UM1CA186107, P50CA127003, P01CA87969, R01CA49449, and UM1 CA167552. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure
PMU and ØM report that they are members of the steering board of the nonprofit Foundation to Promote Research into Functional Vitamin B12 Deficiency. All remaining authors have declared no conflicts of interest.
References
- 1. Siegel RL, Miller KD, Jemal A.. Cancer Statistics, 2017. CA Cancer J Clin 2017; 67(1): 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Torre LA, Bray F, Siegel RL. et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65(2): 87–108. [DOI] [PubMed] [Google Scholar]
- 3. Hirsch FR, Scagliotti GV, Mulshine JL. et al. Lung cancer: current therapies and new targeted treatments. Lancet 2017; 389(10066): 299–311. [DOI] [PubMed] [Google Scholar]
- 4. Grivennikov SI, Greten FR, Karin M.. Immunity, inflammation, and cancer. Cell 2010; 140(6): 883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ridker PM, MacFadyen JG, Thuren T. et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 2017; 390(10105): 1833–1842. [DOI] [PubMed] [Google Scholar]
- 6. Mocellin S, Briarava M, Pilati P.. Vitamin B6 and cancer risk: a field synopsis and meta-analysis. J Natl Cancer Inst 2017; 109(3): 1–9. [DOI] [PubMed] [Google Scholar]
- 7. Johansson M, Relton C, Ueland PM. et al. Serum B vitamin levels and risk of lung cancer. JAMA 2010; 303(23): 2377–2385. [DOI] [PubMed] [Google Scholar]
- 8. Fanidi A, Muller DC, Yuan JM. et al. Circulating folate, vitamin B6, and methionine in relation to lung cancer risk in the Lung Cancer Cohort Consortium (LC3). J Natl Cancer Inst 2018; 110(1): 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hartman TJ, Woodson K, Stolzenberg-Solomon R. et al. Association of the B-vitamins pyridoxal 5'-phosphate (B(6)), B(12), and folate with lung cancer risk in older men. Am J Epidemiol 2001; 153(7): 688–694. [DOI] [PubMed] [Google Scholar]
- 10. Ueland PM, Ulvik A, Rios-Avila L. et al. Direct and functional biomarkers of vitamin B6 status. Annu Rev Nutr 2015; 35: 33–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ulvik A, Midttun O, Pedersen ER. et al. Evidence for increased catabolism of vitamin B-6 during systemic inflammation. Am J Clin Nutr 2014; 100(1): 250–255. [DOI] [PubMed] [Google Scholar]
- 12. Ueland PM, McCann A, Midttun O, Ulvik A.. Inflammation, vitamin B6 and related pathways. Mol Aspects Med 2017; 53: 10–27. [DOI] [PubMed] [Google Scholar]
- 13. Zuo H, Ueland PM, Midttun O. et al. Results from the European prospective investigation into cancer and nutrition link vitamin B6 catabolism and lung cancer risk. Cancer Res 2018; 78(1): 302–308. [DOI] [PubMed] [Google Scholar]
- 14. Zuo H, Ueland PM, Eussen SJ. et al. Markers of vitamin B6 status and metabolism as predictors of incident cancer: the Hordaland Health Study. Int J Cancer 2015; 136(12): 2932–2939. [DOI] [PubMed] [Google Scholar]
- 15. Midttun O, Theofylaktopoulou D, McCann A. et al. Circulating concentrations of biomarkers and metabolites related to vitamin status, one-carbon and the kynurenine pathways in US, Nordic, Asian, and Australian populations. Am J Clin Nutr 2017; 105: 1314–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Midttun O, Hustad S, Ueland PM.. Quantitative profiling of biomarkers related to B-vitamin status, tryptophan metabolism and inflammation in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 2009; 23(9): 1371–1379. [DOI] [PubMed] [Google Scholar]
- 17. Midttun O, Kvalheim G, Ueland PM.. High-throughput, low-volume, multianalyte quantification of plasma metabolites related to one-carbon metabolism using HPLC-MS/MS. Anal Bioanal Chem 2013; 405(6): 2009–2017. [DOI] [PubMed] [Google Scholar]
- 18. Levey AS, Stevens LA, Schmid CH. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150(9): 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Riley RD, Lambert PC, Abo-Zaid G.. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ 2010; 340: c221.. [DOI] [PubMed] [Google Scholar]
- 20. Higgins JP, Thompson SG, Deeks JJ, Altman DG.. Measuring inconsistency in meta-analyses. BMJ 2003; 327(7414): 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Theofylaktopoulou D, Midttun O, Ueland PM. et al. Impaired functional vitamin B6 status is associated with increased risk of lung cancer. Int J Cancer 2018; 142(12): 2425–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shiels MS, Katki HA, Freedman ND. et al. Cigarette smoking and variations in systemic immune and inflammation markers. J Natl Cancer Inst 2014; 106(11): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hustad S, Eussen S, Midttun O. et al. Kinetic modeling of storage effects on biomarkers related to B vitamin status and one-carbon metabolism. Clin Chem 2012; 58(2): 402–410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.