Abstract
Micro-computed tomography (CT) enables three-dimensional (3D) imaging of complex soft tissue structures, but current protocols used to achieve this goal preclude cellular and molecular phenotyping of the tissue. Here we describe a radiolucent cryostage that permits micro-CT imaging of unfixed frozen human lung samples at an isotropic voxel size of (11 µm)3 under conditions where the sample is maintained frozen at −30°C during imaging. The cryostage was tested for thermal stability to maintain samples frozen up to 8 h. This report describes the methods used to choose the materials required for cryostage construction and demonstrates that whole genome mRNA integrity and expression are not compromised by exposure to micro-CT radiation and that the tissue can be used for immunohistochemistry. The new cryostage provides a novel method enabling integration of 3D tissue structure with cellular and molecular analysis to facilitate the identification of molecular determinants of disease.
NEW & NOTEWORTHY The described micro-CT cryostage provides a novel way to study the three-dimensional lung structure preserved without the effects of fixatives while enabling subsequent studies of the cellular matrix composition and gene expression. This approach will, for the first time, enable researchers to study structural changes of lung tissues that occur with disease and correlate them with changes in gene or protein signatures.
Keywords: cryostage, gene expression, lung, micro-computed tomography, soft tissue imaging
to study the micropathology of large and complex organs such as the human lung with three-dimensional (3D) micro-computed tomography (CT) imaging, existing protocols require tissue sampling and a form of tissue fixation (6, 13, 15, 24, 25). A series of methods for soft tissue imaging involving fixation with formalin, ethanol, or glutaraldehyde and staining with heavy metals for high-contrast micro-CT imaging have demonstrated outstanding image quality ideal for structural assessment (5, 15–18, 21). Staining with heavy metals precludes further immunohistochemical staining while chemical fixatives dehydrate the tissue, which results in uncontrolled shrinkage of the sample. In addition, the use of fixatives fragments RNA and cross-links proteins making the imaged tissues unusable for polymerase chain reaction-based analysis of gene expression or proteomic analysis. Hence it has been difficult to combine morphological and molecular studies to identify the molecular determinants responsible for the alterations of tissue structure which occur in disease. Recently, Tang et al. have demonstrated that micro-CT can be used for imaging nonfixed breast cancer specimens (22). However, to avoid sample movement caused by tissue drying, their approach relied on a fast scanning protocol (<7 min) which led to significant loss in image resolution and contrast. Traditionally, micro-CT has been used to study bone structures, and Maran et al. (14) have previously demonstrated the feasibility of analyzing the three-dimensional architecture of bone biopsies using a custom-made cryogenic micro-CT stage (12) followed by gene expression analysis of the same specimen. More recently, Kampshulte et al. have shown that it is possible to scan frozen bone specimen using a dry ice-filled radiolucent container if a scan can be completed before the dry ice evaporates (11).
A previous unique study from our group demonstrated that it is possible to determine a gene expression signature related to tissue destruction by combining morphometric measurements obtained by micro-CT with gene expression analysis on adjacent tissue samples from the same subject (4). The limitation of this protocol was that the gene expression analysis had to be performed on adjacent (not fixed) tissue samples, with the assumption that the disease severity was comparable. To accurately relate 3D micropathology measured with micro-CT to molecular and cellular changes, the micro-CT scanning must be performed on frozen tissue samples. Hullar et al. have recently proposed a cost-effective, compact cold stage for micro-CT imaging of chilled or frozen ice samples (7). The aim of the present study was to develop and test a cryostage suitable for high-resolution micro-CT imaging of frozen lung tissue samples that does not affect protein and RNA integrity.
METHODS
Lung tissue and sampling.
A nontransplantable donor lung released for research was obtained by the James Hogg Lung Registry from the Gift of Life Program, Philadelphia, Pennsylvania. Written informed consent was obtained from the next of kin, and the study was approved by the University of British Columbia Providence Health Care Ethics Committee.
The lung was inflated with air to a positive pressure of 30 cmH2O for alveolar recruitment, followed by a slow deflation to 10 cmH2O, which was maintained while the lung was placed in liquid nitrogen vapor until frozen solid. Eight sets of paired cylindrical tissue cores (16 mm in diameter and 20-mm high) were then extracted from random, uniformly distributed locations throughout the frozen lung using a custom-made hollow punch cylinder. The lung-processing protocol we used has previously been described in detail (15, 26). The main difference between the previous studies and the present one was that in the previous studies the tissue samples were fixed in 1% glutaraldehyde in acetone and critically point dried to enable imaging via micro-CT; however, this process destroys RNA and proteins. In the present study, tissue samples were stored at −80°C after sample extraction until they were scanned while being maintained frozen using the custom-built micro-CT cryostage.
Custom-built micro-CT cryostage for soft tissue imaging.
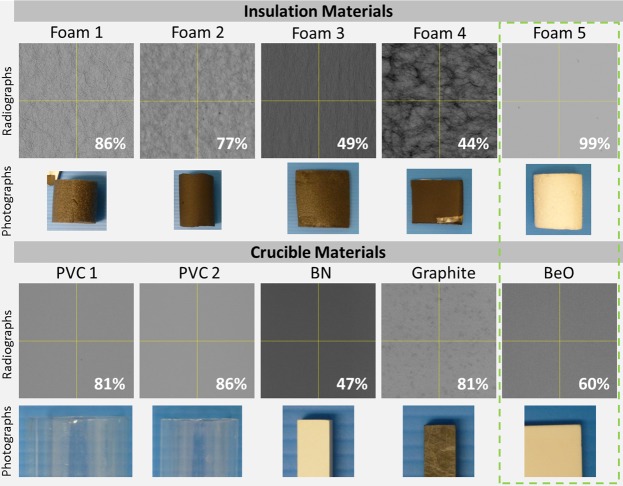
We adapted a previous cryostage design presented by Hullar et al. through collaboration with Paige Instruments (Woodland, CA), to enable high-contrast imaging of soft tissue samples (7). Briefly, multiple materials including polyvinyl chloride (PVC), boron nitride (as used by Hullar et al.), pure graphite, and beryllium oxide (beryllia) were tested for use as a thermal conductive crucible. As shown by the data in Fig. 1, beryllia (American Beryllia) with an X-ray transmission rate of 60% and high thermal conductivity (218 W·m−1·K−1) was chosen for the final design. To insulate the crucible, four open cell foams (thickness ranges 2–5 mm) and one type of extruded polystyrene foam (Styrofoam) were tested as shown in Fig. 1. Because of Styrofoam having the lowest transmission rate it was used to manufacture a custom insulation sleeve. We manufactured a polystyrene cylinder with an inner diameter of 26 mm and a wall thickness of 5 mm, which was fitted over the final crucible.
Fig. 1.
Material testing: five different insulating foams (see photographs in 2nd row) and five materials (depicted in the photographs of the 4th row) that could be used as sample holders were tested for their individual radiolucency. All radiographs of the materials were acquired at 45 kV, 350 µA, 500-ms exposure, and a gain of 24 dB. The X-ray transmission is indicated for each material as a percent of air. The green dashed box indicates the final material combination used for the cold stage with a combined transmission of 59%. Images highlight the difference in absorption levels and patterns generated by inhomogenous particle distribution in the different materials.
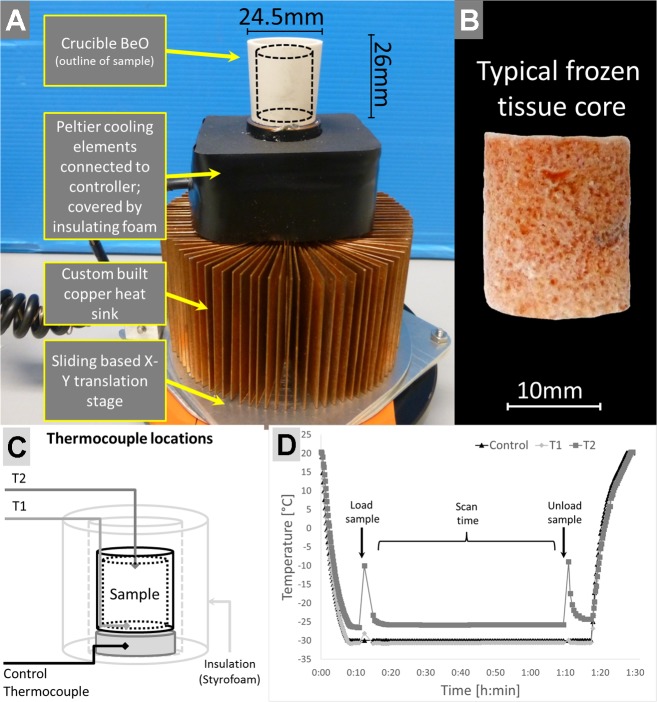
As shown in Fig. 2A the Beryllia crucible was fixed with a thermally conducting epoxy resin to a solid copper cylinder (4-mm high and 26-mm diameter) which was soldered to a copper plate to provide an efficient thermal connection to the Peltier cooling elements. This design raised the crucible from the rest of the stage enabling imaging of the crucible without having to image through the Peltier cooler, which means the sample holder could be brought closer to the X-ray source to enable higher-resolution and higher-contrast imaging. The two-stage Peltier element (model 19012-5L031-02CQQ, 40 × 40 mm; Custom Thermoelectric, Bishopville, MD) was mounted between the copper plate and a custom-built circular finned copper heat sink to dissipate the waste heat from the Peltier cooler. The Peltier elements were controlled by a custom control module, attached to the copper cylinder under the crucible, which continuously measured the temperature of the crucible via a thermocouple probe (K-type) and proportioned the power output to the Peltier elements to maintain the system temperature correspondingly within ±0.2°C of the setting. Two external directional fans (Brushless Blower Cooling Fan; Fugetek, Houston, TX) powered by the same control module were placed on either side of the heat sink to efficiently cool it.
Fig. 2.
Cooling stage design, example sample, and testing. A: the cooling stage is composed of a beryllia crucible mounted on a small copper stub which enables the Peltier elements to draw heat away from the sample. The Peltier elements are directly mounted on top of a custom-made heat sink. The entire assembly is mounted on an X-Y translation stage which enabled a precise positioning in the scanner. B: a typical frozen lung tissue core which was extracted from a whole air-inflated frozen lung; the diameter is ~16 mm, and it is 20-mm high. C: schematic of stage indicating the location of additional temperature probes (T1, T2) within the sample holder. D: temperature plot over the duration of one experiment including precooling time, scan time, and warm-up phase after scan completion and sample removal.
Integration with the micro-CT scanner.
The cryostage, fans, and control unit were placed inside the micro-CT scanner cabinet. The cryostage was firmly attached to the existing micro-CT sample stage with a centering screw. The crucible of the cryostage was centered in the field of view using multiple radiographs from different angles. The two directional fans were then placed on opposite sides of the cryostage to enable airflow onto the heat sink but away from the crucible to avoid any sample movement due to vibrations during scanning. The wires leading to the cold stage were attached to a wire guide which enabled the stage to rotate freely up to 360°.
Micro-CT protocol.
Prior to a scan, a shading correction was generated by using the empty crucible to improve image contrast. The cryostage was then set to −30°C and allowed to cool for 8–10 min, after which a tissue core was transferred into the center of the crucible. The slow movement of the stage did not require any additional sample stabilization.
A Nikon HMX 225ST micro-CT scanner with a molybdenum X-ray target, a high-transparency beryllium X-ray window, and a high-sensitivity PerkinElmer detector (EH1200) was used to acquire the 3D data set. This configuration enabled a high signal-to-noise ratio during the scanning of the frozen tissue cores. The following parameters were used to achieve an isotropic voxel size of (11 µm)3 and a high contrast between the tissue and air: 40 kV, 350 µA, 500-ms exposure time, and a gain of 32 dB. To limit scanning time to 1 h, only 1,800 projections over a rotation of 360° were acquired. Transverse serial images (2,000 × 2,000 pixels) were reconstructed at a voxel size of 11 μm using the Nikon reconstruction program CT Pro version 3.4.3 resulting in a data set of 6.91 GB. After scan completion the tissue samples were transferred back to −80°C storage. The tissue could then be used for either histological preparation or RNA extraction as detailed below.
Image segmentation.
By using previously developed image segmentation algorithms (24), individual components of the lung parenchyma could be segmented. In brief, a series of image-processing steps were performed: image filtering using an unsharp mask to enhance contrast between tissue and air; a threshold that separated tissue and air; application of a connected-components algorithm to eliminate noise; and, last, inverting the complete tissue segmentation to obtain an air mask. The segmentation of all air spaces was then separated into individual components by applying a topomorphologic opening algorithm. The seed-based separation provided individual segmentation masks of the parenchymal features of conducting airways, arteries, and veins as well as the separation of pulmonary acini. Visualization of the combined structures was performed with the 3D viewer plug-in in ImageJ (20).
Histological preparation.
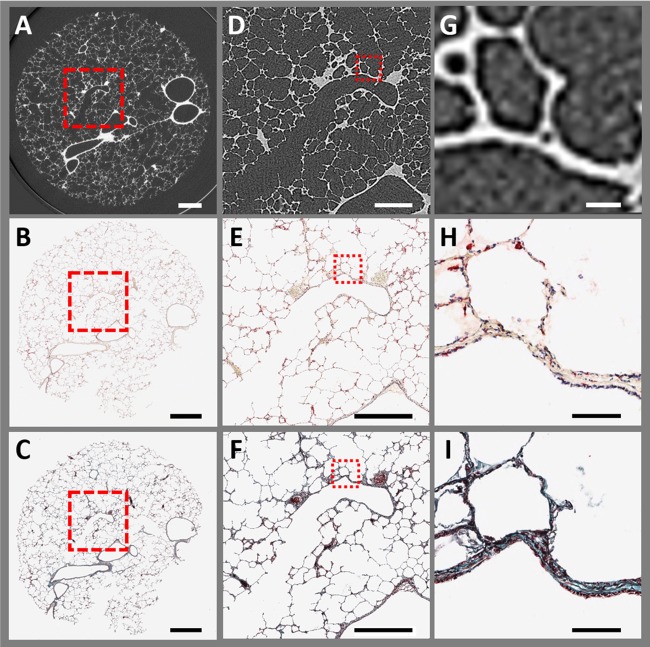
To identify regions of interest (ROI) within the sample that could be assessed by histology, the 3D micro-CT scan was used to determine the exact location of the ROI. The tissue core was then sampled to obtain the ROI in a 5-mm-thick section. To prevent tissue collapse and preserve the local architecture, the section was fixed with a precooled alcohol-based formalin (mixture of 1:9) (2) at approximately −20°C overnight and subsequently warmed to room temperature. The 5-mm section was then placed in a tissue processor (Leica ASP 6025) for paraffin infiltration. Last, the tissue section was embedded in paraffin, and 4-μm-thick sections were cut with a microtome. A set of three initial sections were used to confirm the location within the micro-CT scan. This reference location was used to compute the distance to the site of interest. The corresponding amount of tissue was cut off with the microtome. At the site of interest, serial sections were taken. As shown in Fig. 3B, one section was stained with antibodies for CD68-positive cells (catalog no. M0876, clone PG-M1, lot no. 00061489, and a dilution of 1:200; Dako) with an automatic tissue stainer (Leica Bond RX). A second section was stained with Mason’s trichrome chemical stain (Fig. 3C). Sections were then scanned with an automatic slide scanner (ScanScope XT; Aperio) at ×40 magnification. The area of the section as well as the alveolar wall thickness were measured in the matched micro-CT images and the histological sections to determine the amount of shrinkage induced by the tissue processing.
Fig. 3.
Comparison of frozen micro-CT image with matched histological sections: A–C: large field of view of a representative micro-CT image at a pixel size of 11 µm (A) and ×1 magnifications of the matched histological serial sections stained with CD68+ (B) and Mason’s trichrome (C). Micro-CT as well as histology clearly show airways, blood vessels, and alveoli. Rigid 3D image registration was performed to match the micro-CT scan with the histological sections. During tissue processing, a small part of the histological section (lower right corner) detached from the section. The red highlighted areas indicate the location of magnified areas. D–F: magnification of the region of interest centered on a terminal bronchiole. G–I: further magnified images of the bronchiolar wall and alveolar structure. The stained histological sections (×10 magnification) show a greater level of detail than the micro-CT because of the higher optical magnification vs. digital zooming into the micro-CT image. (Scale bar sizes: A–C, 2 mm; D–F, 1 mm; and G–I, 100 µm).
Sample preparation for RNA quantification.
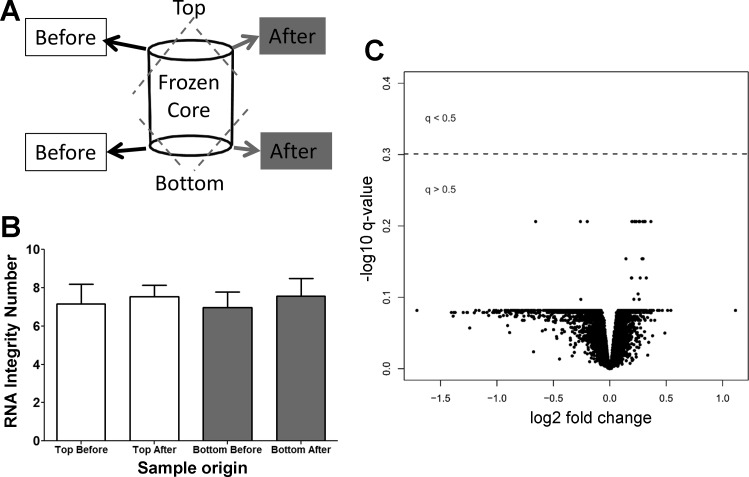
To assess the preservation of RNA integrity during the micro-CT scan, a pair of small tissue samples were taken from three tissue cores before scanning, and an additional paired sample was taken after each scan. From each sample pair, one of the tissue samples was taken from the top of the core, and one was taken from the bottom (as indicated in Fig. 5A). Each of the tissue samples was chopped very finely with a precooled razor blade on dry ice and transferred into precooled RNA extraction tubes. An RNeasy Mini Kit (Qiagen) was used to extract the RNA from the tissue samples. Subsequently, the Agilent 2100 Bioanalyzer was used to determine the RNA integrity number, and statistical difference was tested using a paired Student’s t-test. A P value of <0.01 was considered statistically significant.
Fig. 5.
RNA integrity testing and gene expression comparison. A: four tissue samples were taken from three test samples for RNA integrity test. Two samples were taken before scanning in the cryostage, and two samples were taken afterward. B: there was no significant difference in the RNA integrity before and after the scan and between the top and bottom of the samples. Additionally, the gene expression was measured in 16 paired tissue samples, of which 8 were scanned frozen with the micro-CT scanner while the adjacent samples were not scanned. C: there were no gene expression profiles associated with micro-CT exposure (q < 0.5). Fold change was calculated using the “limma” package and represents the mean difference of the log 2-transformed expression profiles between the micro-CT-exposed and unexposed groups for each gene. P values were corrected by the Benjamini-Hochberg method, log 10 transformed and multiplied by −1 so that the most significant genes are represented at the top of the plot.
Gene expression measurement.
To ensure that gene expression levels were not changed by the cryoimaging protocol or X-ray exposure, we profiled RNA from eight pairs of tissue samples (total of 16 samples) that were uniformly distributed from the top to the bottom of a whole frozen air-inflated lung. From each pair, one of the samples was imaged frozen using the cryostage, and then both samples were profiled for whole genome RNA using an Affymetrix Human Exon 1.0 ST microarray (Affymetrix, Santa Clara, CA). The robust multichip array (RMA) algorithm, from the “affy” package (v1.36.1), and the BrainArray v17 chip definition file (CDF) were used to generate log 2 normalized gene expression profiles using the R v2.15.3 programing language (3, 8, 9). The “limma” package (v3.22.7) (19) was used within R v3.1.1 (23) to perform differential expression analysis to identify gene expression that was affected by X-ray exposure. The Benjamini-Hochberg adjustment was used to adjust for multiple comparisons. A false discovery rate q value <0.5 was considered statistically significant.
RESULTS
Cryostage material testing.
The cryostage was built to maintain tissue samples frozen at −30°C to preserve RNA quality suitable for subsequent gene expression analysis and enable imaging at high resolution (10–11-µm isotropic voxel side length) with a micro-CT scanner. To maintain samples at a constant temperature of −30°C during a micro-CT scan, a well-insulated and highly thermal-conductive crucible was required. Furthermore, the crucible and the insulation had to be radiolucent to enable high-contrast micro-CT imaging of low-density tissue samples. Five insulating foams as well as five different crucible materials were tested for radiolucency and thermal conductivity. As can be seen in Fig. 1, open cell foams 1–4, which typically have higher thermal resistance, also had a relatively high X-ray absorption rate. This was due to carbon particles mixed within the open cell foams causing texturing within the radiographic projections which interferes with the imaging protocol. Foam 5 (extruded polystyrene foam) had an X-ray transmission rate of 99% and gave no texturing because of its homogenous nature and was therefore chosen to insulate the crucible. The materials tested for use as crucible were polyvinyl chloride (PVC), boron nitride [as used by Hullar et al. (7)], pure graphite, and beryllium oxide (beryllia). As can be seen in Fig. 1, the PVC and graphite had very high transmission rates (81–86%). However, the thermal conductivity of PVC (0.19 W·m−1·K−1) and graphite (65–95 W·m−1·K−1) was relatively low and therefore would not keep the specimen cold and at a uniform temperature. Boron nitride (BN), previously used by Hullar et al., had a lower transmission rate (47%) and a low thermal conductivity (30 W·m−1·K−1), which made it also unsuitable for our experiments. Consequently, the material of choice for building the crucible was beryllia with a relatively good transmission rate of 60% and a superior thermal conductivity of 218 W·m−1·K−1. In addition, beryllia generated a clear 3D tomographic image because of its high purity and homogenous structure, which enabled the crucible itself to be used as a shading correction filter to improve image contrast. The final sample holder was constructed from the beryllia crucible and polystyrene foam, which together had an X-ray transmission rate of 59%.
Stage design.
Figure 2A shows a photograph of the final cold stage, and Fig. 2B shows an example of a frozen lung tissue core. To maintain the crucible at −30°C, two serially mounted Peltier cooling elements were attached to a custom-made copper heat sink. A thermocouple probe (K-type), mounted at the base of the crucible (see diagram in Fig. 2C), provided a feedback to the controller of the Peltier elements once the desired temperature was reached. The Peltier elements were covered by a layer of closed cell insulating foam and PVC tape to avoid buildup of frost. To ensure that the Peltier elements were not in the field of view during micro-CT scanning, the crucible was mounted on top of a solid copper cylinder (4-mm high and 26-mm diameter) mounted above the Peltier elements, which also provided excellent heat transfer away from the crucible. A custom-made X-Y translation stage enabled centering the cryostage in the field of view of the scanner. Last, two directional fans were used to dissipate the heat built up from the heat sink. All components are inexpensive and readily available electronic parts such as the Peltier elements, insulating materials, and thermocouple probes, or are custom made from readily available materials such as the copper sheets used for the heat sink.
Temperature-testing results.
To test if the required temperature was maintained throughout the duration of a scan, two thermocouple probes (K-type) were inserted into the crucible. As shown in Fig. 2C the first thermocouple was attached to the bottom of the crucible (T1), while the second thermocouple (T2) was suspended at the top of the crucible so that it would touch the top of the tissue sample. During a scanning protocol, as shown in Fig. 2D, without a sample inside the crucible, the T1 probe at the bottom of the crucible reached a temperature of −30°C within 8 min while at the top of the crucible, the air temperature reached approximately −25°C. When the tissue sample was placed inside the crucible, the temperature of the T2 probe spiked up because of the warming up of the probe while taken out of the crucible. The increase in temperature of the bottom probe was negligible if the sample was quickly placed inside and the insulation was restored. Over the course of the developed 60-min imaging protocol the temperature remained constant across the tissue sample (Fig. 2D), and further tests showed that the cryostage could maintain its temperature for up to 8 h without significant buildup of frost (data not shown) allowing for either multiple scans or long scanning protocols. The graph in Fig. 2D shows that once the cooling stage controller is turned off, the temperature reaches room temperature very quickly because of the reverse effect of the heat transfer from the warm (+35°C) heat sink to the very cold crucible (−30°C). Therefore, if a controller failed to maintain power to the Peltier cooling elements, the sample would need to be removed within 1 min before it would start to defrost.
High-contrast lung tissue imaging protocol.
As shown by the micro-CT image in Fig. 3A the micro-CT scanning protocol used with the cryostage enabled scanning of frozen human lung tissue samples at a resolution of 11 µm. The red dashed box in Fig. 3A indicates an enlarged view of a terminal bronchiole shown in Fig. 3B, which was further enlarged to visualize individual alveoli at a resolution comparable with ×20 light microscopy imaging. Figure 3C demonstrates the high contrast between the frozen tissue and air which is possible due to the preserved water content within the frozen tissue sample. Tissue volume calculated from micro-CT images of frozen lung tissue samples vs. the same sample after fixation with a precooled solution of acetone with 1% glutaraldehyde and critical point drying [replicating from the McDonough et al. (15) and Verleden et al. (26) protocols] demonstrated a 20 ± 8% (SD) shrinkage (ranging from 2 to 35%) in the fixed and dehydrated samples, which needs to be corrected for. Furthermore, the time required to image a fixed-dried tissue sample at a comparable resolution and image contrast was significantly greater than that for the frozen core (3 vs. 1 h).
The 3D micro-CT data set allowed us to identify a region of interest inside the tissue sample and use those coordinates to precisely section the sample for immunohistochemical analysis. The representative light microscopy images in Fig. 3, B, E, and H, and Fig. 3, C, F, and I, show the matching histological sections to the micro-CT scan (Fig. 3, A, D, and G) after the tissue was fixed in precooled alcohol-based formalin, paraffin embedded, and stained with an antibody for the macrophage receptor CD68 (Fig. 3B) and the adjacent tissue section was stained with Mason trichrome stain (Fig. 3C) to show the tissue composition. This demonstrates that our cryoimaging protocol enables subsequent analysis of the cellular and extracellular matrix composition using immunohistochemistry, as proteins are not destroyed by the tissue preservation normally required for previous micro-CT soft tissue imaging methods. A comparison of tissue area and alveolar wall thickness in the frozen sample imaged by micro-CT vs. matched histological sections captured by light microscopy indicated a 40% shrinkage in tissue area and ~60% thinner alveolar walls after fixation with precooled alcohol-based formalin and paraffin embedding (Fig. 3, B and C).
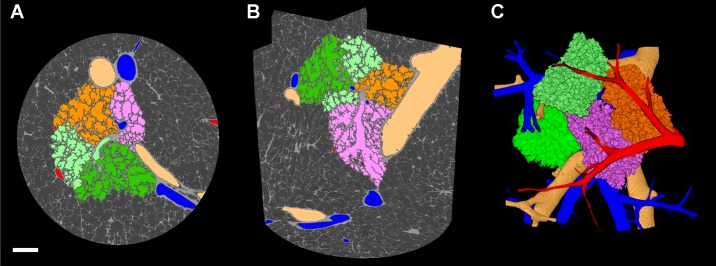
Micro-CT image segmentation.
The high-contrast imaging achieved using the cryostage imaging protocol enabled previously developed image segmentation algorithms (24) to be applied to segment conducting airways and vasculature and, for the first time, separate individual human pulmonary acini as shown in Fig. 4, A–C. To view the full segmentation in 3D, see the Supplemental Video (Supplemental Material for this article is available online at the Journal of Applied Physiology Web site).
Fig. 4.
Image segmentation of parenchymal components in a scan of a frozen tissue sample. A: micro-CT image with segmentation overlays of airways (beige), arteries (blue), veins (red), and multiple acini (orange, light green, dark green, and pink; 2-mm scale bar). B: three-plane orthogonal view of micro-CT scan highlighting segmentations of parenchymal components using the same color scheme. C: three-dimensional reconstruction of the segmented components enables the visualization of the complex parenchymal structure. A 3D animated video of B and C is available online in the Supplemental Video.
RNA integrity.
To ensure that RNA quantity and integrity were maintained during the cryoimaging protocol, RNA was assessed before and after scanning, both at the top and at the bottom of the tissue sample as shown in Fig. 5A. We found no difference in RNA concentration obtained from either the top or the bottom of the extracted samples before (top, 522 ± 53 ng; bottom, 556 ± 89 ng) or after (top, 586 ± 188 ng; bottom, 536 ± 140 ng) the scanning protocol. As shown in Fig. 5B, there was no significant difference in the RNA quality as demonstrated by the RNA integrity number values of the samples before and after scanning. Importantly, to ensure that the expression of genes was not changed by the cryoimaging protocol, we profiled whole genome RNA from eight pairs of tissue samples with and without radiation. Paired t-tests were used to determine if X-ray exposure affected gene expression profiles and were corrected using the Benjamini-Hochberg method. As shown in the volcano plot in Fig. 5C, no genes were differentially expressed at a false discovery rate corrected q value <0.5.
DISCUSSION
The described cryostage for soft tissue micro-CT imaging provides a comprehensive workflow optimized for imaging soft tissue structures with high contrast and resolution while preserving RNA and protein for further investigation. This strategy differs substantially from conventional micro-CT imaging protocols which require tissue fixation to prevent motion artifacts and staining with heavy metals to enable high contrast between tissue types. The presented cryostage was easily integrated with the Nikon HMX-225 micro-CT scanner and could be used in micro-CT scanners from other manufacturers that use upright sample holders. We demonstrate that the cold stage can maintain soft tissue samples frozen at a stable temperature of −30°C for 1 h to complete the micro-CT scanning protocol. However, longer scans of up to 8 h could be conducted as we tested continuous use of the stage until buildup of frost around the crucible was noticeable.
Previously developed cryogenic stages, either custom made (7, 10–12) or commercially available (Deben UK, Suffolk, United Kingdom, or Bruker, Kontich, Belgium), have focused on scanning solid tissue samples such as bone, tumors, and ice. In contrast, air-inflated lung tissue samples have a large air-to-tissue ratio, which means they are prone to thawing due to the rapid heat transfer between the tissue and the surrounding air temperature. Therefore producing a cryogenic stage capable of maintaining a stable temperature for extended periods of time was an important design component of the discussed stage. In addition, currently available commercial cryostages are expensive and do not have the volume capacity to hold the size of lung tissue samples required for our studies. Hence the custom cryostage proposed in this manuscript was designed.
The developed cryostage and micro-CT imaging protocol enabled scanning of frozen lung tissue samples in 1 h at a resolution of 11 μm. To achieve the same high contrast-to-noise ratio and resolution using a fixed and dried lung tissue sample, a 3-h micro-CT imaging protocol is required, which is a substantial time and cost disadvantage compared with scanning frozen samples. Here we demonstrate that previously developed algorithms for segmenting lung parenchymal structures can be applied to the micro-CT images of frozen lung samples. Using such segmentation tools, we were able, for the first time, to visualize the 3D complexity of the human lung parenchyma by separating the small airways, acinar structures, and pulmonary vasculature from each other. Thus the imaging of frozen lung tissue samples using the cryostage presented here provides the methodology to quantify acinar structures which could be used to assess tissue pathology in lung diseases such as chronic obstructive pulmonary disease.
High-resolution images of frozen tissue samples can be utilized to detect regions of interest, such as disease lesions. To further analyze cellular and extracellular matrix composition, histological preparation is necessary. Unlike tissue samples of muscle, brain, or solid organs, lung tissues are difficult to preserve in their natural inflated state because of their inherent elastic properties. The lung needs to be inflated with positive pressure while being frozen to preserve its in vivo inflated shape. Cutting sections from a frozen specimen such as the lung is only possible if samples are embedded in a support medium such as optimum cutting temperature (OCT) cryomatrix. Our group has shown that it is possible to infiltrate air-filled lung tissue cores with OCT; however, such a procedure requires defrosting the tissue to near melting point, which does affect the lung structure. In general, the processes of fixation and embedding are known to affect the structure of tissue samples, especially the lung. As shown in Fig. 3, B and C, the lower right corner of the histological sections is missing a small area of tissue following tissue fixation and processing, compared with the intact tissue imaged by micro-CT (Fig. 3A). Furthermore, uneven warping of formalin-fixed samples during paraffin embedding, caused by the exposure to hot paraffin and cooling during solidification, can prevent perfect registration of micro-CT and histological images. Here we demonstrate that structural changes such as shrinkage induced by tissue processing can be determined when micro-CT images of the frozen sample are matched with histological sections, enabling application of correction factors for the accurate measurement of cellular and matrix composition in tissues. One limitation of our protocol is that when scanning objects at the resolution limit of a scanner, the partial volume effects can influence structural measurements. Hence absolute measurement of thin structures such as septal wall thickness scanned at a voxel size of (11 µm)3 will result in thicker measurements which should be taken into consideration.
Fixatives such as glutaraldehyde and contrast agents such as heavy metals prevent staining of tissues with antibodies. Here we demonstrate that following micro-CT scanning of frozen tissue, samples can be fixed with alcohol-based formalin at −20°C and paraffin embedded for immunohistochemistry analysis of cells such as CD68+ macrophages. This protocol offers substantial advantages over existing protocols as tissue can now be assessed at the cellular level following 3D assessment of tissue morphology.
A further advantage of maintaining tissue samples frozen is that RNA is preserved. We have previously demonstrated that matching gene expression to tissue morphology in separate but adjacent samples enables identification of molecular determinants of disease (4). To more accurately study the molecular changes associated with alterations in tissue structure, it is necessary to first image the tissue and subsequently extract the RNA for gene expression analysis. Previous micro-CT imaging protocols using fixation and drying of samples prevented such molecular studies as RNA is destroyed during the tissue preparation process. Several publications have also discussed the topic of molecular alterations within a sample following long exposures to high radiation, and it has been concluded that no significant alterations occur because of the heating or microthawing caused by high X-ray energies (1, 10). Here we confirm that the micro-CT scanning protocol used in combination with the cryostage did not affect the quantity or quality of extracted mRNA and did not produce any artifactual differential expression of genes. This methodology therefore enables, for the first time, studies that can correlate gene expression changes with structural alterations assessed by micro-CT in lung tissues.
A current limitation of our cryostage is that samples larger than 25 mm in diameter and 26 mm in height cannot be scanned with the current design. However, this limitation can be overcome by using larger crucibles that fit within the size of the Peltier elements (40 × 40 mm).
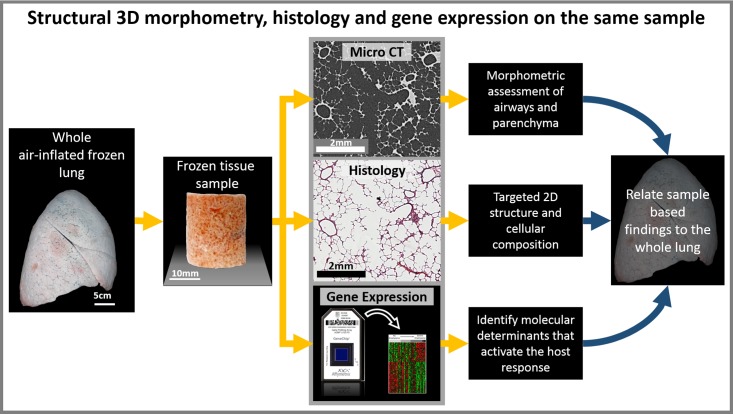
In conclusion, we have demonstrated that our novel cryostage and micro-CT imaging protocol enabled us to design a multiplex workflow (Fig. 6) that allows for comprehensive assessment of tissue morphometry, cellular composition, and gene expression within the human lung. Although not demonstrated here, the same workflow and methods can be applied to other frozen tissue samples, which opens up new investigative possibilities for integrating morphometrical and biological studies.
Fig. 6.
Whole organ investigative workflow: Multiplex study workflow for investigating whole large organs. The workflow describes how the 3D morphometry, the cellular composition by histology, and the gene expression can be studied on the same samples from a whole air inflated frozen lung. When frozen tissue samples are imaged by micro-CT using the described cryostage, the 3D morphology can then be related to histological assessment and RNA and protein analysis on the same sample. This workflow provides a method for analyzing molecular determinants of disease.
GRANTS
D. M. Vasilescu was supported in part by the following grants: British Columbia Lung Association, Alpha-1 Foundation, and IMPACT-Canadian Institutes of Health Research fellowship program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.M.V. conceived and designed research; D.M.V., A.B.P., N.T., D.K., D.F.P., G.L., H.L., N.F., and T.-L.H. performed experiments; D.M.V., N.T., D.K., J.J.K., G.L., H.L., N.F., and T.-L.H. analyzed data; D.M.V., D.K., J.J.K., N.F., A.S., and T.-L.H. interpreted results of experiments; D.M.V. and J.J.K. prepared figures; D.M.V. and T.-L.H. drafted manuscript; D.M.V., A.B.P., N.T., D.K., D.F.P., J.J.K., N.F., S.E.V., C.S.S., T.-L.H., and J.C.H. edited and revised manuscript; D.M.V., A.B.P., N.T., D.K., D.F.P., J.J.K., H.L., N.F., S.E.V., B.M.V., M.E.L., C.S.S., A.S., J.D.C., T.-L.H., and J.C.H. approved final version of manuscript.
Supplemental Data
ACKNOWLEDGMENTS
We thank Fanny Chu and Amrit Samra for their technical expertise in histological sample preparation. Furthermore, we acknowledge the help of Dr. Aaron Barlow for providing support in micro-CT scanning in the Biophysics Imaging Core at the Centre for Heart Lung Innovation. We extend special thanks to Dr. Peter Paré for his invaluable revisions. We also acknowledge Dr. Feng Xu for his help with the statistical analysis. We are grateful to Jeffrey Brundage of American Beryllia Inc., Haskell, New Jersey, for supplying the beryllium oxide crucible.
REFERENCES
- 1.Alpen EL. Modification of the radiation response. In: Radiation Biophysics (2nd ed.). San Diego: Academic, 1998, chapt. 9, p. 194–221. doi: 10.1016/B978-012053085-4/50011-9. [DOI] [Google Scholar]
- 2.Bancroft JD, Stevens A. Theory and Practice of Histological Techniques. Philadelphia, PA: Churchill Livingstone, 1996. [Google Scholar]
- 3.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics : 185–193, 2003. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 4.Campbell JD, McDonough JE, Zeskind JE, Hackett TL, Pechkovsky DV, Brandsma CA, Suzuki M, Gosselink JV, Liu G, Alekseyev YO, Xiao J, Zhang X, Hayashi S, Cooper JD, Timens W, Postma DS, Knight DA, Lenburg ME, Hogg JC, Spira A. A gene expression signature of emphysema-related lung destruction and its reversal by the tripeptide GHK. Genome Med : 67, 2012. doi: 10.1186/gm367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de S e Silva JM, Zanette I, Noël PB, Cardoso MB, Kimm MA, Pfeiffer F. Three-dimensional non-destructive soft-tissue visualization with X-ray staining micro-tomography. Sci Rep : 14088, 2015. doi: 10.1038/srep14088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haberthür D, Barré SF, Tschanz SA, Yao E, Stampanoni M, Schittny JC. Visualization and stereological characterization of individual rat lung acini by high-resolution X-ray tomographic microscopy. J Appl Physiol (1985) : 1379–1387, 2013. doi: 10.1152/japplphysiol.00642.2013. [DOI] [PubMed] [Google Scholar]
- 7.Hullar T, Paige DF, Rowland DJ, Anastasio C. Compact cold stage for micro-computerized tomography imaging of chilled or frozen samples. Rev Sci Instrum : 043708, 2014. doi: 10.1063/1.4871473. [DOI] [PubMed] [Google Scholar]
- 8.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res : e15, 2003. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics : 249–264, 2003. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 10.Jorgensen SM, Blank B, Ritman EL. Cryostatic micro-CT imaging of transient processes. Proc. SPIE Int Soc Opt Eng : 140–145, 2002. doi: 10.1117/12.452837. [DOI] [Google Scholar]
- 11.Kampschulte M, Erdmann G, Sender J, Martels G, Böcker W, ElKhassawna T, Heiß C, Langheinrich AC, Roeb E, Roderfeld M, Krombach GA. The development and validation of micro-CT of large deep frozen specimens. Scanning : 63–72, 2015. doi: 10.1002/sca.21180. [DOI] [PubMed] [Google Scholar]
- 12.Kantor B, Jorgensen SM, Lund PE, Chmelik MS, Reyes DA, Ritman EL. Cryostatic micro-computed tomography imaging of arterial wall perfusion. Scanning : 186–190, 2002. doi: 10.1002/sca.4950240405. [DOI] [PubMed] [Google Scholar]
- 13.Litzlbauer HD, Korbel K, Kline TL, Jorgensen SM, Eaker DR, Bohle RM, Ritman EL, Langheinrich AC. Synchrotron-based micro-CT imaging of the human lung acinus. Anat Rec (Hoboken) : 1607–1614, 2010. doi: 10.1002/ar.21161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maran A, Khosla S, Riggs BL, Zhang M, Ritman EL, Turner RT. Measurement of gene expression following cryogenic mu-CT scanning of human iliac crest biopsies. J Musculoskelet Neuronal Interact : 83–88, 2003. [PubMed] [Google Scholar]
- 15.McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, Wright AC, Gefter WB, Litzky L, Coxson HO, Paré PD, Sin DD, Pierce RA, Woods JC, McWilliams AM, Mayo JR, Lam SC, Cooper JD, Hogg JC. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med : 1567–1575, 2011. doi: 10.1056/NEJMoa1106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metscher BD. Biological applications of X-ray microtomography: imaging microanatomy, molecular expression and organismal diversity. Microsc Anal : 13–16, 2013. [Google Scholar]
- 17.Metscher BD. MicroCT for comparative morphology: simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiol : 11, 2009. doi: 10.1186/1472-6793-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metscher BD. MicroCT for developmental biology: a versatile tool for high-contrast 3D imaging at histological resolutions. Dev Dyn : 632–640, 2009. doi: 10.1002/dvdy.21857. [DOI] [PubMed] [Google Scholar]
- 19.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res : e47, 2015. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmid B, Schindelin J, Cardona A, Longair M, Heisenberg M. A high-level 3D visualization API for Java and ImageJ. BMC Bioinformatics : 274, 2010. doi: 10.1186/1471-2105-11-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith DB, Bernhardt G, Raine NE, Abel RL, Sykes D, Ahmed F, Pedroso I, Gill RJ. Exploring miniature insect brains using micro-CT scanning techniques. Sci Rep : 21768, 2016. doi: 10.1038/srep21768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang R, Buckley JM, Fernandez L, Coopey S, Aftreth O, Michaelson J, Saksena M, Lei L, Specht M, Gadd M, Yagi Y, Rafferty E, Brachtel E, Smith BL. Micro-computed tomography (Micro-CT): a novel approach for intraoperative breast cancer specimen imaging. Breast Cancer Res Treat : 311–316, 2013. doi: 10.1007/s10549-013-2554-6. [DOI] [PubMed] [Google Scholar]
- 23.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2014. http://www.R-project.org/
- 24.Vasilescu DM, Gao Z, Saha PK, Yin L, Wang G, Haefeli-Bleuer B, Ochs M, Weibel ER, Hoffman EA. Assessment of morphometry of pulmonary acini in mouse lungs by nondestructive imaging using multiscale microcomputed tomography. Proc Natl Acad Sci U S A : 17105–17110, 2012. doi: 10.1073/pnas.1215112109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasilescu DM, Knudsen L, Ochs M, Weibel ER, Hoffman EA. Optimized murine lung preparation for detailed structural evaluation via micro-computed tomography. J Appl Physiol 1985), : 159–166, 2012. doi: 10.1152/japplphysiol.00550.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verleden SE, Vasilescu DM, Willems S, Ruttens D, Vos R, Vandermeulen E, Hostens J, McDonough JE, Verbeken EK, Verschakelen J, Van Raemdonck DE, Rondelet B, Knoop C, Decramer M, Cooper J, Hogg JC, Verleden GM, Vanaudenaerde BM. The site and nature of airway obstruction after lung transplantation. Am J Respir Crit Care Med : 292–300, 2014. doi: 10.1164/rccm.201310-1894OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.