Abstract
Vascular aging, characterized by endothelial dysfunction and large elastic arterial stiffening, is a major risk factor for age-associated cardiovascular disease (CVD). Although women have a lower prevalence of CVD until midlife, prevalence rates increase rapidly coincident with the menopausal transition to match those observed in men. The menopausal transition, or perimenopause, is a chaotic period that is associated with increased symptoms (e.g., hot flashes, depressed mood, anxiety, sleep disturbances) and CVD risk factors due to changes in the hormonal environment. Because these quality of life factors and CVD risk factors also change with aging, the arteries of women appear to endure a double insult. Our laboratory has been investigating how changes in gonadal function and hormone levels with the menopause transition impacts the vascular aging process in healthy women. Our work has shown that vascular endothelial function progressively declines, and large elastic arterial stiffness is greater across the stages of the menopausal transition. This acceleration in vascular aging may be due to the loss of vasodilatory, antioxidant, anti-inflammatory, and antiproliferative effects of estradiol on the vascular wall. This minireview discusses the impact of changes in gonadal function and hormones with the menopausal transition on vascular aging in women and areas for investigations to further our understanding of the intersection between gonadal function and vascular aging.
Keywords: aging, sex hormones, vascular biology, women
INTRODUCTION
Despite declining mortality rates, cardiovascular disease (CVD) is still the leading cause of death in women, accounting for more deaths than cancer, chronic lower respiratory disease, and diabetes combined (6). Aging is the number one risk factor for CVD, and although prevalence is lower in women than in men until the sixth decade, the rate of increase in prevalence is greater in women from the second to the sixth decade (6). The reason for this disparity is unclear but could be related to accelerated vascular aging due to hormonal changes with the menopause transition. Vascular aging, featuring endothelial dysfunction and large artery stiffening, is a major risk factor for age-associated CVD (19). Sex differences in the trajectory of vascular aging are observed, suggesting influences of gonadal hormones.
Menopause is often considered a single event that precipitates estrogen deficiency, and as such, much focus has been on the impact of menopause on vascular health. However, menopause is a process occurring over several years that typically begins in the mid to late 40s, intersecting with aging. The menopausal transition, or perimenopause, is associated with profound changes in gonadal hormones and symptoms, including hot flashes, sleep disturbances, depression, increased anxiety, cognitive difficulties, and reduced quality of life. Evidence is accumulating that the aging vasculature may be more vulnerable during the perimenopausal transition, specifically, the late perimenopausal stage, where menopausal symptoms and CVD risk factors are increasing (15, 29, 37). Thus, a better understanding of the complex interplay between aging and changes in the hormonal environment during this chaotic period and the mechanisms underlying vascular aging are needed.
Our laboratory is interested in understanding how changes in gonadal function and hormone levels affect biological processes within the vasculature in women and how the effects of changing gonadal function interact with aging. In this minireview I provide a brief overview of our work and others' on the influence of gonadal function on vascular aging in women, including underlying mechanisms and areas needed for future investigation.
GONADAL CHANGES ACROSS THE MENOPAUSAL TRANSITION
In 2001, the Stages of Reproductive Aging Workshop (STRAW) published nomenclature and a staging system for ovarian aging across the adult life span in healthy women (41). Ovarian aging was classified into three phases: reproductive, menopausal transition, and postmenopause that were subdivided into seven stages. The reproductive phase was divided into early, peak and late, with the last stage characterized by regular menstrual cycles but with increasing follicle-stimulating hormone (FSH) levels. The menopausal transition phase was divided into early (menstrual cycle changes of ≥7 days) and late (≥2 mo of amenorrhea) perimenopause, and the postmenopause phase was divided into early and late stages, all characterized by a persistent elevation in FSH levels.
The STRAW staging system was updated in 2012 (STRAW + 10) with revised criteria for the onset of late reproductive life, early menopausal transition, and postmenopause staging, and includes other endocrine markers of ovarian aging and ovarian reserve [i.e., antimullerian hormone (AMH), inhibin B] (14). The late reproductive stage is subdivided into −3b/−3a with regular menstrual cycles, normal FSH, AMH and inhibin B (−3b), followed by subtle changes in menstrual cycle flow length, variable early follicular FSH, and low AMH and inhibin B (−3b). The early perimenopause stage is characterized by variable cycles (similar criteria as STRAW) and FSH levels and low AMH, and generally starts around the age of 47 years. The late perimenopause stage is considered the “speed bump” of the menopausal transition and occurs around the age of 49 years (36). This stage is distinguished by extreme fluctuations of hormone levels, increased anovulation (cycles ≥60 days apart), symptoms (e.g., hot flashes), and FSH levels (>25 IU/l) and decreased AMH and inhibin B. The early postmenopause period is now divided into three substages (+1a, +1b, +1c), where +1a and +1b each last about one year, and +1c marks the end of a 12-mo period of amenorrhea and the end of perimenopause. During these two years, FSH continues to rise, progesterone is no longer produced, and estradiol continues to decrease. The remainder of the early postmenopausal period lasts around three to six years, and FSH and estradiol levels stabilize. Finally, during the late postmenopause period (Stage +2; >6 yr postmenopause) changes in endocrine function are limited. Note that the hormone changes discussed above may not be applicable to women of various body composition, lifestyle, and health status, and the trajectory of hormone changes may differ accordingly.
GONADAL HORMONE CHANGES AND VASCULAR AGING
Vascular Endothelial Dysfunction
The vascular endothelium is a single cell layer that lines the arterial wall and synthesizes and releases a variety of pleiotropic molecules that influence the function and structure of arteries and other tissues, including heart, skeletal muscle, bone, and brain. An important molecule released from the vascular endothelium is nitric oxide (NO), a potent vasodilator with anti-inflammatory, antiproliferative, and anticoagulative properties. A measureable characteristic of endothelial dysfunction is impaired endothelial-dependent vasodilation, due to reduced NO bioavailability. Macrovascular endothelial function is commonly assessed via brachial artery flow-mediated dilation (FMD), a noninvasive ultrasound procedure. Microvascular endothelial function can be assessed pharmacologically by administering endothelial-dependent agents (e.g., acetylcholine) into the brachial artery and measuring the forearm blood flow response. Both procedures evoke the activation of endothelial nitric oxide synthase (eNOS), causing NO to be synthesized and released to the medial layer of the arterial wall, where it produces cyclic guanosine monophosphate (cGMP) from guanylate cyclase, causing vascular smooth muscle cell dilation. It is important to note that other vasoactive substances are released during brachial artery FMD, including vasodilators (i.e., prostacyclin and endothelium-derived hyperpolarizing factor) and vasoconstrictors (i.e., endothelin-1 and norepinephrine), and as such, it has been suggested that the total FMD response is likely explained by an interplay of vasodilator and vasoconstrictor stimuli (12).
Aging is associated with a progressive decline in both macro- and microvascular endothelial function (7, 46). In women, vascular aging also appears to be modulated by the menopause transition and declines in gonadal hormones, specifically estradiol. The first evidence to suggest that menopause influences age-associated macro- and microvascular endothelial function showed that the age-related decline in endothelial-dependent vasodilation is attenuated in premenopausal women compared with age-matched men but rapidly declines in postmenopausal women such that no sex difference is observed after the sixth decade of life (7, 46). Our work investigating how hormonal fluctuations during the perimenopausal years interacts with aging and contributes to endothelial dysfunction expanded on these studies. We found that, although endothelial function (measured via brachial artery FMD) was impaired in early perimenopausal compared with premenopausal women, the level of impairment in late perimenopausal women (~34%) was twice that of their age-matched early perimenopausal peers (~17%) (29). A lower brachial artery FMD was strongly correlated with reduced estradiol and higher FSH levels. Because menopausal stage is highly correlated with age, we compared brachial artery FMD across early and late perimenopausal and postmenopausal women aged 50–59 years. FMD was lower in late perimenopausal and postmenopausal women compared with early perimenopausal women (29). Importantly, FMD in the early perimenopausal women was healthy, indeed higher than that typically reported in young men (39). Collectively, these data support the idea that changes in gonadal function and hormone levels across the menopause transition, particularly during late perimenopause, contribute to age-associated endothelial dysfunction in women (Fig. 1).
Fig. 1.
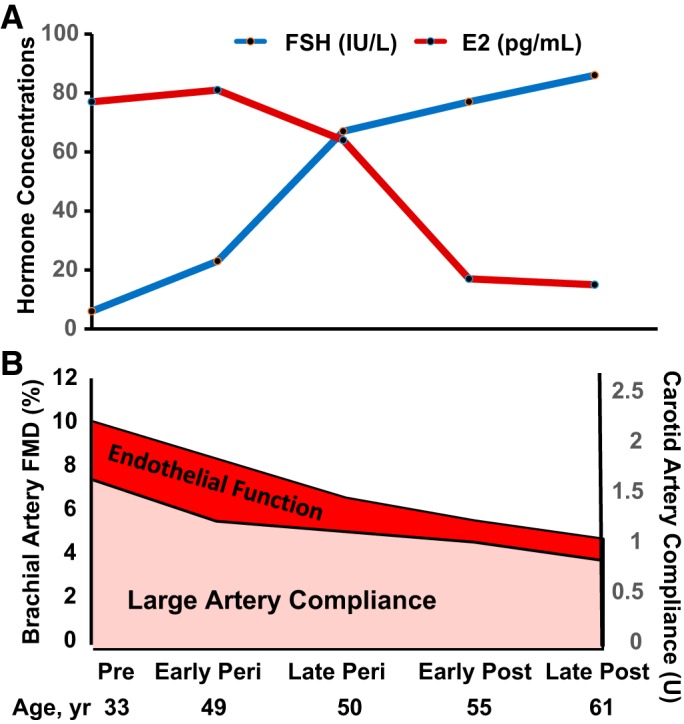
Gonadal function and vascular aging across the stages of the menopausal transition. The decline in endothelial function [brachial artery flow-mediated dilation (FMD), bottom] accelerates in late perimenopausal women, coincident with declines in mean estradiol (E2) and elevated follicle-stimulating hormone (FSH) concentrations (top) (29). The decline in large elastic artery compliance (carotid artery compliance) is mediated largely by aging; however, changes in gonadal function likely contibute to this process (15). Pre, premenopausal; Peri, early- and late-perimenopausal, Post, postmenopausal.
Arterial Stiffness
Large elastic arteries in the cardiothoracic circulation (i.e., aorta and carotid arteries) expand and recoil with cardiac ejection and relaxation and act to “buffer” the rise in systolic pressure by storing a portion of the ejected stroke volume during systole while maintaining a continuous and steady blood flow across capillary beds. Large elastic artery compliance (inverse of stiffness) is primarily determined by the intrinsic elastic properties of the artery, including the endothelium, smooth muscle cells, elastin, collagen, and other extracellular matrix proteins. Changes within these structural elements or the functioning of these components can result in a reduced “buffering capacity” and lead to increases in systolic blood pressure and aortic impedance, left ventricular (LV) hypertrophy, and a decline in LV diastolic function, leading to the development of cardiovascular disorders (19). Large elastic arterial stiffness is commonly measured by aortic pulse wave velocity (PWV), the speed the pulse wave travels over a given distance (typically carotid to femoral artery), and by measuring the compliance (inverse of stiffness) of the carotid artery using ultrasound.
Aortic PWV increases and carotid artery compliance is reduced with advancing age even in the absence of clinical CVD (4, 15, 27, 48, 50). Some evidence suggests that in women the age-associated increased arterial stiffening is more rapid after menopause (43, 53, 55), although this is not a universal finding (4, 34, 50). The discordant findings may be due to differences in methodologies to assess arterial stiffness, failure to adjust for sex differences in body/vessel size or CVD risk factors, or not considering women’s menopausal status.
We (15) showed a progressive reduction in carotid artery compliance across the stages of the menopausal transition. The effect of menopause stage was still present after adjusting for CVD risk factors including blood pressure, lipids, and adiposity. Similar to endothelial function, larger rates of decline in carotid artery compliance from premenopausal levels were observed in late perimenopausal compared with age-matched early perimenopausal women. In contrast, PWV was not different across menopausal transition stages after adjusting for age (34). However, as mentioned above, age and menopausal stage are highly correlated; thus, it is difficult to uncouple the tight association between the two. It may be that aging has a larger impact on large artery stiffening that is accelerated by changing gonadal function and hormones with the menopause transition. In this regard, we (27) and others (33, 38) reported that age-associated reductions in arterial compliance are attenuated in postmenopausal women on chronic estrogen-based hormone therapy (HT) with or without progestins compared with non-HT users.
EXPERIMENTAL MODELS TO DISTINGUISH THE EFFECTS OF GONADAL HORMONE DEFICIENCY FROM AGING ON VASCULAR FUNCTION
Although cross-sectional studies have provided strong evidence for the impact of the menopausal transition and changes in gonadal hormones on vascular aging, there are important limitations to cross-sectional study designs that warrant discussion. For example, cross-sectional designs preclude any conclusions about causality and conclusions. It is possible that genetic and other constitutional factors may have influenced vascular aging, and because the studies from our work included only healthy women without overt disease, these findings can be generalized only to these populations. Additionally, studies that compared chronic HT users to nonusers could introduce a health bias effect, because women who use HT are often thought to be healthier and more educated than nonusers. Finally, because menopause is a function of gonadal aging, the effects of changes in gonadal function and hormone levels on vascular function cannot be completely discerned from aging in cross-sectional study designs.
To overcome limitations of cross-sectional study designs, experimental intervention approaches, including sex hormone withdrawal and add-back, have been used to determine sex hormone effects on vascular function in women. We (30, 32), and others (30, 40, 45) have shown that acute and chronic estradiol treatment improves endothelial function and decreases large artery stiffening in postmenopausal women, although not to premenopausal levels. In contrast, progesterone treatment, particularly medroxyprogesterone acetate (MPA), may attenuate estradiol effects (23, 24). The effects of testosterone on vascular function in women are unclear; declines in testosterone may contribute to endothelial dysfunction in postmenopausal women (25), but testosterone may also be proatherogenic in women (22, 29).
Because many physiological changes (e.g., body composition, blood pressure) occur with more prolonged sex hormone deficiency that could exaggerate or even mitigate the effects of gonadal hormones on vascular function, we have employed acute, reversible, short-term gonadal suppression interventions [e.g., gonadotropin-releasing hormone antagonist (GnRHant)] to distinguish the independent effects of sex hormone deficiency from other factors (e.g., increased adiposity and blood pressure) that influence vascular function during aging and/or more chronic sex hormone withdrawal. As highlighted in a review article by Stachenfeld and Taylor (42), gonadal suppression models provide the most controlled environment to permit causal inferences about gonadal hormone effects on physiological function. Others using this model showed greater brachial artery FMD in premenopausal women treated with a GnRHant with estradiol add-back compared with GnRHant alone or with estradiol plus MPA or micronized progesterone add-back (23, 24). Moreover, we found that GnRHant decreased FMD in premenopausal women to perimenopausal levels, and in perimenopausal women to postmenopausal levels, whereas no change was observed in postmenopausal women (unpublished data). Collectively, these data support the idea that changing gonadal function and estradiol levels may initiate endothelial dysfunction during the perimenopausal years. However, studying vascular function after short-term manipulations of sex hormone concentrations may not reflect changes seen with chronic declines in estradiol with the menopause transition, nor does it enable the separation of the long-term effect of menopause from those of aging. Effectively separating these effects would require a long-term intervention study, following women through the menopause transition and well into menopause.
MECHANISMS UNDERLYING VASCULAR AGING ACROSS THE MENOPAUSAL TRANSITION: OXIDATIVE STRESS AND INFLAMMATION
Although the mechanisms underlying age-associated vascular aging have been intensively studied, there is a paucity of research into how changes in gonadal function and hormones with the menopausal transition contribute to vascular aging in women. In general, oxidative stress and inflammation have been identified as key mechanisms underlying age-associated endothelial dysfunction and large elastic arterial stiffening (39, 54). Evidence suggests that the menopause transition is associated with a prooxidant, proinflammatory phenotype due to a shift in redox balance and inflammatory cytokines in the late perimenopausal to early postmenopausal period, presumably related to the loss of antioxidant and anti-inflammatory properties of estradiol (Fig. 2) (1, 5, 15, 47). Other factors that have been reported to be associated with vascular aging (e.g., increased sympathetic nervous system activity, endothelin-1, and angiotensin II) likely contribute to vascular aging in women. Below, the mechanism by which oxidative stress and inflammation contribute to vascular aging in women is discussed in more detail.
Fig. 2.
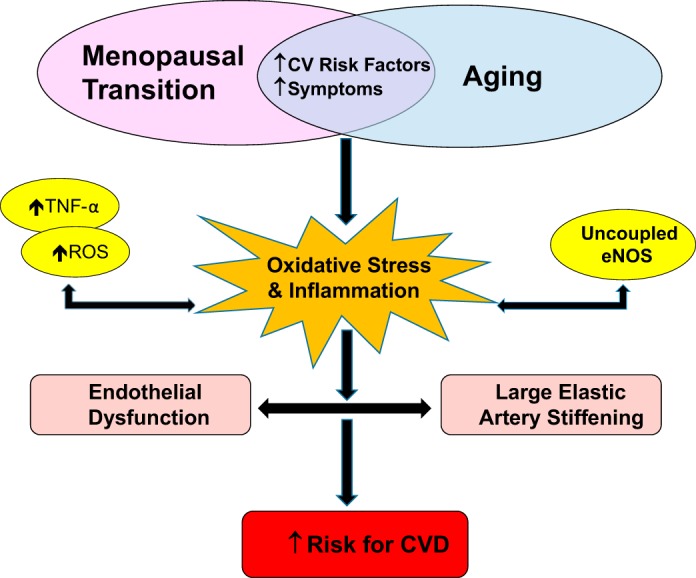
Schematic of vascular aging across the menopausal transition in women. Vascular aging, including vascular endothelial dysfunction and large elastic artery stiffening, increases the risk for age-associated cardiovascular disease (CVD) in that it combines with other known risk factors (e.g., increased adiposity, cholesterol, depression). In women, arteries are also exposed to increased risk factors (i.e., increased adiposity, cholesterol, depression) during the menopause transition when they are vulnerable to damage mediated by changes in the hormonal environment. Changes in the hormonal milieu during the menopause transition may be associated with a prooxidant, proinflammatory phenotype due to a shift in redox balance and inflammatory cytokines in the late perimenopausal to early postmenopausal period. Excessive reactive oxygen species (ROS) and inflammatory cytokines impair endothelial function and increases arterial stiffness by scavenging nitric oxide (NO) and decreasing its biosynthesis by oxidizing endothelial nitric oxide synthase (eNOS) and its cofactors [i.e., tetrahydrobioptern (BH4)], resulting in uncoupled eNOS, which produces more ROS. ROS and inflammatory cytokines also contribute to arterial stiffening by altering arterial wall structure, including intimal thickening, elastin fragmentation, collagen and extracellular matrix deposition. TNFα, tumor necrosis factor-α.
Oxidative Stress
Oxidative stress represents the imbalance between the production and destruction of reactive oxygen species (ROS). Excessive ROS impairs endothelial function and increases arterial stiffness by scavenging NO and decreasing its biosynthesis by oxidizing eNOS and its cofactors [i.e., tetrahydrobioptern (BH4)], resulting in uncoupled eNOS, which produces more ROS (18). ROS also increases vasoconstrictors (e.g., endothelin-1, angiotensin II) and modulates sympathetic α-adrenergic vasoconstrictor tone (13, 16), resulting in increased vascular smooth muscle cell vasoconstrictor tone, consequently increasing arterial stiffness. ROS also alters arterial wall structure, including intimal thickening, elastin fragmentation, collagen and extracellular matrix deposition, and formation of advanced glycation end products (10, 20, 49).
Preclinical models of menopause show elevated ROS and reduced endothelial-dependent vasodilation in ovariectomized compared with intact and ovariectomized animals treated with estradiol (17, 44). In human studies, infusion of the antioxidant vitamin C improved brachial artery FMD and carotid artery compliance in estrogen-deficient postmenopausal women and reversed oophorectomy-induced microvascular endothelial dysfunction in premenopausal women with leiomyoma (15, 28, 32, 51). Vitamin C had no effect on vascular function in premenopausal controls (15, 28, 32, 51) or in oophorectomized women in whom microvascular endothelial function was restored after 3 mo of estradiol treatment (51). We (15) demonstrated that the oxidative stress appears to develop during the late perimenopausal period and worsens during the postmenopausal years. Infusion of vitamin C improved brachial artery FMD (unpublished data) and carotid artery compliance in late perimenopausal and postmenopausal women (15) but had no effect in premenopausal and early perimenopausal women, possibly because circulating estradiol was adequate to protect against oxidative damage. Collectively, these data support the idea that changing gonadal function and hormones with the menopause transition results in oxidative stress that tonically suppresses vascular function, presumably due to the loss of the antioxidant properties of estradiol.
Inflammation
Vascular inflammation is a potent mediator of endothelial dysfunction and large elastic arterial stiffening and works collaboratively with oxidative stress (35, 52). Proinflammatory cytokines, including tumor necrosis factor-α (TNFα) activates the nuclear factor (NF)-κB signaling pathway, resulting in a downregulation and inactivation of eNOS (57) and upregulation of other inflammatory cytokines (e.g., inducible NOS), vasoconstrictors (e.g., endothelin-1, angiotensin II, sympathetic nervous system) and ROS (8, 9, 21). Estradiol antagonizes the proinflammatory effects of TNFα via estrogen receptor (ER)α (56) and inhibits inflammation and the release of ROS (e.g., NADPH oxidase) through controlling NF-κB signaling (11). These data suggest that changes in gonadal function and hormones with the menopause transition promote a proinflammatory phenotype.
Preclinical studies show that the endothelial dysfunction in ovariectomized rats is associated with higher serum TNFα, greater vascular NADPH oxidase, and reduced vascular eNOS levels (2, 3). Serum TNFα was lower in ovariectomized rats treated with either estradiol or the TNFα blocker etanercept (2, 3). Vascular NADPH oxidase was lower and eNOS and enhanced endothelial-dependent vasodilation was greater in ovariectomized rats treated with etanercept (2). Consistent with this, we (26) showed improvements in brachial artery FMD and carotid artery compliance in estrogen-deficient postmenopausal women treated with either acute transdermal estradiol, or acute etanercept. Conversely, in the estradiol-treated women and in premenopausal controls, acute etanercept had no effect on vascular function. Collectively, these observations suggest that estrogen deficiency with menopause results in the development of vascular inflammation that contributes to age-associated endothelial dysfunction and large elastic artery stiffening in women. Whether inflammation contributes to vascular aging during perimenopause is unclear.
CONCLUSIONS AND FUTURE AREAS OF STUDY
CVD remains a major health problem in women, with vascular aging, including endothelial dysfunction and large elastic arterial stiffening, as critical etiological factors. Changes in gonadal function and the hormonal milieu with the menopause transition may be an added insult to the vascular aging process in women. The phenotypic shift from antioxidative/anti-inflammatory to prooxidant/proinflammatory observed during late perimenopause and postmenopause, coincident with declines in estradiol, increases the susceptibility of middle-aged and older arteries to oxidative and inflammatory damage. Future research should investigate the underlying sources of ROS and inflammation, and the specific timing of their accumulation to determine whether interventions with antioxidants and anti-inflammatory agents can target these sources before they reach toxic levels that cause vascular damage. Additionally, research is needed on the biological effects of changes in other hormones on vascular aging, including testosterone, progesterone, and FSH and/or sex hormone ratios. Investigations are also needed into the influence of “life” factors associated with increased CVD risk that could alter the trajectory of vascular aging, such as complicated pregnancies, cancer survivorship, trauma, and posttraumatic stress disorder.
With current life expectancies, women will spend one-third of their life span in the postmenopausal period. It is crucial that we continue to increase our knowledge and understanding of the impact of the changing hormonal environment during the menopause transition on the vulnerability of the aging vasculature to the initiation and progression of CVD to guide therapeutic strategies targeting the vasculature in aging women. Furthermore, as highlighted in a recent review (31), there is increasing evidence that lifestyle interventions, including regular exercise, may not be as effective at attenuating or reversing the effects of vascular aging in estrogen-deficient postmenopausal women as it does in older men. As such, it will be important to understand how the changing hormone environment during the menopause transition impacts the ability of the aging artery to respond to therapeutic interventions in women.
GRANTS
This work was supported by the National Institutes of Health awards AG-027678, AG-049762, R56 HL-114073, AG-20683, P30 DK-048520, and UL1 TR-001082, University of Colorado Denver (UCD) Center for Women’s Health Research, and Eastern Colorado Veterans Administration GRECC.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
K.L.M. conceived and designed research; K.L.M. prepared figures; K.L.M. drafted manuscript; K.L.M. edited and revised manuscript; K.L.M. approved final version of manuscript.
ACKNOWLEDGMENTS
I thank my collaborators and colleagues within the IMAGE (Investigators in Metabolism, Aging, Gender and Exercise) Research Group for their support.
REFERENCES
- 1.Abu-Taha M, Rius C, Hermenegildo C, Noguera I, Cerda-Nicolas J-M, Issekutz AC, Jose PJ, Cortijo J, Morcillo EJ, Sanz M-J. Menopause and ovariectomy cause a low grade of systemic inflammation that may be prevented by chronic treatment with low doses of estrogen or losartan. J Immunol 183: 1393–1402, 2009. doi: 10.4049/jimmunol.0803157. [DOI] [PubMed] [Google Scholar]
- 2.Arenas IA, Armstrong SJ, Xu Y, Davidge ST. Chronic tumor necrosis factor-alpha inhibition enhances NO modulation of vascular function in estrogen-deficient rats. Hypertension 46: 76–81, 2005. doi: 10.1161/01.HYP.0000168925.98963.ef. [DOI] [PubMed] [Google Scholar]
- 3.Arenas IA, Armstrong SJ, Xu Y, Davidge ST. Tumor necrosis factor-alpha and vascular angiotensin II in estrogen-deficient rats. Hypertension 48: 497–503, 2006. doi: 10.1161/01.HYP.0000235865.03528.f1. [DOI] [PubMed] [Google Scholar]
- 4.Avolio AP, Deng FQ, Li WQ, Luo YF, Huang ZD, Xing LF, O’Rourke MF. Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation 71: 202–210, 1985. doi: 10.1161/01.CIR.71.2.202. [DOI] [PubMed] [Google Scholar]
- 5.Bechlioulis A, Naka KK, Kalantaridou SN, Kaponis A, Papanikolaou O, Vezyraki P, Kolettis TM, Vlahos AP, Gartzonika K, Mavridis A, Michalis LK. Increased vascular inflammation in early menopausal women is associated with hot flush severity. J Clin Endocrinol Metab 97: E760–E764, 2012. doi: 10.1210/jc.2011-3151. [DOI] [PubMed] [Google Scholar]
- 6.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 135: e146–e603, 2017. [Erratum in Circulation 135: e646, 2017.] doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 8.Clapp BR, Hingorani AD, Kharbanda RK, Mohamed-Ali V, Stephens JW, Vallance P, MacAllister RJ. Inflammation-induced endothelial dysfunction involves reduced nitric oxide bioavailability and increased oxidant stress. Cardiovasc Res 64: 172–178, 2004. doi: 10.1016/j.cardiores.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-tumor necrosis factor-alpha treatment in aging. Am J Pathol 170: 388–398, 2007. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleenor BS, Sindler AL, Eng JS, Nair DP, Dodson RB, Seals DR. Sodium nitrite de-stiffening of large elastic arteries with aging: role of normalization of advanced glycation end-products. Exp Gerontol 47: 588–594, 2012. doi: 10.1016/j.exger.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghisletti S, Meda C, Maggi A, Vegeto E. 17Beta-estradiol inhibits inflammatory gene expression by controlling NF-kappaB intracellular localization. Mol Cell Biol 25: 2957–2968, 2005. doi: 10.1128/MCB.25.8.2957-2968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green DJ, Dawson EA, Groenewoud HMM, Jones H, Thijssen DHJ. Is flow-mediated dilation nitric oxide mediated? A meta-analysis. Hypertension 63: 376–382, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02044. [DOI] [PubMed] [Google Scholar]
- 13.Hao L, Nishimura T, Wo H, Fernandez-Patron C. Vascular responses to α1-adrenergic receptors in small rat mesenteric arteries depend on mitochondrial reactive oxygen species. Arterioscler Thromb Vasc Biol 26: 819–825, 2006. doi: 10.1161/01.ATV.0000204344.90301.7c. [DOI] [PubMed] [Google Scholar]
- 14.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ; STRAW 10 Collaborative Group . Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause 19: 387–395, 2012. doi: 10.1097/gme.0b013e31824d8f40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hildreth KL, Kohrt WM, Moreau KL. Oxidative stress contributes to large elastic arterial stiffening across the stages of the menopausal transition. Menopause 21: 624–632, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kähler J, Ewert A, Weckmüller J, Stobbe S, Mittmann C, Köster R, Paul M, Meinertz T, Münzel T. Oxidative stress increases endothelin-1 synthesis in human coronary artery smooth muscle cells. J Cardiovasc Pharmacol 38: 49–57, 2001. doi: 10.1097/00005344-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Keaney JF Jr, Shwaery GT, Xu A, Nicolosi RJ, Loscalzo J, Foxall TL, Vita JA. 17 beta-estradiol preserves endothelial vasodilator function and limits low-density lipoprotein oxidation in hypercholesterolemic swine. Circulation 89: 2251–2259, 1994. doi: 10.1161/01.CIR.89.5.2251. [DOI] [PubMed] [Google Scholar]
- 18.Kojda G, Harrison D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res 43: 562–571, 1999. doi: 10.1016/S0008-6363(99)00169-8. [DOI] [PubMed] [Google Scholar]
- 19.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003. doi: 10.1161/01.CIR.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 20.Lalu MM, Cena J, Chowdhury R, Lam A, Schulz R. Matrix metalloproteinases contribute to endotoxin and interleukin-1beta induced vascular dysfunction. Br J Pharmacol 149: 31–42, 2006. doi: 10.1038/sj.bjp.0706823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madge LA, Pober JS. TNF signaling in vascular endothelial cells. Exp Mol Pathol 70: 317–325, 2001. doi: 10.1006/exmp.2001.2368. [DOI] [PubMed] [Google Scholar]
- 22.Maturana MA, Breda V, Lhullier F, Spritzer PM. Relationship between endogenous testosterone and cardiovascular risk in early postmenopausal women. Metabolism 57: 961–965, 2008. doi: 10.1016/j.metabol.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Meendering JR, Torgrimson BN, Miller NP, Kaplan PF, Minson CT. Estrogen, medroxyprogesterone acetate, endothelial function, and biomarkers of cardiovascular risk in young women. Am J Physiol Heart Circ Physiol 294: H1630–H1637, 2008. doi: 10.1152/ajpheart.01314.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miner JA, Martini ER, Smith MM, Brunt VE, Kaplan PF, Halliwill JR, Minson CT. Short-term oral progesterone administration antagonizes the effect of transdermal estradiol on endothelium-dependent vasodilation in young healthy women. Am J Physiol Heart Circ Physiol 301: H1716–H1722, 2011. doi: 10.1152/ajpheart.00405.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montalcini T, Gorgone G, Gazzaruso C, Sesti G, Perticone F, Pujia A. Endogenous testosterone and endothelial function in postmenopausal women. Coron Artery Dis 18: 9–13, 2007. doi: 10.1097/01.mca.0000236290.79306.d1. [DOI] [PubMed] [Google Scholar]
- 26.Moreau KL, Deane KD, Meditz AL, Kohrt WM. Tumor necrosis factor-α inhibition improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Atherosclerosis 230: 390–396, 2013. doi: 10.1016/j.atherosclerosis.2013.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreau KL, Donato AJ, Seals DR, DeSouza CA, Tanaka H. Regular exercise, hormone replacement therapy and the age-related decline in carotid arterial compliance in healthy women. Cardiovasc Res 57: 861–868, 2003. doi: 10.1016/S0008-6363(02)00777-0. [DOI] [PubMed] [Google Scholar]
- 28.Moreau KL, Gavin KM, Plum AE, Seals DR. Ascorbic acid selectively improves large elastic artery compliance in postmenopausal women. Hypertension 45: 1107–1112, 2005. doi: 10.1161/01.HYP.0000165678.63373.8c. [DOI] [PubMed] [Google Scholar]
- 29.Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab 97: 4692–4700, 2012. doi: 10.1210/jc.2012-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreau KL, Meditz A, Deane KD, Kohrt WM. Tetrahydrobiopterin improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Am J Physiol Heart Circ Physiol 302: H1211–H1218, 2012. doi: 10.1152/ajpheart.01065.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moreau KL, Ozemek C. Vascular adaptations to habitual exercise in older adults: time for the sex talk. Exerc Sport Sci Rev 45: 116–123, 2017. doi: 10.1249/JES.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreau KL, Stauffer BL, Kohrt WM, Seals DR. Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J Clin Endocrinol Metab 98: 4507–4515, 2013. doi: 10.1210/jc.2013-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagai Y, Earley CJ, Kemper MK, Bacal CS, Metter EJ. Influence of age and postmenopausal estrogen replacement therapy on carotid arterial stiffness in women. Cardiovasc Res 41: 307–311, 1999. doi: 10.1016/S0008-6363(98)00219-3. [DOI] [PubMed] [Google Scholar]
- 34.O’Neill SM, Liu J, O’Rourke MF, Khoo SK. The menopausal transition does not appear to accelerate age-related increases in arterial stiffness. Climacteric 16: 62–69, 2012. doi: 10.3109/13697137.2012.739220. [DOI] [PubMed] [Google Scholar]
- 35.Pleiner J, Mittermayer F, Schaller G, Marsik C, MacAllister RJ, Wolzt M. Inflammation-induced vasoconstrictor hyporeactivity is caused by oxidative stress. J Am Coll Cardiol 42: 1656–1662, 2003. doi: 10.1016/j.jacc.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Santoro N. Perimenopause: from research to practice. J Womens Health (Larchmt) 25: 332–339, 2016. doi: 10.1089/jwh.2015.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santoro N, Sutton-Tyrrell K. The SWAN song: Study of Women’s Health Across the Nation’s recurring themes. Obstet Gynecol Clin North Am 38: 417–423, 2011. doi: 10.1016/j.ogc.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scuteri A, Lakatta EG, Bos AJ, Fleg JL. Effect of estrogen and progestin replacement on arterial stiffness indices in postmenopausal women. Aging (Milano) 13: 122–130, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Seals DR. Edward F. Adolph Distinguished Lecture: The remarkable anti-aging effects of aerobic exercise on systemic arteries. J Appl Physiol (1985) 117: 425–439, 2014. doi: 10.1152/japplphysiol.00362.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sherwood A, Bower JK, McFetridge-Durdle J, Blumenthal JA, Newby LK, Hinderliter AL. Age moderates the short-term effects of transdermal 17beta-estradiol on endothelium-dependent vascular function in postmenopausal women. Arterioscler Thromb Vasc Biol 27: 1782–1787, 2007. doi: 10.1161/ATVBAHA.107.145383. [DOI] [PubMed] [Google Scholar]
- 41.Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, Woods N. Stages of Reproductive Aging Workshop (STRAW). J Womens Health Gend Based Med 10: 843–848, 2001. doi: 10.1089/152460901753285732. [DOI] [PubMed] [Google Scholar]
- 42.Stachenfeld NS, Taylor HS. Challenges and methodology for testing young healthy women in physiological studies. Am J Physiol Endocrinol Metab 306: E849–E853, 2014. doi: 10.1152/ajpendo.00038.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Staessen JA, van der Heijden-Spek JJ, Safar ME, Den Hond E, Gasowski J, Fagard RH, Wang JG, Boudier HA, Van Bortel LM. Menopause and the characteristics of the large arteries in a population study. J Hum Hypertens 15: 511–518, 2001. doi: 10.1038/sj.jhh.1001226. [DOI] [PubMed] [Google Scholar]
- 44.Sudoh N, Toba K, Akishita M, Ako J, Hashimoto M, Iijima K, Kim S, Liang YQ, Ohike Y, Watanabe T, Yamazaki I, Yoshizumi M, Eto M, Ouchi Y. Estrogen prevents oxidative stress-induced endothelial cell apoptosis in rats. Circulation 103: 724–729, 2001. doi: 10.1161/01.CIR.103.5.724. [DOI] [PubMed] [Google Scholar]
- 45.Sumino H, Ichikawa S, Kasama S, Takahashi T, Kumakura H, Takayama Y, Kanda T, Kurabayashi M. Different effects of oral conjugated estrogen and transdermal estradiol on arterial stiffness and vascular inflammatory markers in postmenopausal women. Atherosclerosis 189: 436–442, 2006. doi: 10.1016/j.atherosclerosis.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 46.Taddei S, Virdis A, Ghiadoni L, Mattei P, Sudano I, Bernini G, Pinto S, Salvetti A. Menopause is associated with endothelial dysfunction in women. Hypertension 28: 576–582, 1996. doi: 10.1161/01.HYP.28.4.576. [DOI] [PubMed] [Google Scholar]
- 47.Taleb-Belkadi O, Chaib H, Zemour L, Fatah A, Chafi B, Mekki K. Lipid profile, inflammation, and oxidative status in peri- and postmenopausal women. Gynecol Endocrinol 32: 982–985, 2016. doi: 10.1080/09513590.2016.1214257. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation 102: 1270–1275, 2000. doi: 10.1161/01.CIR.102.11.1270. [DOI] [PubMed] [Google Scholar]
- 49.Thakore AH, Guo CY, Larson MG, Corey D, Wang TJ, Vasan RS, D’Agostino RB Sr, Lipinska I, Keaney JF Jr, Benjamin EJ, O’Donnell CJ. Association of multiple inflammatory markers with carotid intimal medial thickness and stenosis (from the Framingham Heart Study). Am J Cardiol 99: 1598–1602, 2007. doi: 10.1016/j.amjcard.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 50.Vaitkevicius PV, Fleg JL, Engel JH, O’Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation 88: 1456–1462, 1993. doi: 10.1161/01.CIR.88.4.1456. [DOI] [PubMed] [Google Scholar]
- 51.Virdis A, Ghiadoni L, Pinto S, Lombardo M, Petraglia F, Gennazzani A, Buralli S, Taddei S, Salvetti A. Mechanisms responsible for endothelial dysfunction associated with acute estrogen deprivation in normotensive women. Circulation 101: 2258–2263, 2000. doi: 10.1161/01.CIR.101.19.2258. [DOI] [PubMed] [Google Scholar]
- 52.Vlachopoulos C, Dima I, Aznaouridis K, Vasiliadou C, Ioakeimidis N, Aggeli C, Toutouza M, Stefanadis C. Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation 112: 2193–2200, 2005. doi: 10.1161/CIRCULATIONAHA.105.535435. [DOI] [PubMed] [Google Scholar]
- 53.Waddell TK, Dart AM, Gatzka CD, Cameron JD, Kingwell BA. Women exhibit a greater age-related increase in proximal aortic stiffness than men. J Hypertens 19: 2205–2212, 2001. doi: 10.1097/00004872-200112000-00014. [DOI] [PubMed] [Google Scholar]
- 54.Wang M, Monticone RE, Lakatta EG. Proinflammation of aging central arteries: a mini-review. Gerontology 60: 519–529, 2014. doi: 10.1159/000362548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Westendorp IC, Bots ML, Grobbee DE, Reneman RS, Hoeks AP, Van Popele NM, Hofman A, Witteman JC. Menopausal status and distensibility of the common carotid artery. Arterioscler Thromb Vasc Biol 19: 713–717, 1999. doi: 10.1161/01.ATV.19.3.713. [DOI] [PubMed] [Google Scholar]
- 56.Xing D, Feng W, Miller AP, Weathington NM, Chen YF, Novak L, Blalock JE, Oparil S. Estrogen modulates TNF-alpha-induced inflammatory responses in rat aortic smooth muscle cells through estrogen receptor-beta activation. Am J Physiol Heart Circ Physiol 292: H2607–H2612, 2007. doi: 10.1152/ajpheart.01107.2006. [DOI] [PubMed] [Google Scholar]
- 57.Yoshizumi M, Perrella MA, Burnett JC Jr, Lee ME. Tumor necrosis factor downregulates an endothelial nitric oxide synthase mRNA by shortening its half-life. Circ Res 73: 205–209, 1993. doi: 10.1161/01.RES.73.1.205. [DOI] [PubMed] [Google Scholar]


