Abstract
Gd-based MRI contrast agents (GBCAs) have come under intense regulatory scrutiny due to concerns of Gd retention and delayed toxicity. Three GBCAs comprising acyclic Gd chelates, the class of GBCA most prone to Gd release, are no longer marketed in Europe. Of particular concern are the acyclic chelates that remain available for liver scans, where there is an unmet diagnostic need and no replacement technology. To address this concern, we evaluated our previously reported Mn-based MRI contrast agent, Mn-PyC3A, and nine newly synthesized derivatives as liver specific MRI contrast agents. Within this focused library the transient liver uptake and rate of blood clearance are directly correlated with logP. The complex Mn-PyC3A-3-OBn emerged as the lead candidate due to a combination of high relaxivity, rapid blood clearance, and avid hepatocellular uptake. Mn-PyC3A-3-OBn rendered liver tumors conspicuously hypo-intense in a murine model and is wholly eliminated within 24 h of injection.
INTRODUCTION
Gd-based MRI contrast agents (GBCAs) are heavily relied upon for the radiologic diagnosis of tissue and vascular abnormalities.1–9 However, regulatory agencies are currently re-assessing the risk vs. benefit of GBCAs in response to well-documented safety concerns related to long-term Gd retention.10–15 In 2017, the FDA announced a new class warning for all GBCAs and the European Medicines Agency suspended marketing authorizations for three of the five Gd-based agents comprised of acyclic chelators, which are more prone to Gd release than the class of agents comprised of macrocyclic chelators (Figure S1).15–18 However, long-term Gd retention is also documented in patients that have exclusively received macrocyclic GBCAs.19–21 EMA has allowed two acyclic agents, Gd-DTPA-EOB and Gd-BOPTA, to remain available but according to the EMA 2017 statement these agents are now restricted “to be used for liver scans because they are taken up in the liver and meet an important diagnostic need.”15 Liver-specific agents enable high-sensitivity detection of liver metastases, differential diagnosis of malignant tissue from benign abnormalities, and staging of hepatocellular lesions.22, 23 There are no liver specific macrocyclic GBCAs or iodinated computed tomography (CT) contrast agents. Differential diagnosis cannot be made using ultrasound imaging, CT, or contrast-free MRI.
The rising concerns over GBCA safety have underscored a pressing urgency to develop Gd-free MRI contrast agents. Most research to date has focused on developing extracellular agents. 24–33 Considerably less effort has been placed on developing Gd-free liver-specific agents despite the fact that liver cancer and metastatic liver disease are among the leading causes of cancer related death.34, 35 In this regard, we sought to develop a Gd-free liver-specific contrast agent. We posit that a Mn complex is the most likely GBCA alternative to succeed as a clinically viable agent. Mn2+ is one of the few ions that can generate MRI contrast that is comparable to Gd3+.36 Unlike Gd, Mn is a trace nutritional element that the human body can incorporate or excrete as required, avoiding concerns of long-term accumulation.37
A new liver-specific agent should be a direct replacement for the GBCA standard of care. The liver specificity of Gd-DTPA-EOB and Gd-BOPTA is imparted by peripheral lipophilic functional groups that promote recognition by organic anion transporting peptides (OATPs) that are abundantly expressed on the cell membranes of hepatocytes lining biliary sinusoids.38 Both liver tumors and benign abnormalities such as focal nodular hyperplasia can be avidly enhanced by contrast agents during the arterial, venous, or extracellular phases of distribution, but OATP-mediated hepatocellular uptake enables differential diagnosis during what is referred to as the delayed phase (15–60 min post injection) when the blood signal is diminished but liver enhancement is strong.22, 23 Malignancies, which are comprised of non-OATP expressing cells (ie. metastases) or OATP under-expressing cells (primary liver cancers), are rendered conspicuously hypo-intense against normal liver during the delayed phase.
A number of design criteria must be met in developing a suitable Mn complex for liver-specific imaging. We require a Mn complex that possesses relaxivity (r1 = (Δ1/T1) normalized to mM contrast agent) that is comparable or better than GBCAs. The complex must be inert to Mn release so that it can be safely delivered as a bolus injection in order to see dynamic phase tumor enhancement. The complex must also exhibit a combination of rapid blood clearance and preferential hepatocyte uptake in order to provide differential contrast enhancement between benign and malignant tissue within minutes of injection. The complex must also be rapidly eliminated as the probability of exposure to dissociated metal ions increases with dwell time.39
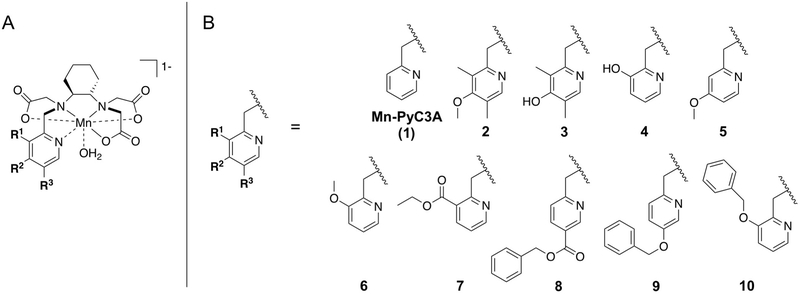
We hypothesized that a lipophilic, anionic Mn complex based on the previously reported Mn-PyC3A chelator could meet the criteria required for a successful liver-targeting agent. It has been demonstrated that Mn-PyC3A possesses relaxivity that is comparable to GBCAs and is also highly resistant to Mn release in vivo.40, 41 However, it is difficult to predict the molecular attributes needed to impart the pharmacokinetics and biodistribution requisite for a liver-targeting agent (transient hepatocellular uptake, rapid blood clearance). Liver-targeting via increased lipophilicity can promote rapid elimination from the bloodstream,42 but on the other hand increased lipophilicity can also promote greater affinity for plasma proteins which has been shown in many cases to prolong the circulatory lifetime of MRI contrast agents.43, 44 Slow blood clearance will blunt the capability of a liver targeting agent to aid in differential diagnoses, or prolong the time to delayed phase imaging. In developing a liver-specific agent, it is critical to understand the structure-activity relationships (SAR) that influence the protein binding, relaxivity, pharmacokinetics, biodistribution, and elimination. In this regard, we examined SAR for a focused library of anionic Mn complexes comprising Mn-PyC3A (referred to as 1 from here on) and nine newly synthesized derivatives of varying lipophilicity (2-10, Chart 1) in order to identify a lead candidate Mn-based contrast agent for liver imaging.
Chart 1. A focused library of ten structurally related Mn-PyC3A-derived complexes were evaluated as liver-specific MRI contrast agents.
(A) Structures of Mn-PyC3A and Mn-PyC3A-derived complexes, (B) derivatives of varying lipophilicity were prepared via modifications to the 2-methylpyridine arm of the Mn chelates.
RESULTS
Synthesis.
Complexes 2-10 were derived from Mn-PyC3A using the pyridyl-N donor group as the modular handle (Chart 1). We targeted modifications that were unlikely to negatively impact relaxivity or stability, were amenable to further modulation, and can be easily scaled up for large batch preparations. Substitutions were thus introduced at the 3, 4, and 5 positions of the N-pyridyl donor group, remote from the rapidly exchanging water co-ligand that is key for high-relaxivity.40 Pyridyl functionalization does not introduce any new chirality and the –OR or -COOR functional groups present in 2-10 are amenable to further modulation.
The 2-methylpyridine-derived donor arms of 2 and 3 were introduced to the ligand as the commercially available 2-(chloromethane)-3,5-dimethyl-5-hydroxypyridine. The 2-methane(pyridine)-derived arms of 4-10 were introduced as the corresponding 2-(bromomethane)pyridine synthons (14d-i) which were synthesized as described in Scheme 1 and Table 1. Briefly, the 2-methylpyridine derived starting material was converted to the corresponding N-oxide (12d-i) by mCPBA oxidation before heating in neat acetic anhydride to generate the corresponding pyridine-2-ylmethyl acetate derivative (13d-i). In most instances the starting 2-methylpyridine derivatives were purchased from commercial sources, but 11g-i were prepared by O-benzylation of the methyl nicotinate or the corresponding 5- and 3-hydroxy-2-methylpyridines, respectively. The acetyl-O group of 13d-i was then removed to yield pyridine-2-ylmethanol derived 14d-i either by saponification with KOH or by treatment with acetyl chloride. Compounds 14d-i were converted to the 15d-i by treatment with PBr3. 2-(chloromethane)-3,5-dimethyl-5-hydroxypyridine and 15d-i were then coupled with the common synthon, N,N’,N’-trans-1,2-cyclohexylenediaminetri-tert-butylacetate, to yield O-protected ligand 16a,d-i. Subsequent O-deprotection yielded ligands 17a-i. Complexes 2-10 were prepared by addition of 1 mol equiv. MnCl2 to an aqueous solution of ligand and then adjusted the pH to 6.5, as previously described.40
Scheme 1. Synthesis of complexes 2-10a-e.
aReagents and conditions: (i) mCBPA, CH2Cl2, RT, 16 h, (ii) Ac2O, reflux, 5-7 h, (iii) 5:1 THF: 2M NaOH, RT, 2-7 h, (iv) Acetyl chloride, MeOH, RT, 16h, (v) PBr3, CH2Cl2, RT, 5 h. (vi). N,N,N’-trans-1,2-diaminocyclohexanetri-tert-butylacetate, KI, K2CO3, MeCN, reflux, 20 h. (vii) TFA. RT, 5 h, (viii) BBr3, CH2Cl2, −78 °C to RT, 16 h, (ix) MnCl2, H2O, adjust to pH 6.5. b11g-i were prepared by benzylation of methyl nicotinate, 5- or 3-hydroxy-2-methylpyridines, respectively. c12d-f were prepared from commercially available 2-methylpyridine derivatives. d16a was prepared from 2-(chloromethane)-3,5-dimethyl-5-hydroxypyridine. eBoth 17a and 17b prepared from 16a, both 17c and 17e prepared from 16e.
Table 1.
Guide to intermediates synthesized en route to complexes 2-10.
| Complex | Corresponding intermediates denoted by: |
R1 | R2 | R3 |
|---|---|---|---|---|
| 1 | −H | −H | −H | |
| 2 | a | −CH3 | −OCH3 | −CH3 |
| 3 | b | −CH3 | −OH | −CH3 |
| 4 | c | −OH | −H | −H |
| 5 | d | −H | −OCH3 | −H |
| 6 | e | −OCH3 | −H | −H |
| 7 | f | −C(O)OEt | −H | −H |
| 8 | g | −H | −H | −C(O)OBn |
| 9 | h | −H | −H | −OBn |
| 10 | i | −OBn | −H | −H |
Physical characterization.
The relative polarities of complexes 1-10 are described by the estimated 1-octanol/ water partition co-efficient (est. log P) values determined from reverse phase HPLC measurements. HPLC retention times were cast as est. log P using a calibration curve generated from 6 reference compounds of known log P45 (Figure S2). The est. log P of 1-10 range from 0.455 to 1.15, respectively (Table 2). Estimations of plasma protein binding by ultrafiltration assay indicate that 1-10 have moderate plasma protein binding affinity ranging from Ka = 0.13 to 0.94 mM−1 (Table 2). Protein binding is strongly and positively correlated with est. log P (Figure 1A). A parallel ultrafiltration assay performed in 4.5% wt/vol human serum albumin (HSA) indicates that plasma protein binding is largely attributable to HSA, which is present in plasma at 600–700 µM and is a known binding target for lipophilic anions (Figure S12).
Table 2.
Retention time (tR, method 2), est. logP, r1 and r2 in pH 7.4 Tris buffer and human blood plasma, and protein association constants (Ka) in human blood plasma. tR corresponds to the major diastereomer of 1-10, the tR of the minor diastereomer is typically within <0.2 min of the major diastereomer (Figures S3-11). Relaxivity measurements were performed at 1.4T and 37 °C. Protein binding was estimated via ultra-filtration assay. Data reported as mean±SD.
|
tR (min) |
est. logP |
r1 pH 7.4 Tris (mM−1s−1) |
r2 pH 7.4 Tris (mM−1s−1) |
r1 plasma (mM−1s−1) |
r2 plasma (mM−1s−1) |
Ka plasma (mM−1) |
|
|---|---|---|---|---|---|---|---|
| 1 | 6.96 | 0.575 | 2.1±0.1 | 5.1±0.4 | 3.5±0.5 | 8.2±2.1 | 0.13±0.095 |
| 2 | 8.05 | 0.922 | 2.2±0.5 | 5.1±0.2 | 10.4±0.9 | 34.5±3.8 | 0.63±0.062 |
| 3 | 7.03 | 0.601 | 2.5±0.2 | 5.2±0.2 | 5.1±0.5 | 12.4±2.1 | 0.22±0.077 |
| 4 | 6.61 | 0.455 | 2.3±0.2 | 5.4±0.3 | 5.2±1.5 | 14.2±1.7 | 0.22±0.12 |
| 5 | 7.40 | 0.748 | 2.4±0.1 | 5.5±0.2 | 6.6±0.5 | 23.8±1.8 | 0.60±0.040 |
| 6 | 7.48 | 0.750 | 2.4±0.1 | 5.4±0.1 | 7.3±1.1 | 16.3±1.4 | 0.41±0.18 |
| 7 | 8.01 | 0.909 | 2.6±0.2 | 6.0±0.8 | 6.4±0.7 | 11.6±4.2 | 0.58±0.080 |
| 8 | 8.63 | 1.09 | 3.0±0.2 | 6.4±0.5 | 12.2±1.2 | 25.3±2.1 | 0.60±0.12 |
| 9 | 8.57 | 1.07 | 2.6±0.1 | 5.6±0.5 | 9.8±1.0 | 28.5±2.3 | 0.94±0.067 |
| 10 | 8.86 | 1.15 | 2.6±0.2 | 6.4±0.5 | 9.0±1.4 | 29.1±3.7 | 0.87±0.23 |
Figure 1.
Plasma protein affinity and plasma relaxivity (1.4T, 37 °C) of 1-10 are tightly correlated with estimated log P. Data reported as mean±SD, solid lines represent fits to the data. (A) Plasma protein Ka determined by ultrafiltration assay plotted as a function of est. log P (r = 0.9083, P = 0.0003), (B) Plasma r1 plotted as a function of est. log P (r = 0.8446, P = 0.0021), (C) Plasma r1 plotted as a function of Ka (r = 0.7527, P = 0.0120), (D) Plasma r2 plotted as a function of est. log P (r = 0.7243, P = 0.0178), and (E) Plasma r2 plotted as a function of Ka (r = 0.8016, P = 0.0053). The plasma relaxivity data plotted as a function of Ka confirms that the r1 and r2 dependence on est. log P is due to an increased affinity for plasma proteins.
The r1 values of complexes 1-10 range between 2.1 to 3.0 mM−1s−1 in pH 7.4 Tris buffer (1.4T, 37 °C) and are consistent with ternary complexes possessing a rapidly exchanging water co-ligand (Table 2). However, the r1 values in human blood plasma are significantly greater and range from 3.5 to 12.2 mM−1s-1. The plasma r1 values represent between a 70% and 360% increase over the values recorded in buffer. Blood plasma r1 correlates strongly with both est. log P (Figure 1B) and plasma protein binding (Figure 1C). The r1 increase observed upon protein binding arises from coupling of the rotational motions of the Mn complexes to more slowly tumbling protein macromolecules.46, 47
The r2 values range between 5.1 and 6.4 mM−1s−1 in pH 7.4 Tris buffer but can increase by 60% to 440% in blood plasma due to protein binding interactions. Consistent with the r1 data, plasma r2 is also strongly and positively correlated with est. log P (r = 0.7243, P = 0.0178) and with plasma Ka (r = 0.8016, P = 0.0053) (Figures 1D,E).
Dynamic MRI.
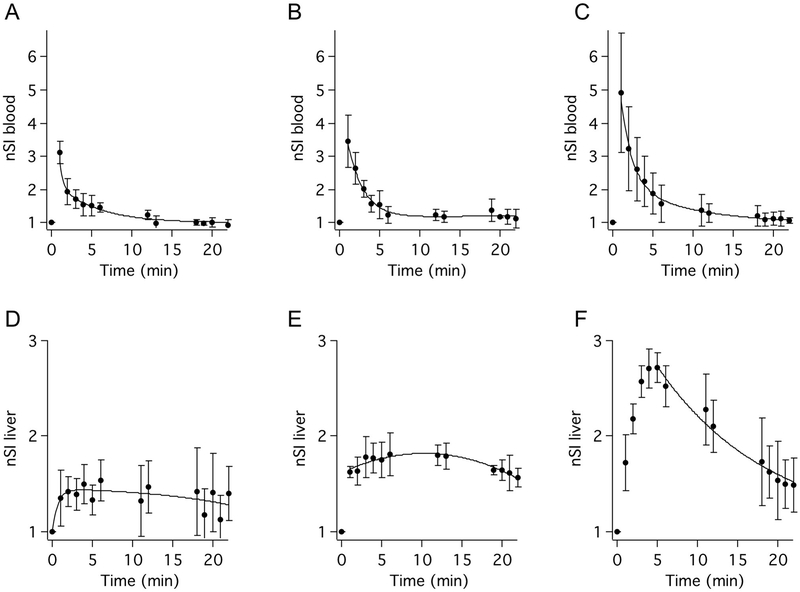
In order to study the influence of changing polarity on pharmacokinetics and biodistribution of the Mn complexes we examined the in vivo behavior of three representative complexes in mice by dynamic MRI using a 4.7T small bore imager. We chose complexes 4 (R1 = -OH, R2 = R3 = -H), 6 (R1 = -OCH3, R2 = R3 = -H), and 10 (R1 = -OBn, R2 = R3 = -H) for evaluation, which represent the complexes of lowest, intermediate, and greatest lipophilicity. Mice receiving 0.1 mmol/kg of Mn complex intravenously were scanned dynamically starting pre-injection and up to 22 min post-injection using a 3D T1-weighted fast low-angle low-shot (FLASH) sequence. Representative 3D maximum intensity projections of T1-weighted images recorded at baseline, 1 min, 5 min, 12 min, and 22 min post-injection are shown in Figure S13. To compare liver uptake between the three agents we included in the scanning protocol a 2D T1-weighted FLASH acquisition capturing the liver and adjacent psoas muscle at 7 min post injection (delayed phase), at which point blood signal is diminished but liver signal is near its peak (Figure 2).
Figure 2.
MR signal as a function of time in the blood (A-C) and liver (D-F) for three representative Mn-based contrast agents. Signal intensity normalized to pre-injection image (nSI) in the blood and liver are plotted as a function of time following injection of complex 4 (A,D), complex 6 (B,E), and complex 10 (C,F). nSI reported as mean±SD, solid lines represent best fits to the data. (N=4 for each complex).
Blood signal recorded in the vena cava normalized to pre-contrast values (nSI) plotted as a function of time reveal bi-phasic blood elimination consistent with extracellular distribution and rapid contrast agent washout (Figures 2A-C, Table 3). Mean blood nSI during the 0 to 6 min time points is greater for the more lipophilic agents. We attribute this effect to the logP dependence on plasma protein affinity, which will result in higher intravascular concentrations of the more lipophilic contrast agents during the initial distribution equilibrium time points.
Table 3.
The pharmacokinetics of complexes 4, 6, and 10 were estimated from the dynamic MRI data, N =4 each.
| logP | elimination t1/2 (min) |
AUC0-22 liver (nSI*min) |
AUC0-22 kidney (nSI*min) |
|
|---|---|---|---|---|
| 4 | 0.46 | 5.7±1.0 | 10.2±6.52 | 21.3±8.30 |
| 6 | 0.75 | 3.6±1.4* | 21.1±12.2 | 20.4±4.50 |
| 10 | 1.15 | 2.4±0.4** | 23.5±4.71 | 14.2±4.70 |
Data reported as mean±SD.
P = 0.0419 vs. complex 4,
P = 0.0030 vs. complex 4.
The elimination half-lives (t1/2) estimated from the blood signal in the dynamic scans indicates that blood half-life correlates inversely with est. log P. Complexes 4, 6, and 10 exhibit t1/2 of 5.7±1.0 min, 3.6±1.4 min, and 2.4±0.4 min, respectively, Table 3. The elimination t1/2 of 10 is significantly faster than that recorded for 4 and 6. The increasingly rapid blood clearance of the more lipophilic complexes reflects increased hepatobiliary contribution to excretion. Liver nSI of complex 10 is 105% and 55% greater than that generated by 6 and 4 at 5 min post injection (Figures 2D-F). Clearance into the bile is evidenced by an increase in gall bladder signal observed during liver washout, shown in Figures S13. This observation is consistent with liver accumulation via a hepatocellular mechanism and with hepatobiliary excretion. Quantification of liver and kidney nSI area under the curve (AUC) recorded up to 22 min post-injection is also consistent with increased fractional excretion via the hepatobiliary path for the more lipophilic agents (Table 3).
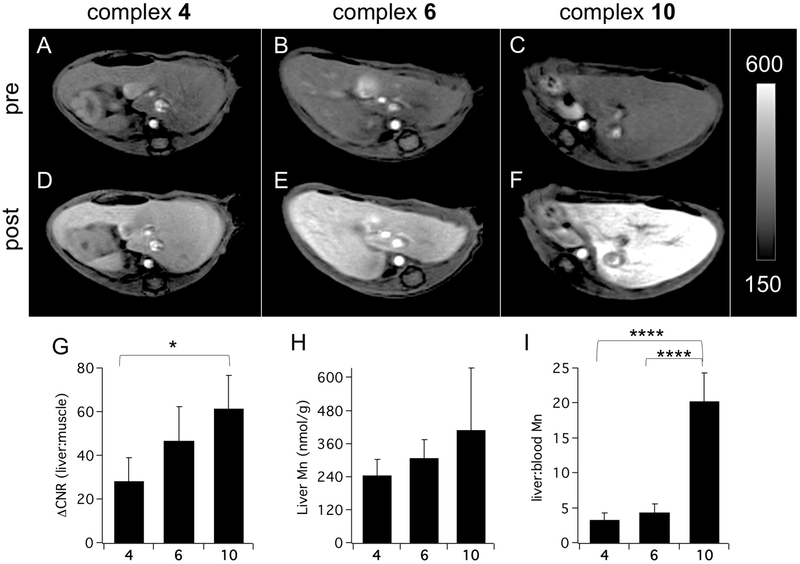
The increase in delayed phase liver vs. muscle contrast-to-noise ratio (ΔCNR) generated by 10 (61.6±15.4) is greater than that generated by 6 (48.2±15.2) and significantly greater than that generated by 4 (22.4±16.7) (Figures 3A-G). Ex vivo analysis of liver and blood Mn content 10 min post injection are also consistent with delayed phase contrast resulting from transient hepatocyte uptake and confirm that liver uptake increases with relative lipophilicity (Figure 3H, Table S1, Figure S14). Complex 10 provides the strongest liver enhancement of the three complexes tested but the real advantage for liver tumor imaging is evidenced by comparing liver-to-blood Mn content ratios 10 min after injection (Figure 3I). The delayed phase liver-to-blood ratio measured for 10 is 360% and 520% greater than for 6 and 4, respectively. The rapid blood clearance and strong delayed liver enhancement effect of 10 make this complex the lead candidate for liver imaging indications.
Figure 3.
(A-F) Axial T1-weighted images recorded at 4.7T capturing the liver and psoas muscle collected prior to (A-C) and 7 min after (D-F) injection of 4 (A,D), 6 (B,E), and 10 (C,F). (G) Liver vs. muscle ΔCNR recorded 7 min post-injection indicates that liver enhancement is greatest for the most lipophilic complex 10. (ΔCNR for 10 vs. 4, P = 0.0164, N=4 for 4 and 6, N=7 for 10). (H) Injected Mn recovered from the liver 10 min after injection is greatest for 10 (N=4 each). Note that significant washout of 10 has already occurred by 10 min (see Fig 3F) (I) Liver:blood Mn ratio 10 min after injection is significantly is higher for 10 than for 4 or 6 (P < 0.0001 for both). The rapid blood clearance and strong transient liver uptake of 10 are ideal properties for an agent designed to detect liver malignancies. Data reported as mean±SD. *P < 0.05, **** P < 0.0001.
Ex vivo quantitation of Mn content.
The three complexes tested in mice are efficiently excreted. The rates of whole body elimination reflect the blood clearance half-lives. Ex vivo analysis of tissue Mn content recorded in 9 tissues (blood, bone, brain, heart, kidney, liver, lung, muscle, spleen) 90 min post injection yielded 9.0±4.5% and 2.1±0.81% recovery of the injected dose following injection of 4 and 6, respectively (Figure 4A, Tables S2–3). No significant Mn recovery was observed following injection of the liver targeting agent 10. Mn levels had returned to baseline within 24h of injection for all three complexes (Figure 4B, Table S4).
Figure 4.
Mn levels in mice recorded 90 min (A) or 24 h (B) after injection 0.1 mmol/kg of complex 4 (red bars), 6 (green bars), or 10 (blue bars). Endogenous Mn levels in naïve mice are depicted by black bars. Data reported as mean±SD.
Evaluation of lead candidate in a murine liver tumor model.
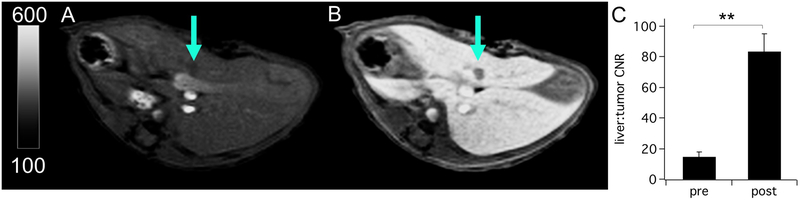
The efficacy of complex 10 to detect liver tumors was tested in a mouse model. Figures 5A,B show axial T1-weighted images of the liver at the level of the tumor prior to and during the delayed phase following injection of 10. The small (<1.3 mm), hepatocyte-devoid lesion is barely perceptible in the pre-contrast image but is rendered conspicuously hypo-intense after contrast enhancement (ΔCNR = 69.1±8.5 for pre- vs. post-injection CNR, Figure 5C). The contrast enhanced imaging results were independently confirmed by a T2-weighted pathology scan recorded at baseline and by ex vivo histopathologic examination of the liver (Figure S15).
Figure 5.
Liver imaging of an orthotopic mouse model of colorectal liver metastasis with complex 10 at 4.7T. (A and B) T1-weighted axial images of the liver at the level of the tumor (arrow) prior to and during the hepatocellular phase after injection of 10, respectively. (C) Liver parenchyma vs. tumor CNR prior to and following treatment with 10 are 14.8±3.1 and 83.8±11.5, respectively, (N=3, P = 0.005, paired t-test). Data reported as mean±SD.
DISCUSSION
We evaluated a focused library of ten mono-anionic Mn complexes comprising Mn-PyC3A and nine amphiphilic derivatives of varying polarity as liver specific MRI contrast agents. Across this series of molecules the plasma protein binding affinity, rate of elimination, hepatocellular accumulation, and delayed phase liver enhancement increase with est. logP. The blood plasma relaxivity at 1.4T also correlates with est. logP due to increased a protein binding, but relaxation enhancement due to protein binding is a low field phenomenon and the effect will be largely attenuated at field strength >3.0T. Each of the three complexes evaluated provided strong contrast enhancement of the blood vessels and strong delayed phase liver specific contrast in vivo. The most lipophilic contrast agent in the series, complex 10, demonstrated the optimal combination of properties desired for a liver specific agent – high-relaxivity, rapid blood clearance, and avid hepatocellular accumulation.
The SAR generated during this study suggests that the liver specific properties of Mn-PyC3A-derived chelates can be reliably tuned by modulating est. logP. However, we have not yet determined whether there is a threshold at which point increasing est. logP yields diminishing returns. We also note that our SAR evaluation focuses exclusively on mono-anionic complexes and that the est. logP dependence on protein binding, pharmacokinetics, and hepatocellular uptake may differ for complexes of different charge. Regardless, the lead candidate agent identified in this study represents a viable candidate for development as a direct replacement for the liver specific GBCAs, The SAR outlined by this study provides an accompanying playbook for further optimization if necessary.
Other non-GBCA liver-specific MRI contrast agents have been developed but are not directly compatible with well-established and heavily relied upon clinical liver imaging protocols. The Mn-based liver targeting agent Mn-DPDP was approved in the US and Europe but is not currently marketed, largely due to its drawbacks in comparison to GBCAs. Mn-DPDP undergoes rapid and significant dissociation upon intravenous injection and thus must be administered as a slow infusion in order to avoid toxicity associated with exposure to large amounts of free Mn2+.48–50 A protein-based agent named ProCA32 has also been developed as a liver specific agent.51 The safety advantage of ProCA32 over commercial GBCAs is that the protein is engineered to bind Gd3+ with 1011-fold higher specificity than for endogenously competing metal ions like Zn2+. ProCA32 exhibits slow blood clearance and the time to delayed phase imaging is long relative to GBCAs but T2/T1 ratiometric processing of the imaging data enables clear visualization of liver tumors within minutes of injection. The agent Mn-EDTA-BTA was recently reported to provide strong delayed phase liver enhancement,52 but >50% of the injected Mn is retained in mice 24h after injection whereas no detectable Mn is recovered 24h after injection of the Mn complexes evaluated in this work.
Conclusion.
Mn-PyC3A-3-OBn (10) is a liver-specific MRI contrast agent that offers a potentially direct replacement for the GBCA standard of care for liver scans. In addition to developing Mn-PyC3A-3-OBn, we identified SAR that govern relaxivity, protein binding, pharmacokinetics, and transient hepatocellular uptake of structurally related Mn complexes. This SAR can be applied to guide further optimization of Mn-based liver-specific contrast agents, if required.
EXPERIMENTAL
General.
All chemicals and solvents were purchased commercially and used without further purification. All biologically evaluated compounds were isolated in ≥95% purity. Purity was assessed by HPLC using Method A2 described under Analytical HPLC Methods.
NMR.
NMR spectra were recorded on a 500 MHz Varian spectrometer at 25 °C. Chemical shifts are reported in δ (ppm). For 1H and 13C NMR spectra, the residual solvent peaks were used as internal reference except for 13C NMR recorded in D2O where dioxane was used as the internal references, respectively.53 Relaxivity measurements were performed on a Bruker mq60 Minispec, 1.41 T and 37 °C. Longitudinal (T1) relaxation was acquired via an inversion recovery experiment using 10 inversion times of duration ranging between 0.05 × T1 and 5 × T1. Relaxivity (r1) was determined from the slope of a plot of 1/T1 vs. [Mn] for at least 4 concentrations.
Analytical HPLC methods.
Liquid chromatography-mass spectrometry (LC-MS) was performed using an Agilent 1100 Series apparatus with an LC/MSD trap and Daly conversion dynode detector with UV detection at 220, 254, and 280 nm. The reverse phase high pressure liquid chromatography (RP-HPLC) methods used on this system are as follows: (A1) Luna C18 column (100 × 2 mm 100 Å); eluent A: H2O/0.1 % formic acid, eluent B: MeCN/0.1 % formic acid; gradient: 5 % B to 95 % B from 0–3 min, 95 % B from 3–4.5 min, 95 to 5% B from 4.5–5 min, 5% B from 5–7 min; flow rate at 0.7 mL/min (used for characterization of organic molecules). (A2) Phenomenex C4 column (150 × 4.6 mm 100 Å); eluent C: 10 mM ammonium acetate in water, eluent D: 90 % MeCN and 10 % 10 mM ammonium acetate in water; gradient: 5 % D from 0–1 min, 5 to 50 % D from 1–10 min, 50 to 95 % D from 10–11 min, 95% D from 11–12 min, 95 to 5% D from 12–13 min, 5% D from 13–15 min; flow rate at 0.7 mL/min (used for characterization of manganese complexes).
Reverse-phase semi-preparative purification was performed on an Agilent 1260 Infinity preparative HPLC system with UV detection at 220, 254, and 280 nm. The reverse phase high pressure liquid chromatography (RP-HPLC) methods used on this system are as follows: (P1) Phenomenex C18 column (250 × 21.8 cm); eluent A: H2O/0.1 % TFA, eluent B: MeCN/0.1 % TFA; gradient: 5% B from 0–4 min, 5% to 95 % B from 4–20 min, 95% B from 20–24 min, 95% to 5% B from 24–25, 5% B from 25–29 min, flow rate at 20 mL/min (used for separation of organic molecules). (P2) Phenomenex C5 column (250 × 21.8 cm); eluent C: 10 mM ammonium acetate in water, eluent D: 90 % MeCN and 10 % 10 mM ammonium acetate in water; gradient: 5% D from 0–3 min, 5% to 95 % D from 3–23 min, 95% D from 23–26 min, 95% to 5% D from 26–28 min, 5% D from 27–31 min, flow rate at 15 mL/min (used for separation of manganese complexes).
ICP-MS.
Metal concentrations were determined using an Agilent 8800-QQQ ICP-MS system. All samples were diluted with 0.1 % Triton X-100 in 5 % nitric acid. A linear calibration curve for each metal ranging from 0.1 ppb to 200 ppb was generated daily for the quantification.
Estimates of 1-octanol:water partition coefficient (est. log P).
The est. log P values were estimated using a gradient elution RP-HPLC assay that is similar to previously described methods.54, 55 A linear calibration curve correlating logP of 6 standards (DMSO, DMF, nitrobenzene, nitromethane, phenol, pyridine)45 to HPLC retention time (tR) under method A2 conditions was generated (Figure S2). The est log P values of complexes 1-10 were estimated from their corresponding tR using this calibration curve.
Estimates of plasma protein binding.
Measurements were performed on 50 µM solutions of complexes 1-10 in human blood plasma (MGH blood bank) or 4.5% wt/vol human serum albumin. 150 µL of each solution was placed within a Millipore Ultra Free MC 3 kDa cutoff filtration vessel and ~10 µL of the solution was forced through the filter by centrifugation. Mn content in each unfiltered solution and filtrate were quantified by ICP-MS. The percentage of Mn complex bound to protein was estimated from the difference in Mn concentrations between unfiltered solution and filtrate. For consistency, all measurements used the same source or plasma or albumin and were run side-by-side simultaneously. Our prior experience with this assay teaches that protein binding can vary significantly between plasma samples acquired from different donors. In order to account for any loss of free complex due to adherence to plastics or the filter membrane, control experiments were performed in PBS buffer and protein binding was corrected for retention measured under control conditions. All measurements were performed in triplicate.
Orthotopic mouse model of colorectal liver metastasis.
All experiments were performed in accordance with the National Institutes of Health’s Guide for the Care of Use of Laboratory Animals and approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee. Eight-to-ten-week old immunocompetent male BALB/c mice were obtained from Charles River Labs (Wilmington, MA, USA).
The mc26 cell line was generously provided by the laboratory of Dr. Khalid Shah at Harvard Medical School. Cells were plated at low density in T75 uncoated plastic flasks and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (high glucose 4.5g/L) supplemented with 10% fetal bovine serum (FBS) and 1% Pen/Strep (100 U/ml Penicillin, 100 ug/ml Streptomycin). Cells were grown at 37° C in a humidified, 5% CO2 incubator until they reached approximately 70% confluence. All experiments were performed between passage 1 and 8.
Mice were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). Fur was shaved in the region of the incision using an electric clipper. The surgical field was sterilized with betadyne, and was allowed to dry for 30 seconds. Orthotopic liver tumors were created by injecting 104 cells in 10 µL Matrigel into the left lobe of the liver. Mice were monitored daily for disease progression by abdominal palpation, as well as for morbidity, evidenced by decreased grooming, hunched posture, and reduced movement.
MR imaging of mice.
Mice (balb/C, Charles River Laboratories, Cambridge, MA) were imaged at 4.7T using a small bore animal scanner (Bruker Biospec) with a custom-built volume coil. The imaging protocol at baseline consisted of multislice 2D T1-weighted fast low-angle shot (FLASH) sequence to delineate anatomy and a 3D T1-weighted FLASH sequence. For the liver tumor mice, a baseline 2D T2-weighted rapid acquisition with refocused echo (RARE) sequence was also applied. After tail vein administration of 0.1 mmol/kg of Mn complex (formulated as 50 mM aqueous solution), the 3D T1-weighted FLASH sequence used at baseline was repeated every 1 minute out to 6 min following contrast delivery, the 2D T1-weighted FLASH imaging was applied at 6 min, and then the T1-weighted FLASH imaging continued in order to further monitor clearance of the agent. 3D T1-weighted FLASH imaging parameters: coronal orientation, TR/TE/flip angle=15 ms/2.46 ms/25°, in-plane FOV = 48×28 mm, matrix=85×70, 50 slices, slice thickness = 0.4 mm, voxel size = 0.4 × 0.4 × 0.4 mm, 1 average, and acquisition time = 0 min, 52 s. 2D T1-weighted FLASH imaging parameters: axial orientation, TR/TE/flip angle=125 ms/2.93 ms/60°, in-plane FOV = 33×33 mm, matrix=140×140, 9 slices, slice thickness = 1.0 mm, voxel size = 0.24 × 0.24 × 1.0 mm, 16 averages, and acquisition time = 4 min, 40s. 2D T2-weighted RARE imaging parameters: axial orientation, TR/TE/flip angle=1286 ms/27 ms/90°, in-plane FOV = 33×33 mm, matrix=192×192, 9 slices, slice thickness = 1.0 mm, voxel size = 0.17 × 0.17 × 1.0 mm, 4 averages, and acquisition time = 2 min, 3 s.
Images were analyzed in Horos by drawing regions of interest (ROI) and measuring signal intensity (SI) of the kidneys, liver, vena cava, and muscle. Noise was estimated from the standard deviation (SD) of an ROI taken in the air outside the animal. Normalized SI (nSI) was calculated by dividing SI at each time point post probe injection by the SI prior to injection. Liver-to-muscle contrast-to-noise ratio (CNR) was calculated by dividing the absolute difference in SI between liver and muscle by noise.
Pharmacokinetics.
Blood pharmacokinetics (distribution and elimination rates, kdist and kel, respectively) were estimated by plotting blood nSI recorded from the dynamic 3D T1-FLASH acquisitions as a function of time and fitting the decay to a bi-exponential function. The distribution and elimination half-lives (t1/2) were estimated by dividing ln(2) by kdist and kel, respectively
Ex vivo Mn quantification.
The mice were euthanized 90 min and 24h after injection of the Mn complex and tissues harvested for analysis. Tissues were dissolved in a defined weight of concentrated nitric acid, and then an aliquot of digest of defined weight was diluted into a known weight of ICP dilutent for ICP-MS analysis.
Histopathology.
A cardiac terminal blood withdrawal was performed at the time of sacrifice. Liver and tumors were extracted from mice, and tissues were formalin fixed for 48 hours prior to paraffin embedding. Sections were cut at 5 um thickness. Representative sections were stained with Hematoxylin & Eosin (H&E). Slides were digitally scanned for analysis using a Hamamastu NanoZoomer-SQ Digital slide scanner.
Statistical Analysis.
All statistical analysis was performed using GraphPad Prism 7. Correlations between corresponding physical properties were determined by calculating the Pearson product-moment correlation coefficient followed by a two-tailed t-test. Comparisons between dynamic MRI data and 2D liver imaging data recorded following injection of complexes 4, 6, and 10 were made by one-way multiple comparisons ANOVA followed by a Tukey’s post-hoc test. Comparisons between basal Mn levels and Mn levels recorded after injection of complexes 4, 6, and 10 were made by one-way multiple comparisons ANOVA followed by a Tukey’s post-hoc test. Comparisons between liver vs. tumor CNR following prior to and after injection of complex 10 were made by a paired t-test. For all correlations and comparisons P < 0.05 was considered significant.
Synthesis.
General method for compounds 11g-i.
To a batch of the 2-methylpyridine derivative (6.0 mmol) in THF (27 mL) was added KOH (7.0 mmol) and tetra-N-butylammonium bromide (TBAB 2.0 mmol). The mixture was stirred at room temperature for 10 min followed by addition of benzyl bromide (7.2 mmol). The reaction mixture was stirred at room temperature for 48 hours. The solvents were removed under reduced pressure, and the resulting residue was suspended in water and extracted with DCM three times. The combined organics were washed with brine, dried over MgSO4, filtered, and dried under reduced pressure. The crude material was adsorbed onto a plug of silica gel and purified by chromatography, eluting with eluting with 20% EtOAc in hexanes to give the benzyl protected pyridine derivatives 11g-i in 92–97% yield as colorless to light yellow oils.
Benzyl 6-methylnicotinate (11g) was isolated in 92% yield as a light yellow oil. 1H NMR (500 MHz, CDCl3) δ 9.15 (d, J = 2.1 Hz, 1H), 8.20 (dd, J = 8.1, 2.2 Hz, 1H), 7.45 (dd, J = 4.4, 3.4 Hz, 2H), 7.41 (dd, J = 11.6, 4.6 Hz, 2H), 7.38 – 7.33 (m, 1H), 7.23 (d, J = 8.1 Hz, 1H), 5.39 (s, 2H), 2.63 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 165.3, 163.3, 150.6, 137.4, 135.7, 128.6, 128.4, 128.2, 123.3, 122.9, 66.9, 24.8. LC-MS (method A1): tR = 3.57 min, m/z = 228.1[M + H]+.
5-(benzyloxy)-2-methylpyridine (11h) was isolated in 97% yield as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 8.27 (s, 1H), 7.54 – 7.35 (m, 4H), 7.33 (m, 1H), 7.16 (d, J = 8.5 Hz, 1H), 7.05 (d, J = 8.5 Hz, 1H), 5.07 (s, 2H), 2.49 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 150.3, 148.1, 134.5, 133.8, 126.1, 125.6, 124.9, 120.8, 119.9, 67.9, 20.8. LC-MS (method A1): tR = 2.86 min, m/z = 200.1 [M + H]+.
3-(Benzyloxy)-2-methylpyridine (11i) was isolated in 95% yield as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 8.09 (dd, J = 4.7, 1.2 Hz, 1H), 7.47 – 7.37 (m, 4H), 7.34 (t, J = 7.0 Hz, 1H), 7.12 (d, J = 8.2 Hz, 1H), 7.07 (dd, J = 8.2, 4.8 Hz, 1H), 5.09 (s, 2H), 2.54 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 152.9, 149.2, 140.5, 136.5, 128.7, 128.1, 127.1, 121.7, 117.9, 69.9, 19.5. LC-MS (method A1): tR = 2.81 min, m/z = 200.1 [M + H]+.
General method for compounds 12d-i.
To a batch of the corresponding 2-methylpyridine derivative 11 (5.0 mmol) in DCM (20 mL) was added m-CPBA (6.0 mmol) at room temperature. The resulting mixture was stirred overnight. The crude material was adsorbed onto a plug of silica gel and purified by chromatography, eluting with a gradient of 0−20% MeOH in EtOAc, to provide pyridine N-oxide intermediates 12d-i in 95–99% yield.
4-Methoxy-2-methylpyridine 1-oxide (12d) was isolated in 97% yield as a white solid. 1H NMR (500 MHz, CDCl3) δ 7.95 (d, J = 7.2 Hz, 1H), 6.59 (d, J = 3.4 Hz, 1H), 6.51 (dd, J = 7.2, 3.4 Hz, 1H), 3.63 (s, 3H), 2.29 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 157.4, 149.6, 139.9, 111.5, 109.8, 55.8, 18.2. ESI-MS: tR = 0.50 min, m/z = 140.1[M + H]+.
3-Methoxy-2-methylpyridine 1-oxide (12e) was isolated in 99% yield as a white solid. 1H NMR (500 MHz, CDCl3) δ 7.82 (d, J = 6.5 Hz, 1H), 6.93 (m, 1H), 6.69 (d, J = 8.6 Hz, 1H), 3.75 (s, 3H), 2.32 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 155.8, 140.5, 132.3, 121.6, 107.6, 56.2, 10.4. ESI-MS: m/z = 140.0 [M + H]+.
3-(Ethoxycarbonyl)-2-methylpyridine 1-oxide (12f) was isolated in 95% yield as a white solid. 1H NMR (500 MHz, CDCl3) δ 8.35 (d, J = 6.4 Hz, 1H), 7.66 (d, J = 8.0 Hz, 1H), 7.16 (dd, J = 7.5, 6.9 Hz, 1H), 4.35 (q, J = 7.1 Hz, 2H), 2.73 (s, 3H), 1.36 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 164.8, 151.1, 141.5, 130.1, 126.8, 122.2, 62.1, 14.9, 14.1. LC-MS (method A1): tR = 2.74 min, m/z = 182.0 [M + H]+.
5-((Benzyloxy)carbonyl)-2-methylpyridine 1-oxide (12g) was isolated in 95% yield as a white solid. 1H NMR (500 MHz, CDCl3) δ 8.81 (dd, J = 1.1, 0.5 Hz, 1H), 7.73 (dd, J = 8.1, 1.6 Hz, 1H), 7.39 – 7.28 (m, 6H), 5.32 (s, 2H), 2.51 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 162.9, 153.3, 140.3, 134.9, 128. 7, 128.7, 128.4, 127.5, 126.2, 125.9, 67.7), 18.1. LC-MS (method A1): tR = 3.47 min, m/z = 244.1 [M + H]+.
5-(Benzyloxy)-2-methylpyridine 1-oxide (12h) was isolated in 98% yield as a white solid. 1H NMR (500 MHz, CDCl3) δ 8.09 (d, J = 2.4 Hz, 1H), 7.43 – 7.38 (m, 4H), 7.38 – 7.30 (m, 1H), 7.11 (d, J = 8.8 Hz, 1H), 6.88 (dd, J = 8.7, 2.4 Hz, 1H), 5.05 (s, 2H), 2.45 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 155.2, 141.9, 135.2, 128.8, 128.5, 128.1, 127.5, 125.8, 114.6, 70.9, 16.9. LC-MS (method A1): tR = 3.52 min, m/z = 216.1 [M + H]+.
3-(Benzyloxy)-2-methylpyridine 1-oxide (12i) was isolated in 97% yield as a white solid. 1H NMR (500 MHz, CDCl3) δ 7.93 (d, J = 6.5 Hz, 1H), 7.49 – 7.32 (m, 4H), 7.32 (td, J = 8.6, 4.2 Hz, 1H), 7.02 – 6.93 (m, 1H), 6.82 (d, J = 8.6 Hz, 1H), 5.08 (s, 2H), 2.48 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 154.9, 141.1, 135.5, 132.7, 128.7, 128.4, 127.2, 121.7, 109.3, 71.1, 10.8. LC-MS (method A1): tR = 3.11 min, m/z = 216.1 [M + H]+.
General method for the synthesis of compounds 13d-i.
A batch of 2-methylpyridine N-oxide intermediate (4.0 mmol) was dissolved in acetic anhydride (7.0 mL) and the resulting mixture was refluxed for 5–7 hours. The reaction was cooled to room temperature and 10 mL of MeOH was then added slowly, and the mixture was stirred at room temperature overnight. The solvent was removed under reduced pressure, and the resulting residue was suspended in water and extracted with DCM three times. The combined organics were washed with brine, dried over MgSO4, filtered, and dried under reduced pressure. The crude material was adsorbed onto a plug of silica gel and purified by chromatography, eluting with 30% EtOAc in hexanes to give the pyridine-2-ylmethyl acetate derivatives 13d-i in 82–95% yields.
(4-methoxypyridin-2-yl)methyl acetate (13d) was isolated in 94% yield as a light yellow oil. 1H NMR (500 MHz, CDCl3) δ 8.41 (s, 1H), 6.87 (s, 1H), 6.75 (s, 1H), 5.16 (s, 2H), 3.86 (s, 4H), 2.16 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 170.6, 166.4, 157.3, 150.8, 108.8, 108.1, 66.7, 55.2, 20.9. ESI-MS: m/z = 182.1 [M + H]+.
(3-methoxypyridin-2-yl)methyl acetate (13e) was isolated in 95% yield as a light yellow oil. 1H NMR (500 MHz, CDCl3) δ 8.18 (s, 1H), 7.26 – 7.20 (m, 1H), 7.17 (d, J = 8.2 Hz, 1H), 5.26 (s, 2H), 3.84 (s, 3H), 2.12 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 170.7, 153.9, 144.7, 140.8, 123.9, 117.6, 62.6, 55.4, 20.9. LC-MS (method A1): tR = 2.27 min, m/z = 182.1 [M + H]+.
Ethyl 2-(acetoxymethyl)nicotinate (13f) was isolated in 89% yield as a light yellow oil. 1H NMR (500 MHz, CDCl3) δ 8.59 (d, J = 5.0 Hz, 1H), 8.14 (dt, J = 7.9, 1.4 Hz, 1H), 7.22 (dd, J = 8.25, 5.0 Hz, 1H), 5.47 (s, 2H), 4.27 (qd, J = 7.1, 1.3 Hz, 2H), 2.04 (s, 3H), 1.28 (td, J = 7.1, 1.3 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 170.5, 165.5, 156.1, 151.8, 138.5, 125.2, 122.5, 65.6, 61.5, 20.7, 14.1. LC-MS (method A1): tR = 3.47 min, m/z = 224.0 [M + H]+.
Benzyl 6-(acetoxymethyl)nicotinate (13g) was isolated in 82% yield as a white solid. 1H NMR (500 MHz, CDCl3) δ 9.27 – 9.20 (m, 1H), 8.34 (dd, J = 8.2, 2.1 Hz, 1H), 7.42 (ddd, J = 20.7, 11.9, 7.3 Hz, 6H), 5.41 (s, 2H), 5.29 (s, 2H), 2.20 (s, 3H). 13C NMR (126 MHz, CDCcl3) δ 170.4, 164.9, 160.3, 150.8, 138.0, 135.5, 128.7, 128.5, 128.3, 125.2, 120.9, 77.27 (s), 77.02 (s), 76.76 (s), 67.1, 66.3, 20.8. LC-MS (method A1): tR = 4.32 min, m/z = 286.0 [M + H]+.
(5-(benzyloxy)pyridin-2-yl)methyl acetate (13h) was isolated in 95% yield as a light yellow oil. 1H NMR (500 MHz, CDCl3) δ 8.22 (s, 1H), 7.32 – 7.21 (m, 4H), 7.21 – 7.15 (m, 1H), 7.11 (dd, J = 18.9, 8.5 Hz, 2H), 5.00 (s, 2H), 4.95 (s, 2H), 1.97 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 170.7, 154.4, 147.8, 138.1, 135.9, 128.7, 128.3, 127.5, 123.0, 121.9, 70.4, 66.7, 20.9. LC-MS (method A1): tR = 3.78 min, m/z = 258.0 [M + H]+.
(3-(benzyloxy)pyridin-2-yl)methyl acetate (13i) was isolated in 92% yield as a light yellow oil. 1H NMR (500 MHz, CDCl3) δ 8.18 (dd, J = 4.6, 1.3 Hz, 1H), 7.42 – 7.32 (m, 4H), 7.33 – 7.27 (m, 1H), 7.20 (dd, J = 8.3, 1.2 Hz, 1H), 7.16 (dd, J = 8.3, 4.6 Hz, 1H), 5.32 (s, 2H), 5.08 (s, 2H), 2.09 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 170.9, 153.0, 145.1, 141.2, 136.0, 128.7, 128.2, 127.1, 123.9, 119.1, 70.1, 62.8, 20.9. LC-MS (method A1): tR = 3.90 min, m/z = 258.1 [M + H]+.
General method for compounds 14d-i.
Saponification procedure.
A batch of pyridine-2-ylmethyl acetate derivative 13d,e,h-i (3.0 mmol) was dissolved in THF (10 mL) followed by addition of 2 mL of KOH solution (2.0 M). The reaction mixture was stirred at room temperature for between 2–7 hours. The solvents were removed under reduced pressure, and the resulting residue was suspended in water and extracted with EtOAc three times. The combined organics were washed with brine, dried over MgSO4, filtered, and dried under reduced pressure. The crude material was adsorbed onto a plug of silica gel and purified by chromatography, eluting with eluting with 50% EtOAc in hexanes to give the pyridin-2-ylmethanol derivatives 14d,e,h-i in 97–99% yield.
Acetyl chloride deprotection.
A batch of Pyridine-2-ylmethyl acetate derivative 13f,g (3.0 mmol) was dissolved in MeOH (10 mL) followed by addition of 1.2 equivalent of acetyl chloride. The the mixture was stirred at room temperature overnight. The solvent was removed under reduced pressure, and the resulting residue was suspended in water and extracted with EtOAc three times. The combined organics were washed with brine, dried over MgSO4, filtered, and dried under reduced pressure. The crude material was adsorbed onto a plug of silica gel and purified by chromatography, eluting with eluting with 50% EtOAc in hexanes to give the pyridin-2-ylmethanol derivatives 14f,g in 90–92% yield.
(4-methoxypyridin-2-yl)methanol (14d) was isolated in 97% yield as a white solid using the saponification procedure. 1H NMR (500 MHz, CDCl3) δ 8.32 (d, J = 3.3 Hz, 1H), 8.26 – 8.17 (m, 1H), 6.85 (s, 1H), 6.73 (d, J = 2.4 Hz, 1H), 4.71 (s, 2H), 3.85 (d, J = 0.9 Hz, 3H). 13C NMR (126 MHz, CD3OD) δ 166.6, 161.4, 149.3, 109.2, 106.2, 64.2, 55.3. ESI-MS: m/z = 140.1 [M + H]+.
(3-methoxypyridin-2-yl)methanol (14e) was isolated in 99% yield as a white solid using the saponification procedure. 1H NMR (500 MHz, CDCl3) δ 8.10 (d, J = 4.7 Hz, 1H), 7.16 (dd, J = 8.2, 4.8 Hz, 1H), 7.09 (d, J = 8.2 Hz, 1H), 4.70 (s, 2H), 3.81 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 152.3, 148.4, 139.3, 122.6, 116.5, 59.9, 55.2. ESI-MS: m/z = 140.1 [M + H]+.
Ethyl 2-(hydroxymethyl)nicotinate (14f) was isolated in 82% yield as a light yellow oil following treatment with acetyl chloride. 1H NMR (500 MHz, CDCl3) δ 8.70 (dd, J = 4.8, 1.0 Hz, 1H), 8.33 (dd, J = 7.8, 1.4 Hz, 1H), 7.33 (dd, J = 7.5, 4.9 Hz, 1H), 5.05 (s, 2H), 4.78 (s, 1H), 4.37 (q, J = 7.1, 2H), 1.39 (t, J = 7.1, 3H). 13C NMR (126 MHz, CDCl3) δ 165.2, 160.1, 150.9, 139.1, 123.5, 121.9, 63.4, 61.5, 14.2. LC-MS (method A1): tR = 0.90 min, m/z = 182.1 [M + H]+.
Benzyl 6-(hydroxymethyl)nicotinate (14g) was isolated in 92% yield as a white solid following treatment with acetyl chloride. 1H NMR (500 MHz, CDCl3) δ 9.20 (s, 1H), 8.32 (d, J = 8.2 Hz, 1H), 7.46 (d, J = 7.7 Hz, 2H), 7.43 – 7.31 (m, 4H), 5.40 (s, 2H), 4.84 (s, 2H). 13C NMR (126 MHz, CDCl3) δ 164.9, 163.7, 149.9, 137.9, 135.5, 128.7, 128.5, 128.3, 124.9, 120.1, 67.1, 64.2. LC-MS (method A1): RT = 3.44 min, m/z = 244.0 [M + H]+.
(5-(benzyloxy)pyridin-2-yl)methanol (14h) was isolated in 97% yield as a white solid following the saponification procedure. 1H NMR (500 MHz, CDCl3) δ 8.33 (d, J = 2.7 Hz, 1H), 7.49–7.39 (m, 4H), 7.36 (t, J = 6.9 Hz, 1H), 7.32 – 7.26 (m, 1H), 7.22 (d, J = 8.6 Hz, 1H), 5.13 (s, 2H), 4.72 (s, 2H). 13C NMR (126 MHz, CDCl3) δ 154.1, 151.6, 136.4, 136.0, 128.7, 128.4, 127.5, 123.0, 121.2, 70.6, 63.9. LC-MS (method A1): tR = 3.26 min, m/z = 216.1 [M + H]+.
(3-(benzyloxy)pyridin-2-yl)methanol (14i) was isolated in 98% yield as a white solid following the saponification procedure. 1H NMR (500 MHz, CDCl3) δ 8.70 (dd, J = 4.8, 1.6 Hz, 1H), 8.36 (dd, J = 7.9, 1.6 Hz, 1H), 7.47 – 7.41 (m, 2H), 7.41 – 7.37 (m, 2H), 7.37 – 7.34 (m, 1H), 7.32 (dd, J = 7.8, 4.9 Hz, 1H), 5.36 (s, 2H), 5.08 (s, 2H). 13C NMR (126 MHz, CDCl3) δ 164.9, 160.3, 151.1, 139.2, 135.3, 128.7, 128.6, 128.5, 123.2, 122.1, 67.3, 63.5. LC-MS (method A1): tR = 2.52 min, m/z = 216.1 [M + H]+.
General method for the synthesis of compounds 15.
To a batch of of pyridin-2-ylmethanol derivatives 14 (2.0 mmol) in DCM (10.0 mL) was added PBr3 (812.1 mg, 3.0 mmol). And the resulting solution was stirred at room temperature for 5 hours. Then the reaction mixture was extracted with saturated sodium carbonate and washed with brine, dried over MgSO4 and filtered. Then 1.2 equivalent HBr was added and the solvents were removed under reduced pressure to give 2-(bromomethyl) pyridine hydrogen bromide salt derivatives 15 as white solids in quantitative yield.
2-(bromomethyl)-4-methoxypyridine hydrogen bromide (15d).
1H NMR (500 MHz, CD3OD) δ 8.64 (dd, J = 6.9, 4.7 Hz, 1H), 7.67 (dd, J = 5.4, 2.7 Hz, 1H), 7.53 – 7.45 (m, 1H), 4.78 (s, 2H), 4.17 (s, 3H). 13C NMR (126 MHz, CD3OD) δ 172.9, 153.2, 143.5, 112.7, 111.8, 57.3, 24.5. ESI-MS: m/z = 203.9 [M + H]+.
2-(bromomethyl)-3-methoxypyridine hydrogen bromide (15e).
1H NMR (500 MHz, CD3OD) δ 8.45 (d, J = 5.7 Hz, 1H), 8.36 (d, J = 8.8 Hz, 1H), 8.05 (dd, J = 8.6, 5.9 Hz, 1H), 4.83 (s, 2H), 4.16 (s, 3H). 13C NMR (126 MHz, CD3OD) δ 155.9, 141.3, 132.9, 128.7, 128.2, 56.9, 19.7. LC-MS (method A1): tR = 2.22 min, m/z = 203.9 [M + H]+.
Ethyl 2-(bromomethyl)nicotinate hydrogen bromide (15f).
1H NMR (500 MHz, CD3OD) δ 9.03 (d, J = 7.0 Hz, 1H), 8.99 (d, J = 5.0 Hz, 1H), 8.09 (t, d, J = 8.0 Hz, 1H), 5.19 (s, 2H), 4.51 (q, J = 7.1 Hz, 2H), 1.45 (t, J = 7.1 Hz, 3H).13C NMR (126 MHz, CD3OD) δ 162.3, 153.6, 147.3, 145.9, 126.5, 62.9, 24.2, 12.9. LC-MS (method A1): tR = 3.63 min, m/z = 245.9 [M + H]+.
benzyl 6-(bromomethyl)nicotinate hydrogen bromide (15g).
1H NMR (500 MHz, CD3OD) δ 9.30 (d, J = 2.0 Hz, 1H), 8.96 (dd, J = 8.3, 2.0 Hz, 1H), 8.19 (d, J = 8.3 Hz, 1H), 7.55 – 7.47 (m, 2H), 7.44 – 7.34 (m, 3H), 5.48 (s, 2H), 4.91 (s, 2H). 13C NMR (126 MHz, CD3OD) δ 161.9, 156.5, 145.4, 144.9, 135.1, 128.5, 128.4, 128.4, 126.9, 67.9, 25.5. LC-MS (method A1): tR = 4.93 min, m/z = 308.0 [M + H]+.
5-(benzyloxy)-2-(bromomethyl)pyridine hydrogen bromide (15h).
1H NMR (500 MHz, CD3OD) δ 8.65 (d, J = 2.7 Hz, 1H), 8.24 (dd, J = 9.0, 2.8 Hz, 1H), 8.07 (d, J = 9.0 Hz, 1H), 7.64 – 7.48 (m, 2H), 7.41 (ddd, J = 14.1, 7.0, 3.8 Hz, 3H), 5.37 (s, 2H), 4.84 (s, 2H). 13C NMR (126 MHz, CD3OD) δ 156.9, 144.0, 134.7, 132.3, 130.6, 128.40 (t, J = 10.5 Hz), 127.8, 71.8, 24.4. LC-MS (method A1): tR = 4.66 min, m/z =278.0 [M + H]+.
3-(benzyloxy)-2-(bromomethyl)pyridine hydrogen bromide (15i).
1H NMR (500 MHz, CD3OD) δ 8.43 (dt, J = 5.7, 1.1 Hz, 1H), 8.38 (d, J = 8.8 Hz, 1H), 8.04 – 7.96 (m, 1H), 7.59 – 7.54 (m, 2H), 7.44 (td, J = 7.1, 1.0 Hz, 2H), 7.42 – 7.33 (m, 1H), 5.49 (s, 2H), 4.84 (s, 2H). 13C NMR (126 MHz, CD3OD) δ 154.9, 141.6, 134.6, 133.1, 129.7, 128.5, 127.9, 127.6, 71.9, 19.8. LC-MS (method A1): tR = 3.88 min, m/z = 278.0 [M + H]+.
General method for synthesis of compounds 16a,b-d-i.
A batch N,N,N’-trans-1,2-diaminocyclohexanetri-tert-butylacetate of (0.50 mmol) and either -(chloromethane)-3,5-dimethyl-5-hydroxypyridine or 2-(bromomethyl) pyridine hydrobromide derivative 15d-i (0.50 mmol) were dissolved in dry acetonitrile (10 mL) followed by addition of potassium iodide (0.50 mmol) and potassium carbonate (0.50 mmol). The resulting mixture was refluxed for 20 min to give the corresponding compounds 16a,d-i in 89–95% yield.
Di-tert-butyl trans-2,2’-((2-((2-(tert-butoxy)-2-oxoethyl)((4-methoxy-3,5-dimethylpyridin-2-yl) methyl)amino)cyclohexyl)azanediyl)diacetate (16a) was isolated in 89% yield. 1H NMR (500 MHz, CDCl3) δ 8.28 (s, 1H), 4.16 (d, J = 14.7 Hz, 1H), 4.00 (d, J = 14.2 Hz, 1H), 3.88 (s, 3H), 3.59 – 2.91 (m, 6H), 2.76 (t, J = 11.4 Hz, 1H), 2.65 – 2.51 (m, 1H), 2.41 (s, 3H), 2.31 (s, 3H), 2.19 (d, J = 11.5 Hz, 1H), 2.02 (d, J = 12.0 Hz, 1H), 1.75 (m, 2H), 1.40 (s, 9H), 1.29 – 1.18 (m, 1H), 1.11 (m, 2H), 1.09 – 0.97 (m, 1H). 13C NMR (126 MHz, CDCl3) δ 173.7, 171.2, 160.8, 160.6, 127.5, 80.8, 62.1, 60.5, 54.1, 52.6, 28.1, 28.0, 26.2, 25.6, 25.5, 21.7, 13.7, 11.0. LC-MS (method A1): tR = 5.44 min, m/z = 606.3[M + H]+.
Di-tert-butyl trans-2,2’-((2-((2-(tert-butoxy)-2-oxoethyl)((4-methoxypyridin-2-yl)methyl)amino) cyclohexyl)azanediyl)diacetate (16d) was isolated in 90% yield. 1H NMR (500 MHz, CDCl3) δ 8.58 (s, 1H), 8.41 (d, J = 6.2 Hz, 1H), 7.52 (s, 1H), 6.87 (d, J = 4.5 Hz, 1H), 4.16 (d, J = 15.4 Hz, 1H), 3.99 (s, 3H), 3.80 (d, J = 15.5 Hz, 1H), 3.53 (d, J = 17.0 Hz, 2H), 3.40–3.25 (m, 4H), 2.77 (t, J = 9.9 Hz, 1H), 2.60 (t, J = 9.3 Hz, 1H), 2.06 (dd, J = 22.0, 11.0 Hz, 2H), 1.75 (br, 2H), 1.39 (s, 18H), 1.37 (s, 9H), 1.27 – 0.93 (m, 4H). 13C NMR (126 MHz, CDCl3) δ 171.2, 170.9, 168.7, 166.6, 160.9, 145.8, 110.4, 109.4, 80.9, 80.7, 62.7, 62.2, 56.4, 54.3, 53.1, 52.4, 28.1, 28.1, 27.0, 25.7, 25.6. LC-MS (method A1): tR = 3.63 min, m/z = 578.3[M + H]+
Di-tert-butyl 2,2’-((2-((2-(tert-butoxy)-2-oxoethyl)((3-methoxypyridin-2-yl)methyl)amino) cyclohexyl)azanediyl)diacetate (16e) was isolated in 92% yield. 1H NMR (500 MHz, CDCl3) δ 8.56 (s, 1H), 8.16 (d, J = 4.4 Hz, 1H), 7.27 (d, J = 4.8 Hz, 1H), 4.12 (dd, J = 33.3, 14.2 Hz, 2H), 3.89 (s, 3H), 3.51 (q, J = 17.3 Hz, 2H), 3.39 – 3.16 (m, 4H), 2.78 – 2.64 (m, 2H), 2.10 – 1.97 (m, 2H), 1.75–1.64 (m, 2H), 1.40 (s, 9H), 1.39 (s, 18H),1.27–1.17 (m, 1H), 1.17–1.07 (m, 3H). 13C NMR (126 MHz, CDCl3) δ 171.4, 171.2, 166.5, 154.6, 147.9, 138.8, 123.6, 118.6, 80.7, 80.5, 63.1, 62.3, 55.5, 53.2, 51.6, 29.3, 28.1, 28.1, 27.7, 25.6, 25.5. LC-MS (method A1): tR = 3.59 min, m/z = 578.3 [M + H]+.
Di-tert-butyl trans-2,2’-((2-((2-(tert-butoxy)-2-oxoethyl)((3-(ethoxycarbonyl)pyridin-2-yl)methyl)amino)cyclohexyl)azanediyl)diacetate (16f) was isolated in 90% yield. 1H NMR (500 MHz, CDCl3) δ 8.63 (br, 1H), 8.14 (d, J = 7.2 Hz, 1H), 7.27 (br, 1H), 4.61 (d, J = 13.8 Hz, 1H), 4.46 (d, J = 13.6 Hz, 1H), 4.40 (dd, J = 12.9, 6.2 Hz, 2H), 3.55 (s, 2H), 3.29 (s, 3H), 2.70 (d, J = 31.4 Hz, 2H), 2.12 (br, 1H), 2.06 (s, 1H), 2.02 (d, J = 7.8 Hz, 1H), 1.68 (br, 2H), 1.41 (s, 9H), 1.39 (s, 3H), 1.11 (br, 4H). 13C NMR (126 MHz, CDCl3) δ 175.2, 171.4, 166.6, 150.6, 138.4, 127.2, 121.9, 80.3, 62.8, 62.6, 61.5, 56.6, 53.3, 52.9, 29.5, 29.0, 28.1, 28.0, 25.7, 25.6, 21.5, 14.2. LC-MS (method A1): tR = 3.96 min, m/z = 620.4 [M + H]+.
Di-tert-butyl trans-2,2’-((2-(((5-((benzyloxy)carbonyl)pyridin-2-yl)methyl)(2-(tert-butoxy)-2-oxoethyl)amino)cyclohexyl)azanediyl)diacetate (16g) was isolated in 92% yield. 1H NMR (500 MHz, CDCl3) δ 9.11 (d, J = 2.1 Hz, 1H), 8.28 (dd, J = 8.2, 2.1 Hz, 1H), 8.04 (d, J = 8.2 Hz, 1H), 7.56 – 7.41 (m, 2H), 7.39 (dd, J = 9.7, 4.7 Hz, 2H), 7.38 – 7.29 (m, 1H), 5.38 (s, 2H), 4.22 (d, J = 15.4 Hz, 1H), 3.89 (d, J = 15.3 Hz, 1H), 3.67 – 3.26 (m, 6H), 2.74 (t, J = 9.6 Hz, 1H), 2.66 – 2.57 (m, 1H), 2.06 (br, 2H), 1.69 (br, 2H), 1.42 (d, J = 4.0 Hz, 19H), 1.35 (d, J = 60.3 Hz, 9H), 1.29–1.09 (m, 4H). 13C NMR (126 MHz, CDCl3) δ 171.4, 166.1, 165.4, 149.7, 137.5, 135.7, 128.6, 128.6, 128.3, 128.2, 124.2, 123.6, 80.6, 80.4, 66.8, 63.6, 62.0, 56.1, 53.8, 52.8, 28.6, 28.1, 28.1, 27.9, 25.8, 25.7. LC-MS (method A1): tR = 3.75 min, m/z = 682.3 [M + H]+.
Di-tert-butyl trans-2,2’-((2-(((5-(benzyloxy)pyridin-2-yl)methyl)(2-(tert-butoxy)-2-oxoethyl)amino) cyclohexyl)azanediyl)diacetate (16h) was isolated in 95% yield. 1H NMR (500 MHz, CDCl3) δ 8.25 (d, J = 2.2 Hz, 1H), 7.82 (d, J = 8.6 Hz, 1H), 7.43 (d, J = 7.4 Hz, 2H), 7.39 (t, J = 7.4 Hz, 2H), 7.34 (t, J = 7.1 Hz, 1H), 7.30 (dd, J = 8.6, 2.5 Hz, 1H), 5.09 (s, 2H), 4.08 (d, J = 13.9 Hz, 1H), 3.90 – 3.78 (m, 1H), 3.47 (dd, J = 36.7, 16.9 Hz, 5H), 3.36 (d, J = 16.8 Hz, 1H), 2.75 (m, 1H), 2.70 – 2.57 (m, 1H), 2.14 – 2.02 (m, 2H), 1.70 (br, 2H), 1.43 (s, 18H), 1.42 (s, 9H), 1.20 – 1.07 (m, 4H). 13C NMR (126 MHz, CDCl3) δ 171.5, 153.9, 136.3, 135.9, 128.6, 128.2, 127.5, 124.8, 122.8, 80.4, 70.5, 63.1, 61.8, 55.4, 53.4, 53.0, 29.0, 28.1, 28.1, 27.3, 25.8, 25.6. LC-MS (method A1): tR = 3.69 min, m/z = 654.4 [M + H]+.
Di-tert-butyl trans-2,2’-((2-(((3-(benzyloxy)pyridin-2-yl)methyl)(2-(tert-butoxy)-2-oxoethyl)amino) cyclohexyl)azanediyl)diacetate (16i) was isolated in 94% yield. 1H NMR (500 MHz, CDCl3) δ 8.58 (s, 1H), 8.20 (d, J = 4.8 Hz, 1H), 7.46 (d, J = 7.4 Hz, 2H), 7.39 (t, J = 7.4 Hz, 2H), 7.34 (t, J = 7.3 Hz, 1H), 7.28 (d, J = 8.0 Hz, 1H), 5.19 (s, 2H), 4.20 (q, J = 14.6 Hz, 2H), 3.61 (d, J = 17.4 Hz, 1H), 3.47 (d, J = 17.4 Hz, 1H), 3.31 (d, J = 17.2 Hz, 2H), 3.25 (d, J = 17.1 Hz, 2H), 2.89 – 2.59 (m, 2H), 2.08 (d, J = 12.3 Hz, 1H), 2.01 (d, J = 12.1 Hz, 1H), 1.68 (d, J = 8.4 Hz, 2H), 1.39 (s, 9H), 1.38–1.13 (m, 2H), 1.10–1.03 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 171.2, 166.5, 153.7, 147.9, 138.6, 135.9, 128.7, 128.3, 127.5, 123.7, 120.5, 80.9, 80.7, 70.5, 62.8, 62.2, 53.4, 52.9, 51.1, 28.1, 28.1, 27.8, 25.5, 25.4. LC-MS (method A1): tR = 5.45 min, m/z = 654.3[M + H]+.
General protocols for synthesis of compounds 17a-i
BBr3 deprotection.
To a solution of compounds 16b,e (115 mg, 0.2 mmol) in dry DCM (7 mL) under a nitrogen atmosphere was added BBr3 (2 mmol) at −78˚C. The resulting mixture was warmed to room temperature slowly and stirred at room temperature overnight. Then the reaction mixture was quenched by addition of water (2 ml) and neutralized with sodium carbonate. After removing the organic solvents, the resulting mixture was purified by C18 preparative HPLC (Method P1) to give the corresponding final compounds 17b,c in 50–52% yield.
TFA deprotection.
Compounds 16a,d,f-i (0.02 mmol) were dissolved in 2.0 ml TFA, and the resulting mixture was stirred at room temperature for 5 h. The corresponding ligand 17d,f-i was isolated as a white solid in 87–92% yield after removal of TFA en vacuo.
PyC3A-4-OMe-3,5-diMe (17a) was isolated in 92% yield. 1H NMR (500 MHz, D2O) δ 7.97 (s, 1H), 3.64 (d, J = 13.0 Hz, 1H), 3.60 (s, 3H), 3.28 (d, J = 13.0 Hz, 1H), 3.15 (d, J = 15.9 Hz, 1H), 2.88 (d, J = 15.9 Hz, 1H), 2.79 (d, J = 15.9 Hz, 1H), 2.62 (d, J = 15.6 Hz, 1H), 2.28 (d, J = 15.2 Hz, 1H), 2.11 (td, J = 11.4, 2.7 Hz, 1H), 2.07 (s, 3H), 2.02 (s, 3H), 1.93 (dd, J = 18.3, 10.3 Hz, 2H), 1.67 (d, J = 10.5 Hz, 2H), 1.54 (d, J = 10.1 Hz, 1H), 1.44 (d, J = 10.5 Hz, 1H), 1.02 (dd, J = 23.1, 11.1 Hz, 1H), 0.83 (dq, J = 24.7, 12.4 Hz, 2H), 0.64 (dd, J = 22.4, 10.3 Hz, 1H). 13C NMR (126 MHz, D2O) δ 180.3, 180.2, 180.1, 178.9, 163.4, 156.6, 150.4, 126.2, 125.5, 60.1, 59.8, 57.2, 56.6, 53.6, 52.5, 51.4, 25.2, 25.0, 23.8, 23.2, 12.4, 10.2. LC-MS (method A1): tR = 2.46 min, m/z = 438.1 [M + H]+.
PyC3A-4-OH-3,5-diMe (17b) was isolated in 50% yield. 1H NMR (500 MHz, D2O) δ 8.13 (s, 1H), 7.42 (s, 1H), 3.37 (d, J = 12.0 Hz, 1H), 2.97 (dd, J = 22.1, 14.0 Hz, 2H), 2.71 (d, J = 14.8 Hz, 1H), 2.59 (d, J = 15.8 Hz, 1H), 2.41 (d, J = 15.4 Hz, 1H), 2.10 (d, J = 15.2 Hz, 1H), 1.90 (d, J = 7.9 Hz, 2H), 1.75 (s, 3H), 1.64 (s, 3H), 1.49 (d, J = 12.7 Hz, 2H), 1.33 (d, J = 39.8 Hz, 2H), 0.84–0.78 (m, 1H), 0.72–0.63 (m, 2H), 0.51–0.45 m, 1H). 13C NMR (126 MHz, d2o) δ 180.3, 180.2, 179.1, 171.6, 171.1, 171.1, 168.3, 153.3, 147.9, 147.7, 122.6, 121.2, 59.7, 56.3, 56.0, 53.8, 52.9, 51.1, 25.2, 24.9, 23.9, 23.1, 13.9, 13.9, 11.1. LC-MS (method A1): tR = 2.14 min, m/z = 424.0 [M + H]+.
PyC3A-3-OH (17c) was isolated 52% yield. 1H NMR (500 MHz, D2O) δ 7.31 (d, J = 4.6 Hz, 1H), 6.72 (dd, J = 8.2, 4.6 Hz, 1H), 6.61 (d, J = 8.2 Hz, 1H), 3.67 (d, J = 12.5 Hz, 1H), 3.04 (s, 1H), 3.01 (d, J = 3.8 Hz, 1H), 2.80 (d, J = 15.4 Hz, 1H), 2.67 (d, J = 15.7 Hz, 1H), 2.54 (d, J = 15.7 Hz, 1H), 2.23 (d, J = 16.2 Hz, 1H), 1.98 (d, J = 9.3 Hz, 2H), 1.87 (d, J = 11.0 Hz, 1H), 1.83 – 1.74 (m, 1H), 1.57 (d, J = 12.2 Hz, 1H), 1.38 (d, J = 22.2 Hz, 2H), 0.90 – 0.81 (m, 1H), 0.80 – 0.64 (m, 2H), 0.69–0.55 (m, 1H). 13C NMR (126 MHz, D2O) δ 180.5, 179.4, 168.3, 162.0, 148.4, 134.4, 125.9, 123.7, 60.1, 56.9, 56.7, 53.8, 51.4, 50.5, 25.2, 25.0, 23.5, 23.1. LC-MS (method A1): tR = 1.61 min, m/z = 396.1 [M + H]+.
PyC3A-4-OMe (17d) was isolated in 90% yield. 1H NMR (500 MHz, D2O) δ 7.98 (d, J = 5.8 Hz, 1H), 6.60 (d, J = 2.3 Hz, 1H), 6.55 (dd, J = 5.8, 2.5 Hz, 1H), 3.56 (s, 3H), 3.29 (d, J = 13.2 Hz, 1H), 3.23 (d, J = 12.8 Hz, 1H), 3.00 (d, J = 16.1 Hz, 1H), 2.75 (br, 1H), 2.65 (d, J = 15.4 Hz, 1H), 2.51 (br, 1H), 2.18 (br, 1H), 2.05 – 1.90 (m, 2H), 1.77–1.72 (m, 2H), 1.55 (d, J = 11.5 Hz, 1H), 1.37 (d, J = 9.2 Hz, 1H), 1.32–1.29 (m, 1H), 0.84 (m, 1H), 0.75 – 0.62 (m, 2H), 0.51 (m, 1H). 13C NMR (126 MHz, D2O) δ 179.8, 178.8, 168.3, 165.9, 160.0, 151.3, 110.4, 108.4, 59.6, 57.0, 56.4, 55.4, 55.3, 53.2, 51.5, 24.9, 24.8, 22.9, 22.9. LC-MS (method A1): tR = 1.63 min, m/z = 410.1 [M + H]+.
PyC3A-3-OMe (17e) was isolated in 92%. 1H NMR (500 MHz, D2O) δ 7.86 (d, J = 4.6 Hz, 1H), 7.25 (d, J = 8.3 Hz, 1H), 7.18 (dd, J = 8.4, 4.8 Hz, 1H), 3.73 (s, 3H), 3.66 (d, J = 11.8 Hz, 1H), 3.33 (d, J = 11.8 Hz, 1H), 3.16 (d, J = 15.8 Hz, 1H), 2.86 (d, J = 16.0 Hz, 1H), 2.73 (d, J = 15.6 Hz, 1H), 2.61 (d, J = 16.0 Hz, 1H), 2.19 (d, J = 16.4 Hz, 1H), 2.17 – 2.02 (m, 2H), 1.95 (d, J = 12.2 Hz, 1H), 1.66 (d, J = 12.7 Hz, 1H), 1.62 – 1.45 (m, 2H), 1.43 (br, 1H), 1.02 (dd, J = 23.3, 11.4 Hz, 1H), 0.83 (p, J = 11.7 Hz, 2H), 0.59 (dd, J = 18.5, 10.7 Hz, 1H). 13C NMR (126 MHz, D2O) δ 179.9, 178.9, 163.0, 162.8, 154.7, 146.8, 139.9, 124.6, 120.6, 59.2, 56.9, 56.1, 54.3, 51.9, 50.9, 25.2, 24.9, 23.1, 22.9. LC-MS (method A1): tR = 1.58 min, m/z = 410.1 [M + H]+.
PyC3A-3-COOEt (17f) was isolated in 90% yield. 1H NMR (500 MHz, CD3OD) δ 8.76 (d, J = 4.9 Hz, 1H), 8.49 (dd, J = 7.9, 1.3 Hz, 1H), 7.56 (dd, J = 7.8, 5.0 Hz, 1H), 5.00 (br, 1H), 4.42 (q, J = 7.1 Hz, 2H), 4.22 (d, J = 16.5 Hz, 1H), 4.1–3.3 (m, 6H), 3.16 (d, J = 10.0 Hz, 1H), 2.37 (d, J = 11.8 Hz, 1H), 2.20 (d, J = 8.4 Hz, 1H), 1.93 (d, J = 10.0 Hz, 1H), 1.84 (br, 1H), 1.66 (dd, J = 22.4, 10.5 Hz, 1H), 1.42 (t, J = 7.1 Hz, 3H), 1.40 – 1.04 (m, 4H). 13C NMR (126 MHz, CD3OD) δ 168.1, 164.8, 151.1, 139.5, 124.8, 123.2, 64.7, 61.6, 60.3, 52.2, 24.6, 24.3, 24.1, 13.1. LC-MS (method A1): tR = 2.73 min, m/z = 452.1 [M + H]+.
PyC3A-5-COOBn (17g) was isolated in 87% yield. 1H NMR (500 MHz, CD3OD) δ 9.10 (d, J = 2.1 Hz, 1H), 8.40 (dd, J = 8.1, 2.0 Hz, 1H), 7.94 (d, J = 8.2 Hz, 1H), 7.48 (d, J = 7.3 Hz, 2H), 7.40 (t, J = 7.5 Hz, 2H), 7.36 (d, J = 7.1 Hz, 1H), 5.40 (s, 2H), 4.48 (d, J = 15.0 Hz, 1H), 4.01 (br, 1H), 3.72 (br, 4H), 3.55 (d, J = 17.0 Hz, 1H), 3.46 (br, 2H), 3.22 (t, J = 10.2 Hz, 2H), 2.30 (d, J = 12.7 Hz, 1H), 2.23 (d, J = 12.2 Hz, 1H), 1.88 (d, J = 8.3 Hz, 2H), 1.47 (m, 2H), 1.42 – 1.24 (m, 4H). 13C NMR (126 MHz, CD3OD) δ 171.3, 169.7, 164.7, 149.3, 138.2, 128.2, 128.0, 127.9, 125.4, 123.9, 66.7, 61.5, 48.1, 24.6, 24.3, 24.2, 24.0. LC-MS (method A1): tR = 3.04 min, m/z = 514.1 [M + H]+.
PyC3A-5-OBn (17h) was isolated in 91% yield. 1H NMR (500 MHz, D2O) δ 8.43 (s, 1H), 7.54 (d, J = 7.0 Hz, 2H), 7.50 (t, J = 7.2 Hz, 2H), 7.45 (d, J = 7.1 Hz, 1H), 7.35 (d, J = 8.2 Hz, 1H), 7.23 (d, J = 8.3 Hz, 1H), 5.26 (s, 2H), 3.85 (d, J = 13.1 Hz, 1H), 3.63 (d, J = 13.3 Hz, 1H), 3.53 (s, 1H), 3.47 (d, J = 16.1 Hz, 1H), 3.08 (d, J = 15.9 Hz, 1H), 2.99 (br, 1H), 2.70 (br, 2H), 2.51 (m, 1H), 2.27 (m, 1H), 2.01 (br, 2H), 1.77 – 1.66 (m, 2H), 1.28 – 1.20 (m, 1H), 1.13 (m, 1H), 0.92 (m, 2H). 13C NMR (126 MHz, D2O) δ 179.9, 179.7, 169.1, 153.9, 151.6, 139.2, 139.1, 136.7, 128.9, 128.7, 128.3, 128.1, 124.9, 124.7, 124.0, 71.4, 60.9, 58.2, 56.4, 56.2, 55.1, 53.9, 25.6, 25.4, 23.8, 23.6. LC-MS (method A1): tR = 2.72 min, m/z = 486.2 [M + H]+.
PyC3A-3-OBn (17i) was isolated in 92% yield. 1H NMR (500 MHz, D2O) δ 7.81 (s, 1H), 6.98 (d, J = 7.4 Hz, 2H), 6.93 (s, 2H), 6.87 (t, J = 7.3 Hz, 2H), 6.78 (t, J = 7.1 Hz, 1H), 4.56 (s, 2H), 3.58 (d, J = 13.1 Hz, 1H), 3.08 – 2.99 (m, 1H), 2.95 (d, J = 15.8 Hz, 1H), 2.75 (d, J = 17.0 Hz, 1H), 2.48 (d, J = 16.4 Hz, 2H), 2.16 (d, J = 15.2 Hz, 1H), 1.96 – 1.85 (m, 1H), 1.71 – 1.53 (m, 2H), 1.45 (s, 2H), 1.06 (dd, J = 23.4, 11.6 Hz, 2H), 0.62 (d, J = 11.1 Hz, 1H), 0.51 (br, 1H), 0.28 (m, 2H). 13C NMR (126 MHz, D2O) δ 180.1, 179.1, 178.7, 168.3, 152.9, 147.9, 142.3, 136.4, 128.3, 127.7, 127.3, 123.5, 120.1, 69.7, 59.7, 57.5, 56.7, 53.2, 51.7, 49.5, 25.3, 24.8, 23.3, 23.2. LC-MS (method A1): tR = 2.78 min, m/z = 486.1 [M + H]+.
General procedures complexes 2–10.
The syntheses were adapted from previously published procedures.40, 56 MnCl2 was titrated into buffered solutions of ligand 17a-i and complex formation was monitored by LC-MS (to check for any free chelate) and T2 1H NMR (any excess Mn(II) results in a sharp r2 increase). Physicochemical mesurements were performed on solutions containing a slight excess of ligand (<1%) to ensure no interferences from the free Mn ion. Complexes used for in vivo studies (4, 6, and 10) were isolated as white solids as follows: A batch of ligand 17c, 17e or 17i (70.0 µmol) and MnCl2•4H2O (0.020 g, 101 µmol) were combined in 5 mL H2O and the pH adjusted to neutral. The mixture was purified by RP-HPLC using MethodP2. The fractions containing pure product were freeze dried to yield pure complex 4, 6, and 10 as white solids in 48%, 38%, and 34% yield, respectively. Purity and identity of 2-10 was determined by LC-MS using Method A2 recording at 254 nm. All complexes were isolated in 95% or greater purity. LC-MS traces showing purity, corresponding m/z, and tR of complexes 2-10 are shown in Figures S3–11.
Supplementary Material
ACKNOWLEDGMENT
We kindly thank Dr. Khalid Shah (Harvard Medical School) for providing mc26 cells.
Funding Sources
This work was supported by grants from the National Institutes of Health: HL128899, HL119145, EB022804, EB009062, RR014075, RR023385, and OD010650.
ABBREVIATIONS
- MRI
magnetic resonance imaging
- GBCA
gadolinium-based MRI contrast agent
- OATP
Organic anion transporting peptides
- PyC3A
N-picolyl- N, N′, N′- trans-1,2-cyclohexylenediaminetriacetate
- FLASH
fast angle low shot
- RARE
rapid acquisition with refocused echo
Footnotes
Conflict of Interest Disclosure
P.C. and E.M.G. are co-founders and hold equity in Reveal Pharmaceuticals, a company that is developing a Mn-based MRI contrast agent.
Supporting Information
Compounds 1 through 17i as molecular-formula strings. Structures of commercially available GBCAs, linear calibration curve of tR vs. log P of standards used to determined est. log P, LC-MS traces showing purity and tR of tested complexes 2-10, a plot of plasma Ka vs. HSA Ka, plots of r2 as a function of logP and plasma protein Ka, plots correlating delayed liver enhancement and 10 min liver uptake of Mn complexes to est. logP, T2-weighted imaging of liver tumors and histopathology confirming the presence of liver tumors, tabulated r2 data, and tabulated biodistribution data are available in as Supporting Information available free of charge on the ACS Publications website.
REFERENCES
- 1.Moon M; Cornfield D; Weinreb J Dynamic Contrast-Enhanced Breast MR Imaging. Magn. Reson. Imaging Clin. N. Am 2009, 2. [DOI] [PubMed] [Google Scholar]
- 2.Essig M; Dinkel J; Gutierrez JE Use of Contrast Media in Neuroimaging. Magn. Reson. Imaging Clin. N. Am 2012, 20, 633–648. [DOI] [PubMed] [Google Scholar]
- 3.Yang S; Law M; Zagzag D; Wu HH; Cha S Dynamic Contrast-Enhanced Perfusion MR Imaging Measurements of Endothelial Permeability: Differentiation between Atypical and Typical Meningiomas. Am. J. Neuroradiol 2003, 24, 1554–1559. [PMC free article] [PubMed] [Google Scholar]
- 4.Chandra T; Pukenas B; Mohan S; Melhem E Contrast-Enhanced Magnetic Resonance Angiography. Magn. Reson. Imaging Clin. N. Am 2012, 20, 687–698. [DOI] [PubMed] [Google Scholar]
- 5.Leiner T; Michaely H Advances in Contrast Enhanced MR Angiography of the Renal Arteries. Magn. Reson. Imaging Clin. N. Am 2008, 16, 561–572. [DOI] [PubMed] [Google Scholar]
- 6.Keston P; Murray AD; Jackson A Cerebral Perfusion Imaging using Contrast-Enhanced MRI. Clin. Radiology 2003, 58, 505–513. [DOI] [PubMed] [Google Scholar]
- 7.Lima JAC Myocardial Viability Assessment by Contrast-Enhanced Magnetic Resonance Imaging. J. Am. Coll. Cardiol 2003, 42, 902–904. [DOI] [PubMed] [Google Scholar]
- 8.Catalano OA; Manfredi R; Vanzulli A; Tomei E; Napolitano M; Esposito A; Resnick D MR Arthrography of the Glenohumeral Joint: Modified Posterior Approach without Imaging Guidance. Radiology 2007, 242, 550–554. [DOI] [PubMed] [Google Scholar]
- 9.Andreisek G; Duc SR; Froehlich JM; Hodler J; Weishaupt D MR Arthrography of the Shoulder, Hip, and Wrist: Evaluation of Contrast Dynamics and Image Quality with Increasing Injection-to-Imaging Time. Am. J. Roentgenol 2007, 188, 1081–1088. [DOI] [PubMed] [Google Scholar]
- 10.Grobner T; Prischl FC Gadolinium and Nephrogenic Systemic Fibrosis. Kidney Int 2007, 72, 260–264. [DOI] [PubMed] [Google Scholar]
- 11.Kanda T; Fukusato T; Matsuda M; Toyoda K; Oba H; Kotoku J; Haruyama T; Kitajima K; Furui S Gadolinium-Based Contrast Agent Accumulates in the Brain Even in Subjects Without Severe Renal Dysfunction: Evaluation of Autopsy Brain Specimens with Inductively Coupled Plasma Mass Spectroscopy. Radiology 2015, 276, 228–232. [DOI] [PubMed] [Google Scholar]
- 12.Kanda T; ishii K; Kawaguchi H; Kitajima K; Takenaka D High Signal Intensity in the Dentate Nucleus and Globus Pallidus on Unenhanced T1-Weighted MR Images: Relationship with Increasing Cumulative Dose of a Gadolinium-Based Contrast Material. Radiology 2014, 270, 834–841. [DOI] [PubMed] [Google Scholar]
- 13.Burke LMB; Ramalho M; AlObaidy M; Chang E; Jay M; Semelka RC Self-Reported Gadolinium Toxicity: A Survey of Patients with Chronic Symptoms. Magn Reson Imaging 2016, 34, 1078–1080. [DOI] [PubMed] [Google Scholar]
- 14.Semelka RC; Ramalho J; Vakharia A; AlObaidy M; Burke LM; Jay M; Ramalho M Gadolinium Deposition Disease: Initial Description of a Disease That has been Around for a While. Magnetic Resonance Imaging 2016, 34, 1383–1390. [DOI] [PubMed] [Google Scholar]
- 15.PRAC Confirms Restrictions on the Use of Linear Gadolinium Agents. In EMA, Ed. 2017. [Google Scholar]
- 16.FDA. FDA Drug Safety Communication: FDA Warns That Gadolinium-based Contrast Agents (GBCAs) are Retained in the Body; Requires New Class Warnings. In FDA, Ed. 2017. [Google Scholar]
- 17.Kanda T; Osawa M; Oba H; Toyoda K; Kotoku J; Haruyama T High Signal Intensity in Dentate Nucleus on Unenhanced T1-Weighted MR Images:Association with Linear Versus Macrocyclic Gadolinium Chelate Administration. Radiology 2015, 275, 803–809. [DOI] [PubMed] [Google Scholar]
- 18.Radbruch A; Weberling LD; Kieslich PJ; Eidel O; Burth S; Kickingereder P; Heiland S; Wick W; Schlemmer HP; Bendszus M Gadolinium Retention in the Dentate Nucleus and Globus Pallidus is Dependent on the Class of Contrast Agent. Radiology 2015, 275, 783–791. [DOI] [PubMed] [Google Scholar]
- 19.Splendiani A; Perri M; Marsecano C; Vellucci V; Michelini G; Barile A; Di Cesare E Effects of Serial Macrocyclic-Based Contrast Materials Gadoterate Meglumine and Gadobutrol Administrations on Gadolinium-Related Dentate Nuclei Signal Increases in Unenhanced T1-Weighted Brain: a Retrospective Study in 158 Multiple Sclerosis (MS) Patients. Radiologia Medica 2018, 123, 125–134. [DOI] [PubMed] [Google Scholar]
- 20.Lord ML; Chettle DR; Grafe JL; Noseworthy MD; McNeill FE Observed Deposition of Gadolinium in Bone Using a New Noninvasive in Vivo Biomedical Device: Results of a Small Pilot Feasibility Study. Radiology 2018, 287, 96–103. [DOI] [PubMed] [Google Scholar]
- 21.Murata N; Gonzalez-Cuyar LF; Murata K; Fligner C; Dills R; Hippe D; Maravilla KR Macrocyclic and Other Non–Group 1 Gadolinium Contrast Agents Deposit Low Levels of Gadolinium in Brain and Bone Tissue Preliminary Results From 9 Patients With Normal Renal Function. Invest. Radiol 2016, 51, 447–453. [DOI] [PubMed] [Google Scholar]
- 22.Choi JY; Lee JM; Sirlin CB CT and MR Imaging Diagnosis and Staging of Hepatocellular Carcinoma. Part II. Extracellular Agents, Hepatobiliary Agents, and Ancillary Imaging Features. Radiology 2014, 273, 30–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi JY; Lee JM; Sirlin CB CT and MR Imaging Diagnosis and Staging of Hepatocellular Carcinoma: Part I. Development, Growth, and Spread: Key Pathologic and Imaging Aspects. Radiology 2014, 272, 634–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ekanger LA; Polin LA; Shen YM; Haacke EM; Allen MJ Evaluation of Eu-II-based Positive Contrast Enhancement after Intravenous, Intraperitoneal, and Subcutaneous Injections. Contrast Media & Molecular Imaging 2016, 11, 299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng W; Haedicke IE; Nofiele J; Martinez F; Beera K; Scholl TJ; Cheng H-LM; Zhang X.-a. Complementary Strategies for Developing Gd-Free High-Field T1 MRI Contrast Agents Based on Mn(III) Porphyrins. J. Med. Chem 2014, 57, 516–520. [DOI] [PubMed] [Google Scholar]
- 26.Anemone A; Consolino L; Longo DL MRI-CEST Assessment of Tumour Perfusion Using X-ray Iodinated Agents: Comparison with a Conventional Gd-Based Agent. European Radiology 2017, 27, 2170–2179. [DOI] [PubMed] [Google Scholar]
- 27.Paech D; Schuenke P; Koehler C; Windschuh J; Mundiyanapurath S; Bickelhaupt S; Bonekamp D; Baumer P; Bachert P; Ladd ME; Bendszus M; Wick W; Unterberg A; Schlemmer HP; Zaiss M; Radbruch A T1 rho- Weighted Dynamic Glucose-enhanced MR Imaging in the Human Brain. Radiology 2017, 285, 914–922. [DOI] [PubMed] [Google Scholar]
- 28.Rao MR; Stewart NJ; Griffiths PD; Norquay G; Wild JM Imaging Human Brain Perfusion with Inhaled Hyperpolarized Xe-129 MR Imaging. Radiology 2018, 286, 659–665. [DOI] [PubMed] [Google Scholar]
- 29.Boehm-Sturm P; Haeckel A; Hauptmann R; Mueller S; Kuhl CK; Schellenberger EA Low-Molecular-Weight Iron Chelates May Be an Alternative to Gadolinium-based Contrast Agents for T1-weighted Contrast-enhanced MR Imaging. Radiology 2018, 286, 537–546. [DOI] [PubMed] [Google Scholar]
- 30.Gale EM; Caravan P Gadolinium-Free Contrast Agents for Magnetic Resonance Imaging of the Central Nervous System. ACS Chem. Neurisci 2018, 9, 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen HVT; Chen QX; Paletta JT; Harvey P; Jiang Y; Zhang H; Boska MD; Ottaviani MF; Jasanoff A; Rajca A; Johnson JA Nitroxide-Based Macromolecular Contrast Agents with Unprecedented Transverse Relaxivity and Stability for Magnetic Resonance Imaging of Tumors. Acs Central Science 2017, 3, 800–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toth GB; Varallyay CG; Horvath A; Bashir MR; Choyke PL; Daldrup-Link HE; Dosa E; Finn JP; Gahramanov S; Harisinghani M; Macdougall I; Neuwelt A; Vasanawala SS; Ambady P; Barajas R; Cetas JS; Ciporen J; DeLoughery TJ; Doolittle ND; Fu RW; Grinstead J; Guimaraes AR; Hamilton BE; Li X; McConnell HL; Muldoon LL; Nesbit G; Netto JP; Petterson D; Rooney WD; Schwartz D; Szidonya L; Neuwelt EA Current and Potential Imaging Applications of Ferumoxytol for Magnetic Resonance Imaging. Kidney International 2017, 92, 47–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drahoš B; Lukeš I; Tóth E Mn(II) Complexes as Potential Contrast Agents for MRI. Eur. J. Inorg. Chem 2012, 2012, 1975–1986. [Google Scholar]
- 34.Mahnken AH; Pereira PL; de Baere T Interventional Oncologic Approaches to Liver Metastases. Radiology 2013, 266, 407–430. [DOI] [PubMed] [Google Scholar]
- 35.Ferlay J; Soerjomataram I; Dikshit R; Eser S; Mathers C; Rebelo M; Parkin DM; Forman D; Bray F Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. International Journal of Cancer 2015, 136, E359–E386. [DOI] [PubMed] [Google Scholar]
- 36.Caravan P; Farrar CT; Frullano L; Uppal R Influence of Molecular Parameters and Increasing Magnetic Field Strength on Relaxivity of Gadolinium- and Manganese-Based T1-Contrast Agents. Contrast Media. Mol. Imag 2009, 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aschner JL; Aschner M Nutritional Aspects of Manganese Homeostasis. Mol. Aspects. Med 2005, 26, 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Montfoort JE; Stieger B; Meijer DKF; Weinmann HJ; Meier PJ; Fattinger KE Hepatic Uptake of the Magnetic Resonance Imaging Contrast Agent Gadoxetate by the Organic Anion Transporting Polypeptide OATP1. Journal of Pharmacology and Experimental Therapeutics 1999, 290, 153–157. [PubMed] [Google Scholar]
- 39.Yang L; Krefting I; Gorovets A; Marzella L; Kaiser J; Boucher R; Rieves D Nephrogenic Systemic Fibrosis and Class Labeling of Gadolinium-based Contrast Agents by the Food and Drug Administration. Radiology 2012, 265, 248–253. [DOI] [PubMed] [Google Scholar]
- 40.Gale EM; Atanasova I; Blasi F; Ay I; Caravan P A Manganese Alternative to Gadolinium for MRI Contrast. J. Am. Chem. Soc 2015, 137, 15548–15557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gale EM; Wey HY; Ramsay I; Yen YF; Sosnovik D; Caravan P A Manganese-Based Alternative to Gadolinium: Contrast Enhanced MR Angiography, Pharmacokinetics, and Metabolism. Radiology 2018, 286, 865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schuhmann-Giampieri G; Schmitt-Willich H; Press WR; Negishi C; Weinmann HJ; Speck U Preclinical Evaluation of GD-EOB-DTPA as a Contrast Agent in MR Imaging of the Hepatobiliary System. Radiology 1992, 183, 59–64. [DOI] [PubMed] [Google Scholar]
- 43.Caravan P Protein-Targeted Gadolinium-Based Magnetic Resonance Imaging (MRI) Contrast Agents: Design and Mechanism of Action. Acc. Chem. Res 2009, 42, 851–862. [DOI] [PubMed] [Google Scholar]
- 44.Lorusso V; Anelli PL; Morisetti A Gadocoletic Acid Trisodium Salt (B22956/1): A New Blood Pool Magnetic Resonance Contrast Agent With Application in Coronary Angiography. Invest Radiol 2006, 41. [DOI] [PubMed] [Google Scholar]
- 45.Sangster J Octanol-Water Partition Coefficients of Simple Organic Compounds. J. Phys. Chem. Ref. Data 1989, 18, 1111–1229. [Google Scholar]
- 46.Aime S; Anelli PL; Botta M; Brocchetta M; Canton S; Fedeli F; Gianolio E; Terreno E Relaxometric Evaluation of Novel Manganese(II) Complexes for Application as Contrast Agents in Magnetic Resonance Imaging. J. Biol. Inorg. Chem 2002, 7, 58–67. [DOI] [PubMed] [Google Scholar]
- 47.Troughton JS; Greenfield MT; Greenwood JM; Dumas S; Wiethoff AJ; Wang J; Spiller M; McMurry TJ; Caravan P Synthesis and Evaluation of a High Relaxivity Manganese(II)-based MRI Contrast Agent. Inorg. Chem 2004, 43, 6313–6323. [DOI] [PubMed] [Google Scholar]
- 48.Toft KG; Hustvedt SO; Grant D; Martinsen I; Gordon PB; Friisk GA; Korsmo a. J.; Skotland T Metabolism and Pharmacokinetics of Mn-DPDP in Man. Acta Radiolog. 1997, 38, 677–689. [DOI] [PubMed] [Google Scholar]
- 49.Torres CG; Lundby B; Sterud AT; McGill S; Gordon PB; Bjerknes HS MnDPDP for MR imaging of the liver - Results from the European phase III studies. Acta Radiologica 1997, 38, 631–637. [DOI] [PubMed] [Google Scholar]
- 50.Hamm B; Vogl TJ; Branding G; Schnell B; Taupitz M; Woft K-J; LIssner J Focal Liver Lesions: MR Imaging with MnDPDP Initial Clinical Results in 40 Patients. Radiology 1992, 182, 167–174. [DOI] [PubMed] [Google Scholar]
- 51.Xue SH; Yang H; Qiao JJ; Pu F; Jiang J; Hubbard K; Hekmatyar K; Langley J; Salarian M; Long RC; Bryant RG; Hu XP; Grossniklaus HE; Liu ZR; Yang JJ Protein MRI Contrast Agent with Unprecedented Metal Selectivity and Sensitivity for Liver Cancer Imaging. Proceedings of the National Academy of Sciences of the United States of America 2015, 112, 6607–6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Islam MK; Kim S; Kim HK; Park S; Lee GH; Kang HJ; Jung JC; Park JS; Kim TJ; Chan Y Manganese Complex of Ethylenediaminetetraacetic Acid (EDTA)-Benzothiazole Aniline (BTA) Conjugate as a Potential Liver-Targeting MRI Contrast Agent. Journal of Medicinal Chemistry 2017, 60, 2993–3001. [DOI] [PubMed] [Google Scholar]
- 53.Fulmer GR; Miller AJM; Sherden NH; Gottlieb HE; Nudelman A; Stoltz BM; Bercaw JE; Goldberg KI NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar]
- 54.Minick DJ; Frenz JH; Patrick MA; Brent DA A Comprehensive Method for Determining Hydrophobicity Constants by Reversed-Phase High-Performance Liquid Chromatography. J. Med. Chem 1988, 31, 1923–1933. [DOI] [PubMed] [Google Scholar]
- 55.Krass JD; Jastorff B; Genieser H-G Determination of Lipophilicity by Gradient Elution High-Performance Liquid Chromatography. Anal. Chem 1997, 69, 2575–2581. [DOI] [PubMed] [Google Scholar]
- 56.Zhu J; Gale EM; Atanasova I; Rietz TA; Caravan P Hexameric Mn(II) dendrimer as MRI contrast agent. Chem. Eur. J 2014, 20, 14507–14513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.