Abstract
Background:
Pregnancy is associated with improvement in immunoregulation that persists into the geriatric phase. Impaired immunoregulation is implicated in Alzheimer’s disease (AD) pathogenesis. Hence, we investigate the relationship between pregnancy and AD.
Methods:
Cross-sectional cohort of British women (N = 95). Cox proportional hazards modeling assessed the putative effects of cumulative months pregnant on AD risk and the mutually adjusted effects of counts of first and third trimesters on AD risk.
Results:
Cumulative number of months pregnant, was associated with lower AD risk (β = −1.90, exp(β) = 0.15, P = .02). Cumulative number of first trimesters was associated with lower AD risk after adjusting for third trimesters (β = −3.83, exp(β) = 0.02, P < .01), while the latter predictor had no significant effect after adjusting for the former.
Conclusions:
Our observation that first trimesters (but not third trimesters) conferred protection against AD is more consistent with immunologic effects, which are driven by early gestation, than estrogenic exposures, which are greatest in late gestation. Results may justify future studies with immune biomarkers.
Keywords: reproductive history, pregnancy, parity, Alzheimer’s disease, immunoregulation, adaptive immunity, autoimmunity
Introduction
Inflammatory processes are implicated in the pathogenesis of Alzheimer’s disease (AD). 1,2 Reproductive life history is known to influence inflammatory pathways and affect inflammatory disease activity, both in terms of short-term symptomology (eg, asthma, 3 rheumatoid arthritis, 4,5 multiple sclerosis 6 ) and long-term risk (eg, allergies, 7,8 systemic sclerosis, 9,10 rheumatoid arthritis 4,11,12 ). Pregnancy is an especially important modifier of women’s inflammatory activity. Inflammation as a possible link warrants an investigation of whether a woman’s pregnancy history influences her AD risk. Only a small number of studies have addressed the possibility that aspects of reproductive life history might influence AD risk, and authors largely ignore inflammation in discussing those results. We critically evaluate these studies and further discuss how reproductive life history affects risk of other maladies with similar etiologies. Using data collected from our cross-sectional study of British women, we explore the possibility that women’s pregnancy life history influences risk of Alzheimer’s-type dementia.
Alzheimer’s and Adaptive Immunity
There is abundant evidence to support the concept that AD is a systemic inflammatory disease. 13,14 A full review of inflammation in AD is beyond the scope of this article, but given the ways in which pregnancy modifies the adaptive immune system, it is important to highlight the role of T-cells in AD etiology. T-cells are more numerous in the AD brain than healthy brains, 15,16 potentially as a result of the blood–brain barrier dysregulation that is typical of AD neuropathy. 17 Participants with AD exhibit more activated T-cells both in the periphery and the brain compared to age-matched controls. 18 This increase in T-cells has been attributed to the CD4+ compartment, 14,19 with greater concentrations of effector memory cells (CD45RA−CCR7−), specifically late differentiated cells (CD28−CD27−), and lower concentrations of early differentiated (CD28+CD27+) and naive CD4+ cells (CD45RA−CCR7+). 20,21
Characterization of the upregulated CD4+ cells in AD reveals that among individuals with AD, excessive inflammation is exhibited that is type 1 dominant, with elevated levels of TH1-associated cytokines. 2,22 –24 TH1 cells can influence AD pathogenesis both from within the brain and from the periphery: Pro-inflammatory cytokines secreted by activated TH1 cells in the periphery can cross the blood–brain barrier and activate dendritic cells, microglia, and astrocytes, and amyloid-β has an activating effect on microglia and astrocytes, stimulating preferential TH1 proliferation. 16 Importantly, while individuals with AD exhibit proliferation of effector CD4+ cells, this is not the case for the CD4+ cells with a suppressive phenotype, regulatory T-cells (TRegs; CD25+FoxP3+CD127low). 21 In healthy individuals, sufficient TReg supply regulates effector T-cell activity and prevents excessive inflammation. It has been suggested that insufficient TReg repositories may contribute to AD pathogenesis. 20,21
Converging evidence suggests that alterations in CD4+ subset concentrations may be an early hallmark of AD, potentially contributing to the pathological cascade. 25,26 Upregulation of late differentiated T-cells and depletion of naive T-cells and TRegs are apparent during the preclinical (mild cognitive impairment [MCI]) and early stages of AD symptomology 19 and do not appear to change over the course of AD progression. 21 Rodent models of AD support the notion that the immunological changes characteristic of AD occur before neurocognitive deterioration, 26 and experimental amplification of TReg response delays onset 27 and can even reverse AD-like cognitive impairment. 28 These AD-characteristic immune profiles described here do not occur in individuals with other dementias, 19,29 supporting the likelihood that this inflammatory profile is specific to AD. Individuals with AD do not exhibit greater degrees of immunosenescence than age-matched controls, 30 suggesting that neurotypical “inflammaging” is not responsible for these changes. Inflammaging is a concept developed by Franceschi et al 31 to describe typical, age-related, chronic, low-grade inflammation characterized by immunosenescence, but patients with AD do not exhibit higher concentrations of pro-inflammatory biomarkers C-reactive protein and interleukin 6 compared to controls. 30
Pregnancy and Adaptive Immunity
In a woman’s (postnatal) life span, the most dramatic increases in TRegs occur during the first trimester of pregnancy. Evidence suggests there are exponential TReg increases in pre- and early pregnancy, stable levels across late pregnancy, and mild increases postpartum, which plausibly could persist for the rest of the life span. Specifically, pregnancy is maintained by an increase in TReg cells that, some evidence suggests, could begin as early as coitus in response to seminal fluid exposure in preparation for embryo implantation. 32 -36 The TReg levels rise further at implantation and during the first 2 pregnancy trimesters. 32,37 Pregnancy-induced TRegs are generated in the periphery 38 and migrate to the fetal–maternal interface, leading to lower detectable levels of TRegs in the maternal periphery during pregnancy. 6 In human pregnancy (unlike murine pregnancy 38 ), elevated TReg levels are maintained postpartum. 39,40 Somerset et al demonstrated that maternal TReg concentrations showed a significant increase from prepregnancy to 6 to 8 weeks postpartum (4.4% vs 7.5% of peripheral lymphocytes). The TReg levels continue to rise for a year throughout the postpartum phase at a rate of 4% increase per month, 41 and so maternal peripheral TReg frequency is significantly higher postpartum compared with during pregnancy. 6 There is evidence that within the TReg proliferation that occurs with pregnancy, the TRegs specific for fetal antigens are expelled with decidual tissue and those without such specificity are retained in the maternal body. 39,42,43 It remains unknown how long these changes in T-cell subsets persist beyond 1 year postpartum.
While pregnancy induces increases in TRegs that suppress effector T-cells, there is still immune activity during pregnancy, and immunosuppression is not complete. The effector cells that are upregulated in pregnancy are TH2 dominant, suppressing TH1 inflammation. In addition to the maternal immune modifications, the conceptus secretes TH2 cytokines that downregulate TH1 cytokines, 44 which may influence the maternal compartment.
In sum, the changes that occur in the CD4+ compartment during pregnancy (proliferation of TRegs, downregulation of type 1 inflammation) are in direct contrast with the typical profile of the CD4+ compartment in AD (depletion of TRegs, upregulation of type 1 inflammation). We speculate that if pregnancy’s immunologic alterations persist across the life span, we might expect that women with more pregnancies should benefit from greater protection against AD pathogenesis.
Hypotheses
This study tests the overarching hypothesis that women who spend more cumulative time pregnant in their lives will experience reduction in AD risk via improvement in immunoregulation. To test this hypothesis, our study considers women’s cumulative number of months pregnant with relation to AD risk. If pregnancy were to protect against AD via greater repositories of TRegs, then cumulative number of months pregnant would be a better predictor of AD risk than parity. Cumulative number of first trimesters would be a better predictor than parity because the major changes in TReg concentrations during pregnancy occur in the early phase, so regardless of whether the pregnancy lasted to completion, the benefit from increased concentrations of TRegs might persist.
We acknowledge the possibility that even if we observe that cumulative time pregnant is correlated with AD risk, an alternative explanation for this relationship could be related to estrogen exposure. Cumulative months pregnant could be a proxy for duration of estrogen exposure (via longer reproductive span) or quantity of estrogen exposure (via pregnancy-associated high concentrations of estrogen). Estrogen levels rise exponentially during pregnancy, with typical plasma concentrations during the third trimester of pregnancy approximately 85 times levels typical during an ovulatory menstrual cycle (calculated from Tulchinsky and Little 45 ). Several in vitro and animal studies have demonstrated estrogen’s role in inhibiting and reversing AD-specific brain insults, 46 -50 and human studies have investigated how lifetime duration of endogenous estrogen exposure may influence later-life cognitive performance 51 -55 and AD risk. 56 -58 It could be hypothesized that this higher dose of estrogen exposure might confer reduction in AD risk.
We adopted 2 strategies for distinguishing between an immunologic versus estrogenic explanation for pregnancy’s hypothesized effect on AD risk. Firstly, more pregnancies could be associated with longer reproductive span, defined as the time between menarche and menopause, which has been used as a proxy measure of duration estrogen exposure. 59,60 We addressed the possibility of cumulative time pregnant acting as a proxy for reproductive span by adjusting for reproductive span in all analyses. Secondly, more pregnancies could be associated with greater quantity of estrogen exposure. If pregnancy were to protect against AD via greater concentrations of estrogen, later pregnancy would exert a more potent anti-AD effect than early pregnancy because of the exponential nature of estrogen’s increase across the course of pregnancy. We addressed the possibility of cumulative months pregnant acting as a proxy for high doses of estrogen exposure by conducting 2 separate analyses of the reliance of AD risk upon a woman’s cumulative number of first trimesters (proxy for immunoregulation) and the reliance of AD risk upon a woman’s cumulative number of third trimesters (proxy for estrogenic neuroprotection).
A summary of our hypotheses is that we anticipate (1) cumulative months pregnant will be negatively associated with AD risk, (2) cumulative months pregnant will be a better predictor of AD risk than parity, and (3) cumulative number of first trimesters will be a better predictor of AD risk than cumulative number of third trimesters.
Methods
Cohort
Women aged 70 to 100 years along with family member(s) and/or carer(s) were recruited for participation through nursing homes, churches, community centers, the Alzheimer’s Society, and a retired employee community from 2010 to 2012. Participants received a modest gift voucher as incentive. The protocol had approval from the University of Cambridge Human Biology Research Ethics Committee. Participants were informed of research purpose, activities, and confidentiality. Proband, informant (family member or carer), and, when necessary, legally authorized representative provided written informed consent.
Procedures
Each session consisted of an interview collecting information about reproductive history and factors that would potentially confound the relationship of dementia status with reproductive history, including use of contraceptive and menopause hormone therapies. Information was collected through detailed interviews with probands, family members, carers, nursing home staff, and written records, when necessary and available. Exclusionary criteria included self, informant, or carer report of proband having non-Alzheimer’s-type dementia (eg, vascular, Parkinsonian) or any possible external injury to the brain (eg, head impact injury, brain tumor). Ten cases were excluded from the analysis because of these criteria (Table S1). A weakness in the study design was lack of information gathered about immunopathology. Dementia status was measured by the Clinical Dementia Rating (CDR) scale, consisting of a 60- to 90-minute interview conducted in 2 parts, one with the proband and the other with an informant, that is, her relative or carer. In the CDR, probands are evaluated in 6 categories: memory, orientation, judgment and problem–solving, home and hobbies, community affairs, and personal care. The “sum of boxes (SOB)” was used as a continuous variable, as has become standard in clinical trials, 61,62 computed from the sum of each category score creating a scale from 0 to 18. Cases and controls were not distinguished until CDR-SOB scores were calculated, at which time individuals scoring “0” were designated as controls. Details of the study protocol are described in previous publications. 58,63
Variable Calculations
Age at Alzheimer’s onset
For the purposes of the Cox model, the time-to-event was defined as years between age 50 and CDR-SOB score turning from 0 to 0.5, indicating onset of AD symptoms. This was estimated based on CDR-SOB score at the time of interview. Using published AD progression norms 64 (typical number of years spent in each dementia phase), a scale was created to estimate age at onset for each possible CDR-SOB score by interpolating CDR-SOB scores between the end points of other scales’ categories. Year at which CDR-SOB score would have progressed from 0 to 0.5 was back-extrapolated from the observed degree of dementia at the time of interview. Details of this methodology are described in Supplementary Methods.
Predictive variables
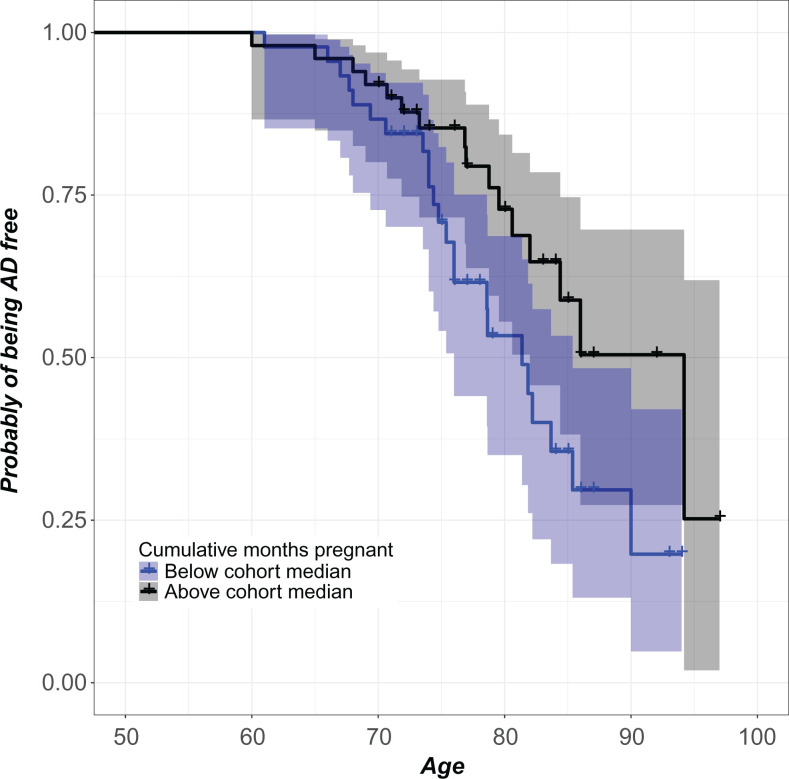
Cumulative months pregnant was calculated in a comprehensive manner, such that all pregnancies including miscarriages and medical terminations were included. Information was collected about the trimester at which spontaneous and elective abortions occurred. For calculating cumulative months pregnant, number of first trimesters and number of third trimesters, we considered a first-trimester pregnancy termination to be equivalent to 3 months spent pregnant, and second trimester was considered 6 months (there were no third-trimester terminations in this cohort). All child-yielding pregnancies, including stillbirths, were included in the variable calculation as 9 months. Parity was calculated as each woman’s total number of delivered births, including live births and stillbirths. Predictive variables were natural logarithm transformed to improve the symmetry of the distributions. Quantification of covariates follows standard procedures and is described in Table S2. When necessary, continuous covariates were transformed to improve symmetry of distribution. All effect size coefficients were back-transformed for interpretability (Figure 1).
Figure 1.
Women with more cumulative months pregnant had lower AD risk. For each age, the plot reports the covariate-adjusted probability of being AD-free for women with total lifetime number of months pregnant below the cohort median (lower curve) and above the cohort median (upper curve). Pointwise 95% confidence bands are also shown. The purpose of this plot is to give a visual sense of the magnitude of the effect by dichotomizing the number of cumulative months pregnant variable. Cox regression of the reliance of AD risk on median-split dichotomous characterization of cumulative months pregnant demonstrates that women above the cohort median exhibit 37.01% lower AD risk compared with women below the cohort median (β = −.99, exp(β) = .37, se(β) = .40, P = .01, 95% CI = 0.17-0.81). The Cox model reported in Table 2 represents a more meaningful analysis by utilizing the continuous cumulative months pregnant variable. AD indicates Alzheimer’s disease; CI, confidence interval.
Statistical Tests
In a main effects analysis, each predictive variable contributed into the Cox model a coefficient, “coef,” whose value is estimated on the basis of the data. When exponentiated, “exp(coef),” this parameter yields the ratio of hazards (probability of AD onset per unit time) between 2 hypothetical women who are identical except for a unit difference between their respective values of the predictive variable. “Alzheimer’s disease-free time” was defined as the retrospectively estimated number of years in excess of age 50, prior to the interview, during which the woman was free from AD. The AD-free time for those women who were judged to be free from AD at the time of the interview was treated as right censored, as is common practice in survival analysis. The dependency of AD-free time on the predictive variables was analyzed via Cox proportional hazards model. Plots of the martingale residuals revealed that the model fits were not unduly influenced by particular cases (Figures S1 and S2).
We undertook a 2-step process to select covariates for the Cox models. Firstly, we identified all variables that might confound the statistical relationship between pregnancy history and AD risk (Table S2). Each of these variables was independently tested for covariance with each predictive and outcome variable. Secondly, those variables that exhibited significant (P < .10) relationships with both a predictive and an outcome variable were included in models. Additionally, 2 interaction terms were included to investigate whether subsets of women exhibited different relationships between pregnancy history and AD risk based upon (1) whether or not they breastfed and (2) whether or not they had a first-degree relative with dementia. These 2 interaction terms were selected because of previously reported importance of breastfeeding history and family history of dementia with AD risk in this cohort. 63
Additionally, for cases (CDR-SOB > 0), we performed a linear regression to check whether incomplete pregnancies were statistically related to the degree of dementia (CDR-SOB score) at interview, in order to determine whether our ability to detect incomplete pregnancies was biased related to proband memory impairment.
Results
Cohort Statistics
To investigate the role of pregnancy history on AD risk in a cohort of British women, a subset of 95 women from the total cohort of 133 women were included in the analyses. All women were of white British ethnic identity, currently residing in England. Ten probands from the initial cohort of 133 were excluded from analyses due to factors that would cause non-Alzheimer’s-type dementia (eg, stroke) or could obscure the effects of reproductive history (eg, ovarian cancer). Twenty-eight more participants were excluded due to missing information (Table S1).
Degree of dementia at the time of interview varied across the full range of possible CDR-SOB scores, with some of the participants judged to have no sign of cognitive impairment (N = 56) and some assessed to have CDR-SOB scores of 0.5 or higher (N = 39) at interview (Table 1). Of the controls, 83% were born in England and 86% were educated to age 16 or less, and of the cases, 82% were born in England and 92% were educated to age 16 or less. Comparing reproductive patterns between the control group and the case group, we observed identical median ages at menarche, menopause, similar reproductive spans, and ages at first birth. No statistically significant differences existed between cases and controls for any reproductive life history variables (Table 1). We investigated potential detection bias in the case sample and found no significant relationship between severity of dementia and number of incomplete pregnancies in the subset of cases (linear model results: R2 = 0.0, F1, 48 = 0.9, P = .34). This null result suggests that detection of incomplete pregnancies was not biased due to the memory loss associated with degree of dementia. Our methods involving interview of informants and consultation of written records may have contributed to greater accuracy than participants with dementia could have provided on their own. There were significant differences in age at interview, education, and occupation between the case and control subsets of the cohort (Table 1). Each of these variables was investigated for covariate status (Table S2). Education was found to be correlated with both predictive and outcome variables and was therefore included in multivariate models (Table S2).
Table 1.
Cohort Characteristics.a
| Controls, N = 56 | Cases, N = 39 | Controls Versus Cases | |
|---|---|---|---|
| Global CDR score, median (SD) | NA | 3.0 (1.1) | NA |
| CDR-SOB score, median (SD) | NA | 15.0 (6.5) | NA |
| Age at menarche, median (SD), years | 13 (1.54) | 13 (1.70) | t test P = 0.2, NS |
| Age at menopause, median (SD), years | 50 (5.63) | 50 (6.49) | t test P = 0.6, NS |
| Age at natural menopause only, N = 88, median (SD), years | 50 (5.26) | 50 (5.60) | t test P = 0.9, NS |
| Reproductive span, median (SD), years | 38 (5.49) | 37 (6.98) | t test P = 0.4, NS |
| Age at first birth, median (SD), years | 26 (4.14) | 25 (3.48) | t test P = 0.4, NS |
| Parity, continuous, median (SD) | 2 (1.20) | 2 (1.81) | t test P = 0.2, NS |
| Cumulative months pregnant, median (SD), months | 26 (11.81) | 21 (16.70) | t test P = 0.5, NS |
| Cumulative breastfeeding duration, median (SD); months | 9 (11.40) | 6 (11.55) | t test P = 0.5, NS |
| Hysterectomy, n (%) | χ2 test P = 0.2, NS | ||
| No | 23 (41) | 28 (72) | |
| Yes | 19 (34) | 11 (28) | |
| Unknown | 14 (25) | 0 (0) | |
| Bilateral oophorectomy, n (%) | χ2 test P = 0.4, NS | ||
| No | 52 (93) | 36 (92) | |
| Yes | 7 (13) | 3 (8) | |
| Parity, binary, n (%) | χ2 test P = 0.8, NS | ||
| Nulliparous | 5 (9) | 2 (5) | |
| Parous | 51 (91) | 37 (95) | |
| Miscarriages, n (%) | χ2 test P = 0.2, NS | ||
| None | 37 (66) | 33 (85) | |
| One | 12 (21) | 4 (10) | |
| Two | 5 (9) | 0 (0) | |
| Three | 1 (2) | 1 (3) | |
| Four | 1 (2) | 1 (3) | |
| Medical abortions, n (%) | χ2 test P = 1.0, NS | ||
| None | 53 (95) | 36 (92) | |
| One | 0 (0) | 3 (8) | |
| Two | 3 (5) | 0 (0) | |
| Age at interview, median (SD), years | 77 (6.68) | 86 (5.79) | t(88.5) = −5.2, P = 0.00b |
| Place born, n (%) | χ2 test P = 0.2, NS | ||
| Cambridge | 13 (23) | 6 (15) | |
| London | 14 (25) | 5 (13) | |
| Other Southern England | 15 (27) | 12 (31) | |
| Northern England | 5 (9) | 9 (23) | |
| Scotland, Wales, Ireland | 7 (13) | 6 (15) | |
| Outside United Kingdom | 2 (4) | 1 (3) | |
| Education, n (%) | t(148.1) = 3.9, P = 0.00b | ||
| To age 16 or less | 48 (86) | 36 (92) | |
| Past age 16 | 8 (14) | 3 (8) | |
| Occupation, n (%) | χ2 (8) = 17.4, P = 0.03c | ||
| No work | 1 (2) | 3 (8) | |
| Telephonist, technician | 2 (4) | 7 (18) | |
| Secretary, clerical, post office | 23 (41) | 21 (54) | |
| High office job, artist, design, fashion, retail | 8 (14) | 2 (5) | |
| Teacher, librarian | 10 (18) | 1 (3) | |
| Nurse | 7 (13) | 1 (3) | |
| Social worker, consultant, architect | 2 (4) | 2 (5) | |
| Factory, land, odd jobs | 3 (5) | 2 (5) | |
| Smoking history, n (%) | χ2 test P = 0.2, NS | ||
| Never or <1 year | 31 (55) | 19 (49) | |
| 1-10 years | 5 (9) | 2 (5) | |
| 11-20 years | 3 (5) | 4 (10) | |
| >20 years | 7 (13) | 12 (31) | |
| Unknown | 10 (18) | 2 (5) | |
| Alcohol consumption, n (%) | χ2 test P = 1.0, NS | ||
| ≤2 servings per day | 43 (77) | 34 (92) | |
| >2 servings per day | 3 (5) | 3 (8) |
Abbreviations: CDR, Clinical Dementia Rating; NA, not applicable; NS, not significant P > .10; SD, standard deviation; SOB, sum of boxes.
a While age at interview and education differed significantly between cases and controls, neither was significantly correlated with any predictors, and therefore, these potential covariates were dropped from model design (Table S2). Occupation was included as a covariate in all models.
b P < .001, P < .05.
Hypothesis 1: More Cumulative Months Pregnant Is Associated With Lower Alzheimer’s Risk
In a Cox proportional hazards model adjusting for age at first birth, reproductive span, and history of breastfeeding, marriages, and occupation, we found that AD risk had a significant dependence on cumulative months pregnant, with more months pregnant associated with lower AD risk (Table 2). For example, a woman who spent 3% more total months pregnant than another (otherwise identical) woman would have approximately 5.50% (25th-75th percentiles 3.9-7.0%) lower in AD risk (P = .02), i.e., this would apply to two (otherwise identical) women who had spent 34 versus 33 months pregnant. Similarly, 2.8% more total months pregnant was associated with 5.5% (25th-75th percentiles 0.5-10.3%) lower AD risk (P = .03). These results are consistent with our prediction that pregnancy may exert long-term protective effects against AD risk potentially due to the benefits of pregnancy-induced TReg proliferation but does not rule out other plausible biomechanisms of neuroprotection.
Table 2.
Cox Models Measuring Relationship Between Pregnancy History by Months and Parity and Alzheimer’s Disease Risk.a
| Model Number | Parameter, Natural Log Transformed | Coef | Exp(coef) | Se(coef) | P Value | 95% CI |
|---|---|---|---|---|---|---|
| 1 | Cumulative months pregnant | −1.901 | 0.1495 | 0.821 | .021b | 0.030-0.747 |
| 2 | Cumulative months pregnant, adjusted for parity | −2.049 | 0.1289 | 0.958 | .032b | 0.020-0.843 |
| 3 | Parity | 0.666 | 1.947 | 0.526 | .205 (NS) | 0.695-5.458 |
| 4 | Parity, adjusted for cumulative months pregnant | 1.693 | 5.436 | 1.067 | .113 (NS) | 0.671-44.019 |
Abbreviations: CI, confidence interval; NS, not significant.
a Cox model analysis of the dependence of AD risk on cumulative months pregnant and parity defined as number of full-term pregnancies. All models are adjusted for age at first birth, reproductive span, and history of breastfeeding, marriages, and occupation. The table reports the partial likelihood point estimate for the effect of the parameter, the corresponding exponentiated value, the standard error, the P value for the relative sharp null hypothesis, and the 95% confidence interval for exp(coef). The partial likelihood ratio test P value for the null hypothesis of no effect for model 1 was .005, model 2 was .008, model 3 was .038, and model 4 was .039. The score log-rank test P value for model 1 was .004, model 2 was .007, model 3 was .055, and model 4 was .047. Models were fitted on the basis of 95 sample individuals (10 observations deleted due to ineligibility and 28 observations deleted due to lack of data), for a total of 39 observed failure events.
b P < .05.
Hypothesis 2: Cumulative Months Pregnant Is a Better Predictor of AD Risk Than Parity
In a Cox proportional hazards model controlling for age at first birth, reproductive span, and history of breastfeeding, marriages, and occupation, we found that the number of births (both live childbearing and stillbirths, ie, “parity”) was not significantly associated with the risk of AD (P = .21, and P = .11 adjusted for cumulative months pregnant; Table 2). This metric, parity, is a less precise reflection of a woman’s full pregnancy history than cumulative months pregnant and has been the construct of interest in previous studies.
Hypothesis 3: Cumulative Number of First Trimesters Is a Better Predictor of AD Risk Than Third Trimesters
In separate Cox proportional hazards models, all controlling for age at first birth, reproductive span, and history of breastfeeding, marriages, and occupation, we assessed AD risk reliance on first trimesters alone and third trimesters alone, first while adjusting for third trimesters and third while adjusting for first trimesters. We found that AD risk had a significant dependence on cumulative number of first trimesters and no significant dependence on cumulative number of third trimesters (Table 3). For example, a woman who had 20% more first trimesters than another (otherwise identical) woman would have approximately 30% (25th-75th percentiles 22.4-36.4%) lower AD risk (P = .02) i.e., this would apply to two (otherwise identical) women who had 6 versus 5 total first trimesters. Models that measure the reliance of AD risk on first trimesters while adjusting for third trimesters, and vice versa, demonstrated similar results (Table 3). Furthermore, the 95% confidence interval for the exponentiated coefficients do not overlap (models 6 and 8 in Table 3), suggesting that the reduction in AD risk brought by the first 3 months of a new pregnancy is greater than that brought by the final 3 months of an ongoing pregnancy (Table 3). These data provide evidence against the idea that greater quantity of estrogen exposure explains pregnancy’s protective effect against AD risk, which would be most dependent on third trimesters, and instead support the possibility of an immunoregulatory mechanism, which would be most dependent on first trimesters.
Table 3.
Cox Models Measuring Relationship Between Pregnancy History by Trimester and Alzheimer’s Disease Risk.a
| Model Number | Parameter, Natural Log Transformed | Coef | Exp(coef) | Se(coef) | P Value | 95% CI |
|---|---|---|---|---|---|---|
| 5 | First trimesters | −1.936 | 0.144 | 1.807 | .016c | 0.030-0.702 |
| 6 | First trimesters, adjusted for third trimesters | −3.834 | 0.022 | 1.292 | .003d | 0.002-0.272 |
| 7 | Third trimesters | −8.024 | 0.448 | 0.703 | .254 (NS) | 0.113-1.777 |
| 8 | Third trimesters, adjusted for first trimesters | 1.386 | 4.000 | 1.358 | .307 (NS) | 0.280-57.226 |
Abbreviations: CI, confidence interval; NS, not significant.
a Cox model analysis of the dependence of AD risk on cumulative number of first and third trimesters. All models are adjusted for age at first birth, reproductive span, and history of breastfeeding, marriages, and occupation.
The table reports the partial likelihood point estimate for the effect of the parameter, the corresponding exponentiated value, the standard error, the P value for the relative sharp null hypothesis, and the 95% confidence interval for exp(coef). The partial likelihood ratio test P value for the null hypothesis of no effect for model 5 was .058, model 6 was .002, model 7 was .122, and model 8 was .002. The score log-rank test P value for model 5 was .023, model 6 was .001, model 7 was .061, and model 8 was .002. The models were fitted on the basis of 95 sample individuals (10 observations omitted due to ineligibility and 28 observations omitted due to lack of data), for a total of 39 observed failure events.
bP < .05.
cP < .01.
Discussion
We find that women who spent more months of life pregnant exhibited a significant, dose-dependent reduction in AD risk. Our results support the possibility that pregnancy protects against later-life AD onset, potentially due to pregnancy’s characteristic increase in TReg proliferation. Previous studies found effects in the opposite direction, with higher parity associated with earlier onset of AD, 55,65 and one study reported that women who had 3 or more pregnancies had triple the AD risk. 55 It is possible that the inconsistent results for the reliance of AD risk between our study versus studies of parity could be due to those studies’ neglect of the considerable variation in breastfeeding rates and incomplete pregnancies.
Other studies have explored the relationship between women’s parity and geriatric cognitive performance (which may or may not be indicative of AD risk) with mixed results. One study found that higher parity was associated with better memory ability in elderly women, 53 and others have observed that estrogen replacement therapy’s beneficial effect on cognitive function improved with increasing parity, although this effect was not statistically significant. 66 However, there has been more robust evidence for the opposite trend: One study found higher parity associated with worse cognitive function, 54 and another found that women who had 5 or more pregnancies had worse cognitive impairment compared with those who had fewer pregnancies. 51 It should be noted that pregnancy’s potential anti-AD effect due to improvement in immunoregulation may not be relevant for non-AD-related cognitive decline. Therefore, in these studies of non-AD cognitive decline, 51,53,54,66 the mechanisms and pathways responsible for differences in cognitive performance may be considerably different and potentially variable between individuals and study cohorts. Further research is necessary to resolve whether failure to consider incomplete pregnancies and breastfeeding in studies of AD, as well as whether there may be contradictory risk factors for AD and non-AD cognitive decline, accounts for some inconsistencies in previous study results.
Immunoregulatory or Estrogenic Pathway?
Our observation that more cumulative months pregnant is associated with reduced AD risk could potentially be attributable to a number of explanations. We suggest the most likely explanation is related to immunoregulation. Other possible explanations could be that cumulative months pregnant is a proxy for duration of estrogen exposure (longer reproductive span) or quantity of estrogen exposure (pregnancy-associated high concentrations of estrogen). We address these possibilities in 2 ways. Firstly, we attended to duration of estrogen exposure by controlling for reproductive span in all models and still found that AD had a significant reliance on cumulative months pregnant (Table 2). Secondly, we compared cumulative number of first trimesters (mean [M] = 3.0, standard deviation [SD] = 1.8) to third trimesters (M = 2.5, SD = 1.5). If pregnancy exerts its anti-AD effect via recruitment of TRegs, then first trimesters would be expected to exert the stronger effect because the most dramatic acceleration in TReg recruitment occurs from the nonpregnant to early pregnant state. 40,41 Conversely, if pregnancy exerts its anti-AD effect via quantity of estrogen exposure, then third trimesters would be expected to exert the stronger effect because estrogen levels rise exponentially during pregnancy. Our results are more consistent with an immunologic explanation and less with an estrogenic mechanism.
Pregnancy and Autoimmunity
Alzheimer’s disease is characterized by a number of immunologic similarities with autoimmune diseases, including not only TH1 dominance and insufficient TRegs but also the presence of autoantibodies. 67,68 There is abundant evidence that pregnancy induces protection and relief from autoimmune diseases. Such evidence is consistent with the idea that pregnancy induces increases in TRegs that suppress effector T-cells and mildly upregulates TH2 inflammation. It has been known since 1938 that pregnancy is associated with symptom relief in rheumatoid arthritis, 4,5 with many people going into remission during pregnancy. 9 While previous authors have interpreted this effect with relation to estradiol or cortisol, there has been no evidence for such an association, 69,70 consistent with the possibility that proliferation of TRegs is responsible for the suppression of inflammation characteristic of rheumatoid arthritis. 71 We posit the protective effect of TRegs in pregnancy may have long-term advantages in protection against developing rheumatoid arthritis due to increases in TReg cell quantity or activity that are sustained beyond pregnancy. Nulliparous women have twice the risk of rheumatoid arthritis compared to parous women. 4,11,12 In spondyloarthropathy, another form of inflammatory arthritis, as well as autoimmune hepatitis, pregnancy usually has beneficial effects. 9,72,73 Additionally, nulliparas have increased risk of systemic sclerosis compared with parous women, 9,10 representing further evidence for a long-term protective effect. Multiple sclerosis is a particularly relevant disease to consider in light of AD because it is characterized by neuroinflammation. There is significant symptom reduction during pregnancy among women with multiple sclerosis. 6 A recent study found that individuals with amnesic MCI and multiple sclerosis exhibited similar levels of CD45+ T-cells and pro-inflammatory cytokines in cerebrospinal fluid, suggesting similar central inflammatory profiles that manifest before AD neurocognitive impairment. 25
There is also evidence that pregnancy induces protection and relief from atopies, which are TH2 dominant. This evidence is consistent with the idea that pregnancy induces increases in TRegs that suppress effector T-cells. Pregnancy may induce relief from asthma and improvement in bronchial hyperresponsiveness. 3 It is noteworthy that the improvement in asthma symptoms was observed from preconception through the second trimester, which is when TReg cells proliferate, and then there was no statistically significant change between the second and third trimester. Interpretation of this trend has not previously included discussion of TRegs but rather speculated on the role of sex steroids in asthma symptomology. 74 There is evidence that increasing parity has a beneficial effect in diminishing maternal allergies, 7,8 further evidence that pregnancy-induced change in adaptive immunity may have long-term effects for the mother.
Further evidence for pregnancy-induced long-term improvements in immunoregulation comes from studies of fetal microchimerism. Fetal cells are semi-allogeneic to the mother’s genetic identity, and after a pregnancy, fetal cells remain in the mother. It is thought that such cells persist in the mother’s body for the duration of her lifetime, 75 and thus a woman with multiple pregnancies would carry fetal microchimeric cells of multiple genetic identities. It has been postulated that maternal lymph nodes might contain increased levels of TRegs in order to sustain an immunosuppressed environment to facilitate tolerance of these populations of semiallogeneic cells, 75 as has been demonstrated in fetal lymph nodes to sustain tolerance of alloantigens. 76
Research Considerations
Limitations of this study include the small sample size, potential for recall inaccuracies or biases which may be higher than in other cohorts due to this cohort’s age range and dementia status, lack of biomarker data, lack of full medical history, and lack of information on causes of miscarriage, although it would be nearly impossible to find causal information from miscarriages that occurred as early as the 1920s. Furthermore, our data cannot test (or rule out) the possibility that estrogenic neuroprotection requires merely a mild elevation in estrogen concentration, and so the extremely high concentrations of third-trimester estrogen may be irrelevantly above the necessary threshold for reduction of AD risk. We also cannot rule out the possibility that another unknown biological pathway connects pregnancy and AD etiology.
The relationship between miscarriage and inflammation is unlikely to confound our model. Firstly, we address the issue of inflammation as a cause of incomplete pregnancies in this cohort. Sporadic miscarriage (occurring after a missed period and therefore known to the proband) is a common and normal part of a woman’s reproductive experience, affecting an estimated 1 in 4 pregnancy-attemptant women 77 and 15% of pregnancies. 78,79 There are myriad causes of sporadic miscarriage, most often and nonexclusively chromosomal abnormalities (observed in 75% of cases) and fetal malformation (observed in 85% of cases), 80 in addition to uterine abnormalities, cervical compromise, endocrine dysregulation, and toxic exposure. 79 In some cases, infection can cause inflammation that directly causes miscarriage, but there is no reason to suspect that women who experience inflammation-induced miscarriage fail to benefit from the increase in TReg concentrations that occur with seminal fluid exposure, 32 -35 conception, and implantation, 32,37 albeit insufficient immunosuppression to maintain the pregnancy to completion.
Recurrent miscarriage, defined as 3 or more consecutive miscarriages, is a rarer condition affecting an estimated 1% of pregnancy-attemptant women and can be caused by endocrine, autoimmune, or thrombotic abnormalities, 77 with the latter, sometimes, possibly caused by cytokine degradation of vasculature. 81 Because only 4% (N = 4) of the women in our cohort experienced a total of 3 or more miscarriages (Table 1), we suspect a low rate of recurrent miscarriage and thus a low degree to which incomplete pregnancy rates would be caused by the immunodysfunction associated with recurrent miscarriage. Similar to the argument above, there is no reason to suspect that women who experience inflammation-induced miscarriage fail to benefit from the increase in TReg concentrations that characterize the early stages of pregnancy, even if those changed are insufficient for successful gestational maintenance. The degree to which TReg proliferation occurs and is sustained in incomplete pregnancies is a question that requires further study. For now, there is no evidence to predict that experiencing a miscarriage would undermine our hypothesis of long-term immunoregulatory benefits of gestation.
Future research should expand upon our understanding of how reproductive history affects inflammatory mechanisms in the long term. More information is especially needed on the effects of pregnancy on T-cell activity in mothers with and without inflammatory diseases. It will be important for studies to consider the presence of pro-inflammatory alleles in understanding how immune system development, pregnancy, and other inflammation-related mechanisms affect AD risk. Further research is also needed to elucidate whether each pregnancy in a woman’s life history confers equivalent long-term changes to immune and endocrine systems. 82
Conclusion
Using data from a cohort of elderly British women, we calculated cumulative time each woman spent pregnant and fit Cox models to test the statistical dependence of AD risk on pregnancy history. We found that more months pregnant in the lifetime was associated with reduced risk of AD. The more typically consulted but less comprehensive construct, parity (number of deliveries), exhibited no significant effect. Cumulative number of first—but not third—trimesters conferred a protective effect against AD risk. These observations are consistent with a protective effect of pregnancy-induced proliferation of TRegs. Reproductive life history has an effect on maternal immune function, and there may be long-term impacts from immune cell proliferation that occurred during a woman’s reproductive years. Pregnancy is characterized by an immunosuppressive profile, and the increase in concentration of regulatory immune cells may have implications for inflammatory propensity in later life. We hope our findings prompt further study of this previously overlooked mechanism as a possible link between women’s reproductive life history and AD.
Supplemental Material
Supplementary_Material_26dec17 for Women’s Pregnancy Life History and Alzheimer’s Risk: Can Immunoregulation Explain the Link? by Molly Fox, Carlo Berzuini, Leslie A. Knapp, and Laura M. Glynn in American Journal of Alzheimer's Disease & Other Dementias
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: M.F. was supported by the Gates Cambridge Trust, Gonville & Caius College, and NIH grant K01 DK105110.
ORCID iD: Molly Fox  http://orcid.org/0000-0001-9219-8971
http://orcid.org/0000-0001-9219-8971
Supplemental Material: Supplemental material for this article is available online.
References
- 1. McNaull B, Todd S, McGuinness B, Passmore A. Inflammation and anti-inflammatory strategies for Alzheimer’s disease—a mini-review. Gerontology. 2010;56(1):3–14. [DOI] [PubMed] [Google Scholar]
- 2. Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease—a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med. 2012;2(1):a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Juniper EF, Daniel EE, Roberts RS, Kline PA, Hargreave FE, Newhouse MT. Improvement in airway responsiveness and asthma severity during pregnancy: a prospective study. Am J Respir Crit Care Med. 1989;140(4):924–931. [DOI] [PubMed] [Google Scholar]
- 4. Buyon JP. The effects of pregnancy on autoimmune diseases. J Leukoc Biol. 1998;63(3):281–287. [DOI] [PubMed] [Google Scholar]
- 5. Hench PS. The ameliorating effect of pregnancy on chronic atrophic (infectious rheumatoid) arthritis, fibrositis, and intermittent hydrathrosis. Proc Staff Meet Mayo Clinic. 1938;13:161–167. [Google Scholar]
- 6. Neuteboom RF, Verbraak E, Wierenga-Wolf AF, et al. Pregnancy-induced fluctuations in functional T-cell subsets in multiple sclerosis patients. Mult Scler. 2010;16(9):1073–1078. [DOI] [PubMed] [Google Scholar]
- 7. Forastiere F, Sunyer J, Farchi S, et al. Number of offspring and maternal allergy. Allergy. 2005;60(4):510–514. [DOI] [PubMed] [Google Scholar]
- 8. Doull I. Does pregnancy prevent atopy? Clin Exp Allergy. 2001;31(9):1335–1337. [DOI] [PubMed] [Google Scholar]
- 9. Gordon C. Pregnancy and autoimmune diseases. Best Pract Res Clin Rheumatol. 2004;18(3):359–379. [DOI] [PubMed] [Google Scholar]
- 10. Edith Pisa F, Bovenzi M, et al. Reproductive factors and the risk of scleroderma: an Italian case–control study. Arthritis Rheum. 2002;46(2):451–456. [DOI] [PubMed] [Google Scholar]
- 11. Spector TD, Roman E, Silman AJ. The pill, parity, and rheumatoid arthritis. Arthritis Rheum. 1990;33(6):782–789. [DOI] [PubMed] [Google Scholar]
- 12. Hazes J, Dijkmans B, Vandenbroucke J, Vries RRPD, Cats A. Pregnancy and the risk of developing rheumatoid arthritis. Arthritis Rheum. 1990;33(12):1770–1775. [DOI] [PubMed] [Google Scholar]
- 13. Britschgi M, Wyss-Coray T. Systemic and acquired immune responses in Alzheimer’s disease. Int Rev Neurobiol. 2007;82:205–233. [DOI] [PubMed] [Google Scholar]
- 14. Zhang R, Miller RG, Madison C, et al. Systemic immune system alterations in early stages of Alzheimer’s disease. J Neuroimmunol. 2013;256(1):38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Togo T, Akiyama H, Iseki E, et al. Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. J Neuroimmunol. 2002;124(1):83–92. [DOI] [PubMed] [Google Scholar]
- 16. Town T, Tan J, Flavell RA, Mullan M. T-cells in Alzheimer’s disease. Neuromol Med. 2005;7(3):255–264. [DOI] [PubMed] [Google Scholar]
- 17. Fiala M, Liu Q, Sayre J, et al. Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer’s disease brain and damange the blood–brain barrier. Eur J Clin Invest. 2002;32(5):360–371. [DOI] [PubMed] [Google Scholar]
- 18. Li M, Shang DS, Zhao WD, et al. Amyloid β interaction with receptor for advanced glycation end products up-regulates brain endothelial CCR5 expression and promotes T cells crossing the blood–brain barrier. J Immunol. 2009;182(9):5778–5788. [DOI] [PubMed] [Google Scholar]
- 19. Lueg G, Gross CC, Lohmann H, et al. Clinical relevance of specific T-cell activation in the blood and cerebrospinal fluid of patients with mild Alzheimer’s disease. Neurobiol Aging. 2015;36(1):81–89. [DOI] [PubMed] [Google Scholar]
- 20. Larbi A, Pawelec G, Witkowski JM, et al. Dramatic shifts in circulating CD4 but not CD8 T cell subsets in mild Alzheimer’s disease. J Alzheimers Dis. 2009;17(1):91–103. [DOI] [PubMed] [Google Scholar]
- 21. Pellicano M, Larbi A, Goldeck D, et al. Immune profiling of Alzheimer patients. J Neuroimmunol. 2012;242(1-2):52–59. [DOI] [PubMed] [Google Scholar]
- 22. Heneka MT, O’Banion MK. Inflammatory processes in Alzheimer’s disease. J Neuroimmunol. 2007;184(1):69–91. [DOI] [PubMed] [Google Scholar]
- 23. Swardfager W, Lanctot K, Rothenburg L, Wong A, Cappell J, Herrmann N. A meta-analysis of cytokines in Alzheimer’s disease. Biol Psychiatry. 2010;68(10):930–941. [DOI] [PubMed] [Google Scholar]
- 24. Huberman M, Shalit F, Roth-Deri I, Gutman B, Brodie C, Kott E, Sredni B. Correlation of cytokine secretion by mononuclear cells of Alzheimer patients and their disease stage. J Neuroimmunol. 1994;52(2):147–152. [DOI] [PubMed] [Google Scholar]
- 25. Monson NL, Ireland SJ, Ligocki AJ, et al. Elevated CNS inflammation in patients with preclinical Alzheimer’s disease. J Cereb Blood Flow Metab. 2014;34(1):30–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marchese M, Cowan D, Head E, et al. Autoimmune manifestations in the 3xTg-AD model of Alzheimer’s disease. J Alzheimers Dis. 2014;39(1):191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dansokho C, Ait Ahmed D, Tolyndour C, et al. Beneficial role of regulatory T cells in a mouse model of Alzheimer’s disease. J Neuroimmunol. 2014;275(1):124. [Google Scholar]
- 28. Bing L, Feng M, Xiaoxia F, Yihua Q. Interleukin-10 prevents rats from okadaic acid-induced Alzheimer’s disease. Acta Univ Med Nanjing (Nat Sci). 2013;7:17. [Google Scholar]
- 29. Lanuti P, Ciccocioppo F, Bonanni L, et al. Amyloid-specific T-cells differentiate Alzheimer’s disease from Lewy body dementia. Neurobiol Aging. 2012;33(11):2599–2611. [DOI] [PubMed] [Google Scholar]
- 30. Westman G, Lidehall AK, Magnusson P, et al. Decreased proportion of cytomegalovirus specific CD8 T-cells but no signs of general immunosenescence in Alzheimer’s disease. PLos One. 2013;8(10):e77921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Franceschi C, Campisi J. Chronic inflammation (Inflammaging) and its potential contribution to age-associated diseases. The Journals of Gerontology: Series A. 69(Suppl1): S4–S9. 10.1093/gerona/glu057 [DOI] [PubMed] [Google Scholar]
- 32. Guerin LR, Prins JR, Robertson SA. Regulatory T-cells and immune tolerance in pregnancy: a new target for infertility treatment? Hum Reprod Update. 2009;15(5):517–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Robertson SA. Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res. 2005;322(1):43–52. [DOI] [PubMed] [Google Scholar]
- 34. Robertson SA, Sharkey DJ. The role of semen in induction of maternal immune tolerance to pregnancy. Semin Immunol. 2001;13(4):243–254. [DOI] [PubMed] [Google Scholar]
- 35. Robertson SA, Prins JR, Sharkey DJ, Moldenhauer LM. Seminal fluid and the generation of regulatory T cells for embryo implantation. Am J Reprod Immunol. 2013;69(4):315–330. [DOI] [PubMed] [Google Scholar]
- 36. Sharkey DJ, Tremellen KP, Jasper MJ, Gemzell-Danielsson K, Robertson SA. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol. 2012;188(5):2445–2454. [DOI] [PubMed] [Google Scholar]
- 37. Kahn DA, Baltimore D. Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. PNAS. 2010;107(20):9299–9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao J, Zeng Y, Liu Y. Fetal alloantigen is responsible for the expansion of the CD4+ CD25+ regulatory T cell pool during pregnancy. J Reprod Immunol. 2007;75(2):71–81. [DOI] [PubMed] [Google Scholar]
- 39. Heikkinen J, Mottonen M, Alanen A, Lassila O. Phenotypic characterization of regulatory T cells in the human decidua. Clin Exp Immunol. 2004;136(2):373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112(1):38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wegienka G, Havstad S, Bobbitt KR, et al. Within-woman change in regulatory T cells from pregnancy to the postpartum period. J Reprod Immunol. 2011;88(1):58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tilburgs T, Roelen D, Van Der Mast B, et al. Differential distribution of CD4+ CD25bright and CD8+ CD28-T-cells in decidua and maternal blood during human pregnancy. Placenta. 2006;27(suppl A):S47–S53. [DOI] [PubMed] [Google Scholar]
- 43. Tilburgs T, Roelen DL, van der Mast BJ, et al. Evidence for a selective migration of fetus-specific CD4+ CD25bright regulatory T cells from the peripheral blood to the decidua in human pregnancy. J Immunol. 2008;180(8):5737–5745. [DOI] [PubMed] [Google Scholar]
- 44. Belderbos M, Houben M, van Bleek G, et al. Breastfeeding modulates neonatal innate immune responses: a prospective birth cohort study. Pediatr Allergy Immunol. 2012;23(1):65–74. [DOI] [PubMed] [Google Scholar]
- 45. Tulchinsky D, Little AB. Maternal–Fetal Endocrinology. 2nd ed. Philadelphia, PA: W.B. Saunders; 1994. [Google Scholar]
- 46. Bruce-Keller AJ, Keeling JL, Keller JN, Huang FF, Camondola S, Mattson MP. Antiinflammatory effects of estrogen on microglial activation. Endocrinology. 2000;141(10):3646–3656. [DOI] [PubMed] [Google Scholar]
- 47. Rogers J, Strohmeyer R, Kovelowski C, Li R. Microglia and inflammatory mechanisms in the clearance of amyloid β peptide. Glia. 2002;40(2):260–269. [DOI] [PubMed] [Google Scholar]
- 48. Henderson VW. Alzheimer’s disease: review of hormone therapy trials and implications for treatment and prevention after menopause. J Steroid Biochem Mol Biol. 2014;142:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pike CJ. Estrogen modulates neuronal Bcl-xl expression and β-amyloid-induced apoptosis. J Neurochem. 1999;72(4):1552–1563. [DOI] [PubMed] [Google Scholar]
- 50. Stoltzner SE, Berchtold NC, Cotman CW, Pike CJ. Estrogen regulates Bcl-x expression in rat hippocampus. Neuroreport. 2001;12(13):2797. [DOI] [PubMed] [Google Scholar]
- 51. Rasgon NL, Magnusson C, Johansson AL, Pedersen NL, Elman S, Gatz M. Endogenous and exogenous hormone exposure and risk of cognitive impairment in Swedish twins: a preliminary study. Psychoneuroendocrinology. 2005;30(6):558–567. [DOI] [PubMed] [Google Scholar]
- 52. Ryan J, Carriere I, Scali J, Ritchie K, Ancelin ML. Life-time estrogen exposure and cognitive functioning in later life. Psychoneuroendocrinology. 2009;34(2):287–298. [DOI] [PubMed] [Google Scholar]
- 53. Henderson V, Guthrie J, Dudley E, Burger H, Dennerstein L. Estrogen exposures and memory at midlife: a population-based study of women. Neurology. 2003;60(8):1369–1371. [DOI] [PubMed] [Google Scholar]
- 54. Heys M, Jiang C, Cheng KK, et al. Life long endogenous estrogen exposure and later adulthood cognitive function in a population of naturally postmenopausal women from Southern China: the Guangzhou Biobank Cohort Study. Psychoneuroendocrinology. 2011;36(6):864–873. [DOI] [PubMed] [Google Scholar]
- 55. Colucci M, Cammarata S, Assini A, et al. The number of pregnancies is a risk factor for Alzheimer’s disease. Eur J Neurol. 2006;13(12):1374–1377. [DOI] [PubMed] [Google Scholar]
- 56. Hong X, Zhang X, Li H. A case–control study of endogenous estrogen and risk of Alzheimer’s disease. Zhonghua Liu Xing Bing Xue Za Zhi. 2001;22(5):379–382. [PubMed] [Google Scholar]
- 57. Paganini-Hill A, Henderson VW. Estrogen deficiency and risk of Alzheimer’s disease in women. Am J Epidemiol. 1994;140(3):256–261. [DOI] [PubMed] [Google Scholar]
- 58. Fox M, Berzuini C, Knapp LA. Cumulative estrogen exposure, number of menstrual cycles, and Alzheimer’s risk in a cohort of British women. Psychoneuroendocrinology. 2013;38(12):2973–2982. [DOI] [PubMed] [Google Scholar]
- 59. Johnston SL. Associations with age at natural menopause in Blackfeet women. Am J Hum Biol. 2001;13(4):512–520. [DOI] [PubMed] [Google Scholar]
- 60. Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13(11):1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. O’Bryant SE, Waring SC, Cullum CM, et al. Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: a Texas Alzheimer’s Research Consortium Study. Arch Neurol. 2008;65(8):1091–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Coley N, Andrieu S, Jaros M, Weiner M, Cedarbaum J, Vellas B. Suitability of the Clinical Dementia Rating-Sum of Boxes as a single primary endpoint for Alzheimer’s disease trials. Alzheimers Dement. 2011;7(6):602–610. [DOI] [PubMed] [Google Scholar]
- 63. Fox M, Berzuini C, Knapp LA. Maternal breastfeeding history and Alzheimer’s disease risk. J Alzheimers Dis. 2013;37(4):809–821. [DOI] [PubMed] [Google Scholar]
- 64. Reisberg B, Jamil I, Kham S, et al. Staging dementia. In: Abou-Saleh MT, Katona CLE, Kumar A, eds. Principles and Practice of Geriatric Psychiatry. Chichester, UK: John Wiley & Sons; 2010:162–169. [Google Scholar]
- 65. Sobow T, Kutter EP, Kloszewska I. Hormonal decline indicator in women (age at menopause) modifies age of onset in sporadic Alzheimer’s disease. Alzheimer Report. 1999;2:27–30. [Google Scholar]
- 66. Dunkin J, Rasgon N, Wagner-Steh K, David S, Altshuler L, Rapkin A. Reproductive events modify the effects of estrogen replacement therapy on cognition in healthy postmenopausal women. Psychoneuroendocrinology. 2005;30(3):284–296. [DOI] [PubMed] [Google Scholar]
- 67. D’Andrea MR. Add Alzheimer’s disease to the list of autoimmune diseases. Med Hypotheses. 2005;64(3):458–463. [DOI] [PubMed] [Google Scholar]
- 68. D’Andrea MR. Evidence linking neuronal cell death to autoimmunity in Alzheimer’s disease. Brain Res. 2003;982(1):19–30. [DOI] [PubMed] [Google Scholar]
- 69. Østensen M. Glucocorticosteroids in pregnant patients with rheumatoid arthritis. Z Rheumatol. 2000;59(suppl 2):II/70–74. [PubMed] [Google Scholar]
- 70. Østensen M, Villiger PM. The remission of rheumatoid arthritis during pregnancy. Seminars in Immunopathology 2007;29(2):185–91. [DOI] [PubMed] [Google Scholar]
- 71. Amin S, Peterson EJ, Reed AM, Mueller DL. Pregnancy and rheumatoid arthritis: insights into the immunology of fetal tolerance and control of autoimmunity. Curr Rheumatol Rep. 2011;13(5):449–455. [DOI] [PubMed] [Google Scholar]
- 72. Buchel E, Van Steenbergen W, Nevens F, Fevery J. Improvement of autoimmune hepatitis during pregnancy followed by flare-up after delivery. Am J Gastroenterol. 2002;97(12):3160–3165. [DOI] [PubMed] [Google Scholar]
- 73. Uribe M, Chavez-Tapia NC, Mendez-Sanchez N. Pregnancy and autoimmune hepatitis. Ann Hepatol. 2006;5(3):187–189. [PubMed] [Google Scholar]
- 74. Kwon HL, Belanger K, Bracken MB. Effect of pregnancy and stage of pregnancy on asthma severity: a systematic review. Am J Obstet Gynecol. 2004;190(5):1201–1210. [DOI] [PubMed] [Google Scholar]
- 75. Groer MW, Manion M, Szekeres C, El-Badri NS. Fetal microchimerism and women’s health. Biol Res Nurs. 2011;13(4):346–350. [DOI] [PubMed] [Google Scholar]
- 76. Mold JE, Michaelsson J, Burt TD, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322(5907):1562–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Regan L, Rai R. Epidemiology and the medical causes of miscarriage. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14(5):839–854. [DOI] [PubMed] [Google Scholar]
- 78. Wilcox AJ, Weinberg CR, O’Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319(4):189–194. [DOI] [PubMed] [Google Scholar]
- 79. Larsen EC, Christiansen OB, Kolte AM, Macklon N. New insights into mechanisms behind miscarriage. BMC Med. 2013;11(1):154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Philipp T, Philipp K, Reiner A, Beer F, Kalousek D. Embryoscopic and cytogenetic analysis of 233 missed abortions: factors involved in the pathogenesis of developmental defects of early failed pregnancies. Hum Reprod. 2003;18(8):1724–1732. [DOI] [PubMed] [Google Scholar]
- 81. Clark DA, Coulam CB, Daya S, Chaouat G. Unexplained sporadic and recurrent miscarrage in the new millennium: a critical analysis of immune mechanisms and treatments. Hum Reprod Update. 2001;7(5):501–511. [DOI] [PubMed] [Google Scholar]
- 82. Fox M, Sandman CA, Davis EP, Glynn LM. Intra-individual consistency in endocrine profiles across successive pregnancies. J Clin Endocrinol Metab. 2015;100(12):4637–4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_Material_26dec17 for Women’s Pregnancy Life History and Alzheimer’s Risk: Can Immunoregulation Explain the Link? by Molly Fox, Carlo Berzuini, Leslie A. Knapp, and Laura M. Glynn in American Journal of Alzheimer's Disease & Other Dementias