Abstract
Background:
Fatigue and cognitive dysfunction are two common symptoms experienced by patients with multiple sclerosis (MS). The relationship between subjective and objective fatigue (fatigability) in MS is poorly understood. Cognitive control tasks might be more conducive to fatigability and more likely to show associations between subjective and objective cognitive fatigue in MS.
Objective:
To study the association between objective fatigability, as induced by a cognitive control task called the Blocked Cyclic Naming Task (BCNT), subjective fatigue and baseline cognitive functioning in patients with MS.
Methods:
Twenty-one patients with MS completed baseline questions about their disease, the Montreal Cognitive Assessment (MoCA) battery and self-reported questionnaires on trait fatigue, sleep and depression. Disability was captured using the expanded disability status scale (EDSS). Participants then performed the BCNT and were asked about their level of state momentary fatigue before and after the BCNT. The BCNT consists of several blocks of either related or unrelated pictures that participants name as quickly as possible. The pictures cycled 4 times in each block and the difference in the response times (RTs) between related and unrelated blocks was captured. Data were analyzed using repeated measures analysis of variance and Pearson correlations.
Results:
MS participants’ performance declined for the related, but not unrelated blocks. The difference in RTs between related and unrelated conditions increased with repetition across cycles (p < 0.001). Participants also showed objective fatigability with less repetition priming (p = 0.02) in the 4th quarter and with greater differences between related and unrelated conditions in the later part of the task. Objective fatigability was strongly associated with participants’ assessment of their level of momentary state fatigue (r =0.612, p =0.007).
Conclusion:
Using the appropriate tools, this study showed an association between subjective and objective cognitive fatigue in people with MS. The BCNT and cognitive control are useful tools in assessing patients with MS and should be explored in future, larger studies in this population.
Keywords: Multiple Sclerosis, Multiple Sclerosis Relapsing Remitting, Fatigue, Mental Fatigue, Cognitive Dysfunction, Repetition Priming
1. Introduction
Fatigue and cognitive dysfunction are two common and often disabling symptoms in patients with multiple sclerosis (MS).1–5 Uncovering the association between fatigue, its subtypes, and cognitive dysfunction in MS has been the focus of numerous studies,6–11 but their complex relationship remains poorly understood6–11 likely due to the choice of tools that fail to capture the multifaceted nature of fatigue in MS.4, 10, 12–14
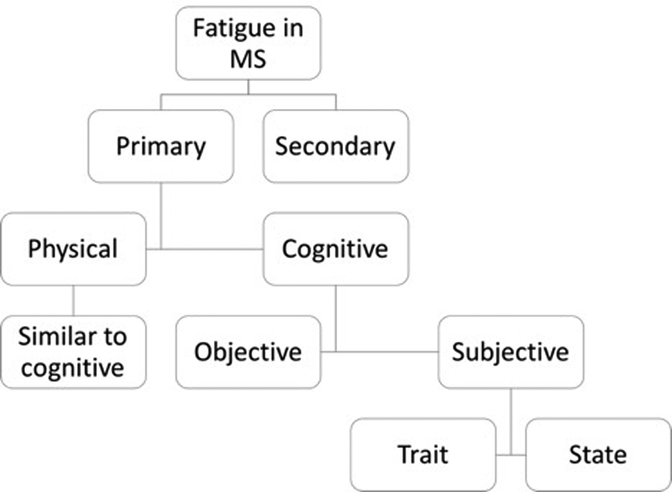
MS fatigue can be physical or cognitive and can be further described as subjective fatigue or objective fatigability.3, 10 Subjective fatigue can be thought of as a trait (long-term) or state (momentary) fatigue3, 10 (Figure 1). Whereas objective fatigability is measured as the quantitative decline in physical or cognitive performance over time, subjective fatigue is measured using patient reported instruments like the Fatigue Severity Scale (FSS)15 and the Modified Fatigue Impact Scale (MFIS)1 that assess trait fatigue and the Visual Analogue Scale (VAS) that assesses state fatigue.3, 10
Figure 1.
Flowchart of the subsets of fatigue in MS. Fatigue can either be primary or secondary to other causes. Primary fatigue can be divided into physical or cognitive components. Each component can then be subdivided into objective (performance fatigability) and subjective (individual’s perception). Furthermore, subjective fatigue can be divided into trait (long-term) and state (momentary).
In cognitive fatigue research, there is no consensus on a choice of a cognitive task (or task attributes) that captures objective fatigability and cognitive dysfunction adequately.4, 10, 12–14 Furthermore, there is often limited understanding of the differences and associations between subjective and objective cognitive fatigue10–12, 14 often resulting in negative results.6–11
We posit that selection of the right cognitive task and fatigue subtype can lead to improved understanding of subjective and objective cognitive fatigue, ultimately resulting in better treatment options. In this study, we used the Blocked Cyclic Naming Task (BCNT), a task that taps cognitive control processes, to explore MS patients’ cognitive fatigability and its association with subjective fatigue.
Cognitive control5, 16–18 is the combination of processes that allow information processing to quickly adapt and change depending on current goals.19, 20 The Stroop test21 can best illustrate this: participants are asked to focus on one stimulus and respond appropriately (name the color of the font of the written word) while simultaneously suppressing a competing stimulus (naming the written word).22 The Stroop test has revealed significant differences between MS patients and controls and between MS types, but has not captured fatigability.13, 22
The BCNT is a simple naming task that manipulates cognitive control demands by requiring participants to repeatedly name pictures presented in the context of other, semantically related (e.g. DOG, CAT, BEAR) versus unrelated pictures (e.g., DOG, CAR, RUG). Typically, participants are slower and more error prone when naming related versus unrelated items (hereafter, interference effect), which is thought to reflect competition during word retrieval due to the notion that retrieving a word activates related words from the same category. Further, pictures within each context are presented repeatedly (across a number of cycles) within a block of trials, resulting in faster response times (RTs) across cycles due to repetition priming.23–25 However, when compared to the unrelated condition, repetition priming in the related condition is attenuated due to the build-up of competition across cycles, resulting in an increasing interference effect with repetition across the cycles (hereafter, slope of the interference effect). The BCNT is used in cognitive and language research26 to understand the competitive dynamics of word retrieval, and, in turn, the processes behind semantic interference.25–28 As it pertains to the current study, a crucial finding from prior research is that variability in cognitive control function is related to the size of the semantic interference effect.24, 29, 30 In a large study of neurotypical speakers, increased semantic interference in the BCNT was associated with performance on the Stroop task.29 Studies in aphasic speakers secondary to stroke have shown that damage to a region in the prefrontal cortex implicated in cognitive control results in increased semantic interference effects when compared to aphasic speakers with lesions that spare cognitive control regions30–33 and healthy older adults.24 In particular, prefrontal cortex damage results in an exaggerated slope of the interference effect, indicating that this measure of semantic interference reflects an inability to recruit cognitive control processes required to resolve competition.30 Interestingly, semantic interference effects are comparable in younger and older adults,29, 34 suggesting that faulty cognitive control, rather than general cognitive decline due to aging, underlies the increase in semantic interference with repetition (across cycles). Taken together, these findings indicate that the BCNT can capture faulty cognitive control (in both neurologically healthy and impaired individuals) by looking at variations in performance contrasted across both context manipulations (i.e., related/unrelated) and task duration (i.e., first vs. fourth quarter of trials). These inherent qualities of the BCNT, and its relatively simplicity, make it easy to use in MS and more likely to show fatigability.
In this study, we hypothesized that MS participants will perform poorly on the related (compared to the unrelated) blocks especially later in the task, reflecting objective fatigability. We also hypothesized that this objective fatigability will be associated with subjective momentary fatigue.
2. Materials and Methods
2.1. Participants
Participants were recruited from the Neurology clinic at the University of Pennsylvania. Inclusion criteria included age older than 18 years, a confirmed diagnosis of MS, irrespective of the level of disability or MS subtype, and the ability to consent for the study. Disease modifying treatments (DMT), stimulants and neuro-depressive medication usage were recorded. This study was approved by the Institutional Review Board of the University of Pennsylvania.
2.2. Baseline cognitive function (MoCA)
The Montreal Cognitive Assessment (MoCA) is a sensitive and reliable 10-minute cognitive screening test.35, 36 It has been used successfully as a screening tool for cognitive impairment in MS.36 Higher scores indicated better performance and a score ≥26 out of 30 is considered normal.
2.3. Fatigue
Subjective trait fatigue was assessed using the MFIS and FSS. The MFIS assesses cognitive, physical and psychosocial fatigue over the past 4 weeks. Scores range from 0–84, higher scores indicate worse fatigue and participants are considered fatigued if their score is ≥38.1, 3, 37 The FSS assesses trait fatigue over the past week. Scores range from 0–7, higher scores indicate worse fatigue, and participants are considered fatigued if their score is ≥4. Subjective state fatigue was assessed using the VAS. Participants mark their current level of fatigue on a 10-cm line from 0 (no fatigue) to 10 (severe fatigue).3 The difference between the VAS immediately before (VAS1) and after (VAS2) the BCNT was calculated. The Patient-Reported Outcomes Measurement Information System (PROMIS) Sleep Disturbance Form 8a38 and the PROMIS Depression Short Form 8a39 were administered to account for other causes of MS-related fatigue.
2.4. Neurologic disability
The Expanded Disability Status Scale (EDSS) was used to assess neurologic disability.40 Scores range from 0–10, with higher scores indicating greater disability.
2.5. BCNT
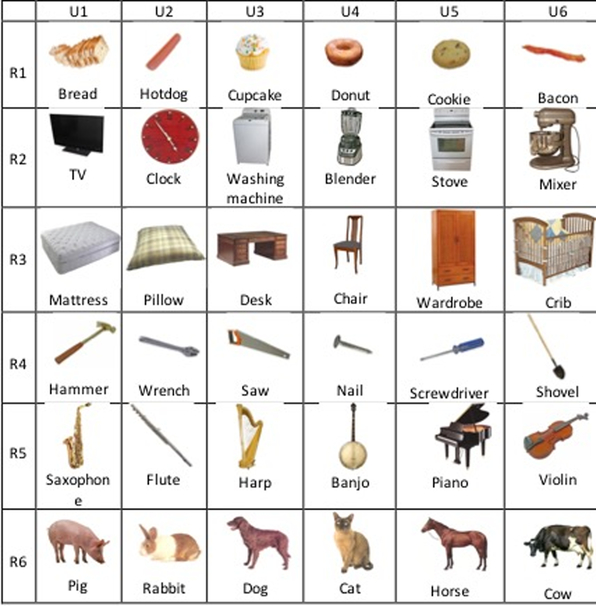
Stimuli were 72 pictures comprising 6 exemplars drawn from 12 semantic categories. Each picture appeared an equal number of times in the related and unrelated sets (Figure 2), which controls for item-specific factors that may affect naming, independent of the manipulation of interest.29, 30 One item from 6 of the categories was grouped together to form 6 unrelated blocks of 6 items each, and one item from the remaining 6 categories was group together to form an additional 6 unrelated blocks of 6 items each. There were 12 related and 12 unrelated 6-item sets, for a total of 24 blocks. Within each context condition, the 6-item sets of pictures repeated four times (cycles) in a different pseudorandom order such that no item appeared on two consecutive trials, forming a block of 24 trials. Thus, the task consisted of 576 trials. The 24 blocks were presented in pseudorandom order with the constraint that no more than three blocks of a given context condition (related/unrelated) appeared consecutively. Pseudorandomization of stimuli was carried out using the software Mix.41
Figure 2.
Example of the structure of the Blocked Cyclic Naming Task. In this illustration, there are 6 sets each of related and unrelated items (the actual experiment contained 12 sets). The rows represent examples of related blocks (R1–R6) and the columns are blocks of unrelated items (U1–U6). The same items repeat between the related and unrelated blocks.
Participants were instructed to name each picture as quickly and accurately as possible after pressing a button to begin the test. The experiment began immediately following a training session. Each image remained on the screen for 2 seconds or until the participant made a response. A fixation cross appeared on the screen for 1 second between images.
2.6. Study Procedures
Following informed consent, participants completed a demographics form, followed by the MoCA, the MFIS, FSS and the sleep and depression forms. A 10-minute optional break was offered to all participants but none required it. Participants rated their current state fatigue (VAS), then proceeded to perform the BCNT after which they were again asked to rate their current state fatigue (VAS). The EDSS was completed during the office visit that preceded or followed the study visit.
2.7. Statistical Analysis
Participants had very few naming errors, thus only RTs were included in the analysis. Statistical analysis was performed using the JASP software (version 0.9.0.1).42 At the group level, we performed within-subject repeated measures analyses of variance (ANOVA) using RTs as the dependent variable to (1) establish that participants exhibit interference effects in this task; and (2) determine whether interference and the slope of the effect increases over the course of performing the task, indicative of cognitive fatigue. In the analysis of overall performance on the BCNT, fixed factors included condition (related, unrelated) and cycle (1–4). In the BCNT objective fatigability analysis, we filtered the data to include only the first 144 trials (hereafter, 1st quarter) and the last 144 trials (hereafter, 4th quarter). We then added to the above-described ANOVA the fixed factor of quarter (1st, 4th).
Pearson correlation analyses and chi-square tests were used to study the association between key variables and two measures of performance on the BCNT: (1) overall performance, calculated as the average difference in RTs between conditions, collapsed across cycles, i.e. the interference effect and slope of the interference effect; and (2) BCNT objective fatigability, calculated as the difference between the slope of the interference effect for the 4th minus 1st quarter.
One participant was not included in the analysis because of missing data (due to a technical error) and two participants with secondary progressive MS (SPMS) were unable to complete the BCNT because of fatigue, they were included in the overall analysis but not in any analysis that involved data from the 4th quarter (i.e. fatigability analysis).
Only 3 participants had abnormalities on the PROMIS sleep and depression scales. Sleep and depression measures did not affect any of the relationships in this study and were not included in the final analysis.
3. Results
3.1. Participants
Twenty-one participants with MS were enrolled and 20 were included in the analysis. All participants provided written informed consent. Nineteen participants had relapsing-remitting MS (RRMS) and 2 had SPMS, 76% were female and the median disease duration was 13 years. Participants’ mean age was 43. Eighty-one percent of the participants were on treatment. Seventy-six percent of the participants had fatigue as indicated by the FSS and 57% had fatigue as indicated by the MFIS (cutoff score of ≥ 38) (Table 1).1, 15, 37 Eighty-one percent of the participants were on DMT but none were taking stimulants or medications that could negatively affect their performance.
Table 1 –
Characteristics of study participants
| Age | 42.95 ± 9.75 |
| Years with MS | 13.33 ± 8.66 |
| Sex | 5 males (23.80%) 16 females (76.20%) |
| On DMT | 17 (81%) on DMT |
| Mean EDSS Scores | 3.5 ± 1.6 |
| Mean MoCA Scores | 25.3 ± 2.5 |
| Participants with abnormal MoCA scores | 10 (47.6%) |
| Mean FSS Scores | 4.8 ± 1.3 |
| Participants with abnormal FSS scores | 16 (76.2%) |
| Mean MFIS Scores | 42.7 ± 15.5 |
| Participants with abnormal MFIS scores | 12 (57.14%) |
| VAS Difference | 2.7 ± 2.14 |
3.2. Overall performance on the BCNT
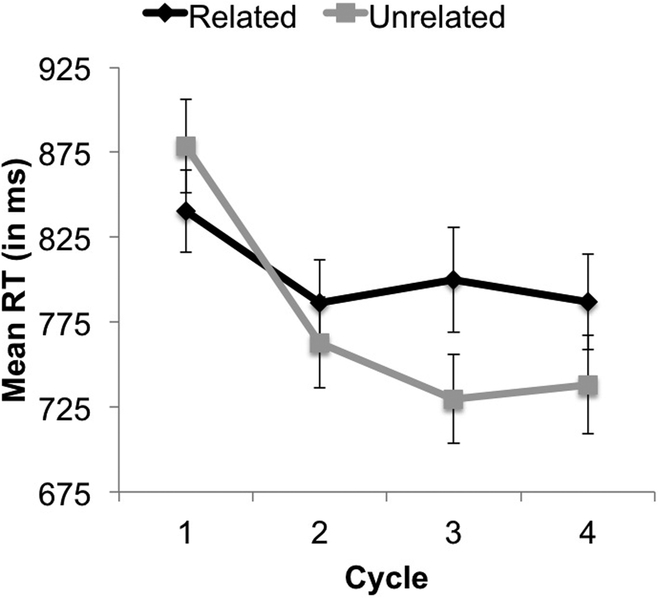
Participants had very few naming errors (mean 16, 3.4% of trials, range [0.2–15.8%]) in both the related and unrelated blocks. Analyses conducted on RTs revealed main effects of condition (slower RTs for related vs. unrelated blocks, 803 vs. 777 ms, respectively, p = 0.005) and cycle (faster RTs with repetition across cycles in a block, p < 0.001) (Figure 3). We also found a significant interaction between condition and cycle (p < 0.001), indicating that the difference in RTs between related and unrelated conditions increased with repetition across the cycles (Figure 3). These findings demonstrate that participants with MS exhibit the typical pattern results seen in previous studies using the BCNT:24, 25, 30 an interference effect when naming related compared to unrelated items that increases with repetition.
Figure 3.
Overall response times (RTs) on the Blocked Cyclic Naming Task for the related (black) and unrelated (gray) condition across the four cycles. As expected, participants became faster with repetition (across cycles) in both conditions, but this effect was attenuated in the related condition, making the difference in RTs between conditions increase across the cycles (i.e., significant condition by cycle interaction, p < 0.001). Error bars represent the standard error of the mean.
3.3. Relationship between overall performance on the task and disease variables
Participants with SPMS exhibited larger interference effects on the overall test when compared to RRMS (mean difference 62 ms). SPMS participants were unable to complete the task in its entirety due to self-reported severe fatigue. The overall performance on the BCNT was not associated with disease duration, disability level, trait fatigue or changes in state fatigue.
3.4. Fatigability and subjective fatigue
When comparing performance in the 1st and 4th quarters, there was a significant main effect of quarter (RTs were slower in 4th (794 ms), compared to the 1st (742 ms) quarter, p = 0.006), condition (p < 0.001), and cycle (p < 0.001). There were significant interactions between quarter and cycle (p = 0.023), indicating less repetition priming in the 4th compared to the 1st quarter (i.e., 1st quarter RTs: 826, 734, 711, and 701 ms vs. 4th quarter RTs: 854, 774, 770, and 779 ms) and significant interactions between condition and cycle (p < 0.001) indicating that the differences in RTs between related and unrelated conditions increased with repetition across cycles. The interaction between quarter and condition and the three-way interaction between quarter, condition, and cycle were not significant (p’s > .13).
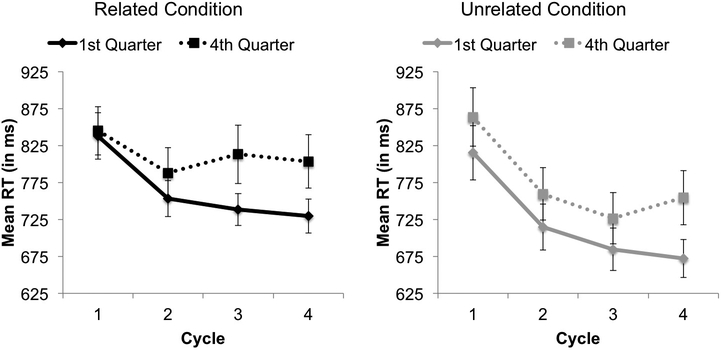
We also analyzed RTs in the 1st vs. 4th quarter across cycles in each condition separately. Within both the related and unrelated items, we found significant effects of quarter (p = .018, p = .009 respectively) and cycle (p < .001 for both). Notably, we found a significant interaction between quarter and cycle for the related (p = 0.019) but not unrelated (p = 0.201) blocks, revealing that the degree to which participants became faster with repetition (across cycles) was larger for related blocks in the 1st vs. 4th quarters (i.e., 838, 753, 738, and 729 ms vs. 845, 788, 813, and 803 ms, respectively) but was comparable across the 1st and 4th quarters (i.e., 839, 737, 705, and 713 ms and 863, 760, 726, and 754 ms, respectively) for the unrelated blocks. This suggests that, in addition to experiencing increasing competition across the experiment, participants were also exhibiting BCNT objective fatigability in the related items (Figure 4).
Figure 4.
Response times (RTs) on the Blocked Cyclic Naming Task for the 1st quarter (solid) and the 4th quarter (dashed) across the four cycles separated by the related condition (left) and unrelated condition (right). Error bars represent the standard error of the mean.
We compared the slopes of the related/unrelated difference across cycles for each context condition in the 1st vs. 4th quarter. The slope of the interference effect in the 1st quarter was 12 ms (rate of repetition priming for related vs. unrelated items across cycles was −34 vs. −46, respectively), whereas the slope of the interference effect in the 4th quarter was 26 ms (rate of repetition priming for related vs. unrelated items across cycles was −10 vs. −36 ms, respectively).
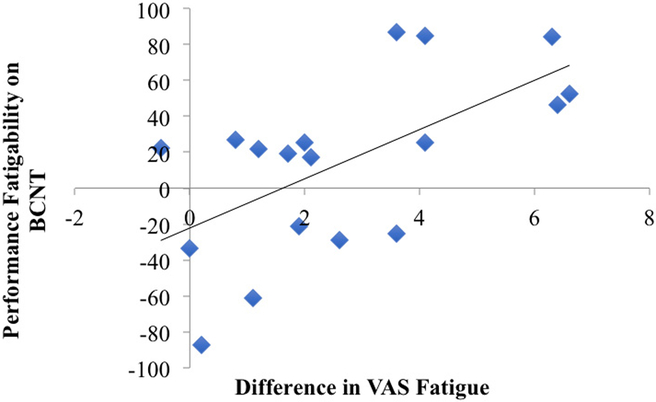
We computed the difference in the slope of the interference effect for the 4th minus the 1st quarter and assessed whether this measure correlated with differences in subjective fatigability (VAS difference). Participants rated their level of momentary fatigue as worse following the completion of the BCNT, with a VAS difference of 2.7 ± 2.14 (Table 1). Critically, BCNT objective fatigability was correlated with changes in state fatigue (r = 0.61, p = 0.007) but not with trait fatigue, disease duration or disability levels (Figure 5, Table 2).
Figure 5.
Scatterplot of the relationship between the difference in VAS (before and after the BCNT) and performance fatigability on the BCNT (difference in the slope of the interference effect in the 4th vs. 1st quarter). The correlation was significant (r=0.612, p=0.007).
Table 2 –
Fatigability and VAS correlations
| Type | Correlation | P Value |
|---|---|---|
| Fatigability x disability level (EDSS) | R = 0.263 | P = 0.292 |
| Fatigability x years with MS | R = −0.302 | P = 0.222 |
| Fatigability x VAS Diff | R = 0.612 | P = 0.007 |
| Fatigability x FSS Total | R = 0.268 | P = 0.282 |
| Fatigability x MFIS Total | R = −0.119 | P = 0.638 |
| Fatigability x MoCA Total | R = 0.768 | P = 0.002 |
| MFIS Total x VAS Diff | R = −0.181 | P = 0.472 |
| FSS Total x VAS Diff | R = 0.004 | P = 0.989 |
3.5. Baseline Cognitive screening (MoCA)
Participants scored well on the MoCA (average score of 25.3 ± 2.5) (Table 1). MoCA scores were not associated with performance on the task overall and had an inverse association with BCNT objective fatigability (r = 0.628 p = 0.005), indicating better performance on the MoCA correlated with worsening fatigability. MoCA scores were associated with pretest state fatigue (VAS1) (r = −0.53 p = 0.017) but not with state fatigue changes or with measures of trait fatigue.
4. Discussion
Our study showed that the BCNT, a measure not previously used in MS, uncovered impairment in cognitive control mechanisms and produced fatigability in patients with MS. Not only was the performance on related blocks slower compared to unrelated blocks across cycles, but this difference was larger in the 4th relative to the 1st quarter of the task for the related blocks. Our study also showed that fatigability was associated with patients’ assessment of worsening levels of fatigue after the task.
That the BCNT could elicit cognitive fatigability and show associations with subjective fatigue in a relatively small sample size is very encouraging. Cognitive fatigability and subjective fatigue are separate but clinically important entities that can negatively affect quality of life,10, 12 though their association has been difficult to prove.8, 10 Sandry et al (2014) showed that time on task was related to cognitive fatigability but did not find any association with subjective fatigue.10 Krupp et al (2000) uncovered cognitive fatigability by showing that MS patients had a decline in performance on cognitive measures following a continuous effortful mental task,8 however, subjective fatigue in this study was independent of fatigability.8
We postulate that our positive results were partly due to the choice of the assessment tools. To our knowledge, our study was the first to both employ a cognitive control task and show associations with subjective fatigue. Prior studies have shown that MS patients perform poorly on the Stroop task,13, 22 furthermore, time on task was found to be an important factor to induce fatigability.13 The BCNT, in addition to tapping into cognitive control, has inherent qualities that distinguish it from the Stroop and other similar tasks. The BCNT is simpler and easier to perform. Its length and ability to test the additive effects of fatigue due to time on task and demand on cognitive control (the related versus unrelated comparisons in the first and fourth quarter), make it intrinsically conducive to fatigability. Interestingly, the Stroop interference effect is correlated with the slope of the RTs across cycles in the related, but not unrelated, conditions,29 suggesting that the BCNT’s ability to compare across conditions allows for better understanding of the demands on cognitive control (the participants act as their own controls, in a way) and how it changes with task duration.29
Participants with SPMS performed worse on the BCNT. While the two SPMS participants had greater levels of disability, EDSS scores were not associated with task performance. This could be due to the relative small sample size and the fact that the EDSS is weighted more towards physical impairment.43 A striking observation is the inability of the SPMS participants to complete the task due to increasing levels of fatigue, although no conclusions can be drawn from this observation since the performance of only two SPMS participants may not reflect that of all SPMS patients. We propose that future studies specifically target the recruitment of more patients with progressive MS.
Interestingly, participants with worse baseline cognitive functioning showed less fatigability across the task. Participants with greater cognitive impairment could have worse performance throughout the task, making it more difficult to show fatigability. MoCA scores were not associated with overall performance on the test or with changes in state fatigue, but were associated with pre-test state fatigue (participants with more cognitive impairment possibly experienced greater subjective fatigue after completing the MoCA). It is difficult to make assumptions about these relationships given the relatively good performance on the MoCA (although half the participants had an abnormal score (less than 26), none had scores indicating severe cognitive impairment).
Our study is limited by a relatively small sample size. Nevertheless, our study elicited fatigability on the BCNT and showed a significant correlation with subjective fatigue. Also, our study did not have a control group, however, our results are in line with data from healthy volunteers from other studies: Healthy older adults’ response time was shown to be slower in the related versus unrelated conditions (815 versus 782 ms)24 and age did not have an effect on task performance.27, 29 Also, by including related and unrelated conditions, the test inherently compares performance within each subject (participants are acting as their own controls). Lastly, our finding of a correlation between performance variability (i.e. fatigability) and subjective fatigue in this group suggest that the test is able to capture heterogeneity within MS patients.
We cannot draw conclusions on the clinical meaningfulness of the millisecond difference between conditions on the BCNT. However, the captured fatigability is likely clinically meaningful because it mirrors real life (due to the more ecologically valid nature of the task when compared to traditional cognitive control tasks such as the Stroop test) and is strongly associated with participants’ own feeling or worsening fatigue.
The BCNT’s length may be its most important feature, yet may limit its usability. Although the test is only 20–30 minutes and its results are easy to analyze, we propose, after validation of our findings in larger future studies, to compare the BCNT to other tests like the Stroop task and to manipulate the length of the test to find the duration that balances usability in clinical settings with the ability to produce fatigability.
In conclusion, patients with MS had cognitive fatigability on the BCNT that was associated with increased subjective momentary fatigue, an association that has proven elusive in other studies of MS. This study showed that the BCNT can be a useful task in cognitive research in MS.
Highlights.
Blocked Cyclic Naming Task captures cognitive fatigability in MS
MS patients performed worse on related conditions especially later in the task
Objective fatigability was associated with state, not trait, fatigue
Funding:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations:
- FSS
Fatigue Severity Scale
- VAS
Modified Fatigue Impact Scale (MFIS) Visual Analog Scale
- BCNT
Blocked Cyclic Naming Task
- RT
Response Time
- MoCA
Montreal Cognitive Assessment
- PROMIS
Patient-Reported Outcomes Measurement Information System
- EDSS
Expanded Disability Status Scale
- ANOVA
Analyses of Variance
Footnotes
Declaration of Conflicting Interests:
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References:
- 1.Fisk JD, Pontefract A, Ritvo PG, Archibald CJ and Murray TJ. The impact of fatigue on patients with multiple sclerosis. Can J Neurol Sci. 1994; 21: 9–14. [PubMed] [Google Scholar]
- 2.Julian LJ. Cognitive functioning in multiple sclerosis. Neurol Clin. 2011; 29: 507–25. [DOI] [PubMed] [Google Scholar]
- 3.Krupp LB, Serafin DJ and Christodoulou C. Multiple sclerosis-associated fatigue. Expert Rev Neurother. 2010; 10: 1437–47. [DOI] [PubMed] [Google Scholar]
- 4.Langdon DW. Cognition in multiple sclerosis. Curr Opin Neurol. 2011; 24: 244–9. [DOI] [PubMed] [Google Scholar]
- 5.Lovera J and Kovner B. Cognitive impairment in multiple sclerosis. Curr Neurol Neurosci Rep. 2012; 12: 618–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andreasen AK, Spliid PE, Andersen H and Jakobsen J. Fatigue and processing speed are related in multiple sclerosis. Eur J Neurol. 2010; 17: 212–8. [DOI] [PubMed] [Google Scholar]
- 7.Bol Y, Duits AA, Hupperts RM, Verlinden I and Verhey FR. The impact of fatigue on cognitive functioning in patients with multiple sclerosis. Clin Rehabil. 2010; 24: 854–62. [DOI] [PubMed] [Google Scholar]
- 8.Krupp LB and Elkins LE. Fatigue and declines in cognitive functioning in multiple sclerosis. Neurology. 2000; 55: 934–9. [DOI] [PubMed] [Google Scholar]
- 9.Niino M, Mifune N, Kohriyama T, et al. Apathy/depression, but not subjective fatigue, is related with cognitive dysfunction in patients with multiple sclerosis. BMC Neurol. 2014; 14: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandry J, Genova HM, Dobryakova E, DeLuca J and Wylie G. Subjective cognitive fatigue in multiple sclerosis depends on task length. Front Neurol. 2014; 5: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey A, Channon S and Beaumont JG. The relationship between subjective fatigue and cognitive fatigue in advanced multiple sclerosis. Mult Scler. 2007; 13: 73–80. [DOI] [PubMed] [Google Scholar]
- 12.Kluger BM, Krupp LB and Enoka RM. Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology. 2013; 80: 409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chinnadurai SA, Venkatesan SA, Shankar G, Samivel B and Ranganathan LN. A study of cognitive fatigue in Multiple Sclerosis with novel clinical and electrophysiological parameters utilizing the event related potential P300. Mult Scler Relat Disord. 2016; 10: 1–6. [DOI] [PubMed] [Google Scholar]
- 14.Walker LA, Berard JA, Berrigan LI, Rees LM and Freedman MS. Detecting cognitive fatigue in multiple sclerosis: method matters. J Neurol Sci. 2012; 316: 86–92. [DOI] [PubMed] [Google Scholar]
- 15.Krupp LB, LaRocca NG, Muir-Nash J and Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989; 46: 1121–3. [DOI] [PubMed] [Google Scholar]
- 16.Hoorelbeke K, Koster EH, Demeyer I, Loeys T and Vanderhasselt MA. Effects of cognitive control training on the dynamics of (mal)adaptive emotion regulation in daily life. Emotion. 2016; 16: 945–56. [DOI] [PubMed] [Google Scholar]
- 17.Chrysikou EG, Novick JM, Trueswell JC and Thompson-Schill SL. The other side of cognitive control: can a lack of cognitive control benefit language and cognition? Top Cogn Sci. 2011; 3: 253–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richter FR and Yeung N. Memory and cognitive control in task switching. Psychol Sci. 2012; 23: 1256–63. [DOI] [PubMed] [Google Scholar]
- 19.Leshem R Relationships between trait impulsivity and cognitive control: the effect of attention switching on response inhibition and conflict resolution. Cogn Process. 2016; 17: 89–103. [DOI] [PubMed] [Google Scholar]
- 20.Shin G and Kim C. Neural correlates of cognitive style and flexible cognitive control. Neuroimage. 2015; 113: 78–85. [DOI] [PubMed] [Google Scholar]
- 21.Ridley DR, Johnson DE and Braisted PD. The color-word connotative incongruity effect. Percept Mot Skills. 1978; 46: 939–46. [DOI] [PubMed] [Google Scholar]
- 22.Dobryakova E, Rocca MA, Valsasina P, et al. Abnormalities of the executive control network in multiple sclerosis phenotypes: An fMRI effective connectivity study. Hum Brain Mapp. 2016; 37: 2293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oppenheim GM, Dell GS and Schwartz MF. The dark side of incremental learning: a model of cumulative semantic interference during lexical access in speech production. Cognition. 2010; 114: 227–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schnur TT, Schwartz MF, Brecher A and Hodgson C. Semantic Interference during blocked-cyclic naming: Evidence from aphasia. Journal of Memory and Language. 2006; 54: 199–227. [Google Scholar]
- 25.Hughes JW and Schnur TT. Facilitation and interference in naming: A consequence of the same learning process? Cognition. 2017; 165: 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Damian MF and Als LC. Long-lasting semantic context effects in the spoken production of object names. J Exp Psychol Learn Mem Cogn. 2005; 31: 1372–84. [DOI] [PubMed] [Google Scholar]
- 27.Belke E and Stielow A. Cumulative and non-cumulative semantic interference in object naming: evidence from blocked and continuous manipulations of semantic context. Q J Exp Psychol (Hove). 2013; 66: 2135–60. [DOI] [PubMed] [Google Scholar]
- 28.Harvey DY and Schnur TT. Different Loci of Semantic Interference in Picture Naming vs. Word-Picture Matching Tasks. Front Psychol. 2016; 7: 710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crowther JE and Martin RC. Lexical selection in the semantically blocked cyclic naming task: the role of cognitive control and learning. Front Hum Neurosci. 2014; 8: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harvey DY and Schnur TT. Distinct loci of lexical and semantic access deficits in aphasia: Evidence from voxel-based lesion-symptom mapping and diffusion tensor imaging. Cortex. 2015; 67: 37–58. [DOI] [PubMed] [Google Scholar]
- 31.Biegler KA, Crowther JE and Martin RC. Consequences of an inhibition deficit for word production and comprehension: evidence from the semantic blocking paradigm. Cogn Neuropsychol. 2008; 25: 493–527. [DOI] [PubMed] [Google Scholar]
- 32.Ries SK, Greenhouse I, Dronkers NF, Haaland KY and Knight RT. Double dissociation of the roles of the left and right prefrontal cortices in anticipatory regulation of action. Neuropsychologia. 2014; 63: 215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schnur TT, Schwartz MF, Kimberg DY, Hirshorn E, Coslett HB and Thompson-Schill SL. Localizing interference during naming: convergent neuroimaging and neuropsychological evidence for the function of Broca’s area. Proc Natl Acad Sci U S A. 2009; 106: 322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belke E and Meyer AS. Single and multiple object naming in healthy ageing. Language and Cognitive Processes. 2007; 22: 1178–211. [Google Scholar]
- 35.Dagenais E, Rouleau I, Demers M, et al. Value of the MoCA test as a screening instrument in multiple sclerosis. Can J Neurol Sci. 2013; 40: 410–5. [DOI] [PubMed] [Google Scholar]
- 36.Freitas S, Batista S, Afonso AC, et al. The Montreal Cognitive Assessment (MoCA) as a screening test for cognitive dysfunction in multiple sclerosis. Appl Neuropsychol Adult. 2016: 1–14. [DOI] [PubMed] [Google Scholar]
- 37.Flachenecker P, Kumpfel T, Kallmann B, et al. Fatigue in multiple sclerosis: a comparison of different rating scales and correlation to clinical parameters. Mult Scler. 2002; 8: 523–6. [DOI] [PubMed] [Google Scholar]
- 38.Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMIS sleep disturbance and Sleep-Related Impairment item banks. Behav Sleep Med. 2011; 10: 6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pilkonis PA, Yu L, Dodds NE, Johnston KL, Maihoefer CC and Lawrence SM. Validation of the depression item bank from the Patient-Reported Outcomes Measurement Information System (PROMIS) in a three-month observational study. J Psychiatr Res. 2014; 56: 112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983; 33: 1444–52. [DOI] [PubMed] [Google Scholar]
- 41.van Casteren M and Davis MH. Mix, a program for pseudorandomization. Behav Res Methods. 2006; 38: 584–9. [DOI] [PubMed] [Google Scholar]
- 42.JASP. JASP Team version 0.9.0.1, 2018.
- 43.Cohen JA, Reingold SC, Polman CH, Wolinsky JS and International Advisory Committee on Clinical Trials in Multiple S. Disability outcome measures in multiple sclerosis clinical trials: current status and future prospects. Lancet Neurol. 2012; 11: 467–76. [DOI] [PubMed] [Google Scholar]