Abstract
Mac‐2 binding protein glycosylation isomer (M2BPGi) is a novel glycoprotein biomarker that correlates with liver fibrosis. It has been investigated in East Asian populations as a hepatocellular carcinoma (HCC) biomarker. We assessed M2BPGi as an HCC biomarker in an ethnically diverse cohort of patients with chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infection. We enrolled 947 treatment‐naive patients mono‐infected with HBV or HCV without HCC at baseline. Biomarker levels were measured from baseline sera and correlated with longitudinal clinical data. The primary outcome was HCC occurrence during long‐term follow‐up. Median M2BPGi was significantly higher among patients with cirrhosis (2.67 versus 0.80; P < 0.001) and patients who developed HCC (3.22 versus 1.16; P < 0.001). The area under the receiver operating characteristic (AUROC) for M2BPGi and alpha‐fetoprotein (AFP) was similar overall (0.77 versus 0.72; P = 0.15), but M2BPGi outperformed AFP among patients with HBV (0.84 versus 0.75; P = 0.02). M2BPGi performed poorly among patients with HCV (AUROC, 0.51). M2BPGi was an independent predictor of HCC among patients with HBV but not among patients with HCV. M2BPGi performed better in patient subgroups with a lower prevalence of cirrhosis. Conclusion: In our HBV cohort, M2BPGi was more effective than AFP in predicting HCC and was an independent predictor of HCC. However, M2BPGi had limited predictive value in our HCV cohort, likely due to a high cirrhosis burden in this cohort. Further studies are needed to evaluate M2BPGi as an HCC biomarker in broader patient populations with more diverse disease etiology, non‐Asian ethnicity, and more advanced fibrosis.
Abbreviations
- AFP
alpha‐fetoprotein
- AUROC
area under the receiver operating characteristic curve
- BMI
body mass index
- CI
confidence interval
- COI
cutoff index
- FIB‐4
fibrosis‐4
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HR
hazard ratio
- M2BPGi
Mac‐2 binding protein glycosylation isomer
- NAFLD
nonalcoholic fatty liver disease
- ROC
receiver operating characteristic
Hepatocellular carcinoma (HCC) was responsible for more than 800,000 deaths in 2015 and is the fourth leading cause of cancer deaths around the world.1 Early detection improves survival, and several HCC biomarkers have been studied for this purpose. However, aside from alpha‐fetoprotein (AFP), none have entered broad clinical use globally.2
In 2013, Kuno et al.3 identified a glycosylation isoform of Mac‐2 binding protein that correlated with liver fibrosis in patients with chronic hepatitis C virus (HCV) infection. Mac‐2 binding protein is produced by various cell types, including hepatocytes, and changes in its glycosylation pattern in the setting of liver disease are the basis for its use as a biomarker.
The glycan‐based immunoassay described by Kuno et al.3 is now available in Japan and has been used to study this marker in a variety of contexts. In addition to HCV, the Mac‐2 binding protein glycosylation isomer (M2BPGi) has been studied as a marker for fibrosis in chronic hepatitis B virus (HBV) infection, nonalcoholic fatty liver disease (NAFLD), and primary biliary cirrhosis, among others.4, 5, 6, 7 It has also been evaluated as a biomarker for HCC in HBV, HCV, and NAFLD, with favorable results.8, 9, 10, 11
Several gaps in the literature regarding M2BPGi remain and should be addressed prior to widespread adoption of the biomarker. First, M2BPGi has thus far only been evaluated in East Asian populations. Data from other populations are necessary to establish its generalizability. Second, as an HCC biomarker, M2BPGi has thus far been studied in cohorts with a single underlying liver disease, whether HBV, HCV, or NAFLD. In routine clinical practice, health care providers serve populations with a mix of liver diseases, all of whom may be at risk of HCC. As such, data from mixed cohorts are needed to compare M2BPGi performance across various liver diseases.
We designed this multicenter study to assess M2BPGi as a biomarker for HCC in an ethnically diverse and international cohort of patients with HBV and HCV from Asia and California.
Patients and Methods
Study Design and Setting
This cohort study involved 947 adult patients (18 years or older) with chronic viral hepatitis (HBV or HCV) who were enrolled and observed from April 2001 to October 2017 at three teaching hospitals in the United States and Taiwan (Stanford University Medical Center, Palo Alto, California; E‐Da Hospital, Kaohsiung, Taiwan; Kaohsiung Medical University Hospital, Kaohsiung, Taiwan). The primary outcome was occurrence of HCC during long‐term follow‐up.
Baseline clinical characteristics and sera were collected at enrollment. Routine laboratory tests were conducted at each institution, whereas measurement of M2BPGi was conducted at a single laboratory at the Department of Virology and Liver Unit, Nagoya City University, Nagoya, Japan. The laboratory personnel performing the M2BPGi assay were blinded to clinical information associated with each sample. Clinical and laboratory data were submitted to the data center at Stanford University Medical Center, Stanford, CA, for data management and analysis.
All patients gave written informed consent prior to blood collection. The study was approved by the institutional review board at each participating institution.
Patient Population
Patients 18 years or older who were mono‐infected with HBV or HCV, antiviral treatment naive, and had at least 1 year of follow‐up were included. Patients were excluded if they were diagnosed with HCC within 6 months of entering the study as these may have been prevalent rather than incident cases. Patients with HBV–HCV coinfection or nonviral chronic liver diseases were also excluded.
Diagnoses of HBV and HCV were established by serologic testing and nucleic acid tests for viremia. HCC was diagnosed based on histology or noninvasive imaging criteria as recommended by the American Association for the Study of Liver Diseases.12 Cirrhosis was diagnosed based on imaging, clinical, and histopathologic findings. Patients with HCV were considered treated if they achieved sustained virologic response between enrollment and censoring; patients with HBV were considered treated if they received any antiviral treatment between enrollment and censoring.
Data Collection and M2BPGi Measurement
Baseline demographic, clinical, laboratory, pathologic, and radiographic data were extracted from the medical records at each site and compiled into a common database with uniform definitions.
Noninvasive scores based on routine laboratory tests were also calculated as indicators of liver fibrosis or dysfunction. We used the following equations: fibrosis‐4 (FIB‐4) = (aspartate aminotransferase [U/L] × age [years]) / (alanine aminotransferase [U/L]1/2 × platelet count [103/μL])13; Model for End‐Stage Liver Disease = 3.78 × ln(serum bilirubin [mg/dL]) + 11.2 × ln(international normalized ratio) + 9.57 × ln(serum creatinine [mg/dL]) + 6.43.14
Serum M2BPGi levels were measured from archived sera using an automated analyzer applying the lectin‐antibody sandwich immunoassay (HISCL‐2000i; Sysmex Corporation, Hyogo, Japan), as described.15 M2BPGi levels were expressed as a cutoff index (COI), which was calculated according to the following formula: COI = ([M2BPGi]sample – [M2BPGi]negative control) / ([M2BGi]positive control – [M2BPGi]negative control).
Statistical Analysis
M2BPGi values were reported as medians with interquartile ranges and compared across patient subgroups using the Mann‐Whitney U test. Other descriptive statistics were reported as proportions (%), mean with SD, or medians with interquartile ranges and compared using the Student t test, the chi‐square test, or the Mann‐Whitney U test as appropriate. Statistical significance was defined as two‐tailed P < 0.05.
Time‐dependent receiver operating characteristic (ROC) curves were generated in R (R Foundation, Vienna, Austria) using the timeROC package. Comparisons of paired ROC curves were performed using the timeROC package, and unpaired comparisons were performed in MedCalc (MedCalc Software, Ostend, Belgium).
Univariate and multivariate survival models were constructed using Cox proportional hazards models in Stata, version 14 (Stata Corporation, College Station, TX). The primary outcome was incident HCC diagnosis. The primary predictor variable was M2BPGi level. Secondary predictors were sex, age, cirrhosis status, and treatment status at baseline. To account for patient heterogeneity, models were adjusted for site as a random effect, and separate models were constructed for patients with HBV and HCV.
Results
Baseline Characteristics
The baseline clinical characteristics of patients in this study are described in Table 1. Patient populations with HCV and HBV differed in several important respects. Patients with HCV were older on average, had a higher body mass index (BMI), were more likely to be female, were less likely to be Asian, were more likely to come from the U.S. cohort, and were more likely to have cirrhosis. Median M2BPGi levels were higher in the HCV group (2.28 versus 1.09; P < 0.001). Median length of follow‐up in the overall cohort was 6.8 years and not significantly different between the HBV and HCV groups.
Table 1.
Patient Clinical Characteristics at Baseline and Follow‐up
| HCV (n = 233) | HBV (n = 714) |
Overall (n = 947) |
P Value | |
|---|---|---|---|---|
| BASELINE | ||||
| Age at entry, years | 55.6 ± 9.2 | 45.1 ± 12.1 | 47.7 ± 12.3 | <0.001 |
| Female | 113 (48.7%) | 205 (28.8%) | 318 (33.7%) | <0.001 |
| Caucasian | 66 (28.6%) | 5 (0.7%) | 71 (7.5%) | <0.001 |
| Asian | 141 (61.0%) | 707 (99.2%) | 848 (89.8%) | <0.001 |
| Other ethnicities | 24 (10.4%) | 1 (0.1%) | 25 (2.7%) | <0.001 |
| U.S. cohort | 118 (50.6%) | 79 (11.1%) | 197 (20.8%) | <0.001 |
| BMI (kg/m2) | 27.8 ± 5.4 | 24.9 ± 3.4 | 25.7 ± 4.5 | <0.001 |
| Diabetes mellitus | 60 (26.8%) | 73 (12.9%) | 133 (16.9%) | <0.001 |
| Cirrhosis | 204 (87.6%) | 259 (36.3%) | 463 (48.9%) | <0.001 |
| Antiviral therapy | 107 (48.4%) | 443 (62.0%) | 550 (58.8%) | <0.001 |
| Aspartate aminotransferase, U/L | 78 (52‐118.5) | 57 (34‐111.5) | 63 (37‐115.5) | <0.001 |
| Alanine aminotransferase, U/L | 84.5 (56‐132.5) | 81 (39‐178) | 82 (43‐156) | 0.27 |
| Bilirubin, mg/dL | 0.9 (0.6‐1.6) | 1.1 (0.74‐1.73) | 1.08 (0.7‐1.7) | 0.01 |
| Platelet count, 103/μL | 126 (84‐176) | 171.5 (113.5‐217) | 158 (102‐210) | <0.001 |
| Albumin, g/dL | 3.6 (2.9‐4) | 4.04 (3.6‐4.4) | 3.92 (3.4‐4.3) | <0.001 |
| MELD | 9 (7‐13) | 9 (7‐12) | 9 (7‐12) | 0.64 |
| FIB‐4 | 4.07 (2.19‐6.78) | 1.91 (1.01‐4.20) | 2.39 (1.19‐5.33) | <0.001 |
| AFP, ng/mL | 8.9 (4.9‐17) | 4.44 (2.35‐9.16) | 5.2 (2.7‐11.4) | <0.001 |
| M2BPGi, COI | 2.28 (1.05‐4.81) | 1.09 (0.59‐2.84) | 1.27 (0.64‐3.4) | <0.001 |
| FOLLOW‐UP | ||||
| Follow‐up, years | 7.48 (4.16‐9.96) | 6.48 (3.21‐11.19) | 6.80 (3.37‐10.81) | 0.25 |
| Age at HCC diagnosis, years | 62.4 ± 7.7 | 59.8 ± 9.3 | 60.9 ± 8.7 | 0.16 |
| 5‐year cumulative HCC incidence | 22 (9.4%) | 28 (3.9%) | 50 (5.3%) | 0.001 |
| 10‐year cumulative HCC incidence | 38 (16.3%) | 47 (6.6%) | 85 (9.0%) | <0.001 |
Data are reported as proportions (%), means ± standard deviations, or medians with interquartile ranges, as appropriate.
Abbreviation: MELD, Model for End‐Stage Liver Disease.
Patient characteristics stratified by site and underlying disease are reported in Supporting Tables S1A,B. Patients from the United States had a higher average BMI (27.4 kg/m2 versus 25.2 kg/m2; P < 0.001) and were more likely to have diabetes (21.3% versus 15.4%; P = 0.053). All patients from Taiwan were Asian compared to 50.5% of patients from the United States. Rates of antiviral therapy for either HBV or HCV were higher in Taiwan than the United States (62% versus 46.0%; P < 0.001). The 10‐year incidence of HCC was higher among patients from Taiwan than from the United States (10.3% versus 4.1%; P = 0.007). Within each site, the differences between HBV and HCV patients were generally similar to the overall cohort.
Median M2BPGi was markedly higher among patients with cirrhosis than those without (2.67 versus 0.80; P < 0.001) and among those who developed HCC than those who did not (3.22 versus 1.16; P < 0.001) (Fig. 1A,B). When stratifying by both cirrhosis and HCC, median M2BPGi was still higher among patients with cirrhosis who developed HCC than among patients with cirrhosis who did not develop HCC, although the difference was less prominent (3.31 versus 2.63; P = 0.04) (Fig. 1C). There was no significant difference in median M2BPGi levels between patients without cirrhosis who developed HCC and those who did not (0.31 versus 0.81; P = 0.18); however, only 3 patients without cirrhosis developed HCC (Fig. 1C).
Figure 1.
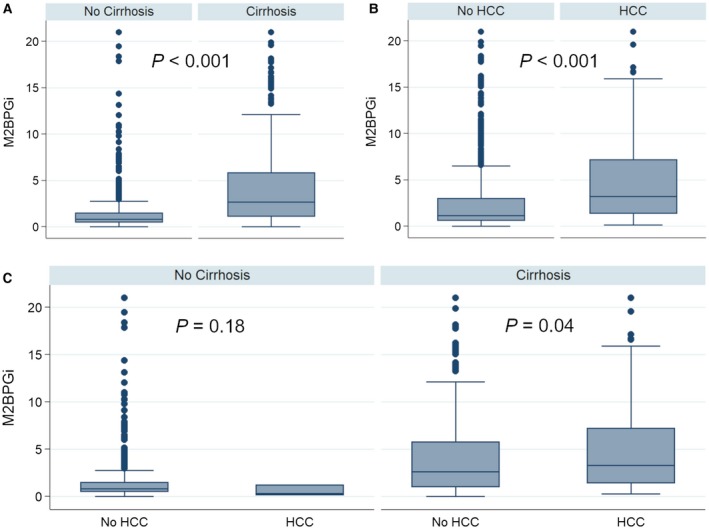
Stratification of M2BPGi levels. M2BPGi levels were stratified by (A) cirrhosis status, (B) HCC status, and (C) both cirrhosis and HCC status. Each box represents the 25th to 75th percentiles. Whiskers extend to the upper and lower adjacent values.
M2BPGi Compared to AFP
To assess the performance of M2BPGi as a biomarker for the prediction of future HCC, we compared M2BPGi to AFP using the area under the ROC curve (AUROC) (Fig. 2). M2BPGi and AFP had similar AUROC values in the overall cohort (0.77 versus 0.72; P = 0.15), among patients with cirrhosis (0.60 versus 0.57; P = 0.50), and among patients with HCV (0.51 versus 0.59; P = 0.34). M2BPGi had a significantly higher AUROC value than AFP among patients with HBV (0.84 versus 0.75; P = 0.02). Time‐dependent AUROC values for AFP and M2BPGi at 3, 5, and 10 years are reported in Table 2A,B.
Figure 2.
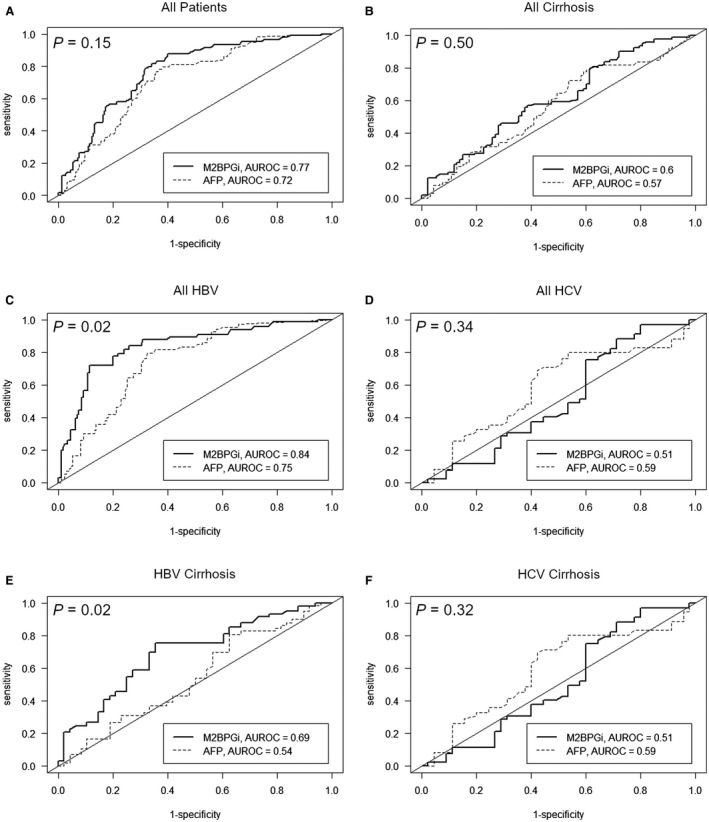
Time‐dependent ROC curves for M2BPGi and AFP as predictors of HCC within 10 years. (A) all patients, (B) patients with cirrhosis, (C) patients with HBV, (D) patients with HCV, (E) patients with HBV and cirrhosis, (F) patients with HCV and cirrhosis.
Table 2A.
AFP: Time‐dependent Auroc Values for Predicting HCC at Different Time Points Across Patient Subgroups
| Time (year) |
All Patients (n = 879) |
All Cirrhosis (n = 433) |
All HBV (n = 664) |
All HCV (n = 215) |
HBV Cirrhosis (n = 239) |
HCV Cirrhosis (n = 194) |
|---|---|---|---|---|---|---|
| 3 |
0.67 (0.59‐0.76) |
0.55 (0.44‐0.66) |
0.66 (0.54‐0.77) |
0.63 (0.53‐0.74) |
0.49 (0.31‐0.66) |
0.61 (0.50‐0.72) |
| 5 |
0.69 (0.62‐0.75) |
0.57 (0.48‐0.65) |
0.71 (0.62‐0.79) |
0.60 (0.48‐0.72) |
0.55 (0.42‐0.67) |
0.58 (0.46‐0.71) |
| 10 |
0.72 (0.66‐0.78) |
0.57 (0.48‐0.66) |
0.75 (0.68‐0.82) |
0.59 (0.46‐0.73) |
0.54 (0.42‐0.66) |
0.59 (0.46‐0.73) |
Values are AUROC (95% CI) at the specified time point.
Table 2B.
M2BPGi: Time‐dependent Auroc Values for Predicting HCC at Different Time Points Across Patient Subgroups
| Time (year) |
All Patients (n = 879) |
All Cirrhosis (n = 433) |
All HBV (n = 664) |
All HCV (n = 215) |
HBV Cirrhosis (n = 239) |
HCV Cirrhosis (n = 194) |
|---|---|---|---|---|---|---|
| 3 |
0.68 (0.58‐0.78) |
0.60 (0.48‐0.71) |
0.72 (0.56‐0.87) |
0.55 (0.43‐0.68) |
0.65 (0.48‐0.84) |
0.54 (0.42‐0.67) |
| 5 |
0.71 (0.64‐0.78) |
0.62 (0.53‐0.70) |
0.76 (0.66‐0.86) |
0.56 (0.46‐0.66) |
0.66 (0.54‐0.79) |
0.56 (0.46‐0.66) |
| 10 |
0.77 (0.71‐0.83) |
0.60 (0.52‐0.69) |
0.84 (0.77‐0.91) |
0.51 (0.38‐0.63) |
0.69 (0.59‐0.80) |
0.51 (0.38‐0.63) |
Values are AUROC (95% CI) at the specified time point.
We attempted to improve predictive performance by combining AFP and M2BPGi in a logistic regression model. The model’s performance did not significantly differ from that of M2BPGi because the coefficient for AFP was small (Supporting Fig. S1).
M2BPGi in Predictive Models of HCC
Univariate and multivariate Cox proportional hazards models were separately constructed for patients with HBV and HCV. Covariates included were sex, age, cirrhosis, treatment status, and M2BPGi. Site was controlled for as a random effect. Cirrhosis was omitted from the HCV model as all patients with HCV who developed HCC had cirrhosis. Among patients with HBV, M2BPGi and cirrhosis were both independent predictors of HCC (Table 3A). Among patients with HCV, M2BPGi was not an independent predictor of HCC (Table 3B). Sensitivity analyses to diabetes in the multivariate models did not change the results with regard to M2BPGi (hazard ratio [HR] 1.11, P = 0.001 for HBV; HR 0.97, P = 0.53 for HCV).
Table 3A.
Predictors of HCC Among Patients with Chronic Hepatitis B
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| (n = 692) | ||||
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Male sex | 1.31 (0.67‐2.58) | 0.428 | 1.94 (0.93‐4.02) | 0.077 |
| Age* | 1.42 (1.25‐1.62) | <0.001 | 1.34 (1.12‐1.61) | 0.002 |
| Cirrhosis | 24.01 (7.45‐77.4) | <0.001 | 16.19 (3.78‐69.32) | <0.001 |
| Antiviral therapy | 4.57 (1.93‐10.84) | 0.001 | 1.30 (0.49‐3.45) | 0.603 |
| M2BPGi (COI) | 1.16 (1.11‐1.21) | <0.001 | 1.11 (1.05‐1.18) | <0.001 |
5‐year increments.
Table 3B.
Predictors of HCC Among Patients with Chronic Hepatitis C
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| (n = 218) | ||||
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Male sex | 1.20 (0.63‐2.27) | 0.576 | 1.29 (0.67‐2.48) | 0.447 |
| Age* | 1.17 (0.98‐1.41) | 0.089 | 1.19 (0.98‐1.45) | 0.078 |
| Cirrhosis† | ‐ | ‐ | ‐ | ‐ |
| Antiviral therapy | 0.63 (0.33‐1.20) | 0.158 | 0.53 (0.27‐1.04) | 0.067 |
| M2BPGi (COI) | 0.96 (0.88‐1.05) | 0.409 | 0.96 (0.87‐1.07) | 0.492 |
5‐year increments.
Omitted due to complete correlation with HCC.
Interactions Between M2BPGi, Cirrhosis, and Underlying Liver Disease
When comparing the performance of M2BPGi among various subsets of patients, we observed that M2BPGi performance was better in groups with a lower proportion of patients with cirrhosis. For example, M2BPGi performed significantly better among patients with HBV (36.3% cirrhosis) than patients with HCV (87.6% cirrhosis) (AUROC, 0.84 versus 0.51; P < 0.001). Similarly, M2BPGi performed significantly better among patients with HBV overall compared to patients with HBV with cirrhosis (AUROC, 0.84 versus 0.69; P = 0.02) and among the overall cohort compared to patients with cirrhosis only (AUROC, 0.77 versus 0.60; P = 0.001). Stratifying the cohort into tertiles by the FIB‐4 index produced a similar pattern; the AUROC for M2BPGi was higher in the tertiles with lower FIB‐4 scores and less cirrhosis (Supporting Figs. S2 and S3; Supporting Tables S2 and S3).
Because M2BPGi was an independent predictor of HCC among patients with HBV but not patients with HCV, we postulated that there might be an interaction between M2BPGi and the underlying liver disease (HBV or HCV) independent of the prevalence of cirrhosis in each group. A multivariate model, including an interaction term for underlying liver disease and M2BPGi, found that the interaction was significant (HR, 1.15; 95% confidence interval [CI], 1.04‐1.28; P = 0.009), even after controlling for cirrhosis (Table 4).
Table 4.
Multivariate Model for Predictors of HCC at 10 Years Among All Patients, Including an Interaction Term for Underlying Liver Disease and M2BPGi
| n = 921 | ||
|---|---|---|
| Variable | HR (95% CI) | P Value |
| Male sex | 1.49 (0.93‐2.39) | 0.100 |
| Age* | 1.25 (1.09‐1.42) | 0.001 |
| Asian | 2.24 (0.87‐5.78) | 0.095 |
| HBV versus HCV | 0.49 (0.25‐0.95) | 0.036 |
| M2BPGi (COI) | 0.96 (0.88‐1.06) | 0.420 |
| HBV × M2BPGi† | 1.15 (1.04‐1.28) | 0.009 |
| Cirrhosis | 19.21 (4.60‐80.28) | <0.001 |
5‐year increments.
Interaction between etiology and M2BPGi.
Discussion
This is the first study to evaluate M2BPGi as a predictor of HCC in non‐Asian patients and patients outside of East Asia. Among patients with HBV, we found that M2BPGi was a more informative biomarker than AFP and was an independent predictor of HCC, even after accounting for cirrhosis. This recapitulates the findings of other studies and extends those findings to include a sample of Asian patients living outside East Asia. However, we did not find that M2BPGi was a useful predictor of HCC in our HCV cohort. This differs from what has been reported from exclusively East Asian populations.
This discrepancy could be related to a number of factors. First, our HCV cohort differed significantly from previously tested populations. More than one third of our cohort was non‐Asian, and approximately half were living in the United States. All other studies to date looking at M2BPGi as an HCC biomarker in patients with HCV have been conducted in Japan. It is possible that genetic or environmental differences may lead to alternative glycosylation patterns of M2BP, which the current assay does not detect. The current assay uses the lectin Wisteria floribunda agglutinin, which was chosen as the best candidate out of a screen looking for M2BP lectins that correlated with liver fibrosis stage in 125 Japanese patients with HCV.3 It is possible that alternative lectins could improve the test’s performance in non‐Japanese HCV populations. For HBV, M2BPGi has been successfully used in Korean and Chinese populations in addition to Japanese populations, demonstrating that the test is robust to some changes in patient population.9, 10
Another consideration is M2BPGi’s close association with fibrosis and cirrhosis. As noted above, M2BPGi was originally identified in a screen for a fibrosis marker not an HCC marker. However, cirrhosis is a powerful risk factor for HCC regardless of liver disease etiology. Thus, there is a risk of cirrhosis confounding analyses of M2BPGi as an HCC biomarker. We observed that M2BPGi was less effective when applied to patient populations with a higher burden of cirrhosis or fibrosis. This may have played a role in our results as cirrhosis was present in the vast majority (87.6%) of our HCV cohort and all HCV–HCC cases in the cohort were in patients who had cirrhosis at baseline. In comparison, in their study of 707 Japanese patients with HCV, Yamasaki et al.8 observed F4 fibrosis at baseline in only 17% of patients. Similarly, Sasaki et al.16 observed F4 fibrosis at baseline in only 10.1% of their patients. Whereas Yamasaki et al. and Sasaki et al. were able to use M2BPGi to stratify for HCC risk within each fibrosis stage, including F4, we found that neither M2BPGi nor AFP had much predictive power in our HCV cohort. It may be that cirrhosis was relatively prevalent and severe in our cohort, thus limiting the predictive power of both AFP and M2BPGi.
There may also be systematic differences in the prevalence of cirrhosis between Eastern and Western populations with chronic liver disease who present for medical attention.17 The HCC BRIDGE study, which examined the clinical characteristics of patients diagnosed with HCC in Asia, Europe, and North America, found that approximately 90% of Japanese and Taiwanese patients with HCC were Child‐Pugh A compared to approximately 70% of North American or European patients.18 Japan has implemented nationwide screening and surveillance programs for HCV and HCC, which have led to declining rates of HCC incidence and mortality.19, 20 Screening efforts in the United States may not be as consistent, resulting in patients with more advanced cirrhosis by the time they connect with the medical system.21, 22, 23 Indeed, prior studies have reported a cirrhosis prevalence of 85% to 90% among patients with HCV–HCC in the United States.24, 25, 26
Additional considerations are alcohol consumption patterns and ethnic differences in disease progression. Comorbid alcoholic liver disease may influence the prevalence and severity of cirrhosis in these populations as well as the risk of HCC.27, 28, 29 The 2010 World Health Organization estimates for annual per capita alcohol consumption were 10.4 liters in Japan and 13.3 liters in the United States.30 Ethnic differences in the risk of cirrhosis and HCC have also been reported among patients with HCV in the United States, including more severe cirrhosis in non‐Asian compared to Asian patients.24, 31, 32 One study also reported a higher risk of HCC among Asian patients with HCV and cirrhosis compared to non‐Asian individuals with HCV and cirrhosis.31 It is not well established whether Asian ethnicity is an independent risk factor for HCV–HCC, but it is notable that the cohorts of Sasaki et al.16 and Yamasaki et al.8 had relatively high incidences of HCC (6.8% and 15.6%, respectively), despite having relatively low rates of F4 fibrosis (10% and 17%, respectively). In our HCV cohort, 16.3% of patients developed HCC and 87.6% of patients had cirrhosis.
This study raises a number of questions for future investigations. First, it is increasingly apparent that M2BPGi is not solely a marker of liver fibrosis. M2BPGi levels are elevated in the setting of nonspecific acute liver injury, and M2BPGi levels decline quickly with antiviral treatment for HBV and HCV, too quickly to be due to regression of fibrosis.33, 34, 35, 36, 37 Bekki et al.38 demonstrated that hepatic stellate cells produce M2BPGi in vitro and that M2BPGi signaling between hepatic stellate cells and Kupffer cells may promote a fibrogenic program. More work is needed to understand the biology of M2BPGi.
The effect of underlying liver disease on M2BPGi performance should also be evaluated in greater depth. M2BPGi has been shown to be predictive of HCC in East Asian cohorts with HCV, HBV, and NAFLD but with different optimal threshold values in each.8, 9, 11 We found that there was a significant interaction between underlying liver disease and M2BPGi in our cohort, even after taking cirrhosis into account. This has implications for the real‐world application of M2BPGi because health care providers serve clinical populations with diverse liver pathologies and individual patients may have more than one underlying liver disease.
In particular, the performance of M2BPGi in patients with NAFLD should be investigated further. NAFLD is a common HCC risk factor. About one third of NAFLD–HCC cases develop in patients without clinically apparent cirrhosis.39 There is currently no reliable way to identify patients with NAFLD but without cirrhosis who are at high risk of HCC. Because we observed that M2BPGi performed better in patient populations with lower burdens of cirrhosis, M2BPGi may be a useful marker in this population. One retrospective study of 331 Japanese patients with biopsy‐proven NAFLD found an AUROC of 0.81 for M2BPGi predicting HCC.11 However, among patients with F2 or less fibrosis, M2BPGi values did not discriminate between those who developed HCC and those who did not. More studies into this population are needed.
The strengths of our study include the multicenter design and the inclusion of patient populations in which M2BPGi had not previously been studied. The use of a blinded central laboratory with experience in the conduct of the gold‐standard assay is also a strength. Furthermore, our statistical analysis is bolstered by stratification for cirrhosis to address possible confounding.
There are a number of limitations in our study. Although we have included a diverse patient population, it should be noted that our HBV cohort is still predominantly East Asian and from Taiwan. Larger studies involving more patients from outside Asia are needed to confirm the broader applicability of M2BPGi as an HBV–HCC biomarker. As described above, our HCV cohort had relatively prevalent and severe cirrhosis, which may have limited the predictive performance of M2BPGi. Although we do not have liver biopsy data to precisely stage fibrosis, we were able to use the FIB‐4 score to stratify patients by predicted degree of fibrosis. It will be useful to study M2BPGi in larger non‐Asian HCV cohorts with a balance of patients with and without cirrhosis.
In conclusion, M2BPGi performed better than AFP for predicting HCC in our HBV cohort. The performance of M2BPGi was limited in our HCV cohort, perhaps due to a high burden of cirrhosis and fibrosis. Further studies are needed to evaluate M2BPGi as an HCC biomarker in broader patient populations, including a greater variety of liver diseases, non‐Asian patients, and those with high burdens of liver fibrosis.
Supporting information
Acknowledgment
We thank An Le, Joseph Hoang, and Samuel Trinh from the Division of Gastroenterology and Hepatology, Stanford University Medical Center, Palo Alto, CA, for technical assistance and support in statistical analysis, database management, and biosample management.
Potential conflict of interest: Dr. Nguyen advises for and received grants from Bristol Myers Squibb, Janssen, Gilead, Laboratory for Advanced Medicine, and Exact Science; she advises for Intercept, Roche, Dynavax, Alnylam, Novartis, Eisai Science, and Bayer and received grants from NCI and the BK.K. Kee Foundation. Dr. Tanaka reports honoraria from Sysmex Corp and Fujurebio Inc. The other authors have nothing to report.
Contributor Information
Yasuhito Tanaka, Email: ytanaka@med.nagoya-cu.ac.jp.
Mindie H. Nguyen, Email: mindiehn@stanford.edu
References
- 1. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability‐adjusted life‐years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol 2017;3:524‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chaiteerakij R, Addissie BD, Roberts LR. Update on biomarkers of hepatocellular carcinoma. Clin Gastroenterol Hepatol 2015;13:237‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kuno A, Ikehara Y, Tanaka Y, Ito K, Matsuda A, Sekiya S, et al. A serum “sweet‐doughnut” protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci Rep 2013;3:1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Toshima T, Shirabe K, Ikegami T, Yoshizumi T, Kuno A, Togayachi A, et al. A novel serum marker, glycosylated Wisteria floribunda agglutinin‐positive Mac‐2 binding protein (WFA(+)‐M2BP), for assessing liver fibrosis. J Gastroenterol 2015;50:76‐84. [DOI] [PubMed] [Google Scholar]
- 5. Zou X, Zhu M‐Y, Yu D‐M, Li W, Zhang D‐H, Lu F‐J, et al. Serum WFA+ ‐M2BP levels for evaluation of early stages of liver fibrosis in patients with chronic hepatitis B virus infection. Liver Int 2017;37:35‐44. [DOI] [PubMed] [Google Scholar]
- 6. Abe M, Miyake T, Kuno A, Imai Y, Sawai Y, Hino K, et al. Association between Wisteria floribunda agglutinin‐positive Mac‐2 binding protein and the fibrosis stage of non‐alcoholic fatty liver disease. J Gastroenterol 2015;50:776‐784. [DOI] [PubMed] [Google Scholar]
- 7. Umemura T, Joshita S, Sekiguchi T, Usami Y, Shibata S, Kimura T, et al. Serum Wisteria floribunda Agglutinin‐Positive Mac‐2‐Binding Protein Level Predicts Liver Fibrosis and Prognosis in Primary Biliary Cirrhosis. Am J Gastroenterol 2015;110:857‐864. [DOI] [PubMed] [Google Scholar]
- 8. Yamasaki K, Tateyama M, Abiru S, Komori A, Nagaoka S, Saeki A, et al. Elevated serum levels of Wisteria floribunda agglutinin‐positive human Mac‐2 binding protein predict the development of hepatocellular carcinoma in hepatitis C patients. Hepatology 2014;60:1563‐1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim SU, Heo JY, Kim BK, Park JY, Kim DY, Han K‐H, et al. Wisteria floribunda agglutinin‐positive human Mac‐2 binding protein predicts the risk of HBV‐related liver cancer development. Liver Int 2017;37:879‐887. [DOI] [PubMed] [Google Scholar]
- 10. Liu J, Hu H‐H, Lee M‐H, Korenaga M, Jen C‐L, Batrla‐Utermann R, et al. Serum levels of M2BPGi as short‐term predictors of hepatocellular carcinoma in untreated chronic hepatitis B patients. Sci Rep 2017;7:14352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kawanaka M, Tomiyama Y, Hyogo H, Koda M, Shima T, Tobita H, et al. Wisteria floribunda agglutinin‐positive Mac‐2 binding protein predicts the development of hepatocellular carcinoma in patients with nonalcoholic fatty liver disease. Hepatol Res 2018;48:521‐528. [DOI] [PubMed] [Google Scholar]
- 12. Bruix J, Sherman M; American Association for the Study of Liver Diseases . Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vallet‐Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin‐Venier V, et al. FIB‐4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007;46:32‐36. [DOI] [PubMed] [Google Scholar]
- 14. Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end‐stage liver disease. Hepatology 2001;33:464‐470. [DOI] [PubMed] [Google Scholar]
- 15. Kuno A, Sato T, Shimazaki H, Unno S, Saitou K, Kiyohara K, et al. Reconstruction of a robust glycodiagnostic agent supported by multiple lectin‐assisted glycan profiling. Proteomics Clin Appl 2013;7:642‐647. [DOI] [PubMed] [Google Scholar]
- 16. Sasaki R, Yamasaki K, Abiru S, Komori A, Nagaoka S, Saeki A, et al. Serum Wisteria Floribunda agglutinin‐positive Mac‐2 binding protein values predict the development of hepatocellular carcinoma among patients with chronic hepatitis C after sustained virological response. PLoS One 2015;10:e0129053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Choo SP, Tan WL, Goh BKP, Tai WM, Zhu AX. Comparison of hepatocellular carcinoma in Eastern versus Western populations. Cancer 2016;122:3430‐3446. [DOI] [PubMed] [Google Scholar]
- 18. Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int 2015;35:2155‐2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kudo M. Surveillance, diagnosis, treatment, and outcome of liver cancer in Japan. Liver Cancer 2015;4:39‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chung H, Ueda T, Kudo M. Changing trends in hepatitis C infection over the past 50 years in Japan. Intervirology 2010;53:39‐43. [DOI] [PubMed] [Google Scholar]
- 21. Coffin PO, Reynolds A. Ending hepatitis C in the United States: the role of screening. Hepatic Med 2014;6:79‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roblin DW, Smith BD, Weinbaum CM, Sabin ME. HCV screening practices and prevalence in an MCO, 2000‐2007. Am J Manag Care 2011;17:548‐555. [PubMed] [Google Scholar]
- 23. Davila JA, Henderson L, Kramer JR, Kanwal F, Richardson PA, Duan Z, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus‐infected veterans in the United States. Ann Intern Med 2011;154:85‐93. [DOI] [PubMed] [Google Scholar]
- 24. Yip B, Wantuck JM, Kim LH, Wong RJ, Ahmed A, Garcia G, et al. Clinical presentation and survival of Asian and non‐Asian patients with HCV‐related hepatocellular carcinoma. Dig Dis Sci 2014;59:192‐200. [DOI] [PubMed] [Google Scholar]
- 25. Mittal S, Sada YH, El‐Serag HB, Kanwal F, Duan Z, Temple S, et al. Temporal trends of nonalcoholic fatty liver disease‐related hepatocellular carcinoma in the veteran affairs population. Clin Gastroenterol Hepatol 2015;13:594‐601.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Franssen B, Alshebeeb K, Tabrizian P, Marti J, Pierobon ES, Lubezky N, et al. Differences in surgical outcomes between hepatitis B‐ and hepatitis C‐related hepatocellular carcinoma: a retrospective analysis of a single North American center. Ann Surg 2014;260:650‐656. [DOI] [PubMed] [Google Scholar]
- 27. Takada A, Takase S, Tsutsumi M. Characteristic features of alcoholic liver disease in Japan: a review. Gastroenterol Jpn 1993;28:137‐148. [DOI] [PubMed] [Google Scholar]
- 28. Peters MG, Terrault NA. Alcohol use and hepatitis C. Hepatology 2002;36(Suppl.):S220‐S225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liangpunsakul S, Haber P, McCaughan G. Alcoholic liver disease in Asia, Europe, and North America. Gastroenterology 2016;150:1786‐1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. World Health Organization . Global Status Report on Alcohol and Health, 2014. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 31. Nguyen MH, Whittemore AS, Garcia RT, Tawfeek SA, Ning J, Lam S, et al. Role of ethnicity in risk for hepatocellular carcinoma in patients with chronic hepatitis C and cirrhosis. Clin Gastroenterol Hepatol 2004;2:820‐824. [DOI] [PubMed] [Google Scholar]
- 32. Nguyen LH, Nguyen MH. Systematic review: Asian patients with chronic hepatitis C infection. Aliment Pharmacol Ther 2013;37:921‐936. [DOI] [PubMed] [Google Scholar]
- 33. Shinkai N, Nojima M, Iio E, Matsunami K, Toyoda H, Murakami S, et al. High levels of serum Mac‐2‐binding protein glycosylation isomer (M2BPGi) predict the development of hepatocellular carcinoma in hepatitis B patients treated with nucleot(s)ide analogues. J Gastroenterol 2018;53:883‐889. [DOI] [PubMed] [Google Scholar]
- 34. Kawaguchi K, Honda M, Ohta H, Terashima T, Shimakami T, Arai K, et al. Serum Wisteria floribunda agglutinin‐positive Mac‐2 binding protein predicts hepatocellular carcinoma incidence and recurrence in nucleos(t)ide analogue therapy for chronic hepatitis B. J Gastroenterol 2018;53:740‐751. [DOI] [PubMed] [Google Scholar]
- 35. Nagata H, Nakagawa M, Nishimura‐Sakurai Y, Asano Y, Tsunoda T, Miyoshi M, et al.; Ochanomizu Liver Conference Study Group . Serial measurement of Wisteria floribunda agglutinin positive Mac‐2‐binding protein is useful for predicting liver fibrosis and the development of hepatocellular carcinoma in chronic hepatitis C patients treated with IFN‐based and IFN‐free therapy. Hepatol Int 2016;10:956‐964. [DOI] [PubMed] [Google Scholar]
- 36. Morio K, Imamura M, Daijo K, Teraoka Y, Honda F, Nakamura Y, et al. Wisteria floribunda agglutinin positive Mac‐2‐binding protein level increases in patients with acute liver injury. J Gastroenterol 2017;52:1252‐1257. [DOI] [PubMed] [Google Scholar]
- 37. Hsu Y‐C, Jun T, Huang Y‐T, Yeh M‐L, Lee C‐L, Ogawa S, et al. Serum M2BPGi level and risk of hepatocellular carcinoma after oral anti‐viral therapy in patients with chronic hepatitis B. Aliment Pharmacol Ther 2018;48:1128‐1137. [DOI] [PubMed] [Google Scholar]
- 38. Bekki Y, Yoshizumi T, Shimoda S, Itoh S, Harimoto N, Ikegami T, et al. Hepatic stellate cells secreting WFA+ ‐M2BP: its role in biological interactions with Kupffer cells. J Gastroenterol Hepatol 2017;32:1387‐1393. [DOI] [PubMed] [Google Scholar]
- 39. Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non‐alcoholic fatty liver disease: an emerging menace. J Hepatol 2012;56:1384‐1391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


