Abstract
Background:
Some individuals control HIV replication without antiretroviral (ARV) therapy.
Objective:
To analyze viral suppression in young women in rural South Africa enrolled in a trial evaluating a behavioral intervention for HIV prevention.
Methods:
Plasma samples were obtained from women ages 13–24 (81 infected at enrollment, 164 seroconverters). ARV testing was performed using an assay that detects 20 ARV drugs. Women were classified as viremic controllers if they were virally suppressed for ≥12 months with no ARV drug use.
Results:
Samples from 216/245 (88.2%) women had no ARV drugs detected at their first HIV-positive visit. Thirty-four (15.7%) of the 216 women had a viral load <2,000 copies/mL. Fifteen of the 34 women were followed for ≥12 months; 12 were virally suppressed with no ARV drugs detected during follow-up. These women were classified as viremic controllers (overall: 12/216=5.6%). The median CD4 cell count at the first HIV-positive visit was higher among the 12 controllers than among the 204 women who were not using ARV drugs (759 vs. 549 cells/mm3, p=0.02). Some women had a viral load <40 copies/mL at a single study visit, but none were classified as elite controllers (viral load <40 copies/mL for ≥12 months with no ARV drug use).
Conclusions:
In this cohort, 5.6% of women who were not using ARV drugs had sustained viral suppression. This represents a minimum estimate of the frequency of viremic controllers in this cohort, since some women were not followed long enough to meet the criteria for classification.
Keywords: HIV, viremic controllers, natural control, young women, South Africa
Introduction
Some HIV-infected individuals are able to maintain low or undetectable levels of HIV RNA without antiretroviral therapy (ART).1–3 These individuals often have low levels of viremia during acute infection, normal or slightly higher CD4 cell counts, normal levels of immune activity, and slower progression to AIDS.4–6 The criteria used to classify these individuals as elite or viremic controllers vary, but are usually based on the degree of viral suppression and the duration of viremic control in the absence of ART.7,8
Several factors have been associated with natural control of HIV infection.3,9,10 The ability to control viral replication to very low levels may reflect differences in viral genetics, host genetics, or innate, humoral, or adaptive immune responses.3,9,10 Most reports have not found an association between natural control of HIV infection and race, gender, age, or route of HIV acquisition.1,10
Most studies of HIV controllers rely on self-report or medical records to identify individuals who are not using antiretroviral (ARV) drugs.11–13 However, previous studies in both HIV-infected and HIV–uninfected cohorts have demonstrated that self-report of ARV drug use may be unreliable.14,15 We developed a high-throughput assay that detects 20 ARV drugs in five drug classes. This assay provides an objective method for assessing ARV drug use. In this report, we used the multi-drug assay to identify HIV controllers in a cohort of young women enrolled in a clinical trial (HIV Prevention Trials Network [HPTN] 068) in rural South Africa that evaluated a behavioral intervention for HIV prevention.16,17 HIV viral load is associated with HIV transmission risk and HIV treatment outcomes. Information on viremic control of HIV infection may help inform the design and interpretation of HIV treatment and prevention studies and may also be helpful for understanding the dynamics of the HIV epidemic in some populations.
Methods
Study cohort
Samples were obtained from women aged 13–24 who were enrolled in the HPTN 068 study (2011–2015).16,17 HPTN 068 evaluated whether a cash transfer conditional on school attendance reduced HIV incidence.16,17 Participants were followed until their expected school graduation date or study completion. A subset of participants had a single follow-up visit 1–2 years after their expected study exit (post-intervention follow-up study). At the time when HPTN 068 study was conducted, ART was recommended in the study region for individuals with CD4 cell counts ≤200 cells/mm3; earlier ART initiation was recommended for pregnant women and those with tuberculosis (CD4 cell count ≤350 cells/mm3).18
Laboratory testing
HIV testing was performed at enrollment (all participants) and at annual follow-up visits for participants who were HIV uninfected at enrollment. CD4 cell count testing was performed at study sites for HIV-infected participants at their first HIV-positive visit and follow-up visits.16 Other laboratory testing described in this report was performed retrospectively at the HPTN Laboratory Center (Johns Hopkins University School of Medicine, Baltimore, MD, USA). HIV testing was performed to confirm results from site testing and to confirm HIV seroconversion events.17 HIV viral load testing was performed using the FDA-cleared RealTime HIV-1 Viral Load assay (Abbott Molecular, Abbott Park, IL; limit of quantification: 40 copies/mL). Rigorous procedures for sample collection, processing, storage, shipping, and testing were used throughout the HPTN 068 trial and in this study, to minimize any risk of sample degradation. ARV drug testing was performed using a qualitative multi-drug assay based on high-performance liquid chromatography coupled with high-resolution accurate mass mass spectrometry.19,20 HIV genotyping was performed using the ViroSeq HIV-1 Genotyping System v2.8 (Abbott Molecular, Des Plaines, IL). HIV sequences generated with the ViroSeq system were used for HIV subtyping using the REGA HIV-1 Subtyping tool.21 Sequences were submitted to GenBank (Accession numbers: KY883695–KY883762, KY888784–KY888875, KY921717–KY921757).
Statistical analysis
HIV controllers were defined as having a viral load below a fixed cutoff (<40 copies/mL for elite controllers; <2,000 copies/mL for viremic controllers) at their first HIV-positive visit and one or more annual study visits (i.e., for at least 12 months), with no ARV drugs detected at those visits. Those who had a single viral load measurement ≥2,000 copies/mL during follow-up were included in this group. The viral load cutoffs used to define elite and viremic controllers in this report are used by International HIV Controller Consortium (www.hivcontrollers.org) and have been used in other reports.22–24 Statistical analysis was performed using the SAS software (SAS 9.4). Associations between viremic controllers and participant characteristics were examined using Wilcoxon rank sum tests.
Ethical considerations
Study participants and their parents/guardians provided written consent for participation in the HPTN 068 study. Written assent was obtained for participants younger than 18 years. The HPTN 068 study was approved by ethical review boards at participating institutions.
Results
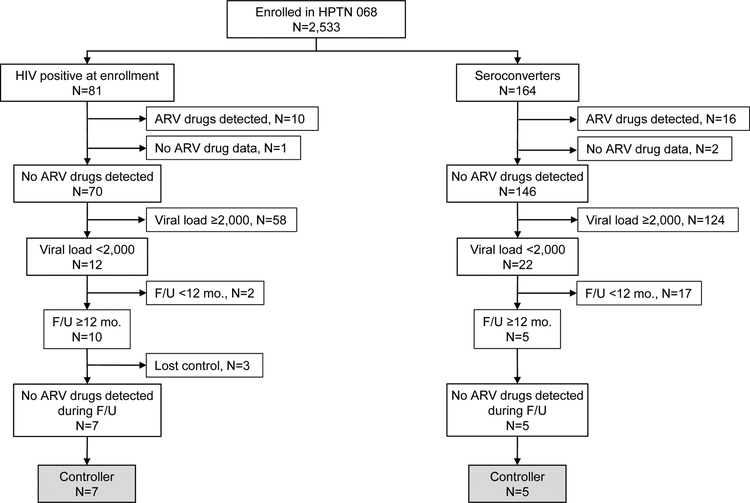
The HPTN 068 cohort included 245 HIV-infected women; 81 were HIV-infected at enrollment and 164 acquired HIV infection during the study (seroconverters, Figure). ARV testing was performed using samples collected at the first HIV-positive visit; results were obtained for 242 of the 245 HIV-infected women. ARV drugs were detected in samples from 26 (10.7%) of the 242 women (10 infected at enrollment; 16 seroconverters, Figure). The 26 women with ARV drugs detected and the three women without ARV drug testing results were excluded from further analysis. Thirty-four (15.7%) of the remaining 216 women had a viral load <2,000 copies/mL at their first HIV-positive visit (12 infected at enrollment; 22 seroconverters); three of the 34 women had a viral load <40 copies/mL. The median viral load among the 34 women was 492 copies/mL (range: <40–1,479).
Figure. Study cohort and identification of viremic controllers in the HPTN 068 cohort.
The figure shows the number of HPTN 068 participants who were HIV-infected at study enrollment and the number of women who acquired HIV infection during study follow-up (F/U). For each group, the figure shows the number of those participants who had no antiretroviral (ARV) drugs detected at their first HIV-positive visit (enrollment or seroconversion), the number of participants with viral load levels <2,000 copies/mL at their first HIV-positive visit, the number of participants with at least 12 months of follow-up, and the number identified as viremic controllers. Loss of virologic control was defined as having two or more HIV RNA measurements >2,000 copies/mL during study follow-up.
Abbreviations: mo.: month; F/U: follow-up.
Fifteen (44.1%) of the 34 women who had an initial viral load <2,000 copies/mL were followed for at least 12 months (median follow-up period: 23 months, range: 13–51 months). Twelve of 15 participants had sustained viral suppression (viral load <2,000 copies/mL for at least 12 months; median follow-up: 20 months, range 13–42; one of the 12 women had a single elevated viral load result of 2,693 copies/mL during this period; Supplemental Figure). None of the 12 women had a sustained viral load <40 copies/mL (i.e., no elite controllers were identified in this study). ARV drugs were not detected in samples from any of the 12 women at any follow-up visit. These 12 women were classified as viremic controllers.
Age, viral load, and CD4 cell count were compared for the 12 viremic controllers and the other 204 women who had no ARV drugs detected at their first HIV-positive visit (control group). The median age at the first HIV-positive visit was lower for the viremic controllers (17.5 years [range: 16–19] vs. 19 years [range: 13–24], p=0.04, Table). This most likely reflects selection bias, since the viremic controller group only included women with at least 12 months of follow-up; women with <12 months follow-up who were enrolled in HPTN 068 acquired HIV infection in the year before their last study visit. The median viral load at the first HIV-positive visit was lower for the viremic controllers than the control group, as expected (1,063 copies/mL [range: 61–1,479] vs.17,911 copies/mL [range: <40–5,667,096]). The median CD4 cell count at the first HIV-positive visit was higher for the viremic controllers than the control group (759 cells/mm3 [range: 448–1,817] vs. 549 cells/mm3 [range: 13–1,440], p=0.02), and remained above 500 cells/mm3 in seven of the 12 viremic controllers throughout the follow-up period (Supplemental Figure). There was no significant difference between the median CD4 cell count during the follow-up period among viremic controllers and the control group (542 cells/mm3 vs. 538 cells/mm3, respectively, p=0.42).
Table.
Characteristics of HIV-infected participants who had no ARV drugs detected at their first HIV-positive visit.
| Parameters | Viremic controllersa N=12 |
Other women N=204 |
P value |
|---|---|---|---|
| First HIV-positive visit | |||
| Median (range) viral load, copies/mL | 1,063 (61–1,479) | 17,911 (40–5,667,096) | NAb |
| Median (range) age, years | 17.5 (16–19) | 19 (13–24) | 0.04 |
| Median (range) CD4 cell count, cells/mm3 | 759 (448–1817) | 549 (13–1440) | 0.02 |
| During follow-up | |||
| Median (range) CD4 cell count, cells/mm3 | 542 (394–1130) | 538 (77–1404) | 0.42 |
Participants who had a HIV viral load <2,000 copies/mL at their first HIV-positive visit with sustained viral suppression for at least 12 months (see Methods).
A p value is not reported for this comparison, since viral load was used to identify viremic controllers (NA: not applicable).
HIV genotyping was performed for 212 (92.3%) of the 245 HIV-positive women; 33 samples were not tested (viral load <400 copies/mL or no sample available for testing). Results were obtained for 201 (96.7%) of the 212 samples (11 samples failed testing). HIV genotyping results from the full HPTN 068 cohort are presented in a separate report.25 HIV drug resistance results were obtained for five viremic controllers; (Y181C) was detected in only one of these cases. HIV subtyping was performed previously using pol region sequences obtained from HIV genotyping. Two-hundred women had subtype C infection, including five of 12 viremic controllers; one woman had subtype A infection; the other seven viremic controllers did not have genotyping results (most due to low viral load).26
Discussion
This report analyzed viral suppression in young women in rural South Africa. In this cohort, 216 (88.2%) of the 245 HIV-infected women were not taking ARV drugs at their first HIV-positive visit (enrollment or seroconversion). Some women had a viral load <40 copies/mL at a single study visit, but none met the criteria for elite controllers. Twelve (5.6%) of the 216 women had sustained viral suppression in the absence of ARV drug use and were classified as viremic controllers. This provides a minimum estimate of the frequency of viremic controllers in this cohort, because more than half of the 34 women who had an initial low viral load value and were not taking ARV drugs were not classified as viremic controllers because they were followed for <12 months. Based on results obtained for the women with ≥12 months follow-up, we estimate that the true frequency of viremic controllers in this cohort is 12.6% ([34 women with initial viral suppression] × [80% sustained viral suppression] × [100] / 216 women with no ARV drugs detected). A previous study from South Africa reported a high prevalence (17.2%) of individuals who had viral loads <50 copies/mL in the absence of ART.27 In other studies, the portion of individuals with natural control of HIV infection ranged from <1% to 7.7%.2,3,8,11–13,22,28,29 These studies used different criteria to define HIV controllers. Two of these studies used the same viral load cutoff to define viremic controllers that was used in this report (2,000 copies/mL); those studies reported a prevalence of viremic controllers of 2.7%22 and 3.34%.2 ARV drug use was identified in some studies based on self-report or medical records;11–13 some studies did not indicate how ARV drug use was assessed.2,3,8,28,29 Our previous studies of men who have sex with men,14,30,31 heterosexual couples,32 and women at high risk of HIV infection,15 demonstrate that some individuals may not disclose ARV drug use. Therefore, the frequency of HIV controllers may be lower than reported in studies that rely on self-report to assess ARV drug use.
Higher CD4 cell counts have been observed in HIV controllers in other studies.3,8,33 In this report, among women who were not taking ARV drugs, the median CD4 cell count was significantly higher among the viremic controllers compared to women with higher viral loads. However, we did not observe a significant difference in CD4 cell counts between these groups over time. Previous studies have showed an association of natural control of HIV infection with specific HLA class I alleles.3,9,10 However, the frequency of these alleles in HIV controllers varies from 15% to 86% in different populations,1,3,34 and some of the putatively “protective” HLA class I alleles were also identified in HIV progressors who were not virally suppressed.34 Therefore, it seems unlikely that HLA class I genotype alone leads to natural control of infection. Viral (HIV) genetics may also play a role in natural control of HIV infection.
Some HIV drug resistance mutations that have been associated with lower viral fitness (e.g., M184I/V)10,35 have been identified in some HIV controllers. In other cases, HIV controllers were infected with high-fitness viruses,9,36 that did not have mutations considered to be protective genetic factors.35,37 M184I/V was not detected in this study in the five viremic controllers who had HIV genotyping results. One viremic controller had HIV with the Y181C mutation; this mutation has a minimal impact on HIV replication capacity38 and has not been associated with lower viral load levels.35 Because drug resistance data were only obtained for five viremic controllers in the HPTN 068 study, we were not able evaluate whether drug resistance played a role in viremic control in this cohort. The HPTN 068 study did not obtain consent to perform host genetic testing, and phenotypic studies of HIV replication capacity could not be performed in this study because insufficient plasma was available for analysis.
Most prior reports have found no association of viremic control with gender, age, or race,1,10 though these associations have been reported in some studies.11 In our study, there was no age difference between women who had viral loads <2,000 copies/mL vs. >2,000 copies/mL at their first HIV-positive visit. Our finding that the median age of viremic controllers was lower than other women (p=0.04) most likely reflects the requirement for a 12-month follow-up for classification of viremic control. Women with longer follow-up after their first HIV-positive visit were more likely to have enrolled in the HPTN 068 study during an earlier school grade, when they were younger.
Previous studies found an association of disease progression with HIV subtype.39 For example, HIV subtype D infection has been associated with faster CD4 cell decline, frequent virologic rebound during ART, and higher rate of mortality compared to subtypes A, B and C.39,40 In one cohort, slower disease progression was observed in women with HIV subtype C infection compared to those with subtype A and D infection.41 HIV subtype was determined for the majority (82%) of the HIV-infected women in HPTN 068; all but one had subtype C infection, including all of the viremic controllers who had genotyping results.
One potential limitation of this report is that it included both women who were infected at study entry and those who acquired HIV infection during the study. Several factors make it less likely that were significant differences between the two study groups that might impact viremic control. HIV seroconverters were identified at annual study visits; therefore, these women were infected in the year prior to the seroconversion visit. Because the study enrolled women as young as 13 years of age, some women who were HIV infected at study entry were also likely to have been recently infected. The enrollment criteria for HPTN 068 were also not likely to have impacted enrollment of viremic controllers vs. non-controllers. There was no incentive for enrollment of HIV-uninfected women, and no cap or other disincentive for enrollment of those with known HIV infection or a history of ARV drug exposure. Overall, only 2% of young women and 5% of parent/guardians refused participation in the trial,16 and the baseline prevalence of HIV infection in HPTN 068 was 3.2%, which is similar to the prevalence of HIV in another study performed in population of school students in rural South Africa in the same age category (3.8%).42
In conclusion, in this cohort of young women from rural South Africa, we identified a high portion of women who were virally suppressed in the absence of ARV drug use (N=34, 15.7%). Approximately one-third of these women had sustained viral suppression with no ARV drug use. The actual frequency of viremic controllers in this cohort may have been higher, since HIV infection in some women could not be fully characterized due to limited study follow-up. Based on results from women who were followed for at least one year, the prevalence of viremic controllers in this cohort was 12.6%. The relatively high frequency of viremic controllers in this population suggests that ARV drug testing may be useful in addition to HIV viral load testing for evaluating treatment outcomes in future studies, to distinguish between those with natural vs. ARV-induced viral suppression. This information may also be useful for studies of HIV transmission and dynamics among young women in similar settings, since viremic controllers are less likely to transmit HIV to others.
Supplementary Material
Acknowledgements
The authors thank the HPTN 068 study participants and study team for providing samples and data for this study. The authors also thank the laboratory staff at the study sites and the HPTN Laboratory Center for their assistance with sample management and laboratory testing.
This work was supported by (1) UM1-AI068613 (Eshleman), UM1-AI068619 (Cohen/El-Sadr), and UM1-AI068617 (Donnell), from the National Institute of Allergy and Infectious Diseases, the National Institute of Mental Health and the National Institute on Drug Abuse of the National Institutes of Health; (2) R01-AI095068 (Eshleman); (3) R01-MH087118 (Pettifor); and (4) the Carolina Population Center and its NIH Center grant, P2C-HD050924.
Footnotes
Note: Some of the work in this manuscript was presented as an abstract at the Conference on Retroviruses and Opportunistic Infections (CROI) in Boston, MA, USA (March, 2018)
Declaration of Interests and Source of Funding:
None of the authors has a conflict of interest or potential conflict of interest, with the following exceptions: Susan Eshleman has collaborated on research studies with investigators from Abbott Laboratories; Abbott Laboratories has provided reagents for collaborative research studies.
Contributor Information
Mariya V. Sivay, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, USA, 410-614-6498, msivay1@jhmi.edu.
Jessica M. Fogel, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, USA, 410-614-6498, jfogel@jhmi.edu.
Jing Wang, Statistical Center for HIV/AIDS Research & Prevention (SCHARP), Seattle, WA, USA, 206-667-6357, jwang2@scharp.org .
Yinfeng Zhang, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, USA, 410-614-6498, yzhan249@jhmi.edu.
Estelle Piwowar-Manning, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, USA, 410-614-6736, epiwowa@jhmi.edu.
William Clarke, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, USA, 410-502-7692, wclarke@jhmi.edu.
Autumn Breaud, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, USA, 443-287-7662, abreaud1@jhmi.edu.
Joel Blankson, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA, 410-955-7757, jblanks@jhmi.edu.
Erica L. Hamilton, Science Facilitation Department, FHI 360, Durham, NC, USA, 919-544-7040, ehamilton@fhi360.org.
Kathleen Kahn, MRC/Wits Rural Public Health and Health, Transitions Research Unit (Agincourt), School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa, 27-11-717-2617, kathleen.kahn@wits.ac.za.
Amanda Selin, University of North Carolina at Chapel Hill, Carolina Population Center, Chapel Hill, NC, USA, 919-360-5988, aselin@unc.edu.
F. Xavier Gomez-Olive, MRC/Wits Rural Public Health and Health, Transitions Research Unit (Agincourt), School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa, 27-13-795-5076 ext 128, F.Gomez-OliveCasas@wits.ac.za.
Catherine MacPhail, School of Health and Society, University of Wollongong, Australia; MRC/Wits Rural Public Health and Health, Transitions Research Unit (Agincourt), School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa, 61-242-214-112, cmacphai@uow.edu.au.
James P. Hughes, Department of Biostatistics, University of Washington, Seattle, WA, USA, 206-744-3633, jphughes@u.washington.edu.
Audrey Pettifor, Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, NC; MRC/Wits Rural Public Health and Health, Transitions Research Unit (Agincourt), School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa, 919-966-7439, apettif@email.unc.edu.
Susan H. Eshleman, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, USA, 410-614-4734, seshlem@jhmi.edu.
References
- 1.Okulicz JF, Lambotte O. Epidemiology and clinical characteristics of elite controllers. Curr Opin HIV AIDS. 2011;6(3):163–168. [DOI] [PubMed] [Google Scholar]
- 2.Okulicz JF, Marconi VC, Landrum ML, et al. Clinical outcomes of elite controllers, viremic controllers, and long-term nonprogressors in the US Department of Defense HIV natural history study. J Infect Dis. 2009;200(11):1714–1723. [DOI] [PubMed] [Google Scholar]
- 3.Pereyra F, Addo MM, Kaufmann DE, et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis. 2008;197(4):563–571. [DOI] [PubMed] [Google Scholar]
- 4.Taborda NA, Rugeles MT, Montoya CJ. Spontaneous control of HIV replication, but not HAART-induced viral suppression, is associated with lower activation of immune cells. J Acquir Immune Defic Syndr. 2014;66(4):365–369. [DOI] [PubMed] [Google Scholar]
- 5.Miura T, Brumme ZL, Brockman MA, et al. Impaired replication capacity of acute/early viruses in persons who become HIV controllers. J Virol. 2010;84(15):7581–7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bello G, Velasco-de-Castro CA, Bongertz V, et al. Immune activation and antibody responses in non-progressing elite controller individuals infected with HIV-1. J Med Virol. 2009;81(10):1681–1690. [DOI] [PubMed] [Google Scholar]
- 7.Olson AD, Meyer L, Prins M, et al. An evaluation of HIV elite controller definitions within a large seroconverter cohort collaboration. PLoS One. 2014;9(1):e86719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crowell TA, Hatano H. Clinical outcomes and antiretroviral therapy in ‘elite’ controllers: a review of the literature. J Virus Erad. 2015;1(2):72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blankson JN. Effector mechanisms in HIV-1 infected elite controllers: highly active immune responses? Antiviral Res. 2010;85(1):295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Theze J, Chakrabarti LA, Vingert B, Porichis F, Kaufmann DE. HIV controllers: a multifactorial phenotype of spontaneous viral suppression. Clin Immunol. 2011;141(1):15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang OO, Cumberland WG, Escobar R, Liao D, Chew KW. Demographics and natural history of HIV-1-infected spontaneous controllers of viremia. AIDS. 2017;31(8):1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowell TA, Gebo KA, Blankson JN, et al. Hospitalization rates and reasons among HIV elite controllers and persons with medically controlled HIV infection. J Infect Dis. 2015;211(11):1692–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandalia S, Westrop SJ, Beck EJ, Nelson M, Gazzard BG, Imami N. Are long-term non-progressors very slow progressors? Insights from the Chelsea and Westminster HIV cohort, 1988–2010. PLoS One. 2012;7(2):e29844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marzinke MA, Clarke W, Wang L, et al. Nondisclosure of HIV status in a clinical trial setting: antiretroviral drug screening can help distinguish between newly diagnosed and previously diagnosed HIV infection. Clin Infect Dis. 2014;58(1):117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen I, Clarke W, Ou SS, et al. Antiretroviral drug use in a cohort of HIV-uninfected women in the United States: HIV Prevention Trials Network 064. PLoS One. 2015;10(10):e0140074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pettifor A, MacPhail C, Selin A, et al. HPTN 068: A randomized control trial of a conditional cash transfer to reduce HIV infection in young women in South Africa-study design and baseline results. AIDS Behav. 2016;20(9):1863–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pettifor A, MacPhail C, Hughes JP, et al. The effect of a conditional cash transfer on HIV incidence in young women in rural South Africa (HPTN 068): a phase 3, randomised controlled trial. Lancet Glob Health. 2016;4(12):e978–e988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.South African National Department of Health. Clinical guidelines for the managements of HIV & AIDS in adults and adolescents. 2010; http://www.who.int/hiv/pub/guidelines/south_africa_art.pdf. Accessed August 16, 2018.
- 19.Marzinke MA, Breaud A, Parsons TL, et al. The development and validation of a method using high-resolution mass spectrometry (HRMS) for the qualitative detection of antiretroviral agents in human blood. Clin Chim Acta. 2014;433:157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Clarke W, Marzinke MA, et al. Evaluation of a multidrug assay for monitoring adherence to a regimen for HIV preexposure prophylaxis in a clinical study, HIV Prevention Trials Network 073. Antimicrob Agents Chemother. 2017;61(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Oliveira T, Deforche K, Cassol S, Rambaut A, Vandamme A-M. REGA HIV-1 subtyping tool. 2014; http://dbpartners.stanford.edu:8080/RegaSubtyping/stanford-hiv/typingtool/. Accessed December 7, 2016.
- 22.Groves KC, Bibby DF, Clark DA, et al. Disease progression in HIV-1-infected viremic controllers. J Acquir Immune Defic Syndr. 2012;61(4):407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ndhlovu ZM, Chibnik LB, Proudfoot J, et al. High-dimensional immunomonitoring models of HIV-1-specific CD8 T-cell responses accurately identify subjects achieving spontaneous viral control. Blood. 2013;121(5):801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.International HIV Controllers Study, Pereyra F, Jia X, et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2010;330(6010):1551–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Sivay MV, Hudelson SE, et al. Antiretroviral drug use and HIV drug resistance among young women in rural South Africa: HPTN 068. J Acquir Immune Defic Syndr. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sivay MV, Hudelson SE, Wang J, et al. HIV-1 diversity among young women in rural South Africa: HPTN 068. Plos One. 2018;13(7):e0198999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kranzer K, Lawn SD, Johnson LF, Bekker LG, Wood R. Community viral load and CD4 count distribution among people living with HIV in a South African Township: implications for treatment as prevention. J Acquir Immune Defic Syndr. 2013;63(4):498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laeyendecker O, Redd AD, Lutalo T, et al. Frequency of long-term nonprogressors in HIV-1 seroconverters From Rakai Uganda. J Acquir Immune Defic Syndr. 2009;52(3):316–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goujard C, Chaix ML, Lambotte O, et al. Spontaneous control of viral replication during primary HIV infection: when is “HIV controller” status established? Clin Infect Dis. 2009;49(6):982–986. [DOI] [PubMed] [Google Scholar]
- 30.Chen I, Connor MB, Clarke W, et al. Antiretroviral drug use and HIV drug resistance among HIV-infected black men who have sex with men: HIV Prevention Trials Network 061. J Acquir Immune Defic Syndr. 2015;69(4):446–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fogel JM, Zhang Y, Guo X, et al. Reliability of self-reported HIV status among African MSM screened for HPTN 075 Paper presented at: Conference on retroviruses and opportunistic infections; 4–7 March, 2018; Boston. [Google Scholar]
- 32.Fogel JM, Wang L, Parsons TL, et al. Undisclosed antiretroviral drug use in a multinational clinical trial (HIV Prevention Trials Network 052). J Infect Dis. 2013;208(10):1624–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunt PW, Brenchley J, Sinclair E, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197(1):126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker BD, Yu XG. Unravelling the mechanisms of durable control of HIV-1. Nat Rev Immunol. 2013;13(7):487–498. [DOI] [PubMed] [Google Scholar]
- 35.Harrison L, Castro H, Cane P, et al. The effect of transmitted HIV-1 drug resistance on pre-therapy viral load. AIDS. 2010;24(12):1917–1922. [DOI] [PubMed] [Google Scholar]
- 36.Lamine A, Caumont-Sarcos A, Chaix ML, et al. Replication-competent HIV strains infect HIV controllers despite undetectable viremia (ANRS EP36 study). AIDS. 2007;21(8):1043–1045. [DOI] [PubMed] [Google Scholar]
- 37.Blankson JN, Bailey JR, Thayil S, et al. Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J Virol. 2007;81(5):2508–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez-Picado J, Martinez MA. HIV-1 reverse transcriptase inhibitor resistance mutations and fitness: a view from the clinic and ex vivo. Virus Res 2008;134(1–2):104–123. [DOI] [PubMed] [Google Scholar]
- 39.Easterbrook PJ, Smith M, Mullen J, et al. Impact of HIV-1 viral subtype on disease progression and response to antiretroviral therapy. J Int AIDS Soc. 2010;13:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiwanuka N, Laeyendecker O, Robb M, et al. Effect of human immunodeficiency virus Type 1 (HIV-1) subtype on disease progression in persons from Rakai, Uganda, with incident HIV-1 infection. J Infect Dis. 2008;197(5):707–713. [DOI] [PubMed] [Google Scholar]
- 41.Venner CM, Nankya I, Kyeyune F, et al. Infecting HIV-1 subtype predicts disease progression in women of sub-Saharan Africa. EBioMedicine. 2016;13:305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kharsany AB, Buthelezi TJ, Frohlich JA, et al. HIV infection in high school students in rural South Africa: role of transmissions among students. AIDS Res Hum Retroviruses. 2014;30(10):956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.