Abstract
Exposure to drugs of abuse produces maladaptive changes in cost-benefit decision-making, including the evaluation of time and risk. Studies probing the effects of drug exposure on such evaluations have primarily used experimenter-administered drug regimens. Similarly, while much is known about the neural bases of effort, there have been relatively fewer investigations of the effects of drug experience on effort-based choices. We recently reported that experimenter-administered methamphetamine (meth) resulted in steeper discounting of effort for food rewards in rats, when assessed in protracted withdrawal. Here, we studied rats that underwent withdrawal from weeks of meth intravenous self-administration that later could freely select between a high effort, preferred option (progressive ratio lever pressing for sucrose pellets) versus a low effort, less preferred option (freely-available lab chow). We found decreased effort for the preferred reward and changes in a behavioral economic index demonstrating an increased sensitivity to effort in meth-experienced rats. Critically, the decreased effort for the preferred option was only present in the context of a competing option, not when it was the only option. We also confirmed rats preferred sucrose pellets over chow when both were freely available. These long-lasting changes were accompanied by decreased c-Fos activation in ventral striatum and basolateral amygdala, regions known to be important in effort-based choices. Taken together with our previous observations, these results suggest a robust and enduring effect of meth on value-based decision-making, and point to the underlying neural mechanisms that support the evaluation of an effort cost.
Keywords: Methamphetamine, Effort, Decision-making, Striatum, Amygdala
1. Introduction
Withdrawal from drugs of abuse leads to changes in motivated behavior and decision-making (Groman et al., 2012; Lee et al., 2009; Olausson et al., 2006; Stolyarova et al., 2015; Thompson et al., 2015, 2017) A prominent feature of withdrawal from psychostimulants is fatigue (Lago and Kosten, 1994; McGregor et al., 2005), which may be related to mechanisms of decreased effort or altered cost-benefit decision-making involving effort (Salamone and Correa, 2012). The neural basis of effort has been well studied in rodent models and in humans, but fewer investigations have probed drug withdrawal experiences that may also contribute to changes in effort-based choice. Here we studied the long-term effects of psychostimulant withdrawal on choices involving physical effort for food rewards, to be distinguished from cognitive or attentional effort that is also probed in rats (Cocker et al., 2012; Hosking et al., 2014, 2015, 2016).
Recent work investigating motivated behavior following weeks of exposure to methamphetamine (meth) showed that rats exert more physical effort for natural rewards such as food (Stolyarova et al., 2015) and exercise (Thompson et al., 2015) in acute and protracted withdrawal from drug. Moreover, others have similarly reported increased lever pressing for grain pellets following withdrawal from psychostimulants like nicotine, MDMA, cocaine, and amphetamine (Olausson et al., 2006). Importantly, the effects mentioned above occur in the context of a single reward-type and/ or only one response option. However, organisms face environments where there are qualitatively different options to choose from, each with different effort requirements. To model self-paced, free choice between qualitatively different options, we adopted an effort-based cost benefit decision-making task (Randall et al., 2012) where rats can select between a high effort, preferred reward (progressive ratio lever pressing for sucrose pellets) and a low effort, less preferred alternative (freely, concurrently available lab chow). We previously reported that rats given experimenter-administered meth and tested in protracted withdrawal exert less effort for a preferred sucrose reward over freely-available chow (Thompson et al., 2017). This altered cost-benefit decision-making (i.e. increased sensitivity to effort costs) was not due to decreased reward sensitivity, anhedonia, or decreased preference for the preferred reinforcer, as determined by control experiments.
Studies investigating the lasting effects of drug experience on effort-based decision-making often use experimenter-administered methods (Floresco and Whelan, 2009; Kosheleff et al., 2012a; Olausson et al., 2006; Thompson et al., 2017). Additionally, studies that have probed related behaviors, such as delay discounting (Floresco and Whelan, 2009) or reversal learning (Groman et al., 2012; Izquierdo et al., 2010; Jentsch et al., 2002; Kosheleff et al., 2012b) have also frequently used experimenter-administered drug. Yet, several groups have also examined self-administered drug effects on delay discounting (Mendez et al., 2010) and other measures of cognitive flexibility (Cox et al., 2016). However, it is presently unknown if withdrawal from meth self-administration results in long-lasting alterations in effort-based decision making involving food rewards.
Behavioral economic indices can be used to assess drug taking and seeking similarly in humans and in experimental animals (Bentzley et al., 2013; Hursh, 1980; Hursh and Silberberg, 2008) and may have high translational value. Using these economic models, demand curves are generated that allow us to estimate consumption at the lowest effort cost (Q0), the rate of decline in consumption as a function of increasing effort cost (α), and an index of demand inelasticity derived from α, essential value (EV). These measures have been shown to predict addiction-like behaviors (Bentzley and Aston-Jones, 2015; Galuska et al., 2011; Gray and MacKillop, 2014; Murphy et al., 2009; Petry, 2001) and decreased food demand (Galuska et al., 2011). For these reasons, we fit an exponential model (Hursh and Silberberg, 2008) to our effort-based choice data.
The present experiment also sought to investigate the effects of intravenous self-administration (IVSA) of meth and activation of brain regions that are known to be important in effortful choice. We assessed c-Fos in anterior cingulate cortex (ACC), ventral striatum (VS), dorsal striatum (DS), and basolateral amygdala (BLA) approximately 30 days following their last drug (or yoked saline) infusion. We predicted that rats withdrawn from meth IVSA would exhibit reduced lever pressing for sucrose, similar to what we have recently shown following experimenter-administered drug (Thompson et al., 2017). We also expected this effect would be accompanied by changes in activation of brain regions previously shown to support this behavior. And finally, we hypothesized that steeper discounting of the more effortful, preferred option would also be revealed as a long-lasting change in demand elasticity (α).
2. Materials and methods
Subjects.
A timeline of all procedures is shown in Fig. 1. Subjects were 32 (n = 19 meth IVSA, n = 13 yoked saline) adult male Long-Evans rats (Charles River Laboratories, Hollister, CA), singly-housed for all phases of experiments with the exception of the acclimation period and handling. A subset of these rats (n = 14; n = 8 meth and n = 6 saline) were used for c-Fos experiments (details below). All animals were handled for 10 min in pairs for 5 d after a brief acclimation period (3 d). Rats weighed an average of 300 g at the beginning of the experiment. One subject was not included in the final data analyses due to premature death, and three others were omitted due to early (i.e., less than 7 IVSA sessions) catheter patency loss. The vivarium was maintained under a 12/12 h reverse light cycle at 22°C, and lab chow and water were available ad libitum prior to behavioral testing. Training and testing were conducted during the early portion of the dark cycle (~0800–1200 H). Experiments were conducted 5–7 d per week, and animals were fed once daily on weekends when testing was not conducted. All procedures were approved by the Chancellor’s Animal Research Committee at the University of California, Los Angeles.
Fig. 1.
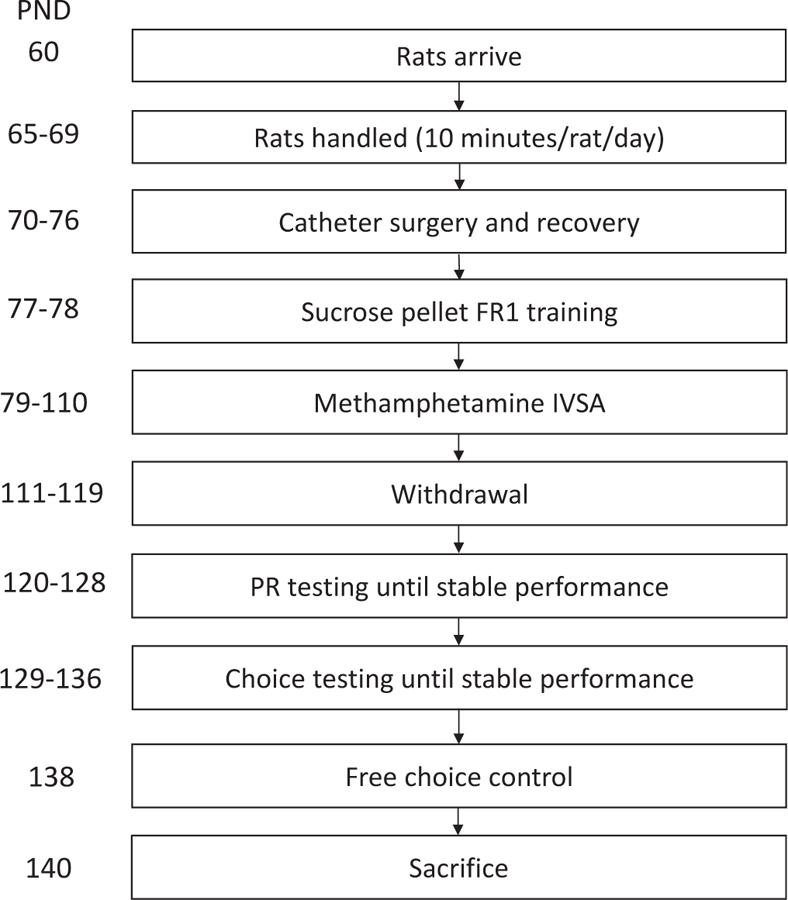
Timeline of events. Sequence of handling, surgery, testing, and euthanasia are depicted from top to bottom order in postnatal day (PND).
Food restriction.
One day before behavioral testing began, the amount of chow given to each rat was reduced to 12 g/d, and rats were given ~10 sucrose pellets (45-mg dustless precision sucrose pellets; Bio-Serv, Frenchtown NJ) in their home cage to acclimate them to the food rewards. Following the initial 2 d of sucrose pellet training, rats were switched to free feeding for the duration of self-administration and withdrawal. Prior to the beginning of the progressive ratio phase of training, rats were restricted to 12 g of chow per d. Finally, rats performing the choice task were given 8 g of chow per d, in addition to the food they consumed during testing. At the time of euthanasia, rats weighed on average ~350 g.
Surgery and FR1 sucrose pellet training.
Implantation of chronic indwelling intravenous jugular catheters took place prior to all behavioral testing. Catheters were custom made and consisted of a 14 cm Silastic tubing fit to a 28-gauge 11 mm cannula (PlasticsOne, Roanoke, VA) bent to 90° and encased in dental cement with a mesh base. Rats were anesthetized with isoflurane (5% induction, 2% maintenance in 2 L/min O2), after which incisions were made on the right dorsolateral surface of the back and the right anterolateral surface of the neck. The cannula of the catheter was inserted through a small incision on the dorsal surface of the back between the scapulae through the back incision. The catheter tubing was passed subcutaneously from the dorsal surface of the back to the ventral incision, the jugular vein was isolated, and the catheter tip was inserted and secured with surgical silk sutures. Following a patency test with heparinized (30 USP units/mL) saline, incisions were sutured closed, and the rats were placed on a heating pad and kept in recovery until ambulatory. Post-operative care consisted of five daily injections of carprofen (5 mg/kg, s. c.). Following a 7-d free-feeding recovery period, rats were restricted to 12 g of food daily following testing and given 2 d of active lever pressing on a fixed ratio-1 schedule for sucrose pellets (45 mg, Bioserv, Frenchtown, NJ). Sessions lasted until 30 min had elapsed or 30 pellets had been earned. The first session was conducted untethered, and the second session was conducted with the steel leash attached to the catheter to acclimate animals to this procedure. Following surgery, catheters were flushed daily with 0.1 mL heparinized saline and for the duration of IVSA.
Apparatus.
All behavioral testing was conducted in chambers outfitted with a house light, internal stimulus lights, a food-delivery magazine, 2 retractable levers positioned to the left and right of the chamber wall opposite the magazine, a syringe pump, and a liquid swivel attached to a steel leash for drug self-administration. All hardware was controlled by a PC running Med-PC IV (Med-Associates, St. Albans, VT).
Meth intravenous self-administration (IVSA) and withdrawal.
Rats were given food ad-libitum for the duration of the 27 sessions of IVSA. Catheter patency was tested the day prior to beginning IVSA with 0.2 mL propofol solution (PropoFlo, Zoetis, Parsippany-Troy Hills, NJ). Rats were allowed to self-administer meth hydrochloride (Sigma, St. Louis, MO) for 2-h sessions 6–7 d per week, during which active and inactive lever presses were recorded. The active lever was the opposite from that used during the initial sucrose pellet training. Each infusion consisted of 0.1 mg/kg freebase meth/ 0.0875 mL saline and lasted for 5 s with a 20 s timeout between infusions. Catheters were flushed before and after sessions with 0.05 mL heparinized saline. Rats were given 6 sessions of FR1 IVSA, 14 sessions of FR3 IVSA, and 7 sessions of FR5 IVSA where 1, 3, or 5 active lever presses were required to earn a single infusion, respectively. Pressing of the inactive lever had no programmed consequences. Saline animals were yoked to meth animals to receive an equal number and volume of saline infusions. Following IVSA, rats were left undisturbed for 9 d to withdraw from the drug prior to beginning progressive ratio testing.
Progressive ratio and effortful choice testing.
Following withdrawal, rats were restricted to 12 g food daily (to no less than 85% free-feeding weight) following testing on the PR schedule for sucrose pellets. The lever that earned pellets was opposite that of the active IVSA lever. The required number of presses increased according to the formula ni=5e(i/5)-5, where ni is equal to the number of presses required on the ith ratio, rounded to the nearest whole number, after 5 successive schedule completions. No time-out was imposed. Rats were tested on the PR schedule until stable performance was achieved (~6 sessions), defined as the number of lever presses across three days of testing falling between 80% and 120% of the mean of those three days. This testing was performed to assess the willingness to work for sucrose pellets in the absence of freely available chow. Upon meeting criteria for stable PR performance, a ceramic ramekin containing 18 g of lab chow was introduced (modified from (Randall et al., 2012)) during testing. Rats were free to choose between consuming freely-available but less preferred chow or lever pressing for preferred sucrose pellets and tested until stable lever pressing performance was achieved as described above. Detailed methods have been published recently (Hart et al., 2017; Hart and Izquierdo, 2017).
Freely-available choice.
Following effortful choice testing, we conducted a test wherein both sucrose pellets and chow were freely-available. Rats were given 30 min of free access to pre-weighed amounts of sucrose pellets and lab chow (~18 g each) in standard empty cages, but different from their home cages. Following the 30-min period, remaining food was collected and weighed to determine rats’ food preferences.
Euthanasia.
Rats in both pretreatment groups (meth and saline) were given a final effortful choice testing session. One hour following this session, animals were humanely euthanized by Euthasol overdose (Euthasol, 0.8 mL, 390 mg/mL pentobarbital, 50 mg/mL phenytoin; Virbac, Fort Worth, TX), and brains were removed for histological processing.
Histological processing and immunohistochemistry.
Following removal, brains were fixed in 10% buffered formalin acetate for 24 h followed by 30% sucrose for 5 d 50 mm sections of ACC, striatum, and BLA were collected and stained for c-Fos. Staining was performed by incubation for 24 h at 4°C in a primary antibody consisting of 1:2000 rabbit polyclonal to c-Fos (Abcam, Cambridge, MA), 3% normal goat serum (Abcam, Cambridge, MA), and 0.5% Triton-X (Sigma, St. Louis, MO) in PBS, followed by four 5-min washes in PBS. Secondary antibody incubations were 2 h at 20 °C in the 0.5% Triton-X/5% normal goat serum/PBS solution with 1:500 goat anti-rabbit Alexa 488 (Abcam, Cambridge, MA) replaced for the primary antibody, followed by four 5-min washes in PBS. Slides were subsequently mounted and cover-slipped with fluoroshield DAPI mounting medium (Abcam, Cambridge, MA). Slices were visualized using a BZ-X710 microscope (Keyence, Itasca, IL), and analyzed with BZ-X Viewer and analysis software. Images for quantification of c-Fos immunoreactivity were taken with a 20× objective with a 724 mm by 543 mm field of view and converted to cells per millimeter squared. For each region, three images were taken from two or three separate slices from both hemispheres at the same approximate AP coordinate (ACC +2.0 mm; VS +2.0 mm; DS 1.6 mm; BLA-3.0 mm), and final cell counts were based on the averages of the three images.
Data analyses.
Data were analyzed using GraphPad Prism v.7 (La Jolla, CA) and SPSS v.25 (Armonk, NY). An alpha level for significance was set to 0.05. Two-way repeated measures ANOVA was used to compare the number of active and inactive lever presses across the 27 IVSA sessions. Independent samples t-tests (reported as means ± SEM) on data from the last of the 3 stable sessions were conducted to analyze group differences on PR and on the choice task. Multivariate ANOVAs with Pillai’s Trace corrections were conducted to analyze group differences on the density of c-Fos immunoreactive cells as well as group differences on behavioral economic indices (described below). A two-way ANOVA was used to test for group differences on food preference, and number of sessions to stable performance. Behavioral economic indices were generated by fitting an exponential model (Hursh and Silberberg, 2008) to our data:
where Q = consumption at a given price C, or cost (FR value), Q0 = demand intensity, consumption at lowest price, k = constant parameter reflecting the range of consumption values in log10 units, α = demand elasticity, or the derived demand parameter reflecting the rate of consumption decline associated with increasing price. Q0 and α were calculated based on the number of pellets earned at each ratio. Because the number of pellets earned at each ratio in our task is necessarily restricted to five, differences in α would be due to animals reaching higher/lower ratios or earning more/fewer pellets at a given ratio. For each rat, we also calculated Essential Value (EV) to quantify the ‘strength’ of the reinforcer (i.e. sucrose pellets) (Kearns et al., 2017), based on the value of the exponential rate constant. As indicated above, the value of α is the rate of consumption decline and k is the consumption range for each rat in the following:
All behavioral economic indices were correlated with brain activation data, and multiple linear regression analyses were conducted to predict indices from c-Fos immunoreactivity.
3. Results
IVSA.
Rats acquired and maintained meth self-administration and increased active lever pressing as a function of increased work requirement. There was a significant main effect of lever (F(1,28) = 22.99 p < 0.001), significant main effect of session (F(26,728) = 4.71 p < 0.0001), and significant lever by session interaction (F(26,728) 4.89 p < 0.001) (Fig. 2A). These press rates yielded relatively stable levels of meth intake across the 27 sessions (Fig. 2B), average dose of 1.16 mg/kg/session. Yoked saline controls had no preference for the active lever over the inactive lever. There was no significant main effect of lever (F(1,24) = 0.83, p = 0.37). There was a significant main effect of session due to extinction (rats had previously received 2 d of FR1 for sucrose pellets) (F(26,624) = 4.76, p < 0.001) and no significant lever by session interaction (F(26,624) = 1.46, p = 0.07) (data not shown).
Fig. 2.
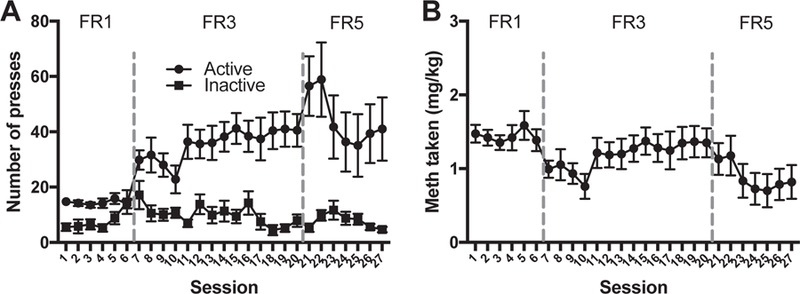
Meth IVSA. (A) Number of active and inactive lever presses on each of the 27 IVSA sessions that had FR1, FR3, and FR5 work requirements for each infusion. Animals showed a preference for the active lever over the inactive lever (p < 0.0001). (B) Amount of meth taken (mg/kg) on each of the 27 IVSA sessions. Points denote mean ± SEM.
Progressive Ratio.
There were no group differences in the number of lever presses on the PR schedule for sucrose pellets. An independent samples t-test on the number of lever presses on the last of the three stable days of PR testing revealed no significant difference between groups (t(26) = 0.91 p = 0.37; saline = 582.3 ± 68.8; meth = 480.5 ± 85.29) (Fig. 3A).
Fig. 3.
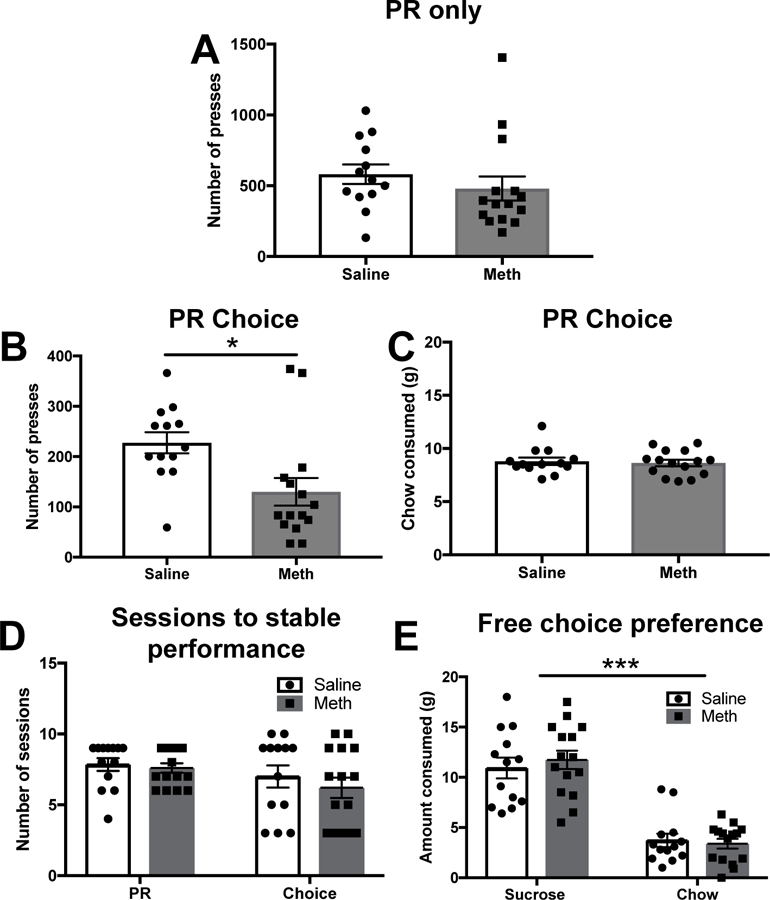
Effects of meth IVSA withdrawal on effort for food rewards. (A) Number of lever presses for sucrose pellets on the last of the three stable PR testing sessions in yoked saline and meth IVSA groups. Groups did not differ in the number of lever presses on the PR schedule. (B) Number of lever presses for sucrose pellets on the last of the three stable choice testing sessions in yoked saline and meth IVSA groups. Meth exposed animals emitted fewer lever presses compared to yoked saline controls (p < 0.05). (C) Amount of chow consumed on the last of the three stable choice testing sessions in yoked saline and meth IVSA groups. Groups did not differ in the amount of chow they consumed. (D) Number of sessions required to reach stable performance on the PR and choice tasks in yoked saline and meth IVSA animals. Groups did not differ in the number of sessions required to reach stable performance on either schedule. (E) In the free choice control task where both options were freely available, both groups preferred the sucrose pellets to the lab chow (p < 0.001). Points denote mean ± SEM, *p < 0.05, ***p < 0.001.
Effortful Choice Testing.
After allowing several sessions to achieve stable performance, a significant difference in number of lever presses on the last of the three stable sessions was revealed by an independent samples t-test (t(26) = 2.74 p = 0.01; saline 227.4 ± 21.00; meth = 130.00 ± 27.59): meth-experienced rats lever pressed significantly less for sucrose pellets than yoked saline controls (Fig. 3B). There was no significant difference in the amount of chow consumed between these conditions (t(26) = 0.36 p = 0.72; saline = 8.8 ± 0.35; meth = 8.63 ± 0.31) (Fig. 3C). Groups did not differ in the number of sessions required to reach stable performance on the PR or choice task. A two-way ANOVA conducted on the number of sessions to stable performance in the PR and choice tasks revealed no significant effect of session type (F(1,52) = 3.54 p = 0.07), no significant effect of condition (F(1,52) = 0.769 p = 0.38), and no session type by condition interaction (F(1,52) = 0.21 p = 0.64) (Fig. 3D).
Freely-available Choice.
A test wherein sucrose pellets and lab chow were concurrently freely-available was conducted to confirm that meth withdrawal effects on PR effortful choice were not due to decreased preference for sucrose. Analysis of amount of food consumed (g) using a two-way ANOVA with food type (sucrose pellet, chow) and condition (saline, meth) as fixed factors revealed a significant effect of food type (F(1,52) = 94.14 p < 0.001; sucrose = 11.36 ± 0.68; chow = 3.55 ± 0.40). No significant food type by condition interaction (F(1,52) = 0.51 p = 0.47), or effect of condition (F(1,52) 0.09 p 0.76) was found (Fig. 3E).
Behavioral economic indices.
Because we detected no group differences on progressive ratio testing in the absence of freely available chow, behavioral economic measures were taken from the last session of choice testing. An independent samples t-test on the number of pellets earned on the last of the three stable sessions revealed a significant difference between yoked saline and meth IVSA groups (t(26) = 3.27 p < 0.01; saline = 32.92 ± 1.42; meth 24.4 ± 2.09) (Fig. 4A). A similar pattern of effects was observed for the highest ratio achieved, or breakpoint: yoked saline animals reached a higher breakpoint than meth animals (t(26) = 2.58 p = 0.02; saline = 15.38 ± 1.12; meth = 10.53 ± 1.46) (Fig. 4B). After fitting Hursh’s exponential model (Hursh and Silberberg, 2008) to the
Fig. 4.
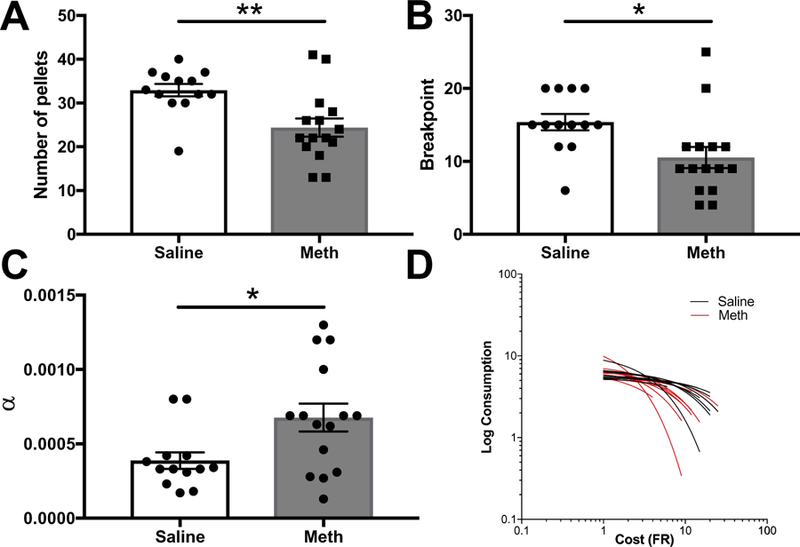
Effects of meth IVSA withdrawal on behavioral economic indices. (A) Number of pellets earned on the last of the three stable PR testing sessions in yoked saline and meth IVSA groups. Meth exposed animals earned fewer pellets compared to yoked saline controls (p < 0.01). (B) Breakpoint (highest ratio achieved) on the last of the three stable choice testing sessions in yoked saline and meth IVSA groups. Meth exposed animals reached lower ratios compared to yoked saline controls (p < 0.05). (C) α values calculated from Hursh and Silberberg’s exponential model. Meth exposed animals had steeper rates of decline, as indicated by higher α (p < 0.05). (D) Individual demand curves generated from Hursh and Silberberg’s exponential model. Points denote mean ± SEM, *p < 0.05, **p < 0.01.
number of pellets consumed at each ratio and generating individual demand curves Q0 and α values for each subject, a multivariate ANOVA with Pillai’s Trace correction revealed a significant effect of group (F(3,24) = 3.39, p = 0.03). Specifically, meth IVSA animals consumed fewer pellets at higher ratios, as indicated by significantly higher α values than yoked saline controls (p = 0.02; saline = 3.9e—4 ± 5.5 e—5; meth = 6.8 e—4 ± 9.4 e—5) (Fig. 4C). Q0 and EV values did not differ between groups (p=0.75 and p=0.13, respectively) (data not shown). Individual demand curves are shown in Fig. 4D.
c-Fos immunoreactivity.
Following the final effortful choice test, brains were collected 1 h after the termination of testing and processed for c-Fos immunoreactivity. A multivariate ANOVA with Pillai’s Trace correction revealed a significant effect of group (F(4,9) = 8.403, p < 0.01) In VS, there was a significant difference in the number of c-Fos immunoreactive cells with meth animals exhibiting reduced expression (p = 0.04; saline = 66.7 ± 9.68; meth = 40.53 ± 6.69) (Fig. 5A). In BLA, there was also a significant difference in the number of c-Fos immunoreactive cells with meth animals exhibiting reduced expression (p = 0.02; saline = 67.27 ± 6.23; meth = 46.83 ± 4.43) (Fig. 5B). In ACC, there was a trend toward a significant decrease in the number of c-Fos immunoreactive cells in the meth rats (p = 0.09; saline = 64.28 ± 8.96; meth = 44.37 ± 6.52) (Fig. 5C). In DS, there was no significant difference in the number of c-Fos immunoreactive cells (p = 0.39; saline = 76.37 ± 15.15; meth = 57.39 ± 14.39) (Fig. 5D).
Fig. 5.
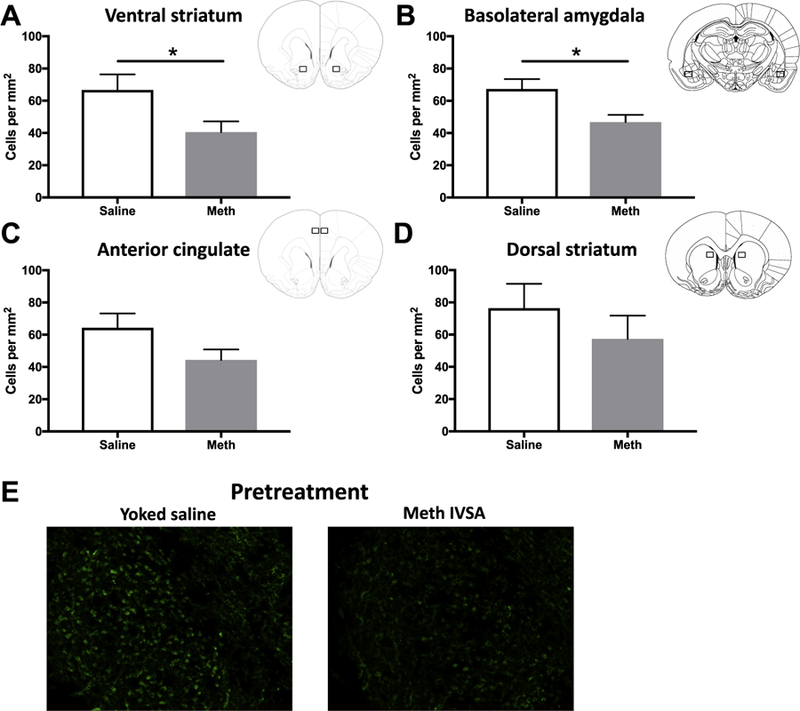
c-Fos activity following behavior (A) Number c-Fos positive cells/mm2 in VS. Meth IVSA pretreatment decreased c-Fos immunoreactivity (p < 0.05). (B) Number c-Fos positive cells/mm2 in BLA. Meth IVSA pretreatment decreased c-Fos immunoreactivity (p < 0.05). (C) Number c-Fos positive cells/mm2 in ACC. Meth IVSA pretreatment had a marginal effect on c-Fos immunoreactivity (p = 0.09). (D) Number c-Fos positive cells/mm2 in DS. Meth IVSA pretreatment had no effect on c-Fos immunoreactivity in this region. (E) Representative photomicrographs of c-Fos immunoreactivity taken at 20x in BLA. Insets represent ROIs for image capture. Points denote mean ± SEM, *p < 0.05.
A Pearson r-correlation matrix of behavioral economic indices and brain activation (c-Fos) data for all animals is shown in Table 1. Interestingly, only VS c-Fos was found to be significantly correlated with Q0 and α. As might be expected, α and Q0 were significantly correlated, as were α and EV. Multiple linear regression analyses were conducted to predict behavioral economic indices based on ROI c-Fos immunoreactivity. Based on the results of our correlation matrix, we employed a forward selection model with VS entered first, followed by BLA, ACC, and DS c-Fos. We found that only VS c-Fos significantly predicted α (F(1,12) = 5.244, p = 0.04, R2 = 0.304) and Q0 (F(1,12) = 7.480, p = 0.018, R2 = 0.384). Results were the same if BLA c-Fos was entered in the model first.
Table 1.
Correlation matrix of behavioral economic indices and regional c-Fos immunoreactivity. Demand elasticity (α) and demand intensity at lowest cost (Q0) were significantly correlated, as were α and essential value (EV). Only VS cFos was negatively correlated with Q0 and α.
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | ||
|---|---|---|---|---|---|---|---|---|
| 1. | Q0 | |||||||
| 2. | α | 0.684** | ||||||
| 3. | EV | −0.341 | −0.770** | |||||
| 4. | ACC cFos | 0.059 | −0.097 | −0.145 | ||||
| 5. | VS cFos | −0.620* | −0.551* | 0.226 | −0.276 | |||
| 6. | DS cFos | −0.005 | −0.279 | 0.226 | 0.141 | 0.155 | ||
| 7. | BLA cFos | 0.012 | −0.104 | −0.007 | 0.345 | 0.234 | 0.178 |
p < 0.05
p < 0.01.
4. Discussion
Exposure to drugs of abuse produces marked changes in cost-benefit decision-making, often increasing discounting of time and effort costs (Mendez et al., 2010; Thompson et al., 2017). Here we show that IVSA of meth and subsequent withdrawal also results in steeper effort discounting. Importantly, these changes are accompanied by long-term adaptations in VS and BLA, regions known to be important in effort-based choice. We also demonstrate that meth withdrawal results in a persistent change in the demand elasticity (α), without affecting the demand intensity (Q0) or essential value (EV) of a preferred sucrose reward.
4.1. Meth experience produces long-lasting changes in relative reward value
Our group previously showed decreased effort following binge exposure to meth assessed in a T-maze task (Kosheleff et al., 2012a). Critically, that pattern of dosing is likely not as relevant to the human condition and is well outside the dose range that rats self-administer. In the present experiment, rats readily acquired and maintained IVSA of relatively low sensitizing doses (~1 mg/kg) (Frey et al., 1997), consistent with doses others report (Winkler et al., 2018). We also did not observe escalating doses, likely due to the short 2-h sessions (Orio et al., 2010). Nevertheless, we replicate here what we have previously demonstrated following experimenter-administered escalating meth: steeper effort discounting in the presence of a lower cost option that is not due to reduced reward sensitivity or changes in food preference (Thompson et al., 2017). Importantly, rats’ steep effort discounting for a preferred reward was similarly not due to a general lack of motivation, learning or memory impairment, or changes in the hedonic value of the preferred reward. Taken together, the finding that both experimenter- and self-administered meth produce the same long-lasting effects on (relative) food reward value points to a robust drug effect.
Our group previously showed meth treated animals exert more effort in the form of wheel running (Thompson et al., 2015) and will choose to climb a higher barrier to earn more food reward (Stolyarova et al., 2015) in withdrawal, and others have found increased progressive ratio lever pressing for food in amphetamine withdrawal (Olausson et al., 2006). Task differences and effects of stimulants on delay discounting (Hoffman et al., 2006) may explain this potential discrepancy. Those tasks all imposed a single response option (i.e. running, barrier climbing, lever pressing) rather than providing animals a choice of actions. Given that exposure to meth increases delay discounting (Hoffman et al., 2006) it is plausible that, when faced with a single option, sensitivity to delay costs exerts stronger effects on behavior than sensitivity to physical effort costs, and drug-treated animals will maximize the number of rewards acquired per unit of time by responding more vigorously. Ostensibly, when an option is introduced that is less costly in terms of both time and effort, it becomes the preferred choice in meth-experienced animals, despite being of lesser hedonic value. Indeed, we found that meth-experienced rats reached lower stable levels of lever pressing when exposed to the choice phase, and we found no effect on PR responding in the absence of an alternative option, though deficits in effort were revealed when a lower cost, concurrently available option was introduced.
4.2. Meth experience increases sensitivity to effort cost in withdrawal
Behavioral economic analysis of our data yielded several interesting results. Consistent with previous reports, we found that exposure to meth IVSA decreased food demand (Galuska et al., 2011). Although those authors found slight effects on Q0 as well as a, our task differences may explain the divergent effects. Rather than increasing ratios (costs) across sessions, our task progressively increases the cost every five reinforcers within a single session. Therefore, it is not surprising that Q0 was not different between groups: consumption is necessarily equal at the lowest cost, as all animals earned more than five reinforcers. Thus, although we report Q0 values, their interpretation in the context of a progressive ratio task is unclear, and further clarification would require a traditional behavioral economics paradigm (Bentzley et al., 2013; Cox et al., 2017; Galuska et al., 2011). Yet the lack of effect on EV, together with our control tasks involving free-choice between sucrose pellets vs. chow, does also provide a point of convergence: rats express intact hedonic valuation of the preferred sucrose reinforcer. Critically, we observed effects of meth IVSA and withdrawal on α: rats exhibited greater elasticity of demand for food and were less willing to work for sucrose pellets, particularly at higher ratios, consistent with effects others report (Bentzley et al., 2013; Galuska et al., 2011). Recent behavioral economic analysis of meth seeking found that oxytocin decreases meth demand, and this effect is accompanied by decreased VS activation (Cox et al., 2017). Future work should determine whether meth and food demand are mediated by distinct mechanisms. Indeed, moving this research area forward are several interesting studies directly comparing the elasticity of demand between drug and non-drug reinforcers (Kearns et al., 2011, 2017).
4.3. Meth experience results in persistent brain activation changes
On the last day of behavioral testing we collected brains to determine whether there were regional brain adaptations following prolonged meth experience and withdrawal. The pattern of effects we observed in our c-Fos expression points to clear roles of VS and BLA in withdrawal effects on effort. We found that meth-withdrawn animals showed reduced c-Fos expression in BLA and VS, not DS and ACC. Our results are consistent with others showing greater immediate early gene expression in VS in animals that lever press more for high carbohydrate pellets (Randall et al., 2012). These findings are also consistent with a general role of VS (Ghods-Sharifi and Floresco, 2010) and BLA (Ghods-Sharifi et al., 2009; Hart and Izquierdo, 2017) in effort-based choice. Interestingly, though group differences did not reach statistical significance (p = 0.09), there was a trend for a decrease in c-Fos expression in ACC (Cai and Padoa-Schioppa, 2012; Hart et al., 2017; Klein-Flugge et al., 2016; Rudebeck et al., 2008; Schweimer and Hauber, 2005; Walton et al., 2003).
Different models of meth exposure produce varying effects on brain and behavior (Kosheleff et al., 2012b). The chronic IVSA regimen here resulted in the same behavioral effects as chronic escalating doses previously administered in our laboratory (Thompson et al., 2017). However, neither of these regimens results in neurotoxicity (Belcher et al., 2008); it is therefore unlikely the reduced c-Fos expression we observe here is due to fewer cells overall.
Analyses revealed only VS activation correlated significantly with Q0 and α. These findings are consistent with the wealth of evidence implicating VS in effort (Cousins and Salamone, 1994; Ghods-Sharifi and Floresco, 2010; Nunes et al., 2013). Although BLA and ACC activation did not correlate with behavioral economic indices, we did find reduced activation in BLA and a trend in ACC in meth withdrawn animals, providing support for the idea that BLA, ACC, and VS interact in the regulation of effort (Floresco and Ghods-Sharifi, 2007; Hart et al., 2017; Hart and Izquierdo, 2017; Hauber and Sommer, 2009; Salamone et al., 2007). Further, the lack of effect of withdrawal on DS activation is consistent with several studies showing DS dopamine receptor blockade and related manipulations have no effects on effort-based choice (Farrar et al., 2010; Font et al., 2008; Nunes et al., 2013). While we did not examine orbitofrontal cortex (OFC), its role in effort should be further elucidated. While OFC neurons do encode economic value and choice between options (Padoa-Schioppa and Assad, 2006), OFC lesions do not result in impaired effort in a T-maze task (Ostrander et al., 2011; Rudebeck et al., 2008). Notably, there is a recent report of OFC lesions and pharmacological inhibition actually increasing progressive ratio responding and selection of a high effort choice (Munster and Hauber, 2017). However, to our knowledge, OFC has not been probed in an effort-based choice task involving qualitatively different options and thus it would be useful to compare its role to that of other cortical regions, like ACC (Hart et al., 2017; Rudebeck et al., 2006; Walton et al., 2003).
4.4. Concluding remarks
Overall, we found that meth IVSA and withdrawal leads to long-term changes in effort-based choice, a behavior that is associated with several conditions, including depression (Treadway and Zald, 2011) Parkinson’s disease (Friedman et al., 2010), and schizophrenia (Gold et al., 2013). These findings are consistent with the clinical literature showing decreased effort is one of the most prominent symptoms in amphetamine withdrawal (McGregor et al., 2005), even in the absence of depressed mood (Volkow et al., 2001), further supporting the idea that mechanisms underlying the motivation to obtain rewards versus the primary hedonic response to rewards are dissociable (Salamone and Correa, 2012; Treadway and Zald, 2011). Overall, the pattern is in keeping with an increased elasticity of demand for food reward (i.e. greater sensitivity to effort cost) that has been reported following neurotoxic dopamine lesions, dopamine depletion, and dopamine receptor blockade (Aberman and Salamone, 1999; Salamone et al., 2017). The behavioral effect we report here was accompanied by changes in activation of several regions known to be important in effortful decision making. This finding contributes to the understanding of persistent, value-based decision-making changes that occur in drug withdrawal, and that may subvert long-term effort toward sobriety.
Acknowledgments
We thank Dr. Lara Ray and Dr. David Kearns for helpful feedback on preliminary data, the Kennedy lab for guidance on self-administration surgeries, and Dr. J. David Jentsch for donating the Med-Associates operant chambers. We also thank Alexandra Stolyarova and Joseph Munier for comments on an earlier version of this manuscript and Yolanda Segura for assistance with cell counting.
Funding and disclosure
This work was supported by UCLA’s Division of Life Sciences Recruitment and Retention fund (Izquierdo), the Training program in Translational Neuroscience of Drug Abuse (T32 DA024635, London), and the Training program in Neural Microcircuits (T32 NS058280, Feldman). We thank the Staglin Center for Brain and Behavioral Health for additional support.
Footnotes
Conflicts of interest
Authors report no conflict of interest.
References
- Aberman JE, Salamone JD, 1999. Nucleus accumbens dopamine depletions make rats more sensitive to high ratio requirements but do not impair primary food reinforcement. Neuroscience 92, 545–552. [DOI] [PubMed] [Google Scholar]
- Belcher AM, Feinstein EM, O’Dell SJ, Marshall JF, 2008. Methamphetamine influences on recognition memory: comparison of escalating and single−day dosing regimens. Neuropsychopharmacology 33, 1453–1463. [DOI] [PubMed] [Google Scholar]
- Bentzley BS, Aston−Jones G, 2015. Orexin−1 receptor signaling increases motivation for cocaine−associated cues. Eur. J. Neurosci 41, 1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Fender KM, Aston−Jones G, 2013. The behavioral economics of drug self−administration: a review and new analytical approach for within−session procedures. Psychopharmacology (Berl) 226, 113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Padoa−Schioppa C, 2012. Neuronal encoding of subjective value in dorsal and ventral anterior cingulate cortex. J. Neurosci 32, 3791–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocker PJ, Hosking JG, Benoit J, Winstanley CA, 2012. Sensitivity to cognitive effort mediates psychostimulant effects on a novel rodent cost/benefit decision−making task. Neuropsychopharmacology 37, 1825–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins MS, Salamone JD, 1994. Nucleus accumbens dopamine depletions in rats affect relative response allocation in a novel cost/benefit procedure. Pharmacol. Biochem. Behav 49, 85–91. [DOI] [PubMed] [Google Scholar]
- Cox BM, Bentzley BS, Regen−Tuero H, See RE, Reichel CM, Aston−Jones G, 2017. Oxytocin acts in nucleus accumbens to attenuate methamphetamine seeking and demand. Biol. Psychiatr 81, 949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BM, Cope ZA, Parsegian A, Floresco SB, Aston−Jones G, See RE, 2016. Chronic methamphetamine self−administration alters cognitive flexibility in male rats. Psychopharmacology (Berl) 233, 2319–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar AM, Segovia KN, Randall PA, Nunes EJ, Collins LE, Stopper CM, Port RG, Hockemeyer J, Muller CE, Correa M, Salamone JD, 2010. Nucleus accumbens and effort−related functions: behavioral and neural markers of the interactions between adenosine A2A and dopamine D2 receptors. Neuroscience 166, 1056–1067. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Ghods−Sharifi S, 2007. Amygdala−prefrontal cortical circuitry regulates effort−based decision making. Cerebr. Cortex 17, 251–260. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Whelan JM, 2009. Perturbations in different forms of cost/benefit decision making induced by repeated amphetamine exposure. Psychopharmacology (Berl) 205, 189–201. [DOI] [PubMed] [Google Scholar]
- Font L, Mingote S, Farrar AM, Pereira M, Worden L, Stopper C, Port RG, Salamone JD, 2008. Intra−accumbens injections of the adenosine A2A agonist CGS 21680 affect effort−related choice behavior in rats. Psychopharmacology (Berl) 199, 515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey K, Kilbourn M, Robinson T, 1997. Reduced striatal vesicular monoamine transporters after neurotoxic but not after behaviorally−sensitizing doses of methamphetamine. Eur. J. Pharmacol 334, 273–279. [DOI] [PubMed] [Google Scholar]
- Friedman JH, Alves G, Hagell P, Marinus J, Marsh L, Martinez−Martin P, Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins G, Schrag A, 2010. Fatigue rating scales critique and recommendations by the Movement Disorders Society task force on rating scales for Parkinson’s disease. Mov. Disord 25, 805–822. [DOI] [PubMed] [Google Scholar]
- Galuska CM, Banna KM, Willse LV, Yahyavi−Firouz−Abadi N, See RE, 2011. A comparison of economic demand and conditioned−cued reinstatement of methamphetamine−seeking or food−seeking in rats. Behav. Pharmacol 22, 312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghods−Sharifi S, Floresco SB, 2010. Differential effects on effort discounting induced by inactivations of the nucleus accumbens core or shell. Behav. Neurosci 124, 179–191. [DOI] [PubMed] [Google Scholar]
- Ghods−Sharifi S, Onge JR St, Floresco SB, 2009. Fundamental contribution by the basolateral amygdala to different forms of decision making. J. Neurosci 29, 5251–5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, Frank MJ, 2013. Negative symptoms of schizophrenia are associated with abnormal effort−cost computations. Biol. Psychiatr 74, 130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JC, MacKillop J, 2014. Interrelationships among individual differences in alcohol demand, impulsivity, and alcohol misuse. Psychol. Addict. Behav 28, 282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, Lee B, Seu E, James AS, Feiler K, Mandelkern MA, London ED, Jentsch JD, 2012. Dysregulation of D(2)−mediated dopamine transmission in monkeys after chronic escalating methamphetamine exposure. J. Neurosci 32, 5843–5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart EE, Gerson JO, Zoken Y, Garcia M, Izquierdo A, 2017. Anterior cingulate cortex supports effort allocation towards a qualitatively preferred option. Eur. J. Neurosci 46, 1682–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart EE, Izquierdo A, 2017. Basolateral amygdala supports the maintenance of value and effortful choice of a preferred option. Eur. J. Neurosci 45, 388–397. [DOI] [PubMed] [Google Scholar]
- Hauber W, Sommer S, 2009. Prefrontostriatal circuitry regulates effort−related decision making. Cerebr. Cortex 19, 2240–2247. [DOI] [PubMed] [Google Scholar]
- Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH, 2006. Neuropsychological function and delay discounting in methamphetamine−dependent individuals. Psychopharmacology (Berl) 188, 162–170. [DOI] [PubMed] [Google Scholar]
- Hosking JG, Cocker PJ, Winstanley CA, 2014. Dissociable contributions of anterior cingulate cortex and basolateral amygdala on a rodent cost/benefit decision−making task of cognitive effort. Neuropsychopharmacology 39, 1558–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosking JG, Cocker PJ, Winstanley CA, 2016. Prefrontal cortical inactivations decrease willingness to expend cognitive effort on a rodent cost/benefit decision−making task. Cerebr. Cortex 26, 1529–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosking JG, Floresco SB, Winstanley CA, 2015. Dopamine antagonism decreases willingness to expend physical, but not cognitive, effort: a comparison of two rodent cost/benefit decision−making tasks. Neuropsychopharmacology 40, 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, 1980. Economic concepts for the analysis of behavior. J. Exp. Anal. Behav 34, 219–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A, 2008. Economic demand and essential value. Psychol. Rev 115, 186–198. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Belcher AM, Scott L, Cazares VA, Chen J, O’Dell SJ, Malvaez M, Wu T, Marshall JF, 2010. Reversal−specific learning impairments after a binge regimen of methamphetamine in rats: possible involvement of striatal dopamine. Neuropsychopharmacology 35, 505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, De La Garza R 2nd, Taylor JR, 2002. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology 26, 183–190. [DOI] [PubMed] [Google Scholar]
- Kearns DN, Gomez−Serrano MA, Tunstall BJ, 2011. A review of preclinical research demonstrating that drug and non−drug reinforcers differentially affect behavior. Curr. Drug Abuse Rev 4, 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns DN, Kim JS, Tunstall BJ, Silberberg A, 2017. Essential values of cocaine and non−drug alternatives predict the choice between them. Addiction Biol 22, 1501–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein−Flugge MC, Kennerley SW, Friston K, Bestmann S, 2016. Neural signatures of value comparison in human cingulate cortex during decisions requiring an effort−reward trade−off. J. Neurosci 36, 10002–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosheleff AR, Grimes M, O’Dell SJ, Marshall JF, Izquierdo A, 2012a. Work aversion and associated changes in dopamine and serotonin transporter after methamphetamine exposure in rats. Psychopharmacology (Berl) 219, 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosheleff AR, Rodriguez D, O’Dell SJ, Marshall JF, Izquierdo A, 2012b. Comparison of single−dose and extended methamphetamine administration on reversal learning in rats. Psychopharmacology (Berl) 224, 459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lago JA, Kosten TR, 1994. Stimulant withdrawal. Addiction 89, 1477–1481. [DOI] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA, 2009. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J. Neurosci 29, 14734–14740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Wongtan T, White JM, 2005. The nature, time course and severity of methamphetamine withdrawal. Addiction 100, 1320–1329. [DOI] [PubMed] [Google Scholar]
- Mendez IA, Simon NW, Hart N, Mitchell MR, Nation JR, Wellman PJ, Setlow B, 2010. Self−administered cocaine causes long−lasting increases in impulsive choice in a delay discounting task. Behav. Neurosci 124, 470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster A, Hauber W, 2017. Medial orbitofrontal cortex mediates effort−related responding in rats. Cerebr. Cortex 1–11. [DOI] [PubMed]
- Murphy JG, MacKillop J, Skidmore JR, Pederson AA, 2009. Reliability and validity of a demand curve measure of alcohol reinforcement. Exp. Clin. Psychopharmacol 17, 396–404. [DOI] [PubMed] [Google Scholar]
- Nunes EJ, Randall PA, Hart EE, Freeland C, Yohn SE, Baqi Y, Muller CE, Lopez−Cruz L, Correa M, Salamone JD, 2013. Effort−related motivational effects of the VMAT−2 inhibitor tetrabenazine: implications for animal models of the motivational symptoms of depression. J. Neurosci 33, 19120–19130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Tronson N, Neve RL, Nestler EJ, Taylor JR, 2006. DeltaFosB in the nucleus accumbens regulates food−reinforced instrumental behavior and motivation. J. Neurosci 26, 9196–9204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orio L, Wee S, Newman AH, Pulvirenti L, Koob GF, 2010. The dopamine D3 receptor partial agonist CJB090 and antagonist PG01037 decrease progressive ratio responding for methamphetamine in rats with extended−access. Addiction Biol 15, 312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrander S, Cazares VA, Kim C, Cheung S, Gonzalez I, Izquierdo A, 2011. Orbitofrontal cortex and basolateral amygdala lesions result in suboptimal and dissociable reward choices on cue−guided effort in rats. Behav. Neurosci 125, 350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa−Schioppa C, Assad JA, 2006. Neurons in the orbitofrontal cortex encode economic value. Nature 441, 223–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, 2001. A behavioral economic analysis of polydrug abuse in alcoholics: asymmetrical substitution of alcohol and cocaine. Drug Alcohol Depend 62, 31–39. [DOI] [PubMed] [Google Scholar]
- Randall PA, Pardo M, Nunes EJ, Lopez Cruz L., Vemuri VK, Makriyannis A, Baqi Y, Muller CE, Correa M, Salamone JD, 2012. Dopaminergic modulation of effort−related choice behavior as assessed by a progressive ratio chow feeding choice task: pharmacological studies and the role of individual differences. PLoS One 7, e47934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Behrens TE, Kennerley SW, Baxter MG, Buckley MJ, Walton ME, Rushworth MF, 2008. Frontal cortex subregions play distinct roles in choices between actions and stimuli. J. Neurosci 28, 13775–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MF, 2006. Separate neural pathways process different decision costs. Nat. Neurosci 9, 1161–1168. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, 2012. The mysterious motivational functions of mesolimbic dopamine. Neuron 76, 470–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM, 2007. Effort−related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 191, 461–482. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Yohn SE, Yang J−H, Somerville M, Rotolo RA, Presby RE, 2017. Behavioral activation, effort−based choice, and elasticity of demand for motivational stimuli: basic and translational neuroscience approaches. Motivation Science 3, 208–229. [Google Scholar]
- Schweimer J, Hauber W, 2005. Involvement of the rat anterior cingulate cortex in control of instrumental responses guided by reward expectancy. Learn. Mem 12, 334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolyarova A, Thompson AB, Barrientos RM, Izquierdo A, 2015. Reductions in frontocortical cytokine levels are associated with long−lasting alterations in reward valuation after methamphetamine. Neuropsychopharmacology 40, 1234–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AB, Gerson J, Stolyarova A, Bugarin A, Hart EE, Jentsch JD, Izquierdo A, 2017. Steep effort discounting of a preferred reward over a freely−available option in prolonged methamphetamine withdrawal in male rats. Psychopharmacology (Berl) 234, 2697–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AB, Stolyarova A, Ying Z, Zhuang Y, Gomez−Pinilla F, Izquierdo A, 2015. Methamphetamine blocks exercise effects on Bdnf and Drd2 gene expression in frontal cortex and striatum. Neuropharmacology 99, 658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Zald DH, 2011. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci. Biobehav. Rev 35, 537–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido−Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN, 2001. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am. J. Psychiatr 158, 377–382. [DOI] [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Alterescu K, Rushworth MF, 2003. Functional specialization within medial frontal cortex of the anterior cingulate for evaluating effort−related decisions. J. Neurosci 23, 6475–6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler MC, Greager EM, Stafford J, Bachtell RK, 2018. Methamphetamine self−administration reduces alcohol consumption and preference in alcohol−preferring P rats. Addiction Biol 23, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]


