Abstract
3-Phenoxybenzoic acid (3-PBA), 1(Figure 1) is a human urinary metabolite of many pyrethroid insecticides and can be used as a biomarker to monitor human exposure to these pesticides. A rapid and sensitive direct competitive fluorescence enzyme immunoassay (dc-FEIA) for 3-PBA based on a nanobody (Nb)-alkaline phosphatase (AP) fusion protein was developed. The anti-3-PBA Nb-AP fusion protein was expressed and purificated. The 50% inhibitory concentration (IC50) and the detection limit of the dc-FEIA were 0.082 and 0.011 ng/mL, respectively, with a linear range of 0.015–0.447 ng/mL. The IC50 of the one-step dc-FEIA was improved by nearly ten times compared with that of the one-step and three-step dc-ELISA. This assay was also compared with LC-MS for detecting the spiked urine samples, and the results indicated the reliability of Nb-AP fusion protein-based dc-FEIA for monitoring 3-PBA in urine.
Keywords: 3-PBA, nanobody-alkaline phosphatase fusion protein, one-step competitive fluorescence enzyme immunoassay
Graphical Abstract
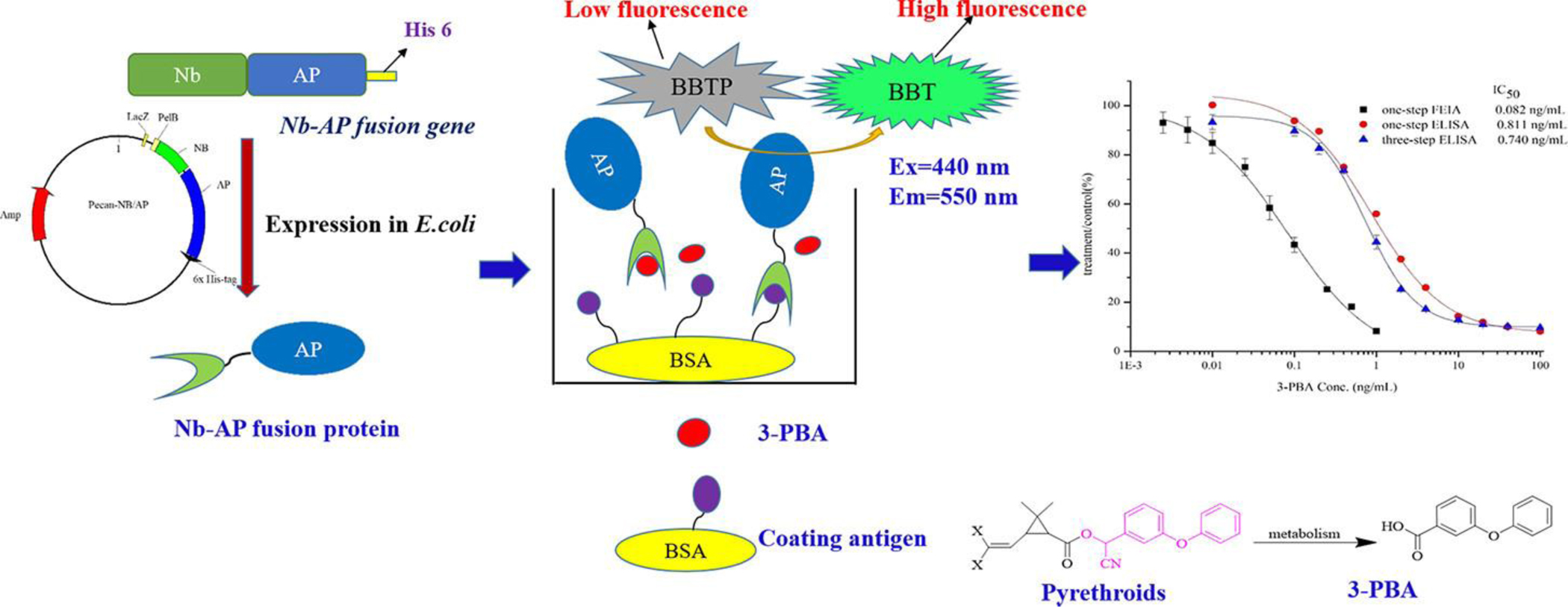
Introduction
Pyrethroids, one of the most widely used insecticide classes in the world, are mainly applied in agriculture, forestry and horticulture for controlling insect pests. 1 Also, pyrethroids can be used as insecticides in indoor environments and are found in medication used for treating for scabies and topical louse infestations. 2 The pyrethroids are among the safest insecticides marketed. However, because of the numerous applications and increasing worldwide use of pyrethroids, a variety of people are exposed to pyrethroids at levels that could be harmful to humans and particularly to vulnerable subpopulations such as children. 3 For example, the potential effects include endocrine disruption, 4 DNA damage in human sperm, 5 primary ovarian insufficiency, 6 liver injury, 7 sensory alterations, 8 and others. 9, 10 Therefore, it is important to develop a rapid, sensitive, and efficient analytical method for environmental monitoring and assessment of human exposure to pyrethroids and to aid in proper use of these compounds. Most pyrethroids contain a phenoxybenzyl alcohol or cyanohydrin (Figure 1) and both of these esters can be hydrolyzed and converted to the corresponding acid. Thus, 3-PBA is a human urinary metabolite or breakdown product of many pyrethroid insecticides. 11 The concentration of 3-PBA in urine provides information about a person’s recent exposure to pyrethroid insecticides. Higher urine concentrations generally indicate more exposure, therefore, 3-PBA can be used as a biomarker to monitor human exposure to pyrethroid insecticides.12, 13
Figure 1.
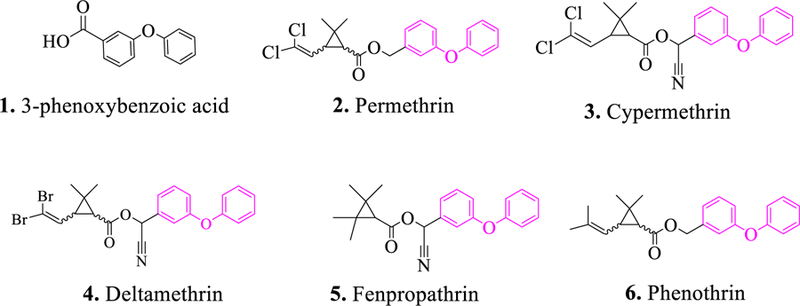
Structure of 3-phenoxybenzoic acid, 1, and pyrethroids containing a phenoxybenzyl group, 2–6.
At present, the detection methods for 3-PBA include supercritical fluid chromatography, 14 gas chromatography, 15 high-performance liquid chromatography, 16 immunoassays, 17, 18 and optical sensing. 19 Among the above methods, immunoassay stands out because of its unique ability to routinely handle many samples and because it also does not require time-consuming procedures and sophisticated equipment when compared with the instrumental methods. Most of the previously reported immunoassays for 3-PBA are based on a monoclonal antibody or a polyclonal antibody 11, 20 and secondary or tertiary antibodies are required in the process. Due to the advances in recombinant DNA technology, construction of an antibody-AP fusion has been proposed and used for simple and rapid immunoassay analysis. 21 One study on the one-step detection of O,O-diethyl organophosphorus pesticides based on the recombinant fusion protein of single chain variable fragment (scFv) and AP has been reported. 22 The single variable domain of a heavy chain (VHH) antibodies, also called as nanobodies (Nbs), with properties such as small size, high solubility and stability, has become as an attractive alternative to conventional scFv. It has been reported that the fusions of AP to Nbs were applied in the detection of proteins 23 as well as small molecules, such as mycotoxins 24 and tetrabromobisphenol A. 25 Until now, there have been few reports on the application of Nb-AP fusions for the detection of pesticides or their metabolites. Recently, many fluorescent phosphatase substrates have been developed and commercialized. Most of those new fluorescent substrates are highly sensitive, maintaining a low fluorescence signal until enzymatically acted upon and even yielding detection of AP as low as 0.1 attomole.26 Based on the highly sensitive substrate, the fluorescence assay allowed for more sensitive detection than the colorimetric assay. 27 Hence, Nb-AP fusions could be a powerful reagent for trace detection of pesticides or their metabolites.
In our previous work, a phage VHH library was constructed based on an immunized alpaca, and seven VHH clones were selected by competitive binding with 3-PBA. 28 In this study, the VHH which showed the highest sensitivity was used to construct the Nb-AP fusion. The Nb gene was cloned into the expression vector pecan45, which contained the AP gene 29 to produce the Nb-AP fusion protein. After the characterization by sodium dodecyl sulfate polyacrylamide gel electrophoresis, the purified fusion protein was used to develop a rapid, simple, and sensitive direct competitive fluorescence enzyme immunoassay for detection of 3-PBA in urine.
Materials and Methods
Chemicals and Reagents.
T4 DNA ligase and restriction enzyme SfiI were obtained from New England Biolabs, Inc. (Beverly, MA). Phusion High-Fidelity DNA Polymerase, bacterial protein extraction reagent (B-PER), HisPur Ni-NTA resin, NuPAGE Bis-Tris Gel (the precast polyacrylamide gels designed to give optimal separation for a wide range of molecular weight proteins during gel electrophoresis) and chemically competent cells of E. coli BL21 (DE3) pLysS were from Thermo Fisher Scientific (Rockford, IL). Standards (3-phenoxybenzoic acid, 1, and its analogs, 3-phenoxybeneyl aldehyde and 3-phenoxybenzyl alcohol; permethrin, 2; cypermethrin, 3; deltamethrin, 4; fenpropathrin, 5; and phenothrin, 6) (Figure 1)), isopropyl-β-D-thiogalactopyranoside (IPTG), and p-nitrophenyl phosphate (pNPP) substrate were from Sigma (St. Louis, MO). The AttoPhos AP fluorescent substrate system was purchased from Roche (Pleasanton, CA). The vector pecan 45 containing AP gene was a generous gift from Dr. Jinny L. Liu and Dr. Ellen R. Goldman (Naval Research Laboratory, Center for Bio/Molecular Science and Engineering, Washington, DC). The AP-conjugated goat anti-mouse lgG was purchased from Jackson ImmunoResearch Inc. (West Grove, PA).
Construction of the Recombinant Plasmid, Expression and Identification of Nb-AP Fusion Protein.
The recombinant plasmid encoding the Nb-AP fusion protein with a 6X His tag at its C-terminal end was constructed. Briefly, primers AP-F and AP-R were used to amplify the Nb gene, followed digestion with SfiI restriction enzyme (forward primer: GAG GAG GAG GTG GCC CAG CCG GCC CAG GTG CAG CTC GTG GAG TCT GGG GGA; reverse primer: GAG GAG GAG CTG GCC CCC GAG GCC GCG TCT TGT GGT TTT GGT GTC TTG GG). The Nb fragment was then ligated into the similarly digested expression vector pecan45 containing AP gene at a 10:1 molar ratio using T4 DNA ligase, followed by transforming the ligation products into the chemically competent cells of the E. coli strain BL21(DE3) pLysS by heat shock (42 °C, 30 s). The transformed bacteria were seeded on super broth (SB) agar plates containing 50 μg/mL ampicillin (Amp), and positive clones were picked for plasmid extraction and DNA sequencing (Division of Biological Sciences, Automated DNA Sequencing Facility, University of California, Davis).
The colony containing the recombinant plasmid was cultured in 10 mL SB medium with 50 μg/mL Amp at 37 °C overnight. Then, the overnight culture was inoculated 1: 100 (v/v) in 1 L of SB medium containing 50 μg/mL Amp and incubated at 37 °C until the OD 600 reached approximately 0.6. The culture was then induced with 0.1 mM IPTG at 25 °C and was shaken at 250 rpm overnight. The bacterial cells were collected by centrifugation at 10 000 g for 20 min, and the soluble fusion protein was extracted by B-PER method according to the manufacturer’s instructions.
Purification of the Anti-3-PBA Nb-AP Fusion Protein.
The extracted Nb-AP fusion protein, which contains a 6X His tag, was first filtered through a 0.22 μm sterile filter (MilliporeSigma, Temecula, CA), followed by loading onto a high-capacity nickel immobilized metal ion affinity chromatography (IMAC) resin column for purification. After being washed with six resin-bed volumes of wash buffer (10 mM PBS containing 25 mM imidazole, pH 7.4), the Nb-AP fusion protein was eluted with 6 mL of elution buffer (10 mM PBS containing 100 mM imidazole, pH 7.4). After dialysis with 10 mM PBS (pH 7.4) at 4 °C for 72 h, the obtained Nb-AP fusion protein was stored at −20 °C until use. The purity of the resulting Nb-AP fusion protein was evaluated by sodium dodecyl sulfate polyacrylamide gel electrophoresis using the NuPAGE Bis-Tris Gels according to the manufacturer’s instructions.
The AP enzyme activity of the Nb-AP fusion protein. 27
Colorimetric analysis is the most common method to measure the AP enzyme activity. In order to compare the sensitivity of colorimetric analysis with the fluorometric analysis, here, we measured the AP enzyme activity using the two analyses according to the reported method. 27 Briefly, serially diluted Nb-AP fusion protein was added into 96-well microplate, followed by addition of substrates. The mixture was incubated for 30 min and 15 min at room temperature for colorimetric analysis and fluorometric analysis, respectively. The reaction for colorimetric analysis was stopped with 3 M NaOH and then the absorbance was measured at 405 nm. While, the fluorescence was measured at 440 nm excitation wavelength and 550 nm emission wavelength. Generally, the colorimetric analysis used the ordinary 96-well microplate while the fluorometric analysis using the black opaque 96-well microplate. The substrates for colorimetric analysis and fluorometric analysis were p-nitrophenyl phosphate and 2′-(2-benzothiazoyl)-6′-hydroxybenzothiazole phosphate, respectively.
One-step competitive immunoassay for 3-PBA Based on Nb-AP Fusion Protein.
Colorimetric Enzyme Immunoassay
3-Phenoxybenzoic acid/bovine serum albumin conjugate in PBS (10 mM, pH 7.4) (400 ng/mL, 100 μL/well) was coated in microplates overnight at 4 °C and blocked with 200 μL/well 3% skim milk in PBS (10 mM, pH 7.4) for 1 h at room temperature. After washing with PBST (10 mM, pH 7.4, 0.5% Tween 20) three times, 500 ng/mL of Nb-AP (50 μL/well) and various concentrations of 3-PBA standard (0.01, 0.1, 0.2, 0.4, 1, 2, 4, 10, 20, 40, 100 ng/mL, 50 μL /well), both diluted with PBS (10 mM, pH 7.4) were added; the mixture in the plate was incubated at 25 °C for 1 h. After washing five times with PBST, the enzyme activity in the wells was determined with the colorimetric assay described above, and standard curve was established by plotting the value of B/B0 (%) against the 3-PBA concentration, where B is the absorbance in the presence of 3-PBA and B0 is the absorbance in their absence.
For comparison, three-step competitive ELISA (colorimetric enzyme immunoassay) for the parental anti-3-PBA VHH fused with C-terminal hemagglutinin (HA)-tag was also performed. Microwells were coated with 100 μL/well 50 ng/mL 1/BSA conjugate and then incubated overnight at 4 °C. After blocking with 3% skim milk (200 μL/well) in 10 mM PBS for 1 h at room temperature and then washing with PBST, each serial concentration of 3-PBA (0.01, 0.1, 0.2, 0.4, 1, 2, 4, 10, 20, 40, 100 ng/mL, 50 μL /well) equally with 450 ng/mL VHH was added to the wells and incubated at 25 °C for 1 h. Following washing five times with PBST, 1:5000 dilution of anti-HA-tag mouse monoclonal antibody (100 μL) was incubated in the wells at 25 °C for 30 min. After washing five times with PBST, in order to use the same substrate with the one-step competitive immunoassay (colorimetric enzyme immunoassay), 1:5000 dilution of AP-conjugated goat anti-mouse lgG (100 μL) was incubated in the wells at 25 °C for 30 min. Finally, AP substrate pNPP (150 μL) was added to the wells for 15 min and the reaction was stopped with 3M NaOH. The optical density at 405 nm was determined on a microplate reader.
Fluorescence Enzyme Immunoassay
For this assay, a black opaque 96-well microplate was incubated with 100 μL/well of 25 ng/mL 1/BSA conjugate in PBS (10 mM, pH 7.4) at 4 °C overnight. After blocked with 3% skim milk in 10 mM PBS (200 μL/well) at room temperature for 1 h, the plate was washed three times with PBST. Subsequently, 50 μL/well of 20 ng/mL Nb-AP fusion protein diluted in 10 mM PBS was added and incubated with 50 μL/well of serial concentrations of 3-PBA standards (0, 0.001, 0.005, 0.01, 0.025, 0.05, 0.1, 0.25, 0.5, and 1 ng/mL in 10 mM PBS) at 25 °C for 1 h. After five washings with PBST, the plate was incubated with 100 μL/well of the 2′-(2-benzothiazoyl)-6′-hydroxybenzothiazole phosphate at room temperature for 15 min. The fluorescent signal was measured as described above, and standard curve was established by plotting the value of F/F0 (%) against the 3-PBA concentration, where F is the fluorescence intensity in the presence of 3-PBA and F0 is the fluorescence intensity in its absence.
Analysis and Validation of Spiked Urine Sample based on Direct Competitive Fluorescence Enzyme Immunoassay (dc-FEIA) and LC-MS.
Urine samples used for the spike recovery study were collected from person with no known exposure to pyrethroid insecticides and were confirmed to be free of 3-PBA by LC-MS. Urine samples fortified with 3-PBA (1, 2, and 4 ng/mL) were diluted with 10 mM PBS to reach a final percentage of 5% urine/PBS (v/v). After complete mixing, the diluted samples were subjected to the one-step fluorescence enzyme immunoassay. LC-MS analysis was performed on Waters Acquity UPLC system, coupled to Xevo TQ-S Triple Quadrupole LC-MS. The above spiked urine samples (50 μL with the 3-PBA of 1, 2, and 4 ng/mL) were mixed with 50 µL 200 nM 12-(3-cyclohexyl-ureido)-dodecanoic acid in methanol, which was used as an internal standard to account for ion suppression, before detection. All data were acquired and processed using Masslynx 4.1 software with TargetLynx.
Results and Discussion
Expression, Purification, and Characterization of the Nb-AP Fusion Protein.
To create a convenient one-step detection for 3-PBA, the plasmid pecan45-Nb-AP was constructed by inserting the Nb gene into the expression vector pecan45 containing AP gene. The positive recombinant plasmid was confirmed by colony PCR and DNA sequencing. Resulting plasmid was then transformed into E. coli strain BL21(DE3) pLysS for the expression of the soluble fusion protein. The periplasmic protein was extracted by the B-PER reagent and was purified by the Ni-NTA affinity column. Protein size and integrity were characterized by sodium dodecyl sulfate polyacrylamide gel electrophoresis (Figure 2). The gels of selected purified Nb-AP fusion protein showed the expected band of approximately 65 kDa for the 1:1 fusion of Nb and AP.
Figure 2.
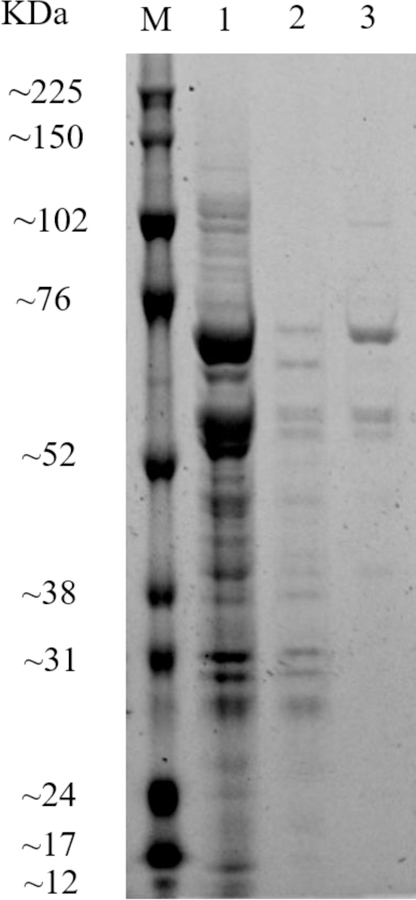
Sodium dodecyl sulfate polyacrylamide gel electrophoresis analysis of expression of the Nb-AP fusion protein. Blots were stained with SYPRO Ruby protein gel stain. Key: lane M, PageRuler unstained protein ladder and spectrum multicolor broad-range protein ladder. lane 1, the whole cell extract of Nb-AP under induced conditions; lane 2, the wash buffer from the Ni-NTA purification; lane 3, Nb-AP fusion protein following the Ni-NTA column.
AP Enzyme Activity and Anti-3-PBA Reactivity of Nb-AP Fusion Protein.
The AP enzyme activity of Nb-AP fusion protein was evaluated with colorimetric and fluorometric analysis. As shown in Figure 3, the signal intensity decreased as the amount of Nb-AP fusion protein decreased in both the fluorometric assay and colorimetric assay. The limit of detection (signal to noise>3) for AP enzyme activity in the fluorometric assays (dilution factor 4000 times) was approximately 20 times lower than that in the colorimetric assay (dilution factor 200 times), indicating the higher sensitivity of fluorometric assay compared with colorimetric assay.
Figure 3.
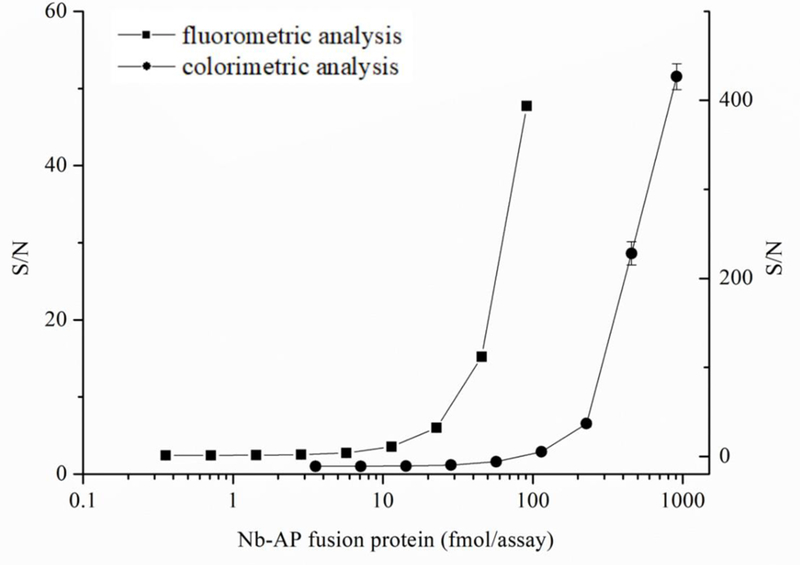
Dose-response curves for AP enzyme activity of the purified Nb-AP fusion protein in colorimetric (●) and fluorometric (■) assays. n = 3.
To evaluate the anti-3-PBA reactivity of Nb-AP fusion protein and compare its sensitivity with the parental VHH, one-step and three-step direct competitive ELISA were carried out. The concentrations of coating antigen and antibody were determined by checkerboard titration. As shown in Figure 4, it is obvious that the binding between the antibody and coating antigen can be inhibited by free 3-PBA, and the 50% inhibition concentrations are 0.811 ng/mL and 0.740 ng/mL for one-step ELISA and three-step ELISA, respectively, showing the similar sensitivity in 3-PBA detection. This result indicated that Nb-AP fusion protein had similar binding properties to the parental VHH in the immunoassay, while the one-step immunoassay based on Nb-AP fusion protein significantly reduced the assay time to 1 h compared with three-step ELISA that require more than 3 h.
Figure 4.
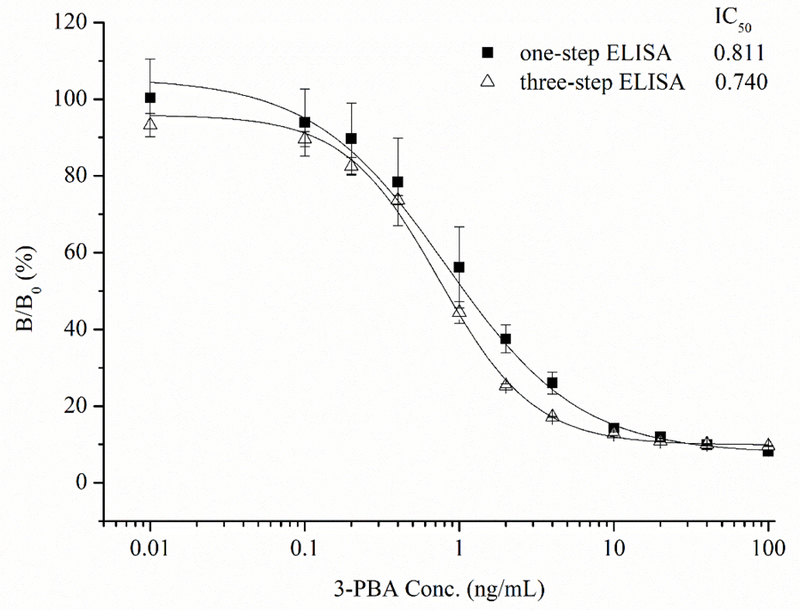
Standard curves for one-step and three-step colorimetric immunoassays (ELISA).
FEIA for 3-PBA Using Nb-AP Fusion Protein as the Probe
The fact that fluorometric assay was 20 times more sensitive than colorimetric assay for the detection of AP enzyme activity (Figure 3) indicated the higher sensitivity in FEIA could be attained compared to the conventional immunoassay. Therefore, the direct competitive FEIA based on Nb-AP was developed and the conditions for best performance of dc-FEIA were investigated in this study. Since many assay parameters can influence immunoreactions, 30, 31 optimization of the concentration of coating antigen and antibody, ionic strength and pH value were important to increase the sensitivity of 3-PBA detection. The working concentrations of coating antigen 1/BSA conjugate (25 μg/mL) and Nb-AP fusion protein (20 ng/mL) were first determined by a checkerboard titration. To evaluate the effect of the ionic strength on the assay performance four different concentrations of PBS (5, 10, 25, and 50 mM) were tested. As can be seen from Figure 5A, the fluorescence intensity decreased as the ionic strength increased. Although the IC50 were not significantly different with different concentrations of PBS buffer, the lowest IC50 of 0.076 ng/mL was observed at 10 mM PBS. The influence of the pH on FEIA was evaluated in the range pH 4.0–11.0 (Figure 5B). The FEIA was most influenced at pH 6.0 and 11.0, with the IC50 being 0.411 and 0.480 ng/mL, respectively. The maximum relative fluorescence unit (RFUmax) was observed abnormally at very low and high pH, and the performance of Nb-AP did not exhibit best activity. The best assay performance was obtained at pH 7.4, while, pH values between 7.4 and 9.0 are equally suitable for the assay.
Figure 5.
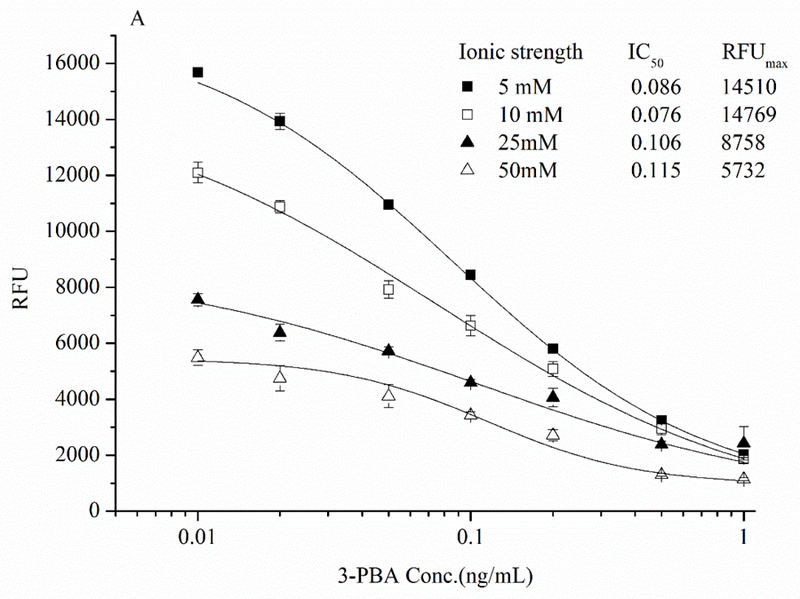
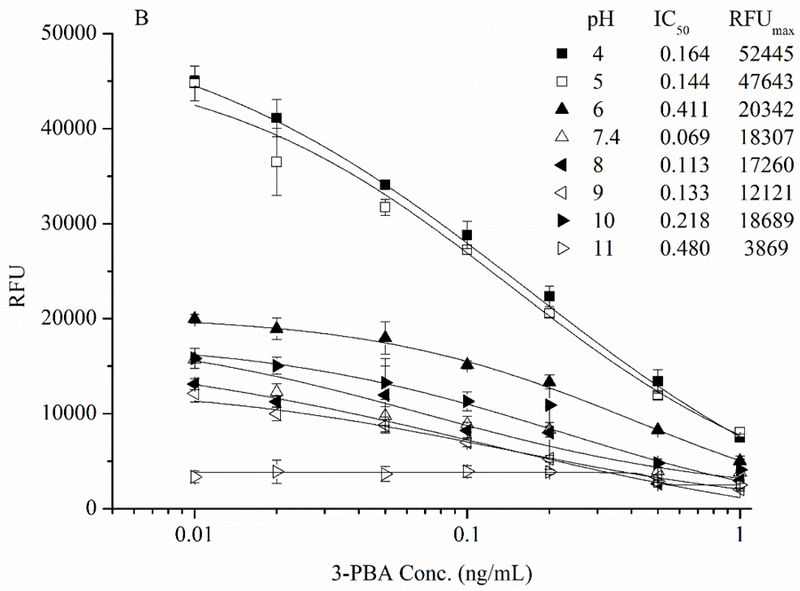
Effects of (A) ionic strength and (B) pH on the performance of dc-FEIA for 3-PBA. n = 3.
A direct competitive Nb-AP fusion protein-based FEIA standard curve was established using the optimal conditions (Figure 6). The standard curve exhibited a good correlation coefficient of 0.994 with the limit of detection of 0.011 ng/mL. The assay has a linear range of 0.015~0.447 ng/mL and an IC50 of 0.082 ng/mL. The IC50 of the one-step competitive FEIA was improved by almost ten times compared to the one-step competitive colorimetric ELISA (IC50 =0.811 ng/mL) and the three-step competitive colorimetric ELISA (IC50 =0.740 ng/mL).
Figure 6.
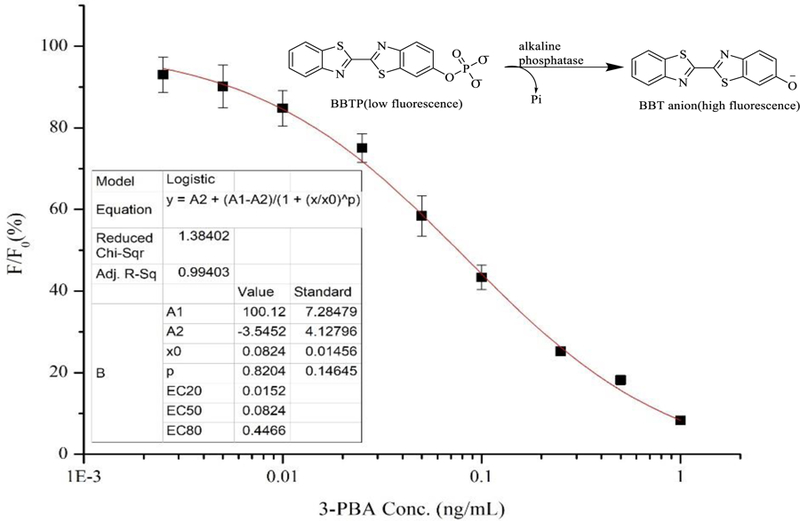
Standard competitive binding curve of Nb-AP fusion protein-based dc-FEIA for 3-PBA under optimized parameters. CcAg = 25 ng/mL. CNb-AP = 20 ng/mL. n = 3.
Cross-Reactivity.
The specificity of the assay was tested for Nb-AP fusion protein, using two 3-PBA analogues and five parent pyrethroids (Table 1). The cross-reactivity was determined using the formula:
Table 1.
Cross Reactivity of Nb-AP Fusion Protein with 3-PBA Structural Analogues and Five Parent Pyrethroids
| Analytes | Cross-reactivity (%) |
|---|---|
| 3-PBA | 100 |
| 3-Phenoxybeneyl aldehyde | 22.6 |
| 3-Phenoxybenzyl alcohol | <0.01 |
| Permethrin | <0.01 |
| Cypermethrin | <0.01 |
| Deltamethrin | <0.01 |
| Fenpropathrin | <0.01 |
| Phenothrin | <0.01 |
[(IC50 of 3-PBA)/(IC50 of cross-reacting compounds)] ×100.
As shown in Table 1, 3-phenoxybenzyl aldehyde had a highest cross reactivity of 22.6%. The result was consistent with the cross-reactivity pattern for the parental VHH,28 indicating that Nb-AP fusion preserved recognition features of the original VHH after fusion manipulation. Negligible cross reactivity with other 3-PBA analogues and parent pyrethroids demonstrated good selectivity of Nb-AP fusion protein in dc-FEIA effectively guaranteed the detection of 3-PBA.
Matrix Effect.
Matrix effects are common challenges for immunoassays, because they can not only cause false positive and lower sensitivity but also can reduce the specificity of the assay. 32 The matrix effects can be reduced in many ways. Sample cleanup procedures such as solid-phase extraction are very useful for the removal of interferences. 33–35 Otherwise, dilution of the sample with assay buffer is another common method to reduce the matrix effects on immunoassay. 36 Since the main intended use of the developed FEIA method is for the evaluation of human/animal exposure to pyrethroids by detecting their common urinary metabolite 3-PBA, urine was selected for the matrix effect evaluation. Urine samples were collected from healthy volunteers and confirmed to be free of 3-PBA by LC-MS (LOD=0.01 ng/mL, LOQ=0.034 ng/mL) analysis. Undiluted urine, urine diluted 5-, 10-, 20-, 40- and 50-fold in the assay buffer and assay buffer were used to prepare serial concentrations of 3-PBA standards for the dc-FEIA. As shown in Figure 7, undiluted urine sample and the 5-fold diluted urine sample showed low fluorescence and did not have an IC50 value, which indicated that the high concentration of urine had a significant influence on the performance of Nb-AP fusion. No significant reduction of maximum fluorescence intensity was observed among the 10-, 20-, 40-, 50-fold diluted urine samples and assay buffer, but the sensitivity was slightly affected in the 10-fold diluted urine sample, indicating the Nb-AP fusion protein was resistant to matrix effects caused by low concentrations of urine. Considering variation among different urine samples, a 20-fold dilution was chosen as an optimal dilution for the developed assay. In general, dilution with assay buffer is a simple and effective method for reduction/elimination of the matrix effect in immunoassay, but the disadvantage of dilution is that it results in lower sensitivity. In our study, even with a 20-fold dilution factor, the final sensitivity of dc-FEIA for urine sample was maintained in the acceptable range due to the high sensitivity of fluorometric analysis format.
Figure 7.
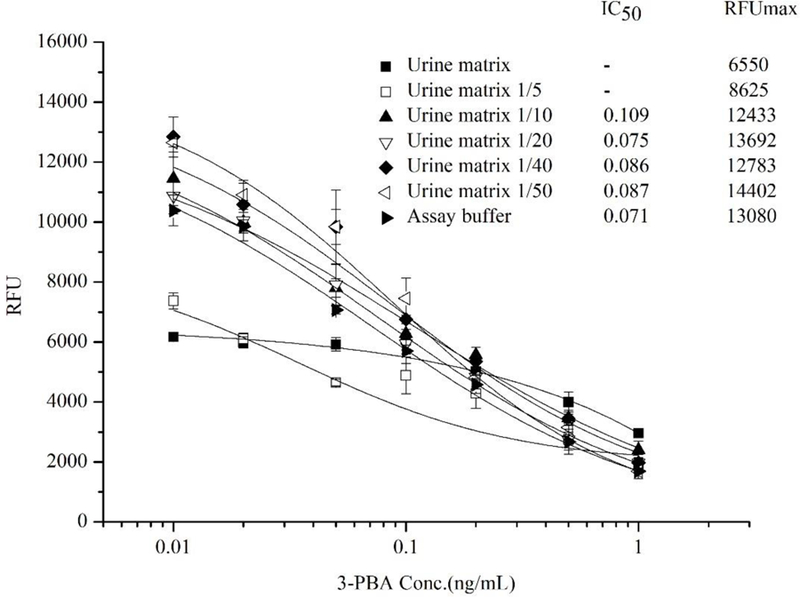
The effect of urine matrix on the performance of Nb-AP fusion protein-based dc-FEIA. n = 3.
Validation Study.
To evaluate the effectiveness of the assay for 3-PBA analysis, the Nb-AP fusion protein-based FEIA was performed to detect 3-PBA in the spiked urine samples. Before the spike and recovery study, all urine samples were confirmed to be free of 3-PBA by LC-MS. Urine samples spiked with three different concentrations of 3-PBA (1, 2 and 4 ng/mL) were prepared for analysis. As shown in Table 2, the average recoveries ranged from 84% to 109% for the FEIA assay, with the coefficient of variation ranging from 0.046 to 0.136, and the average recoveries ranging from 102% to 119% for the LC-MS assay, with the coefficient of variation ranging from 0.076 to 0.154. These results indicate the reliability, accuracy and reproducibility of the Nb-AP fusion protein-based dc-FEIA for 3-PBA detection in urine samples.
Table 2.
Recoveries of 3-PBA from Spiked Urine Samples
| 3-PBA added(ng/mL) | FEIA(ng/mL) | Average recovery(%) | CV(%) | LC-MS (ng/mL) | Average recovery(%) | CV(%) |
|---|---|---|---|---|---|---|
| 1 | 1.04±0.05 | 104 | 4.59 | 1.11±0.17 | 102 | 15.37 |
| 2 | 1.92±0.09 | 96 | 4.87 | 2.47±0.19 | 119 | 7.56 |
| 4 | 4.35±0.41 | 109 | 9.46 | 4.63±0.47 | 114 | 10.08 |
Note: Each assay was performed in triplicate on the same day.
In this study, a rapid and sensitive one-step competitive fluorometric enzyme immunoassay for detecting 3-PBA in urine samples was successfully developed based on a novel Nb-AP fusion protein. The fluorometric detection exhibited remarkable advantages in terms of sensitivity compared to conventional colorimetric techniques. The IC50 of the one-step competitive fluorometric enzyme immunoassay was nearly ten times higher compared with that of the one-step and three-step competitive colorimetric enzyme immunoassays. The high sensitivity of dc-FEIA compensated for the loss of sensitivity during the dilution of urine sample. In addition, one-step detection format based on Nb-AP fusion protein speeds up the assay procedure because no secondary or tertiary antibodies are required for the one-step dc-FEIA. With these advantages, all the procedure for 3-PBA detection could be accomplished in 2 h from sample dilution to data analysis. These results indicate that the construction of Nb-AP fusions can be considered as an attractive and powerful reagent for immunoassay analysis and the development of one-step competitive fluorometric enzyme immunoassay can be a simple and rapid analytical tool for quantitative determinations of pesticides or their metabolites.
Supplementary Material
ACKNOWLEDGMENT
This work was financially supported by the National Institute of Environmental Health Science Superfund Research Program (P42ES004699), the National Nature Science Foundation of China (No. 31471786) and the National Academy of Sciences, NAS Subaward (No. 2000009144).
Footnotes
Supporting Information
The synthetic gene for Nb-AP fusion protein, optimized parameters of LC and MS and structure of immunization antigen and coating antigen were supplied in the supporting information. This material is available free of charge via the Internet at http://pubs.acs.org.
Reference
- (1).Kaneko H Pyrethroids: Mammalian metabolism and toxicity. J. Agric. Food Chem 2011, 59, 2786–2791. [DOI] [PubMed] [Google Scholar]
- (2).Saillenfait AM; Ndiaye D; Sabaté JP Pyrethroids: Exposure and health effects-An update. Int. J. Hyg. Environ. Health 2015, 218, 281–292. [DOI] [PubMed] [Google Scholar]
- (3).Perkins A; Walters F; Sievert J; Rhodes B; Morrissey B; Karr CJ Home use of a pyrethroid-containing pesticide and facial paresthesia in a toddler: A case report. Int. J. Environ. Res. Public Health 2016, 13, 829–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Go V; Garey J; Wolff MS; Pogo GT Estrogenic potential of certain pyrethroid compounds in the MCF-7 human breast carcinoma cell line. Environ. Health Perspect 1999, 107, 173–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Jurewicz J; Radwan M; Wielgomas B; Sobala W; Piskunowicz M; Radwan P; Bochenek M; Hanke W The effect of environmental exposure to pyrethroids and DNA damage in human sperm. Syst. Biol. Reprod. Med 2015, 61, 37–43. [DOI] [PubMed] [Google Scholar]
- (6).Li CM; Cao MF; Ma LJ; Ye XQ; S, Y.; Pan WY; Xu ZF; Ma XC; Lan YB; Chen PQ; Liu WP; Liu J; Zhou JH Pyrethroid pesticide exposure and risk of primary ovarian insufficiency in Chinese women. Environ. Sci. Technol 2018, 52, 3240–3248 [DOI] [PubMed] [Google Scholar]
- (7).Aouey B; Derbali M; Chtourou Y; Bouchard M; Khabir A; Fetoui H Pyrethroid insecticide lambda-cyhalothrin and its metabolites induce liver injury through the activation of oxidative stress and proinflammatory gene expression in rats following acute and subchronic exposure. Environ. Sci. Pollut. Res 2017, 24, 5841–5856. [DOI] [PubMed] [Google Scholar]
- (8).Castellanos A; Andres A; Bernal L; Callejo G; Comes N; Gual A; Giblin JP; Roza C; Gasull X Pyrethroids inhibit K2P channels and activate sensory neurons: basis of insecticide- induced paraesthesias. Pain 2018, 159, 92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).He F; Sun J; Han K; Wu Y; Yao P Effects of pyrethroid insecticides on subjects engaged in packaging pyrethroids. Br. J. Ind. Med 1988, 45, 548–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Richardson JR; Taylor MM; Shalat SL; Guillot TS; Caudle WM; Hossain MM; Mathews TA; Jones SR; Cory-Slechta DA; Miller GW Developmental pesticide exposure reproduces features of attention deficit hyperactivity disorder. FASEB J 2015, 29, 1960–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Shan GM; Huang HZ; Stoutamire DW; Gee SJ; Leng G; Hammock BD A sensitive class specific immunoassay for the detection of pyrethroid metabolites in human urine. Chem. Res. Toxicol 2004, 17, 218–225. [DOI] [PubMed] [Google Scholar]
- (12).Noort D; van Zuylen A; Fidder A; van Ommen B; Hulst AG Protein adduct formation by glucuronide metabolites of permethrin. Chem. Res. Toxicol 2008, 21, 1396–1406. [DOI] [PubMed] [Google Scholar]
- (13).Chen L; Zhao TF; Pan CP; Ross JH; Krieger RI Preformed biomarkers including dialkylphosphates (DAPs) in produce may confound biomonitoring in pesticide exposure and risk assessment. J. Agric. Food Chem 2012, 60, 9342–9351. [DOI] [PubMed] [Google Scholar]
- (14).El-Saeid MH; Khan HA Determination of pyrethroid insecticides in crude and canned vegetable samples by supercritical fluid chromatography. Int. J. Food Prop 2015, 18, 1119–1127. [Google Scholar]
- (15).Mudiam MKR; Jain R; Singh A; Khan HA; Parmar D Development of ultrasound-assisted dispersive liquid-liquid microextraction-large volume injection-gas chromatography-tandem mass spectrometry method for determination of pyrethroid metabolites in brain of cypermethrin-treated rats. Forensic Toxicol 2014, 32, 19–29. [Google Scholar]
- (16).Ding Y; White CA; Muralidhara S; Bruckner JV; Bartlett MG Determination of deltamethrin and its metabolite 3-phenoxybenzoic acid in male rat plasma by high-performance liquid chromatography. J. Chromatogr. B 2004, 810, 221–227. [DOI] [PubMed] [Google Scholar]
- (17).Liu Y; Wu AH; Hu J; Lin MM; Wen MT; Zhang X; Xu CX; Hu XD; Zhong JF; Jiao LX; Xie YJ; Zhang CZ; Yu XY; Liang Y; Liu XJ Detection of 3-phenoxybenzoic acid in river water with a colloidal gold-based lateral flow immunoassay. Anal. Biochem 2015, 483, 7–11. [DOI] [PubMed] [Google Scholar]
- (18).Thiphom S; Prapamontol T; Chantara S; Mangklabruks A; Suphavilai C; Ahn KC; Gee SJ; Hammock BD Determination of the pyrethroid insecticide metabolite 3-PBA in plasma and urine samples from farmer and consumer groups in northern Thailand. J. Environ. Sci. Health, Part B 2014, 49, 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Pandey V; Chauhan A; Pandey G; Mudiam MKR Optical sensing of 3-phenoxybenzoic acid as a pyrethroid pesticides exposure marker by surface imprinting polymer capped on manganese-doped zinc sulfide quantum dots. Anal. Chem. Res 2015, 5, 21–27. [Google Scholar]
- (20).Ahn KC; Gee SJ; Kim HJ; Aronov PA; Vega H; Krieger RI; Hammock BD Immunochemical analysis of 3-phenoxybenzoic acid, a biomarker of forestry worker exposure to pyrethroid insecticides. Anal. Bioanal. Chem 2011, 401, 1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Rau D; Kramer K; Hock B Single-chain FV antibody-alkaline phosphatase fusion proteins produced by one-step cloning as rapid detection tools for ELISA. J. immunoassay Immunochem 2002, 23, 129–143. [DOI] [PubMed] [Google Scholar]
- (22).Xu ZL; Dong JX; Wang H; Li ZF; Beier RC; Jiang YM; Lei HT; Shen YD; Yang JY; Sun YM Production and characterization of a single-chain variable fragment linked alkaline phosphatase fusion protein for detection of o,o-diethyl organophosphorus pesticides in a one-step enzyme-linked immunosorbent assay. J. Agric. Food Chem 2012, 60, 5076–5083. [DOI] [PubMed] [Google Scholar]
- (23).Swain MD; Anderson GP; Serrano-González J; Liu JL; Zabetakis D; Goldman ER Immunodiagnostic reagents using llama single domain antibody-alkaline phosphatase fusion proteins. Anal. Biochem 2011, 417, 188–194. [DOI] [PubMed] [Google Scholar]
- (24).Tang ZW; Wang XR; Lv JW; Hu XR; Liu X One-step detection of ochratoxin A in cereal by dot immunoassay using a nanobody-alkaline phosphatase fusion protein. Food Control 2018, 92, 430–436. [Google Scholar]
- (25).Wang J; Majkova Z; Bever CRS; Yang J; Gee SJ; Li J; Xu T; Hammock BD One-step immunoassay for tetrabromobisphenol A using a camelid single domain antibody-alkaline phosphatase fusion protein. Anal. Chem 2015, 87, 4741–4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Cano R; Torres MJ; Klem RE; Palomares JC DNA hybridization assay using ATTOPHOS(TM), a fluorescent substrate for alkaline phosphatase. Biotechniques 1992, 12, 264–269. [PubMed] [Google Scholar]
- (27).Liu X; Xu Y; Wan DB; Xiong YH; He ZY; Wang XX; Gee SJ; Ryu D; Hammock BD Development of a nanobody-alkaline phosphatase fusion protein and its application in a highly sensitive direct competitive fluorescence enzyme immunoassay for detection of ochratoxin A in cereal. Anal. Chem 2015, 87, 1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Kim HJ; McCoy MR; Majkova Z; Dechant JE; Gee SJ; Rosa ST; González-Sapienza GG; Hammock BD Isolation of alpaca anti-hapten heavy chain single domain antibodies for development of sensitive immunoassay. Anal. Chem 2012, 84, 1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Liu JL; Zabetakis D; Lee AB; Goldman ER; Anderson GP Single domain antibody-alkaline phosphatase fusion proteins for antigen detection--analysis of affinity and thermal stability of single domain antibody. J. Immunol. Methods 2013, 393, 1–7. [DOI] [PubMed] [Google Scholar]
- (30).Lei JW; Li PW; Zhang Q; Wang YR; Zhang ZW; Ding XX; Zang W Anti-idiotypic nanobody-phage based real-time immuno-PCR for detection of hepatocarcinogen aflatoxin in grains and feedstuffs, Anal. Chem 2014, 86, 10841–10846. [DOI] [PubMed] [Google Scholar]
- (31).Qiu YL; Li P; Dong S; Zhang XS; Yang QR; Wang YL; Ge J; Hammock BD; Zhang CZ; Liu XJ Phage-mediated competitive chemiluminescent immunoassay for detecting Cry1Ab toxin by using an anti-idiotypic camel nanobody. J. Agric. Food Chem 2018, 66, 950–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).McCoy MR; Yang Z; Fu X; Ahn KC; Gee SJ; Bom DC; Zhong P; Chang D; Hammock BD Monitoring of total type II pyrethroid pesticides in citrus oils and water by converting to a common product 3-phenoxybenzoic acid. J. Agric. Food Chem 2012, 60, 5065–5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Koeber R; Fleischer C; Lanza F; Boos KS; Sellergren B; Barceló D Evaluation of a multidimensional solid-phase extraction platform for highly selective on-line cleanup and high-throughput LC-MS analysis of triazines in river water samples using molecularly imprinted polymers. Anal. Chem 2001, 73, 2437–2444. [DOI] [PubMed] [Google Scholar]
- (34).Auréa AE; Moisés C; Valérie LC; Víctor C Solid-phase extraction of organic compounds: A critical review (Part I). TrAC, Trends Anal. Chem 2016, 80, 641–654. [Google Scholar]
- (35).Fang Y; Tian W; Pei F; Li P; Shao XL; Fan Y; Hu QH Simultaneous determination of pesticide residues and antioxidants in blended oil using a liquid-liquid extraction combined with dispersive solid phase extraction method. Food Chem 2017, 229, 347–353. [DOI] [PubMed] [Google Scholar]
- (36).Watanabe E; Yamasaki T; Hirakawa Y; Harada A; Iwasa S; Miyake S Organic solvent-free immunoassay for quantitative detection of neonicotinoid acetamiprid residues in agricultural products. Anal. Methods 2018, DOI: 10.1039/C8AY01061G. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


