Abstract
The anterior cingulate cortex (ACC) is known to be involved in effortful choice, yet its role in cost-benefit evaluation of qualitatively different rewards (more/less preferred), beyond magnitude differences (larger/smaller), is poorly understood. Selecting between qualitatively different options is a decision type commonly faced by humans. Here, we assessed the role of ACC on a task that has primarily been used to probe striatal function in motivation. Rats were trained to stable performance on a progressive ratio schedule for sucrose pellets and were then given sham surgeries (control) or excitotoxic NMDA lesions of ACC. Subsequently, a choice was introduced: chow was concurrently available while animals could work for the preferred sucrose pellets. ACC lesions produced a significant decrease in lever presses for sucrose pellets compared to control, whereas chow consumption was unaffected. Lesions had no effect on sucrose pellet preference when both options were freely available. When laboratory chow was not concurrently available, ACC-lesioned rats exhibited similar lever pressing as controls. During a test under specific satiety for sucrose pellets, ACC-lesioned rats also showed intact devaluation effects. The effects of ACC lesions in our task are not mediated by decreased appetite, a change in food preference, a failure to update value or a learning deficit. Taken together, we found that ACC lesions decreased effort for a qualitatively preferred option. These results are discussed with reference to effects of striatal manipulations and our recent report of a role for basolateral amygdala in effortful choice.
Keywords: amygdala, cost-benefit, devaluation, medial prefrontal cortex, progressive ratio
Introduction
Organisms must frequently overcome physical effort costs in the pursuit of rewards. The striatum has been identified as a key region in regulating effort-related functions (Salamone et al., 1991, 2001, 2007; Nowend et al., 2001; Nunes et al., 2013). The basolateral amygdala (Floresco & Ghods-Sharifi, 2007) and cortical regions, namely the medial frontal cortex and specifically the anterior cingulate cortex (ACC; Walton et al., 2002, 2003, 2007; Rudebeck et al., 2006), have also been assigned important roles in decisions involving different effort costs when choosing between whether to work for more reward or to forego the effort cost and select the lower magnitude reward instead.
In seminal work by Walton et al. (2002), it was found that lesions encompassing prelimbic cortex, infralimbic cortex and ACC (i.e. the medial wall) biased animals’ choices towards the low effort (low magnitude) option in a T-maze task. This effect was not mediated by an inability to physically perform the task, a memory impairment for the arm-reward magnitude assignment (i.e. animals chose the higher magnitude rewards when effort costs were equivalent) or a reduction in pursuing the rewards when they were freely available. In a subsequent study by this same group, lesions were specific to PL, IL or ACC. Lesions of PL and IL failed to produce any effect on effort-related choice behaviour, and ACC lesions reproduced the effects of the large lesions administered in the earlier study (Walton et al., 2003). Other work replicated this effect and further probed the neurochemical signalling and circuitry mediating this effect: Schweimer & Hauber (2006) found that blockade of dopamine D1-like receptors in the ACC impaired effort in a barrier climbing task and that disconnection of the ventral striatum and ACC similarly resulted in the work-averse phenotype following either ACC or ventral striatal lesions alone (Hauber & Sommer, 2009). Similarly, disconnection of the basolateral amygdala and ACC impairs effort allocation (Floresco & Ghods-Sharifi, 2007). Thus, these regions have already been identified as critical components of the brain circuitry that regulates effort in the context of choice between options of different magnitudes.
Previous work investigating the role of ACC in effortful choice in rodents made use of T-maze tasks in which the rat selected among different magnitudes of the same reward identity (more vs. less). We previously reported that basolateral amygdala supports the maintenance of value and effortful choice of a qualitatively preferred option (working for pellets vs. freely available chow; Hart & Izquierdo, 2017). It remains unknown whether ACC has a similar function in choosing between qualitatively different reinforcers. A task that involves qualitatively different reinforcers may more closely match that of the human condition where we are faced with different options that are more/less preferred, not differences in magnitude of the same reward. Additionally, previous studies probing regions other than the striatum (e.g. amygdala, ACC) in physical effort have primarily used T-maze tasks. Therefore, a thorough investigation of ACC effects in a task where animals can self-titrate the amount of effort they are willing to exert allows direct comparison with striatal manipulations and further understanding of the circuitry that regulates effort.
To address this question, we tested the effects of ACC lesions in the same effortful choice task as (Hart & Izquierdo, 2017) that required animals to choose between working for a preferred reward vs. consuming a concurrently available, lower cost, but less preferred reward. We assessed the role of ACC on (i) progressive ratio (PR) pressing for sucrose pellets (i.e. general motivation), and (ii) PR pressing in the presence of a freely available alternative (i.e. effortful decision-making: choosing between working for sucrose pellets vs. concurrently available laboratory chow). We also tested the effects of ACC on the choice between sucrose pellets vs. chow when these reinforcers were both freely available. And finally, we assessed sensitivity to devaluation via satiety with the preferred sucrose pellet reward. Given that ACC lesions produced work aversion for rewards of greater magnitude and effort cost in previous studies, it was hypothesized that this manipulation would similarly decrease effort for the qualitatively preferred option in our task. It was also predicted that ACC lesions would not impair sensitivity to devaluation, consistent with a previous report in monkeys (Chudasama et al., 2013).
Materials and methods
Subjects
A timeline of all procedures is shown in Fig. 1. Subjects were 16 (n = 8 sham, n = 8 lesion) adult male Long–Evans rats (Charles River Laboratories, Hollister, CA), singly housed for all phases of experiments with the exception of the acclimation period and handling. All rats had previous experience in a behavioural radial arm maze task in an undergraduate laboratory class prior to experiments. All animals were handled for 10 min in pairs for 5 days after a brief acclimation period in the vivarium. Rats weighed an average of 300 g at the beginning of the experiments. One lesioned rat was omitted from analysis due to a target miss confirmed by histological verification. All animals weighed between 300 and 350 g at the time of euthanasia and brain collection. The vivarium was maintained under a 12/12-h reverse light cycle at 22 °C. All procedures were approved by the Chancellor’s Animal Research Committee at the University of California, Los Angeles.
Fig. 1.
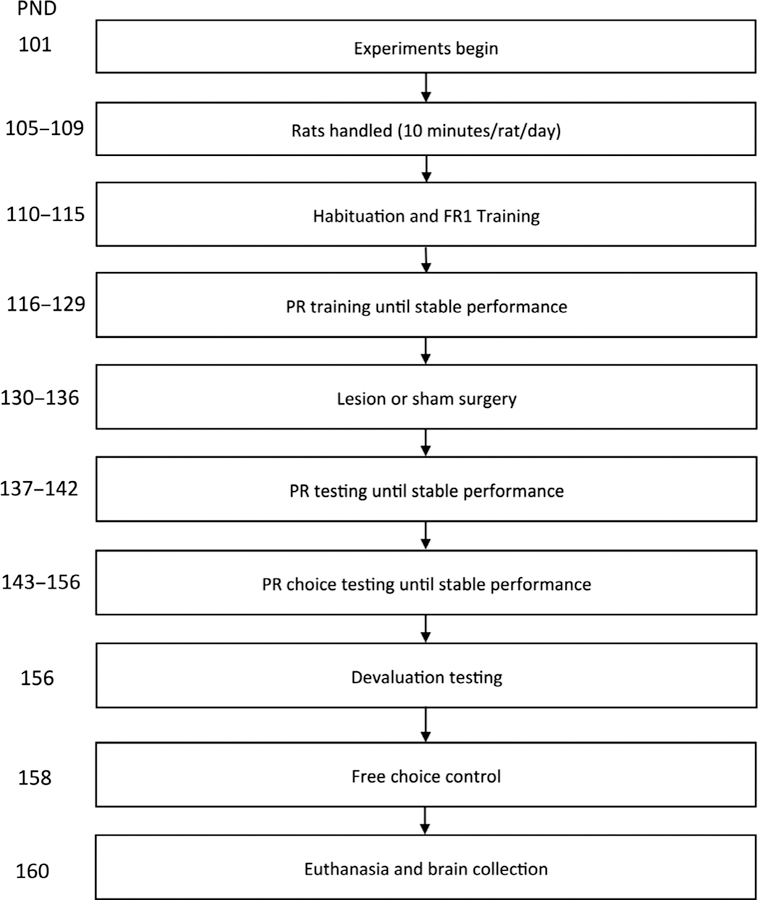
Timeline of events. Sequence of handling, surgery, testing and euthanasia are depicted from top to bottom order.
Progressive ratio training and surgery
Rats were handled, food restricted and given magazine training in Med-Associate operant chambers with hardware controlled by a PC running Med-PC IV (Med-Associates, St. Albans, VT, USA), as previously reported (Hart & Izquierdo, 2017). After this training, 30-min behavioural sessions were administered wherein rats learned to press on a PR schedule for 45 mg sucrose pellets (Bioserv, Frenchtown, NJ, USA) where the required number of presses increased according to the formula ni = 5e(i/5)−5, where ni is equal to the number of presses required on the ith ratio, rounded to the nearest whole number, after five successive schedule completions (Hart & Izquierdo, 2017). General surgical procedures were the same as recently published (Hart & Izquierdo, 2017). Animals were anesthetized with isoflurane (5% induction, 2% maintenance in 2 L/min O2). Burr holes were drilled bilaterally on the skull for insertion of 22-gauge guide cannulae (Plastics One, Roanoke, VA, USA), after which 28-gauge internal cannulae (Plastics One) were inserted. Sham (control) surgery animals were infused with 0.5 μL saline at a flow rate of 0.25 μL/min after which injectors were left in place for one additional minute to allow for diffusion of solution. Lesion surgery animals were infused with 0.5 μL of 20 mg/mL NMDA (Sigma, St. Louis, MO, USA) dissolved in saline. The coordinates used for the guide cannulae targeting ACC were as follows: AP = +2.0 mm, ML = ±0.7 mm, DV = −1.9 mm from Bregma. Injectors extended 1 mm beyond the tip of the cannula. Following the 1-min diffusion time, the cannulae and injectors were removed, incisions were stapled closed, and the rats were placed on a heating pad and kept in recovery until ambulatory before being returned to the vivarium. Post-operative care consisted of five daily injections of carprofen (5 mg/kg, s.c.). Following a 7-days free-feeding recovery period, rats were put back on food restriction and PR training resumed. Following surgery, all animals were allowed to reach stable PR performance (85–115% of mean lever pressing across three consecutive days) as in Ref. Hart & Izquierdo (2017). Data from this phase of testing were used to assess the effects of ACC lesions on general willingness to work or primary motivation (Fig. 2B).
Fig. 2.
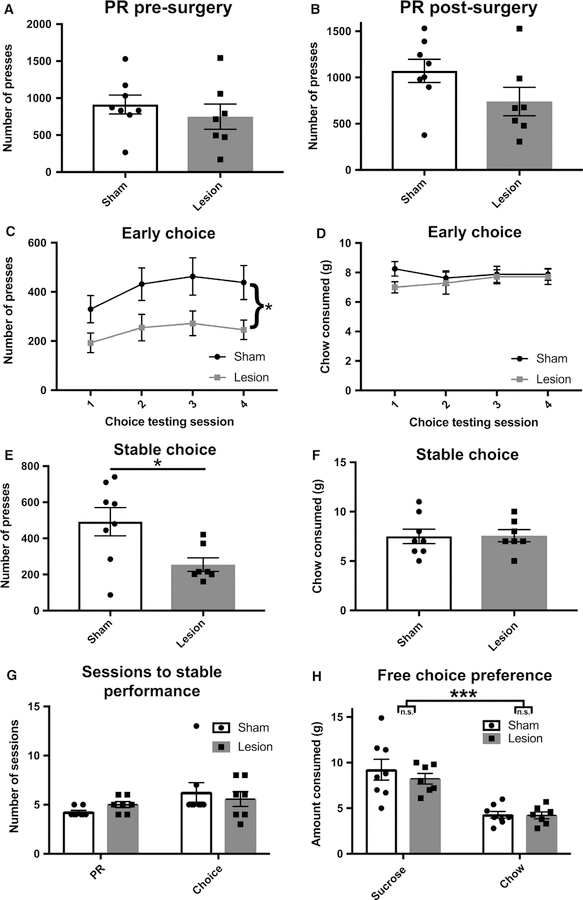
Effect of ACC lesions on PR responding, effortful choice, number of sessions to stable performance and food preference. (A) Number of lever presses on the last of the three stable PR testing sessions prior to surgery. There were no pre-existing differences in effort output. (B) Number of lever presses on the last of the three stable PR testing sessions post-surgery. ACC-lesioned animals showed a slight tendency towards attenuated lever pressing, although the difference did not reach statistical significance. (C) Number of lever presses on first four choice testing sessions, in the presence of freely available chow. ACC-lesioned animals showed significantly fewer lever presses. (D) Amount of chow consumed on the first four choice testing sessions. Groups did not differ in the amount of chow they consumed. (E) Number of lever presses on the last of the three stable choice testing sessions, in the presence of freely available chow. ACC-lesioned animals showed significantly fewer lever presses. (F) Amount of chow consumed on the last of the three stable choice testing sessions. Groups did not differ in the amount of chow they consumed. (G) Number of sessions required to reach stable PR performance and stable choice performance. Groups did not differ in the number of sessions to reach criterion. (H) In the free choice test where both sucrose pellets and chow were freely available (equivalent effort), there was a main effect of food type, but no effect of sham or lesion condition and no food type × condition interaction. Bars denote mean SEM, *P < 0.05, ***P < 0.001.
Effortful choice testing and devaluation
Upon meeting criteria for stable PR performance, effortful choice testing with concurrently freely available laboratory chow was conducted and was administered until stable lever pressing performance was achieved. Subsequently, to assess the effect of incentive state and the role of ACC on effortful choice, all animals underwent a satiation procedure for sucrose pellets following previously published methods (Hart & Izquierdo, 2017). To determine how sensitive animals were to this procedure, data were analysed by calculating difference scores (the session preceding devaluation testing – the devaluation testing session) for the number of lever presses and amount of chow (g) consumed.
Freely available choice
We also conducted a test wherein both sucrose pellets and chow were freely available to assess potential effects of ACC lesions on food preference, and to ensure that sucrose pellets would still be preferred over chow when both rewards had equal work requirements. Following devaluation testing and on a separate test day at least 48 h later, rats were given this test as previously described (Hart & Izquierdo, 2017).
Histology
Following behavioural testing, animals were killed by pentobarbital overdose (Euthasol, 0.8 mL, 390 mg/mL pentobarbital, 50 mg/mL phenytoin; Virbic, Fort Worth, TX, USA) and transcardial perfusion (Hart & Izquierdo, 2017). Brains were post-fixed in 10% buffered formalin acetate for 24 h followed by 30% sucrose for 5 days. 50μm sections were stained for NeuN, visualized using a BZ-X710 microscope (Keyence, Itasca, IL, USA) and analysed with BZ-X Viewer software. Lesions were determined by comparison with a standard rat brain atlas (Paxinos & Watson 1997). NeuN staining was performed by incubation for 24 h at 4 °C in a primary antibody consisting of 1 : 1000 rabbit polyclonal to NeuN (Abcam, Cambridge, MA, USA), 10% normal goat serum (Abcam) and 0.5% Triton X (Sigma) in PBS, followed by three 5-min washes in PBS. Secondary antibody incubations were performed for 2 h at 20 °C in the same solution with 1 : 500 goat anti-rabbit Alexa 594 (Abcam) replaced for the primary antibody, followed by three 5-min washes in PBS. Slides were subsequently mounted and cover-slipped with DAPI mounting medium (Abcam). Reconstructions of lesions are shown in Fig. 4.
Fig. 4.
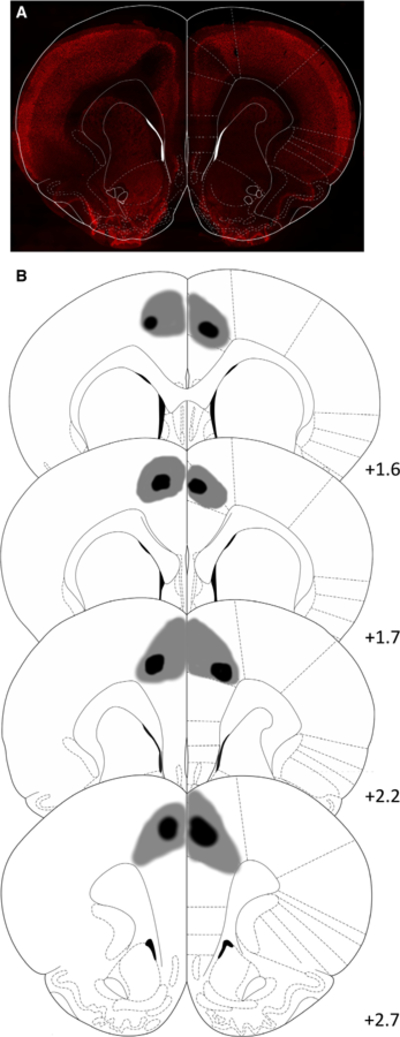
Reconstructions of lesions. (A) Representative photomicrograph of NeuN stained coronal section showing ACC lesions (AP +2.0 mm). (B) Depictions of coronal sections adapted from Paxinos & Watson (1997). Lesion areas arranged from anterior to posterior where numerals on the lower right of each matched section represent the anterior-posterior distance (mm) from Bregma. Grey and black represent maximum and minimum lesion area, respectively. [Colour figure can be viewed at wileyonlinelibrary.com].
Data analyses
Data were analysed using SPSS (IBM Corp, Chicago, IL, USA) and plotted using GRAPHPAD PRISM (La Jolla, CA, USA). An alpha level for significance was set to P < 0.05. Two-way repeated-measures ANOVA was used to compare number of lever presses and amount of chow consumed on the first four choice testing sessions. Unpaired t-tests on data from the last of the three stable sessions were conducted to analyse group differences on PR and on the choice task, and two-way ANOVAS for group differences on food preference and number of sessions to stable performance. Devaluation effects were determined by calculating difference scores (number of lever presses on the session preceding devaluation minus number of lever presses on the devaluation test session), and analysed with unpaired t-tests to probe group differences.
Results
Progressive ratio
There were no pre-surgical differences when rats stabilized on PR prior to surgery (t13 = 0.7835, P = 0.447; control = 913 ± 127.8; lesion = 749.3 ± 169.3; Fig. 2A). Following surgery, after allowing several days of PR testing until stable performance was reached, lesioned animals showed a slight tendency towards reduced lever pressing for sucrose pellets, although this did not reach statistical significance (t13 = 1.689, P = 0.115; control = 1072 ± 125.1; lesion = 740.1 ± 153.7; Fig. 2B). Animals were allowed several sessions of effortful choice testing until stable lever pressing performance was achieved.
Effortful choice testing
Group differences in effort appeared from the start of exposure to the choice task. A two-way repeated-measures ANOVA on number of lever presses during each of the first four choice sessions revealed a significant main effect of condition (F1,13 = 5.006, P = 0.0434), significant main effect of session (F3,39 = 6.361, P = 0.0013) and no significant session-by-condition interaction (F3,39 = 0.4987, P = 0.6854; Fig. 2C). We found no group differences in amount of chow consumed during the first four choice sessions: there was no effect of condition (F1,13 = 0.6833, P = 0.4234), session (F3,39 = 0.3982, P = 0.7550) or session-by- condition interaction (F3,39 = 1.027, P = 0.3912; Fig. 2D). These differences in effortful choice persisted once all animals reached stable levels of performance: a significant difference in number of lever presses in the ACC-lesioned animals was revealed by an unpaired t-test (t13 = 2.616, P = 0.021; control = 492.3 ± 77.89; lesion = 255.1 ± 37.52) – animals pressed significantly less in the ACC lesion condition than in the control condition (Fig. 2E). Importantly, there was no significant difference in the amount of chow consumed between these conditions (t13 = 0.0736, P = 0.9425; control = 7.5 ± 0.7319; lesion = 7.571 ± 0.6117; Fig. 2F). Groups did not differ in the number of sessions required to reach stable performance during PR testing. A two-way ANOVA with session type (PR, choice) and condition (control, lesion) as fixed factors revealed no significant effect of session type (F1,26 = 3.788, P = 0.0625; PR = 4.60 ± 0.190; choice = 5.933 ± 0.621), no effect of condition (F1,26 = 0.0029, P = 0.9573) and no significant session type-by-condition interaction (F1,26 = 1.169, P = 0.2895; Fig. 2G).
Freely available choice
A test wherein both sucrose pellets and laboratory chow were concurrently freely available was conducted to confirm that ACC lesion effects on PR responding were not due to decreased preference for sucrose. Analysis of amount of food consumed (g) using a two-way ANOVA with food type (sucrose pellet, chow) and condition (control, lesion) as fixed factors revealed a significant effect of food type (F1,26 = 38.79, P = 0.0001; sucrose = 8.727 ± 0.501; chow = 4.194 ± 0.484). No significant food type-by-condition interaction (F1,26 = 0.4226, P = 0.5213) or effect of condition (F1,26 = 0.5394, P = 0.4693) was found (Fig. 2H).
Devaluation
Analysis of difference scores by unpaired t-test revealed that there was no significant difference between groups pre-post devaluation (t13 = 1.739, P = 0.1056; control = 332 ± 74.48; lesion = 179.3 ± 38.88; Fig. 3A). Difference scores were calculated for chow consumption as well. An unpaired t-test revealed that difference scores for chow consumption were not different between control and ACC lesion conditions (t13 = 1.104, P = 0.2896; control = 2.5 ± 0.6268; lesion = 1.429 ± 0.7514; Figs 3B).
Fig. 3.
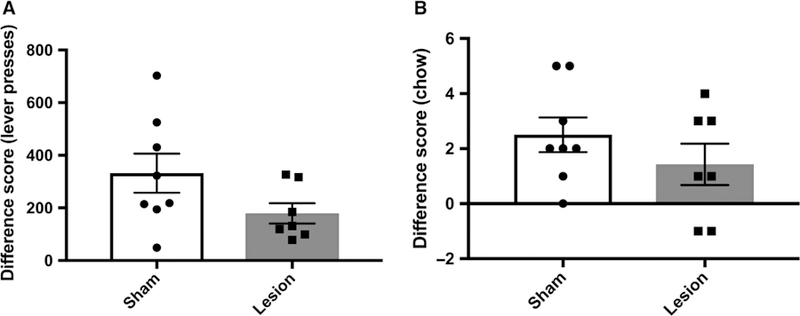
Effects of ACC lesions on sensitivity to sucrose pellet devaluation. (A) Difference scores were calculated by subtracting the number of lever presses on the day of devaluation testing from the session preceding devaluation testing for the control and ACC lesion groups. Difference scores were not significantly different between the two groups. (B) Difference scores were calculated by subtracting the amount of chow consumed on the day of devaluation testing from the session preceding devaluation testing for the control and ACC lesion groups. Groups did not differ in the amount of chow they consumed on the devaluation test relative to the preceding testing session. Bars denote mean ± SEM.
Discussion
We found that ACC lesions resulted in fewer lever presses on the PR schedule when laboratory chow was presented as a competing choice, an effect which was apparent from the beginning of choice testing that persisted even when performance was stable. This suggests that ACC is generally important in supporting the cost calculation involved in choosing how much effort to exert for a reward when a less costly alternative is concurrently available. ACC lesions had no effect on the consumption of freely available laboratory chow during effortful choice testing, providing evidence that this manipulation did not reduce appetite.
We conducted several control measures to rule out factors that could have accounted for ACC effects on lever pressing. One potential explanation for our findings was that ACC lesions simply decreased preference for the sucrose pellets. In a free-feeding choice task, we found a significant main effect of food type (sucrose pellets preferred over chow) and no interaction, indicating that ACC lesions did not change food preferences when effort requirements were equal. We also allowed animals to stabilize on the PR requirement prior to introduction of choice to investigate ACC effects on general willingness to exert effort. In our recent study of task performance after basolateral amygdala inactivations (Hart & Izquierdo, 2017), we saw a clearer dissociation between PR and choice (reduced lever pressing only in the latter condition following basolateral amygdala inactivation). Here, with ACC lesions we saw a tendency for reduced lever pressing in post-surgery PR, but with group differences only reaching significance in both early and stable choice performance. This indicates that ACC may have a broader role than basolateral amygdala in exertion to reward. Groups did not differ in the number of days required to reach stable performance on PR or the choice task, indicating that none of our effects were due to a learning deficit.
In support of related investigations of effort costs (Walton et al., 2002, 2003, 2007; Floresco & Ghods-Sharifi, 2007), here we also report effects of ACC lesions on effortful choice. The pattern of effects we obtained resembles that of D1 or D2 receptor antagonism (Randall et al., 2012) or dopamine depletion (Randall et al., 2014). Given that ACC sends excitatory projections to ventral striatum, it is possible that these projections mediate our effects. Indeed, disconnection of ACC and ventral striatum also reduces choice of the high-effort option in a T-maze task (Hauber & Sommer, 2009). However, another group has previously reported a null effect on PR performance when chow was freely available (Schweimer & Hauber, 2005). One potential explanation is that the experiment by Schweimer & Hauber (2005) assessed animals that had prior experience in another effort task, which may have allowed enough time and/or experience to improve performance. Indeed, recovery of function has been demonstrated after cortical lesions wherein the behavioural effects resolved spontaneously, leading to full recovery presumably by adaptations or ‘repurposing’ of unaffected circuits (Otchy et al., 2015). Such a consideration is a limitation for direct comparison of our present work (NMDA lesions) with recent work (baclofen/muscimol inactivations, Hart & Izquierdo, 2017). However, the finding that ACC-lesioned rats showed significantly reduced lever pressing in effortful choice over 2 weeks after surgery argues against this recovery of function.
Given the nature of PR, where each successive reinforcer requires more effort in addition to more time to earn, it is plausible that our effects on lever pressing could be due to a time-averse phenotype. This is likely not the case, as ACC lesions similarly impaired selection of a high-effort option in T-maze tasks where the time to reward delivery is more equivalent for both options (Walton et al., 2003; Schweimer & Hauber, 2006).
One recently proposed idea is that both cognitive and physical effort discounting share overlapping neurocomputational signatures, particularly those coding reward value (Chong et al., 2017), but that the substrates may segregate depending on the cost type (whether physical or cognitive; Hosking et al., 2014). We found that difference scores in a test of sucrose devaluation – a measure of how well animals are able to use a change in motivational state to guide effortful behaviour – to be unaffected by ACC lesions, similar to a previous report in monkeys (Chudasama et al., 2013). These stand in contrast to effects of basolateral amygdala, which had an impact on both the valuation and effortful choice of the preferred option (Hart & Izquierdo, 2017). Taken together, our results support the idea that ACC, unlike basolateral amygdala, supports a more general effort to reward, without an update to value (i.e. valued vs. devalued responses were intact after ACC lesion). One possibility is that once preference is established through pre-surgery exposure with both preferred and non-preferred foods, ACC may be involved in supporting effort allocation using only, or primarily, reward identity information (sucrose pellets vs. chow), not value. Recent proposals suggest this may be similar to the role of other cortical regions, namely orbitofrontal cortex (Stalnaker et al., 2014). A question for future research using both rodent and primate effort paradigms would be to determine the conditions in which ACC and orbitofrontal cortex support action value in effortful choice, given that both cortical regions have now been ascribed roles in this process (Kolling et al., 2016; Winstanley & Floresco, 2016; Fiuzat et al., 2017).
Acknowledgements
This work was supported by UCLA’s Division of Life Sciences Recruitment and Retention fund (Izquierdo) and the Training Program in Translational Neuroscience of Drug Abuse (T32 DA024635, London).
Footnotes
Conflict of interest
Authors report no conflict of interest.
Data accessibility
Data will be uploaded on Figshare with a CC-Zero licence.
References
- Chong TT, Apps M, Giehl K, Sillence A, Grima LL & Husain M (2017) Neurocomputational mechanisms underlying subjective valuation of effort costs. PLoS Biol, 15, e1002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Daniels TE, Gorrin DP, Rhodes SE, Rudebeck PH & Murray EA (2013) The role of the anterior cingulate cortex in choices based on reward value and reward contingency. Cereb. Cortex, 23, 2884–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiuzat EC, Rhodes SE & Murray EA (2017) The role of orbitofrontal-amygdala interactions in updating action-outcome valuations in macaques. J. Neurosci, 37, 2463–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB & Ghods-Sharifi S (2007) Amygdala-prefrontal cortical circuitry regulates effort-based decision making. Cereb. Cortex, 17, 251–260. [DOI] [PubMed] [Google Scholar]
- Hart EE & Izquierdo A (2017) Basolateral amygdala supports the maintenance of value and effortful choice of a preferred option. Eur. J. Neurosci, 45, 388–397. [DOI] [PubMed] [Google Scholar]
- Hauber W & Sommer S (2009) Prefrontostriatal circuitry regulates effort-related decision making. Cereb. Cortex, 19, 2240–2247. [DOI] [PubMed] [Google Scholar]
- Hosking JG, Cocker PJ & Winstanley CA (2014) Dissociable contributions of anterior cingulate cortex and basolateral amygdala on a rodent cost/benefit decision-making task of cognitive effort. Neuropsychopharmacol, 39, 1558–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolling N, Behrens T, Wittmann MK & Rushworth M (2016) Multiple signals in anterior cingulate cortex. Curr. Opin. Neurobiol, 37, 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowend KL, Arizzi M, Carlson BB & Salamone JD (2001) D1 or D2 antagonism in nucleus accumbens core or dorsomedial shell suppresses lever pressing for food but leads to compensatory increases in chow consumption. Pharmacol. Biochem. Be, 69, 373–382. [DOI] [PubMed] [Google Scholar]
- Nunes EJ, Randall PA, Hart EE, Freeland C, Yohn SE, Baqi Y, Muller CE, Lopez-Cruz L et al. (2013) Effort-related motivational effects of the VMAT-2 inhibitor tetrabenazine: implications for animal models of the motivational symptoms of depression. J. Neurosci, 33, 19120–19130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otchy TM, Wolff SB, Rhee JY, Pehlevan C, Kawai R, Kempf A, Gobes SM & Olveczky BP (2015) Acute off-target effects of neural circuit manipulations. Nature, 528, 358–363. [DOI] [PubMed] [Google Scholar]
- Paxinos G & Watson C (1997). The Rat Brain in Stereotaxic Coordinates Academic Press, Cambridge, MA. [Google Scholar]
- Randall PA, Pardo M, Nunes EJ, Lopez Cruz L, Vemuri VK, Makriyannis A, Baqi Y, Muller CE et al. (2012) Dopaminergic modulation of effort-related choice behavior as assessed by a progressive ratio chow feeding choice task: pharmacological studies and the role of individual differences. PLoS One, 7, e47934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall PA, Lee CA, Nunes EJ, Yohn SE, Nowak V, Khan B, Shah P, Pandit S et al. (2014) The VMAT-2 inhibitor tetrabenazine affects effort-related decision making in a progressive ratio/chow feeding choice task: reversal with antidepressant drugs. PLoS One, 9, e99320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Walton ME, Smyth AN, Bannerman DM & Rushworth MF (2006) Separate neural pathways process different decision costs. Nat. Neurosci, 9, 1161–1168. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D & Mahan K (1991) Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology, 104, 515–521. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Wisniecki A, Carlson BB & Correa M (2001) Nucleus accumbens dopamine depletions make animals highly sensitive to high fixed ratio requirements but do not impair primary food reinforcement. Neuroscience, 105, 863–870. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A & Mingote SM (2007) Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology, 191, 461–482. [DOI] [PubMed] [Google Scholar]
- Schweimer J & Hauber W (2005) Involvement of the rat anterior cingulate cortex in control of instrumental responses guided by reward expectancy. Learn Memory, 12, 334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweimer J & Hauber W (2006) Dopamine D1 receptors in the anterior cingulate cortex regulate effort-based decision making. Learn Memory, 13, 777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnaker TA, Cooch NK, McDannald MA, Liu TL, Wied H & Schoenbaum G (2014) Orbitofrontal neurons infer the value and identity of predicted outcomes. Nat. Commun, 5, 3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Bannerman DM & Rushworth MF (2002) The role of rat medial frontal cortex in effort-based decision making. J. Neurosci, 22, 10996–11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Alterescu K & Rushworth MF (2003) Functional specialization within medial frontal cortex of the anterior cingulate for evaluating effort-related decisions. J. Neurosci, 23, 6475–6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Rudebeck PH, Bannerman DM & Rushworth MF (2007) Calculating the cost of acting in frontal cortex. Ann. N. Y. Acad. Sci, 1104, 340–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA & Floresco SB (2016) Deciphering decision making: variation in animal models of effort- and uncertainty-based choice reveals distinct neural circuitries underlying core cognitive processes. J. Neurosci, 36, 12069–12079. [DOI] [PMC free article] [PubMed] [Google Scholar]