Abstract
Objectives
To evaluate the evidence for morphine and ziconotide as firstline intrathecal (IT) analgesia agents for patients with chronic pain.
Methods
Medline was searched (through July 2017) for “ziconotide” or “morphine” AND “intrathecal” AND “chronic pain,” with results limited to studies in human populations.
Results
The literature supports the use of morphine (based primarily on noncontrolled, prospective, and retrospective studies) and ziconotide (based on randomized controlled trials and prospective observational studies) as first-choice IT therapies. The 2016 Polyanalgesic Consensus Conference (PACC) guidelines recommended both morphine and ziconotide as firstline IT monotherapy for localized and diffuse chronic pain of cancer-related and non–cancer-related etiologies; however, one consensus point emphasized ziconotide use, unless contraindicated, as firstline IT therapy in patients with chronic non–cancer-related pain. Initial IT therapy choice should take into consideration individual patient characteristics (e.g., pain location, response to previous therapies, comorbid medical conditions, psychiatric history). Trialing is recommended to assess medication efficacy and tolerability. For both morphine and ziconotide, the PACC guidelines recommend conservative initial dosing strategies. Due to its narrow therapeutic window, ziconotide requires careful dose titration. Ziconotide is contraindicated in patients with a history of psychosis. IT morphine administration may be associated with serious side effects (e.g., respiratory depression, catheter tip granuloma), require dose increases, and cause dependence over time.
Conclusion
Based on the available evidence, morphine and ziconotide are recommended as firstline IT monotherapy for cancer-related and non–cancer-related pain. The choice of first-in-pump therapy should take into consideration patient characteristics and the advantages and disadvantages of each medication.
Keywords: Cancer Pain, Firstline Intrathecal Therapy, Morphine, Noncancer Pain, Polyanalgesic Consensus Conference, Ziconotide
Introduction
Intrathecal (IT) drug delivery offers proven benefits over oral analgesics for the treatment of patients with chronic intractable pain [1]. IT therapy delivers analgesic medication directly to the site of action on the dorsal horn of the spinal cord, thereby bypassing first-pass metabolism and the blood–brain barrier [1,2]. This direct delivery allows a lower effective dose and less interaction with systemic receptors, thus decreasing systemic adverse effects [1–3]. The advantages of IT drug delivery may be relevant given the opioid abuse epidemic in the United States, which has led to an excessive number of deaths (33,091 opioid-related overdose deaths in 2015, including >15,000 deaths related to prescription opioids) [4–6], a growing population of opioid-dependent patients [7], continuing drug diversion [7], and patients whose pain is refractory due to opioid-induced hyperalgesia [8].
The Polyanalgesic Consensus Conference (PACC) panel was formed in 2000 to review evidence pertaining to the efficacy and safety of IT therapies and provide published guidelines regarding their use [9]. Recently, the PACC panel reconvened to examine the foremost evidence on IT therapy and update the 2012 PACC guidelines, with the aim of providing clinically appropriate, evidence-based treatment recommendations that enhance both analgesia and patient safety [9]. The 2016 consensus conference, from which the updated guidelines were developed and published in 2017, did not delineate pain treatment recommendations by pain type (i.e., nociceptive, neuropathic), because many patients with chronic pain syndromes experience both nociceptive and neuropathic pain, but instead provided separate guidance for localized and diffuse pain [9,10]. In addition, the latest PACC group considered the disease status of patients with cancer, decried the use of IT therapy only as salvage treatment, and applied a validated evidence ranking system (US Preventive Services Task Force [USPSTF] hierarchy of studies and degrees of recommendation) to guide the new recommendations (Table 1) [9].
Table 1.
PACC 2016 updates that enhance analgesia and patient safety [9]
| Treatment Recommendations by Pain Etiology and Breadth | Classification of Cancer Patients Based on Disease State and Prognosis | Recognition that Previous Treatment May Influence Response | Ranking System Used to Grade Evidence | |
|---|---|---|---|---|
|
|
|
IT analgesia should be considered only as salvage therapy after failure of high doses of systemic opioids |
|
Figure adapted with permission from: Deer TR, Pope JE, Hayek S, et al. The Polyanalgesic Consensus Conference (PACC): Recommendations on intrathecal drug infusion systems best practices and guidelines. Neuromodulation 2017;20(2):96–132 [9].
IT = intrathecal; PACC = Polyanalgesic Consensus Conference; USPSTF = United States Preventive Services Task Force.
Consensus grading performed when >80% of the PACC members were present.
Morphine (a µ-opioid receptor agonist; Infumorph, West-Ward Pharmaceuticals Corp., Eatontown, NJ, USA) and ziconotide (a nonopioid calcium channel blocker; Prialt, Jazz Pharmaceuticals, Palo Alto, CA, USA) are the only agents approved by the US Food and Drug Administration (FDA) as IT therapies for chronic pain [9,11,12]. Though both medications have demonstrated efficacy, each has unique pharmacologic properties, limitations, and monitoring requirements; thus, proper use of these IT therapies requires a thorough understanding of medication pharmacokinetics and pharmacodynamics, appropriate patient selection, and proper administration and monitoring [1]. This review considers the evidence for the use of morphine and ziconotide as firstline IT therapies in the management of chronic pain, summarizes the PACC 2016 guidelines for these medications, and provides guidance for appropriate patient selection, dosing, and monitoring for each agent.
Methods
Searches of the Medline database were conducted in July 2017 using the search terms “ziconotide” or “morphine” AND “intrathecal” AND “chronic pain” and limited to human research articles. These searches returned 59 English-language articles for ziconotide and 138 for morphine. Of these, 31 were duplicates. An additional 52 articles were excluded for nonrelevance based on the title (e.g., focused on mechanism of action, nonchronic pain population). The remaining articles were reviewed and included if they reported clinical research data or other relevant information pertaining to use of morphine or ziconotide as firstline IT therapies. The reference lists of these articles were further reviewed for additional pertinent articles.
Efficacy of Morphine and Ziconotide as Firstline Intrathecal Therapies
Morphine
Morphine is a µ-opioid receptor agonist that is available as a preservative-free solution for IT administration [11]. Given its effectiveness [13] and long history of IT use [14], it has been considered the standard for IT medication. The efficacy of IT morphine for the relief of cancer-related and non–cancer-related pain has been demonstrated primarily in noncontrolled, prospective, and retrospective studies, and in many studies it was unclear whether morphine was the first agent used in the IT pump [15–28]. However, given that these reports describe initial IT pump implantation in patients with either inadequate response to or intolerable side effects from conservative treatment (eg, systemic nonopioid and opioid analgesics, tricyclic antidepressants, antiepileptic drugs) [15–18,20–28], morphine seems most likely to be the first IT medication used in the majority of these studies. The results of these studies suggest that morphine administered as the initial IT therapy provides clinically relevant analgesia in patients with cancer-related and non–cancer-related pain (Supplementary Data). For example, in a prospective open-label study of 119 patients with refractory cancer-related pain, IT morphine (delivered in patient-controlled bolus doses) was associated with a 31% decrease in mean pain scores on a numeric analog scale (NAS) after one month of treatment (P < 0.01) [22]. Similarly, in a prospective study of 30 patients with refractory non–cancer-related pain, IT morphine (delivered via continuous infusion) was associated with a 37% decrease in visual analog scale (VAS) pain score after three months of treatment [20].
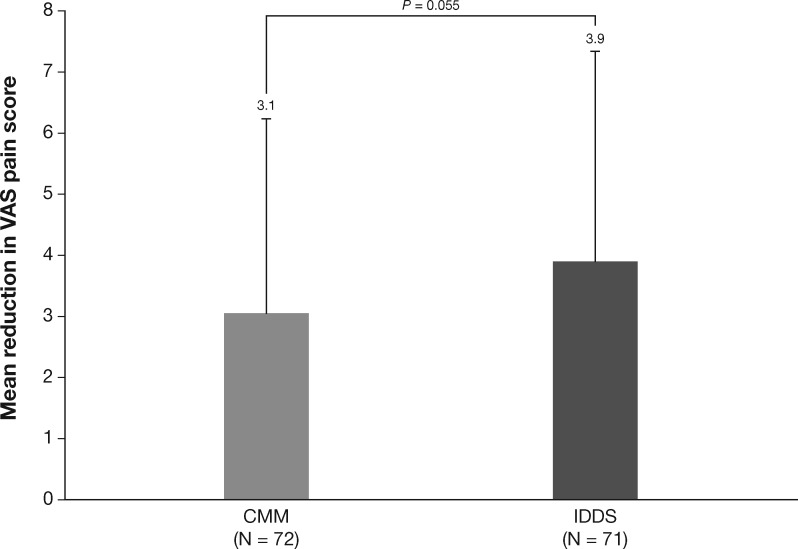
Only two randomized controlled studies specified the use of morphine as the first IT therapy administered, one study for cancer pain [29] and one for noncancer pain [30]. In IT-naïve patients with advanced cancer and refractory pain, pain (as assessed by VAS) was reduced after four weeks of therapy with both IT morphine (delivered by programmable infusion pump) plus comprehensive medical management (N = 101) and comprehensive medical management alone (N = 99). Greater pain relief was observed with IT morphine (12.4% between-group difference), although the difference was not significant (P = 0.055) (Figure 1) [29]. The IT morphine group also had a nonsignificant improvement in survival at six months (53.9%) vs comprehensive medical management alone (37.2%; P = 0.06). In a small (N = 15) randomized, double-blind, dose reduction study, patients with chronic non–cancer-related pain who had received IT morphine for ≥12 months were randomly assigned either to undergo reduction of their morphine dose or to maintain their morphine dose [30]. In the morphine dose reduction group (reduction from 1.6 mg/d to 1.15 mg/d; decrease of 36% in IT opioid dose), 70% of patients discontinued the study, mostly due to worsening of pain, whereas no patients in the dose maintenance group discontinued. Taken together, these results suggest that IT morphine provides meaningful analgesia in patients with noncancer pain.
Figure 1.
Pain reduction with intrathecal (IT) opioids as the first agent in pump in patients with cancer pain [29]. Patients with cancer pain received comprehensive medical management (CMM; all pain therapy except spinally administered medications, cordotomy, or other similar neurosurgical interventions) or IT morphine or hydromorphone therapy for four weeks. After four weeks of treatment, patients who received IT opioids had a nonsignificantly greater reduction in pain, as measured on a continuous visual analog scale ranging from 0 (no pain) to 10 (worst pain imaginable), than those who received CMM. Figure created with data from: Smith TJ, Staats PS, Deer T, et al. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: Impact on pain, drug-related toxicity, and survival. J Clin Oncol 2002;20(19):4040–9 [29]. CMM = comprehensive medical management; IDDS = implantable drug delivery system; VAS = visual analog scale.
Ziconotide
Ziconotide is a nonopioid analgesic agent that selectively and reversibly binds to N-type voltage-sensitive calcium channels and appears to produce analgesia by blocking pronociceptive neurotransmitter release from nociceptive afferent nerves in the dorsal horn of the spinal cord [31]. Three randomized, placebo-controlled studies of patients with chronic, refractory cancer-related and non–cancer-related pain [32–34] have demonstrated effective analgesia with IT ziconotide, with sustained efficacy in long-term open-label extension studies (Supplementary Data) [35,36]. In the first two randomized placebo-controlled studies (fast-titration studies), the starting dose was reduced (9.6–2.4 mcg/d) and the titration schedule altered during the study because of tolerability concerns; therefore, the efficacy results from these studies are not included in the label [12,32,33]. The label describes the efficacy results from the third randomized placebo-controlled study (slow titration study), which used a lower starting dose (2.4 mcg/d) and a slower titration schedule [12,34].
In a retrospective Italian registry study of IT ziconotide in patients with cancer-related (N = 32) or non–cancer-related (N = 72) pain, pain intensity was reduced within one month after initiating ziconotide in the overall population and among patients with cancer or noncancer pain [37]. Patients with cancer-related and non–cancer-related pain attained pain reduction (20%–50%) after a mean of one and three months of treatment, respectively. Although most (53%) of the patients in this study received ziconotide as the first IT therapy, a subanalysis of these patients was not conducted; therefore, definitive conclusions regarding ziconotide as the first agent in the IT pump were not possible. A more recent retrospective review of 15 patients, all of whom received ziconotide as their initial IT therapy, showed that 53% of patients were classified as treatment responders (defined as ≥30% improvement in numeric pain rating scale [NPRS] score and/or physician-observed, clinically significant increases in activities of daily living) after approximately two months of conservatively dosed ziconotide (mean initial dose of 1.1 mcg/d [range = 0.6–1.4 mcg/d] with titration at one- to four-week intervals to a mean dose of 2.8 mcg/d [range = 1.8–3.8 mcg/d]) [38].
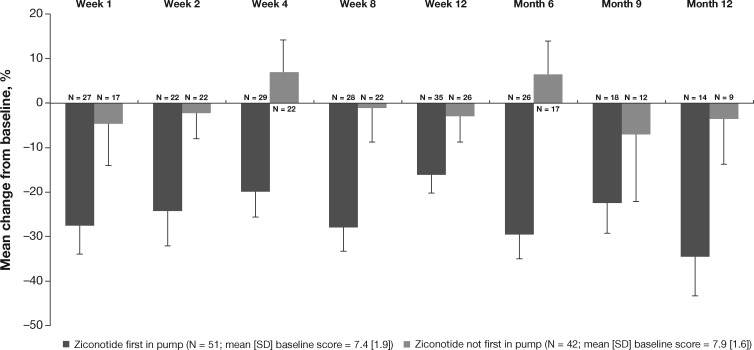
These positive findings were supported by the results of an interim analysis of the open-label, prospective, US-based Patient Registry of Intrathecal Ziconotide Management (PRIZM) study [10]. The preliminary data from the study were analyzed based on patients’ use of different IT therapies as the first agent in pump (N = 51 patients with ziconotide as the first agent in pump, N = 42 patients with ziconotide as the second or later IT agent). From baseline to week 12, the mean percentage change in NPRS score was greater in patients for whom ziconotide was the first in pump (–16.0%) compared with patients for whom ziconotide was not the first in pump (–2.8%); this pattern of results was observed through month 12 (–34.4% vs –3.4%, respectively) (Figure 2) [10]. In a subgroup analysis of patients enrolled for ≥12 months with NPRS scores at months 3, 6, 9, and 12, the percentage improvement at month 12 was 32.7% for ziconotide first-in-pump patients (N = 13) and 5.4% for ziconotide not first-in-pump patients (N = 8). Clinically significant improvement in pain (defined as ≥30% reduction from baseline NPRS score) was also observed in a greater percentage of patients with ziconotide first-in-pump vs not first-in-pump (20.0% vs 11.5% at week 12; 50.0% vs 11.1% at month 12) [10].
Figure 2.
Mean percentage change from baseline in numeric pain rating scale (NPRS). Error bars represent 1 standard error of the mean. Sample sizes represent observed cases with an NPRS score at each assessment [10]. Lower scores indicate reduction in pain. Figure adapted with permission from: Deer T, Rauck RL, Kim P, et al. Effectiveness and safety of intrathecal ziconotide: Interim analysis of the Patient Registry of Intrathecal Ziconotide Management (PRIZM). Pain Pract 2018;18(2):230–8 [10].
Based on the the available evidence, the PACC 2016 guidelines advocate the use of morphine or ziconotide as firstline IT therapy for both cancer-related and non–cancer-related pain (Table 2) [9,39,40].
Table 2.
| Statement | USPSTF Evidence Level* | USPSTF Recommendation Grade† | PACC Consensus Level‡ |
|---|---|---|---|
| IT therapy with opioids should be utilized for active cancer-related pain | I | A | Strong |
| IT therapy with ziconotide should be utilized for active cancer-related pain | I | A | Strong |
| IT therapy with opioids should be utilized for active noncancer pain | III | B | Strong |
| IT therapy with ziconotide should be utilized for active noncancer pain | I | A | Strong |
Figure adapted with permission from: Deer et al, The Polyanalgesic Consensus Conference (PACC): Recommendations on intrathecal drug infusion systems best practices and guidelines. Neuromodulation 2017;20(2):96–132 [9]. Additional data reprinted from: Harris RP, Helfand M, Woolf SH, et al. Current methods of the US Preventive Services Task Force: A review of the process. Am J Prev Med 2001;20(3 Suppl):21–35 [40].
IT = intrathecal; PACC = Polyanalgesic Consensus Conference; USPSTF = United States Preventive Services Task Force.
Evidence grades: I, at least one controlled and randomized clinical trial, properly designed; II-1, well-designed, controlled, nonrandomized clinical trials; II-2, cohort or case studies and well-designed controls, preferably multicenter; II-3, multiple series compared over time, with or without intervention and surprising results in noncontrolled experiences; III, clinical experience–based opinions, descriptive studies, clinical observations, or reports of expert committees.
Recommendation grades: A, extremely recommendable (good evidence that the measure is effective and benefits outweigh the harms); B, recommendable (at least moderate evidence that the measure is effective and benefits exceed harms); C, neither recommendable nor inadvisable (at least moderate evidence that the measure is effective, but benefits are similar to harms and a general recommendation cannot be justified); D, inadvisable (at least moderate evidence that the measure is ineffective or that the harms exceed the benefits); I, insufficient, low-quality, or contradictory evidence (the balance between benefit and harms cannot be determined).
Level of consensus among members of the PACC: strong, >80% consensus; moderate, 50% to 79% consensus; weak, <49% consensus.
Safety of Firstline Morphine and Ziconotide Intrathecal Therapies
IT therapy may be associated with complications related to many factors, including those related to the IT pump itself (e.g., mechanical failure), pump pocket fills, issues surrounding preparation of the IT medication and programming of the pump for medication release, and complications following pump implantation surgery [41–44]. Such events are relatively rare and typically unrelated to the specific medication within the pump, so they do not directly influence the choice of firstline IT therapy. However, when selecting the recommended therapeutic agent to use first in the IT pump (i.e., morphine or ziconotide), the safety of the medications and their potential limitations should be considered [14].
Morphine
Morphine administered as IT therapy may be less likely to elicit the systemic effects associated with oral opioid therapy [1,3] and therefore may be beneficial for patients with refractory pain or those who are intolerant to oral opioids. For example, a woman with neuropathic cancer-related pain that was refractory to oral opioids experienced improved analgesia, increased alertness, and reduced side effects with IT morphine in a case report [45]. However, there is limited evidence for this approach, and changing the route of administration may not be sufficient to address opioid-related issues (e.g., tolerance, adverse effects, opioid-induced hyperalgesia) in some patients, and a nonopioid analgesic, such as ziconotide, may be considered [46].
IT morphine may also be used as an add-on therapy for patients with breakthrough pain who are receiving oral opioids [3]. There is little evidence regarding the safety of this combination (IT opioids + oral opioids), but initiating IT therapy may reduce the required oral opioid dose, which could ameliorate some of the side effects associated with these medications [29,41,47]. It is the recommendation of the authors that continued use of oral opioids should be limited. Medical professionals should have the goal of removing oral opioids from the treatment regimen once IT agents are initiated.
Although the 2016 PACC guidelines consider IT delivery of opioids such as morphine to be a relatively safe therapeutic option, this strategy is not without concerns (Table 3) [39]. IT morphine has been associated with serious adverse events including respiratory depression that could lead to death, the formulation of inflammatory masses (granulomas), and myoclonus [3,48,49]. Respiratory depression occurs most often during the initiation or restarting of IT opioid use and when used in combination with central nervous system depressants [39]. Because of this, caution should be used when initiating or restarting IT morphine to avoid overdose [13]. The development of granulomas around the catheter tip may occur with morphine (either as monotherapy or in combination with non-FDA-approved agents) and can cause serious neurologic deficits (if impingement on the spinal cord occurs) and disrupt opioid delivery (resulting in abrupt withdrawal) [39,46]; however, the association between granuloma formation and IT medication dose remains unclear [39]. For example, of 208 patients who presented over a 34-week period for a refill of their IT pump and had imaging studies performed, six had granulomas [50]. Of these, three were receiving morphine, all at different maximum concentrations (10, 25, and 50 mg/mL). However, in a retrospective study of 56 patients receiving long-term IT therapy, both average opioid dose and dose escalation were associated with granuloma formation [51]. Additionally, in a canine model, granuloma formation has been associated with higher doses of IT morphine [52]. Routine imaging for granuloma formation is not necessary. However, if a patient presents with new-onset axial or radicular pain, a granuloma should be considered within the differential diagnosis, using imaging for confirmation. If a patient presents with focal neurologic deterioration, new-onset extremity numbness or weakness, or urinary or bowel incontinence, then a granuloma should be suspected and imaging ordered in the appropriate timeline. Imaging for detecting a granuloma should include magnetic resonance imaging (MRI) with and without contrast, or computed tomography (CT) myelogram or CT scan if MRI is contraindicated [39].
Table 3.
PACC 2016 safety recommendations for IT opioid therapy [39]
| Statement | USPSTF Evidence Level* | USPSTF Recommendation Grade† | PACC Consensus Strength‡ |
|---|---|---|---|
| IT opioid delivery is a relatively safe and effective method for chronic infusion to treat cancer-related and non–cancer-related pain | II-2 | A | Strong |
| Respiratory depression can occur with IT opioid administration, and careful dosing is critical to avoid this complication | II-3 | B | Strong |
| Concurrent use of sedative medications in patients receiving opioids should be minimized or avoided | II-2 | A | Strong |
| Single-shot trialing with IT opioids is a safe strategy, with an observation period of ≥6 hours in an outpatient or inpatient site of service; outpatients should have continued observation after discharge with a responsible adult | II-3 | B | Moderate |
| Endocrinopathic side effects are a consequence of IT opioids, and preoperative surveillance and monitoring are recommended | II-3 | A | Strong |
| Lower extremity edema can occur by an unknown mechanism and can be mitigated by transition to a more lipophilic opioid | III | C | Strong |
| Urinary retention is a complication that may be mitigated by the administration of parasympathomimetic medications | III | C | Moderate |
| Nausea, vomiting, and pruritus are consequences of IT delivery of opioids and, although they typically resolve with time, should be considered when employing opioids for chronic infusion | III | C | Moderate |
| Consideration of patient candidacy for IT opioid therapy is crucial, and evaluation should consider the pain generator(s), patient age, location and type of pain, previous opioid exposure, and patient comorbidities | II-2 | B | Strong |
Adapted from: Deer TR, Pope JE, Hayek S, et al. The Polyanalgesic Consensus Conference (PACC): Recommendations on intrathecal drug infusion systems best practices and guidelines. Neuromodulation 2017;20(2):96–132 [9]; and Deer TR, Pope JE, Hayek S, et al. The Polyanalgesic Consensus Conference (PACC): Recommendations for intrathecal drug delivery: Guidance for improving safety and mitigating risks. Neuromodulation 2017;20(2):155–76 [39].
IT = intrathecal; PACC = Polyanalgesic Consensus Conference; USPSTF = United States Preventive Services Task Force.
Evidence grades: I, at least one controlled and randomized clinical trial, properly designed; II-1, well-designed, controlled, nonrandomized clinical trials; II-2, cohort or case studies and well-designed controls, preferably multicenter; II-3, multiple series compared over time, with or without intervention and surprising results in noncontrolled experiences; III, clinical experience–based opinions, descriptive studies, clinical observations, or reports of expert committees.
Recommendation grades: A, extremely recommendable (good evidence that the measure is effective and benefits outweigh the harms); B, recommendable (at least moderate evidence that the measure is effective and benefits exceed harms); C, neither recommendable nor inadvisable (at least moderate evidence that the measure is effective, but benefits are similar to harms and a general recommendation cannot be justified); D, inadvisable (at least moderate evidence that the measure is ineffective or that the harms exceed the benefits); I, insufficient, low-quality or contradictory evidence (the balance between benefit and harms cannot be determined).
Level of consensus among members of the PACC: strong, >80% consensus; moderate, 50% to 79% consensus; weak, <49% consensus.
Less serious but bothersome adverse events such as constipation, nausea, vomiting, urinary retention, peripheral edema, neuroendocrine disruption (e.g., suppression of the hypothalamic–pituitary–adrenal axis), and pruritus may also occur with IT morphine administration [39,53]. As with oral opioids, tolerance may develop with IT morphine, particularly in younger patients [54] and those whose pain is refractory to high doses of oral opioids, who may need higher medication doses and would be at increased risk for adverse events [14]. There is also the possibility of dependence and opioid withdrawal symptoms if the IT infusion is disrupted (e.g., following development of catheter kinks or tears or after the pump motor stalls) [13,14]. In addition, care must be taken when lowering the dose or discontinuing IT morphine to reduce the likelihood of withdrawal symptoms [13].
Safety data from prospective studies evaluating morphine as the initial IT therapy report adverse events typical of morphine, including nausea/vomiting, sleep disorders, drowsiness, urinary retention, pruritus, fatigue, and sweating [21,22,24,27]. These side effects typically occurred early in therapy administration and improved over time, either spontaneously or with medical management [21,22,24,27]. Results from the only published randomized controlled trial of IT morphine with safety information showed a significantly greater reduction of toxicity from baseline to week 4 (as evaluated using the Common Toxicity Criteria) with a combination of IT morphine and comprehensive medical management (which consisted of all pain therapies except spinally administered medications) compared with comprehensive medical management alone (P = 0.004) [29]. In particular, fatigue and reduced consciousness significantly improved with IT morphine plus comprehensive medical management vs comprehensive medical management alone (P < 0.05 for both) [29]. Notably, the median daily systemic morphine oral equivalent dose was similar between groups at randomization (comprehensive medical management, 272 mg; IT morphine, 250 mg), but by week 4 it had increased to 290 mg in the comprehensive management group and decreased to 50 mg in the IT morphine group [29].
Ziconotide
Strategic dosing to maximize the efficacy and safety of IT ziconotide is required because of its narrow therapeutic window and because its tolerability profile correlates less with the actual dose administered than with the rate of dosage increase [31,39]. PACC 2016 recommendations for ziconotide safety are presented in Table 4 [39]. The most frequently reported adverse events with ziconotide were dizziness, nausea, confusion, and repetitive rapid eye movements (nystagmus) [12]. The tolerability of ziconotide is generally better with the improved dosing suggestions, including nocturnal dosing [55,56]. Less frequently reported but common adverse events included asthenia, somnolence, abnormal gait, vomiting, and diarrhea [12,53]. Cognitive impairment (e.g., mental slowing, impaired memory and speech, confusion, psychosis, changes in consciousness) was reported with use of high starting doses and aggressive titration schedules but appears to be less frequent with slower titration protocols [46]. However, IT ziconotide remains contraindicated for patients with a history of psychosis [12]. Cognitive adverse events typically have a delayed onset (e.g., occurring several weeks after therapy initiation) but may occur within 10 days of the beginning of ziconotide treatment and require dose adjustment or discontinuation of the medication [12,57]. Because of this, caregivers should vigilantly monitor and report any cognitive-related adverse events displayed by patients.
Table 4.
PACC 2016 safety recommendations for IT ziconotide therapy [39]
| Statement | USPSTF Evidence Level* | USPSTF Recommendation Grade† | PACC Consensus Strength‡ |
|---|---|---|---|
| Ziconotide has no cardiopulmonary side effects when delivered intrathecally | I | A | Strong |
| Ziconotide use is contraindicated in patients with a history of psychosis | I | A | Strong |
| Ziconotide can cause predictable increases in creatinine kinase; it is recommended to perform baseline laboratory testing before initiation and repeat testing if muscle-related symptoms occur | I | B | Strong |
| It is recommended that ziconotide therapy be introduced initially if appropriate (or “first in pump”) and not as an adjuvant therapy | I | A | Strong |
| Ziconotide needs to be titrated slowly with recommended amounts of <1 mcg/d each week | II | B | Moderate |
| If side effects occur, and depending on their severity, titration to half the dose with continued infusion may be helpful | III | C | Strong |
Adapted from: Deer TR, Pope JE, Hayek S, et al. The Polyanalgesic Consensus Conference (PACC): Recommendations for intrathecal drug delivery: Guidance for improving safety and mitigating risks. Neuromodulation 2017;20(2):155–76 [39].
IT = intrathecal; PACC = Polyanalgesic Consensus Conference; USPSTF = United States Preventive Services Task Force.
Evidence grades: I, at least one controlled and randomized clinical trial, properly designed; II-1, well-designed, controlled, nonrandomized clinical trials; II-2, cohort or case studies and well-designed controls, preferably multicenter; II-3, multiple series compared over time, with or without intervention and surprising results in noncontrolled experiences; III, clinical experience–based opinions, descriptive studies, clinical observations, or reports of expert committees.
Recommendation grades: A, extremely recommendable (good evidence that the measure is effective and benefits outweigh the harms); B, recommendable (at least moderate evidence that the measure is effective and benefits exceed harms); C, neither recommendable nor inadvisable (at least moderate evidence that the measure is effective, but benefits are similar to harms and a general recommendation cannot be justified); D, inadvisable (at least moderate evidence that the measure is ineffective or that the harms exceed the benefits); I, insufficient, low-quality, or contradictory evidence (the balance between benefit and harms cannot be determined).
Level of consensus among members of the PACC: strong, >80% consensus; moderate, 50% to 79% consensus; weak, <49% consensus.
Elevated serum creatine kinase (CK) levels are fairly common with IT ziconotide (experienced by 40% of patients in clinical studies) and are typically observed within the first two months of therapy [12]. Checking CK levels should be considered at baseline (before initiation of ziconotide) and periodically during therapy because sometimes elevated CK levels can be asymptomatic. Regular monitoring of CK levels in asymptomatic patients has not been shown to be needed but could be considered on an annual basis or more frequently in patients with muscle weakness at baseline. For patients presenting with symptoms of elevated CK (e.g., myalgias, muscle weakness, nausea/vomiting, tea-colored urine) during treatment, CK levels should be checked at the time of presentation and at more frequent intervals subsequently [57]. Reducing the dose and stopping ziconotide treatment are options in the presence of this condition (Table 5). Only a few cases of rhabdomyolysis have been reported [12,58], making a direct association between ziconotide use and rhabdomyolysis difficult to confirm [12,58].
Table 5.
Suggested actions to address side effects of IT ziconotide
| Side Effect | Suggested Action |
|---|---|
| Dizziness | These side effects can generally be reduced or avoided using nocturnal dosing or slower titration protocols [46,55,56]. If these side effects occur and depending on their severity, titrate to half the dose with continued infusion [39]. If side effects persist or are severe, reduce dose further or discontinue medication. |
| Nausea | |
| Nystagmus | |
| Asthenia | |
| Somnolence | |
| Abnormal gait | |
| Vomiting | |
| Diarrhea | |
| Confusion | Patient with confusion should be assessed for other signs of cognitive impairment. If confusion is not severe enough to warrant discontinuation and other severe cognitive symptoms are not present, dose should be reduced [57]. |
| Cognitive impairment | Reduce dose or discontinue medication. Cognitive-related side effects are dose dependent; therefore, the severity of symptoms should be considered when managing dosing during a cognitive change evaluation. In the event that changes occur and it is uncertain if they are related to the drug, neurology consultation should be considered once the drug has been eliminated. |
| CK elevations | For symptomatic patients with CK elevations, reduce dose or discontinue medication. For asymptomatic patients with CK elevations, continue monitoring [12,57]. |
CK = creatine kinase; IT = intrathecal.
IT ziconotide does not cause respiratory depression or physical dependence due to its unique mechanism of action, and no withdrawal symptoms have been observed after treatment interruption or abrupt discontinuation [3]. Also, no incidences of granulomas [31,46] or lethal overdoses [31] have been reported. If adverse events do occur, dose reduction or rapid discontinuation is possible and may alleviate symptoms [39].
In an interim analysis of PRIZM registry data, the adverse event profile was generally consistent with the ziconotide prescribing information. In patients who received ziconotide as the first IT agent in pump, the most common adverse events were nausea, dizziness, and peripheral edema [10]. In a retrospective review of 15 patients receiving firstline IT ziconotide monotherapy, notable adverse events included dizziness in two patients (which resolved with dose reduction) and transient urinary retention in one patient (which resolved without additional intervention) [38].
The advantages, disadvantages, and considerations for IT morphine and ziconotide are summarized in Table 6.
Table 6.
Advantages and disadvantages/considerations for IT morphine and ziconotide
| Morphine | Ziconotide | |
|---|---|---|
| Advantages |
|
|
| Disadvantages/considerations |
|
|
AE = adverse event; CNS = central nervous system; IT = intrathecal.
Combination Therapy
The PACC 2016 guidelines recommend that off-label medications and combination IT therapy be considered only after the failure of IT morphine or ziconotide monotherapy [9]. The only prospective study that examined the combination of morphine and ziconotide as initial IT agents was an observational study of patients with chronic cancer–related pain (bone metastasis) refractory to oral opioids [59]. The therapy combination improved overall pain scores from day 7 to day 28 (mean percentage change from baseline in visual analog scale of pain intensity [VASPI] score = 51% at day 7 and 62% at day 28) with concomitant increases in the morphine (mean dose = 0.82 mg/d at day 1 vs 1.2 mg/d at day 28) and ziconotide (2.4 mcg/d at day 1 vs 4.8 mcg/d at day 28) doses. Additional high-quality research (e.g., randomized active comparator [morphine and ziconotide monotherapy]) is needed to thoroughly evaluate the benefits and risks associated with the morphine and ziconotide combination as initial IT therapy.
Patient Selection Considerations
Good candidates for IT therapy typically include patients with either nociceptive or neuropathic pain or with mixed nociceptive and neuropathic pain (e.g., postlaminectomy syndrome or cancer pain) that is well localized and has a clear diagnosis [13]. Again, the intention of the PACC 2016 guidelines was to move away from nociceptive or neuropathic pain as an algorithmic determiner, as diffuse vs localized pain and appropriate catheter location seemed to play a larger role in treatment efficacy [9]. IT therapy may not be a good option for patients with widespread pain; headaches or facial pain; ischemic heart disease, heart failure, or cerebrovascular disease; inadequate caregiver support or transportation; or cancer with less than three months’ life expectancy. Chronic noncancer pain indications for targeted drug delivery include, but are not limited to, postlaminectomy syndrome, chronic compression fractures, spinal stenosis, spondylosis, spondylolisthesis, complex regional pain syndrome, neuropathies, rheumatoid arthritis, and chronic pancreatitis [9]. Patients do not need to have failed a trial of oral opioid management to be considered for IT therapy. Furthermore, patients do not need to have failed a trial of neurostimulation to be considered candidates for targeted drug delivery. There is strong evidence for the efficacy of IT therapy for localized pain, but it has also been shown to be effective for diffuse pain [9]. Although the 2016 PACC guidelines recommend ziconotide over morphine as the first agent used in the IT pump for patients with non–cancer-related pain [9], the choice of firstline therapy should take into consideration multiple patient characteristics (e.g., age, pain location, response to previous therapies, anatomic catheter location) and psychological characteristics (e.g., history of psychosis, anxiety, depression, personality disorders) [9,13]. The higher rate of dose escalation that occurs with IT opioids in patients <50 years of age should also be taken into consideration when choosing to initiate therapy with ziconotide vs morphine [54].
IT morphine may be beneficial as the first therapy used in the pump for patients with intractable chronic pain, particularly for patients who have contraindications to ziconotide [46]. However, IT morphine would be detrimental for those whose pain is refractory to high-dose oral opioids and those with substance abuse problems, pulmonary disease, and/or central or obstructive sleep apnea [13,46]. It has become increasingly clear that weaning strategies, as defined by Grider et al., may be important when preparing to employ IT opioids [60,61]. Given the risk of exacerbating respiratory depression and subsequent death with IT morphine [3,48,53,62], patients should be evaluated for cardiopulmonary and respiratory status [13] and use of medications that may impact these factors [39]. The use of IT morphine may be associated with adverse cardiopulmonary effects. Therefore, the risks and benefits of use of IT morphine in patients with advanced age, morbid obesity, and sleep apnea should be considered before trialing [13,46,63]. The effects of IT morphine on the neuroendocrine system necessitate consideration, particularly if a patient is younger, because these disruptions may alter sex hormones (e.g., testosterone, estrogen) [63]. In patients receiving IT opioids, neuroendocrine dysfunction should be monitored based on patient symptomatology and physical examination findings (e.g., low energy, low libido, gynecomastia). Care should also be taken in younger patients, given reports of increased tolerance in this patient population [46,54,64]. Finally, patients who experienced inadequate efficacy, opioid-related adverse events, or hyperalgesia with systemic opioids may not be good candidates for IT morphine, as these effects may also occur with IT opioid therapy [46].
Ziconotide is not associated with an increased risk of respiratory depression, even at supratherapeutic doses [12] and, therefore, is not subject to many of the patient concerns associated with use of IT morphine. The cardiopulmonary safety profile of IT ziconotide is particularly advantageous [39]. However, ziconotide is contraindicated for patients with histories of psychosis [12], and careful monitoring is required for patients taking concomitant central nervous system (CNS)–active medications (e.g., antiepileptics, neuroleptics, sedatives), as these may increase the risk of CNS-related adverse events (e.g., reduced consciousness, dizziness, confusion) [46].
Trialing and Dosing of Morphine and Ziconotide as Firstline Intrathecal Therapies
Trialing
Trialing of IT medications before implanting the IT device is recommended to provide clinically relevant information about the efficacy and tolerability of specific medications in a given patient, although evidence to support the predictive validity of such trials is limited [65,66]. Trials of IT morphine can be performed using bolus injection(s), continuous epidural infusion, or continuous IT infusion [66,67]. In contrast, ziconotide trialing is performed via bolus IT injections(s) and continuous IT infusion only [42,66]. Based on the current limited evidence, no trialing method is regarded as superior [66,68]. The choice of trialing method should consider many factors, including available resources, the chronic IT infusion strategy to be chosen for the patient, site of service, and physician preference and familiarity with the technique [41,47,69]. In a 2005 survey of interventional pain physicians (N = 205 physicians), continuous IT (∼40% of physicians) and continuous epidural (∼35% of physicians) administration were the preferred methods for trialing opioid medications [70]. Trialing outcomes may be more informative when the trialing method (bolus vs continuous infusion) matches the planned long-term IT delivery regimen [66].
Although no standardized method of trialing IT opioids has been established [9], several considerations should be noted. When performing bolus trials, the dose for IT opioids should be minimized to reduce the risks of complications or death (e.g., respiratory depression), with duration of post-trial monitoring based on medication dose and half-life and patient comorbidities (eg, sleep apnea, pulmonary compromise) [66]. Also, improved efficacy of IT opioids has been demonstrated when oral opioids are discontinued or doses are reduced before the trial [60,61,66]. Physician survey data regarding the methods of trialing used for ziconotide are not available, but in the PRIZM patient registry, bolus injections were used in >90% of patients who received a trial of ziconotide [10]. Guidance on the trialing of ziconotide is available in at least two published protocols [56,71]. These protocols used sequential bolus injections over several days or weeks, with dose adjustments based on efficacy and side effects. In light of these recent studies, the 2016 PACC recommended bolus trialing for ziconotide at doses ranging from 1 to 5 mcg [66].
An important factor in trialing IT medications is defining their levels of success, because no standardized definition of a “successful” trial exists [66]. In clinical terms, a trial may be considered successful if pain is reduced to an acceptable level and side effects are minimal or tolerable. Medications that provide analgesia with some side effects may still be appropriate therapies if the dose is reduced. In such cases, the patient should be trialed again using the lower dose of the medication.
Dosing
Once trialing of medication has been successful, the IT pump may be implanted and drug administration initiated [13]. Standard administration of IT medication occurs via intermittent or continuous infusion, as programmed by the clinician, with dose adjustments made as needed at clinic visits to maximize efficacy and tolerability [39,42]. Bolus and patient-controlled IT administration dosing strategies have also been reported with both morphine and ziconotide [22,27,56,72–76]. In the 2016 PACC guidelines, recommended starting doses for continuous IT administration are 0.1–0.5 mg/d for morphine in opioid-naïve patients and 0.5–1.2 mcg/d (up to 2.4 mcg/d) for ziconotide [9,66].
The starting dose of IT morphine in patients who have developed a tolerance to oral opioids is variable (range = 1–10 mg/d), and caution is required with administering starting doses >20 mg/d [11,13]. Opioid dose and the occurrence of adverse events are directly related; therefore, morphine doses should be kept as low as possible [13]. Maintenance of analgesia may require increases in IT morphine doses over time as tolerance develops. However, if analgesic efficacy is not observed after multiple dose escalations approaching 50% of the PACC maximum limit (i.e., 20 mg/mL or 15 mg/d), the integrity of the device should be investigated [9]. The lowest possible effective dose of IT morphine therapy is preferred to limit many of the adverse effects of chronic IT opioid administration, including the development of tolerance and opioid-induced hyperalgesia [9,13]. In small, noncontrolled studies, initiating a low-dose IT opioid therapy (0.25–0.5 mg/d and approximately 250 morphine equivalents/d) after an oral opioid weaning protocol has shown promise [61,77], but randomized, placebo-controlled trials are needed to establish the effectiveness of low-dose therapy. In the outpatient setting, practitioners are cautioned to use the lowest dose of IT morphine possible, with no more than 0.15 mg suggested for bolus administration, and an observation period of at least six to eight hours [66].
Dosing of ziconotide delivered continuously, based on its prescribing information, should begin low (≤2.4 mcg/d), followed by slow upward titration (dose increases of ≤2.4 mcg/d every two to four days to a maximum dose of 19.2 mcg/d) to minimize occurrence of adverse events [12,13]. However, to further improve tolerability, some recommend an even more conservative dose/titration schedule for ziconotide (starting dose of ≤0.5 mcg/d with dose increases of ≤0.5 mcg/d no more often than once weekly until effective analgesia and tolerability is reached) (Table 7) [53,57,78]. Tolerability may also be enhanced by altering the concentration of ziconotide in the pump reservoir or changing the flow rate; however, altering the flow rate may impact dosing of any concomitant IT therapies [57]. In contrast to IT delivery of morphine and other opioids, tolerance does not develop with ziconotide; therefore, dose escalation is typically unnecessary and doses may even be reduced over time, particularly in patients who receive IT ziconotide as the first treatment in their IT pump [10,35,36]. Consistent with this point, in the interim analysis of the PRIZM registry study, the ziconotide dose was 1.6 mcg/d at baseline and 1.5 mcg/d at month 12 in patients who received ziconotide as the first agent in their IT pump.
Table 7.
Dosing and titration schemes for IT ziconotide
| Dosing/Titration Scheme | Summary |
| Continuous dosing per prescribing information [12] |
|
| Low dose/slow titration [53,57,78] |
|
| Night time bolus (flex) dosing [55] |
|
| Patient-controlled analgesia [55] |
|
Portions of this table were adapted with permission from: McDowell GC, Pope JE. Intrathecal ziconotide: Dosing and administration strategies in patients with refractory chronic pain. Neuromodulation 2016;19(5):522–32; via a Creative Commons Attribution-NonCommercial-NoDerivs License [55].
AE = adverse event; IT = intrathecal; PTM = personal therapy manager.
Recent publications describe two novel dosing paradigms designed to improve the safety and efficacy of ziconotide: night time bolus (flex) dosing and patient-controlled administration (Table 7) [55,56]. These dosing strategies take into account preliminary data suggesting that the distribution of ziconotide within the cerebrospinal fluid may be greater with bolus administration than with continuous infusion [79]. Night time–weighted bolus dosing makes use of the flex mode feature of the IT pump to deliver a clinician-programmed bolus dose of ziconotide in the evening (with or without around-the-clock, low-dose continuous ziconotide infusion) [55,80,81]. Successful use of night time bolus dosing was demonstrated in a case series of 16 patients with chronic non–cancer-related pain [56]. In this study, patients who successfully completed a ziconotide bolus trial were initiated on ziconotide using continuous infusion flex dosing at a concentration of 5 mcg/mL or 10 mcg/mL, delivered as a 2-mcg bolus over a 30–45-minute period at night (11 pm). Six months after initiation of therapy, 70% of patients remained on this dosing schedule.
Patient-controlled administration of IT medication is also available for commercial pumps in the United States [81,82]. Preliminary data from a case series (three patients on IT ziconotide monotherapy) support the use of supplemental, patient-controlled, ziconotide bolus dosing (within limits programmed by the clinician) in addition to continuous background infusion [55]. Further support derives from the results obtained from the PRIZM study site with the highest number of enrolled patients. At this location, 12 patients received ziconotide as the first IT agent in pump, with supplemental, patient-controlled dosing enabled for 11 of them [83]. The mean pain reduction (NPRS) score in 10 ziconotide first-in-pump patients assessed at week 12 (the primary study end point) was –35% [83]. It should be noted that prescriber labeling for some devices states that the patient-activated dosing feature should not be used with IT ziconotide [84] and that ziconotide is FDA-approved only for continuous infusion [12]. However, the rationale for patient-administered dosing is supported by the routine use of bolus doses in the trialing of ziconotide without serious safety issues [66,71] and the absence of serious side effects even after massive accidental overdoses of ziconotide [85].
PACC 2016 Guidelines: Highlights of Changes in Recommendations for Morphine and Ziconotide
Morphine and ziconotide are recommended in the 2016 PACC guidelines as firstline IT monotherapies for localized and diffuse pain of cancer-related and non–cancer-related etiologies [9]. However, caution is warranted when considering IT treatment of global pain, as the evidence is less well defined and suggests that such treatment is inadvisable [9]. Off-label IT monotherapy or combination therapy is not recommended until FDA-approved drugs have been tried and failed. Exceptions to this recommendation may be made when the patient has a contraindication to the labeled drug or in special circumstances, such as end-of-life care [9].
Based on clinical evidence regarding efficacy and safety, ziconotide, unless contraindicated, is recommended as the first choice in the treatment of patients with cancer-related pain [39] and as the first IT medication selected for patients with non–cancer-related pain [9]. The recommended starting dose for ziconotide (0.5–1.2 mcg/d) [9] is lower than in the previous PACC guidelines (0.5–2.4 mcg/d) and the prescribing information, which recommends that ziconotide be initiated at no more than 2.4 mcg/d [12]. The 2016 guidelines specifically recommend a trial of ziconotide before using it in the pump (although trialing with ziconotide is considered off label), noting that a single-shot (bolus) trial seems adequate in most instances. In light of the interim PRIZM study results showing that first-in-pump use of ziconotide may confer a therapeutic advantage, the 2016 PACC guidelines note that “first-in-patient trialing of this medication is an attractive option” [66].
Similar to ziconotide, morphine is recommended as a first choice treatment for patients with cancer-related and non–cancer-related pain; however, the level of evidence and recommendation grade are lower for IT opioids in non–cancer-related pain [9]. New to the 2016 PACC guidelines is a discussion of low-dose IT opioid therapy (also referred to as microdosing), for which additional evidence is needed to support recommendations, preferably from high-quality controlled trials [9,66]. For trialing of IT opioids in an outpatient setting with conservative dosing, a shorter duration of post-trial monitoring (six to eight hours, compared with 24 hours in the previous PACC guidelines) is generally recommended; physiochemical properties of the specific medication and individual patient risk factors should be considered in discharge decisions [66].
Conclusions
After its 2016 meeting, the PACC published updated guidelines to enhance patient safety and decrease the risk of complications related to IT therapy for pain relief [9]. These guidelines recommended morphine and ziconotide as firstline monotherapy for cancer-related and non–cancer-related pain [9]. Ziconotide was recommended ahead of morphine (barring a contraindication for ziconotide) in patients with non–cancer-related pain [9]. Ultimately, the choice of first-in-pump therapy should take into consideration patient characteristics and the advantages and disadvantages of each medication. Applying the PACC 2016 guidelines in clinical practice—particularly the use of ziconotide as firstline IT therapy for patients with noncancer pain—may improve the efficacy and safety of IT therapy for patients with chronic pain. The interim analysis data of the PRIZM registry suggest sustained effectiveness when ziconotide is used as the first agent in the pump [10]; however, increased patient numbers and additional analyses of these data will contribute to our knowledge of and comfort in using nonopioid IT analgesics. In addition, data are needed to further understand the benefits and risks associated with the choice of initial IT medication (i.e., morphine or ziconotide) in diverse chronic pain populations.
Authors’ Contributions
Conception and design: TRD, JEP, MCH, GCM; acquisition of data: TRD, JEP, MCH, GCM; analysis and interpretation of data: TRD, JEP, MCH, GCM; drafting the article: TRD, JEP, MCH, GCM; revising it for intellectual content: TRD, JEP, MCH, GCM; final approval of the completed article: TRD, JEP, MCH, GCM.
Supplementary Material
Acknowledgments
The authors thank Jillian Gee, PhD, and Nancy Holland, PhD, of Synchrony Medical Communications, LLC, and Megan Knagge, PhD, of MedErgy for providing medical writing support and Synchrony Medical Communications for editorial assistance in formatting, proofreading, copy editing, and fact checking, which was funded by Jazz Pharmaceuticals in accordance with Good Publication Practice guidelines (http://www.ismpp.org/gpp3).
Jazz Pharmaceuticals also reviewed the manuscript. Although Jazz Pharmaceuticals was involved in the review of the manuscript, the content of this manuscript, the ultimate interpretation, and the decision to submit it for publication in Pain Medicine were made by the authors independently.
Supplementary Data
Supplementary Data may be found online at http://painmedicine.oxfordjournals.org.
Funding sources: Jazz Pharmaceuticals provided funding to Synchrony Medical Communications, LLC, and MedErgy for support in writing and editing this manuscript.
Disclosure: Although Jazz Pharmaceuticals (the sponsor) was involved in the design, collection, analysis, interpretation, and fact-checking of information, the content of the manuscript, the ultimate interpretation, and the decision to submit for publication in Pain Medicine were determined by the authors independently.
Conflicts of interest: TRD is a member of the Polyanalgesic Consensus Conference (PACC) and an investigator for the Patient Registry of Intrathecal Ziconotide Management (PRIZM) study; served as a consultant for Abbot, Axonics Modulation Technologies, Bioness, Inc., Flowonix Medical, Jazz Pharmaceuticals, Nalu, Saluda, SpineThera, Vertiflex Inc., and Vertos Medical, Inc.; served on the speakers’ bureau for Jazz Pharmaceuticals; received meeting travel support from Jazz Pharmaceuticals and Medtronic, Inc.; is a stockholder of Axonics Modulation Technologies, Bioness, Inc., SpineThera, Vertiflex Inc., and Vertos Medical, Inc. JEP is a member of the Polyanalgesic Consensus Conference (PACC); has served as a consultant for Abbott, SpineThera, Vertiflex Inc., Saluda, Jazz Pharmaceuticals, and Flowonix Medical; served on the speakers’ bureau for Jazz Pharmaceuticals. MCH served as a consultant for Medtronic, Inc., and Abbott. GCM is a member of the Polyanalgesic Consensus Conference (PACC) and an investigator for the Patient Registry of Intrathecal Ziconotide Management (PRIZM) study; received research grants from Flowonix Medical, Jazz Pharmaceuticals, Mallinckrodt Pharmaceuticals, and Medtronic, Inc.; served as a consultant for Flowonix Medical, Jazz Pharmaceuticals, and Medtronic, Inc.; served on the speakers’ bureau for Jazz Pharmaceuticals and Medtronic, Inc.
References
- 1. Hayek SM, Hanes MC.. Intrathecal therapy for chronic pain: Current trends and future needs. Curr Pain Headache Rep 2014;18(1):388.. [DOI] [PubMed] [Google Scholar]
- 2. Pope JE, Deer TR.. Intrathecal drug delivery for pain: A clinical guide and future directions. Pain Manag 2015;5(3):175–83. [DOI] [PubMed] [Google Scholar]
- 3. Webster LR. The relationship between the mechanisms of action and safety profiles of intrathecal morphine and ziconotide: A review of the literature. Pain Med 2015;16(7):1265–77. [DOI] [PubMed] [Google Scholar]
- 4. Rudd RA, Seth P, David F, Scholl L.. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep 2016;65(5051):1445–52. [DOI] [PubMed] [Google Scholar]
- 5. Paulozzi LJ, Jones CM, Mack KA, Rudd RA.. Vital signs: Overdoses of prescription opioid pain relievers–United States, 1999-2008. MMWR Morb Mortal Wkly Rep 2011;60:1487–92. [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention. Prescription opioid overdose data. 2016. http://www.cdc.gov/drugoverdose/data/overdose.html (accessed December 2016).
- 7. Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med 2015;372(3):241–8. [DOI] [PubMed] [Google Scholar]
- 8. Zhang Y, Ahmed S, Vo T, et al. Increased pain sensitivity in chronic pain subjects on opioid therapy: A cross-sectional study using quantitative sensory testing. Pain Med 2015;16(5):911–22. [DOI] [PubMed] [Google Scholar]
- 9. Deer TR, Pope JE, Hayek S, et al. The Polyanalgesic Consensus Conference (PACC): Recommendations on intrathecal drug infusion systems best practices and guidelines. Neuromodulation 2017;20(2):96–132. [DOI] [PubMed] [Google Scholar]
- 10. Deer T, Rauck RL, Kim P, et al. Effectiveness and safety of intrathecal ziconotide: Interim analysis of the Patient Registry of Intrathecal Ziconotide Management (PRIZM). Pain Pract 2018;18(2):230–8. [DOI] [PubMed] [Google Scholar]
- 11. Infumorph 200, Infumorph 500 (Preservative-Free Morphine Sulfate Sterile Solution) [Package Insert]. Eatontown, NJ: West-Ward Pharmaceuticals; 2016. [Google Scholar]
- 12. Prialt (Ziconotide) Solution, Intrathecal Infusion [Package Insert]. Palo Alto, CA: Jazz Pharmaceuticals, Inc; 2013. [Google Scholar]
- 13. Pope JE, Deer TR, Bruel BM, Falowski S.. Clinical uses of intrathecal therapy and its placement in the pain care algorithm. Pain Pract 2016;16(8):1092–106. [DOI] [PubMed] [Google Scholar]
- 14. Bruel BM, Burton AW.. Intrathecal therapy for cancer-related pain. Pain Med 2016;17(12):2404–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Penn RD, Paice JA.. Chronic intrathecal morphine for intractable pain. J Neurosurg 1987;67(2):182–6. [DOI] [PubMed] [Google Scholar]
- 16. Onofrio BM, Yaksh TL.. Long-term pain relief produced by intrathecal morphine infusion in 53 patients. J Neurosurg 1990;72(2):200–9. [DOI] [PubMed] [Google Scholar]
- 17. Follett KA, Hitchon PW, Piper J, et al. Response of intractable pain to continuous intrathecal morphine: A retrospective study. Pain 1992;49(1):21–5. [DOI] [PubMed] [Google Scholar]
- 18. Tutak U, Doleys DM.. Intrathecal infusion systems for treatment of chronic low back and leg pain of noncancer origin. South Med J 1996;89(3):295–300. [DOI] [PubMed] [Google Scholar]
- 19. Winkelmüller M, Winkelmüller W.. Long-term effects of continuous intrathecal opioid treatment in chronic pain of nonmalignant etiology. J Neurosurg 1996;85(3):458–67. [DOI] [PubMed] [Google Scholar]
- 20. Anderson VC, Burchiel KJ.. A prospective study of long-term intrathecal morphine in the management of chronic nonmalignant pain. Neurosurgery 1999;44(2):289–300. [DOI] [PubMed] [Google Scholar]
- 21. Kumar K, Kelly M, Pirlot T.. Continuous intrathecal morphine treatment for chronic pain of nonmalignant etiology: Long-term benefits and efficacy. Surg Neurol 2001;55(2):79–86. [DOI] [PubMed] [Google Scholar]
- 22. Rauck RL, Cherry D, Boyer MF, et al. Long-term intrathecal opioid therapy with a patient-activated, implanted delivery system for the treatment of refractory cancer pain. J Pain 2003;4(8):441–7. [DOI] [PubMed] [Google Scholar]
- 23. Becker G, Galandi D, Blum HE.. Peripherally acting opioid antagonists in the treatment of opiate-related constipation: A systematic review. J Pain Symptom Manage 2007;34(5):547–65. [DOI] [PubMed] [Google Scholar]
- 24. Saltari MR, Shaladi A, Piva B, et al. The management of pain from collapse of osteoporotic vertebrae with continuous intrathecal morphine infusion. Neuromodulation 2007;10(2):167–76. [DOI] [PubMed] [Google Scholar]
- 25. Duse G, Davià G, White PF.. Improvement in psychosocial outcomes in chronic pain patients receiving intrathecal morphine infusions. Anesth Analg 2009;109(6):1981–6. [DOI] [PubMed] [Google Scholar]
- 26. Rauck R, Deer T, Rosen S, et al. Accuracy and efficacy of intrathecal administration of morphine sulfate for treatment of intractable pain using the Prometra® Programmable Pump. Neuromodulation 2010;13(2):102–8. [DOI] [PubMed] [Google Scholar]
- 27. Lara NA Jr, Teixeira MJ, Fonoff ET.. Long term intrathecal infusion of opiates for treatment of failed back surgery syndrome. Acta Neurochir Suppl 2011;108:41–7. [DOI] [PubMed] [Google Scholar]
- 28. Kim JH, Jung JY, Cho MS.. Continuous intrathecal morphine administration for cancer pain management using an intrathecal catheter connected to a subcutaneous injection port: A retrospective analysis of 22 terminal cancer patients in Korean population. Korean J Pain 2013;26(1):32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smith TJ, Staats PS, Deer T, et al. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: Impact on pain, drug-related toxicity, and survival. J Clin Oncol 2002;20(19):4040–9. [DOI] [PubMed] [Google Scholar]
- 30. Raphael JH, Duarte RV, Southall JL, Nightingale P, Kitas GD.. Randomised, double-blind controlled trial by dose reduction of implanted intrathecal morphine delivery in chronic non-cancer pain. BMJ Open 2013;3(7):e003061.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pope JE, Deer TR, Amirdelfan K, McRoberts WP, Azeem N.. The pharmacology of spinal opioids and ziconotide for the treatment of non-cancer pain. Curr Neuropharmacol 2017;15(2):206–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Staats PS, Yearwood T, Charapata SG, et al. Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: A randomized controlled trial. JAMA 2004;291(1):63–70. [DOI] [PubMed] [Google Scholar]
- 33. Wallace MS, Charapata SG, Fisher R, et al. Intrathecal ziconotide in the treatment of chronic nonmalignant pain: A randomized, double-blind, placebo-controlled clinical trial. Neuromodulation 2006;9(2):75–86. [DOI] [PubMed] [Google Scholar]
- 34. Rauck RL, Wallace MS, Leong MS, et al. A randomized, double-blind, placebo-controlled study of intrathecal ziconotide in adults with severe chronic pain. J Pain Symptom Manage 2006;31(5):393–406. [DOI] [PubMed] [Google Scholar]
- 35. Ellis DJ, Dissanayake S, McGuire D, et al. Continuous intrathecal infusion of ziconotide for treatment of chronic malignant and nonmalignant pain over 12 months: A prospective, open-label study. Neuromodulation 2008;11(1):40–9. [DOI] [PubMed] [Google Scholar]
- 36. Webster LR, Fisher R, Charapata S, Wallace MS.. Long-term intrathecal ziconotide for chronic pain: An open-label study. J Pain Symptom Manage 2009;37(3):363–72. [DOI] [PubMed] [Google Scholar]
- 37. Raffaeli W, Sarti D, Demartini L, Sotgiu A, Bonezzi C.. Italian registry on long-term intrathecal ziconotide treatment. Pain Physician 2011;14(1):15–24. [PubMed] [Google Scholar]
- 38. Prusik J, Argoff C, Peng S, Pilitsis JG.. Use of low dose ziconotide as first-line intrathecal monotherapy. Neuromodulation 2017;20(4):386–91. [DOI] [PubMed] [Google Scholar]
- 39. Deer TR, Pope JE, Hayek S, et al. The Polyanalgesic Consensus Conference (PACC): Recommendations for intrathecal drug delivery: Guidance for improving safety and mitigating risks. Neuromodulation 2017;20(2):155–76. [DOI] [PubMed] [Google Scholar]
- 40. Harris RP, Helfand M, Woolf SH, et al. Current methods of the US Preventive Services Task Force: A review of the process. Am J Prev Med 2001;20(3 Suppl):21–35. [DOI] [PubMed] [Google Scholar]
- 41. Deer T, Winkelmüller W, Erdine S, Bedder M, Burchiel K.. Intrathecal therapy for cancer and nonmalignant pain: Patient selection and patient management. Neuromodulation 2002;2(2):55–66. [DOI] [PubMed] [Google Scholar]
- 42. Bottros MM, Christo PJ.. Current perspectives on intrathecal drug delivery. J Pain Res 2014;7:615–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Staats PS. Complications of intrathecal therapy. Pain Med 2008;9(Suppl 1):S102–7. [Google Scholar]
- 44. Naumann C, Erdine S, Koulousakis A, Buyten J-P, Schuchard M.. Drug adverse events and system complications of intrathecal opioid delivery for pain: Origins, detection, manifestations, and management. Neuromodulation 2002;2(2):92–107. [DOI] [PubMed] [Google Scholar]
- 45. Gogia V, Chaudhary P, Ahmed A, et al. Intrathecal morphine pump for neuropathic cancer pain: A case report. Am J Hosp Palliat Med 2012;29(5):409–11. [DOI] [PubMed] [Google Scholar]
- 46. Saulino M, Kim PS, Shaw E.. Practical considerations and patient selection for intrathecal drug delivery in the management of chronic pain. J Pain Res 2014;7:627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Deer TR, Smith HS, Burton AW, et al. Comprehensive consensus based guidelines on intrathecal drug delivery systems in the treatment of pain caused by cancer pain. Pain Physician 2011;14:E283–312. [PubMed] [Google Scholar]
- 48. Ruan X. Drug-related side effects of long-term intrathecal morphine therapy. Pain Physician 2007;10(2):357–65. [PubMed] [Google Scholar]
- 49. McNicol E, Horowicz-Mehler N, Fisk RA, et al. Management of opioid side effects in cancer-related and chronic noncancer pain: A systematic review. J Pain 2003;4(5):231–56. [DOI] [PubMed] [Google Scholar]
- 50. Deer T, Chapple I, Classen A, et al. Intrathecal drug delivery for treatment of chronic low back pain: Report from the National Outcomes Registry for Low Back Pain. Pain Med 2004;5(1):6–13. [DOI] [PubMed] [Google Scholar]
- 51. Duarte RV, Raphael JH, Southall JL, Baker C, Hanu-Cernat D.. Intrathecal inflammatory masses: Is the yearly opioid dose increase an early indicator? Neuromodulation 2010;13(2):109–13. [DOI] [PubMed] [Google Scholar]
- 52. Yaksh TL, Horais KA, Tozier NA, et al. Chronically infused intrathecal morphine in dogs. Anesthesiology 2003;99(1):174–87. [DOI] [PubMed] [Google Scholar]
- 53. Prager J, Deer T, Levy R, et al. Best practices for intrathecal drug delivery for pain. Neuromodulation 2014;17(4):354–72. [DOI] [PubMed] [Google Scholar]
- 54. Hayek SM, Veizi IE, Narouze SN, Mekhail N.. Age-dependent intrathecal opioid escalation in chronic noncancer pain patients. Pain Med 2011;12(8):1179–89. [DOI] [PubMed] [Google Scholar]
- 55. McDowell GC, Pope JE.. Intrathecal ziconotide: Dosing and administration strategies in patients with refractory chronic pain. Neuromodulation 2016;19(5):522–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pope JE, Deer TR.. Intrathecal pharmacology update: Novel dosing strategy for intrathecal monotherapy ziconotide on efficacy and sustainability. Neuromodulation 2015;18(5):414–20. [DOI] [PubMed] [Google Scholar]
- 57. Mitchell AA, Sapienza-Crawford AJ, Hanley KL, et al. Administering ziconotide and monitoring patients treated with ziconotide: Expert opinions. Pain Manag Nurs 2013;14(3):e84–94. [DOI] [PubMed] [Google Scholar]
- 58. Horazeck C, Huh AS, Huh BK.. Acute rhabdomyolysis in a patient with long-term exposure to intrathecal ziconotide: A case report. Pain Pract 2015;15(3):E34–9. [DOI] [PubMed] [Google Scholar]
- 59. Alicino I, Giglio M, Manca F, Bruno F, Puntillo F.. Intrathecal combination of ziconotide and morphine for refractory cancer pain: A rapidly acting and effective choice. Pain 2012;153(1):245–9. [DOI] [PubMed] [Google Scholar]
- 60. Grider JS, Harned ME, Etscheidt MA.. Patient selection and outcomes using a low-dose intrathecal opioid trialing method for chronic nonmalignant pain. Pain Physician 2011;14:343–51. [PubMed] [Google Scholar]
- 61. Grider JS, Etscheidt MA, Harned ME, et al. Trialing and maintenance dosing using a low-dose intrathecal opioid method for chronic nonmalignant pain: A prospective 36-month study. Neuromodulation 2016;19(2):206–19. [DOI] [PubMed] [Google Scholar]
- 62. Coffey RJ, Owens ML, Broste SK, et al. Mortality associated with implantation and management of intrathecal opioid drug infusion systems to treat noncancer pain. Anesthesiology 2009;111(4):881–91. [DOI] [PubMed] [Google Scholar]
- 63. Abs R, Verhelst J, Maeyaert J, et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab 2000;85(6):2215–22. [DOI] [PubMed] [Google Scholar]
- 64. Buntin-Mushock C, Phillip L, Moriyama K, Palmer PP.. Age-dependent opioid escalation in chronic pain patients. Anesth Analg 2005;100(6):1740–5. [DOI] [PubMed] [Google Scholar]
- 65. Deer TR, Prager J, Levy R, et al. Polyanalgesic Consensus Conference–2012: Recommendations on trialing for intrathecal (intraspinal) drug delivery: Report of an interdisciplinary expert panel. Neuromodulation 2012;15(5):420–35. [DOI] [PubMed] [Google Scholar]
- 66. Deer TR, Hayek S, Pope JE, et al. The Polyanalgesic Consensus Conference (PACC): Recommendations for trialing of intrathecal drug delivery infusion therapy. Neuromodulation 2017;20(2):133–54. [DOI] [PubMed] [Google Scholar]
- 67. Paice JA, Penn RD, Shott S.. Intraspinal morphine for chronic pain: A retrospective, multicenter study. J Pain Symptom Manage 1996;11(2):71–80. [DOI] [PubMed] [Google Scholar]
- 68. Anderson VC, Burchiel KJ, Cooke B.. A prospective, randomized trial of intrathecal injection vs. epidural infusion in the selection of patients for continuous intrathecal opioid therapy. Neuromodulation 2003;6(3):142–52. [DOI] [PubMed] [Google Scholar]
- 69. Prager JP. Neuraxial medication delivery: The development and maturity of a concept for treating chronic pain of spinal origin. Spine (Phila Pa 1976) 2002;27:2593–605. [DOI] [PubMed] [Google Scholar]
- 70. Ahmed SU, Martin NM, Chang Y.. Patient selection and trial methods for intraspinal drug delivery for chronic pain: A national survey. Neuromodulation 2005;8(2):112–20. [DOI] [PubMed] [Google Scholar]
- 71. Mohammed SI, Eldabe S, Simpson KH, et al. Bolus intrathecal injection of ziconotide (Prialt®) to evaluate the option of continuous administration via an implanted intrathecal drug delivery (ITDD) system: A pilot study. Neuromodulation 2013;16(6):576–81. [DOI] [PubMed] [Google Scholar]
- 72. Maeyaert J, Buchser E, Van Buyten JP, Rainov NG, Becker R.. Patient-controlled analgesia in intrathecal therapy for chronic pain: Safety and effective operation of the Model 8831 Personal Therapy Manager with a pre-implanted SynchroMed Infusion System. Neuromodulation 2003;6(3):133–41. [DOI] [PubMed] [Google Scholar]
- 73. Ilias W, le Polain B, Buchser E, Demartini L.. Patient-controlled analgesia in chronic pain patients: Experience with a new device designed to be used with implanted programmable pumps. Pain Pract 2008;8(3):164–70. [DOI] [PubMed] [Google Scholar]
- 74. Brogan SE, Winter NB, Okifuji A.. Prospective observational study of patient-controlled intrathecal analgesia: Impact on cancer-associated symptoms, breakthrough pain control, and patient satisfaction. Reg Anesth Pain Med 2015;40(4):369–75. [DOI] [PubMed] [Google Scholar]
- 75. McDowell GC. Use of the personal therapy manager with Prialt® (ziconotide intrathecal infusion) for patient-controlled analgesia: Case series. Presented at: 14th Annual Meeting of the North American Neuromodulation Society; December 2–5, 2010; Las Vegas, NV. http://www.slideserve.com/uri/use-of-the-personal-therapy-manager-with-prialt-ziconotide-intrathecal-infusion-for-patient-controlled-analgesia-case (accessed June 29, 2018).
- 76. Bolash RB, Niazi T, Kumari M, Azer G, Mekhail N.. Efficacy of a targeted drug delivery on-demand bolus option for chronic pain. Pain Pract 2018;18(3):305.. [DOI] [PubMed] [Google Scholar]
- 77. Hamza M, Doleys D, Wells M, et al. Prospective study of 3-year follow-up of low-dose intrathecal opioids in the management of chronic nonmalignant pain. Pain Med 2012;13(10):1304–13. [DOI] [PubMed] [Google Scholar]
- 78. Fisher R, Hassenbusch S, Krames E, et al. A consensus statement regarding the present suggested titration for prialt (ziconotide). Neuromodulation 2005;8(3):153–4. [DOI] [PubMed] [Google Scholar]
- 79. Flack SH, Bernards CM.. Cerebrospinal fluid and spinal cord distribution of hyperbaric bupivacaine and baclofen during slow intrathecal infusion in pigs. Anesthesiology 2010;112(1):165–73. [DOI] [PubMed] [Google Scholar]
- 80. Medtronic Inc. N’Vision® Clinician Programmer with Software [Programmer Guide]. Minneapolis, MN: Medtronic Inc; 2016. http://manuals.medtronic.com/wcm/groups/mdtcom_sg/@emanuals/@era/@neuro/documents/documents/contrib_240051.pdf (accessed May 2017). [Google Scholar]
- 81. Prometra® II Programmable Pump for Use with Intrathecal Catheter [Package Insert]. Mount Olive, NJ: Flowonix Medical Inc; 2012. [Google Scholar]
- 82. Medtronic Inc. Personal Therapy Manager [Patient Manual]. Minneapolis, MN: Medtronic Inc; 2007. [Google Scholar]
- 83. McDowell G, Saulino MF, Rauck RL, et al. Effectiveness and safety of intrathecal ziconotide: Interim analysis results from a single center of the Patient Registry of Intrathecal Ziconotide Management. Presented at: International Conference on Neurology and Brain Disorders; June 26–28, 2018; Valencia, Spain.
- 84. SynchroMed® Programmable Infusion Systems [Clinical Reference Guide]. Minneapolis, MN: Medtronic Inc; 2013. [Google Scholar]
- 85. Charapata SG, Ellis D.. Unintentional overdose with intrathecal ziconotide. Pain Med 2002;3(2):189–90. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.