Abstract
Background:
To predict the presence of asthma in adult patients with respiratory symptoms, we developed a scoring algorithm using clinical parameters.
Methods:
We prospectively analysed 566 adult outpatients who visited Kinki University Hospital for the first time with complaints of nonspecific respiratory symptoms. Asthma was comprehensively diagnosed by specialists using symptoms, signs, and objective tools including bronchodilator reversibility and/or the assessment of bronchial hyperresponsiveness (BHR). Multiple logistic regression analysis was performed to categorise patients and determine the accuracy of diagnosing asthma.
Results:
A scoring algorithm using the symptom-sign score was developed, based on diurnal variation of symptoms (1 point), recurrent episodes (2 points), medical history of allergic diseases (1 point), and wheeze sound (2 points). A score of ≥3 had 35% sensitivity and 97% specificity for discriminating between patients with and without asthma and assigned a high probability of having asthma (accuracy 90%). A score of 1 or 2 points assigned intermediate probability (accuracy 68%). After providing additional data of forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) ratio <0.7, the post-test probability of having asthma was increased to 93%. A score of 0 points assigned low probability (accuracy 31%). After providing additional data of positive reversibility, the post-test probability of having asthma was increased to 88%.
Conclusions:
This pragmatic diagnostic algorithm is useful for predicting the presence of adult asthma and for determining the appropriate time for consultation with a pulmonologist.
Keywords: algorithm, asthma, diagnosis, scoring
Introduction
Asthma is a clinical syndrome characterised by episodes of wheezing at night and/or in the early morning. Global asthma guidelines specify that asthma diagnoses should be based on both symptoms and objective physiological evidence of variable airflow obstruction, bronchial hyperresponsiveness (BHR) and/or airway inflammation.1–3 However, recent studies have shown that many of the diagnoses of asthma in primary care rely solely on clinical evaluation and/or response to treatment without any objective examinations.4 Less than half of patients with physician-diagnosed asthma do not have asthma when assessed using objective examinations.5,6
General physicians can mistakenly make the diagnosis of asthma if they only use a suspected symptom such as wheeze, breathlessness, chest tightness, and cough because the symptom often mimics other conditions. International guidelines state that symptom patterns as key indicators including ‘recurrent/episodic’, ‘occur/worsen at night or early in the morning’, ‘occur/worsen upon exposure to allergens or irritants’, and ‘respond to appropriate asthma therapy’ are useful for suggesting asthma.2
The 2012 BTS/SIGN guideline recommends an integrated approach to diagnosis using the probability of having asthma by focusing on the patient's presenting symptoms and signs.3 In this paper we identified useful indicators and constructed a scoring algorithm to be combined with these symptoms and signs, and then assessed the pre-test probability of predicting the presence of adult asthma. We then assessed the post-test probability by combining the pre-test probability and positive likelihood ratio (LR+) of objective examinations, and propose an integer scoring algorithm for predicting the presence of adult asthma.
Methods
Participants
This prospective study was performed on adult outpatients who presented to our university hospital clinics during the period from January 2008 to September 2011 with non-specific respiratory symptoms including wheeze, shortness of breath, and cough. During the study period 4,129 patients seen by pulmonologists at our hospital were referred for enrolment in the study (Figure 1). Eligible patients aged 18–88 years were ultimately divided into two groups, asthma and non-asthma. The diagnosis of asthma was ascertained according to the physician decision based on a relevant symptom history (present in all patients) and then established by a positive finding for reversibility with a bronchodilator and/or a positive test for BHR. These were defined as: an increase in forced expiratory volume in 1 second (FEV1) both >200ml and 12% above the pre-bronchodilator FEV17 and provocative challenge concentration of methacholine resulting in a 20% fall (PC20) in FEV1 of <8mg/mL.8 The study was approved by our institutional ethics committee and all subjects provided informed consent.
Figure 1.
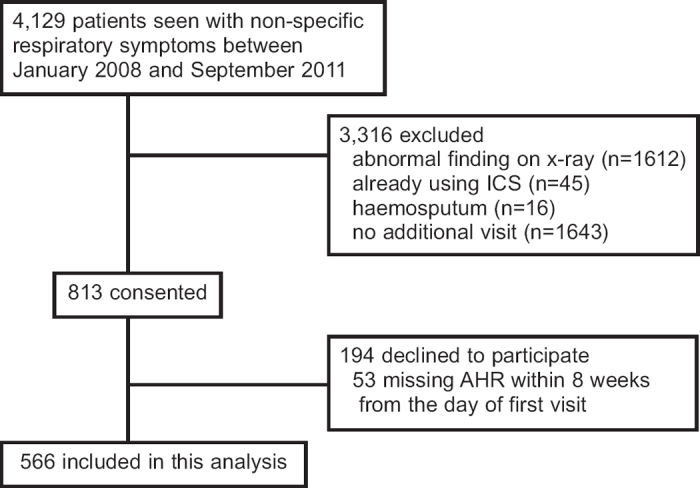
Study flow. AHR=airway hyperresponsiveness, ICS=inhaled corticosteroids
Exclusion criteria
Exclusion criteria included the following: (1) pregnant and breastfeeding; (2) current diagnosis of pneumonia, pneumothorax, atelectasis, pulmonary fibrotic disease, chronic bronchitis, or any other lower respiratory abnormalities; (3) current use of systemic or inhaled corticosteroids (ICS), β-blockers, or angiotensin converting enzyme inhibitors; (4) symptoms of chest pain or haemosputum; or (5) abnormal findings on chest x-ray.
Procedure
At the first visit patients who met the inclusion criteria completed a routine interview and underwent a general physical examination in addition to the Asthma Screening Questionnaire (ASQ).9 Lung function tests were performed and the fraction of exhaled nitric oxide (FeNO) was measured in a manner that was blinded with respect to the interview and the questionnaire. Blood samples were obtained from each patient independent of the interview and the questionnaire. Each pulmonologist made the diagnosis based on this information and prescribed medications accordingly. If the pulmonologist diagnosed the patient as having asthma or suspected that he/she had asthma at visit 1, treatment with ICS was started before the methacholine challenge test (MCT) was completed. At a second visit (within 8 weeks), all eligible patients underwent the MCT.
Data sources
Symptoms and signs
The patients were required to undergo a routine interview as well as five additional questions as follows: “Have you ever had any experiences of wheezing?”; “Did your symptoms occur in the early morning or at night (diurnal variation)?”; “Have you had similar episodes of respiratory symptoms (recurrent episodes)?”; “Have you had any medical history of allergic diseases such as asthma, atopic dermatitis, and allergic rhinitis?”; and “Do you have any close relatives with allergic diseases?”. All these findings were recorded in the computer. The patients subsequently underwent a physical examination and the finding of ‘wheeze sound’ was especially recorded.
Blood samples
Blood was collected for measurement of total serum IgE and complete blood count at visit 1. Total and specific IgE were measured with a third-generation chemiluminescent enzyme immunoassay (IMMULITE 2000; Siemens Medical, Deerfield, Illinois, USA). Atopy was defined as any specific IgE to common allergen ≥0.35kU/L.
Pulmonary function tests
At visit 1, spirometry was performed using a standard spirometer (Minato, Tokyo) according to the ATS guidelines.10 The results were expressed as the best FEV1. Reversibility of the reduction in FEV1 was measured 10 min after administration of 20μg inhaled procaterol.
Fraction of exhaled nitric oxide (FeNO)
At visit 1, FeNO was measured online by chemiluminescence at a constant expiratory flow rate (50mL/s) before any forced expiratory manoeuvres, consistent with published guidelines.11 All readings were obtained by technical staff.
Methacholine challenge test
At the second visit (within 8 weeks) the MCT was performed using the five-breath dosimeter method, in accordance with the ATS guidelines.12 The test was administered at least 8 hrs after the last dose of short-acting bronchodilators and 24 hrs after ICS and/or long-acting bronchodilators.
Definition of non-asthma
Asthma, chronic obstructive pulmonary disease (COPD), and non-asthmatic eosinophilic bronchitis often manifest with an overlapping clinical picture. When the post-bronchodilator FEV1/FVC ratio was <0.7, computed tomography and measurement of diffusing capacity for carbon monoxide adjusted by the alveolar volume (DLCO/VA) were also performed. Emphysema can be diagnosed by macroscopic emphysema using computed tomography or DLCO/VA adjusted for haemoglobin less than the lower limit of normal.13
After differentiating from other diseases due to cough, eosinophilic bronchitis was judged as chronic cough in patients with no objective evidence of variable airflow obstruction, normal BHR, and FeNO ≥25ppb instead of sputum eosinophilia.14 Allergic rhinitis and Sjógren's syndrome were ultimately diagnosed by specialists.
Statistical analysis
Data were abstracted from the patients' hospital files and computer records and the clinical and laboratory parameters of those who fulfilled the diagnostic criteria for adult asthma were compared. In order to analyse the binary data, a cut-off level was set using receiver operating characteristic (ROC) curves. Differences of continuous variables between the groups were analysed by the Mann-Whitney U test; for categorical variables the χ2 test (or Fisher's exact test for small proportions) was used.
Logistic regression analysis was performed to investigate the independent effects of the significant discriminating variables between the groups. Statistical significance was assessed using the Wald test in the multivariate analysis. Each point was assigned by the regression coefficient, which was obtained from the result of logistic regression analysis. The score was then constructed by the simple arithmetic sum of the points. All analyses were performed using SPSS software version 19.0 (SPSS Inc, Chicago, Illinois, USA).
We next calculated the pre-test probability of constructed symptom-sign scores for predicting the presence of adult asthma. According to Bayes' theorem, the post-test odds that a patient has a disease is obtained by multiplying the pre-test odds by the likelihood ratio (LR) of the test.15 In order to limit the number of possible test combinations, we chose a LR point estimate threshold of 2.0 due to the fact that LRs ≥2.0 reflect small but sometimes important changes in post-test probability.16 The product of pre-test odds and the LR is the post-test odds, which is then converted into post-test probability [probability = odds/(1+ odds)].
Results
During the study period 4,129 patients with non-specific respiratory symptoms who were seen by pulmonologists at our hospital were referred for enrolment in the study (Figure 1). We excluded those with abnormal x-ray findings and those with other causes. Of these, 813 consented to participate in the study. We excluded those who did not complete the MCT, leaving 566 subjects for inclusion in the analysis. Among the 566 eligible patients who presented at our hospital with non-specific respiratory symptoms, 367 and 199 were diagnosed with asthma and non-asthma, respectively. There were 345 women and 221 men with a median age of 52 years (range 18–88). Asthma was comprehensively diagnosed not only by relevant symptoms and signs but also by bronchodilator reversibility and/or BHR. Table 1 summarises the demographic and clinical features of the study population. Patients with asthma were younger, there was a higher proportion of females, and they had a greater prevalence of some typical indicators including symptoms and signs, findings of blood samples, and respiratory function than the non-asthma group.
Table 1. Clinical characteristics of adult asthma and non-asthma.
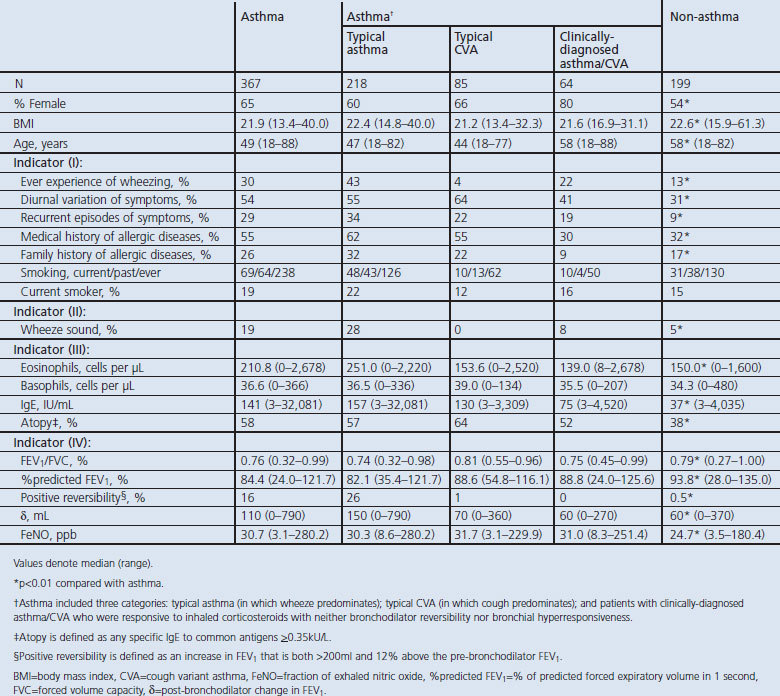
Asthma included three categories: typical asthma (in which wheeze predominated, n=217), typical cough variant asthma (CVA, in which cough predominated, n=85); and patients with clinically-diagnosed asthma/CVA (n=64) who were responsive to ICS with neither bronchodilator reversibility nor BHR.
Of the eligible patients, 34% were deemed not to have asthma. The final diagnosis for these patients was as follows: eosinophilic bronchitis (n=45), COPD (n=20), upper airway cough syndrome (post-nasal drip syndrome) (n=26), gastro-oesophageal reflux disease-related cough (n=17), post-infectious cough (n=34), bronchiectasis (n=3), cigarette-associated cough (n=3), irritable larynx (n=3), heart failure (n=4), idiopathic cough (n=8), non-specific intestinal pneumonia (n=2), old tuberculosis (n=1), non-tuberculous mycobacteria (n=1), rheumatoid arthritis-associated lung disease (n=1), pulmonary embolism (n=1), and chronic hypersensitive pneumonia (n=1).
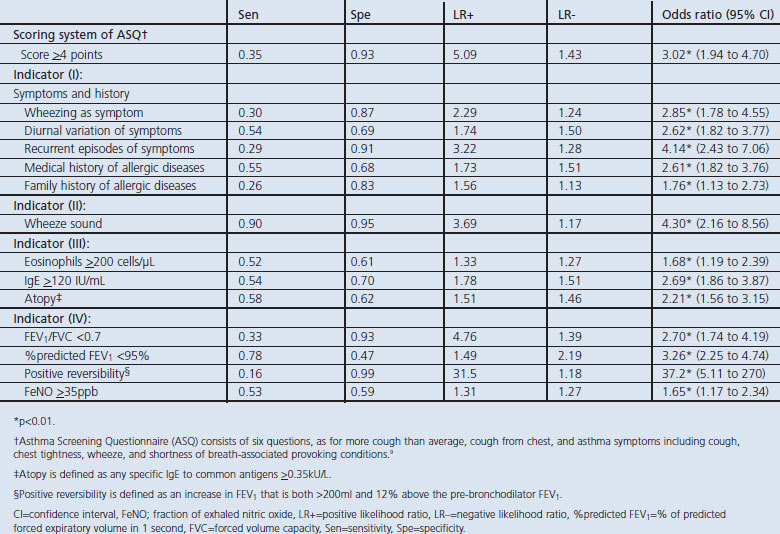
Each of the indicators was evaluated with respect to the obtained sensitivity, specificity, LR+ and LR− (Table 2). In order to obtain these measures, a cut-off level had to be selected based on ROC analysis. In Table 2 almost all the indicators had lower values of LR+ (<5.0) except for the ASQ score (LR+ 5.09). While only bronchodilator reversibility showed an LR+ value of 31.5, it was determined to be the most useful indicator for predicting the presence of asthma. Table 2 also shows the results of univariate logistic regression models to distinguish between patients with and without asthma. Some items had significant ability to discriminate between asthma and non-asthma patients. Objective examinations, including positive bronchodilator reversibility, were able to distinguish patients with asthma from non-asthma patients.
Table 2. Sensitivity, specificity and likelihood rates of each indicator for predicting the presence of adult asthma and univariate logistic regression models to distinguish patients with asthma from non-asthma.
Next, multivariable logistic regression models were used to determine the scoring system that was constructed from the symptoms and signs (Table 3). All patients with non-specific respiratory symptoms were assigned the symptom-sign score for four variables of symptoms and signs that remained statistically significant in the multivariate logistic regression model. As the differences in regression coefficients ranged from 0.17 to 1.32, for the sake of simplicity 1 point was assigned for diurnal variation of symptoms (odds ratio (OR) 2.47) and a medical history of allergic diseases (OR 2.39) and 2 points were assigned for recurrent episodes of symptoms (OR 3.72) and wheeze sound (OR 3.68) (Table 3). The symptom-sign score, which was calculated by adding up these points, ranged from 0 to 6.
Table 3. Multivariate logistic regression model for developing the symptom-sign score.
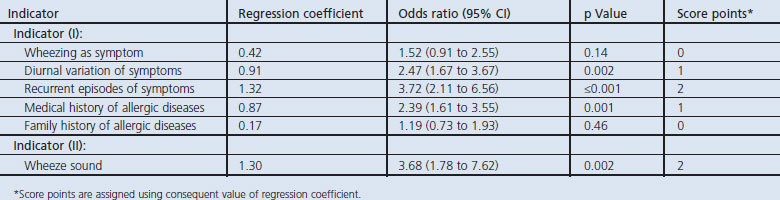
Table 4 indicates the three levels of probability that were classified using the symptom-sign score. When any one of the four indicators (diurnal variation of symptoms, recurrent episodes of symptoms, medical history of allergy diseases, or wheezing sound) was fulfilled, the pre-test probability of asthma was 75% compared with a probability of 41% in patients who did not exhibit any of these indicators (LR+ 1.41). When a symptom-sign score of ≥3 points was assigned, the probability of the accuracy for having asthma was 89% with 12.6 of LR+ (Figure 2). On the other hand, when an ACQ score of ≥4 points was assigned, the probability of the accuracy for diagnosing asthma was 81% with 5.09 of LR+.
Table 4. Pre-test probability of asthma.
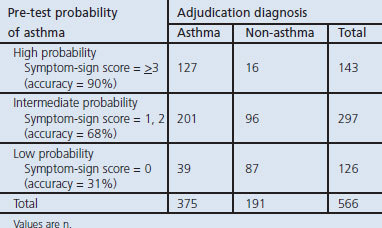
Figure 2.
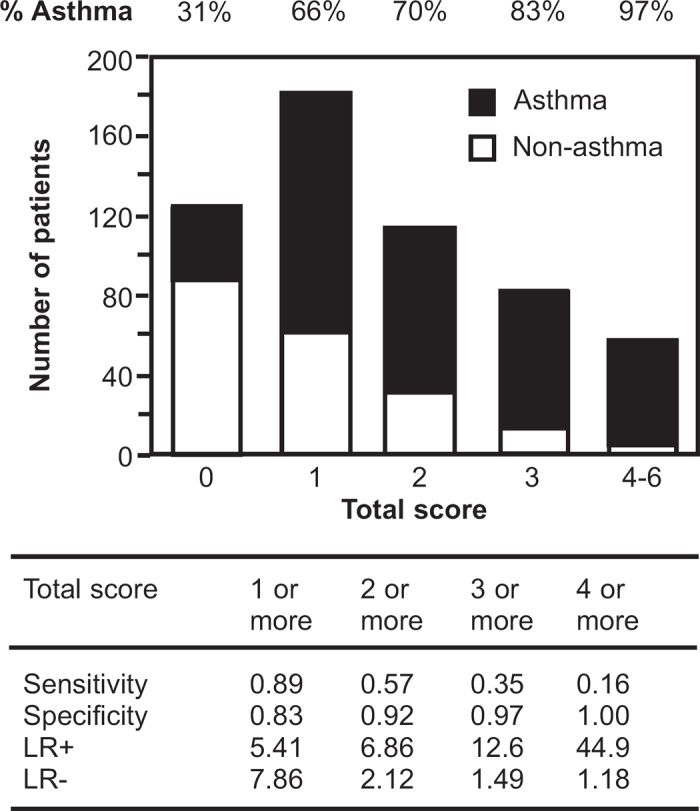
Observed numbers of asthma (closed column) and non-asthma patients (open column) in relation to the symptom-sign score (based on diurnal variation of symptoms, recurrent episodes of symptoms, medical history with allergic diseases, and wheeze sound) in 566 patients who complained of non-specific respiratory symptoms. LR+=positive likelihood ratio, LR−=negative likelihood ratio
According to Bayes' theorem, the post-test probability was calculated (Table 5) and a scoring algorithm was developed (Figure 3) based on the multivariable logistic regression model. In the 143 patients who had a high probability based on the symptom-sign score, the additional information of FEV1/FVC (LR+ 11.4), serum IgE levels (LR+ 3.2), and bronchodilator reversibility (LR+ 3.1) increased the post-test probability to 99%, 96%, and 96%, respectively. In the 297 patients who had intermediate probability in the symptom-sign score, adding the information regarding bronchodilator reversibility (LR+ 12.4), FEV1/FVC (LR+ 6.0), and FeNO levels (LR+ 2.2) increased the post-test probability to 96%, 93%, and 82%, respectively. In the 126 patients who had a low probability based on the symptom-sign score, the additional information of bronchodilator reversibility (LR+ 15.6), FEV1/FVC (LR+ 3.0), and FeNO levels (LR+ 2.6) increased the post-test probability to 88%, 57%, and 54%, respectively.
Table 5. Diagnostic algorithms for adult asthma.
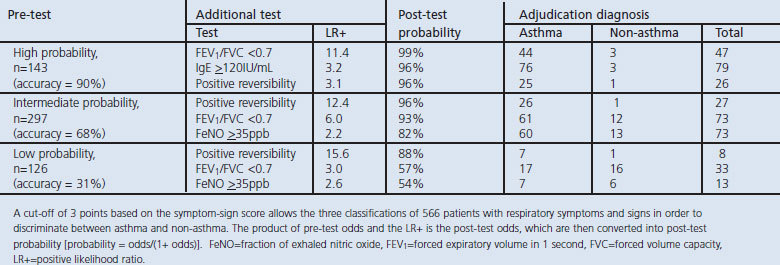
Figure 3.
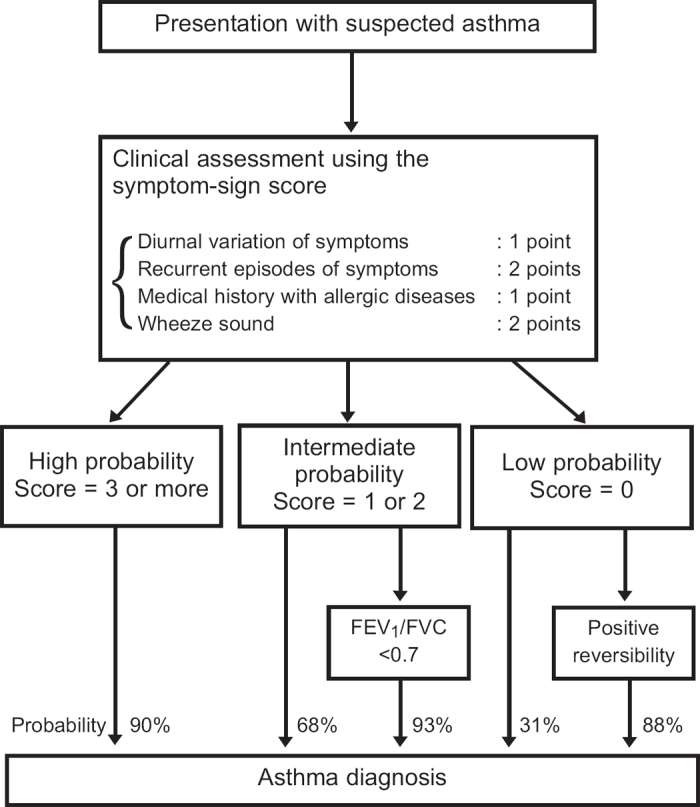
Flow diagram showing the diagnostic process in adults in relation to the initial clinical assessment using the symptom-sign score and probability of asthma. FEV1=forced expiratory volume in 1 second, FVC=forced vital capacity
Discussion
Main findings
A new integer scoring algorithm based on the clinical parameters of the symptom-sign score was developed which may help to predict the presence of asthma in patients with non-specific respiratory symptoms. This study was intended to aid in the early stage diagnosis of adult asthma at the clinician level.
In this study, an integer scoring algorithm was defined based on the regression coefficient in the final multivariable logistic regression model. The probability of post-tests was then calculated by LR+ of objective tests, including lung function tests, using Bayes' theorem. The Bayesian skills might be acquired through clinical experience — that is, through regular exposure to the interpretation of diagnostic tests.17,18 The 2012 BTS/SIGN guideline recommended that it should be possible to determine the probability of a diagnosis of asthma based on a clinical assessment.3 To our knowledge, this is the first report to respond to this requirement using statistical analysis. An integrated approach was adopted previously by the International Primary Care Respiratory Group.19,20 The guideline therefore encourages clinicians to use their knowledge and deductive skills to determine the probability of someone having asthma when they present with symptoms and signs.3 In this study, our symptom-sign score was able to classify three levels of probability of having asthma and to discriminate asthma from non-asthma in adult patients with non-specific respiratory symptoms.
Interpretation of findings in relation to previously published work
The most commonly used indicators in the diagnosis of asthma are symptom data — specifically wheeze, breathlessness, chest tightness, and cough.21 Several studies have validated asthma questionnaires against physicians' diagnoses of asthma.9,22,23 Shin and colleagues showed that the ASQ score was a useful tool for diagnosing asthma.9 The ASQ score has certainly proved useful, but it is of limited use in screening patients to discriminate those with asthma from healthy controls. When the ASQ score was used to diagnose adult asthma in our real-world clinical situation, the accuracy of the ASQ score was limited with a low LR+ (5.09) and a probability of 81% for having asthma. As LR+ ≥10 was considered to be adequate as a screening indicator, our symptom-sign score cut-off value of ≥3 optimally could discriminate between patients with and without asthma (LR+ 12.6).
Over- or under-statement of symptoms to physicians can also produce differences in responses to questionnaires. Large variations in the perception of respiratory symptoms, particularly dyspnoea, have been observed among patients with asthma, thus limiting the value of questionnaires.24 In this study we assessed the post-test probability in combination with the pre-test probability of symptom-sign scores and LR+ of objective examinations. LR+ values are able to summarise in a single number how the initial assessment of the likelihood of disease (‘pre-test probability’) is modified by a test result (‘post-test probability).25 In our results, information on bronchodilator reversibility and FEV1/FVC increased the post-test probability.
Our results might support the appropriate time for consultation with pulmonologists who would also assess BHR. In almost three-quarters of patients with non-specific respiratory symptoms it is possible to judge whether they have asthma based on the combination of the symptom-sign score and lung function tests according to our algorithm. Because the MCT has a high negative value, it is more useful in ruling out asthma (if the result is negative) than in proving it (if the result is positive).26,27 When a patient does not complain of any typical symptoms (i.e. symptom-sign score 0 points) and is suspected of having asthma, clinicians should consult with pulmonologists to rule out asthma by the assessment of BHR.
Strengths and limitations of this study
Several limitations of our analyses should be acknowledged. First, attrition bias, which is caused by the exclusion of patients, is an important issue in this study. A consequence of attrition is biasing of samples that leads to a lack of generalisability to the target population. However, since the prevalence of asthma — upon which the pre-test probability is dependent — is the same as in previous reports,28,29 our pre- and post-test probability is assumed to be generalisable to the diagnosis of asthma in patients with suspected symptoms of asthma. Second, our scoring system requires external validation in a different patient population. As asthma is a syndrome with heterogeneous aspects — for example, inflammatory cells, origin of environmental factors, origin of genomic backgrounds — our scoring algorithm should be tested under a variety of clinical situations. Third, there is a problem in confirming the MCT as one of the tools for the diagnosis of asthma. Several studies have reported that BHR is enhanced in patients with allergic rhinitis in the absence of asthma.30,31 BHR was performed after ICS had been started in the patients who had been diagnosed or suspected of having asthma at the first visit (n=421). This might influence the finding that patients with clinically-diagnosed asthma/CVA, who were responsive to ICS with neither bronchodilator reversibility nor BHR, accounted for almost 20% of the patients with asthma, because it was reported that 23% of patients with physician-diagnosed asthma receiving ICS had a negative test result.32
Implications for future research, policy and practice
We have adopted a pragmatic approach based on the scoring algorithm of the symptom-sign score for predicting the presence of adult asthma. The combination of the symptom-sign score and the objective examinations described in this paper was designed to screen adult patients with asthma without testing for BHR. This conclusion is in line with guideline recommendations. However, approximately one-quarter of patients with asthma required BHR testing due to the heterogeneous features of adult asthma. Future work is required to search for useful biomarkers to help in the diagnosis of asthma.
Conclusions
This pragmatic diagnostic algorithm is useful for predicting the presence of adult asthma and for determining the appropriate time for consultation with a pulmonologist.
Acknowledgments
Handling editor Gopal Netuveli
Statistical review Gopal Netuveli
The authors would like to thank Ms Mami Ueno Ms. Nahoko Yamashita and Ms Miki Katoh for their assistance in collecting the data. We also thank Ms Chizu Oka for assisting with lung function testing.
Funding None.
Footnotes
None of the authors has a financial relationship with a commercial entity that has an interest in the contents of this manuscript.
References
- Global Initiative for Asthma (GINA). The global strategy for asthma management and prevention, 2011. Available from: http://www.ginasthma.org/ (accessed 28 July 2012).
- Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. 2007. Available from: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf (accessed 28 July 2012).
- British Thoracic Society/Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. Updated 2012. Available from: http://www.britthoracic.org.uk (accessed 28 July 2012).
- Caramori G, Bettoncelli G, Carone M, et al. Degree of control of physician-diagnosed asthma and COPD in Italy. Monaldi Arch Chest Dis 2007;67(1):15–22. [DOI] [PubMed] [Google Scholar]
- Linden Smith J, Morrison D, Deveau C, Hernandez P. Overdiagnosis of asthma in the community. Can Respir J 2004;11(2):111–16. [DOI] [PubMed] [Google Scholar]
- Gershon AS, Victor JC, Guan J, Aaron SD, To T. Pulmonary function testing in the diagnosis of asthma: population study. Chest 2012;141(5):1190–6. http://dx.doi.org/10.1378/chest.11-0831 [DOI] [PubMed] [Google Scholar]
- Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26(5):948–68. http://dx.doi.org/10.1183/09031936.05.00035205 [DOI] [PubMed] [Google Scholar]
- Malo JL, Pineau L, Cartier A, Martin RR. Reference values of the provocative concentrations of methacholine that cause 6% and 20% changes in forced expiratory volume in one second in a normal population. Am Rev Respir Dis 1983;128(1):8–11. [DOI] [PubMed] [Google Scholar]
- Shin B, Cole SL, Park S-J, Ledford DL, Lockey RF. A new symptom-based questionnaire for predicting the presence of asthma. J Investig Allergol Clin Immunol 2010;20(1):27–34. [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26(2):319–38. http://dx.doi.org/10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- American Thoracic Society and European Respiratory Society. Recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. Am J Respir Crit Care Med 2005;171:912–30. http://dx.doi.org/10.1164/rccm.200406-710ST [DOI] [PubMed] [Google Scholar]
- American Thoracic Society. Guidelines for methacholine and exercise challenge testing-1999. Am J Respir Crit Care Med 2000;161:309–29. [DOI] [PubMed] [Google Scholar]
- MacIntyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005;26(4):720–35. http://dx.doi.org/10.1183/09031936.05.00034905 [DOI] [PubMed] [Google Scholar]
- Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 2011;184(5):602–15. http://dx.doi.org/10.1164/rccm9120-11ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks JJ, Altman DG. Diagnostic tests, 4: Likelihood ratios. BMJ 2004;329(7458):168–9. http://dx.doi.org/10.1136/bmj.329.7458.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinksy LE, Wipf JE, Ramsey SD. Evidence-based medicine glossary. In: Geyman JP, Deyo RA, Ramsey SD, eds. Evidence-Based Clinical Practice: Concepts and Approaches. Boston, MA: Butterworth-Heinemann, 2000. pp 165–72. [Google Scholar]
- Reid MC, Lane DA, Feinstein AR. Academic calculations versus clinical judgments: practising physicians’ use of quantitative measures of test accuracy. Am J Med 1998;104(4):374–80. http://dx.doi.org/10.1016/S0002-9343(98)00054-0 [DOI] [PubMed] [Google Scholar]
- Gill CJ, Sabin L, Schmid CH. Why clinicians are natural Bayesians. BMJ 2005;330(7499):1080–3. http://dx.doi.org/10.1136/bmj.330.7499.1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schayck CPO, Levy ML, Stephenson P, Sheikh A. The IPCRG guidelines: developing guidelines for managing chronic respiratory diseases in primary care. Prim Care Respir J 2006;15(1):1–4. http://dx.doi.org/10.1016/j.pcrj.2005.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy ML, Fletcher M, Price DB, Hausen T, Halbert RJ, Yawn BP. International Primary Care Respiratory Group (IPCRG) guidelines: diagnosis of respiratory diseases in primary care. Prim Care Respir J 2006;15(1):20–34. http://dx.doi.org/10.1016/j.pcrj.2005.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor GT, Weiss ST. Clinical and symptom measures. Am J Respir Crit Care Med 1994;149(2Pt2):S21–8 [DOI] [PubMed] [Google Scholar]
- Burney PG, Laitinen LA, Perdrizet S, et al. Validity and repeatability for the IUATLD bronchial symptoms questionnaire: an international comparison. Eur Respir J 1984;2(10):940–5 [PubMed] [Google Scholar]
- Bansal A, Farnham JM, Crapo RO, Hughes DC, Jensen RL, Cannon-Aibright LA. A simple diagnostic index for asthma. Clin Exp Allergy 2001;31(5):756–60. http://dx.doi.org/10.1046/j.1365-2222.2001.01065.x [DOI] [PubMed] [Google Scholar]
- Magadle R, Berar-Yanay N, Weiner P. The risk of hospitalization and near-fatal and fatal asthma in relation to the perception of dyspnea. Chest 2002;121(2):329–33. http://dx.doi.org/10.1378/chest.121.2.329 [DOI] [PubMed] [Google Scholar]
- Simel DL, Rennie D, Bossuyt PM. The STARD statement for reporting diagnostic accuracy studies: application to the history and physical examination. J Gen Intern Med 2008;23(6):768–74. http://dx.doi.org/10.1007/s11606-008-0583-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert R, Auchincloss JH. Post-test probability of asthma following methacholine challenge. Chest 1990;97(3):562–5. http://dx.doi.org/10.1378/chest.97.3.562 [DOI] [PubMed] [Google Scholar]
- Perpiñá M, Pellicer C, de Diego A, et al. Diagnostic value of the bronchial provocation test with methacholine in asthma. A Bayesian analysis approach. Chest 1993;104(1):149–54. http://dx.doi.org/10.1378/chest.104.1.149 [DOI] [PubMed] [Google Scholar]
- Löwhagen O, Arvidsson M, Pettersson K. Asthma and asthma-like disorder, a 5-year follow-up study. Respir Med 2002;96(12):1040–4. http://dx.doi.org/10.1053/rmed.2002.1383 [DOI] [PubMed] [Google Scholar]
- Hirsch S, Frank TL, Shapiro JL, Hazell ML, Frank PI. Development of a questionnaire weighted scoring system to target diagnostic examinations for asthma in adults: a modelling study. BMC Fam Pract 2004;5(1):30. http://dx.doi.org/10.1186/1471-2296-5-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braman SS, Barrows AA, DeCotiis BA, Settipane GA, Corrao WM. Airway hyperresponsiveness in allergic rhinitis. A risk factor for asthma. Chest 1987;91(5):671–4. http://dx.doi.org/10.1378/chest.91.5.671 [DOI] [PubMed] [Google Scholar]
- Polosa R, Li Gotti F, Mangano G, Mastruzzo C, Pistorio MP, Crimi N. Monitoring of seasonal variability in bronchial hyper-responsiveness and sputum cell counts in non-asthmatic subjects with rhinitis and effect of specific immunotherapy. Clin Exp Allergy 2003;33(7):873–81. http://dx.doi.org/10.1046/j.1365-2222.2003.01676.x [DOI] [PubMed] [Google Scholar]
- Sumino K, Sugar EA, Irvin CG, et al. Methacholine challenge test: diagnostic characteristics in asthmatic patients receiving controller medications. J Allergy Clin Immunol 2012;130(1):69–75. http://dx.doi.org/10.1016/j.jaci.2012.02.025 [DOI] [PubMed] [Google Scholar]


