Abstract
Research during the last decade has generated numerous insights on the presence, phenotype, and function of myeloid cells in cardiovascular organs. Newer tools with improved detection sensitivities revealed sizable populations of tissue-resident macrophages in all major healthy tissues. The heart and blood vessels contain robust numbers of these cells; for instance, 8% of noncardiomyocytes in the heart are macrophages. This number and the cell’s phenotype change dramatically in disease conditions. While steady-state macrophages are mostly monocyte independent, macrophages residing in the inflamed vascular wall and the diseased heart derive from hematopoietic organs. In this review, we will highlight signals that regulate macrophage supply and function, imaging applications that can detect changes in cell numbers and phenotype, and opportunities to modulate cardiovascular inflammation by targeting macrophage biology. We strive to provide a systems-wide picture, i.e., to focus not only on cardiovascular organs but also on tissues involved in regulating cell supply and phenotype, as well as comorbidities that promote cardiovascular disease. We will summarize current developments at the intersection of immunology, detection technology, and cardiovascular health.
I. HISTORICAL PERSPECTIVE
Macrophages, the first immune cells to appear in an organism’s development, are also the most important: they not only play a key role in immunity (in steady state and inflammation), but also help regulate organ development and function. These large cells are present in all tissues and can clear cellular debris and pathogens, present antigens to T cells, and produce cytokines to alert cells about ongoing damage and later promote tissue healing. In 1882, Élie Metchnikoff (293) was the first to fully appreciate and describe macrophages’ ingestion of particles or cells, called phagocytosis, as a protective immune response. For this concept of “cellular innate immunity,” he received the Nobel Prize in Physiology or Medicine in 1908, together with Paul Ehrlich, who received it for the concept of humoral immunity. Metchnikoff also coined the term macrophage (from Greek, meaning “big eaters”). In the following years, however, the research community mostly focused on humoral and cellular components of adaptive immunity, as these were assumed to be more important. Macrophages were simply regarded as phagocytes that controlled the spread of invading microorganisms until adaptive immune cells arrived. Indeed, it took more than 70 yr for researchers to turn their attention to innate immune cells. Studies by a limited group of laboratories found that adherent cells in spleen cell cultures were needed to induce an adaptive immune response, and that both antigen presentation and costimulation had to be provided by the same cell in vivo (74, 202, 219, 333). These cells were identified as antigen-presenting cells: macrophages and the more recently discovered dendritic cells (DCs) (428). In 1989, Charles Janeway (202) argued that the innate immune system controls the adaptive immune system, and that antigen-presenting cells have pattern recognition receptors (PRRs) to distinguish foreign vs. host antigens. Subsequent research then focused on unraveling the specific microbial antigens, i.e., pathogen-associated molecular patterns (PAMPs), and PRRs required to recognize antigens and process them for presentation. Moreover, several laboratories investigated the secondary signals (costimulatory receptors and cytokines) provided by macrophages and DCs to activate T cells. The discovery of CD80 and CD86 (and many other costimulatory molecules) has further unraveled the complex interaction between antigen-presenting cells and T cells (134, 232, 267). It should be noted that, while macrophages can present antigen to T cells, they are much less efficient in doing so.
Today, we define macrophages by their function (phagocytosis, immunity), specific markers (F4/80, CD64, MertK), morphology (e.g., phagosome inclusions), and location in specific tissues. However, we are starting to appreciate that macrophages are highly plastic and dynamic: on activation, their morphology and protein expression can rapidly change, resulting in expression of markers specific for other cells, and the cells may migrate to sites of inflammation, for example, upon brain or liver injury (450, 483). To add to this complexity, other cell types, such as smooth muscle cells and DCs, may express macrophage markers. As we are currently limited to the use of specific markers and observations of morphology to identify macrophages in tissues, it becomes increasingly important to understand macrophage dynamics in steady state and disease. New techniques and approaches, such as cytometer time-of-flight (CyTOF) mass cytometry, transcriptional profiling, fate mapping studies, and in silico/computational modeling approaches, are undoubtedly going to be of great help. Additionally, a better understanding of the actual proteins we are using as markers, for example, F4/80, which has been suggested to be involved in immunomodulation, is crucial (144). However, the question remains, if there really is a very defined cell type that is a macrophage, or if these are rather a family of distinct subtypes, which depend on tissue location, function, and disease state. With our growing knowledge of the complexity of the immune system and how cells can adopt markers and functions of other cells in specific settings, it may be time to reconsider the importance of names and put more emphasis on specific cell identifiers. For instance, including more than a handful of markers when assessing a macrophage’s function, or, conversely, investigating macrophages’ functions when describing different subsets should become self-evident. We will discuss macrophages, their subset classification, and their plasticity in more detail later in this review. Despite this immense complexity in macrophage biology, we have come a long way in understanding macrophage biology since macrophages were first described ~130 yr ago (293). We have made many new and exciting discoveries in terms of macrophage function (e.g., their role in electrical conduction, iron recycling, and synaptic pruning) and origin (embryonic vs. adult), but there is still much to be learned about this versatile immune cell type.
In this review, we will discuss some of the earliest discoveries concerning the origin, phenotype, and function of monocytes and macrophages, as these provide the groundwork for our current understanding. Furthermore, we will first discuss the dogma of framing mononuclear phagocytes as a linear system progressing from pro-monocyte to monocyte to macrophage and then expand it using our current knowledge of myeloid origin and heterogeneity. Since both macrophage origins and phenotypes are highly heterogenic, we will examine macrophages’ immune system functions, organ development and maintenance roles, interactions with other cells, and plasticity. We will emphasize exciting new discoveries, particularly in cardiovascular diseases, and explore how we can potentially use this new understanding of macrophage biology to define new drug targets and therapies. We will mainly focus on murine monocytes and macrophages, but we will also mention important findings regarding DCs and Langerhans cells, as well as findings in human settings, where appropriate. Other excellent current reviews discuss DCs (81, 93) and Langerhans cells (215).
II. THE MONONUCLEAR PHAGOCYTE SYSTEM
Early studies on macrophages sought to understand their role in clearing microorganisms. Many such studies examined peritoneal macrophages that had been exposed to a variety of pathogens (276, 492). Interestingly, one early study observed that, in mice, resistance to secondary infection was mediated by macrophages (277). Such resistance was later shown to depend on macrophages producing IFN-γ, which in turn activates macrophages and enhances their oxidative metabolism (323). This early description of trained immunity preceded a vibrant field of investigation.
Overall, early conclusions about macrophage phenotype and location were largely drawn from cytochemical procedures, especially histological staining for phagocyte-specific enzymes, such as nonspecific esterase, a technique still used to identify monocytic differentiation in leukemia. However, the discovery of F4/80 (20) and CD68 (365) as specific macrophage markers in the 1980s allowed researchers to fully appreciate macrophages’ tremendous heterogeneity in various tissues, at different ages and in different diseases. These markers became tools through which researchers could investigate macrophage behavior. Moreover, the development of fraktalkine receptor (CX3CR1) reporter mice, by Littman’s group in 2000 (209), and Csf1 reporter mice, by Hume’s group in 2003 (394), enabled a better understanding of the myeloid system. More recently, Mertk and CD64 were added as markers of tissue-resident macrophages (124).
A serendipitous discovery in 1966 by Bradley and Metcalf (49), who had sought to culture leukemia cells, led to the establishment of an agar culture that, by coculturing bone marrow and leukemia cells, could enumerate hematopoietic progenitors from the bone marrow. Leukemia cells produce colony-stimulating factors (CSFs) that are necessary for hematopoietic cell development. The subsequent discoveries of M-CSF (macrophage-CSF, also called Csf1) (427) and GM-CSF (granulocyte macrophage-CSF, also called Csf2) (54) in the 1970s and G-CSF (granulocyte-CSF, also called Csf3) in 1983 (330) enabled a better understanding of myeloid cell development. Osteopetrotic (op/op) mice and toothless rats (tl/tl), which lack M-CSF, and mice lacking its receptor CD115 (also called Csf1r) have severe monocytopenia and macrophage deficiency that demonstrate M-CSF’s crucial role in monocyte/macrophage development and maintenance (490, 493). However, while these animals lack most macrophages, such as peritoneal macrophages, others, such as macrophages in the intestinal lamina propria, are present at normal levels, suggesting a difference in M-CSF dependency among macrophage populations (83, 494, 499). Mice lacking G-CSF or its receptor appear healthy but have severe neutropenia and reduced macrophage numbers (264, 268). Intriguingly, GM-CSF-deficient mice show no striking phenotype. They have reduced peritoneal macrophages (264) and eventually develop an abnormal lung phenotype, as alveolar macrophages are highly dependent on GM-CSF (30, 426). While these studies clearly show M-CSF, G-CSF, and GM-CSF play crucial roles in myeloid cell development, they also demonstrate the complexity of the myeloid system. Other studies have identified additional factors, such as IL-3 (487), IL-33 (279), IL-34 (265), and IFN-γ (53), that can increase myelopoiesis in steady state, inflammation, or cancer.
In the 1970s, van Furth et al. (467) coined the term “mononuclear phagocyte system,” which encompasses early myeloid precursors in the bone marrow, monocytes, and macrophages. DCs were included after their discovery in 1973 (428). This classification was based on myeloid cells’ common origin, morphology, and function. The general concept at the time was that promonocytes in the bone marrow give rise to monocytes that differentiate to tissue-resident macrophages. This framework was based on several observations in mice and rats. Studies showed that precursors in the bone marrow give rise to circulating monocytes, as assessed by radioactive labeling or bone marrow transplantation (466, 479, 480). Furthermore, bone marrow transplantation studies revealed that bone marrow precursor-derived monocytes were also a source of macrophages (218, 479, 480). Monocytes differentiate to macrophages after being recruited to sites of inflammation, for example, the peritoneal cavity after injection of newborn calf serum (466) or lipopolysaccharide (LPS) (70). Evaluation after bone marrow transplantation in humans also established that alveolar macrophages could be derived from donor-origin monocytes (453), further supporting the idea that tissue-resident macrophages have bone marrow/monocyte origins.
However, it should be noted that early observations challenging this dogma, such as monocyte depletion’s lack of effects on peritoneal, alveolar, and liver macrophages in the steady state (398, 507), that monocytopenia does not influence macrophages in leukemia patients (138), or the repopulation of alveolar macrophages by host macrophage proliferation after irradiation in mice (448), did not receive much attention. Several early studies also observed macrophage appearance before monocyte development: in the fetus, macrophages develop in the yolk sac on days 9 and 10 of gestation, before the initiation of hepatic hematopoiesis, and do not pass through a (pro-)monocyte stage (321, 423, 443). This was initially explained by the assumption that embryonic macrophage development is simply different, and that embryonic macrophages would gradually be replaced by monocyte-derived macrophages in the adult mouse. Additionally, some early studies found that macrophages proliferated locally (122, 469), and it was thought that macrophage progenitors can seed tissues and give rise to macrophages locally. Recently, important fate-mapping studies have revealed that adult mice still have some macrophages of embryonic origin without going through a monocyte stage, while other macrophages are indeed derived from recruited monocytes. It has also become clear that macrophages can proliferate locally, as we will discuss in more detail later.
III. HEMATOPOIESIS AND MONOCYTE ORIGIN
In mice, monocytes are identified by their expression of CD11b and CD115 and represent ~2–4% of blood leukocytes. In humans, monocytes can represent 10–20% of peripheral blood mononuclear cells and are identified by their expression of CD14. Monocytes are released from the bone marrow into the blood, where they can circulate for several days. Moreover, both mouse and human spleens have a marginal monocyte pool that can expand on inflammation (117, 437, 496). Monocytes are produced in the fetal liver during embryonic development and in the bone marrow during adult hematopoiesis. More specifically, monocytes derive from hematopoietic stem cells (HSCs) that differentiate to common myeloid progenitors (CMPs), which are negative for markers of mature hematopoietic cell lineages and express CD117 (also known as c-kit), Sca-1, and CD34. The CMPs increase CD16/32 and differentiate to granulocyte-macrophage progenitors (GMPs) (8). Researchers recently described a monocyte-macrophage DC precursor (MDP) (112) and a common monocyte progenitor (cMoP) (176). MDPs upregulate CD115 and CX3CR1, whereas cMoP downregulate CD135 [also known as fms-like tyrosine kinase 3 (Flt3), or fetal liver kinase-2 (Flk2)]. While it was assumed that CMPs give rise to GMPs, then to MDPs and then cMoPs, which differentiate to monocytes, a recent study indicates that MDPs can arise from CMPs independent of GMPs (510). The authors suggest that monocytes with distinct phenotypes arise directly from either MDPs or GMPs, and that this helps to adapt the combination of myeloid cell types to environmental needs. MDPs can give rise to not only cMoPs, but also common DC precursors (CDPs) (269, 474). Whether GMPs give rise to MDPs and whether MDPs in turn give rise to DCs has been challenged (396, 510). Furthermore, MDPs can give rise to Langerhans cells and DCs independently from monocytes (269). cMoP, however, cannot generate common DCs or plasmacytoid DCs, only monocytes and their descendants, and CDPs in turn produce the DC lineage specifically (269). This linear development from a long-term HSC to a monocyte was a widely accepted model for >30 yr, but recent observations suggest that precursor populations are more heterogeneous (356, 497), and that lineage commitment occurs much earlier than previously assumed (351, 356). One can imagine several feedback mechanisms that control and limit hematopoiesis. For example, macrophages in the bone marrow express the receptor for M-CSF and can thus eliminate this growth factor from their environment, possibly reducing monocyte production in the bone marrow (27). Other cells that influence hematopoiesis, and thereby monocyte production, include leptin receptor-expressing cells, CXCL12-abundant reticular (CAR) cells, nestin-expressing cells, osteolineage cells, endothelial cells (ECs), and adipocytes. These cells can express several factors, such as stem cell factor and CXCL12, that influence stem cell quiescence and modulate leukocyte retention, respectively, in the bone marrow. For a detailed review of the bone marrow niche and its influence on hematopoiesis, see the excellent recent reviews by Crane et al. (77), Hoggatt et al. (182), and Mendelson and Frenette (291). Under certain inflammatory conditions, such as atherosclerosis and myocardial infarction (MI), monocytes can also be produced by extramedullary hematopoiesis in the spleen (95, 256, 379, 437).
Numerous transcription factors function as key regulators of hematopoiesis, and some specifically determine differentiation to a specific lineage (e.g., myeloid vs. lymphoid). PU.1 is a central driver of myelopoiesis, is needed to generate CMPs, and is the first transcription factor to promote myeloid-specific gene transcription (325, 406). PU.1 deficiency results in a complete lack of common lymphoid and myeloid progenitors and thus monocytes/macrophages and B cells (288, 406). Conversely, decreased PU.1 is essential for CMPs to differentiate to megakaryocyte-erythroid progenitors and common lymphoid progenitors to T cells (14, 334, 383). Transcription regulation by PU.1 is complex, as evidenced by both its initial differentiation of progenitors that can still give rise to lymphoid and erythroid lineages and its involvement in HSC self-renewal (197). Thus a fine balance of low PU.1 signaling, which is essential for B-cell and granulocyte development, and high PU.1 signaling, which is essential for monocyte development, is needed to guide hematopoiesis (13, 82). Interestingly, conditionally deleting PU.1 in adult mice affects only downstream GMP differentiation, which is skewed toward granulopoiesis (84). The transcription factor CCAAT enhancer binding protein-α gene (C/EBP-α) promotes HSCs’ capacity to self-renew and is essential for producing GMPs (165, 366). However, after the GMP stage, C/EBP-α expression promotes granulopoiesis and must decrease before differentiation toward monocytes (522, 523). The balance between PU.1 and C/EBP-α is crucial, as higher PU.1 favors monocytes/macrophages and higher C/EBP-α favors neutrophils (82). This balance is regulated by two sets of transcriptional repressors: Early growth response 2 (Egr-2)/NGFI-A-binding protein 2 (Nab-2) is activated by PU.1, and growth factor independent-1 transcriptional repressor (Gfi-1) is activated by C/EBP-α. These repressors provide positive feedback by limiting the expression of either monocyte-specific genes (on activation of C/EBP-α and Gfi-1) or neutrophil-specific genes (on activation of PU.1 and Egr-2/Nab-2) (246). Gfi-1, like PU.1, also promotes HSC self-renewal (179, 197, 520). The transcription factor interferon regulatory factor 8 (IRF8; also called interferon consensus sequence binding protein) is also needed for GMPs to differentiate to monocytes and DCs. It inhibits C/EBP-α and thereby prevents differentiation toward neutrophils (242). Lack of IRF8 results in a myeloproliferative disorder that skews toward granulocytes rather than monocytes/macrophages in mice and resembles human chronic myeloid leukemia (183). IRF8 may enable signaling downstream of CD115 by inducing proteolytic degradation of c-Cbl, a ubiquitin ligase that targets activated CD115 for degradation, so that its degradation prevents CD115 degradation (212). Overall, HSC differentiation toward monocytes is highly regulated by a finely balanced array of transcription factors that, if mutated or dysregulated at any step, can result in several types of leukemia (384). Please see Zhu et al. (527) for a more detailed review of transcription factors involved in myelopoiesis.
IV. MONOCYTE PHENOTYPE AND FUNCTION IN STEADY STATE
Monocytes are heterogeneous in phenotype, function, and size. In the steady state, they surveil blood vessels and nonlymphoid tissues (127, 201), take up antigen and recirculate to draining lymph nodes (201, 370), and maintain certain tissue-resident macrophages and DCs (21, 444). During inflammation, monocytes can acquire a macrophage or DC phenotype (21, 410). Within inflamed tissues, such as the skin and intestine, monocytes can be exposed to pathogens and may take up antigen and migrate to lymph nodes either with (370) or without (201) differentiating to DCs. Tacke et al. (440) have shown that immature monocytes in the bone marrow can also acquire antigen and present it to T cells after maturation in the periphery. While monocytes can differentiate to macrophages and DCs in different conditions, it is unclear if there are specific monocyte subsets that give rise to specific subsets of macrophages or DCs. Our understanding of monocyte subsets is currently limited to the distinction between two subsets in mice and three subsets in humans, but this is likely an oversimplification. In mice, monocytes are divided into a Ly-6Chi subset (which also expresses high levels of CCR2 and CD62L and intermediate levels of CX3CR1) and a Ly-6Clow subset (which also expresses high levels of CX3CR1 and low levels of CCR2 and CD62L) (127); each of these two subsets accounts for ~50% of circulating monocytes in C57BL/6 mice. In humans, three distinct monocyte subsets can be distinguished by their expression of CD14 and CD16: classical (CD14++ CD16−; ~90% of all monocytes), intermediate (CD14++ CD16+), and nonclassical (CD14+ CD16++) monocytes (347). Gene expression profiling established that CD16− monocytes are the human equivalent of Ly-6Chi monocytes and that CD16+ monocytes are the equivalent of Ly-6Clow monocytes. Interestingly, several genes, such as CD36, TREM-1, and IL-1β (193), were differentially expressed between the two species. It should be noted that, in this comparison, the CD14++ CD16+ intermediate monocytes were included in the CD16+ fraction (500). Recent profiling of circulating monocytes in humans by CyTOF (454) and single-cell RNA sequencing (RNA-seq) (478) indeed revealed substantial heterogeneity in the intermediate monocyte population and indicates that we can certainly discover more about monocyte functions.
Recent studies suggest that, under steady-state conditions, MDPs and cMoPs mostly give rise to Ly-6Chi monocytes, which then undergo differentiation to Ly-6Clow monocytes. For example, transplanted MDP and cMoP give rise initially to Ly-6Chi monocytes and subsequently to Ly-6Clow monocytes (18, 176). Similarly, BrdU incorporation after injection can be first observed in Ly-6Chi monocytes and later in Ly-6Clow monocytes (176, 474, 516). This indicates that Ly-6Clow monocytes do not or only minimally proliferate, whereas progenitors of Ly-6Chi monocytes readily incorporate BrdU. Ly-6Chi monocytes then retain the label while differentiating to Ly-6Clow monocytes (474, 516). Indeed, in the absence of Ly-6Chi monocytes, Ly-6Clow monocytes do not get labeled with BrdU (516), further suggesting their Ly-6Chi origin. Interestingly, a recent study found that administering M-CSF-blocking antibodies reduces Ly-6Clow monocytes and tissue-resident macrophages, but increases Ly-6Chi monocytes and their recruitment. This suggests that, in adult mice, M-CSF is specifically needed for the maturation and production of Ly-6Clow monocytes (275). Moreover, the transcription factor Nr4a1 (also called Nur77) and CX3CR1 are crucial for the development of Ly-6Clow monocytes (127, 160). Monocytes additionally receive crucial survival signals from CX3CR1, providing a potential explanation why this subset of monocytes, which expresses this receptor highly, lives longer (245). Some studies, however, suggest that Ly-6Clow monocytes are directly produced in the bone marrow (160), and, indeed, reducing Ly-6Chi monocytes via loss of certain transcription factors or chemokine receptors does not affect Ly-6Clow monocytes as dramatically (11, 58, 241, 516). Yet in these situations, Ly-6Clow monocytes, which usually have a half-life of ∼2 days in mice, likely extend their lifespan to compensate for the reduced number of circulating monocytes (516). The conversion of Ly6Chi monocytes to Ly6Clow monocytes was initially assumed to occur mainly in the circulation, but there is emerging evidence that this can also occur at other sites, such as the bone marrow (474, 516). Furthermore, during sterile hepatic injury, Ly-6Chi monocytes locally differentiate to Ly-6Clow monocytes, and this is necessary for optimal tissue repair (85), a result that suggests monocyte conversion could also occur at other sites of inflammation, such as during MI. A recent study by Patel et al. (348) shows that monocyte conversion also occurs in humans: classical monocytes circulate for ∼1 day before they give rise to intermediate and then nonclassical monocytes, which circulate for ∼4 and 7 days, respectively.
Ly-6Chi monocytes, also called “classical monocytes” or “inflammatory monocytes,” have a short half-life in steady state of ~20 h in mice (348, 516), upon which they either transition to Ly6Clow monocytes, leave the blood, or potentially die. As previously mentioned, monocyte production in the bone marrow results in mostly Ly-6Chi monocytes, which are then released from the bone marrow in a CCR2/CCL2/CCL7-dependent manner (409, 415, 458). Accordingly, CCL2-deficient and CCR2-deficient mice show increased monocyte numbers in the bone marrow but dramatically fewer in the periphery (409). In infections, CCL2 is produced locally in the bone marrow by mesenchymal stromal cells and CAR cells near sinusoids in response to low doses of circulating Toll-like receptor (TLR) ligands (415). This locally produced CCL2 promotes monocyte release.
Inflammatory sites recruit Ly-6Chi monocytes mainly through the cells’ expression of the chemokine receptor CCR2 and the inflamed tissues’ production of CCL2 and CCL7 (239). CCL2 enables these monocytes to adhere firmly to the endothelium and to transmigrate (130). While CCR2 may play an important role during early inflammation (458), it may become dispensable as inflammation progresses (409). The antimicrobial peptide LL-37 and the heparin-binding protein, both produced by neutrophils at sites of inflammation, are also needed for proper recruitment of Ly-6Chi monocytes (422). In addition to their expression of chemokines, ECs guide monocyte extravasation (also called diapedesis) by expressing several adhesion molecules [e.g., selectins, vascular adhesion molecule-1 (VCAM-1), ICAM-1]. For an excellent review of the adhesion mechanisms involved in monocyte extravasation, see Imhof and Aurrand-Lions (192).
Diurnal circadian rhythms, regulated by the circadian transcription factor Bmal1 (also called Arntl), tightly control the bone marrow’s Ly-6Chi monocyte release, which peaks 4–8 h after light onset (328). This “anticipatory inflammation” likely evolved to enable a better response to potential infections. Interestingly, both patients (178, 374, 434) and mice (94, 401) exhibit circadian differences in acute MI onset and outcome, with peak incidence and infarct size during the sleep-to-wake transition phase. In mice, this has been linked to the cardiomyocyte circadian clock (94) and to increased neutrophil production and recruitment to the infarct (401). However, temporary complete disruption of the circadian rhythm after MI also worsens outcome by reducing neutrophil infiltration, among other effects (12), indicating a delicate balance between beneficial and destructive innate immune cell recruitment.
Ly-6Clow monocytes extravasate less often and rarely differentiate to macrophages and DCs under steady-state conditions (18). Indeed, Ly6Clow monocytes likely represent the most mature and terminally differentiated monocyte stage. They are also termed “patrolling monocytes” as they slowly crawl (~4–20 μm/min), in a chemokine-independent and LFA1-ICAM1/2-dependent manner, along the luminal side of vascular endothelium, patrolling for endothelial integrity (18). Ly-6Clow monocytes are retained on the endothelium at sites of injury and inflammation, which is mediated by expression of fraktalkine (CX3CL1) by endothelium and CX3CR1 and Mac1 (CD11b/CD18) by monocytes, where they rapidly extravasate (18, 58). Early after extravasation, Ly-6Clow monocytes produce a variety of inflammatory mediators, such as TNF-α, CXCL1, and CCL7, while later upregulating genes involved in tissue remodeling (18, 58). Due to their early production of chemokines and inflammatory mediators, Ly-6Clow monocytes likely contribute to the early neutrophil recruitment. Interestingly, Ly-6Clow monocytes sense damage via TLR-7, whereas TLR-4 does not seem to play a role (58). Similarly, human nonclassical monocytes rely on TLR-7 and TLR-8 for surveillance (79). Ly-6Clow monocytes appear enriched in the microvasculature of host-environment interfaces, such as the skin, kidneys, and lungs (58, 161), further supporting the idea that patrolling is an important function of this subset. Deleting a specific subdomain in the Nr4a1 enhancer results in a loss of Ly-6Clow monocytes without disrupting Nr4a1 gene expression in macrophages (455). This uncoupling of inflammatory macrophage gene expression from Ly-6Clow monocyte differentiation provides an interesting tool for further analysis of Ly-6Clow monocyte function.
Monocytes’ overall function is to sense their environment and fight infections, partly by giving rise to macrophages and DCs in inflamed tissues. Studies in the early 1970s showed that, in humans after sepsis and in mice after intraperitoneal injection of newborn calf serum, monocyte production and release into the circulation were significantly increased, resulting in monocytosis (294, 468). This is also the case in atherosclerosis and MI, wherein monocytosis significantly contributes to disease (95, 320, 379). It is widely accepted that monocytes are educated in the periphery by local cues after being recruited to the site of infection, for example, by IFN-γ production by natural killer cells (140), upon which they differentiate to macrophages or DCs. Monocyte-derived macrophages/DCs differ from their tissue-resident counterparts, which are derived from embryonic progenitors, but they often have identical functions and phenotypes (444). Recent studies comparing tissue-resident vs. recruited macrophages or DCs have shown that only a very limited gene set was specific to either one of these populations (104, 132). The current assumption is that, under inflammatory conditions, monocytes are recruited to aid tissue-resident macrophages that often undergo apoptosis on PRR activation by either pathogens or endogenous ligands, as in MI (320) or atherosclerosis (408). Our improved understanding of the difference between monocyte-derived and tissue-resident phagocytes could be therapeutically useful, as recruited monocytes are more easily accessible, for example, by nanoparticle-mediated delivery of siRNA, and could thus be therapeutically modulated via, for instance, their cytokine responses or phagocytosis activity. Targeting monocytes by nanoparticles to silence CCR2 has been previously explored (254), resulting in reduced monocyte recruitment and infiltration, as well as improved outcome in the setting of atherosclerosis and MI.
Recent evidence indicates that, in addition to being shaped by environmental cues in the periphery, Ly-6Chi monocytes can be instructed in the bone marrow, before they enter inflammatory sites, and serve as effector cells. During viral infections, for example, Ly-6Chi monocytes in the bone marrow can directly sense viral antigens and produce type I interferons before differentiating in the periphery (26). Similarly, after MI, Ly-6Chi monocytes in the blood already show increased proteolytic and inflammatory functions before they enter ischemic myocardium (320, 386). Additionally, during gastrointestinal infection, bone marrow natural killer cells can sense distal IL-12 production and produce IFN-γ, which reprograms cMoPs (17). These studies illustrate that Ly-6Chi monocytes or their direct progenitors can either directly or indirectly sense danger signals from the periphery, and such sensing results in their reprogramming and preparation for upcoming battles. Similar mechanisms have been described for DCs (400). This education in the periphery, however, also has further biological relevance, as it will also affect monocyte responses to future insults. For instance, MI and stroke both accelerate atherosclerosis by increasing monocyte production and recruitment to plaques (95). Interestingly, plaque monocytes show increased proinflammatory gene expression after MI, indicating that ischemic events may (re)educate monocytes toward a more proinflammatory phenotype.
Some recent studies suggest that, in addition to simply assisting mononuclear phagocytes, monocytes may also have their own cell-specific functions. For example, Ly6Chi monocytes may enter tissues for a short period, without differentiating, and acquire antigen for transport to the lymph nodes. While some monocytes differentiate to DCs in the lymph nodes (370), other monocytes can retain their phenotype but lose their capacity to recirculate in the blood; thus they have lost some monocyte features (201). Monocytes may also assist in angiogenesis (198). Moreover, it is currently debated whether monocytes can give rise to fibroblasts after MI: while some studies suggest they can (168, 305), others show no contribution (214, 511). In an attempt to resolve this issue, a current lineage tracing approach found no contribution of bone marrow-derived cells to cardiac fibroblasts after MI (306). However, in certain instances, myeloid cells may acquire some fibroblast functions, as has been suggested in angiotensin-induced cardiac hypertrophy (167). Further studies are thus warranted to address monocyte subsets and their function in steady state and disease.
V. MONOCYTES IN CARDIOVASCULAR DISEASE
Monocyte numbers increase on inflammation, and they are crucial for host defense (239). This is also true in cardiovascular diseases, during which monocyte numbers correlate with atherosclerosis disease severity (438) and myocardial infarct size (278, 386, 459). In humans, CD14++ CD16− monocytes negatively correlate with left ventricular function post-MI (278, 459), whereas intermediate CD14++ CD16+ monocytes correlate with cardiovascular disease severity (33, 381, 495).
Cholesterol levels directly correlate with circulating monocyte numbers (435). Atherosclerotic mice have increased hematopoiesis and thus monocyte production, likely as a direct consequence of cholesterol accumulation in hematopoietic stem and progenitor cells (HSPCs). Augmented cholesterol content due to inefficient cholesterol efflux [mediated by apolipoprotein E (ApoE), ABCA1, and ABCG1] increases plasma membrane lipid raft formation and expression of the common β-subunit of the IL-3/GM-CSF receptor on the surface of HSPCs. This results in increased IL-3 and GM-CSF signaling and thereby expansion of HSPCs (314, 517). Independently of cholesterol metabolism, mutations of Tet methylcytosine dioxygenase 2 (Tet2), an epigenetic regulator catalyzing the conversion of 5-methylcytosine to 5-hydroxymethylcytosine, rise with age and predispose an individual to hematopoietic malignancies and cardiovascular disease (199, 504). Tet2 mutations result in increased HSPC self-renewal and myeloid transformation (310) that accelerate atherosclerosis (119). Furthermore, sympathetic activation of the bone marrow niche and increased myelopoiesis occur after MI and stroke (76, 95). Similarly, chronic mild stress increases, via the β3-adrenergic receptor, hematopoiesis, and bone marrow myeloid output (173), a correlation also observed in humans (449). Chronic stress, MI, and stroke can all contribute to atherosclerotic plaque progression due to increased myelopoiesis (76, 95, 173). Other signals to the bone marrow, such as IFN-γ and IFN-α, produced during inflammation, could further activate HSPCs (23, 107). In addition to increased hematopoiesis in the bone marrow, extramedullary hematopoiesis in the spleen, which depends on GM-CSF- and IL-3-producing innate response activator B cells, is also elevated in atherosclerosis (373, 379).
Atherosclerosis, a chronic disease of the arteries, is characterized by inflammation and lipid accumulation in the vessel wall that results in plaque build-up. Vessel areas with disturbed flow are prone to developing atherosclerosis. Low shear stress in the inner curvatures of coronary arteries or oscillatory shear stress around bifurcations induce vascular EC dysfunction. This results in altered gene expression and a related change in EC cell morphology that raises permeability for macromolecules, such as low-density lipoprotein (LDL). LDL can then diffuse through the disturbed endothelial layer and is retained in the intima by interactions with proteoglycans (420). Trapped LDL then undergoes modifications, for example, lipolysis, proteolysis, and oxidation. Evidence from animal models suggests that oxidation is a crucial step in LDL conversion into an atherogenic particle. Oxidation is likely facilitated by lipoxygenases, myeloperoxidases, inducible nitric oxide synthase (iNOS), and NADPH oxidases found within lesions (259). Oxidized LDL (oxLDL) further amplifies the activation of ECs, which increase their expression of adhesion molecules, cytokines, and growth factors (e.g., M-CSF) (309). Altered shear stress also elevates vascular EC turnover and expression of chemokines and adhesion molecules for leukocytes, such as CCL2 and VCAM-1 (524). VCAM-1, detected by noninvasive PET-CT imaging on inflamed atheroma-prone vessels, correlates with local inflammation and could help identify vulnerable patients and monitor their treatment (317). Monocyte recruitment to atherosclerotic lesions via this “leaky” endothelium, as measured, for example, by Evans blue, was already observed in the late 1970s (129). It is now well established that, in the early phases of lesion development, Ly-6Chi monocyte recruitment via activated endothelium plays a predominant role, and circulating Ly-6Chi monocyte numbers correlate with lesion size and serum cholesterol levels (435, 438, 439). However, it should be noted that Ly-6Clow monocytes can sometimes also enter lesions, upon which they express the DC marker CD11c, indicating that infiltrating monocyte phenotypes may be linked to lesional phagocyte phenotypes (439). Ly-6Chi monocytes have been thought to differentiate to inflammatory (M1) macrophages in the lesion, whereas Ly-6Clow monocytes differentiate to reparative (M2) macrophages, but this has never been convincingly shown (308, 309), likely because M1/M2 macrophage phenotypes are primarily observed in vitro.
As in other diseases, during atherosclerosis, monocytes produced in the bone marrow and spleen are recruited to the plaques via CCL2/CCL7-CCR2 (46, 145, 152, 439). CX3CR1 also plays a role (253, 439), and conflicting evidence exists regarding CCR5 (244, 361, 439, 521). After recruitment to the plaques, monocytes differentiate to macrophages, which engulf lipids and become foam cells. Indeed, most macrophages in early plaques originate directly from circulating monocytes that are recruited and exposed to growth factors inducing their differentiation (438). It is well established that either preventing monocyte recruitment to lesions (71, 145, 152, 154, 254, 380), or depleting monocytes (380, 432) significantly reduces foam cell formation and lesion development. Similarly, op/op mice on an LDLr knockout (KO) or ApoE KO background show reduced atherosclerosis (368, 421). Statins also reduce monocytosis and thus lesion progression (435). On the other hand, mice deficient in Nr4a1 have elevated atherosclerosis, presumably due to increased inflammatory Ly-6Chi monocytes (155, 162). Interestingly, a monoclonal antibody against CCR2 reduces C-reactive protein, a risk factor for atherosclerosis, for up to 3 mo in patients in a phase II clinical trial (133).
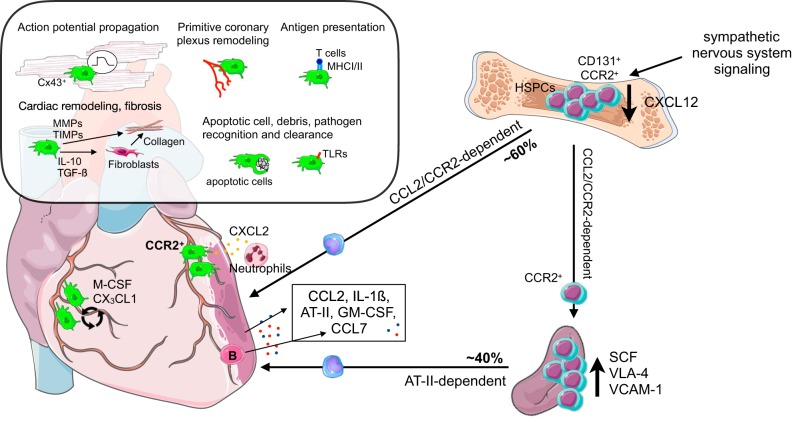
MI is caused by a ruptured atherosclerotic plaque that results in a thrombus, which obstructs blood flow to the heart and thereby induces cardiomyocyte death and damage to the myocardial tissue. As previously mentioned, this injury activates myelopoiesis: autonomic β3-adrenoceptor-dependent signaling to the bone marrow niche decreases several quiescence and retention factors, for example, CXCL12, resulting in increased production and release of not only myeloid cells, but also HSPCs (95). In addition to autonomic signaling, soluble factors, such as IL-1β, which is higher in circulation after MI, also increase hematopoiesis in the bone marrow (390). Not only is mobilization of HSPCs a phenomenon observed in mice, but patients with acute MI also have more circulating progenitors (286). In mice, these HSPCs subsequently seed the spleen, resulting in local proliferation and extramedullary hematopoiesis (95). In patients, circulating progenitors could also result in extramedullary hematopoiesis, as has been observed in other conditions, for example, osteopetrosis and invasive lung carcinoma (51). Indeed, increased splenic activity measured by glucose uptake, which could suggest increased proliferation, is observed in patients with acute MI using 18FDG-PET imaging (101, 226), and marginal monocyte pools have been described in humans (117). Further studies in humans addressing if this phenomenon occurs to the same extent as in mice, which readily show extramedullary hematopoiesis in various inflammatory settings (51), are warranted. Interestingly, Dutta et al. (97) showed that CCR2 is expressed on a subset of HSCs with increased proliferation rates and myeloid bias after MI, and that these HSPCs preferentially seed the spleen. Patients undergoing open heart surgery had similar CCR2+ HSCs in their sternal marrow that showed increased proliferation compared with CCR2− HSC (97). Stem cell factor and VLA-4 expression mediate HSPC retention in the spleen (95). Additionally, VCAM-1 expression by red pulp macrophages plays a key role in splenic HSPC retention (96). Splenic monocyte release depends on angiotensin II and is mediated by B cells; once released, splenic monocytes are recruited to the injured myocardium via CCL2-CCR2 (89, 290, 437). Cardiac ECs strongly upregulate several adhesion molecules and cytokines, specifically CCL2, directly after MI, which, in addition to an initial burst of angiotensin II, results in the recruitment of Ly-6Chi monocytes to the myocardium (89, 254, 437). Indeed, inhibiting angiotensin-converting enzyme and CCR2 reduces monocyte release from the spleen and can improve MI outcome (255, 280). B cells have been implicated in monocyte recruitment to the injured heart, as they produce CCL7 (528), which is also recognized by CCR2. Approximately 40% of monocytes found in the myocardium are recruited from the spleen (437). Interestingly, splenic monocytes show a more proinflammatory gene expression, with a 60-fold higher IL-1β expression than bone marrow-derived monocytes (95). Recent work by Anzai et al. (15) shows that, after MI, GM-CSF is produced by fibroblasts in the infarct. GM-CSF promotes local recruitment of myeloid cells, by inducing CCL2 production, and stimulates proliferation and differentiation of CD131+ myeloid-biased progenitors in the bone marrow. Interestingly, in the setting of autoimmunity, GM-CSF induces a pathogenic proinflammatory gene signature in Ly-6Chi monocytes and their progeny (80). Similarly, MI in Csf2rb KO mice reduces expression of inflammatory genes in the infarct and improves survival (15). Recently, DCs were shown to play a role post-MI in activation of autoreactive CD4+ T cells (45), and it will be interesting to determine whether locally produced GM-CSF induces monocyte-to-DC differentiation, DC proliferation, or affects DC and macrophage phenotypes.
CX3CR1+ cells accumulate in the murine heart within minutes after ischemia onset (207). These early monocytes/macrophages could represent either early patrolling Ly-6Clow monocytes, which extravasate and aid neutrophil recruitment in inflammation (18, 58), or locally attracted cardiac macrophages that sense tissue injury, similar to what has been described for microglia (450). Indeed CCR2+ monocyte-derived cardiac macrophages enable neutrophil extravasation (261). Neutrophils appear later in the infarct area and peak on day 1, while monocytes aggregate over days (207). In mice, monocyte accumulation occurs in two sequential waves: first Ly-6Chi monocytes (days 1–4 post-MI) and then Ly-6Clow monocytes (days 4–7) (320). In humans, classical CD14+CD16− monocytes are found mostly in the infarct border zone during the acute phase, and both CD16− and CD16+ monocytes are found in the infarct core post-MI, indicating that two successive waves may also exist in humans (464). Since these analyses are based on histological examination, it is impossible to dissect the roles of intermediate vs. nonclassical monocytes in patients. Interestingly, in humans, monocytes are also recruited from both the bone marrow and the spleen (464). These strong similarities between mice and humans suggest that mouse models are a valuable tool for studying MI.
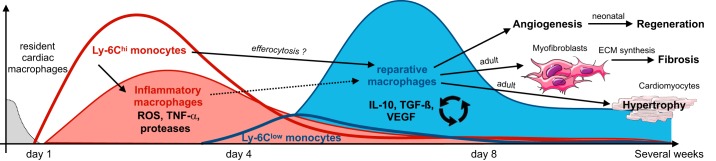
Ly-6Chi monocytes, unlike Ly-6Clow monocytes, can give rise to cardiac macrophages, which proliferate locally and replenish the cardiac-resident macrophages initially lost after MI (177). Together with Ly-6Chi monocytes, cardiac macrophages release inflammatory mediators and thereby propagate local inflammation. While Ly-6Chi monocytes are crucial to myocardial infarct healing, as they are responsible for digesting damaged tissue, they can also be detrimental due to excess production of inflammatory mediators. A rise in Ly-6Chi recruitment, as is the case in ApoE KO mice with chronically elevated Ly-6Chi monocyte levels, promotes an inflammatory cascade and post-MI heart failure (342).
In the second phase, post-MI, infiltrating Ly-6Chi monocytes differentiate to Ly-6Clow macrophages, mediated by Nr4a1 (177). Additionally, low numbers of Ly-6Clow monocytes are also directly recruited (177, 186, 320). At this stage, reparative macrophages predominate in the lesion and promote angiogenesis and fibrosis (436). Overall, studies show that the presence of both monocytes and macrophages is critical to balance between an initial inflammatory phase, needed for cellular debris clearance, and a subsequent reparative phase, needed for efficient fibrosis and angiogenesis (177, 320). The latter prevents ensuing heart failure, as we will describe in more detail when discussing macrophages in the context of MI. A better understanding of monocyte plasticity and function in inflammatory tissues may thus reveal novel therapeutic targets and strategies.
Stroke is similar to MI and atherosclerosis in that monocytes, but also neutrophils, strongly increase in circulation after ischemia. Their production in the bone marrow rises due to increased sympathetic tone and peaks 4 days after stroke (76). Extramedullary hematopoiesis in the spleen further contributes to increased monocytosis (95, 256). Monocyte recruitment from the spleen occurs within hours after stroke (256) and is highest after 7 days (128). CCL2 is again the main chemokine involved, and its deficiency results in reduced monocyte infiltration to the ischemic area, decreased infarction size, and fewer neurological deficits after stroke (90).
VI. GENERAL DESCRIPTION OF MACROPHAGES
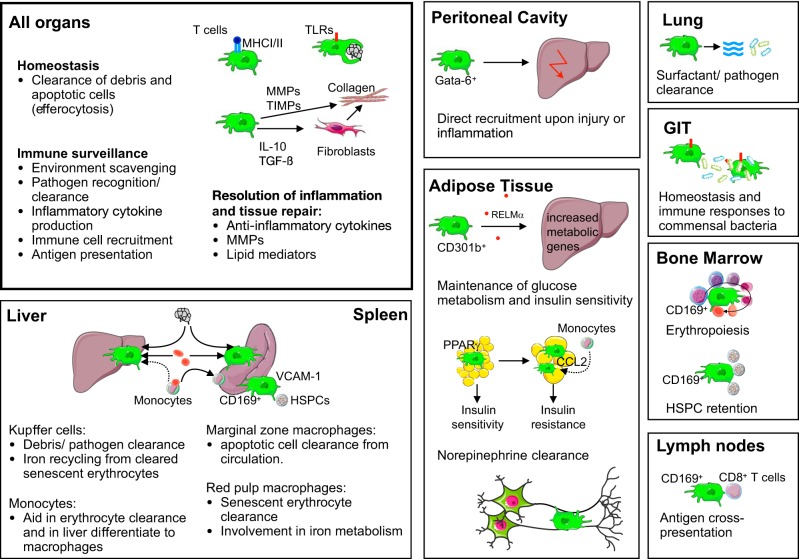
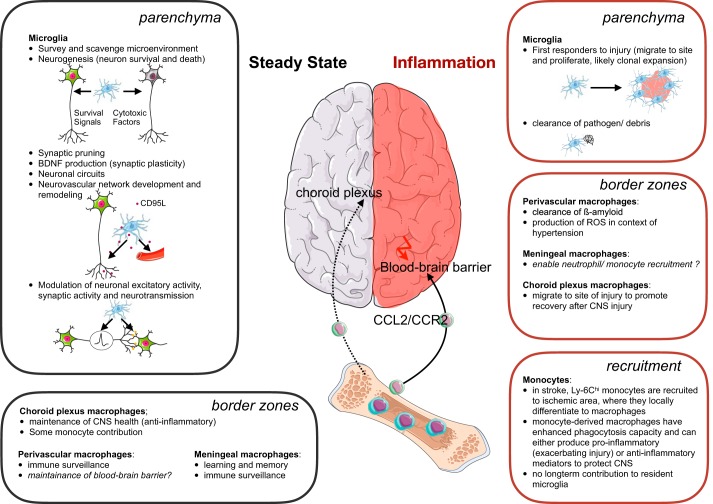
Tissue-resident macrophages are highly abundant in all organs, including the skin, gastrointestinal tract, lymphoid organs, and lungs (98). In the brain, for example, macrophages make up 5–15% of all cells (354). According to their location and function, macrophages have different names, such as Kupffer cells in the liver, microglia in the brain, and alveolar macrophages in the lung. Overall, all macrophages share several important functions: they play a role in tissue development and maintenance (e.g., by clearing apoptotic cells and debris), tissue surveillance and sampling, pathogen clearance, antigen presentation, inflammation resolution, and tissue repair. Depending on the organ or tissue localization, macrophages can fulfill several of these tasks and, as such, comprise a highly heterogeneous cell population with various phenotypes and functions (124) (FIGURE 1). One example of macrophage heterogeneity in steady state occurs in the spleen, which contains white pulp macrophages, red pulp macrophages, marginal zone macrophages, and metallophilic macrophages (44). With wider use of mass cytometry and single-cell RNA-seq, we will soon have a better understanding of myeloid heterogeneity at the cellular level in steady state. A recent CyTOF analysis of the liver reported two macrophage (Kupffer cell) populations, two infiltrating monocyte populations, two granulocyte populations, and four DC populations (86), while a CyTOF analysis of the brain revealed four additional previously unrecognized border zone macrophage subsets, which line the meninges, choroid plexus, and perivascular spaces in the brain (312).
FIGURE 1.
Plethora of macrophage functions. Macrophages have a vast number of functions in different tissues, in addition to their main functions they share in all organs. We highlight some new and exciting discoveries. GIT, gastrointestinal tract; HSPCs, hematopoietic stem and progenitor cells; MHC, major histocompatibility complex; MMPs, matrix metalloproteinases; PPAR, peroxisome proliferator-activated receptor; RELM, resistin-like molecule; TIMPs, tissue inhibitors of matrix metalloproteinases; TLRs, Toll-like receptors; VCAM-1, vascular cell adhesion molecule 1.
Two decades ago, the terms “M1” and “M2” were proposed to classify macrophage subsets in a way that described and simplified their phenotype and function in accordance with T-cell polarization. These terms were mainly based on the observation that macrophages from C57BL/6 mice, which have Th1-dominated immune responses, and Balb/c mice, which have Th2-dominated immune responses, had different responses to LPS and IFN-γ: M1 macrophages from C57BL/6 mice produced nitric oxide (NO) from arginine via iNOS, and M2 macrophages from Balb/c mice produced ornithine via arginase. Two types of macrophages, the thinking went, could be clearly distinguished based on their arginine metabolism (302). Ornithine promotes cell proliferation and is produced by macrophages in the context of wound healing or cancer, whereas macrophages produce NO to inhibit or kill pathogens or nearby cells. Thus a simple balance of arginine metabolism by macrophages greatly influences inflammation vs. its resolution; this explains why the M1/M2 paradigm proved helpful. Over time, the same classification became synonymous with classical (M1) and alternative (M2) macrophage polarization. M1 macrophages are induced by LPS and IFN-γ in a STAT1- and aerobic glycolysis-dependent manner (164), and M2 macrophages are induced by IL-4 in a STAT6-dependent manner that seems to rely on fatty acid oxidation (187, 475). Some well-accepted markers for these cells are iNOS, TNF-α, IL-1β, IL-12, and CD68 for M1 macrophages, and arginase 1, CD206, transforming growth factor (TGF)-β, IL-10, VEGF, Ym1, and Rtnla for M2 macrophages (311, 316). Interestingly, TGF-β itself regulates the balance between NO and ornithine production (99).
This macrophage classification was based on in vitro observations after stimulation with specific stimuli and assumed that macrophage polarization was linear with two extremes that went hand in hand with T-cell subsets: proinflammatory M1 macrophages that activate and are activated by Th1 cells, and anti-inflammatory M2 macrophages that activate and are activated by Th2 cells. This simplified classification has several shortcomings when translating it to in vivo observations. First, the pro- vs. anti-inflammatory classification is misleading. More specifically, M2 macrophages play a role in the inflammatory settings of wounds and allergies, and NO, when overproduced, can inhibit immune responses (301). Second, categorizing macrophages by a mere handful of markers ignores their complex nature and may even prevent proper identification of their phenotype and function. Third, macrophages in tissues never exist with only one phenotype. Lastly, macrophages may not clonally expand, and many heterogeneous “in-between” or even “out-of-spectrum” phenotypes exist, depending on the local tissue situation. Indeed, as macrophages in more complex settings and diseases were analyzed and more markers were profiled, it became clear that a dualistic M1/M2 macrophage classification is inappropriate. In an attempt to better categorize and describe macrophages, more macrophage phenotypes were suggested (e.g., M2a, M2b, M2c, and Mox) and placed along a linear spectrum between the M1 and M2 extremes (211, 282, 284, 506). Rather than clarifying macrophage phenotypes, however, these additional labels may have increased confusion, as many laboratories now use their preferred marker for the same subset, and macrophages are described by their activation/stimulation while their function is oversimplified as either inflammatory or anti-inflammatory. Furthermore, researchers were beginning to ask whether macrophages could shift their phenotype between a proinflammatory M1 macrophage and an inflammation-resolving repair M2 macrophage, or whether these are distinct, fully differentiated cells. Indeed, macrophage polarization is fluid, and many subsets express similar markers; put simply, there are no well-defined macrophage subsets in vivo. Over time it has been acknowledged that macrophages are highly plastic (433) and should be thought of as colors on a color wheel with many different “shades” (311). Three years ago, several renowned macrophage biologists suggested adopting a common macrophage-activation nomenclature based on macrophage origin, activation, and an agreed-upon collection of markers (315). This classification solved one shortcoming of the general M1 vs. M2 classification by including the stimuli. This at least addressed differences between M1 macrophages receiving different stimuli (e.g., LPS vs. IL-1β). However, while stimuli are readily identifiable in vitro, the situation in vivo is much more complex, with numerous stimuli and macrophages of different origins in the same tissue. This complexity has been efficiently demonstrated during disease, for example, by transcriptional analysis of human alveolar macrophages in the context of chronic obstructive pulmonary disease (506), as well as CyTOF analysis identifying 17 different macrophage subsets in clear cell renal cancer (62). Replacing M1 with M(LPS) and M2 with M(IL-4) is thus not the ultimate solution for in vivo contexts. Perhaps we should abandon such a classification altogether, as it seems impossible to accurately “label” macrophages because doing so fails to describe their actual context-dependent functions. Most importantly, an agreed-upon collection of markers runs the risk that researchers will simply check for these specific markers, give macrophages a label, and then fail to further analyze their function. Recent advances in understanding the impact of metabolic signals, circadian rhythm, and central influences on macrophages, as well as the ability to examine macrophages’ entire genome and transcriptome, even on a single-cell level, should underscore that experiments can only show a snapshot of macrophages at a specific time, and findings only capture a fraction of the myriad of functions a macrophage can adopt.
Overall, the real problem will remain how to accurately name in vivo stimulated macrophages. It may prove most beneficial to characterize macrophages by some of their functional attributes to refocus on the roles they play, homeostatic, resolving, reparative, in addition to their origin and the stressor to which they might have been exposed. As M1 has become synonymous with inflammatory and M2 with reparative macrophages, we expect this classification will persist, but caution should be taken when using it. More broadly, whatever classification we choose, we should take it for what it is: a helpful tool to grasp the almost infinite complexity of macrophage biology. For more detailed information on current macrophages classifications and a critical review thereof, see earlier reviews by Martinez and Gordon (284), Ginhoux et al. (137), Nahrendorf and Swirski (319).
VII. MACROPHAGE ORIGIN, HETEROGENEITY, AND FUNCTION IN THE STEADY STATE
According to earlier thinking, in the steady state tissue-resident macrophages are constantly replenished by circulating monocytes. We now know this holds only true for specific tissues and conditions. Intestinal lamina propria macrophages and uterine macrophages, for example, have a half-life of about three weeks and are constantly renewed from circulating Ly-6Chi monocytes (21, 378, 441). Overall, two key findings have challenged the model of the mononuclear phagocyte system: macrophages appear prenatally before definitive hematopoiesis is established, and they renew by local proliferation independent of monocytes (106, 418). In the healthy heart, for instance, <2% (105), in the aorta <17% (104), and in the lungs <5% (153) of macrophages are partially replenished from circulating monocytes, whereas microglia are not replenished at all by circulating monocytes (6, 135, 298). However, in inflammatory settings, this can substantially increase, and abundant Ly-6Chi monocytes invade inflamed tissues to give rise to macrophages.
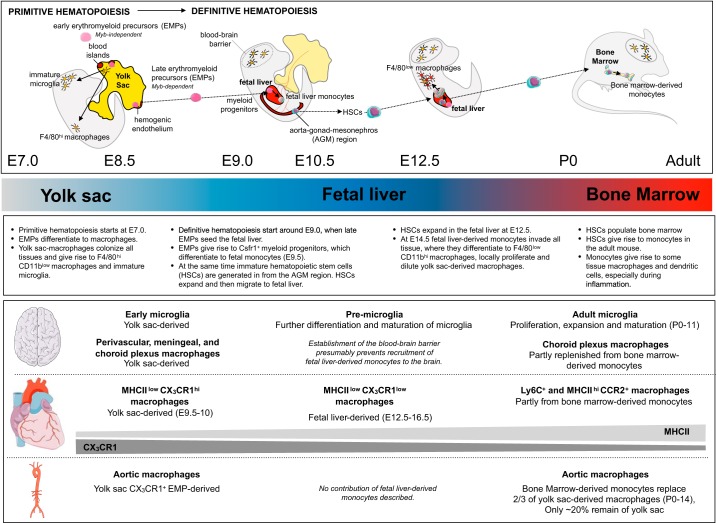
Embryonic hematopoiesis is conserved among species and is similar in zebrafish as well as humans (34, 228, 296, 424, 473). It can be divided into two main phases (FIGURE 2). First, early primitive hematopoiesis occurs in the extraembryonic yolk sac on embryonic days (E) 6.5 to E8.5 and is restricted to myeloid and erythroid development, giving rise directly to macrophages. Second, definitive hematopoiesis ensues in the fetal liver at around E9.0 and gives rise to macrophages via monocyte progenitors (355). The earliest progenitors to emerge have only myeloid or erythroid potential, presumably arise directly from the posterior plate mesoderm (yolk sac blood islands) in a Runx1-dependent manner around E7.0 and are distinct from blood island lining ECs. These myeloid progenitors are restricted to the yolk sac in their expansion and differentiation and have been suggested to give rise to early MYB-independent c-kit+CD115+ erythro-myeloid progenitors (EMPs). Alternatively, or additionally, late MYB-dependent c-kit+AA4.1+ EMPs could derive from yolk sac hemogenic endothelium around E8.5. The exact origin and temporal appearance of EMPs, however, is still not fully understood (260, 363). Yolk sac-derived macrophages seed the brain and all other peripheral tissues, where they have been suggested to be F4/80hi, they do not go through a monocyte precursor, and they rely on the transcription factor PU.1 (313, 403). After appearing in the yolk sac, EMPs can give rise to fetal macrophages and, as early as E9.0, start seeding the fetal liver, where they expand and acquire the potential to additionally differentiate to erythrocytes, megakaryocytes, granulocytes, mast cells, and monocytes. These late EMPs are the first definitive progenitors but lack characteristics of HSCs. Specifically, EMPs cannot self-renew, cannot give rise to the lymphoid lineage, and lack Sca-1. At this point, most tissues have already been colonized by early yolk sac-derived fetal macrophages (403). Fetal liver monocytes begin to strongly proliferate around E12.5 and colonize all peripheral tissues except the brain around E14.5, a process that has been found to depend on plasmalemmal vesicle-associated protein (372). On recruitment to tissues, monocyte-derived macrophages locally proliferate and dilute yolk sac-derived macrophages in tissues (403), as has been described for the heart (105). Interestingly, Langerhans cells are almost completely replaced by fetal liver-derived monocytes that persist throughout adult life (181). Simultaneously, at around E8.5, immature HSCs are produced in the para-aortic splanchnopleural region, which develops into the aorta-gonad-mesonephros region. These definite HSCs then migrate to the fetal liver at around E10.0, where they, in addition to the EMPs, generate monocytes (43, 87, 393, 529). Definite HSCs, which possess long-term self-renewal capacity, start to expand and differentiate in the fetal liver, and from E11.0 the fetal liver becomes the main site of embryonic hematopoiesis. Now all hematopoietic lineages, including monocytes, are being produced in the fetal liver. After birth, HSCs seed the bone marrow, which now becomes the main site of hematopoiesis (338). Adult definitive HSCs can then give rise to macrophages by going through a monocyte stage after birth in a process that depends on Flt3, among others (47).
FIGURE 2.
Macrophage origin in the brain and cardiovascular organs. The cartoon depicts important steps in the development of monocytes and tissue-resident macrophages. The main organ of hematopoiesis (yolk sac, fetal liver, and bone marrow) is indicated. The main steps in tissue-resident macrophage ontogeny, as well as the origin of specific macrophage subsets in brain, heart, and aorta, are highlighted below the schematic. Density gradients of major histocompatibility complex II (MHCII) and CX3CR1 in cardiac macrophages indicate that these decrease or increase with age, respectively. Overall proliferation capacity of cardiac macrophages decreases with age, and they are increasingly replenished by monocytes. AGM, aorta-gonad-mesonephros; E, embryonic day; EMPs, early erythromyeloid precursors; HSCs, hematopoietic stem cells. P, postnatal day.
Extensive fate mapping studies in the past 5 yr have clearly established that most adult tissue-resident macrophages are of embryonic origin and in the steady state are mainly maintained by local self-renewal, while monocytes contribute minimally to tissue-resident macrophages (37, 166, 180, 403, 516). Early indications of embryonic origin of some macrophages came from op/op mice, which lack M-CSF. While these mice have normal hematopoiesis until birth (331), they lack macrophages in many tissues. Microglia and splenic red pulp cells are mostly unaffected (40, 508), which already indicated at least a partial independence from not only M-CSF, but also adult hematopoiesis. Initial studies after irradiation showed that tissue-resident macrophages were derived from donor bone marrow, although some tissue-resident macrophages from the host may survive irradiation, then expand and repopulate tissues in the absence of donor macrophage contribution (166). This indicates that tissue repopulation by donor monocyte-derived macrophages is only possible by temporarily impairing host-derived, tissue-resident macrophages, as is also the case during inflammation, which we will discuss later. By fractioning the irradiation and thus reducing its effect, a larger proportion of macrophages remain of host origin (448). Further indication that macrophages in adults are not replenished by monocytes comes from parabiosis experiments, in which partner monocytes barely contribute to resident macrophages in the other mouse’s brain (6, 139, 166), heart (105, 172), aorta (104), lung (153, 166), bone marrow (166), and spleen (166) over the course of several months. Furthermore, fate mapping with mice that have conditional CX3CR1 promoter-driven Cre recombinase enables fluorescent labeling of bone marrow MDPs at a given time point. Postnatal labeling resulted in robustly labeled monocytes, but almost no label in CX3CR1− tissue-resident macrophages, further confirming that these macrophages in steady state are not derived from adult monocytes (516). Some subsets of macrophages and DCs in the intestine (21), skin (56, 444), and dermis (444) are constantly replenished by monocytes. Fate-mapping studies are difficult in humans; as such, insights into the origins of human tissue-resident macrophages are mostly made from observations after transplantations or in disease. Resident dermal and alveolar macrophages, for instance, seem to only minimally receive monocyte input, as after sex-mismatched bone marrow transplantation about one-fourth of recipient skin-resident macrophages remain up to 1 yr after transplantation, and their numbers are normal in patients with monocytopenia (37, 102, 159). This is analogous to mice (444) and indicates that similarities exist between mice and humans. Whether the same is true for cardiovascular macrophages is currently not known, but could, for example, be assessed after sex-mismatched heart transplantations.
Deficiency of PU.1, which is required for primitive macrophages, results in complete lack of tissue macrophages, while deficiency of Myb, required for definitive and adult hematopoiesis, omits HSCs without affecting F4/80hi macrophages in tissues, thereby suggesting that F4/80hi macrophages originate from the yolk sac (403). Yet fetal liver-derived macrophages could also give rise to F4/80hi macrophages, something not further addressed in the study by Schulz et al. (403). Fetal liver-derived progenitors may populate tissues and first give rise to more “naive” F4/80low macrophages, which are absent in Myb-deficient mice, that then differentiate to F4/80hi macrophages and outcompete yolk sac-derived macrophages. In the absence of definitive hematopoiesis, yolk sac-derived macrophages could simply persist longer. The fact that F4/80hi macrophages express markers of mature macrophages, while F4/80low macrophages express monocyte markers, could be evidence of this process. Genomewide expression analysis indicates that F4/80hi macrophages in tissues indeed cluster with yolk sac-derived-macrophages and not with F4/80low tissue macrophages, which are absent in Myb-deficient mice (403). Unfortunately, this study did not explore clustering with fetal liver-derived macrophages. Overall, current studies clearly show that most tissue macrophages are derived from fetal hematopoiesis, although the precise developmental stage of origin (hemogenic endothelium of the yolk sac or aorta-gonad-mesonephros) still needs to be elucidated. Some studies have further tried to resolve this. A recent study by Gomez Perdiguero et al. (141) made use of Tie2MeriCreMer mice crossed to Rosa26YFP, which express YFP in Tie2-expressing cells upon tamoxifen treatment, enabling fate mapping of their progeny. Because Tie2 is expressed by all progenitors regardless of yolk sac vs. fetal liver origin, administering tamoxifen on serial embryonic days labels the progeny of different embryonic progenitors. Interestingly, the later tamoxifen was given, the fewer resident macrophages were YFP positive, thereby indicating that tissue macrophages have early embryonic origin, specifically from a pre-E10.5 progenitor that loses its Tie2 expression thereafter. This progenitor was identified as the EMPs (141, 180). However, whether these EMPs give rise to macrophages when in the yolk sac or the fetal liver has not been clearly defined. A study by Hoeffel et al. (180) suggests that early Myb-independent EMPs from the yolk sac give rise to microglia, while later Myb-dependent EMPs from the fetal liver give rise to all other tissue-resident macrophages. Nonetheless, whether EMPs depend on Myb according to their location remains to be confirmed, especially since Myb-deficient mice have normal fetal tissue-derived macrophages (403). So far, only the origin of microglia and Langerhans cells has been clearly established: microglia derive from yolk sac EMPs (135, 224), and Langerhans cells derive from fetal liver monocytes (181). A recent study by Thion et al. (452) adds another dimension to macrophage ontogeny: the maternal microbiome influences microglia development in offspring, and this effect is sex specific. Maternal microbiome depletion affects microglia of male offspring more profoundly immediately after birth, whereas microglia of female offspring are more affected in adult life. Not only origin, but also environmental circumstances during development, thus shape macrophages.
Recent studies clearly demonstrate not only that steady-state, tissue-resident macrophages are mostly embryonic in origin, but also that macrophages proliferate locally for maintenance in the adult organism. BrdU incorporation studies show tissue-resident macrophage turnover largely depends on location. For example, substantial macrophage turnover in bone marrow, lungs, and peritoneum occurs within 3 wk (166), whereas turnover in the heart, aorta, and brain is slower (6, 104, 304). After partial depletion, resident macrophages in the brain and the lung quickly repopulates by local proliferation without monocyte input (6, 104, 166). M-CSF is crucial to bone marrow and peritoneal macrophage repopulation, whereas alveolar macrophages depend on GM-CSF in steady state and IL-4 during parasitic infections (166, 203). It seems that context, specifically the tissue and inflammatory status, determines which growth factors and cytokines are essential for local macrophage proliferation.
Although we know that tissue-resident macrophages have embryonic origins and are maintained by local proliferation in adulthood, the mechanisms orchestrating this are still not fully understood. Furthermore, it is unclear whether all macrophages in one tissue have the same proliferative capacity, or whether some cells have more stem cell-like features. Some tissues may contain an intrinsic “macrophage stem cell” that can give rise to local macrophages when needed, for example, after injury (100, 136). Considering recent studies suggesting that the environment shapes macrophage phenotype (132, 405), specific niches in tissues could maintain these stem cell-like macrophages. Clonal expansion has indeed been observed in Langerhans cells (131) and microglia (450), and it would be interesting to determine if a certain degree of clonality exists among cardiovascular macrophages.
Recent work has also addressed to what extent local microenvironment vs. cell origin contributes to the tissue-resident macrophages’ phenotype. Tissue-resident macrophages locally proliferate in the steady state, but during inflammation they can be replaced by monocytes. Monocyte-derived macrophages then acquire similar tissue-specific functions (86, 405). Interestingly, the tissue microenvironment directly modulates macrophages regardless of origin by inducing specific enhancer-associated histone modification landscapes (146, 247). Thus the tissue directly dictates a specific macrophage phenotype. For example, peritoneal macrophages transferred into the alveolar cavity (247) or macrophages restimulated ex vivo with factors from another tissue (146) can be reprogrammed to adopt the enhancer signature and phenotype of the new microenvironment. The open chromatin structure correlates with a specific tissue-resident macrophage expression of transcription factors: Mef2c for microglia, Lxra for Kupffer cells, Gata6 for peritoneal macrophages, and Pparg for alveolar macrophages (247). Notably, Gata6 has been described as specific to peritoneal macrophages (124, 337) and is required for peritoneal cell self-renewal and proliferation (382), suggesting that macrophages’ distinct transcriptional profiles are closely linked to important cell-specific functions.
A. Macrophages in Blood Vessels
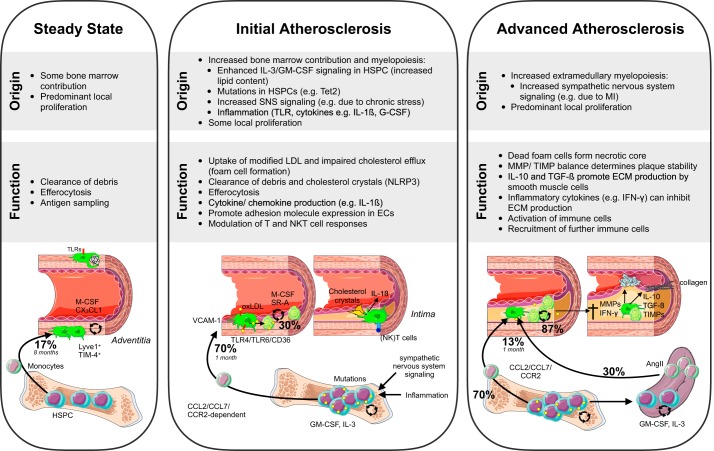
Resident macrophages found in the adventitia of the murine aorta develop in the embryo from yolk sac-derived CX3CR1+ EMPs. Circulating monocytes derived from Flt3-dependent postnatal definitive hematopoiesis replace these macrophages in the first 2 wk after birth (104). At birth, ~60% of all arterial macrophages are yolk sac derived, whereas, in the adult mouse, only ~20% are still yolk sac derived (104) (FIGURE 2). In adult mice, steady-state arterial resident macrophages are maintained mainly by local self-renewal, which depends on M-CSF and CX3CL1 produced by ECs and mesenchymal cells, while a small fraction, <17% of macrophages, derive from infiltrating monocytes over the course of 8 mo (104). Yolk sac- and bone marrow-derived arterial macrophages have similar self-renewal capacities. Interestingly, resident arterial macrophages, regardless of origin, are Lyve-1 positive. They show overall less phagocytic activity than macrophages derived from recruited monocytes during inflammation (104) (FIGURE 3). Isolating arterial macrophages in large numbers has been difficult, and initial analysis of arterial macrophages was limited to qPCR on total aorta and histology. The establishment of flow cytometry protocols for aortic macrophages (55, 120) enabled a better understanding of these cells; the recent advent of new techniques such as mass cytometry (38, 498) and single cell RNA-seq (69, 498) has already furthered a more detailed understanding of macrophage heterogeneity and function in the arterial wall.
FIGURE 3.
Macrophage origin and function in steady-state and diseased vessels. Aortic macrophage ontogeny and role during steady-state and atherosclerosis initiation and progression are illustrated for each stage. For simplicity, only the role of macrophages is depicted. The role of other immune cells, endothelial cells, and vascular smooth muscle cells is not shown. Percentages indicate contribution of monocyte recruitment or local proliferation to macrophage origin over the indicated amount of months. AngII, angiotensin II; ECs, endothelial cells; ECM, extracellular matrix; GM-CSF, granulocyte macrophage-CSF; HSPCs, hematopoietic stem and progenitors cells; LDL, low-density lipoprotein; M-CSF, macrophage-colony-stimulating factor; MHC, major histocompatibility complex; MI, myocardial infarction; MMPs, matrix metalloproteinases; NK, natural killer; NLRP, NACHT, LRR, and PYD, domains containing protein; oxLDL, oxidized LDL; SR-A, scavenger receptor A; Tet2, Tet methylcytosine dioxygenase 2; TGF, transforming growth factor; TIMPs, tissue inhibitors of matrix metalloproteinases; TLRs, Toll-like receptors.
The aortas in both mice (66) and humans (300) also contain large amounts of CD11c+ MHCII+ DCs, particularly in areas prone to atherosclerosis (120, 204). DCs in mice are in the subintimal space and can sample antigens from the vessel lumen, whereas macrophages are found in the adventitia in steady state. Two DC subsets exist in steady-state aortas: tolerogenic Flt3-signaling-dependent CD103+ Langerin+ CD11b− F4/80− CD8− CD205− CX3CR1− 33D1− and inflammatory M-CSF-dependent CD14+ CD11b+ F4/80+ CX3CR1+ TLR4+ DCs (65). DCs in the aorta expand during normal aging processes (270). Since this preferentially occurs in areas of disturbed blood flow, proliferation could be induced by local flow-associated stress, similar to macrophage proliferation induced by mechanical strain in the heart after MI (391). M-CSF-dependent DCs are monocyte derived, and their accumulation depends on CX3CR1 (65, 270), while Flt3 signaling-dependent DCs are monocyte-independent (65) and likely have embryonic origins. We still need fate mapping studies delineating DC development in the aorta and heart. Macrophages can also express CD11c, especially in hypercholesterolemic conditions, and DCs can also express CD11b and CX3CR1, making it difficult to distinguish these cells merely by surface markers. The difference in location may help to differentiate between the two cell types, at least in the steady state in vivo, but future studies on macrophage heterogeneity will need to take these cells into account as well.
B. Macrophages in the Heart
Cardiac macrophages are spindlelike cells in the interstitial space between myocytes, fibroblasts, and ECs (188). They are located throughout the entire heart, in both atria and ventricles, are closely associated to vessels, and enrich in the conducting system (188, 358). In the steady state, four main cardiac resident macrophage subsets can be distinguished by their expression of major histocompatibility complex (MHC) II, Ly-6C, CCR2, and CD11c. These are as follows: 1) Ly-6C− MHCIIhi CX3CR1hi CD206int MerTK+ CD11clow CCR2− CD64+ macrophages; 2) Ly-6C− MHCIIlow CX3CR1int CD206hi MerTK+ CD11clow CCR2− CD64+ macrophages; 3) Ly6C+ MHCIIhi/low CX3CR1hi CD206hi/int MerTK+ CD11clow CCR2− CD64+ macrophages; and 4) Ly-6C− MHCIIhi CX3CR1hi CD206int MerTK+ CD11chi CCR2+ CD103− CD64+ macrophages. Subsets 1 and 2 are the most abundant in the steady state. Subset 4 can also be considered a subfraction of subset 1 and constitutes 5–15% of these cells. Subset 3 is the smallest fraction of cardiac macrophages and only accounts for 2% of macrophages in the steady state. These subsets differ in origin (105).
MHCIIlow CX3CR1hi macrophages seed the heart as early as E9.5–10.5 and are followed by fetal liver-derived MHCIIlow CX3CR1low macrophages that seed the heart around E12.5–16.5; both postnatally upregulate their MHCII expression (FIGURE 2). Fate-mapping studies suggest that yolk sac- and fetal liver-derived macrophages can give rise to all cardiac macrophage subsets, with slightly more contribution to the MHCIIlow subset (304). Only MHCIIhi CD11chi CCR2+ and Ly6C+ macrophages are replenished by both local proliferation and monocyte recruitment (105). Expression of CX3CR1 and the number of positive macrophages decreases with age, while MHCIIhi macrophage levels increase with age. At birth, almost all macrophages are MHCIIlow CX3CR1hi, maintained by local proliferation, and estimated to turn over every month, but with age this subset is progressively lost, whereas all other subsets expand (105, 172, 304).
As mice age, the proliferation capacity of local cardiac macrophages diminishes and is, for example, 10-fold lower in mice that are over 2 mo old vs. newborns (304). Cardiac macrophages are gradually, albeit very slowly, replenished by circulating monocytes, which preferentially differentiate to MHCIIhi macrophages (304). Since monocyte contribution to the local macrophage pool seems to depend on local niche availability (405), mechanical stresses in the heart may be the cause for local macrophage turnover in steady state. Vast depletion of local macrophages can open niches that cannot be replaced by macrophage proliferation and result in monocytes recruitment to the heart, where they can give rise to self-sustaining, monocyte-derived macrophages (105). Monocytes give rise to MHCIIhi macrophages, whereas embryonic macrophages are mostly MHCIIlow. This shift coincides with the heart’s age-induced loss of regeneration capacity, indicating that embryonic macrophages may be better at tissue repair. The risk of heart failure increases with age; the change in cardiac resident macrophages may contribute to this risk. For instance, monocyte-derived macrophages may assume more profibrotic phenotypes (304). Interestingly, like the brain, lung, aorta, and liver, the heart contains yolk sac-derived macrophages into adulthood (105, 304).
Global gene expression analysis that compared cardiac macrophages to CX3CR1+ cells from spleen and brain revealed that cardiac macrophages have enriched genes involved in immunomodulation, tissue homeostasis, angiogenesis, and phagocytosis (358) (FIGURE 4). In development, embryonically derived CCR2− cardiac macrophages play a crucial role in vascular remodeling of the primitive coronary plexus (252). In the steady-state adult mouse heart, functional analysis of cardiac macrophages showed their overall function to be clearing debris and pathogens (172). MHCIIlow macrophages are the most efficient phagocytes, whereas MHCIIhi macrophages are most efficient in antigen presentation and T-cell activation. Of the MHCIIhi macrophages, CD11chi macrophages specifically produce IL-1β (105). This indicates that MHCIIlow macrophages may play a predominant role in tissue homeostasis and innate immunity, whereas MHCIIhi macrophages are important in immune surveillance and shaping adaptive immunity, further illustrating why a deeper understanding of macrophage heterogeneity is important for developing novel therapeutic strategies.
FIGURE 4.
Macrophage origin and function in the steady-state and inflamed heart. Cardiac macrophage ontogeny and function during steady state and inflammation are depicted in the inset. For simplicity, only the role of macrophages is depicted. Macrophage turnover in the heart is governed by macrophage-colony-stimulating factor (M-CSF) and CX3CL1. A variety of soluble factors are increased in circulation after myocardial infarction, listed in the box, which result in monocyte recruitment and further increase hematopoiesis, in addition to autonomic signaling. Proliferation and differentiation of CD131+ and CCR2+ hematopoietic stem and progenitors cells (HSPCs) is increased in the bone marrow. A decrease of retention factors, for example, CXCL12, allows for mobilization of CCR2+ HSPCs to the spleen, where they are retained, for example, via vascular cell adhesion molecule 1 (VCAM-1), and further proliferate and differentiate. Monocyte release from the bone marrow is CCL2/CCR2 dependent, while release from the spleen is angiotensin II dependent. Percentages indicate contribution of bone marrow and spleen to monocytes recruited to the infarct area. CCR2+ macrophages have been indicated to recruit neutrophils, whereas B cells were found to recruit monocytes via CCL7. AT-II, angiotensin II; Cx, connexin; M-CSF, macrophage-colony-stimulating factor; MHC, major histocompatibility complex; MMPs, matrix metalloproteinases; TIMPs, tissue inhibitors of matrix metalloproteinases; TLRs, Toll-like receptors.
DCs are also present in the heart. There are three types of DCs: IRF8-dependent conventional, IRF4-dependent conventional, and monocyte-derived DCs (45). During MI, DCs increase in the heart, and IRF4-dependent conventional DCs exacerbate MI by inducing autoreactive T cells in draining lymph nodes (45). Interestingly, adoptively transferred tolerogenic, infarcted myocardium-primed DCs can induce regulatory T cells, thereby shifting macrophages toward a reparative phenotype and increasing infarct healing (67). These studies indicate that targeting DCs could have therapeutic potential in the setting of MI, and future studies will also need to address the complexity of DC phenotypes in the heart and their relationship to macrophages to better understand how we could harness them for therapy.
Hulsmans et al. recently showed that cardiac macrophages residing in the conducting system facilitate action potential propagation in the murine heart (188). Macrophages are directly electrotonically coupled with cardiomyocytes’ cell plasma via connexin 43-containing gap junctions that enable electrical charge exchanges. This affects both cells: macrophages coupled to cardiomyocytes are rhythmically depolarized, and resting cardiomyocytes show a more positive depolarized resting membrane potential. The interaction reduces the action potential upstroke and overshoot, enables an earlier repolarization, and consequently shortens the cardiomyocyte action potential and refractory period. Macrophage-cardiomyocyte coupling, therefore, facilitates higher rates of conducted beats, but may also support an electrical charge exchange between noncoupled cardiomyocytes by bridging through macrophages. While macrophages in the atrioventricular node comprise all cardiac macrophage subsets, it would be interesting to know whether a specific subset is enriched for connexin-43 and possibly facilitates establishment of the conducting system in development. Moreover, we do not yet know how inflammation affects macrophage’s involvement in conduction and whether the macrophages that are present in the human conduction system (188) have similar functions to those in mice. This is another example of macrophages’ ability to adopt tissue-specific functions, as has been described for iron recycling from stressed and senescent erythrocytes by monocyte-derived macrophages (451), surfactant clearance by alveolar macrophages (416), maintenance of glucose metabolism by CD301b+ macrophages (237), and synaptic pruning by microglia (343). Thermoregulation by adipose tissue macrophages remains controversial (111, 329). Recent work delineating adipose tissue macrophages indicates that, while macrophages may not produce norepinephrine themselves, a subpopulation of adipose tissue macrophages associated with sympathetic neurons may function as “norepinephrine sinks” and could thereby directly contribute to obesity. Preventing norepinephrine uptake by these sympathetic neuron-associated macrophages reduces energy storage and increases thermogenesis, results that show macrophages can influence thermogenesis via modulated norepinephrine availability and, consequently, adipocyte metabolism (359).
C. Macrophages in the Brain
Microglia are found in the embryonic brain, which is the first organ to be populated by macrophages (417, 423). As previously mentioned, microglia are, as far as we know, the only macrophage population that derives entirely from yolk sac EMPs, which depend on the transcription factors PU.1, IRF8, and Runx1 and are independent of MYB (135, 141, 180, 224, 363, 414). Microglia development begins as early as E7.5 (364) and c-kit+ CD45low EMPs give rise to a macrophage progenitor population that is initially low in CX3CR1 and F4/80 and then upregulates these proteins (260, 363) (FIGURE 2). Assay for transposase-accessible chromatin followed by sequencing (ATAC-seq), chromatin immunoprecipitation followed by sequencing (ChIP-seq), and RNA-seq has revealed at least three temporal stages of microglia development and phenotype, with, for example, microglial MAFB being important in adult brain homeostasis (287). In steady state, an intact blood-brain barrier prevents monocytes from entering the central nervous system (CNS), and, throughout the life of an organism, microglia completely self-renew at a very low rate (6, 139) that can be substantially increased after an insult (450). A recent study by Tay et al. (450) found that, while microglia expansion is low in steady state, individual microglia populations show heterogeneity: microglia from the hippocampus and olfactory bulb have higher turnover rates than those from the cortex, hypothalamus, midbrain, and cerebellum. Early studies indicated that irradiation results in a slow repopulation of microglia by bone marrow donor cells, but it must be noted that these donor-derived microglia were barely found in the parenchyma (6, 222, 233). These results accord with the current concept that Ly-6Chi monocytes contribute to macrophage maintenance in the choroid plexus only (139). Additionally, irradiation creates several artifacts, such as impairment of local microglia proliferation and blood-brain barrier defects. Using the alkylating agent busulfan, which does not disrupt the blood-brain barrier, only a small recruitment of bone marrow-derived cells to the brain can be observed (225). Microglia also depend on Csf1r (CD115) signaling (100, 135). Inhibiting Csf1r signaling almost completely reduces microglia, which completely repopulate in a very short time once Csf1r signaling is restored. IL-34-deficient mice also have reduced microglia, but no changes in monocytes or other macrophages (149, 485). Similarly, microglia depletion by the CX3CR1CreER:iDTR system results in a fast bone marrow-independent repopulation of microglia within 1 wk, which occurs in clusters and is dependent on IL-1 receptor signaling (52). Microglia are thus in steady state independent of bone marrow hematopoiesis and have a slow turnover rate (249), which can dramatically increase after their depletion or an injury to the brain. The dramatic increase in proliferation occurs in clusters and likely relies on clonal expansion, as has been observed in the context of facial nerve injury (450).
Microglia play a crucial role in brain development and homeostasis as they actively survey and scavenge their microenvironment (332) (FIGURE 5). They provide survival signals for neurons (460), but also produce cytotoxic factors to induce neuronal cell death (283) and thereby modulate postnatal neurogenesis. Microglia are also actively involved in the synaptic pruning that ensures proper synaptic maturation and postnatal brain development (343, 503). In addition to postnatal pruning, microglia produce brain-derived neurotrophic factor, which regulates and enables learning-dependent synapse plasticity, postnatally, in early adulthood (345) and in response to exercise (501). Additionally, macrophage-derived CD95L promotes the establishment of a proper neurovascular network during development (61). In adults, microglia produce several signaling molecules that influence neuronal excitatory activity (262, 346), as well as synaptic activity and neurotransmission (29). Aberrant microglia function can result in several neurodegenerative disorders (503). Genetic variations of Trem2 or CD33, which are specifically expressed on myeloid cells, hamper microglia responses to amyloid plaques in Alzheimer’s disease and worsen disease outcome (150, 442, 513). Other mutations associated with microgliopathies include Hoxb8, USP18, and Csf1r (363).
FIGURE 5.
Macrophage origin and function in the steady-state and injured brain. Microglia and border zone macrophage functions are illustrated in steady state and inflammation. Monocytes do not contribute to most central nervous system (CNS) macrophages in the steady state, with the exception of choroid plexus macrophages. After injury or stroke, the blood-brain barrier is disrupted, and monocytes can be recruited in large numbers and transiently give rise to macrophages. BDNF, brain-derived neurotrophic factor; ROS, reactive oxygen species.
While the brain parenchyma is filled with microglia, brain border zones host other macrophage types. These include meningeal, perivascular (between endothelium and astrocytic end feet), and choroid plexus macrophages (139, 371). Similar to microglia, these other macrophages are initially derived from the yolk sac (139, 363), but could be further derived from fetal liver monocytes, as is the case in zebrafish (180), and are mostly distinguished by their localization and morphology, as there are no known markers for each subset. While microglia have predominantly cytosolic MHCII molecules, the brain border zone macrophages sample their environment, express MHCII molecules and costimulatory molecules, and can thus present antigens to T cells (371). Perivascular and meningeal macrophages are maintained through local gradual self-renewal, while choroid plexus macrophages can derive from circulating monocytes and show more substantial turnover (139). Only a few functions of border zone macrophages are currently known (FIGURE 5). Perivascular macrophages are involved in clearing β-amyloid from the CNS (169) and may also help maintain the blood-brain barrier by contributing to vessel barrier integrity, similar to macrophages found around mesenteric capillaries (171). Meningeal macrophages support learning and memory by sustaining an anti-inflammatory environment, and choroid plexus macrophages seem to help maintain overall CNS health (175). Macrophages in the choroid plexus have an anti-inflammatory phenotype, and since the choroid plexus is a neuroimmunological interface and the site of education for infiltrating immune cells (175), macrophages therein may play a substantial role in immunomodulation. Interestingly, monocytes/macrophages originating from the choroid plexus migrate to sites of injury in the CNS to promote recovery after spinal cord damage (412) or brain injury (404).
Recent studies employing CyTOF analysis demonstrate the heterogeneity of macrophages and immune cell populations in the brain, clearly showing we still have much to learn about different macrophage populations and their functions (7, 230, 312). Indeed, a recent transcriptomic and epigenomic analysis of microglia from humans and mice revealed a core microglia signature of 881 genes (147). Comparison of these genes to microglia in different diseases revealed disease-specific genes, proving this could be a valuable tool to establish microglia involvement in certain diseases and to enable drug discovery. Single-cell RNA-seq can further help to identify specific markers for CNS macrophage subsets, such as the recently described transmembrane protein 119 (TMEM119) for microglia (32), or help to classify microglia populations with specific functions, such as the newly described Alzheimer’s disease-restricting microglia (223). These novel insights will aid future research into diverse roles of these subsets. For a more thorough review of current literature on myeloid cells in the CNS, see Prinz et al. (363), Réu et al. (376), and Li and Barres (260).
VIII. MACROPHAGE ORIGIN, HETEROGENEITY, AND FUNCTION IN CARDIOVASCULAR DISEASE
In contrast to steady state, upon inflammation, tissue injury, or (artificial) macrophage depletion, monocytes are readily recruited to tissues and can give rise to monocyte-derived macrophages, replacing embryonically derived macrophages that undergo local cell death (39, 105, 166, 172, 451). Whether these monocyte-derived macrophages eventually integrate into tissues seems to depend on the tissue they colonize and the type of injury: while monocyte-derived macrophages do not integrate into the CNS after injury (5), they do integrate into the resident macrophage pool of the peritoneal cavity after thioglycollate challenge (516), the cardiac macrophage pool after MI (172), and the liver after specific Clec4f+ Kupffer cell depletion (405). However, monocyte-derived Kupffer cells’ proliferation was in competition with remaining embryonically derived Kupffer cells when these were not fully depleted (405). This study demonstrates that monocyte differentiation to macrophages and population of tissues during inflammation may depend on several components, such as growth factors and cytokines available in their niche. This could also explain why monocytes readily repopulate resident macrophages’ empty niches after lethal irradiation.
In addition to monocyte recruitment, a recent study showed a subset of Gata-6+ peritoneal cavity macrophages were recruited to help resolve inflammation in the setting of sterile liver injury (483). This adds another dimension to macrophage origin, as these macrophages, like monocytes, can be directly and quickly recruited to sites of inflammation without having to go through circulation. Such a backup battalion of macrophages may exist not only in the peritoneal cavity but perhaps also in the pericardial and pleural space. Whether these peritoneal macrophages have embryonic origins likely depends on age: after birth, peritoneal macrophages are increasingly replaced by bone marrow-derived monocytes with high proliferative capacity (21).
A. Macrophages in Vascular Disease and Atherosclerosis
Macrophages present in the normal arterial wall can contribute locally to homeostasis but also respond to stressors. Any kind of injury to vessels results in endothelial activation and increases monocytes and macrophages. For example, hemodynamic stress in the arterial wall leads to a large CXCR3-dependent spike in perivascular adventitial macrophages, which are likely, at least partly, to be derived from recruited monocytes. These macrophages are crucial to vascular remodeling (525). During exposure to LPS or sepsis, arterial macrophages initially diminish, perhaps providing room for infiltrating monocytes that have better phagocytic capacity, and help clear the infection, only to later repopulate (104). Such behavior may enable a rapid and drastic change to the prevalent macrophage phenotype, as recruiting inflammatory monocyte-derived macrophages could be faster than reprogramming tissue-resident macrophages on a large scale.
The most studied arterial disease is atherosclerosis (FIGURE 3). Macrophages are present in all disease stages (148) and play a central role by promoting lipid accumulation, increasing inflammation, and eventually contributing to plaque destabilization. Mice that either lack monocyte/macrophages or have had these cells depleted show dramatically less disease initiation and progression (421, 432), exemplifying macrophages’ fundamental role in the disease. Macrophage origin during the early stages of disease development has been intensively dissected. As previously discussed, monocyte infiltration and differentiation to foam cells are early hallmarks of atherosclerotic plaques in mice and humans (41). Circulating monocyte numbers directly correlate with early plaque size, and multiple studies over the last decades have shown that preventing monocyte recruitment reduces atherosclerotic plaques and macrophage content (46, 71, 152). Because many years of research focused on early lesion development, it was long assumed that monocytes were the only source of lesional macrophages, and that atherosclerosis could be prevented by simply inhibiting monocyte recruitment. While this is true for early lesion development (438) and perhaps an inflammatory burst that leads to rupture and erosion, we are starting to appreciate that atherosclerosis is more complex. As Western society becomes increasingly obese, and young children already present with atherosclerotic lesions, the field is now focusing on better understanding lesion progression. Early indications that local macrophage proliferation may dominate in advanced atherosclerotic lesions came from studies showing that preventing monocyte recruitment in late stages of the disease could not halt or reduce lesion progression (154). In 1948, foam cells were found to be dividing in rabbit atherosclerotic lesions (289), and studies in the 1990s confirmed macrophage proliferation also occurs in humans (50, 143, 217). Interestingly, investigating overall immune cell infiltrate shows macrophages predominantly proliferate in advanced atherosclerotic lesions (258, 375). With new fate-mapping techniques available, Robbins et al. addressed the contribution of local macrophage proliferation at different stages of atherosclerotic plaque development (380). While most macrophages (~70%) in early lesions are directly derived from monocytes, macrophages in established plaques depend less on monocyte recruitment, as ~87% are derived from local macrophage proliferation (380, 432). However, even in established lesions, all macrophages are descendants of recruited monocytes (380). This suggests that longer or more dramatic monocyte reduction could affect established lesions, which may explain why some studies find that monocyte recruitment affects lesion progression (407), while others do not (154, 432). The role of monocyte recruitment and macrophage turnover in lesion regression still needs further examination. On the one hand, treating ApoE KO mice with an apoE-encoding adenoviral vector prevents monocyte recruitment and induces lesion regression (362). On the other hand, in an aortic transplantation model, recipient Ly-6Chi monocyte-derived macrophages are vital to inducing plaque regression by producing anti-inflammatory mediators (367). Additionally, after transplantation, monocyte-derived cells have been proposed to contribute to regression by emigrating from lesions (271). However, as both models have clear caveats, future studies will need to address whether these observations are artifacts of these models. We will need to further decipher monocyte/macrophage dynamics in settings of atherosclerotic lesion regression, a topic with high clinical relevance. It would also be interesting to determine whether, and when, embryonic macrophages are completely lost, and whether this affects plaque composition. Moreover, studies employing novel techniques, such as mass cytometry, will likely shed light on macrophage population complexity in the plaque, including the question of whether or not clonality exists, and to what degree. Recent studies associate clonal hematopoiesis of indeterminate potential (CHIP) with coronary heart disease and identify specific mutations (TET2, DNMT3A, ASZL1, and JAK2) that increase HSPC expansion, clonality, and ultimately atherosclerosis (119, 200); perhaps myeloid cell clonality also increases at the lesional level.
Lesional macrophages’ microenvironment may influence their proliferation. For example, oxLDL promotes macrophage proliferation in vitro (157). Furthermore, lesional macrophage proliferation in ApoE KO mice on a high-cholesterol diet is higher than in ApoE KO mice on a chow diet, and proliferation lowers in scavenger receptor (SR)-A-deficient macrophages (380). Interestingly, a later study by Lhotak et al. (258) confirmed local macrophage proliferation in atherosclerotic lesions, but found that in ApoE KO mice on a chow diet, macrophage proliferation is more predominant in early stages of lesion development. This adds another dimension to macrophage proliferation: it initiates lesions early on, but later exacerbates lesion progression in a high-cholesterol setting. Future studies will need to address how translatable this dynamic is to human disease, in which arteries already have diffuse intimal thickening (322), so that local early intimal macrophage proliferation may contribute more substantially to plaque initiation than in the mouse. Indeed, DNA synthesis, mostly corresponding to macrophages, has a biphasic pattern in human plaques: an initial increase in (early) type II lesions in foam cell-rich areas, and an increase in late (complicated, ruptured) type VI lesions, mostly in the rupture-prone shoulder regions (273). Despite the potential species differences in proliferation peaks, inhibiting macrophage proliferation is a promising strategy to reduce lesion progression. High-density lipoprotein (HDL) nanoparticle-based delivery of simvastatin to macrophages reduced lesion progression in advanced atherosclerosis by decreasing macrophage proliferation (446). However, as simvastatin also has anti-inflammatory effects, further studies are warranted.
Macrophages in atherosclerotic lesions play a crucial role in clearing debris, such as modified lipids. OxLDL can be efficiently cleared by macrophages, which recognize it via SRs, including SR-A and CD36 (309), which together mediate up to 90% of OxLDL uptake in vitro (238). Interestingly, native LDL particles (236) and cholesterol crystals (91) can also be taken up by macrophages and contribute to foam cell formation. Cholesterol crystals also potently activate the inflammasome, resulting in strong IL-1β responses (91) that further promote local inflammation. These foam cells eventually undergo apoptosis and, due to inefficient clearance by local macrophages, contribute to necrotic core formation and lesion progression (308, 309). Because of its central role in lesion initiation and progression, LDL is accepted as the key contributor in the pathogenesis of atherosclerosis. OxLDL, once cleared by macrophages, is delivered to lysosomes, where lysosomal acid lipase facilitates the hydrolysis of cholesterol esters to free cholesterol and fatty acids. Free cholesterol is then either effluxed or reesterified by acyl-CoA cholesterol ester transferase (ACAT) and stored in cytosolic lipid droplets, if an appropriate acceptor is lacking (309). In atherosclerotic lesions, where efflux is not sufficient, cholesterol esters are stored in lipid droplets to maintain proper cell function. Lipid droplet cholesterol in foam cells undergoes repeated esterification and hydrolysis cycles that are mediated by ACAT and neutral hydrolases. Furthermore, lipid droplets can be engulfed by autophagy and delivered to the lysosome, resulting in a breakdown by lysosomal acid lipase and cholesterol availability (339). This ensures that, when cholesterol is needed for cell membranes or a cholesterol acceptor outside the cell is finally present, cholesterol can be used or effluxed out of the cell. At the same time, as abundant cholesterol accumulates in macrophages, negative feedback mechanisms reduce cholesterol synthesis and uptake (340). Excessive cholesterol also results in oxysterols (oxygenated derivatives of cholesterol) binding to liver X receptor (LXR) and activating downstream signaling. LXR signaling induces three classes of target genes to help eliminate excess cholesterol: ATP-binding cassette (ABC) transporter genes and ApoE both promote cholesterol efflux, and fatty acid synthesis genes promote cholesterol reesterification. Cholesterol efflux has been extensively studied, and the best described transporters are ABCA1 and ABCG1 (308). ApoA-I, the major apolipoprotein of HDL, can accept cholesterol esters from ABCA1 to form nascent HDL. Lecithin cholesterol acyltransferase then mediates free-cholesterol esterification and converts nascent HDL to mature HDL, which can accept free cholesterol from ABCG1 and SR-B1. HDL facilitates the transport of cholesterol esters back to the liver for either excretion via the bile or reuse during lipoprotein assembly.
Cholesterol efflux importance in macrophages has been demonstrated by reconstituting LDLr KO mice with ABCA1 KO or ABCA1 ABCG1 double KO bone marrow (518). These mice show increased foam cell formation and atherosclerosis. In contrast, the consequences of ABCG1 deficiency alone are less clear (22, 295, 369), perhaps due to compensatory upregulation of ABCA1. Targeting LXR has been extensively studied, and agonists, such as T0901317 and GW3965, can lower cholesterol levels and thereby reduce atherosclerosis development (10, 206). However, these agonists induce triglyceridemia and hepatic steatosis, and thus either more specific agonists or more specific targeting should prove beneficial. The latter has been addressed in a recent study in which GW3965 delivered to macrophages by nanoparticles decreased their cholesterol loading without toxic effects on the liver (445). Indeed, reducing macrophages’ lipid storage (350, 360) or increasing cholesterol efflux (445, 447) attenuates atherosclerosis. Infusion of either apoA-I or HDL can also increase cholesterol excretion and reduce coronary artery disease in humans (447).
Interestingly, LXR activation provides beneficial anti-inflammatory effects independent of myeloid ABCA1 and ABCG1 (1, 216). Desmosterol, a cholesterol-precursor present in oxLDL, induces this anti-inflammatory macrophage phenotype through LXR activation (425). Additionally, independent of LXRs, but dependent on NRF2, exposing macrophages to oxLDL reduced proinflammatory cytokine transcription in the late stages of TLR-induced responses, further supporting the idea that oxLDL itself may have anti-inflammatory effects (205). Plaque macrophages’ microenvironment thus clearly provides proinflammatory signals that supersede these beneficial effects.
In addition to oxLDL, macrophages clear cellular debris. Uptake of apoptotic cells, also called efferocytosis, is enabled by recognition of so-called “eat-me” signals on apoptotic cells’ membrane. These signals consist of modified membrane molecules, such as altered carbohydrates and oxidized molecules that resemble oxLDL (60), and newly exposed molecules, such as phosphatidylserine (PS) (109). Efferocytosis results in elevated LXR signaling, which in turn increases Mertk and promotes efferocytosis (1). Clearing apoptotic cells itself induces an anti-inflammatory phenotype in macrophages (481). Efferocytosis is impaired during atherosclerosis for many reasons (402): 1) apoptotic cells and oxLDL are recognized by the same receptors, and excess oxLDL may thus reduce efferocytosis; 2) because oxLDL contains a high amount of lysophosphatidylcholine, a chemoattractant that recruits phagocytes to apoptotic cells, oxLDL could effectively distract from apoptotic cells; 3) efferocytosis occurring in an inflammatory environment may skew cell responses toward inflammation; and 4) phagocytes may be exposed to maturation signals and could lose their phagocytic capacities. If apoptotic cells are not cleared, they undergo secondary necrosis, which further promotes inflammation (402). Human lesions have high numbers of apoptotic cells that are not cleared by adjacent phagocytes (402), thereby indicating that human plaques lack proper efferocytosis. Proof-of-concept studies have shown that lack of receptors (Mertk, milk fat globule-epidermal growth factor 8, C1qa, and TIM-3) involved in recognizing apoptotic cells results in increased plaque development (3, 4, 113, 456). Conversely, administering apoptotic cells or PS-containing liposomes slows disease development and progression (118, 184). Similarly, blocking CD47, a key “do-not-eat-me” signal, restores efferocytosis and reduces atherosclerosis (229).
In addition to their roles in lipid metabolism and clearing cellular debris in the arterial wall, macrophages are also involved in modulating local immune responses. Macrophages express several PRRs by which they can sense PAMPs or DAMPs in lesions. Upon sensing, macrophages are activated and produce proinflammatory cytokines. TLR-4 in particular has been implicated in inducing proinflammatory cytokine production in lesional macrophages by recognition of oxLDL, in conjunction with CD36 and TLR-6 (429) and minimally modified LDL via CD14 and MD-2 (299). Interestingly, lipids trigger increased TLR-4, possibly linking lipid metabolism to inflammation (505). Macrophages can also present antigens on MHCI and MHCII molecules and interact with memory/effector CD8+ T cells and CD4+ T cells, respectively (263). Additionally, foam cells express CD1d, which presents lipid antigens and allows interaction with NKT cells (470).
Macrophages further influence the plaque environment and plaque stability by their production of matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs (TIMPs). In advanced plaques, an increased amount of MMPs and a lack of TIMPs can promote extracellular matrix degradation and fibrous cap thinning (327). The latter often occurs in plaque shoulder regions, which are enriched for monocytes/macrophages (327). The role of MMPs and TIMPs in vascular disease has been previously extensively reviewed (327, 484). Macrophages can instigate collagen production by smooth muscle cells, for example, by production of TGF-β (309). In addition, dying macrophages release tissue factor, which, upon plaque rupture, initiates the coagulation cascade and thrombus formation (308). Currently, however, commonly used mouse models do not develop true plaque rupture and mainly address plaque collagen content and fibrous cap thickness. We currently can thus rely mostly on histopathological samples from patients to identify characteristics of unstable vs. stable plaques. Mice with spontaneous plaque rupture, such as the HypoE/SR-BI double KO mice (174), will enable analysis of mechanisms underlying plaque rupture.
After years of unravelling how macrophages shape atherosclerotic lesions and immune responses, we are now taking on a new task: understanding how the microenvironment affects and shapes macrophages themselves. There has been considerable recent interest in how clearing debris, oxLDL, and other DAMPs affects macrophages, specifically their metabolic and epigenetic reprogramming. For example, metabolic pathway reprogramming occurs after TLR-4 stimulation. LPS promotes a switch from oxidative phosphorylation to aerobic glycolysis (also called the Warburg effect) and decreases tricarboxylic acid (TCA) cycle activity in phagocytes (234), which enables a quick, although inefficient, ATP production to meet the macrophages’ demand (221). Similarly, hypoxia induces glycolysis (221). Increased glycolysis is observed in inflammatory macrophages, while anti-inflammatory macrophages rely on oxidative phosphorylation, indicating that metabolic state is linked to phenotype (125). Macrophages in atherosclerotic plaques are exposed to multiple stimuli that induce metabolic changes. In addition to changing their metabolism, macrophages’ encounters with DAMPs result in long-term innate immune memory known as “trained immunity.” Epigenetic changes, for example, methylation and acetylation of histones, after a first encounter induce a lasting phenotypic change that enables macrophages to respond more vigorously to a second encounter (387). Intriguingly, TCA cycle intermediates act as antagonists of DNA demethylases (430). Efforts to better decipher what induces metabolic and epigenetic reprogramming in plaque macrophages and how this affects macrophage phenotype and proliferation will uncover new therapeutic opportunities. For an extensive review on immune cell metabolism, see O’Neill et al. (335), and for discussions of metabolism, trained immunity, and their interplay in atherosclerosis, see Groh et al. (151), Stienstra et al. (430), and Phan et al. (357).
Macrophage phenotype can vary dramatically within the same lesion. Proinflammatory macrophages localize to rupture-prone regions and the necrotic core (431), and anti-inflammatory macrophages are located in the adventitia (63, 251). In patients with unstable angina, lesions contained more proinflammatory macrophages (465). While transcriptional profiling of total lesional macrophages may indicate how macrophages change overall in response to increased lipid content and inflammation (142), single-cell resolution by single-cell RNA-seq or mass cytometry will allow us to fully understand the complexity of macrophages in atherosclerotic plaques. Two recent studies by Cochain et al. (69) and Winkels et al. (498) describe leukocyte heterogeneity, i.e., 11–13 distinct leukocyte populations, in atherosclerotic plaques by single-cell RNA-seq and CyTOF. Winkels et al. (498) further described the presence of two macrophage populations in atherosclerotic lesions that could be distinguished by their expression of Lyve-1. Of interest, the study by Cochain et al. (69) identified, in addition to resident and inflammatory macrophages, a third macrophage population, which highly expresses TREM2. TREM2 is involved in the clearance of harmful debris and recognizes lipoproteins. TREM2hi macrophages enriched for genes involved in lipid metabolism. It is interesting to note that TREM2 loss of function promotes Alzheimer’s disease (513), and one could thus speculate that loss of TREM2 may also exacerbate atherosclerosis.
Smooth muscle cells can also become macrophage-like cells in atherosclerotic lesions (9, 110), and DCs share many features with macrophages (64, 126, 352, 502). As previously mentioned, macrophages and DCs are found in the adventitia and subintimal space, respectively, but whether these locations change during atherosclerosis development needs to be addressed. Since DCs are found where LDL initially accumulates and is modified, they may be the first cell type to respond in atherosclerosis. Moreover, they can activate naive T cells and could be responsible for later T cell responses. Indeed, DCs can form stable contacts with T cells, mostly in the shoulder and rupture-prone regions (42), and can activate T cells (66, 341, 486), suggesting that T-cell activation can in fact occur within the atherosclerotic lesion. In line with this, DCs in advanced lesions have a mature phenotype (514). Several studies find that DCs are crucial for atherosclerosis to begin and progress (123, 352, 388). In examining aortas, Choi et al. (65) observe both pro- and anti-atherogenic DC subsets, both of which expand during disease: tolerogenic regulatory T-cell-inducing Flt3-signaling-dependent DCs and inflammatory M-CSF-dependent DCs are both capable of inducing T-cell responses in vitro. Ccl17-expressing DCs are another subset that induces proinflammatory CD4+ T-cell responses (486), but whether these are distinct from M-CSF-dependent DCs remains to be seen. GM-CSF in plaques results in monocyte-to-DC differentiation and local DC expansion independent of monocyte input (515, 526). GM-CSF deficiency results in a loss of lesional DCs without affecting any other cell type. A future challenge will be to decipher the relationships among various DC and macrophage subsets in the complex atherosclerotic plaque. More specific markers, such as the classical DC-specific transcription factor Zbtb46 (292, 397), and novel techniques will help further research in these areas. For an extensive review comparing the function of DCs and macrophages in atherogenesis, see Cybulsky et al. (81).
An important challenge is to translate findings on macrophage biology from animal models to human disease, as macrophage markers do not fully overlap and are partly not reliable in humans (41). Additionally, mouse models have aggravated inflammatory responses, exacerbated hyperlipidemia, and mostly lack plaque rupture, possibly going hand in hand with different macrophage responses. For further literature on macrophages in human atherosclerotic plaques, we refer to excellent current reviews (36, 41).
B. Macrophages in MI
Permanent coronary ligation is the most commonly used model of MI. In response to ischemia, as discussed earlier, initial neutrophil recruitment is followed by two sequential waves of monocytes that are recruited to the injured myocardium. Interestingly, intravital two-photon imaging of transplanted and reperfused hearts following 1 h of ischemia revealed a role for CCR2+ monocyte-derived macrophages in neutrophil extravasation (261). CCR2+ cardiac macrophages are important for initiating inflammation (105, 248) and in this setting produce CXCL2 and CXCL5 in a TLR-9-Myd88-dependent manner (261) (FIGURE 4). These responses were observed only a few hours after reperfusion, and it would be interesting to investigate if similar phenomena occur in other ischemia settings. Since local macrophages are still present in the first few hours after MI, they may help initiate inflammation. Those macrophages then die within 1 day after ischemia, giving way to newly recruited monocytes and monocyte-derived macrophages (172, 320) (FIGURE 6). In the first inflammatory wave, Ly-6Chi monocytes give rise to inflammatory macrophages, which are highly phagocytic and clear debris but also secrete proteases, reactive oxygen species, and inflammatory cytokines, such as TNF-α (320). A recent study by King et al. (227) found that self-DNA/IRF3-dependent activation of cardiac macrophages results in type I interferon responses, which in turn activate a specific macrophage subset that is monocyte derived and positive for MHCII and CCR2. This initial phase is essential for debris clearance and infarct healing, as is evident from either depleting monocytes and macrophages (105, 250, 320, 462) or preventing monocyte recruitment (89). Impaired infarct healing results in persistent cardiac inflammation, increased necrotic debris, reduced ventricular function, and, ultimately, heart failure. Recruited inflammatory monocytes, on the other hand, must also be kept in check so that inflammation does not become exaggerated and chronic, thereby impairing healing. Indeed, monocytosis in mice (68, 95, 342) and patients (278) impairs cardiac function. In settings with high systemic inflammation, reducing Ly-6Chi monocyte recruitment is beneficial. Several studies illustrated the advantageous effects of dampening monocytosis by administering specific nanoparticles (254, 389) or antibodies (170, 419, 509), for example, anti-CD20 antibodies to deplete the B cells needed for monocyte recruitment (528). Additionally, inhibiting IRF3 signaling or blocking the type I interferon receptor after MI may produce therapeutic benefits by lowering Ly-6Chi monocyte infiltration levels. Such a reduction in turn decreases inflammatory cytokines, chemokines, and adhesion molecules in the heart, thereby reducing cardiac injury and increasing survival (227).
FIGURE 6.
Monocyte and macrophage kinetics during myocardial infarction. The time course of the biphasic monocyte and macrophage response after myocardial infarction is depicted. Resident cardiac macrophages are initially lost, and recruited monocytes give rise to new macrophages. Ly-6Chi monocytes can give rise to both inflammatory macrophages in the initial inflammatory phase and the ensuing reparative macrophages in the reparative phase. The role of Ly-6Clow monocytes is not currently known. Mediators produced by the different macrophages are shown. Reparative macrophages can induce angiogenesis, which results in cardiac restoration in neonatal mice. This regenerative ability is lost with age. In adults, reparative macrophages induce fibrosis via induction of collagen production by myofibroblasts and cardiomyocyte hypertrophy. A fine balance of these phases is crucial to prevent heart failure: On the one hand, apolipoprotein E (ApoE) knockout (KO) mice with increased inflammatory monocytes/macrophages show increased cardiac inflammation, increased necrotic debris, and reduced ventricular function. Increased reparative macrophages or chronic responses, on the other hand, can result in increased cardiac hypertrophy, fibrosis, and reduced contractile function. ECM, extracellular matrix; ROS, reactive oxygen species; VEGF, vascular endothelial growth factor.
In a second reparative phase, which begins about 3 days after MI, Ly-6Chi monocytes differentiate to reparative macrophages, which produce TGF-β, VEGF, and IL-10 (177). This differentiation depends on Nr4a1, the deficiency of which results in abnormally inflammatory macrophages, poor healing, increased fibrosis, and signs of heart failure (177). The switch from Ly-6Chi to Ly-6Clow monocytes is also critical in other disease settings. For example, in both sterile hepatic injury (85) and skin wounds (78), this switch is needed for proper wound healing. At later stages post-MI, monocyte-derived macrophages can remain in the heart for weeks and depend on local proliferation (391). Ly-6Clow monocyte/macrophages promote wound repair and scar formation: TGF-β and IL-10 induce collagen production by myofibroblasts, VEGF induces angiogenesis, and their production of MMPs and TIMPs regulates the extracellular matrix network. This environment promotes myofibroblast proliferation and migration into the infarct area, where they synthesize initially collagen III and later collagen I (114, 208). Once the collagen matrix is established in the infarct area, some myofibroblasts in the heart persist, possibly reflecting the beating heart’s ongoing need for collagen production (463). Intriguingly, mineralocorticoid antagonists exert cardioprotective effects by inducing a reparative macrophage phenotype (461). Similarly, modulating macrophage function by nanoparticle-mediated silencing of the transcription factor IRF5, thereby inhibiting inflammatory gene expression, promotes infarct healing and decreases post-MI heart failure (75). The precise functions of Ly-6Clow monocytes, which are recruited in the second reparative phase in lower numbers, still need to be explored in more detail (177, 186, 320).
The macrophage shift from inflammatory to reparative phenotypes may also be a direct consequence of macrophage function: efferocytosis, the uptake of apoptotic cells (e.g., apoptotic cardiomyocytes, neutrophils, and macrophages), induces an anti-inflammatory cell phenotype that downregulates inflammatory cytokines and upregulates anti-inflammatory cytokines, such as IL-10 and TGF-β (108, 481). Interestingly, the SR CD36 is essential for not only clearing dying cardiomyocytes, but also inducing Nr4a1 and Mertk (88), a dynamic that further shows how inflammatory monocyte/macrophages transition to reparative macrophages (266, 274). Deficient efferocytosis results in larger infarct size, greater fibrotic area, and reduced angiogenesis due to lack of VEGF (185, 482); furthermore, administering PS-containing liposomes, which mimic apoptotic cells, improves infarct healing in rodents (163). SR-A, which is important in macrophages’ transition to a reparative phenotype in MI (186), signals via Mertk and is also involved in efferocytosis (457). Interestingly, neonatal hearts can fully regenerate after MI. Not only do these hearts have higher numbers of resident macrophages, but they are also uniquely capable of inducing neovascularization and supporting cardiac regeneration without producing inflammatory mediators or promoting fibrosis (19). Understanding these neonatal macrophages better could lead to novel therapeutic strategies.
A large myocardial infarct is often associated with ventricular dilation, hypertrophy of the remote myocardium, reduced contractile function, and diminished cardiac output, eventually resulting in heart failure, in which the heart is unable to pump a sufficient volume of blood to meet the demand of all tissues. Non-reperfused MI initially thins the cardiac wall due to loss of cardiomyocytes. The cardiac wall can, to some extent, be strengthened by myofibroblast scar formation, which is initially beneficial because it partially secures integrity of the injured ventricular wall. However, contractility in the infarcted area remains lost (391). The infarct healing quality and infarct size determine the extent of remodeling and the likelihood of developing heart failure later on.
C. Macrophages in Heart Failure and Hypertension
MI, cardiomyopathies, valve disease, and viral infections can result in heart failure with reduced ejection fraction (HFrEF, systolic heart failure), while systemic inflammation induced by comorbidities, such as hypertension, obesity, diabetes mellitus, chronic obstructive pulmonary disease, renal dysfunction, and aging, lead to heart failure with preserved ejection fraction (HFpEF, diastolic heart failure) (481). The current paradigm assumes that HFrEF is caused by death or diminished contraction of cardiomyocytes, and HFpEF is caused by a systemic proinflammatory state that triggers coronary microvascular inflammation (oxidative stress originating in the endothelial layer), which results in cardiomyocyte hypertrophy and stiffness (353). Collagen production by myofibroblasts and ventricular hypertrophy are observed in both HFrEF and HFpEF. Both humans and mice with different types of heart failure have more circulating leukocytes, higher plasma TNF-α levels, and elevated cardiac macrophage levels (103, 105, 189, 196, 257, 391), thereby demonstrating that inflammation occurs in both types of heart failure. In patients, TNF-α levels predict impaired cardiac function and mortality (92).
Fate mapping revealed that, in heart failure after MI, cardiac macrophages in the remote, nonischemic myocardium derive from both recruited monocytes, produced in bone marrow and spleen, and local macrophage proliferation, induced by mechanical strain in the ventricular wall (391). Other stressors, such as hypoxia due to reduced capillary density, not only worsen contractile dysfunction, but may also promote local macrophage proliferation (156, 336). Interestingly, monocyte-derived macrophages in the myocardium produce VEGF, possibly in an attempt to induce angiogenesis to increase oxygen supply (391). Monocyte-derived and local macrophages show distinct phenotypes and thus can be targeted separately (391). In mice after MI, recruited monocytes and their macrophage progeny promote heart failure; perhaps these cells can themselves injure myocardium (196). Nanoparticle-delivered siRNA silencing five adhesion molecules involved in monocyte recruitment (ICAM-1, ICAM-2, VCAM-1, E-selectin, and P-selectin) 1 wk after MI significantly reduces macrophages and adverse cardiac remodeling (391). Similarly, splenectomy protects against heart failure in mice (196), which may indicate that monocytes from splenic extramedullary hematopoiesis have significant influence in this setting. However, as T cells also play a crucial role in heart failure (326), the splenectomy effect may partly result from a reduction in T cells or other splenocytes. Furthermore, a recent study using a model of nonischemic angiotensin II-induced cardiomyopathy suggests B cells may also be active in heart failure (72).
Cardiac macrophage origin in hypertension, which associates with HFpEF (231), includes both monocyte recruitment and local macrophage proliferation. Cardiac pressure overload models that result in heart failure include transverse aortic constriction (mimicking pressure overload due to aortic stenosis), genetically inbred hypertensive rodent strains, and administration of angiotensin II, aldosterone, or deoxycorticosterone acetate. Increased macrophage proliferation found in angiotensin II models could result from mechanical stresses in the heart caused by hypertension (105, 391). Additionally, angiotensin II directly raises HSCP proliferation in the spleen (73) and induces rapid monocyte influx to the heart and concomitant differentiation (105). These CCR2+ monocyte-derived cardiac macrophages show a strongly inflammatory gene signature associated with NLRP3 inflammasome activation, including upregulated IL-1β, which contributes to systolic dysfunction and heart failure (48, 105). Higher levels of cardiac macrophages are also found in a mouse model that mimics clinical manifestations of HFpEP (189). In this mouse model, aldosterone is administered by osmotic minipumps, one kidney is removed, and mice drink salty water, which increases cardiac macrophages through elevated monocyte recruitment and hematopoiesis in bone marrow and spleen. Interestingly, in humans with HFpEF, circulating myeloid cells are more frequent, and splenic 18F-FDG PET/CT imaging signal correlates with echocardiographic indexes of diastolic dysfunction (189), thereby indicating that similar mechanisms may occur in humans and mice. Local macrophage proliferation in this model was not addressed but likely also contributes to increased macrophage numbers. Like hypertension, advanced age and concomitant diastolic dysfunction in mice lead to higher numbers of cardiac macrophages and associate with increased hematopoiesis (189).
Almost all forms of heart disease involve fibrosis. Monocytes and macrophages can affect the extracellular matrix either directly (by producing MMPs and TIMPs) or indirectly (by producing, e.g., IL-10 and TGF-β) (190). The aftermath of MI raises TGF-β (477), which is vital to inducing angiotensin II-induced cardiomyocyte hypertrophy (385), as well as fibroblast proliferation and differentiation to collagen-producing myofibroblasts frequently found near macrophages. In patients with HFpEF, CD11a+ inflammatory cells with increased TGF-β production are also noted in the heart (491), pointing to a parallel between humans and mice. In a murine model mimicking HFpEF, increased numbers of cardiac macrophages exacerbate heart failure by producing IL-10 (189), which, in collaboration with TGF-β, indirectly promotes collagen deposition by myofibroblasts. Going forward, researchers should determine whether this holds true in other heart failure settings and whether therapeutic targeting IL-10 or TGF-β in monocytes can prevent heart failure. In contrast, administering IL-10 prevents cardiac remodeling after pressure overload (476) and MI (235), again indicating that finding the right balance, location, and timing for this cytokine may be crucial to successfully using it as a therapeutic. Strikingly, loss of either CCL2 (167) or IL-4 (213) decreases fibrosis after cardiac stress by angiotensin-II-induced cardiac hypertrophy or transverse aortic constriction, respectively; results that support the idea that monocytes and macrophages are fundamental to cardiac fibrosis development. For a more detailed review of cardiac macrophages and fibroblasts in cardiac remodeling, see Hulsmans et al. (190) and Frangogiannis (115), respectively.
We should be cautious regarding strategies that target all monocytes and macrophages in heart failure since myeloid cell responses are diverse and may aid cardiac repair. Indeed, depleting monocytes and macrophages in hypertensive rats results in earlier onset of myocardial dysfunction with increased cardiomyocyte loss and enhanced CD4+ T-cell responses (519). Interestingly, T cells are central to nonischemic heart failure, and depleting T cells in mice after transverse aortic constriction prevents heart failure (326). Thus MHCIIhi cardiac macrophages, which are most efficient in antigen presentation and T-cell activation, may play a different role than MHCIIlow macrophages. As the CCR2+ CD11chi subset of MHCIIhi cardiac macrophages is derived from circulating monocytes, specifically modulating this subset could be a promising approach to altering T-cell responses. In contrast, in a rat model of suprarenal aortic constriction, an antibody against CCL2 reduced fibrosis, cardiac stiffness, local TGF-β production, and fibroblast-to-myofibroblast differentiation, thereby improving diastolic dysfunction (243). Preventing macrophages from producing IL-10 and TGF-β may curtail myofibroblasts’ collagen production, but this may negatively affect T-cell and other inflammatory responses. In the setting of MI, monocyte and macrophage depletion results in impaired resolution of inflammation and reduced collagen production that increase cardiac dysfunction (116, 320, 462); thus macrophages are beneficial for cardiac healing. On the other hand, an exacerbated monocyte response, as observed in ApoE KO mice or wild-type mice exposed to LPS, lowers ejection fraction more quickly (342); thus overly strong myeloid responses are detrimental. These studies clearly indicate the need for a balanced approach. Careful patient selection and monitoring, perhaps by imaging, are likely needed to avoid the harmful effects of any treatment targeting cardiac macrophages. Furthermore, macrophages not only contribute to extracellular matrix production, but also produce matrix metalloprotease inhibitors. Monocytes and macrophages may also have differential functions in the heart and the vessel wall. In the hypertensive aorta, increased macrophages are predominantly derived from recruited monocytes and show a proinflammatory phenotype. Depleting monocytes or preventing their recruitment to the vessel wall reduces oxidative stress, medial thickness, vessel stiffness, fibrosis, and hypertension (195, 243, 307, 489). Future studies should thus address which monocyte/macrophage subsets and/or which of the factors they produce are beneficial in the setting of heart failure, focusing specifically on their temporal and spatial roles in the cardiovascular system. Clearly, a better understanding of macrophage heterogeneity in cardiovascular diseases is needed.
D. Macrophages in CNS Inflammation and Stroke
In the brain, microglia are the first responders to any type of insult and readily detect and clear cellular debris or pathogens. After facial nerve axotomy, in which the blood-brain barrier remains intact, microglia rapidly migrate to the site of injury and expand clonally. After insult cessation, they disappear via egress and apoptosis (450). Whether this clonal expansion also occurs when the blood-brain barrier is disrupted and allows monocytes to enter, as in stroke, remains unknown.
In both stroke and experimental autoimmune encephalomyelitis (EAE) Ly-6Chi monocytes are recruited to the brain via CCL2 (128, 139, 297) (FIGURE 5). These recruited monocytes can partially differentiate to macrophages (139). Yet after a full recovery phase in EAE and stroke, the microglia pool does not include monocyte-derived macrophages, thereby indicating that these cells only play a transient role during brain inflammation (and possibly recovery) (5, 399). Whether monocyte recruitment is beneficial seems to depend on the type of inflammation. In the context of spinal cord injury, for example, monocyte recruitment is essential for recovery (411), while during acute intracerebral hemorrhage, monocyte recruitment worsens outcome (158). Interestingly, monocyte-derived macrophages show enhanced phagocytic capacity and can provide anti-inflammatory mediators to protect the nervous system and reduce microglia activation (210, 272, 411). In CNS inflammation, then, microglia seem to be important for the first clearance phase, as they are rapidly recruited from surrounding nonischemic areas and clear debris. Monocyte-derived macrophages play a role in both the first phase, as they help microglia to clear debris and can produce inflammatory mediators, and the resolution phase in a biphasic dynamic analogous to the distinct waves observed after MI (210). Targeting monocytes to control stroke pathologies could therefore be potentially therapeutic, as with other CNS pathologies (413), but targeting microglia should also be considered.
Microglia function and turnover poststroke remains unclear. Microglia can either acquire a proinflammatory phenotype and exacerbate injury or acquire an anti-inflammatory phenotype and help repair the CNS, which heavily depends on the signals microglia receive (349). Mass et al. (285) further established that vast inflammatory microglia expansion due to BRAF mutations induced at the EMP stage resulted in increased neuronal loss and damage, thereby showing that inflammatory MAPK signaling in microglia is linked to neurodegeneration. In the peripheral nervous system, macrophages are key to nerve regeneration. Macrophages produce VEGF, which promotes vasculature formation across the new tissue. These vessels are used by Schwann cells as tracks to migrate across the wound while directing regrowing axons (59). These studies clearly indicate that we are far from understanding the complexity of microglia in not only stroke, but also other CNS diseases, in part because we largely lack strategies to specifically assess or target microglia, monocytes, and other nonparenchymal macrophages. A recent study by Ajami et al. (7) sought to shed some light on the complexity of myeloid cells in the brain in neuroinflammation by comparing cells observed after EAE to the neurodegenerative diseases Huntington’s disease and amyotrophic lateral sclerosis. By employing mass cytometry, the authors discovered that CD49e was specifically expressed in EAE on Ly-6C+ CNS-resident myeloid cells. This suggest that CD49e-fibronectin interactions are crucial in enabling these cells to enter the brain. Similar strategies to compare stroke to steady-state as well as other inflammatory brain diseases by mass cytometry and single-cell transcriptomic analysis could potentially reveal interesting therapeutic targets.
Overall, it is becoming evident that myeloid cells facilitate an initial inflammatory phase, resulting in debris clearance and a later healing phase, resulting in resolution and repair. If we can better understand what cells and factors regulate these phases, we can harness them for therapies to (re)establish proper system balance in stroke patients.
IX. MACROPHAGE IMAGING, TARGETING, AND MODULATION IN CARDIOVASCULAR DISEASE
In all cardiovascular diseases, monocytes are initially recruited to sites of inflammation. This temporary monocyte recruitment may help tissue-resident macrophages fight a pathogen or perceived danger, since tissue-resident macrophages often undergo apoptosis, likely in an attempt to neutralize the detected and ingested pathogen. However, if inflammation is not resolved and the situation becomes chronic, persistent monocyte recruitment can become detrimental, as is the case in atherosclerosis.
Tissue-resident macrophages, as discussed previously, can have diverse origins. Despite the efforts put into deciphering macrophage provenance, we do not yet understand the regulation of differential hematopoietic contributions by the yolk sac, fetal liver, and bone marrow to tissue-resident macrophages, nor how these dynamics affect macrophage phenotype and function. Embryonic-derived macrophages may have more tissue-protective functions, as they are present during development, while monocyte-derived macrophages focus on defense. Tissue-resident macrophages could thus be killed during inflammation to enable monocyte-derived macrophages to take over local defense against pathogens. Moreover, skin and gut macrophages, which are continuously exposed to microorganisms, are predominantly monocyte-derived, further suggesting these cells may be primarily defensive. Two recent studies compared gene expression among macrophages of different origins and found no striking differences. Gibbings et al. (132) compared alveolar macrophages of embryonic vs. adult origin and found that only ∼0.1% of genes were determined by the cell origin. Only 24 genes were specific for macrophages of embryonic origin (e.g., MARCO, Lepr), and 12 genes were specific for macrophages of adult origin (e.g., C1qb, Plbd1, H2-M2, Retnla). Similarly, Scott et al. (405) found that monocyte-derived Kupffer cells showed similar gene expression profiles to embryonic-derived Kupffer cells, with only 54 genes being differentially expressed; for example, Tim-4 and CD163 were specific for embryonic-derived Kupffer cells. Monocyte-derived Kupffer cells seem better at pathogen clearance, whereas embryonic-derived Kupffer cells seem better at lipoprotein clearance (28). This indicates that macrophage phenotype is predominantly determined by the environment, but also suggests that monocyte-derived macrophages in inflammatory settings will have some different functions than steady-state macrophages. Going forward, we should determine whether newly recruited monocyte-derived macrophages can maintain tissue homeostasis like their embryonic-derived counterparts, and whether their response to another infection or injury will be altered, i.e., whether they are epigenetically modified.
One strategy to monitor monocyte and macrophage distribution, phenotype, and behavior in vivo is to image these cells in fluorescent reporter mice like CX3CR1-GFP (209), Csf1r-EGFP (395), or hCD68-GFP (194). Alternately, using nanoparticles does not rely on protein overexpression and is translatable to humans (Table 1). Modulating nanoparticle material and size greatly alters their behavior. Recent work has focused on improving surface properties (e.g., dextran coating) to make nanoparticles better “pan-macrophage” agents. One well-studied nanoparticle uses cross-linked dextran iron oxide, which is cleared via macropinocytosis and specifically taken up by monocytes, macrophages, and immature DCs. That nanoparticles are taken up preferentially by Ly-6Chi rather than Ly-6Clow monocytes supports the use of nanoparticles in inflammatory settings. As no particular macrophage subset seems to preferentially incorporate nanoparticles, this approach should be improved to target a certain macrophage subpopulation. Nanoparticles can be either compatible with optical imaging and flow cytometry or compatible with MRI, PET, and SPECT imaging, depending on their label. Not only can the use of nanoparticles improve in vivo understanding of monocyte and macrophage kinetics in preclinical mouse models, they also have diagnostic potential in the clinic. Furthermore, in addition to enabling noninvasive evaluation of inflammation and disease status, nanoparticles can be used to assess therapy efficacy. For example, nanoparticles have been combined with PET/MRI to understand monocyte and macrophage kinetics and treatment effects during MI (220, 318) and atherosclerosis (281, 512). Recently, hyaluronan nanoparticles were found to preferentially target plaque-associated macrophages (31). Nanoparticles can be used not only for imaging inflammation during atherosclerosis, but also to specifically deliver therapeutic payloads, for example, siRNAs, to plaque macrophages. One limitation is that nanoparticles are not specific to cardiovascular macrophages, although strategies of conjugating specific affinity ligands to nanoparticles may increase target specificity. For a thorough review of nanoparticles, as well as their use in cardiovascular diseases, see Weissleder et al. (488), Vandoorne and Nahrendorf (472), and Zupančič et al. (530).
Table 1.
Models and techniques to address macrophage phenotype and function
| Models/Techniques | Examples | |
|---|---|---|
| Macrophage origin | Bone marrow transplantation | UBC-GFP into C57BL/6 mouse |
| Parabiosis | CD45.1 with CD45.2 mouse | |
| Adoptive transfer of progenitors | Fluorescent-labeled HSPCs or monocytes | |
| (Inducible) Cre-lox mouse | Cx3cr1CreER, Flt3Cre, Runx1CreER, crossed to loxP-flanked stop tdTomato or YFP | |
| Macrophage imaging and detection | Fluorescent reporter mice | Cx3cr1GFP/+, Csf1r-EGFP, hCD68-GFP |
| Antibodies | Fluorescent/radioisotope-labeled (microscopy, flow cytometry, PET, scintillation counting) | |
| Nanoparticles and liposomes | Gadolinium-based (MRI), iron oxide core (MRI), 18F and 89Zr label (PET) | |
| Macrophage depletion | Liposomes | Encapsulated bisphosphonates (e.g., clodronate) |
| (Inducible) (Cre) mouse models | CD11bDTR, Csf1rCreER:iDTR, Cx3cr1CreER:iDTR, CD169DTR, MaFIA | |
| Macrophage isolation and culture | Isolation by magnetic beads and/or FACS | CD11b+ F4/80+ tissue-resident macrophages |
| Bone marrow-derived macrophages | Culture with M-CSF | |
| Macrophage function | Imaging agents | MMPSense (MMP activity), ProSense (pan-cathepsin activity), fluorescent-labeled latex beads or dextran, pHrodo particle labeling, fluorescent bacteria |
| Cytokine responses | In vitro stimulation followed by ELISA, qPCR | |
| Metabolism | BioProfile FLEX analyzer, Seahorse XF analyzer | |
| Macrophage targeting and modulation | Cre-lox mouse models | Inducible Cre (e.g., Cx3cr1CreER, Csf1rCre) crossed to mouse with loxP-flanked gene of interest |
| Nanoparticles and liposomes | siRNAs, drugs | |
| (Ant)agonistic antibodies | anti-CD40, anti-CD47, anti-IL-1β, anti-IL-4 | |
| Small molecule inhibitors | Receptor tyrosine kinase inhibitor of Csf1r (PLX3397), inhibitor of CD40-TRAF6 interaction (TRAF-STOP) |
A caveat with current models and techniques is that they are never truly specific. For example, CD11b is expressed on all myeloid cells and some dendritic cells; and Cx3cr1 and Csf1r are also expressed on some dendritic cell subsets. LysM and Csf1r also target granulocytes. PLX3397 also inhibits, although with lower affinity, kit and Flt3. There are currently no strategies to exclusively target tissue-resident macrophages in a specific cardiovascular organ. DTR, diphtheria toxin receptor; ELISA, enzyme-linked immunosorbent assay; FACS, fluorescence-activated cell sorting; GFP, green fluorescent protein; HSPCs, hematopoietic stem and progenitor cells; MaFIA, macrophage Fas-induced apoptosis; M-CSF, macrophage-colony-stimulating factor; MMP, matrix metalloproteinases; MRI, magnetic resonance imaging; PET, positron emission tomography; TLR, Toll-like receptor; TRAF, tumor necrosis factor receptor-associated factor; UBC, ubiquitin.
To more specifically understand monocyte and macrophage contributions to cardiovascular diseases, these cells can be specifically depleted at a certain time point to analyze the effects on disease initiation and progression (Table 1). Diphtheria toxin receptor (DTR)-mediated conditional depletion of myeloid cells (e.g., CD11bDTR and CX3CR1CreER:iDTR) may help elucidate these cells’ contributions to disease (25, 345, 392, 432). Depleting monocytes and, less efficiently, macrophages can also be achieved using clodronate liposomes, which induce apoptosis by binding and, consequently, disabling intracellular ATP (471). Although monocytes are the main target of intravenous administration, tissue-specific administration has been attempted to specifically deplete macrophages in certain tissues, for example, intratracheal administration depletes tissue-resident alveolar macrophages (303), and intracerebroventricular injection depletes microglia (16). Experiments using CD11bDTR mice (432) and clodronate liposomes (380) in the setting of atherosclerosis demonstrated that plaque development depends on monocyte recruitment. Similarly, intravenous application of liposome-encapsulated bisphosphonates (LABR-312) improved restenosis in patients with stent implantation in a phase II clinical trial (24), further indicating the therapeutic potential of this approach for atherosclerosis.
Certain proteins’ roles in monocytes and macrophages can be more precisely addressed using mice that express a Cre recombinase under the control of monocyte/macrophage-specific promoters (CX3CR1Cre and Csf1rCre) to deplete floxed alleles. Moreover, by using tamoxifen, fate-mapping models (CX3CR1CreER and Csf1rMercreMer) advantageously allow depletion at a defined time. Note that the CX3CR1CreER mouse exists in a combination with YFP-expression under the endogenous CX3CR1 promoter (CX3CR1CreER/YFP) to enable simultaneous detection of monocytes and macrophages, which supports not only analysis of their in vivo behavior and phenotype, but also ex vivo histology or flow cytometry. These animal models allow us to address the function of a protein in monocytes/macrophages at a given disease stage. Additionally, by temporarily inducing depletion in all monocytes/macrophages by administering tamoxifen and waiting until monocytes turned over from hematopoietic progenitors, these models can specifically address a protein’s function in tissue-resident macrophages during disease. Despite their great potential for helping us find and validate targets for cardiovascular therapies, these approaches do have limitations. As with all models currently available, there is no 100% specificity (e.g., certain DCs will be affected), and there is currently no way to exclusively target macrophages in certain organs or of a certain subset. Also, monocytes will always be affected by this sort of approach, making it hard to decipher whether monocytes or macrophages are important in the observed phenotype in the inflammatory disease setting.
So why do macrophages’ origin, plasticity, and function in a specific context matter therapeutically? Understanding macrophage vs. monocyte functions will improve targeting specificity. In atherosclerosis and MI, for instance, modulating monocytes could alter the functions of monocyte-derived macrophages at inflammatory sites. Nanoparticles and liposomes can carry cargo that targets myeloid cell phenotypes. Liposomes enriched with PS mimic apoptotic cell recognition, which induces anti-inflammatory responses in macrophages, and these can be used therapeutically. In MI, these PS-containing liposomes improved infarct healing (163). Correspondingly, administering apoptotic cells or PS-containing liposomes reduced atherosclerotic plaque development and progression (118, 184). Interestingly, unmodified liposome accumulation in infarct areas was initially used as an MRI contrast agent (57), so that liposomes may be able to specifically deliver treatment to myocardial infarcts. Further optimization, such as PEG-coating and antibody incorporation, has improved liposomal targeting. As previously described, nanoparticles loaded with specific siRNAs that lower the expression of adhesion molecules, chemokine receptors, or transcription factors can also be used to dampen inflammatory responses in atherosclerosis and MI (75, 254, 281, 389, 391). Additionally, it is possible to combine multiple siRNAs (389). Nanoparticles can also be used to target microglia (191, 324, 344), thereby opening new possibilities for differentially modulating inflammation in stroke.
Indirect modulation of myeloid cell function can be achieved by administering antibodies that block cytokines or chemokines to affect cell production, development, or function (Table 1). An antibody against IL-1β, for example, reduces HSC proliferation after MI, thereby lowering monocyte production, cardiac inflammation, and heart failure (390). Furthermore, inhibiting IL-1β in atherosclerosis prevents lesion progression in mice (35), and the CANTOS (Canakinumab Anti-inflammatory Thrombosis Outcomes Study) trial showed that Canakinumab has therapeutic potential in humans (377). IL-4-neutralizing antibodies, which inhibit macrophage conversion to a reparative phenotype, curtail macrophage proliferation to decrease cardiac fibrosis and cardiac remodeling in a transverse aortic constriction model (213). Like IL-10 and TGF-β, neutralizing IL-4 could thus be of potential therapeutic interest in heart failure.
Since specificity improves treatment efficacy while reducing harmful off-target effects, it is crucial to understand monocyte and macrophage kinetics and behavior to determine when and how treatment is beneficial or detrimental. Dampening the initial inflammatory monocyte response benefits mice after MI, as demonstrated by blocking monocyte recruitment with nanoparticles encapsulating siRNAs against CCR2 (254, 280). These nanoparticles also limit atherosclerotic lesion development (254). To address local macrophage proliferation in addition to monocyte recruitment in more advanced lesions, a more recent strategy used HDL nanoparticle-based delivery of simvastatin to macrophages (446). In this approach, while monocyte recruitment was not affected, macrophages’ local proliferation and inflammatory cytokine production were both reduced. As atherosclerosis is a disease driven by both high cholesterol and inflammation propelled by monocytes and macrophages, a combination strategy may be the most beneficial. Indeed, combining simvastatin nanoparticles with oral simvastatin treatment showed better treatment potential than nanoparticles alone (446).
While these studies implicate the potential therapeutic benefits of monocyte/macrophage-targeted approaches, we are still lacking such therapies in the clinic. Administration of anakinra (an anti-IL-1 receptor antagonist) reduced adverse remodeling after MI in 10 patients (2). This suggests that improving infarct remodeling by reducing inflammation and supporting macrophages’ reparative functions may be a feasible target. Effectively targeting monocytes and macrophages in MI will require finding the right balance between its two phases: monocyte recruitment and proinflammatory macrophages clearing debris in the first stages, and monocyte recruitment and reparative macrophages promoting myocardial healing in later stages. Combining circulating biomarker assessment with imaging may provide opportunities to select patients and monitor therapy by determining inflammation levels and biomarkers tied to outcomes such as fibrosis and angiogenesis. For example, patients with significantly elevated leukocyte levels may benefit from inhibition of monocyte recruitment, whereas patients with extensive cardiac fibrosis may benefit from inhibition of macrophage proliferation or IL-4 neutralization. Since none of our approaches to date is specific to a cell type that does not also have beneficial purposes, any therapy will likely affect other potentially protective inflammatory responses, as indicated by the CANTOS trial. This dynamic necessitates caution and a careful risk/benefit analysis. Ideally, therapeutic approaches will be tailored specifically to the patient and disease stage, perhaps focusing on patient cohorts with exacerbated inflammatory responses.
X. SUMMARY AND CONCLUSIONS
We progressed a great deal from regarding macrophages as simply the body’s waste disposal system. We now know these cells are vital to organ development, homeostasis, tolerance, and immune responses. During steady state, macrophages primarily have organ maintenance functions, such as clearing debris and inducing tolerance. Macrophages can also have organ-specific functions, such as support of electrical conduction in the heart. Fate-mapping studies have revealed that, in steady state, macrophages in the heart and aorta have mainly embryonic origins and entirely embryonic origins in the brain. Monocytes recruited during inflammation can locally differentiate to macrophages that are often found to be more phagocytic, proinflammatory, and destructive, as in MI and atherosclerosis. However, monocytes recruited in late phases of cardiovascular disease may be beneficial, as they give rise to reparative macrophages that can induce infarct healing and scar formation. Nonetheless, if monocyte recruitment and macrophage responses become chronic, it can be destructive, as in heart failure after MI or lesion progression in atherosclerosis. In summary, embryonic macrophages seem to maintain tissue homeostasis, while monocyte-derived macrophages clear debris and may induce resolution of inflammation, depending on the tissue environment.
Mouse models can greatly help us better understand the complexity of monocyte and macrophage function and phenotype in cardiovascular diseases. Such models can reveal the monocyte/macrophage populations’ origins and dynamics, define the molecular mechanisms involved in their recruitment and proliferation, and identify markers for specific cell subsets. By depleting monocytes/macrophages at specific disease stages, knocking out certain proteins or inhibiting certain molecules involved in their function, we begin to understand how they function. Yet many questions remain unanswered: Does it matter whether macrophages are yolk sac-derived or fetal liver-derived? Which signals direct local macrophage proliferation vs. monocyte recruitment? Do macrophages clonally expand during aging or inflammation? To what extent are tissue-resident macrophages replaced by monocyte-derived macrophages during aging and inflammation? Does inflammation affect the origins of specific tissue-resident macrophage subsets, and what does this mean? Do specific macrophage subsets in cardiovascular organs play distinct roles? What governs the transition of inflammatory macrophages to reparative macrophages?
Currently, we are limited in answering these questions due to a lack of more specific markers for macrophage subsets in specific organs. Moreover, some markers we use, such as MHCII and CCR2, have functions themselves and may thus change during inflammation. In the future, more detailed transcriptomic analysis of macrophages on a single-cell level in different organs, as well as different disease stages, will likely help to address the previous questions more accurately. As we further unravel macrophage subsets and their roles in cardiovascular diseases, we will not only uncover new therapeutic targets, but also discover how to temporarily and spatially modulate myeloid cells.
GRANTS
This work was funded in part by federal funds from the National Institutes of Health Grants NS-084863, HL-128264, HL-117829, HL-096576, and HL-131495; the European Union’s Horizon 2020 research and innovation program under Grant Agreement 667837; and the Massachusetts General Hospital Research Scholar Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We gratefully acknowledge Kaley Joyes for editing the manuscript. The Figures were made using Servier Medical Art.
Address for reprint requests and other correspondence: V. Frodermann and M. Nahrendorf, Center for Systems Biology, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114 (e-mail: vfrodermann@mgh.harvard.edu; mnahrendorf@mgh.harvard.edu).
REFERENCES
- 1.A-Gonzalez N, Bensinger SJ, Hong C, Beceiro S, Bradley MN, Zelcer N, Deniz J, Ramirez C, Díaz M, Gallardo G, Ruiz de Galarreta CR, Salazar J, Lopez F, Edwards P, Parks J, Andujar M, Tontonoz P, Castrillo A. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity 31: 245–258, 2009. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbate A, Kontos MC, Grizzard JD, Biondi-Zoccai GG, Van Tassell BW, Robati R, Roach LM, Arena RA, Roberts CS, Varma A, Gelwix CC, Salloum FN, Hastillo A, Dinarello CA, Vetrovec GW; VCU-ART Investigators . Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study). Am J Cardiol 105: 1371–1377.e1, 2010. doi: 10.1016/j.amjcard.2009.12.059. [DOI] [PubMed] [Google Scholar]
- 3.Ait-Oufella H, Kinugawa K, Zoll J, Simon T, Boddaert J, Heeneman S, Blanc-Brude O, Barateau V, Potteaux S, Merval R, Esposito B, Teissier E, Daemen MJ, Lesèche G, Boulanger C, Tedgui A, Mallat Z. Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation 115: 2168–2177, 2007. doi: 10.1161/CIRCULATIONAHA.106.662080. [DOI] [PubMed] [Google Scholar]
- 4.Ait-Oufella H, Pouresmail V, Simon T, Blanc-Brude O, Kinugawa K, Merval R, Offenstadt G, Lesèche G, Cohen PL, Tedgui A, Mallat Z. Defective mer receptor tyrosine kinase signaling in bone marrow cells promotes apoptotic cell accumulation and accelerates atherosclerosis. Arterioscler Thromb Vasc Biol 28: 1429–1431, 2008. doi: 10.1161/ATVBAHA.108.169078. [DOI] [PubMed] [Google Scholar]
- 5.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci 14: 1142–1149, 2011. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 6.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci 10: 1538–1543, 2007. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 7.Ajami B, Samusik N, Wieghofer P, Ho PP, Crotti A, Bjornson Z, Prinz M, Fantl WJ, Nolan GP, Steinman L. Single-cell mass cytometry reveals distinct populations of brain myeloid cells in mouse neuroinflammation and neurodegeneration models. Nat Neurosci 21: 541–551, 2018. doi: 10.1038/s41593-018-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404: 193–197, 2000. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 9.Albarrán-Juárez J, Kaur H, Grimm M, Offermanns S, Wettschureck N. Lineage tracing of cells involved in atherosclerosis. Atherosclerosis 251: 445–453, 2016. doi: 10.1016/j.atherosclerosis.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Alberti S, Schuster G, Parini P, Feltkamp D, Diczfalusy U, Rudling M, Angelin B, Björkhem I, Pettersson S, Gustafsson JA. Hepatic cholesterol metabolism and resistance to dietary cholesterol in LXRbeta-deficient mice. J Clin Invest 107: 565–573, 2001. doi: 10.1172/JCI9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alder JK, Georgantas RW III, Hildreth RL, Kaplan IM, Morisot S, Yu X, McDevitt M, Civin CI. Kruppel-like factor 4 is essential for inflammatory monocyte differentiation in vivo. J Immunol 180: 5645–5652, 2008. doi: 10.4049/jimmunol.180.8.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alibhai FJ, Tsimakouridze EV, Chinnappareddy N, Wright DC, Billia F, O’Sullivan ML, Pyle WG, Sole MJ, Martino TA. Short-term disruption of diurnal rhythms after murine myocardial infarction adversely affects long-term myocardial structure and function. Circ Res 114: 1713–1722, 2014. doi: 10.1161/CIRCRESAHA.114.302995. [DOI] [PubMed] [Google Scholar]
- 13.Anderson KL, Smith KA, Pio F, Torbett BE, Maki RA. Neutrophils deficient in PU.1 do not terminally differentiate or become functionally competent. Blood 92: 1576–1585, 1998. [PubMed] [Google Scholar]
- 14.Anderson MK, Weiss AH, Hernandez-Hoyos G, Dionne CJ, Rothenberg EV. Constitutive expression of PU.1 in fetal hematopoietic progenitors blocks T cell development at the pro-T cell stage. Immunity 16: 285–296, 2002. doi: 10.1016/S1074-7613(02)00277-7. [DOI] [PubMed] [Google Scholar]
- 15.Anzai A, Choi JL, He S, Fenn AM, Nairz M, Rattik S, McAlpine CS, Mindur JE, Chan CT, Iwamoto Y, Tricot B, Wojtkiewicz GR, Weissleder R, Libby P, Nahrendorf M, Stone JR, Becher B, Swirski FK. The infarcted myocardium solicits GM-CSF for the detrimental oversupply of inflammatory leukocytes. J Exp Med 214: 3293–3310, 2017. doi: 10.1084/jem.20170689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T, Wolozin B, Butovsky O, Kügler S, Ikezu T. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci 18: 1584–1593, 2015. doi: 10.1038/nn.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Askenase MH, Han SJ, Byrd AL, Morais da Fonseca D, Bouladoux N, Wilhelm C, Konkel JE, Hand TW, Lacerda-Queiroz N, Su XZ, Trinchieri G, Grainger JR, Belkaid Y. Bone-marrow-resident NK cells prime monocytes for regulatory function during infection. Immunity 42: 1130–1142, 2015. doi: 10.1016/j.immuni.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317: 666–670, 2007. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 19.Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA, Olson EN. Macrophages are required for neonatal heart regeneration. J Clin Invest 124: 1382–1392, 2014. doi: 10.1172/JCI72181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol 11: 805–815, 1981. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- 21.Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, Guilliams M, Malissen B, Agace WW, Mowat AM. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol 6: 498–510, 2013. doi: 10.1038/mi.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baldán A, Pei L, Lee R, Tarr P, Tangirala RK, Weinstein MM, Frank J, Li AC, Tontonoz P, Edwards PA. Impaired development of atherosclerosis in hyperlipidemic Ldlr−/− and ApoE−/− mice transplanted with Abcg1−/− bone marrow. Arterioscler Thromb Vasc Biol 26: 2301–2307, 2006. doi: 10.1161/01.ATV.0000240051.22944.dc. [DOI] [PubMed] [Google Scholar]
- 23.Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature 465: 793–797, 2010. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banai S, Finkelstein A, Almagor Y, Assali A, Hasin Y, Rosenschein U, Apruzzese P, Lansky AJ, Kume T, Edelman ER. Targeted anti-inflammatory systemic therapy for restenosis: the Biorest Liposomal Alendronate with Stenting sTudy (BLAST)-a double blind, randomized clinical trial. Am Heart J 165: 234–240.e1, 2013. doi: 10.1016/j.ahj.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baranska A, Shawket A, Jouve M, Baratin M, Malosse C, Voluzan O, Vu Manh TP, Fiore F, Bajénoff M, Benaroch P, Dalod M, Malissen M, Henri S, Malissen B. Unveiling skin macrophage dynamics explains both tattoo persistence and strenuous removal. J Exp Med 215: 1115–1133, 2018. doi: 10.1084/jem.20171608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barbalat R, Lau L, Locksley RM, Barton GM. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat Immunol 10: 1200–1207, 2009. doi: 10.1038/ni.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartocci A, Mastrogiannis DS, Migliorati G, Stockert RJ, Wolkoff AW, Stanley ER. Macrophages specifically regulate the concentration of their own growth factor in the circulation. Proc Natl Acad Sci USA 84: 6179–6183, 1987. doi: 10.1073/pnas.84.17.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beattie L, Sawtell A, Mann J, Frame TCM, Teal B, de Labastida Rivera F, Brown N, Walwyn-Brown K, Moore JWJ, MacDonald S, Lim EK, Dalton JE, Engwerda CR, MacDonald KP, Kaye PM. Bone marrow-derived and resident liver macrophages display unique transcriptomic signatures but similar biological functions. J Hepatol 65: 758–768, 2016. doi: 10.1016/j.jhep.2016.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Béchade C, Cantaut-Belarif Y, Bessis A. Microglial control of neuronal activity. Front Cell Neurosci 7: 32, 2013. doi: 10.3389/fncel.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Becher B, Tugues S, Greter M. GM-CSF: from growth factor to central mediator of tissue inflammation. Immunity 45: 963–973, 2016. doi: 10.1016/j.immuni.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 31.Beldman TJ, Senders ML, Alaarg A, Pérez-Medina C, Tang J, Zhao Y, Fay F, Deichmöller J, Born B, Desclos E, van der Wel NN, Hoebe RA, Kohen F, Kartvelishvily E, Neeman M, Reiner T, Calcagno C, Fayad ZA, de Winther MPJ, Lutgens E, Mulder WJM, Kluza E. Hyaluronan nanoparticles selectively target plaque-associated macrophages and improve plaque stability in atherosclerosis. ACS Nano 11: 5785–5799, 2017. doi: 10.1021/acsnano.7b01385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB, Mulinyawe SB, Bohlen CJ, Adil A, Tucker A, Weissman IL, Chang EF, Li G, Grant GA, Hayden Gephart MG, Barres BA. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci USA 113: E1738–E1746, 2016. doi: 10.1073/pnas.1525528113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berg KE, Ljungcrantz I, Andersson L, Bryngelsson C, Hedblad B, Fredrikson GN, Nilsson J, Björkbacka H. Elevated CD14++CD16− monocytes predict cardiovascular events. Circ Cardiovasc Genet 5: 122–131, 2012. doi: 10.1161/CIRCGENETICS.111.960385. [DOI] [PubMed] [Google Scholar]
- 34.Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 464: 108–111, 2010. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhaskar V, Yin J, Mirza AM, Phan D, Vanegas S, Issafras H, Michelson K, Hunter JJ, Kantak SS. Monoclonal antibodies targeting IL-1 beta reduce biomarkers of atherosclerosis in vitro and inhibit atherosclerotic plaque formation in Apolipoprotein E-deficient mice. Atherosclerosis 216: 313–320, 2011. doi: 10.1016/j.atherosclerosis.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 36.Biessen EAL, Wouters K. Macrophage complexity in human atherosclerosis: opportunities for treatment? Curr Opin Lipidol 28: 419–426, 2017. doi: 10.1097/MOL.0000000000000447. [DOI] [PubMed] [Google Scholar]
- 37.Bigley V, Haniffa M, Doulatov S, Wang XN, Dickinson R, McGovern N, Jardine L, Pagan S, Dimmick I, Chua I, Wallis J, Lordan J, Morgan C, Kumararatne DS, Doffinger R, van der Burg M, van Dongen J, Cant A, Dick JE, Hambleton S, Collin M. The human syndrome of dendritic cell, monocyte, B and NK lymphoid deficiency. J Exp Med 208: 227–234, 2011. doi: 10.1084/jem.20101459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bjornson ZB, Nolan GP, Fantl WJ. Single-cell mass cytometry for analysis of immune system functional states. Curr Opin Immunol 25: 484–494, 2013. doi: 10.1016/j.coi.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blériot C, Dupuis T, Jouvion G, Eberl G, Disson O, Lecuit M. Liver-resident macrophage necroptosis orchestrates type 1 microbicidal inflammation and type-2-mediated tissue repair during bacterial infection. Immunity 42: 145–158, 2015. doi: 10.1016/j.immuni.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 40.Blevins G, Fedoroff S. Microglia in colony-stimulating factor 1-deficient op/op mice. J Neurosci Res 40: 535–544, 1995. doi: 10.1002/jnr.490400412. [DOI] [PubMed] [Google Scholar]
- 41.Bobryshev YV, Ivanova EA, Chistiakov DA, Nikiforov NG, Orekhov AN. Macrophages and Their Role in Atherosclerosis: Pathophysiology and Transcriptome Analysis. BioMed Res Int 2016: 9582430, 2016. doi: 10.1155/2016/9582430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bobryshev YV, Lord RS. Mapping of vascular dendritic cells in atherosclerotic arteries suggests their involvement in local immune-inflammatory reactions. Cardiovasc Res 37: 799–810, 1998. doi: 10.1016/S0008-6363(97)00229-0. [DOI] [PubMed] [Google Scholar]
- 43.Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature 464: 116–120, 2010. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- 44.Borges da Silva H, Fonseca R, Pereira RM, Cassado AA, Álvarez JM, D’Império Lima MR. Splenic macrophage subsets and their function during blood-borne infections. Front Immunol 6: 480, 2015. doi: 10.3389/fimmu.2015.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van der Borght K, Scott CL, Nindl V, Bouché A, Martens L, Sichien D, Van Moorleghem J, Vanheerswynghels M, De Prijck S, Saeys Y, Ludewig B, Gillebert T, Guilliams M, Carmeliet P, Lambrecht BN. Myocardial infarction primes autoreactive T cells through activation of dendritic cells. Cell Reports 18: 3005–3017, 2017. doi: 10.1016/j.celrep.2017.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 394: 894–897, 1998. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 47.Boyer SW, Schroeder AV, Smith-Berdan S, Forsberg EC. All hematopoietic cells develop from hematopoietic stem cells through Flk2/Flt3-positive progenitor cells. Cell Stem Cell 9: 64–73, 2011. doi: 10.1016/j.stem.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bracey NA, Beck PL, Muruve DA, Hirota SA, Guo J, Jabagi H, Wright JR Jr, Macdonald JA, Lees-Miller JP, Roach D, Semeniuk LM, Duff HJ. The Nlrp3 inflammasome promotes myocardial dysfunction in structural cardiomyopathy through interleukin-1β. Exp Physiol 98: 462–472, 2013. doi: 10.1113/expphysiol.2012.068338. [DOI] [PubMed] [Google Scholar]
- 49.Bradley TR, Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci 44: 287–300, 1966. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- 50.Brandl R, Richter T, Haug K, Wilhelm MG, Maurer PC, Nathrath W. Topographic analysis of proliferative activity in carotid endarterectomy specimens by immunocytochemical detection of the cell cycle-related antigen Ki-67. Circulation 96: 3360–3368, 1997. doi: 10.1161/01.CIR.96.10.3360. [DOI] [PubMed] [Google Scholar]
- 51.Bronte V, Pittet MJ. The spleen in local and systemic regulation of immunity. Immunity 39: 806–818, 2013. doi: 10.1016/j.immuni.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruttger J, Karram K, Wörtge S, Regen T, Marini F, Hoppmann N, Klein M, Blank T, Yona S, Wolf Y, Mack M, Pinteaux E, Müller W, Zipp F, Binder H, Bopp T, Prinz M, Jung S, Waisman A. Genetic cell ablation reveals clusters of local self-renewing microglia in the mammalian central nervous system. Immunity 43: 92–106, 2015. doi: 10.1016/j.immuni.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 53.Buechler MB, Teal TH, Elkon KB, Hamerman JA. Cutting edge: type I IFN drives emergency myelopoiesis and peripheral myeloid expansion during chronic TLR7 signaling. J Immunol 190: 886–891, 2013. doi: 10.4049/jimmunol.1202739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burgess AW, Camakaris J, Metcalf D. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J Biol Chem 252: 1998–2003, 1977. [PubMed] [Google Scholar]
- 55.Butcher MJ, Herre M, Ley K, Galkina E. Flow cytometry analysis of immune cells within murine aortas. J Vis Exp 53: 2848, 2011. doi: 10.3791/2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Capucha T, Mizraji G, Segev H, Blecher-Gonen R, Winter D, Khalaileh A, Tabib Y, Attal T, Nassar M, Zelentsova K, Kisos H, Zenke M, Seré K, Hieronymus T, Burstyn-Cohen T, Amit I, Wilensky A, Hovav AH. Distinct murine mucosal langerhans cell subsets develop from pre-dendritic cells and monocytes. Immunity 43: 369–381, 2015. doi: 10.1016/j.immuni.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 57.Caride VJ, Zaret BL. Liposome accumulation in regions of experimental myocardial infarction. Science 198: 735–738, 1977. doi: 10.1126/science.910155. [DOI] [PubMed] [Google Scholar]
- 58.Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, Hedrick CC, Cook HT, Diebold S, Geissmann F. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell 153: 362–375, 2013. doi: 10.1016/j.cell.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cattin AL, Burden JJ, Van Emmenis L, Mackenzie FE, Hoving JJ, Garcia Calavia N, Guo Y, McLaughlin M, Rosenberg LH, Quereda V, Jamecna D, Napoli I, Parrinello S, Enver T, Ruhrberg C, Lloyd AC. Macrophage-induced blood vessels guide schwann cell-mediated regeneration of peripheral nerves. Cell 162: 1127–1139, 2015. doi: 10.1016/j.cell.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang MK, Bergmark C, Laurila A, Hörkkö S, Han KH, Friedman P, Dennis EA, Witztum JL. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc Natl Acad Sci USA 96: 6353–6358, 1999. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen S, Tisch N, Kegel M, Yerbes R, Hermann R, Hudalla H, Zuliani C, Gülcüler GS, Zwadlo K, von Engelhardt J, Ruiz de Almodóvar C, Martin-Villalba A. CNS Macrophages Control Neurovascular Development via CD95L. Cell Reports 19: 1378–1393, 2017. doi: 10.1016/j.celrep.2017.04.056. [DOI] [PubMed] [Google Scholar]
- 62.Chevrier S, Levine JH, Zanotelli VRT, Silina K, Schulz D, Bacac M, Ries CH, Ailles L, Jewett MAS, Moch H, van den Broek M, Beisel C, Stadler MB, Gedye C, Reis B, Pe’er D, Bodenmiller B. An immune atlas of clear cell renal cell carcinoma. Cell 169: 736–749.e18, 2017. doi: 10.1016/j.cell.2017.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chinetti-Gbaguidi G, Colin S, Staels B. Macrophage subsets in atherosclerosis. Nat Rev Cardiol 12: 10–17, 2015. doi: 10.1038/nrcardio.2014.173. [DOI] [PubMed] [Google Scholar]
- 64.Cho HJ, Shashkin P, Gleissner CA, Dunson D, Jain N, Lee JK, Miller Y, Ley K. Induction of dendritic cell-like phenotype in macrophages during foam cell formation. Physiol Genomics 29: 149–160, 2007. doi: 10.1152/physiolgenomics.00051.2006. [DOI] [PubMed] [Google Scholar]
- 65.Choi JH, Cheong C, Dandamudi DB, Park CG, Rodriguez A, Mehandru S, Velinzon K, Jung IH, Yoo JY, Oh GT, Steinman RM. Flt3 signaling-dependent dendritic cells protect against atherosclerosis. Immunity 35: 819–831, 2011. doi: 10.1016/j.immuni.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 66.Choi JH, Do Y, Cheong C, Koh H, Boscardin SB, Oh YS, Bozzacco L, Trumpfheller C, Park CG, Steinman RM. Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. J Exp Med 206: 497–505, 2009. doi: 10.1084/jem.20082129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choo EH, Lee JH, Park EH, Park HE, Jung NC, Kim TH, Koh YS, Kim E, Seung KB, Park C, Hong KS, Kang K, Song JY, Seo HG, Lim DS, Chang K. Infarcted myocardium-primed dendritic cells improve remodeling and cardiac function after myocardial infarction by modulating the regulatory T cell and macrophage polarization. Circulation 135: 1444–1457, 2017. doi: 10.1161/CIRCULATIONAHA.116.023106. [DOI] [PubMed] [Google Scholar]
- 68.Cochain C, Auvynet C, Poupel L, Vilar J, Dumeau E, Richart A, Récalde A, Zouggari Y, Yin KY, Bruneval P, Renault G, Marchiol C, Bonnin P, Lévy B, Bonecchi R, Locati M, Combadière C, Silvestre JS. The chemokine decoy receptor D6 prevents excessive inflammation and adverse ventricular remodeling after myocardial infarction. Arterioscler Thromb Vasc Biol 32: 2206–2213, 2012. doi: 10.1161/ATVBAHA.112.254409. [DOI] [PubMed] [Google Scholar]
- 69.Cochain C, Vafadarnejad E, Arampatzi P, Pelisek J, Winkels H, Ley K, Wolf D, Saliba AE, Zernecke A. Single-cell RNA-Seq reveals the transcriptional landscape and heterogeneity of aortic macrophages in murine atherosclerosis. Circ Res 122: 1661–1674, 2018. doi: 10.1161/CIRCRESAHA.117.312509. [DOI] [PubMed] [Google Scholar]
- 70.Cohn ZA, Benson B. The differentiation of mononuclear phagocytes. morphology, cytochemistry, and biochemistry. J Exp Med 121: 153–170, 1965. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Combadière C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation 117: 1649–1657, 2008. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 72.Cordero-Reyes AM, Youker KA, Trevino AR, Celis R, Hamilton DJ, Flores-Arredondo JH, Orrego CM, Bhimaraj A, Estep JD, Torre-Amione G. Full expression of cardiomyopathy is partly dependent on B-cells: a pathway that involves cytokine activation, immunoglobulin deposition, and activation of apoptosis. J Am Heart Assoc 5: 5, 2016. doi: 10.1161/JAHA.115.002484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cortez-Retamozo V, Etzrodt M, Newton A, Ryan R, Pucci F, Sio SW, Kuswanto W, Rauch PJ, Chudnovskiy A, Iwamoto Y, Kohler R, Marinelli B, Gorbatov R, Wojtkiewicz G, Panizzi P, Mino-Kenudson M, Forghani R, Figueiredo JL, Chen JW, Xavier R, Swirski FK, Nahrendorf M, Weissleder R, Pittet MJ. Angiotensin II drives the production of tumor-promoting macrophages. Immunity 38: 296–308, 2013. doi: 10.1016/j.immuni.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cosenza H, Leserman LD, Rowley DA. The third cell type required for the immune response of spleen cells in vitro. J Immunol 107: 414–421, 1971. [PubMed] [Google Scholar]
- 75.Courties G, Heidt T, Sebas M, Iwamoto Y, Jeon D, Truelove J, Tricot B, Wojtkiewicz G, Dutta P, Sager HB, Borodovsky A, Novobrantseva T, Klebanov B, Fitzgerald K, Anderson DG, Libby P, Swirski FK, Weissleder R, Nahrendorf M. In vivo silencing of the transcription factor IRF5 reprograms the macrophage phenotype and improves infarct healing. J Am Coll Cardiol 63: 1556–1566, 2014. doi: 10.1016/j.jacc.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Courties G, Herisson F, Sager HB, Heidt T, Ye Y, Wei Y, Sun Y, Severe N, Dutta P, Scharff J, Scadden DT, Weissleder R, Swirski FK, Moskowitz MA, Nahrendorf M. Ischemic stroke activates hematopoietic bone marrow stem cells. Circ Res 116: 407–417, 2015. doi: 10.1161/CIRCRESAHA.116.305207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Crane GM, Jeffery E, Morrison SJ. Adult haematopoietic stem cell niches. Nat Rev Immunol 17: 573–590, 2017. doi: 10.1038/nri.2017.53. [DOI] [PubMed] [Google Scholar]
- 78.Crane MJ, Daley JM, van Houtte O, Brancato SK, Henry WL Jr, Albina JE. The monocyte to macrophage transition in the murine sterile wound. PLoS One 9: e86660, 2014. doi: 10.1371/journal.pone.0086660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, Jais JP, D’Cruz D, Casanova JL, Trouillet C, Geissmann F. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 33: 375–386, 2010. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Croxford AL, Lanzinger M, Hartmann FJ, Schreiner B, Mair F, Pelczar P, Clausen BE, Jung S, Greter M, Becher B. The cytokine GM-CSF drives the inflammatory signature of CCR2+ monocytes and licenses autoimmunity. Immunity 43: 502–514, 2015. doi: 10.1016/j.immuni.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 81.Cybulsky MI, Cheong C, Robbins CS. Macrophages and dendritic cells: partners in atherogenesis. Circ Res 118: 637–652, 2016. doi: 10.1161/CIRCRESAHA.115.306542. [DOI] [PubMed] [Google Scholar]
- 82.Dahl R, Walsh JC, Lancki D, Laslo P, Iyer SR, Singh H, Simon MC. Regulation of macrophage and neutrophil cell fates by the PU.1:C/EBPalpha ratio and granulocyte colony-stimulating factor. Nat Immunol 4: 1029–1036, 2003. doi: 10.1038/ni973. [DOI] [PubMed] [Google Scholar]
- 83.Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, Sylvestre V, Stanley ER. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood 99: 111–120, 2002. doi: 10.1182/blood.V99.1.111. [DOI] [PubMed] [Google Scholar]
- 84.Dakic A, Metcalf D, Di Rago L, Mifsud S, Wu L, Nutt SL. PU.1 regulates the commitment of adult hematopoietic progenitors and restricts granulopoiesis. J Exp Med 201: 1487–1502, 2005. doi: 10.1084/jem.20050075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dal-Secco D, Wang J, Zeng Z, Kolaczkowska E, Wong CH, Petri B, Ransohoff RM, Charo IF, Jenne CN, Kubes P. A dynamic spectrum of monocytes arising from the in situ reprogramming of CCR2+ monocytes at a site of sterile injury. J Exp Med 212: 447–456, 2015. doi: 10.1084/jem.20141539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.David BA, Rezende RM, Antunes MM, Santos MM, Freitas Lopes MA, Diniz AB, Sousa Pereira RV, Marchesi SC, Alvarenga DM, Nakagaki BN, Araújo AM, Dos Reis DS, Rocha RM, Marques PE, Lee WY, Deniset J, Liew PX, Rubino S, Cox L, Pinho V, Cunha TM, Fernandes GR, Oliveira AG, Teixeira MM, Kubes P, Menezes GB. Combination of mass cytometry and imaging analysis reveals origin, location, and functional repopulation of liver myeloid cells in mice. Gastroenterology 151: 1176–1191, 2016. doi: 10.1053/j.gastro.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 87.de Bruijn MF, Speck NA, Peeters MC, Dzierzak E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J 19: 2465–2474, 2000. doi: 10.1093/emboj/19.11.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dehn S, Thorp EB. Myeloid receptor CD36 is required for early phagocytosis of myocardial infarcts and induction of Nr4a1-dependent mechanisms of cardiac repair. FASEB J 32: 254–264, 2018. doi: 10.1096/fj.201700450R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res 96: 881–889, 2005. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 90.Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke 38: 1345–1353, 2007. doi: 10.1161/01.STR.0000259709.16654.8f. [DOI] [PubMed] [Google Scholar]
- 91.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464: 1357–1361, 2010. [Erratum in Nature 466: 652, 2010.] doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dunlay SM, Weston SA, Redfield MM, Killian JM, Roger VL. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation 118: 625–631, 2008. doi: 10.1161/CIRCULATIONAHA.107.759191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Durai V, Murphy KM. Functions of murine dendritic cells. Immunity 45: 719–736, 2016. doi: 10.1016/j.immuni.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Durgan DJ, Pulinilkunnil T, Villegas-Montoya C, Garvey ME, Frangogiannis NG, Michael LH, Chow CW, Dyck JR, Young ME. Short communication: ischemia/reperfusion tolerance is time-of-day-dependent: mediation by the cardiomyocyte circadian clock. Circ Res 106: 546–550, 2010. doi: 10.1161/CIRCRESAHA.109.209346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HW, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M. Myocardial infarction accelerates atherosclerosis. Nature 487: 325–329, 2012. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dutta P, Hoyer FF, Grigoryeva LS, Sager HB, Leuschner F, Courties G, Borodovsky A, Novobrantseva T, Ruda VM, Fitzgerald K, Iwamoto Y, Wojtkiewicz G, Sun Y, Da Silva N, Libby P, Anderson DG, Swirski FK, Weissleder R, Nahrendorf M. Macrophages retain hematopoietic stem cells in the spleen via VCAM-1. J Exp Med 212: 497–512, 2015. doi: 10.1084/jem.20141642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dutta P, Sager HB, Stengel KR, Naxerova K, Courties G, Saez B, Silberstein L, Heidt T, Sebas M, Sun Y, Wojtkiewicz G, Feruglio PF, King K, Baker JN, van der Laan AM, Borodovsky A, Fitzgerald K, Hulsmans M, Hoyer F, Iwamoto Y, Vinegoni C, Brown D, Di Carli M, Libby P, Hiebert SW, Scadden DT, Swirski FK, Weissleder R, Nahrendorf M. Myocardial infarction activates CCR2(+) hematopoietic stem and progenitor cells. Cell Stem Cell 16: 477–487, 2015. doi: 10.1016/j.stem.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dzik JM. The ancestry and cumulative evolution of immune reactions. Acta Biochim Pol 57: 443–466, 2010. [PubMed] [Google Scholar]
- 99.Dzik JM. Evolutionary roots of arginase expression and regulation. Front Immunol 5: 544, 2014. doi: 10.3389/fimmu.2014.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, Kitazawa M, Matusow B, Nguyen H, West BL, Green KN. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82: 380–397, 2014. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Emami H, Singh P, MacNabb M, Vucic E, Lavender Z, Rudd JH, Fayad ZA, Lehrer-Graiwer J, Korsgren M, Figueroa AL, Fredrickson J, Rubin B, Hoffmann U, Truong QA, Min JK, Baruch A, Nasir K, Nahrendorf M, Tawakol A. Splenic metabolic activity predicts risk of future cardiovascular events: demonstration of a cardiosplenic axis in humans. JACC Cardiovasc Imaging 8: 121–130, 2015. doi: 10.1016/j.jcmg.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Emile JF, Geissmann F, Martin OC, Radford-Weiss I, Lepelletier Y, Heymer B, Espanol T, de Santes KB, Bertrand Y, Brousse N, Casanova JL, Fischer A. Langerhans cell deficiency in reticular dysgenesis. Blood 96: 58–62, 2000. [PubMed] [Google Scholar]
- 103.Engström G, Melander O, Hedblad B. Leukocyte count and incidence of hospitalizations due to heart failure. Circ Heart Fail 2: 217–222, 2009. doi: 10.1161/CIRCHEARTFAILURE.108.827071. [DOI] [PubMed] [Google Scholar]
- 104.Ensan S, Li A, Besla R, Degousee N, Cosme J, Roufaiel M, Shikatani EA, El-Maklizi M, Williams JW, Robins L, Li C, Lewis B, Yun TJ, Lee JS, Wieghofer P, Khattar R, Farrokhi K, Byrne J, Ouzounian M, Zavitz CC, Levy GA, Bauer CM, Libby P, Husain M, Swirski FK, Cheong C, Prinz M, Hilgendorf I, Randolph GJ, Epelman S, Gramolini AO, Cybulsky MI, Rubin BB, Robbins CS. Self-renewing resident arterial macrophages arise from embryonic CX3CR1(+) precursors and circulating monocytes immediately after birth. Nat Immunol 17: 159–168, 2016. doi: 10.1038/ni.3343. [DOI] [PubMed] [Google Scholar]
- 105.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, Schilling JD, Schwendener R, Sergin I, Razani B, Forsberg EC, Yokoyama WM, Unanue ER, Colonna M, Randolph GJ, Mann DL. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 40: 91–104, 2014. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity 41: 21–35, 2014. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature 458: 904–908, 2009. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- 108.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest 101: 890–898, 1998. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol 148: 2207–2216, 1992. [PubMed] [Google Scholar]
- 110.Feil S, Fehrenbacher B, Lukowski R, Essmann F, Schulze-Osthoff K, Schaller M, Feil R. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ Res 115: 662–667, 2014. doi: 10.1161/CIRCRESAHA.115.304634. [DOI] [PubMed] [Google Scholar]
- 111.Fischer K, Ruiz HH, Jhun K, Finan B, Oberlin DJ, van der Heide V, Kalinovich AV, Petrovic N, Wolf Y, Clemmensen C, Shin AC, Divanovic S, Brombacher F, Glasmacher E, Keipert S, Jastroch M, Nagler J, Schramm KW, Medrikova D, Collden G, Woods SC, Herzig S, Homann D, Jung S, Nedergaard J, Cannon B, Tschöp MH, Müller TD, Buettner C. Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nat Med 23: 623–630, 2017. doi: 10.1038/nm.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science 311: 83–87, 2006. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 113.Foks AC, Ran IA, Wasserman L, Frodermann V, Ter Borg MN, de Jager SC, van Santbrink PJ, Yagita H, Akiba H, Bot I, Kuiper J, van Puijvelde GH. T-cell immunoglobulin and mucin domain 3 acts as a negative regulator of atherosclerosis. Arterioscler Thromb Vasc Biol 33: 2558–2565, 2013. doi: 10.1161/ATVBAHA.113.301879. [DOI] [PubMed] [Google Scholar]
- 114.Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol Rev 92: 635–688, 2012. doi: 10.1152/physrev.00008.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Frangogiannis NG. Fibroblasts and the extracellular matrix in right ventricular disease. Cardiovasc Res 113: 1453–1464, 2017. doi: 10.1093/cvr/cvx146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Frantz S, Hofmann U, Fraccarollo D, Schäfer A, Kranepuhl S, Hagedorn I, Nieswandt B, Nahrendorf M, Wagner H, Bayer B, Pachel C, Schön MP, Kneitz S, Bobinger T, Weidemann F, Ertl G, Bauersachs J. Monocytes/macrophages prevent healing defects and left ventricular thrombus formation after myocardial infarction. FASEB J 27: 871–881, 2013. doi: 10.1096/fj.12-214049. [DOI] [PubMed] [Google Scholar]
- 117.Freedman MH, Saunders EF. Hematopoiesis in the human spleen. Am J Hematol 11: 271–275, 1981. doi: 10.1002/ajh.2830110307. [DOI] [PubMed] [Google Scholar]
- 118.Frodermann V, van Puijvelde GH, Wierts L, Lagraauw HM, Foks AC, van Santbrink PJ, Bot I, Kuiper J, de Jager SC. Oxidized low-density lipoprotein-induced apoptotic dendritic cells as a novel therapy for atherosclerosis. J Immunol 194: 2208–2218, 2015. doi: 10.4049/jimmunol.1401843. [DOI] [PubMed] [Google Scholar]
- 119.Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu CL, Sano S, Muralidharan S, Rius C, Vuong J, Jacob S, Muralidhar V, Robertson AA, Cooper MA, Andrés V, Hirschi KK, Martin KA, Walsh K. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 355: 842–847, 2017. doi: 10.1126/science.aag1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med 203: 1273–1282, 2006. doi: 10.1084/jem.20052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gartner JG, Adamson IY, Bowden DH. Proliferation of pulmonary macrophages during the early phase of an acute graft-versus-host reaction in mice. Exp Lung Res 14: 781–796, 1988. doi: 10.3109/01902148809087844. [DOI] [PubMed] [Google Scholar]
- 123.Gautier EL, Huby T, Saint-Charles F, Ouzilleau B, Pirault J, Deswaerte V, Ginhoux F, Miller ER, Witztum JL, Chapman MJ, Lesnik P. Conventional dendritic cells at the crossroads between immunity and cholesterol homeostasis in atherosclerosis. Circulation 119: 2367–2375, 2009. doi: 10.1161/CIRCULATIONAHA.108.807537. [DOI] [PubMed] [Google Scholar]
- 124.Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma’ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, Randolph GJ; Immunological Genome Consortium . Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol 13: 1118–1128, 2012. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Geeraerts X, Bolli E, Fendt SM, Van Ginderachter JA. Macrophage metabolism as therapeutic target for cancer, atherosclerosis, and obesity. Front Immunol 8: 289, 2017. doi: 10.3389/fimmu.2017.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol 10: 453–460, 2010. doi: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19: 71–82, 2003. doi: 10.1016/S1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 128.Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, Arumugam TV, Orthey E, Gerloff C, Tolosa E, Magnus T. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 40: 1849–1857, 2009. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- 129.Gerrity RG, Naito HK, Richardson M, Schwartz CJ. Dietary induced atherogenesis in swine. Morphology of the intima in prelesion stages. Am J Pathol 95: 775–792, 1979. [PMC free article] [PubMed] [Google Scholar]
- 130.Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA Jr, Luster AD, Luscinskas FW, Rosenzweig A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature 398: 718–723, 1999. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 131.Ghigo C, Mondor I, Jorquera A, Nowak J, Wienert S, Zahner SP, Clausen BE, Luche H, Malissen B, Klauschen F, Bajénoff M. Multicolor fate mapping of Langerhans cell homeostasis. J Exp Med 210: 1657–1664, 2013. doi: 10.1084/jem.20130403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gibbings SL, Goyal R, Desch AN, Leach SM, Prabagar M, Atif SM, Bratton DL, Janssen W, Jakubzick CV. Transcriptome analysis highlights the conserved difference between embryonic and postnatal-derived alveolar macrophages. Blood 126: 1357–1366, 2015. doi: 10.1182/blood-2015-01-624809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gilbert J, Lekstrom-Himes J, Donaldson D, Lee Y, Hu M, Xu J, Wyant T, Davidson M; MLN1202 Study Group . Effect of CC chemokine receptor 2 CCR2 blockade on serum C-reactive protein in individuals at atherosclerotic risk and with a single nucleotide polymorphism of the monocyte chemoattractant protein-1 promoter region. Am J Cardiol 107: 906–911, 2011. doi: 10.1016/j.amjcard.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 134.Gimmi CD, Freeman GJ, Gribben JG, Sugita K, Freedman AS, Morimoto C, Nadler LM. B-cell surface antigen B7 provides a costimulatory signal that induces T cells to proliferate and secrete interleukin 2. Proc Natl Acad Sci USA 88: 6575–6579, 1991. doi: 10.1073/pnas.88.15.6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330: 841–845, 2010. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol 14: 392–404, 2014. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- 137.Ginhoux F, Schultze JL, Murray PJ, Ochando J, Biswas SK. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat Immunol 17: 34–40, 2016. doi: 10.1038/ni.3324. [DOI] [PubMed] [Google Scholar]
- 138.Golde DW, Finley TN, Cline MJ. The pulmonary macrophage in acute leukemia. N Engl J Med 290: 875–878, 1974. doi: 10.1056/NEJM197404182901603. [DOI] [PubMed] [Google Scholar]
- 139.Goldmann T, Wieghofer P, Jordão MJ, Prutek F, Hagemeyer N, Frenzel K, Amann L, Staszewski O, Kierdorf K, Krueger M, Locatelli G, Hochgerner H, Zeiser R, Epelman S, Geissmann F, Priller J, Rossi FM, Bechmann I, Kerschensteiner M, Linnarsson S, Jung S, Prinz M. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol 17: 797–805, 2016. doi: 10.1038/ni.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Goldszmid RS, Caspar P, Rivollier A, White S, Dzutsev A, Hieny S, Kelsall B, Trinchieri G, Sher A. NK cell-derived interferon-γ orchestrates cellular dynamics and the differentiation of monocytes into dendritic cells at the site of infection. Immunity 36: 1047–1059, 2012. doi: 10.1016/j.immuni.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald HR. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 518: 547–551, 2015. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Goo YH, Yechoor VK, Paul A. Transcriptional profiling of foam cells in response to hypercholesterolemia. Genom Data 9: 37–39, 2016. doi: 10.1016/j.gdata.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gordon D, Reidy MA, Benditt EP, Schwartz SM. Cell proliferation in human coronary arteries. Proc Natl Acad Sci USA 87: 4600–4604, 1990. doi: 10.1073/pnas.87.12.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gordon S, Hamann J, Lin HH, Stacey M. F4/80 and the related adhesion-GPCRs. Eur J Immunol 41: 2472–2476, 2011. doi: 10.1002/eji.201141715. [DOI] [PubMed] [Google Scholar]
- 145.Gosling J, Slaymaker S, Gu L, Tseng S, Zlot CH, Young SG, Rollins BJ, Charo IF. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Invest 103: 773–778, 1999. doi: 10.1172/JCI5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gosselin D, Link VM, Romanoski CE, Fonseca GJ, Eichenfield DZ, Spann NJ, Stender JD, Chun HB, Garner H, Geissmann F, Glass CK. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 159: 1327–1340, 2014. doi: 10.1016/j.cell.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gosselin D, Skola D, Coufal NG, Holtman IR, Schlachetzki JCM, Sajti E, Jaeger BN, O’Connor C, Fitzpatrick C, Pasillas MP, Pena M, Adair A, Gonda DD, Levy ML, Ransohoff RM, Gage FH, Glass CK. An environment-dependent transcriptional network specifies human microglia identity. Science 356: eaal3222, 2017. doi: 10.1126/science.aal3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gown AM, Tsukada T, Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol 125: 191–207, 1986. [PMC free article] [PubMed] [Google Scholar]
- 149.Greter M, Lelios I, Pelczar P, Hoeffel G, Price J, Leboeuf M, Kündig TM, Frei K, Ginhoux F, Merad M, Becher B. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity 37: 1050–1060, 2012. doi: 10.1016/j.immuni.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Griciuc A, Serrano-Pozo A, Parrado AR, Lesinski AN, Asselin CN, Mullin K, Hooli B, Choi SH, Hyman BT, Tanzi RE. Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron 78: 631–643, 2013. doi: 10.1016/j.neuron.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Groh L, Keating ST, Joosten LAB, Netea MG, Riksen NP. Monocyte and macrophage immunometabolism in atherosclerosis. Semin Immunopathol 40: 203–214, 2018. doi: 10.1007/s00281-017-0656-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell 2: 275–281, 1998. doi: 10.1016/S1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 153.Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, Deswarte K, Malissen B, Hammad H, Lambrecht BN. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med 210: 1977–1992, 2013. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Guo J, de Waard V, Van Eck M, Hildebrand RB, van Wanrooij EJ, Kuiper J, Maeda N, Benson GM, Groot PH, Van Berkel TJ. Repopulation of apolipoprotein E knockout mice with CCR2-deficient bone marrow progenitor cells does not inhibit ongoing atherosclerotic lesion development. Arterioscler Thromb Vasc Biol 25: 1014–1019, 2005. doi: 10.1161/01.ATV.0000163181.40896.42. [DOI] [PubMed] [Google Scholar]
- 155.Hamers AA, Vos M, Rassam F, Marinković G, Kurakula K, van Gorp PJ, de Winther MP, Gijbels MJ, de Waard V, de Vries CJ. Bone marrow-specific deficiency of nuclear receptor Nur77 enhances atherosclerosis. Circ Res 110: 428–438, 2012. doi: 10.1161/CIRCRESAHA.111.260760. [DOI] [PubMed] [Google Scholar]
- 156.Hamilton JA, Lacey DC, Turner A, de Kok B, Huynh J, Scholz GM. Hypoxia enhances the proliferative response of macrophages to CSF-1 and their pro-survival response to TNF. PLoS One 7: e45853, 2012. doi: 10.1371/journal.pone.0045853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hamilton JA, Myers D, Jessup W, Cochrane F, Byrne R, Whitty G, Moss S. Oxidized LDL can induce macrophage survival, DNA synthesis, and enhanced proliferative response to CSF-1 and GM-CSF. Arterioscler Thromb Vasc Biol 19: 98–105, 1999. doi: 10.1161/01.ATV.19.1.98. [DOI] [PubMed] [Google Scholar]
- 158.Hammond MD, Taylor RA, Mullen MT, Ai Y, Aguila HL, Mack M, Kasner SE, McCullough LD, Sansing LH. CCR2+ Ly6C(hi) inflammatory monocyte recruitment exacerbates acute disability following intracerebral hemorrhage. J Neurosci 34: 3901–3909, 2014. doi: 10.1523/JNEUROSCI.4070-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Haniffa M, Ginhoux F, Wang XN, Bigley V, Abel M, Dimmick I, Bullock S, Grisotto M, Booth T, Taub P, Hilkens C, Merad M, Collin M. Differential rates of replacement of human dermal dendritic cells and macrophages during hematopoietic stem cell transplantation. J Exp Med 206: 371–385, 2009. doi: 10.1084/jem.20081633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, Geissmann F, Hedrick CC. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nat Immunol 12: 778–785, 2011. doi: 10.1038/ni.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hanna RN, Cekic C, Sag D, Tacke R, Thomas GD, Nowyhed H, Herrley E, Rasquinha N, McArdle S, Wu R, Peluso E, Metzger D, Ichinose H, Shaked I, Chodaczek G, Biswas SK, Hedrick CC. Patrolling monocytes control tumor metastasis to the lung. Science 350: 985–990, 2015. doi: 10.1126/science.aac9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hanna RN, Shaked I, Hubbeling HG, Punt JA, Wu R, Herrley E, Zaugg C, Pei H, Geissmann F, Ley K, Hedrick CC. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ Res 110: 416–427, 2012. doi: 10.1161/CIRCRESAHA.111.253377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Harel-Adar T, Ben Mordechai T, Amsalem Y, Feinberg MS, Leor J, Cohen S. Modulation of cardiac macrophages by phosphatidylserine-presenting liposomes improves infarct repair. Proc Natl Acad Sci USA 108: 1827–1832, 2011. doi: 10.1073/pnas.1015623108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Haschemi A, Kosma P, Gille L, Evans CR, Burant CF, Starkl P, Knapp B, Haas R, Schmid JA, Jandl C, Amir S, Lubec G, Park J, Esterbauer H, Bilban M, Brizuela L, Pospisilik JA, Otterbein LE, Wagner O. The sedoheptulose kinase CARKL directs macrophage polarization through control of glucose metabolism. Cell Metab 15: 813–826, 2012. doi: 10.1016/j.cmet.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hasemann MS, Lauridsen FKB, Waage J, Jakobsen JS, Frank AK, Schuster MB, Rapin N, Bagger FO, Hoppe PS, Schroeder T, Porse BT. C/EBPα is required for long-term self-renewal and lineage priming of hematopoietic stem cells and for the maintenance of epigenetic configurations in multipotent progenitors. PLoS Genet 10: e1004079, 2014. doi: 10.1371/journal.pgen.1004079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, García-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38: 792–804, 2013. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Haudek SB, Cheng J, Du J, Wang Y, Hermosillo-Rodriguez J, Trial J, Taffet GE, Entman ML. Monocytic fibroblast precursors mediate fibrosis in angiotensin-II-induced cardiac hypertrophy. J Mol Cell Cardiol 49: 499–507, 2010. doi: 10.1016/j.yjmcc.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, Pilling D, Gomer RH, Trial J, Frangogiannis NG, Entman ML. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci USA 103: 18284–18289, 2006. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hawkes CA, McLaurin J. Selective targeting of perivascular macrophages for clearance of β-amyloid in cerebral amyloid angiopathy. Proc Natl Acad Sci USA 106: 1261–1266, 2009. doi: 10.1073/pnas.0805453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hayashidani S, Tsutsui H, Shiomi T, Ikeuchi M, Matsusaka H, Suematsu N, Wen J, Egashira K, Takeshita A. Anti-monocyte chemoattractant protein-1 gene therapy attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation 108: 2134–2140, 2003. doi: 10.1161/01.CIR.0000092890.29552.22. [DOI] [PubMed] [Google Scholar]
- 171.He H, Mack JJ, Güç E, Warren CM, Squadrito ML, Kilarski WW, Baer C, Freshman RD, McDonald AI, Ziyad S, Swartz MA, De Palma M, Iruela-Arispe ML. Perivascular Macrophages Limit Permeability. Arterioscler Thromb Vasc Biol 36: 2203–2212, 2016. doi: 10.1161/ATVBAHA.116.307592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Heidt T, Courties G, Dutta P, Sager HB, Sebas M, Iwamoto Y, Sun Y, Da Silva N, Panizzi P, van der Laan AM, Swirski FK, Weissleder R, Nahrendorf M. Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ Res 115: 284–295, 2014. [Erratum in Circ Res 115: e95, 2014.] doi: 10.1161/CIRCRESAHA.115.303567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von Zur Muhlen C, Bode C, Fricchione GL, Denninger J, Lin CP, Vinegoni C, Libby P, Swirski FK, Weissleder R, Nahrendorf M. Chronic variable stress activates hematopoietic stem cells. Nat Med 20: 754–758, 2014. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hermann S, Kuhlmann MT, Starsichova A, Eligehausen S, Schäfers K, Stypmann J, Tiemann K, Levkau B, Schäfers M. Imaging reveals the connection between spontaneous coronary plaque ruptures, atherothrombosis, and myocardial infarctions in HypoE/SRBI−/− mice. J Nucl Med 57: 1420–1427, 2016. doi: 10.2967/jnumed.115.171132. [DOI] [PubMed] [Google Scholar]
- 175.Herz J, Filiano AJ, Smith A, Yogev N, Kipnis J. Myeloid cells in the central nervous system. Immunity 46: 943–956, 2017. doi: 10.1016/j.immuni.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hettinger J, Richards DM, Hansson J, Barra MM, Joschko AC, Krijgsveld J, Feuerer M. Origin of monocytes and macrophages in a committed progenitor. Nat Immunol 14: 821–830, 2013. doi: 10.1038/ni.2638. [DOI] [PubMed] [Google Scholar]
- 177.Hilgendorf I, Gerhardt LM, Tan TC, Winter C, Holderried TA, Chousterman BG, Iwamoto Y, Liao R, Zirlik A, Scherer-Crosbie M, Hedrick CC, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res 114: 1611–1622, 2014. doi: 10.1161/CIRCRESAHA.114.303204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hjalmarson A, Gilpin EA, Nicod P, Dittrich H, Henning H, Engler R, Blacky AR, Smith SC Jr, Ricou F, Ross J Jr. Differing circadian patterns of symptom onset in subgroups of patients with acute myocardial infarction. Circulation 80: 267–275, 1989. doi: 10.1161/01.CIR.80.2.267. [DOI] [PubMed] [Google Scholar]
- 179.Hock H, Hamblen MJ, Rooke HM, Schindler JW, Saleque S, Fujiwara Y, Orkin SH. Gfi-1 restricts proliferation and preserves functional integrity of haematopoietic stem cells. Nature 431: 1002–1007, 2004. doi: 10.1038/nature02994. [DOI] [PubMed] [Google Scholar]
- 180.Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, See P, Beaudin AE, Lum J, Low I, Forsberg EC, Poidinger M, Zolezzi F, Larbi A, Ng LG, Chan JK, Greter M, Becher B, Samokhvalov IM, Merad M, Ginhoux F. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 42: 665–678, 2015. doi: 10.1016/j.immuni.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, Leboeuf M, Low D, Oller G, Almeida F, Choy SH, Grisotto M, Renia L, Conway SJ, Stanley ER, Chan JK, Ng LG, Samokhvalov IM, Merad M, Ginhoux F. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med 209: 1167–1181, 2012. doi: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hoggatt J, Kfoury Y, Scadden DT. Hematopoietic stem cell niche in health and disease. Annu Rev Pathol 11: 555–581, 2016. doi: 10.1146/annurev-pathol-012615-044414. [DOI] [PubMed] [Google Scholar]
- 183.Holtschke T, Löhler J, Kanno Y, Fehr T, Giese N, Rosenbauer F, Lou J, Knobeloch KP, Gabriele L, Waring JF, Bachmann MF, Zinkernagel RM, Morse HC III, Ozato K, Horak I. Immunodeficiency and chronic myelogenous leukemia-like syndrome in mice with a targeted mutation of the ICSBP gene. Cell 87: 307–317, 1996. doi: 10.1016/S0092-8674(00)81348-3. [DOI] [PubMed] [Google Scholar]
- 184.Hosseini H, Li Y, Kanellakis P, Tay C, Cao A, Tipping P, Bobik A, Toh BH, Kyaw T. Phosphatidylserine liposomes mimic apoptotic cells to attenuate atherosclerosis by expanding polyreactive IgM producing B1a lymphocytes. Cardiovasc Res 106: 443–452, 2015. doi: 10.1093/cvr/cvv037. [DOI] [PubMed] [Google Scholar]
- 185.Howangyin KY, Zlatanova I, Pinto C, Ngkelo A, Cochain C, Rouanet M, Vilar J, Lemitre M, Stockmann C, Fleischmann BK, Mallat Z, Silvestre JS. Myeloid-epithelial-reproductive receptor tyrosine kinase and milk fat globule epidermal growth factor 8 coordinately improve remodeling after myocardial infarction via local delivery of vascular endothelial growth factor. Circulation 133: 826–839, 2016. doi: 10.1161/CIRCULATIONAHA.115.020857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hu Y, Zhang H, Lu Y, Bai H, Xu Y, Zhu X, Zhou R, Ben J, Xu Y, Chen Q. Class A scavenger receptor attenuates myocardial infarction-induced cardiomyocyte necrosis through suppressing M1 macrophage subset polarization. Basic Res Cardiol 106: 1311–1328, 2011. doi: 10.1007/s00395-011-0204-x. [DOI] [PubMed] [Google Scholar]
- 187.Huang SC, Everts B, Ivanova Y, O’Sullivan D, Nascimento M, Smith AM, Beatty W, Love-Gregory L, Lam WY, O’Neill CM, Yan C, Du H, Abumrad NA, Urban JF Jr, Artyomov MN, Pearce EL, Pearce EJ. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol 15: 846–855, 2014. doi: 10.1038/ni.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hulsmans M, Clauss S, Xiao L, Aguirre AD, King KR, Hanley A, Hucker WJ, Wülfers EM, Seemann G, Courties G, Iwamoto Y, Sun Y, Savol AJ, Sager HB, Lavine KJ, Fishbein GA, Capen DE, Da Silva N, Miquerol L, Wakimoto H, Seidman CE, Seidman JG, Sadreyev RI, Naxerova K, Mitchell RN, Brown D, Libby P, Weissleder R, Swirski FK, Kohl P, Vinegoni C, Milan DJ, Ellinor PT, Nahrendorf M. Macrophages facilitate electrical conduction in the heart. Cell 169: 510–522.e20, 2017. doi: 10.1016/j.cell.2017.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hulsmans M, Sager HB, Roh JD, Valero-Muñoz M, Houstis NE, Iwamoto Y, Sun Y, Wilson RM, Wojtkiewicz G, Tricot B, Osborne MT, Hung J, Vinegoni C, Naxerova K, Sosnovik DE, Zile MR, Bradshaw AD, Liao R, Tawakol A, Weissleder R, Rosenzweig A, Swirski FK, Sam F, Nahrendorf M. Cardiac macrophages promote diastolic dysfunction. J Exp Med 215: 423–440, 2018. doi: 10.1084/jem.20171274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hulsmans M, Sam F, Nahrendorf M. Monocyte and macrophage contributions to cardiac remodeling. J Mol Cell Cardiol 93: 149–155, 2016. doi: 10.1016/j.yjmcc.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hutter E, Boridy S, Labrecque S, Lalancette-Hébert M, Kriz J, Winnik FM, Maysinger D. Microglial response to gold nanoparticles. ACS Nano 4: 2595–2606, 2010. doi: 10.1021/nn901869f. [DOI] [PubMed] [Google Scholar]
- 192.Imhof BA, Aurrand-Lions M. Adhesion mechanisms regulating the migration of monocytes. Nat Rev Immunol 4: 432–444, 2004. doi: 10.1038/nri1375. [DOI] [PubMed] [Google Scholar]
- 193.Ingersoll MA, Spanbroek R, Lottaz C, Gautier EL, Frankenberger M, Hoffmann R, Lang R, Haniffa M, Collin M, Tacke F, Habenicht AJ, Ziegler-Heitbrock L, Randolph GJ. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood 115: e10–e19, 2010. doi: 10.1182/blood-2009-07-235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Iqbal AJ, McNeill E, Kapellos TS, Regan-Komito D, Norman S, Burd S, Smart N, Machemer DE, Stylianou E, McShane H, Channon KM, Chawla A, Greaves DR. Human CD68 promoter GFP transgenic mice allow analysis of monocyte to macrophage differentiation in vivo. Blood 124: e33–e44, 2014. doi: 10.1182/blood-2014-04-568691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ishibashi M, Hiasa K, Zhao Q, Inoue S, Ohtani K, Kitamoto S, Tsuchihashi M, Sugaya T, Charo IF, Kura S, Tsuzuki T, Ishibashi T, Takeshita A, Egashira K. Critical role of monocyte chemoattractant protein-1 receptor CCR2 on monocytes in hypertension-induced vascular inflammation and remodeling. Circ Res 94: 1203–1210, 2004. doi: 10.1161/01.RES.0000126924.23467.A3. [DOI] [PubMed] [Google Scholar]
- 196.Ismahil MA, Hamid T, Bansal SS, Patel B, Kingery JR, Prabhu SD. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ Res 114: 266–282, 2014. doi: 10.1161/CIRCRESAHA.113.301720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Iwasaki H, Somoza C, Shigematsu H, Duprez EA, Iwasaki-Arai J, Mizuno S, Arinobu Y, Geary K, Zhang P, Dayaram T, Fenyus ML, Elf S, Chan S, Kastner P, Huettner CS, Murray R, Tenen DG, Akashi K. Distinctive and indispensable roles of PU.1 in maintenance of hematopoietic stem cells and their differentiation. Blood 106: 1590–1600, 2005. doi: 10.1182/blood-2005-03-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jaipersad AS, Lip GY, Silverman S, Shantsila E. The role of monocytes in angiogenesis and atherosclerosis. J Am Coll Cardiol 63: 1–11, 2014. doi: 10.1016/j.jacc.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 199.Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, Higgins JM, Moltchanov V, Kuo FC, Kluk MJ, Henderson B, Kinnunen L, Koistinen HA, Ladenvall C, Getz G, Correa A, Banahan BF, Gabriel S, Kathiresan S, Stringham HM, McCarthy MI, Boehnke M, Tuomilehto J, Haiman C, Groop L, Atzmon G, Wilson JG, Neuberg D, Altshuler D, Ebert BL. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 371: 2488–2498, 2014. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, Baber U, Mehran R, Fuster V, Danesh J, Frossard P, Saleheen D, Melander O, Sukhova GK, Neuberg D, Libby P, Kathiresan S, Ebert BL. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med 377: 111–121, 2017. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, Ivanov S, Duan Q, Bala S, Condon T, van Rooijen N, Grainger JR, Belkaid Y, Ma’ayan A, Riches DW, Yokoyama WM, Ginhoux F, Henson PM, Randolph GJ. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity 39: 599–610, 2013. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol 54: 1–13, 1989. doi: 10.1101/SQB.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 203.Jenkins SJ, Ruckerl D, Thomas GD, Hewitson JP, Duncan S, Brombacher F, Maizels RM, Hume DA, Allen JE. IL-4 directly signals tissue-resident macrophages to proliferate beyond homeostatic levels controlled by CSF-1. J Exp Med 210: 2477–2491, 2013. doi: 10.1084/jem.20121999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jongstra-Bilen J, Haidari M, Zhu SN, Chen M, Guha D, Cybulsky MI. Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J Exp Med 203: 2073–2083, 2006. doi: 10.1084/jem.20060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Jongstra-Bilen J, Zhang CX, Wisnicki T, Li MK, White-Alfred S, Ilaalagan R, Ferri DM, Deonarain A, Wan MH, Hyduk SJ, Cummins CL, Cybulsky MI. Oxidized low-density lipoprotein loading of macrophages downregulates TLR-induced proinflammatory responses in a gene-specific and temporal manner through transcriptional control. J Immunol 199: 2149–2157, 2017. doi: 10.4049/jimmunol.1601363. [DOI] [PubMed] [Google Scholar]
- 206.Joseph SB, McKilligin E, Pei L, Watson MA, Collins AR, Laffitte BA, Chen M, Noh G, Goodman J, Hagger GN, Tran J, Tippin TK, Wang X, Lusis AJ, Hsueh WA, Law RE, Collins JL, Willson TM, Tontonoz P. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc Natl Acad Sci USA 99: 7604–7609, 2002. doi: 10.1073/pnas.112059299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Jung K, Kim P, Leuschner F, Gorbatov R, Kim JK, Ueno T, Nahrendorf M, Yun SH. Endoscopic time-lapse imaging of immune cells in infarcted mouse hearts. Circ Res 112: 891–899, 2013. [Erratum in Circ Res 112: e155, 2013.] doi: 10.1161/CIRCRESAHA.111.300484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Jung M, Ma Y, Iyer RP, DeLeon-Pennell KY, Yabluchanskiy A, Garrett MR, Lindsey ML. IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic Res Cardiol 112: 33, 2017. doi: 10.1007/s00395-017-0622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol 20: 4106–4114, 2000. doi: 10.1128/MCB.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Jung S, Schwartz M. Non-identical twins—microglia and monocyte-derived macrophages in acute injury and autoimmune inflammation. Front Immunol 3: 89, 2012. doi: 10.3389/fimmu.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kadl A, Meher AK, Sharma PR, Lee MY, Doran AC, Johnstone SR, Elliott MR, Gruber F, Han J, Chen W, Kensler T, Ravichandran KS, Isakson BE, Wamhoff BR, Leitinger N. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res 107: 737–746, 2010. doi: 10.1161/CIRCRESAHA.109.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kallies A, Rosenbauer F, Scheller M, Knobeloch KP, Horak I. Accumulation of c-Cbl and rapid termination of colony-stimulating factor 1 receptor signaling in interferon consensus sequence binding protein-deficient bone marrow-derived macrophages. Blood 99: 3213–3219, 2002. doi: 10.1182/blood.V99.9.3213. [DOI] [PubMed] [Google Scholar]
- 213.Kanellakis P, Ditiatkovski M, Kostolias G, Bobik A. A pro-fibrotic role for interleukin-4 in cardiac pressure overload. Cardiovasc Res 95: 77–85, 2012. doi: 10.1093/cvr/cvs142. [DOI] [PubMed] [Google Scholar]
- 214.Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, Brody MJ, Lin SC, Aronow BJ, Tallquist MD, Molkentin JD. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun 7: 12260, 2016. doi: 10.1038/ncomms12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kaplan DH. Ontogeny and function of murine epidermal Langerhans cells. Nat Immunol 18: 1068–1075, 2017. doi: 10.1038/ni.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kappus MS, Murphy AJ, Abramowicz S, Ntonga V, Welch CL, Tall AR, Westerterp M. Activation of liver X receptor decreases atherosclerosis in Ldlr−/− mice in the absence of ATP-binding cassette transporters A1 and G1 in myeloid cells. Arterioscler Thromb Vasc Biol 34: 279–284, 2014. doi: 10.1161/ATVBAHA.113.302781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Katsuda S, Coltrera MD, Ross R, Gown AM. Human atherosclerosis. IV. Immunocytochemical analysis of cell activation and proliferation in lesions of young adults. Am J Pathol 142: 1787–1793, 1993. [PMC free article] [PubMed] [Google Scholar]
- 218.Katz SI, Tamaki K, Sachs DH. Epidermal Langerhans cells are derived from cells originating in bone marrow. Nature 282: 324–326, 1979. doi: 10.1038/282324a0. [DOI] [PubMed] [Google Scholar]
- 219.Kawakami K, Yamamoto Y, Kakimoto K, Onoue K. Requirement for delivery of signals by physical interaction and soluble factors from accessory cells in the induction of receptor-mediated T cell proliferation. Effectiveness of IFN-gamma modulation of accessory cells for physical interaction with T cells. J Immunol 142: 1818–1825, 1989. [PubMed] [Google Scholar]
- 220.Keliher EJ, Ye YX, Wojtkiewicz GR, Aguirre AD, Tricot B, Senders ML, Groenen H, Fay F, Perez-Medina C, Calcagno C, Carlucci G, Reiner T, Sun Y, Courties G, Iwamoto Y, Kim HY, Wang C, Chen JW, Swirski FK, Wey HY, Hooker J, Fayad ZA, Mulder WJ, Weissleder R, Nahrendorf M. Polyglucose nanoparticles with renal elimination and macrophage avidity facilitate PET imaging in ischaemic heart disease. Nat Commun 8: 14064, 2017. doi: 10.1038/ncomms14064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kelly B, O’Neill LA. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res 25: 771–784, 2015. doi: 10.1038/cr.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kennedy DW, Abkowitz JL. Kinetics of central nervous system microglial and macrophage engraftment: analysis using a transgenic bone marrow transplantation model. Blood 90: 986–993, 1997. [PubMed] [Google Scholar]
- 223.Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, Itzkovitz S, Colonna M, Schwartz M, Amit I. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169: 1276–1290.e17, 2017. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 224.Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, Perdiguero EG, Wieghofer P, Heinrich A, Riemke P, Hölscher C, Müller DN, Luckow B, Brocker T, Debowski K, Fritz G, Opdenakker G, Diefenbach A, Biber K, Heikenwalder M, Geissmann F, Rosenbauer F, Prinz M. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci 16: 273–280, 2013. doi: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- 225.Kierdorf K, Katzmarski N, Haas CA, Prinz M. Bone marrow cell recruitment to the brain in the absence of irradiation or parabiosis bias. PLoS One 8: e58544, 2013. doi: 10.1371/journal.pone.0058544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kim EJ, Kim S, Kang DO, Seo HS. Metabolic activity of the spleen and bone marrow in patients with acute myocardial infarction evaluated by 18f-fluorodeoxyglucose positron emission tomograpic imaging. Circ Cardiovasc Imaging 7: 454–460, 2014. doi: 10.1161/CIRCIMAGING.113.001093. [DOI] [PubMed] [Google Scholar]
- 227.King KR, Aguirre AD, Ye YX, Sun Y, Roh JD, Ng RP Jr, Kohler RH, Arlauckas SP, Iwamoto Y, Savol A, Sadreyev RI, Kelly M, Fitzgibbons TP, Fitzgerald KA, Mitchison T, Libby P, Nahrendorf M, Weissleder R. IRF3 and type I interferons fuel a fatal response to myocardial infarction. Nat Med 23: 1481–1487, 2017. doi: 10.1038/nm.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 464: 112–115, 2010. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- 229.Kojima Y, Volkmer JP, McKenna K, Civelek M, Lusis AJ, Miller CL, Direnzo D, Nanda V, Ye J, Connolly AJ, Schadt EE, Quertermous T, Betancur P, Maegdefessel L, Matic LP, Hedin U, Weissman IL, Leeper NJ. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 536: 86–90, 2016. doi: 10.1038/nature18935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Korin B, Ben-Shaanan TL, Schiller M, Dubovik T, Azulay-Debby H, Boshnak NT, Koren T, Rolls A. High-dimensional, single-cell characterization of the brain’s immune compartment. Nat Neurosci 20: 1300–1309, 2017. doi: 10.1038/nn.4610. [DOI] [PubMed] [Google Scholar]
- 231.Kostis JB, Davis BR, Cutler J, Grimm RH Jr, Berge KG, Cohen JD, Lacy CR, Perry HM Jr, Blaufox MD, Wassertheil-Smoller S, Black HR, Schron E, Berkson DM, Curb JD, Smith WM, McDonald R, Applegate WB; SHEP Cooperative Research Group . Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. JAMA 278: 212–216, 1997. doi: 10.1001/jama.1997.03550030052033. [DOI] [PubMed] [Google Scholar]
- 232.Koulova L, Clark EA, Shu G, Dupont B. The CD28 ligand B7/BB1 provides costimulatory signal for alloactivation of CD4+ T cells. J Exp Med 173: 759–762, 1991. doi: 10.1084/jem.173.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Krall WJ, Challita PM, Perlmutter LS, Skelton DC, Kohn DB. Cells expressing human glucocerebrosidase from a retroviral vector repopulate macrophages and central nervous system microglia after murine bone marrow transplantation. Blood 83: 2737–2748, 1994. [PubMed] [Google Scholar]
- 234.Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ, Cross JR, Jung E, Thompson CB, Jones RG, Pearce EJ. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood 115: 4742–4749, 2010. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Krishnamurthy P, Rajasingh J, Lambers E, Qin G, Losordo DW, Kishore R. IL-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of STAT3 and suppression of HuR. Circ Res 104: e9–e18, 2009. doi: 10.1161/CIRCRESAHA.108.188243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kruth HS. Receptor-independent fluid-phase pinocytosis mechanisms for induction of foam cell formation with native low-density lipoprotein particles. Curr Opin Lipidol 22: 386–393, 2011. doi: 10.1097/MOL.0b013e32834adadb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kumamoto Y, Camporez JPG, Jurczak MJ, Shanabrough M, Horvath T, Shulman GI, Iwasaki A. CD301b(+) mononuclear phagocytes maintain positive energy balance through secretion of resistin-like molecule alpha. Immunity 45: 583–596, 2016. doi: 10.1016/j.immuni.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kunjathoor VV, Febbraio M, Podrez EA, Moore KJ, Andersson L, Koehn S, Rhee JS, Silverstein R, Hoff HF, Freeman MW. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem 277: 49982–49988, 2002. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 239. Kurihara T, Warr G, Loy J, Bravo R. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J Exp Med 186: 1757–1762, 1997. doi: 10.1084/jem.186.10.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kurotaki D, Osato N, Nishiyama A, Yamamoto M, Ban T, Sato H, Nakabayashi J, Umehara M, Miyake N, Matsumoto N, Nakazawa M, Ozato K, Tamura T. Essential role of the IRF8-KLF4 transcription factor cascade in murine monocyte differentiation. Blood 121: 1839–1849, 2013. doi: 10.1182/blood-2012-06-437863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kurotaki D, Yamamoto M, Nishiyama A, Uno K, Ban T, Ichino M, Sasaki H, Matsunaga S, Yoshinari M, Ryo A, Nakazawa M, Ozato K, Tamura T. IRF8 inhibits C/EBPα activity to restrain mononuclear phagocyte progenitors from differentiating into neutrophils. Nat Commun 5: 4978, 2014. doi: 10.1038/ncomms5978. [DOI] [PubMed] [Google Scholar]
- 243.Kuwahara F, Kai H, Tokuda K, Takeya M, Takeshita A, Egashira K, Imaizumi T. Hypertensive myocardial fibrosis and diastolic dysfunction: another model of inflammation? Hypertension 43: 739–745, 2004. doi: 10.1161/01.HYP.0000118584.33350.7d. [DOI] [PubMed] [Google Scholar]
- 244.Kuziel WA, Dawson TC, Quinones M, Garavito E, Chenaux G, Ahuja SS, Reddick RL, Maeda N. CCR5 deficiency is not protective in the early stages of atherogenesis in apoE knockout mice. Atherosclerosis 167: 25–32, 2003. doi: 10.1016/S0021-9150(02)00382-9. [DOI] [PubMed] [Google Scholar]
- 245.Landsman L, Bar-On L, Zernecke A, Kim KW, Krauthgamer R, Shagdarsuren E, Lira SA, Weissman IL, Weber C, Jung S. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood 113: 963–972, 2009. doi: 10.1182/blood-2008-07-170787. [DOI] [PubMed] [Google Scholar]
- 246.Laslo P, Spooner CJ, Warmflash A, Lancki DW, Lee HJ, Sciammas R, Gantner BN, Dinner AR, Singh H. Multilineage transcriptional priming and determination of alternate hematopoietic cell fates. Cell 126: 755–766, 2006. doi: 10.1016/j.cell.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 247.Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159: 1312–1326, 2014. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, Ornitz DM, Randolph GJ, Mann DL. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci USA 111: 16029–16034, 2014. [Erratum in Proc Natl Acad Sci USA 113: E1414, 2016.] doi: 10.1073/pnas.1406508111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lawson LJ, Perry VH, Gordon S. Turnover of resident microglia in the normal adult mouse brain. Neuroscience 48: 405–415, 1992. doi: 10.1016/0306-4522(92)90500-2. [DOI] [PubMed] [Google Scholar]
- 250.Leblond AL, Klinkert K, Martin K, Turner EC, Kumar AH, Browne T, Caplice NM. Systemic and cardiac depletion of M2 macrophage through CSF-1R signaling inhibition alters cardiac function post myocardial infarction. PLoS One 10: e0137515, 2015. doi: 10.1371/journal.pone.0137515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Legein B, Temmerman L, Biessen EA, Lutgens E. Inflammation and immune system interactions in atherosclerosis. Cell Mol Life Sci 70: 3847–3869, 2013. doi: 10.1007/s00018-013-1289-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Leid J, Carrelha J, Boukarabila H, Epelman S, Jacobsen SE, Lavine KJ. Primitive embryonic macrophages are required for coronary development and maturation. Circ Res 118: 1498–1511, 2016. doi: 10.1161/CIRCRESAHA.115.308270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lesnik P, Haskell CA, Charo IF. Decreased atherosclerosis in CX3CR1−/− mice reveals a role for fractalkine in atherogenesis. J Clin Invest 111: 333–340, 2003. doi: 10.1172/JCI15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Donahoe JS, Courties G, Lee KM, Kim JI, Markmann JF, Marinelli B, Panizzi P, Lee WW, Iwamoto Y, Milstein S, Epstein-Barash H, Cantley W, Wong J, Cortez-Retamozo V, Newton A, Love K, Libby P, Pittet MJ, Swirski FK, Koteliansky V, Langer R, Weissleder R, Anderson DG, Nahrendorf M. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol 29: 1005–1010, 2011. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Leuschner F, Panizzi P, Chico-Calero I, Lee WW, Ueno T, Cortez-Retamozo V, Waterman P, Gorbatov R, Marinelli B, Iwamoto Y, Chudnovskiy A, Figueiredo JL, Sosnovik DE, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ Res 107: 1364–1373, 2010. doi: 10.1161/CIRCRESAHA.110.227454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y, Sena B, Chudnovskiy A, Panizzi P, Keliher E, Higgins JM, Libby P, Moskowitz MA, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med 209: 123–137, 2012. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 323: 236–241, 1990. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 258.Lhoták Š, Gyulay G, Cutz JC, Al-Hashimi A, Trigatti BL, Richards CD, Igdoura SA, Steinberg GR, Bramson J, Ask K, Austin RC. Characterization of proliferating lesion-resident cells during all stages of atherosclerotic growth. J Am Heart Assoc 5: e003945, 2016. doi: 10.1161/JAHA.116.003945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Li AC, Glass CK. The macrophage foam cell as a target for therapeutic intervention. Nat Med 8: 1235–1242, 2002. doi: 10.1038/nm1102-1235. [DOI] [PubMed] [Google Scholar]
- 260.Li Q, Barres BA. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol 18: 225–242, 2018. doi: 10.1038/nri.2017.125. [DOI] [PubMed] [Google Scholar]
- 261.Li W, Hsiao HM, Higashikubo R, Saunders BT, Bharat A, Goldstein DR, Krupnick AS, Gelman AE, Lavine KJ, Kreisel D. Heart-resident CCR2(+) macrophages promote neutrophil extravasation through TLR9/MyD88/CXCL5 signaling. JCI Insight 1: e87315, 2016. doi: 10.1172/jci.insight.87315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Li Y, Du XF, Liu CS, Wen ZL, Du JL. Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev Cell 23: 1189–1202, 2012. doi: 10.1016/j.devcel.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 263.Lichtman AH, Binder CJ, Tsimikas S, Witztum JL. Adaptive immunity in atherogenesis: new insights and therapeutic approaches. J Clin Invest 123: 27–36, 2013. doi: 10.1172/JCI63108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Lieschke GJ, Grail D, Hodgson G, Metcalf D, Stanley E, Cheers C, Fowler KJ, Basu S, Zhan YF, Dunn AR. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood 84: 1737–1746, 1994. [PubMed] [Google Scholar]
- 265.Lin H, Lee E, Hestir K, Leo C, Huang M, Bosch E, Halenbeck R, Wu G, Zhou A, Behrens D, Hollenbaugh D, Linnemann T, Qin M, Wong J, Chu K, Doberstein SK, Williams LT. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science 320: 807–811, 2008. doi: 10.1126/science.1154370. [DOI] [PubMed] [Google Scholar]
- 266.Lindsey ML, Saucerman JJ, DeLeon-Pennell KY. Knowledge gaps to understanding cardiac macrophage polarization following myocardial infarction. Biochim Biophys Acta 1862: 2288–2292, 2016. doi: 10.1016/j.bbadis.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Linsley PS, Brady W, Grosmaire L, Aruffo A, Damle NK, Ledbetter JA. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med 173: 721–730, 1991. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Liu F, Wu HY, Wesselschmidt R, Kornaga T, Link DC. Impaired production and increased apoptosis of neutrophils in granulocyte colony-stimulating factor receptor-deficient mice. Immunity 5: 491–501, 1996. doi: 10.1016/S1074-7613(00)80504-X. [DOI] [PubMed] [Google Scholar]
- 269.Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, Chu FF, Randolph GJ, Rudensky AY, Nussenzweig M. In vivo analysis of dendritic cell development and homeostasis. Science 324: 392–397, 2009. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Liu P, Yu YR, Spencer JA, Johnson AE, Vallanat CT, Fong AM, Patterson C, Patel DD. CX3CR1 deficiency impairs dendritic cell accumulation in arterial intima and reduces atherosclerotic burden. Arterioscler Thromb Vasc Biol 28: 243–250, 2007. doi: 10.1161/ATVBAHA.107.158675. [DOI] [PubMed] [Google Scholar]
- 271.Llodrá J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci USA 101: 11779–11784, 2004. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.London A, Cohen M, Schwartz M. Microglia and monocyte-derived macrophages: functionally distinct populations that act in concert in CNS plasticity and repair. Front Cell Neurosci 7: 34, 2013. doi: 10.3389/fncel.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Lutgens E, de Muinck ED, Kitslaar PJ, Tordoir JH, Wellens HJ, Daemen MJ. Biphasic pattern of cell turnover characterizes the progression from fatty streaks to ruptured human atherosclerotic plaques. Cardiovasc Res 41: 473–479, 1999. doi: 10.1016/S0008-6363(98)00311-3. [DOI] [PubMed] [Google Scholar]
- 274.Ma Y, Mouton AJ, Lindsey ML. Cardiac macrophage biology in the steady-state heart, the aging heart, and following myocardial infarction. Transl Res 191: 15–28, 2018. doi: 10.1016/j.trsl.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.MacDonald KP, Palmer JS, Cronau S, Seppanen E, Olver S, Raffelt NC, Kuns R, Pettit AR, Clouston A, Wainwright B, Branstetter D, Smith J, Paxton RJ, Cerretti DP, Bonham L, Hill GR, Hume DA. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood 116: 3955–3963, 2010. doi: 10.1182/blood-2010-02-266296. [DOI] [PubMed] [Google Scholar]
- 276.Mackaness GB. The phagocytosis and inactivation of staphylococci by macrophages of normal rabbits. J Exp Med 112: 35–53, 1960. doi: 10.1084/jem.112.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Mackaness GB. Cellular resistance to infection. J Exp Med 116: 381–406, 1962. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Maekawa Y, Anzai T, Yoshikawa T, Asakura Y, Takahashi T, Ishikawa S, Mitamura H, Ogawa S. Prognostic significance of peripheral monocytosis after reperfused acute myocardial infarction:a possible role for left ventricular remodeling. J Am Coll Cardiol 39: 241–246, 2002. doi: 10.1016/S0735-1097(01)01721-1. [DOI] [PubMed] [Google Scholar]
- 279.Mager LF, Riether C, Schürch CM, Banz Y, Wasmer MH, Stuber R, Theocharides AP, Li X, Xia Y, Saito H, Nakae S, Baerlocher GM, Manz MG, McCoy KD, Macpherson AJ, Ochsenbein AF, Beutler B, Krebs P. IL-33 signaling contributes to the pathogenesis of myeloproliferative neoplasms. J Clin Invest 125: 2579–2591, 2015. doi: 10.1172/JCI77347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Majmudar MD, Keliher EJ, Heidt T, Leuschner F, Truelove J, Sena BF, Gorbatov R, Iwamoto Y, Dutta P, Wojtkiewicz G, Courties G, Sebas M, Borodovsky A, Fitzgerald K, Nolte MW, Dickneite G, Chen JW, Anderson DG, Swirski FK, Weissleder R, Nahrendorf M. Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice. Circulation 127: 2038–2046, 2013. doi: 10.1161/CIRCULATIONAHA.112.000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Majmudar MD, Yoo J, Keliher EJ, Truelove JJ, Iwamoto Y, Sena B, Dutta P, Borodovsky A, Fitzgerald K, Di Carli MF, Libby P, Anderson DG, Swirski FK, Weissleder R, Nahrendorf M. Polymeric nanoparticle PET/MR imaging allows macrophage detection in atherosclerotic plaques. Circ Res 112: 755–761, 2013. doi: 10.1161/CIRCRESAHA.111.300576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25: 677–686, 2004. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 283.Marín-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M. Microglia promote the death of developing Purkinje cells. Neuron 41: 535–547, 2004. doi: 10.1016/S0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- 284.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6: 13, 2014. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Mass E, Jacome-Galarza CE, Blank T, Lazarov T, Durham BH, Ozkaya N, Pastore A, Schwabenland M, Chung YR, Rosenblum MK, Prinz M, Abdel-Wahab O, Geissmann F. A somatic mutation in erythro-myeloid progenitors causes neurodegenerative disease. Nature 549: 389–393, 2017. doi: 10.1038/nature23672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Massa M, Rosti V, Ferrario M, Campanelli R, Ramajoli I, Rosso R, De Ferrari GM, Ferlini M, Goffredo L, Bertoletti A, Klersy C, Pecci A, Moratti R, Tavazzi L. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood 105: 199–206, 2005. doi: 10.1182/blood-2004-05-1831. [DOI] [PubMed] [Google Scholar]
- 287.Matcovitch-Natan O, Winter DR, Giladi A, Vargas Aguilar S, Spinrad A, Sarrazin S, Ben-Yehuda H, David E, Zelada González F, Perrin P, Keren-Shaul H, Gury M, Lara-Astaiso D, Thaiss CA, Cohen M, Bahar Halpern K, Baruch K, Deczkowska A, Lorenzo-Vivas E, Itzkovitz S, Elinav E, Sieweke MH, Schwartz M, Amit I. Microglia development follows a stepwise program to regulate brain homeostasis. Science 353: aad8670, 2016. doi: 10.1126/science.aad8670. [DOI] [PubMed] [Google Scholar]
- 288.McKercher SR, Torbett BE, Anderson KL, Henkel GW, Vestal DJ, Baribault H, Klemsz M, Feeney AJ, Wu GE, Paige CJ, Maki RA. Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. EMBO J 15: 5647–5658, 1996. [PMC free article] [PubMed] [Google Scholar]
- 289.McMILLAN GC, Duff GL. Mitotic activity in the aortic lesions of experimental cholesterol atherosclerosis of rabbits. Arch Pathol (Chic) 46: 179–182, 1948. [PubMed] [Google Scholar]
- 290.Mellak S, Ait-Oufella H, Esposito B, Loyer X, Poirier M, Tedder TF, Tedgui A, Mallat Z, Potteaux S. Angiotensin II mobilizes spleen monocytes to promote the development of abdominal aortic aneurysm in Apoe−/− mice. Arterioscler Thromb Vasc Biol 35: 378–388, 2015. doi: 10.1161/ATVBAHA.114.304389. [DOI] [PubMed] [Google Scholar]
- 291.Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med 20: 833–846, 2014. doi: 10.1038/nm.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Meredith MM, Liu K, Darrasse-Jeze G, Kamphorst AO, Schreiber HA, Guermonprez P, Idoyaga J, Cheong C, Yao KH, Niec RE, Nussenzweig MC. Expression of the zinc finger transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. J Exp Med 209: 1153–1165, 2012. doi: 10.1084/jem.20112675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Metchnikoff E. Uber den Kampf der Zellen gegen Erysipel-kokken. Arch Pathol Anat Physiol Klin Med 107: 209–249, 1887. doi: 10.1007/BF01926053. [DOI] [Google Scholar]
- 294.Meuret G, Bammert J, Hoffmann G. Kinetics of human monocytopoiesis. Blood 44: 801–816, 1974. [PubMed] [Google Scholar]
- 295.Meurs I, Lammers B, Zhao Y, Out R, Hildebrand RB, Hoekstra M, Van Berkel TJ, Van Eck M. The effect of ABCG1 deficiency on atherosclerotic lesion development in LDL receptor knockout mice depends on the stage of atherogenesis. Atherosclerosis 221: 41–47, 2012. doi: 10.1016/j.atherosclerosis.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 296.Migliaccio G, Migliaccio AR, Petti S, Mavilio F, Russo G, Lazzaro D, Testa U, Marinucci M, Peschle C. Human embryonic hemopoiesis. Kinetics of progenitors and precursors underlying the yolk sac—liver transition. J Clin Invest 78: 51–60, 1986. doi: 10.1172/JCI112572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Mildner A, Mack M, Schmidt H, Brück W, Djukic M, Zabel MD, Hille A, Priller J, Prinz M. CCR2+Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain 132: 2487–2500, 2009. doi: 10.1093/brain/awp144. [DOI] [PubMed] [Google Scholar]
- 298.Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, Heikenwalder M, Brück W, Priller J, Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci 10: 1544–1553, 2007. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- 299.Miller YI, Viriyakosol S, Binder CJ, Feramisco JR, Kirkland TN, Witztum JL. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J Biol Chem 278: 1561–1568, 2003. doi: 10.1074/jbc.M209634200. [DOI] [PubMed] [Google Scholar]
- 300.Millonig G, Niederegger H, Rabl W, Hochleitner BW, Hoefer D, Romani N, Wick G. Network of vascular-associated dendritic cells in intima of healthy young individuals. Arterioscler Thromb Vasc Biol 21: 503–508, 2001. doi: 10.1161/01.ATV.21.4.503. [DOI] [PubMed] [Google Scholar]
- 301.Mills CD. Macrophage arginine metabolism to ornithine/urea or nitric oxide/citrulline: a life or death issue. Crit Rev Immunol 21: 399–425, 2001. doi: 10.1615/CritRevImmunol.v21.i5.10. [DOI] [PubMed] [Google Scholar]
- 302.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 164: 6166–6173, 2000. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 303.Misharin AV, Morales-Nebreda L, Reyfman PA, Cuda CM, Walter JM, McQuattie-Pimentel AC, Chen CI, Anekalla KR, Joshi N, Williams KJN, Abdala-Valencia H, Yacoub TJ, Chi M, Chiu S, Gonzalez-Gonzalez FJ, Gates K, Lam AP, Nicholson TT, Homan PJ, Soberanes S, Dominguez S, Morgan VK, Saber R, Shaffer A, Hinchcliff M, Marshall SA, Bharat A, Berdnikovs S, Bhorade SM, Bartom ET, Morimoto RI, Balch WE, Sznajder JI, Chandel NS, Mutlu GM, Jain M, Gottardi CJ, Singer BD, Ridge KM, Bagheri N, Shilatifard A, Budinger GRS, Perlman H. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J Exp Med 214: 2387–2404, 2017. doi: 10.1084/jem.20162152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Molawi K, Wolf Y, Kandalla PK, Favret J, Hagemeyer N, Frenzel K, Pinto AR, Klapproth K, Henri S, Malissen B, Rodewald HR, Rosenthal NA, Bajenoff M, Prinz M, Jung S, Sieweke MH. Progressive replacement of embryo-derived cardiac macrophages with age. J Exp Med 211: 2151–2158, 2014. doi: 10.1084/jem.20140639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Möllmann H, Nef HM, Kostin S, von Kalle C, Pilz I, Weber M, Schaper J, Hamm CW, Elsässer A. Bone marrow-derived cells contribute to infarct remodelling. Cardiovasc Res 71: 661–671, 2006. doi: 10.1016/j.cardiores.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 306.Moore-Morris T, Cattaneo P, Guimarães-Camboa N, Bogomolovas J, Cedenilla M, Banerjee I, Ricote M, Kisseleva T, Zhang L, Gu Y, Dalton ND, Peterson KL, Chen J, Pucéat M, Evans SM. Infarct fibroblasts do not derive from bone marrow lineages. Circ Res 122: 583–590, 2018. doi: 10.1161/CIRCRESAHA.117.311490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Moore JP, Vinh A, Tuck KL, Sakkal S, Krishnan SM, Chan CT, Lieu M, Samuel CS, Diep H, Kemp-Harper BK, Tare M, Ricardo SD, Guzik TJ, Sobey CG, Drummond GR. M2 macrophage accumulation in the aortic wall during angiotensin II infusion in mice is associated with fibrosis, elastin loss, and elevated blood pressure. Am J Physiol Heart Circ Physiol 309: H906–H917, 2015. doi: 10.1152/ajpheart.00821.2014. [DOI] [PubMed] [Google Scholar]
- 308.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol 13: 709–721, 2013. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell 145: 341–355, 2011. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Moran-Crusio K, Reavie L, Shih A, Abdel-Wahab O, Ndiaye-Lobry D, Lobry C, Figueroa ME, Vasanthakumar A, Patel J, Zhao X, Perna F, Pandey S, Madzo J, Song C, Dai Q, He C, Ibrahim S, Beran M, Zavadil J, Nimer SD, Melnick A, Godley LA, Aifantis I, Levine RL. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell 20: 11–24, 2011. doi: 10.1016/j.ccr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8: 958–969, 2008. [Erratum in Nat Rev Immunol 10: 460, 2010.] doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Mrdjen D, Pavlovic A, Hartmann FJ, Schreiner B, Utz SG, Leung BP, Lelios I, Heppner FL, Kipnis J, Merkler D, Greter M, Becher B. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 48: 380–395.e6, 2018. [Erratum in Immunity 48: 599, 2018.] doi: 10.1016/j.immuni.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 313.Mucenski ML, McLain K, Kier AB, Swerdlow SH, Schreiner CM, Miller TA, Pietryga DW, Scott WJ Jr, Potter SS. A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell 65: 677–689, 1991. doi: 10.1016/0092-8674(91)90099-K. [DOI] [PubMed] [Google Scholar]
- 314.Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, Bochem AE, Kuivenhoven JA, Yvan-Charvet L, Tall AR. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest 121: 4138–4149, 2011. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41: 14–20, 2014. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 11: 723–737, 2011. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Nahrendorf M, Keliher E, Panizzi P, Zhang H, Hembrador S, Figueiredo JL, Aikawa E, Kelly K, Libby P, Weissleder R. 18F-4V for PET-CT imaging of VCAM-1 expression in atherosclerosis. JACC Cardiovasc Imaging 2: 1213–1222, 2009. doi: 10.1016/j.jcmg.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Nahrendorf M, Sosnovik DE, Waterman P, Swirski FK, Pande AN, Aikawa E, Figueiredo JL, Pittet MJ, Weissleder R. Dual channel optical tomographic imaging of leukocyte recruitment and protease activity in the healing myocardial infarct. Circ Res 100: 1218–1225, 2007. doi: 10.1161/01.RES.0000265064.46075.31. [DOI] [PubMed] [Google Scholar]
- 319.Nahrendorf M, Swirski FK. Abandoning M1/M2 for a network model of macrophage function. Circ Res 119: 414–417, 2016. doi: 10.1161/CIRCRESAHA.116.309194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 204: 3037–3047, 2007. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Naito M, Takahashi K, Nishikawa S. Development, differentiation, and maturation of macrophages in the fetal mouse liver. J Leukoc Biol 48: 27–37, 1990. doi: 10.1002/jlb.48.1.27. [DOI] [PubMed] [Google Scholar]
- 322.Nakashima Y, Fujii H, Sumiyoshi S, Wight TN, Sueishi K. Early human atherosclerosis: accumulation of lipid and proteoglycans in intimal thickenings followed by macrophage infiltration. Arterioscler Thromb Vasc Biol 27: 1159–1165, 2007. doi: 10.1161/ATVBAHA.106.134080. [DOI] [PubMed] [Google Scholar]
- 323.Nathan CF, Murray HW, Wiebe ME, Rubin BY. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med 158: 670–689, 1983. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Nemeth CL, Drummond GT, Mishra MK, Zhang F, Carr P, Garcia MS, Doman S, Fatemi A, Johnston MV, Kannan RM, Kannan S, Wilson MA. Uptake of dendrimer-drug by different cell types in the hippocampus after hypoxic-ischemic insult in neonatal mice: Effects of injury, microglial activation and hypothermia. Nanomedicine (Lond) 13: 2359–2369, 2017. doi: 10.1016/j.nano.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Nerlov C, Graf T. PU.1 induces myeloid lineage commitment in multipotent hematopoietic progenitors. Genes Dev 12: 2403–2412, 1998. doi: 10.1101/gad.12.15.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Nevers T, Salvador AM, Grodecki-Pena A, Knapp A, Velázquez F, Aronovitz M, Kapur NK, Karas RH, Blanton RM, Alcaide P. Left ventricular t-cell recruitment contributes to the pathogenesis of heart failure. Circ Heart Fail 8: 776–787, 2015. doi: 10.1161/CIRCHEARTFAILURE.115.002225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Newby AC, George SJ, Ismail Y, Johnson JL, Sala-Newby GB, Thomas AC. Vulnerable atherosclerotic plaque metalloproteinases and foam cell phenotypes. Thromb Haemost 101: 1006–1011, 2009. [PMC free article] [PubMed] [Google Scholar]
- 328.Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science 341: 1483–1488, 2013. doi: 10.1126/science.1240636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 480: 104–108, 2011. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Nicola NA, Metcalf D, Matsumoto M, Johnson GR. Purification of a factor inducing differentiation in murine myelomonocytic leukemia cells. Identification as granulocyte colony-stimulating factor. J Biol Chem 258: 9017–9023, 1983. [PubMed] [Google Scholar]
- 331.Nilsson SK, Bertoncello I. The development and establishment of hemopoiesis in fetal and newborn osteopetrotic (op/op) mice. Dev Biol 164: 456–462, 1994. doi: 10.1006/dbio.1994.1215. [DOI] [PubMed] [Google Scholar]
- 332.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308: 1314–1318, 2005. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 333.Nussenzweig MC, Steinman RM. Contribution of dendritic cells to stimulation of the murine syngeneic mixed leukocyte reaction. J Exp Med 151: 1196–1212, 1980. doi: 10.1084/jem.151.5.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Nutt SL, Metcalf D, D’Amico A, Polli M, Wu L. Dynamic regulation of PU.1 expression in multipotent hematopoietic progenitors. J Exp Med 201: 221–231, 2005. doi: 10.1084/jem.20041535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.O’Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol 16: 553–565, 2016. doi: 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Oka T, Akazawa H, Naito AT, Komuro I. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ Res 114: 565–571, 2014. doi: 10.1161/CIRCRESAHA.114.300507. [DOI] [PubMed] [Google Scholar]
- 337.Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell 157: 832–844, 2014. doi: 10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell 132: 631–644, 2008. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Ouimet M, Franklin V, Mak E, Liao X, Tabas I, Marcel YL. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab 13: 655–667, 2011. doi: 10.1016/j.cmet.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Ouimet M, Marcel YL. Regulation of lipid droplet cholesterol efflux from macrophage foam cells. Arterioscler Thromb Vasc Biol 32: 575–581, 2012. doi: 10.1161/ATVBAHA.111.240705. [DOI] [PubMed] [Google Scholar]
- 341.Packard RR, Maganto-García E, Gotsman I, Tabas I, Libby P, Lichtman AH. CD11c(+) dendritic cells maintain antigen processing, presentation capabilities, and CD4(+) T-cell priming efficacy under hypercholesterolemic conditions associated with atherosclerosis. Circ Res 103: 965–973, 2008. doi: 10.1161/CIRCRESAHA.108.185793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Panizzi P, Swirski FK, Figueiredo JL, Waterman P, Sosnovik DE, Aikawa E, Libby P, Pittet M, Weissleder R, Nahrendorf M. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol 55: 1629–1638, 2010. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science 333: 1456–1458, 2011. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 344.Papa S, Ferrari R, De Paola M, Rossi F, Mariani A, Caron I, Sammali E, Peviani M, Dell’Oro V, Colombo C, Morbidelli M, Forloni G, Perale G, Moscatelli D, Veglianese P. Polymeric nanoparticle system to target activated microglia/macrophages in spinal cord injury. J Control Release 174: 15–26, 2014. doi: 10.1016/j.jconrel.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 345.Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR III, Lafaille JJ, Hempstead BL, Littman DR, Gan WB. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155: 1596–1609, 2013. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Pascual O, Ben Achour S, Rostaing P, Triller A, Bessis A. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc Natl Acad Sci USA 109: E197–E205, 2012. doi: 10.1073/pnas.1111098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 74: 2527–2534, 1989. [PubMed] [Google Scholar]
- 348.Patel AA, Zhang Y, Fullerton JN, Boelen L, Rongvaux A, Maini AA, Bigley V, Flavell RA, Gilroy DW, Asquith B, Macallan D, Yona S. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J Exp Med 214: 1913–1923, 2017. doi: 10.1084/jem.20170355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Patel AR, Ritzel R, McCullough LD, Liu F. Microglia and ischemic stroke: a double-edged sword. Int J Physiol Pathophysiol Pharmacol 5: 73–90, 2013. [PMC free article] [PubMed] [Google Scholar]
- 350.Paul A, Chang BH, Li L, Yechoor VK, Chan L. Deficiency of adipose differentiation-related protein impairs foam cell formation and protects against atherosclerosis. Circ Res 102: 1492–1501, 2008. doi: 10.1161/CIRCRESAHA.107.168070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Paul F, Arkin Y, Giladi A, Jaitin DA, Kenigsberg E, Keren-Shaul H, Winter D, Lara-Astiaso D, Gury M, Weiner A, David E, Cohen N, Lauridsen FK, Haas S, Schlitzer A, Mildner A, Ginhoux F, Jung S, Trumpp A, Porse BT, Tanay A, Amit I. Transcriptional heterogeneity and lineage commitment in myeloid progenitors. Cell 163: 1663–1677, 2015. [Erratum in Cell 164: 325, 2016.] doi: 10.1016/j.cell.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 352.Paulson KE, Zhu SN, Chen M, Nurmohamed S, Jongstra-Bilen J, Cybulsky MI. Resident intimal dendritic cells accumulate lipid and contribute to the initiation of atherosclerosis. Circ Res 106: 383–390, 2010. doi: 10.1161/CIRCRESAHA.109.210781. [DOI] [PubMed] [Google Scholar]
- 353.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62: 263–271, 2013. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 354.Pelvig DP, Pakkenberg H, Stark AK, Pakkenberg B. Neocortical glial cell numbers in human brains. Neurobiol Aging 29: 1754–1762, 2008. doi: 10.1016/j.neurobiolaging.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 355.Perdiguero EG, Geissmann F. The development and maintenance of resident macrophages. Nat Immunol 17: 2–8, 2016. doi: 10.1038/ni.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Perié L, Duffy KR, Kok L, de Boer RJ, Schumacher TN. The branching point in erythro-myeloid differentiation. Cell 163: 1655–1662, 2015. doi: 10.1016/j.cell.2015.11.059. [DOI] [PubMed] [Google Scholar]
- 357.Phan AT, Goldrath AW, Glass CK. Metabolic and epigenetic coordination of T cell and macrophage immunity. Immunity 46: 714–729, 2017. doi: 10.1016/j.immuni.2017.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Pinto AR, Paolicelli R, Salimova E, Gospocic J, Slonimsky E, Bilbao-Cortes D, Godwin JW, Rosenthal NA. An abundant tissue macrophage population in the adult murine heart with a distinct alternatively-activated macrophage profile. PLoS One 7: e36814, 2012. doi: 10.1371/journal.pone.0036814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Pirzgalska RM, Seixas E, Seidman JS, Link VM, Sánchez NM, Mahú I, Mendes R, Gres V, Kubasova N, Morris I, Arús BA, Larabee CM, Vasques M, Tortosa F, Sousa AL, Anandan S, Tranfield E, Hahn MK, Iannacone M, Spann NJ, Glass CK, Domingos AI. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat Med 23: 1309–1318, 2017. doi: 10.1038/nm.4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Pols TW, Nomura M, Harach T, Lo Sasso G, Oosterveer MH, Thomas C, Rizzo G, Gioiello A, Adorini L, Pellicciari R, Auwerx J, Schoonjans K. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab 14: 747–757, 2011. doi: 10.1016/j.cmet.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Potteaux S, Combadière C, Esposito B, Lecureuil C, Ait-Oufella H, Merval R, Ardouin P, Tedgui A, Mallat Z. Role of bone marrow-derived CC-chemokine receptor 5 in the development of atherosclerosis of low-density lipoprotein receptor knockout mice. Arterioscler Thromb Vasc Biol 26: 1858–1863, 2006. doi: 10.1161/01.ATV.0000231527.22762.71. [DOI] [PubMed] [Google Scholar]
- 362.Potteaux S, Gautier EL, Hutchison SB, van Rooijen N, Rader DJ, Thomas MJ, Sorci-Thomas MG, Randolph GJ. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe−/− mice during disease regression. J Clin Invest 121: 2025–2036, 2011. doi: 10.1172/JCI43802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Prinz M, Erny D, Hagemeyer N. Ontogeny and homeostasis of CNS myeloid cells. Nat Immunol 18: 385–392, 2017. doi: 10.1038/ni.3703. [DOI] [PubMed] [Google Scholar]
- 364.Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci 15: 300–312, 2014. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- 365.Pulford KA, Rigney EM, Micklem KJ, Jones M, Stross WP, Gatter KC, Mason DY. KP1: a new monoclonal antibody that detects a monocyte/macrophage associated antigen in routinely processed tissue sections. J Clin Pathol 42: 414–421, 1989. doi: 10.1136/jcp.42.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Radomska HS, Huettner CS, Zhang P, Cheng T, Scadden DT, Tenen DG. CCAAT/enhancer binding protein α is a regulatory switch sufficient for induction of granulocytic development from bipotential myeloid progenitors. Mol Cell Biol 18: 4301–4314, 1998. doi: 10.1128/MCB.18.7.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Rahman K, Vengrenyuk Y, Ramsey SA, Vila NR, Girgis NM, Liu J, Gusarova V, Gromada J, Weinstock A, Moore KJ, Loke P, Fisher EA. Inflammatory Ly6Chi monocytes and their conversion to M2 macrophages drive atherosclerosis regression. J Clin Invest 127: 2904–2915, 2017. doi: 10.1172/JCI75005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Rajavashisth T, Qiao JH, Tripathi S, Tripathi J, Mishra N, Hua M, Wang XP, Loussararian A, Clinton S, Libby P, Lusis A. Heterozygous osteopetrotic (op) mutation reduces atherosclerosis in LDL receptor- deficient mice. J Clin Invest 101: 2702–2710, 1998. doi: 10.1172/JCI119891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Ranalletta M, Wang N, Han S, Yvan-Charvet L, Welch C, Tall AR. Decreased atherosclerosis in low-density lipoprotein receptor knockout mice transplanted with Abcg1−/− bone marrow. Arterioscler Thromb Vasc Biol 26: 2308–2315, 2006. doi: 10.1161/01.ATV.0000242275.92915.43. [DOI] [PubMed] [Google Scholar]
- 370.Randolph GJ, Inaba K, Robbiani DF, Steinman RM, Muller WA. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity 11: 753–761, 1999. doi: 10.1016/S1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- 371.Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature 468: 253–262, 2010. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- 372.Rantakari P, Jäppinen N, Lokka E, Mokkala E, Gerke H, Peuhu E, Ivaska J, Elima K, Auvinen K, Salmi M. Fetal liver endothelium regulates the seeding of tissue-resident macrophages. Nature 538: 392–396, 2016. doi: 10.1038/nature19814. [DOI] [PubMed] [Google Scholar]
- 373.Rauch PJ, Chudnovskiy A, Robbins CS, Weber GF, Etzrodt M, Hilgendorf I, Tiglao E, Figueiredo JL, Iwamoto Y, Theurl I, Gorbatov R, Waring MT, Chicoine AT, Mouded M, Pittet MJ, Nahrendorf M, Weissleder R, Swirski FK. Innate response activator B cells protect against microbial sepsis. Science 335: 597–601, 2012. doi: 10.1126/science.1215173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Reiter R, Swingen C, Moore L, Henry TD, Traverse JH. Circadian dependence of infarct size and left ventricular function after ST elevation myocardial infarction. Circ Res 110: 105–110, 2012. doi: 10.1161/CIRCRESAHA.111.254284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Rekhter MD, Gordon D. Active proliferation of different cell types, including lymphocytes, in human atherosclerotic plaques. Am J Pathol 147: 668–677, 1995. [PMC free article] [PubMed] [Google Scholar]
- 376.Réu P, Khosravi A, Bernard S, Mold JE, Salehpour M, Alkass K, Perl S, Tisdale J, Possnert G, Druid H, Frisén J. The lifespan and turnover of microglia in the human brain. Cell Reports 20: 779–784, 2017. doi: 10.1016/j.celrep.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group . Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 377: 1119–1131, 2017. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 378.Rivollier A, He J, Kole A, Valatas V, Kelsall BL. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J Exp Med 209: 139–155, 2012. doi: 10.1084/jem.20101387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo JL, Iwamoto Y, Gorbatov R, Etzrodt M, Weber GF, Ueno T, van Rooijen N, Mulligan-Kehoe MJ, Libby P, Nahrendorf M, Pittet MJ, Weissleder R, Swirski FK. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation 125: 364–374, 2012. doi: 10.1161/CIRCULATIONAHA.111.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, Zavitz CC, Shikatani EA, Parsons M, van Rooijen N, Lin HY, Husain M, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med 19: 1166–1172, 2013. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Rogacev KS, Cremers B, Zawada AM, Seiler S, Binder N, Ege P, Große-Dunker G, Heisel I, Hornof F, Jeken J, Rebling NM, Ulrich C, Scheller B, Böhm M, Fliser D, Heine GH. CD14++CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiography. J Am Coll Cardiol 60: 1512–1520, 2012. doi: 10.1016/j.jacc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 382.Rosas M, Davies LC, Giles PJ, Liao CT, Kharfan B, Stone TC, O’Donnell VB, Fraser DJ, Jones SA, Taylor PR. The transcription factor Gata6 links tissue macrophage phenotype and proliferative renewal. Science 344: 645–648, 2014. doi: 10.1126/science.1251414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Rosenbauer F, Owens BM, Yu L, Tumang JR, Steidl U, Kutok JL, Clayton LK, Wagner K, Scheller M, Iwasaki H, Liu C, Hackanson B, Akashi K, Leutz A, Rothstein TL, Plass C, Tenen DG. Lymphoid cell growth and transformation are suppressed by a key regulatory element of the gene encoding PU.1. Nat Genet 38: 27–37, 2006. doi: 10.1038/ng1679. [DOI] [PubMed] [Google Scholar]
- 384.Rosenbauer F, Tenen DG. Transcription factors in myeloid development: balancing differentiation with transformation. Nat Rev Immunol 7: 105–117, 2007. doi: 10.1038/nri2024. [DOI] [PubMed] [Google Scholar]
- 385.Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res 63: 423–432, 2004. doi: 10.1016/j.cardiores.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 386.Ruparelia N, Godec J, Lee R, Chai JT, Dall’Armellina E, McAndrew D, Digby JE, Forfar JC, Prendergast BD, Kharbanda RK, Banning AP, Neubauer S, Lygate CA, Channon KM, Haining NW, Choudhury RP. Acute myocardial infarction activates distinct inflammation and proliferation pathways in circulating monocytes, prior to recruitment, and identified through conserved transcriptional responses in mice and humans. Eur Heart J 36: 1923–1934, 2015. doi: 10.1093/eurheartj/ehv195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Saeed S, Quintin J, Kerstens HH, Rao NA, Aghajanirefah A, Matarese F, Cheng SC, Ratter J, Berentsen K, van der Ent MA, Sharifi N, Janssen-Megens EM, Ter Huurne M, Mandoli A, van Schaik T, Ng A, Burden F, Downes K, Frontini M, Kumar V, Giamarellos-Bourboulis EJ, Ouwehand WH, van der Meer JW, Joosten LA, Wijmenga C, Martens JH, Xavier RJ, Logie C, Netea MG, Stunnenberg HG. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345: 1251086, 2014. doi: 10.1126/science.1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Sage AP, Murphy D, Maffia P, Masters LM, Sabir SR, Baker LL, Cambrook H, Finigan AJ, Ait-Oufella H, Grassia G, Harrison JE, Ludewig B, Reith W, Hansson GK, Reizis B, Hugues S, Mallat Z. MHC Class II-restricted antigen presentation by plasmacytoid dendritic cells drives proatherogenic T cell immunity. Circulation 130: 1363–1373, 2014. doi: 10.1161/CIRCULATIONAHA.114.011090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Sager HB, Dutta P, Dahlman JE, Hulsmans M, Courties G, Sun Y, Heidt T, Vinegoni C, Borodovsky A, Fitzgerald K, Wojtkiewicz GR, Iwamoto Y, Tricot B, Khan OF, Kauffman KJ, Xing Y, Shaw TE, Libby P, Langer R, Weissleder R, Swirski FK, Anderson DG, Nahrendorf M. RNAi targeting multiple cell adhesion molecules reduces immune cell recruitment and vascular inflammation after myocardial infarction. Sci Transl Med 8: 342ra80, 2016. doi: 10.1126/scitranslmed.aaf1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Sager HB, Heidt T, Hulsmans M, Dutta P, Courties G, Sebas M, Wojtkiewicz GR, Tricot B, Iwamoto Y, Sun Y, Weissleder R, Libby P, Swirski FK, Nahrendorf M. Targeting interleukin-1β reduces leukocyte production after acute myocardial infarction. Circulation 132: 1880–1890, 2015. doi: 10.1161/CIRCULATIONAHA.115.016160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Sager HB, Hulsmans M, Lavine KJ, Moreira MB, Heidt T, Courties G, Sun Y, Iwamoto Y, Tricot B, Khan OF, Dahlman JE, Borodovsky A, Fitzgerald K, Anderson DG, Weissleder R, Libby P, Swirski FK, Nahrendorf M. Proliferation and recruitment contribute to myocardial macrophage expansion in chronic heart failure. Circ Res 119: 853–864, 2016. doi: 10.1161/CIRCRESAHA.116.309001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Saito M, Iwawaki T, Taya C, Yonekawa H, Noda M, Inui Y, Mekada E, Kimata Y, Tsuru A, Kohno K. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nat Biotechnol 19: 746–750, 2001. doi: 10.1038/90795. [DOI] [PubMed] [Google Scholar]
- 393.Samokhvalov IM, Samokhvalova NI, Nishikawa S. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature 446: 1056–1061, 2007. doi: 10.1038/nature05725. [DOI] [PubMed] [Google Scholar]
- 394.Sasmono RT, Oceandy D, Pollard JW, Tong W, Pavli P, Wainwright BJ, Ostrowski MC, Himes SR, Hume DA. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood 101: 1155–1163, 2003. doi: 10.1182/blood-2002-02-0569. [DOI] [PubMed] [Google Scholar]
- 395.Sasmono RT, Williams E. Generation and characterization of MacGreen mice, the Cfs1r-EGFP transgenic mice. Methods Mol Biol 844: 157–176, 2012. doi: 10.1007/978-1-61779-527-5_11. [DOI] [PubMed] [Google Scholar]
- 396.Sathe P, Metcalf D, Vremec D, Naik SH, Langdon WY, Huntington ND, Wu L, Shortman K. Lymphoid tissue and plasmacytoid dendritic cells and macrophages do not share a common macrophage-dendritic cell-restricted progenitor. Immunity 41: 104–115, 2014. doi: 10.1016/j.immuni.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 397.Satpathy AT, Kc W, Albring JC, Edelson BT, Kretzer NM, Bhattacharya D, Murphy TL, Murphy KM. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J Exp Med 209: 1135–1152, 2012. doi: 10.1084/jem.20120030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Sawyer RT, Strausbauch PH, Volkman A. Resident macrophage proliferation in mice depleted of blood monocytes by strontium-89. Lab Invest 46: 165–170, 1982. [PubMed] [Google Scholar]
- 399.Schilling M, Besselmann M, Leonhard C, Mueller M, Ringelstein EB, Kiefer R. Microglial activation precedes and predominates over macrophage infiltration in transient focal cerebral ischemia: a study in green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol 183: 25–33, 2003. doi: 10.1016/S0014-4886(03)00082-7. [DOI] [PubMed] [Google Scholar]
- 400.Schlitzer A, Sivakamasundari V, Chen J, Sumatoh HR, Schreuder J, Lum J, Malleret B, Zhang S, Larbi A, Zolezzi F, Renia L, Poidinger M, Naik S, Newell EW, Robson P, Ginhoux F. Identification of cDC1- and cDC2-committed DC progenitors reveals early lineage priming at the common DC progenitor stage in the bone marrow. Nat Immunol 16: 718–728, 2015. doi: 10.1038/ni.3200. [DOI] [PubMed] [Google Scholar]
- 401.Schloss MJ, Horckmans M, Nitz K, Duchene J, Drechsler M, Bidzhekov K, Scheiermann C, Weber C, Soehnlein O, Steffens S. The time-of-day of myocardial infarction onset affects healing through oscillations in cardiac neutrophil recruitment. EMBO Mol Med 8: 937–948, 2016. doi: 10.15252/emmm.201506083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol 25: 1256–1261, 2005. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- 403.Schulz C, Perdiguero EG, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336: 86–90, 2012. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 404.Schwartz M, Baruch K. The resolution of neuroinflammation in neurodegeneration: leukocyte recruitment via the choroid plexus. EMBO J 33: 7–22, 2014. doi: 10.1002/embj.201386609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Scott CL, Zheng F, De Baetselier P, Martens L, Saeys Y, De Prijck S, Lippens S, Abels C, Schoonooghe S, Raes G, Devoogdt N, Lambrecht BN, Beschin A, Guilliams M. Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat Commun 7: 10321, 2016. doi: 10.1038/ncomms10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Scott EW, Simon MC, Anastasi J, Singh H. Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science 265: 1573–1577, 1994. doi: 10.1126/science.8079170. [DOI] [PubMed] [Google Scholar]
- 407.Secchiero P, Candido R, Corallini F, Zacchigna S, Toffoli B, Rimondi E, Fabris B, Giacca M, Zauli G. Systemic tumor necrosis factor-related apoptosis-inducing ligand delivery shows antiatherosclerotic activity in apolipoprotein E-null diabetic mice. Circulation 114: 1522–1530, 2006. doi: 10.1161/CIRCULATIONAHA.106.643841. [DOI] [PubMed] [Google Scholar]
- 408.Seimon TA, Obstfeld A, Moore KJ, Golenbock DT, Tabas I. Combinatorial pattern recognition receptor signaling alters the balance of life and death in macrophages. Proc Natl Acad Sci USA 103: 19794–19799, 2006. doi: 10.1073/pnas.0609671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol 7: 311–317, 2006. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 410.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 19: 59–70, 2003. doi: 10.1016/S1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 411.Shechter R, London A, Varol C, Raposo C, Cusimano M, Yovel G, Rolls A, Mack M, Pluchino S, Martino G, Jung S, Schwartz M. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med 6: e1000113, 2009. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Shechter R, Miller O, Yovel G, Rosenzweig N, London A, Ruckh J, Kim KW, Klein E, Kalchenko V, Bendel P, Lira SA, Jung S, Schwartz M. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity 38: 555–569, 2013. doi: 10.1016/j.immuni.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Shechter R, Schwartz M. Harnessing monocyte-derived macrophages to control central nervous system pathologies: no longer ‘if’ but ‘how’. J Pathol 229: 332–346, 2013. doi: 10.1002/path.4106. [DOI] [PubMed] [Google Scholar]
- 414.Sheng J, Ruedl C, Karjalainen K. Most tissue-resident macrophages except microglia are derived from fetal hematopoietic stem cells. Immunity 43: 382–393, 2015. doi: 10.1016/j.immuni.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 415.Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity 34: 590–601, 2011. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Shibata Y, Berclaz PY, Chroneos ZC, Yoshida M, Whitsett JA, Trapnell BC. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity 15: 557–567, 2001. doi: 10.1016/S1074-7613(01)00218-7. [DOI] [PubMed] [Google Scholar]
- 417.Sierra A, de Castro F, Del Río-Hortega J, Rafael Iglesias-Rozas J, Garrosa M, Kettenmann H. The “Big-Bang” for modern glial biology: Translation and comments on Pío del Río-Hortega 1919 series of papers on microglia. Glia 64: 1801–1840, 2016. doi: 10.1002/glia.23046. [DOI] [PubMed] [Google Scholar]
- 418.Sieweke MH, Allen JE. Beyond stem cells: self-renewal of differentiated macrophages. Science 342: 1242974, 2013. doi: 10.1126/science.1242974. [DOI] [PubMed] [Google Scholar]
- 419.Simpson PJ, Todd RF III, Fantone JC, Mickelson JK, Griffin JD, Lucchesi BR. Reduction of experimental canine myocardial reperfusion injury by a monoclonal antibody (anti-Mo1, anti-CD11b) that inhibits leukocyte adhesion. J Clin Invest 81: 624–629, 1988. doi: 10.1172/JCI113364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Skålén K, Gustafsson M, Rydberg EK, Hultén LM, Wiklund O, Innerarity TL, Borén J. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature 417: 750–754, 2002. doi: 10.1038/nature00804. [DOI] [PubMed] [Google Scholar]
- 421.Smith JD, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci USA 92: 8264–8268, 1995. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Soehnlein O, Zernecke A, Eriksson EE, Rothfuchs AG, Pham CT, Herwald H, Bidzhekov K, Rottenberg ME, Weber C, Lindbom L. Neutrophil secretion products pave the way for inflammatory monocytes. Blood 112: 1461–1471, 2008. doi: 10.1182/blood-2008-02-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Sorokin SP, Hoyt RF Jr, Blunt DG, McNelly NA. Macrophage development: II. Early ontogeny of macrophage populations in brain, liver, and lungs of rat embryos as revealed by a lectin marker. Anat Rec 232: 527–550, 1992. doi: 10.1002/ar.1092320410. [DOI] [PubMed] [Google Scholar]
- 424.Soza-Ried C, Hess I, Netuschil N, Schorpp M, Boehm T. Essential role of c-myb in definitive hematopoiesis is evolutionarily conserved. Proc Natl Acad Sci USA 107: 17304–17308, 2010. doi: 10.1073/pnas.1004640107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Spann NJ, Garmire LX, McDonald JG, Myers DS, Milne SB, Shibata N, Reichart D, Fox JN, Shaked I, Heudobler D, Raetz CR, Wang EW, Kelly SL, Sullards MC, Murphy RC, Merrill AH Jr, Brown HA, Dennis EA, Li AC, Ley K, Tsimikas S, Fahy E, Subramaniam S, Quehenberger O, Russell DW, Glass CK. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell 151: 138–152, 2012. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JA, Maher DW, Cebon J, Sinickas V, Dunn AR. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci USA 91: 5592–5596, 1994. doi: 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Stanley ER, Cifone M, Heard PM, Defendi V. Factors regulating macrophage production and growth: identity of colony-stimulating factor and macrophage growth factor. J Exp Med 143: 631–647, 1976. doi: 10.1084/jem.143.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med 137: 1142–1162, 1973. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol 11: 155–161, 2010. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Stienstra R, Netea-Maier RT, Riksen NP, Joosten LAB, Netea MG. Specific and complex reprogramming of cellular metabolism in myeloid cells during innate immune responses. Cell Metab 26: 142–156, 2017. doi: 10.1016/j.cmet.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 431.Stöger JL, Gijbels MJ, van der Velden S, Manca M, van der Loos CM, Biessen EA, Daemen MJ, Lutgens E, de Winther MP. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis 225: 461–468, 2012. doi: 10.1016/j.atherosclerosis.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 432.Stoneman V, Braganza D, Figg N, Mercer J, Lang R, Goddard M, Bennett M. Monocyte/macrophage suppression in CD11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circ Res 100: 884–893, 2007. doi: 10.1161/01.RES.0000260802.75766.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol 76: 509–513, 2004. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Suárez-Barrientos A, López-Romero P, Vivas D, Castro-Ferreira F, Núñez-Gil I, Franco E, Ruiz-Mateos B, García-Rubira JC, Fernández-Ortiz A, Macaya C, Ibanez B. Circadian variations of infarct size in acute myocardial infarction. Heart 97: 970–976, 2011. doi: 10.1136/hrt.2010.212621. [DOI] [PubMed] [Google Scholar]
- 435.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest 117: 195–205, 2007. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 339: 161–166, 2013. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325: 612–616, 2009. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Swirski FK, Pittet MJ, Kircher MF, Aikawa E, Jaffer FA, Libby P, Weissleder R. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc Natl Acad Sci USA 103: 10340–10345, 2006. doi: 10.1073/pnas.0604260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest 117: 185–194, 2007. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Tacke F, Ginhoux F, Jakubzick C, van Rooijen N, Merad M, Randolph GJ. Immature monocytes acquire antigens from other cells in the bone marrow and present them to T cells after maturing in the periphery. J Exp Med 203: 583–597, 2006. doi: 10.1084/jem.20052119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Tagliani E, Shi C, Nancy P, Tay CS, Pamer EG, Erlebacher A. Coordinate regulation of tissue macrophage and dendritic cell population dynamics by CSF-1. J Exp Med 208: 1901–1916, 2011. doi: 10.1084/jem.20110866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med 201: 647–657, 2005. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Takahashi K, Yamamura F, Naito M. Differentiation, maturation, and proliferation of macrophages in the mouse yolk sac: a light-microscopic, enzyme-cytochemical, immunohistochemical, and ultrastructural study. J Leukoc Biol 45: 87–96, 1989. doi: 10.1002/jlb.45.2.87. [DOI] [PubMed] [Google Scholar]
- 444.Tamoutounour S, Guilliams M, Montanana Sanchis F, Liu H, Terhorst D, Malosse C, Pollet E, Ardouin L, Luche H, Sanchez C, Dalod M, Malissen B, Henri S. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity 39: 925–938, 2013. doi: 10.1016/j.immuni.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 445.Tang J, Baxter S, Menon A, Alaarg A, Sanchez-Gaytan BL, Fay F, Zhao Y, Ouimet M, Braza MS, Longo VA, Abdel-Atti D, Duivenvoorden R, Calcagno C, Storm G, Tsimikas S, Moore KJ, Swirski FK, Nahrendorf M, Fisher EA, Pérez-Medina C, Fayad ZA, Reiner T, Mulder WJ. Immune cell screening of a nanoparticle library improves atherosclerosis therapy. Proc Natl Acad Sci USA 113: E6731–E6740, 2016. doi: 10.1073/pnas.1609629113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Tang J, Lobatto ME, Hassing L, van der Staay S, van Rijs SM, Calcagno C, Braza MS, Baxter S, Fay F, Sanchez-Gaytan BL, Duivenvoorden R, Sager H, Astudillo YM, Leong W, Ramachandran S, Storm G, Pérez-Medina C, Reiner T, Cormode DP, Strijkers GJ, Stroes ES, Swirski FK, Nahrendorf M, Fisher EA, Fayad ZA, Mulder WJ. Inhibiting macrophage proliferation suppresses atherosclerotic plaque inflammation. Sci Adv 1: e1400223, 2015. doi: 10.1126/sciadv.1400223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Tardif JC. Emerging high-density lipoprotein infusion therapies: fulfilling the promise of epidemiology? J Clin Lipidol 4: 399–404, 2010. doi: 10.1016/j.jacl.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 448.Tarling JD, Lin HS, Hsu S. Self-renewal of pulmonary alveolar macrophages: evidence from radiation chimera studies. J Leukoc Biol 42: 443–446, 1987. doi: 10.1002/jlb.42.5.443. [DOI] [PubMed] [Google Scholar]
- 449.Tawakol A, Ishai A, Takx RA, Figueroa AL, Ali A, Kaiser Y, Truong QA, Solomon CJ, Calcagno C, Mani V, Tang CY, Mulder WJ, Murrough JW, Hoffmann U, Nahrendorf M, Shin LM, Fayad ZA, Pitman RK. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet 389: 834–845, 2017. doi: 10.1016/S0140-6736(16)31714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Tay TL, Mai D, Dautzenberg J, Fernández-Klett F, Lin G, Sagar, Datta M, Drougard A, Stempfl T, Ardura-Fabregat A, Staszewski O, Margineanu A, Sporbert A, Steinmetz LM, Pospisilik JA, Jung S, Priller J, Grün D, Ronneberger O, Prinz M. A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat Neurosci 20: 793–803, 2017. doi: 10.1038/nn.4547. [DOI] [PubMed] [Google Scholar]
- 451.Theurl I, Hilgendorf I, Nairz M, Tymoszuk P, Haschka D, Asshoff M, He S, Gerhardt LM, Holderried TA, Seifert M, Sopper S, Fenn AM, Anzai A, Rattik S, McAlpine C, Theurl M, Wieghofer P, Iwamoto Y, Weber GF, Harder NK, Chousterman BG, Arvedson TL, McKee M, Wang F, Lutz OM, Rezoagli E, Babitt JL, Berra L, Prinz M, Nahrendorf M, Weiss G, Weissleder R, Lin HY, Swirski FK. On-demand erythrocyte disposal and iron recycling requires transient macrophages in the liver. Nat Med 22: 945–951, 2016. doi: 10.1038/nm.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Thion MS, Low D, Silvin A, Chen J, Grisel P, Schulte-Schrepping J, Blecher R, Ulas T, Squarzoni P, Hoeffel G, Coulpier F, Siopi E, David FS, Scholz C, Shihui F, Lum J, Amoyo AA, Larbi A, Poidinger M, Buttgereit A, Lledo PM, Greter M, Chan JKY, Amit I, Beyer M, Schultze JL, Schlitzer A, Pettersson S, Ginhoux F, Garel S. Microbiome influences prenatal and adult microglia in a sex-specific manner. Cell 172: 500–516.e16, 2018. doi: 10.1016/j.cell.2017.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Thomas ED, Ramberg RE, Sale GE, Sparkes RS, Golde DW. Direct evidence for a bone marrow origin of the alveolar macrophage in man. Science 192: 1016–1018, 1976. doi: 10.1126/science.775638. [DOI] [PubMed] [Google Scholar]
- 454.Thomas GD, Hamers AAJ, Nakao C, Marcovecchio P, Taylor AM, McSkimming C, Nguyen AT, McNamara CA, Hedrick CC. Human blood monocyte subsets: a new gating strategy defined using cell surface markers identified by mass cytometry. Arterioscler Thromb Vasc Biol 37: 1548–1558, 2017. doi: 10.1161/ATVBAHA.117.309145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Thomas GD, Hanna RN, Vasudevan NT, Hamers AA, Romanoski CE, McArdle S, Ross KD, Blatchley A, Yoakum D, Hamilton BA, Mikulski Z, Jain MK, Glass CK, Hedrick CC. Deleting an Nr4a1 super-enhancer subdomain ablates Ly6Clow monocytes while preserving macrophage gene function. Immunity 45: 975–987, 2016. doi: 10.1016/j.immuni.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Thorp E, Cui D, Schrijvers DM, Kuriakose G, Tabas I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe−/− mice. Arterioscler Thromb Vasc Biol 28: 1421–1428, 2008. doi: 10.1161/ATVBAHA.108.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Todt JC, Hu B, Curtis JL. The scavenger receptor SR-A I/II (CD204) signals via the receptor tyrosine kinase Mertk during apoptotic cell uptake by murine macrophages. J Leukoc Biol 84: 510–518, 2008. doi: 10.1189/jlb.0307135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest 117: 902–909, 2007. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Tsujioka H, Imanishi T, Ikejima H, Kuroi A, Takarada S, Tanimoto T, Kitabata H, Okochi K, Arita Y, Ishibashi K, Komukai K, Kataiwa H, Nakamura N, Hirata K, Tanaka A, Akasaka T. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J Am Coll Cardiol 54: 130–138, 2009. doi: 10.1016/j.jacc.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 460.Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, Ishii M, Yamashita T. Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci 16: 543–551, 2013. doi: 10.1038/nn.3358. [DOI] [PubMed] [Google Scholar]
- 461.Usher MG, Duan SZ, Ivaschenko CY, Frieler RA, Berger S, Schütz G, Lumeng CN, Mortensen RM. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J Clin Invest 120: 3350–3364, 2010. doi: 10.1172/JCI41080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.van Amerongen MJ, Harmsen MC, van Rooijen N, Petersen AH, van Luyn MJ. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J Pathol 170: 818–829, 2007. doi: 10.2353/ajpath.2007.060547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.van den Borne SW, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol 7: 30–37, 2010. doi: 10.1038/nrcardio.2009.199. [DOI] [PubMed] [Google Scholar]
- 464.van der Laan AM, Ter Horst EN, Delewi R, Begieneman MP, Krijnen PA, Hirsch A, Lavaei M, Nahrendorf M, Horrevoets AJ, Niessen HW, Piek JJ. Monocyte subset accumulation in the human heart following acute myocardial infarction and the role of the spleen as monocyte reservoir. Eur Heart J 35: 376–385, 2014. doi: 10.1093/eurheartj/eht331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.van der Wal AC, Becker AE. Atherosclerotic plaque rupture—pathologic basis of plaque stability and instability. Cardiovasc Res 41: 334–344, 1999. doi: 10.1016/S0008-6363(98)00276-4. [DOI] [PubMed] [Google Scholar]
- 466.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med 128: 415–435, 1968. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.van Furth R, Cohn ZA, Hirsch JG, Humphrey JH, Spector WG, Langevoort HL. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ 46: 845–852, 1972. [PMC free article] [PubMed] [Google Scholar]
- 468.Van Furth R, Diesselhoff-den Dulk MC, Mattie H. Quantitative study on the production and kinetics of mononuclear phagocytes during an acute inflammatory reaction. J Exp Med 138: 1314–1330, 1973. doi: 10.1084/jem.138.6.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.van Furth R, Diesselhoff-den Dulk MM. Dual origin of mouse spleen macrophages. J Exp Med 160: 1273–1283, 1984. doi: 10.1084/jem.160.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.van Puijvelde GHM, Kuiper J. NKT cells in cardiovascular diseases. Eur J Pharmacol 816: 47–57, 2017. doi: 10.1016/j.ejphar.2017.03.052. [DOI] [PubMed] [Google Scholar]
- 471.van Rooijen N, Hendrikx E. Liposomes for specific depletion of macrophages from organs and tissues. Methods Mol Biol 605: 189–203, 2010. doi: 10.1007/978-1-60327-360-2_13. [DOI] [PubMed] [Google Scholar]
- 472.Vandoorne K, Nahrendorf M. Multiparametric imaging of organ system interfaces. Circ Cardiovasc Imaging 10: e005613, 2017. doi: 10.1161/CIRCIMAGING.116.005613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Vanhee S, De Mulder K, Van Caeneghem Y, Verstichel G, Van Roy N, Menten B, Velghe I, Philippé J, De Bleser D, Lambrecht BN, Taghon T, Leclercq G, Kerre T, Vandekerckhove B. In vitro human embryonic stem cell hematopoiesis mimics MYB-independent yolk sac hematopoiesis. Haematologica 100: 157–166, 2015. doi: 10.3324/haematol.2014.112144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, Kalchenko V, Geissmann F, Jung S. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med 204: 171–180, 2007. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Greaves DR, Wagner RA, Murray PJ, Chawla A. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab 4: 13–24, 2006. [Erratum in Cell Metab 4: 255, 2006.] doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Verma SK, Krishnamurthy P, Barefield D, Singh N, Gupta R, Lambers E, Thal M, Mackie A, Hoxha E, Ramirez V, Qin G, Sadayappan S, Ghosh AK, Kishore R. Interleukin-10 treatment attenuates pressure overload-induced hypertrophic remodeling and improves heart function via signal transducers and activators of transcription 3-dependent inhibition of nuclear factor-κB. Circulation 126: 418–429, 2012. doi: 10.1161/CIRCULATIONAHA.112.112185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Vilahur G, Juan-Babot O, Peña E, Oñate B, Casaní L, Badimon L. Molecular and cellular mechanisms involved in cardiac remodeling after acute myocardial infarction. J Mol Cell Cardiol 50: 522–533, 2011. doi: 10.1016/j.yjmcc.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 478.Villani AC, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, Griesbeck M, Butler A, Zheng S, Lazo S, Jardine L, Dixon D, Stephenson E, Nilsson E, Grundberg I, McDonald D, Filby A, Li W, De Jager PL, Rozenblatt-Rosen O, Lane AA, Haniffa M, Regev A, Hacohen N. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 356: eaah4573, 2017. doi: 10.1126/science.aah4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Virolainen M. Hematopoietic origin of macrophages as studied by chromosome markers in mice. J Exp Med 127: 943–952, 1968. doi: 10.1084/jem.127.5.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Volkman A, Gowans JL. The origin of macrophages from bone marrow in the rat. Br J Exp Pathol 46: 62–70, 1965. [PMC free article] [PubMed] [Google Scholar]
- 481.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature 390: 350–351, 1997. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 482.Wan E, Yeap XY, Dehn S, Terry R, Novak M, Zhang S, Iwata S, Han X, Homma S, Drosatos K, Lomasney J, Engman DM, Miller SD, Vaughan DE, Morrow JP, Kishore R, Thorp EB. Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ Res 113: 1004–1012, 2013. doi: 10.1161/CIRCRESAHA.113.301198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Wang J, Kubes P. A reservoir of mature cavity macrophages that can rapidly invade visceral organs to affect tissue repair. Cell 165: 668–678, 2016. doi: 10.1016/j.cell.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 484.Wang X, Khalil RA. Matrix metalloproteinases, vascular remodeling, and vascular disease. Adv Pharmacol 81: 241–330, 2018. doi: 10.1016/bs.apha.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, Barrow AD, Diamond MS, Colonna M. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol 13: 753–760, 2012. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Weber C, Meiler S, Döring Y, Koch M, Drechsler M, Megens RT, Rowinska Z, Bidzhekov K, Fecher C, Ribechini E, van Zandvoort MA, Binder CJ, Jelinek I, Hristov M, Boon L, Jung S, Korn T, Lutz MB, Förster I, Zenke M, Hieronymus T, Junt T, Zernecke A. CCL17-expressing dendritic cells drive atherosclerosis by restraining regulatory T cell homeostasis in mice. J Clin Invest 121: 2898–2910, 2011. doi: 10.1172/JCI44925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Weber GF, Chousterman BG, He S, Fenn AM, Nairz M, Anzai A, Brenner T, Uhle F, Iwamoto Y, Robbins CS, Noiret L, Maier SL, Zönnchen T, Rahbari NN, Schölch S, Klotzsche-von Ameln A, Chavakis T, Weitz J, Hofer S, Weigand MA, Nahrendorf M, Weissleder R, Swirski FK. Interleukin-3 amplifies acute inflammation and is a potential therapeutic target in sepsis. Science 347: 1260–1265, 2015. doi: 10.1126/science.aaa4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Weissleder R, Nahrendorf M, Pittet MJ. Imaging macrophages with nanoparticles. Nat Mater 13: 125–138, 2014. doi: 10.1038/nmat3780. [DOI] [PubMed] [Google Scholar]
- 489.Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, Karbach SH, Schwenk M, Yogev N, Schulz E, Oelze M, Grabbe S, Jonuleit H, Becker C, Daiber A, Waisman A, Münzel T. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation 124: 1370–1381, 2011. doi: 10.1161/CIRCULATIONAHA.111.034470. [DOI] [PubMed] [Google Scholar]
- 490.Van Wesenbeeck L, Odgren PR, MacKay CA, D’Angelo M, Safadi FF, Popoff SN, Van Hul W, Marks SCJ Jr. The osteopetrotic mutation toothless (tl) is a loss-of-function frameshift mutation in the rat Csf1 gene: evidence of a crucial role for CSF-1 in osteoclastogenesis and endochondral ossification. Proc Natl Acad Sci USA 99: 14303–14308, 2002. doi: 10.1073/pnas.202332999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, von Schlippenbach J, Skurk C, Steendijk P, Riad A, Poller W, Schultheiss HP, Tschöpe C. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail 4: 44–52, 2011. doi: 10.1161/CIRCHEARTFAILURE.109.931451. [DOI] [PubMed] [Google Scholar]
- 492.Whitby JL, Rowley D. The role of macrophages in the elimination of bacteria from the mouse peritoneum. Br J Exp Pathol 40: 358–370, 1959. [PMC free article] [PubMed] [Google Scholar]
- 493. Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW Jr, Ahmed-Ansari A, Sell KW, Pollard JW, Stanley ER. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci USA 87: 4828–4832, 1990. [Erratum in Proc Natl Acad Sci USA 88: 5937, 1991.] doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Wiktor-Jedrzejczak WW, Ahmed A, Szczylik C, Skelly RR. Hematological characterization of congenital osteopetrosis in op/op mouse. Possible mechanism for abnormal macrophage differentiation. J Exp Med 156: 1516–1527, 1982. doi: 10.1084/jem.156.5.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Wildgruber M, Aschenbrenner T, Wendorff H, Czubba M, Glinzer A, Haller B, Schiemann M, Zimmermann A, Berger H, Eckstein HH, Meier R, Wohlgemuth WA, Libby P, Zernecke A. The “Intermediate” CD14++CD16+ monocyte subset increases in severe peripheral artery disease in humans. Sci Rep 6: 39483, 2016. doi: 10.1038/srep39483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Wilkins BS, Green A, Wild AE, Jones DB. Extramedullary haemopoiesis in fetal and adult human spleen: a quantitative immunohistological study. Histopathology 24: 241–247, 1994. doi: 10.1111/j.1365-2559.1994.tb00516.x. [DOI] [PubMed] [Google Scholar]
- 497.Wilson NK, Kent DG, Buettner F, Shehata M, Macaulay IC, Calero-Nieto FJ, Sánchez Castillo M, Oedekoven CA, Diamanti E, Schulte R, Ponting CP, Voet T, Caldas C, Stingl J, Green AR, Theis FJ, Göttgens B. Combined single-cell functional and gene expression analysis resolves heterogeneity within stem cell populations. Cell Stem Cell 16: 712–724, 2015. doi: 10.1016/j.stem.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Winkels H, Ehinger E, Vassallo M, Buscher K, Dinh H, Kobiyama K, Hamers A, Cochain C, Vafadarnejad E, Saliba AE, Zernecke A, Pramod A, Ghosh A, Anto Michel N, Hoppe N, Hilgendorf I, Zirlik A, Hedrick C, Ley K, Wolf D. Atlas of the immune cell repertoire in mouse atherosclerosis defined by single-cell RNA-sequencing and mass cytometry. Circ Res 122: 1675–1688, 2018. doi: 10.1161/CIRCRESAHA.117.312513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Witmer-Pack MD, Hughes DA, Schuler G, Lawson L, McWilliam A, Inaba K, Steinman RM, Gordon S. Identification of macrophages and dendritic cells in the osteopetrotic (op/op) mouse. J Cell Sci 104: 1021–1029, 1993. [DOI] [PubMed] [Google Scholar]
- 500.Wong KL, Tai JJ, Wong WC, Han H, Sem X, Yeap WH, Kourilsky P, Wong SC. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood 118: e16–e31, 2011. doi: 10.1182/blood-2010-12-326355. [DOI] [PubMed] [Google Scholar]
- 501.Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab 18: 649–659, 2013. doi: 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Wu H, Gower RM, Wang H, Perrard XY, Ma R, Bullard DC, Burns AR, Paul A, Smith CW, Simon SI, Ballantyne CM. Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation 119: 2708–2717, 2009. doi: 10.1161/CIRCULATIONAHA.108.823740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Wu Y, Dissing-Olesen L, MacVicar BA, Stevens B. Microglia: dynamic mediators of synapse development and plasticity. Trends Immunol 36: 605–613, 2015. doi: 10.1016/j.it.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Xie M, Lu C, Wang J, McLellan MD, Johnson KJ, Wendl MC, McMichael JF, Schmidt HK, Yellapantula V, Miller CA, Ozenberger BA, Welch JS, Link DC, Walter MJ, Mardis ER, Dipersio JF, Chen F, Wilson RK, Ley TJ, Ding L. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med 20: 1472–1478, 2014. doi: 10.1038/nm.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Xu XH, Shah PK, Faure E, Equils O, Thomas L, Fishbein MC, Luthringer D, Xu XP, Rajavashisth TB, Yano J, Kaul S, Arditi M. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation 104: 3103–3108, 2001. doi: 10.1161/hc5001.100631. [DOI] [PubMed] [Google Scholar]
- 506.Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, De Nardo D, Gohel TD, Emde M, Schmidleithner L, Ganesan H, Nino-Castro A, Mallmann MR, Labzin L, Theis H, Kraut M, Beyer M, Latz E, Freeman TC, Ulas T, Schultze JL. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 40: 274–288, 2014. doi: 10.1016/j.immuni.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Yamada M, Naito M, Takahashi K. Kupffer cell proliferation and glucan-induced granuloma formation in mice depleted of blood monocytes by strontium-89. J Leukoc Biol 47: 195–205, 1990. doi: 10.1002/jlb.47.3.195. [DOI] [PubMed] [Google Scholar]
- 508.Yamamoto T, Kaizu C, Kawasaki T, Hasegawa G, Umezu H, Ohashi R, Sakurada J, Jiang S, Shultz L, Naito M. Macrophage colony-stimulating factor is indispensable for repopulation and differentiation of Kupffer cells but not for splenic red pulp macrophages in osteopetrotic (op/op) mice after macrophage depletion. Cell Tissue Res 332: 245–256, 2008. doi: 10.1007/s00441-008-0586-8. [DOI] [PubMed] [Google Scholar]
- 509.Yamazaki T, Seko Y, Tamatani T, Miyasaka M, Yagita H, Okumura K, Nagai R, Yazaki Y. Expression of intercellular adhesion molecule-1 in rat heart with ischemia/reperfusion and limitation of infarct size by treatment with antibodies against cell adhesion molecules. Am J Pathol 143: 410–418, 1993. [PMC free article] [PubMed] [Google Scholar]
- 510.Yáñez A, Coetzee SG, Olsson A, Muench DE, Berman BP, Hazelett DJ, Salomonis N, Grimes HL, Goodridge HS. Granulocyte-monocyte progenitors and monocyte-dendritic cell progenitors independently produce functionally distinct monocytes. Immunity 47: 890–902.e4, 2017. doi: 10.1016/j.immuni.2017.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Yano T, Miura T, Ikeda Y, Matsuda E, Saito K, Miki T, Kobayashi H, Nishino Y, Ohtani S, Shimamoto K. Intracardiac fibroblasts, but not bone marrow derived cells, are the origin of myofibroblasts in myocardial infarct repair. Cardiovasc Pathol 14: 241–246, 2005. doi: 10.1016/j.carpath.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 512.Ye YX, Calcagno C, Binderup T, Courties G, Keliher EJ, Wojtkiewicz GR, Iwamoto Y, Tang J, Pérez-Medina C, Mani V, Ishino S, Johnbeck CB, Knigge U, Fayad ZA, Libby P, Weissleder R, Tawakol A, Dubey S, Belanger AP, Di Carli MF, Swirski FK, Kjaer A, Mulder WJ, Nahrendorf M. Imaging Macrophage and hematopoietic progenitor proliferation in atherosclerosis. Circ Res 117: 835–845, 2015. doi: 10.1161/CIRCRESAHA.115.307024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Yeh FL, Hansen DV, Sheng M. TREM2, microglia, and neurodegenerative diseases. Trends Mol Med 23: 512–533, 2017. doi: 10.1016/j.molmed.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 514.Yilmaz A, Lochno M, Traeg F, Cicha I, Reiss C, Stumpf C, Raaz D, Anger T, Amann K, Probst T, Ludwig J, Daniel WG, Garlichs CD. Emergence of dendritic cells in rupture-prone regions of vulnerable carotid plaques. Atherosclerosis 176: 101–110, 2004. doi: 10.1016/j.atherosclerosis.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 515.Yona S, Jung S. Monocytes: subsets, origins, fates and functions. Curr Opin Hematol 17: 53–59, 2010. doi: 10.1097/MOH.0b013e3283324f80. [DOI] [PubMed] [Google Scholar]
- 516. Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38: 79–91, 2013. [Erratum in Immunity 38: 1073–1079, 2013.] doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science 328: 1689–1693, 2010. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, Welch C, Tall AR. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest 117: 3900–3908, 2007. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Zandbergen HR, Sharma UC, Gupta S, Verjans JW, van den Borne S, Pokharel S, van Brakel T, Duijvestijn A, van Rooijen N, Maessen JG, Reutelingsperger C, Pinto YM, Narula J, Hofstra L. Macrophage depletion in hypertensive rats accelerates development of cardiomyopathy. J Cardiovasc Pharmacol Ther 14: 68–75, 2009. doi: 10.1177/1074248408329860. [DOI] [PubMed] [Google Scholar]
- 520.Zeng H, Yücel R, Kosan C, Klein-Hitpass L, Möröy T. Transcription factor Gfi1 regulates self-renewal and engraftment of hematopoietic stem cells. EMBO J 23: 4116–4125, 2004. doi: 10.1038/sj.emboj.7600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Zernecke A, Liehn EA, Gao JL, Kuziel WA, Murphy PM, Weber C. Deficiency in CCR5 but not CCR1 protects against neointima formation in atherosclerosis-prone mice: involvement of IL-10. Blood 107: 4240–4243, 2006. doi: 10.1182/blood-2005-09-3922. [DOI] [PubMed] [Google Scholar]
- 522.Zhang DE, Zhang P, Wang ND, Hetherington CJ, Darlington GJ, Tenen DG. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein alpha-deficient mice. Proc Natl Acad Sci USA 94: 569–574, 1997. doi: 10.1073/pnas.94.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Zhang P, Iwasaki-Arai J, Iwasaki H, Fenyus ML, Dayaram T, Owens BM, Shigematsu H, Levantini E, Huettner CS, Lekstrom-Himes JA, Akashi K, Tenen DG. Enhancement of hematopoietic stem cell repopulating capacity and self-renewal in the absence of the transcription factor C/EBP α. Immunity 21: 853–863, 2004. doi: 10.1016/j.immuni.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 524.Zhou J, Li YS, Chien S. Shear stress-initiated signaling and its regulation of endothelial function. Arterioscler Thromb Vasc Biol 34: 2191–2198, 2014. doi: 10.1161/ATVBAHA.114.303422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Zhou J, Tang PC, Qin L, Gayed PM, Li W, Skokos EA, Kyriakides TR, Pober JS, Tellides G. CXCR3-dependent accumulation and activation of perivascular macrophages is necessary for homeostatic arterial remodeling to hemodynamic stresses. J Exp Med 207: 1951–1966, 2010. doi: 10.1084/jem.20100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Zhu SN, Chen M, Jongstra-Bilen J, Cybulsky MI. GM-CSF regulates intimal cell proliferation in nascent atherosclerotic lesions. J Exp Med 206: 2141–2149, 2009. doi: 10.1084/jem.20090866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Zhu YP, Thomas GD, Hedrick CC. 2014 Jeffrey M. Hoeg award lecture: transcriptional control of monocyte development. Arterioscler Thromb Vasc Biol 36: 1722–1733, 2016. doi: 10.1161/ATVBAHA.116.304054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Zouggari Y, Ait-Oufella H, Bonnin P, Simon T, Sage AP, Guérin C, Vilar J, Caligiuri G, Tsiantoulas D, Laurans L, Dumeau E, Kotti S, Bruneval P, Charo IF, Binder CJ, Danchin N, Tedgui A, Tedder TF, Silvestre JS, Mallat Z. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med 19: 1273–1280, 2013. doi: 10.1038/nm.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Zovein AC, Turlo KA, Ponec RM, Lynch MR, Chen KC, Hofmann JJ, Cox TC, Gasson JC, Iruela-Arispe ML. Vascular remodeling of the vitelline artery initiates extravascular emergence of hematopoietic clusters. Blood 116: 3435–3444, 2010. doi: 10.1182/blood-2010-04-279497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Zupančič E, Fayad ZA, Mulder WJM. Cardiovascular Immunotherapy and the Role of Imaging. Arterioscler Thromb Vasc Biol 37: e167–e171, 2017. doi: 10.1161/ATVBAHA.117.309227. [DOI] [PMC free article] [PubMed] [Google Scholar]