Abstract
The unique architecture of the mammalian lung is required for adaptation to air breathing at birth and thereafter. Understanding the cellular and molecular mechanisms controlling its morphogenesis provides the framework for understanding the pathogenesis of acute and chronic lung diseases. Recent single-cell RNA sequencing data and high-resolution imaging identify the remarkable heterogeneity of pulmonary cell types and provides cell selective gene expression underlying lung development. We will address fundamental issues related to the diversity of pulmonary cells, to the formation and function of the mammalian lung, and will review recent advances regarding the cellular and molecular pathways involved in lung organogenesis. What cells form the lung in the early embryo? How are cell proliferation, migration, and differentiation regulated during lung morphogenesis? How do cells interact during lung formation and repair? How do signaling and transcriptional programs determine cell-cell interactions necessary for lung morphogenesis and function?
I. INTRODUCTION
The unique architecture of the mammalian lung is required for adaptation to air breathing at birth and thereafter. Identifying the cellular and molecular mechanisms controlling normal lung morphogenesis provides the framework for understanding the pathogenesis of acute and chronic lung diseases. Recent single cell RNA sequencing data and high-resolution imaging identifies the remarkable heterogeneity of pulmonary cell types and provides insights into cell-selective gene regulating networks underlying lung development. We will address fundamental issues related to the diversity of pulmonary cells involved in formation and function of the mammalian lung. We will review recent advances regarding the cellular and molecular pathways involved in lung organogenesis. What cells form the lung in the early embryo? How are cell proliferation, migration, and differentiation regulated during lung morphogenesis? How do cells interact during lung formation and repair? How do signaling and transcriptional programs determine cell-cell interactions necessary for lung morphogenesis and function?
II. A COMPLEX STRUCTURE SUPPORTS THE FUNCTION OF THE VERTEBRATE LUNG
Adaptation of vertebrates to air breathing depends on the structure of the large and complex organ that enables the efficient transfer of oxygen and carbon dioxide necessary for oxidative metabolism. The respiratory tract is a remarkably complex machine consisting of semi-rigid conducting airway tubes that bifurcate, branch, and taper, from the trachea, bronchi, and bronchioles, leading to highly vascularized saccules or alveoli, where respiratory gases are exchanged. The respiratory tract comprises multiple cell types derived from embryonic neuroectoderm, mesoderm, and endoderm. A great diversity of cell types is found in precise numbers and positions to create the architectural features upon which ventilation depends (FIGURE 1). Tubules of the conducting airways and alveolar saccules are lined by distinct epithelial cell types that vary along the cephalo-caudal axis of the lung. Airways are supported by cartilage, smooth muscle, and a complex extracellular matrix. Conducting airways lead to the alveoli, where the dynamic process of inflation and deflation is enabled by a remarkable network of flexible collagen and elastin fibers. This complex structure is protected from continuous exposure to particles, pathogens, and toxicants by the process of mucociliary clearance and by a robust innate and acquired immune system. Mucociliary clearance depends on precise regulation of surface fluids and electrolytes, and mechanical activity of ciliated and secretory cells to clear pathogens and particles (353). The lung is innervated, responding to central and peripheral inputs that influence cough and fluid secretion and integrate neural control of oxygen, carbon dioxide, and pH sensing (13, 350). Conducting airways lead to an alveolar region that provides a vast epithelial lined surface, covered primarily by alveolar type 1 (AT1) cells, which are in close contact with endothelial cells of the pulmonary capillaries. Oxygen is taken up by erythrocytes within the vessels, and carbon dioxide diffuses into alveolar gases and is exhaled. Pulmonary blood flow is supplied from the right ventricle via the pulmonary arteries and drains into the left atrium via the pulmonary veins. An extensive lymphatic system controls pulmonary fluid balance critical for alveolar gas exchange.
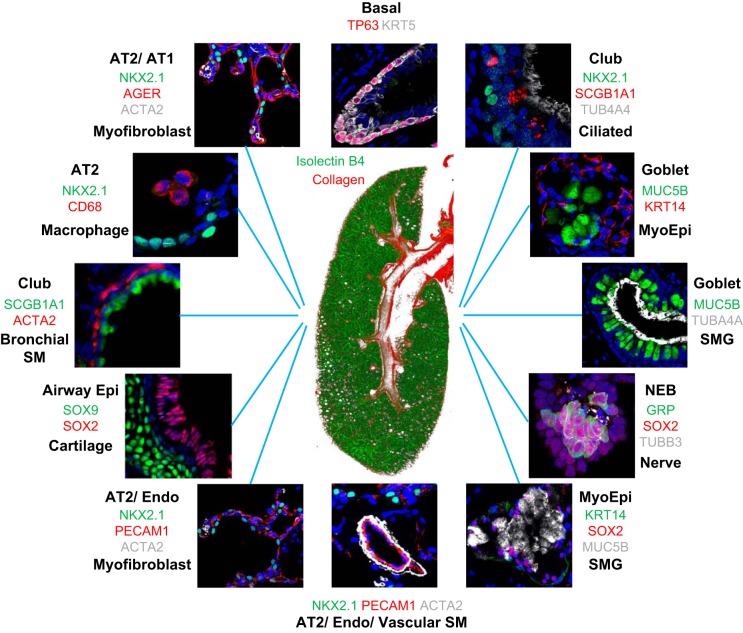
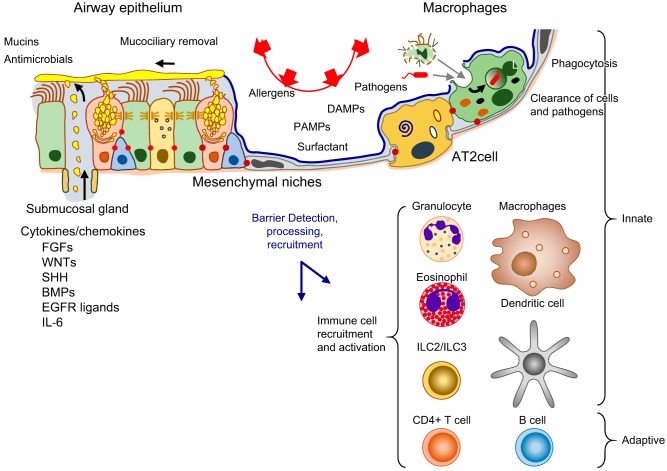
FIGURE 1.
Diverse cells and structures of the mammalian lung. At the center is an image of the right lobe of the mouse lung on PN3, in the early alveolar period of morphogenesis. Green indicates endothelial cells of the pulmonary vasculature, and red marks the second harmonic image of collagen in the main bronchus, subsegmental bronchi, and pulmonary artery (red) at the center of the figure. Diverse pulmonary cell types and their niches are shown by fluorescence antibody staining as indicated by the colors that correspond to the antibodies used to stain each cell type. Images are available on the LungImage website (https://research.cchmc.org/lungimage/?page_id=21726) and include examples of cells and structures shared by mouse and human pulmonary tissues.
Skeletal muscles of the diaphragm and chest walls create the mechanical bellows that inflate and deflate the alveoli during each ventilatory cycle. Precise control of capillary blood flow and alveolar-capillary permeability is necessary for proper gas exchange. The hydrated alveolar surfaces are in direct contact with inhaled gases creating surface tension and collapsing forces. These forces are minimized by the production of pulmonary surfactant lipids and proteins by specialized alveolar type 2 cells (AT2 cells) that keep peripheral saccules from atelectasis during the ventilatory cycle. Cells of the innate and acquired immune systems are abundant. Since the singular role of this remarkable organ is to mediate efficient gas exchange, its function is entirely dependent on its architecture, created and maintained by the interactions of a myriad of cells (284). The complexity of this remarkable organ raises fundamental questions regarding its formation and its repair following injury, topics recently addressed in a number of reviews (76, 126, 166, 303, 310). We will address recent advances related to and the cellular and molecular processes that control lung formation during embryogenesis, the perinatal period of respiratory adaptation and during regeneration. Traditionally, lung formation is divided into five distinct periods based on structure: embryonic, pseudoglandular, canalicular, saccular, and alveolar periods which are shared among mammalian species. In this review, we will prioritize findings in human and mouse lung.
III. THE DIVERSITY OF CELLS THAT FORM THE MAMMALIAN LUNG
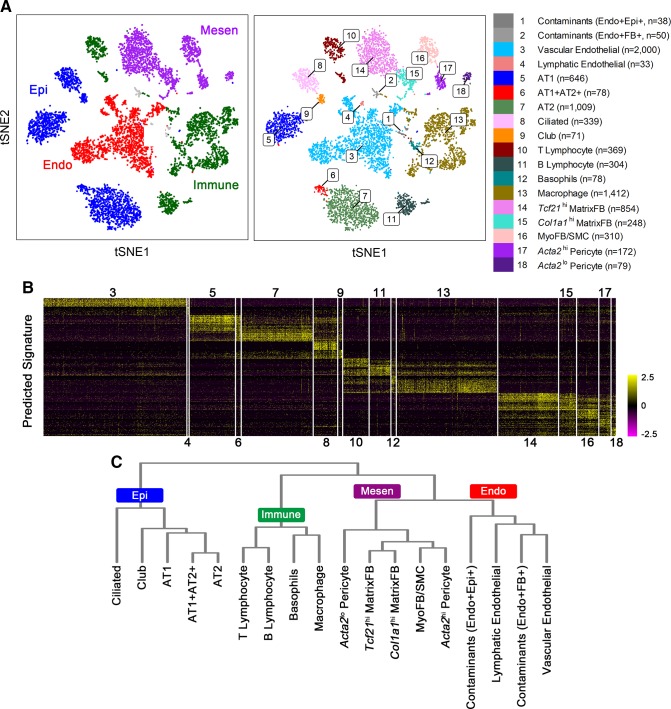
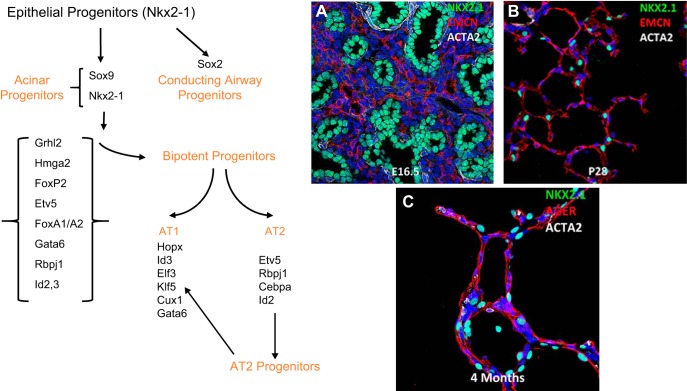
Perhaps consideration of the cellular mechanisms contributing to the formation of the lung begins with identifying and understanding the many cells and architectural features that contribute to its structures and functions (FIGURE 1). The respiratory tract conducts air from the nares and pharynx to the trachea and conducting airways that end in the alveoli where gases are exchanged. The precise numbers and distinct pulmonary cell types, their lineages, and differentiation change dynamically during lung morphogenesis and are now being appreciated from single cell transcriptomic studies and lineage tracing studies that are providing an even more detailed insight into lung formation and repair (73, 74, 110, 171, 183, 327, 377). Advances in proteomics, while not yet at single cell level, complement RNA studies providing insights into both transcriptional and posttranscriptional control of lung formation (226). Historically, morphological and ultrastructural studies were used to estimate the diversity of cell types forming the lung; however, the recent development of single cell RNA sequencing methods and advances in imaging are enabling new insights into the diversity of cell types, lineage relationships, cell-cell interactions, and gene expression patterns accompanying embryogenesis, organogenesis, and disease pathogenesis (109, 110, 175, 360, 377). After tissue digestion, single cells are readily purified by fluorescence activated cell sorting (FACS), microfluidics or in microdroplets from which RNA is prepared, barcoded, and amplified for next generation DNA sequencing. With the use of iterative hierarchical and graph-based clustering strategies, cells are statistically subclassified into major cell types and subtypes from lung samples from different developmental stages, as shown in FIGURE 2. Based on these newly defined cell types, we performed binomial or negative probability testing to identify differentially expressed genes and predict signature genes characteristic of each cell type (FIGURES 2 and 3). Cell types are readily clustered on the basis of RNA profiles that, together with antibody, lineage tracing, and in situ hybridization, validate cell type specificity provided by the transcriptomic data. Since multiple cell types interact and differentiate during organogenesis, distinct cell “states” of differentiation, proliferation, and cellular responses are evident, even within a seemingly homogeneous populations of cells. Sequential relatedness of cells is predicted statistically in “pseudotime” or ordered by single cell entropy. New computational approaches for lineage prediction from single cell RNA analysis provide insights into the kinetic properties of individual cells (109, 326). FIGURE 4 illustrates a model predicting the lineage trajectory of epithelial progenitor cells during mouse lung development using SLICE, an algorithm developed to determine cell differentiation and lineage based on single cell entropy (109). At E16.5 of mouse lung gestation, many epithelial cells are highly proliferative and remain relatively poorly differentiated. Pro-AT1 and pro-AT2 lineages have begun to differentiate and can be separated into differentiated AT1, AT2, and lesser numbers of “bipotential” progenitors, the latter expressing both AT1 and AT2 selective RNAs (67, 327). Lung formation and function depend on precise interactions and communications among diverse cell types that can be inferred by the combinatorial patterns of ligands, receptors, and transcription factors active in individual cells, providing insights into signaling processes among individual cells. Single cell RNA analyses can be utilized to predict cell type contributions to bulk RNA data available for the developing lung (74, 226).
FIGURE 2.
Single cell RNA analysis identifies multiple pulmonary cell types. A: four major cell types (left panel) and 18 subtypes (right panel) were identified by RNA analysis using “Drop-seq” of single cells (n = 8,090) from mouse lung at postnatal day 3 (PND3). B: heatmap shows the expression of the predicted cell type signature genes corresponding to cell type. Numerical values 3–18 represent cell clusters defined in A. C: hierarchical clustering reconstructs major lung cell types predicted from the RNA data. Endo, endothelial cells; Mesen, mesenchymal cells; Epi, epithelial cells; Immune, immune cells; FB, fibroblast cells; AT1, alveolar type 1 cells; AT2, alveolar type 2 cells; MyoFB, myofibroblast cells; SMC, smooth muscle cells; MatrixFB, matrix fibroblast cells.
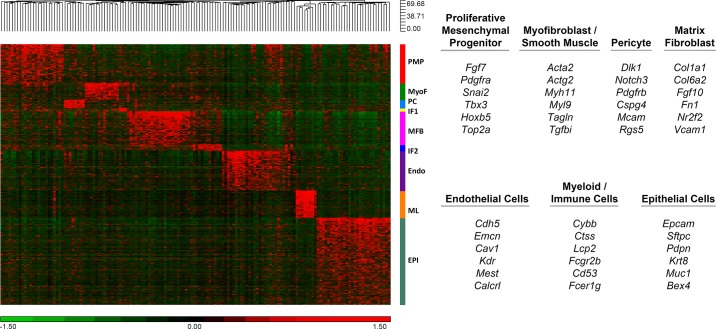
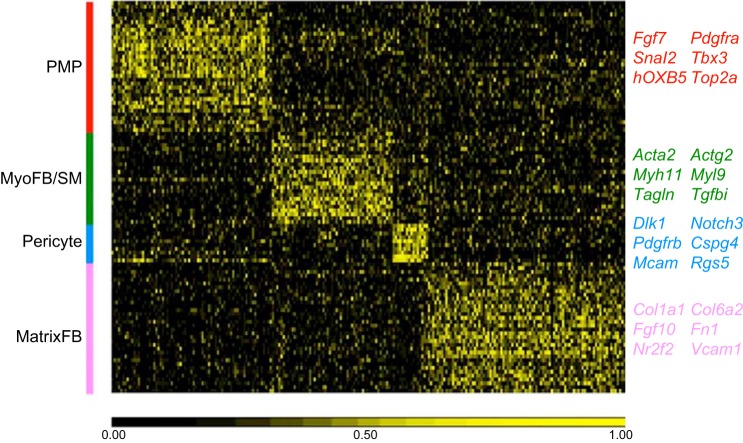
FIGURE 3.
Heatmap of gene expression patterns from single cell RNA-sequencing. Signature genes identifying major lung cell types were predicted from single cell RNA-seq analysis from E16.5 fetal mouse lungs and represented in 2D heatmaps (data are available at https://research.cchmc.org/pbge/lunggens/celltype_E16_p3.html). Nine major cell clusters are labeled with the color bar at the right side of the heatmap. PMP, proliferative mesenchymal progenitor; MyoF, myofibroblast; IF1, intermediate fibroblast 1; MFB, matrix fibroblast; Endo, endothelial cell; ML, myeloid cell; Epi, epithelial cell. Representative signature genes identifying seven major cell types are listed in the right panel.
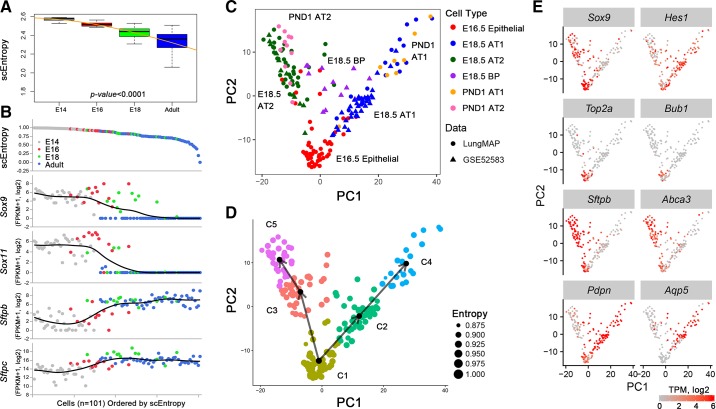
FIGURE 4.
SLICE reconstructs cell differentiation lineages using single-cell RNA-seq data. A: single cell entropy (scEntropy) of mouse alveolar type 2 (AT2, n = 101) cells decreased during the perinatal period. RNA data from epithelial cells from E14.5, E16.5, E18.5, and adult mouse lung (327) were used to compute entropy. B: the decrease in scEntropies of AT2 cells correlates with increased AT2 cell differentiation. Expression of early progenitor cell markers (Sox9 and Sox11) and mature AT2 markers (Sftpb and Sftpc) were used to validate the order predicted by scEntropies. C: predicted differentiation path of AT1 and AT2 cells from E16.5, E18.5, and postnatal day 1 (PND1) is shown. D: a branched differentiation model of AT1 and AT2 cell differentiation from bipotent progenitors was inferred using SLICE (109). E: an inferred differentiation model was produced using known cell selective marker genes.
IV. FORMATION OF THE TRACHEA AND LUNG BUDS IN THE EMBRYONIC PERIOD OF LUNG DEVELOPMENT (HUMAN, 3–6 WK PC)
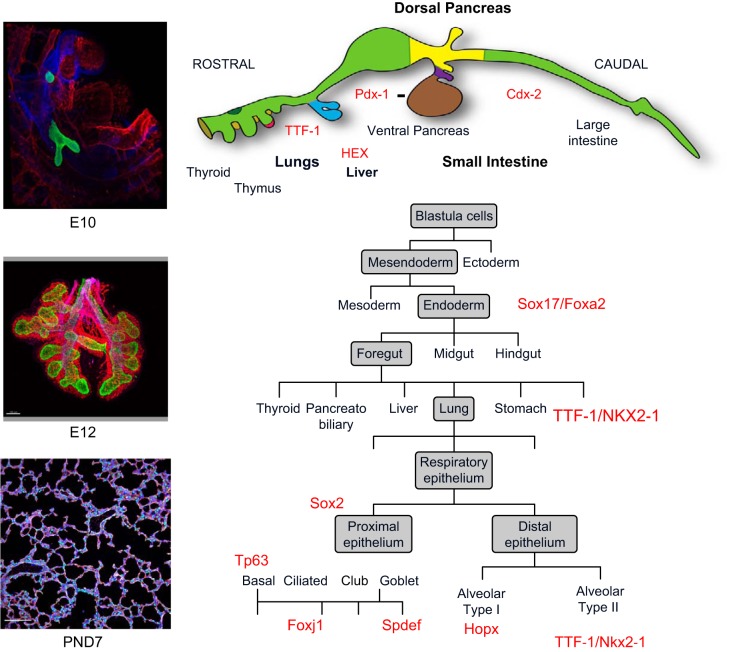
Formation of the lung begins with the early specification of the germ layers as the ectoderm, mesoderm, and endoderm are formed near the time of blastocyst implantation. Thereafter, sequential signaling among mesenchymal and epithelial cells directs transcriptional programs to produce foregut endoderm, identified by expression of SRY-related HMG box (SOX) 17 and forkhead box (FOX) A2 (385) (FIGURE 5). The embryonic period of lung morphogenesis, from 3 to 6 wk postconception (PC) in humans, begins as pulmonary progenitors from the anterior-ventral foregut endoderm are committed to lung epithelial lineages marked by NK2 homeobox 1 [NKX2–1; or thyroid transcription factor-1 (TTF-1)], a transcription factor critical for lung formation and epithelial differentiation (160). During the embryonic period, trachea, main stem, lobar, and segmental bronchi are formed and the trachea and esophagus separate. Lung progenitor cells are found in distinct conducting airway and acinar regions, their lineages committed to proximal or peripheral cell fates even before the appearance of lung buds or the tracheal stalk (256). Sequential and reciprocal signaling between the mesoderm and endoderm mediate growth and differentiation of the respiratory tubes into the splanchnic mesenchyme. Insights into the mechanisms involved in early lung development are provided by studies both in mouse and frog which implicate multiple cell-cell interactions via wingless-type mouse mammary tumor virus integration site (WNT), bone morphogenetic protein (BMP), retinoic acid (RA), sonic hedgehog (SHH), and fibroblast growth factor (FGF) signaling processes that collaborate in creating branched tubules (68, 71, 76, 123, 126, 205, 266, 310).
FIGURE 5.
Transcriptional network initiating pulmonary morphogenesis and differentiation. Lung buds and the tracheal stalk form between E9 and E10 from the ventral region of the foregut endoderm of the mouse embryo. The transcription factor TTF-1 (NKX2–1), shown at E10 (green), marks cells that form the initial lung buds. Transcription factors SOX17 and FOXA2 mark the differentiation of the early endoderm before lung bud specification. During branching morphogenesis (E12), epithelial cells migrate and proliferate to form the major conducting airways, indicated by expression of SOX2. SOX9 and high levels of TTF-1 mark peripheral acinar bud epithelial cells that will form the alveoli after birth. Alveolarization (shown at PN7) occurs from birth to ~28 days in the mouse, creating the extensive gas-exchange region typical of the mammalian lung. During the canalicular-saccular period of development, airway epithelial cell differentiation, influenced by the transcription factors in red, produce ciliated, basal, goblet, and club cells. Alveolar AT2 (TTF-1) and AT1 cells (HOPX) are derived from SOX9 expressing progenitors. [A part of this figure was created by Dr. John Shannon and used with permission. Another part of this figure, used with permission, was from Whitsett et al. (354).]
V. FORMATION AND EARLY BRANCHING OF THE EMBRYONIC LUNG
Signaling and transcriptional programs mediating the initial lung buds are highly conserved among vertebrates and are mediated by many of the same gene networks active during formation of other branched organs (266, 275). After differentiation of the germ layers and gastrulation, the initial endodermal tube that will give rise to the anterior and posterior foregut forms. Commitment to endoderm is dependent on the expression of FOXA1, FOXA2, and SOX17 (FIGURE 6, A and B). The anterior foregut cells, expressing SOX2, T-box (TBX) 1, and paired box (PAX) 9, are distinct from the posterior region where high levels of hepatocyte nuclear factor 1-β (HNF1β), GATA binding protein 6 (GATA6), caudal type homeobox 2 (CDX2), and pancreatic and duodenal homeobox 1 (PDX1) define the gut tube primordia (385). Endodermal cells lining the foregut tube are surrounded by mesodermal cells of the splanchnic mesenchyme, septum transversus mesoderm, and dorsal mesenchymal protrusion from which critical instructive signals establish the dorsal and ventral domains of the foregut. Specification of the dorsal region depends on the inhibition of BMP signaling, mediated by the expression of Noggin from the notochord, enabling endodermal expression of SOX2 in the dorsal esophageal domain. Conversely, high levels of BMP, RA, and expression of WNT2/2b by the ventral mesenchyme induce ventral expression of NKX2–1 within the lung buds. RA plays diverse and critical roles in the mesenchyme to activate paracrine networks dependent on SHH signaling from the endoderm to mesenchymal targets including glioma-associated oncogene family zinc finger (GLI) 1/2/3, FOXF1, and TBX family members. The specificity and outgrowth of the primordial lung bud depends on mesenchymal WNT4 (45); regulating WNT2/2B, BMP4, and HOX family members; and the expression of FGF10. At the onset of branching morphogenesis (128), WNT, BMP, and FGF10 gradients are regulated by TBX family members, FOXF1, GATA6, and odd-skipped related transciption factor 1 (OSR1) in the mesenchyme, creating signaling centers required for the outgrowth of the tracheal stalk and primordial bronchi (9). Prior to separation of the trachea and esophagus, high levels of SOX2 are expressed by the dorsal, esophageal region of the common foregut tube. Following separation of esophagus and lung, SOX2 expression is established in conducting airways, whereas the most distal epithelial regions express SOX9 and inhibitor of DNA binding (ID) 2 (45, 259, 267). During the embryonic and pseudoglandular period, airways branch and SOX9 expressing cells in the peripheral bronchial tubules proliferate and migrate. Branching of the lung tubules is controlled in part by the refinement of FGF10 signaling by mesenchymal cells that signal to the epithelial cells expressing FGFR2. BMP, WNT, and RA signaling define the FGF10 gradients upon which branching depends (FIGURE 6C). A paracrine feedback loop regulated by ETS variant 5 (ETV5) controls the periodicity of branching (71). ETV5 regulates SHH production in epithelial cells, in turn, activating GLI1,2,3 in mesenchymal cells that inhibits FGF10 production by the mesenchyme, thus limiting responses by the epithelium. During later stages of branching morphogenesis, SOX9/ID2 defines the acinar buds that will form the alveoli. Recent studies in the human support the presence of bi-potential SOX2/SOX9 expressing epithelial cells located between conducting and peripheral region of the tubules (62, 242).
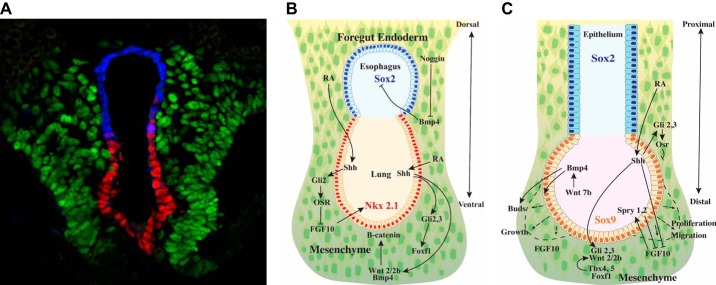
FIGURE 6.
Differentiation of the embryonic foregut endoderm. A: foregut endodermal cells respond to bone morphogenetic protein 4 (BMP4), sonic hedgehog (SHH), and fibroblast growth factor (FGF) signaling along the dorsal-ventral axis of the common esophageal (SOX2 in blue) and lung (NKX2–1 in red) tubules. The tubules migrate into the splanchnic mesenchyme (FOXF1 in green). B: Noggin, from the notochord, inhibits BMP4, maintaining SOX2 expression in esophageal cells. Retinoic acid mediates SHH signaling that activates Gli2/3 in the splanchnic mesenchyme, activating Wnt2/2b and BMP4 that maintains NKX2–1 expression in the epithelium required for lung specification. Maintenance of the lung bud requires FGF10 produced by the mesenchyme and β-catenin signaling in the epithelium that regulate the patterning of the mesenchyme (green) and the epithelium (red). C: complex paracrine signaling regulates branching morphogenesis. During the embryonic to canalicular periods of lung development, respiratory epithelial cells migrate and proliferate as airways and peripheral acini are formed. Epithelial cells from the peripheral lung buds proliferate and migrate in response to FGF10 gradients produced by the mesenchyme that are counterregulated by Spry1,2 to limit proliferation. FGF, WNT, SHH, and BMP signaling regulates growth and patterning of the lung buds in a transcriptional network by which ETV5 regulates SHH in the epithelium, activating Gli2/3, FoxF1, and TBX proteins in the mesenchyme, to control expression of Wnt2/2b and FGF-10. Retinoic acid influences SHH and renders the endoderm responsive to NKX2–1. After separation of trachea and esophagus, SOX2 is re-expressed in conducting airways. SOX9 marks peripheral acinar cells that ultimately differentiate into AT1 and AT2 cells to form the alveoli. (A courtesy of Dr. Aaron Zorn, used with permision.)
VI. BRANCHING MORPHOGENESIS: PSEUDOGLANDULAR PERIOD OF LUNG DEVELOPMENT (6–17 WK PC)
The process of branching morphogenesis is shared throughout the plant and animal kingdoms and is exemplified by the remarkably branched structure of the mammalian lung. Changes in lung growth and architecture that occur during branching morphogenesis, sacculation, and alveolarizaion are shown in FIGURE 7. Branching morphogenesis occurs primarily in the late embryonic and pseudoglandular period, the latter from 6 to 17 wk in the human lung and from embryonic day (E) 12–16 in the mouse (220). The rapid advances in knowledge regarding the molecular and cellular networks regulating branching morphogenesis of the lung were recently reviewed. Many of the genes and processes involved in growth of the lung bud are reutilized during branching morphogenesis (126, 310). FIGURE 6B provides a schematic of some of the many genes involved in branching morphogenesis. While branching of tubular structures has been actively studied by anatomists for centuries, present models include 1) mathematical modeling defining the fractal dimensions of branching; 2) reaction-diffusion gradients mediating cell responses to changes in concentrations of signaling molecules, their diffusion, or attachment to matrices which provide temporal and spatial information regulating cell proliferation, differentiation, and migration; and 3) directional stress forces creating branches based on biophysical models as tubes elongate within tissues of distinct densities (335). Pioneering anatomic work by Weibel and Gomez (349) provided data supported by a fractal model for the structure of the human lung with its 23 generations of dichotomous branches that lead to each alveolus. When this model was constrained by the pleura, diaphragm, and skeleton, mathematical rules predicted a structure that is highly similar to that of the human lung (162). Recent imaging studies demonstrated a remarkable diversity of branching strategies (termed domain, planar, and orthogonal bifurcation and branching) that contribute to the complexity of the developing mouse lung (195, 220, 284). Mathematical models can be reconsidered in the light of more recent data regarding signaling and transcription centers that determine the interactions between diverse embryonic lung cells, e.g., SHH, FGF, BMP, vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and WNT signaling pathways, to name a few. Temporal and regional expression of signaling molecules, their diffusion or assembly along matrices, and temporal and stochastic regulation of cellular responses, underlies the present Turing instability model of branching morphogenesis (225). Branching of the fetal lung is also influenced by the transmural pressure in the chest cavity and by smooth muscle contractions that influence the synchronization of branching events, linking biomechanical forces to the process of branching (237). HIPPO/YAP signaling regulates myosin light chain kinase activity, creating mechanical forces influencing cell shape required for branching morphogenesis (192). Noncanonical WNT signaling also influences cell shape and migration during branching morphogenesis (145). Complex airway branching is closely associated with growth of the pulmonary vasculature that occurs by angiogenesis and vasculogenesis, connecting the microcirculation of the peripheral lung with larger pulmonary arteries and veins that enter the heart (66). While the pulmonary vasculature is not required for initiation or branching of the embryonic lung buds (116), their survival and growth is dependent on the pulmonary circulation later in development. An extensive microvasculature is well established during the pseudoglandular/canalicular period of lung morphogenesis as formation of bronchioles and peripheral respiratory bronchioles is completed. During the canalicular period of development, epithelial cells differentiate and begin to produce the multiple cell types that line airways and peripheral acinar regions of the lung.
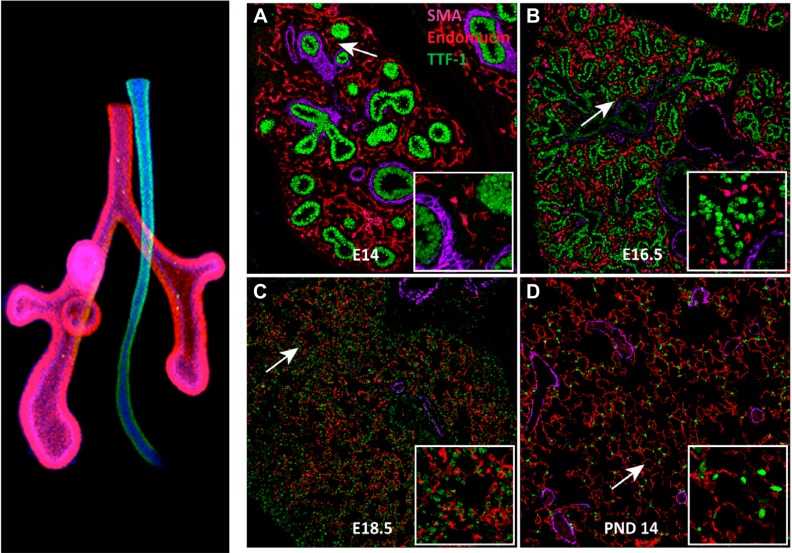
FIGURE 7.
Stages of branching morphogenesis. Left panel shows a posterior view of the mouse lung (red) and the esophagus (blue) at E10. Images of embryonic and postnatal mouse lung from E14 to the alveolar period on postnatal day 14 are shown in A–D. Major stages of lung morphogenesis are shared in the human and mouse. In A–D, lung epithelial cells (thyroid transcription factor-1, TTF-1) are shown in green, endothelial cells in red (endomucin), and smooth muscle myofibroblasts in purple (α-smooth muscle actin, SMA). Lung morphogenesis proceeds from a solid branched organ to the open alveolar structures after birth. Major conducting airways are formed by branching morphogenesis from the embryonic to canalicular period of fetal development. Sacculation and alveolarization are completed in the perinatal and postnatal period, creating the gas exchange region. Insets show higher magnifications. (Left image courtesy of Dr. Aaron Zorn, used with permission.)
VII. GENETIC DISORDERS AFFECTING BRANCHING MORPHOGENESIS
Congenital malformations of the lung are a relatively common cause of morbidity and mortality in newborn infants. Since lung function is not required for fetal development, but is critical for postnatal survival, infants with severe lung malformations usually present with respiratory distress or failure at the time of birth. Defects in lung formation are associated with mutations in genes that play important roles during lung branching morphogenesis, including transcription factors and signaling networks directing both epithelial and mesenchymal activities, and their interactions. Mutations disrupting the SHH pathway, e.g., Pallister-Hall, Smith-Lemli-Opitz (155), NKX2–1 (brain, thyroid, lung) (236), FOXF1 (alveolar capillary dysplasia) (288), TBX4 (acinar hypoplasia) (312), SOX2 (TE fistula and anophthalmia) (191), SOX9 (campomelic dysplasia) (127), and FGFR2 (lung and tracheal-bronchial malformation) are among severe malformations encountered in newborn infants related to genes critical for lung formation and branching morphogenesis. Loss of amniotic fluid, oligohydramnios, skeletal abnormalities affecting the rib cage and space filling lesions, e.g., congenital diaphragmatic hernia, embryonic tumors, and congenital pulmonary airway malformations, impair branching and can cause lung hypoplasia, presenting with respiratory distress following birth (311).
VIII. CONDUCTING AIRWAY EPITHELIAL DIFFERENTIATION DURING THE CANALICULAR-SACCULAR PERIOD OF DEVELOPMENT (16–36 WK PC)
In the mature human lung, conducting airways are lined by a pseudostratified epithelium consisting primarily of basal and ciliated cells, and lesser numbers of various secretory cells (FIGURE 8). The predominance of ciliated cells in the human contrasts sharply with the nearly equal abundance of secretory (club) and ciliated cells lining airways in the mouse. Secretory cells, including brush, goblet, club, and neuroepithelial cells, are present in varying numbers along the airways and submucosal glands. Distinct epithelial cells lining conducting airways are readily distinguished by their morphology, molecular signatures, and their functions which are regulated by cell specific transcriptional networks. Cartilaginous airways contain extensive submucosal glands, themselves formed by a diversity of epithelial cell types, including myoepithelial, basal, ciliated, goblet, and other secretory cells that produce fluid, electrolytes, mucus, and host defense proteins required for mucociliary clearance and innate defense. Differentiation and functions of airway epithelial cells become increasingly defined during the transition between the canalicular (16–26 wk PC) and saccular (26–36 wk PC) periods of human fetal lung development. The branched structure of the conducting airways, e.g., trachea, bronchi, and bronchioles, is completed during the canalicular period of development. During the canalicular-saccular period, distinct epithelial cell types differentiate and are distinguished by their morphology, expression of cell-selective RNAs, and proteins directed by cell selective transcriptional networks. In the human lung, cartilaginous airways are lined by a pseudostratified epithelium in which basal cells, variably expressing TP63, SOX2, and cytokeratins, e.g., KRT5 and KRT14. In the adult lung, basal cells serve as airway epithelial progenitors from which ciliated, goblet, secretory (club) cells, and other basal cells are derived in transcriptional networks dependent on SOX2 (FIGURE 8). In the mouse, most of the conducting airways are lined by various “club” and ciliated cells, club cells serving as airway progenitor cells that differentiate into ciliated and goblet cells. During lung morphogenesis, each epithelial cell type is located in precise anatomic positions and numbers, their differentiation being strongly influenced by levels of NOTCH signaling between neighboring cells.
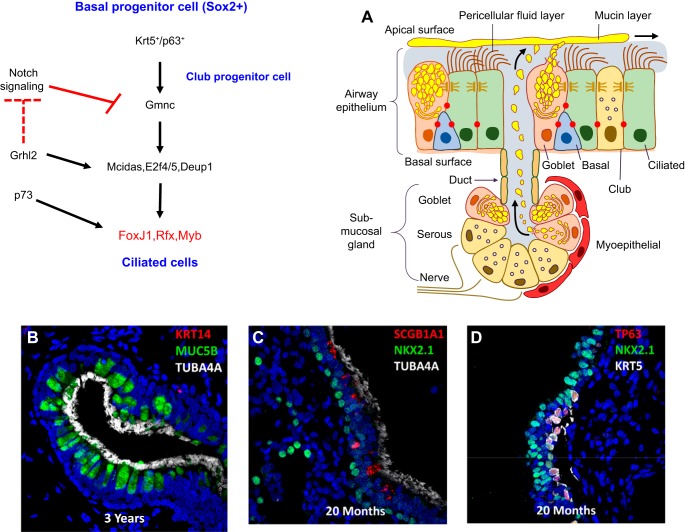
FIGURE 8.
Genetic networks regulating ciliated cell differentiation in conducting airways and submucosal glands. Basal and club progenitor cells differentiate into ciliated cells that line the majority of the conducting airways and the ducts of submucosal glands (A). In the absence of NOTCH, Gmnc is induced, activating a transcriptional network that directs centriole replication and the synthesis of the structural proteins forming motile cilia. Expression and assembly of ciliary proteins is regulated by Foxj1, and associated transcription factors, Rfx and Myb. B and C: confocal images of human lung. Ciliated cells are shown in the ducts of submucosal glands and along the conducting airways identified by TubA4A (in white). Club cells, marked by SCGB1A1 (red), are less abundant in human than mouse airways. Basal cells expressing TP63 and KRT5 or KRT14 (B and D) are progenitors of ciliated cells in the human airways. [A from Whitsett and Alenghat (353).]
A. Basal Cells Are Progenitors in the Conducting Airways
Basal cells serve as important progenitor cells in the adult conducting airways (FIGURE 8). In the absence of NOTCH activation, basal cells produce ciliated cells; differentiation of secretory cells (club) requires NOTCH, and high levels of NOTCH signaling cause goblet cell differentiation (227, 228, 295). Proliferation and differentiation of basal cells are regulated by interactions between FGF10 producing mesenchymal cells, the HIPPO pathway, and other factors that influence their proliferation and differentiation (178, 192, 202, 314, 338, 382).
B. Ciliated Cell Differentiation
Basal and secretory progenitor cells (e.g., club cells) differentiate into ciliated cells whose activities are critical for mucociliary clearance (FIGURE 8). Ciliated cells are readily recognized by the multiple cilia that are present on their apical surfaces. Generation of multiciliated cells such as those in mammalian airway epithelium requires remarkable amplification of centrioles that generate basal bodies for multiciliogenesis (364). While single duplications of centrioles are required for mitosis, massive centriole amplification that drives multiciliogenesis is initiated after exit from the cell cycle.
Amplification of the centrioles is mediated by a complex gene network, in which centrosomal protein (Cep) 63, deuterosome assembly protein 1 (Deup1), and Cep152 recruit polo-like kinase 4 to initiate centriole duplication and assembly (163, 318, 380). The transcriptional program regulating ciliated cell fate represents an ancient network of genes and proteins evolving from single-cell organisms. Inhibition of NOTCH activates a regulatory gene network that includes Gemc1 (also called Gmnc), multicilin (also called Mcidas), E2F4, Myb1, Rfx2, and FoxJ1, which together regulate a number of largely conserved structural proteins that comprise the cilia (7, 42, 69, 260, 308, 384). Basal cells expressing TP73 and TP63 are destined to become multiciliated cells expressing FOXJ1, a transcription factor required for the organization of the apical ciliary apparatus, and a direct target of TP63 and TP73 (207, 238). The evolutionarily conserved grainyhead like 2 (Grhl2) transcription factor promotes ciliated cell fate during airway regeneration by activating genes involved in multiciliogenesis. Grhl2 differentially regulates expression of upstream NOTCH ligands, activating NOTCH1, Jag1, and Jag2, but inhibiting NOTCH3, to influence ciliated cell fate through modulation of the NOTCH pathway (95, 96). A schematic of a hierarchical transcriptional network mediating ciliated cell differentiation is indicated in FIGURE 8. Individuals with biallelic nonsense mutations in the multicillin gene (MCIDAS) develop chronic lung disease caused by defects in mucociliary clearance (29). Ciliated cell differentiation mediated by multicillin is inhibited by TH2 cytokines independently of NOTCH signaling (100).
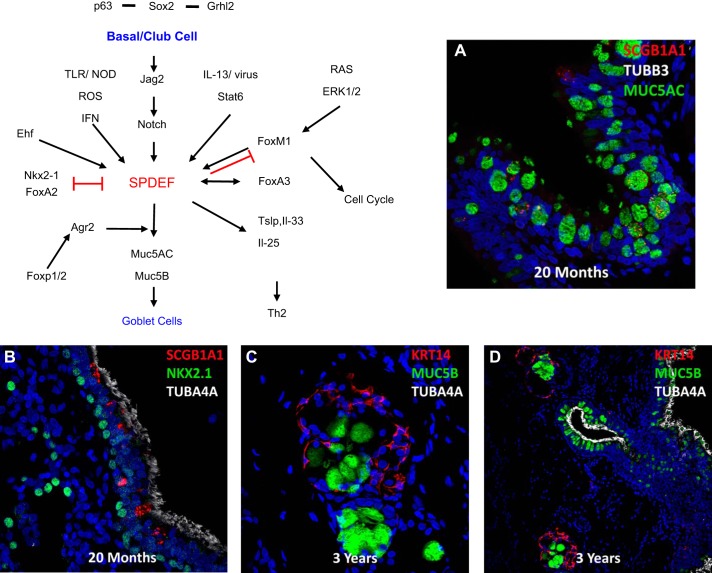
C. Goblet Cells
Goblet cells differentiate from basal and club cells and are recognized by their characteristic globular shape and accumulation of mucins that are secreted into the airway surfaces from ducts of submucosal glands and from goblet cells lining conducting airways (FIGURE 9). Goblet cells are prominent in human airways in the canalicular/saccular period of development and thereafter. The numbers as well as production and secretion of mucins and other innate defense molecules are highly responsive to environmental stimuli. Differentiation of goblet cells from basal or other secretory cells requires high levels of NOTCH signaling. Goblet cell differentiation is induced by toxicants, particles, pathogens, innate immune signals, and neural stimuli that influence mucus production and secretion. Goblet cells also influence responses to environmental stimuli by expression of cytokines and chemokines that recruit and educate innate immune cells, including dendritic, innate lymphoid (ILC2) cells, and eosinophils that contribute to Th2 immune responses typical of asthma (50, 263). Goblet cell metaplasia and mucus hyperproduction are characteristic of Th2-mediated inflammation, as well as non-Th2-mediated inflammation [e.g., in cystic fibrosis (CF), chronic obstructive lung disease (COPD), and idiopathic pulmonary fibrosis (IPF)]. As in other airway epithelial cells, differentiation of goblet cells is controlled by transcriptional networks that, in the airway, depend on the expression of SOX2 and Sam-pointed domain Ets-like factor (SPDEF), an atypical Ets family transcription factor, required for goblet cell differentiation in normal airways. Activation of SPDEF in club cells causes goblet cell metaplasia, rather than hyperplasia, and is rapidly reversible. SPDEF is dependent on SOX2, and its expression is controlled, at least in part, by NOTCH. Mucus metaplasia and expression of SPDEF respond to Th2 cytokines, including interleukin (IL)-4 and IL-13, via the activation of STAT6 and depend on FOXM1 (271, 309). Likewise, respiratory virus, e.g., rhinoviruses, activates SPDEF and FOXA3 during goblet cell metaplasia mediated by STAT1/2 signaling (49). While FOXA2 and NKX2–1 inhibit SPDEF and goblet cell differentiation, FOXA3 and FOXM1 induce SPDEF, activating its transcriptional targets, including mucin (MUC) 5AC and MUC5B, glycosyltransferases, ion transporters, and AGR2, all involved in the packaging of mucins, water and electrolyte transport critical for hydration of the airway surface, and mucociliary clearance (49, 50, 223, 263). A schematic of a gene regulatory network directing goblet cell differentiation is provided by FIGURE 9. Mucus hyperproduction plays an important role in the pathogenesis of common pulmonary disorders. Exposure to toxicants, particles, viral and bacterial pathogens, parasites, and allergens causes goblet cell differentiation and mucus hyperproduction that contributes to innate defense and mucociliary clearance, but complicates common acute and chronic pulmonary disorders (340). While NOTCH signaling plays an important role in goblet cell metaplasia, activation via JAK/STAT provides an alternative mechanism (370). Abnormalities in the hydration of the airways impair mucociliary clearance, causing mucus inspissation and airway obstruction, e.g., in CF, primary ciliary dyskinesia (PCD), COPD, and IPF (see Refs. 80, 353, 355 for review).
FIGURE 9.
Genetic networks regulating goblet cell differentiation. Goblet cells produce mucins, e.g., MUC5B and MUC5AC (green), in submucosal glands and airways (A–D). Basal and club cells expressing Sox2, TP63, and Grhl2 are the primary progenitors from which goblet cells differentiate in response to environmental, infectious, and inflammatory signals. Active NOTCH signaling and JAK/STAT, in part via STAT6, activates SPDEF (or Sam pointed domain Ets-like factor) that regulates gene expression and differentiation of goblet cells and production of mucins. Factors activating or inhibiting SPDEF and goblet cell differentiation are shown. Goblet cells express cytokines and chemokines regulating Th2 innate immunity in the lung.
While goblet cell metaplasia and hyperplasia represent a common response to airway and environmental injury, goblet cells and their major products, MUC5AC and MUC5B, the “gel forming mucins,” play direct roles in innate immune responses in the lung. In mice, SPDEF is required for Th2 sensitization of the airways to common allergens (263). Spdef gene deleted mice lack goblet cells in both submucosal glands and in the airways, and do not recruit dendritic cells, ILC2, or Th2 lymphocytes following allergen challenge (50, 263). In postnatal mice, SPDEF activates FOXA3 in airway cells causing mucus metaplasia and enhances expression of thymic stromal lymphopoietin (TSLP), IL-33, and IL-25 in a cytokine network regulating Th2 lymphocytic responses (49). Thus the goblet cell plays a direct role in establishing the innate immune system in the developing lung. Hypomethylation of the SPDEF gene locus may underlie the hypersecretion of mucus in COPD (298). MUC5B and MUC5AC are coexpressed or are independently expressed in goblet cells and in lesser amounts in club cells lining the airway epithelium (278). MUC5B is the most abundant gel-forming mucin produced by goblet cells in the submucosal glands from which it is secreted as organized rafts of MUC5B coated by MUC5AC (79, 247). Conducting airway epithelial goblet cells express both MUC5B and MUC5AC in response to inflammatory signaling. MUC5B is required for mucociliary clearance in the mouse. In mice, both airways and nasal passages fill with debris and inflammatory cells and are chronically infected in the absence of MUC5B (277). While MUC5AC is not required for mucociliary clearance, it plays an important role in allergic responses. Deletion of the mouse Muc5ac gene inhibits airway hyperreactivity (AHR), but does not influence inflammation to common allergens (81). Mucus hyperproduction, dehydration, and goblet cell metaplasia impair mucociliary clearance contributing to the pathogenesis of CF, IPF, and other chronic pulmonary disorders (80).
D. Club Cells
Club cells are relatively columnar, secretory cells whose numbers vary among mammalian species, during development, and along the proximal-peripheral and dorsal-ventral axes of the airways (138, 180, 208). In the mouse, peripheral regions of the conducting airways are lined by nearly equal numbers of ciliated and club cells in sharp contrast to the relative paucity of club cells in human and non-human primate (54) (FIGURES 8 and 9). Club cells express high levels of the cytochrome P-450 detoxifying enzyme CYP2F and innate immune proteins, including secretaglobins, SCGB1A1, SCGB3A1, lactoferrin, defensins, and surfactant proteins (SP-A, SP-B, and SP-D). Distinct subsets of club-secretory cells are located in selective niches near neuroepithelial bodies (NEBs), along the ducts of submucosal glands, and in the bronchoalveolar ductal regions. Variation in expression of SCGB1A1, SCGB3A1, and uroplakin (UPK3a) identify club cells with distinct capacities for proliferation and differentiation (261). UPK3a positive cells represent a relatively rare subset of airway secretory cells that proliferate and selectively differentiate into ciliated cells after injury. UPK3a positive club cells are prominent in the interstitial lung disorder neuroendocrine hyperplasia (106). Club cell differentiation begins in the canalicular period of development, with many club cell proteins, e.g., SCGB1A1, increasing with advancing gestation. Club cells proliferate and differentiate into goblet or ciliated cells in response to injury and other environmental signals. Club cells concentrate and metabolize a variety of chemical toxicants (e.g., naphthalene) that are metabolized to toxic compounds selectively killing subsets of club cells, a strategy commonly used to deplete airway club cells for study of airway epithelial regeneration in the mouse (180). Club cells express SOX2, NKX2–1, and a number of ETS family transcription factors, including ETS homologous factor (EHF) and E74 like ETS transcription factor 3 (ELF3), as well as FOXA1, FOXA2, and FOXP1/2. Like basal cells, proliferation and differentiation of club cells are influenced by NOTCH and SOX2 (FIGURES 2, 8, and 9). A list of club cell signature genes is shown in FIGURE 2.
E. Neuroendocrine Cells and Neuroepithelial Bodies
Neuroendocrine (NE) cells represent <1% of the epithelial cells lining the human airways and are readily identified by gene expression patterns and their innervation (FIGURE 1). While recognized by their distinct morphology, developmental origins and functions of NE cells and NEBs have remained relatively mysterious. NE cells in the mature lung are innervated and primarily found as groups of cells, termed neuroendocrine bodies (NEBs), which are usually located near airway branch points, perhaps at sites of low airflow where stasis enhances sensory inputs from inhaled gases. NE cells are identified by expression of distinct peptides and chemical markers, including calcitonin gene-related peptide (CGRP), serotonin, gastric releasing peptide, and others that are localized in secretory granules in the cytoplasm of NE cells. NE cells are found as isolated cells in conducting airways during the canalicular and pseudoglandular periods of embryonic lung development. Later in development, NE cells cluster and form NEBs that are “capped” by subsets of airway secretory club cells. NEBs are formed by directed migration of NE cells to airway branch points, where they serve as sensors for oxygen, CO2, and other environmental stimuli (60, 175, 243). Migration of neuroepithelial cells is controlled by Robo-Slit. Slit, expressed in airway smooth muscle cells, interacts with CGRP+ epithelial cells expressing Robo1/2 (33). As in other airway epithelial cells, NE cell differentiation is controlled by the NOTCH pathway; loss of Hes1 induces NEB differentiation from endodermal precursors (139). NEBs serve as airway sensors, and their clustering is required for appropriate innate immune inflammatory responses (33). NEBs signal via neurons in the carotid body, to coordinate responses to oxygen, pH, and CO2. NEBs respond to hypoxia via hypoxia inducible factor 1α (HIF1α) to activate K+ channels causing secretion of neuropeptides (e.g., CGRP) that influence inflammation (see Ref. 60 for review). CGRP inhibits the release of proinflammatory chemokines and cytokines to regulate airway mucus production after birth (33). NEBs are conserved across animal phyla and tissues, likely representing an ancient mechanism for environmental sensing that integrates epithelial receptors with innate immunity. Neuroendocrine hyperplasia of infancy (NEHI) is a chronic disorder causing interstitial lung disease in infants and children. While the genetic causes underlying NEHI are poorly understood, heterozygous mutations in NKX2–1 were associated with NEHI in a single family (240). Increased numbers of NE cells are associated with a wide range of congenital and infantile lung disorders.
F. Brush or “Tuft” Cells
Brush (also called Tuft) cells were recognized decades ago in ultrastructural studies of intestinal, nasal, and airway epithelial cells (167). Brush cells are found in multiple organs, e.g., trachea, pancreas, and intestine (98). Brush cells share a dense, apical microtubular network attached to filamentous “brushlike” microvilli that extend onto the airway surface. Cell selective markers are used to identify brush cells, including α-gustducin, TRMP5, and DCKL1. The transcription factor POU2F3 is required for formation and differentiation of Tuft (brush) cells (98). While the functions of airway brush cells are not known, recent evidence from studies in the intestine support the important role of these cells in sensing microorganisms and parasites. Intestinal Tuft cells may serve as “taste” sensors, responding to helminths and other cellular products released by parasites. Brush cells secrete IL-25 which recruits and activates ILC2 innate immune cells (99) to influence production of IL-13 and other cytokines required for expulsion of parasites from the intestine. Whether innate immune functions of airway brush cells influence the pathogenesis of chronic lung diseases will be of further interest.
IX. DIFFERENTIATION OF THE ALVEOLAR EPITHELIUM IN THE SACCULAR PERIOD (26–36 WK PC)
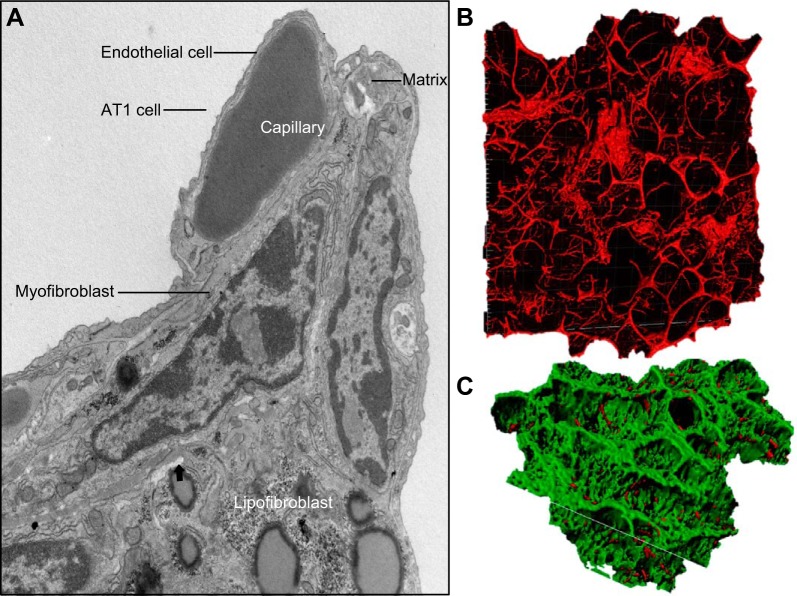
The function of the gas exchange region of the lung at birth is dependent on the creation of extensive alveolar epithelial surface created primarily by squamous AT1 cells, which forms in close apposition to capillary endothelial cells of the pulmonary microcirculation. The remarkable efficient exchange of oxygen and CO2 is accomplished at the interface between the AT1 epithelial cells and capillary endothelial cells (365) (FIGURE 10). In the mature lung, the alveolar surface consists primarily of AT1 cells (~95% of the surface area), the remaining covered by AT2 cells, which produce surfactant lipids and proteins required to reduce surface tension between alveolar gases and the hydrated epithelial cell surfaces of the alveoli (353, 354). The terminal respiratory ducts and alveoli are supported by an extensive, elastin- and collagen-rich network, which is produced primarily by diverse fibroblasts and myofibroblasts. This flexible scaffold enables the dynamic expansion and compression of alveoli during the respiratory cycle (FIGURE 10). While the signaling and transcriptional mechanisms controlling differentiation of AT1 and AT2 cells are far from complete, single cell transcriptomic and lineage tracing are providing increasing clarity to the process of alveolar epithelial cell proliferation and differentiation (FIGURE 10).
FIGURE 10.
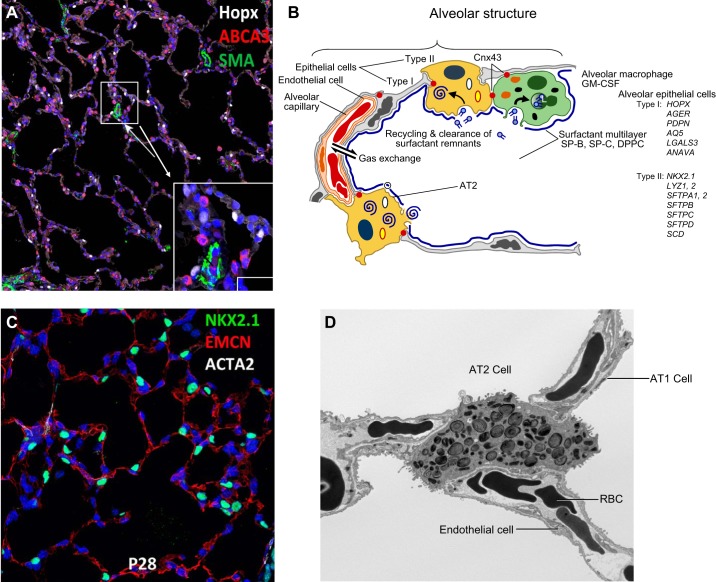
Transcriptional networks regulating differentiation of alveolar epithelial cells during the saccular stage of mouse lung development. A: NKX2–1 (aka TTF-1, green) identifies epithelial cells in acinar tubules at the canalicular stage of mouse lung development (E16.5). Endothelial cells of the pulmonary microvasculature are shown by EMCN (red) (A and B). NKX2–1 (blue-green) identifies AT2 cells in mouse alveoli at PN28 (B) at which time alveolar capillary networks are stained by EMCN (red) (C). AT1 (AGER in red) and AT2 cells (NKX2–1, green) are shown in human lung tissues at 4 mo of age. Acta2 (white) stains myofibroblasts seen at alveolar tips (C). Transcription factors influencing AT2 and AT1 differentiation are shown in the schematic.
Progenitor cells that form the lung periphery are specified from those lining conducting airways in the embryonic lung before the emergence of the lung bud from the foregut endoderm (256). These cells represent the progenitors that form the peripheral acinar buds that are highly proliferative during branching morphogenesis and sacculation. In the latter third of embryonic lung development, the peripheral tubules dilate, the pulmonary mesenchyme thins, and capillaries form in closer contact to the lung saccules. During the saccular (26–36 wk PC) period, epithelial cell progenitors differentiate to produce AT1 and AT2 cells. Single cell RNA studies support cell lineage models in which subsets of AT1 and AT2 cell progenitors are distinguished early in morphogenesis. Later in the canalicular-saccular period, a subset of “bipotent” progenitors proliferate and produce both AT1 and AT2 cells (67, 91, 109, 327) (FIGURE 4). Differentiation of AT1 and AT2 cells becomes more established in the early postnatal period. As postnatal alveolarization proceeds, mature AT2 cells serve as the primary epithelial progenitors. Cell turnover is remarkably low in the normal mature lung consistent with low rates of epithelial proliferation (91). Recent studies demonstrate that a subset of Axin2+, WNT-responsive AT2 cells are important contributors to alveologenesis and to regeneration of the mature alveoli after injury (91, 234, 375). Likewise, epidermal growth factor receptor (EGFR) and FGF receptor (FGFR) signaling pathways play an important role in AT2 progenitor cells proliferation (67). Proliferative capacity is maintained in AT2 cells after birth, while AT1 cells are essentially non-proliferative even after injury (15). Some of the signaling and transcriptional networks controlling alveolarization and differentiation of AT2 cells in the saccular-alveolar transition are shown in FIGURE 10 (361, 362).
AT2 cells produce pulmonary surfactant lipids, primarily phosphatidylcholine, and surfactant proteins which reduce alveolar surface tension, preventing atelectasis during the respiratory cycle (354) (FIGURE 11). Since survival of preterm infants depends on surfactant activity, the control of AT2 cell maturation has been of considerable clinical interest. AT2 cells synthesize and recycle surfactant lipids, predominately palmitoyl-phosphatidylcholine, and surfactant proteins SFTPA, SFTPB, SFTPC, and SFTPD, each protein serving innate immune, biophysical, and regulatory functions (353, 354). As in early lung morphogenesis, AT2 cell differentiation is dependent on NKX2–1 which directly activates expression of surfactant proteins, lipid synthetic enzymes, and genes controlling electrolyte and water balance (64). NKX2–1 expression is regulated by the noncoding RNA NANCI that is required for normal AT2 cell differentiation (121). Disruption of Nkx2–1 in the mouse caused respiratory failure and decreased expression of a network of genes involved in perinatal lung adaptation to air breathing (64). NKX2–1, FOXA family members, KLF5, GATA6, STAT3, ETV5, FOXM1, and FOXP2 interact in gene networks active during the differentiation of AT2 cells (148, 362). Sterol regulatory element-binding protein (SREBP) and NKX2–1 interact to regulate surfactant lipid homeostasis, (e.g., ABCA3, LPCAT1, and SLC34a2) (21, 22). NKX2–1 mediates surfactant protein gene expression and the proteolytic enzymes which process the proprotein to the active SP-B and SP-C peptides required for surfactant function at birth. Since the maturation of AT2 cells occurs relatively late in gestation, preterm infants frequently suffer from lung disease caused by surfactant deficiency. Exogenous surfactant replacement preparations are used to provide surfactant lipids and proteins (SP-B and SP-C) that support the infant until endogenous surfactant synthesis by AT2 cells is sufficient to maintain ventilation after birth.
FIGURE 11.
Structure of the alveolar gas-exchange region. A: gas exchange is facilitated by creation of a vast surface area lined primarily by AT1 cells (HOPX, in white) and AT2 cells (ABCA3 in red) or NKX2–1 in green (C). AT2 cells synthesize and secrete pulmonary surfactant lipids and proteins that reduce surface tension in the alveoli after birth. SMA (green) marks vascular smooth muscle. B: surfactant components are secreted into the alveoli and recycled by AT2 epithelial cells. Surfactant is catabolized by alveolar macrophages by processes regulated by GM-CSF. Proteins selectively expressed by AT1 or AT2 cells are listed on the right of the panel. D: an electron micrograph demonstrates lamellar bodies containing surfactant lipids and proteins in AT2 epithelial cells. AT1 cells line the majority of the alveolar gas exchange surface, coming into close contact with endothelial cells in the pulmonary microvasculature to facilitate transport of O2 and CO2 to red blood cells (RBC). [Modified from Whitsett and Alenghat (353).]
A. Genetic Disorders of Surfactant Homeostasis
Advances in molecular biology and genetics have identified a number of gene mutations causing acute and chronic interstitial lung diseases in newborn infants and children. Mutations in genes critical for surfactant homeostasis, including ABCA3, SFTPB, SFTPC, NKX2–1, filamin A, and SLC34a (a phosphate transport disorder causing alveolar microlithiasis), cause respiratory failure or chronic lung disease (236, 292, 300, 354).
B. Differentiating Induced Pluripotent Stem Cells Into Pulmonary Epithelial Lineages
The groundbreaking work of Yamanaka and colleagues (316) demonstrating reprogramming somatic cells into multiple cell lineages provides the framework for the recent studies, seeking to differentiate induced pluripotent stem cells (iPSCs) into endoderm foregut, and then into pulmonary cell types. Knowledge of the signaling and transcriptional regulators of foregut and pulmonary differentiation was applied to produce both conducting airway and peripheral epithelial cells (32, 53, 75, 130, 131, 137). Protocols directing pulmonary cell type differentiation begin with the formation of definitive endoderm marked by expression of FOXA2 and SOX17. Endodermal differentiation is induced by exposure of pluripotent stem cells to activin A and Wnt3a. Inhibition of BMP signaling, via Noggin or with SB431542, causes foregut differentiation. Precisely timed treatment with BMP4 and WNT3a produces a mixture of both thyroid and pulmonary foregut lineages. Cell labeling with SOX2-green fluorescent protein (GFP) or NKX2–1-GFP can be used to purify cells differentiating into lung or thyroid lineages (166, 302). RNA profiles from lung-directed iPSCs share similarities to normal airway cells and form organoids in Matrigel. “Organoids” from iPSCs can be grown in vitro and implanted in vivo for study of lung morphogenesis, disease, and drug testing. Similarly, organoids formed with epithelial cells from lung tissues are useful for study of cell-cell interactions and functions (242). These remarkable advances in cell biology enable the generation of patient-specific pulmonary cells and creation of mutation specific lung cells from iPSCs, e.g., by gene editing, for study of disease pathogenesis.
C. Noncoding RNAs in Lung Morphogenesis and Disease
There is increasing recognition that noncoding RNAs play pleotropic and important roles in regulating cellular processes, including pulmonary formation and disease (reviewed in Refs. 4, 59). MicroRNAs (miRs) are small, generally <22 nucleotides, noncoding RNAs that mediate a diversity of transcriptional processes, primarily by suppression of RNA translation. miRs are produced from larger precursors by the actions of Dicer and Argonaute that mediate the cleavage and maturation of the miRs. Dicer plays a critical role in lung morphogenesis. Mutations in Dicer underlie the pathogenesis of pulmonary pleuroblastoma (122). A number of miRs have been identified that play roles in lung morphogenesis. For example, miR-302/367, a target of GATA6, regulates the expansion of epithelial progenitor cells in the prenatal lung. Similarly, miR-326 is a regulator of SHH signaling (140). miR-200 regulates TTF-1 and surfactant protein and lipid homeostasis in the perinatal lung (19). A number of long noncoding RNAs (lncRNAs) are located near genes critical for lung develoment, including GATA6, FOXA2, NKX2–1, and FOXF1 (120, 121).
X. REPAIR OF THE RESPIRATORY EPITHELIUM
While epithelial cell turnover and proliferation in the conducting airways, alveoli, and submucosal glands are quiescent in the normal lung, the respiratory epithelium is capable of rapid and extensive regenerative responses following injury (67). The important role of airway basal and alveolar AT2 cell in repair of the respiratory epithelium was well-recognized decades ago (82–84), wherein pulsed DNA labeling studies identified proliferative basal and AT2 cells and their subsequent differentiation after injury. The lung is constantly exposed to toxicants, particles, microbial pathogens, and mechanical trauma which cause acute and chronic epithelial cell injury. Loss of epithelial cells is followed by rapid cell migration that maintains cell junctions and barrier functions. Proliferation and redifferentiation of progenitor cells then restores lung homeostasis. Diverse “stem” and progenitor cells are present in distinct niches throughout the lung that mediate region-specific and graded responses to injuries (FIGURE 12). Some subsets of progenitor cells are intrinsically resistant to injury or are located in protected sites. Capacities for proliferation and differentiation vary greatly among epithelial subtypes. In general, AT1, goblet, and ciliated cells are not proliferative, while AT2 cells in the alveoli, and basal and secretory (club) cells in the conducting airways, serve as multipotent progenitors. A number of recent reviews link developmental and molecular processes with lung regeneration (58, 76, 126, 166, 319, 344). Many of the signaling and transcriptional programs regulating lung epithelial development are reutilized during repair of the respiratory epithelium. Consistent with the processes mediating lung morphogenesis, repair depends on interactions of epithelial progenitor cells with multiple cell types, including fibroblasts (52) as well as smooth muscle (337), immune (43, 181, 334), and endothelial cells (43, 182, 183, 377). In chronic pulmonary disease, failure of normal epithelial repair causes tissue remodeling, disrupts interactions among diverse cell types, and causes inflammation, fibrosis, and epithelial hyperplasia/metaplasia.
FIGURE 12.
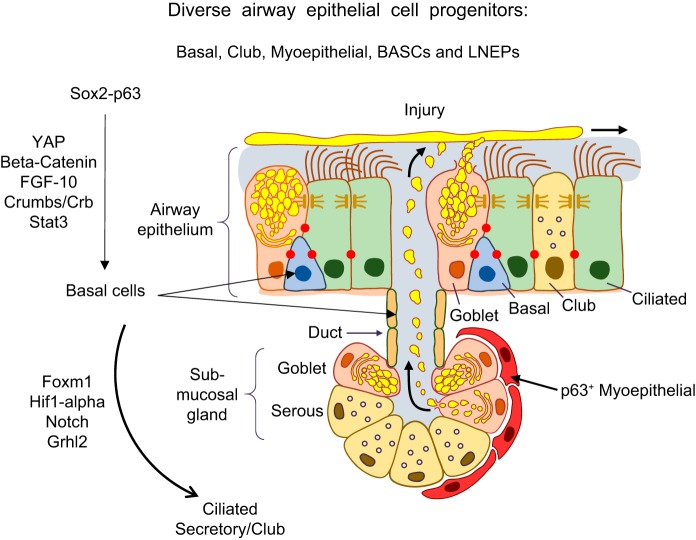
Diverse progenitor cells repair the conducting airway epithelium. The conducting airway surface and submucosal glands are shown. Basal cells (SOX2, TP63) are the primary progenitor-stem cells in the pseudostratified regions of the conducting airways. Basal cells in the ducts of submucosal glands and myoepithelial cells in the submucosal glands also serve as progenitors from which ciliated, basal, and secretory cells are produced. Secretory (club) cells also serve as proliferative progenitors following injury. Signaling and transcriptional networks regulating cell migration, proliferation, and redifferentiation following lung injury are shown. Bronchoalveolar stem cells (BASCs) and lineage negative epithelial progenitors (LNEPs) represent additional progenitor cells types (not shown).
A. Repair of the Conducting Airway Epithelium
While basal and secretory cells are the primary progenitor cells that proliferate and differentiate after epithelial injury, there is increasing evidence for region-specific diversity of airway progenitor cells of varying proliferative and differentiation capacities (FIGURE 12). Basal cells, located below the surface of the epithelium, are relatively resistant to injury. A diversity of basal cells is found in distinct niches along the airways and within submucosal glands. Basal cells are able to rapidly proliferate, migrate, restore barrier function, and differentiate into ciliated or secretory cells. Distinct basal cell types are identified by varied expression of cytokeratins, e.g., KRT8, KRT5, and KRT14, and key transcription factors TP63, SOX2, TTF-1, along the airways and in submucosal glands (30, 117). Cytokeratin 5 expressing myoepithelial cells in the submucosal glands can serve as multipotent progenitors which differentiate into multiple epithelial cell types, including serous, mucus, and ductal cell types (5). Submucosal gland progenitors are WNT responsive and may have greater capacity for regeneration of the airway epithelium than some airway basal progenitors (198). SOX2 regulates TP63 in basal cells and is required for airway epithelial proliferation and redifferentiation during repair. A number of signaling networks regulating epithelial cell proliferation are activated following injury (FIGURES 12 and 13). For example, signaling from EGF-R (34, 58), PDGFRA (52), FGF (183), WNT (198), BMP4 (182), and HIPPO/Yes-associated protein (YAP) (178) are active during repair of the airway epithelium. Transcription factors, β-catenin (198), p53, SOX2 (268), GATA6 (379), GRHL2 (95), STAT3 (156, 315), CEBPα (281), and FOXM1 (330, 343) interact to activate then inhibit the cell cycle, enabling the precise temporal sequence of cell migration, proliferation, and redifferentiation. Restoration of cell polarity is also critical for epithelial repair. Proteins involved in planar polarity, for example, HIPPO/YAP and cell polarity complex component (CRUMBS), influence progenitor cell differentiation and repolarization during development and repair (61, 202, 314). As during lung morphogenesis, NOTCH signaling plays diverse and important roles in airway repair. Basal cells with high NOTCH activity produce secretory cells, while those expressing MYB, differentiate into ciliated cells during airway repair (248).
FIGURE 13.
Intracellular signaling during repair of the respiratory epithelium. The respiratory tract is constantly exposed to microbial pathogens, particles, and toxicants and is capable of remarkable regeneration to maintain and restore the lung structure and function following injury. Mucociliary clearance, barrier function, production of antimicrobial proteins, and robust innate and acquired immune systems protect the lung from injury. Epithelial cells, innate immune lymphocytes, macrophages, monocytes, lymphocytes, and neutrophils respond to pathogens with robust and diverse cytokine, chemokine, and growth factor expression to recruit inflammatory cells, in turn, enhancing lung repair. Paracrine signaling and direct cell-cell interactions among multiple cell types mediate responses to injury. A diversity of signaling molecules are generated during lung injury and repair which activate proliferation, migration, and differentiation to maintain epithelial barriers and restore lung function and homeostasis.
Lineage analysis and single-cell transcriptional studies have enabled the identification of an increasing diversity of “stem and progenitor” cells in the respiratory epithelium that influence both normal and abnormal repair of the airways and alveoli (see Ref. 166 for review). In addition to club, basal, and AT2 cells, known to be primary epithelial progenitors, “bronchoalveolar stem cells” (157), lineage negative epithelial progenitors (LNEPs) (359), TP63, and KRT5 positive basal stem cells (172, 386) are proposed as alternative epithelial progenitor cells activated during repair. Whether these diverse cells are distinct cell types in the quiescent lung or represent intermediate cell “states” of differentiating progenitor cells is unresolved at present. Airway cells are remarkably “flexible” or “plastic” and can migrate, transdifferentiate, and redifferentiate rapidly during regeneration (122, 211, 319). The severity and chronicity of injury impacts the repair process in both conducting and peripheral airways and alveoli. While complete repair often occurs after time-limited injuries, continued epithelial injury may fail to restore homeostasis, as seen in severe influenza A or after repeated epithelial injury caused by conditional diphtheria toxin (255) or bleomycin (72). Repeated injury leads to failed repair and loss of normal lung architecture (336). After severe lung injury, SOX2/KRT5/TP63 basal cells migrate and proliferate forming alveolar “pods.” These abnormal basal cells fail to reestablish normal alveolar cell differentiation and likely represent scar tissue that does not restore lung function. Proximal airway cells migrate into the alveolar parenchyma in the human disorders, such as IPF (268). In a mouse model of influenza A infection, HIF-1α and NOTCH interactions mediate basal cell expansion into the alveoli (359). In IPF, remodeled regions of the lung parenchyma are lined by diverse and abnormal conducting airway epithelial cells. Single-cell transcriptomic studies revealed abnormal gene expression characteristics typical of proximal rather than alveolar epithelial cell types in IPF (360).
B. Paracrine and Cell-Cell Interactions Regulating Lung Repair
Acute lung injury, whether related to infection, toxicants, or barotrauma, activates multiple inflammatory responses to induce innate and acquired immunity, remove tissue debris, and initiate regenerative programs to restore lung structure and function (23, 165) (FIGURE 13). Many of the cellular and genetic responses active in formation of the lung parenchyma are reutilized during regeneration after birth. The maturation of the innate immune system adds to the complexity of cell-cell interactions that are active during repair of the lung. Injury elicits signals that recruit and activate cells from the hematopoietic system. Macrophages, monocytes, and dendritic cells all play important roles in the clearance of pathogens and tissue debris (see Ref. 165 for review) (FIGURE 13). Distinct subpopulations of macrophages colonize the lung during development, each contributing distinct innate immune functions (317). Alveolar macrophages, monocytes, innate lymphocytes (ILCs), and neutrophils are recruited to the lung during infection and injury, and produce a wide variety of growth factors, chemokines, and cytokines that interact with lung epithelial cell receptors to enhance repair (24, 133, 165, 241). M2-like macrophages and ILC2 cells expressing IL-13 are recruited to the lung following unilateral pneumonectomy in response to C-C motif chemokine ligand 2 (CCL2) and C-C motif chemokine receptor 2 (CCR2) signaling, that activate Th2-like immune response required for full regeneration (181). During influenza infection, cytokines, including IL-6 and granulocyte-macrophage colony-stimulating factor (GM-CSF), recruit and activate macrophage phagocytosis (136). IL-6 enhances transforming growth factor (TGF)-β production, suppressing fibroblast proliferation (366). Following lung injury, GM-CSF, produced by AT2 cells in response to tumor necrosis factor (TNF) and hepatocyte growth factor (HGF), activates differentiation and recruitment of lung monocytes and dendritic cells that protect the lung during infection (276). While alveolar macrophages play important paracrine roles by secreting innate immune regulatory molecules, direct interactions between AT2 cells and macrophages were demonstrated following TLR4 activation in the lung (24, 351). After lipopolysaccharide (LPS) exposure, alveolar macrophages form cell synapses with AT2 cells via connexin 43, establishing synchronized intercellular calcium transients that suppress inflammation via inhibition of cytokine and chemokine production, e.g., C-X-C motif chemokine ligand (CXCL) 1, MIP-1α, CXCL5, IL-1β, and IL-6. Direct transfer of mitochondria from microvesicles of bone marrow-derived stromal cells via connexin 43 increased AT2 cell ATP, mitigating mortality from LPS-induced acute lung injury (135).
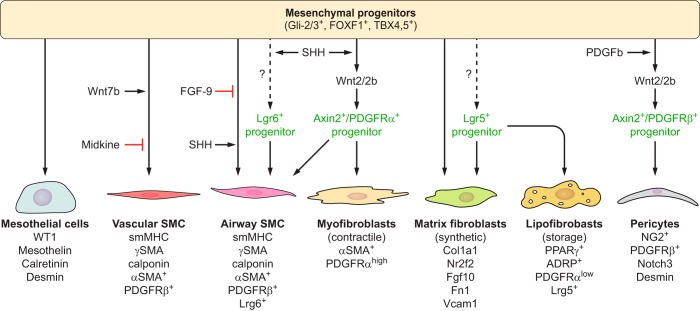
Recent studies are providing ever deeper insights into the complex interactions among regenerating epithelial cells, stromal and endothelial cells that interact via WNT, BMP, IL-6, FGF, and SHH signaling during lung regeneration. SHH is required for maintenance of normal lung homeostasis, being produced by the epithelium and signaling to the underlying mesenchyme to suppress myofibroblast remodeling (252). Recent single cell RNA analyses identified subsets of lung myofibroblasts and stromal cells and their complex interactions with bronchiolar and AT2 cells, functioning within distinct mesenchymal niches that influence normal and pathological repair. Subsets of mesenchymal cells expressing WNT2 and PDGFR2 activate WNT signaling in Axin2+ AT2 cells to enhance repair. Axin2+ cells serve as alveolar progenitor cells activated by WNT signals in both developing and mature lung (234, 375). Similarly, WNT responses in mesenchymal cells (Axin2+ myofibrogenic precursors) contribute to myofibroblast differentiation and pathological tissue remodeling following injury of the airways (377). These mesenchymal-alveolar niche cells respond to injury by activating IL-6, BMP, and FGF signaling to promote alveolar repair and expression of FGF-10. Similarly, the expression of WNT from airway progenitor cells to subsets of Lgr6+ myofibroblasts produces FGF-10 critical for bronchiolar repair (183). Ablation of Lgr6+ smooth muscle cells impairs airway epithelial regeneration. A distinct Lgr5+ subset of mesenchymal cells in the alveoli produces Wnt3a and Wnt5a, enhancing alveolar differentiation and repair. Inhibition of WNT signaling increases bronchiolar cell differentiation in lung organoid cultures, while activation of canonical WNT signaling in lung fibroblasts enhances AT2 cell differentiation in vitro. Conversely, epithelial production of Wnt7b activates FGF-10 expression in airway smooth muscle cells to mediate repair of the airway epithelium (338). Together, these recent findings highlight the importance of reciprocal signaling, in precise anatomic niches, among stromal and epithelial cells coordinating repair of the respiratory epithelium.
XI. FORMATION OF THE PULMONARY VASCULATURE
A. Heterogeneity of Pulmonary Endothelial Cells
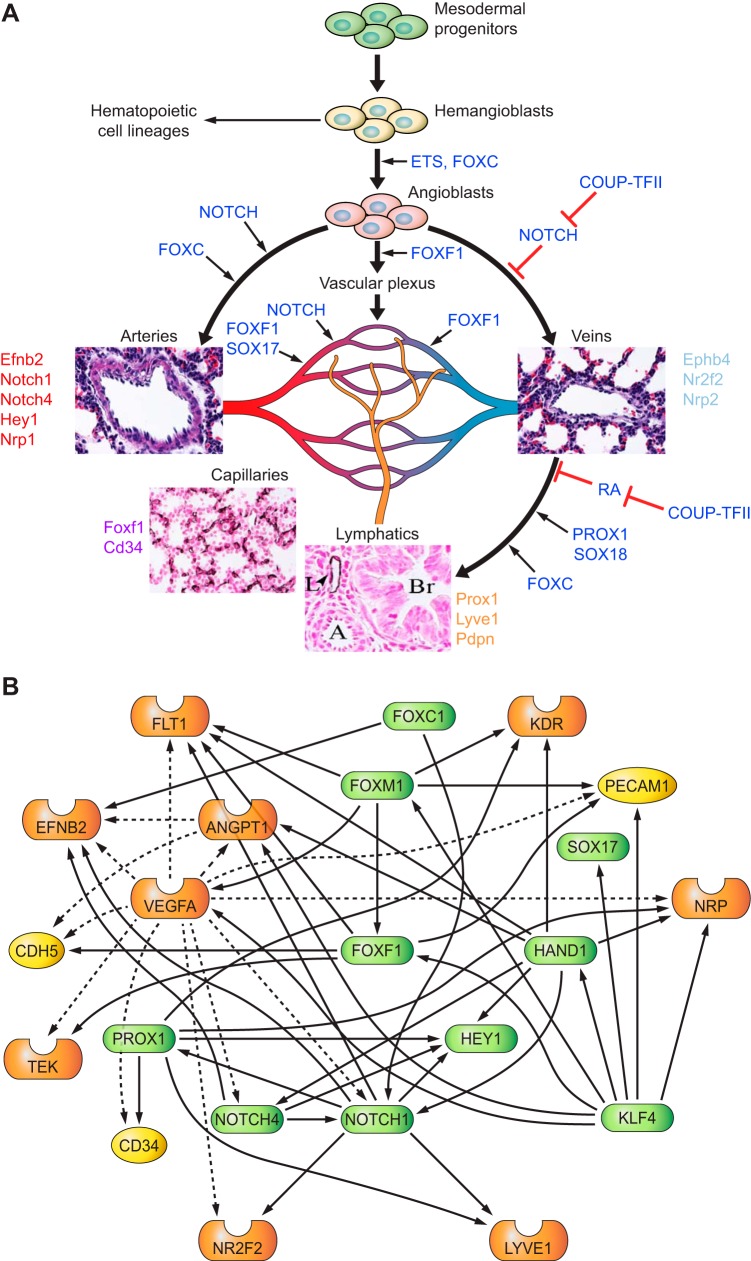
Endothelial cells play key roles in lung morphogenesis by secreting morphogens and providing nutrients and oxygen to support developmental processes. The pulmonary vascular network can be subdivided into proximal and peripheral circulations that contain multiple vascular-endothelial cell types (FIGURE 14A). The proximal circulation consists of pulmonary veins and arteries, whereas the peripheral circulation consists of microvascular-capillary networks facilitating gas exchange in the alveoli. The lymphatic circulation regulates pulmonary fluid homeostasis in the alveoli and drains into the thoracic duct. Endothelial cells of blood and lymphatic vessels share the expression of CD31 (Pecam-1) and VE-cadherin (Cdh5). Arterial endothelial cells express ephrin B2 (Efnb2), neuropilin 1 (Nrp1), NOTCH 1 and 4, and the NOTCH target gene Hey1, whereas venous endothelial cells express ephrin receptor B4 (Ephb4), neuropilin 2 (Nrp2), and the transcription factor COUP-TFII (Nr2f2) (reviewed in Refs. 55, 65, 323). Lymphatic endothelial cells are derived from pulmonary veins and express Prox1, Lyve1, and Pdpn. Pulmonary capillary endothelial cells express FOXF1 transcription factor, CD34 and common endothelial markers (FIGURE 14A). While Foxf1 is expressed in fibroblasts and visceral smooth muscle cells (150, 151, 272), coexpression of Pecam1+/CD45−/CD34+/FOXF1+ is specific to capillary endothelial cells in the adult lung. Loss of Foxf1 in mice and humans causes alveolar capillary dysplasia (ACD) without affecting the vasculature of other organs (151, 304), indicating a critical role of FOXF1 in the growth and differentiation of alveolar capillary endothelial cells.
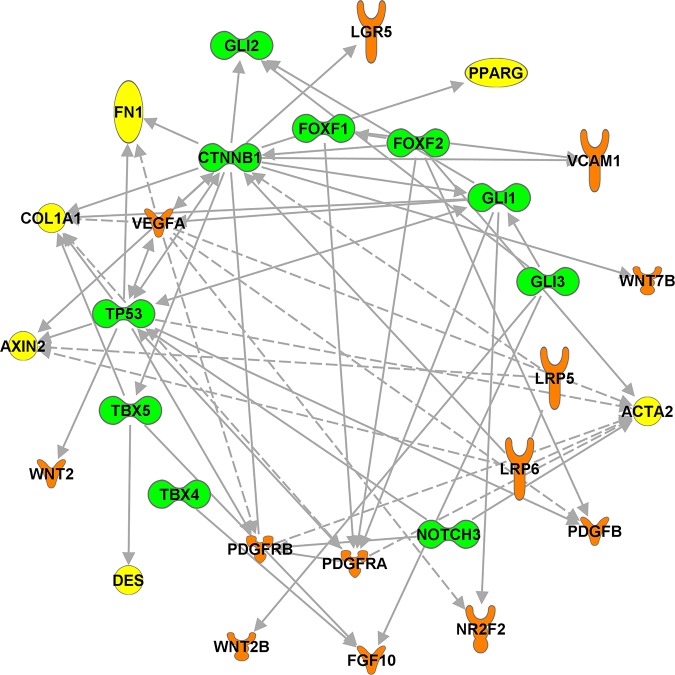
FIGURE 14.
Signaling and transcriptional networks regulating endothelial cell lineages. A: heterogeneity of pulmonary endothelial cells. Mesoderm-derived bipotential hemangioblasts differentiate into hematopoietic and endothelial progenitor cells (angioblasts). Angioblasts give a rise to arterial, venal, lymphatic, and capillary endothelial cell lineages. Arterial endothelial cell fate is promoted by NOTCH activation. Chicken ovalbumin in upstream promoter transcription factor 2 (COUP-TFII) inhibits NOTCH, enhances venous endothelial differentiation, and cooperates with PROX1, FOXC1/2, and SOX18 to stimulate lymphatic endothelial differentiation. Development of pulmonary capillary (microvascular) endothelial cells is promoted by FOXF1, SOX17, and NOTCH. B: transcriptional network regulating pulmonary endothelial development. Predicted endothelial cell regulatory gene network consists of representative transcription factors (green), signaling molecules (orange), and target genes (yellow). The regulatory relationships between key regulators and their predicted targets are identified through literature mining using the Ingenuity knowledge base. Solid lines indicate direct regulations. Dashed lines indicate indirect regulation.
B. Vasculogenesis and Angiogenesis Direct the Formation of the Pulmonary Circulation in the Embryonic Lung
The embryonic lung vasculature is formed by vasculogenesis (de novo formation of blood vessels) and angiogenesis (branching of preexisting blood vessels). Vasculogenesis, as occurs in the yolk sac, allantois, placenta, and dorsal aorta, is a process in which bipotential hemangioblasts differentiate into hematopoietic and endothelial cells to form primitive blood vessels filled with hematopoietic cells and lined by endothelial cells (66, 97, 306). Angiogenesis involves formation of new blood vessels by branching and expansion of preexisting vessels. Proximal pulmonary vascular structures are formed via angiogenesis, whereas differentiation of endothelial precursors from splanchnic mesenchyme forms the microvasculature. Anastomoses between pulmonary arteries and veins and the pulmonary microvasculature occurs during the pseudoglandular stage (E13.5) of lung morphogenesis in the mouse (66, 97, 306). Pulmonary blood flow, without vascular leak, occurs as early as E11.5 (249, 287), supporting the concept that formation of pulmonary capillaries occurs, at least in part, via vascular remodeling of preexisting vessels. Sprouting (budding) and intussusception mediate the process of angiogenesis. Sprouting is initiated by pro-angiogenic stimuli to form new vascular branches and their elongation. Vessel walls and basal lamina are disassembled, followed by migration and proliferation of endothelial cells at sites of vascular remodeling. Lumen are formed by coalescence of endothelial cells. Pericytes and smooth muscle cells are recruited at sites of new vascular branches to stabilize endothelial sprouts. Intussusception, a process during which lumen of preexisting blood vessels are subdivided by the insertion of interstitial cells to redistribute blood flow during vascular remodeling, plays important roles in formation of the pulmonary circulation (169, 170, 284). Circulating endothelial progenitor cells contribute to the formation of pulmonary blood vessels (372, 373).
Main pulmonary arteries and veins that establish connections between the heart and lung are produced primarily by vasculogenesis (283). Endothelial, smooth muscle, and pericyte-like cells are derived from cardiopulmonary progenitor cells during formation of large pulmonary vessels (253). The finding that pulmonary arteries and veins develop in a model of lung agenesis indicates that lung endoderm is not required for the initial formation of pulmonary arteries and veins (253). However, endoderm-derived pulmonary epithelium is critical for growth of embryonic microvasculature by producing VEGF-A. Angiopoietin/TIE2, PDGF, phosphatidylinositol 3'-kinase (PI3K)-protein kinase B (AKT), TGF-β, SHH, WNT and NOTCH, and transcription factors including FOX, SOX, HOX, GATA, KLF, ETS, basic helix loop helix (bHLH), TBX, MEF2, nuclear receptor and zinc finger family members, all contribute to lung vasculogenesis (reviewed in Refs. 10, 28, 63, 321). Temporal and spatial coordination of a diversity of these signaling and transcriptional networks regulates the interactions among multiple cell types during pulmonary vasculogenesis.
C. Forming the Pulmonary Circulation During the Pseudoglandular and Saccular Period
Extensive remodeling of lung structure occurs during the saccular stage of lung development. Numbers of peripheral saccules increase and the vascular network expands to the periphery (46, 56, 120, 164, 230, 284, 345). Pulmonary arteries develop in close relationship to conducting airways. A separate bronchial circulation develops, and extensive vascular lymphatic vessels form (35). In the human lung, the bronchial vessels, supplied from the aorta, provide oxygen and nutrients to conducting airways. The pulmonary circulation, distinct from the systemic circulation, delivers deoxygenated blood from the right ventricle via pulmonary arteries and returns oxygenated blood to the left atrium via the pulmonary veins. An extensive lymphatic circulation develops during the saccular stage of lung development to regulate pulmonary fluid homeostasis.
D. Signaling and Transcriptional Networks Regulating Formation of the Pulmonary Vasculature
Morphogenesis of the pulmonary vasculature is influenced by a remarkable diversity of signaling networks. FGF10, 9, 18, 8 and their receptors FGFR2b and FGFR3/4 are expressed in the embryonic lung where they influence morphogenesis (46, 97). Formation of the bronchial buds, branching of the bronchioles, and pulmonary vasculature all require FGF signaling (120, 230, 352). PDGFs, VEGFs, and RA signaling influence the formation of the capillary network in the peripheral lung (reviewed in Refs. 120, 164, 173, 284). Angiogenesis and vasculogenesis require signaling mediated by VEGFs. In mammals, the VEGF family consists of five members: VEGF-A, -B, -C, and -D and the placenta growth factor (PGF). VEGF-A is the primary proangiogenic mediator of lung morphogenesis, whereas VEGF-C and VEGF-D play important roles in formation of the lymphatics. VEGF-A is mainly produced by respiratory epithelial cells and acts via tyrosine kinase receptors Flk1 (VEGF receptor type II or KDR) and Flt1 (VEGF receptor type I) to stimulate vascular development. Inactivation of the mouse Vegfa gene impairs blood-island formation and delays differentiation of endothelial cells (47, 87). Flk1−/− and Flt1−/− embryos fail to form mature blood vessels and die in utero at midsomite stages (89, 291). Increased expression of VEGF-A, under control of the lung epithelial-specific SFTPC promoter, accelerated pulmonary vascular development but disrupted epithelial differentiation (376), indicating that precise levels of VEGF-A are required for lung morphogenesis.
SHH, produced by epithelial cells in the lung buds, regulates FGF10 expression by the lung mesenchyme (173, 346) and is required for branching morphogenesis and development of pulmonary vasculature (230, 332). Deletion of Shh impaired differentiation of vascular and bronchial smooth muscle and caused mispatterning of tracheal-bronchial mesenchyme required for formation of cartilage rings in conducting airways (224, 332). Consistent with a critical role of BMP signaling in pulmonary vascular development, mice lacking the Id1 (inhibitor of differentiation and DNA binding) and Id3, both targets of the BMP signaling pathway, develop multiple defects in the pulmonary vasculature (197, 250).
NOTCH plays an important role in pulmonary vascular development (FIGURE 14A), stabilizing angiogenic sprouts which are essential for expansion of the pulmonary vascular network (185). NOTCH regulates Vegfa (57) and activates Hey1 and Hey2 increasing vascular branching (88, 250). NOTCH regulates SOX17 in pulmonary endothelial cells (185), the latter playing a critical role in angiogenesis (154). Conditional deletion of Sox17 from endothelial cells impairs formation of the pulmonary microvasculature and causes alveolar simplification, whereas endothelial-specific expression of Sox17 increases angiogenic sprouting (185). Deletion of Sox17 in the mesenchyme using Dermo1-Cre causes alveolar simplification associated with severe defects in formation of the peripheral pulmonary microvasculature (177).
E. Noncoding RNAs in Development of Pulmonary Vasculature
miRs and lncRNAs have emerged as critical regulators of lung vascular development. Deletion of either Dicer or Drosha, genes involved in processing and splicing of miRs, inhibited endothelial sprouting. Epithelial-specific inactivation of Dicer decreased epithelial branching (120, 206). miR-27b promoted specification of endothelial tip cells during branching of the vascular plexus (206). Mice deficient in the lncRNA Fendrr, located near the Foxf1 gene, develop hypoplastic lungs and enlarged alveoli, similar to findings in Foxf1+/− mice (152, 282). Both miR-126 and miR-221 are proangiogenic (206).
F. Gene Networks Regulating Arterial, Venous, and Lymphatic Cell Differentiation
Growth, differentiation, and branching of the pulmonary vasculature are controlled by complex transcriptional and signaling networks, shown in FIGURE 14B. Arterial endothelial cell fate is promoted by NOTCH, whereas COUP-TFII inhibits NOTCH and enhances venous endothelial differentiation (FIGURE 14A). COUP-TFII and PROX1 regulate lymphatic endothelial differentiation in the saccular stage of development (93, 254, 356). COUP-TFII inhibits RA signaling, suppressing venous and enhancing lymphatic cell differentiation (161) (FIGURE 14A). SOX18 enhances both lymphatic and venous endothelial cell differentiation (90, 250), while FOXC2 stimulates lymphatic development (250, 258). Inactivation of both FOXC1 and FOXC2 is embryonic lethal, disrupting vascular remodeling (174, 250). FOXF1 plays a critical role in pulmonary vasculature, activating Flk1, Flt1, Cdh5, and Pecam 1 (41, 272) (FIGURE 14, A and B). Paired-class homeobox PRX1 and PRX2 induce tenascin-C in vascular smooth muscle cells (141). Prx1−/− mice die after birth due to respiratory insufficiency associated with reduced angiogenesis and decreased expression of Flk1 and Vcam1 (134, 142). RUNX3 inhibits lung angiogenesis, by decreasing expression of CD31, VEGF, and von Willebrand factor (184). ETS1, expressed in endothelial cells, regulates Tie1/2, Flt1, Flk1, and Cdh5, but is not required for pulmonary angiogenesis, perhaps related to compensation by other ETS transcription factors, e.g., FLI1 and ERG1, that regulate Cdh5 and Flt1 (105, 186).
XII. BUILDING THE ALVEOLAR MICROCIRCULATION DURING THE SACCULAR-ALVEOLAR PERIOD OF LUNG DEVELOPMENT (36 WK PC AND POSTNATAL PERIOD)
A. Development of Alveolar Capillary Network
During the saccular stage of lung development, pulmonary capillaries are embedded in a thick mesenchyme, located relatively far from developing acinar cells lining peripheral lung tubules. During the saccular-alveolar transition, capillary endothelial cells are in close apposition to AT1 cells covering the majority of the peripheral saccules. VEGF signaling is required for pulmonary angiogenesis and alveolar septation (1). Neonatal exposure to hyperoxia reduced capillary density and caused alveolar simplification, which was restored by treatment with VEGF and angiopoietin (320). TGF-β signaling, phosphorylation of Smad2, and TGF-β receptors 1 and 2, Smad1 and Smad4 were increased during exposure to hypoxia (2, 235). Inhibition of TGF-β restored alveologenesis in hyperoxia-treated mouse lungs; likewise, increased expression of TGF-β, reduced septation, alveolar-capillary networks, and gas-exchange surface area (212, 231, 235), indicating that precise levels of TGF-β are required for alveolarization.
B. Role of FOXF1 in Pulmonary Vasculogenesis
Alveolar capillary dysplasia with misalignment of pulmonary veins (ACD/MPV) is a fatal congenital disorder of neonates and infants, associated with lung hypoplasia, fusion of the lung lobes and pulmonary vessels, and malposition of pulmonary veins (25). Heterozygous deletion and point mutations in the FOXF1 gene were found in a majority of ACD/MPV cases (288, 304). In mice, loss of pulmonary capillaries was associated with decreased NOTCH and VEGF signaling after deletion of Foxf1 (149, 152, 153). SHH activates GLI transcription factors regulating FOXF1 (158, 199, 201). Mutations in GLI-binding sites in the FOXF1 gene promoter caused ACD/MPV in spite of preservation of the FOXF1 coding region (159, 313). Mesenchymal-specific inactivation of Pten reduced FOXF1 and caused lung hypoplasia (321). Likewise, inactivation of endothelium-derived nitric oxide synthase (eNOS) or disruption of semaphorin-3 (Sema3)-neuropilin-1 (Nrp1) protein-protein interactions in Nrp1Sema- mice, reduced density of alveolar capillaries and caused misalignment of pulmonary veins (114, 143). Together, these findings indicate that FOXF1 signaling through NOTCH, VEGF, PTEN, SEMA3, NRP1, and eNOS regulates pulmonary vasculature morphogenesis.
XIII. REPAIR OF THE PULMONARY VASCULATURE
Repair of the pulmonary vasculature requires coordinated signaling among all cells in the vascular wall, including endothelial cells, pericytes, smooth muscle cells, and interstitial fibroblasts. Circulating immune cells actively participate in the repair process, secreting various cytokines and chemokines that stimulate proliferation and migration of endothelial and stromal cells to restore the vessel architecture. VEGF, produced by epithelial, smooth muscle, and immune cells at sites of injury, plays multiple roles in repair of the pulmonary circulation. While VEGFA enhances endothelial permeability and disrupts endothelial junctions (1), treatment with VEGFA promoted lung angiogenesis and decreased alveolar damage after hyperoxia-induced lung injury (320) in association with increased endothelial proliferation, migration, and survival via Flk1 mediated by activation of Ras/ERK and PI3K/AKT signaling pathways. Endothelial proliferation is increased after acute and chronic lung injury caused by environmental toxicants and pathogens, including influenza virus (172, 265). FOXM1, a downstream target of RAS/ERK pathway (341, 342), regulates endothelial proliferation by increasing expression of cell cycle regulatory genes cyclin B1, Plk1, and aurora B kinase (150, 383). FOXF1 increases expression of Cdh5 (VE-cadherin) and S1pr1, serving to maintain endothelial adherens junctions after lung injury (41, 146). FOXF1 enhances endothelial proliferation by repressing cell cycle inhibitors Cdkn1a and Cdkn2b (27).
Regeneration of the pulmonary vasculature occurs during compensatory lung growth following partial pneumonectomy (70). Endothelial cells produce matrix metallopeptidase (MMP) 14, releasing active EGF-like fragments from heparin-binding EGF-like growth factor and the laminin 5γ2 subunit. EGF-like fragments produced by MMP14 in response to VEGF and FGF signaling act via EGFR to stimulate epithelial proliferation during lung regeneration (70). Endothelial cells support airway epithelial repair by expression of extracellular matrix (ECM) protein thrombospondin-1 (TSP1) (182). TSP1 expression in endothelial cells was dependent on the calcineurin/NFATc1 signaling induced by BMP4 at sites of injury. Taken together, these studies demonstrate active cross-talk between pulmonary endothelial and epithelial cells during lung regeneration.
A. Resident Progenitor Cells Mediate Repair of the Pulmonary Circulation
In the adult lung, repair of the pulmonary circulation is mediated by proliferation of resident endothelial cells, with little contribution from bone marrow-derived circulating endothelial progenitor cells (EPCs). Bone marrow-derived EPCs have a low capacity to engraft but may enhance vascular repair via paracrine mechanisms (378). EPCs were originally defined as mononuclear blood cells isolated from peripheral blood that proliferated in vitro and expressed CD34 and FLK1 cell surface markers (11). EPCs formed endothelial-like sprouts, expressed Pecam 1 (CD31), and were pro-angiogenic in various ischemia models, but did not engraft to replace pulmonary endothelial cells perhaps acting by local release of VEGF and HGF (269). Myeloid-derived EPCs transiently expressed endothelial markers and did not serve as progenitor cells (378). In contrast, a rare population of circulating cells expressing endothelial cell-specific surface markers and lacking hematopoietic markers formed tube-like networks in Matrigel, bound Ulex europaeus lectin, and took up acetylated low-density lipoprotein. These cells self-renewed in vitro and contributed to de novo vessel formation in mouse models (3, 373). Since the endothelial colony forming cells proliferate without losing the progenitor properties in vitro, they may have therapeutic potential (3).
Vascular repair is dependent on proliferation of resident EPCs (378). While a diversity of endothelial cells may be active during regeneration of the pulmonary microvasculature, lineage markers specific to lung resident EPCs have been lacking. Recent studies by Fang et al. (85) identified vascular endothelial stem cells (VESC) expressing CD31+CD105+Sca1+c-Kit+ which produce functional blood vessels and exhibit long-term self-renewal capacity, consistent with properties of stem cells. While VESCs and endothelial colony forming cells (372, 373) may represent endothelial progenitor cells, additional studies are needed to determine whether these cells can be used for vascular repair after lung injury.
XIV. FORMATION OF THE PULMONARY MESENCHYME
Mesenchymal cells of the early embryo provide the information critical for formation of the body plan and create supporting tissues that define our body structure. Fundamental to the organization of the lung in the early embryonic period is the establishment of the anterior-posterior (A-P), dorsal-ventral, and left-right axes. Signaling and transcriptional programs determining axial organization depend on the precisely timed expression of genes controlling segmentation that are driven by homeotic (HOX) genes located in clusters of genes on several chromosomes. The position of the HOX genes is ordered in relationship to regional control of gene expression that regulates segmentation along the A-P axis. Gene networks, sequentially regulated by the HOX genes, provide spatial cues primarily via mesenchymal cells that influence sites of organogenesis. Mesenchymal progenitors from the lateral plate mesoderm and splanchnic mesenchyme signal to the foregut endoderm to control lung formation and serve as the cellular source of stromal components of pulmonary tissues, including cartilage, blood vessels, mesothelium, adventitia, and connective tissues that support the trachea, conducting airways, and alveoli.
A. Heterogeneity of Pulmonary Mesenchymal Cells
The embryonic lung mesenchyme provides the progenitors that form the cartilage, smooth muscle, stromal, and alveolar supporting structures. Mature lung fibroblasts include distinct matrix-, myo-, and lipofibroblast populations (16). During development, diverse fibroblastic cells, recognized by their characteristic ultrastructure and gene expression patterns, interact with multiple pulmonary cell types in diverse anatomic niches (FIGURES 15 and 16). During alveologenesis, lipofibroblasts, identified by expression of ADRP (Plin1), peroxisome proliferator activated receptor (PPAR)-γ, and low levels of platelet-derived growth factor receptor α (PDGFRα), store neutral lipids and retinoids and support growth and differentiation of AT2 epithelial cells (213, 322). Lipofibroblasts are located in close proximity to AT2 cells and endothelial cells in alveolar septae (37) (FIGURE 17). Matrix fibroblasts actively synthesize collagen and elastic fibers supporting the alveoli (78). Interstitial myofibroblasts, expressing α-smooth muscle actin (α-SMA) and high levels of PDGFRα, contain myofibrils oriented parallel to the alveolar walls (38, 51, 213) and are distinct from smooth muscle cells surrounding conducting airways and smooth muscle in the pulmonary vasculature (FIGURES 16 and 17).
FIGURE 15.
Identification of diverse pulmonary mesenchymal cells. Signature genes of 4 major lung mesenchymal cell types from E16.5 mouse lung identified via single cell RNA-seq analysis were represented in a 2-dimensional heatmap. Each row represents a single cell. Each column represents a signature gene. Cell clusters were labeled in the color bar at the left side of the heatmap. PMP, proliferative mesenchyme progenitors; MyoFB/SM, myofibroblast/smooth muscle; MatrixFB, matrix fibroblast. Representative signature genes were listed in the right panel colored as the corresponding cell types. Data are available online: https://research.cchmc.org/pbge/lunggens/celltype_E16_p3.html.
FIGURE 16.
Signaling networks regulating mesenchymal cell differentiation. Mesenchymal progenitors expressing glioma-associated oncogene family zinc finger 2/3 (Gli2/3), forkhead box F1 (FOXF1), and T-box 4/5 (TBX4/5) differentiate into vascular smooth muscle cells (SMC), airway SMC, myofibroblasts, lipofibroblasts, matrix fibroblasts, pericytes, and mesothelial cells. Cell-selective markers for each of the mesenchymal cell types are provided. Differentiation of airway SMC and myofibroblasts occurs, in part, via intermediate progenitors expressing Lgr6 and Axin2/PDGFRα. Lgr5+ mesenchymal progenitors differentiate into lipofibroblasts and matrix fibroblasts. Axin2+/PDGFRβ+ progenitors give rise to pericytes. Differentiation of mesenchymal cell types is regulated by the wingless (WNT), sonic hedgehog (SHH), fibroblast growth factor (FGF), and platelet-derived growth factor (PDGF) signaling pathways as indicated in diagram.
FIGURE 17.
Diverse fibroblasts build the alveolar scaffold. A: the electron micrograph shows the ultrastructure of an alveolar septae from the adult mouse lung. Most of the alveolar surface is covered by AT1 epithelial cell(s). Gas exchange occurs across the AT1-endothelial interface with alveolar capillaries. Matrix-, lipo-, and myofibroblasts are seen in the septal wall. A collagen-elastin bundle (matrix) is seen at the septal tip. B and C: confocal imaging of the mouse lung (PND3) is shown after injection with isolectin B4 visualizing endothelial cells of the alveolar capillary network. Surfaces of the alveoli were rendered from confocal images after staining the endothelium with isolectin B4 lectin after acquisition at 585–635 nm (green). Second harmonic imaging shows collagen bundles in red. (Figure courtesy of Dr. Matt Kofron, used with permission).
B. Heterogeneity of Pulmonary Mesenchymal Cells Predicted by Single Cell RNA Sequencing
Diverse mesenchymal cells, including fibroblasts, smooth muscle cells, endothelial cells, lipofibroblasts, myofibroblasts, and bone marrow-derived cells all contribute to the formation and function of the lung. While lineage relationships among epithelial cells are becoming increasingly understood, progress toward defining distinct mesenchymal cell subsets, their hierarchical relationships, and associated functions has been challenging due in part to the paucity of cell-specific markers and inability to isolate and characterize mesenchymal cells throughout lung development. Recent technological advances, including scRNA-seq, ChIP-seq, and high-resolution scanning confocal microscopy, are beginning to reveal the heterogeneity of lung mesenchymal cells and their importance in lung development and repair (183, 189, 253, 377).
Analytic pipelines “Sincera” and “SLICE” were developed for scRNA-seq analyses, and both software code and interpretation are freely accessible (109, 110). With the use of “Sincera” and “SLICE,” single cells isolated from mouse lung during development were used to identify cell type heterogeneity and their ontogenetic changes. Six distinct mesenchymal cell subtypes were identified for single cell RNA sequencing. Cells and signature genes associated with each mesenchymal subtype are shown in FIGURES 3 and 15. Functional enrichment analysis of signature genes from diverse fibroblastic subtypes revealed distinct (subtype specific) functionalities. A subset of fibroblasts enriched in “mitotic cell cycle” and “chromosome modification” associated processes and were termed “proliferative mesenchymal progenitors” (PMP); their signature RNAs include transcription factors (Foxm1, Hoxb5, Snai2, and Tbx3), signaling molecules (Fgf7 and Pdgfra), and cell cycle/chromatin modifiers (Top2a and Hmgb2). “Myofibroblasts” (MyoFB) were identified by RNAs predicting “actin binding,” “smooth muscle contraction,” and “extra-cellular matrix,” e.g., Acta2, Actg2, Myh11, Myl9, Tgfbi, and Tagln. “Pericyte”-like cells (PC) coexpressed Pdgfrb, Dlk1, NOTCH3, Rgs5, Cspg4, and Mcam. “Matrix fibroblasts” (MatrixFB) expressed RNAs associated with “extracellular matrix” and “cell adhesion,” e.g., Fn1, Nr2f2, Col1a1, Col6a2, Vcam, and Fgf10. Single cell RNA analyses extend the diversity of lung mesenchymal progenitor cells and provide new RNA markers useful for their study. As evidenced by the broad similarities of RNA expression by mesenchymal cells, the clustering of closely related cell types is more challenging than for the epithelial cells. Shared transcriptional programs among mesenchymal cells and their common origin, primarily from splanchnic mesenchyme, make clear cell type assignments difficult. Using lineage tracing and clonal analysis, Peng et al. (253) identified a population of multipotent cardiopulmonary mesoderm progenitors expressing Wnt2, Gli1, and Isl1 that serve as progenitors of cardiomyocytes, pulmonary vascular and airway smooth muscle, proximal vascular endothelium, and pericyte-like cells (132). Selective expression of Pdgfra, Wnt2, Gli1, and Twist2 mRNAs in cells with highest average single cell entropy support the concept that PMP represent important progenitors of multiple mesenchymally derived cell types.
Pericytes are distinguished by their extensive cellular processes that form along capillary basement membranes in close contact with the endothelial cells (8, 16). Pericytes are contractile, sharing characteristics of vascular smooth muscle cells and myofibroblasts and are generally identified by coexpression of platelet-derived growth factor receptor β (PDGFRβ), chondroitin-sulfate proteoglycan (NG2), and desmin (274) (FIGURES 15 and 16). Desmin is expressed in pleural mesothelial cells which form the lining of the thoracic cavity, producing a lubricating fluid to decrease resistance between lung and thoraic wall during respiration. In addition to desmin, mesothelial cells express Wilm's tumor susceptibility gene 1 (WT1), mesothelin, and calretinin (108, 144).
Differentiated smooth muscle cells in the lung are divided into two major subtypes: vascular smooth muscle (VSM) and airway smooth muscle cells (ASM) that express similar contractile proteins, share regulatory networks controlling smooth muscle contractility (86), and express smooth muscle myosin heavy chain, γ-SMA and smooth muscle-specific calponin (FIGURE 16). Vascular smooth muscle in the pulmonary vasculature regulates blood flow. ASM regulates airway constriction and relaxation.
C. Role of Pulmonary Mesenchyme in Branching Morphogenesis
Interactions among multiple signaling molecules, including SHH, GLI2/3, FGF signaling, and WNT, mediate crosstalk between the lung epithelium and mesenchyme during branching morphogenesis, as described earlier in this section. SHH produced primarily by the lung buds activates Gli2/3 to regulate cells in the mesenchyme. Deletion of either Gli2 or Gli3 blocks branching morphogenesis (56, 104, 232), whereas mice with Gli1 deletion lack lung malformations (251). Compound mutations in Gli1/2 or Gli2/3 in mice cause severe lung hypoplasia (56, 104, 232). Gli2 and 3 stimulate FGF10 expression in subsets of pulmonary mesenchymal progenitor cells, activating FGF-R2 in the epithelial cells to control branching morphogenesis (77).
A number of mesenchymal transcription factors interact in a network with GLI2/3 to regulate branching pulmonary morphogenesis (FIGURE 18). TBX4 controls mesenchymal signaling critical for lung sacculation. Mutations in TBX4 cause acinar hypoplasia/dysplasia, a rare congenital lung disorder characterized by the growth arrest at the pseudoglandular stage of morphogenesis (312). TBX2 and TBX3 act downstream of SHH to maintain mesenchymal WNT signaling by repression of WNT antagonists FRZB (196). Forkhead box (FOX) proteins, expressed in the embryonic pulmonary mesenchyme, are critical transcriptional regulators of branching morphogenesis and work in a genetic network with TBX family members. FOXM1 and FOXF1 play important roles in both proliferation and differentiation of the mesenchymal progenitors during branching morphogenesis. FOXM1 activates the transcription of cell cycle regulatory genes, e.g., Cdc25B, Plk1, Aurora B kinase, cyclin B1, and Jnk1 (147). Deletion of Foxm1 causes G1 arrest and reduces cell proliferation in the embryonic lung mesenchyme (158). SHH via patched tumor suppressor 1 (PTCH1) and GLI signaling activates expression of FOXF1 in the pulmonary mesenchyme (200) to regulate branching morphogenesis. Haploinsufficiency of the Foxf1 gene inhibits branching morphogenesis and causes alveolar capillary dysplasia (152, 200). PTEN, an inhibitor of the PI3K/AKT/mTOR pathway, activates FOXF1 required for lung mesenchymal proliferation. In mice, deletion of Pten causes lung hypoplasia and respiratory insufficiency at birth (321). The tuberous sclerosis complex (TSC), a negative regulator of mTOR, plays an important role during postnatal alveologenesis (270). Mesenchyme-specific inactivation of the glucocorticoid sensing nuclear receptor Nr3c1 impairs lung maturation causing respiratory failure at birth (111). The homeobox transcription factors Irx1,2,3–5, primarily expressed in lung epithelial cells, play pleiotropic roles during branching morphogenesis, inhibiting genes regulating both vascular branching and differentiation of airway epithelial cells (333). While broadly expressed in various lung cells, Ezh2 restricts the smooth muscle gene expression in developing mesothelium, repressing Tbx18 and myocardin (297). Zinc finger transcription factor Osr1, a downstream target of SHH, is required for branching morphogenesis and mesenchyme differentiation towards smooth muscle and cartilage cell lineages (113). Mesenchymal-specific inactivation of Apc, an inhibitor of canonical WNT signaling, increased proliferation of lung mesenchyme and inhibited differentiation of airway and vascular smooth muscle cells, causing pulmonary hemorrhage (189).
FIGURE 18.
A predicted transcriptional network regulating differentiation of the pulmonary mesenchyme. A predicted mesenchymal cell gene regulatory network consists of representative transcription factors (green), signaling molecules (orange), and target genes (yellow). Regulatory relationships between key transcriptional regulators and signaling predicted targets were identified through literature mining using the Ingenuity knowledge database. Solid lines indicate direct regulatory relationships. Dashed lines indicate indirect regulatory relationships.
D. Smooth Muscle Cells During Lung Morphogenesis
While pulmonary cells are highly proliferative during the embryonic, pseudoglandular, and canalicular periods, cell proliferation decreases dramatically during the saccular period as both mesenchymal and epithelial cells differentiate before birth (18). In the mouse, ASM are derived from mesenchymal progenitors located in close proximity to SHH-producing epithelial cells (348). SHH, produced by respiratory epithelial cells, stimulates differentiation of ASM from lung mesenchyme (224). On the other hand, FGF9, expressed by mesothelial cells, inhibits ASM differentiation (348). Loss of FGF9 or conditional inactivation of FGF receptors 1 and 2 causes ectopic differentiation of ASM (371). Complex paracrine signaling from the mesothelium via FGF9 and epithelial SHH controls ASM differentiation in the conducting airway in close proximity to epithelial basement membranes (352). ASM differentiation is regulated by laminins in the basement membrane (368) and by physical forces (369). SHH inhibits miR-206, increasing expression of brain-derived neurotrophic factor (BDNF), an essential ASM-derived signal that mediates recruitment of nerve cells critical for innervation of the lung (262).
Differentiation of VSM requires interactions among Wnt7a expressing airway epithelial cells and the lung mesenchyme. Deletion of Wnt7b, enhanced formation of VSM without altering peribronchial smooth muscle, causing postnatal lung hemorrhage (293). Expression of midkine, normally produced by the respiratory epithelium, caused VSM hyperplasia and increased muscularization of pulmonary blood vessels independently of bronchiolar ASM (273). Proliferation of VSM is highly active before birth and decreases in the perinatal period of development (18) and is regulated by FOXM1 (168, 264). Conditional inactivation of Foxm1 in smooth muscle cells decreased VSM cell proliferation and disrupted integrity of arterial walls, mediated in part by the inhibition of cell cycle regulatory genes (329). Thus FOXM1 functions in a cell autonomous manner to regulate proliferation of VSM. Inhibition of IRX homeobox transcription factors reduced the thickness of VSM layers surrounding pulmonary arteries (333).
E. Pulmonary Fibroblasts Build the Alveoli
Alveolarization requires complex interactions among differentiating AT1 and AT2 epithelial cells and diverse mesenchymal progenitors that form the collagen and elastin-rich scaffold that define alveolar structure (189) (FIGURE 17). Embryonic PDGFRα expressing mesenchymal cells are progenitors of both lipofibroblasts (LFs) and myofibroblasts (MFs) in processes mediated by SHH and FGF signaling (189, 214, 257). During postnatal alveolarization, PDGFRα+ myofibroblasts within the alveolar septae produce the elastin and collagen that forms the scaffolds supporting alveolar septa (31, 230) (FIGURE 16). In the adult lung, MFs are rare α-SMA-positive cells embedded in the peripheral lung parenchyma. PDGFα enhances MF differentiation and stimulates production of elastin, fibrillin, and other ECM proteins (31, 193). The ECM binds growth factors coordinating lung morphogenesis (239). Contractile MFs are recognized by a SMA-rich cytoskeleton that connects cells with tensile and elastic elements in the ECM needed to accommodate the dynamic mechanical forces created during the ventilatory cycle. Interactions among MFs and endothelial cells, mediated in part by ephrin B2, are required for normal matrix disposition and septation (357).
During the saccular-alveolar transition in the perinatal period, LFs are recognized by their abundant lipid droplets and expression of PLIN2A, PDGFRα, and THY-1. Lipofibroblasts enhance differentiation of epithelial progenitors when cultured in the presence of FGF-2 (217). A subpopulation of SCA1+ and PDGFRα+ fibroblasts produces SHH, a survival signal for lung mesenchyme (216). SCA1+ fibroblasts consist of two distinct subpopulations: CD166−/THY-1+ cells, that differentiate into LFs when exposed to BMP-4, and CD166+/THY-1− cells, which differentiate into MFs in response to TGF-β1 (217). Both LFs and matrix fibroblasts produce FGF10, supporting expansion and differentiation of epithelial cells in vitro (15). Lineage tracing studies indicate that FGF10-expressing mesenchymal cells differentiate into LFs rather than MFs (76) in a process inhibited by ALK5 (188).
F. Pericytes Stabilize the Pulmonary Vasculature
Vasulogenesis and angiogenesis are critical for the survival of the embryo and are precisely regulated by a diversity of signaling networks that depend on interactions of pericytes and endothelial cells. Proliferative fibroblasts serve as progenitors of pericytes that stabilize vascular endothelial cells (215). Pulmonary pericytes are recognized by the close association with endothelial cells and by expression of NG2 and PDGFRβ. PDGFRβ signaling is required for recruitment of mesenchymal progenitor cells to the periendothelial niche where they differentiate into pericytes (118). PDGF-B, secreted by endothelial cells, binds to PDGFRβ expressed on the surface of pericyte progenitors, activating mitogen-activated protein kinase (MAPK) signaling, causing pericyte migration into angiogenic sprouts. In mice, deletion of either Pdgfb or Pdgfrb blocks pericyte differentiation, disrupts pulmonary vascular function, and causes death after birth (187). TGF-β and angiopoietin/TIE-2 play important roles in vascular development and stability (reviewed in Refs. 8, 212). TGF-β activates ALK-5, critical for endothelial-pericyte interactions, and promotes vessel maturation. TGF-β signaling, through ALK-1, inhibits vessel maturation and promotes endothelial proliferation and migration (102, 245). Genetic deletion of several genes critical for TGF-β signaling pathway, such as Tgfb1 (103), Alk1 (328), Alk5 (179), Tgfbr2 (246), Smad4 (176), Smad5 (48, 367), and endoglin (190), cause severe vascular abnormalities, including remodeling defects in the yolk sac vasculature, arteriovenous anastomoses, and abnormal pericyte coverage of blood vessels (101). In humans, mutations in ENDOGLIN, ALK1, and SMAD4 cause hereditary hemorrhagic telangiectasia, an autosomal-dominant disorder causing arteriovenous anastomoses (20, 94, 210).
Angiopoietin 1 (ANG1), produced by pericytes, acts via the endothelial TIE-2 receptor TEK to enhance maturation of blood vessels (339). Ang1−/− and Tie2−/− mice fail to stabilize blood vessels and lack normal pericytes. ANG2, produced by endothelial cells, antagonizes TIE-2, causing vessel destabilization and loss of pericytes (203). Paracrine signaling between pericytes and endothelial cells via ephrins (Ephs) and their receptors stimulates angiogenesis and maintains endothelial permeability. Although pericytes are sparsely distributed along endothelial cells of the pulmonary microvasculature, pericytes influence capillary permeability, regulate blood flow, and contribute to vascular surface area required for gas exchange. Deletion of EphrinB2 in mice or inhibition of EphrinB2 in rats decreased numbers of pulmonary blood vessels and caused alveolar simplification (331, 357). Intranasal administration of EphrinB2 ameliorated the loss of pulmonary vasculature and prevented alveolar simplification after hyperoxia. Lineage tracing using Foxd1-Cre labeled a subset of mesenchymal cells in E10.5–12.5 embryos (132); Foxd1 labeled cells differentiated into pericytes, expressed PDGFRβ and NG2.
XV. REPAIRING THE PULMONARY STROMA AFTER INJURY
A. Myofibroblast Activation and Fibrogenic Pathways Following Lung Injury
Diverse fibroblasts, including myofibroblasts, lipofibroblasts, and matrix fibroblasts, comprise the stromal components of the lung. Like the respiratory epithelium, the stromal compartments of the lung are stable at homeostasis but capable of remarkable cell proliferation and regeneration following acute lung injury. Failure of lung regeneration occurs in common chronic lung diseases and causes tissue remodeling and fibrosis mediated by the activation of myofibroblasts from various cellular sources. Resident lung fibroblasts, bone-marrow-derived fibrocytes, and local epithelial-mesenchymal (EMT) or endothelial-mesenchymal transition (EndMT) serve as progenitors of activated myofibroblasts (126, 299). A number of signaling pathways activate fibroblasts following injury, including TGF-β, HIPPO/YAP, PI3K, mTOR, WNT, EGFR, and SHH. Following injury, TGF-β recruits myofibroblasts, enhances proliferation, and deposits abnormal collagen and extracellular matrix, mediated by the activation of SMAD3 (347, 381). TGF-β signaling plays major roles in the pathological remodeling in chronic fibrotic pulmonary diseases, e.g., IPF, COPD, and asthma (12, 280). While SHH signaing is quiescent in the normal adult lung, SHH production by lung epithelial cells is induced in chronic lung diseases, activating PTCH and GLI in the stromal fibroblasts. PTCH-1, SMO, and GLI1 are increased in fibroblasts from IPF lungs (26). Pulmonary stromal cell proliferation and activation following injury is influenced by a number of growth factors, cytokines, and chemokines. Recent single cell transcriptomic analyses of epithelial cells in IPF predicts the activation of TGF-β, WNT, PI3K/mTOR, and HIPPO/YAP pathways in IPF (360). Activation of WNT, PDGF, and NOTCH signaling has been associated with fibroblast proliferation and differentiation in the setting of pulmonary fibrosis (36, 194, 244, 358). Crosstalk among diverse pulmonary cells via these signaling pathways likely reinforces the activation of myofibroblasts and expression of α-SMA, type Iα1 collagen, and connective tissue growth factor (290, 301).
B. Progenitors of Smooth Muscle Cells in Repair
The heterogeneity of vascular smooth muscle cells (SMCs) subtypes and their origin are supported by lineage mapping and functional studies (204, 307, 374). Neural crest, proepicardium, mesothelium, and the secondary heart field all provide progenitors forming vascular smooth muscle (204). SMC morphology and gene expression change dynamically during lung formation and repair (92). While best characterized in vascular smooth muscle in the systemic circulation, much less is known regarding vascular cells in the pulmonary arteries and veins. Distinct characteristics of the SMC differentiation are readily apparent during perinatal development and the adjustment to airbreating at birth when the pulmonary vaculature is highly responsive to hypoxemia and hyperoxia (305). Progenitors of SMC in adult arteries were identified in cytometry of cells from the vascular intima and media and were not found in adventitial tissues (279). Exposure of SMC progenitors to TGF-β or PDGF-β enhanced their differentiation into smooth muscle cells while VEGFA caused their differentiation into endothelial cells. However, SMC progenitors also are present in arterial adventitia and are highly abundant in the aortic root (129). Thus distinct SMC progenitors reside in the artery walls in adult tissues and respond to injury by differentiating into SMCs. Cells capable of differentiating into SMCs are present in the systemic circulation and are recruited from bone marrow under pathological conditions (44, 218, 219). Thus vascular SMCs can be formed from progenitor cells located within vessel walls or recruited from the systemic circulation.
C. Role of Alveolar Macrophages in Repair of the Alveoli
Macrophages are one of the most abundant cell types in the healthy lung (40). Alveolar macrophages (AMs) and interstitial macrophages (IMs) are the most abundant resident macrophages. Monocyte-derived macrophages are recruited to the lung during inflammation. AMs, located in the airway lumen, express CD11c, but lack CD11b. IMs, in the lung parenchyma, express CD11b and low levels of CD11c. AMs play critical roles in innate immunity and are generally weak antigen presenting cells, but are active in phagocytosis and production of host defense molecules, including nitric oxide and cytokines, e.g., TNF-α, IL-1, and interferon (IFN)-γ (39). AMs play a central role in the degradation of lipids and proteins in the alveolus, the disruption of which causes the disease pulmonary alveolar proteinosis (PAP). IMs are efficient antigen-presenting cells and secrete cytokines associated with adaptive immune response, including IL-10 (17).
Lung macrophages are highly “plastic” and may exist in different activation states in response to injury, micropathogens, and toxicants (6). Macrophages are subclassified according to their activation status as M1 macrophages respond to IFN-γ, LPS, and/or TNF-α, and contribute to host defense against intracellular pathogens by generation of reactive nitric oxide. M1 macrophages produce proinflammatory cytokines, including IL-1β, IL-12β, IL-23, and TNF-α, and promote a local Th1 immune environment. M2 macrophages respond to IL-4, IL-13, TGF-β, and IL-10 (233) and participate in type 2 (Th2) immune responses. M2 macrophages have been further subdivided into M2a, M2b, and M2c subsets. For example, M2a macrophages facilitate parasite encapsulation and destruction, whereas M2b macrophages are immunoregulatory. Tissue remodeling and matrix deposition are activated by M2c macrophages (6). Since pulmonary macrophages may coexpress both M1 and M2 markers, this classification has been limited in defining disease states. Th2 inflammatory responses mediated by ILC2 cells (innate lymphocyte cells) were required for lung regeneration following unilateral pneumonectomy in mice (181).
D. Cellular Origins of Pulmonary Macrophages
Lineage tracing studies during embryonic development demonstrated that yolk sac macrophages give rise to pulmonary macrophages independently of hematopoietic cells in the bone marrow (286). While alveolar macrophages and monocytes are present in the lung before birth, the numbers increase dramatically in the perinatal period when the lung is colonized by fetal monocytes (107). Depletion and fate-mapping studies support the concept that AMs are derived from progenitors from the yolk sac and fetal liver macrophages in the embryonic period (124, 285). In general, AMs are long-lived cells, and under homeostatic conditions, repopulation of AMs occurs by in situ proliferation rather than by immigration from bone marrow (107, 115).
E. Regulators of Macrophage Activation
Resident AMs provide sentinel functions against inhaled pathogens, modulating inflammatory and immune responses, and surfactant clearance (125, 353). Activation of AMs is tightly controlled through cellular interactions and soluble mediators (133, 209). CD200 receptor (CD200R) inhibits macrophage activation, as depletion of CD200 increases the number of AMs and causes their spontaneous activation (296). CD200 on lung epithelial cells binds to CD200R and recruits docking protein 2 (DOK2) and RAS GTPase-activating protein RASA2 (RASGAP), inhibiting ERK, p38, and JNK signaling pathways (221, 222). AMs express signal regulatory protein-α (SIRPα; also known as SHPS1) (14). Phosphorylation of tyrosine residues in the cytoplasmic part of SIRPα recruits and activates either SH2 domain-containing protein tyrosine phosphatase 1 (SHP1) or SHP2. SHP1 is a negative regulator of cytokine-receptor signaling in macrophages (294). Shp1-deficient mice die from pneumonia caused by accumulation of macrophages and neutrophils in the lungs (294). Pulmonary surfactant-associated protein A (SP-A) and SP-D negatively regulate macrophage activation, inhibiting interactions of TLR2/4 with their co-receptors MD2 and CD14, to reduce activation of NF-κB (363). AMs are activated in the absence of SP-D resulting in inflammation and alveolar remodeling (112, 256). TGF-β is activated through αvβ6 integrin and inhibits MMP12 production by macrophages (229). Mice deficient in αvβ6 develop spontaneous pulmonary emphysema caused by the induction of MMP12 (229). GM-CSF is required for differentiation of monocyte progenitors into mature AMs and associated macrophage functions including phagocytosis, clearance of microbial pathogens, and cytokine responses (324). GM-CSF signaling induces clearance and metabolism of surfactant lipids and proteins by AMs (325). Injury to the respiratory epithelium activates secretion of early-response cytokines including TNF-α and IL-1β, which stimulate release of chemokines by alveolar cells to recruit neutrophils, monocytes, and lymphocytes (119). AMs also play important roles during resolution of inflammation. Phagocytosis of apoptotic cells suppresses macrophage activation causing release of TGF-β, IL-10, and prostaglandin E2 (289).
XVI. CONCLUSIONS
It is a remarkable time in biology and medicine during which advances in imaging and high-throughput technologies are enabling ever more detailed views of the diverse cells and processes that form and maintain complex organs like the lung. The application of the “omics” to the study of normal lung formation and function will provide the scientific framework needed to understand lung malformations and chronic lung diseases affecting individuals of all ages. A great challenge for the field will be the integration and interpretation of the ever more complex data defining the cellular and molecular processes determining the structures and functions of the normal lung and those that contribute to the pathogenesis of pulmonary diseases.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL122642, HL122638 (LungMap), and HL134745 (to J. A. Whitsett and Y. Xu); HL84151, HL141174, and HL123490 (to V. V. Kalinichenko); and HL132849 (to T. V. Kalin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Drs. Matt Kofron, Aaron Zorn, Minzhe Guo, Yina Du, and Mr. Joseph Kitzmiller for images and bioinformatics analyses; Ann Maher for manuscript preparation; and Drs. Samriddha Ray, Daniel Swarr, Sheila Bell, and Scott Rankin for helpful editing and discussions.
Address for reprint requests and other correspondence: V. V. Kalinichenko and J. A. Whitsett, Cincinnati Children's Hospital Medical Center, Perinatal Institute, Division of Neonatology, Perinatal and Pulmonary Biology, 3333 Burnet Ave., MLC 7029, Cincinnati, OH 45229–3039 (e-mail: vladimir.kalinichenko@cchmc.org and jeff.whitsett@cchmc.org).
REFERENCES
- 1.Akeson AL, Cameron JE, Le Cras TD, Whitsett JA, Greenberg JM. Vascular endothelial growth factor-A induces prenatal neovascularization and alters bronchial development in mice. Pediatr Res 57: 82–88, 2005. doi: 10.1203/01.PDR.0000148070.89006.3F. [DOI] [PubMed] [Google Scholar]
- 2.Alejandre-Alcázar MA, Kwapiszewska G, Reiss I, Amarie OV, Marsh LM, Sevilla-Pérez J, Wygrecka M, Eul B, Köbrich S, Hesse M, Schermuly RT, Seeger W, Eickelberg O, Morty RE. Hyperoxia modulates TGF-beta/BMP signaling in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 292: L537–L549, 2007. doi: 10.1152/ajplung.00050.2006. [DOI] [PubMed] [Google Scholar]
- 3.Alphonse RS, Vadivel A, Zhong S, McConaghy S, Ohls R, Yoder MC, Thébaud B. The isolation and culture of endothelial colony-forming cells from human and rat lungs [Correction in Nat Protoc 11: 192, 2016.]. Nat Protoc 10: 1697–1708, 2015. doi: 10.1038/nprot.2015.107. [DOI] [PubMed] [Google Scholar]
- 4.Ameis D, Khoshgoo N, Iwasiow BM, Snarr P, Keijzer R. MicroRNAs in Lung Development and Disease. Paediatr Respir Rev 22: 38–43, 2017. doi: 10.1016/j.prrv.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Anderson PJ, Lynch TJ, Engelhardt JF. Multipotent Myoepithelial Progenitor Cells Are Born Early during Airway Submucosal Gland Development. Am J Respir Cell Mol Biol 56: 716–726, 2017. doi: 10.1165/rcmb.2016-0304OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anthony D, McQualter JL, Bishara M, Lim EX, Yatmaz S, Seow HJ, Hansen M, Thompson M, Hamilton JA, Irving LB, Levy BD, Vlahos R, Anderson GP, Bozinovski S. SAA drives proinflammatory heterotypic macrophage differentiation in the lung via CSF-1R-dependent signaling. FASEB J 28: 3867–3877, 2014. doi: 10.1096/fj.14-250332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arbi M, Pefani DE, Kyrousi C, Lalioti ME, Kalogeropoulou A, Papanastasiou AD, Taraviras S, Lygerou Z. GemC1 controls multiciliogenesis in the airway epithelium. EMBO Rep 17: 400–413, 2016. doi: 10.15252/embr.201540882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21: 193–215, 2011. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Arora R, Metzger RJ, Papaioannou VE. Multiple roles and interactions of Tbx4 and Tbx5 in development of the respiratory system. PLoS Genet 8: e1002866, 2012. doi: 10.1371/journal.pgen.1002866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arora R, Papaioannou VE. The murine allantois: a model system for the study of blood vessel formation. Blood 120: 2562–2572, 2012. doi: 10.1182/blood-2012-03-390070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–966, 1997. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 12.Aschner Y, Downey GP. Transforming Growth Factor-β: Master Regulator of the Respiratory System in Health and Disease. Am J Respir Cell Mol Biol 54: 647–655, 2016. doi: 10.1165/rcmb.2015-0391TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Audrit KJ, Delventhal L, Aydin Ö, Nassenstein C. The nervous system of airways and its remodeling in inflammatory lung diseases. Cell Tissue Res 367: 571–590, 2017. doi: 10.1007/s00441-016-2559-7. [DOI] [PubMed] [Google Scholar]
- 14.Barclay AN. Signal regulatory protein alpha (SIRPalpha)/CD47 interaction and function. Curr Opin Immunol 21: 47–52, 2009. doi: 10.1016/j.coi.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BL. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest 123: 3025–3036, 2013. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barron L, Gharib SA, Duffield JS. Lung Pericytes and Resident Fibroblasts: Busy Multitaskers. Am J Pathol 186: 2519–2531, 2016. doi: 10.1016/j.ajpath.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bedoret D, Wallemacq H, Marichal T, Desmet C, Quesada Calvo F, Henry E, Closset R, Dewals B, Thielen C, Gustin P, de Leval L, Van Rooijen N, Le Moine A, Vanderplasschen A, Cataldo D, Drion PV, Moser M, Lekeux P, Bureau F. Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice. J Clin Invest 119: 3723–3738, 2009. doi: 10.1172/JCI39717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belknap JK, Weiser-Evans MC, Grieshaber SS, Majack RA, Stenmark KR. Relationship between perlecan and tropoelastin gene expression and cell replication in the developing rat pulmonary vasculature. Am J Respir Cell Mol Biol 20: 24–34, 1999. doi: 10.1165/ajrcmb.20.1.3321. [DOI] [PubMed] [Google Scholar]
- 19.Benlhabib H, Guo W, Pierce BM, Mendelson CR. The miR-200 family and its targets regulate type II cell differentiation in human fetal lung. J Biol Chem 290: 22409–22422, 2015. doi: 10.1074/jbc.M114.636068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berg JN, Gallione CJ, Stenzel TT, Johnson DW, Allen WP, Schwartz CE, Jackson CE, Porteous ME, Marchuk DA. The activin receptor-like kinase 1 gene: genomic structure and mutations in hereditary hemorrhagic telangiectasia type 2. Am J Hum Genet 61: 60–67, 1997. doi: 10.1086/513903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Besnard V, Wert SE, Stahlman MT, Postle AD, Xu Y, Ikegami M, Whitsett JA. Deletion of Scap in alveolar type II cells influences lung lipid homeostasis and identifies a compensatory role for pulmonary lipofibroblasts. J Biol Chem 284: 4018–4030, 2009. doi: 10.1074/jbc.M805388200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Besnard V, Xu Y, Whitsett JA. Sterol response element binding protein and thyroid transcription factor-1 (Nkx2.1) regulate Abca3 gene expression. Am J Physiol Lung Cell Mol Physiol 293: L1395–L1405, 2007. doi: 10.1152/ajplung.00275.2007. [DOI] [PubMed] [Google Scholar]
- 23.Bhattacharya J, Matthay MA. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu Rev Physiol 75: 593–615, 2013. doi: 10.1146/annurev-physiol-030212-183756. [DOI] [PubMed] [Google Scholar]
- 24.Bhattacharya J, Westphalen K. Macrophage-epithelial interactions in pulmonary alveoli. Semin Immunopathol 38: 461–469, 2016. doi: 10.1007/s00281-016-0569-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bishop NB, Stankiewicz P, Steinhorn RH. Alveolar capillary dysplasia. Am J Respir Crit Care Med 184: 172–179, 2011. doi: 10.1164/rccm.201010-1697CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolaños AL, Milla CM, Lira JC, Ramírez R, Checa M, Barrera L, García-Alvarez J, Carbajal V, Becerril C, Gaxiola M, Pardo A, Selman M. Role of Sonic Hedgehog in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 303: L978–L990, 2012. doi: 10.1152/ajplung.00184.2012. [DOI] [PubMed] [Google Scholar]
- 27.Bolte C, Flood HM, Ren X, Jagannathan S, Barski A, Kalin TV, Kalinichenko VV. FOXF1 transcription factor promotes lung regeneration after partial pneumonectomy. Sci Rep 7: 10690, 2017. doi: 10.1038/s41598-017-11175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolte C, Whitsett JA, Kalin TV, Kalinichenko VV. Transcription Factors Regulating Embryonic Development of Pulmonary Vasculature. Adv Anat Embryol Cell Biol 228: 1–20, 2018. doi: 10.1007/978-3-319-68483-3_1. [DOI] [PubMed] [Google Scholar]
- 29.Boon M, Wallmeier J, Ma L, Loges NT, Jaspers M, Olbrich H, Dougherty GW, Raidt J, Werner C, Amirav I, Hevroni A, Abitbul R, Avital A, Soferman R, Wessels M, O’Callaghan C, Chung EM, Rutman A, Hirst RA, Moya E, Mitchison HM, Van Daele S, De Boeck K, Jorissen M, Kintner C, Cuppens H, Omran H. MCIDAS mutations result in a mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat Commun 5: 4418, 2014. doi: 10.1038/ncomms5418. [DOI] [PubMed] [Google Scholar]
- 30.Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol 24: 662–670, 2001. doi: 10.1165/ajrcmb.24.6.4217. [DOI] [PubMed] [Google Scholar]
- 31.Boström H, Willetts K, Pekny M, Levéen P, Lindahl P, Hedstrand H, Pekna M, Hellström M, Gebre-Medhin S, Schalling M, Nilsson M, Kurland S, Törnell J, Heath JK, Betsholtz C. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell 85: 863–873, 1996. doi: 10.1016/S0092-8674(00)81270-2. [DOI] [PubMed] [Google Scholar]
- 32.Brafman DA, Moya N, Allen-Soltero S, Fellner T, Robinson M, McMillen ZL, Gaasterland T, Willert K. Analysis of SOX2-expressing cell populations derived from human pluripotent stem cells. Stem Cell Rep 1: 464–478, 2013. doi: 10.1016/j.stemcr.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Branchfield K, Nantie L, Verheyden JM, Sui P, Wienhold MD, Sun X. Pulmonary neuroendocrine cells function as airway sensors to control lung immune response. Science 351: 707–710, 2016. doi: 10.1126/science.aad7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brechbuhl HM, Li B, Smith RW, Reynolds SD. Epidermal growth factor receptor activity is necessary for mouse basal cell proliferation. Am J Physiol Lung Cell Mol Physiol 307: L800–L810, 2014. doi: 10.1152/ajplung.00201.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bridges JP, Weaver TE. Use of transgenic mice to study lung morphogenesis and function. ILAR J 47: 22–31, 2006. doi: 10.1093/ilar.47.1.22. [DOI] [PubMed] [Google Scholar]
- 36.Brigstock DR. Connective tissue growth factor (CCN2, CTGF) and organ fibrosis: lessons from transgenic animals. J Cell Commun Signal 4: 1–4, 2010. doi: 10.1007/s12079-009-0071-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brody JS, Kaplan NB. Proliferation of alveolar interstitial cells during postnatal lung growth. Evidence for two distinct populations of pulmonary fibroblasts. Am Rev Respir Dis 127: 763–770, 1983. [DOI] [PubMed] [Google Scholar]
- 38.Bruce MC, Honaker CE, Cross RJ. Lung fibroblasts undergo apoptosis following alveolarization. Am J Respir Cell Mol Biol 20: 228–236, 1999. doi: 10.1165/ajrcmb.20.2.3150. [DOI] [PubMed] [Google Scholar]
- 39.Byrne AJ, Maher TM, Lloyd CM. Pulmonary Macrophages: A New Therapeutic Pathway in Fibrosing Lung Disease? Trends Mol Med 22: 303–316, 2016. doi: 10.1016/j.molmed.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Byrne AJ, Mathie SA, Gregory LG, Lloyd CM. Pulmonary macrophages: key players in the innate defence of the airways. Thorax 70: 1189–1196, 2015. doi: 10.1136/thoraxjnl-2015-207020. [DOI] [PubMed] [Google Scholar]
- 41.Cai Y, Bolte C, Le T, Goda C, Xu Y, Kalin TV, Kalinichenko VV. FOXF1 maintains endothelial barrier function and prevents edema after lung injury. Sci Signal 9: ra40, 2016. doi: 10.1126/scisignal.aad1899. [DOI] [PubMed] [Google Scholar]
- 42.Campbell EP, Quigley IK, Kintner C. Foxn4 promotes gene expression required for the formation of multiple motile cilia. Development 143: 4654–4664, 2016. doi: 10.1242/dev.143859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao Z, Lis R, Ginsberg M, Chavez D, Shido K, Rabbany SY, Fong GH, Sakmar TP, Rafii S, Ding BS. Targeting of the pulmonary capillary vascular niche promotes lung alveolar repair and ameliorates fibrosis. Nat Med 22: 154–162, 2016. doi: 10.1038/nm.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caplice NM, Doyle B. Vascular progenitor cells: origin and mechanisms of mobilization, differentiation, integration, and vasculogenesis. Stem Cells Dev 14: 122–139, 2005. doi: 10.1089/scd.2005.14.122. [DOI] [PubMed] [Google Scholar]
- 45.Caprioli A, Villasenor A, Wylie LA, Braitsch C, Marty-Santos L, Barry D, Karner CM, Fu S, Meadows SM, Carroll TJ, Cleaver O. Wnt4 is essential to normal mammalian lung development. Dev Biol 406: 222–234, 2015. doi: 10.1016/j.ydbio.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cardoso WV, Lü J. Regulation of early lung morphogenesis: questions, facts and controversies. Development 133: 1611–1624, 2006. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- 47.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380: 435–439, 1996. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 48.Chang H, Huylebroeck D, Verschueren K, Guo Q, Matzuk MM, Zwijsen A. Smad5 knockout mice die at mid-gestation due to multiple embryonic and extraembryonic defects. Development 126: 1631–1642, 1999. [DOI] [PubMed] [Google Scholar]
- 49.Chen G, Korfhagen TR, Karp CL, Impey S, Xu Y, Randell SH, Kitzmiller J, Maeda Y, Haitchi HM, Sridharan A, Senft AP, Whitsett JA. Foxa3 induces goblet cell metaplasia and inhibits innate antiviral immunity. Am J Respir Crit Care Med 189: 301–313, 2014. doi: 10.1164/rccm.201306-1181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen G, Korfhagen TR, Xu Y, Kitzmiller J, Wert SE, Maeda Y, Gregorieff A, Clevers H, Whitsett JA. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest 119: 2914–2924, 2009. doi: 10.1172/JCI39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen H, Matsumoto K, Brockway BL, Rackley CR, Liang J, Lee JH, Jiang D, Noble PW, Randell SH, Kim CF, Stripp BR. Airway epithelial progenitors are region specific and show differential responses to bleomycin-induced lung injury. Stem Cells 30: 1948–1960, 2012. doi: 10.1002/stem.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen L, Acciani T, Le Cras T, Lutzko C, Perl AK. Dynamic regulation of platelet-derived growth factor receptor α expression in alveolar fibroblasts during realveolarization. Am J Respir Cell Mol Biol 47: 517–527, 2012. doi: 10.1165/rcmb.2012-0030OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen YW, Huang SX, de Carvalho ALRT, Ho SH, Islam MN, Volpi S, Notarangelo LD, Ciancanelli M, Casanova JL, Bhattacharya J, Liang AF, Palermo LM, Porotto M, Moscona A, Snoeck HW. A three-dimensional model of human lung development and disease from pluripotent stem cells. Nat Cell Biol 19: 542–549, 2017. doi: 10.1038/ncb3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coppens JT, Van Winkle LS, Pinkerton K, Plopper CG. Distribution of Clara cell secretory protein expression in the tracheobronchial airways of rhesus monkeys. Am J Physiol Lung Cell Mol Physiol 292: L1155–L1162, 2007. doi: 10.1152/ajplung.00454.2006. [DOI] [PubMed] [Google Scholar]
- 55.Corada M, Morini MF, Dejana E. Signaling pathways in the specification of arteries and veins. Arterioscler Thromb Vasc Biol 34: 2372–2377, 2014. doi: 10.1161/ATVBAHA.114.303218. [DOI] [PubMed] [Google Scholar]
- 56.Costa RH, Kalinichenko VV, Lim L. Transcription factors in mouse lung development and function. Am J Physiol Lung Cell Mol Physiol 280: L823–L838, 2001. doi: 10.1152/ajplung.2001.280.5.L823. [DOI] [PubMed] [Google Scholar]
- 57.Crivellato E. The role of angiogenic growth factors in organogenesis. Int J Dev Biol 55: 365–375, 2011. doi: 10.1387/ijdb.103214ec. [DOI] [PubMed] [Google Scholar]
- 58.Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol 298: L715–L731, 2010. doi: 10.1152/ajplung.00361.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cushing L, Jiang Z, Kuang P, Lü J. The roles of microRNAs and protein components of the microRNA pathway in lung development and diseases. Am J Respir Cell Mol Biol 52: 397–408, 2015. doi: 10.1165/rcmb.2014-0232RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cutz E. Hyperplasia of pulmonary neuroendocrine cells in infancy and childhood. Semin Diagn Pathol 32: 420–437, 2015. doi: 10.1053/j.semdp.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Dai Y, Jablons D, You L. Hippo pathway in lung development. J Thorac Dis 9: 2246–2250, 2017. doi: 10.21037/jtd.2017.07.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Danopoulos S, Alonso I, Thornton ME, Grubbs BH, Bellusci S, Warburton D, Al Alam D. Human lung branching morphogenesis is orchestrated by the spatiotemporal distribution of ACTA2, SOX2, and SOX9. Am J Physiol Lung Cell Mol Physiol 314: L144–L149, 2018. doi: 10.1152/ajplung.00379.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Val S, Black BL. Transcriptional control of endothelial cell development. Dev Cell 16: 180–195, 2009. doi: 10.1016/j.devcel.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DeFelice M, Silberschmidt D, DiLauro R, Xu Y, Wert SE, Weaver TE, Bachurski CJ, Clark JC, Whitsett JA. TTF-1 phosphorylation is required for peripheral lung morphogenesis, perinatal survival, and tissue-specific gene expression. J Biol Chem 278: 35574–35583, 2003. doi: 10.1074/jbc.M304885200. [DOI] [PubMed] [Google Scholar]
- 65.Dela Paz NG, D’Amore PA. Arterial versus venous endothelial cells. Cell Tissue Res 335: 5–16, 2009. doi: 10.1007/s00441-008-0706-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.DeMello DE, Sawyer D, Galvin N, Reid LM. Early fetal development of lung vasculature. Am J Respir Cell Mol Biol 16: 568–581, 1997. doi: 10.1165/ajrcmb.16.5.9160839. [DOI] [PubMed] [Google Scholar]
- 67.Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 507: 190–194, 2014. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Desai TJ, Malpel S, Flentke GR, Smith SM, Cardoso WV. Retinoic acid selectively regulates Fgf10 expression and maintains cell identity in the prospective lung field of the developing foregut. Dev Biol 273: 402–415, 2004. doi: 10.1016/j.ydbio.2004.04.039. [DOI] [PubMed] [Google Scholar]
- 69.Didon L, Zwick RK, Chao IW, Walters MS, Wang R, Hackett NR, Crystal RG. RFX3 modulation of FOXJ1 regulation of cilia genes in the human airway epithelium. Respir Res 14: 70, 2013. doi: 10.1186/1465-9921-14-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ding BS, Nolan DJ, Guo P, Babazadeh AO, Cao Z, Rosenwaks Z, Crystal RG, Simons M, Sato TN, Worgall S, Shido K, Rabbany SY, Rafii S. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell 147: 539–553, 2011. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Domyan ET, Ferretti E, Throckmorton K, Mishina Y, Nicolis SK, Sun X. Signaling through BMP receptors promotes respiratory identity in the foregut via repression of Sox2. Development 138: 971–981, 2011. doi: 10.1242/dev.053694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dovey JS, Zacharek SJ, Kim CF, Lees JA. Bmi1 is critical for lung tumorigenesis and bronchioalveolar stem cell expansion. Proc Natl Acad Sci USA 105: 11857–11862, 2008. doi: 10.1073/pnas.0803574105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Du Y, Guo M, Whitsett JA, Xu Y. ‘LungGENS’: a web-based tool for mapping single-cell gene expression in the developing lung. Thorax 70: 1092–1094, 2015. doi: 10.1136/thoraxjnl-2015-207035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Du Y, Kitzmiller JA, Sridharan A, Perl AK, Bridges JP, Misra RS, Pryhuber GS, Mariani TJ, Bhattacharya S, Guo M, Potter SS, Dexheimer P, Aronow B, Jobe AH, Whitsett JA, Xu Y. Lung Gene Expression Analysis (LGEA): an integrative web portal for comprehensive gene expression data analysis in lung development. Thorax 72: 481–484, 2017. doi: 10.1136/thoraxjnl-2016-209598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dye BR, Hill DR, Ferguson MA, Tsai YH, Nagy MS, Dyal R, Wells JM, Mayhew CN, Nattiv R, Klein OD, White ES, Deutsch GH, Spence JR. In vitro generation of human pluripotent stem cell derived lung organoids. eLife 4: e05098, 2015. doi: 10.7554/eLife.05098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.El Agha E, Bellusci S. Walking along the Fibroblast Growth Factor 10 Route: A Key Pathway to Understand the Control and Regulation of Epithelial and Mesenchymal Cell-Lineage Formation during Lung Development and Repair after Injury. Scientifica (Cairo) 2014: 538379, 2014. doi: 10.1155/2014/538379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.El Agha E, Herold S, Al Alam D, Quantius J, MacKenzie B, Carraro G, Moiseenko A, Chao CM, Minoo P, Seeger W, Bellusci S. Fgf10-positive cells represent a progenitor cell population during lung development and postnatally. Development 141: 296–306, 2014. doi: 10.1242/dev.099747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Endale M, Ahlfeld S, Bao E, Chen X, Green J, Bess Z, Weirauch MT, Xu Y, Perl AK. Temporal, spatial, and phenotypical changes of PDGFRα expressing fibroblasts during late lung development. Dev Biol 425: 161–175, 2017. doi: 10.1016/j.ydbio.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ermund A, Meiss LN, Rodriguez-Pineiro AM, Bähr A, Nilsson HE, Trillo-Muyo S, Ridley C, Thornton DJ, Wine JJ, Hebert H, Klymiuk N, Hansson GC. The normal trachea is cleaned by MUC5B mucin bundles from the submucosal glands coated with the MUC5AC mucin. Biochem Biophys Res Commun 492: 331–337, 2017. doi: 10.1016/j.bbrc.2017.08.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Evans CM, Fingerlin TE, Schwarz MI, Lynch D, Kurche J, Warg L, Yang IV, Schwartz DA. Idiopathic Pulmonary Fibrosis: A Genetic Disease That Involves Mucociliary Dysfunction of the Peripheral Airways. Physiol Rev 96: 1567–1591, 2016. doi: 10.1152/physrev.00004.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Evans CM, Raclawska DS, Ttofali F, Liptzin DR, Fletcher AA, Harper DN, McGing MA, McElwee MM, Williams OW, Sanchez E, Roy MG, Kindrachuk KN, Wynn TA, Eltzschig HK, Blackburn MR, Tuvim MJ, Janssen WJ, Schwartz DA, Dickey BF. The polymeric mucin Muc5ac is required for allergic airway hyperreactivity. Nat Commun 6: 6281, 2015. doi: 10.1038/ncomms7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol 22: 142–150, 1975. doi: 10.1016/0014-4800(75)90059-3. [DOI] [PubMed] [Google Scholar]
- 83.Evans MJ, Moller PC. Biology of airway basal cells. Exp Lung Res 17: 513–531, 1991. doi: 10.3109/01902149109062862. [DOI] [PubMed] [Google Scholar]
- 84.Evans MJ, Shami SG, Cabral-Anderson LJ, Dekker NP. Role of nonciliated cells in renewal of the bronchial epithelium of rats exposed to NO2. Am J Pathol 123: 126–133, 1986. [PMC free article] [PubMed] [Google Scholar]
- 85.Fang S, Wei J, Pentinmikko N, Leinonen H, Salven P. Generation of functional blood vessels from a single c-kit+ adult vascular endothelial stem cell. PLoS Biol 10: e1001407, 2012. doi: 10.1371/journal.pbio.1001407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fernandes DJ, McConville JF, Stewart AG, Kalinichenko V, Solway J. Can we differentiate between airway and vascular smooth muscle? Clin Exp Pharmacol Physiol 31: 805–810, 2004. doi: 10.1111/j.1440-1681.2004.04084.x. [DOI] [PubMed] [Google Scholar]
- 87.Ferrara N. Vascular endothelial growth factor. Eur J Cancer 32: 2413–2422, 1996. doi: 10.1016/S0959-8049(96)00387-5. [DOI] [PubMed] [Google Scholar]
- 88.Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev 18: 901–911, 2004. doi: 10.1101/gad.291004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 376: 66–70, 1995. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 90.François M, Caprini A, Hosking B, Orsenigo F, Wilhelm D, Browne C, Paavonen K, Karnezis T, Shayan R, Downes M, Davidson T, Tutt D, Cheah KS, Stacker SA, Muscat GE, Achen MG, Dejana E, Koopman P. Sox18 induces development of the lymphatic vasculature in mice. Nature 456: 643–647, 2008. doi: 10.1038/nature07391. [DOI] [PubMed] [Google Scholar]
- 91.Frank DB, Peng T, Zepp JA, Snitow M, Vincent TL, Penkala IJ, Cui Z, Herriges MJ, Morley MP, Zhou S, Lu MM, Morrisey EE. Emergence of a Wave of Wnt Signaling that Regulates Lung Alveologenesis by Controlling Epithelial Self-Renewal and Differentiation. Cell Reports 17: 2312–2325, 2016. doi: 10.1016/j.celrep.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Frid MG, Shekhonin BV, Koteliansky VE, Glukhova MA. Phenotypic changes of human smooth muscle cells during development: late expression of heavy caldesmon and calponin. Dev Biol 153: 185–193, 1992. doi: 10.1016/0012-1606(92)90104-O. [DOI] [PubMed] [Google Scholar]
- 93.Galambos C, deMello DE. Molecular mechanisms of pulmonary vascular development. Pediatr Dev Pathol 10: 1–18, 2007. doi: 10.2350/06-06-0122.1. [DOI] [PubMed] [Google Scholar]
- 94.Gallione CJ, Repetto GM, Legius E, Rustgi AK, Schelley SL, Tejpar S, Mitchell G, Drouin E, Westermann CJ, Marchuk DA. A combined syndrome of juvenile polyposis and hereditary haemorrhagic telangiectasia associated with mutations in MADH4 (SMAD4). Lancet 363: 852–859, 2004. doi: 10.1016/S0140-6736(04)15732-2. [DOI] [PubMed] [Google Scholar]
- 95.Gao X, Bali AS, Randell SH, Hogan BL. GRHL2 coordinates regeneration of a polarized mucociliary epithelium from basal stem cells. J Cell Biol 211: 669–682, 2015. doi: 10.1083/jcb.201506014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gao X, Vockley CM, Pauli F, Newberry KM, Xue Y, Randell SH, Reddy TE, Hogan BL. Evidence for multiple roles for grainyhead-like 2 in the establishment and maintenance of human mucociliary airway epithelium [corrected]. Proc Natl Acad Sci USA 110: 9356–9361, 2013. doi: 10.1073/pnas.1307589110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gao Y, Cornfield DN, Stenmark KR, Thébaud B, Abman SH, Raj JU. Unique aspects of the developing lung circulation: structural development and regulation of vasomotor tone. Pulm Circ 6: 407–425, 2016. doi: 10.1086/688890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gerbe F, Jay P. Intestinal tuft cells: epithelial sentinels linking luminal cues to the immune system. Mucosal Immunol 9: 1353–1359, 2016. doi: 10.1038/mi.2016.68. [DOI] [PubMed] [Google Scholar]
- 99.Gerbe F, Sidot E, Smyth DJ, Ohmoto M, Matsumoto I, Dardalhon V, Cesses P, Garnier L, Pouzolles M, Brulin B, Bruschi M, Harcus Y, Zimmermann VS, Taylor N, Maizels RM, Jay P. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 529: 226–230, 2016. doi: 10.1038/nature16527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gerovac BJ, Fregien NL. IL-13 Inhibits Multicilin Expression and Ciliogenesis via Janus Kinase/Signal Transducer and Activator of Transcription Independently of Notch Cleavage. Am J Respir Cell Mol Biol 54: 554–561, 2016. doi: 10.1165/rcmb.2015-0227OC. [DOI] [PubMed] [Google Scholar]
- 101.Goumans MJ, Mummery C. Functional analysis of the TGFbeta receptor/Smad pathway through gene ablation in mice. Int J Dev Biol 44: 253–265, 2000. [PubMed] [Google Scholar]
- 102.Goumans MJ, Valdimarsdottir G, Itoh S, Lebrin F, Larsson J, Mummery C, Karlsson S, ten Dijke P. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signaling. Mol Cell 12: 817–828, 2003. doi: 10.1016/S1097-2765(03)00386-1. [DOI] [PubMed] [Google Scholar]
- 103.Goumans MJ, Zwijsen A, van Rooijen MA, Huylebroeck D, Roelen BA, Mummery CL. Transforming growth factor-beta signalling in extraembryonic mesoderm is required for yolk sac vasculogenesis in mice. Development 126: 3473–3483, 1999. [DOI] [PubMed] [Google Scholar]
- 104.Grindley JC, Bellusci S, Perkins D, Hogan BL. Evidence for the involvement of the Gli gene family in embryonic mouse lung development. Dev Biol 188: 337–348, 1997. doi: 10.1006/dbio.1997.8644. [DOI] [PubMed] [Google Scholar]
- 105.Grzenda A, Shannon J, Fisher J, Arkovitz MS. Timing and expression of the angiopoietin-1-Tie-2 pathway in murine lung development and congenital diaphragmatic hernia. Dis Model Mech 6: 106–114, 2013. doi: 10.1242/dmm.008821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guha A, Deshpande A, Jain A, Sebastiani P, Cardoso WV. Uroplakin 3a+ Cells Are a Distinctive Population of Epithelial Progenitors that Contribute to Airway Maintenance and Post-injury Repair. Cell Reports 19: 246–254, 2017. doi: 10.1016/j.celrep.2017.03.051. [DOI] [PubMed] [Google Scholar]
- 107.Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, Deswarte K, Malissen B, Hammad H, Lambrecht BN. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med 210: 1977–1992, 2013. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gulyás M, Hjerpe A. Proteoglycans and WT1 as markers for distinguishing adenocarcinoma, epithelioid mesothelioma, and benign mesothelium. J Pathol 199: 479–487, 2003. doi: 10.1002/path.1312. [DOI] [PubMed] [Google Scholar]
- 109.Guo M, Bao EL, Wagner M, Whitsett JA, Xu Y. SLICE: determining cell differentiation and lineage based on single cell entropy. Nucleic Acids Res 45: e54, 2017. doi: 10.1093/nar/gkw1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guo M, Wang H, Potter SS, Whitsett JA, Xu Y. SINCERA: A Pipeline for Single-Cell RNA-Seq Profiling Analysis. PLOS Comput Biol 11: e1004575, 2015. doi: 10.1371/journal.pcbi.1004575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Habermehl D, Parkitna JR, Kaden S, Brügger B, Wieland F, Gröne HJ, Schütz G. Glucocorticoid activity during lung maturation is essential in mesenchymal and less in alveolar epithelial cells. Mol Endocrinol 25: 1280–1288, 2011. doi: 10.1210/me.2009-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Haczku A. Protective role of the lung collectins surfactant protein A and surfactant protein D in airway inflammation. J Allergy Clin Immunol 122: 861–879, 2008. doi: 10.1016/j.jaci.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Han L, Xu J, Grigg E, Slack M, Chaturvedi P, Jiang R, Zorn AM. Osr1 functions downstream of Hedgehog pathway to regulate foregut development. Dev Biol 427: 72–83, 2017. doi: 10.1016/j.ydbio.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Han RN, Babaei S, Robb M, Lee T, Ridsdale R, Ackerley C, Post M, Stewart DJ. Defective lung vascular development and fatal respiratory distress in endothelial NO synthase-deficient mice: a model of alveolar capillary dysplasia? Circ Res 94: 1115–1123, 2004. doi: 10.1161/01.RES.0000125624.85852.1E. [DOI] [PubMed] [Google Scholar]
- 115.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, García-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38: 792–804, 2013. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Havrilak JA, Melton KR, Shannon JM. Endothelial cells are not required for specification of respiratory progenitors. Dev Biol 427: 93–105, 2017. doi: 10.1016/j.ydbio.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hegab AE, Ha VL, Darmawan DO, Gilbert JL, Ooi AT, Attiga YS, Bisht B, Nickerson DW, Gomperts BN. Isolation and in vitro characterization of basal and submucosal gland duct stem/progenitor cells from human proximal airways. Stem Cells Transl Med 1: 719–724, 2012. doi: 10.5966/sctm.2012-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hellström M, Kalén M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126: 3047–3055, 1999. [DOI] [PubMed] [Google Scholar]
- 119.Herold S, von Wulffen W, Steinmueller M, Pleschka S, Kuziel WA, Mack M, Srivastava M, Seeger W, Maus UA, Lohmeyer J. Alveolar epithelial cells direct monocyte transepithelial migration upon influenza virus infection: impact of chemokines and adhesion molecules. J Immunol 177: 1817–1824, 2006. doi: 10.4049/jimmunol.177.3.1817. [DOI] [PubMed] [Google Scholar]
- 120.Herriges M, Morrisey EE. Lung development: orchestrating the generation and regeneration of a complex organ. Development 141: 502–513, 2014. doi: 10.1242/dev.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Herriges MJ, Tischfield DJ, Cui Z, Morley MP, Han Y, Babu A, Li S, Lu M, Cendan I, Garcia BA, Anderson SA, Morrisey EE. The NANCI-Nkx2.1 gene duplex buffers Nkx2.1 expression to maintain lung development and homeostasis. Genes Dev 31: 889–903, 2017. doi: 10.1101/gad.298018.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hill JJ, Tremblay TL, Fauteux F, Li J, Wang E, Aguilar-Mahecha A, Basik M, O’Connor-McCourt M. Glycoproteomic comparison of clinical triple-negative and luminal breast tumors. J Proteome Res 14: 1376–1388, 2015. doi: 10.1021/pr500987r. [DOI] [PubMed] [Google Scholar]
- 123.Hines EA, Sun X. Tissue crosstalk in lung development. J Cell Biochem 115: 1469–1477, 2014. doi: 10.1002/jcb.24811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, See P, Beaudin AE, Lum J, Low I, Forsberg EC, Poidinger M, Zolezzi F, Larbi A, Ng LG, Chan JK, Greter M, Becher B, Samokhvalov IM, Merad M, Ginhoux F. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 42: 665–678, 2015. doi: 10.1016/j.immuni.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hoffmann F, Ender F, Schmudde I, Lewkowich IP, Köhl J, König P, Laumonnier Y. Origin, Localization, and Immunoregulatory Properties of Pulmonary Phagocytes in Allergic Asthma. Front Immunol 7: 107, 2016. doi: 10.3389/fimmu.2016.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hogan BL, Barkauskas CE, Chapman HA, Epstein JA, Jain R, Hsia CC, Niklason L, Calle E, Le A, Randell SH, Rock J, Snitow M, Krummel M, Stripp BR, Vu T, White ES, Whitsett JA, Morrisey EE. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell 15: 123–138, 2014. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Houston CS, Opitz JM, Spranger JW, Macpherson RI, Reed MH, Gilbert EF, Herrmann J, Schinzel A. The campomelic syndrome: review, report of 17 cases, and follow-up on the currently 17-year-old boy first reported by Maroteaux et al in 1971. Am J Med Genet 15: 3–28, 1983. doi: 10.1002/ajmg.1320150103. [DOI] [PubMed] [Google Scholar]
- 128.Hrycaj SM, Dye BR, Baker NC, Larsen BM, Burke AC, Spence JR, Wellik DM. Hox5 Genes Regulate the Wnt2/2b-Bmp4-Signaling Axis during Lung Development. Cell Reports 12: 903–912, 2015. doi: 10.1016/j.celrep.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, Xu Q. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest 113: 1258–1265, 2004. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huang SX, Green MD, de Carvalho AT, Mumau M, Chen YW, D’Souza SL, Snoeck HW. The in vitro generation of lung and airway progenitor cells from human pluripotent stem cells. Nat Protoc 10: 413–425, 2015. doi: 10.1038/nprot.2015.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huang SX, Islam MN, O’Neill J, Hu Z, Yang YG, Chen YW, Mumau M, Green MD, Vunjak-Novakovic G, Bhattacharya J, Snoeck HW. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol 32: 84–91, 2014. doi: 10.1038/nbt.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hung C, Linn G, Chow YH, Kobayashi A, Mittelsteadt K, Altemeier WA, Gharib SA, Schnapp LM, Duffield JS. Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med 188: 820–830, 2013. doi: 10.1164/rccm.201212-2297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol 14: 81–93, 2014. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 134.Ihida-Stansbury K, McKean DM, Gebb SA, Martin JF, Stevens T, Nemenoff R, Akeson A, Vaughn J, Jones PL. Paired-related homeobox gene Prx1 is required for pulmonary vascular development. Circ Res 94: 1507–1514, 2004. doi: 10.1161/01.RES.0000130656.72424.20. [DOI] [PubMed] [Google Scholar]
- 135.Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med 18: 759–765, 2012. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ito Y, Correll K, Zemans RL, Leslie CC, Murphy RC, Mason RJ. Influenza induces IL-8 and GM-CSF secretion by human alveolar epithelial cells through HGF/c-Met and TGF-α/EGFR signaling. Am J Physiol Lung Cell Mol Physiol 308: L1178–L1188, 2015. doi: 10.1152/ajplung.00290.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jacob A, Morley M, Hawkins F, McCauley KB, Jean JC, Heins H, Na CL, Weaver TE, Vedaie M, Hurley K, Hinds A, Russo SJ, Kook S, Zacharias W, Ochs M, Traber K, Quinton LJ, Crane A, Davis BR, White FV, Wambach J, Whitsett JA, Cole FS, Morrisey EE, Guttentag SH, Beers MF, Kotton DN. Differentiation of Human Pluripotent Stem Cells into Functional Lung Alveolar Epithelial Cells. Cell Stem Cell 21: 472–488.e10, 2017. doi: 10.1016/j.stem.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ji CM, Plopper CG, Pinkerton KE. Clara cell heterogeneity in differentiation: correlation with proliferation, ultrastructural composition, and cell position in the rat bronchiole. Am J Respir Cell Mol Biol 13: 144–151, 1995. doi: 10.1165/ajrcmb.13.2.7626284. [DOI] [PubMed] [Google Scholar]
- 139.Jia S, Wildner H, Birchmeier C. Insm1 controls the differentiation of pulmonary neuroendocrine cells by repressing Hes1. Dev Biol 408: 90–98, 2015. doi: 10.1016/j.ydbio.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 140.Jiang Z, Cushing L, Ai X, Lü J. miR-326 is downstream of Sonic hedgehog signaling and regulates the expression of Gli2 and smoothened. Am J Respir Cell Mol Biol 51: 273–283, 2014. doi: 10.1165/rcmb.2013-0127OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jones FS, Meech R, Edelman DB, Oakey RJ, Jones PL. Prx1 controls vascular smooth muscle cell proliferation and tenascin-C expression and is upregulated with Prx2 in pulmonary vascular disease. Circ Res 89: 131–138, 2001. doi: 10.1161/hh1401.093582. [DOI] [PubMed] [Google Scholar]
- 142.Jones PL. Homeobox genes in pulmonary vascular development and disease. Trends Cardiovasc Med 13: 336–345, 2003. doi: 10.1016/j.tcm.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 143.Joza S, Wang J, Fox E, Hillman V, Ackerley C, Post M. Loss of semaphorin-neuropilin-1 signaling causes dysmorphic vascularization reminiscent of alveolar capillary dysplasia. Am J Pathol 181: 2003–2017, 2012. doi: 10.1016/j.ajpath.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 144.Kachali C, Eltoum I, Horton D, Chhieng DC. Use of mesothelin as a marker for mesothelial cells in cytologic specimens. Semin Diagn Pathol 23: 20–24, 2006. doi: 10.1053/j.semdp.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 145.Kadzik RS, Cohen ED, Morley MP, Stewart KM, Lu MM, Morrisey EE. Wnt ligand/Frizzled 2 receptor signaling regulates tube shape and branch-point formation in the lung through control of epithelial cell shape. Proc Natl Acad Sci USA 111: 12444–12449, 2014. doi: 10.1073/pnas.1406639111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kalin TV, Meliton L, Meliton AY, Zhu X, Whitsett JA, Kalinichenko VV. Pulmonary mastocytosis and enhanced lung inflammation in mice heterozygous null for the Foxf1 gene. Am J Respir Cell Mol Biol 39: 390–399, 2008. doi: 10.1165/rcmb.2008-0044OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kalin TV, Ustiyan V, Kalinichenko VV. Multiple faces of FoxM1 transcription factor: lessons from transgenic mouse models. Cell Cycle 10: 396–405, 2011. doi: 10.4161/cc.10.3.14709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kalin TV, Wang IC, Meliton L, Zhang Y, Wert SE, Ren X, Snyder J, Bell SM, Graf L Jr, Whitsett JA, Kalinichenko VV. Forkhead Box m1 transcription factor is required for perinatal lung function. Proc Natl Acad Sci USA 105: 19330–19335, 2008. doi: 10.1073/pnas.0806748105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kalinichenko VV, Gusarova GA, Kim IM, Shin B, Yoder HM, Clark J, Sapozhnikov AM, Whitsett JA, Costa RH. Foxf1 haploinsufficiency reduces Notch-2 signaling during mouse lung development. Am J Physiol Lung Cell Mol Physiol 286: L521–L530, 2004. doi: 10.1152/ajplung.00212.2003. [DOI] [PubMed] [Google Scholar]
- 150.Kalinichenko VV, Gusarova GA, Shin B, Costa RH. The forkhead box F1 transcription factor is expressed in brain and head mesenchyme during mouse embryonic development. Gene Expr Patterns 3: 153–158, 2003. doi: 10.1016/S1567-133X(03)00010-3. [DOI] [PubMed] [Google Scholar]
- 151.Kalinichenko VV, Lim L, Shin B, Costa RH. Differential expression of forkhead box transcription factors following butylated hydroxytoluene lung injury. Am J Physiol Lung Cell Mol Physiol 280: L695–L704, 2001. doi: 10.1152/ajplung.2001.280.4.L695. [DOI] [PubMed] [Google Scholar]
- 152.Kalinichenko VV, Lim L, Stolz DB, Shin B, Rausa FM, Clark J, Whitsett JA, Watkins SC, Costa RH. Defects in pulmonary vasculature and perinatal lung hemorrhage in mice heterozygous null for the Forkhead Box f1 transcription factor. Dev Biol 235: 489–506, 2001. doi: 10.1006/dbio.2001.0322. [DOI] [PubMed] [Google Scholar]
- 153.Kalinichenko VV, Zhou Y, Shin B, Stolz DB, Watkins SC, Whitsett JA, Costa RH. Wild-type levels of the mouse Forkhead Box f1 gene are essential for lung repair. Am J Physiol Lung Cell Mol Physiol 282: L1253–L1265, 2002. doi: 10.1152/ajplung.00463.2001. [DOI] [PubMed] [Google Scholar]
- 154.Kanai-Azuma M, Kanai Y, Gad JM, Tajima Y, Taya C, Kurohmaru M, Sanai Y, Yonekawa H, Yazaki K, Tam PP, Hayashi Y. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development 129: 2367–2379, 2002. [DOI] [PubMed] [Google Scholar]
- 155.Katheria AC, Masliah E, Benirschke K, Jones KL, Kim JH. Idiopathic persistent pulmonary hypertension in an infant with Smith-Lemli-Opitz syndrome. Fetal Pediatr Pathol 29: 373–379, 2010. doi: 10.3109/15513815.2010.512045. [DOI] [PubMed] [Google Scholar]
- 156.Kida H, Mucenski ML, Thitoff AR, Le Cras TD, Park KS, Ikegami M, Müller W, Whitsett JA. GP130-STAT3 regulates epithelial cell migration and is required for repair of the bronchiolar epithelium. Am J Pathol 172: 1542–1554, 2008. doi: 10.2353/ajpath.2008.071052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 121: 823–835, 2005. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 158.Kim IM, Ramakrishna S, Gusarova GA, Yoder HM, Costa RH, Kalinichenko VV. The forkhead box m1 transcription factor is essential for embryonic development of pulmonary vasculature. J Biol Chem 280: 22278–22286, 2005. doi: 10.1074/jbc.M500936200. [DOI] [PubMed] [Google Scholar]
- 159.Kim IM, Zhou Y, Ramakrishna S, Hughes DE, Solway J, Costa RH, Kalinichenko VV. Functional characterization of evolutionarily conserved DNA regions in forkhead box f1 gene locus. J Biol Chem 280: 37908–37916, 2005. doi: 10.1074/jbc.M506531200. [DOI] [PubMed] [Google Scholar]
- 160.Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM, Gonzalez FJ. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev 10: 60–69, 1996. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- 161.Kimura Y, Suzuki T, Kaneko C, Darnel AD, Moriya T, Suzuki S, Handa M, Ebina M, Nukiwa T, Sasano H. Retinoid receptors in the developing human lung. Clin Sci (Lond) 103: 613–621, 2002. doi: 10.1042/cs1030613. [DOI] [PubMed] [Google Scholar]
- 162.Kitaoka H, Takaki R, Suki B. A three-dimensional model of the human airway tree. J Appl Physiol (1985) 87: 2207–2217, 1999. doi: 10.1152/jappl.1999.87.6.2207. [DOI] [PubMed] [Google Scholar]
- 163.Klos Dehring DA, Vladar EK, Werner ME, Mitchell JW, Hwang P, Mitchell BJ. Deuterosome-mediated centriole biogenesis. Dev Cell 27: 103–112, 2013. doi: 10.1016/j.devcel.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kool H, Mous D, Tibboel D, de Klein A, Rottier RJ. Pulmonary vascular development goes awry in congenital lung abnormalities. Birth Defects Res C Embryo Today 102: 343–358, 2014. doi: 10.1002/bdrc.21085. [DOI] [PubMed] [Google Scholar]
- 165.Kopf M, Schneider C, Nobs SP. The development and function of lung-resident macrophages and dendritic cells. Nat Immunol 16: 36–44, 2015. doi: 10.1038/ni.3052. [DOI] [PubMed] [Google Scholar]
- 166.Kotton DN, Morrisey EE. Lung regeneration: mechanisms, applications and emerging stem cell populations. Nat Med 20: 822–832, 2014. doi: 10.1038/nm.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Krasteva G, Kummer W. “Tasting” the airway lining fluid. Histochem Cell Biol 138: 365–383, 2012. doi: 10.1007/s00418-012-0993-5. [DOI] [PubMed] [Google Scholar]
- 168.Krupczak-Hollis K, Wang X, Kalinichenko VV, Gusarova GA, Wang IC, Dennewitz MB, Yoder HM, Kiyokawa H, Kaestner KH, Costa RH. The mouse Forkhead Box m1 transcription factor is essential for hepatoblast mitosis and development of intrahepatic bile ducts and vessels during liver morphogenesis. Dev Biol 276: 74–88, 2004. doi: 10.1016/j.ydbio.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 169.Kubis N, Levy BI. Vasculogenesis and angiogenesis: molecular and cellular controls. Part 1: growth factors. Interv Neuroradiol 9: 227–237, 2003. doi: 10.1177/159101990300900301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kubis N, Levy BI. Vasculogenesis and Angiogenesis: Molecular and Cellular Controls. Part 2: Interactions between Cell and Extracellular Environment. Interv Neuroradiol 9: 239–248, 2003. doi: 10.1177/159101990300900302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kumar ME, Bogard PE, Espinoza FH, Menke DB, Kingsley DM, Krasnow MA. Mesenchymal cells. Defining a mesenchymal progenitor niche at single-cell resolution. Science 346: 1258810, 2014. doi: 10.1126/science.1258810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, Sun Y, Joo LS, Dagher R, Zielonka EM, Wang Y, Lim B, Chow VT, Crum CP, Xian W, McKeon F. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell 147: 525–538, 2011. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kumar VH, Lakshminrusimha S, El Abiad MT, Chess PR, Ryan RM. Growth factors in lung development. Adv Clin Chem 40: 261–316, 2005. doi: 10.1016/S0065-2423(05)40007-4. [DOI] [PubMed] [Google Scholar]
- 174.Kume T, Jiang H, Topczewska JM, Hogan BL. The murine winged helix transcription factors, Foxc1 and Foxc2, are both required for cardiovascular development and somitogenesis. Genes Dev 15: 2470–2482, 2001. doi: 10.1101/gad.907301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kuo CS, Krasnow MA. Formation of a Neurosensory Organ by Epithelial Cell Slithering. Cell 163: 394–405, 2015. doi: 10.1016/j.cell.2015.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lan Y, Liu B, Yao H, Li F, Weng T, Yang G, Li W, Cheng X, Mao N, Yang X. Essential role of endothelial Smad4 in vascular remodeling and integrity. Mol Cell Biol 27: 7683–7692, 2007. doi: 10.1128/MCB.00577-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lange AW, Haitchi HM, LeCras TD, Sridharan A, Xu Y, Wert SE, James J, Udell N, Thurner PJ, Whitsett JA. Sox17 is required for normal pulmonary vascular morphogenesis. Dev Biol 387: 109–120, 2014. doi: 10.1016/j.ydbio.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lange AW, Sridharan A, Xu Y, Stripp BR, Perl AK, Whitsett JA. Hippo/Yap signaling controls epithelial progenitor cell proliferation and differentiation in the embryonic and adult lung. J Mol Cell Biol 7: 35–47, 2015. doi: 10.1093/jmcb/mju046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Larsson J, Goumans MJ, Sjöstrand LJ, van Rooijen MA, Ward D, Levéen P, Xu X, ten Dijke P, Mummery CL, Karlsson S. Abnormal angiogenesis but intact hematopoietic potential in TGF-beta type I receptor-deficient mice. EMBO J 20: 1663–1673, 2001. doi: 10.1093/emboj/20.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lawson GW, Van Winkle LS, Toskala E, Senior RM, Parks WC, Plopper CG. Mouse strain modulates the role of the ciliated cell in acute tracheobronchial airway injury-distal airways. Am J Pathol 160: 315–327, 2002. doi: 10.1016/S0002-9440(10)64375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lechner AJ, Driver IH, Lee J, Conroy CM, Nagle A, Locksley RM, Rock JR. Recruited Monocytes and Type 2 Immunity Promote Lung Regeneration following Pneumonectomy. Cell Stem Cell 21: 120–134.e7, 2017. doi: 10.1016/j.stem.2017.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lee JH, Bhang DH, Beede A, Huang TL, Stripp BR, Bloch KD, Wagers AJ, Tseng YH, Ryeom S, Kim CF. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell 156: 440–455, 2014. doi: 10.1016/j.cell.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lee JH, Tammela T, Hofree M, Choi J, Marjanovic ND, Han S, Canner D, Wu K, Paschini M, Bhang DH, Jacks T, Regev A, Kim CF. Anatomically and Functionally Distinct Lung Mesenchymal Populations Marked by Lgr5 and Lgr6. Cell 170: 1149–1163.e12, 2017. doi: 10.1016/j.cell.2017.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lee JM, Kwon HJ, Lai WF, Jung HS. Requirement of Runx3 in pulmonary vasculogenesis. Cell Tissue Res 356: 445–449, 2014. doi: 10.1007/s00441-014-1816-x. [DOI] [PubMed] [Google Scholar]
- 185.Lee SH, Lee S, Yang H, Song S, Kim K, Saunders TL, Yoon JK, Koh GY, Kim I. Notch pathway targets proangiogenic regulator Sox17 to restrict angiogenesis. Circ Res 115: 215–226, 2014. doi: 10.1161/CIRCRESAHA.115.303142. [DOI] [PubMed] [Google Scholar]
- 186.Lelièvre E, Lionneton F, Soncin F, Vandenbunder B. The Ets family contains transcriptional activators and repressors involved in angiogenesis. Int J Biochem Cell Biol 33: 391–407, 2001. doi: 10.1016/S1357-2725(01)00025-5. [DOI] [PubMed] [Google Scholar]
- 187.Levéen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev 8: 1875–1887, 1994. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- 188.Li A, Ma S, Smith SM, Lee MK, Fischer A, Borok Z, Bellusci S, Li C, Minoo P. Mesodermal ALK5 controls lung myofibroblast versus lipofibroblast cell fate. BMC Biol 14: 19, 2016. doi: 10.1186/s12915-016-0242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Li C, Li M, Li S, Xing Y, Yang CY, Li A, Borok Z, De Langhe S, Minoo P. Progenitors of secondary crest myofibroblasts are developmentally committed in early lung mesoderm. Stem Cells 33: 999–1012, 2015. doi: 10.1002/stem.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, Boak BB, Wendel DP. Defective angiogenesis in mice lacking endoglin. Science 284: 1534–1537, 1999. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- 191.Li L, Wei J. A newborn with anophthalmia and pulmonary hypoplasia (the Matthew-Wood syndrome). Am J Med Genet A 140: 1564–1566, 2006. doi: 10.1002/ajmg.a.31298. [DOI] [PubMed] [Google Scholar]
- 192.Lin C, Yao E, Zhang K, Jiang X, Croll S, Thompson-Peer K, Chuang PT. YAP is essential for mechanical force production and epithelial cell proliferation during lung branching morphogenesis. eLife 6: e21130, 2017. doi: 10.7554/eLife.21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lindahl P, Karlsson L, Hellström M, Gebre-Medhin S, Willetts K, Heath JK, Betsholtz C. Alveogenesis failure in PDGF-A-deficient mice is coupled to lack of distal spreading of alveolar smooth muscle cell progenitors during lung development. Development 124: 3943–3953, 1997. [DOI] [PubMed] [Google Scholar]
- 194.Liu T, Hu B, Choi YY, Chung M, Ullenbruch M, Yu H, Lowe JB, Phan SH. Notch1 signaling in FIZZ1 induction of myofibroblast differentiation. Am J Pathol 174: 1745–1755, 2009. doi: 10.2353/ajpath.2009.080618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lovric G, Mokso R, Arcadu F, Vogiatzis Oikonomidis I, Schittny JC, Roth-Kleiner M, Stampanoni M. Tomographic in vivo microscopy for the study of lung physiology at the alveolar level. Sci Rep 7: 12545, 2017. doi: 10.1038/s41598-017-12886-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lüdtke TH, Rudat C, Wojahn I, Weiss AC, Kleppa MJ, Kurz J, Farin HF, Moon A, Christoffels VM, Kispert A. Tbx2 and Tbx3 Act Downstream of Shh to Maintain Canonical Wnt Signaling during Branching Morphogenesis of the Murine Lung. Dev Cell 39: 239–253, 2016. doi: 10.1016/j.devcel.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 197.Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O’Reilly R, Bader BL, Hynes RO, Zhuang Y, Manova K, Benezra R. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature 401: 670–677, 1999. doi: 10.1038/44334. [DOI] [PubMed] [Google Scholar]
- 198.Lynch TJ, Anderson PJ, Xie W, Crooke AK, Liu X, Tyler SR, Luo M, Kusner DM, Zhang Y, Neff T, Burnette DC, Walters KS, Goodheart MJ, Parekh KR, Engelhardt JF. Wnt Signaling Regulates Airway Epithelial Stem Cells in Adult Murine Submucosal Glands. Stem Cells 34: 2758–2771, 2016. doi: 10.1002/stem.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Madison BB, McKenna LB, Dolson D, Epstein DJ, Kaestner KH. FoxF1 and FoxL1 link hedgehog signaling and the control of epithelial proliferation in the developing stomach and intestine. J Biol Chem 284: 5936–5944, 2009. doi: 10.1074/jbc.M808103200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mahlapuu M, Enerbäck S, Carlsson P. Haploinsufficiency of the forkhead gene Foxf1, a target for sonic hedgehog signaling, causes lung and foregut malformations. Development 128: 2397–2406, 2001. [DOI] [PubMed] [Google Scholar]
- 201.Mahlapuu M, Ormestad M, Enerbäck S, Carlsson P. The forkhead transcription factor Foxf1 is required for differentiation of extra-embryonic and lateral plate mesoderm. Development 128: 155–166, 2001. [DOI] [PubMed] [Google Scholar]
- 202.Mahoney JE, Mori M, Szymaniak AD, Varelas X, Cardoso WV. The hippo pathway effector Yap controls patterning and differentiation of airway epithelial progenitors. Dev Cell 30: 137–150, 2014. doi: 10.1016/j.devcel.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277: 55–60, 1997. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 204.Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol 27: 1248–1258, 2007. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- 205.Malpel S, Mendelsohn C, Cardoso WV. Regulation of retinoic acid signaling during lung morphogenesis. Development 127: 3057–3067, 2000. [DOI] [PubMed] [Google Scholar]
- 206.Marcelo KL, Goldie LC, Hirschi KK. Regulation of endothelial cell differentiation and specification. Circ Res 112: 1272–1287, 2013. doi: 10.1161/CIRCRESAHA.113.300506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Marshall CB, Mays DJ, Beeler JS, Rosenbluth JM, Boyd KL, Santos Guasch GL, Shaver TM, Tang LJ, Liu Q, Shyr Y, Venters BJ, Magnuson MA, Pietenpol JA. p73 Is Required for Multiciliogenesis and Regulates the Foxj1-Associated Gene Network. Cell Reports 14: 2289–2300, 2016. doi: 10.1016/j.celrep.2016.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Massaro GD, Singh G, Mason R, Plopper CG, Malkinson AM, Gail DB. Biology of the Clara cell. Am J Physiol 266: L101–L106, 1994. [DOI] [PubMed] [Google Scholar]
- 209.Mayer AK, Bartz H, Fey F, Schmidt LM, Dalpke AH. Airway epithelial cells modify immune responses by inducing an anti-inflammatory microenvironment. Eur J Immunol 38: 1689–1699, 2008. doi: 10.1002/eji.200737936. [DOI] [PubMed] [Google Scholar]
- 210.McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, Helmbold EA, Markel DS, McKinnon WC, Murrell J, McCormick MK, Pericak-Vance MA, Heutink P, Oostra BA, Haitjema T, Westerman CJJ, Porteous ME, Guttmacher AE, Letarte M, Marchuk DA. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet 8: 345–351, 1994. doi: 10.1038/ng1294-345. [DOI] [PubMed] [Google Scholar]
- 211.McConnell AM, Yao C, Yeckes AR, Wang Y, Selvaggio AS, Tang J, Kirsch DG, Stripp BR. p53 Regulates Progenitor Cell Quiescence and Differentiation in the Airway. Cell Reports 17: 2173–2182, 2016. doi: 10.1016/j.celrep.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 212.McGowan SE. Paracrine cellular and extracellular matrix interactions with mesenchymal progenitors during pulmonary alveolar septation. Birth Defects Res A Clin Mol Teratol 100: 227–239, 2014. doi: 10.1002/bdra.23230. [DOI] [PubMed] [Google Scholar]
- 213.McGowan SE, Grossmann RE, Kimani PW, Holmes AJ. Platelet-derived growth factor receptor-alpha-expressing cells localize to the alveolar entry ring and have characteristics of myofibroblasts during pulmonary alveolar septal formation. Anat Rec (Hoboken) 291: 1649–1661, 2008. doi: 10.1002/ar.20764. [DOI] [PubMed] [Google Scholar]
- 214.McGowan SE, McCoy DM. Fibroblast growth factor signaling in myofibroblasts differs from lipofibroblasts during alveolar septation in mice. Am J Physiol Lung Cell Mol Physiol 309: L463–L474, 2015. doi: 10.1152/ajplung.00013.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.McGowan SE, McCoy DM. Fibroblasts expressing PDGF-receptor-alpha diminish during alveolar septal thinning in mice. Pediatr Res 70: 44–49, 2011. doi: 10.1203/PDR.0b013e31821cfb5a. [DOI] [PubMed] [Google Scholar]
- 216.McGowan SE, McCoy DM. Platelet-derived growth factor-A and sonic hedgehog signaling direct lung fibroblast precursors during alveolar septal formation. Am J Physiol Lung Cell Mol Physiol 305: L229–L239, 2013. doi: 10.1152/ajplung.00011.2013. [DOI] [PubMed] [Google Scholar]
- 217.McQualter JL, Brouard N, Williams B, Baird BN, Sims-Lucas S, Yuen K, Nilsson SK, Simmons PJ, Bertoncello I. Endogenous fibroblastic progenitor cells in the adult mouse lung are highly enriched in the sca-1 positive cell fraction. Stem Cells 27: 623–633, 2009. doi: 10.1634/stemcells.2008-0866. [DOI] [PubMed] [Google Scholar]
- 218.Metharom P, Caplice NM. Vascular disease: a new progenitor biology. Curr Vasc Pharmacol 5: 61–68, 2007. doi: 10.2174/157016107779317215. [DOI] [PubMed] [Google Scholar]
- 219.Metharom P, Liu C, Wang S, Stalboerger P, Chen G, Doyle B, Ikeda Y, Caplice NM. Myeloid lineage of high proliferative potential human smooth muscle outgrowth cells circulating in blood and vasculogenic smooth muscle-like cells in vivo. Atherosclerosis 198: 29–38, 2008. doi: 10.1016/j.atherosclerosis.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 220.Metzger RJ, Klein OD, Martin GR, Krasnow MA. The branching programme of mouse lung development. Nature 453: 745–750, 2008. doi: 10.1038/nature07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Mihrshahi R, Barclay AN, Brown MH. Essential roles for Dok2 and RasGAP in CD200 receptor-mediated regulation of human myeloid cells. J Immunol 183: 4879–4886, 2009. doi: 10.4049/jimmunol.0901531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Mihrshahi R, Brown MH. Downstream of tyrosine kinase 1 and 2 play opposing roles in CD200 receptor signaling. J Immunol 185: 7216–7222, 2010. doi: 10.4049/jimmunol.1002858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Milewski D, Balli D, Ustiyan V, Le T, Dienemann H, Warth A, Breuhahn K, Whitsett JA, Kalinichenko VV, Kalin TV. FOXM1 activates AGR2 and causes progression of lung adenomas into invasive mucinous adenocarcinomas. PLoS Genet 13: e1007097, 2017. doi: 10.1371/journal.pgen.1007097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Miller LA, Wert SE, Clark JC, Xu Y, Perl AK, Whitsett JA. Role of Sonic hedgehog in patterning of tracheal-bronchial cartilage and the peripheral lung. Dev Dyn 231: 57–71, 2004. doi: 10.1002/dvdy.20105. [DOI] [PubMed] [Google Scholar]
- 225.Miura T. Models of lung branching morphogenesis. J Biochem 157: 121–127, 2015. doi: 10.1093/jb/mvu087. [DOI] [PubMed] [Google Scholar]
- 226.Moghieb A, Clair G, Mitchell HD, Kitzmiller J, Zink EM, Kim YM, Petyuk V, Shukla A, Moore RJ, Metz TO, Carson J, McDermott JE, Corley RA, Whitsett JA, Ansong C. Time-resolved proteome profiling of normal lung development. Am J Physiol Lung Cell Mol Physiol 315: L11–L24, 2018. doi: 10.1152/ajplung.00316.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Morimoto M, Liu Z, Cheng HT, Winters N, Bader D, Kopan R. Canonical Notch signaling in the developing lung is required for determination of arterial smooth muscle cells and selection of Clara versus ciliated cell fate. J Cell Sci 123: 213–224, 2010. doi: 10.1242/jcs.058669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Morimoto M, Nishinakamura R, Saga Y, Kopan R. Different assemblies of Notch receptors coordinate the distribution of the major bronchial Clara, ciliated and neuroendocrine cells. Development 139: 4365–4373, 2012. doi: 10.1242/dev.083840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Morris DG, Huang X, Kaminski N, Wang Y, Shapiro SD, Dolganov G, Glick A, Sheppard D. Loss of integrin alpha(v)beta6-mediated TGF-beta activation causes Mmp12-dependent emphysema. Nature 422: 169–173, 2003. doi: 10.1038/nature01413. [DOI] [PubMed] [Google Scholar]
- 230.Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell 18: 8–23, 2010. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Morty RE, Königshoff M, Eickelberg O. Transforming growth factor-beta signaling across ages: from distorted lung development to chronic obstructive pulmonary disease. Proc Am Thorac Soc 6: 607–613, 2009. doi: 10.1513/pats.200908-087RM. [DOI] [PubMed] [Google Scholar]
- 232.Motoyama J, Liu J, Mo R, Ding Q, Post M, Hui CC. Essential function of Gli2 and Gli3 in the formation of lung, trachea and oesophagus. Nat Genet 20: 54–57, 1998. doi: 10.1038/1711. [DOI] [PubMed] [Google Scholar]
- 233.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. [Correction in Immunity 41: 339–340, 2014.] Immunity 41: 14–20, 2014. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Nabhan AN, Brownfield DG, Harbury PB, Krasnow MA, Desai TJ. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science 359: 1118–1123, 2018. doi: 10.1126/science.aam6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Nakanishi H, Sugiura T, Streisand JB, Lonning SM, Roberts JD Jr. TGF-beta-neutralizing antibodies improve pulmonary alveologenesis and vasculogenesis in the injured newborn lung. Am J Physiol Lung Cell Mol Physiol 293: L151–L161, 2007. doi: 10.1152/ajplung.00389.2006. [DOI] [PubMed] [Google Scholar]
- 236.Nattes E, Lejeune S, Carsin A, Borie R, Gibertini I, Balinotti J, Nathan N, Marchand-Adam S, Thumerelle C, Fauroux B, Bosdure E, Houdouin V, Delestrain C, Louha M, Couderc R, De Becdelievre A, Fanen P, Funalot B, Crestani B, Deschildre A, Dubus JC, Epaud R. Heterogeneity of lung disease associated with NK2 homeobox 1 mutations. Respir Med 129: 16–23, 2017. doi: 10.1016/j.rmed.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 237.Nelson CM, Gleghorn JP, Pang MF, Jaslove JM, Goodwin K, Varner VD, Miller E, Radisky DC, Stone HA. Microfluidic chest cavities reveal that transmural pressure controls the rate of lung development. Development 144: 4328–4335, 2017. doi: 10.1242/dev.154823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Nemajerova A, Kramer D, Siller SS, Herr C, Shomroni O, Pena T, Gallinas Suazo C, Glaser K, Wildung M, Steffen H, Sriraman A, Oberle F, Wienken M, Hennion M, Vidal R, Royen B, Alevra M, Schild D, Bals R, Dönitz J, Riedel D, Bonn S, Takemaru K, Moll UM, Lizé M. TAp73 is a central transcriptional regulator of airway multiciliogenesis. Genes Dev 30: 1300–1312, 2016. doi: 10.1101/gad.279836.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet 33: 407–411, 2003. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- 240.Nevel RJ, Garnett ET, Worrell JA, Morton RL, Nogee LM, Blackwell TS, Young LR. Persistent Lung Disease in Adults with NKX2.1 Mutation and Familial Neuroendocrine Cell Hyperplasia of Infancy. Ann Am Thorac Soc 13: 1299–1304, 2016. doi: 10.1513/AnnalsATS.201603-155BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Newton AH, Cardani A, Braciale TJ. The host immune response in respiratory virus infection: balancing virus clearance and immunopathology. Semin Immunopathol 38: 471–482, 2016. doi: 10.1007/s00281-016-0558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Nikolić MZ, Caritg O, Jeng Q, Johnson JA, Sun D, Howell KJ, Brady JL, Laresgoiti U, Allen G, Butler R, Zilbauer M, Giangreco A, Rawlins EL. Human embryonic lung epithelial tips are multipotent progenitors that can be expanded in vitro as long-term self-renewing organoids. eLife 6: e26575, 2017. doi: 10.7554/eLife.26575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Noguchi M, Sumiyama K, Morimoto M. Directed Migration of Pulmonary Neuroendocrine Cells toward Airway Branches Organizes the Stereotypic Location of Neuroepithelial Bodies. Cell Reports 13: 2679–2686, 2015. doi: 10.1016/j.celrep.2015.11.058. [DOI] [PubMed] [Google Scholar]
- 244.Noseda M, Fu Y, Niessen K, Wong F, Chang L, McLean G, Karsan A. Smooth Muscle alpha-actin is a direct target of Notch/CSL. Circ Res 98: 1468–1470, 2006. doi: 10.1161/01.RES.0000229683.81357.26. [DOI] [PubMed] [Google Scholar]
- 245.Orlova VV, Liu Z, Goumans MJ, ten Dijke P. Controlling angiogenesis by two unique TGF-β type I receptor signaling pathways. Histol Histopathol 26: 1219–1230, 2011. doi: 10.14670/HH-26.1219. [DOI] [PubMed] [Google Scholar]
- 246.Oshima M, Oshima H, Taketo MM. TGF-beta receptor type II deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev Biol 179: 297–302, 1996. doi: 10.1006/dbio.1996.0259. [DOI] [PubMed] [Google Scholar]
- 247.Ostedgaard LS, Moninger TO, McMenimen JD, Sawin NM, Parker CP, Thornell IM, Powers LS, Gansemer ND, Bouzek DC, Cook DP, Meyerholz DK, Abou Alaiwa MH, Stoltz DA, Welsh MJ. Gel-forming mucins form distinct morphologic structures in airways. Proc Natl Acad Sci U S A 114: 6842–6847, 2017. doi: 10.1073/pnas.1703228114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Pardo-Saganta A, Law BM, Tata PR, Villoria J, Saez B, Mou H, Zhao R, Rajagopal J. Injury induces direct lineage segregation of functionally distinct airway basal stem/progenitor cell subpopulations. Cell Stem Cell 16: 184–197, 2015. doi: 10.1016/j.stem.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Parera MC, van Dooren M, van Kempen M, de Krijger R, Grosveld F, Tibboel D, Rottier R. Distal angiogenesis: a new concept for lung vascular morphogenesis. Am J Physiol Lung Cell Mol Physiol 288: L141–L149, 2005. doi: 10.1152/ajplung.00148.2004. [DOI] [PubMed] [Google Scholar]
- 250.Park C, Kim TM, Malik AB. Transcriptional regulation of endothelial cell and vascular development. Circ Res 112: 1380–1400, 2013. doi: 10.1161/CIRCRESAHA.113.301078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Park HL, Bai C, Platt KA, Matise MP, Beeghly A, Hui CC, Nakashima M, Joyner AL. Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development 127: 1593–1605, 2000. [DOI] [PubMed] [Google Scholar]
- 252.Peng T, Frank DB, Kadzik RS, Morley MP, Rathi KS, Wang T, Zhou S, Cheng L, Lu MM, Morrisey EE. Hedgehog actively maintains adult lung quiescence and regulates repair and regeneration. Nature 526: 578–582, 2015. doi: 10.1038/nature14984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Peng T, Tian Y, Boogerd CJ, Lu MM, Kadzik RS, Stewart KM, Evans SM, Morrisey EE. Coordination of heart and lung co-development by a multipotent cardiopulmonary progenitor. Nature 500: 589–592, 2013. doi: 10.1038/nature12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Pereira FA, Qiu Y, Zhou G, Tsai MJ, Tsai SY. The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev 13: 1037–1049, 1999. doi: 10.1101/gad.13.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Perl AK, Riethmacher D, Whitsett JA. Conditional depletion of airway progenitor cells induces peribronchiolar fibrosis. Am J Respir Crit Care Med 183: 511–521, 2011. doi: 10.1164/rccm.201005-0744OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci USA 99: 10482–10487, 2002. doi: 10.1073/pnas.152238499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Perl AK, Zhang L, Whitsett JA. Conditional expression of genes in the respiratory epithelium in transgenic mice: cautionary notes and toward building a better mouse trap. Am J Respir Cell Mol Biol 40: 1–3, 2009. doi: 10.1165/rcmb.2008-0011ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Petrova TV, Karpanen T, Norrmén C, Mellor R, Tamakoshi T, Finegold D, Ferrell R, Kerjaschki D, Mortimer P, Ylä-Herttuala S, Miura N, Alitalo K. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med 10: 974–981, 2004. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- 259.Que J, Luo X, Schwartz RJ, Hogan BL. Multiple roles for Sox2 in the developing and adult mouse trachea. Development 136: 1899–1907, 2009. doi: 10.1242/dev.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Quigley IK, Kintner C. Rfx2 Stabilizes Foxj1 Binding at Chromatin Loops to Enable Multiciliated Cell Gene Expression. PLoS Genet 13: e1006538, 2017. doi: 10.1371/journal.pgen.1006538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Rackley CR, Stripp BR. Building and maintaining the epithelium of the lung. J Clin Invest 122: 2724–2730, 2012. doi: 10.1172/JCI60519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Radzikinas K, Aven L, Jiang Z, Tran T, Paez-Cortez J, Boppidi K, Lu J, Fine A, Ai X. A Shh/miR-206/BDNF cascade coordinates innervation and formation of airway smooth muscle. J Neurosci 31: 15407–15415, 2011. doi: 10.1523/JNEUROSCI.2745-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Rajavelu P, Chen G, Xu Y, Kitzmiller JA, Korfhagen TR, Whitsett JA. Airway epithelial SPDEF integrates goblet cell differentiation and pulmonary Th2 inflammation. J Clin Invest 125: 2021–2031, 2015. doi: 10.1172/JCI79422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Ramakrishna S, Kim IM, Petrovic V, Malin D, Wang IC, Kalin TV, Meliton L, Zhao YY, Ackerson T, Qin Y, Malik AB, Costa RH, Kalinichenko VV. Myocardium defects and ventricular hypoplasia in mice homozygous null for the Forkhead Box M1 transcription factor. Dev Dyn 236: 1000–1013, 2007. doi: 10.1002/dvdy.21113. [DOI] [PubMed] [Google Scholar]
- 265.Ramasamy SK, Kusumbe AP, Adams RH. Regulation of tissue morphogenesis by endothelial cell-derived signals. Trends Cell Biol 25: 148–157, 2015. doi: 10.1016/j.tcb.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Rankin SA, Han L, McCracken KW, Kenny AP, Anglin CT, Grigg EA, Crawford CM, Wells JM, Shannon JM, Zorn AM. A Retinoic Acid-Hedgehog Cascade Coordinates Mesoderm-Inducing Signals and Endoderm Competence during Lung Specification. Cell Reports 16: 66–78, 2016. doi: 10.1016/j.celrep.2016.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Rawlins EL, Clark CP, Xue Y, Hogan BL. The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development 136: 3741–3745, 2009. doi: 10.1242/dev.037317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Ray S, Chiba N, Yao C, Guan X, McConnell AM, Brockway B, Que L, McQualter JL, Stripp BR. Rare SOX2+ Airway Progenitor Cells Generate KRT5+ Cells that Repopulate Damaged Alveolar Parenchyma following Influenza Virus Infection. Stem Cell Rep 7: 817–825, 2016. doi: 10.1016/j.stemcr.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 107: 1164–1169, 2003. doi: 10.1161/01.CIR.0000058702.69484.A0. [DOI] [PubMed] [Google Scholar]
- 270.Ren S, Luo Y, Chen H, Warburton D, Lam HC, Wang LL, Chen P, Henske EP, Shi W. Inactivation of Tsc2 in Mesoderm-Derived Cells Causes Polycystic Kidney Lesions and Impairs Lung Alveolarization. Am J Pathol 186: 3261–3272, 2016. doi: 10.1016/j.ajpath.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Ren X, Shah TA, Ustiyan V, Zhang Y, Shinn J, Chen G, Whitsett JA, Kalin TV, Kalinichenko VV. FOXM1 promotes allergen-induced goblet cell metaplasia and pulmonary inflammation. Mol Cell Biol 33: 371–386, 2013. doi: 10.1128/MCB.00934-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ren X, Ustiyan V, Pradhan A, Cai Y, Havrilak JA, Bolte CS, Shannon JM, Kalin TV, Kalinichenko VV. FOXF1 transcription factor is required for formation of embryonic vasculature by regulating VEGF signaling in endothelial cells. Circ Res 115: 709–720, 2014. doi: 10.1161/CIRCRESAHA.115.304382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Reynolds PR, Mucenski ML, Le Cras TD, Nichols WC, Whitsett JA. Midkine is regulated by hypoxia and causes pulmonary vascular remodeling. J Biol Chem 279: 37124–37132, 2004. doi: 10.1074/jbc.M405254200. [DOI] [PubMed] [Google Scholar]
- 274.Rock JR, Gao X, Xue Y, Randell SH, Kong YY, Hogan BL. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell 8: 639–648, 2011. doi: 10.1016/j.stem.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Rock JR, Hogan BL. Developmental biology. Branching takes nerve. Science 329: 1610–1611, 2010. doi: 10.1126/science.1196016. [DOI] [PubMed] [Google Scholar]
- 276.Rösler B, Herold S. Lung epithelial GM-CSF improves host defense function and epithelial repair in influenza virus pneumonia-a new therapeutic strategy? Mol Cell Pediatr 3: 29, 2016. doi: 10.1186/s40348-016-0055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, Alexander SN, Bellinghausen LK, Song AS, Petrova YM, Tuvim MJ, Adachi R, Romo I, Bordt AS, Bowden MG, Sisson JH, Woodruff PG, Thornton DJ, Rousseau K, De la Garza MM, Moghaddam SJ, Karmouty-Quintana H, Blackburn MR, Drouin SM, Davis CW, Terrell KA, Grubb BR, O’Neal WK, Flores SC, Cota-Gomez A, Lozupone CA, Donnelly JM, Watson AM, Hennessy CE, Keith RC, Yang IV, Barthel L, Henson PM, Janssen WJ, Schwartz DA, Boucher RC, Dickey BF, Evans CM. Muc5b is required for airway defence. Nature 505: 412–416, 2014. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Roy MG, Rahmani M, Hernandez JR, Alexander SN, Ehre C, Ho SB, Evans CM. Mucin production during prenatal and postnatal murine lung development. Am J Respir Cell Mol Biol 44: 755–760, 2011. doi: 10.1165/rcmb.2010-0020RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Sainz J, Al Haj Zen A, Caligiuri G, Demerens C, Urbain D, Lemitre M, Lafont A. Isolation of “side population” progenitor cells from healthy arteries of adult mice. Arterioscler Thromb Vasc Biol 26: 281–286, 2006. doi: 10.1161/01.ATV.0000197793.83391.91. [DOI] [PubMed] [Google Scholar]
- 280.Saito A, Nagase T. Hippo and TGF-β interplay in the lung field. Am J Physiol Lung Cell Mol Physiol 309: L756–L767, 2015. doi: 10.1152/ajplung.00238.2015. [DOI] [PubMed] [Google Scholar]
- 281.Sato A, Xu Y, Whitsett JA, Ikegami M. CCAAT/enhancer binding protein-α regulates the protease/antiprotease balance required for bronchiolar epithelium regeneration. Am J Respir Cell Mol Biol 47: 454–463, 2012. doi: 10.1165/rcmb.2011-0239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Sauvageau M, Goff LA, Lodato S, Bonev B, Groff AF, Gerhardinger C, Sanchez-Gomez DB, Hacisuleyman E, Li E, Spence M, Liapis SC, Mallard W, Morse M, Swerdel MR, D’Ecclessis MF, Moore JC, Lai V, Gong G, Yancopoulos GD, Frendewey D, Kellis M, Hart RP, Valenzuela DM, Arlotta P, Rinn JL. Multiple knockout mouse models reveal lincRNAs are required for life and brain development. eLife 2: e01749, 2013. doi: 10.7554/eLife.01749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Schachtner SK, Wang Y, Scott Baldwin H. Qualitative and quantitative analysis of embryonic pulmonary vessel formation. Am J Respir Cell Mol Biol 22: 157–165, 2000. doi: 10.1165/ajrcmb.22.2.3766. [DOI] [PubMed] [Google Scholar]
- 284.Schittny JC. Development of the lung. Cell Tissue Res 367: 427–444, 2017. doi: 10.1007/s00441-016-2545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Schneider C, Nobs SP, Kurrer M, Rehrauer H, Thiele C, Kopf M. Induction of the nuclear receptor PPAR-γ by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat Immunol 15: 1026–1037, 2014. doi: 10.1038/ni.3005. [DOI] [PubMed] [Google Scholar]
- 286.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336: 86–90, 2012. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 287.Schwarz MA, Caldwell L, Cafasso D, Zheng H. Emerging pulmonary vasculature lacks fate specification. Am J Physiol Lung Cell Mol Physiol 296: L71–L81, 2009. doi: 10.1152/ajplung.90452.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Sen P, Yang Y, Navarro C, Silva I, Szafranski P, Kolodziejska KE, Dharmadhikari AV, Mostafa H, Kozakewich H, Kearney D, Cahill JB, Whitt M, Bilic M, Margraf L, Charles A, Goldblatt J, Gibson K, Lantz PE, Garvin AJ, Petty J, Kiblawi Z, Zuppan C, McConkie-Rosell A, McDonald MT, Peterson-Carmichael SL, Gaede JT, Shivanna B, Schady D, Friedlich PS, Hays SR, Palafoll IV, Siebers-Renelt U, Bohring A, Finn LS, Siebert JR, Galambos C, Nguyen L, Riley M, Chassaing N, Vigouroux A, Rocha G, Fernandes S, Brumbaugh J, Roberts K, Ho-Ming L, Lo IF, Lam S, Gerychova R, Jezova M, Valaskova I, Fellmann F, Afshar K, Giannoni E, Muhlethaler V, Liang J, Beckmann JS, Lioy J, Deshmukh H, Srinivasan L, Swarr DT, Sloman M, Shaw-Smith C, van Loon RL, Hagman C, Sznajer Y, Barrea C, Galant C, Detaille T, Wambach JA, Cole FS, Hamvas A, Prince LS, Diderich KE, Brooks AS, Verdijk RM, Ravindranathan H, Sugo E, Mowat D, Baker ML, Langston C, Welty S, Stankiewicz P. Novel FOXF1 mutations in sporadic and familial cases of alveolar capillary dysplasia with misaligned pulmonary veins imply a role for its DNA binding domain. Hum Mutat 34: 801–811, 2013. doi: 10.1002/humu.22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol 6: 1191–1197, 2005. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 290.Shafer SL, Towler DA. Transcriptional regulation of SM22alpha by Wnt3a: convergence with TGFbeta(1)/Smad signaling at a novel regulatory element. J Mol Cell Cardiol 46: 621–635, 2009. doi: 10.1016/j.yjmcc.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376: 62–66, 1995. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 292.Shelmerdine SC, Semple T, Wallis C, Aurora P, Moledina S, Ashworth MT, Owens CM. Filamin A (FLNA) mutation-A newcomer to the childhood interstitial lung disease (ChILD) classification. Pediatr Pulmonol 52: 1306–1315, 2017. doi: 10.1002/ppul.23695. [DOI] [PubMed] [Google Scholar]
- 293.Shu W, Jiang YQ, Lu MM, Morrisey EE. Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development 129: 4831–4842, 2002. [DOI] [PubMed] [Google Scholar]
- 294.Shultz LD, Rajan TV, Greiner DL. Severe defects in immunity and hematopoiesis caused by SHP-1 protein-tyrosine-phosphatase deficiency. Trends Biotechnol 15: 302–307, 1997. doi: 10.1016/S0167-7799(97)01060-3. [DOI] [PubMed] [Google Scholar]
- 295.Siebel C, Lendahl U. Notch Signaling in Development, Tissue Homeostasis, and Disease. Physiol Rev 97: 1235–1294, 2017. doi: 10.1152/physrev.00005.2017. [DOI] [PubMed] [Google Scholar]
- 296.Snelgrove RJ, Goulding J, Didierlaurent AM, Lyonga D, Vekaria S, Edwards L, Gwyer E, Sedgwick JD, Barclay AN, Hussell T. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat Immunol 9: 1074–1083, 2008. doi: 10.1038/ni.1637. [DOI] [PubMed] [Google Scholar]
- 297.Snitow M, Lu M, Cheng L, Zhou S, Morrisey EE. Ezh2 restricts the smooth muscle lineage during mouse lung mesothelial development. Development 143: 3733–3741, 2016. doi: 10.1242/dev.134932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Song J, Heijink IH, Kistemaker LEM, Reinders-Luinge M, Kooistra W, Noordhoek JA, Gosens R, Brandsma CA, Timens W, Hiemstra PS, Rots MG, Hylkema MN. Aberrant DNA methylation and expression of SPDEF and FOXA2 in airway epithelium of patients with COPD. Clin Epigenetics 9: 42, 2017. doi: 10.1186/s13148-017-0341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Sorrell JM, Caplan AI. Fibroblasts-a diverse population at the center of it all. Int Rev Cell Mol Biol 276: 161–214, 2009. doi: 10.1016/S1937-6448(09)76004-6. [DOI] [PubMed] [Google Scholar]
- 300.Spagnolo P, Bush A. Interstitial Lung Disease in Children Younger Than 2 Years. Pediatrics 137: e20152725, 2016. doi: 10.1542/peds.2015-2725. [DOI] [PubMed] [Google Scholar]
- 301.Spanjer AI, Baarsma HA, Oostenbrink LM, Jansen SR, Kuipers CC, Lindner M, Postma DS, Meurs H, Heijink IH, Gosens R, Königshoff M. TGF-β-induced profibrotic signaling is regulated in part by the WNT receptor Frizzled-8. FASEB J 30: 1823–1835, 2016. doi: 10.1096/fj.201500129. [DOI] [PubMed] [Google Scholar]
- 302.Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, Wells JM. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470: 105–109, 2011. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Stabler CT, Morrisey EE. Developmental pathways in lung regeneration. Cell Tissue Res 367: 677–685, 2017. doi: 10.1007/s00441-016-2537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Stankiewicz P, Sen P, Bhatt SS, Storer M, Xia Z, Bejjani BA, Ou Z, Wiszniewska J, Driscoll DJ, Maisenbacher MK, Bolivar J, Bauer M, Zackai EH, McDonald-McGinn D, Nowaczyk MM, Murray M, Hustead V, Mascotti K, Schultz R, Hallam L, McRae D, Nicholson AG, Newbury R, Durham-O’Donnell J, Knight G, Kini U, Shaikh TH, Martin V, Tyreman M, Simonic I, Willatt L, Paterson J, Mehta S, Rajan D, Fitzgerald T, Gribble S, Prigmore E, Patel A, Shaffer LG, Carter NP, Cheung SW, Langston C, Shaw-Smith C. Genomic and genic deletions of the FOX gene cluster on 16q24.1 and inactivating mutations of FOXF1 cause alveolar capillary dysplasia and other malformations. [Correction in Am J Hum Genet 85: 537, 2009.] Am J Hum Genet 84: 780–791, 2009. doi: 10.1016/j.ajhg.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 99: 675–691, 2006. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 306.Stenmark KR, Mecham RP. Cellular and molecular mechanisms of pulmonary vascular remodeling. Annu Rev Physiol 59: 89–144, 1997. doi: 10.1146/annurev.physiol.59.1.89. [DOI] [PubMed] [Google Scholar]
- 307.Stevens T, Phan S, Frid MG, Alvarez D, Herzog E, Stenmark KR. Lung vascular cell heterogeneity: endothelium, smooth muscle, and fibroblasts. Proc Am Thorac Soc 5: 783–791, 2008. doi: 10.1513/pats.200803-027HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Stubbs JL, Vladar EK, Axelrod JD, Kintner C. Multicilin promotes centriole assembly and ciliogenesis during multiciliate cell differentiation. Nat Cell Biol 14: 140–147, 2012. doi: 10.1038/ncb2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Sun L, Ren X, Wang IC, Pradhan A, Zhang Y, Flood HM, Han B, Whitsett JA, Kalin TV, Kalinichenko VV. The FOXM1 inhibitor RCM-1 suppresses goblet cell metaplasia and prevents IL-13 and STAT6 signaling in allergen-exposed mice. Sci Signal 10: eaai8583, 2017. doi: 10.1126/scisignal.aai8583. [DOI] [PubMed] [Google Scholar]
- 310.Swarr DT, Morrisey EE. Lung endoderm morphogenesis: gasping for form and function. Annu Rev Cell Dev Biol 31: 553–573, 2015. doi: 10.1146/annurev-cellbio-100814-125249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Swarr DT, Peranteau WH, Pogoriler J, Frank DB, Adzick S, Hedrick H, Morley M, Zhou S, Morrisey EE. Novel Molecular and Phenotypic Insights into Congenital Lung Malformations. Am J Respir Crit Care Med 197: 1328–1339, 2018. doi: 10.1164/rccm.201706-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Szafranski P, Coban-Akdemir ZH, Rupps R, Grazioli S, Wensley D, Jhangiani SN, Popek E, Lee AF, Lupski JR, Boerkoel CF, Stankiewicz P. Phenotypic expansion of TBX4 mutations to include acinar dysplasia of the lungs. Am J Med Genet A 170: 2440–2444, 2016. doi: 10.1002/ajmg.a.37822. [DOI] [PubMed] [Google Scholar]
- 313.Szafranski P, Dharmadhikari AV, Brosens E, Gurha P, Kolodziejska KE, Zhishuo O, Dittwald P, Majewski T, Mohan KN, Chen B, Person RE, Tibboel D, de Klein A, Pinner J, Chopra M, Malcolm G, Peters G, Arbuckle S, Guiang SF III, Hustead VA, Jessurun J, Hirsch R, Witte DP, Maystadt I, Sebire N, Fisher R, Langston C, Sen P, Stankiewicz P. Small noncoding differentially methylated copy-number variants, including lncRNA genes, cause a lethal lung developmental disorder. Genome Res 23: 23–33, 2013. doi: 10.1101/gr.141887.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Szymaniak AD, Mahoney JE, Cardoso WV, Varelas X. Crumbs3-Mediated Polarity Directs Airway Epithelial Cell Fate through the Hippo Pathway Effector Yap. Dev Cell 34: 283–296, 2015. doi: 10.1016/j.devcel.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Tadokoro T, Wang Y, Barak LS, Bai Y, Randell SH, Hogan BL. IL-6/STAT3 promotes regeneration of airway ciliated cells from basal stem cells. Proc Natl Acad Sci USA 111: E3641–E3649, 2014. doi: 10.1073/pnas.1409781111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872, 2007. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 317.Tan SY, Krasnow MA. Developmental origin of lung macrophage diversity. Development 143: 1318–1327, 2016. doi: 10.1242/dev.129122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Tang TK. Centriole biogenesis in multiciliated cells. Nat Cell Biol 15: 1400–1402, 2013. doi: 10.1038/ncb2892. [DOI] [PubMed] [Google Scholar]
- 319.Tata PR, Rajagopal J. Plasticity in the lung: making and breaking cell identity. Development 144: 755–766, 2017. doi: 10.1242/dev.143784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Thébaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, Hashimoto K, Harry G, Haromy A, Korbutt G, Archer SL. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation 112: 2477–2486, 2005. doi: 10.1161/CIRCULATIONAHA.105.541524. [DOI] [PubMed] [Google Scholar]
- 321.Tiozzo C, Carraro G, Al Alam D, Baptista S, Danopoulos S, Li A, Lavarreda-Pearce M, Li C, De Langhe S, Chan B, Borok Z, Bellusci S, Minoo P. Mesodermal Pten inactivation leads to alveolar capillary dysplasia- like phenotype. J Clin Invest 122: 3862–3872, 2012. doi: 10.1172/JCI61334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Torday JS, Rehan VK. On the evolution of the pulmonary alveolar lipofibroblast. Exp Cell Res 340: 215–219, 2016. doi: 10.1016/j.yexcr.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Townsley MI. Structure and composition of pulmonary arteries, capillaries, and veins. Compr Physiol 2: 675–709, 2012. doi: 10.1002/cphy.c100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Trapnell BC, Carey BC, Uchida K, Suzuki T. Pulmonary alveolar proteinosis, a primary immunodeficiency of impaired GM-CSF stimulation of macrophages. Curr Opin Immunol 21: 514–521, 2009. doi: 10.1016/j.coi.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Trapnell BC, Whitsett JA, Nakata K. Pulmonary alveolar proteinosis. N Engl J Med 349: 2527–2539, 2003. doi: 10.1056/NEJMra023226. [DOI] [PubMed] [Google Scholar]
- 326.Trapnell C, Cacchiarelli D, Grimsby J, Pokharel P, Li S, Morse M, Lennon NJ, Livak KJ, Mikkelsen TS, Rinn JL. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat Biotechnol 32: 381–386, 2014. doi: 10.1038/nbt.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Treutlein B, Brownfield DG, Wu AR, Neff NF, Mantalas GL, Espinoza FH, Desai TJ, Krasnow MA, Quake SR. Reconstructing lineage hierarchies of the distal lung epithelium using single-cell RNA-seq. Nature 509: 371–375, 2014. doi: 10.1038/nature13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Urness LD, Sorensen LK, Li DY. Arteriovenous malformations in mice lacking activin receptor-like kinase-1. Nat Genet 26: 328–331, 2000. doi: 10.1038/81634. [DOI] [PubMed] [Google Scholar]
- 329.Ustiyan V, Wang IC, Ren X, Zhang Y, Snyder J, Xu Y, Wert SE, Lessard JL, Kalin TV, Kalinichenko VV. Forkhead box M1 transcriptional factor is required for smooth muscle cells during embryonic development of blood vessels and esophagus. Dev Biol 336: 266–279, 2009. doi: 10.1016/j.ydbio.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Ustiyan V, Wert SE, Ikegami M, Wang IC, Kalin TV, Whitsett JA, Kalinichenko VV. Foxm1 transcription factor is critical for proliferation and differentiation of Clara cells during development of conducting airways. Dev Biol 370: 198–212, 2012. doi: 10.1016/j.ydbio.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Vadivel A, van Haaften T, Alphonse RS, Rey-Parra GJ, Ionescu L, Haromy A, Eaton F, Michelakis E, Thébaud B. Critical role of the axonal guidance cue EphrinB2 in lung growth, angiogenesis, and repair. Am J Respir Crit Care Med 185: 564–574, 2012. doi: 10.1164/rccm.201103-0545OC. [DOI] [PubMed] [Google Scholar]
- 332.Van Tuyl M, Groenman F, Wang J, Kuliszewski M, Liu J, Tibboel D, Post M. Angiogenic factors stimulate tubular branching morphogenesis of sonic hedgehog-deficient lungs. Dev Biol 303: 514–526, 2007. doi: 10.1016/j.ydbio.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 333.Van Tuyl M, Liu J, Groenman F, Ridsdale R, Han RN, Venkatesh V, Tibboel D, Post M. Iroquois genes influence proximo-distal morphogenesis during rat lung development. Am J Physiol Lung Cell Mol Physiol 290: L777–L789, 2006. doi: 10.1152/ajplung.00293.2005. [DOI] [PubMed] [Google Scholar]
- 334.Vannella KM, Wynn TA. Mechanisms of Organ Injury and Repair by Macrophages. Annu Rev Physiol 79: 593–617, 2017. doi: 10.1146/annurev-physiol-022516-034356. [DOI] [PubMed] [Google Scholar]
- 335.Varner VD, Nelson CM. Computational models of airway branching morphogenesis. Semin Cell Dev Biol 67: 170–176, 2017. doi: 10.1016/j.semcdb.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Vaughan AE, Brumwell AN, Xi Y, Gotts JE, Brownfield DG, Treutlein B, Tan K, Tan V, Liu FC, Looney MR, Matthay MA, Rock JR, Chapman HA. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature 517: 621–625, 2015. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Volckaert T, Dill E, Campbell A, Tiozzo C, Majka S, Bellusci S, De Langhe SP. Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury. J Clin Invest 121: 4409–4419, 2011. doi: 10.1172/JCI58097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Volckaert T, Yuan T, Chao CM, Bell H, Sitaula A, Szimmtenings L, El Agha E, Chanda D, Majka S, Bellusci S, Thannickal VJ, Fässler R, De Langhe SP. Fgf10-Hippo Epithelial-Mesenchymal Crosstalk Maintains and Recruits Lung Basal Stem Cells. Dev Cell 43: 48–59.e5, 2017. doi: 10.1016/j.devcel.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res 312: 623–629, 2006. doi: 10.1016/j.yexcr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 340.Voynow JA, Rubin BK. Mucins, mucus, and sputum. Chest 135: 505–512, 2009. doi: 10.1378/chest.08-0412. [DOI] [PubMed] [Google Scholar]
- 341.Wang IC, Snyder J, Zhang Y, Lander J, Nakafuku Y, Lin J, Chen G, Kalin TV, Whitsett JA, Kalinichenko VV. Foxm1 mediates cross talk between Kras/mitogen-activated protein kinase and canonical Wnt pathways during development of respiratory epithelium. Mol Cell Biol 32: 3838–3850, 2012. doi: 10.1128/MCB.00355-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Wang IC, Ustiyan V, Zhang Y, Cai Y, Kalin TV, Kalinichenko VV. Foxm1 transcription factor is required for the initiation of lung tumorigenesis by oncogenic Kras(G12D.). Oncogene 33: 5391–5396, 2014. doi: 10.1038/onc.2013.475. [DOI] [PubMed] [Google Scholar]
- 343.Wang IC, Zhang Y, Snyder J, Sutherland MJ, Burhans MS, Shannon JM, Park HJ, Whitsett JA, Kalinichenko VV. Increased expression of FoxM1 transcription factor in respiratory epithelium inhibits lung sacculation and causes Clara cell hyperplasia. Dev Biol 347: 301–314, 2010. doi: 10.1016/j.ydbio.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Wansleeben C, Barkauskas CE, Rock JR, Hogan BL. Stem cells of the adult lung: their development and role in homeostasis, regeneration, and disease. Wiley Interdiscip Rev Dev Biol 2: 131–148, 2013. doi: 10.1002/wdev.58. [DOI] [PubMed] [Google Scholar]
- 345.Warburton D, El-Hashash A, Carraro G, Tiozzo C, Sala F, Rogers O, De Langhe S, Kemp PJ, Riccardi D, Torday J, Bellusci S, Shi W, Lubkin SR, Jesudason E. Lung organogenesis. Curr Top Dev Biol 90: 73–158, 2010. doi: 10.1016/S0070-2153(10)90003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Warburton D, Schwarz M, Tefft D, Flores-Delgado G, Anderson KD, Cardoso WV. The molecular basis of lung morphogenesis. Mech Dev 92: 55–81, 2000. doi: 10.1016/S0925-4773(99)00325-1. [DOI] [PubMed] [Google Scholar]
- 347.Warburton D, Shi W, Xu B. TGF-β-Smad3 signaling in emphysema and pulmonary fibrosis: an epigenetic aberration of normal development? Am J Physiol Lung Cell Mol Physiol 304: L83–L85, 2013. doi: 10.1152/ajplung.00258.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Weaver M, Batts L, Hogan BL. Tissue interactions pattern the mesenchyme of the embryonic mouse lung. Dev Biol 258: 169–184, 2003. doi: 10.1016/S0012-1606(03)00117-9. [DOI] [PubMed] [Google Scholar]
- 349.Weibel ER, Gomez DM. Architecture of the human lung. Use of quantitative methods establishes fundamental relations between size and number of lung structures. Science 137: 577–585, 1962. doi: 10.1126/science.137.3530.577. [DOI] [PubMed] [Google Scholar]
- 350.West PW, Canning BJ, Merlo-Pich E, Woodcock AA, Smith JA. Morphologic Characterization of Nerves in Whole-Mount Airway Biopsies. Am J Respir Crit Care Med 192: 30–39, 2015. doi: 10.1164/rccm.201412-2293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Westphalen K, Gusarova GA, Islam MN, Subramanian M, Cohen TS, Prince AS, Bhattacharya J. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature 506: 503–506, 2014. doi: 10.1038/nature12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.White AC, Xu J, Yin Y, Smith C, Schmid G, Ornitz DM. FGF9 and SHH signaling coordinate lung growth and development through regulation of distinct mesenchymal domains. Development 133: 1507–1517, 2006. doi: 10.1242/dev.02313. [DOI] [PubMed] [Google Scholar]
- 353.Whitsett JA, Alenghat T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat Immunol 16: 27–35, 2015. doi: 10.1038/ni.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Whitsett JA, Wert SE, Weaver TE. Diseases of pulmonary surfactant homeostasis. Annu Rev Pathol 10: 371–393, 2015. doi: 10.1146/annurev-pathol-012513-104644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Widdicombe JH, Wine JJ. Airway Gland Structure and Function. Physiol Rev 95: 1241–1319, 2015. doi: 10.1152/physrev.00039.2014. [DOI] [PubMed] [Google Scholar]
- 356.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell 98: 769–778, 1999. doi: 10.1016/S0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 357.Wilkinson GA, Schittny JC, Reinhardt DP, Klein R. Role for ephrinB2 in postnatal lung alveolar development and elastic matrix integrity. Dev Dyn 237: 2220–2234, 2008. doi: 10.1002/dvdy.21643. [DOI] [PubMed] [Google Scholar]
- 358.Wollin L, Maillet I, Quesniaux V, Holweg A, Ryffel B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J Pharmacol Exp Ther 349: 209–220, 2014. doi: 10.1124/jpet.113.208223. [DOI] [PubMed] [Google Scholar]
- 359.Xi Y, Kim T, Brumwell AN, Driver IH, Wei Y, Tan V, Jackson JR, Xu J, Lee DK, Gotts JE, Matthay MA, Shannon JM, Chapman HA, Vaughan AE. Local lung hypoxia determines epithelial fate decisions during alveolar regeneration. Nat Cell Biol 19: 904–914, 2017. doi: 10.1038/ncb3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Xu Y, Mizuno T, Sridharan A, Du Y, Guo M, Tang J, Wikenheiser-Brokamp KA, Perl AT, Funari VA, Gokey JJ, Stripp BR, Whitsett JA. Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight 1: e90558, 2016. doi: 10.1172/jci.insight.90558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Xu Y, Wang Y, Besnard V, Ikegami M, Wert SE, Heffner C, Murray SA, Donahue LR, Whitsett JA. Transcriptional programs controlling perinatal lung maturation. PLoS One 7: e37046, 2012. doi: 10.1371/journal.pone.0037046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Xu Y, Zhang M, Wang Y, Kadambi P, Dave V, Lu LJ, Whitsett JA. A systems approach to mapping transcriptional networks controlling surfactant homeostasis. BMC Genomics 11: 451, 2010. doi: 10.1186/1471-2164-11-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Yamada C, Sano H, Shimizu T, Mitsuzawa H, Nishitani C, Himi T, Kuroki Y. Surfactant protein A directly interacts with TLR4 and MD-2 and regulates inflammatory cellular response. Importance of supratrimeric oligomerization. J Biol Chem 281: 21771–21780, 2006. doi: 10.1074/jbc.M513041200. [DOI] [PubMed] [Google Scholar]
- 364.Yan X, Zhao H, Zhu X. Production of Basal Bodies in bulk for dense multicilia formation. F1000 Res 5: 1533, 2016. doi: 10.12688/f1000research.8469.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Yang J, Hernandez BJ, Martinez Alanis D, Narvaez del Pilar O, Vila-Ellis L, Akiyama H, Evans SE, Ostrin EJ, Chen J. The development and plasticity of alveolar type 1 cells. Development 143: 54–65, 2016. doi: 10.1242/dev.130005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Yang ML, Wang CT, Yang SJ, Leu CH, Chen SH, Wu CL, Shiau AL. IL-6 ameliorates acute lung injury in influenza virus infection. Sci Rep 7: 43829, 2017. doi: 10.1038/srep43829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Yang X, Castilla LH, Xu X, Li C, Gotay J, Weinstein M, Liu PP, Deng CX. Angiogenesis defects and mesenchymal apoptosis in mice lacking SMAD5. Development 126: 1571–1580, 1999. [DOI] [PubMed] [Google Scholar]
- 368.Yang Y, Palmer KC, Relan N, Diglio C, Schuger L. Role of laminin polymerization at the epithelial mesenchymal interface in bronchial myogenesis. Development 125: 2621–2629, 1998. [DOI] [PubMed] [Google Scholar]
- 369.Yang Y, Relan NK, Przywara DA, Schuger L. Embryonic mesenchymal cells share the potential for smooth muscle differentiation: myogenesis is controlled by the cell’s shape. Development 126: 3027–3033, 1999. [DOI] [PubMed] [Google Scholar]
- 370.Yew-Booth L, Birrell MA, Lau MS, Baker K, Jones V, Kilty I, Belvisi MG. JAK-STAT pathway activation in COPD. Eur Respir J 46: 843–845, 2015. doi: 10.1183/09031936.00228414. [DOI] [PubMed] [Google Scholar]
- 371.Yi L, Domyan ET, Lewandoski M, Sun X. Fibroblast growth factor 9 signaling inhibits airway smooth muscle differentiation in mouse lung. Dev Dyn 238: 123–137, 2009. doi: 10.1002/dvdy.21831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Yoder MC. Endothelial stem and progenitor cells (stem cells): (2017 Grover Conference Series). Pulm Circ 8: 2045893217743950, 2018. doi: 10.1177/2045893217743950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 109: 1801–1809, 2007. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Yoshida T, Owens GK. Molecular determinants of vascular smooth muscle cell diversity. Circ Res 96: 280–291, 2005. doi: 10.1161/01.RES.0000155951.62152.2e. [DOI] [PubMed] [Google Scholar]
- 375.Zacharias WJ, Frank DB, Zepp JA, Morley MP, Alkhaleel FA, Kong J, Zhou S, Cantu E, Morrisey EE. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature 555: 251–255, 2018. doi: 10.1038/nature25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Zeng X, Wert SE, Federici R, Peters KG, Whitsett JA. VEGF enhances pulmonary vasculogenesis and disrupts lung morphogenesis in vivo. Dev Dyn 211: 215–227, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 377.Zepp JA, Zacharias WJ, Frank DB, Cavanaugh CA, Zhou S, Morley MP, Morrisey EE. Distinct Mesenchymal Lineages and Niches Promote Epithelial Self-Renewal and Myofibrogenesis in the Lung. Cell 170: 1134–1148.e10, 2017. doi: 10.1016/j.cell.2017.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Zhang M, Malik AB, Rehman J. Endothelial progenitor cells and vascular repair. Curr Opin Hematol 21: 224–228, 2014. doi: 10.1097/MOH.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Zhang Y, Goss AM, Cohen ED, Kadzik R, Lepore JJ, Muthukumaraswamy K, Yang J, DeMayo FJ, Whitsett JA, Parmacek MS, Morrisey EE. A Gata6-Wnt pathway required for epithelial stem cell development and airway regeneration. Nat Genet 40: 862–870, 2008. doi: 10.1038/ng.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Zhao H, Zhu L, Zhu Y, Cao J, Li S, Huang Q, Xu T, Huang X, Yan X, Zhu X. The Cep63 paralogue Deup1 enables massive de novo centriole biogenesis for vertebrate multiciliogenesis. Nat Cell Biol 15: 1434–1444, 2013. doi: 10.1038/ncb2880. [DOI] [PubMed] [Google Scholar]
- 381.Zhao J, Shi W, Wang YL, Chen H, Bringas P Jr, Datto MB, Frederick JP, Wang XF, Warburton D. Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol 282: L585–L593, 2002. doi: 10.1152/ajplung.00151.2001. [DOI] [PubMed] [Google Scholar]
- 382.Zhao R, Fallon TR, Saladi SV, Pardo-Saganta A, Villoria J, Mou H, Vinarsky V, Gonzalez-Celeiro M, Nunna N, Hariri LP, Camargo F, Ellisen LW, Rajagopal J. Yap tunes airway epithelial size and architecture by regulating the identity, maintenance, and self-renewal of stem cells. Dev Cell 30: 151–165, 2014. doi: 10.1016/j.devcel.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Zhao YY, Gao XP, Zhao YD, Mirza MK, Frey RS, Kalinichenko VV, Wang IC, Costa RH, Malik AB. Endothelial cell-restricted disruption of FoxM1 impairs endothelial repair following LPS-induced vascular injury. J Clin Invest 116: 2333–2343, 2006. doi: 10.1172/JCI27154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Zhou F, Narasimhan V, Shboul M, Chong YL, Reversade B, Roy S. Gmnc Is a Master Regulator of the Multiciliated Cell Differentiation Program. Curr Biol 25: 3267–3273, 2015. [Correction in Curr Biol 27: 305–307, 2017.] doi: 10.1016/j.cub.2015.10.062. [DOI] [PubMed] [Google Scholar]
- 385.Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol 25: 221–251, 2009. doi: 10.1146/annurev.cellbio.042308.113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Zuo W, Zhang T, Wu DZ, Guan SP, Liew AA, Yamamoto Y, Wang X, Lim SJ, Vincent M, Lessard M, Crum CP, Xian W, McKeon F. p63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature 517: 616–620, 2015. doi: 10.1038/nature13903. [DOI] [PMC free article] [PubMed] [Google Scholar]