Abstract
Wound healing is one of the most complex processes in the human body. It involves the spatial and temporal synchronization of a variety of cell types with distinct roles in the phases of hemostasis, inflammation, growth, re-epithelialization, and remodeling. With the evolution of single cell technologies, it has been possible to uncover phenotypic and functional heterogeneity within several of these cell types. There have also been discoveries of rare, stem cell subsets within the skin, which are unipotent in the uninjured state, but become multipotent following skin injury. Unraveling the roles of each of these cell types and their interactions with each other is important in understanding the mechanisms of normal wound closure. Changes in the microenvironment including alterations in mechanical forces, oxygen levels, chemokines, extracellular matrix and growth factor synthesis directly impact cellular recruitment and activation, leading to impaired states of wound healing. Single cell technologies can be used to decipher these cellular alterations in diseased states such as in chronic wounds and hypertrophic scarring so that effective therapeutic solutions for healing wounds can be developed.
I. INTRODUCTION TO WOUND HEALING
The skin is the largest organ by surface area in the human body. It is the critical structure that shields internal tissues from mechanical damage, microbial infection, ultraviolet radiation, and extreme temperature. This makes it highly susceptible to injury with significant impact to both individual patients and the healthcare economy. In the United States alone, nonhealing wounds account for approximately $50 billion, scars from surgical incisions and trauma account for nearly $12 billion, and burns account for $7.5 billion in healthcare costs each year (111, 235). Patients with diabetes, the elderly, and patients with genetic disorders such as sickle cell disease are especially predisposed to abnormal wound healing leading to long-term sequela. Astonishingly, the interventions that exist have not significantly impacted the situation. While several therapies for wound healing are available, these are only moderately effective. Thus there is a need for more effective therapies for healing wounds.
Skin repair requires the intricate synchronization of several different cell types in sequential steps. In the uninjured skin, the epidermis is the outer, impermeable layer that withstands the harsh external environment. The epidermis also contains the sebaceous glands, sweat glands, and hair follicles. The dermis is rich in extracellular matrix (ECM), vasculature, and mechanoreceptors and provides the skin with strength, nutrients, and immunity. The subcutaneous adipose tissue underlies the dermis and functions as an energy reserve. It is also a constant source of growth factors to the dermis. In addition to these cell types, each layer contains resident immune cells that are constantly surveying the skin for damage. When the skin is wounded, multiple cell types within these three layers need to coordinate at precise stages to bring about healing. These stages of hemostasis, inflammation, angiogenesis, growth, re-epithelialization, and remodeling occur in a temporal sequence but also overlap (167). Thus skin repair is among the most complex processes in the human body.
The first response to a wound is constriction of the injured blood vessels and activation of platelets to form a fibrin clot (63). The fibrin clot ceases blood flow and provides a scaffold for incoming inflammatory cells. Neutrophils are immediately recruited to the clot as a first line of defense against bacteria (453). Monocytes are recruited within 48–96 h after injury and transform into tissue-activated macrophages at the wound site (307). The adaptive immune system comprising Langerhans cells, dermal dendritic cells, and T cells are also activated to combat self and foreign antigens. There is an increased interest in understanding the heterogeneity within these immune cell populations, especially how specific subsets are involved in clearance of cellular debris versus resolution of infection (78, 79).
As the inflammatory phase ends, angiogenesis occurs. Angiogenesis involves endothelial cell proliferation, migration, and branching to form new blood vessels. Concurrent with proliferation of endothelial cells, pericytes within the basal lamina are activated (9) which scaffold and provide structural integrity to the endothelial cells (10). Some groups suggest that these activated pericytes are mesenchymal stromal cells with increased plasticity (73). In addition to the local cells, circulating progenitor cells from the bone marrow are also found to support new blood vessel formation during wound healing (12, 53, 225, 412). New blood vessel formation involves several cell types with most of the cellular diversity occurring within the perivascular space.
While new blood vessels emerge, resident fibroblasts proliferate and invade the clot to form contractile granulation tissue. Here, some fibroblasts differentiate into myofibroblasts, drawing the wound margins together (263). The dividing fibroblasts deposit ECM and shift the wound microenvironment from the inflammatory to the growth state (445). Re-epithelialization simultaneously occurs and involves the proliferation of both unipotent epidermal stem cells from the basement membrane and de-differentiation of terminally differentiated epidermal cells (90). Repair of the epidermal layer also involves reconstruction of the skin appendages. Tissue-resident stem cells for sebaceous glands, sweat glands, and hair follicles have also been discovered, which can activate local appendage repair (9, 24, 125). While these epidermal stem cells are mostly unipotent in homeostasis, they become highly plastic in response to injury and can give rise to other cell types to rapidly repair the epidermis during wound healing.
Within the subcutaneous adipose tissue, stromal vascular cells and their subsets have been well characterized (330). These cells release growth factors and cytokines critical for neovascularization and wound repair. Inflammatory cells within the subcutaneous tissue have also generated attention especially in conditions of obesity and type 2 diabetes since increased inflammation can alter the outcome of wound repair.
In most cases, healing restores barrier function and close to normal tensile strength of the skin. However, unlike prenatal wound healing, which is a regenerative process that recapitulates the original skin architecture, wound healing in adults results in a fibrotic scar that serves as a rapid patch for the wound (167). Excessive scarring shifts the balance towards fibrotic states of hypertrophic scarring and keloid formation (436). There is growing evidence that scarring is a result of differential cellular responses towards mechanical stress within the healing skin (96, 464). Impairments in the wound healing response can also lead to chronic wounds (211). Chronic wounds are common in diabetes, vascular disease, and aging as well as in patients suffering from hemoglobinopathies. Improper care of these wounds result in recurrence and can lead to limb amputations and mortality.
It is important to note that our current knowledge of skin repair and the cellular architecture of healing wounds has largely been derived using surgically constructed models of skin injury in rodents. Murine models have been used more often than porcine models of skin injury since it is easier to establish impaired wound states in mice such as those displayed in diabetes, aging, and hemoglobinopathies. However, rodent skin unlike human or porcine skin is elastic, lacks adherence to underlying structures, and closes by contraction stimulated by the panniculus carnosus or the striated muscle (132). Human skin in contrast heals by granulation tissue formation and re-epithelialization. There has been an increased shift into studying wound healing in human skin using ex vivo cultures of human skin, organotypic cultures, and debrided skin specimens (310). When murine models for wound healing are employed, there is an emphasis on application of silicone stents around the excised skin, which prevents contraction and allows for healing through granulation tissue formation and re-epithelialization (132).
This review will detail the role of the various cell types involved in wound healing and the current advances in single cell technologies that reveal phenotypic and functional heterogeneity within these cell types. Where relevant, the differences between murine and human skin have been identified. This review also addresses alterations of cells of the skin that lead to diseased states of fibrosis and nonhealing wounds. Finally, we describe available wound healing treatments and suggest avenues for therapeutic interventions including development of cell-based therapies.
II. CELLULAR RESPONSES DURING WOUND HEALING
A. Hemostasis
Hemostasis marks the first stage of wound healing (320) that stops bleeding after vascular damage. It occurs in three steps: vasoconstriction, primary hemostasis, and secondary hemostasis. The critical cell involved in this process is the platelet; the critical matrix component is fibrinogen. In the uninjured state, platelets are protected from untimely activation by the healthy endothelial cell monolayer (349). Platelets in uninjured skin do not attach to the vessel wall or aggregate with each other. Fibrinogen (factor 1) is produced by hepatocytes and circulates in the blood (411). It is also present within platelets but is not cleaved into fibrin fibers, which is an essential component of the blood clot (212).
When the skin is wounded, the immediate response to stop bleeding is vasoconstriction of the vessel walls. Next, primary hemostasis and secondary hemostasis occur via two concurrent and mechanistically intertwined pathways (129). Primary hemostasis involves platelet aggregation and platelet plug formation that is elicited by exposure of collagen within the subendothelial matrix. Secondary hemostasis refers to the activation of the coagulation cascade where soluble fibrinogen is converted to insoluble strands that make up the fibrin mesh. The platelet plug and the fibrin mesh combine to form the thrombus, which stops bleeding, releases complements and growth factors, and provides a provisional scaffold for infiltrating cells necessary for wound healing (320).
1. Vasoconstriction
Following injury, vessels constrict rapidly to reduce bleeding from ruptured microvasculature. This is achieved by reflexive contracture of the vascular smooth muscle and is triggered by vasoconstrictors such as endothelin, released from the damaged endothelium. Additionally, circulating catecholamines, epinephrine, norepinephrine, and prostaglandins released from injured cells regulate vasoconstriction (145). Platelets themselves produce platelet-derived growth factor (PDGF) which preferentially activates mesenchymal cells, especially smooth muscles in the vessel walls causing contraction (20, 315). However, initial reflexive contraction only temporarily reduces bleeding. This is because increasing hypoxia and acidosis of the wound results in passive relaxation of the muscle, and causes bleeding to resume (320). Subsequent activation of the coagulation cascade is needed to further regulate vasoconstriction through the mediators bradykinin, fibrinopeptide, serotonin, and thromboxane A2 (410) and resolve bleeding in the long term (392).
2. Formation of the platelet plug (primary hemostasis)
Platelets were first discovered by Schultze in 1865 (34). Bizzozero in 1881 named these cells “piastrine” or “little plates,” which led to the term platelets (34). He was also the first to note that platelets were involved in thrombus formation (388). Platelets are anucleate cells that bud off from megakaryocytes. Morphologically, they contain a unique structure of open canalicular system (OCS), which are tunneling invaginations of the cell membrane (122). Platelets also consist of secretory granules, of which α-granules are the most important for platelet activity (22). During homeostasis, these cells circulate in proximity to endothelial cells. However, the intact endothelial cell monolayer exhibits anti-thrombotic properties where it produces nitric oxide, prostacyclin, and negatively charged heparin-like glycosaminoglycans that prevent platelet activation, attachment, and aggregation (149).
Following wounding and blood vessel rupture, the thrombogenic subendothelial matrix is exposed (152). Platelets, through G protein-coupled receptors on their surface, bind this matrix and activate the inside-out signaling pathway (321), which causes integrin activation and increased attachment of the platelets to other platelets and the surrounding ECM. αIIbβ3 is the most abundant integrin on platelets that mediates attachment to fibrinogen, fibronectin, and von Willebrand factor (vWF) through RGD sequences (432). α2β1 Integrin is the second most abundant integrin on platelets and mediates attachment to collagen (351). Next, activation of the outside-in signaling pathway occurs, which increases platelet activation and modulates the actin cytoskeleton. Filamentous actin is the most abundant protein in the platelet comprising 40% of the cellular protein in the resting state but increasing to ~70% of the platelet protein content in the activated state (118). Change in actin conformation transforms the free-floating disk-shaped platelet into a rounded structure and finally to a fried-egg-shaped cell containing pseudopodia and lamellipodia, which strongly attaches to the ECM, contracts, and mechanically seals the blood vessel (388).
The activated platelet also has a large surface area due to fusion of the intracellular granules with the plasma membrane or the surface connected membranes of the OCS. These intracellular granules secrete more than 300 active substances (149) such as ADP, serotonin, calcium, and histamine that are required for platelet activation as well as vWF and integrins, which are required for primary and secondary hemostasis (22).
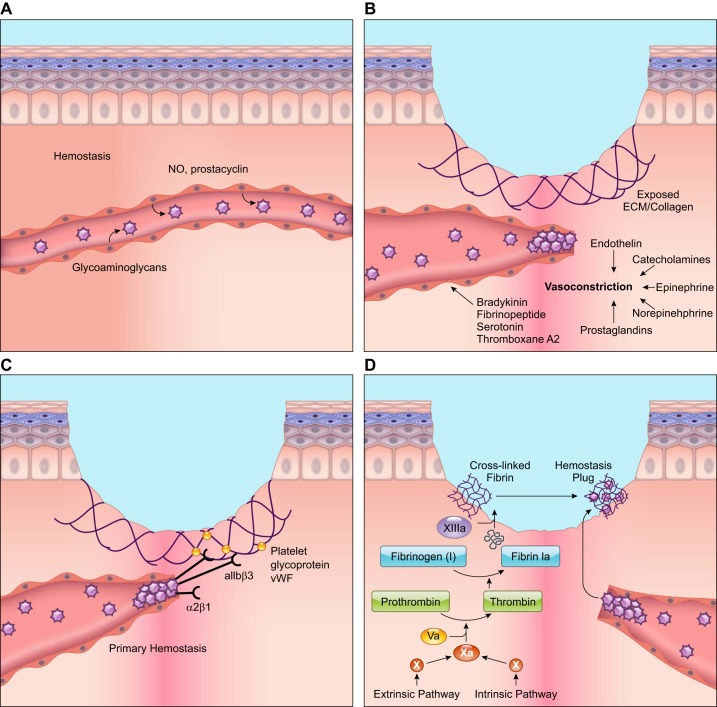
In addition to integrins, several other cell surface receptors are actively involved in the platelet aggregation process. The platelet glycoprotein Ib-IX-V and glycoprotein VI, for example, bind immobilized vWF and collagen in the subendothelial matrix, respectively, causing platelet aggregation and attachment to the subendothelial matrix (21). Activated platelets also release compounds such as thromboxane A2 that increase platelet aggregation (112). Together, these interactions result in forming the “platelet plug” (FIGURE 1).
FIGURE 1.
Cellular responses during the hemostasis phase of wound healing. A: during hemostasis, platelets circulate in close proximity to the vessel wall. However, anti-thrombotic agents such as nitric oxide (NO) and prostacyclin released from endothelial cells prevent platelet attachment to the endothelial lining and platelet aggregation. B: wounding stimulates injured cells to rapidly release vasoconstrictors that cause reflexive contracture of the smooth muscle and temporary stoppage of bleeding. C: blood vessel rupture during wound healing exposes the subendothelial matrix. Platelets bind this subendothelial matrix and to each other using G protein-coupled receptors, integrins, and glycoproteins on their surface. von Willebrand factor (vWF) released by platelets also attaches to the subendothelial matrix. Platelets bind extracellular vWF through their surface receptors, strengthening the platelet plug. D: the extrinsic and intrinsic pathways lead to the activation of Factor X, which ultimately results in the cleavage of fibrinogen to fibrin. Cross-linked fibrin binds the aggregated platelet plug to form the thrombus that stops blood flow and provides a provisional matrix for healing. The illustration is a simplified rendering based on current knowledge.
Platelets within the plug release growth factors and cytokines such as PDGF, transforming growth factor-β (TGF-β), epidermal growth factor (EGF), and insulin growth factor (IGF) (320), which are important cellular mediators for the subsequent phases of healing. The release of platelet factors is most intense within the first hour of platelet activation, but activated platelets continue to release these factors for up to 7 days (365), exerting paracrine effects on other cell types in the wound, including smooth muscle cells, endothelial cells, monocytes, and fibroblasts. Due to the abundant presence of growth factors released by activated platelets, platelet-rich plasma (PRP) has widely been tested in small and large animal models for the treatment of various injuries including wounds (346), with positive outcomes. PRP contains roughly five times the normal blood platelet count in humans, is devoid of red blood cells, and may or may not contain leukocytes. Two methods for isolating PRP were United States Food and Drug Administration (FDA)-approved in 2009, but the outcome in patients treated with PRP has been varied.
Platelets demonstrate heterogeneity based on α-granule secretion, most likely due to variations in shear stress (80). Platelets also display spatial heterogeneity based on their location within the growing thrombus. The core of the thrombus usually contains highly activated P-selectin expressing platelets, and the borders of the thrombus contain fewer activated P-selectin negative platelets (80).
While platelets are essential for hemostasis, their absence does not bring about critical defects in wound healing. Murine experiments have indicated that thrombocytopenia, or low platelet count, does not have a profound effect on healing wounds. Mice with thrombocytopenia show increased macrophage and T cell numbers, but no changes in growth factor release, rate of wound re-epithelialization, collagen synthesis, or angiogenesis compared with normal mice (399).
3. Coagulation and reinforcement of the platelet plug
Platelets provide the surface for the assembly and activation of coagulation complexes. The classic coagulation pathways are the intrinsic and extrinsic pathways, both of which are activated by exposure of the subendothelial matrix, and lead to factor X activation. Following activation of factor X by either pathway, prothrombin gets converted into thrombin, which cleaves fibrinogen into fibrin. Factor XIII covalently crosslinks fibrin, which binds the aggregated platelet plug forming a definitive secondary hemostasis plug or the thrombus. The thrombus also serves as the provisional wound matrix for the infiltration of other cells in the subsequent stages of healing.
B. The Inflammatory Phase of Wound Healing
1. Mechanisms of inflammatory cell recruitment
Since wound healing involves several steps, it is important to understand the first signals that activate the cellular response in the wounded tissue. Activation of the transcriptional machinery takes time. Thus the wound first turns on transcription-independent pathways that can readily be activated. These include Ca2+ waves, reactive oxygen species (ROS) gradients, and purigenic molecules. Increase of intracellular Ca2+ occurs at the wound edges within the first few minutes of injury and propagates to the center of the wound (230). Damage-associated molecular patterns (DAMPs), hydrogen peroxide (H2O2), lipid mediators, and chemokines released by injured cells also provide signals for the recruitment of inflammatory cells, especially neutrophils. DAMP molecules include DNA, peptides, ECM components, ATP, and uric acid. Studies across various organisms have displayed that rapid production of H2O2 in the wound minimizes infections, activates keratinocyte regeneration, recruits neutrophils, and promotes new vessel formation (428).
Chemokines are small 8- to 10-kDa proteins that contain cysteines in their molecular structure. The most prominent cytokines are the CC cytokines that contain two adjacent cysteines and the CXC cytokines, in which the two cysteines are separated by one amino acid. The CXC cytokines are ELR+ if they have glutamic acid (E), leucine (L), or arginine (R) in front of the first cysteine residue. ELR+ chemokines preferentially attract neutrophils and are angiogenic (264). ELR- chemokines do not contain the ELR residue and preferentially attract lymphocytes (264). To date, 47 chemokines have been identified. Chemokines bind to cells on G protein-coupled receptors (GPCRs) called chemokine receptors. Some chemokine receptors are exclusive to one chemokine, but many bind more than one chemokine, triggering activation of downstream pathways that lead to directional cell movement, or chemotaxis (264). The production of chemokines in the wound is time- and dose-dependent and is initiated by the presence of bacteria, cleaved fibrin or pro-inflammatory factors such as tumor necrosis factor (TNF)-α.
In addition to purigenic molecules, mast cells have been discovered to recruit inflammatory cells immediately after injury. These cells, which otherwise are involved in allergic responses, contain various mediators in their granules. Immediately after injury, mast cells release factors that include inflammatory cytokines, vasodilation agents, vascular permeability factors, and proteases that enhance the recruitment of immune cells into the wound (303). Mice lacking specific mast cell enzymes such as mast cell proteases 4 and 5 display reduced neutrophil recruitment, indicating the significance of mast cells in early inflammatory cell recruitment during wound healing (480).
2. Neutrophils in wound healing
Neutrophils are usually not observed in the normal skin. They are produced in the bone marrow from promyelocytes and are recruited as “first responders” from the bone marrow in response to “find me” signals including DAMPs, hydrogen peroxide, lipid mediators, and chemokines released from regions of injury or infection (393). There are more than 30 different surface receptors including GPCRs, Fc receptors, integrins, and pattern recognition receptors that aid the neutrophil in detecting these injury signals. On the day following injury, neutrophils constitute 50% of all cells in the wound (142). Activated neutrophils can release factors to prolong and amplify further neutrophil infiltration (393). Neutrophils destroy infectious threats by releasing toxic granules, producing an oxidative burst, initiating phagocytosis, and generating neutrophil extracellular traps (NETs).
Neutrophils develop unique granules during various stages of development, each containing a specific set of antimicrobial agents geared with a specific task. Azurophilic (or primary) granules are the first granules to form, while the neutrophil is still in the bone marrow (393). They are also the last granules to undergo exocytosis and mainly destroy bacteria intracellularly, by fusing with the phagolysosome. Azurophilic granules contain myeloperoxidase, azurocidin, lysozyme, bacterial permeability increasing protein, and serine proteases such as capthepsin G, elastase, and protease 3 (453). Secondary granules or specific granules are the next to form and contain human cationic antimicrobial protein (hCAP-18), lactoferrin, matrix metalloprotease 8 (MMP-8), and collagenase-2 (453). Gelatinase granules contain MMPs with gelatinase activity. Secretory vesicles are the last to form. They contain integrins, growth factors, and cytokine receptors that are released rapidly from the cell.
Proteases comprise a major portion of all the toxic granules. Proteases are important for both antimicrobial activity and break down of the basement membrane and ECM, allowing neutrophils to leave blood vessels and enter the injured tissue (327). The main serine proteases are cathepsin G, elastase, and protease 3, which are stored in the azurophilic granules, and break down elastin, fibronectin, laminin, vitronectin, and collagen IV (453). They also activate MMPs and inhibit protease inhibitors exacerbating the proteolytic response. Elastase knockout mice show reduced effectiveness of clearing bacteria (17), suggesting its importance in wound healing. However, increased production of neutrophil-derived proteolytic enzymes, as evidenced in chronic wounds, can lead to cleavage of growth factors, growth factor receptors, and ECM, impairing vascular processes and blood flow as well as causing tissue damage (363).
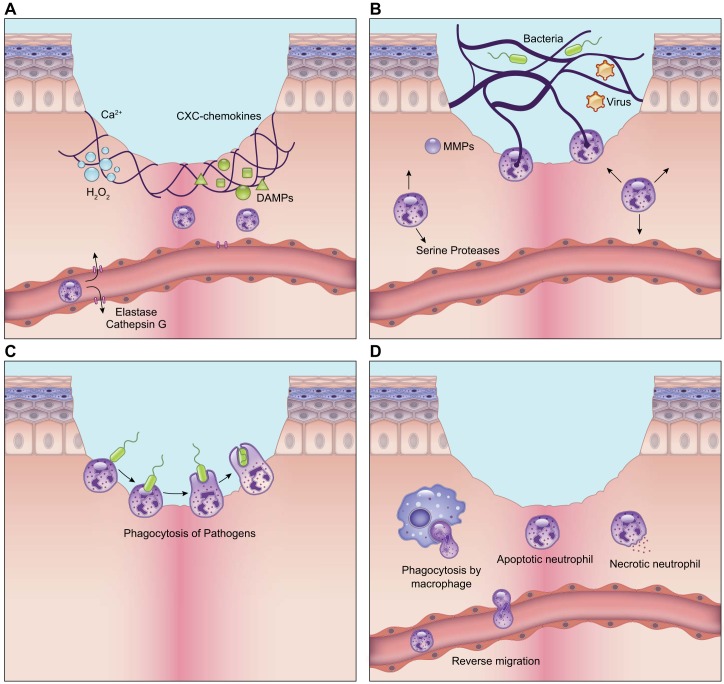
Activated neutrophils additionally produce NETs, which are chromatin filaments that extend into the extracellular space and are coated with histones, cytosolic proteins, and proteases for the capture and elimination of exogenous bacteria, fungi, and viruses (209, 479) (FIGURE 2). There are two ways by which these traps are released into the extracellular space. In the first method, elastase and myeloperoxidase produced in the azurophilic granules lyse parts of the nuclear and plasma membrane, unfolding chromatin and releasing it into the cytosol and the extracellular space. This process causes the rupture of the neutrophil membranes and death of the cell (suicidal NETosis) (209). The second method called vital NETosis is activated by Toll-like receptor activation of platelets and CD11a activation on the neutrophil, resulting in nuclear budding and release of the NET through vesicles (209). Vital NETosis allows the neutrophils to stay alive and participate in subsequent functions such as phagocytosis.
FIGURE 2.
Role of neutrophils in wound healing. A: neutrophils are recruited to the wound in response to calcium waves, damage-associated molecular patterns (DAMPs), hydrogen peroxide, lipid mediators, and chemokines that are released by resident cells immediately after wounding. B: neutrophils combat pathogens through release of proteases from their intracellular granules. They also produce neutrophil extracellular traps (NETs) that capture pathogens through a process called NETosis. In this process, neutrophils extend chromatin filaments coated with proteases outside of the cell to aid in the elimination of pathogens. C: neutrophils also perform phagocytosis in the wound. They probe antigens using surface receptors and integrins and form a phagocytic cup that engulfs antigens. Internalized antigens are degraded by proteases within the neutrophil granules. D: timely clearance of neutrophils is critical for the resolution of inflammation. They are either engulfed by macrophages through efferocytosis or they can re-enter the circulation and leave the wound through a process called reverse migration. The illustration is a simplified rendering based on current knowledge.
Neutrophils can engulf and destroy bacteria and cell debris by phagocytosis. Neutrophils display the same stages of phagocytosis as macrophages but contain a combination of phagocytic receptors distinct from macrophages that allow for the recognition of certain pathogens preferentially (242). The antigens that neutrophils recognize can be both opsonized and non-opsonized. Opsonized antigens are recognized and bound by Fc receptors CD32, CD16, and CD64, as well as the β2 integrin CD11b (MAC1) (239). Once the neutrophil has probed the antigen, these Fc receptors and integrins cluster and activate downstream Src and Rho-GTPases that lead to an actin-driven extension of the plasma membrane around the pathogen, forming a phagocytic cup. Activation of phospholipase C (PLC)-γ leads to changes in calcium gradients and activation of myosin, which helps the neutrophil seal the phagocytic cup (239). The nascent phagosome that is formed then undergoes maturation.
In contrast to macrophages that contain abundant early endosomes, late endosomes, and lysosomes, maturation of the phagosome in neutrophils occurs via fusion with the various granules containing antimicrobial agents and proteases (28). The fusion of the phagosome with the neutrophil granules is a calcium-dependent process and leads to the degradation of the pathogen.
Clearance of neutrophils begins by apoptosis or necrosis and subsequent engulfment by macrophages, or efferocytosis (33). The matricellular protein CCN1 has been found to be important for neutrophil efferocytosis (210). It binds phosphatidylserine on the neutrophil on one end and to integrins on macrophages on the other, creating a bridge that activates efferocytosis through a Rac1-dependent pathway (210). Failure to activate neutrophil efferocytosis can lead to secondary necrosis where the neutrophils lyse, resulting in the release of pro-inflammatory and cytotoxic molecules, and increased tissue damage (33). Interestingly, not all neutrophils are cleared by macrophages. A subset of neutrophils has been shown to leave the wound site through interstitial migration within the tissue or reentry into the vasculature in a process called “neutrophil reverse migration” (82). Timely clearance of neutrophils is critical since the disappearance of neutrophils begins the resolution of inflammation. Neutrophil persistence leads to a prolonged inflammatory state and the emergence of chronic wounds as seen in diabetic foot ulcers, pressure ulcers, and venous leg ulcers (56).
3. Macrophages in wound healing
Macrophages are commonly identified by the surface marker expression CD45+/ CD11b+/ F480+ in mice and CD45+/ Cd11b+/ CD66B- in humans. Wounding induces an accumulation of macrophages within the first 24–48 h at the site of injury (176). In young healthy mice, where wounds are re-epithelialized and closed by day 14, the number of macrophages in the wound peaks roughly at day 3, decreases around day 5, and reaches near-baseline levels by day 10 (477). The increase in macrophage number can occur through both increases in local tissue-resident macrophages and by monocyte recruitment from the bone marrow.
There is sparse information about the role of tissue-resident macrophages in wound healing. It is likely that the tissue-resident macrophages persist in the skin from embryonic development and proliferate during wound healing to increase in number, but this remains to be definitively demonstrated. Most studies focus on monocytes, which differentiate into macrophages within the wound (88). Monocytes are recruited in response to platelet and mast cell degranulation and in response to increase in hypoxia-inducible factors and chemokines such as stromal-derived factor 1 (SDF1/ CXCL12). Macrophages within the wound can also recruit additional monocytes and exacerbate the macrophage inflammatory response by producing potent chemoattractants such as monocyte chemoattractant protein (MCP)-1 (89).
Macrophages are critical to normal wound healing and tissue regeneration. Murine wounds depleted of macrophages show delayed wound closure (151, 276). The macrophage-depleted wounds demonstrate an influx of neutrophils as a compensatory immune response and a decrease in angiogenesis, granulation tissue, collagen deposition, and growth factor release (254, 486). Conversely, increasing the number of monocytes or macrophages in the wound can significantly accelerate both normal and diabetic murine wound healing (189). These observations in mice are consistent with other organisms. In salamander, for example, systemic depletion of macrophages after limb amputation results in the permanent failure to regenerate the limb (146). Fascinatingly, replenishment of endogenous macrophages following amputation in these macrophage-depleted salamanders restores limb regeneration (146).
In the early stages of wound healing, macrophages are microbicidal and pro-inflammatory, expressing TNF-α and interleukins (IL)-6 and IL-1β (105) (FIGURE 3). These macrophages are commonly referred to as M1. Pro-inflammatory macrophages recognize and engulf pathogens into intracellular organelles called phagosomes, which are high in ROS and rapidly kill most pathogens (379). Pro-inflammatory macrophages also synthesize MMPs, which allows them to digest the ECM and thrombus to aid in their migration (386). The digested ECM fragments function as immunostimulatory DAMPs, exacerbating the pro-inflammatory state of the wound (386). Pattern recognition receptors on macrophages recognize and bind to these DAMP molecules, activating classical inflammation pathways through Toll-like receptor and inflammasome signaling (401). Apart from being bactericidal, macrophages also perform efferocytosis which is critical in eliminating expended neutrophils within 3–4 days of wounding (263, 357). Improper clearance of neutrophils, as previously described, leads to nonspecific tissue degradation and a persistent inflammatory state.
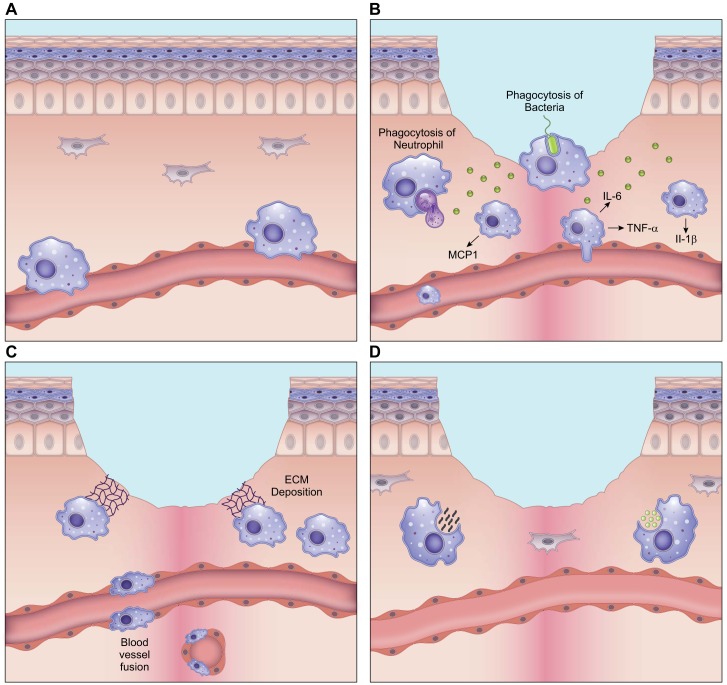
FIGURE 3.
Macrophage phenotypes in wound healing. A: in the uninjured skin, circulating monocytes from the bone marrow are constantly rolling over the inner endothelial wall within the vessel lumen and surveying for damage. The few macrophages that are resident in the skin are prevalent in the perivascular space and can be from embryonic sources. B: following skin injury, during the inflammatory phase of healing, macrophages release pro-inflammatory cytokines such as interleukin (IL)-6, tumor necrosis factor (TNF)-α, and IL-1β to fight infection. Early macrophages in the wound release monocyte chemoattractant protein (MCP)-1 to draw in more monocytes from the bone marrow and heighten the macrophage response. These macrophages also actively participate in phagocytosis of pathogens. At the end of the inflammatory phase, macrophages engulf dying neutrophils, which marks the end of the inflammatory phase of wound healing. C: during the growth stage of wound healing, as granulation tissue forms, macrophages release growth factors such as vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) that are used to signal and activate endothelial cells to perform angiogenesis. Some macrophages deposit extracellular matrix (ECM) at this stage. Unregulated deposition of ECM can lead to fibrosis, and such scar-forming macrophages are called fibrocytes. D: during wound remodeling, macrophages again take on a phagocytic role where they engulf both cell debris and excessive ECM, to bring the healed skin to a homeostatic state. The illustration is a simplified rendering based on current knowledge.
With the resolution of inflammation, the inflammatory macrophage phenotype transitions into an anti-inflammatory cell type called the alternatively activated macrophage or the M2 macrophage (133). Anti-inflammatory macrophages contribute to new vessel formation (240, 280), and increased macrophage numbers during this stage correlate with high microvessel density (69, 297). Pro-vascular macrophages in the wound are found to express the surface marker Tie2 which is also expressed on endothelial cells and some hematopoietic stem cells (108). These macrophages can participate in blood vessel anastomosis by fusing the branching endothelial vessels and connecting them to the systemic vasculature by a process called “vascular mimicry” (108, 304). These cells can also release growth factors such as vascular endothelial growth factor (VEGF) that are important for blood vessel sprouting during angiogenesis (456). Due to their spatial and phenotypic similarities to endothelial cells, the pro-vascular macrophages are commonly mistaken to be circulating endothelial progenitor cells. Macrophages can similarly participate in fusing lymphatic vessels (265). Interestingly, macrophage metabolism also affects neovascularization and determines whether the new vessels are functional and covered by pericytes, or abnormal and leaky (295, 444).
During the proliferation stage, macrophages actively signal to dermal fibroblasts (319). A CD206+/CD301b+ macrophage subset induces the fibroblast to myofibroblast transition in both mice and humans, increasing collagen and α-smooth muscle actin deposition in the wound (369, 485). Macrophages can also transition into fibrotic cells, depositing collagen and other ECM components themselves. These macrophages are referred to as fibrocytes or M2a macrophages (396) and contribute to scar formation (316, 463). It is currently unclear if the provascular (M2) and the profibrotic macrophages (M2a) are unique subsets, since neovascularization and ECM deposition in the wound are temporally congruent. It is possible that macrophages in the wound do not solely exist as one subset but display hybrid phenotypes (336a, 376).
Once re-epithelialization occurs, and the wound proceeds into the remodeling stage, macrophages in the wound regain their phagocytic phenotype and acquire a “fibrolytic” profile. These macrophages, called M2c or Mreg-like (376), release proteases and phagocytize excessive cells and matrix that are no longer required for wound closure (237). Aberrations in macrophage function at this stage can lead to persistence of both excessive ECM and cells, resulting in skin fibrosis.
Altered macrophage numbers, especially in the early inflammatory state of the wound, have been associated with fibrotic diseases such as keloids and hypertrophic scars (30, 463). While early pro-inflammatory macrophages are necessary for normal repair, both increased or decreased numbers of pro-inflammatory macrophages can result in hypertrophic scars (HTS) (42, 228, 426). High throughput transcriptional analysis has demonstrated that there are atypical macrophages that are derived from a common circulating granulocyte and macrophage progenitor that are responsible for scar formation (355). This contrasts with typical macrophages, which are derived from a common macrophage and dendritic cell progenitor. The atypical macrophages increase in numbers approximately at day 13 after injury in mice (355).
Aberrations in macrophage interactions with other cell types can also result in fibrosis. Macrophage activation of T cells has been shown to be important for the onset of HTS (463, 464). The interaction between macrophages and fibroblasts is also critical in determining if the wound heals without a scar. For example, increased expression of the CD47 “don’t eat me” signal on murine fibroblasts prevents them from being phagocytized and eliminated by macrophages (448), leading to excessive matrix deposition.
Macrophage dysfunction is also evident in impaired diabetic wound healing. Diabetic wounds have a temporal lag in expression of chemokines which are required for both monocyte recruitment and macrophage activation (449). The delayed influx of macrophages leads to delayed efferocytosis of neutrophils, ECM, and wound debris, and a delayed onset of the proliferation phase (216). Eventually, a chronic state of inflammation is established with macrophages and apoptotic cells prevailing even in the remodeling stage of healing (449). Aberrant macrophages in diabetic wounds are also compromised in growth factor release and promoting neovascularization (265).
The literature on macrophages in wound healing is vast, yet the diversity of macrophages within the wound and the relationship between macrophage origin (fetal-derived vs. adult bone marrow-derived) and function remains unstudied. The differences between macrophages and dendritic cells in the healing wound have also not been definitively elucidated.
4. Mast cells in wound healing
Mast cells were discovered by Paul Ehrlich in 1978 (103). Progenitors for mast cells are derived in the bone marrow and migrate to perivascular regions of the connective tissue of the skin and mucosa, where they differentiate into mast cells (385). In the skin, they primarily function as effectors of allergic reactions, mediating immunoglobulin E (IgE) reactions and fighting helminth infestations. Whether mast cells are necessary for wound healing is unclear since there are contradictory findings in the wound healing outcomes in mast cell-deficient mice (288, 443). However, mast cells interact with several other cell types during wound healing (11) which will briefly be discussed here. Mast cells are also found to be mechanoresponsive, and there is growing evidence that these cells contribute to the scarring response in conditions such as hypertrophic scarring and scleroderma (7, 114, 218).
In the early stages of wound healing, mast cells release antimicrobial peptides that prevent infections of the skin (84, 372, 438). They synthesize the enzymes chymase and tryptase, important for ECM breakdown, as well as histamines and VEGF inducing vascular permeability and allowing neutrophil influx (98, 480). Mast cell histamine is found to stimulate keratinocyte proliferation and re-epithelialization (443). Mast cell tryptase and histamine enhance fibroblast proliferation and collagen synthesis, which enhances wound contraction (3, 136).
Increased mast cell numbers are implicated in scarring and skin fibrosis (454). Their role in scar formation has been tested using a model of fetal wound healing. This model is based on the paradigm that wounds in mice at embryonic day 15 heal without scar, while wounds at embryonic day 18 display scar. Interestingly, injection of mast cell lysate into wounds at embryonic day 15 can shift scarless healing into scar formation (469). Conversely, knocking out mast cells at embryonic day 18 results in reduced scar formation (469). While these studies provide evidence of the presence of mast cells in the scar response, the exact mechanisms that underlie this process need to be characterized. Similarly, the role of mast cells in chronic wounds remains insufficiently studied (471). While there is evidence that there is reduced mast cell number and reduced degranulation in diabetic wound healing, more studies are needed to characterize the role of these cells in impaired wound healing states (409).
Mast cells from different tissues display heterogeneity in function (326) where their phenotype alters based on alterations in their microenvironment (286). Since the wound microenvironment undergoes several changes, different mast cell subsets with unique functions may exist in the wound, and these remain to be characterized (471).
5. Dendritic cells in wound healing
Dendritic cells (DCs) are antigen presenting cells that are involved in priming T-cell responses. Within the epidermis, they manifest as Langerhans cells. Langerhans cells are named after Paul Langerhans who identified these cells within skin sections in the 1800s and based on their morphology postulated they had a neurologic function (343). Dendritic cells as they are known today were identified and named much later in 1973 by Ralph Steinman (390, 391). Both Langerhans cells and the dermal DCs are found in the skin-draining lymph nodes at the time of infection in the skin.
There has been some debate about DCs being a type of macrophage since both cell types are part of the mononuclear phagocytic system, express common surface markers, and respond to the same growth factors (192). While it can be difficult to distinguish macrophages and DCs based on their surface markers, they are currently characterized based on their primary functions. Macrophages are considered scavenger cells that phagocytize cellular and ECM degradation intermediates and microorganisms (402). They have weaker antigen presenting ability compared with DCs. DCs, when encountered with antigens, present the antigen to T cells within the dermis and migrate into the draining lymph nodes where they continue to activate T-cell responses (402).
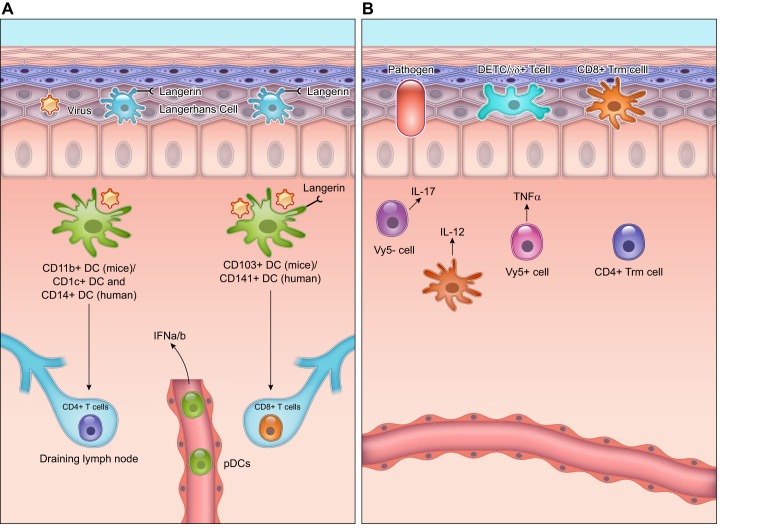
Within the murine dermis, there are usually two subtypes of resident DCs, the CD11b+ DCs and the CD103+ DCs (FIGURE 4). The CD103+ dermal DCs in mice are similar to the CD141+ DCs in humans (40, 170). These cells are also langerin-positive and low for CD11b expression. They are responsible for cross-presenting antigens for induction of CD8+ T-cell responses, identifying dead cells and playing a role in viral immunity. DCs recognize F-actin and other DAMPs on dying cells through their cell surface receptors such as clec9A and prime CD8+ T cells in eliminating these dying cells (6, 483). They recognize viruses through surface receptors such as TLR3, which binds dsRNA, an intermediate of viral infections (100, 178). The CD11b+ DCs in mice correspond to two different subsets of DCs in humans: CD1c+ DCs, which are similar to splenic CD8- DCs, and CD14+ DCs, which are derived from monocytes. The CD11b+ DCs preferentially present antigens to CD4+ T cells during infection (16). They can also present antigens to Treg cells to dampen the adaptive immune response (269).
FIGURE 4.
Dendritic cells (DCs) and T cells in the wound healing response. A: dendritic cells within the murine dermis can be CD103+, which correspond to CD141+ DCs in humans, or CD11b+, which correspond to the CD1c+ and CD14+ subset in humans. The CD103+ DCs mainly activate CD8+ T cells, and the CD11b+ DCs mainly activate the CD4+ T cells in the draining lymph nodes. The wounded skin also contains plasmacytoid dendritic cells (pDCs) that are activated to release interferon (IFN)-α/β that is important to the acute inflammatory response. Within the epidermis, Langerhans cells with high expression of Langerin (CD207) are the main antigen presenting cells. Langerhans cells are derived from early myeloid progenitors in the embryo and persist in adult skin. B: T cells in the skin can either be γδ+ or αβ+. γδ+ T cells of the epidermis are also called dendritic epithelial cells (DETCs). These cells survey the epidermis for infection and produce growth factors that are critical for signaling epidermal cells during wound healing. γδ+ T cells of the dermis are either Vγ5-positive that release tumor necrosis factor (TNF)-α and activate dermal DCs to release interleukin (IL)-12, or they are Vγ5-negative that produce IL-17 following infection. These γδ+ T cells can migrate to the draining lymph nodes and continue to activate DCs. The αβ+ T cells in the dermis mainly consist of CD4+ subsets, while the αβ+ T cells of the epidermis are the CD8+ killer cells. The illustration is a simplified rendering based on current knowledge.
Langerhans cells are involved in surveillance of microorganisms and contact hypersensitivity (271). They are derived in the embryo from early myeloid progenitors (EMPs) that migrate into the epidermis during the embryonic stage (258, 272, 313) and persist into adulthood where their numbers are maintained by signals from adjacent keratinocytes (271, 272). They derive their name from the surface receptor CD207 or Langerin (271). Upon antigen recognition, Langerhans cells downregulate e-cadherin expression that maintains their contact with keratinocytes (403). This allows for their migration from the epidermis through the dermis into the draining lymph nodes where they initiate a T cell-mediated adaptive response (219, 258).
In addition to the resident DCs, plasmacytoid dendritic cells (pDCs) that are absent in the skin under normal conditions are recruited into the healing skin following injury or infection (158). pDCs usually circulate in the bloodstream and are present in the secondary lymphoid organs (459). Following skin injury, there is an early and short-lived infiltration of pDCs into the wound, where in response to self-nucleic acids released by the wounded cells, they are activated to produce interferon (IFN)-α/β through TLR7 and TLR9 (158). Depletion of pDCs during wound healing significantly impairs the acute inflammatory cytokine response and delays wound re-epithelialization (158).
Most of the work on DCs in the skin remains restricted to conditions of infection rather than wound healing. This is most likely because DCs like many other immune cells are difficult to distinguish and isolate based on their surface marker profile. While CD11c is widely used for the isolation of DCs, several other cell types including natural killer cells, activated CD8+ T cells, and certain subsets of macrophages express CD11c. Similarly, several cells in the epidermis including dendritic epithelial T cells (DETCs), intraepithelial lymphocytes, and αβ T cells express markers such as CD103. Single cell technologies will be helpful in determining how these various immune cells in the skin differ from each other based on both surface markers and function (177).
6. T cells in wound healing
There are two variants of T cells in the human epidermis and dermis: γδ+ T cells and αβ+ T cells (29). While human skin mainly contains αβ+ T cells in the dermis, murine epidermis mainly comprises γδ+ T cells, also called DETCs, owing to the morphology of the cells (19, 274). DETCs migrate from the fetal thymus into the epidermis where they survive and proliferate slowly in response to signals from interleukins (especially IL-15) to reach homeostatic numbers (474). They reside in the basal layers of the epidermis and extend their dendrites into the suprabasal layers where they proactively participate in surveillance of ligands that emerge during conditions of epidermal stress such as infections or the presence of transformed cells (61). In the skin, DETCs are nonmigratory (155) (FIGURE 4). Most T-cell studies in wound healing focus on the DETCs most likely since 1) these are the only subtype of T cells that release cytokines and growth factors that act on keratinocytes and facilitate wound re-epithelialization (197, 199), 2) mice lacking DETCs show significant delay in wound closure (197, 215), and 3) DETCs express a canonical T-cell receptor, Vγ3Vδ1, that is specific to T cells in the skin (196).
Upon injury to the skin, wounded keratinocytes upregulate ligands such as SKINTs and CD100, which lead to the activation of DETCs (215, 458). Within 24–48 h following wounding, DETC morphology changes from a dendritic structure to a rounded morphology (197). These activated DETCs release keratinocyte growth factors (KGFs) and other factors, importantly KGF-1, KGF-2, and insulin growth factor-1, which positively impacts proliferation of keratinocytes in the wound (172, 199). Mice lacking DETCs show delayed wound closure through reduced keratinocyte proliferation, delayed infiltration of macrophages, and lesser deposition of ECM such as hyaluronan (197, 198). Impaired wound healing in aged mice is attributed to the dysfunctional signaling between injured keratinocytes and DETCs (215).
The γδ+ T cells of the dermis can cycle in situ and are dependent on IL-7 for their development and survival (397). They are important to the resident cutaneous surveillance program since they positively influence expansion of CD4+ T cells in the skin draining lymph nodes and increase recruitment of neutrophils into the skin following infection (397). γδ+ T cells can be Vγ5-positive or Vγ5-negative. The Vγ5-negative dermal T cells are IL7Rhi CCR6hi (155) and are derived from perinatal thymocytes (155). These dermal T cells are mainly responsible in producing IL-17 in the first hours to days following skin infection (155). The Vγ5-positive dermal T cells can produce TNF-α and stimulate DCs to produce IL-12. They are also migratory and move to the skin-draining lymph nodes following infection, where they enhance DC function (284). γδ+ T cells increase in the skin of patients with psoriasis, and T-cell proliferation in this disease is dependent on the presence of TNF-α in the skin (32, 44).
The αβ+ T cells in the skin display memory and consist of CD4+ helper cells, CD8+ killer cells, and Treg subsets (178). These cells can either be passing through the circulation or have permanent residence in the skin, being disconnected from the circulation. The latter are called tissue-resident memory cells or TRM (178). In murine skin, the CD4+ subsets of both circulating and resident cells are restricted to the dermis, while CD8+ subsets are sequestered in the epidermis (35, 137, 256, 484). When there is an infection, CD8+ cells are sometimes recruited from the epidermis into the dermis where they persist for ~1 month after the resolution of infection, and then return to the epidermis to restore the homeostatic state (137).
Invariant natural killer cells (iNKTs) are a distinct lymphocyte subset that coexpress the αβ T-cell antigen and the NK cell marker. iNKTs have important roles in regulating the allergic response, autoimmune diseases, and protecting against pathogenic infections (144, 423). iNKT cells mediate acute wound healing by producing IFN-γ. Mice lacking iNKT cells show significantly delayed wound closure with lesser collagen and α-smooth muscle actin deposition, and impaired new vessel formation (406). iNKT cells also prevent the prolonged inflammatory response mediated by neutrophils (405).
Aberrations in T-cell function have been correlated with skin fibrosis. Human HTS resulting from burn injuries show an increased presence of T cells (50). Murine studies support this observation and demonstrate that scar formation results from the activation of a TH2 CD4 T-cell response involving the interleukins IL-4, IL-5, and IL-13 (300, 434, 472, 473). The activation of T-cell pathways in HTS is also attributed to increased mechanical forces within the healing skin (463).
Thus there is a lot of diversity in the types of T cells in the skin, yet their role in wound healing and dysregulation in fibrosis needs further elucidation. DETCs remain the most studied of the T-cell subsets, but the absence of the surface markers CD4, CD8, or CD28 on DETCs makes them difficult to isolate and characterize. How the γδ+ T cells differ in function compared with αβ+ T cells during wound healing also needs further elucidation.
C. The Growth Phase of Wound Healing
1. Formation of granulation tissue and neovascularization
During the proliferative phase of wound healing, new connective tissue or granulation tissue is formed concurrently with other healing processes, including re-epithelialization, neovascularization, and immunomodulation. The formation and evolution of granulation tissue was first described by the British surgeon John Hunter in the late 18th century and was characterized in greater detail in the 19th century by the French surgeon Alexis Carrel. Granulation tissue is mainly formed by activated fibroblasts, which synthesize new ECM and help contract the wound (FIGURE 5). It also serves as a scaffold for other cells and components including newly synthesized ECM, new blood vessels, and inflammatory cells. Granulation tissue is eventually replaced by normal connective tissue during wound remodeling (167).
FIGURE 5.
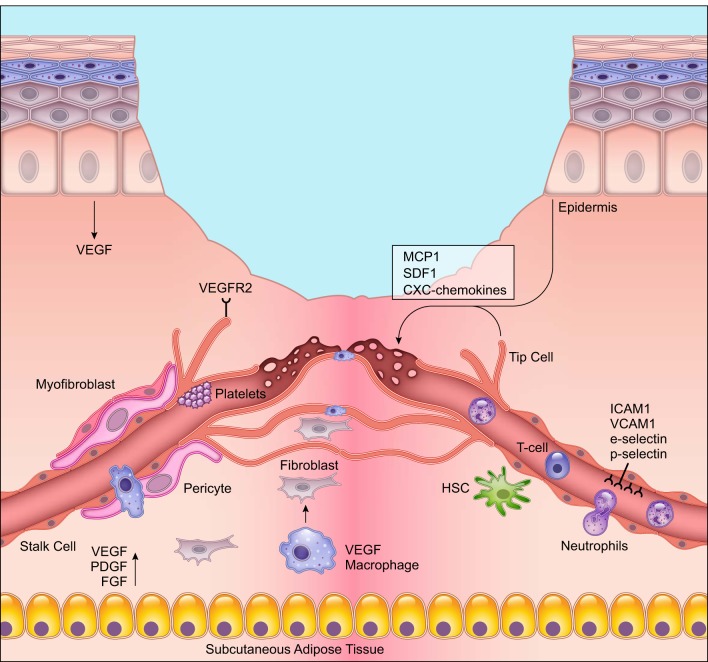
Angiogenesis during wound healing. New blood vessel formation is one of the most important stages of wound healing. Endothelial cells at the leading edge or tip branch out or “sprout” to form new capillaries in response to vascular endothelial growth factor (VEGF) and other growth factor signals from epidermal cells, macrophages, and the subcutaneous adipose tissue. The endothelial cells during angiogenesis are leaky to allow for immune cells and other circulating cells to extravasate from the blood vessel lumen into the wound. Pro-angiogenic macrophages release growth factors for endothelial cell growth and fuse newly forming capillaries. Activated endothelial cells upregulate surface markers intercellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM)-1, E-selectin, and P-selectin that help with cell-cell interactions with leukocytes. Deletion of these surface markers during wound healing impairs wound repair. The illustration is a simplified rendering based on current knowledge.
Neovascularization or new blood vessel formation is critical for efficient wound healing. It is required for the delivery of nutrients and maintenance of oxygen homeostasis, to allow cellular proliferation and tissue regeneration to occur (167). During embryonic development, primitive blood vessels form from mesoderm-derived endothelial precursor cells (EPCs) called angioblasts through a process called vasculogenesis (110). With the discovery of putative EPCs in adult tissue, it was assumed that new blood vessels could also form during adult tissue repair by vasculogenesis, where bone marrow-derived EPCs could home to the repair site and proliferate and differentiate into endothelial cells (12). However, subsequent studies in murine models have demonstrated that putative EPCs are largely monocytes and macrophages, which support neovascularization. After the initial development of the circulatory system, formation of new blood vessels in the adult occurs predominantly through angiogenesis (104).
Angiogenesis involves activation of local microvascular endothelial cells (ECs), which line the inner surface of blood vessels. In the presence of the hypoxic wound environment, ECs respond to hypoxia-responsive growth factors such as VEGF and PDGF. Activated ECs break down ECM in the granulation tissue, proliferate, migrate, form new cell-cell junctions, and branch out to form new capillaries (104). Angiogenesis allows for the delivery of nutrients and maintenance of oxygen homeostasis, which enhances cellular proliferation and tissue regeneration (167). Below, we review the key cells in the angiogenic process, including endothelial cells and pericytes. We describe the current understanding of circulating progenitor cells in wound healing and describe fibroblast subtypes that support granulation tissue formation.
2. Endothelial cells and new vessel formation
Microvascular ECs line the inner surface of blood vessels and are the primary cell type involved in new vessel formation. Activation of ECs requires growth factors from adjoining cells, production of proteolytic enzymes that allow for EC migration within the fibrin/fibronectin-rich clot, intracellular EC response to hypoxia, and EC interactions with adjoining perivascular cells. Endothelial cells initiate angiogenesis by sprouting, where in response to pro-angiogenic signals such as VEGF, fibroblast growth factor (FGF), PDGF-B, TGF-β, and angiopoietins, they proliferate and migrate (419). However, heterogeneity within ECs exists, and during angiogenesis, these cells either take on the role of the lead tip cells or trailing stalk cells. Tip cells extend their filopodia towards pro-angiogenic growth factors and respond to positive and negative guidance cues that ensure that vessel growth is tightly controlled and organized (138). Stalk cells, in turn, trail the tip cells and maintain the integrity of the existing vasculature. Sprouts ultimately become endothelial tubules that connect to other vessels through the formation of new cell-cell junctions and ECM signaling.
The decision of whether endothelial cells become tip or stalk cells is regulated largely by the Notch pathway and its effectors, Delta-like 4 and Jagged1, which have been shown to control cell fate and patterning in several tissues, organisms, and developmental stages (179, 395). Activation of Notch signaling is regulated by VEGF, which is produced by subcutaneous adipose stromal cells, macrophages, and proliferating keratinocytes in the wound microenvironment (138, 246). The role of VEGF-A in angiogenesis and as an initiator of endothelial sprouting is conserved in several species (72, 348, 373), where the tip cells respond by migrating towards an increasing VEGF-A gradient, and stalk cells proliferate in a VEGF-A concentration-dependent manner. In addition to their responsiveness to growth factors, endothelial cells also contain chemokine receptors. Most of the CC and CXC receptors on endothelial cells except for CXCR3 have been shown to promote angiogenesis (26).
Several other endothelial cell receptors play a critical role in angiogenesis. In the unwounded condition, endothelial cells have very few receptors on their adluminal surface, which prevents binding to circulating platelets or immune cells, but allows monocytes to roll and survey the skin (67). In response to wounding and chemokines in the microenvironment, endothelial cells express glycoprotein receptors such as P-selectin and E-selectin that allow for leukocyte adhesion and infiltration into the skin (442). Endothelial cells also upregulate intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 that arrest leukocyte movement (279). Deletion of P-selectin, E-selectin, ICAM-1, or VCAM-1 is found to impede both neovasculatization and wound healing, highlighting the importance of endothelial cell-leukocyte interactions in skin repair (281, 394).
Integrin receptors on endothelial cells, particularly αvβ3, which is the receptor for fibrin, fibronectin, and vitronectin, is critical for angiogenesis (419). Expression of αvβ3 is most pronounced on tip cells that need to migrate through the granulation tissue abundant in these ECM proteins. In addition to its role in angiogenesis, αvβ3 on endothelial cells can attach to extracellular vWF released by platelets during the clotting process, in the initial stage of healing (57, 115). While most of the work on endothelial cells has focused on their adluminal surface and their interactions with hematopoietic cells, endothelial cell interactions with cells on their abluminal surface remain relatively unknown. These latter interactions are primarily endothelial cell-pericyte interactions within the vascular basement membrane. A more thorough understanding of the juxtacrine interactions between these two cellular populations will be important to understand neovascularization in the skin as well as in other tissue types.
3. Pericytes in neovascularization and wound healing
Rouget in 1873 was the first to describe branched, contractile cells that wrapped around blood vessel walls. Fifty years later, Zimmermann found that the Rouget cells aligned themselves along the axis of the blood vessel, and called these cells ‟pericytes.ˮ Pericytes surround endothelial cells in blood vessels and serve critical functions including destabilization and stabilization of microvasculature, regulation of blood flow, and creation of a vascular barrier to bacteria (10, 169, 384, 389). These functions make pericytes indispensible to wound healing.
The current accepted definition of a mature pericyte is a cell embedded within the vascular basement membrane (10). However, this definition is difficult to apply to cells involved in active neovascularization, since other cells including vascular smooth muscle cells, fibroblasts, macrophages, and the circulating progenitor cells also occupy the perivascular space (12). Furthermore, there is no single molecular marker known that can unequivocally distinguish pericytes from these other cell types (10). The identification of pericytes is difficult, if not impossible, in standard tissue sections, and it remains to be seen if new pericytes develop by proliferation of pre-existing tissue-resident pericytes, or if there is systemic recruitment of pericytes from a common reservoir during the formation of new vessels.
Morphologically, pericytes have a large cell body with an elongated cell membrane and can wrap around several endothelial cells. Based on their morphology and physical association with endothelial cells, they are sometimes mistaken as macrophages. There is also no definite distinction between pericytes and smooth muscle cells within the microvasculature that provide tone and integrity to endothelial cells. While smooth muscle cells express α-smooth muscle actin (SMA), pericyte subsets have been found to either be positive or negative for α-SMA (289). Other surface markers such as nestin, NG2, PDGFR-β, and even desmin have been used to define these cells, but these markers need not be coexpressed on all pericytes.
Another hypothesis is that pericytes and mesenchymal stromal cells (MSCs) with multipotent regeneration capacity arise from the same pool of cells (73). While the correlation between the two cell types has not been studied in the skin, MSCs isolated from the bone marrow and adipose tissue have been shown to mimic pericytes by surrounding endothelial cells and accelerating new blood vessel formation both in vitro and in vivo (191, 225). Despite differences in their nomenclature and classification, their spatial proximity to blood vessels, their interactions with both endothelial cells and incoming hematopoietic cells, and their ability to deposit ECM make pericytes important to wound repair. Further studies, fueled by single cell technologies, could help unravel questions related to the ontogeny, identity, and origin of these cells.
4. Circulating progenitor cells in neovascularization and wound healing
Since Asahara and Isner’s initial description of a putative EPC in 1997, there has been much interest and controversy regarding the ability of circulating cells to participate in blood vessel repair (12). Early studies indicated that both hematopoietic stem cells (HSCs) and non-hematopoietic progenitor cells, predominantly EPCs, contribute to regenerating blood vessels (64, 154, 305, 325, 400, 412). These stem and progenitor cells reach the ischemic tissue in three steps. First, chemokines released at the injured tissue cause progenitor cells from the bone marrow to mobilize into the circulation (2, 81). Next, the progenitor cells transit through the circulation towards increasing chemokine gradients and home preferentially to the region of ischemic insult (53). Finally, the progenitor cells incorporate into the sprouting endothelium where the stem and progenitor cells differentiate into endothelial cells (400).
However, subsequent work by several independent groups has cast doubt on whether the circulating cells are endothelial progenitors and are involved in ischemia-responsive vasculogenesis and blood vessel regeneration (165, 220, 297, 322, 329, 334, 340, 424). These studies conducted in vivo in murine models have demonstrated that circulating progenitor cells do not form endothelial cells at sites of injury or tumorigenesis (322, 334). Instead, it has been shown that the circulating cells involved in new blood vessel formation are macrophages that support endothelial cell sprouting, or are pericytes that surround and scaffold endothelial cells (25, 165, 220, 297, 305, 329, 340, 475).
Circulating progenitors have been difficult to characterize since surface markers from the literature may not accurately isolate the cells from the circulation or from the microenvironment of the newly forming blood vessel that contains several other cell types. Single cell technologies will be useful in definitively unraveling the identity and role of circulating progenitor cells in wound healing.
5. Fibroblasts in wound healing
Fibroblasts are ubiquitously present in the connective tissue of every organ system where they deposit and remodel ECM. There is notable heterogeneity among fibroblasts derived from different tissues, during different stages of development and based on their activation status (377). This heterogeneity contributes to substantial phenotypic differences between fibroblast subpopulations that translate to variable functions in wound healing, including ECM deposition and organization, secretion of growth factors and cytokines, and immunomodulation (121). The ability to characterize fibroblasts has been limited until recently due to the absence of defined markers to distinguish fibroblast subpopulations (76). However, advances in marker identification, lineage tracing, and functional assays are improving our understanding of fibroblasts in the skin. With the use of these tools, it has been shown that fibroblasts are comprised of different lineages and are quite plastic and responsive to signals from the epidermis and other cells within the dermis (92).
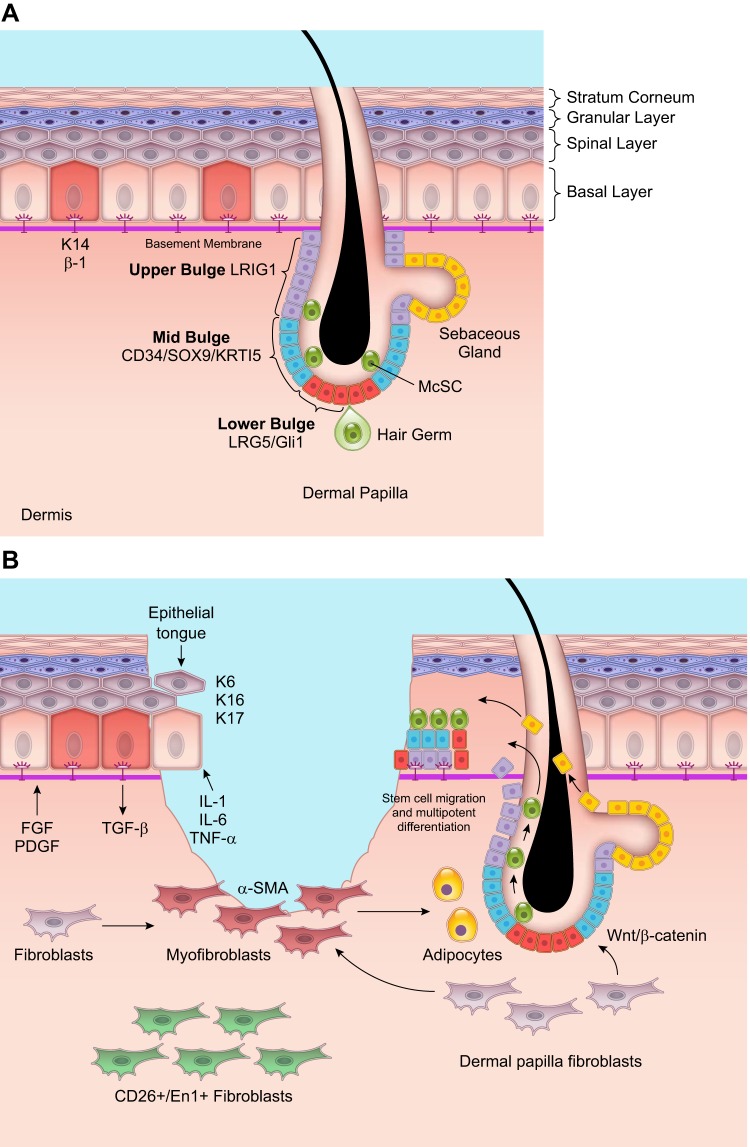
Fibroblast heterogeneity can be positional, defined by their spatial orientation relative to the epidermis and epidermal appendages. It can also be anatomical, defined by their location in various sites within the body (387). In murine skin, the upper (papillary) dermis and lower (reticular) dermis exhibit differences in fibroblast density and organization of collagenous ECM (65). Lineage tracing studies have revealed that initial dermal repair is attributed to the lower lineage fibroblasts that express myofibroblast markers such as α-SMA (92). These cells synthesize a large amount of ECM, which contributes to scarring. The scar-forming fibroblasts are also positive for Engrailed-1 and can be isolated using the surface marker CD26/DPP4 (335). Importantly, inhibition of CD26/DPP4 enzymatic activity during wound healing diminishes scar formation with clinical implications (335).
Fibroblast heterogeneity also affects dermal-epidermal interactions during wound repair. The dermal papilla (DP) fibroblasts, for example, which are at the base of the hair follicle, activate Wnt/β-catenin for hair follicle development (238). Ablation of β-catenin within fibroblasts promotes hair follicle regeneration, while activation of β-catenin in the fibroblasts reduces hair follicle regeneration (339). Conversely, epidermal stem cells in the hair follicle bulge can signal to the adjacent DP fibroblasts and can induce them to differentiate into myofibroblasts or smooth muscle cells (127). More recently, it has been shown that the myofibroblasts within newly forming hair follicles can form dermal adipocytes following wounding, and this myofibroblast-to-adipocyte transition reduces scar formation (318). Dermal adipocytes may prevent scar formation by increasing hair follicle regeneration and activating surrounding fibroblasts (358, 416).
Skin fibroblasts also display anatomical heterogeneity exhibiting distinct patterns of gene expression when isolated from different locations. Differentially expressed genes include those responsible for ECM synthesis, proliferation, and migration, processes that are fundamental for wound healing (54). A major factor behind anatomical heterogeneity might be an outcome of varying fibroblast origins. Fibroblasts in the skin of the face, for example, are derived from the neural crest (205, 335). Fibroblasts in the skin of the ventral body are derived from the lateral plate mesoderm, and fibroblasts from the dorsal skin originate from the dermomyotome (335).
It has been hypothesized that fibroblasts can transdifferentiate into endothelial cells during tissue repair, but this theory has been contested and most endothelial cells during repair have been found to arise from preexisting endothelial cells in tissue (175, 213). It has also been suggested that circulating mesenchymal cells from the bone marrow can contribute to fibroblast populations in the skin during healing (109, 175); however, this remains to be definitively shown. Although fibroblasts do not form cells of other lineages, there is greater plasticity within mesenchymal cells of the skin. In addition to positional and origin specific differences, fibroblasts also demonstrate changes based on their activation status. It is well established that some fibroblasts acquire a more contractile phenotype during wound healing by expressing β- and γ-cytoplasmic actins as well as α-SMA which converts them into contractile myofibroblasts (131, 416) as described below.
6. Myofibroblasts in wound healing
One of the main hallmarks of wound healing is wound contraction, which reduces the surface area of the wound that must be reepithelialized. During this process, collagen fibrils are organized perpendicularly to the wound edges to increase the mechanical strength of the tissue (360). The change in the stiffness of the wound microenvironment converts the fibroblasts migrating towards the center of wound into α-SMA-positive myofibroblasts. Myofibroblasts are a transient cell type that deposit ECM and exhibit characteristics of contractile smooth muscle (181, 416). These cells are formed when fibroblasts acquire bundles of microfilaments in a process regulated by several cellular-ECM interactions, growth factors, and mechanosensory signals within the granulation tissue (130, 162, 353). Given that fibroblasts are a heterogeneous cell population (93, 387), it is possible that only certain fibroblast subpopulations can differentiate into myofibroblasts during wound healing (259, 335). In an injured state, local fibroblasts residing in the uninjured dermis and subcutaneous tissue are considered the main source of myofibroblasts (183), but there is the possibility that myofibroblast subsets derive from other cell types including from fibrocytes (134), MSCs (13), pericytes (359), and epithelial cells.
Fibroblasts from the unwounded dermis and underlying subcutaneous tissue first transition into proto-myofibroblasts with stress fibers in response to increased mechanical stress. Proto-myofibroblasts only display β- and γ-cytoplasmic actin expression (77). The differentiation into α-SMA synthesizing myofibroblasts occurs in response to an interaction of fibroblasts with EDA fibronectin, an alternatively spliced segment of fibronectin (366) in the wound bed via α5β1 and αvβ3 integrins and stimulation by TGF-β (366). The increase of myofibroblasts in murine wounds begins at day 3 and peaks at day 7 following wounding (18) where they produce ECM including collagen type I and III and α-SMA (416). α-SMA is recruited to stress fibers under high tension, which is generated by anchoring stress fibers at sites of focal adhesions called the fibronexus (147, 416). The force exerted by stress fibers frees TGF-β1 from its large latent complex allowing the strained ECM to maintain a feedback loop that regulates persistent fibrotic activity of the myofibroblast (96, 180).
Myofibroblasts also attach to polymerized fibronectin and collagen fibrils via their integrins and pull the fibrils perpendicularly to the wound edge via their actin-rich cytoskeleton, allowing wound contraction to occur (416). Since myofibroblasts are bound to each other via gap junctions, they may work in conjunction when inducing contraction. Interestingly, in the absence of α-SMA in myofibroblasts, smooth muscle γ-actin and skeletal muscle α-actin functionally compensate to bring about contraction (418). Myofibroblasts can also display alternate contractile proteins such as myosin heavy chains or desmin, although α-SMA is expressed far more frequently (416).
Additional factors and ECM components such as hyaluronan, osteopontin, periostin, vitronectin, endothelin, angiotensin, CCN2, and Cx43 have been associated with myofibroblast differentiation and identified as therapeutic targets (234, 375). PDGF has been implicated in regulating proto-myofibroblast motility (163). MMPs have also been shown to mediate myofibroblast differentiation, as MMP inhibitors block myofibroblast differentiation in vivo (275), but their exact mechanism remains unknown. Direct contact of fibroblasts with neuronal processes has also been shown to activate myofibroblast differentiation (128). Finally, certain inflammatory mediators including TNF-α can inhibit differentiation, suggesting that extended inflammation in diseased states may postpone myofibroblast differentiation and contribute to a delay in the wound-healing process (148).
Myofibroblasts ultimately are cleared from the wound site via apoptosis once tissue integrity is sufficiently restored (83). It is unclear if following healing, myofibroblasts can revert to the fibroblast phenotype present in uninjured skin. It is thought that in many forms of fibrosis, such as hypertrophic scarring, myofibroblasts fail to undergo apoptosis and thus lead to scarring conditions (427). Murine studies of hypertrophic scarring support this hypothesis, where following mechanical loading, increased survival of myofibroblasts leads to greater scar tissue. Consequently, myofibroblasts play an important role in the late stages of granulation tissue formation and present a promising target for developing treatments for fibrosis and scarring.
D. Re-epithelialization
The epidermis withstands mechanical stress and protects the body from microorganisms, ultraviolet radiation, water loss, and extreme temperature. It consists of a multilayered epithelium containing keratinocytes that are connected to neighboring keratinocytes by cell-cell junctions called desmosomes. The lowermost layer or the basal layer is attached to a specialized ECM called the basement membrane through hemidesmosomes and focal adhesions. The layers above the basal layer are the spinous layer, granular layer, and the stratum corneum. The stratum corneum consists of impermeable cornified cells that continuously shed. In addition to keratinocytes, the epidermis also contains resident immune cells, hair follicles, sebaceous glands, and sweat glands. Since the epidermis is highly susceptible to injury, resident stem cells are crucial for maintaining both homeostasis and repair of the skin, where stem cell division and differentiation compensates for cell loss.
In the mouse skin, stem cell populations have been found using lineage tracing experiments within the interfollicular epidermis (IFE), hair follicle junction, hair follicle bulge, sebaceous gland, and sweat gland (439). There are also melanocyte stem cells, which in humans are dispersed in the IFE and hair follicles, and in mice are found mostly within hair follicles (292). The prevailing model that explains epidermal regeneration and repair is the epidermal proliferating unit (EPU) model. According to this paradigm, slow cycling and long-lived stem cells of the IFE expressing high levels of K14 and β-1 integrin are present in the basal layer surrounded by ~10 transit amplifying cells (266). IFE stem cells proliferate asymmetrically to generate these transit amplifying cells that are constantly proliferating and differentiating (257). Thus all cells in the basal layer are not stem cells, and under homeostasis, stem cells are not constantly proliferating (441).
An alternative theory suggests that there are no IFE stem cells; instead, all cells in the basal layer are functionally equivalent progenitor cells with equal probabilities of generating a differentiated cell or a progenitor cell (91, 236). This model is dependent on just two parameters: the average cell division rate and the proportions of divisions that lead to an asymmetric fate (207). The progenitor cell hypothesis has been used to describe homeostasis in the mouse tail skin and remains to be demonstrated in other skin structures. However, it could potentially explain the ability to create large sheets of keratinocytes ex vivo and heal superficial wounds. Progenitor cells cannot always survive in full thickness wounds and are not capable of extensive tissue regeneration (266). In contrast, lineage tracing experiments in murine wound healing favor the EPU model. It is shown that wounding leads to the recruitment of IFE stem cells with clones migrating from the periphery to the center of the wound. The IFE stem cells persist in the wound and lead to large numbers of differentiated cells (266).
Interestingly, IFE stem cells possess an inflammatory memory that allows them to respond rapidly to secondary assault. At the time of the primary inflammatory stimulus, cellular alterations lead to increased chromosomal accessibility at transcriptional regions of key stress genes (283). These open-chromatin domains are sustained to rapidly sense and respond to a secondary threat. More than half of the genes induced selectively during wound repair are associated with these open chromatin domains (283).
The epidermal appendages comprise a significant portion of the skin, are subject to external stress, and respond to skin injury. During homeostasis, sebaceous glands turn over constantly. Hair follicles go through bouts of hair growth and degeneration. Sweat glands are mostly quiescent (23). The epidermal stem cells and progenitors for these unique compartments are lineage-restricted and committed to maintaining their own cell type. However, in the event of injury, epidermal stem cells for the appendages can exhibit plasticity and acquire multilineage differentiation potential (23). Stem cells in the hair follicle bulge, for example, do not contribute to the IFE under unwounded conditions. However, after skin injury, cells from the bulge can reprogram and migrate in a linear manner to the center of the wound (FIGURE 6) to reconstitute the IFE where their progeny persist for months following wound healing (194, 243).
FIGURE 6.
Re-epithelialization and fibroblast-epidermal cell interactions during wound healing. A: in the uninjured skin, the epidermis is multi-layered with the lowermost basal layer attached to the basement membrane. This layer contains K14+/β1-integrin positive stem cells that divide and differentiate to form the keratinocytes of the spinal and granular layers. The uppermost layer is the stratum corneum that contains cornified and impermeable cells. The epidermis also contains appendages of which the hair follicle and sebaceous gland are of particular interest since they contain several stem cell subsets with high activity even during homeostasis. The base of the hair follicle and hair germ, which is adjacent to the dermal papilla, contains LRG5+/ Gli1+ stem cells. The mid-bulge contains CD34+/Sox9+/keratin 15+ stem cells, and the upper bulge contains LRIG1+ stem cells. Melanocyte stem cells (McScs) are also dispersed in the hair bulge and germ. There are also stem cells that reconstitute the sebaceous glands. B: following wounding, unipotent stem cells within the hair follicle move in a linear fashion and take on a multipotent differentiation potential to reconstitute various cell types of the epidermis. Fibroblasts in the dermal papilla can signal these stem cells in the hair follicle through the Wnt/β-catenin pathway. In return, the epidermal stem cells signal the fibroblasts, converting them to myofibroblasts, to help in wound contraction. A subset of myofibroblasts become adipocytes, and this switch has been found to reduce scar formation. Another subset of fibroblasts with expression of CD26/En1 preferentially deposit higher levels of ECM and are responsible for fibrosis. Interfollicular stem cells in the basement membrane of the epidermis also proliferate to generate keratinocytes that lose their cell-cell junctions and migrate into the wound forming the epithelial tongue. The proliferation and migration of differentiated keratinocytes and stem cells into the wound, together lead to re-epithelialization of the wound. The illustration is a simplified rendering based on current knowledge.
1. The intrafollicular epidermis during wound healing
The extrinsic signals from the stem cell niche, which includes signals from ECM, growth factors, and surrounding cells, are important in determining stem cell fate. Stem cells of the IFE are clustered rather than distributed as single cells and, since they express higher levels of integrins, are more adhesive compared with transit amplifying cells (208, 441). In homeostasis, integrins are restricted to cells in the basal layer. The main integrins on the basal cells are α2β1 that binds collagen, α3β1 and α6β4 that binds laminin, and αvβ5 that binds vitronectin. α6β4 is concentrated distally on the basement membrane while α3β1 is at the leading apical edge. The other integrins are spread over the basal, lateral, and apical surface of the basal cells (440).
Following wounding, keratinocytes at the wound edge loosen their adhesions to each other and to the basal lamina to close the defect, forming the migrating epithelial tongue. Integrins are also expressed in the suprabasal keratinocytes, which leads to increased Erk-MAPK signaling and inflammatory cytokine synthesis, causing hyperproliferation of keratinocytes and immune cell activation (185). Fibronectin receptors α5β1 and αvβ6 are also upregulated in keratinocytes during wound healing (441). Integrin signaling is critical, and loss of keratinocyte integrins is found to impair wound healing (164, 333).
Several growth factors and proteins modulate keratinocyte migration and proliferation. Protein kinase C (PKC)-α and the transcription factor Slug are known to decrease keratinocyte adhesiveness and increase their motility (356, 435). Migrating and proliferating keratinocytes are responsive to factors from the epidermal growth factor family including EGF, HB-EGF, and TGF-α and fibroblast growth factors, especially FGF2 or KGF (120, 447). These factors upregulate keratins K6, K16, and K17 that are important for migration (309). The cytokines IL-1, IL-6, and TNF-α present in the wound increase keratinocyte motility. In addition to promoting loss of cell adhesions and loss of apical-basal polarity, TNF-α has been shown to induce an epithelial to mesenchymal transition in cells, which if not regulated can lead to a fibrotic state (476). Migrating keratinocytes are also found to express MMPs abundantly at the wound edges that allow these cells to migrate through the fibrin plug and over the granulation tissue (226, 350).
Keratinocytes in the wound are also actively interacting with fibroblasts, endothelial cells, and immune cells in the wound. During inflammation, MCP-1 produced in keratinocytes is important for activating macrophages, neutrophils, and T cells (89, 285). Upregulation of SKINTs and STAT3 in keratinocytes is necessary for activation of DETCs within the epidermis (215). In the early proliferative stage, IL-1 and TNF-α produced by keratinocytes stimulate fibroblasts to produce factors important for keratinocyte proliferation and migration (15, 446). During angiogenesis, VEGF produced in keratinocytes increases vascular permeability within endothelial cells and is critical for new vessel formation (36).
In the later stages of repair, TGF-β produced by activated fibroblasts is found to be essential for reverting activated keratinocytes into the basal cell phenotype by inducing basal cell-specific markers such as K5 and K14 and reducing proliferation (309). Keratinocyte interactions with the other cell types during wound repair is important for complete wound closure, and an imbalance in any of the cellular or molecular mechanisms can shift the wound into fibrosis or chronic wounds.
2. Regeneration of hair follicles
The hair follicle along with the sebaceous gland comprises the pilosebaceous unit and is scattered in the IFE. The hair follicle gives rise to and supports the hair shaft, while the sebaceous gland releases lipids to lubricate the surface of the skin. The hair follicle is unique given that the heterogeneity of its stem cells has been uncovered more than in other organ systems most likely due to its accessibility. The different pools of stem cells are distributed within the vertical and horizontal axis of the hair follicle, each expressing a unique combination of surface markers and responding to distinct signals. In homeostasis, the hair follicle gives rise to the hair shaft in three stages: telogen (resting), anagen (growth), and catagen (destruction). In mice, these stages occur in synchronized bouts allowing for the easy isolation and study of both the cells and the molecular pathways that affect them. While exhibiting some level of plasticity, the various stem cell subsets of the follicle participate in unique functions during the homeostatic stages. However, under conditions of stress or injury, the subsets can substitute for each other to bring about healing.
The base or bulb of the hair follicle is called the bulge. It contains several stem cells based on spatial distribution that can broadly be classified into lower bulge, mid bulge, and upper bulge stem cells. Below the bulge lies the secondary hair germ, which consists of progenitor cells derived from the lower bulge stem cells. The secondary hair germ makes up the outer root sheath and is embedded in the dermal papilla, which contains fibroblasts that are releasing factors critical for hair follicle formation. Vertically above the hair bulge is the junctional zone and the sebaceous gland. The infundibulum is the uppermost layer of the hair follicle and in contact with the IFE.
The lower bulge stem cells are the most proliferative and express the stem cell marker LRG and the Hedgehog transcription factor Gli1+ (14, 37). They are also high in integrin α6. Cells within the secondary hair germ maintain similar markers as the lower bulge stem cells but undergo more divisions in anaphase than the bulge cells due to their proximity to signals from the dermal papilla (156). While the bulge cells usually give rise to the hair germ, the cells of the hair germ can replenish an empty bulge niche, which indicates their ability to compensate for loss of function within the epidermis (344, 345). The mid-bulge stem cells do not express LRG and Gli1 but are positive for CD34, Sox9, and keratin15 (383). Following injury to the skin, the mid-bulge stem cells contribute to healing of the IFE, but do not persist long-term within the IFE following healing (194). The upper bulge stem cells express markers such as Gli1, similar to the lower bulge stem cells. During homeostasis, they are as efficient as the lower bulb stem cells in the anagen phase (383). Upon skin injury, they contribute to the IFE and persist in the IFE long-term following healing (37). Melanocyte stem cells (McSCs) are also dispersed within the bulge and hair germ, and will be described in a subsequent section (294, 323). There is coordination between the cycling of the bulge cells and the McSCs so that mature melanocytes can transfer melanin into the growing hair follicle (124).
Vertically above the bulge region and in the junctional zone are the LRG6 stem cells which maintain the junctional zone and the sebaceous glands, although they can give rise to all epidermal cells including the IFE (382). There are two variants of the LRG6 stem cells, those that express MTS24 and those that do not, and the differences between these two subsets need to be elucidated (204, 291, 382). Further up in the junctional zone are the LRIG1+ stem cells that are responsive to EGF signaling. Under homeostasis, these stem cells contribute to the IFE and sebaceous glands, but not to hair follicles (383). In a wounded condition, these junctional LRIG1+ stem cells can give rise to the IFE, sebaceous glands, and hair follicles (203).
The sebaceous glands are maintained by four subsets of stem cells. These include the bulge stem cells, LRIG1+ stem cells, and LGR6+ stem cells of the junctional zone as well as BLIMP1 expressing cells (126, 277, 302, 407). The sweat glands in the skin are distinct from the pilosebaceous unit that contains hair follicles and the sebaceous glands. Unipotent stem cells have been discovered that maintain the sweat glands under homeostasis, and their role in wound healing needs further elucidation.
3. Melanocytes in wound healing
Partial thickness and deep full thickness injuries, especially those resulting from burns, leave the skin hyper- or hypopigmented, due to changes in melanocyte proliferation and activation (71) Melanocytes are dendritic, neural crest-derived cells that produce melanin, a biopolymer that protects the skin from ultraviolet irradiation and ROS stress. In human skin, melanocytes are dispersed within the IFE and hair follicles (292, 383). In mice, melanocytes are mostly located in the hair follicle except for the ear and tail skin where they are also found in the IFE (312). During homeostasis, melanocytes are repopulated by McSCs, which are in the bulge of the hair follicle. Here, they are influenced by growth factors such as TGF-β, endothelin-2, Notch, and WNT signals from the adjacent hair follicle stem cells (293, 404) and melanocyte repopulation occurs concurrent with the growing phase of the hair cycle (292).
The role of melanocytes in wound healing was observed as early as the 1950s and 1960s when it was found that melanocytes were present in the new epithelial covering within 4–6 days following wounding (381). The melanocytes in the adjacent uninjured skin were found to undergo mitosis and synthesize melanin, but the melanocytes within the repairing epidermis did not have high melanin expression (184). It is now known that following injury, McSCs leave their niche in the bulge of the hair follicle before their initial cell division and migrate into the injured mouse epithelium where they give rise to differentiated, pigment producing melanocytes important for repigmentation of the epidermis (62). This migration might deplete the original McSc pool (62).
The migration of McSCs into the epidermis following injury occurs via activation of the melanocortin 1 receptor (Mc1r). It is interesting that these stem cells can be lured out of their protected niche during times of injury where stem cell differentiation takes precedence over stem cell maintenance. It is thought that repigmentation might be too much of a risk for MsSCs leaving the stem cell niche, and there could be other functions that these stem cells might be involved in, such as regulating keratinocyte differentiation (312). Moreover, their neural crest origin might have a role to play in these stem cells retaining an innate migratory phenotype.
It has been hypothesized that melanocyte-fibroblast interactions during wound healing result in scar formation (135). Per this paradigm, melanocytes are activated in response to cytokines released from fibroblasts (378). Activated melanocytes in return stimulate fibroblasts to deposit ECM. More evidence is needed to confirm melanocyte and fibroblast interactions in wound healing and scar formation.
E. Tissue Maturation and Remodeling in Wound Healing
In most clinical settings, closure of acute and chronic wounds is considered the wound healing end point, but wounds can continue to undergo remodeling or tissue maturation for several months or even years. This last stage of wound healing ultimately determines if scarring will occur or the wound will recur. The remodeling phase consists of regression of the neovasculature, as well as periodic deposition to the ECM and subsequent reconstitution of granulation tissue to scar tissue. Granulation tissue is largely comprised of collagen III, which is partially replaced by the stronger collagen I as remodeling of the wound progresses. This process is a result of concurrent collagen I synthesis and collagen III lysis, which is followed by reorganization of the ECM (167).
Once re-epithelialization occurs, myofibroblasts within the granulation tissue continue to synthesize MMPs and their respective inhibitors (tissue inhibitors of metalloproteinases, TIMPs) (45, 431). MMPs target specific components of the ECM for degradation, an essential step to its remodeling (45). As the MMPs work to reconstitute the ECM, matrix synthesis slows significantly (77). Thus the collagen III found in granulation tissue is gradually decreased and replaced with collagen I (77). Following ECM modification, TIMPs begin to block MMPs, halting further ECM degradation. An imbalance between TIMP and MMP expression can lead to abnormal ECM modification and even chronic wounds (141, 408). As the wound completes the healing process, myofibroblasts undergo mediated apoptosis (182). When cells within the granulation tissue do not undergo apoptosis following wound remodeling, hypertrophic scars tend to form (83).
Macrophages are important during wound remodeling where they take on a fibrolytic phenotype, breaking down excessive ECM and engulfing ECM debris and apoptotic cells. The interaction between macrophages and fibroblasts is also critical. If (myo)fibroblasts overexpress the CD47 “don’t eat me” signal, it prevents them from being phagocytized and eliminated by macrophages (448). This can result in excessive matrix deposition and HTS.
Blood vessels that are generated during angiogenesis are leaky, lacking tight cell-cell contacts and branching out into the granulation tissue. They may also have scant coverage of pericytes to allow immune cells to easily infiltrate into the wound. During remodeling, neovessels undergo pruning to generate stable and well-perfused blood vessels that can resume homeostasis, and to form endothelial cells that are quiescent. Unfortunately, less work has been conducted on vessel regression compared with angiogenesis most likely because in vitro studies allow for new vessel formation, but do not contain negative feedback mechanisms that allow for studying vessel regression. Vessel pruning is found to occur through endothelial cell apoptosis (85), although the mechanisms underlying this process remain unknown. Re-epithelialization may also have a role to play in vessel pruning. With closure of the epithelium, the healed wound bed is no longer in a state of hypoxia, and changes within the hypoxia-responsive elements may contribute to endothelial cell quiescence.
There are negative-feedback mechanisms within endothelial cells such as activation of intracellular Sprouty proteins and Vasohibin that might act as “anti-angiogenic switches” by modulating responsiveness to VEGF (43, 452). Endothelial cells also express CXCR3 during the late stage of wound healing. Upon binding its ligand, CXCL10 that is preferentially expressed during wound remodeling, CXCR3 inhibits endothelial tube formation (27). Mice lacking CXCR3 demonstrate hypertrophic scarring, indicating the importance of this pathway during wound remodeling (478). Uncovering other cellular signaling pathways that are preferentially expressed during wound remodeling will explain why some wounds undergo dystrophic healing such as in keloids and hypertrophic scarring.
III. CELLULAR ALTERATIONS UNDERLYING IMPAIRED WOUND HEALING
A. Differences Between Fetal and Adult Wound Healing
Wound healing outcomes are linked to the developmental stage of the injured organism. In adult cutaneous repair, fibroproliferation rapidly restores the skin barrier to protect the wound from infection and further injury. However, the fibroproliferative response results in deposition of poorly organized collagen and consequently incomplete regeneration of the tissue, including loss of dermal appendages such as hair follicles and sebaceous glands, and reduced tensile strength (167). In comparison, fetal skin possesses the remarkable ability to regenerate tissue without scarring (347), heal at an accelerated rate compared with adult wound healing, and result in complete restoration of the dermis, epidermis and epidermal appendages. The superiority of fetal wound healing over adult wound healing is conserved across several mammalian species, including humans (5, 252).
The transition from fetal scarless wound healing into scar formation occurs at a precise gestational stage, at around 18 days gestation in mice (47) and 24 wk gestation in humans. The factors that contribute to scarless healing in the fetus have been studied extensively, with contributions of multiple cell types, cytokines, growth factors, and ECM components (188).
Differences between fetal and adult wound healing can be noted as early as the inflammatory stage. As previously described, adult wounds are characterized by an influx of neutrophils (299, 354) and macrophages, which secrete cytokines such as CSF-1, TNF-α, and PDGF, promoting a fibrotic response (354). Mast cells are also implicated in adult wound healing (255) where they recruit additional neutrophils and stimulate the fibroblast to myofibroblast transition to exacerbate ECM deposition. In comparison, fetal wounds have fewer neutrophils, macrophages, and mast cells, which contributes to the overall decreased inflammatory response and lesser scar tissue formation (469).
Fibroblasts derived from fetal and adult skin exhibit fundamental differences that contribute to differences in their wound healing ability (335, 436). Fetal fibroblasts migrate faster to the site of injury compared with adult fibroblasts, where they simultaneously proliferate and synthesize collagen (188). In comparison, adult fibroblasts must divide before beginning collagen synthesis, which contributes to delayed wound healing (231). There is an increased interest in understanding which fibroblast subsets preferentially cause the transition into scarring. Engrailed-1 expressing fibroblasts have been found to increase concurrently with the onset of scar formation in the dorsal skin of embryonic mice (335). These fibroblasts can be distinguished by the surface marker CD26, and ATAC-sequencing has been used to determine the exact time points during which the transition into scarring occurs. The discoid domain receptor (DDR) family is also differentially expressed in fetal and adult fibroblasts. Specifically, fetal fibroblasts express a higher level of DDR-1, which regulates cell proliferation, differentiation, and collagen production and expression of this receptor decreases with gestational age (59). Identification of alterations in surface markers such as CD26 and DDR-1 in fibroblasts from various anatomical sites will allow for preferential targeting of scar-forming fibroblasts during adult wound healing.
Fibroblast-mediated collagen synthesis and deposition varies in fetal and adult wounds. Although types I and III collagen are found in skin at both developmental stages, fetal wounds contain a higher ratio of type III to type I collagen, which decreases as the fetus develops. This correlates to a transition from scarless to fibrotic repair (250, 273). Moreover, collagen fibers in the fetal wound are organized to resemble collagen in the uninjured skin, whereas adult wounds form dense collagen bundles parallel to the wound surface. Aging also increases collagen cross-linking, resulting in increased matrix rigidity and scarring (253).
Several other important ECM adhesion proteins and cellular surface receptors are differentially expressed in fetal and adult wounds. Hyaluronic acid (HA) is an important component of the ECM and has been implicated in regulating cell proliferation, motility, and collagen synthesis. In fetal wound healing, sustained deposition of HA occurs more rapidly than in adult wound healing (247) and has a role to play in scarless repair (248). HA levels are regulated by Wnt proteins, which are also differentially regulated in fetal versus adult wounds (298). In addition, fetal fibroblasts express a higher level of surface receptors for HA compared with adult fibroblasts (8, 248) which enhances fetal fibroblast migration into the wound (267).
ECM such as fibronectin and tenascin, which are preferentially expressed in healing wounds compared with uninjured skin, are more highly expressed in fetal wounds (251, 450). These ECM components can activate matrikine signaling to resident cells in the wounds accelerating proliferation and re-epithelialization (48). For example, Tenascin-C contains EGF-like repeats and can bind the EGF receptor present on fibroblasts and keratinocytes, with a low affinity but a high avidity, persistently activating EGFR signaling in these cells (338). Finally, fetal wounds maintain a higher ratio of MMP to TIMP expression than adult wounds, which activates fibrolysis during wound remodeling and precisely resolves wound healing (74).
B. Mechanotransduction and Skin Fibrosis
Historically, skin has been considered a mechanically inert viscoelastic membrane (101, 374), but recent evidence has demonstrated that the skin and its constituent cells are exquisitely mechanoresponsive and that dysregulated mechanotransduction underlies several wound healing disorders (1, 167, 245, 301, 461, 464). Breaks in cutaneous continuity alter the cellular mechanical environment and can influence cells of the wound healing cascade. When cutaneous integrity is re-established, mechanical forces may guide skin remodeling, leading to intact and durable repair. The mechanisms through which these processes occur in normal skin and during wound healing remain unclear. Abnormal wound repair occurs across a spectrum ranging from excessive fibrosis and scarring to underhealing wounds clinically typified by nonhealing chronic ulcers. Both ends of the dysfunctional wound healing and repair spectrum pose a significant healthcare challenge.
Keloids are fibrous lesions characterized by an excessive deposition of ECM components during wound regeneration (168). As such, they superficially resemble HTS and present many of the same burdens, such as pruritis, pain, and psychological discomfort (336). Unlike hypertrophic scars, keloids often grow beyond the original parameters of the wound and rarely regress over time (422). While keloids occur among people throughout the world, certain demographics display a greater incidence than others. Genetics are believed to play a significant role in keloid susceptibility, as populations of darker skinned individuals seem to have a notable predisposition. An estimated 15–20% of those with Hispanic, Asian, or African heritage suffer from keloids (422). Additionally, high frequencies of mutual keloid formation have been found among identical twins, further suggesting a strong genetic component (260). While keloids can develop virtually anywhere on the body, they tend to appear in certain regions, such as the ears, chest, and upper arms (380) with a proclivity to mechanical stress, such as stretching (296).
Hypertrophic scars are a major form of excessive scarring commonly seen as outcomes of surgical procedures, trauma, radiation, and following burn injuries. The sequelae of HTS include airway edema, speech/ swallowing dysfunction, sensory defects, disfigurement, and psychological distress to the patient. Unlike keloids, hypertrophic scars do not extend over the original wound boundaries. The pathophysiology of HTS includes a constitutively active growth phase of wound healing, with a highly vascularized scar tissue abundant with inflammatory cells and fibroblasts depositing excessive and disorganized ECM. Burn wounds that are deep partial thickness or full thickness almost always result in HTS formation. While full thickness burns can be surgically treated using skin grafts, there is currently no standardized treatment for patients with deep partial thickness burns. Thus there is a need to understand the cellular repair pathways following these skin injuries so that treatments that prevent HTS can be developed.
Mechanotransduction pathways are increasingly being implicated in skin and soft tissue pathologies including HTS, keloids, and Dupuytren’s contracture (233, 245, 437, 460). Although a variety of molecular pathways are involved in mechanotransduction, the integrin-FAK molecular pathway is the most well-defined regulator of skin mechanotransduction (96, 245, 461, 464). FAK is expressed in skin cells including keratinocytes and fibroblasts and is important for intracellular signaling in response to mechanical forces. Application of mechanical forces during wound healing leads to activation of FAK in fibroblasts and increased HTS formation (1, 96, 166, 463, 464). This occurs by FAK activation of numerous downstream components involved in fibrogenic responses such as phosphatidylinositol 3-kinase (PI3K)/Akt and mitogen-activated protein kinases (MAPK) (60, 86, 173, 421). In contrast, decreased FAK signaling has been associated with nonhealing wounds (97, 202, 245, 461). Degradation of FAK is accelerated in diabetic wounds, which leads to delayed wound healing and abnormal skin architecture (245). These underscore the significance of FAK-mediated mechanotransduction in skin disorders, specifically in HTS and nonhealing wounds.
Keratinocytes and fibroblasts, the primary cell types in the skin, have both long been known to respond to mechanical forces (96, 462). Fibroblasts have been studied extensively for modulating the ECM in response to changes in their microenvironment (92, 311, 335). Keratinocytes respond to mechanical strain by modulating proliferation and matrix-remodeling proteins (202, 461). However, keratinocyte-fibroblast interactions in skin wound repair that result in changes in overall skin biology are not fully understood. Conflicting reports regarding the role of mechanical strain on keratinocyte-fibroblast interactions have been published in the literature (128, 245, 445, 461, 464). For example, it has been proposed that keratinocytes secrete growth factors that act on fibroblasts and conversely fibroblasts produce proliferation signals for keratinocytes (113, 445). However, an understanding of keratinocyte-fibroblast interactions when integrated into global tissue repair and in response to mechanical forces might provide clues into the cellular and molecular causes underlying scar formation (445).
In addition to resident cells in the skin, cells recruited to the skin from the circulation also respond to mechanical forces. Keloids and hypertrophic scars show distinctive immunophenotypic profiles abundant in T cells, dendritic cells, macrophages, and Langerhans cells (50, 55, 352, 463), but the literature is predominantly focused on the paracrine effects of these cells on keratinocytes and fibroblasts. Little is known about how mechanical strain affects these cells directly. To more fully understand normal and abnormal skin biology, there is a need to uncover and integrate the involvement of cell types apart from keratinocytes and fibroblasts in skin mechanotransduction.
C. Chronic Nonhealing Wounds
Chronic wounds affect 6.5 million people in the United States, costing the healthcare system an estimated 50 billion dollars annually (211, 364). Diabetes, vascular disease, and aging are the main factors contributing to chronic wounds, causing diabetic foot ulcers, venous leg ulcers, and pressure ulcers, respectively (123). With the diabetic population (30 million) rapidly growing in number, chronic wounds are contributing to an increasingly large portion of healthcare costs (51). The nonhealing state leads to morbidity, loss of function, and a significant loss in the quality of life (195, 468). The 5-year mortality for patients following amputation is ~50% (117). While interventions exist, such as growth factors, ECM, engineered skin, and negative pressure wound therapy, many of these treatments are only moderately effective (123). There is a pressing need for new and more effective therapies.
To develop successful therapeutic strategies, it is important to understand the mechanisms that underlie impaired healing. Here, we will briefly address impaired wound healing from the perspective of diabetes since diabetic ulcers are the most common chronic wounds in the United States. The pathophysiology of diabetic wounds is multifactorial. Hyperglycemia alters the microenvironment or the “soil” as well as local and circulating cells or the “seeds” (337). At the molecular level, diabetes brings about impairments in several critical pathways in the skin, which play a role in the outcome of wound healing. One of the major pathways that is affected is the hypoxia response pathway, resulting in impaired neovascularization and poor wound healing outcomes (53, 94, 95, 97, 413, 414).
From a cellular perspective, diabetes impairs progenitor cell recruitment, proliferation, and growth factor release following injury. There is also a chronic inflammatory state and impaired new vessel formation (53, 201, 330, 332, 337). Macrophages are among the most well-studied inflammatory cells in the diabetic wound. As described previously, there is a delay in the production of chemokines and cytokines such as MCP-1 and MIP-2 in the diabetic wound that delays the influx of monocytes and activation of macrophages (449). A lag in macrophage activation causes impaired efferocytosis (216) and a persistence of wound debris, apoptotic cells, and neutrophils. The prevalence of wound debris sets up a constant inflammatory phase, and activated neutrophils continue to release proteases that nonspecifically degrade the wound microenvironment (216). Inflammation persists into the remodeling stage, and the wound never completely resolves healing (449).
Patients with diabetes have microvascular complications and vascular denervation. Endothelial cell dysfunction manifests as nitric oxide production is reduced, ROS levels increase, and expression of chemokines, growth factors, and receptors for these factors are altered. Reduced nitric oxide production alters vascular barrier function, increases platelet coagulation, and alters immune cell responses. While low levels of ROS are present during wound repair and facilitate endothelial function, high ROS impairs neovascularization by impacting endothelial cell proliferation, migration, and apoptosis. The diabetic wound also has reduced VEGF released from macrophages, keratinocytes, and adipose stem cells (ASCs) that impairs endothelial cell branching and angiogenesis.
The reduction in growth factor release from the subcutaneous ASCs is due to the selective depletion of vasculogenic ASC subpopulations in diabetes (330). Murine experiments also demonstrate that diabetes reduces the recruitment of circulating progenitor cells, and this impairment does not revert to normal kinetics even after a “cure” or return to normal blood glucose (201). Fibroblasts and keratinocytes also display impairments in diabetes. Fibroblasts in the diabetic wound are significantly less responsive to hypoxia, produce less VEGF, and highly express MMPs, which break down the ECM produced (241). Keratinocytes in the diabetic wound are also affected by changes in the microenvironment and display alterations in their migration, proliferation, and gap junctions (190).
In addition to these cellular factors, the skin’s surface is also host to a variety of bacteria, viruses, fungi, and other microbes collectively known as the skin microbiome (160). An estimated one billion bacteria occupy each square centimeter of skin, potentially infiltrating into adjacent appendages and glands (159). While many of these organisms are harmless or in some cases even beneficial, there are some that can play harmful roles and cause infection (102, 160). Microbes can prove to be deleterious in individuals that are immunocompromised or that maintain unhealthy or wounded skin (58). Chronic wounds, such as those found in the aged or diabetics, often suffer from the detrimental effects of microbes that colonize and reproduce within the wound bed (58, 102, 221). Burn wounds are also susceptible to certain microbes, with Streptococcus pyogenes, Enterococcus spp., or Pseudomonas aeruginosa (Pa) being frequently observed within the wound (161, 262). Pa is also a particularly devastating pathogen commonly associated with diabetic wounds and is especially difficult to treat due to its propensity to form thick biofilms, which enhance adherence to the wound, immune evasion, and antibiotic resistance (70, 187, 308). These characteristics all encourage the chronicity of recalcitrant wounds.
Collectively, impairments in the microenvironment, cell function, and biofilm deposition lead to abnormal wound healing, reduced tensile strength of the skin, and recurrence of wound (337). These findings may suggest that a “normalization” of the wound microenvironment might revert the dysfunction acquired by the cells. However, cells exposed to prolonged hyperglycemia have been shown to display metabolic “memory,” where even upon return to normoglycemia, cells do not regain normal function (52, 287). Thus it is imperative to address both aberrations in the microenvironment and in the cells to enhance wound healing in impaired states.
A wide variety of products exist for nonhealing wounds such as Promogran, OASIS, Integra, Regranex, Renasys, and OxyHeal1000 but are only modestly effective (143, 317, 331, 342, 430, 451). Bioengineered skin substitutes such as Apligraf, Dermagraft, and Grafix containing living cells have been developed to increase efficacy (87, 261). Randomized controlled multicenter comparative effectiveness studies of these cellular therapies provide level I evidence that cell-based therapies are safe and effective for correcting nonhealing wounds in the diabetic population and other impaired states (87, 261). These therapies are briefly described in the subsequent section.
IV. SKIN SUBSTITUTES AND CELL-BASED THERAPIES FOR WOUND HEALING
Autologous skin grafts are commonly used to promote healing in both partial- and full-thickness wounds. Skin grafts provide the epidermal layer but lack the dermis, resulting in poor contour and reduced survival of the graft (259a). While allograft skin grafts typically take to full-thickness wounds, they are usually ultimately rejected due to the immune response against cells of the epidermis and endothelial and fibroblast cells of the dermis (49, 229, 362). However, the noncellular components of dermis, primarily the ECM proteins and collagen, are relatively nonimmunogenic (481). Consequently, a promising strategy is to bioengineer nonimmunogenic substitutes to promote regeneration of the dermis and provide support for epithelial skin grafts (39, 66). Here we discuss some of the ECM and cell-based therapies and their effectiveness in healing wounds.
A. Extracellular Matrices for Enhanced Wound Healing
Several strategies have been employed to replace the dermis. One particularly desirable strategy is to provide environmental cues to promote dermal regeneration endogenously. This can be achieved using a biodegradable scaffold with or without cells that allows for infiltration of resident cells from the surrounding tissue (214, 398). Because ECM components of the dermis play a primary role in supporting graft retention, scaffolds are typically composed of these components: collagen, glycosaminoglycans (GAGs), and hyaluronic acid. Different scaffolds guide cell behavior in unique ways due to variable physical characteristics that stem from differences in manufacturing techniques, such as decellularization, sterilization, and cross-linking. For example, while chemical cross-linking is used to enhance the strength of the scaffolds, it can exhibit reduced incorporation into the wound, as well as inferior cell infiltration, ECM deposition, and neovascularization compared with non-crosslinked scaffolds. Some of the matrices most commonly utilized for healing are summarized below.
1. Integra
Integra (Johnson & Johnson, New Brunswick, NJ) has several skin substitutes that promote regeneration of the dermis. The Integra Dermal Regeneration Template was FDA-approved in 2002 for treatment of burn injuries. Integra also received approval in 2016 for Omnigraft for treatment of diabetic foot ulcers (DFUs). Their products comprise a slowly degrading dermal component derived from bovine collagen and chondroitin-6-sulfate and a silicone layer, which functions as a provisional epidermis (119). With the scaffold in place, resident cells from the adjacent dermis migrate into the matrix to deposit collagen and support neovascularization. The silicone layer controls water vapor loss, provides a flexible adherent covering, and is removed following dermal regeneration and replaced by an autologous epidermal graft for final wound closure (31, 193).
Integra has demonstrated efficacy and safety in patients with primary full-thickness or deep partial-thickness burn injuries. In studies of primary burn wounds, the neodermis is formed within 2–3 wk after application, after which the silicone is removed and an epidermal autograft placed (75). In addition to favorable clinical outcomes, a major advantage of Integra is that its temporary silicone layer provides immediate coverage of the wound after excision, allowing allografting to be delayed. Moreover, it has excellent biocompatibility and an extended shelf-life. However, when compared with other available skin substitutes, Integra has been shown to induce a greater foreign body response due to it being a chemically cross-linked material. Unlike other non-crosslinked human skin derivatives, the scaffold must be cleared by macrophages for ECM proteins to be deposited (420). Therefore, a strong preference for Integra over other available human skin substitutes demands additional clinical evidence.
2. Epifix
Epifix (MiMedx Group Inc., Marietta, GA) is a dehydrated Human Amnion/Chorion Membrane (dHACM) allograft derived from the placenta. Physiologically, the amniotic membrane holds the developing fetus and amniotic fluid and grows and remodels the ECM over the term of pregnancy (1). This process is mediated by paracrine growth factors that are secreted by the membrane. Microscopic analysis of dHACM confirms that defined amnion and chorion layers identical to those found in the amniotic membrane are maintained and that the allograft comprises a layer of epithelial cells, basement membrane, and an avascular connective tissue matrix (222). Numerous ECM proteins, growth factors, and cytokines known to enhance healing, modulate inflammation, and reduce scar tissue formation are present in and secreted by the allografts, including PDGF, basic fibroblast growth factor (bFGF), EGF, IL-4, IL-6, IL-8, IL-10, and tissue inhibitor of metalloproteinase (TIMP) -1, -2, and -3 (223, 224).
Clinically, Epifix has demonstrated efficacy in promoting the healing of several types of nonhealing wounds resistant to traditional treatments (116). It has also been demonstrated that nonhealing wounds that healed after dHACM treatment did not recur in the long term (367). Additionally, a prospective randomized controlled trial for the treatment of DFUs showed an increased incidence of healing in chronic wounds after 4–6 wk of biweekly treatment of dHACM compared with standard treatment (482). Together, these clinical results support that dHACM is an effective treatment for wound healing, particularly in the context of chronic wounds.
3. OASIS
OASIS Wound Matrix (Cook Biotech, West Lafayette, IN) is a 0.15-mm-thick nonchemically crosslinked cellular biomaterial derived from porcine small intestine submucosa. The material consists primarily of collagen-based ECM but also includes ECM components such as GAGs and fibronectin (278) as well as growth factors including FGF-2, TGF-β, and VEGF (186). It has been cleared for use by the FDA since 1998 and has shown efficacy in multiple preclinical models. Advantages include immediate availability and a shelf life of 2 yr with storage at room temperature (186).
The main indication for the use of OASIS is ulcer treatment. In a randomized controlled study in 120 patients with chronic venous ulcers, it was shown in combination with compression therapy to result in significantly more healed wounds (55%) compared with standard care (34%) (278). Patients with mixed arterial and venous ulcers who were treated with OASIS saw a significantly higher rate of complete wound closure (82%) compared with 46% of ulcers treated with Hyaloskin (Apeldoorn, The Netherlands), a pure hyaluronic acid dermal matrix, with a reported reduction in pain (341). In a separate study, OASIS has also been shown to effectively treat diabetic ulcers, with 49% of wounds healing after 12 wk compared with 29% of ulcers treated with Regranex (Smith and Nephew, Fort Worth, TX), a PDGF-BB gel (290).
4. Alloderm
Alloderm (Allergan, Dublin, Ireland) is a nonchemically crosslinked acellular dermal allograft processed from donated cadaveric skin. Processing removes cellular, infectious, and antigenic components (370), resulting in a dermal matrix comprised of collagen bundles and an intact basement membrane, retaining necessary biochemical and structural components to facilitate new tissue regeneration while avoiding immunogenic rejection. The matrix is typically freeze-dried, allowing for storage at room temperature for several months. A minimally invasive, injectable formula of Alloderm also exists, marketed as Cymetra (Allergan) (361).
Alloderm is used as a dermal substitute in deep partial- and full-thickness burn wounds to improve the take of succeeding autologous split-thickness skin grafts (433). In patients with full-thickness burns, Alloderm effectively supports simultaneous application of overlying meshed split-thickness skin autografts and exhibits host cell infiltration and neovascularization of the allograft, with reduced scarring and contracture of the wound that is commonly observed during allografting. In addition to simultaneous grafting, Alloderm has been successfully used in other procedures, including repair of facial soft-tissue defects (4); prosthesis breast surgery (282); pelvic, abdominal, and chest wall reconstruction (41); lip augmentation (415); and hernia repair (38).
B. Cell-Based Therapies for Enhanced Wound Healing
Despite significant progress in growth factor-based therapies and bioengineered skin grafting, half of chronic wounds that persist for more than a year do not respond to treatment. Cell-based therapies offer a promising avenue for rescuing wound healing defects. Some of the more promising therapies are discussed below.
1. Epicel
Epicel (Genzyme, Cambridge, MA) is a cultured epidermal autograft indicated for the treatment of deep dermal or full-thickness burns covering a total body surface area (TBSA) greater than or equal to 30%. It comprises sheets of autologous keratinocytes that are supported by petrolatum gauze, which is removed 1 wk after grafting (46). Epicel was the first commercially available skin substitute and a significant advance in the treatment of burns (157).
Clinical studies suggest that Epicel is most effective in the treatment of severe burns with a high TBSA. In a study of 30 patients with extensive burns (TBSA >60%), Epicel successfully provided permanent burn coverage of a mean of 26% TBSA, representing a take rate of 69% (46). However, in a 5-yr clinical study of 28 burn patients with a mean TBSA of 52.2%, Epicel had a mean take of 26.9% of the grafted area (457). Treated patients did not experience significantly different mortality, hospitalization time, and number of autograft harvest compared with the untreated matched control population, suggesting that Epicel is inconsistent and may be most useful as a temporary wound dressing. Other known disadvantages of Epicel include its mechanical fragility, hyperkeratosis, contracture, and scarring (429).
2. Apligraf
Apligraf (Organogenesis, Canton, MA), also known as Graftskin, is a bilayer material in which neonatal keratinocytes are seeded onto a dermal layer consisting of bovine type I collagen gel seeded with neonatal fibroblasts (455). Similar to other products, the membrane produces cytokines and growth factors that promote wound healing (150). Additionally, the cells are only transiently present, disappearing after a month, reducing the risk of immune rejection (314). Apligraf can be applied every 4–6 wk and stored at room temperature, although its shelf life is only 5 days. In addition to its short shelf life, disadvantages include its fragility, presence of allogeneic components, and high cost (368, 370).
Apligraf is FDA-approved for the treatment of chronic venous and diabetic leg ulcers (306). When combined with compression therapy, Apligraf treatment doubled the number of healed wounds at 6 mo in chronic venous ulcers (107). Fifty-six percent of patients with chronic diabetic foot ulcers treated with Apligraf reached complete healing by 12 wk compared with 38% in the control group treated with moist gauze dressing. Apligraf has been reported to accelerate healing of several other types of wounds, including surgical wounds (99), full-thickness burns (174), and epidermolysis bullosa (106).
3. Dermagraft
Dermagraft (Organogenesis, Canton, MA) is a cryopreserved monolayer dermal substitute consisting of absorbable PLGA scaffold seeded with neonatal dermal fibroblasts (68). During the manufacturing process, the fibroblasts proliferate to populate the scaffold and secrete collagen, ECM proteins, growth factors, and cytokines, resulting in dynamic human tissue (171). It was first approved by the FDA for the treatment of full-thickness DFUs, but its wound healing properties have been demonstrated in several indications, including epidermolysis bullosa (371) and nonhealing surgical wounds (206).
The primary indication for Dermagraft treatment is chronic lesions, such as diabetic ulcers. In a prospective randomized trial, the efficacy and safety of Dermagraft were demonstrated in the treatment of DFUs. Patients with chronic DFUs who were treated with Dermagraft exhibited a higher percentage of healed wounds (30%) compared with patients treated with standard of care (18.3%) and experienced a faster time to complete wound closure (261). Finally, nonclinical data have demonstrated the proangiogenic properties of Dermagraft, indicating a mechanism of action that supports its wound healing applications.
4. Grafix
Unlike EpiFix, which is a decellularized and dehydrated human amnion/chorion membrane allograft, Grafix (Osiris Therapeutics, Columbia, MD) is cryopreserved and thus contains all of the cellular components of fresh amniotic membrane including neonatal fibroblasts, MSCs, and epithelial cells that are necessary to coordinate wound healing, as well as growth factors and proteins that control scarring, the microbial response, and angiogenesis (18, 34). The presence of MSCs is unique to Grafix among wound healing products. The cryopreservation process used to process Grafix results in >80% cell viability post-thaw (34), compared with less than <50% cell viability using previous methods to recover endogenous cells from placental membranes following cryopreservation. Grafix is FDA approved and indicated for treatment of acute and chronic wounds, including DFUs, venous leg ulcerations (VLUs), pressure ulcers, burns, and epidermolysis bullosa (139).
Grafix has been demonstrated to be safe and effective in two clinical studies (232, 328). In a multicenter, single-blinded, randomized controlled trial of 97 patients, Grafix was shown to be significantly more effective than the standard of care in the treatment of chronic DFUs (232). Sixty-two percents of patients who received Grafix treatment achieved complete wound closure after 12 wk compared with 21.3% of the control group. Moreover, a greater percentage of patients treated with Grafix (62%) saw at least a 50% reduction in wound size by day 28 compared with patients receiving standard of care (40.4%). Patients in the treatment group also experienced significantly fewer wound-related infections (18.0 vs. 36.2%). In a second large, retrospective single-center study of 66 patients with different types of wounds (27 DFUs, 34 VLUs, 6 other), data for all wound care patients receiving at least one Grafix treatment were collected through chart review, including 74.6% of wounds that had failed to heal using other advanced therapies (328). 76.1% of all wounds treated with Grafix achieved closure with a mean healing time of 5.8 wk and an average of 3.2 applications in wounds that healed. No adverse effects were reported, and there were no wound recurrences during an average follow-up time of 20.4 mo.
Compared with the results of other large clinical trials of cell-based products, Grafix has exhibited the greatest efficacy of any therapeutic to date, with treatment benefiting healing of many types of chronic wounds in addition to DFUs, including in difficult-to-treat patient populations.
Despite the promise of current cell-based therapies, they are only moderately effective in correcting chronic wounds. While cell-based therapies are better than the standardized treatment of diabetes or age-related wounds, the products currently available lead to complete healing in only ~50% of patients following 12 wk of treatment (87, 261). Although these therapies are evidence-based, many of the trials exclude patients with multiple medical comorbidities, especially the elderly diabetic population because of a concern for poor compliance or the possibility of increased side effects (153, 244). This limits their usefulness in the patients that need them the most.
V. UNRAVELING CELLULAR HETEROGENEITY AND DEVELOPING THERAPIES USING SINGLE CELL TECHNOLOGIES
The foundation of our understanding of the cellular mechanisms in wound healing has been built on combining RNA or protein from thousands of cells and displaying aggregate expression of replicate samples. Techniques such as polymerase chain reaction (PCR), microarrays, and Western blotting have unraveled transcriptional networks, signal transduction pathways, and cellular metabolism, which has developed our knowledge of skin repair and disease. However, the assumption while using these techniques is that the average cellular output represents the dominant biological state of the cells. In wound healing and in several other regenerative states, this assumption is flawed since there are several cell types that work in unison, including rare cells such as stem cells. Moreover, the cells undergo phenotypic and functional changes based on the temporal nature of the wound in response to the altering wound microenvironment. Population averages are unable to capture these diverse subsets, especially the activity of stem cells. Thus interrogation of cellular heterogeneity is important in understanding disease progression and for designing targeted and effective therapies.
With the evolution of single cell technologies, it is possible to thoroughly evaluate individual cells and understand their role in repair. Additionally, single cell technologies allow for analysis in an unbiased manner, without the use of markers a priori. These technologies provide information about transcript expression, gene fusions, mutations, or single-nucleotide polymorphisms within each cell. Outlier cells are not considered errors in measurement by default, but can for the first time be verified for a unique function. Moreover, cellular heterogeneity can be analyzed by clustering the single cell data into subpopulations of cells. It is possible to identify differences in gene expression, cellular morphology, survival, motility, proliferation, metabolism, and recurrence potential. Single cell technologies have also made it feasible to detect mutational burden and the temporal order of mutations (268). Thus identifying cellular subpopulations has several implications for understanding skin homeostasis, repair, and disease.
Accuracy, precision, and comprehensiveness currently determine which single cell technique to select to answer the biologic question. Most technologies do not offer all three attributes. Accuracy is the measure of certainty or validity that the measured value is close to the true value (466). Precision is the ability to replicate or reproduce a measurement. High precision is associated with a narrow distribution of values (466). Comprehensiveness or sensitivity is the amount of information obtained from each cell (466, 487). In addition to these three criteria, considerations such as cost efficiency, sample-size effects, and false discovery are important in determining the single cell technique that will be used for analysis (467, 487).
In wound healing as in other tissues and regenerative states, single cell technologies can 1) distinguish between seemingly similar cells, 2) identify cellular subsets, and 3) isolate each subset based on distinct surface markers (200). These technologies can also be translated to identify cells for therapy, develop cellular products, and conduct quality control of the cell therapies generated. For example, single-cell RNA sequencing can detect more than 5,000 genes/cell (140) and offers comprehensiveness. While this allows for a thorough understanding of skin biology, the massive amounts of information generated from the RNA of a single cell and subsequent computational analysis of the vast information may compromise accuracy when designing cell-based therapies that will be applied to patients. Single-cell multiplexed qPCR on the other hand is not as comprehensive, and primers for a select number of genes must be selected for probing against a single cell, which can bias analysis towards the genes chosen. However, single-cell qPCR has been shown to have greater accuracy and precision.
Once the data from the single cell assays are obtained, they are combed to determine combination of surface markers which best define cells within the healing wound with transcriptional profiles of interest (377a). These cells can then be isolated by their unique surface marker combinations for functional wound healing assays. Several cell types have been discovered in healing wounds using these technologies in the skin including scar-forming fibroblasts in the skin and growth factor releasing ASCs from the subcutaneous adipose tissue (330, 335). The subset of ASCs displays a higher proliferation and survival ability in the wound, with a better growth factor release profile. Importantly, these cells do not demonstrate toxicity and can revert the time of healing to normal wounds when applied to murine wounds (330). Thus single cell technologies can be extended to determine the heterogeneity within several other cell types within the wound, particularly fibroblasts and immune cells. They can also be used to identify novel stem and progenitor cells during discrete phases of repair so that these cells can be isolated and bioengineered for therapeutic application.
VI. DISCUSSION AND CONCLUSIONS
The skin is the ideal organ to study cellular heterogeneity and stem cell function in homeostasis, wound repair, and disease owing to its large surface area and ease of tissue isolation. Human skin in homeostasis can be studied by obtaining samples which otherwise are discarded following various surgical procedures. Acute wounds can be analyzed by isolating tissue from burn patients and trauma surgeries, while chronic wounds can be studied by analyzing debrided specimens from pressure ulcers, venous leg ulcers, diabetic foot ulcers, or sickle cell ulcers. The cellular and molecular signaling in homeostasis, acute wound healing, and chronic wound healing can then be compared to determine the mechanisms underlying normal tissue repair and aberrations that occur in disease. The robust discovery of these mechanisms in the skin can be used as a model system for other tissue types and can also be compared with cellular mechanisms in other tissues to generate a reliable cell atlas.
It is well-established that the skin in the uninjured state has several resident cell populations, both immune and nonimmune. It is also known that these resident cells are activated at precise temporal stages during wound repair. The resident cells recruit circulating cells from the bone marrow which have definite roles in wound healing. However, the mechanisms that underlie these cellular processes remain unclear. The complexity in the cellular milieu leads to several confounding questions. Each of the resident cell subpopulations may have subtypes that preferentially respond to different wound signals. It is unclear if the subtypes arise from the same source in the adult tissue or have different sources, some of which persist from the time of fetal development.
There are questions about cell-cell interactions, specifically how the resident cell types and subtypes interact with each other and with circulating cells in the wound environment. Furthermore, circulating progenitor cells are elusive and their presence has been contested. It is important to understand the role of these cells in wound healing and what happens to these cells once wound healing resolves. There are also considerations of how surrounding factors such as changes in mechanical forces, oxygen availability, and infection can affect cellular function in wound healing. Additionally, there might be stem cells and progenitor cells in the skin that remain undiscovered.
These questions cannot be answered by analyzing the entire wound by traditional population-averaging techniques due to the multitude of cell types, each activating distinct signaling pathways. Isolating known cell types from the wound tissue by immunostaining with surface markers, followed by population analyses, may not be helpful either since the cellular populations isolated may contain various subsets with very different functions, or may consist of stem cells with outlier functions. For example, the late stage of inflammation in wound healing overlaps with the early stage of angiogenesis. Macrophages isolated at this stage contain both subsets that are performing efferocytosis and those subsets that are releasing growth factors for new vessel formation. Any population assay conducted on this wide group of macrophages would skew results in favor of the larger macrophage subset. Similarly, if all fibroblasts are isolated and analyzed as one group of cells, it is impossible to decipher which subsets are preferentially becoming myofibroblasts, forming adipocytes, or signaling to epidermal cells.
Stem cell diversity and function within the epidermis and its appendages are some of the most well-studied in the body, most likely due to the ease of isolation of the tissue. Lineage tracing in animal models when combined with computational analysis of large single-cell data sets has been an important advancement in identifying these rare cells. The single-cell data sets have been generated by both transcriptional and proteomic assays. High-throughput RNA sequencing has been a revolutionary technology and can comprehensively characterize rare cell subsets. It is also fascinating that stem cells that are unipotent in homeostasis can take on multipotent functions during wound healing. Such mechanisms of healing and regeneration can be extended to identify and study stem cells in other organ systems.
While clinical trials with cell-based therapies have not been successful in several other organ systems, there are several cell-based therapies available that can enhance wound healing. The large surface area and accessibility make it possible to target delivery of cell-based therapies to the skin. These therapies have been used to enhance both acute and chronic wound healing and provide level 1 evidence that cell-based therapies can be effective.
Most cell-based therapies currently available use heterogeneous populations of keratinocytes, fibroblasts, or mesenchymal stromal cells. A robust method of developing more effective cell-based therapies would be to use subsets of these cells that have enhanced wound healing profiles. Accurate and precise single-cell approaches such as multiplexed single-cell qPCR can identify and isolate these important cell subsets. While single-cell qPCR cannot generate large data sets compared with those obtained from RNA sequencing, the smaller data sets of reliable information can aid decision-making from a clinical perspective. Another advantage of designing cell-based therapies for wound healing is that there are reliable and reproducible models of wound healing in both small and large animals, which can be used to test the preclinical effectiveness of the therapies before application on the patient.
Ultimately, a thorough discovery of 1) cellular diversity and function in wound healing and 2) cellular alterations in impaired wound healing states will define our understanding of skin biology. The identification of cell surface markers to isolate the most effective cells for wound healing will aid in generating effective therapies for any skin injury.
GRANTS
Funding for this work has been provided by the Hagey Family Endowed Fund in Stem Cell Research and Regenerative Medicine, Department of Defense Armed Forces Institute of Regenerative Medicine Grants W81XWH-13-2-0052 and W81XWH-13-2-0054, and National Institutes of Health Grants R01-DK074095-13, R01-AG025016, and 5U24DK076169-12.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
Address for reprint requests and other correspondence: G. C. Gurtner, Stanford University School of Medicine, 257 Campus Drive West, GK-201, Stanford, CA 94305 (e-mail: ggurtner@stanford.edu).
REFERENCES
- 1.Aarabi S, Bhatt KA, Shi Y, Paterno J, Chang EI, Loh SA, Holmes JW, Longaker MT, Yee H, Gurtner GC. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J 21: 3250–3261, 2007. doi: 10.1096/fj.07-8218com. [DOI] [PubMed] [Google Scholar]
- 2.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation 110: 3300–3305, 2004. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 3.Abe M, Kurosawa M, Ishikawa O, Miyachi Y. Effect of mast cell-derived mediators and mast cell-related neutral proteases on human dermal fibroblast proliferation and type I collagen production. J Allergy Clin Immunol 106: S78–S84, 2000. doi: 10.1067/mai.2000.106058. [DOI] [PubMed] [Google Scholar]
- 4.Achauer BM, VanderKam VM, Celikoz B, Jacobson DG. Augmentation of facial soft-tissue defects with Alloderm dermal graft. Ann Plast Surg 41: 503–507, 1998. doi: 10.1097/00000637-199811000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Adzick NS, Longaker MT. Scarless fetal healing. Therapeutic implications. Ann Surg 215: 3–30, 1992. doi: 10.1097/00000658-199201000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahrens S, Zelenay S, Sancho D, Hanč P, Kjær S, Feest C, Fletcher G, Durkin C, Postigo A, Skehel M, Batista F, Thompson B, Way M, Reis e Sousa C, Schulz O. F-actin is an evolutionarily conserved damage-associated molecular pattern recognized by DNGR-1, a receptor for dead cells. Immunity 36: 635–645, 2012. doi: 10.1016/j.immuni.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Akimoto S, Ishikawa O, Igarashi Y, Kurosawa M, Miyachi Y. Dermal mast cells in scleroderma: their skin density, tryptase/chymase phenotypes and degranulation. Br J Dermatol 138: 399–406, 1998. doi: 10.1046/j.1365-2133.1998.02114.x. [DOI] [PubMed] [Google Scholar]
- 8.Alaish SM, Yager D, Diegelmann RF, Cohen IK. Biology of fetal wound healing: hyaluronate receptor expression in fetal fibroblasts. J Pediatr Surg 29: 1040–1043, 1994. doi: 10.1016/0022-3468(94)90275-5. [DOI] [PubMed] [Google Scholar]
- 9.Ansell DM, Izeta A. Pericytes in wound healing: friend or foe? Exp Dermatol 24: 833–834, 2015. doi: 10.1111/exd.12782. [DOI] [PubMed] [Google Scholar]
- 10.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21: 193–215, 2011. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Artuc M, Hermes B, Steckelings UM, Grützkau A, Henz BM. Mast cells and their mediators in cutaneous wound healing–active participants or innocent bystanders? Exp Dermatol 8: 1–16, 1999. doi: 10.1111/j.1600-0625.1999.tb00342.x. [DOI] [PubMed] [Google Scholar]
- 12.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–966, 1997. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 13.Barbosa FL, Chaurasia SS, Cutler A, Asosingh K, Kaur H, de Medeiros FW, Agrawal V, Wilson SE. Corneal myofibroblast generation from bone marrow-derived cells. Exp Eye Res 91: 92–96, 2010. doi: 10.1016/j.exer.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barker N, van Es JH, Jaks V, Kasper M, Snippert H, Toftgård R, Clevers H. Very long-term self-renewal of small intestine, colon, and hair follicles from cycling Lgr5+ve stem cells. Cold Spring Harb Symp Quant Biol 73: 351–356, 2008. doi: 10.1101/sqb.2008.72.003. [DOI] [PubMed] [Google Scholar]
- 15.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen 16: 585–601, 2008. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 16.Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, Allan RS, Wojtasiak M, Shortman K, Carbone FR, Brooks AG, Heath WR. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol 10: 488–495, 2009. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 17.Belaaouaj A, McCarthy R, Baumann M, Gao Z, Ley TJ, Abraham SN, Shapiro SD. Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nat Med 4: 615–618, 1998. doi: 10.1038/nm0598-615. [DOI] [PubMed] [Google Scholar]
- 18.Bergmeier V, Etich J, Pitzler L, Frie C, Koch M, Fischer M, Rappl G, Abken H, Tomasek JJ, Brachvogel B. Identification of a myofibroblast-specific expression signature in skin wounds. Matrix Biol 65: 59–74, 2018. doi: 10.1016/j.matbio.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Bergstresser PR, Tigelaar RE, Dees JH, Streilein JW. Thy-1 antigen-bearing dendritic cells populate murine epidermis. J Invest Dermatol 81: 286–288, 1983. doi: 10.1111/1523-1747.ep12518332. [DOI] [PubMed] [Google Scholar]
- 20.Berk BC, Alexander RW, Brock TA, Gimbrone MA Jr, Webb RC. Vasoconstriction: a new activity for platelet-derived growth factor. Science 232: 87–90, 1986. doi: 10.1126/science.3485309. [DOI] [PubMed] [Google Scholar]
- 21.Berndt MC, Shen Y, Dopheide SM, Gardiner EE, Andrews RK. The vascular biology of the glycoprotein Ib-IX-V complex. Thromb Haemost 86: 178–188, 2001. doi: 10.1055/s-0037-1616216. [DOI] [PubMed] [Google Scholar]
- 22.Blair P, Flaumenhaft R. Platelet alpha-granules: basic biology and clinical correlates. Blood Rev 23: 177–189, 2009. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blanpain C, Fuchs E. Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science 344: 1242281, 2014. doi: 10.1126/science.1242281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blanpain C, Horsley V, Fuchs E. Epithelial stem cells: turning over new leaves. Cell 128: 445–458, 2007. doi: 10.1016/j.cell.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blocki A, Wang Y, Koch M, Goralczyk A, Beyer S, Agarwal N, Lee M, Moonshi S, Dewavrin JY, Peh P, Schwarz H, Bhakoo K, Raghunath M. Sourcing of an alternative pericyte-like cell type from peripheral blood in clinically relevant numbers for therapeutic angiogenic applications. Mol Ther 23: 510–522, 2015. doi: 10.1038/mt.2014.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bodnar RJ. Chemokine Regulation of Angiogenesis During Wound Healing. Adv Wound Care (New Rochelle) 4: 641–650, 2015. doi: 10.1089/wound.2014.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bodnar RJ, Yates CC, Wells A. IP-10 blocks vascular endothelial growth factor-induced endothelial cell motility and tube formation via inhibition of calpain. Circ Res 98: 617–625, 2006. doi: 10.1161/01.RES.0000209968.66606.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 89: 3503–3521, 1997. [PubMed] [Google Scholar]
- 29.Bos JD, Teunissen MB, Cairo I, Krieg SR, Kapsenberg ML, Das PK, Borst J. T-cell receptor gamma delta bearing cells in normal human skin. J Invest Dermatol 94: 37–42, 1990. doi: 10.1111/1523-1747.ep12873333. [DOI] [PubMed] [Google Scholar]
- 30.Boyce DE, Ciampolini J, Ruge F, Murison MSC, Harding KG. Inflammatory-cell subpopulations in keloid scars. Br J Plast Surg 54: 511–516, 2001. doi: 10.1054/bjps.2001.3638. [DOI] [PubMed] [Google Scholar]
- 31.Boyce ST, Warden GD. Principles and practices for treatment of cutaneous wounds with cultured skin substitutes. Am J Surg 183: 445–456, 2002. doi: 10.1016/S0002-9610(02)00813-9. [DOI] [PubMed] [Google Scholar]
- 32.Boyman O, Hefti HP, Conrad C, Nickoloff BJ, Suter M, Nestle FO. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-alpha. J Exp Med 199: 731–736, 2004. doi: 10.1084/jem.20031482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bratton DL, Henson PM. Neutrophil clearance: when the party is over, clean-up begins. Trends Immunol 32: 350–357, 2011. doi: 10.1016/j.it.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brewer DB. Max Schultze (1865), G. Bizzozero (1882) and the discovery of the platelet. Br J Haematol 133: 251–258, 2006. doi: 10.1111/j.1365-2141.2006.06036.x. [DOI] [PubMed] [Google Scholar]
- 35.Bromley SK, Yan S, Tomura M, Kanagawa O, Luster AD. Recirculating memory T cells are a unique subset of CD4+ T cells with a distinct phenotype and migratory pattern. J Immunol 190: 970–976, 2013. doi: 10.4049/jimmunol.1202805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown LF, Yeo KT, Berse B, Yeo TK, Senger DR, Dvorak HF, van de Water L. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med 176: 1375–1379, 1992. doi: 10.1084/jem.176.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell 8: 552–565, 2011. doi: 10.1016/j.stem.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buinewicz B, Rosen B. Acellular cadaveric dermis (AlloDerm): a new alternative for abdominal hernia repair. Ann Plast Surg 52: 188–194, 2004. doi: 10.1097/01.sap.0000100895.41198.27. [DOI] [PubMed] [Google Scholar]
- 39.Burke JF, Yannas IV, Quinby WC Jr, Bondoc CC, Jung WK. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann Surg 194: 413–428, 1981. doi: 10.1097/00000658-198110000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH, Hogquist KA. Identification of a novel population of Langerin+ dendritic cells. J Exp Med 204: 3147–3156, 2007. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butler CE, Langstein HN, Kronowitz SJ. Pelvic, abdominal, and chest wall reconstruction with AlloDerm in patients at increased risk for mesh-related complications. Plast Reconstr Surg 116: 1263–1275, 2005. doi: 10.1097/01.prs.0000181692.71901.bd. [DOI] [PubMed] [Google Scholar]
- 42.Butzelaar L, Schooneman DP, Soykan EA, Talhout W, Ulrich MM, van den Broek LJ, Gibbs S, Beelen RH, Mink van der Molen AB, Niessen FB. Inhibited early immunologic response is associated with hypertrophic scarring. Exp Dermatol 25: 797–804, 2016. doi: 10.1111/exd.13100. [DOI] [PubMed] [Google Scholar]
- 43.Cabrita MA, Christofori G. Sprouty proteins, masterminds of receptor tyrosine kinase signaling. Angiogenesis 11: 53–62, 2008. doi: 10.1007/s10456-008-9089-1. [DOI] [PubMed] [Google Scholar]
- 44.Cai Y, Shen X, Ding C, Qi C, Li K, Li X, Jala VR, Zhang HG, Wang T, Zheng J, Yan J. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity 35: 596–610, 2011. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caley MP, Martins VL, O’Toole EA. Metalloproteinases and Wound Healing. Adv Wound Care (New Rochelle) 4: 225–234, 2015. doi: 10.1089/wound.2014.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carsin H, Ainaud P, Le Bever H, Rives J, Lakhel A, Stephanazzi J, Lambert F, Perrot J. Cultured epithelial autografts in extensive burn coverage of severely traumatized patients: a five year single-center experience with 30 patients. Burns 26: 379–387, 2000. doi: 10.1016/S0305-4179(99)00143-6. [DOI] [PubMed] [Google Scholar]
- 47.Cass DL, Bullard KM, Sylvester KG, Yang EY, Longaker MT, Adzick NS. Wound size and gestational age modulate scar formation in fetal wound repair. J Pediatr Surg 32: 411–415, 1997. doi: 10.1016/S0022-3468(97)90593-5. [DOI] [PubMed] [Google Scholar]
- 48.Cass DL, Bullard KM, Sylvester KG, Yang EY, Sheppard D, Herlyn M, Adzick NS. Epidermal integrin expression is upregulated rapidly in human fetal wound repair. J Pediatr Surg 33: 312–316, 1998. doi: 10.1016/S0022-3468(98)90453-5. [DOI] [PubMed] [Google Scholar]
- 49.Castagnoli C, Stella M, Magliacani G, Alasia ST, Richiardi P. Anomalous expression of HLA class II molecules on keratinocytes and fibroblasts in hypertrophic scars consequent to thermal injury. Clin Exp Immunol 82: 350–354, 1990. doi: 10.1111/j.1365-2249.1990.tb05451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castagnoli C, Trombotto C, Ondei S, Stella M, Calcagni M, Magliacani G, Alasia ST. Characterization of T-cell subsets infiltrating post-burn hypertrophic scar tissues. Burns 23: 565–572, 1997. doi: 10.1016/S0305-4179(97)00070-3. [DOI] [PubMed] [Google Scholar]
- 51.CDC National Diabetes Statistics Report (Online). https://www.cdc.gov/diabetes/data/statistics/statistics-report.html. [16 June 2014].
- 52.Cefalu WT, Rosenstock J, LeRoith D, Blonde L, Riddle MC. Getting to the “Heart” of the Matter on Diabetic Cardiovascular Disease: “Thanks for the Memory”. Diabetes Care 39: 664–667, 2016. doi: 10.2337/dc16-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 10: 858–864, 2004. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 54.Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, Brown PO. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci USA 99: 12877–12882, 2002. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Charras G, Sahai E. Physical influences of the extracellular environment on cell migration. Nat Rev Mol Cell Biol 15: 813–824, 2014. doi: 10.1038/nrm3897. [DOI] [PubMed] [Google Scholar]
- 56.Chen WY, Rogers AA. Recent insights into the causes of chronic leg ulceration in venous diseases and implications on other types of chronic wounds. Wound Repair Regen 15: 434–449, 2007. doi: 10.1111/j.1524-475X.2007.00250.x. [DOI] [PubMed] [Google Scholar]
- 57.Cheresh DA, Berliner SA, Vicente V, Ruggeri ZM. Recognition of distinct adhesive sites on fibrinogen by related integrins on platelets and endothelial cells. Cell 58: 945–953, 1989. doi: 10.1016/0092-8674(89)90946-X. [DOI] [PubMed] [Google Scholar]
- 58.Chiller K, Selkin BA, Murakawa GJ. Skin microflora and bacterial infections of the skin. J Investig Dermatol Symp Proc 6: 170–174, 2001. doi: 10.1046/j.0022-202x.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- 59.Chin GS, Lee S, Hsu M, Liu W, Kim WJH, Levinson H, Longaker MT. Discoidin domain receptors and their ligand, collagen, are temporally regulated in fetal rat fibroblasts in vitro. Plast Reconstr Surg 107: 769–776, 2001. doi: 10.1097/00006534-200103000-00018. [DOI] [PubMed] [Google Scholar]
- 60.Chin YR, Toker A. Function of Akt/PKB signaling to cell motility, invasion and the tumor stroma in cancer. Cell Signal 21: 470–476, 2009. doi: 10.1016/j.cellsig.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chodaczek G, Papanna V, Zal MA, Zal T. Body-barrier surveillance by epidermal γδ TCRs. Nat Immunol 13: 272–282, 2012. doi: 10.1038/ni.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chou WC, Takeo M, Rabbani P, Hu H, Lee W, Chung YR, Carucci J, Overbeek P, Ito M. Direct migration of follicular melanocyte stem cells to the epidermis after wounding or UVB irradiation is dependent on Mc1r signaling. Nat Med 19: 924–929, 2013. doi: 10.1038/nm.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clark RA. Fibrin is a many splendored thing. J Invest Dermatol 121: xxi–xxii, 2003. doi: 10.1046/j.1523-1747.2003.12575.x. [DOI] [PubMed] [Google Scholar]
- 64.Cogle CR, Wainman DA, Jorgensen ML, Guthrie SM, Mames RN, Scott EW. Adult human hematopoietic cells provide functional hemangioblast activity. Blood 103: 133–135, 2004. doi: 10.1182/blood-2003-06-2101. [DOI] [PubMed] [Google Scholar]
- 65.Collins CA, Kretzschmar K, Watt FM. Reprogramming adult dermis to a neonatal state through epidermal activation of β-catenin. Development 138: 5189–5199, 2011. doi: 10.1242/dev.064592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Compton CC, Hickerson W, Nadire K, Press W. Acceleration of skin regeneration from cultured epithelial autografts by transplantation to homograft dermis. J Burn Care Rehabil 14: 653–662, 1993. doi: 10.1097/00004630-199311000-00010. [DOI] [PubMed] [Google Scholar]
- 67.Cook-Mills JM, Deem TL. Active participation of endothelial cells in inflammation. J Leukoc Biol 77: 487–495, 2005. doi: 10.1189/jlb.0904554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cooper ML, Hansbrough JF, Spielvogel RL, Cohen R, Bartel RL, Naughton G. In vivo optimization of a living dermal substitute employing cultured human fibroblasts on a biodegradable polyglycolic acid or polyglactin mesh. Biomaterials 12: 243–248, 1991. doi: 10.1016/0142-9612(91)90207-Q. [DOI] [PubMed] [Google Scholar]
- 69.Corliss BA, Azimi MS, Munson JM, Peirce SM, Murfee WL. Macrophages: An Inflammatory Link Between Angiogenesis and Lymphangiogenesis. Microcirculation 23: 95–121, 2016. doi: 10.1111/micc.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 284: 1318–1322, 1999. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 71.Costin GE, Hearing VJ. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J 21: 976–994, 2007. doi: 10.1096/fj.06-6649rev. [DOI] [PubMed] [Google Scholar]
- 72.Covassin LD, Villefranc JA, Kacergis MC, Weinstein BM, Lawson ND. Distinct genetic interactions between multiple Vegf receptors are required for development of different blood vessel types in zebrafish. Proc Natl Acad Sci USA 103: 6554–6559, 2006. doi: 10.1073/pnas.0506886103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3: 301–313, 2008. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 74.Dang CM, Beanes SR, Lee H, Zhang X, Soo C, Ting K. Scarless fetal wounds are associated with an increased matrix metalloproteinase-to-tissue-derived inhibitor of metalloproteinase ratio. Plast Reconstr Surg 111: 2273–2285, 2003. doi: 10.1097/01.PRS.0000060102.57809.DA. [DOI] [PubMed] [Google Scholar]
- 75.Dantzer E, Braye FM. Reconstructive surgery using an artificial dermis (Integra): results with 39 grafts. Br J Plast Surg 54: 659–664, 2001. doi: 10.1054/bjps.2001.3684. [DOI] [PubMed] [Google Scholar]
- 76.Darby IA, Hewitson TD. Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol 257: 143–179, 2007. doi: 10.1016/S0074-7696(07)57004-X. [DOI] [PubMed] [Google Scholar]
- 77.Darby IA, Laverdet B, Bonté F, Desmoulière A. Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol 7: 301–311, 2014. doi: 10.2147/CCID.S50046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol 14: 986–995, 2013. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Davies LC, Rosas M, Jenkins SJ, Liao CT, Scurr MJ, Brombacher F, Fraser DJ, Allen JE, Jones SA, Taylor PR. Distinct bone marrow-derived and tissue-resident macrophage lineages proliferate at key stages during inflammation. Nat Commun 4: 1886, 2013. doi: 10.1038/ncomms2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med 6: 16–26, 2009. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Falco E, Porcelli D, Torella AR, Straino S, Iachininoto MG, Orlandi A, Truffa S, Biglioli P, Napolitano M, Capogrossi MC, Pesce M. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood 104: 3472–3482, 2004. doi: 10.1182/blood-2003-12-4423. [DOI] [PubMed] [Google Scholar]
- 82.De Oliveira S, Rosowski EE, Huttenlocher A. Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol 16: 378–391, 2016. doi: 10.1038/nri.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Desmoulière A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol 146: 56–66, 1995. [PMC free article] [PubMed] [Google Scholar]
- 84.Di Nardo A, Yamasaki K, Dorschner RA, Lai Y, Gallo RL. Mast cell cathelicidin antimicrobial peptide prevents invasive group A Streptococcus infection of the skin. J Immunol 180: 7565–7573, 2008. doi: 10.4049/jimmunol.180.11.7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dimmeler S, Zeiher AM. Endothelial cell apoptosis in angiogenesis and vessel regression. Circ Res 87: 434–439, 2000. doi: 10.1161/01.RES.87.6.434. [DOI] [PubMed] [Google Scholar]
- 86.Ding Q, Gladson CL, Wu H, Hayasaka H, Olman MA. Focal adhesion kinase (FAK)-related non-kinase inhibits myofibroblast differentiation through differential MAPK activation in a FAK-dependent manner. J Biol Chem 283: 26839–26849, 2008. doi: 10.1074/jbc.M803645200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dinh TL, Veves A. The efficacy of Apligraf in the treatment of diabetic foot ulcers. Plast Reconstr Surg 117, Suppl: 152S–157S, 2006. doi: 10.1097/01.prs.0000222534.79915.d3. [DOI] [PubMed] [Google Scholar]
- 88.DiPietro LA, Burdick M, Low QE, Kunkel SL, Strieter RM. MIP-1alpha as a critical macrophage chemoattractant in murine wound repair. J Clin Invest 101: 1693–1698, 1998. doi: 10.1172/JCI1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.DiPietro LA, Polverini PJ, Rahbe SM, Kovacs EJ. Modulation of JE/MCP-1 expression in dermal wound repair. Am J Pathol 146: 868–875, 1995. [PMC free article] [PubMed] [Google Scholar]
- 90.Donati G, Rognoni E, Hiratsuka T, Liakath-Ali K, Hoste E, Kar G, Kayikci M, Russell R, Kretzschmar K, Mulder KW, Teichmann SA, Watt FM. Wounding induces dedifferentiation of epidermal Gata6+ cells and acquisition of stem cell properties. Nat Cell Biol 19: 603–613, 2017. doi: 10.1038/ncb3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Doupé DP, Jones PH. Interfollicular epidermal homeostasis: dicing with differentiation. Exp Dermatol 21: 249–253, 2012. doi: 10.1111/j.1600-0625.2012.01447.x. [DOI] [PubMed] [Google Scholar]
- 92.Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, Ferron SR, Herault Y, Pavlovic G, Ferguson-Smith AC, Watt FM. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 504: 277–281, 2013. doi: 10.1038/nature12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Driskell RR, Watt FM. Understanding fibroblast heterogeneity in the skin. Trends Cell Biol 25: 92–99, 2015. doi: 10.1016/j.tcb.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 94.Duscher D, Januszyk M, Maan ZN, Whittam AJ, Hu MS, Walmsley GG, Dong Y, Khong SM, Longaker MT, Gurtner GC. Comparison of the Hydroxylase Inhibitor Dimethyloxalylglycine and the Iron Chelator Deferoxamine in Diabetic and Aged Wound Healing. Plast Reconstr Surg 139: 695e–706e, 2017. doi: 10.1097/PRS.0000000000003072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Duscher D, Maan ZN, Whittam AJ, Sorkin M, Hu MS, Walmsley GG, Baker H, Fischer LH, Januszyk M, Wong VW, Gurtner GC. Fibroblast-Specific Deletion of Hypoxia Inducible Factor-1 Critically Impairs Murine Cutaneous Neovascularization and Wound Healing. Plast Reconstr Surg 136: 1004–1013, 2015. doi: 10.1097/PRS.0000000000001699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Duscher D, Maan ZN, Wong VW, Rennert RC, Januszyk M, Rodrigues M, Hu M, Whitmore AJ, Whittam AJ, Longaker MT, Gurtner GC. Mechanotransduction and fibrosis. J Biomech 47: 1997–2005, 2014. doi: 10.1016/j.jbiomech.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Duscher D, Neofytou E, Wong VW, Maan ZN, Rennert RC, Inayathullah M, Januszyk M, Rodrigues M, Malkovskiy AV, Whitmore AJ, Walmsley GG, Galvez MG, Whittam AJ, Brownlee M, Rajadas J, Gurtner GC. Transdermal deferoxamine prevents pressure-induced diabetic ulcers. Proc Natl Acad Sci USA 112: 94–99, 2015. doi: 10.1073/pnas.1413445112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dvorak AM. Mast cell-derived mediators of enhanced microvascular permeability, vascular permeability factor/vascular endothelial growth factor, histamine, and serotonin, cause leakage of macromolecules through a new endothelial cell permeability organelle, the vesiculo-vacuolar organelle. Chem Immunol Allergy 85: 185–204, 2005. doi: 10.1159/000086517. [DOI] [PubMed] [Google Scholar]
- 99.Eaglstein WH, Iriondo M, Laszlo K. A composite skin substitute (graftskin) for surgical wounds. A clinical experience. Dermatol Surg 21: 839–843, 1995. doi: 10.1111/j.1524-4725.1995.tb00709.x. [DOI] [PubMed] [Google Scholar]
- 100.Edwards AD, Diebold SS, Slack EM, Tomizawa H, Hemmi H, Kaisho T, Akira S, Reis e Sousa C. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. Eur J Immunol 33: 827–833, 2003. doi: 10.1002/eji.200323797. [DOI] [PubMed] [Google Scholar]
- 101.Edwards C, Marks R. Evaluation of biomechanical properties of human skin. Clin Dermatol 13: 375–380, 1995. doi: 10.1016/0738-081X(95)00078-T. [DOI] [PubMed] [Google Scholar]
- 102.Edwards R, Harding KG. Bacteria and wound healing. Curr Opin Infect Dis 17: 91–96, 2004. doi: 10.1097/00001432-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 103.Ehrlich P. Beitrage zur Theorie und Praxis der histologischen Farbung (Thesis). Leipzig, Germany: Univ. of Leipzig, 1878. [Google Scholar]
- 104.Eilken HM, Adams RH. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr Opin Cell Biol 22: 617–625, 2010. doi: 10.1016/j.ceb.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 105.Englander HR. Fluoridation protects occlusal areas. J Am Dent Assoc 98: 11, 1979. doi: 10.14219/jada.archive.1979.0021. [DOI] [PubMed] [Google Scholar]
- 106.Falabella AF, Valencia IC, Eaglstein WH, Schachner LA. Tissue-engineered skin (Apligraf) in the healing of patients with epidermolysis bullosa wounds. Arch Dermatol 136: 1225–1230, 2000. doi: 10.1001/archderm.136.10.1225. [DOI] [PubMed] [Google Scholar]
- 107.Falanga V, Sabolinski M. A bilayered living skin construct (APLIGRAF) accelerates complete closure of hard-to-heal venous ulcers. Wound Repair Regen 7: 201–207, 1999. doi: 10.1046/j.1524-475X.1999.00201.x. [DOI] [PubMed] [Google Scholar]
- 108.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 116: 829–840, 2010. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, Hocking A, Isik F. Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells 22: 812–822, 2004. doi: 10.1634/stemcells.22-5-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ferguson JE III, Kelley RW, Patterson C. Mechanisms of endothelial differentiation in embryonic vasculogenesis. Arterioscler Thromb Vasc Biol 25: 2246–2254, 2005. doi: 10.1161/01.ATV.0000183609.55154.44. [DOI] [PubMed] [Google Scholar]
- 111.Fife CE, Carter MJ. Wound Care Outcomes and Associated Cost Among Patients Treated in US Outpatient Wound Centers: Data From the US Wound Registry. Wounds 24: 10–17, 2012. [PubMed] [Google Scholar]
- 112.FitzGerald GA. Mechanisms of platelet activation: thromboxane A2 as an amplifying signal for other agonists. Am J Cardiol 68: 11B–15B, 1991. doi: 10.1016/0002-9149(91)90379-Y. [DOI] [PubMed] [Google Scholar]
- 113.Florin L, Maas-Szabowski N, Werner S, Szabowski A, Angel P. Increased keratinocyte proliferation by JUN-dependent expression of PTN and SDF-1 in fibroblasts. J Cell Sci 118: 1981–1989, 2005. doi: 10.1242/jcs.02303. [DOI] [PubMed] [Google Scholar]
- 114.Foley TT, Ehrlich HP. Through gap junction communications, co-cultured mast cells and fibroblasts generate fibroblast activities allied with hypertrophic scarring. Plast Reconstr Surg 131: 1036–1044, 2013. doi: 10.1097/PRS.0b013e3182865c3f. [DOI] [PubMed] [Google Scholar]
- 115.Folkman J, Klagsbrun M. Angiogenic factors. Science 235: 442–447, 1987. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- 116.Forbes J, Fetterolf DE. Dehydrated amniotic membrane allografts for the treatment of chronic wounds: a case series. J Wound Care 21: 290–296, 2012. doi: 10.12968/jowc.2012.21.6.290. [DOI] [PubMed] [Google Scholar]
- 117.Fortington LV, Geertzen JH, van Netten JJ, Postema K, Rommers GM, Dijkstra PU. Short and long term mortality rates after a lower limb amputation. Eur J Vasc Endovasc Surg 46: 124–131, 2013. doi: 10.1016/j.ejvs.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 118.Fox JE, Phillips DR. Inhibition of actin polymerization in blood platelets by cytochalasins. Nature 292: 650–652, 1981. doi: 10.1038/292650a0. [DOI] [PubMed] [Google Scholar]
- 119.Frame JD, Still J, Lakhel-LeCoadou A, Carstens MH, Lorenz C, Orlet H, Spence R, Berger AC, Dantzer E, Burd A. Use of dermal regeneration template in contracture release procedures: a multicenter evaluation. Plast Reconstr Surg 113: 1330–1338, 2004. doi: 10.1097/01.PRS.0000111883.93604.85. [DOI] [PubMed] [Google Scholar]
- 120.Freedberg IM, Tomic-Canic M, Komine M, Blumenberg M. Keratins and the keratinocyte activation cycle. J Invest Dermatol 116: 633–640, 2001. doi: 10.1046/j.1523-1747.2001.01327.x. [DOI] [PubMed] [Google Scholar]
- 121.Fries KM, Blieden T, Looney RJ, Sempowski GD, Silvera MR, Willis RA, Phipps RP. Evidence of fibroblast heterogeneity and the role of fibroblast subpopulations in fibrosis. Clin Immunol Immunopathol 72: 283–292, 1994. doi: 10.1006/clin.1994.1144. [DOI] [PubMed] [Google Scholar]
- 122.Frojmovic MM, Milton JG. Human platelet size, shape, and related functions in health and disease. Physiol Rev 62: 185–261, 1982. doi: 10.1152/physrev.1982.62.1.185. [DOI] [PubMed] [Google Scholar]
- 123.Frykberg RG, Banks J. Challenges in the Treatment of Chronic Wounds. Adv Wound Care (New Rochelle) 4: 560–582, 2015. doi: 10.1089/wound.2015.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fuchs E. Epithelial Skin Biology: Three Decades of Developmental Biology, a Hundred Questions Answered and a Thousand New Ones to Address. Curr Top Dev Biol 116: 357–374, 2016. doi: 10.1016/bs.ctdb.2015.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fuchs E. Finding one’s niche in the skin. Cell Stem Cell 4: 499–502, 2009. doi: 10.1016/j.stem.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fuchs E, Horsley V. Ferreting out stem cells from their niches. Nat Cell Biol 13: 513–518, 2011. doi: 10.1038/ncb0511-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fujiwara H, Ferreira M, Donati G, Marciano DK, Linton JM, Sato Y, Hartner A, Sekiguchi K, Reichardt LF, Watt FM. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell 144: 577–589, 2011. doi: 10.1016/j.cell.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fujiwara T, Kubo T, Kanazawa S, Shingaki K, Taniguchi M, Matsuzaki S, Gurtner GC, Tohyama M, Hosokawa K. Direct contact of fibroblasts with neuronal processes promotes differentiation to myofibroblasts and induces contraction of collagen matrix in vitro. Wound Repair Regen 21: 588–594, 2013. doi: 10.1111/wrr.12059. [DOI] [PubMed] [Google Scholar]
- 129.Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med 359: 938–949, 2008. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 130.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol 200: 500–503, 2003. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 131.Gabbiani G, Ryan GB, Majn0 G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 27: 549–550, 1971. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- 132.Galiano RD, Michaels J V, Dobryansky M, Levine JP, Gurtner GC. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen 12: 485–492, 2004. doi: 10.1111/j.1067-1927.2004.12404.x. [DOI] [PubMed] [Google Scholar]
- 133.Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol 12: 1035–1044, 2011. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Galligan CL, Fish EN. The role of circulating fibrocytes in inflammation and autoimmunity. J Leukoc Biol 93: 45–50, 2013. doi: 10.1189/jlb.0712365. [DOI] [PubMed] [Google Scholar]
- 135.Gao FL, Jin R, Zhang L, Zhang YG. The contribution of melanocytes to pathological scar formation during wound healing. Int J Clin Exp Med 6: 609–613, 2013. [PMC free article] [PubMed] [Google Scholar]
- 136.Garbuzenko E, Nagler A, Pickholtz D, Gillery P, Reich R, Maquart FX, Levi-Schaffer F. Human mast cells stimulate fibroblast proliferation, collagen synthesis and lattice contraction: a direct role for mast cells in skin fibrosis. Clin Exp Allergy 32: 237–246, 2002. doi: 10.1046/j.1365-2222.2002.01293.x. [DOI] [PubMed] [Google Scholar]
- 137.Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, Carbone FR, Mueller SN. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature 477: 216–219, 2011. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- 138.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161: 1163–1177, 2003. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gibbons GW. Grafix®, a Cryopreserved Placental Membrane, for the Treatment of Chronic/Stalled Wounds. Adv Wound Care (New Rochelle) 4: 534–544, 2015. doi: 10.1089/wound.2015.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gierahn TM, Wadsworth MH II, Hughes TK, Bryson BD, Butler A, Satija R, Fortune S, Love JC, Shalek AK. Seq-Well: portable, low-cost RNA sequencing of single cells at high throughput. Nat Methods 14: 395–398, 2017. doi: 10.1038/nmeth.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gill SE, Pape MC, Khokha R, Watson AJ, Leco KJ. A null mutation for tissue inhibitor of metalloproteinases-3 (Timp-3) impairs murine bronchiole branching morphogenesis. Dev Biol 261: 313–323, 2003. doi: 10.1016/S0012-1606(03)00318-X. [DOI] [PubMed] [Google Scholar]
- 142.Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol 69: 513–521, 2001. [PubMed] [Google Scholar]
- 143.Glass GE, Nanchahal J. The methodology of negative pressure wound therapy: separating fact from fiction. J Plast Reconstr Aesthet Surg 65: 989–1001, 2012. doi: 10.1016/j.bjps.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 144.Godfrey DI, Hammond KJ, Poulton LD, Smyth MJ, Baxter AG. NKT cells: facts, functions and fallacies. Immunol Today 21: 573–583, 2000. doi: 10.1016/S0167-5699(00)01735-7. [DOI] [PubMed] [Google Scholar]
- 145.Godo S, Shimokawa H. Endothelial Functions. Arterioscler Thromb Vasc Biol 37: e108–e114, 2017. doi: 10.1161/ATVBAHA.117.309813. [DOI] [PubMed] [Google Scholar]
- 146.Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proc Natl Acad Sci USA 110: 9415–9420, 2013. doi: 10.1073/pnas.1300290110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Goffin JM, Pittet P, Csucs G, Lussi JW, Meister JJ, Hinz B. Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. J Cell Biol 172: 259–268, 2006. doi: 10.1083/jcb.200506179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Goldberg MT, Han YP, Yan C, Shaw MC, Garner WL. TNF-alpha suppresses alpha-smooth muscle actin expression in human dermal fibroblasts: an implication for abnormal wound healing. J Invest Dermatol 127: 2645–2655, 2007. doi: 10.1038/sj.jid.5700890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Golebiewska EM, Poole AW. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev 29: 153–162, 2015. doi: 10.1016/j.blre.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gordley K, Cole P, Hicks J, Hollier L. A comparative, long term assessment of soft tissue substitutes: AlloDerm, Enduragen, and Dermamatrix. J Plast Reconstr Aesthet Surg 62: 849–850, 2009. doi: 10.1016/j.bjps.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 151.Goren I, Allmann N, Yogev N, Schürmann C, Linke A, Holdener M, Waisman A, Pfeilschifter J, Frank S. A transgenic mouse model of inducible macrophage depletion: effects of diphtheria toxin-driven lysozyme M-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes. Am J Pathol 175: 132–147, 2009. doi: 10.2353/ajpath.2009.081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Goto S. Blood constitution: platelet aggregation, bleeding, and involvement of leukocytes. Rev Neurol Dis 5, Suppl 1: S22–S27, 2008. [PubMed] [Google Scholar]
- 153.Gould L, Abadir P, Brem H, Carter M, Conner-Kerr T, Davidson J, DiPietro L, Falanga V, Fife C, Gardner S, Grice E, Harmon J, Hazzard WR, High KP, Houghton P, Jacobson N, Kirsner RS, Kovacs EJ, Margolis D, McFarland Horne F, Reed MJ, Sullivan DH, Thom S, Tomic-Canic M, Walston J, Whitney J, Williams J, Zieman S, Schmader K. Chronic wound repair and healing in older adults: current status and future research. Wound Repair Regen 23: 1–13, 2015. doi: 10.1111/wrr.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Grant MB, May WS, Caballero S, Brown GA, Guthrie SM, Mames RN, Byrne BJ, Vaught T, Spoerri PE, Peck AB, Scott EW. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med 8: 607–612, 2002. doi: 10.1038/nm0602-607. [DOI] [PubMed] [Google Scholar]
- 155.Gray EE, Suzuki K, Cyster JG. Cutting edge: identification of a motile IL-17-producing gammadelta T cell population in the dermis. J Immunol 186: 6091–6095, 2011. doi: 10.4049/jimmunol.1100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, Dela Cruz-Racelis J, Fuchs E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 4: 155–169, 2009. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Green H, Kehinde O, Thomas J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc Natl Acad Sci USA 76: 5665–5668, 1979. doi: 10.1073/pnas.76.11.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gregorio J, Meller S, Conrad C, Di Nardo A, Homey B, Lauerma A, Arai N, Gallo RL, Digiovanni J, Gilliet M. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J Exp Med 207: 2921–2930, 2010. doi: 10.1084/jem.20101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Grice EA, Kong HH, Renaud G, Young AC, Bouffard GG, Blakesley RW, Wolfsberg TG, Turner ML, Segre JA; NISC Comparative Sequencing Program . A diversity profile of the human skin microbiota. Genome Res 18: 1043–1050, 2008. doi: 10.1101/gr.075549.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Grice EA, Segre JA. The skin microbiome. [Corrigendum in Nat Rev Microbiol 9: 626, 2011.] Nat Rev Microbiol 9: 244–253, 2011. doi: 10.1038/nrmicro2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Grice EA, Snitkin ES, Yockey LJ, Bermudez DM, Liechty KW, Segre JA, Mullikin J, Blakesley R, Young A, Chu G, Ramsahoye C, Lovett S, Han J, Legaspi R, Fuksenko T, Reddix-Dugue N, Sison C, Gregory M, Montemayor C, Gestole M, Hargrove A, Johnson T, Myrick J, Riebow N, Schmidt B, Novotny B, Gupti J, Benjamin B, Brooks S, Coleman H, Ho S, Schandler K, Smith L, Stantripop M, Maduro Q, Bouffard G, Dekhtyar M, Guan X, Masiello C, Maskeri B, McDowell J, Park M, Jacques Thomas P; NISC Comparative Sequencing Program . Longitudinal shift in diabetic wound microbiota correlates with prolonged skin defense response. Proc Natl Acad Sci USA 107: 14799–14804, 2010. doi: 10.1073/pnas.1004204107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol 124: 401–404, 1994. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Grinnell F, Petroll WM. Cell motility and mechanics in three-dimensional collagen matrices. Annu Rev Cell Dev Biol 26: 335–361, 2010. doi: 10.1146/annurev.cellbio.042308.113318. [DOI] [PubMed] [Google Scholar]
- 164.Grose R, Hutter C, Bloch W, Thorey I, Watt FM, Fässler R, Brakebusch C, Werner S. A crucial role of beta 1 integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development 129: 2303–2315, 2002. [DOI] [PubMed] [Google Scholar]
- 165.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Yung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells [Correction in Cell 126: 811, 2006.]. Cell 124: 175–189, 2006. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 166.Gurtner GC, Dauskardt RH, Wong VW, Bhatt KA, Wu K, Vial IN, Padois K, Korman JM, Longaker MT. Improving cutaneous scar formation by controlling the mechanical environment: large animal and phase I studies. Ann Surg 254: 217–225, 2011. doi: 10.1097/SLA.0b013e318220b159. [DOI] [PubMed] [Google Scholar]
- 167.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 453: 314–321, 2008. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 168.Halim AS, Emami A, Salahshourifar I, Kannan TP. Keloid scarring: understanding the genetic basis, advances, and prospects. Arch Plast Surg 39: 184–189, 2012. doi: 10.5999/aps.2012.39.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O’Farrell FM, Buchan AM, Lauritzen M, Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508: 55–60, 2014. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Haniffa M, Shin A, Bigley V, McGovern N, Teo P, See P, Wasan PS, Wang XN, Malinarich F, Malleret B, Larbi A, Tan P, Zhao H, Poidinger M, Pagan S, Cookson S, Dickinson R, Dimmick I, Jarrett RF, Renia L, Tam J, Song C, Connolly J, Chan JK, Gehring A, Bertoletti A, Collin M, Ginhoux F. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity 37: 60–73, 2012. doi: 10.1016/j.immuni.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hart CE, Loewen-Rodriguez A, Lessem J. Dermagraft: Use in the Treatment of Chronic Wounds. Adv Wound Care (New Rochelle) 1: 138–141, 2012. doi: 10.1089/wound.2011.0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Havran WL, Jameson JM. Epidermal T cells and wound healing. J Immunol 184: 5423–5428, 2010. doi: 10.4049/jimmunol.0902733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hayashida T, Wu MH, Pierce A, Poncelet AC, Varga J, Schnaper HW. MAP-kinase activity necessary for TGFbeta1-stimulated mesangial cell type I collagen expression requires adhesion-dependent phosphorylation of FAK tyrosine 397. J Cell Sci 120: 4230–4240, 2007. doi: 10.1242/jcs.03492. [DOI] [PubMed] [Google Scholar]
- 174.Hayes DW Jr, Webb GE, Mandracchia VJ, John KJ. Full-thickness burn of the foot: successful treatment with Apligraf. A case report. Clin Podiatr Med Surg 18: 179–188, 2001. [PubMed] [Google Scholar]
- 175.He L, Huang X, Kanisicak O, Li Y, Wang Y, Li Y, Pu W, Liu Q, Zhang H, Tian X, Zhao H, Liu X, Zhang S, Nie Y, Hu S, Miao X, Wang QD, Wang F, Chen T, Xu Q, Lui KO, Molkentin JD, Zhou B. Preexisting endothelial cells mediate cardiac neovascularization after injury. J Clin Invest 127: 2968–2981, 2017. doi: 10.1172/JCI93868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.He L, Marneros AG. Macrophages are essential for the early wound healing response and the formation of a fibrovascular scar. Am J Pathol 182: 2407–2417, 2013. doi: 10.1016/j.ajpath.2013.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Heath WR, Carbone FR. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat Immunol 10: 1237–1244, 2009. doi: 10.1038/ni.1822. [DOI] [PubMed] [Google Scholar]
- 178.Heath WR, Carbone FR. The skin-resident and migratory immune system in steady state and memory: innate lymphocytes, dendritic cells and T cells. Nat Immunol 14: 978–985, 2013. doi: 10.1038/ni.2680. [DOI] [PubMed] [Google Scholar]
- 179.Hellström M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalén M, Gerhardt H, Betsholtz C. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445: 776–780, 2007. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 180.Hinz B. The extracellular matrix and transforming growth factor-β1: tale of a strained relationship. Matrix Biol 47: 54–65, 2015. doi: 10.1016/j.matbio.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 181.Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech 43: 146–155, 2010. doi: 10.1016/j.jbiomech.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 182.Hinz B, Gabbiani G. Cell-matrix and cell-cell contacts of myofibroblasts: role in connective tissue remodeling. Thromb Haemost 90: 993–1002, 2003. doi: 10.1160/TH03-05-0328. [DOI] [PubMed] [Google Scholar]
- 183.Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmoulière A, Varga J, De Wever O, Mareel M, Gabbiani G. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol 180: 1340–1355, 2012. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hirobe T. Proliferation of epidermal melanocytes during the healing of skin wounds in newborn mice. J Exp Zool 227: 423–431, 1983. doi: 10.1002/jez.1402270311. [DOI] [PubMed] [Google Scholar]
- 185.Hobbs RM, Silva-Vargas V, Groves R, Watt FM. Expression of activated MEK1 in differentiating epidermal cells is sufficient to generate hyperproliferative and inflammatory skin lesions. J Invest Dermatol 123: 503–515, 2004. doi: 10.1111/j.0022-202X.2004.23225.x. [DOI] [PubMed] [Google Scholar]
- 186.Hodde JP, Ernst DM, Hiles MC. An investigation of the long-term bioactivity of endogenous growth factor in OASIS Wound Matrix. J Wound Care 14: 23–25, 2005. doi: 10.12968/jowc.2005.14.1.26721. [DOI] [PubMed] [Google Scholar]
- 187.Høiby N, Ciofu O, Johansen HK, Song ZJ, Moser C, Jensen PO, Molin S, Givskov M, Tolker-Nielsen T, Bjarnsholt T. The clinical impact of bacterial biofilms. Int J Oral Sci 3: 55–65, 2011. doi: 10.4248/IJOS11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hu MS, Maan ZN, Wu JC, Rennert RC, Hong WX, Lai TS, Cheung ATM, Walmsley GG, Chung MT, McArdle A, Longaker MT, Lorenz HP. Tissue engineering and regenerative repair in wound healing. Ann Biomed Eng 42: 1494–1507, 2014. doi: 10.1007/s10439-014-1010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hu MS, Walmsley GG, Barnes LA, Weiskopf K, Rennert RC, Duscher D, Januszyk M, Maan ZN, Hong WX, Cheung AT, Leavitt T, Marshall CD, Ransom RC, Malhotra S, Moore AL, Rajadas J, Lorenz HP, Weissman IL, Gurtner GC, Longaker MT. Delivery of monocyte lineage cells in a biomimetic scaffold enhances tissue repair. JCI Insight 2: e96260, 2017. doi: 10.1172/jci.insight.96260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hu SC, Lan CE. High-glucose environment disturbs the physiologic functions of keratinocytes: focusing on diabetic wound healing. J Dermatol Sci 84: 121–127, 2016. doi: 10.1016/j.jdermsci.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 191.Huang S, Lu G, Wu Y, Jirigala E, Xu Y, Ma K, Fu X. Mesenchymal stem cells delivered in a microsphere-based engineered skin contribute to cutaneous wound healing and sweat gland repair. J Dermatol Sci 66: 29–36, 2012. doi: 10.1016/j.jdermsci.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 192.Hume DA. Macrophages as APC and the dendritic cell myth. J Immunol 181: 5829–5835, 2008. doi: 10.4049/jimmunol.181.9.5829. [DOI] [PubMed] [Google Scholar]
- 193.Huss FR, Nyman E, Gustafson CJ, Gisselfält K, Liljensten E, Kratz G. Characterization of a new degradable polymer scaffold for regeneration of the dermis: in vitro and in vivo human studies. Organogenesis 4: 195–200, 2008. doi: 10.4161/org.4.3.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med 11: 1351–1354, 2005. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 195.Jagadish M, McNally MM, Heidel RE, Teffeteller S, Arnold JD, Freeman M, Stevens SL, Grandas OH, Goldman MH. Diabetic Foot Ulcers: The Importance of Patient Comorbidity Recognition and Total Contact Casting in Successful Wound Care. Am Surg 82: 733–736, 2016. [PubMed] [Google Scholar]
- 196.Jameson J, Havran WL. Skin gammadelta T-cell functions in homeostasis and wound healing. Immunol Rev 215: 114–122, 2007. doi: 10.1111/j.1600-065X.2006.00483.x. [DOI] [PubMed] [Google Scholar]
- 197.Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, Havran WL. A role for skin gammadelta T cells in wound repair. Science 296: 747–749, 2002. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 198.Jameson JM, Cauvi G, Sharp LL, Witherden DA, Havran WL. Gammadelta T cell-induced hyaluronan production by epithelial cells regulates inflammation. J Exp Med 201: 1269–1279, 2005. doi: 10.1084/jem.20042057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jameson JM, Cauvi G, Witherden DA, Havran WL. A keratinocyte-responsive gamma delta TCR is necessary for dendritic epidermal T cell activation by damaged keratinocytes and maintenance in the epidermis. J Immunol 172: 3573–3579, 2004. doi: 10.4049/jimmunol.172.6.3573. [DOI] [PubMed] [Google Scholar]
- 200.Januszyk M, Gurtner GC. High-Throughput Single-Cell Analysis for Wound Healing Applications. Adv Wound Care (New Rochelle) 2: 457–469, 2013. doi: 10.1089/wound.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Januszyk M, Sorkin M, Glotzbach JP, Vial IN, Maan ZN, Rennert RC, Duscher D, Thangarajah H, Longaker MT, Butte AJ, Gurtner GC. Diabetes irreversibly depletes bone marrow-derived mesenchymal progenitor cell subpopulations. Diabetes 63: 3047–3056, 2014. doi: 10.2337/db13-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Januszyk M, Kwon SK, Wong VW, Padmanabhan J, Maan ZN, Whittam AJ, Major MR, Gurtner GC. The Role of Focal Adhesion Kinase in Keratinocyte Fibrogenic Gene Expression. Int J Mol Sci 18: 1915, 2017. doi: 10.3390/ijms18091915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jensen KB, Collins CA, Nascimento E, Tan DW, Frye M, Itami S, Watt FM. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell 4: 427–439, 2009. doi: 10.1016/j.stem.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jensen UB, Yan X, Triel C, Woo SH, Christensen R, Owens DM. A distinct population of clonogenic and multipotent murine follicular keratinocytes residing in the upper isthmus. J Cell Sci 121: 609–617, 2008. doi: 10.1242/jcs.025502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Jinno H, Morozova O, Jones KL, Biernaskie JA, Paris M, Hosokawa R, Rudnicki MA, Chai Y, Rossi F, Marra MA, Miller FD. Convergent genesis of an adult neural crest-like dermal stem cell from distinct developmental origins. Stem Cells 28: 2027–2040, 2010. doi: 10.1002/stem.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Joncas V, Prado R, Price Ritchie C, Kaufman LM, Schober-Flores C, Arbuckle HA. The closure of a large chronic abdominal wound in a neonate utilizing a biologic dressing. Adv Skin Wound Care 23: 404–405, 2010. doi: 10.1097/01.ASW.0000383212.95911.f5. [DOI] [PubMed] [Google Scholar]
- 207.Jones P, Simons BD. Epidermal homeostasis: do committed progenitors work while stem cells sleep? Nat Rev Mol Cell Biol 9: 82–88, 2008. doi: 10.1038/nrm2292. [DOI] [PubMed] [Google Scholar]
- 208.Jones PH, Harper S, Watt FM. Stem cell patterning and fate in human epidermis. Cell 80: 83–93, 1995. doi: 10.1016/0092-8674(95)90453-0. [DOI] [PubMed] [Google Scholar]
- 209.Jorch SK, Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat Med 23: 279–287, 2017. doi: 10.1038/nm.4294. [DOI] [PubMed] [Google Scholar]
- 210.Jun JI, Kim KH, Lau LF. The matricellular protein CCN1 mediates neutrophil efferocytosis in cutaneous wound healing. Nat Commun 6: 7386, 2015. doi: 10.1038/ncomms8386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Jung K, Covington S, Sen CK, Januszyk M, Kirsner RS, Gurtner GC, Shah NH. Rapid identification of slow healing wounds. Wound Repair Regen 24: 181–188, 2016. doi: 10.1111/wrr.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kaplan KL, Broekman MJ, Chernoff A, Lesznik GR, Drillings M. Platelet alpha-granule proteins: studies on release and subcellular localization. Blood 53: 604–618, 1979. [PubMed] [Google Scholar]
- 213.Karra R, Walter AO, Wu SM. The relationship between cardiac endothelium and fibroblasts: it’s complicated. J Clin Invest 127: 2892–2894, 2017. doi: 10.1172/JCI95492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kawai K, Suzuki S, Tabata Y, Ikada Y, Nishimura Y. Accelerated tissue regeneration through incorporation of basic fibroblast growth factor-impregnated gelatin microspheres into artificial dermis. Biomaterials 21: 489–499, 2000. doi: 10.1016/S0142-9612(99)00207-0. [DOI] [PubMed] [Google Scholar]
- 215.Keyes BE, Liu S, Asare A, Naik S, Levorse J, Polak L, Lu CP, Nikolova M, Pasolli HA, Fuchs E. Impaired Epidermal to Dendritic T Cell Signaling Slows Wound Repair in Aged Skin. Cell 167: 1323–1338.e14, 2016. doi: 10.1016/j.cell.2016.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Khanna S, Biswas S, Shang Y, Collard E, Azad A, Kauh C, Bhasker V, Gordillo GM, Sen CK, Roy S. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One 5: e9539, 2010. doi: 10.1371/journal.pone.0009539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kim I, Bang SI, Lee SK, Park SY, Kim M, Ha H. Clinical implication of allogenic implantation of adipogenic differentiated adipose-derived stem cells. Stem Cells Transl Med 3: 1312–1321, 2014. doi: 10.5966/sctm.2014-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kischer CW, Bunce H III, Shetlah MR. Mast cell analyses in hypertrophic scars, hypertrophic scars treated with pressure and mature scars. J Invest Dermatol 70: 355–357, 1978. doi: 10.1111/1523-1747.ep12543553. [DOI] [PubMed] [Google Scholar]
- 219.Kissenpfennig A, Henri S, Dubois B, Laplace-Builhé C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, Saeland S, Davoust J, Malissen B. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity 22: 643–654, 2005. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 220.Kokovay E, Li L, Cunningham LA. Angiogenic recruitment of pericytes from bone marrow after stroke. J Cereb Blood Flow Metab 26: 545–555, 2006. doi: 10.1038/sj.jcbfm.9600214. [DOI] [PubMed] [Google Scholar]
- 221.Kong HH, Segre JA. Skin microbiome: looking back to move forward. J Invest Dermatol 132: 933–939, 2012. doi: 10.1038/jid.2011.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Koob TJ, Lim JJ, Massee M, Zabek N, Denozière G. Properties of dehydrated human amnion/chorion composite grafts: Implications for wound repair and soft tissue regeneration. J Biomed Mater Res B Appl Biomater 102: 1353–1362, 2014. doi: 10.1002/jbm.b.33141. [DOI] [PubMed] [Google Scholar]
- 223.Koob TJ, Lim JJ, Massee M, Zabek N, Rennert R, Gurtner G, Li WW. Angiogenic properties of dehydrated human amnion/chorion allografts: therapeutic potential for soft tissue repair and regeneration. Vasc Cell 6: 10, 2014. doi: 10.1186/2045-824X-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Koob TJ, Rennert R, Zabek N, Massee M, Lim JJ, Temenoff JS, Li WW, Gurtner G. Biological properties of dehydrated human amnion/chorion composite graft: implications for chronic wound healing. Int Wound J 10: 493–500, 2013. doi: 10.1111/iwj.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kosaraju R, Rennert RC, Maan ZN, Duscher D, Barrera J, Whittam AJ, Januszyk M, Rajadas J, Rodrigues M, Gurtner GC. Adipose-Derived Stem Cell-Seeded Hydrogels Increase Endogenous Progenitor Cell Recruitment and Neovascularization in Wounds. Tissue Eng Part A 22: 295–305, 2016. doi: 10.1089/ten.tea.2015.0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Krampert M, Bloch W, Sasaki T, Bugnon P, Rülicke T, Wolf E, Aumailley M, Parks WC, Werner S. Activities of the matrix metalloproteinase stromelysin-2 (MMP-10) in matrix degradation and keratinocyte organization in wounded skin. Mol Biol Cell 15: 5242–5254, 2004. doi: 10.1091/mbc.e04-02-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kwon SH, Gurtner GC. Is early inflammation good or bad? Linking early immune changes to hypertrophic scarring. Exp Dermatol 26: 133–134, 2017. doi: 10.1111/exd.13167. [DOI] [PubMed] [Google Scholar]
- 229.Lamke LO. The influence of different “skin grafts” on the evaporative water loss from burns. Scand J Plast Reconstr Surg 5: 82–86, 1971. doi: 10.3109/02844317109042943. [DOI] [PubMed] [Google Scholar]
- 230.Lansdown AB. Calcium: a potential central regulator in wound healing in the skin. Wound Repair Regen 10: 271–285, 2002. doi: 10.1046/j.1524-475X.2002.10502.x. [DOI] [PubMed] [Google Scholar]
- 231.Larson BJ, Longaker MT, Lorenz HP. Scarless fetal wound healing: a basic science review. Plast Reconstr Surg 126: 1172–1180, 2010. doi: 10.1097/PRS.0b013e3181eae781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Lavery LA, Fulmer J, Shebetka KA, Regulski M, Vayser D, Fried D, Kashefsky H, Owings TM, Nadarajah J; Grafix Diabetic Foot Ulcer Study Group . The efficacy and safety of Grafix(®) for the treatment of chronic diabetic foot ulcers: results of a multi-centre, controlled, randomised, blinded, clinical trial. Int Wound J 11: 554–560, 2014. doi: 10.1111/iwj.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Leask A. Integrin β1: A Mechanosignaling Sensor Essential for Connective Tissue Deposition by Fibroblasts. Adv Wound Care (New Rochelle) 2: 160–166, 2013. doi: 10.1089/wound.2012.0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Leask A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res 106: 1675–1680, 2010. doi: 10.1161/CIRCRESAHA.110.217737. [DOI] [PubMed] [Google Scholar]
- 235.Leavitt T, Hu MS, Marshall CD, Barnes LA, Lorenz HP, Longaker MT. Scarless wound healing: finding the right cells and signals. Cell Tissue Res 365: 483–493, 2016. doi: 10.1007/s00441-016-2424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Leblond CP, Greulich RC, Pereira JPM. Relationship of cell formation and cell migration in the renewal of stratified squamous epithelia. Adv Biol Skin 5: 39–57, 1964. [Google Scholar]
- 237.Lech M, Anders HJ. Macrophages and fibrosis: How resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim Biophys Acta 1832: 989–997, 2013. doi: 10.1016/j.bbadis.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 238.Lee J, Tumbar T. Hairy tale of signaling in hair follicle development and cycling. Semin Cell Dev Biol 23: 906–916, 2012. doi: 10.1016/j.semcdb.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Lee WL, Harrison RE, Grinstein S. Phagocytosis by neutrophils. Microbes Infect 5: 1299–1306, 2003. doi: 10.1016/j.micinf.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 240.Leibovich SJ, Polverini PJ, Shepard HM, Wiseman DM, Shively V, Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature 329: 630–632, 1987. doi: 10.1038/329630a0. [DOI] [PubMed] [Google Scholar]
- 241.Lerman OZ, Galiano RD, Armour M, Levine JP, Gurtner GC. Cellular dysfunction in the diabetic fibroblast: impairment in migration, vascular endothelial growth factor production, and response to hypoxia. Am J Pathol 162: 303–312, 2003. doi: 10.1016/S0002-9440(10)63821-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Levin R, Grinstein S, Canton J. The life cycle of phagosomes: formation, maturation, and resolution. Immunol Rev 273: 156–179, 2016. doi: 10.1111/imr.12439. [DOI] [PubMed] [Google Scholar]
- 243.Levy V, Lindon C, Zheng Y, Harfe BD, Morgan BA. Epidermal stem cells arise from the hair follicle after wounding. FASEB J 21: 1358–1366, 2007. doi: 10.1096/fj.06-6926com. [DOI] [PubMed] [Google Scholar]
- 244.Lind KD. Excluding Older, Sicker Patients From Clinical Trials: Issues, Concerns and Solutions. In: Insight on the Issues. Washington, DC: AARP Public Policy Institute, 2011, p. 1–7. [Google Scholar]
- 245.Liu W, Ma K, Kwon SH, Garg R, Patta YR, Fujiwara T, Gurtner GC. The Abnormal Architecture of Healed Diabetic Ulcers Is the Result of FAK Degradation by Calpain 1. J Invest Dermatol 137: 1155–1165, 2017. doi: 10.1016/j.jid.2016.11.039. [DOI] [PubMed] [Google Scholar]
- 246.Liu ZJ, Shirakawa T, Li Y, Soma A, Oka M, Dotto GP, Fairman RM, Velazquez OC, Herlyn M. Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol 23: 14–25, 2003. doi: 10.1128/MCB.23.1.14-25.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Longaker MT, Chiu ES, Adzick NS, Stern M, Harrison MR, Stern R. Studies in fetal wound healing. V. A prolonged presence of hyaluronic acid characterizes fetal wound fluid. Ann Surg 213: 292–296, 1991. doi: 10.1097/00000658-199104000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Longaker MT, Chiu ES, Harrison MR, Crombleholme TM, Langer JC, Duncan BW, Adzick NS, Verrier ED, Stern R. Studies in fetal wound healing. IV. Hyaluronic acid-stimulating activity distinguishes fetal wound fluid from adult wound fluid. Ann Surg 210: 667–672, 1989. doi: 10.1097/00000658-198911000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Longaker MT, Whitby DJ, Adzick NS, Crombleholme TM, Langer JC, Duncan BW, Bradley SM, Stern R, Ferguson MWJ, Harrison MR. Studies in fetal wound healing. VI. Second and early third trimester fetal wounds demonstrate rapid collagen deposition without scar formation. J Pediatr Surg 25: 63–69, 1990. doi: 10.1016/S0022-3468(05)80165-4. [DOI] [PubMed] [Google Scholar]
- 251.Longaker MT, Whitby DJ, Jennings RW, Duncan BW, Ferguson MW, Harrison MR, Adzick NS. Fetal diaphragmatic wounds heal with scar formation. J Surg Res 50: 375–385, 1991. doi: 10.1016/0022-4804(91)90206-2. [DOI] [PubMed] [Google Scholar]
- 252.Lorenz HP, Longaker MT, Perkocha LA, Jennings RW, Harrison MR, Adzick NS. Scarless wound repair: a human fetal skin model. Development 114: 253–259, 1992. [DOI] [PubMed] [Google Scholar]
- 253.Lovvorn HN III, Cheung DT, Nimni ME, Perelman N, Estes JM, Adzick NS. Relative distribution and crosslinking of collagen distinguish fetal from adult sheep wound repair. J Pediatr Surg 34: 218–223, 1999. doi: 10.1016/S0022-3468(99)90261-0. [DOI] [PubMed] [Google Scholar]
- 254.Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Müller W, Roers A, Eming SA. Differential roles of macrophages in diverse phases of skin repair. J Immunol 184: 3964–3977, 2010. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 255.Lunderius-Andersson C, Enoksson M, Nilsson G. Mast cells respond to cell injury through the recognition of IL-33. Front Immunol 3: 82, 2012. doi: 10.3389/fimmu.2012.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, Tscharke DC, Heath WR, Inouye M, Carbone FR, Gebhardt T. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol 14: 1294–1301, 2013. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- 257.Mackenzie IC. Relationship between mitosis and the ordered structure of the stratum corneum in mouse epidermis. Nature 226: 653–655, 1970. doi: 10.1038/226653a0. [DOI] [PubMed] [Google Scholar]
- 258.Malissen B, Tamoutounour S, Henri S. The origins and functions of dendritic cells and macrophages in the skin. Nat Rev Immunol 14: 417–428, 2014. doi: 10.1038/nri3683. [DOI] [PubMed] [Google Scholar]
- 259.Marangoni RG, Korman BD, Wei J, Wood TA, Graham LV, Whitfield ML, Scherer PE, Tourtellotte WG, Varga J. Myofibroblasts in murine cutaneous fibrosis originate from adiponectin-positive intradermal progenitors. Arthritis Rheumatol 67: 1062–1073, 2015. doi: 10.1002/art.38990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259a.Marler JJ, Upton J, Langer R, Vacanti JP. Transplantation of cells in matrices for tissue regeneration. Adv Drug Deliv Rev 33: 165–182, 1998. doi: 10.1016/S0169-409X(98)00025-8. [DOI] [PubMed] [Google Scholar]
- 260.Marneros AG, Norris JE, Olsen BR, Reichenberger E. Clinical genetics of familial keloids. Arch Dermatol 137: 1429–1434, 2001. doi: 10.1001/archderm.137.11.1429. [DOI] [PubMed] [Google Scholar]
- 261.Marston WA, Hanft J, Norwood P, Pollak R; Dermagraft Diabetic Foot Ulcer Study Group . The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care 26: 1701–1705, 2003. doi: 10.2337/diacare.26.6.1701. [DOI] [PubMed] [Google Scholar]
- 262.Martin JM, Zenilman JM, Lazarus GS. Molecular microbiology: new dimensions for cutaneous biology and wound healing. J Invest Dermatol 130: 38–48, 2010. doi: 10.1038/jid.2009.221. [DOI] [PubMed] [Google Scholar]
- 263.Martin P. Wound healing–aiming for perfect skin regeneration. Science 276: 75–81, 1997. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 264.Martins-Green M, Petreaca M, Wang L. Chemokines and Their Receptors Are Key Players in the Orchestra That Regulates Wound Healing. Adv Wound Care (New Rochelle) 2: 327–347, 2013. doi: 10.1089/wound.2012.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Maruyama K, Asai J, Ii M, Thorne T, Losordo DW, D’Amore PA. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol 170: 1178–1191, 2007. doi: 10.2353/ajpath.2007.060018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Mascré G, Dekoninck S, Drogat B, Youssef KK, Broheé S, Sotiropoulou PA, Simons BD, Blanpain C. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature 489: 257–262, 2012. doi: 10.1038/nature11393. [DOI] [PubMed] [Google Scholar]
- 267.Mast BA, Diegelmann RF, Krummel TM, Cohen IK. Hyaluronic acid modulates proliferation, collagen and protein synthesis of cultured fetal fibroblasts. Matrix 13: 441–446, 1993. doi: 10.1016/S0934-8832(11)80110-1. [DOI] [PubMed] [Google Scholar]
- 268.McGranahan N, Swanton C. Clonal Heterogeneity and Tumor Evolution: Past, Present, and the Future. Cell 168: 613–628, 2017. doi: 10.1016/j.cell.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 269.McLachlan JB, Catron DM, Moon JJ, Jenkins MK. Dendritic cell antigen presentation drives simultaneous cytokine production by effector and regulatory T cells in inflamed skin. Immunity 30: 277–288, 2009. doi: 10.1016/j.immuni.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol 8: 935–947, 2008. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 272.Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, Weissman IL, Cyster JG, Engleman EG. Langerhans cells renew in the skin throughout life under steady-state conditions. [Corrigendum in Nat Immunol 4: 92, 2003.] Nat Immunol 3: 1135–1141, 2002. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Merkel JR, DiPaolo BR, Hallock GG, Rice DC. Type I and type III collagen content of healing wounds in fetal and adult rats. Proc Soc Exp Biol Med 187: 493–497, 1988. doi: 10.3181/00379727-187-42694. [DOI] [PubMed] [Google Scholar]
- 274.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol 172: 2731–2738, 2004. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 275.Mirastschijski U, Haaksma CJ, Tomasek JJ, Agren MS. Matrix metalloproteinase inhibitor GM 6001 attenuates keratinocyte migration, contraction and myofibroblast formation in skin wounds. Exp Cell Res 299: 465–475, 2004. doi: 10.1016/j.yexcr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 276.Mirza R, DiPietro LA, Koh TJ. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am J Pathol 175: 2454–2462, 2009. doi: 10.2353/ajpath.2009.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol 22: 411–417, 2004. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 278.Mostow EN, Haraway GD, Dalsing M, Hodde JP, King D; OASIS Venus Ulcer Study Group . Effectiveness of an extracellular matrix graft (OASIS Wound Matrix) in the treatment of chronic leg ulcers: a randomized clinical trial. J Vasc Surg 41: 837–843, 2005. doi: 10.1016/j.jvs.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 279.Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med 178: 449–460, 1993. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer 8: 618–631, 2008. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 281.Nagaoka T, Kaburagi Y, Hamaguchi Y, Hasegawa M, Takehara K, Steeber DA, Tedder TF, Sato S. Delayed wound healing in the absence of intercellular adhesion molecule-1 or L-selectin expression. Am J Pathol 157: 237–247, 2000. doi: 10.1016/S0002-9440(10)64534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Nahabedian MY. AlloDerm performance in the setting of prosthetic breast surgery, infection, and irradiation. Plast Reconstr Surg 124: 1743–1753, 2009. doi: 10.1097/PRS.0b013e3181bf8087. [DOI] [PubMed] [Google Scholar]
- 283.Naik S, Larsen SB, Gomez NC, Alaverdyan K, Sendoel A, Yuan S, Polak L, Kulukian A, Chai S, Fuchs E. Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature 550: 475–480, 2017. doi: 10.1038/nature24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Nakamizo S, Egawa G, Tomura M, Sakai S, Tsuchiya S, Kitoh A, Honda T, Otsuka A, Nakajima S, Dainichi T, Tanizaki H, Mitsuyama M, Sugimoto Y, Kawai K, Yoshikai Y, Miyachi Y, Kabashima K. Dermal Vγ4(+) γδ T cells possess a migratory potency to the draining lymph nodes and modulate CD8(+) T-cell activity through TNF-α production. J Invest Dermatol 135: 1007–1015, 2015. doi: 10.1038/jid.2014.516. [DOI] [PubMed] [Google Scholar]
- 285.Nakamura K, Williams IR, Kupper TS. Keratinocyte-derived monocyte chemoattractant protein 1 (MCP-1): analysis in a transgenic model demonstrates MCP-1 can recruit dendritic and Langerhans cells to skin. J Invest Dermatol 105: 635–643, 1995. doi: 10.1111/1523-1747.ep12324061. [DOI] [PubMed] [Google Scholar]
- 286.Nakano T, Sonoda T, Hayashi C, Yamatodani A, Kanayama Y, Yamamura T, Asai H, Yonezawa T, Kitamura Y, Galli SJ. Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell-deficient W/Wv mice. Evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. J Exp Med 162: 1025–1043, 1985. doi: 10.1084/jem.162.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Nathan DM; DCCT/EDIC Research Group . The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 37: 9–16, 2014. doi: 10.2337/dc13-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Nauta AC, Grova M, Montoro DT, Zimmermann A, Tsai M, Gurtner GC, Galli SJ, Longaker MT. Evidence that mast cells are not required for healing of splinted cutaneous excisional wounds in mice. PLoS One 8: e59167, 2013. doi: 10.1371/journal.pone.0059167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Nehls V, Drenckhahn D. Heterogeneity of microvascular pericytes for smooth muscle type alpha-actin. J Cell Biol 113: 147–154, 1991. doi: 10.1083/jcb.113.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Niezgoda JA, Van Gils CC, Frykberg RG, Hodde JP. Randomized clinical trial comparing OASIS Wound Matrix to Regranex Gel for diabetic ulcers. Adv Skin Wound Care 18: 258–266, 2005. doi: 10.1097/00129334-200506000-00012. [DOI] [PubMed] [Google Scholar]
- 291.Nijhof JG, Braun KM, Giangreco A, van Pelt C, Kawamoto H, Boyd RL, Willemze R, Mullenders LH, Watt FM, de Gruijl FR, van Ewijk W. The cell-surface marker MTS24 identifies a novel population of follicular keratinocytes with characteristics of progenitor cells. Development 133: 3027–3037, 2006. doi: 10.1242/dev.02443. [DOI] [PubMed] [Google Scholar]
- 292.Nishimura EK, Jordan SA, Oshima H, Yoshida H, Osawa M, Moriyama M, Jackson IJ, Barrandon Y, Miyachi Y, Nishikawa S. Dominant role of the niche in melanocyte stem-cell fate determination. Nature 416: 854–860, 2002. doi: 10.1038/416854a. [DOI] [PubMed] [Google Scholar]
- 293.Nishimura EK, Suzuki M, Igras V, Du J, Lonning S, Miyachi Y, Roes J, Beermann F, Fisher DE. Key roles for transforming growth factor beta in melanocyte stem cell maintenance. Cell Stem Cell 6: 130–140, 2010. doi: 10.1016/j.stem.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Nishimura R, Hayashi M, Wu GJ, Kouchi H, Imaizumi-Anraku H, Murakami Y, Kawasaki S, Akao S, Ohmori M, Nagasawa M, Harada K, Kawaguchi M. HAR1 mediates systemic regulation of symbiotic organ development. Nature 420: 426–429, 2002. doi: 10.1038/nature01231. [DOI] [PubMed] [Google Scholar]
- 295.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. [Correction in Immunity 41: 866, 2014.] Immunity 41: 49–61, 2014. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Ogawa R, Akaishi S, Huang C, Dohi T, Aoki M, Omori Y, Koike S, Kobe K, Akimoto M, Hyakusoku H. Clinical applications of basic research that shows reducing skin tension could prevent and treat abnormal scarring: the importance of fascial/subcutaneous tensile reduction sutures and flap surgery for keloid and hypertrophic scar reconstruction. J Nippon Med Sch 78: 68–76, 2011. doi: 10.1272/jnms.78.68. [DOI] [PubMed] [Google Scholar]
- 297.Okuno Y, Nakamura-Ishizu A, Kishi K, Suda T, Kubota Y. Bone marrow-derived cells serve as proangiogenic macrophages but not endothelial cells in wound healing. Blood 117: 5264–5272, 2011. doi: 10.1182/blood-2011-01-330720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Okuse T, Chiba T, Katsuumi I, Imai K. Differential expression and localization of WNTs in an animal model of skin wound healing. Wound Repair Regen 13: 491–497, 2005. doi: 10.1111/j.1067-1927.2005.00069.x. [DOI] [PubMed] [Google Scholar]
- 299.Olutoye OO, Zhu X, Cass DL, Smith CW. Neutrophil recruitment by fetal porcine endothelial cells: implications in scarless fetal wound healing. Pediatr Res 58: 1290–1294, 2005. doi: 10.1203/01.pdr.0000184326.01884.bc. [DOI] [PubMed] [Google Scholar]
- 300.Ong CJ, Ip S, Teh SJ, Wong C, Jirik FR, Grusby MJ, Teh HS. A role for T helper 2 cells in mediating skin fibrosis in tight-skin mice. Cell Immunol 196: 60–68, 1999. doi: 10.1006/cimm.1999.1537. [DOI] [PubMed] [Google Scholar]
- 301.Orgill DP, Manders EK, Sumpio BE, Lee RC, Attinger CE, Gurtner GC, Ehrlich HP. The mechanisms of action of vacuum assisted closure: more to learn. Surgery 146: 40–51, 2009. doi: 10.1016/j.surg.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 302.Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell 104: 233–245, 2001. doi: 10.1016/S0092-8674(01)00208-2. [DOI] [PubMed] [Google Scholar]
- 303.Oskeritzian CA. Mast Cells and Wound Healing. Adv Wound Care (New Rochelle) 1: 23–28, 2012. doi: 10.1089/wound.2011.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Outtz HH, Tattersall IW, Kofler NM, Steinbach N, Kitajewski J. Notch1 controls macrophage recruitment and Notch signaling is activated at sites of endothelial cell anastomosis during retinal angiogenesis in mice. Blood 118: 3436–3439, 2011. doi: 10.1182/blood-2010-12-327015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Ozerdem U, Alitalo K, Salven P, Li A. Contribution of bone marrow-derived pericyte precursor cells to corneal vasculogenesis. Invest Ophthalmol Vis Sci 46: 3502–3506, 2005. doi: 10.1167/iovs.05-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Parenteau N. Skin: the first tissue-engineered products. Sci Am 280: 83–84, 1999. doi: 10.1038/scientificamerican0499-83. [DOI] [PubMed] [Google Scholar]
- 307.Park JE, Barbul A. Understanding the role of immune regulation in wound healing. Am J Surg 187, 5A: 11S–16S, 2004. doi: 10.1016/S0002-9610(03)00296-4. [DOI] [PubMed] [Google Scholar]
- 308.Parsek MR, Singh PK. Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol 57: 677–701, 2003. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- 309.Pastar I, Stojadinovic O, Yin NC, Ramirez H, Nusbaum AG, Sawaya A, Patel SB, Khalid L, Isseroff RR, Tomic-Canic M. Epithelialization in Wound Healing: A Comprehensive Review. Adv Wound Care (New Rochelle) 3: 445–464, 2014. doi: 10.1089/wound.2013.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Pastar I, Wong LL, Egger AN, Tomic-Canic M. Descriptive vs mechanistic scientific approach to study wound healing and its inhibition: Is there a value of translational research involving human subjects? Exp Dermatol 27: 551–562, 2018. doi: 10.1111/exd.13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Paterno J, Vial IN, Wong VW, Rustad KC, Sorkin M, Shi Y, Bhatt KA, Thangarajah H, Glotzbach JP, Gurtner GC. Akt-mediated mechanotransduction in murine fibroblasts during hypertrophic scar formation. Wound Repair Regen 19: 49–58, 2011. doi: 10.1111/j.1524-475X.2010.00643.x. [DOI] [PubMed] [Google Scholar]
- 312.Paus R. Migrating melanocyte stem cells: masters of disaster? Nat Med 19: 818–819, 2013. doi: 10.1038/nm.3264. [DOI] [PubMed] [Google Scholar]
- 313.Perdiguero EG, Geissmann F. The development and maintenance of resident macrophages. Nat Immunol 17: 2–8, 2016. doi: 10.1038/ni.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Phillips TJ, Manzoor J, Rojas A, Isaacs C, Carson P, Sabolinski M, Young J, Falanga V. The longevity of a bilayered skin substitute after application to venous ulcers. Arch Dermatol 138: 1079–1081, 2002. doi: 10.1001/archderm.138.8.1079. [DOI] [PubMed] [Google Scholar]
- 315.Pierce GF, Mustoe TA, Altrock BW, Deuel TF, Thomason A. Role of platelet-derived growth factor in wound healing. J Cell Biochem 45: 319–326, 1991. doi: 10.1002/jcb.240450403. [DOI] [PubMed] [Google Scholar]
- 316.Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One 4: e7475, 2009. doi: 10.1371/journal.pone.0007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Plafki C, Peters P, Almeling M, Welslau W, Busch R. Complications and side effects of hyperbaric oxygen therapy. Aviat Space Environ Med 71: 119–124, 2000. [PubMed] [Google Scholar]
- 318.Plikus MV, Guerrero-Juarez CF, Ito M, Li YR, Dedhia PH, Zheng Y, Shao M, Gay DL, Ramos R, Hsi TC, Oh JW, Wang X, Ramirez A, Konopelski SE, Elzein A, Wang A, Supapannachart RJ, Lee HL, Lim CH, Nace A, Guo A, Treffeisen E, Andl T, Ramirez RN, Murad R, Offermanns S, Metzger D, Chambon P, Widgerow AD, Tuan TL, Mortazavi A, Gupta RK, Hamilton BA, Millar SE, Seale P, Pear WS, Lazar MA, Cotsarelis G. Regeneration of fat cells from myofibroblasts during wound healing. Science 355: 748–752, 2017. doi: 10.1126/science.aai8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Ploeger DT, Hosper NA, Schipper M, Koerts JA, de Rond S, Bank RA. Cell plasticity in wound healing: paracrine factors of M1/ M2 polarized macrophages influence the phenotypical state of dermal fibroblasts. Cell Commun Signal 11: 29, 2013. doi: 10.1186/1478-811X-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Pool JG. Normal hemostatic mechanisms: a review. Am J Med Technol 43: 776–780, 1977. [PubMed] [Google Scholar]
- 321.Pradhan S, Khatlani T, Nairn AC, Vijayan KV. The heterotrimeric G protein Gβ1 interacts with the catalytic subunit of protein phosphatase 1 and modulates G protein-coupled receptor signaling in platelets. J Biol Chem 292: 13133–13142, 2017. doi: 10.1074/jbc.M117.796656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Purhonen S, Palm J, Rossi D, Kaskenpää N, Rajantie I, Ylä-Herttuala S, Alitalo K, Weissman IL, Salven P. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci USA 105: 6620–6625, 2008. doi: 10.1073/pnas.0710516105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Rabbani P, Takeo M, Chou W, Myung P, Bosenberg M, Chin L, Taketo MM, Ito M. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell 145: 941–955, 2011. doi: 10.1016/j.cell.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Rajantie I, Ilmonen M, Alminaite A, Ozerdem U, Alitalo K, Salven P. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood 104: 2084–2086, 2004. doi: 10.1182/blood-2004-01-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Rao KN, Brown MA. Mast cells: multifaceted immune cells with diverse roles in health and disease. Ann N Y Acad Sci 1143: 83–104, 2008. doi: 10.1196/annals.1443.023. [DOI] [PubMed] [Google Scholar]
- 327.Reeves EP, Lu H, Jacobs HL, Messina CG, Bolsover S, Gabella G, Potma EO, Warley A, Roes J, Segal AW. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 416: 291–297, 2002. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- 328.Regulski M, Jacobstein DA, Petranto RD, Migliori VJ, Nair G, Pfeiffer D. A retrospective analysis of a human cellular repair matrix for the treatment of chronic wounds. Ostomy Wound Manage 59: 38–43, 2013. [PubMed] [Google Scholar]
- 329.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 107: 1164–1169, 2003. doi: 10.1161/01.CIR.0000058702.69484.A0. [DOI] [PubMed] [Google Scholar]
- 330.Rennert RC, Januszyk M, Sorkin M, Rodrigues M, Maan ZN, Duscher D, Whittam AJ, Kosaraju R, Chung MT, Paik K, Li AY, Findlay M, Glotzbach JP, Butte AJ, Gurtner GC. Microfluidic single-cell transcriptional analysis rationally identifies novel surface marker profiles to enhance cell-based therapies. Nat Commun 7: 11945, 2016. doi: 10.1038/ncomms11945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Rennert RC, Rodrigues M, Wong VW, Duscher D, Hu M, Maan Z, Sorkin M, Gurtner GC, Longaker MT. Biological therapies for the treatment of cutaneous wounds: phase III and launched therapies. Expert Opin Biol Ther 13: 1523–1541, 2013. doi: 10.1517/14712598.2013.842972. [DOI] [PubMed] [Google Scholar]
- 332.Rennert RC, Sorkin M, Januszyk M, Duscher D, Kosaraju R, Chung MT, Lennon J, Radiya-Dixit A, Raghvendra S, Maan ZN, Hu MS, Rajadas J, Rodrigues M, Gurtner GC. Diabetes impairs the angiogenic potential of adipose-derived stem cells by selectively depleting cellular subpopulations. Stem Cell Res Ther 5: 79, 2014. doi: 10.1186/scrt468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Reynolds LE, Conti FJ, Silva R, Robinson SD, Iyer V, Rudling R, Cross B, Nye E, Hart IR, Dipersio CM, Hodivala-Dilke KM. alpha3beta1 integrin-controlled Smad7 regulates reepithelialization during wound healing in mice. J Clin Invest 118: 965–974, 2008. doi: 10.1172/JCI33538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Rinkevich Y, Lindau P, Ueno H, Longaker MT, Weissman IL. Germ-layer and lineage-restricted stem/progenitors regenerate the mouse digit tip. Nature 476: 409–413, 2011. doi: 10.1038/nature10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Rinkevich Y, Walmsley GG, Hu MS, Maan ZN, Newman AM, Drukker M, Januszyk M, Krampitz GW, Gurtner GC, Lorenz HP, Weissman IL, Longaker MT. Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 348: aaa2151, 2015. doi: 10.1126/science.aaa2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Robles DT, Berg D. Abnormal wound healing: keloids. Clin Dermatol 25: 26–32, 2007. doi: 10.1016/j.clindermatol.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 336a.Rodrigues M, Gurtner GC. Black, White and Gray: Macrophages in Skin Repair and Disease. Curr Pathobiol Rep 5: 333–342, 2017. doi: 10.1007/s40139-017-0152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Rodrigues M, Wong VW, Rennert RC, Davis CR, Longaker MT, Gurtner GC. Progenitor cell dysfunctions underlie some diabetic complications. Am J Pathol 185: 2607–2618, 2015. doi: 10.1016/j.ajpath.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Rodrigues M, Yates CC, Nuschke A, Griffith L, Wells A. The matrikine tenascin-C protects multipotential stromal cells/mesenchymal stem cells from death cytokines such as FasL. Tissue Eng Part A 19: 1972–1983, 2013. doi: 10.1089/ten.tea.2012.0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Rognoni E, Gomez C, Pisco AO, Rawlins EL, Simons BD, Watt FM, Driskell RR. Inhibition of β-catenin signalling in dermal fibroblasts enhances hair follicle regeneration during wound healing. Development 143: 2522–2535, 2016. doi: 10.1242/dev.131797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Rohde E, Malischnik C, Thaler D, Maierhofer T, Linkesch W, Lanzer G, Guelly C, Strunk D. Blood monocytes mimic endothelial progenitor cells. Stem Cells 24: 357–367, 2006. doi: 10.1634/stemcells.2005-0072. [DOI] [PubMed] [Google Scholar]
- 341.Romanelli M, Dini V, Bertone M, Barbanera S, Brilli C. OASIS wound matrix versus Hyaloskin in the treatment of difficult-to-heal wounds of mixed arterial/venous aetiology. Int Wound J 4: 3–7, 2007. doi: 10.1111/j.1742-481X.2007.00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Romanelli M, Dini V, Bertone MS. Randomized comparison of OASIS wound matrix versus moist wound dressing in the treatment of difficult-to-heal wounds of mixed arterial/venous etiology. Adv Skin Wound Care 23: 34–38, 2010. doi: 10.1097/01.ASW.0000363485.17224.26. [DOI] [PubMed] [Google Scholar]
- 343.Romani N, Holzmann S, Tripp CH, Koch F, Stoitzner P. Langerhans cells: dendritic cells of the epidermis. APMIS 111: 725–740, 2003. doi: 10.1034/j.1600-0463.2003.11107805.x. [DOI] [PubMed] [Google Scholar]
- 344.Rompolas P, Deschene ER, Zito G, Gonzalez DG, Saotome I, Haberman AM, Greco V. Live imaging of stem cell and progeny behaviour in physiological hair-follicle regeneration. Nature 487: 496–499, 2012. doi: 10.1038/nature11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Rompolas P, Mesa KR, Greco V. Spatial organization within a niche as a determinant of stem-cell fate. Nature 502: 513–518, 2013. doi: 10.1038/nature12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Roubelakis MG, Trohatou O, Roubelakis A, Mili E, Kalaitzopoulos I, Papazoglou G, Pappa KI, Anagnou NP. Platelet-rich plasma (PRP) promotes fetal mesenchymal stem/stromal cell migration and wound healing process. Stem Cell Rev 10: 417–428, 2014. doi: 10.1007/s12015-013-9494-8. [DOI] [PubMed] [Google Scholar]
- 347.Rowlatt U. Intrauterine wound healing in a 20 week human fetus. Virchows Arch A Pathol Anat Histol 381: 353–361, 1979. doi: 10.1007/BF00432477. [DOI] [PubMed] [Google Scholar]
- 348.Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, Betsholtz C, Shima DT. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev 16: 2684–2698, 2002. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Rumbaut RE, Thiagarajan P. Platelet-Vessel Wall Interactions in Hemostasis and Thrombosis. San Rafael, CA: Morgan & Claypool Life Sciences, 2010. [PubMed] [Google Scholar]
- 350.Salonurmi T, Parikka M, Kontusaari S, Pirilä E, Munaut C, Salo T, Tryggvason K. Overexpression of TIMP-1 under the MMP-9 promoter interferes with wound healing in transgenic mice. Cell Tissue Res 315: 27–37, 2004. doi: 10.1007/s00441-003-0814-1. [DOI] [PubMed] [Google Scholar]
- 351.Santoro SA. Identification of a 160,000 dalton platelet membrane protein that mediates the initial divalent cation-dependent adhesion of platelets to collagen. Cell 46: 913–920, 1986. doi: 10.1016/0092-8674(86)90073-5. [DOI] [PubMed] [Google Scholar]
- 352.Santucci M, Borgognoni L, Reali UM, Gabbiani G. Keloids and hypertrophic scars of Caucasians show distinctive morphologic and immunophenotypic profiles. Virchows Arch 438: 457–463, 2001. doi: 10.1007/s004280000335. [DOI] [PubMed] [Google Scholar]
- 353.Sappino AP, Schürch W, Gabbiani G. Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest 63: 144–161, 1990. [PubMed] [Google Scholar]
- 354.Satish L, Kathju S. Cellular and Molecular Characteristics of Scarless versus Fibrotic Wound Healing. Dermatol Res Pract 2010: 790234, 2010. doi: 10.1155/2010/790234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Satoh T, Nakagawa K, Sugihara F, Kuwahara R, Ashihara M, Yamane F, Minowa Y, Fukushima K, Ebina I, Yoshioka Y, Kumanogoh A, Akira S. Identification of an atypical monocyte and committed progenitor involved in fibrosis. Nature 541: 96–101, 2017. doi: 10.1038/nature20611. [DOI] [PubMed] [Google Scholar]
- 356.Savagner P, Kusewitt DF, Carver EA, Magnino F, Choi C, Gridley T, Hudson LG. Developmental transcription factor slug is required for effective re-epithelialization by adult keratinocytes. J Cell Physiol 202: 858–866, 2005. doi: 10.1002/jcp.20188. [DOI] [PubMed] [Google Scholar]
- 357.Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature 407: 784–788, 2000. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 358.Schmidt BA, Horsley V. Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Development 140: 1517–1527, 2013. doi: 10.1242/dev.087593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Schrimpf C, Duffield JS. Mechanisms of fibrosis: the role of the pericyte. Curr Opin Nephrol Hypertens 20: 297–305, 2011. doi: 10.1097/MNH.0b013e328344c3d4. [DOI] [PubMed] [Google Scholar]
- 360.Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen 19: 134–148, 2011. doi: 10.1111/j.1524-475X.2011.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Sclafani AP, Romo T III, Jacono AA. Rejuvenation of the aging lip with an injectable acellular dermal graft (Cymetra). Arch Facial Plast Surg 4: 252–257, 2002. doi: 10.1001/archfaci.4.4.252. [DOI] [PubMed] [Google Scholar]
- 362.Sedmak DD, Orosz CG. The role of vascular endothelial cells in transplantation. Arch Pathol Lab Med 115: 260–265, 1991. [PubMed] [Google Scholar]
- 363.Segel GB, Halterman MW, Lichtman MA. The paradox of the neutrophil’s role in tissue injury. J Leukoc Biol 89: 359–372, 2011. doi: 10.1189/jlb.0910538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 17: 763–771, 2009. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Senzel L, Gnatenko DV, Bahou WF. The platelet proteome. Curr Opin Hematol 16: 329–333, 2009. doi: 10.1097/MOH.0b013e32832e9dc6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, Gabbiani G. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol 142: 873–881, 1998. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Sheikh ES, Sheikh ES, Fetterolf DE. Use of dehydrated human amniotic membrane allografts to promote healing in patients with refractory non healing wounds. Int Wound J 11: 711–717, 2014. doi: 10.1111/iwj.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Shevchenko RV, James SL, James SE. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J R Soc Interface 7: 229–258, 2010. doi: 10.1098/rsif.2009.0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Shook B, Xiao E, Kumamoto Y, Iwasaki A, Horsley V. CD301b+ Macrophages Are Essential for Effective Skin Wound Healing. J Invest Dermatol 136: 1885–1891, 2016. doi: 10.1016/j.jid.2016.05.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care 20: 493–508, 2007. doi: 10.1097/01.ASW.0000288217.83128.f3. [DOI] [PubMed] [Google Scholar]
- 371.Sibbald RG, Zuker R, Coutts P, Coelho S, Williamson D, Queen D. Using a dermal skin substitute in the treatment of chronic wounds secondary to recessive dystrophic epidermolysis bullosa: a case series. Ostomy Wound Manage 51: 22–46, 2005. [PubMed] [Google Scholar]
- 372.Siebenhaar F, Syska W, Weller K, Magerl M, Zuberbier T, Metz M, Maurer M. Control of Pseudomonas aeruginosa skin infections in mice is mast cell-dependent. Am J Pathol 170: 1910–1916, 2007. doi: 10.2353/ajpath.2007.060770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Siekmann AF, Lawson ND. Notch signalling and the regulation of angiogenesis. Cell Adhes Migr 1: 104–105, 2007. doi: 10.4161/cam.1.2.4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Silver FH, Freeman JW, DeVore D. Viscoelastic properties of human skin and processed dermis. Skin Res Technol 7: 18–23, 2001. doi: 10.1034/j.1600-0846.2001.007001018.x. [DOI] [PubMed] [Google Scholar]
- 375.Simpson RM, Meran S, Thomas D, Stephens P, Bowen T, Steadman R, Phillips A. Age-related changes in pericellular hyaluronan organization leads to impaired dermal fibroblast to myofibroblast differentiation. Am J Pathol 175: 1915–1928, 2009. doi: 10.2353/ajpath.2009.090045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Sindrilaru A, Scharffetter-Kochanek K. Disclosure of the Culprits: Macrophages-Versatile Regulators of Wound Healing. Adv Wound Care (New Rochelle) 2: 357–368, 2013. doi: 10.1089/wound.2012.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Singhal PK, Sassi S, Lan L, Au P, Halvorsen SC, Fukumura D, Jain RK, Seed B. Mouse embryonic fibroblasts exhibit extensive developmental and phenotypic diversity. Proc Natl Acad Sci USA 113: 122–127, 2016. doi: 10.1073/pnas.1522401112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377a.Sinha R, Stanley G, Gulati GS, Ezran C, Travaglini KJ, Wei E, Chan CKF, Nabhan AN, Su T, Morganti RM, Conley SD, Chaib H, Red-Horse K, Longaker MT, Snyder MP, Krasnow MA, Weissman IL. Index switching causes “spreading-of-signal” among multiplexed samples in Illumina HiSeq 4000 DNA sequencing. bioRxiv 2017. doi: 10.1101/125724. [DOI] [Google Scholar]
- 378.Sirimahachaiyakul P, Sood RF, Muffley LA, Seaton M, Lin CT, Qiao L, Armaly JS, Hocking AM, Gibran NS. Race Does Not Predict Melanocyte Heterogeneous Responses to Dermal Fibroblast-Derived Mediators. PLoS One 10: e0139135, 2015. doi: 10.1371/journal.pone.0139135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Slauch JM. How does the oxidative burst of macrophages kill bacteria? Still an open question. Mol Microbiol 80: 580–583, 2011. doi: 10.1111/j.1365-2958.2011.07612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Slemp AE, Kirschner RE. Keloids and scars: a review of keloids and scars, their pathogenesis, risk factors, and management. Curr Opin Pediatr 18: 396–402, 2006. doi: 10.1097/01.mop.0000236389.41462.ef. [DOI] [PubMed] [Google Scholar]
- 381.Snell RS. A study of the melanocytes and melanin in a healing deep wound. J Anat 97: 243–253, 1963. [PMC free article] [PubMed] [Google Scholar]
- 382.Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, van de Wetering M, van den Born M, Begthel H, Vries RG, Stange DE, Toftgård R, Clevers H. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 327: 1385–1389, 2010. doi: 10.1126/science.1184733. [DOI] [PubMed] [Google Scholar]
- 383.Solanas G, Benitah SA. Regenerating the skin: a task for the heterogeneous stem cell pool and surrounding niche. Nat Rev Mol Cell Biol 14: 737–748, 2013. doi: 10.1038/nrm3675. [DOI] [PubMed] [Google Scholar]
- 384.Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol 7: 870–879, 2005. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Sonoda T, Kitamura Y, Haku Y, Hara H, Mori KJ. Mast-cell precursors in various haematopoietic colonies of mice produced in vivo and in vitro. Br J Haematol 53: 611–620, 1983. doi: 10.1111/j.1365-2141.1983.tb07312.x. [DOI] [PubMed] [Google Scholar]
- 386.Sorokin L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol 10: 712–723, 2010. doi: 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- 387.Sorrell JM, Caplan AI. Fibroblast heterogeneity: more than skin deep. J Cell Sci 117: 667–675, 2004. doi: 10.1242/jcs.01005. [DOI] [PubMed] [Google Scholar]
- 388.Sorrentino S, Studt JD, Medalia O, Tanuj Sapra K. Roll, adhere, spread and contract: structural mechanics of platelet function. Eur J Cell Biol 94: 129–138, 2015. doi: 10.1016/j.ejcb.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 389.Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A, Caprioli F, Bottiglieri L, Oldani A, Viale G, Penna G, Dejana E, Rescigno M. A gut-vascular barrier controls the systemic dissemination of bacteria. Science 350: 830–834, 2015. doi: 10.1126/science.aad0135. [DOI] [PubMed] [Google Scholar]
- 390.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med 137: 1142–1162, 1973. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Steinman RM, Gutchinov B, Witmer MD, Nussenzweig MC. Dendritic cells are the principal stimulators of the primary mixed leukocyte reaction in mice. J Exp Med 157: 613–627, 1983. doi: 10.1084/jem.157.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Strecker-McGraw MK, Jones TR, Baer DG. Soft tissue wounds and principles of healing. Emerg Med Clin North Am 25: 1–22, 2007. doi: 10.1016/j.emc.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 393.Su Y, Richmond A. Chemokine Regulation of Neutrophil Infiltration of Skin Wounds. Adv Wound Care (New Rochelle) 4: 631–640, 2015. doi: 10.1089/wound.2014.0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Subramaniam M, Saffaripour S, Van De Water L, Frenette PS, Mayadas TN, Hynes RO, Wagner DD. Role of endothelial selectins in wound repair. Am J Pathol 150: 1701–1709, 1997. [PMC free article] [PubMed] [Google Scholar]
- 395.Suchting S, Freitas C, del Toro R, le Noble F, Benedito R, Breant C, Duarte A, Eichmann A. The Notch ligand Delta-like 4 (Dll4) negatively regulates endothelial tip cell formation and vessel branching. FASEB J 21: A15, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Suga H, Rennert RC, Rodrigues M, Sorkin M, Glotzbach JP, Januszyk M, Fujiwara T, Longaker MT, Gurtner GC. Tracking the elusive fibrocyte: identification and characterization of collagen-producing hematopoietic lineage cells during murine wound healing. Stem Cells 32: 1347–1360, 2014. doi: 10.1002/stem.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Sumaria N, Roediger B, Ng LG, Qin J, Pinto R, Cavanagh LL, Shklovskaya E, Fazekas de St Groth B, Triccas JA, Weninger W. Cutaneous immunosurveillance by self-renewing dermal gammadelta T cells. J Exp Med 208: 505–518, 2011. doi: 10.1084/jem.20101824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Sun G, Zhang X, Shen YI, Sebastian R, Dickinson LE, Fox-Talbot K, Reinblatt M, Steenbergen C, Harmon JW, Gerecht S. Dextran hydrogel scaffolds enhance angiogenic responses and promote complete skin regeneration during burn wound healing. Proc Natl Acad Sci USA 108: 20976–20981, 2011. doi: 10.1073/pnas.1115973108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Szpaderska AM, Egozi EI, Gamelli RL, DiPietro LA. The effect of thrombocytopenia on dermal wound healing. J Invest Dermatol 120: 1130–1137, 2003. doi: 10.1046/j.1523-1747.2003.12253.x. [DOI] [PubMed] [Google Scholar]
- 400.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 5: 434–438, 1999. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 401.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 140: 805–820, 2010. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 402.Tamoutounour S, Guilliams M, Montanana Sanchis F, Liu H, Terhorst D, Malosse C, Pollet E, Ardouin L, Luche H, Sanchez C, Dalod M, Malissen B, Henri S. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity 39: 925–938, 2013. doi: 10.1016/j.immuni.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 403.Tang A, Amagai M, Granger LG, Stanley JR, Udey MC. Adhesion of epidermal Langerhans cells to keratinocytes mediated by E-cadherin. Nature 361: 82–85, 1993. doi: 10.1038/361082a0. [DOI] [PubMed] [Google Scholar]
- 404.Tanimura S, Tadokoro Y, Inomata K, Binh NT, Nishie W, Yamazaki S, Nakauchi H, Tanaka Y, McMillan JR, Sawamura D, Yancey K, Shimizu H, Nishimura EK. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell 8: 177–187, 2011. doi: 10.1016/j.stem.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 405.Tanno H, Kawakami K, Kanno E, Suzuki A, Takagi N, Yamamoto H, Ishii K, Imai Y, Maruyama R, Tachi M. Invariant NKT cells promote skin wound healing by preventing a prolonged neutrophilic inflammatory response. Wound Repair Regen 25: 805–815, 2017. doi: 10.1111/wrr.12588. [DOI] [PubMed] [Google Scholar]
- 406.Tanno H, Kawakami K, Ritsu M, Kanno E, Suzuki A, Kamimatsuno R, Takagi N, Miyasaka T, Ishii K, Imai Y, Maruyama R, Tachi M. Contribution of Invariant Natural Killer T Cells to Skin Wound Healing. Am J Pathol 185: 3248–3257, 2015. doi: 10.1016/j.ajpath.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 407.Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 102: 451–461, 2000. doi: 10.1016/S0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- 408.Telgenhoff D, Shroot B. Cellular senescence mechanisms in chronic wound healing. Cell Death Differ 12: 695–698, 2005. doi: 10.1038/sj.cdd.4401632. [DOI] [PubMed] [Google Scholar]
- 409.Tellechea A, Leal EC, Kafanas A, Auster ME, Kuchibhotla S, Ostrovsky Y, Tecilazich F, Baltzis D, Zheng Y, Carvalho E, Zabolotny JM, Weng Z, Petra A, Patel A, Panagiotidou S, Pradhan-Nabzdyk L, Theoharides TC, Veves A. Mast Cells Regulate Wound Healing in Diabetes. Diabetes 65: 2006–2019, 2016. doi: 10.2337/db15-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Teller P, White TK. The physiology of wound healing: injury through maturation. Surg Clin North Am 89: 599–610, 2009. doi: 10.1016/j.suc.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 411.Tennent GA, Brennan SO, Stangou AJ, O’Grady J, Hawkins PN, Pepys MB. Human plasma fibrinogen is synthesized in the liver. Blood 109: 1971–1974, 2007. doi: 10.1182/blood-2006-08-040956. [DOI] [PubMed] [Google Scholar]
- 412.Tepper OM, Capla JM, Galiano RD, Ceradini DJ, Callaghan MJ, Kleinman ME, Gurtner GC. Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow-derived cells. Blood 105: 1068–1077, 2005. doi: 10.1182/blood-2004-03-1051. [DOI] [PubMed] [Google Scholar]
- 413.Thangarajah H, Vial IN, Grogan RH, Yao D, Shi Y, Januszyk M, Galiano RD, Chang EI, Galvez MG, Glotzbach JP, Wong VW, Brownlee M, Gurtner GC. HIF-1alpha dysfunction in diabetes. Cell Cycle 9: 75–79, 2010. doi: 10.4161/cc.9.1.10371. [DOI] [PubMed] [Google Scholar]
- 414.Thangarajah H, Yao D, Chang EI, Shi Y, Jazayeri L, Vial IN, Galiano RD, Du XL, Grogan R, Galvez MG, Januszyk M, Brownlee M, Gurtner GC. The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proc Natl Acad Sci USA 106: 13505–13510, 2009. doi: 10.1073/pnas.0906670106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Tobin HA, Karas ND. Lip augmentation using an alloderm graft. J Oral Maxillofac Surg 56: 722–727, 1998. doi: 10.1016/S0278-2391(98)90805-9. [DOI] [PubMed] [Google Scholar]
- 416.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3: 349–363, 2002. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 418.Tomasek JJ, Haaksma CJ, Schwartz RJ, Howard EW. Whole animal knockout of smooth muscle alpha-actin does not alter excisional wound healing or the fibroblast-to-myofibroblast transition. Wound Repair Regen 21: 166–176, 2013. doi: 10.1111/wrr.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Tonnesen MG, Feng X, Clark RA. Angiogenesis in wound healing. J Investig Dermatol Symp Proc 5: 40–46, 2000. doi: 10.1046/j.1087-0024.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 420.Truong AT, Kowal-Vern A, Latenser BA, Wiley DE, Walter RJ. Comparison of dermal substitutes in wound healing utilizing a nude mouse model. J Burns Wounds 4: e4, 2005. [PMC free article] [PubMed] [Google Scholar]
- 421.Tsukada S, Westwick JK, Ikejima K, Sato N, Rippe RA. SMAD and p38 MAPK signaling pathways independently regulate alpha1(I) collagen gene expression in unstimulated and transforming growth factor-beta-stimulated hepatic stellate cells. J Biol Chem 280: 10055–10064, 2005. doi: 10.1074/jbc.M409381200. [DOI] [PubMed] [Google Scholar]
- 422.Tuan TL, Nichter LS. The molecular basis of keloid and hypertrophic scar formation. Mol Med Today 4: 19–24, 1998. doi: 10.1016/S1357-4310(97)80541-2. [DOI] [PubMed] [Google Scholar]
- 423.Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol 5: 405–417, 2007. doi: 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- 424.Tura O, Skinner EM, Barclay GR, Samuel K, Gallagher RC, Brittan M, Hadoke PW, Newby DE, Turner ML, Mills NL. Late outgrowth endothelial cells resemble mature endothelial cells and are not derived from bone marrow. Stem Cells 31: 338–348, 2013. doi: 10.1002/stem.1280. [DOI] [PubMed] [Google Scholar]
- 426.Van den Broek LJ, van der Veer WM, de Jong EH, Gibbs S, Niessen FB. Suppressed inflammatory gene expression during human hypertrophic scar compared to normotrophic scar formation. Exp Dermatol 24: 623–629, 2015. doi: 10.1111/exd.12739. [DOI] [PubMed] [Google Scholar]
- 427.Van der Veer WM, Bloemen MC, Ulrich MM, Molema G, van Zuijlen PP, Middelkoop E, Niessen FB. Potential cellular and molecular causes of hypertrophic scar formation. Burns 35: 15–29, 2009. doi: 10.1016/j.burns.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 428.Van der Vliet A, Janssen-Heininger YM. Hydrogen peroxide as a damage signal in tissue injury and inflammation: murderer, mediator, or messenger? J Cell Biochem 115: 427–435, 2014. doi: 10.1002/jcb.24683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Varkey M, Ding J, Tredget EE. Advances in Skin Substitutes-Potential of Tissue Engineered Skin for Facilitating Anti-Fibrotic Healing. J Funct Biomater 6: 547–563, 2015. doi: 10.3390/jfb6030547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Veves A, Sheehan P, Pham HT. A randomized, controlled trial of Promogran (a collagen/oxidized regenerated cellulose dressing) vs standard treatment in the management of diabetic foot ulcers. Arch Surg 137: 822–827, 2002. doi: 10.1001/archsurg.137.7.822. [DOI] [PubMed] [Google Scholar]
- 431.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92: 827–839, 2003. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 432.Wagner CL, Mascelli MA, Neblock DS, Weisman HF, Coller BS, Jordan RE. Analysis of GPIIb/IIIa receptor number by quantification of 7E3 binding to human platelets. Blood 88: 907–914, 1996. [PubMed] [Google Scholar]
- 433.Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns 21: 243–248, 1995. doi: 10.1016/0305-4179(95)93866-I. [DOI] [PubMed] [Google Scholar]
- 434.Wallace VA, Kondo S, Kono T, Xing Z, Timms E, Furlonger C, Keystone E, Gauldie J, Sauder DN, Mak TW, Paige CJ. A role for CD4+ T cells in the pathogenesis of skin fibrosis in tight skin mice. Eur J Immunol 24: 1463–1466, 1994. doi: 10.1002/eji.1830240634. [DOI] [PubMed] [Google Scholar]
- 435.Wallis S, Lloyd S, Wise I, Ireland G, Fleming TP, Garrod D. The alpha isoform of protein kinase C is involved in signaling the response of desmosomes to wounding in cultured epithelial cells. Mol Biol Cell 11: 1077–1092, 2000. doi: 10.1091/mbc.11.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Walmsley GG, Maan ZN, Wong VW, Duscher D, Hu MS, Zielins ER, Wearda T, Muhonen E, McArdle A, Tevlin R, Atashroo DA, Senarath-Yapa K, Lorenz HP, Gurtner GC, Longaker MT. Scarless wound healing: chasing the holy grail. Plast Reconstr Surg 135: 907–917, 2015. doi: 10.1097/PRS.0000000000000972. [DOI] [PubMed] [Google Scholar]
- 437.Wang J, Zhang Y, Zhang N, Wang C, Herrler T, Li Q. An updated review of mechanotransduction in skin disorders: transcriptional regulators, ion channels, and microRNAs. Cell Mol Life Sci 72: 2091–2106, 2015. doi: 10.1007/s00018-015-1853-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Wang Z, Lai Y, Bernard JJ, Macleod DT, Cogen AL, Moss B, Di Nardo A. Skin mast cells protect mice against vaccinia virus by triggering mast cell receptor S1PR2 and releasing antimicrobial peptides. J Immunol 188: 345–357, 2012. doi: 10.4049/jimmunol.1101703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Watt FM. Mammalian skin cell biology: at the interface between laboratory and clinic. Science 346: 937–940, 2014. doi: 10.1126/science.1253734. [DOI] [PubMed] [Google Scholar]
- 440.Watt FM. Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J 21: 3919–3926, 2002. doi: 10.1093/emboj/cdf399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Watt FM. The stem cell compartment in human interfollicular epidermis. J Dermatol Sci 28: 173–180, 2002. doi: 10.1016/S0923-1811(02)00003-8. [DOI] [PubMed] [Google Scholar]
- 442.Weller A, Isenmann S, Vestweber D. Cloning of the mouse endothelial selectins. Expression of both E- and P-selectin is inducible by tumor necrosis factor alpha. J Biol Chem 267: 15176–15183, 1992. [PubMed] [Google Scholar]
- 443.Weller K, Foitzik K, Paus R, Syska W, Maurer M. Mast cells are required for normal healing of skin wounds in mice. FASEB J 20: 2366–2368, 2006. doi: 10.1096/fj.06-5837fje. [DOI] [PubMed] [Google Scholar]
- 444.Wenes M, Shang M, Di Matteo M, Goveia J, Martín-Pérez R, Serneels J, Prenen H, Ghesquière B, Carmeliet P, Mazzone M. Macrophage Metabolism Controls Tumor Blood Vessel Morphogenesis and Metastasis. Cell Metab 24: 701–715, 2016. doi: 10.1016/j.cmet.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 445.Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol 127: 998–1008, 2007. doi: 10.1038/sj.jid.5700786. [DOI] [PubMed] [Google Scholar]
- 446.Werner S, Peters KG, Longaker MT, Fuller-Pace F, Banda MJ, Williams LT. Large induction of keratinocyte growth factor expression in the dermis during wound healing. Proc Natl Acad Sci USA 89: 6896–6900, 1992. doi: 10.1073/pnas.89.15.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Werner S, Smola H, Liao X, Longaker MT, Krieg T, Hofschneider PH, Williams LT. The function of KGF in morphogenesis of epithelium and reepithelialization of wounds. Science 266: 819–822, 1994. doi: 10.1126/science.7973639. [DOI] [PubMed] [Google Scholar]
- 448.Wernig G, Chen SY, Cui L, Van Neste C, Tsai JM, Kambham N, Vogel H, Natkunam Y, Gilliland DG, Nolan G, Weissman IL. Unifying mechanism for different fibrotic diseases. Proc Natl Acad Sci USA 114: 4757–4762, 2017. doi: 10.1073/pnas.1621375114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Wetzler C, Kämpfer H, Stallmeyer B, Pfeilschifter J, Frank S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J Invest Dermatol 115: 245–253, 2000. doi: 10.1046/j.1523-1747.2000.00029.x. [DOI] [PubMed] [Google Scholar]
- 450.Whitby DJ, Longaker MT, Harrison MR, Adzick NS, Ferguson MWJ. Rapid epithelialisation of fetal wounds is associated with the early deposition of tenascin. J Cell Sci 99: 583–586, 1991. [DOI] [PubMed] [Google Scholar]
- 451.Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo-controlled double-blind study. Diabetes Care 21: 822–827, 1998. doi: 10.2337/diacare.21.5.822. [DOI] [PubMed] [Google Scholar]
- 452.Wietecha MS, Cerny WL, DiPietro LA. Mechanisms of vessel regression: toward an understanding of the resolution of angiogenesis. Curr Top Microbiol Immunol 367: 3–32, 2013. doi: 10.1007/82_2012_287. [DOI] [PubMed] [Google Scholar]
- 453.Wilgus TA, Roy S, McDaniel JC. Neutrophils and Wound Repair: Positive Actions and Negative Reactions. Adv Wound Care (New Rochelle) 2: 379–388, 2013. doi: 10.1089/wound.2012.0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Wilgus TA, Wulff BC. The Importance of Mast Cells in Dermal Scarring. Adv Wound Care (New Rochelle) 3: 356–365, 2014. doi: 10.1089/wound.2013.0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Wilkins LM, Watson SR, Prosky SJ, Meunier SF, Parenteau NL. Development of a bilayered living skin construct for clinical applications. Biotechnol Bioeng 43: 747–756, 1994. doi: 10.1002/bit.260430809. [DOI] [PubMed] [Google Scholar]
- 456.Willenborg S, Lucas T, van Loo G, Knipper JA, Krieg T, Haase I, Brachvogel B, Hammerschmidt M, Nagy A, Ferrara N, Pasparakis M, Eming SA. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood 120: 613–625, 2012. doi: 10.1182/blood-2012-01-403386. [DOI] [PubMed] [Google Scholar]
- 457.Williamson JS, Snelling CF, Clugston P, Macdonald IB, Germann E. Cultured epithelial autograft: five years of clinical experience with twenty-eight patients. J Trauma 39: 309–319, 1995. doi: 10.1097/00005373-199508000-00020. [DOI] [PubMed] [Google Scholar]
- 458.Witherden DA, Watanabe M, Garijo O, Rieder SE, Sarkisyan G, Cronin SJ, Verdino P, Wilson IA, Kumanogoh A, Kikutani H, Teyton L, Fischer WH, Havran WL. The CD100 receptor interacts with its plexin B2 ligand to regulate epidermal γδ T cell function. Immunity 37: 314–325, 2012. doi: 10.1016/j.immuni.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Wollenberg A, Wagner M, Günther S, Towarowski A, Tuma E, Moderer M, Rothenfusser S, Wetzel S, Endres S, Hartmann G. Plasmacytoid dendritic cells: a new cutaneous dendritic cell subset with distinct role in inflammatory skin diseases. J Invest Dermatol 119: 1096–1102, 2002. doi: 10.1046/j.1523-1747.2002.19515.x. [DOI] [PubMed] [Google Scholar]
- 460.Wong VW, Akaishi S, Longaker MT, Gurtner GC. Pushing back: wound mechanotransduction in repair and regeneration. J Invest Dermatol 131: 2186–2196, 2011. doi: 10.1038/jid.2011.212. [DOI] [PubMed] [Google Scholar]
- 461.Wong VW, Garg RK, Sorkin M, Rustad KC, Akaishi S, Levi K, Nelson ER, Tran M, Rennert R, Liu W, Longaker MT, Dauskardt RH, Gurtner GC. Loss of keratinocyte focal adhesion kinase stimulates dermal proteolysis through upregulation of MMP9 in wound healing. Ann Surg 260: 1138–1146, 2014. doi: 10.1097/SLA.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 462.Wong VW, Longaker MT, Gurtner GC. Soft tissue mechanotransduction in wound healing and fibrosis. Semin Cell Dev Biol 23: 981–986, 2012. doi: 10.1016/j.semcdb.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 463.Wong VW, Paterno J, Sorkin M, Glotzbach JP, Levi K, Januszyk M, Rustad KC, Longaker MT, Gurtner GC. Mechanical force prolongs acute inflammation via T-cell-dependent pathways during scar formation. FASEB J 25: 4498–4510, 2011. doi: 10.1096/fj.10-178087. [DOI] [PubMed] [Google Scholar]
- 464.Wong VW, Rustad KC, Akaishi S, Sorkin M, Glotzbach JP, Januszyk M, Nelson ER, Levi K, Paterno J, Vial IN, Kuang AA, Longaker MT, Gurtner GC. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med 18: 148–152, 2012. doi: 10.1038/nm.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Wu AR, Neff NF, Kalisky T, Dalerba P, Treutlein B, Rothenberg ME, Mburu FM, Mantalas GL, Sim S, Clarke MF, Quake SR. Quantitative assessment of single-cell RNA-sequencing methods. Nat Methods 11: 41–46, 2014. doi: 10.1038/nmeth.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Wu H, Wang C, Wu Z. PROPER: comprehensive power evaluation for differential expression using RNA-seq. Bioinformatics 31: 233–241, 2015. doi: 10.1093/bioinformatics/btu640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Wukich DK, Raspovic KM. What Role Does Function Play in Deciding on Limb Salvage versus Amputation in Patients With Diabetes? Plast Reconstr Surg 138, Suppl: 188S–195S, 2016. doi: 10.1097/PRS.0000000000002713. [DOI] [PubMed] [Google Scholar]
- 469.Wulff BC, Parent AE, Meleski MA, DiPietro LA, Schrementi ME, Wilgus TA. Mast cells contribute to scar formation during fetal wound healing. J Invest Dermatol 132: 458–465, 2012. doi: 10.1038/jid.2011.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Wulff BC, Wilgus TA. Mast cell activity in the healing wound: more than meets the eye? Exp Dermatol 22: 507–510, 2013. doi: 10.1111/exd.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol 4: 583–594, 2004. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Wynn TA, Cheever AW, Jankovic D, Poindexter RW, Caspar P, Lewis FA, Sher A. An IL-12-based vaccination method for preventing fibrosis induced by schistosome infection. Nature 376: 594–596, 1995. doi: 10.1038/376594a0. [DOI] [PubMed] [Google Scholar]
- 474.Xiong N, Raulet DH. Development and selection of gammadelta T cells. Immunol Rev 215: 15–31, 2007. doi: 10.1111/j.1600-065X.2006.00478.x. [DOI] [PubMed] [Google Scholar]
- 475.Yamaguchi Y, Okazaki Y, Seta N, Satoh T, Takahashi K, Ikezawa Z, Kuwana M. Enhanced angiogenic potency of monocytic endothelial progenitor cells in patients with systemic sclerosis. Arthritis Res Ther 12: R205, 2010. doi: 10.1186/ar3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Yan C, Grimm WA, Garner WL, Qin L, Travis T, Tan N, Han YP. Epithelial to mesenchymal transition in human skin wound healing is induced by tumor necrosis factor-alpha through bone morphogenic protein-2. Am J Pathol 176: 2247–2258, 2010. doi: 10.2353/ajpath.2010.090048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Yanez DA, Lacher RK, Vidyarthi A, Colegio OR. The role of macrophages in skin homeostasis. Pflugers Arch 469: 455–463, 2017. doi: 10.1007/s00424-017-1953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Yates CC, Krishna P, Whaley D, Bodnar R, Turner T, Wells A. Lack of CXC chemokine receptor 3 signaling leads to hypertrophic and hypercellular scarring. Am J Pathol 176: 1743–1755, 2010. doi: 10.2353/ajpath.2010.090564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Yipp BG, Kubes P. NETosis: how vital is it? Blood 122: 2784–2794, 2013. doi: 10.1182/blood-2013-04-457671. [DOI] [PubMed] [Google Scholar]
- 480.Younan G, Suber F, Xing W, Shi T, Kunori Y, Abrink M, Pejler G, Schlenner SM, Rodewald HR, Moore FD Jr, Stevens RL, Adachi R, Austen KF, Gurish MF. The inflammatory response after an epidermal burn depends on the activities of mouse mast cell proteases 4 and 5. J Immunol 185: 7681–7690, 2010. doi: 10.4049/jimmunol.1002803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Yukna RA, Turner DW, Robinson LJ. Variable antigenicity of lyophilized allogeneic and lyophilized xenogeneic skin in guinea pigs. J Periodontal Res 12: 197–203, 1977. doi: 10.1111/j.1600-0765.1977.tb00122.x. [DOI] [PubMed] [Google Scholar]
- 482.Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J 10: 502–507, 2013. doi: 10.1111/iwj.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Zhang JG, Czabotar PE, Policheni AN, Caminschi I, Wan SS, Kitsoulis S, Tullett KM, Robin AY, Brammananth R, van Delft MF, Lu J, O’Reilly LA, Josefsson EC, Kile BT, Chin WJ, Mintern JD, Olshina MA, Wong W, Baum J, Wright MD, Huang DC, Mohandas N, Coppel RL, Colman PM, Nicola NA, Shortman K, Lahoud MH. The dendritic cell receptor Clec9A binds damaged cells via exposed actin filaments. Immunity 36: 646–657, 2012. doi: 10.1016/j.immuni.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 484.Zhu J, Peng T, Johnston C, Phasouk K, Kask AS, Klock A, Jin L, Diem K, Koelle DM, Wald A, Robins H, Corey L. Immune surveillance by CD8αα+ skin-resident T cells in human herpes virus infection. Nature 497: 494–497, 2013. doi: 10.1038/nature12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Zhu Z, Ding J, Ma Z, Iwashina T, Tredget EE. Alternatively activated macrophages derived from THP-1 cells promote the fibrogenic activities of human dermal fibroblasts. Wound Repair Regen 25: 377–388, 2017. doi: 10.1111/wrr.12532. [DOI] [PubMed] [Google Scholar]
- 486.Zhu Z, Ding J, Ma Z, Iwashina T, Tredget EE. Systemic depletion of macrophages in the subacute phase of wound healing reduces hypertrophic scar formation. Wound Repair Regen 24: 644–656, 2016. doi: 10.1111/wrr.12442. [DOI] [PubMed] [Google Scholar]
- 487.Ziegenhain C, Vieth B, Parekh S, Reinius B, Guillaumet-Adkins A, Smets M, Leonhardt H, Heyn H, Hellmann I, Enard W. Comparative analysis of single-cell RNA-sequencing methods. Mol Cell 65: 631–643.e4, 2017. doi: 10.1016/j.molcel.2017.01.023. [DOI] [PubMed] [Google Scholar]