Abstract
Heroin is a highly abused opioid that has reached epidemic status within the United States. Yet, existing therapies to treat addiction are inadequate and frequently result into rates of high recidivism. Vaccination against heroin offers a promising alternative therapeutic option but requires further development to enhance the vaccine’s performance. Hsp70 is a conserved protein with known immunomodulatory properties and is considered an excellent immunodominant antigen. Within an antidrug vaccine context, we envisioned Hsp70 as a potential dual carrier-adjuvant, wherein immunogenicity would be increased by co-localization of adjuvant and antigenic drug hapten. Recombinant Mycobacterium tuberculosis Hsp70 was appended with heroin haptens and the resulting immunoconjugate granted anti-heroin antibody production and blunted heroin-induced antinociception. Moreover, Hsp70 as a carrier protein surpassed our benchmark Her-KLH cocktail through antibody-mediated blockade of 6-acetylmorphine, the main mediator of heroin’s psychoactivity. The work presents a new avenue for exploration in the use of hapten-Hsp70 conjugates to elicit anti-drug immune responses.
Keywords: heroin, opioids, heat shock protein, vaccines, immunotherapy, adjuvant, carrier protein
Graphical Abstract
1. Introduction
Heroin is an acutely harmful and illicit drug worldwide, significantly contributing to an unprecedented increase in global morbidity and mortality. Recently, there has been a marked increase in the prescription of synthetic opioid pain relievers (OPRs) for management of chronic pain. Evidence suggests that abuse of OPRs acts as a “gateway drug” to heroin use, because general access to heroin is easier and substantially less expensive.3 In the face of ever increasing opioid abuse and its associated fatalities, it is critical to find therapies to treat opioid addiction. Current treatments involve patients being subjected to opioid replacement therapy combined with heroin detoxification.4 The addictive capacity of heroin and other opioids, in addition to the adverse effects of withdrawal and the high cost of treatment, lead to a high recidivism rate.4 Antibody-mediated therapies are an attractive alternative because antibodies target the actual drug instead of brain receptors, exhibit fewer side effects, and can impart long-term protection.
There have been some notable advances in the development of vaccines against heroin 1, 5-6 and other drugs of abuse.7-10 These vaccines have been shown to stimulate the immune system to produce antibodies that bind drug molecules in systemic circulation. In general, antibodies are not able to cross the blood-brain barrier (BBB), hence, antibody-bound drug is prevented from entering the brain, thereby blocking the brain reward system, including reinforcing effects of the drug. Anti-drug vaccines against nicotine and cocaine previously failed in human clinical trials. Their lack of success was attributed to a deficiency of an adequate immune response, prompting the need for more immunogenic vaccines.11-12 In the field of vaccine development, new adjuvants are highly sought after that can stimulate a robust humoral immune response.13-14
Heat shock proteins (Hsps) are highly conserved proteins found in all prokaryotes and eukaryotes and play a critical role in cellular homeostasis.15 The most conserved of the Hsps is Hsp70, which is also the most abundantly expressed protein in response to stress.16 From a clinical standpoint, individuals are routinely vaccinated to respond to bacterial Hsps without causing autoimmunity.17 Moreover, a trivalent vaccine against tetanus, diphtheria, and pertussis induces an anti-Hsp70 immune response. Furthermore, approximately 80% of the world’s children are immunized against tuberculosis with live BCG, which contains considerable amounts of Hsp70, yet it presents no evidence of autoimmunity.17 Bacterial Hsps have been examined as vaccine candidates in infectious disease and in cancer treatment, with heat shock based vaccines advancing to Phase II and Phase III clinical trials for melanoma and renal cell carcinoma, respectively.15 Based on these findings, we hypothesized that Mycobacterium tuberculosis Hsp70 would be a strong candidate as a potential carrier-adjuvant in our small molecule vaccines. In this study, we investigated Hsps as dual adjuvant-carrier proteins. Our goal here was to explore their potential as an immunostimulant, wherein drug concentric haptens would be covalently displayed on the protein’s surface.
Despite the wide range of use of Hsp70 in vaccine treatments, recent reports claim that the presence of bacterial lipopolysaccharide (LPS) or endotoxin contamination are responsible for Hsp70’s immune stimulating response and interaction with antigen-presenting cells.16, 18 This controversial study was supported by another account examining Hsp70’s role in dendritic cell activation when free of LPS contamination. Results from this report supported a mechanism wherein Hsp70 exhibited immunosuppressive properties, rather than inflammatory stimulation and that LPS was responsible for the inflammatory response and upregulation of related cytokines.16 Other stringently controlled experiments expressing Hsp70 from nonbacterial sources (i.e., no endogenous source of endotoxin) or LPS-free recombinant Hsp70 recapitulated that Hsp70 retains its immunomodulatory properties.15, 19-21
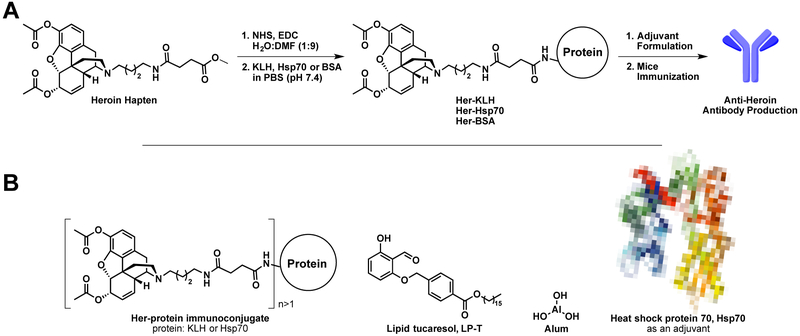
Gram-negative bacterial endotoxins constitute some of the strongest adjuvants known. Their extreme toxicity in small quantities preclude their use as adjuvants in humans and in our current study. To address this gap, monophosphoryl lipid A (MPLA), a detoxified derivative of LPS was isolated, which retains the capacity to act as an adjuvant in mice.22 MPLA is the only non-alum adjuvant approved for use in conjunction with alum in both the US and Europe.23 To observe a potential synergistic immunogenic response with Hsp70 and bacterial LPS, we opted to use an MPLA-type system. From our previous studies with MPLA (Figure 1B),23 we discovered a lipid analogue of tucaresol, LP-T, was a suitable MPLA substitute. In a head-to-head comparison between MPLA and LP-T, LP-T elicited a larger immune response against methamphetamine with superior adjuvancy to MPLA, as indicated by its enhanced anti-methamphetamine antibody titer, affinity, and concentration.23 Thus we also elected to use LP-T as an additional adjuvant in our study to analyze its interaction with the Hsp70 system in comparison to our benchmark second generation heroin hapten-keyhole limpet hemocyanin immunoconjugate (Her-KLH) vaccine (Figure 1A).1 We hypothesized that Her-Hsp70 would perform better than Her-KLH due to its dual carrier-adjuvant properties and strong immunostimulant potential.
Figure 1.
Heroin immunoconjugate vaccine strategy. A) Heroin hapten activation, conjugation, and vaccination strategy; B) Structures of relevant vaccine immunoconjugates and adjuvants. Heroin hapten was synthesized according to literature methods.1-2
2. Materials and methods
2.1. Expression and purification of recombinant Hsp70
The bacterial strain Mycobacterium tuberculosis was purchased from the American Type Culture Collection (ATCC #25618D), and genomic DNA was prepared from bacterial cultures (PureLink Genomic DNA Mini Kit, Invitrogen). The Hsp70 gene H37Rv was amplified by PCR and subcloned into the pET29a expression vector (Novagen) using NdeI and BamHI restriction sites to append a C-terminal His tag as previously described.24 The recombinant Hsp70 protein was overexpressed in E. coli BL21 (DE3) cells and purified using TALON cobalt metal affinity resin (Clontech) under denaturing conditions. In brief, ~7 g of cell paste was resuspended in 50 mL of extraction/wash buffer consisting of 50 mM sodium phosphate, pH 7.0, 6 M guanidine-HCl, and 300 mM NaCl (Buffer A). Following clarification by centrifugation, the supernatant was added to pre-equilibrated TALON resin. After washing with Buffer A, the protein was eluted with 15 mL of elution buffer consisting of 45 mM sodium phosphate, pH 7.0, 5.4 M guanidine-HCl, 270 mM NaCl, and 150 mM imidazole (Buffer B). The eluted protein was dialyzed against phosphate buffered saline (PBS), pH 7.4 (Figure A1).
Protein concentration was determined by BCA assay (Pierce) and characterized by ESI-ToF MS (Figure A6). Endotoxin was removed by performing iterative flow-through endotoxin columns (Thermofisher). The endotoxin level was measured with the Limulus amebocyte lysate assay (Thermo Scientific) and determined to be >6EU/μg of protein before column treatments and <1.1 EU/μg of protein (Table A3).25 The purified protein was stored in PBS with 50% glycerol (v/v) at −80 °C.
2.2. Preparation of heroin immunoconjugates
Heroin was obtained from NIDA. The second generation heroin hapten was synthesized according to literature methods (Figure 1A).1-2 Briefly, after synthesis of the hapten (Figure 1A), the carboxylic acid was activated with NHS using EDC-mediate coupling for several hours at rt and then mixed with BSA, KLH, or Hsp70 (1 mg/mL) in a 1:1 w/w ratio of hapten to protein. For rt conjugation conditions, immunoconjugates were allowed to react at rt for 4 hours, followed by 16 hours at 4 °C, using gentle end-over-end mixing. Alternatively, the 4 °C conjugation conditions consisted of the same overall reaction time but maintained at 4 °C with gentle mixing. Her-KLH and Her-BSA were conjugated using the rt conditions. Following conjugation, the solutions were dialyzed against PBS (pH 7.4) using a 10k MW cut off dialysis cassettes, and buffer was exchanged several times. Immunoconjugates were diluted to 50% (v/v) with glycerol and stored at −80 °C.
2.3. Heroin hapten density analysis by MALDI-ToF
The heroin hapten density for immunoconjugates prepared in this study were quantified using MALDI-ToF and ESI-ToF MS analysis and compared to unmodified Hsp70 or BSA, using the following formula: hapten density = (MWHer-Protein – MWProtein)/ (MWHer – MWwater). Heroin immunoconjugated to BSA (Her-BSA) was used as a surrogate for KLH to quantify the number of heroin haptens (Her) and for ELISAs. The immunoconjugates were run through a PD MiniTrap G-10 desalting column (GE Healthcare) and then analyzed by MALDI-ToF. Spectra can be found in the supplementary information (Figure A6-13). A summary of the hapten density results is listed in Table A2.
2.4. ATPase activity of Hsp70
An ATPase assay was adapted from Ko et al.26 Briefly, a calibration curve of phosphate (0.625 μM – 50 μM) with assay buffer (100 mM Tris-HCl, 20 mM KCl, 6 mM MgCl2, pH 7.4) was made with and without ATP. For samples, Hsp70 (2 μM) was added to Tris buffer. The reaction was initiated by addition of ATP (4 mM) and shaken at 37 °C for 15 minutes, followed by incubation with BiolMol Green (Enzo Life Sciences) at rt for 20 minutes. Absorbance of each well was measured at 625 nm. Calibration samples with and without ATP were each run in duplicate and samples with Hsp70 were run in triplicate.
2.5. Vaccine formulation and administration in animals
Immunoconjugates were stored at −80 °C until the day of immunization. Adjuvants were added to immunoconjugates and allowed to vortex for twenty minutes. Lipid tucaresol (LP-T) was prepared according to literature methods.23 Vaccines were formulated with adjuvants in the following doses per mouse: 40 μg of LP-T, 40 μg Hsp70, and/or 1 mg of alum (Alhydrogel, Invivogen). All studies were performed in compliance with the Scripps Institutional Animal Care and Use Committee and all protocols adhered to the National Institute of Health Guide for the Care and Use of Laboratory Animals. Male Swiss Webster mice (Taconic Farms, Germantown, NY; 6-8 weeks old; 25-30 g) were immunized subcutaneously (s.c.) with 40 μg of heroin-Hsp70 or KLH conjugate vaccines on days 0, 14, and 28. The overall vaccination schedule and behavioral experiments can be viewed in Figure A5.
Mice were bled on day 38 using retro-orbital puncture in order to collect approximately 100-150 μL of whole blood. Groups were composed of 4 mice. Mice were group-housed in an AAALAC-accredited vivarium containing temperature and humidity controlled rooms, and kept on a reverse light cycle (lights on: 9PM-9AM). Mouse weights were measured every week and injection site reactions were measured on the day of antinociception.
2.6. Hot plate and tail immersion antinociceptive testing
Mice were tested for cumulative heroin response in primarily suprapinal (hot plate) and spinal (tail flick) behavioral tests as previously described.2, 27 The heroin doses tested were 2, 4, 6, 8, 10, 14 and 18 mg/kg to generate a full dose-response curve. Testing was repeated at fifteen-minute intervals, following an injection of drug, and this cycle was repeated, increasing cumulative dosing until full antinociception was observed in both assays. Antinociception data were transformed from time to percent maximum possible effect (%MPE), which is calculated as: %MPE = [(test − baseline)/(cutoff − baseline)] × 100. These data were then fit using a log(agonist) vs. normalized response non-linear regression in GraphPad PRISM 6. The ED50 values and 95% confidence intervals were determined for each antinociception test and individual treatment groups to determine ED50 values.
2.7. ELISA Procedure and Antibody Isotyping
Costar 3690 plates with half-area, high-binding 96-well microtiter plates were coated with 50 ng of Her-BSA (25 μL of 2 μg/mL Her-BSA conjugate in PBS) per well overnight at 37 °C. ELISAs were run according to previously published literature procedure, except incubation of the secondary antibody (1:10,000 dilution) was shortened to two hours at rt.2 Serum from non-vaccinated mice did not contain any detectable anti-heroin titers.
For IgG isotyping, costar 3690 plates with half-area, high-binding 96-well microtiter plates were coated with 50 ng of Her-BSA (25 μL of 2 μg/mL Her-BSA conjugate in PBS) per well overnight at 37 °C. Plates were blocked using 5% skim milk in PBS for one and half hours at rt. Mouse sera were diluted in 2% BSA in PBS to their midpoint IgG titer, then 25 μl of diluted sample was added to wells. Plates were incubated at 37 °C in a moist chamber for one and a half hours then washed 10 times using dH2O. Next, 25 μl of appropriate secondary antibody, IgM, IgG1, IgG2a, IgG2b, IgG3 from Isotyping Kit (Southern Biotech at 1:600 dilution) or Gamma-2c chain specific-IgG2c (GAM-igG2c, Southern Biotech at 1:6000 dilution) was added to wells in duplicate, and plates were incubated again for one and half hours at 37 °C in a moist chamber. After washing as described above, plates were developed using TMB and quenched with 2M H2SO4 then read at 450 nm. Raw OD450 values for antibody isotyping are shown in Table A6-7 and averages for the vaccine group and subclass ratios are listed in Table A4 and S5, respectively.
2.8. Blood brain biodistribution
Blood brain biodistribution was determined according to literature procedure with minor modifications.5, 28 A calibration curve for using standard solutions of heroin (H), 6-acetylmorphine (6AM), and morphine (M) was constructed (Figure A14). On Week 7 (3 weeks from last boost), vaccine groups adjuvanted with alum and nonvaccinated control mice (n = 21 and 4, respectively) were injected intraperitoneally (3.0 mg/kg) with heroin. At fifteen minutes following injection the animals were fully anesthetized and then rapidly decapitated using a sharp guillotine. The brain and trunk blood were collected. The trunk blood was collected in a 1:1 ratio with acetate buffer (0.1 M sodium acetate/0.1M acetic acid/50 g/L NaF, pH 6.0), placed on ice for several hours, centrifuged at 10,000 rpm for ten minutes. Brain tissue was immediately flash frozen using a dry ice/acetone bath with 1 mL of acetate buffer. The brain tissue was homogenized using a Bullet Blender with zirconium oxide beads (0.5 mm diameter, Thomas Scientific) and then centrifuged at 2,500 rpm for ten minutes. A 100 μL aliquot of the homogenate or plasma was added to 100 μL of spiked heroin, 6-acetylmorphine, and morphine concentrations (for standard curve, made up in 85:15 ACN:MeOH) or 100 μL of 85:15 ACN:MeOH (for samples), 100 μL of d9-heroin, d3-6-acetylmorphine, and d3-morphine (1 μg/mL in ACN) and 300 μL of ice-cold acetonitrile/methanol (85:15). The mixture was vortexed for a 30 s and stored in the −20 °C freezer for twenty minutes, followed by centrifugation at 2,500 rpm for ten minutes. A 450 μL aliquot was transferred to another test tube and the samples was evaporated using GENEVAC. The dried sample was taken up in acetonitrile, centrifuged at 10,000 rpm for five minutes and then transferred to vials for LCMS analysis.
2.9. Antibody Affinity for Heroin Metabolites
The binding IC50 for mouse serum (day 38) and free heroin was determined by competitive binding assay via surface plasmon resonance using a Biacore 3000 instrument (GE Healthcare) according to literature methods.2, 8 Signal produced by antibody binding to the SPR chip without drug present was used as a reference for 100% binding. IC50 values were determined from a 12 point 6-AM dilution curve and derived from a nonlinear fit of the binding curves in PRISM 6. 3
3. Results
Drugs of abuse vaccines differ fundamentally from traditional vaccines against infectious diseases or cancer because they are designed to induce a humoral response to neutralize drug molecules through antibody production. Small molecule vaccine conjugates are a powerful tool in vaccine design, particularly vaccines against drugs of abuse, enabling the interrogation of any desired hapten (i.e., drug) through chemical synthesis and exploiting a wide array of potentially immunogenic proteins for conjugation. This versatile tool to combat drug abuse uses a combination of chemistry and biochemical techniques to explore new adjuvants and carriers in vivo to direct one arm of the adaptive immune system over the other. To further advance this research initiative, we detail the first example of a small molecule hapten vaccine using Hsp70 as the carrier and adjuvant and heroin as the hapten.
3.1. Hapten-Protein Conjugation
Before conjugation of the recombinant Hsp70, we examined its structure for available surface lysines (PDB: 4RTF, Figure A2). Based on a visual analysis of the only reported crystal structure of M. tuberculosis Hsp70, we found there were a suitable number of lysines for conjugation with our activated heroin hapten (Figure 1A). The protein sequence for Hsp70 indicates a total of 39 lysines, with at least 14 surface lysines available for small molecule conjugation.
During preliminary conjugation procedures, we tested the effect of temperature on the overall hapten density. Recombinant Hsp70 was successfully conjugated to the heroin hapten and showed the same degree of hapten copies for both treatments (12.9 and 12.7 hapten density, respectively, Figure A8-9 and Table A2). These Her-Hsp70 conjugates were combined and used for immunization without alum. Surrogate BSA conjugates for KLH revealed a hapten number of 22 per mole of protein (Figure A12 and Table A2). In the second round of conjugation for the series of vaccinations with alum, the rt method was used and gave a hapten density of ~7 per Hsp70 and 12 for KLH (with BSA surrogate, Figure A10 and 13, respectively).
The positive charge generated from the nitrogen of the heroin hapten may have assisted in the subsequent removal of endotoxin from Her-Hsp70 immunoconjugates below the required level of 0.5 EU/μg (Table A3).21 However, despite iterative endotoxin removal column treatments, endotoxin remained ~1.5 EU/μg protein for unconjugated Hsp70 due to the continuous loss of protein from each pass. We noted the endotoxin levels (Table A3) and carefully monitored mice throughout the course of the study (Figure 2). After both conjugation and endotoxin removal, the ATPase activity of Hsp70 was measured using BioMol Green Reagent to quantify phosphate concentration against unconjugated and conjugated Hsp70. Gratifyingly, we observed no significant difference before and after conjugation in the ATPase activity, indicating Hsp70 retains its structure after conjugation and endotoxin removal conditions (Figure A3).29 With our immunoconjugates in hand, we then tested the efficacy of our heat shock protein based vaccines in a murine model.
Figure 2.
Body weight change over time during vaccination. A) Vaccinated groups without alum; B) vaccinated groups with alum. Data points are means + SEM (n = 4 mice per group). Black arrows indicate time of vaccine injections (s.c.). Her-HSP70 + Alum (blue, dashed line) is the only group that had a single mouse that remained the same weight over the course of five weeks.
3.2. Safety and Efficacy of Vaccines
Weight loss or attenuated growth is a symptom of compromised health in mice, and therefore animal growth was monitored weekly over the course of the vaccine schedule (Figure A5). Fortunately, all vaccines were well tolerated and safe at the selected doses of immunoconjugate and adjuvant per injection. It should be noted that there were no injection site reactions for any of the vaccines in the absence of alum. Despite the higher content of endotoxin in unconjugated Hsp70, mice were not adversely affected by a 40 μg dose (orange lines, Figure 2). All vaccines with alum developed internal bumps at the site of injection; but were not severe or exposed (Figure A4). This phenomenon is commonly associated with alum,30 and a t-test of the average weight gain between vaccine groups treated with or without alum was not found to be statistically significant (Table A1, Figure 2).
3.3. Antinociception
After three boosts, the efficacy of our vaccines was tested for their ability to prevent heroin-induced antinociception using hot plate and tail flick antinociception assays. When we tested the vaccine groups in the absence of alum, the ED50’s were either modest or very low (Figure 3A and 3B, solid bars). As such the vaccine series was repeated in the presence of alum to observe if its depot effect could increase efficacy.30 Indeed, when alum was adjuvanted the ED50 of all groups increased significantly for both tail flick and hot plate antinociception (Figure 3A and 3B, striped bars), except in groups: Her-KLH + LP-T (only hot plate, Figure 3A, olive green bar) and Her-KLH + Hsp70 (both assays, orange bars). A two-way ANOVA was run for both assays. In hot plate, the data were significant with respect to both alum and vaccination treatment group ([F (1, 36) = 510.2, p< 0.0001] and [F (5, 36) = 17.56, p<0.0001], respectively). A similar result was observed for tail flick: alum (F (1, 36) = 1139, p< 0.0001) and vaccination group (F (5, 36) = 79.03, p<0.0001).
Figure 3.
Vaccines against heroin are only effective in behavioral assays with the addition of alum. Effect of adjuvants and carrier proteins on A) hot plate and B) tail flick antinociception assays; C) anti-heroin antibody titers; and D) Th1 and Th2-associated humoral responses. Data points are means + SEM (n = 4 mice per group). Solid bars are vaccine groups without the addition of alum; striped bars represent vaccine groups with the addition of alum (1 mg/injection). Missing solid bars in the antinociception A and B graphs indicate that all individuals within that group maxed out at the lowest dose of heroin (i.e., 2 mg/kg), therefore exhibiting very low or no vaccine efficacy. $ p < 0.001, $$ p < 0.0001 when comparing a vaccine with or without alum (Tukey’s). **** p < 0.0001 for Her-Hsp70 + Alum when compared to all other vaccination groups (Tukey’s).
Vaccines that contained both LP-T and a variation of Hsp70 performed similarly in antinociception assays in the presence of alum (Figure 3A and B, green and purple striped bars). It is possible that LP-T and Hsp70 independently possess immunosuppressive properties at a 40 μg dose, which is reflected in the decrease in ED50 values compared to Her-KLH when either adjuvant is added. Interestingly, when both LP-T and Hsp70 adjuvants are added together the immunosuppressive properties are no longer dominant, but the synergy of LP-T and Hsp70 are not sufficient to add to the overall efficacy of Her-KLH + Alum. In agreement with this hypothesis, the efficacy of the Her-Hsp70 + Alum vaccine also decreases when LP-T is added as an adjuvant (blue and purple striped bars, respectively).
3.4. Antibody titers and class differentiation of IgG antibodies
On average, vaccines with alum (striped bars) gave greater anti-heroin antibody titers than in the absence of alum, which were generally 2- to 4-fold higher than their respective vaccine counterparts (Figure 3C, solid bars). According to the antinociception data, vaccines were only efficacious upon the addition of alum, which is corroborated by the predominant increase in antibody titers. A two-way ANOVA was performed on the midpoint titer data, which found a significant effect from treatment with alum (F (1, 36) = 23.99, p < 0.0001) but not in vaccination group (F (5, 36) = 0.1351, p = 0.9832). After determining adjuvant and carrier protein effects on titer and behavioral data, we were interested in further characterizing the polyclonal antibody response by their specific isotypes.
Adjuvants in vaccine formulation may influence the IgG subclasses and can be used to fine-tune the humoral response to maximize drug molecule neutralization. Both LP-T and Hsp70 have been reported to activate cellular (Th1) and humoral responses (Th2).16, 23 Mice sera were diluted to their midpoint titer, and then examined against different murine secondary antibodies in duplicate (Table A6-7). Overall, the large class distribution toward IgG in the ratios of IgG1 to IgM indicates an efficient IgG memory response to vaccination (Table A5). To assess Th1 and Th2-associated responses, we compared the geometric mean OD450 values of IgG2a (Th1) to IgG1 (Th2) (Figure 3D). As expected, the addition of alum (a Th2 adjuvant) increased the IgG1 response in vaccines with Her-KLH as the immunoconjugate carrier, except when LP-T was used an adjuvant. This may be because tucaresol is known to promote both Th1 response23 and may preferentially induce the response in a dose-dependent manner. It also reduces the overall humoral response against the heroin immunoconjugate potentially in favor of T-cell mediated responses or an overall immunosuppressive effect. In addition, it is known that like LP-T, Hsp70 elicits a Th1-associated response, which is exhibited by the downward trend in Th2 vs Th1 ratios in Figure 3D (orange and green bars), toward a Th1 based response both with and without alum. Nonetheless a Th2 response was predominant among all vaccines and regardless of alum treatment.
The IgG subclass ratio data were analyzed using a two-way ANOVA, which found both the treatment of alum and vaccination groups significant ([F (1, 84) = 8.084, p = 0.0056] and [F (5, 84) = 12.72, P < 0.00010], respectively). Alum significantly altered the Th2:Th1 ratio only in Her-KLH and Her-Hsp70. The Her-Hsp70 and Her-Hsp70 + LP-T without alum showed that IgG1:IgG2a values were drastically inflated by poor IgG2a stimulation. Interestingly, addition of alum increased the IgG2a response and decreased the IgG1 response when only when Hsp70 was employed as a carrier, stimulating a greater Th1 response from the alum depot. One last notable observation was that despite the presence of both Hsp70 and LP-T in both vaccine groups without alum (green and purple bars), there was a significant difference between the IgG1:IgG2a ratio (Tukey’s, p < 0.0001), however addition of alum diminishes this difference.
3.5. Blood brain biodistribution and antibody binding affinity studies
After observing that Hsp70 + Alum exhibited the greatest efficacy in hot plate and tail flick antinociception assays despite a mixed Th1 and Th2 response, it was of interest to determine the blood brain biodistribution of heroin in the mice after a single 3.0 mg/kg dose (i.p.).5 The dynamics of the heroin metabolite binding within the bloodstream were consistent with the known pharmacokinetics of heroin (Figure 4A). Within 15 minutes, the heroin concentration was only detected in minor amounts and only in the brain, coinciding with increased levels of 6AM (Figure 4A). Sequestration of drug in the bloodstream and prevention of its analgesic properties indicate vaccine efficacy. A two-way ANOVA was run on the blood to brain ratios with drugs as the in between factors and adjuvant group as the within factor and both effects were significant (drug [F (1, 36) = 24.02, p < 0.0001] and adjuvant group [F (6, 36) = 7.675, p < 0.0001]). Although there were not substantial differences in the brain levels of heroin and its related metabolites (Figure 4B), there were significant differences in the blood levels clearly showing that vaccine-induced serum antibodies can effectively sequester large amounts of free drug in the blood between vaccinated and nonvaccinated control mice (Figure 4C). These differences induced differential brain to blood ratios, and were significant compared to nonvaccinated control mice, with Hsp70 + Alum having the lowest brain to blood ratio (Figure 4D).
Figure 4.
Blood brain biodistribution of heroin 15 minutes after intraperitoneal injection (3.0 mg/kg) on vaccine groups adjuvanted with alum. A) Metabolic pathway of heroin from carboxylesterase isozymes and their half-lives in humans;31 B) heroin (H), 6-acetylmorphine (6-AM) and morphine (M) in brain; C) drug concentrations found in blood; D) ratio of brain to blood concentrations. Data points are means + SEM (n = 3 or 4 mice per group). *Red asterisk indicates significance of Her-Hsp70 + Alum group to compared to every other group for 6-AM concentrations in blood (p < 0.01, Tukey’s), except with respect to Her-KLH + Hsp70 + Alum (p > 0.05). ****Red asterisks indicate a significant difference of the nonvaccinated control brain and blood ratio to all other vaccine groups for 6-AM, only (p<0.0001, Tukey’s).
An accurate predictor of the efficacy of an active vaccination against heroin should be based upon retention of drug within the bloodstream, limiting distribution to the brain over time. We hypothesized that the sole presence of heroin in samples where Hsp70 was used as a carrier protein may suggest that these antibodies bind tighter and allow a previously “inaccessible” pool of heroin to slowly seep into the brain or provide a slow release, preventing the “bound” heroin from being rapidly processed by serum esterases. To test this hypothesis, we utilized mouse sera and generated antiserum opioid binding curves against heroin and its metabolites to assess affinity of each vaccine group by surface plasmon resonance (SPR).
Hsp70 as a carrier generated IC50s for 6-AM in the range of 67.4 – 86.3 nM, whereas KLH gave values of 75.4 – 119.3 nM (Table 1). A one-way ANOVA was performed on the logIC50 values and determined there was a significant difference between the vaccine groups (F (7, 80) = 9.431, p <0. 0001). A Tukey’s post hoc analysis revealed that although Hsp70 as a carrier generated higher affinity antibodies, the groups were only significantly better when compared to Her-KLH + LP-T + Alum and Her-KLH + LP-T + Hsp70 + Alum (p < 0.01, Table 1). Her-KLH + Alum exhibits higher affinity in the absence of Hsp70 or LP-T adjuvants, which is consistent with the antinociception data. These observations also align with the isotyping results considering both adjuvants promote a Th1 response, whereas addition of alum (Th2) produced tighter binding antibodies within the Her-KLH group. Generating antiserum opioid binding curves for heroin and morphine were attempted, but at the highest dosage (200 μM), the drug concentration did not inhibit IgG binding to chip greater than 50%. Considering that the IC50 for 6-AM ranged in the 70-100 nM range, IC50 values for heroin or morphine would be at least three orders of magnitude larger.
Table 1.
Effects of various immunoconjugate-adjuvant formulation on anti-heroin antibody affinity
| Vaccine | PIC50 ± SEMa | IC50 (nM) |
|---|---|---|
| Her-KLH | 7.013 ± 0.022 | 97.0 |
| Her-Hsp70 | 7.064 ± 0.040 | 86.3 |
| Her-KLH + Alum | 7.123 ± 0.019 | 75.4 |
| Her-KLH + LP-T + Alum | 6.991 ± 0.027 | 102.2 |
| Her-KLH + Hsp70 + Alum | 7.076 ± 0.018 | 83.9 |
| Her-KLH + LP-T + Hsp70 + Alum | 6.923 ± 0.018 | 119.3 |
| Her-Hsp70 + Alum | 7.108 ± 0.034 | 77.9 |
| Her-Hsp70 + LP-T + Alum | 7.172 ± 0.023 | 67.4 |
Mouse sera was also run against heroin and morphine to determine IC50 values by SPR, however we observed less than 50% inhibition at the highest concentration (200 μM) and determined IC50 values for these metabolites was > 200 μM for all vaccine groups.
4. Discussion
To our knowledge, we are the first research group to apply Hsp70 as a dual carrier adjuvant in a drug of abuse vaccine. Her-Hsp70 + Alum was our most efficacious vaccine in both antinociceptive tests and the brain to blood distribution ratio, surpassing our benchmark Her-KLH + Alum. It should be noted that previous studies with alhydrogel revealed that incubation with alum results in adsorption of all vaccine components, including carrier proteins and adjuvants, in the presence of PBS buffer (pH 7.4) at room temperature.2 This adsorption to alum may create a depot effect, contributing to an overall increase in efficacy seen in all vaccine groups.30 When comparing Her-KLH to Her-Hsp70 vaccine performance, addition of Hsp70 to Her-KLH does not enhance its efficacy. We posit that colocalization of antigen and adjuvant contributes to the increase in immune response.32-33 Indeed previous reports have determined that a physical linkage between Hsp and the antigen in question is required for the desired immune response.17, 34 Hsp70 has been shown to enhance tumor antigen presentation through an immune complex formation,20-21 where the complex cross-primes and transfers antigens to antigen-presenting cells (APC). This process may be accelerated by the presence of Hsp receptors on the APC.15 Not only do bacterial Hsp70s facilitate cross-priming, but they also specifically have been shown to process and present antigenic peptides by MHC class II (i.e., humoral mediated response).35
The effect of Hsp70 on antigen-specific CD4+ T cell differentiation could not be justified by direct interaction of Hsp alone or due to endotoxin contamination, but the antigen must form a complex formation with Hsp in order to elicit amplification. Hsp70 as solely an adjuvant had higher endotoxin levels in this study, which has been attributed to immunogenic properties of Hsp70 according to some reports. However, in the case of our vaccine study, higher endotoxin content did not correlate to enhanced immune responses. The current proposed mechanism suggests that Hsp does not increase presentation per se but rather involves the delivery of peptides for processing and presentation to APCs, suggesting the key function is enhanced delivery or processing rather than generalized enhancement of APC function.35 It is hypothesized that the antigen:MHC class II complex is stabilized by HSP and that the MHC class II molecules are displayed for a longer time on the cell surface (slower turn over), which may contribute to the efficacy exhibited by Hsp70 carrier vaccines.21
Adjuvants working in tandem to noncompetitively promote IgG isotypes associated with both Th1 and Th2 responses may improve the efficacy of a vaccine.23, 27 We applied this strategy to our heroin vaccine by formulating both Hsp70 and LP-T with our immunoconjugate, both of which have the potential to stimulate Th1 and Th2 responses. We hypothesized that activation of both Th1 and Th2 humoral responses would be beneficial for increasing heroin-binding capacity over Th2 responses alone. Unfortunately, our substitute for MPLA did not enhance our vaccine system. Instead, we consistently observed the decrease in vaccine efficacy and antibody affinity when using LP-T with alum. Addition of LP-T adjuvant provided an immunosuppressive effect, which may be the result of increased amounts of LP-T used in this study. Only one source has reported an immunosuppressive effect of tucaresol at higher doses, which may be the case considering the vaccine study with methamphetamine used 5.6 μg as opposed to the 40 μg of LP-T used here.23
Dose-dependent studies of Hsp and LP-T may shed more light on the modulation between immunosuppression and activation and their inherent ability to stimulate Th1 and Th2 processes. In a similar vein, Hsp70 has been observed to work in a narrow dose window.15 Low doses of antigen complexed to Hsp70 were superior to stimulating CD 4+ T cells compared to higher doses.21
5. Conclusion
In conclusion, Hsp70 as a carrier protein is an excellent vehicle for antigen delivery and opens the drug of abuse vaccines field to further exploration with this protein. The vaccine may be further improved upon dosing studies and in combination with different adjuvant formulation profiles.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Margaret Olson for the lipid tucaresol. This work was supported by funding from the National Institutes of Health NIDA (UH3DA041146).
Abbreviations:
- Hsp
Heat shocks protein
- BBB
blood-brain barrier
- OPRs
opioid pain relievers
- LPS
lipopolysaccharide
- MPLA
monophosphory lipid A
- LP-T
lipid tucaresol
- ACN
acetonitrile
- MeOH
methanol
- MPE
maximum possible effect
- i.p.
intraperitoneal
- s.c.
subcutaneous
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bremer PT; Schlosburg JE; Banks ML; Steele FF; Zhou B; Poklis JL; Janda KD, Development of a Clinically-Viable Heroin Vaccine. Journal of the American Chemical Society 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hwang CS; Bremer PT; Wenthur CJ; Ho SO; Chiang S; Ellis B; Zhou B; Fujii G; Janda KD, Enhancing Efficacy and Stability of an Antiheroin Vaccine: Examination of Antinociception, Opioid Binding Profile, and Lethality. Mol. Pharm. 2018, 15(3), 1062–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pollini RA; Banta-Green CJ; Cuevas-Mota J; Metzner M; Teshale E; Garfein RS, Problematic use of prescription-type opioids prior to heroin use among young heroin injectors. Substance Abuse and Rehabilitation 2011, 2, 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Principles of Drug Addiction Treatment: A Research-Based Guide, Third Edition. NIH National Institute on Drug Abuse; U.S. Department of Health and Human Services; 2012. [Google Scholar]
- 5.Schlosburg JE; Vendruscolo LF; Bremer PT; Lockner JW; Wade CL; Nunes AAK; Stowe GN; Edwards S; Janda KD; Koob GF, Dynamic vaccine blocks relapse to compulsive intake of heroin. Proc. Natl. Acad. Sci. 2013, 110 (22), 9036–9041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sulima A; Jalah R; Antoline JFG; Torres OB; Imler GH; Deschamps JR; Beck Z; Alving CR; Jacobson AE; Rice KC; Matyas GR, A Stable Heroin Analogue That Can Serve as a Vaccine Hapten to Induce Antibodies That Block the Effects of Heroin and Its Metabolites in Rodents and That Cross-React Immunologically with Related Drugs of Abuse. Journal of Medicinal Chemistry 2018, 61 (1), 329–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimishima A; Wenthur CJ; Zhou B; Janda KD, An Advance in Prescription Opioid Vaccines: Overdose Mortality Reduction and Extraordinary Alteration of Drug Half-Life. ACS Chemical Biology 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bremer PT; Kimishima A; Schlosburg JE; Zhou B; Collins KC; Janda KD, Combatting Synthetic Designer Opioids: A Conjugate Vaccine Ablates Lethal Doses of Fentanyl Class Drugs. Angew. Chem. Int. Ed. Engl. 2016, 55(11), 3772–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pravetoni M; Pentel PR; Potter DN; Chartoff EH; Tally L; LeSage MG, Effects of an oxycodone conjugate vaccine on oxycodone self-administration and oxycodone-induced brain gene expression in rats. PLoS One 2014, 9 (7), e101807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohia-Nwoko O; Kosten TA; Haile CN, Animal Models and the Development of Vaccines to Treat Substance Use Disorders. Int Rev Neurobiol 2016, 126, 263–91. [DOI] [PubMed] [Google Scholar]
- 11.Kosten TR; Domingo CB; Shorter D; Orson F; Green C; Somoza E; Sekerka R; Levin FR; Mariani JJ; Stitzer M; Tompkins DA; Rotrosen J; Thakkar V; Smoak B; Kampman K, Vaccine for cocaine dependence: A randomized double-blind placebo-controlled efficacy trial. Drug and Alcohol Dependence 2014, 140, 42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoogsteder PHJ; Kotz D; van Spiegel PI; Viechtbauer W; van Schayck OCP, Efficacy of the nicotine vaccine 3′-AmNic-rEPA (NicVAX) co-administered with varenicline and counselling for smoking cessation: a randomized placebo-controlled trial. Addiction 2014, 109 (8), 1252–1259. [DOI] [PubMed] [Google Scholar]
- 13.Lockner JW; Eubanks LM; Choi JL; Lively JM; Schlosburg JE; Collins KC; Globisch D; Rosenfeld-Gunn RJ; Wilson IA; Janda KD, Flagellin as carrier and adjuvant in cocaine vaccine development. Mol Pharm 2015, 12 (2), 653–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brimijoin S; Shen X; Orson F; Kosten T, Prospects, promise and problems on the road to effective vaccines and related therapies for substance abuse. Expert Rev Vaccines 2013, 12 (3), 323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Binder RJ, Heat-shock protein-based vaccines for cancer and infectious disease. Expert Review of Vaccines 2008, 7 (3), 383–393. [DOI] [PubMed] [Google Scholar]
- 16.Motta A; Schmitz C; Rodrigues L; Ribeiro F; Teixeira C; Detanico T; Bonan C; Zwickey H; Bonorino C, Mycobacterium tuberculosis heat-shock protein 70 impairs maturation of dendritic cells from bone marrow precursors, induces interleukin-10 production and inhibits T-cell proliferation in vitro. Immunology 2007, 121 (4), 462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzue K; Young RA, Heat shock proteins as immunological carriers and vaccines In Stress-Inducible Cellular Responses, Feige U; Yahara I; Morimoto RI; Folia BS, Eds. Birkhäuser Basel: Basel, 1996; pp 451–465. [DOI] [PubMed] [Google Scholar]
- 18.Stocki P; Wang XN; Dickinson AM, Inducible heat shock protein 70 reduces T cell responses and stimulatory capacity of monocyte-derived dendritic cells. J Biol Chem 2012, 287 (15), 12387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Wijk F; Prakken B, Heat shock proteins: Darwinistic immune modulation on dangerous grounds. J Leukoc Biol 2010, 88 (3), 431–4. [DOI] [PubMed] [Google Scholar]
- 20.Bendz H; Ruhland SC; Pandya MJ; Hainzl O; Riegelsberger S; Braüchle C; Mayer MP; Buchner J; Issels RD; Noessner E, Human Heat Shock Protein 70 Enhances Tumor Antigen Presentation through Complex Formation and Intracellular Antigen Delivery without Innate Immune Signaling. Journal of Biological Chemistry 2007, 282 (43), 31688–31702. [DOI] [PubMed] [Google Scholar]
- 21.Haug M; Dannecker L; Schepp CP; Kwok WW; Wernet D; Buckner JH; Kalbacher H; Dannecker GE; Holzer U, The heat shock protein Hsp70 enhances antigen-specific proliferation of human CD4+ memory T cells. European Journal of Immunology 2005, 35 (11), 3163–3172. [DOI] [PubMed] [Google Scholar]
- 22.Johnson AG; Tomai M; Solem L; Beck L; Ribi E, Characterization of a Nontoxic Monophosphoryl Lipid A. Reviews of Infectious Diseases 1987, 9 (Supplement_5), S512–S516. [DOI] [PubMed] [Google Scholar]
- 23.Collins KC; Schlosburg JE; Lockner JW; Bremer PT; Ellis BA; Janda KD, Lipid tucaresol as an adjuvant for methamphetamine vaccine development. Chem Commun (Camb) 2014, 50 (31), 4079–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehlert A; Young DB, Biochemical and antigenic characterization of the Mycobacterium tuberculosis 71 kD antigen, a member of the 70 kD heat-shock protein family. Molecular Microbiology 1989, 3(2), 125–130. [DOI] [PubMed] [Google Scholar]
- 25.Takemoto S; Nishikawa M; Guan X; Ohno Y; Yata T; Takakura Y, Enhanced Generation of Cytotoxic T Lymphocytes by Heat Shock Protein 70 Fusion Proteins Harboring Both CD8+ T Cell and CD4+ T Cell Epitopes. Molecular Pharmaceutics 2010, 7 (5), 1715–1723. [DOI] [PubMed] [Google Scholar]
- 26.Ko S-K; Kim J; Na Deuk C.; Park S; Park S-H; Hyun Ji Y.; Baek K-H; Kim Nam D.; Kim N-K; Park Young N.; Song K; Shin I, A Small Molecule Inhibitor of ATPase Activity of HSP70 Induces Apoptosis and Has Antitumor Activities. Chemistry & Biology 2015, 22 (3), 391–403. [DOI] [PubMed] [Google Scholar]
- 27.Bremer PT; Schlosburg JE; Lively JM; Janda KD, Injection route and TLR9 agonist addition significantly impact heroin vaccine efficacy. Mol. Pharm. 2014, 11 (3), 1075–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karinen R; Andersen JM; Ripel Å; Hasvold I; Hopen AB; Mørland J; Christophersen AS, Determination of heroin and its main metabolites in small sample volumes of whole blood and brain tissue by reversed-phase liquid chromatography-tandem mass spectrometry. Journal of analytical toxicology 2009, 33 (7), 345–350. [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Buesa P; Pfund C; Craig EA, The biochemical properties of the ATPase activity of a 70-kDa heat shock protein (Hsp70) are governed by the C-terminal domains. Proceedings of the National Academy of Sciences of the United States of America 1998, 95 (26), 15253–15258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hutchison S; Benson RA; Gibson VB; Pollock AH; Garside P; Brewer JM, Antigen depot is not required for alum adjuvanticity. The FASEB Journal 2012, 26 (3), 1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stowe GN; Vendruscolo LF; Edwards S; Schlosburg JE; Misra KK; Schulteis G; Mayorov AV; Zakhari JS; Koob GF; Janda KD, A vaccine strategy that induces protective immunity against heroin. J. Med. Chem. 2011, 54 (14), 5195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shima F; Uto T; Akagi T; Akashi M, Synergistic stimulation of antigen presenting cells via TLR by combining CpG ODN and poly(gamma-glutamic acid)-based nanoparticles as vaccine adjuvants. Bioconjug Chem 2013, 24 (6), 926–33. [DOI] [PubMed] [Google Scholar]
- 33.Moyle PM; Toth I, Modern subunit vaccines: development, components, and research opportunities. ChemMedChem 2013, 8(3), 360–76. [DOI] [PubMed] [Google Scholar]
- 34.Barrios C; Georgopoulos C; Lambert PH; Del Giudice G, Heat shock proteins as carrier molecules: in vivo helper effect mediated by Escherichia coli GroEL and DnaK proteins requires cross-Iinking with antigen. Clinical & Experimental Immunology 1994, 98 (2), 229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tobian AAR; Canaday DH; Harding CV, Bacterial Heat Shock Proteins Enhance Class II MHC Antigen Processing and Presentation of Chaperoned Peptides to CD4+ T Cells. The Journal of Immunology 2004, 173 (8), 5130–5137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.