Abstract
Despite availability of effective drugs for hypertension therapy, significant numbers of hypertensive patients fail to achieve recommended blood pressure (BP) levels while on ≥3 antihypertensive drugs of different classes. These individuals have a high prevalence of adverse cardiovascular events and are defined as having resistant hypertension (RHT) although non-adherence to prescribed antihypertensive medications is common in patients with apparent RHT. Furthermore, apparent and true RHT often display increased sympathetic activity. Based on these findings, technology was developed to treat RHT by suppressing sympathetic activity with electrical stimulation of the carotid baroreflex and catheter-based renal denervation (RDN). Over the last 15 years, experimental and clinical studies have provided better understanding of the physiological mechanisms that account for BP lowering with baroreflex activation (BA) and RDN and, in so doing, have provided insight into which patients in this heterogeneous hypertensive population are most likely to respond favorably to these device-based therapies. Experimental studies have also played a role in modifying device technology after early clinical trials failed to meet key endpoints for safety and efficacy. At the same time, these studies have exposed potential differences between BA and RDN and common challenges that will likely impact antihypertensive treatment and clinical outcomes in patients with RHT. In this review, we emphasize physiological studies that provide mechanistic insights into BP lowering with BA and RDN in the context of progression of clinical studies, which are now at a critical point in determining their fate in RHT management.
Subject Terms: Hypertension, Treatment
Keywords: Blood pressure, sympathetic nervous system, baroreceptors, kidney, angiotensin, clinical trials
INTRODUCTION
Based on the Scientific Statement from the American Heart Association (AHA) published in 2008, resistant hypertension (RHT) is defined as blood pressure (BP) that remains above goal in patients despite concomitant use of ≥3 different classes of antihypertensive drugs, administered at maximally tolerated doses, and including a diuretic.1 A more recent statement and revised version of this definition from the AHA requires excluding inaccurate BP measurement, white-coat-effect, and medication non-adherence in documenting patients with true RHT.2 Of particular significance, patients with RHT are at high risk for having major cardiovascular events.
Non-adherence to prescribed antihypertensive medications is difficult to detect in clinical practice and is a common cause of suboptimal BP control in many patients with apparent RHT. Tomaszewski et al3, using high performance liquid chromatography-tandem mass spectrometry urine analysis to assess antihypertensive drug intake use, reported that 25% of patients were totally or partially non-adherent to their prescribed antihypertensive treatments. The highest prevalence of non-adherence was observed among follow-up patients with inadequate BP control and those referred for consideration of renal denervation (RDN). Other studies using urine analysis have shown even higher (~50%) non-adherence to prescribed antihypertensive medications.4 Incomplete adherence is much more common than complete non-adherence and low adherence appears to be the most common cause of suboptimal BP control in patients with apparent RHT, being twice as frequent as secondary causes of HT.4 de Jager et al5 found that medication compliance, assessed by measured blood levels of prescribed drugs, was especially poor (up to 80% non-adherence) when patients diagnosed with apparent RHT were unaware of monitoring. However, Gupta et al6 reported in a retrospective study of patients in the United Kingdom that most non-adherent patients with apparent RHT were converted to full adherence with good BP control when they were repeatedly screened. Although it is unclear whether these findings can be applied to other populations, it seems likely that BP in many patients with pseudo RHT can be adequately controlled with proper medication prescription and adherence.
Nevertheless, there are significant numbers of patients with HT who cannot be convinced to adhere to prescribed medications or whose BP is not adequately controlled despite adhering to appropriate pharmacological therapy. Because many patients in this heterogeneous population have high sympathetic activity1, 2, 7, 8, there has been considerable interest in the possibility that RHT may be controlled by non-pharmacological neuromodulation. Over the last 15 years substantial attention has been given to the findings from two device-based approaches for treating RHT by chronically suppressing sympathetic activity. The feasibility of controlling HT in humans by devices that modulate sympathetic activity emerged in early 2000 as technology was developed for electrical activation of the carotid baroreflex and catheter-based renal nerve ablation. Both approaches are strongly supported by experimental studies and early clinical trials. However, as clinical trials evolved, critical endpoints for efficacy and safety were not achieved, necessitating modifications in device technology and corrections of deficiencies in trial design. In this review we emphasize experimental studies that provide insights into BP lowering by baroreflex activation (BA) and RDN, and address relevant past and current clinical trials. Both device-based approaches are currently being evaluated for safety and efficacy in critical clinic trials that will determine their fate as viable options for HT therapy when used as a single entity or in combination with antihypertensive medications.
BARORECEPTOR ACTIVATION FOR HYPERTENSION
Carotid Baroreceptor Stimulation with Modern Technology
The idea of treating HT by BA therapy is not new. Clinical studies conducted in the 1960’s-and early 1970’s in patients with severe HT inadequately controlled by medications showed lowering of BP during stimulation of electrodes wrapped around the carotid sinus nerve.9–11 However, the technology was too crude to achieve reliable sustained BA and chronic antihypertensive responses, particularly in the absence of adverse effects. Consequently, controlled clinical trials were not possible. Thus, this device-based approach for treatment of HT was abandoned by mid-1970s owing to technical limitations and the beginning of an era of antihypertensive drug development.
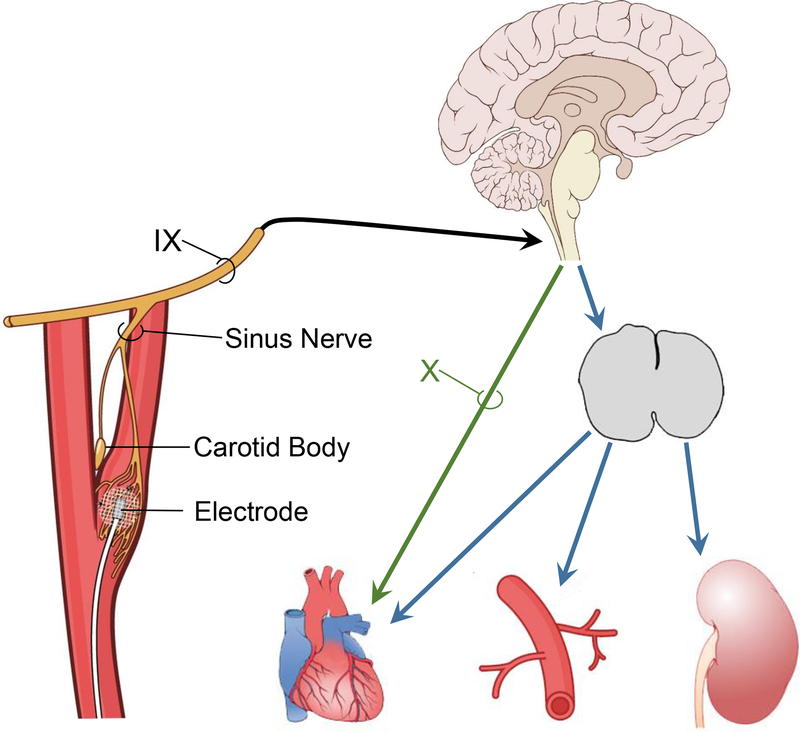
Despite the emergence of tolerable and effective drugs for HT therapy, non-pharmacological control of HT by prolonged activation of the carotid baroreflex was revitalized just after the turn of this century. BA therapy was made possible by technological advances by CVRx, Inc.9–15 The impetus for this device-based approach for HT therapy was and remains patients with RHT. The first-generation system developed by CVRx (Rheos system) addressed several major limitations of the earlier approaches for BA therapy. The Rheos system for carotid BA achieves bilateral electrical field stimulation of the carotid sinus wall with electrodes placed around the carotid sinuses, rather than stimulation of the carotid sinus nerve. The second generation Barostim neo system is based on unilateral carotid sinus stimulation with a smaller disc-shaped electrode sutured to the surface of the carotid sinus (Figure 1).10–15 With either system, there are no measurable sympathoexcitatory and ventilatory responses from co-activation of neighboring carotid body chemoreceptors.16, 17 Electrodes are connected to a pulse generator implanted subcutaneously in a pocket in the anterior chest wall. The implanted pulse generator (IPG) is externally controlled and allows controlled current delivery throughout the day and dose-dependent BP lowering. To date, most of the published findings from experimental and clinical studies have been obtained from studies using the first generation system.
Figure 1.
With the Barostim neo system, a miniaturized stimulating electrode is sutured to the surface of the carotid sinus and is connected to an internally implantable pulse generator that is externally programmable by radiofrequency control. Electrical stimulation of the carotid sinus increases carotid baroreceptor afferent nerve activity to the brain leading to long-term reductions in central sympathetic outflow and increases in cardiac parasympathetic activity. There is no activation of neighboring carotid body chemoreceptors during baroreflex activation (BA). IX, glossopharyngeal nerve; X, vagus nerve. (Redrawn from T.E. Lohmeier and R. Iliescu. Physiology. 2015;30:148–158).
Published clinical trials using BA therapy have typically emphasized antihypertensive responses and safety issues, with little attention given to the actual mechanisms that may account for BP lowering. Regrettably, this is a missed opportunity because the technology developed by CVRx provides a unique tool for better understanding baroreflex physiology, especially the role of arterial baroreflexes in long-term control of BP. Indeed, the findings from early preclinical research played a key role in providing rationale for conducting the first clinical trials using BA therapy. Furthermore, over the last 15 years mechanistic studies in chronically instrumented dogs have continued to influence clinical investigation and, by elucidating mechanisms for BP lowering, have provided insight into which patients are most likely to respond with a favorable antihypertensive response to BA. These experimental studies will be presented in chronological order to coincide with advancement of knowledge provided by clinical trials. Mechanistic insights from experimental studies will be emphasized and less attention will be devoted to an in depth discussion of the clinical trials, which have been reviewed extensively.10–15
Preclinical Studies with the Rheos System
Chronic Electrical Stimulation of the Carotid Baroreflex Causes Sustained Reductions in Sympathetic Activity and BP.
Although it is well established that the baroreflex plays a key role in the acute regulation of BP, the general consensus before the turn of the 21st century was that arterial baroreflexes reset in the direction of the prevailing level of BP and therefore are not important in long-term BP control.18 Most of the evidence in support of this view was based on baroreceptor afferent nerve recordings in various acute preparations designed to mimic physiological BP-baroreceptor interactions. These acute studies demonstrated that changes in baroreceptor activity decrease substantially during sustained BP-induced alterations in vascular stretch, supporting the concept of mechanoreceptor adaptation. Because of technical limitations precluding assessment of baroreflex function in response to long-term changes in BP, these findings have been extrapolated to suggest the unimportance of baroreflexes in the long-term control of sympathetic activity and BP.
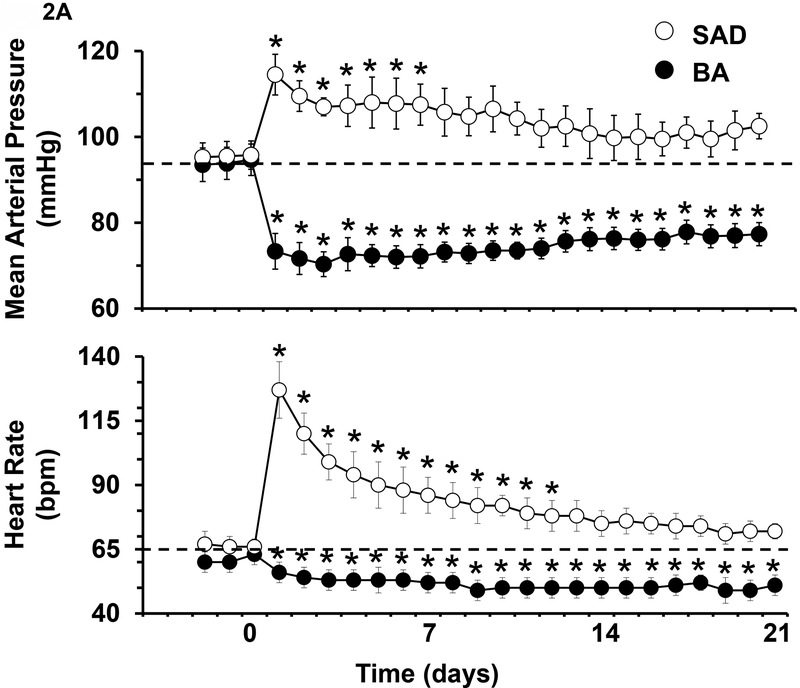
In addition to acute investigations, a key chronic study interpreted to discount the importance of the baroreflex in long-term control of sympathetic activity and BP was based on the BP response to sinoaortic denervation (SAD). As illustrated in Figure 2A, the rise in BP after severing baroreceptor afferents to the brain is largely transient, a response that has been attributed to central neuroplasticity. However, it is unclear from SAD studies if central resetting is a potent mechanism that diminishes changes in sympathetic activity during natural alterations in baroreceptor afferent input. If so, minimal suppression of sympathetic activity and lowering of BP would be expected in response to chronic electrical stimulation of baroreceptor afferents. On the other hand, if resetting is primarily due to mechanical-electrical adaptations that occur at the baroreceptors, then BA may have appreciable sustained sympathoinhibitory effects by directly stimulating baroreceptor afferent fibers.
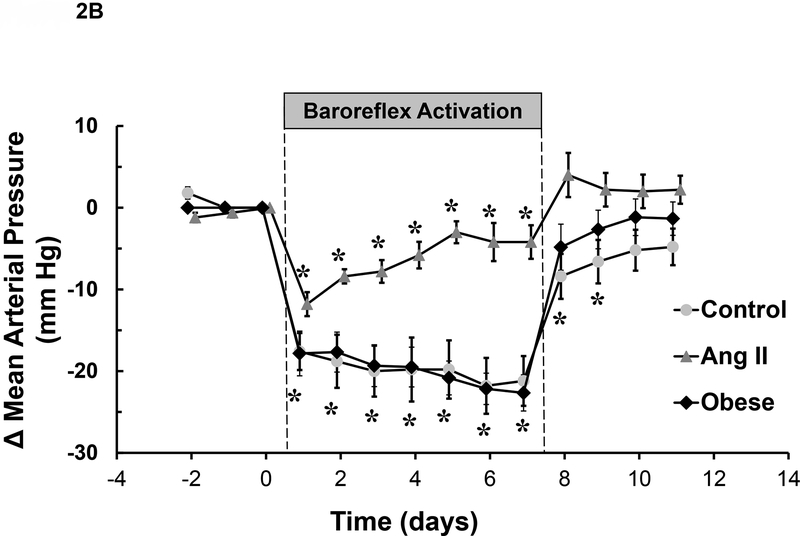
Figure 2.
A. Changes in mean arterial pressure and heart rate after sinoaortic denervation (SAD) and during BA in dogs. Values are means ± SE. P < 0.05 vs. control. (From T.E. Lohmeier et al. Am J Physiol Heart Circ Physiol. 2010;299:H402-H409). B. Changes in mean arterial pressure during BA in dogs before (control) and after induction of ANG II HT and obesity HT. Values are means ± SE. P < 0.05 vs. control. P < 0.05 vs. control. Values for mean arterial pressure before BA: Control=93±1 mmHg, ANG II HT=129±3 mmHg, and obesity HT=110±3 mmHg. (From T. E. Lohmeier et al. Hypertension. 2007;49:1307–1314).
The first long-term preclinical study using the Rheos system was designed to critically evaluate the time-dependent cardiovascular responses to 7 days of bilateral, electrical stimulation of the carotid sinuses in normotensive dogs. BP and heart rate (HR) were recorded continuously, 24h/day, and neurohormonal measurements were made on selected days.19 During BA, the IPG was programed to deliver continuous impulses throughout the cardiac cycle at a frequency that likely activated both myelinated and unmyelinated baroreceptor afferent fibers. The intensity of activation was adjusted to decrease BP ~ 20 mmHg during the first 24 hours of carotid sinus stimulation with no further adjustments in stimulation parameters thereafter. A most impressive and novel finding in this study was that this initial level of BP reduction was sustained throughout the 7 days of BA with little or no time-dependent attenuation in BP lowering. HR decreased in parallel with the fall in BP and was reduced 13 bpm on day 7 of BA. Both BP (e.g., see Figure 2B) and HR increased sharply immediately after termination of BA and by day 4 of the recovery period there was complete restoration to control levels. Along with suppression of BP and HR there were sustained reductions in plasma norepinephrine (NE) concentration throughout 7 days of BA. These responses suggest that by bypassing the normal pressure-encoding step of the baroreceptor, direct electrical stimulation of the afferent limb of carotid baroreflex has potent sustained effects to suppress sympathetic and increase parasympathetic activity. Another notable response was that there were no changes in plasma renin activity (PRA) during BA, despite the pronounced fall in BP. The rapid on- off- transient BP responses, the ease of control, and the ability to achieve graded reductions in BP are additional desirable features of this instrumentation. A final important point was that there were no extraneous stimulation of neighboring muscles and nerves and, although not critically assessed, no effects on respiration.
After first determining the time course of central resetting after SAD (Figure 2A), a follow up study was designed to more critically assess the temporal changes in sympathetic activity, BP, and HR in response to 3 weeks of BA.20 Based on the temporal BP response to SAD, it was surmised that 3 weeks would be sufficient time to allow for full expression of any slowly developing central adaptations that may counteract suppression of sympathetic activity and BP in response to BA. Figure 2A shows substantial differences in the magnitude of the time dependent changes in BP and HR in response to SAD and BA. In marked contrast to SAD, the day 1 reductions in BP and HR were largely sustained throughout the 3 weeks of BA with little or no time-dependent reduction in the magnitude of these responses. Furthermore, as reflected by sustained reductions in plasma NE concentration and whole body plasma NE spillover (to 55% of baseline), the latter an index of centrally generated sympathetic outflow, suppression of sympathetic activity persisted throughout chronic BA. Thus, in contrast to the perspective presented with SAD, which eliminates baroreceptor afferent input into the brain, this study shows that when baroreceptor afferent fibers are intact, central neuroplasticity does not attenuate the initial sympathoinhibition and lowering of BP and HR induced by electrical activation of the carotid baroreflex.
Chronic Electrical Stimulation of the Carotid Baroreflex Abolishes Obesity HT but not Angiotensin II HT.
The above studies clearly demonstrated robust and sustained suppression of sympathetic activity and BP in normotensive dogs during chronic BA. A critical next step relevant to clinical investigation was to determine whether BA also lowers BP in experimental models of HT. To this end, the antihypertensive effects of BA were evaluated in dogs with obesity and angiotensin II (ANG II)-mediated HT, the former associated with high and the latter with low basal sympathetic activity.18, 21, 22 These preclinical studies were conducted at approximately the same time and under similar conditions.
Many subjects with RHT are obese and obesity-related HT has an important neurogenic component.1, 2, 7, 8, 21, 23, 24 Therefore, consideration was given to the possibility that BA may diminish sympathoexcitation and attenuate HT in obese dogs fed a high fat diet, an experimental model that closely mimics the hemodynamic, neurohormonal, renal, and metabolic changes associated with obesity in humans.21 After 4 weeks of feeding dogs a high-fat diet, BP increased ~15 mmHg in association with a 50% increase in weight gain.25 HR also increased substantially, and there were sustained increases in plasma NE concentration. Most significantly, during week 5 of the high-fat diet, BA completely abolished the HT (Figure 2B) concomitant with suppression of plasma NE concentration to lean control levels. The tachycardia was also substantially attenuated. As in normotensive dogs, a striking observation was that PRA did not increase despite the marked fall in BP. Thus, this study showed that BA has a powerful effect to counteract the sympathetic-mediated HT and tachycardia associated with obesity related HT. Based on these observations, it was surmised that had renal sympathoinhibition not offset the stimulation of renin secretion normally attendant with a fall in BP of this magnitude,26 the antinatriuretic effects of increased circulating ANG II would have diminished the antihypertensive response to BA. This hypothesis was tested in a model of HT induced by chronic infusion of ANG II.
The chronic antihypertensive response to BA was vastly different in ANG II HT when compared to obesity-induced HT.25, 27 Following determination of the BP responses to BA under control conditions, identical stimulation parameters were used in the same dogs to activate the baroreflex after induction of ANG II HT (Figure 2B). After 7 days of ANG II infusion at a rate reported to increase plasma levels of the peptide to 3–5 times normal, BP increased 36 mmHg along with a 3-fold increase in plasma aldosterone concentration. As in the normotensive state and in obesity HT, acute BP reductions in response to BA were substantial, likely reflecting the effects of decreased sympathetic activity on peripheral vascular resistance, venous capacitance and cardiac output. However, in contrast to the impressive sustained antihypertensive response in obesity HT, the initial acute fall in BP waned appreciably during prolonged BA in ANG II HT, resulting in only small reductions in BP (Figure 2B).
Because long-term regulation of BP is closely linked to volume homeostasis through pressure natriuresis, the small chronic antihypertensive response to BA suggests that baroreflex-mediated sympathoinhibition had minimal effects to enhance renal excretory function in the presence of high circulating level of the potent sodium-retaining hormones, ANG II and aldosterone. Accordingly, inhibition of the renin-angiotensin-aldosterone system (RAAS) may be a prerequisite for a robust antihypertensive response to BA. However, another consideration is that the pre-existing level of sympathetic activity, which is suppressed in ANG II HT, may also be an important determinant of BP lowering during BA. More specifically, because sympathetic activity is suppressed in this model of HT, further suppression of sympathetic activity by BA would be expected to have little effect to lower BP. The design of this study did not allow differentiation between these two possibilities.
In summary, the marked antihypertensive response to BA in dogs with obesity HT provided sound rational for pursuing clinical trials using the Rheos system for HT therapy in patients with RHT, who are commonly obese. On the other hand, the impaired BP response to BA in the ANG II model of HT indicated that a robust antihypertensive response to BA is not a universal response in all forms of HT. These early preclinical studies also indicate that interactions with the (RAAS) may critically influence BP lowering during BA.
Clinical Studies with the Rheos System
DEBUT-HT Trial and Subgroup Studies.
The DEBuT-HT European trial was a multicenter, non-randomized feasibility study with end points of BP reduction and safety at 3 months post-surgery in 45 patients with RHT systolic BP (SBP) ≥ 160/diastolic BP (DBP) ≥ 90 mmHg.28 Medications were kept constant before and over the 3 months of the trial, but medication adherence was not critically assessed. After 3 months of therapy, there were statistically significant reductions in SBP (−21 mmHg) and HR (−8 bpm). In a cohort that consented to an extended follow-up phase, bradycardia persisted and reductions in SBP exceeded 30 mmHg after 1 and 2 years of therapy.28 At each visit, the device was temporarily turned off to assess BP without activation. At that time, BP increased rapidly toward baseline levels, confirming the sustained antihypertensive effects of BAT and demonstrating the rapid off transient response to deactivation. Shortly thereafter, the Phase III Rheos Pivotal trial was launched (see below).
Buffering of beat-to-beat fluctuations in BP is achieved by dynamic alterations in central input from arterial baroreceptors and compensatory changes in sympathetic and parasympathetic activity. In contrast to pulse-synchronous baroreceptor discharge, the Rheos system delivers continuous non-pulsatile electrical impulses to carotid sinus afferent fibers, which could conceivably disrupt normal physiological function. On the contrary, clinical and experimental studies found just the opposite. In a sub-study of 21 subjects from the DEBut-HT trial, HR and HR variability (HRV) were analyzed using 24-ECG monitoring before and after 3 months of BA therapy.29 Along with decreasing BP, BA therapy lowered HR and actually increased HRV. Frequency-domain analysis suggested improved HRV was associated with increased parasympathetic and decreased sympathetic activity. An experimental study published that same year showed similar findings. In normotensive dogs subjected to 3 weeks of BA, levels of activation producing reductions in BP and HR of 20 mmHg and 15 bpm, respectively, were associated with substantial increases in both cardiac baroreflex sensitivity and HRV.20 Thus, these studies show that chronic BA therapy does not interfere with, but actually enhances, cardiac baroreflex regulation. This may have significance in diminishing the likelihood of arrhythmias in pathophysiological states (see obesity HT below).
Another clinical study focused on cardiac structure and function in 12 patients from feasibility studies.30 After 12 months of BA therapy, the antihypertensive effects of BA were associated were significant improvements in left atrial dimensions and left ventricular mass. Taken together, these studies suggest that BA therapy may have cardioprotective effects.
In another subgroup of 12 patients enrolled in the DEBut-HT study, further insight into neural regulation of BP was achieved using microneurography to determine muscle sympathetic nerve activity (MSNA) during 9 minutes of BA.31 Measurements were made one month after implantation of the Rheos system and before chronic activation. In response to BA, immediate and sharp reductions in MSNA and BP occurred in parallel strongly suggesting that BP lowering can indeed be attributed to suppression of central sympathetic outflow. These acute findings are consistent with measurements showing marked suppression of whole body NE spillover during chronic BA in canines.20
Rheos Pivotal Trial.
Shortly after the encouraging findings from the DEBut-HT study, the U.S. Rheos Pivotal trial was initiated.32 This trial was a randomized, controlled, double-blind trial with end points of safety and BP reduction at 1 year in subjects with RHT. Subjects were implanted with the Rheos system and subsequently 265 patients were randomized 2:1 one month after surgery. Subjects then received BA the first 6 months (immediate BA) or BA was delayed for the first 6 months of the trial (delayed BA). Five co-primary endpoints were established: 1) acute SBP responder rate at 6 months, 2) sustained responder rate at 12 months, 3) procedure safety, 4) BA safety, and 5) device safety. Modification of antihypertensive drugs was allowed during the study, and there was no objective evaluation of drug adherence.
For the immediate BA group, SBP was reduced by 16 mmHg at 6 months and 27 mmHg at 12 months when compared to the 1 month post implant values before activation. For the delayed BA group, reductions in SBP were 9 and 25 mmHg at 6 and 12 months, respectively. The trial was successful in meeting the pre-specified sustained 12 month efficacy endpoint. However, the acute 6 month primary efficacy end point was missed. The failure to meet the pre-specified acute efficacy endpoint was apparently due primarily to a larger and more variable reduction than expected in SBP at 6 months in the group with the inactive implants and likely reflected the less-than-optimal trial design. Beyond efficacy considerations, the pre-specified end point for procedural safety was not met with 9% of patients developing transient or permanent nerve injury and 5% having general surgical complications. However, the majority of the transient adverse events resolved. In contrast to procedural safety, the pre-specified criteria for BA and device safety were exceeded. Thus, the trial did not meet two of the five pre-specified co-primary end points: short-term efficacy and short-term safety. Consequently, CVRx did not receive FDA approval for the use of the Rheos system in subjects with RHT.
After completion of the DEBut-HT and Rheos Pivotal studies, trial participants were enrolled in separate open label, observational follow-up studies. Follow-up findings were reported after patients had completed 2–4 and 5–6 years of therapy.33, 34 In both follow-up studies, the pressure reductions reported in the initial trials were sustained or even enhanced.
Although all patients in the Rheos trial received bilateral implants, the majority of subjects had their devices programed for unilateral activation.35 During regular office visits, the decision to activate either unilaterally or bilaterally was based on acute dose-response tests for BP lowering while adjusting stimulation parameters. To optimize battery longevity, unilateral stimulation was chosen in 75% of the subjects because the acute fall in BP was not greater with bilateral stimulation. Most importantly, the Rheos-HT study showed that unilateral carotid sinus stimulation was sufficient to produce appreciable and sustained reductions in BP in patients with RHT.
In summary, based on the findings from the Rheos Pivotal Trial suggesting efficacy but at the same time recognizing potential concerns with procedural safety, CVRx developed a second generation miniaturized device for unilateral stimulation, the Barostim neo.10–15 Rather than having electrodes wrapped around both carotid sinuses, a smaller disc-shaped electrode implanted on the surface of one sinus decreases the invasiveness of the operation procedure, operation time, and adverse surgical procedure events during implantation (Figure 1). Additionally, unilateral versus bilateral electrode stimulation requires less electrical current for BP reduction, extends battery life, and extends the period required for battery replacement to approximately 3 years. With improved trial design, the Barostim neo trial began in 201136 (see below).
Further Mechanistic Insights from Experimental and Clinical Studies Using the Rheos System
While the DEBut-HT feasibility trial was in progress, findings from two experimental studies were totally unexpected. Both studies addressed potential mechanisms whereby BA chronically lowers BP.
Renal Nerves are not Essential for Chronic BP Lowering During BA.
There is substantial evidence that alterations in renal sympathetic nerve activity (RSNA) have sustained effects on sodium excretion and BP. Further, although RSNA has not been measured during chronic electrical stimulation of the carotid baroreflex, experimental studies in chronically instrumented dogs have clearly demonstrated that natural activation of the baroreflex in HT has sustained effects to suppress RSNA and promote sodium excretion.18, 22 Therefore, it was expected that RDN would attenuate increases in renal excretory function and, therefore, long-term reductions in BP during BA. Remarkably, this hypothesis was not confirmed in an experimental study conducted in dogs.37 During 7 days of BA, reductions in BP, HR, and plasma NE concentration were comparable in the same dogs before and after bilateral surgical renal denervation.
Preliminary observations in normotensive dogs indicate that lowering of BP and HR during BA is associated with a 2–3 fold increase in plasma atrial natriuretic peptide (ANP).37 Given sustained bradycardia, increased vagal activity, and suppression of central sympathetic outflow during BA, increases in ANP secretion may be attributed to autonomic effects on cardiac function that lead to increases in atrial pressure. Although there are no empirical data to support this hypothesis, this possibility is consistent with findings from an established mathematical model of human physiology.38 Despite expected suppression of RSNA, computer simulations showed that clamping cardiac autonomic activity at baseline values during BA abolished increases in ANP secretion and appreciably attenuated BP lowering. In contrast, when suppression of RSNA was prevented while leaving cardiac autonomic control intact, BP lowering was not significantly impaired during BA due to inordinate fluid accumulation and further increases in atrial pressure and ANP secretion. Unfortunately, atrial pressures and plasma ANP concentration are not typically measured during BA. Therefore, while the model predictions indicate that this natriuretic hormone contributes importantly to BP lowering during BA, especially after RDN, this must be considered a hypothesis until formally tested in future studies.
Chronic BA Adds to the BP Lowering Effects of Some Antihypertensive Drugs.
Along with the unforeseen findings with RDN, the long-term BP response to BA during adrenergic blockade was equally surprising.39 After 7 days of α1- and β1,2- blockade in normotensive dogs, BP decreased substantially, as expected, in association with increased plasma NE concentration, presumably reflecting increased central sympathetic outflow due to arterial baroreceptor unloading. Moreover, with continued adrenergic blockade, plasma NE concentration fell to control levels during 7 subsequent days of BA, indicating that electrical stimulation of the carotid sinus was sufficient to totally counteract the reflex-induced sympathoexcitation attendant with the fall in BP. Most significantly, there were further reductions in BP during BA. These findings suggest that reflex-induced increases in sympathetic activity attenuate reductions in BP during chronic administration of adrenergic blocking agents and that inhibition of central sympathetic outflow by prolonged BA lowers BP further by previously undefined mechanisms. Despite the mechanistic uncertainty, acute pharmacological experiments in these same dogs suggested that the additional BP lowering during baroreflex suppression of sympathetic activity could be attributed to diminished activation of vascular α2- receptors. Thus, studies during chronic adrenergic blockade and RDN are remarkable in that they show that well-established mechanisms do not exclusively account for BP lowering during BA.
A similar scenario occurred during chronic administration of another commonly used antihypertensive drug, the calcium channel blocker amlodipine.40 Analogous to the response with adrenergic blockade, the fall in BP during chronic amlodipine administration in normotensive dogs was associated with sustained increases in sympathetic activity, as reflected by increases in plasma NE concentration. Most importantly, during concurrent BA with continued amlodipine administration, BP decreased further along with return of plasma NE concentration to control.
Taken together these studies with adrenergic receptor and calcium channel blockade, suggest that the fall in BP with some classes of antihypertensive drugs, including diuretics, is associated with sustained activation of the sympathetic nervous system, presumably because of the natural unloading of arterial baroreceptors. The ability of BA to counteract drug-induced sympathetic activation by suppressing central sympathetic outflow may contribute to the antihypertensive effects of BA in patients with RHT.
Differential and Common Effects of BA and RDN in Obesity HT.
Because of the importance of the kidneys in long-term control of BP, the efficacy of BA in chronically lowering BP may critically depend on the specific renal mechanisms for increasing renal excretory function. Yet, when the findings from the DEBut-HT and the subsequent Rheos Pivotal trial were published, there was little information on changes in renal function during chronic BA therapy. Because most patients with RHT are obese and obesity HT is commonly associated with sympathetic activation that includes increased sympathetic outflow to the kidneys,1, 2, 7, 8, 21, 23, 24 the effects of global and renal-specific sympathoinhibition were investigated in dogs with obesity HT, a clinically relevant model of human obesity HT.21 The impetus for conducting this study was also driven by the novel observations presented just prior to publication of the DEBut-HT and Rheos trials indicating that that catheter-based endovascular radiofrequency ablation of the renal nerves, like BA, has impressive antihypertensive effects in patients with RHT.41
HT and neurohormonal responses.
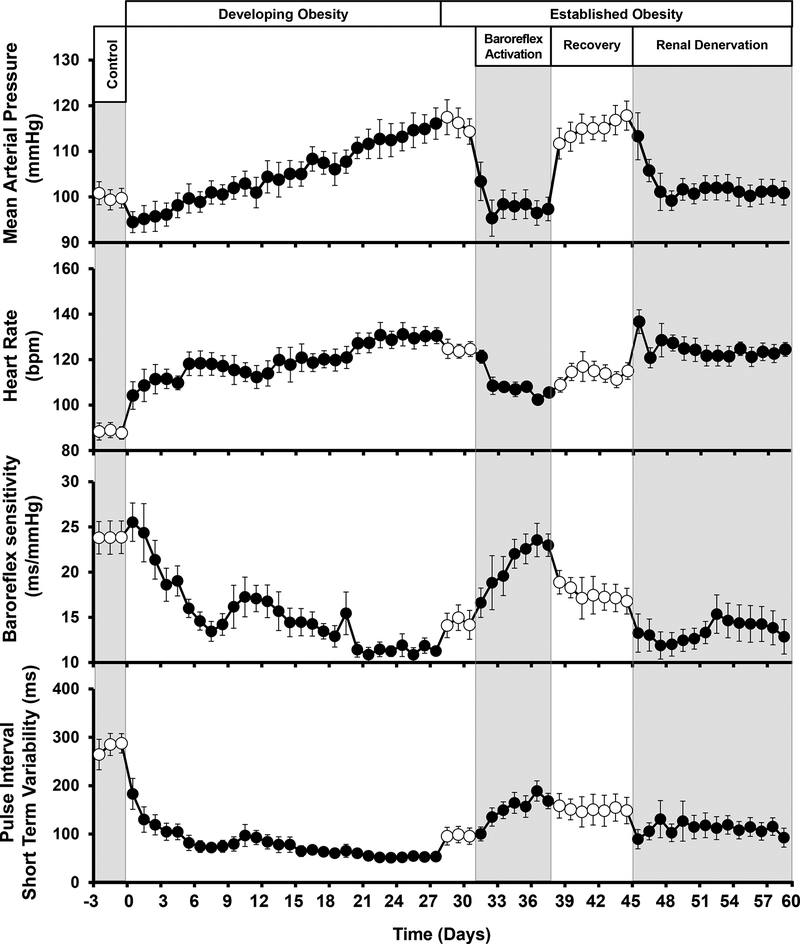
Feeding dogs a high fat diet for 4 weeks led to a progressive increase in body weight and BP until fat intake was reduced thereafter to maintain a target 50% increase in weight gain (Figure 3). HR also increased substantially during development of obesity HT.42 Moreover, as reported previously,25 chronic BA abolished the HT and greatly diminished the tachycardia associated with weight gain (Figure 3). Furthermore, the sustained BP lowering during BA occurred concurrently with significant reductions in plasma NE concentration and PRA. Similarly, RDN abolished the HT (Figure 3) concomitant with suppression of PRA. Taken together, these studies highlight the importance of the renal nerves and neurally-induced renin secretion in the maintenance of obesity HT.
Figure 3.
Changes in mean arterial pressure, heart rate, baroreflex sensitivity and HRV during developing obesity and responses to BA and renal denervation (RDN) after obesity HT was established. Values are means ± SE. During days 1–28, dogs were fed a high fat diet and on day 28 body weight was increased ~ 50%. After day 28, dietary fat was reduced and there were no further changes in body weight for the duration of the study. (Redrawn from R. Iliescu et al. Am J Physiol Cir Physiol. 2013;305:H1080-H1088).
Renal function.
In obese humans, impaired renal excretory function is initially due to increased sodium reabsorption because glomerular filtration rate (GFR) and renal blood flow (RBF) are increased.21, 43 Accordingly, in obese dogs, the HT was associated with a 35% increase in GFR and a reciprocal decrease in fractional sodium excretion.42 Most significantly, the hyperfiltration and reduced fractional sodium excretion of obesity were diminished during BA. Similarly, along with pronounced BP lowering during chronic BA, reductions in GFR of ~ 10% have also been reported in normotensive dogs and in a large cohort of patients included in a follow-up study from the Rheos-HT trial.40, 44 In this follow-up study, substantial BP lowering was sustained between 6 and 12 months of BA therapy without changes in albumin excretion or progressive reductions in GFR from the reduced 6 month values.
Mechanisms that could account for the fall in GFR during BA are the following. First, by reducing the high rate of sodium reabsorption in the proximal tubule and/or the loop of Henle, sites of action of the renal nerves, ANG II, and ANP, increased sodium chloride delivery to the macula densa would favor a tubuloglomerular feedback (TGF) signal to constrict afferent arterioles. This tubular action of BA would likely impair the ability of afferent arterioles to dilate in response to the concurrent fall in BP, resulting in a decrease in GFR attributable to an exaggerated reduction in glomerular pressure. Second, without the normal increase in renin secretion and attendant generation of ANG II induced by the fall in renal perfusion pressure, reduced GFR could also be attributed to compromised constriction of efferent arterioles. Thus, by amplifying reductions in glomerular pressure, the TGF signal leading to constriction of afferent arterioles during BA would add to the clinical benefit of lowering systemic BP in preventing glomerular injury. However, to our knowledge, the impact of BA on TGF has not been directly examined.
HR control.
BA and RDN also had disparate effects on autonomic control of HR.42, 45 Decreases in HRV and cardiac baroreflex sensitivity have been identified as predictors and risk factors for cardiac arrhythmias and mortality in cardiovascular and renal disease, and are common findings in obese individuals.46–52 Increases in HR, and decreases in cardiac baroreflex sensitivity and HRV have been reported in experimental animals and in humans after several weeks of weight gain or in subjects with long-standing obesity.46–48, 52 In accordance with these single time-point determinations made under resting conditions, analyses from continuous 24-hour recordings of spontaneous fluctuations in BP and HR in obese canines indicate that these disturbances in HR control are present during daily activity and throughout the evolution of obesity HT (Figure 3). Furthermore, consistent with previous findings in animals and in human subjects using adrenergic and muscarinic blockers,52, 53 frequency domain analyses indicated that the tachycardia and dysfunction in HR control in obese dogs is primarily due to impairment in parasympathetic activity.42, 45 In this regard, a particularly impressive finding in this study was that BA diminished tachycardia, improved HRV and completely restored cardiac baroreflex sensitivity to control levels measured before weight gain (Figure 3). Frequency domain analyses showed that central effects of BA that counteracted the abnormalities in HR control can be attributed primarily to increased vagal activity and to measured improvement in cardiac baroreflex sensitivity. Another important outcome of this study was that in contrast to BA, RDN did not share these favorable effects on HR control, discounting an influence of afferent signaling from the kidneys on cardiac autonomic activity in obesity.
Thus, these findings suggest that along with decreasing global sympathetic activity and BP, BA may reduce the risk for cardiac arrhythmias, frequently associated with obesity. In the context of long-standing obesity, if reduced mechanical stimulation of baroreceptors due to increased arterial stiffness is a major mechanism that accounts for depressed baroreflex sensitivity, BA would circumvent this disruption in mechanical-electrical coupling by directly stimulating the baroreceptor afferent pathway.
Clinical Studies with the Barostim Neo System
The Barostim Neo Trial.
This trial was a single-arm, open label study that evaluated efficacy and safety of the second generation system for BA in 30 patients with RHT.36 The implant procedure was minimally invasive and required only unilateral suturing of a miniaturized electrode on to the surface of the carotid sinus. The primary efficacy objective of this trial was to describe reductions in office BP through 6 months of BA. The primary safety objective was to describe all procedural and device-related complications during this period of time. Medications were virtually unchanged during the study period, but drug adherence was not critically assessed.
Most significantly, after 3 months of BA, SBP was reduced 26 mmHg. After 6 months of therapy, this fall in BP persisted along with a reduction in HR of ~ 5 bpm. After 6 months of BAT, 43% of the subjects achieved SBPs ≤ 140 mmHg. During the perioperative period 30 days after surgery and before activation, 3 minor complications occurred with no residual effects. Beyond the perioperative period, only one device-related complication was reported. Compared to the Rheos-HT trial, the number of patients who suffered from procedural complications decreased from 25 to 3%. Thus, while long-term device safety was preserved, the procedural safety profile of BA improved substantially relative to that observed in the Rheos Pivotal Trial.
Of particular interest, 6 of the 30 patients enrolled in the Barostim neo trial underwent previous RDN, which was unsuccessful in lowering BP. After 6 months of BA, reductions in SBP and HR were comparable in these patients when compared to the subjects with intact renal innervation.
In summary, in addition to achieving efficacy comparable to that reported in the Rheos-HT trial, the safety concerns raised with the procedural outcomes in the Rheos study were seemingly addressed successfully with the second generation Barostim neo system. Implantation of the Barostim neo device was less invasive, required less surgical time, and the unilateral implant prolonged battery life. Despite the impressive results of the single arm, open label Barostim neo trial, application of the second generation device for RHT therapy in the U.S. will depend on the outcome of the randomized, multicenter Barostim neo HTN Pivotal trial (NCT01679132) in the U.S. Of note, separate randomized, double blind studies of BA in patients with RHT have recently begun in clinical centers in France (NCT02364310) and in Nordic countries.54
Uncontrolled European Clinical Trials with the Barostim neo System.
After CVRx received European CE marketing in 2011 for the use of the Barostim neo system to treat uncontrolled HT, there was a flurry of observational clinical trials evaluating efficacy of this device in patients with RHT. In general, these studies have been uncontrolled and of small sample size. Nonetheless, the findings from these studies are consistent in that they show appreciable lowering of BP in patients with RHT after no less than 6–12 months of therapy.55–60 Acute off transient increases in BP in response to brief device deactivation in patients with chronic BA confirm true BP lowering with the Barostim neo system. In addition, these studies have included patients with prior RDN and protracted HT.55, 56
Studies have also reported favorable effects on BP and renal function in patients with chronic kidney disease (CKD),55, 56 subjects usually excluded from BA trials for fear of worsening renal injury because of reductions in renal perfusion pressure. These latter studies with the Barostim neo system are consistent with observations made in patients and experimental animals with HT and CKD showing that chronic lowering of BP with the Rheos system does not lead to progressive decreases in renal function.44, 61
Despite favorable BP responses to BA in these studies, a cautionary report by Heusser et al. indicated a potential limitation of the second generation system.57 While subjecting patients to 2 minute periods of on/off stimulation at intensities that were tolerable, BP, HR, and MSNA responses were appreciably smaller with the Barostim neo system when compared to their previous findings during acute bilateral carotid sinus stimulation with the Rheos system.31 When stimulation intensities were reduced further to avoid side effects that may be present during chronic activation, measured responses to acute BA were even smaller. On the basis of these acute responses, it was suggested that side effects attributable to electrical stimulation of tissues surrounding the carotid sinus could limit efficacy of the Barostim neo device. Nevertheless, in this same study, SBP decreased 16 mmHg during chronic BA at stimulation intensities that had minimal or no side effects. Furthermore, in most studies the side effects (e.g. paresthesia, dysphagia) reported in patients during BA at higher intensities typically disappear spontaneously or by re-programming and adjustment of stimulating parameters. Thus, with judicious patient monitoring during routine follow-up visits, it appears that sufficient BP lowering can be achieved with the Barostim neo system with few side effects.
Experimental Studies with the Barostim Neo System
Diminished Antihypertensive Effects of BA and RDN in Aldosterone HT.
After production of the Rheos device ended, two canine studies were completed using the Barostim neo system.17, 62 Appreciable BP lowering was achieved in both studies without the side effects reported in clinical studies using Barostim neo. However, in contrast to unilateral electrode implantation in patients, electrodes were implanted bilaterally in the canine studies to allow for simultaneous stimulation of both carotid sinuses. This was done to be consistent with previous canine studies using the Rheos system. If simultaneous unilateral activation combined to have additive effects on BP lowering, the absence of side effects in the canine studies may be attributed to lower stimulation intensities on each side and reduced current spread to adjacent structures.
Although diagnosed primary aldosteronism is an exclusion condition for eligibility in clinical trials using device-based therapy, the proportion of patients with RHT normally screened for this secondary form of HT is unclear, and the effect of aldosterone excess on clinical outcomes with BA is uncertain. Therefore, studies were conducted to determine the antihypertensive responses to 7 days of BA and RDN in the same dogs after a 2 week infusion of aldosterone that increased MAP 20 mmHg.62 Prior to infusion of aldosterone when these same dogs were normotensive the intensity of BA was such that it decreased MAP 16 mmHg. In addition, the time-dependent lowering of BP, HR, plasma NE concentration and absence of suppression of PRA with the Barostim neo system mimicked these same responses previously reported in normotensive dogs using the Rheos system.19, 20, 27, 37, 40 In contrast, whereas bradycardia and suppression of plasma NE concentration were sustained responses to BA after induction of aldosterone HT, initial sharp reductions in BP were not. That is, the time-dependent changes in blood pressure during BA in aldosterone HT were very similar to those in dogs with ANG II hypertension (Figure 2B). More specifically, after substantial acute reductions in blood pressure, MAP gradually increased throughout the 7 days of BA from its early nadir to only 7 mmHg below the initial hypertensive baseline. Thus, when compared to the control response before induction of HT, the fall in BP in response to BA was attenuated 55% during aldosterone HT. Furthermore, after BP returned to previous hypertensive levels after recovery from BA, the subsequent antihypertensive response to RDN was even less impressive than during BA. Indeed, after RDN, there was no fall in MAP in dogs with aldosterone hypertension.
Thus, the similar time-dependent changes in BP and the diminished long-term antihypertensive response to BA in ANG II (Figure 2B) and aldosterone HT, suggest that baroreflex-mediated suppression of sympathetic activity has minimal effects to enhance renal excretory function and lower BP in the presence of elevated circulating levels of these potent sodium-retaining hormones. However, antihypertensive responses are not completely abolished under these conditions. Additionally, as the sympathetic nervous system is not activated in either model of HT, reductions in the BP response to baroreflex-mediated sympathoinhibition may also be attributed to low basal sympathetic activity.
In summary, these findings suggest that aldosterone excess may lead to diminished BP lowering during device-based therapy for global and especially renal-specific sympathoinhibition. An implication of these findings is that there may be diminished antihypertensive responses to device-based therapies in patients with RHT not treated with aldosterone antagonists. This possibility is in line with a post hoc analysis from the Symplicity NTN-3 trial showing that prior treatment with an aldosterone antagonist was a positive predictor for a favorable antihypertensive response to RDN in patients with RHT.63 Finally, these findings add to the evidence that BP lowering during global suppression of sympathetic activity by BA is not exclusively dependent on neurogenic mechanisms that target the renal nerves.
MobiusHD Device for Baroreflex Amplification
An alternate approach for chronically activating the carotid baroreflex is being evaluated in clinical trials. Rather than increasing baroreceptor afferent activity by electrically stimulating the carotid sinus, the concept behind the MobiusHD system (Vascular Dynamics, Inc.) is amplification of the signal sensed by carotid baroreceptors during distortion of their nerve endings by vascular stretch during systole.64 Signal amplification is achieved by a passive, flexible, self-expanding endovascular implant that reshapes the carotid sinus during systole, increasing the radius while preserving pulsatility. In so doing, this increases wall strain and, thus, baroreceptor activation during spontaneous changes in systolic pressure.
Support for this approach comes from an acute study in dogs, but long-term mechanistic studies have not been conducted. Regardless, the device has received CE marking for treatment of RHT in the European Economic Area and in an open label, uncontrolled study in 30 patients with RHT, BP was reduced substantially after 6 months of unilateral endovascular baroreceptor amplification.64 An uncontrolled open label study evaluating the safety and efficacy of the MobiusHD implant in patients with RHT is currently in progress in the U.S. (NCT01831895).
Summary: Baroreceptor Activation for Hypertension Therapy
BA lowers BP chronically in experimental models of HT and in patients with RHT. In addition, through sustained modulation of autonomic activity, BA may have unique BP-independent cardioprotective and nephroprotective effects that add to the clinical beneficial of BP lowering in preserving function in these organs. Furthermore, chronic electrical stimulation of the baroreflex may have some additional advantages over other device-based approaches for HT therapy (Table 1). It is particularly notable that BA lowers BP even in the absence of increased baseline sympathetic activity and after RDN. Nonetheless, as with all antihypertensive treatments, there are non-responders to BA. In this regard, experimental studies have been especially valuable in providing insight into conditions that are favorable/unfavorable for a positive BP response to BA.
Table 1.
Advantages/Limitations of Baroreflex Activation for Treatment of Hypertension
| Advantages |
|
| Limitations |
|
To date, several observational studies have shown that the second generation Barostim neo system is safe and lowers BP without adversely affecting renal function. However, there are potential limitations with using the Barostim neo system for HT therapy (Table 1). Most notably, randomized, controlled trials have not been completed to demonstrate long-term safety and efficacy, but they are ongoing in France and in Nordic countries. It should be noted that the US randomized, multicenter Barostim neo HTN Pivotal trial (NCT01679132) is currently suspended “as company resources will only allow adequate oversight for one pivotal trial at a time.” The findings summarized in this article and reported in experimental animals with heart failure65, 66 indicate unique effects of BA to diminish central sympathetic outflow, improve sympathovagal balance, and favorably affect disease progression. Accordingly, these findings have implications for positively impacting outcome in patients with heart failure, a hypothesis supported by recent preliminary clinical studies.67–69 To this end, CVRx resources are currently committed to sponsoring clinical trials in these high risk patients (NCT02880618, NCT02876042, NCT02627196). Thus, the role of BA in the management of RHT still awaits the findings of randomized, controlled trials.
RENAL DENERVATION FOR HYPERTENSION THERAPY
Rationale for RDN in HT
The renal nerves contain sympathetic efferent and sensory afferent fibers and are recognized as important controllers of kidney function and BP.70 Sympathetic efferent fibers innervate the renal arteries, arterioles, renin-secreting juxtaglomerular cells, veins, and most tubular segments.70 Stimulation of renal sympathetic nerves can lead to increases in renal vascular resistance, tubular reabsorption of NaCl, and renin release depending on stimulation frequency.70 Acute experimental studies in which RSNA was progressively increased by electrical stimulation of renal nerves in rodents or by carotid artery occlusion to unload arterial baroreceptors in conscious dogs suggest that modest increases in RSNA promote renin secretion, increases in tubular NaCl reabsorption, and reductions in NaCl excretion whereas reductions in RBF and GFR occur only at high levels of stimulation70–72. Gross et al.73 reported, for example, that common carotid artery occlusion increased RSNA by 62% but produced little change in RBF in conscious dogs. However, exposure of the dogs to frightening auditory stimuli to induce a 500% increase in RSNA elicited a 40% acute decrease in RBF. Additional studies demonstrated that GFR and RBF do not differ significantly in innervated and denervated kidneys of rabbits74 and dogs75 undergoing normal daily activities; in these experiments, renal hemodynamics were studied in innervated and denervated kidneys of the same animals, controlling for potential effects of changes in BP and hormonal influences. Thus, renal vasoconstriction sufficient to acutely reduce RBF occurs only when RSNA is increased several-fold but even low levels of RSNA can increase tubular NaCl reabsorption and renin release.
Mild to moderate hyperactivity of renal sympathetic nerves, insufficient to directly reduce RBF and GFR, is thought to be involved in development and maintenance of several forms of experimental HT76, 77 (Table 2). For example, RDN consistently reduces BP, although not completely abolishing HT, in spontaneously hypertensive rats (SHR) where increases in RSNA have been clearly documented.81–83 In obese rodents, rabbits, and dogs, increases in RSNA have consistently been demonstrated to contribute to HT associated with increased renal tubular sodium reabsorption.42, 78–80, 102 RDN has also been reported to reduce or abolish HT in several other rodent models, although results have not always been consistent perhaps due to differences in methods of BP measurement, experimental design, age, or stage of HT when RDN was implemented.92, 103, 104
Table 2.
Renal sympathetic nerve activity (RSNA) and blood pressure effects of renal denervation (RDN) in various experimental models and in humans
| Experimental models /Humans | RSNA*/or MSNA | BP Effect of RDN# |
|---|---|---|
| Dietary-induced visceral obesity (dogs, rats, rabbits)42, 78–80 | ↑ | ↓ |
| Spontaneously hypertensive rats (SHR)81–83 | ↑ | ↓ |
| Dahl salt-sensitive rat84 | ? | ↓ |
| Angiotensin II hypertension (dogs, rats, rabbits)27, 85–88 | ↓ | ↔ |
| Aldosterone hypertension (dogs)62 | ? | ↔ |
| Reduced kidney mass, salt loading (rats)89 | ? | ↔ |
| DOCA-salt hypertension (rats)90 | ? | ↓ |
| Goldblatt hypertension (dogs, rats)91, 92 | ? | ↔ |
| Nitric oxide inhibition (rabbits, dogs)93, 94 | ↔ | ↔ |
| Nitric oxide inhibition (rats)95 | ? | ↓ |
| Humans - visceral obesity21, 96, 97 | ↑ | ↓ |
| Humans - subcutaneous/lower body obesity98 | ↔ | ? |
| Humans - Chronic kidney disease99, 100 | ↑ | ↓ |
| Humans - Chronic nephritis101 | ? | ↔ |
RSNA, renal sympathetic nerve activity, assessed directly or indirectly via renal norepinephrine spillover; MSNA, muscle sympathetic nerve activity, measured by microneurography;
BP (blood pressure) effect of renal denervation (RDN), catheter-based methods or surgical
Some models of HT are not associated with increased RSNA and RDN does not significantly lower BP (Table 2). For example, RDN in rats did not attenuate HT caused by chronic ANG II infusion or by salt loading and reduced kidney mass.85, 89 In rabbits, RSNA is suppressed in ANG II HT and RDN does not attenuate the HT.86, 87 In dogs, RSNA is not increased in ANG II HT18, 22 and RDN did not substantially ameliorate HT caused by renal artery stenosis91, 92 or by chronic aldosterone infusion.62 Thus, the renal nerves contribute to experimental HT associated with increased RSNA but there are clearly some forms of HT in which RSNA is not elevated and RDN does not substantially lower BP.
Studies of RDN as a potential therapy for human HT date back to at least the 1930’s when Page and Heuer reported that surgical bilateral RDN had no significant effect on BP in a female patient with primary (essential) HT.105 In 5 patients with chronic nephritis and HT, RDN decreased BP for a few weeks after the operation but BP then returned to the pre-surgical values.101 Studies in the 1950’s and 1960’s demonstrated that thoracolumbar splanchnicectomy, which interrupts RSNA activity, reduced BP and improved survival rates in patients with severe primary HT.106, 107 However, HT was attenuated in only about half of >1000 patients studied. Thus, early studies in patients with HT also suggested that increased RSNA contributes to elevated BP in some, but not all, patients with HT.
Perhaps it seems obvious that RDN should be most effective in reducing BP when RSNA is elevated and least effective in patients with low RSNA. However, it has been challenging to assess RSNA in humans or to determine a priori which patients will have substantial BP reductions after RDN. Microneurography measurements of MSNA, although not practical for most clinical studies, have been used as a surrogate for assessing sympathetic activity in humans even though there are regional differences in SNA in lean and obese subjects with HT.8, 23, 108 Under resting conditions a substantial fraction of patients with primary HT appear to have elevated MSNA.8, 109 For example, hypertensive patients with visceral obesity, obstructive sleep apnea (OSA), and some lean hypertensive patients have elevated MSNA compared to normotensive subjects.8, 24, 97, 110 However, there is considerable variability of MSNA that may relate, in part, to differences in body fat distribution, age, sex, and ethnicity.109 Excess visceral adipose tissue (VAT), for example, appears to be more closely associated with increased MSNA and HT than does overall adiposity.97 Some studies, albeit in a small number of patients with HT, suggest that there is no relationship between baseline MSNA and BP changes after RDN.111 This finding, if confirmed, could imply that MSNA may not be closely related to RSNA in many patients.
Indirect measurements of RSNA, using the renal NE spillover method, also suggest that many patients with primary HT have increased sympathetic activity.8, 109 However, the technical challenges of this method have limited its use in clinical studies and inferences regarding RSNA in hypertensive patients are often based on measurements of MSNA, urinary NE excretion, plasma NE concentration or other indirect indices rather than direct measurements. Additional limitations of current methods for assessing SNA in humans are that 1) measurements are made under quiet, resting conditions rather than normal daily activities where marked changes in SNA may influence BP regulation, and 2) factors that are known to influence SNA (e.g. body fat distribution, sex, age) are often not considered. Therefore, it is perhaps not surprising that there has been considerable variability in BP responses to RDN in hypertensive patients and that the relationship between RSNA and subsequent BP responses to RDN is not well established.
Despite limitations in assessing RSNA, there is evidence that even moderate increases in RSNA, insufficient to cause vasoconstriction, may elevate BP. For example, in experimental models of HT and in primary human HT caused by excess weight gain/obesity, GFR and RBF are often normal or elevated prior to the development of kidney injury21, 42 suggesting that increases in RSNA are insufficient to cause renal vasoconstriction. However, RSNA in obesity is sufficient to increase tubular NaCl reabsorption, perhaps through direct effects on the renal tubules or by stimulating renin release and ANG II formation.21, 42, 112 These combined effects of RSNA to increase renal NaCl reabsorption impair renal-pressure natriuresis and contribute to HT in obesity21 which accounts for 65 to 75% of the risk for human primary HT.113 However, other factors besides increased RSNA may also contribute to activation of the RAAS and HT in obese subjects.114, 115
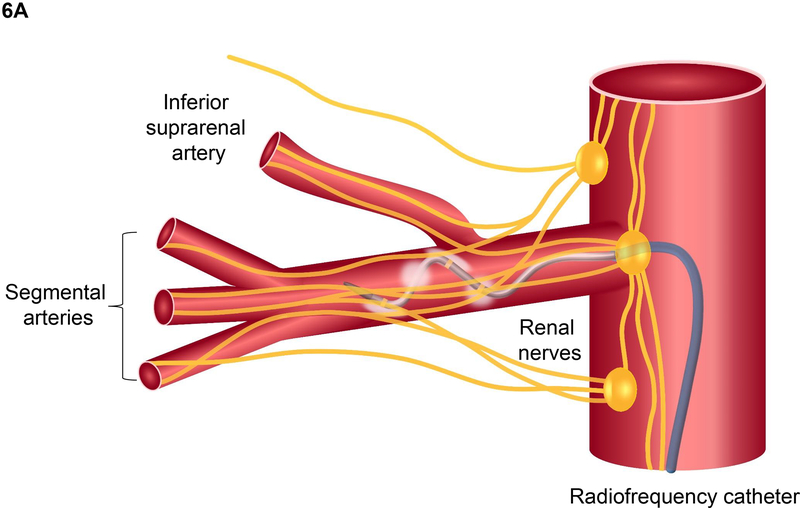
Thus, a long history of studies in experimental animals and in humans provided rationale for RDN as a potential therapy for hypertensive patients whose BP is not adequately controlled with lifestyle changes and pharmacological therapy. To obviate the necessity of major surgery and simplify the RDN procedure, several minimally invasive catheter-based RDN (CB-RDN) methods were developed to employ radiofrequency, ultrasound, or injection of neurotoxic agents such as alcohol to damage the renal afferent and efferent nerves located along the adventitia of the renal arteries.
Clinical Trials of RDN in Patients with RHT
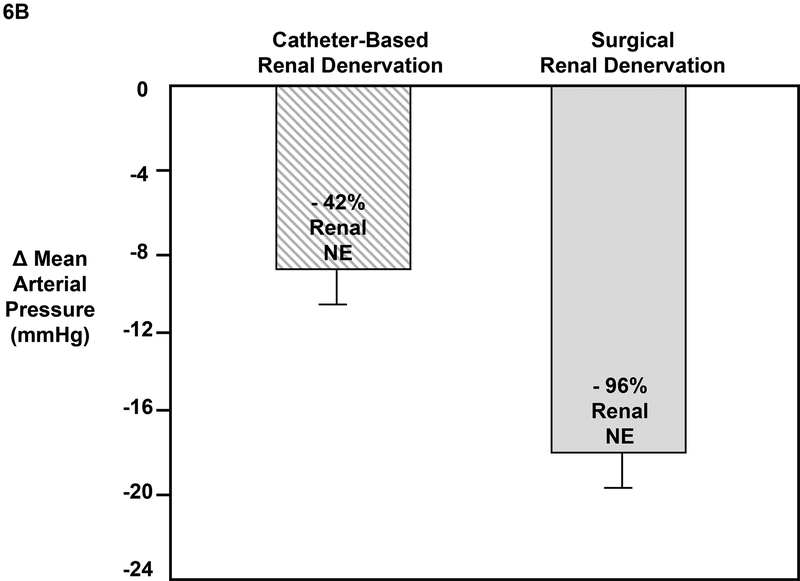
The first CB-RDN studies were conducted in patients with RHT using the radiofrequency method (Symplicity, Ardian, Inc.) to bilaterally ablate the renal nerves running along the main renal arteries.41 This proof-of-principle multi-center study enrolled 45 patients with SBP >160 mmHg and on 3 or more antihypertensive drugs including a diuretic. Reductions in office SBP/DBP after RDN were impressive, averaging −22/−11 and −27/−17 mmHg after 6 and 12 months, respectively. In 10 patients, renal NE spillover, measured using an isotope dilution method, was reduced by an average of 47%, demonstrating that the RDN procedure was moderately effective in ablating the renal nerves. Subsequent follow up of these patients and additional subjects included in SYMPLICITY HTN-1 indicated that BP reductions persisted for up to 3 years (n=88 with complete data).116 These studies suggested that CB-RDN could cause persistent reductions of BP without major complications and paved the way for several prospective randomized clinical trials.
Symplicity HTN-2 and ENLIGHTN 1.
The initial studies of CB-RDN were quickly followed by SYMPLICITY HTN-2, a randomized controlled trial of patients with RHT, including 52 who received RDN and 51 control subjects who were assessed for the primary endpoints of safety and effectiveness of reducing BP at 6 months.117 This trial basically confirmed SYMPLICITY HTN-1 demonstrating −32/−12 mmHg reductions in office SBP/DBP 6 months after CB-RDN with no significant changes in the control group. There were no significant changes in renal function, as assessed by serum creatinine, estimated GFR, and cystatin C concentrations and no serious complications related to the procedure.
These early results further heightened expectations that CB-RDN could be safely used to substantially reduce BP in treatment resistant hypertensive patients. Follow up studies indicated that the antihypertensive effect of CB-RDN persisted for at least 2–3 years.116, 118
The ENLIGHTN 1 trial which used a multielectrode radiofrequency catheter-based system to ablate the renal nerves also demonstrated impressive decreases in BP, measured in the office, at home, or with 24-hour monitoring, following RDN in patients with RHT.119, 120 However, an important criticism of these early trials is that they did not include a sham control group. Also, poor drug adherence is common in treatment RHT but this was not monitored in some cases, making the studies susceptible to the Hawthorne effect (i.e. the possibility that patients altered their behavior and/or medication adherence due to their awareness of being observed).
Symplicity HTN-3.
The first randomized, sham-controlled RDN trial was SYMPLICITY HTN-3 trial which failed to prove superiority of RDN compared with the sham control group.121 A total of 533 patients with RHT were randomly assigned in a 2:1 ratio to undergo CB-RDN or a sham procedure and a constant regimen of 3 or more commonly recommended antihypertensive medications was mandated for both groups. The average decreases in SBP at 6 months were −14.1 and −11.7 mmHg in RDN and sham groups, respectively, with no significant differences in 24-hour ambulatory SBP. After 12 months, the reductions in office BP were similar in the RDN and sham groups.122
Various secondary analyses and editorials have suggested potential reasons for these surprising results. Procedural issues may have influenced the adequacy of RDN since 111 interventionists, many with little procedural experience, performed 364 RDNs, an average of only 3.3 RDN procedures each. Importantly, the effectiveness of RDN was not verified. In a retrospective analysis of angiographic and procedural records, 74% of patients were found to have not even one fully circumferential application of radiofrequency energy to the renal arteries, suggesting that effective RDN may not have been achieved in many patients.123, 124 Also, there were medication changes in 38% of patients in the RDN group and 40% in the sham-control group despite the protocol mandating constant antihypertensive medication regimens. SYMPLICITY HTN-3 also included many patients who likely had low sympathetic activity (e.g. low renin, volume-dependent forms of HT).
A sub-analysis of SYMPLICITY HTN-3 suggested that the failure of RDN to further reduce BP, compared to sham control subjects, was due mainly to large reductions in BP (−18 mmHg) in the sham subgroup of African-American despite mandated regimens of constant antihypertensive medications.63 These limitations led some to conclude that although the design of SYMPLICITY HTN-3 had many strengths compared to earlier studies, including a blinded sham control design and relatively large patient enrollment, execution of the trial was flawed and therefore it cannot be considered definitive.123
DENERHTN trial.
The multicenter randomized controlled Renal Denervation for Hypertension (DENERHTN) trial compared ambulatory BP-lowering efficacy and safety of radiofrequency-based RDN added to a standardized stepped-care antihypertensive treatment (SSAHT) with the same SSAHT alone in patients with RHT.125 The average change in daytime ambulatory SBP at 6 months was −15.8 and −9.9 mmHg in the RDN group (n=53) and the group receiving SSAHT alone (n=53), respectively. Moreover, the number of antihypertensive drugs and drug adherence at 6 months was similar in both groups. The major limitations of this trial are similar to previous trials that did not include a sham RDN control or verify effectiveness of RDN.
SPYRAL HTN-OFF MED and SPYRAL HTN-ON MED trials.
These prospective, randomized, double-blind, sham-controlled studies were designed to assess the impact of RDN in patients with uncontrolled BP and who were medication naïve or had discontinued medication126 and in patients being treated with 1–3 commonly prescribed antihypertensive medications.127 Both studies used a new multielectrode catheter designed to permit reliable circumferential four-quadrant renal nerve ablation. Also, RDN was performed in the main renal arteries and branches, an approach that likely produces more complete renal nerve ablation, as discussed later. Both studies showed that RDN was associated with small but significant reductions in BP through 3 months, compared to sham controls, with no major adverse events.
The primary results from the SPYRAL HTN-OFF MED trial,126 which were obtained in 80 patients (38 RDN and 42 sham controls), showed that RDN significantly reduced ambulatory BP from baseline to 3 months (24-h SBP −5.5 mmHg, 24-h DBP −4.8 mmHg). In contrast to SYMPLICITY HTN-3 in which participants received an average of >5 antihypertensive agents, the effect of the sham procedure was negligible with changes in ambulatory SBP/DBP from baseline of only −0.5/−0.4 mmHg. Besides lowering average 24-h ambulatory and office BP, RDN altered the 24-hour BP profile in the SPYRAL HTN-OFF MED trial.128 Detailed assessment of 24-hour BP patterns using patient-reported wake times revealed consistently lower BP for the RDN group at night and throughout much of the day, with minimal BP reductions throughout the 24-hour period for the sham control patients.128
In the SPYRAL HTN-ON MED trial, medication adherence was about 60% in RDN and control groups and varied for individual patients throughout the study. Eligible patients had an office SBP between 150–180 mmHg, a DBP of ≥90 mmHg, a 24-h ambulatory SBP between 140–170 mm Hg and were on 1–3 antihypertensive drugs (thiazide diuretic, dihydropyridine calcium channel blocker, β-blocker, and an ACE inhibitor or ARB)127 with stable doses for at least 6 weeks. Patients (n=467) underwent renal angiography and were randomly assigned to undergo RDN or sham control. Office and 24-h ambulatory BP decreased significantly from baseline to 6 months in the RDN group, but not in controls, and baseline-adjusted treatment differences in 24-h SBP/DBP averaged −7.0/−4.2 mmHg).
Thus, the SPYRAL HTN-ON MED trial demonstrated in patients receiving antihypertensive medications that RDN extending into segmental branch arteries was associated with clinically relevant reductions in office and ambulatory BPs compared with sham controls. The BP reductions with RDN increased during 6 months of follow up and there were no procedural or intermediate-term adverse safety events reported. Like previous studies, non-adherence to antihypertensive drugs was common in patients with HT.
RADIANCE-HTN SOLO.
This multi-center, single-blind, randomized, sham-controlled trial used an endovascular high-frequency ultrasound device to effect RDN in hypertensive patients who were off antihypertensive drugs.129 The trial showed that RDN caused small but significant reductions in 24-hr SBP/DBP (−7.0/−4.3 mmHg) compared to sham controls. This fall in BP was similar to the antihypertensive response reported in the SPYRAL HTN-OFF MED trial. However, the investigators relied on patient-reported antihypertensive drug use and the extent of RDN was not verified.
RADIOSOUND-HTN.
Fengler et al130 performed a head-to-head comparison of radiofrequency and ultrasound endovascular RDN in 120 patients with RHT who were randomized to receive one of the following treatments: 1) radiofrequency RDN of the main renal arteries; 2) radiofrequency RDN of the main renal arteries, side-branches, and accessory arteries; 3) endovascular ultrasound-based RDN of the main renal artery. The primary outcome was change in daytime ambulatory SBP after 3 months. Ambulatory SBP decreased by an average of −9.5 mmHg in the whole cohort although the reduction was slightly greater in the ultrasound ablation group than in the radiofrequency ablation group of the main renal artery (−13.2 vs. −6.5 mmHg). However, no significant differences in ambulatory SBP reduction were observed when comparing radiofrequency RDN of the main renal arteries, side-branches and accessories (−8.3 mmHg) with the ultrasound RDN group. There was considerable variation in the ambulatory SBP responses in all groups but the frequencies of SBP reductions ≥5 mmHg were not significantly different among the groups. Although these results suggest that ultrasound-based RDN of the main renal arteries may be slightly superior to multipolar radiofrequency RDN of the main renal arteries in reducing ambulatory SBP, the differences were small and laboratory testing of medication adherence was not performed.
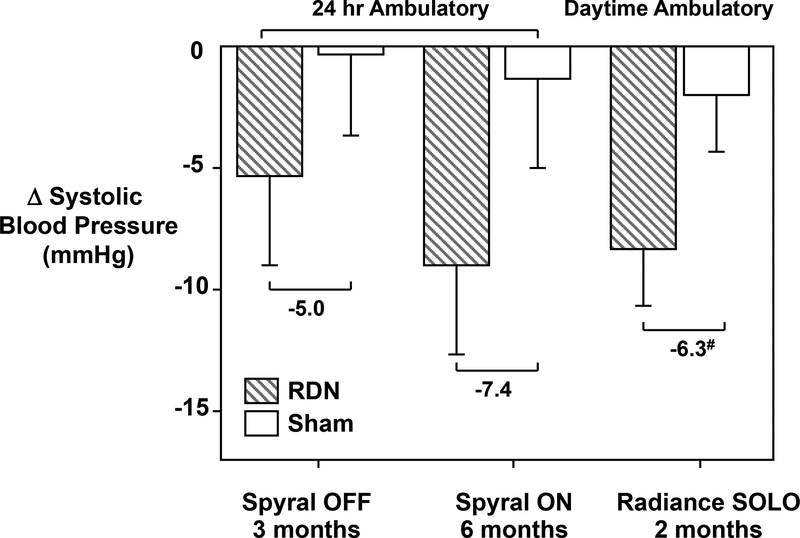
Taken together, the RADIANCE-HTN SOLO, SPYRAL HTN-ON MED, and SPYRAL HTN-OFF MED trials suggest that RDN lowers BP by ~5 to 8 mmHg in RHT subjects in the presence or absence of antihypertensive medications (Figure 4).131 Although the extent of RDN achieved in these trials is uncertain, based on previous studies it seem like that <50% RDN was achieved in most cases. Also, in all of these trials there were responders and non-responders to RDN and some patient subgroups appeared to benefit more than others. Deeper analyses of results from RDN in experimental models of HT and better understanding of the pathophysiology of various forms of HT may help guide future research and clinical trials to determine which patients are likely to receive greatest benefit from RDN.
Figure 4.
Primary endpoints of the recent clinical trials for catheter-based-RDN that included sham controls. #Mean between-group difference was adjusted for baseline systolic blood pressure. All values are means (95% confidence interval). (Redrawn from F. Mafoud et al. Europ. Heart. J. 2018; Sep 14. doi: 10.1093/eurheartj/ehy584. [Epub ahead of print]. PMID:30239686).
In Which Patients is RDN Most Likely to Effectively Reduce BP?
Variability in BP responses to RDN in clinical studies using CB-RDN methods may be partly explained by adequacy of RDN or changes/adherence of prescribed medications. However, variability in RSNA before RDN may also play an important role. Clinical studies suggest that RDN may be less effective in patients with isolated systolic hypertension132, high central pulse pressure133, and increased aortic pulse wave velocity134, although the physiological basis for attenuated BP reductions in these conditions is unclear.
The uncertainty about how to assess effectiveness of RDN and which patients will be responders has driven extensive research to define therapeutic predictors of BP responses to RDN. Along with markers of immediate RDN success (i.e. BP responses to periprocedural renal nerve stimulation, acute changes in RBF, brain derived neurotrophic factor, etc), numerous baseline variables predicting long-term RDN efficacy (i.e., clinic SBP, use of specific antihypertensive drug classes, baroreflex sensitivity, biomarkers [ie, soluble fms-like tyrosine kinase-1 and endothelial adhesion molecules], low pulse wave velocity etc) have all been proposed.135 The reliability of these markers is still uncertain and clinical applicability is limited in most cases.
In a follow-up analysis of the DENER-HTN study, Gosse et al136 identified baseline nighttime BP ≥136 mm Hg as a predictor of daytime BP reduction 6 months after RDN. Ambulatory BP monitoring revealed that the number of patients who had reductions in daytime SBP of ≥20 mm Hg after 6 months follow-up was 2-fold greater in the RDN plus medications group compared with those who only received antihypertensive medications. Higher nighttime BP variability before RDN was also associated with greater daytime SBP lowering at 6 months after RDN in responders. Although these observations suggest that nighttime BP may serve as a marker for predicting responses to RDN, validation is needed with additional well-controlled clinical trials.
As discussed previously, it is unlikely that RDN will be effective in substantially reducing BP in patients with normal or low RSNA. Unfortunately, a reliable and practical method to assess RSNA in everyday clinical practice is not yet available. Therefore, it may be helpful to utilize information gained from studies that have assessed sympathetic activity and/or responses to RDN in various forms of HT. Previous studies in experimental animals have shown that RDN has little effect on BP in models of HT with normal/low sympathetic activity such as HT induced by chronic infusion of ANG II or aldosterone, renal artery stenosis, or salt loading in animals with reduced nephron numbers.62, 85, 87–89, 137 Secondary analyses of data from SYMPLICITY HTN-3 also suggest that RDN may be less effective in patients who have volume-dependent forms of HT.63 In contrast, RDN has been shown to be highly effective in reducing BP in obesity-induced HT which is associated with increased RSNA and may account for 65–75% of the risk for primary HT as well being a major contributor to treatment resistant HT.8, 21, 42, 138–141
The drugs used to treat HT may also influence BP responses to RDN. Experimental studies indicate that the effects of increased RSNA on kidney function are mediated via direct α-adrenergic stimulation of tubular NaCl reabsorption as well as β-adrenergic stimulation of renin release and subsequent ANG II formation which, in turn, has multiple effects to increase NaCl reabsorption. Therefore treatment with angiotensin converting enzyme (ACE) inhibitors or ANG receptor blockers (ARBs) may attenuate the chronic BP effects of RDN. In contrast, treatment with calcium channel blockers and diuretics may increase activity of the SNS and RAAS system and enhance the chronic BP effects of RDN. In most cases of RHT, however, all of these classes of antihypertensive drugs are used in varying doses.
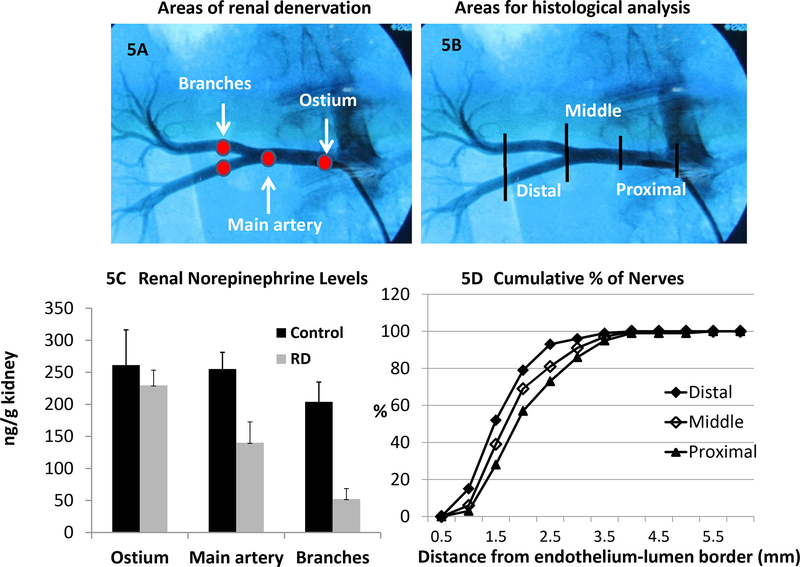
How Effective is Catheter-Based RDN in Ablating Renal Nerves?
Surgical RDN, if done properly, reduces renal tissue NE content by >95%, indicating effective ablation of sympathetic efferent nerves.42, 62, 75, 142 However, in most clinical studies of CB-RDN, denervation efficacy has not been verified. In the few studies where the extent of CB-RDN was assessed, nerve ablation has been much less effective than with surgical RDN. We demonstrated, for example, in obese dogs that CB-RDN reduced renal NE content by ~42% if carefully conducted using a multielectrode device to deliver radiofrequency energy along the wall of the main renal artery prior to any branching.79 In 10 patients with RHT, Krum et al41 measured renal NE spillover using an isotope dilution method before and 15–30 days after radiofrequency CB-RDN and reported an average ~47% decrease in renal NE spillover. Subsequent investigations have shown that RDN achieved with catheter-based procedures is highly variable and more modest, often producing <25% decline in renal NE spillover.8, 123
Various factors can influence the effectiveness of CB-RDN procedures, including the type of procedure used, design of the device, skill of the operator, and variations in anatomy of the renal nerves and the renal artery. Some of these issues have been previously discussed123, 143, 144 and a detailed description of RDN methods is beyond the scope of this review. Many of the early clinical trials used the radiofrequency CB-RDN method with a single electrode, although catheter designs have evolved to incorporate multiple electrodes.
Location of radiofrequency energy delivery in the renal artery also has an important effect on RDN efficacy. We reported that when radiofrequency CB-RDN was performed at the ostium near the origin of the renal artery in pigs, kidney NE content decreased by only 12%.145 In contrast, renal NE decreased by 45% with RDN near the bifurcation in the renal artery and by 74% when RDN was performed in the renal artery branches (Figure 5). Other investigators have reported similar results.146
Figure 5.
Areas of catheter-based denervation along the renal artery. Locations used for RDN (5A) and the approximate areas defined for the histological analysis (5B). Renal tissue NE concentrations (3C) measured from non-denervated kidneys (control) and denervated kidneys (RD). Kidneys were denervated at the ostium, the main artery, or in the branches of the renal artery. Cumulative percentage of nerves (3D) from the renal artery endothelial–lumen interface for the different areas of sections analyzed. (Redrawn from Henegar et al. Am J Hypertens. 2015;28:909–914).
Thus, the efficacy of radiofrequency CB-RDN is greatest when performed in branches of the renal artery close to the kidney or in distal portions of the renal artery near the bifurcation. A likely explanation is that nerves running along the adventitia are closer to the lumen of the smaller branches compared to the main renal artery (Figure 6A). In pigs, 79% of the nerves in distal portions of the renal artery were located within 2.0 mm from the endothelial-lumen interface compared to about 69% in the middle and 57% in proximal sections of the renal artery.145 In the main renal artery of humans, Sakura et al147 found that the 50th, 75th, and 90th percentiles of nerve distance from renal artery lumen were 2.44, 4.28, and 6.39 mm, respectively. Over 98% of the nerves examined were within 3.5 mm measured from the lumen–intima interface. These studies provide insights into the treatment zone that should be targeted by RDN procedures, although there is likely to be significant variability of renal nerve anatomy in humans. As a result of these findings recent CB-RDN studies have targeted nerves in the segmental renal arteries or in distal parts of the renal artery near the bifurcation.
Figure 6.
A. Renal denervation using catheter-based approach. Some renal nerves near the ostium, the inferior suprarenal artery, and accessory arteries (not shown) do not run along the adventitia of the main renal artery and therefore are not susceptible to catheter-based ablation. The radiofrequency catheter is shown in gray. B. Renal tissue NE concentrations in obese dogs after catheter-based RDN (From Henegar et al. Am J Hypertens. 2014;27:1285–1292) or surgical RDN (From Lohmeier et al. Hypertension. 2012;59:331–338).
Verifying RDN efficacy in large clinical studies has been challenging. Although renal NE overflow can be used to assess effectiveness of RDN in humans123, this method is technically difficult and not feasible for periprocedural testing. Therefore, considerable effort has been devoted to developing reliable markers for immediate procedural success. Potential biomarkers that can be measured in plasma (e.g. brain-derived neurotrophic factor, neuropeptide-Y) have been examined after RDN but none have proved to be reliable predictors of therapeutic success.148, 149 Because RDN ablates afferent as well as efferent nerves, BP increases after acute stimulation of the renal nerves have been suggested as a test that ablation has been achieved.148, 150 Electrical stimulation of the renal nerves in dogs, sheep, and humans rapidly raises BP and this response is attenuated after RDN.148 Moreover, reductions in BP 4.5 months after RDN were highly correlated with attenuation of acute BP responses to renal nerve stimulation.151 These observations suggest that assessing BP responses to acute renal nerve stimulation may provide a perioperative test of RDN since catheters can be designed to stimulate as well as ablate the renal nerves. However, randomized controlled trials will be needed to determine the potential clinical usefulness of these tools.
What is the Relationship Between RDN Efficacy and BP responses?
There have been no previous studies, to our knowledge, that have examined the quantitative relationship between renal NE levels and chronic BP reductions after CB-RDN in humans. However, in obese dogs surgical RDN clearly produces a much greater decrease in BP than CB-RDN which produces less complete reductions in renal NE. Kassab et al78 showed that surgical RDN, which decreased renal NE by 92%, almost completely prevented the rise in BP in obese dogs. Lohmeier et al42 found that surgical RDN reduced renal NE content by 96% and completely normalized BP in obese dogs with established HT (Figure 6B). Radiofrequency CB-RDN in obese dogs with established HT, however, caused more modest declines in BP associated with only a 42% fall in renal NE.79 Thus, in obese dogs the fall in BP following RDN is almost directly proportional to effectiveness of sympathetic efferent nerve ablation as assessed by renal tissue norepinephrine concentration several weeks after the procedure (Figure 6B).
Id et al152 reported that patients with accessory renal arteries that were not denervated had less BP reduction following radiofrequency CB-RDN than patients without accessory renal arteries. Thus, it appears that the magnitude of BP reduction following RDN may be related to the degree of renal nerve ablation in humans as well as in experimental animal studies. As discussed previously, CB-RDN procedures are more effective in ablating renal nerves if focused on distal and segmental renal arteries and include the full circumference of both renal arteries. Also, CB-RDN procedures are likely to more effective if the delivered energy penetrates to >3 mm from the lumen-intima interface of the renal arteries since some sympathetic nerves are more remote from the lumen than this.123, 147
Regrowth of Renal Nerves After RDN
The feasibility of using CB-RDN as a major therapy for HT depends partly on its ability to produce durable ablation of renal nerve function. There is considerable evidence that renal sympathetic efferent and sensory afferent nerves eventually regrow after renal denervation.
The time course for restoration of function after RDN is not well defined in humans but may depend on the distance that the renal nerves must regrow. In small animals (e.g. rats) with short renal arteries, regrowth of renal afferent and efferent nerves occurs within a few weeks after RDN.153, 154 Mulder et al154 found that sensory afferent and sympathetic efferent nerves reinnervated the kidneys of rats over the same time course, being complete at 9–12 weeks after RDN. In larger animals, regrowth of the renal nerves may require at least 6 to12 months. In sheep, for example, the functional afferent and efferent responses to electric stimulation were normal 11 months after RDN155; also, immunohistochemical staining for renal efferent (tyrosine hydroxylase) and renal afferent nerves (calcitonin gene–related peptide), as well as renal NE levels, were normal 11 months after RDN. In dogs, there was anatomical evidence for partial renal nerve regeneration between 3–6 months after surgical RDN but restoration of morphology and function of the renal nerves was not complete even after 12 months.156
In humans, the time for regrowth of sensory afferent and sympathetic efferent renal nerves is less clear. Studies in patients receiving kidney transplants suggest that regeneration of periadventitial renal nerves begins as early as 5 months and may be complete in 2 years or less after transplantation157; moreover, the regrowth of renal sympathetic nerves was correlated to development of HT in patients with transplanted kidneys.
In clinical trials the BP lowering effects of RDN have been sustained for up to three years in studies without sham controls or documentation of RDN efficacy. However, the long-term durability of BP reductions is still difficult to assess since most patients in the SPYRAL-OFF and RADIANCE-SOLO trials, which included sham controls, were returned to standardized regimens of antihypertensive medications after primary endpoint data were collected at 2 and 3 months, respectively. These time points were considered to be the longest time that it was safe for hypertensive patients to be without BP medications. Whether patients with RDN will require less intense antihypertensive drug regimens to obtain adequate BP control is still unclear. Studies that are currently underway should reveal the impact of reinnervation on long-term durability of BP responses.
Role of Renal Sympathetic Efferent and Sensory Afferent Nerves in Mediating BP Responses to RDN.
RDN removes sympathetic efferent and afferent renal nerves. Afferent sensory fibers carry information from mechanoreceptors and chemoreceptors to the CNS and have been suggested to mediate some forms of experimental HT.104, 158 The idea that renal afferents may mediate BP effects of CB-RDN in humans was stimulated by a report that whole-body norepinephrine spillover and MSNA were reduced one year after RDN in a single patient.159 Another study reported that RDN slightly decreased MSNA as well as BP in obese hypertensive patients160, a finding interpreted as evidence that interrupting renal afferent pathways may contribute to generalized sympathoinhibition and BP reductions. Witkowski et al161 also suggested that renal nerve ablation in patients with refractory HT produced non-renal systemic effects, including improvements in glucose tolerance and sleep apnea severity.
In contrast to studies supporting a role for renal afferents in mediating BP effects of RDN, Brinkman et al162 failed to observe long-term reductions in MSNA after RDN and BP reductions were not correlated with decreased MSNA; baroreflex control of HR and MSNA as well as HRV and BP variability were analyzed in the time and frequency domain and no changes were observed after RDN. Grassi et al111 observed no changes in MSNA 15 days and 1 month after RDN in 15 patients with RHT; although MSNA decreased slightly at 3 and 6 months, the BP responses to RDN preceded and appeared to be independent of the MSNA changes. Verloop et al163, in studies designed to investigate effects of RDN on insulin sensitivity and BP in patients with metabolic syndrome, found that RDN reduced mean 24-hr SBP/DBP by an average of 6/5 mmHg but had no effect on MSNA, HRV, or insulin sensitivity after 12 months of treatment.
There were several significant limitations in all of these studies that make assessment of the role of afferent sensory and efferent sympathetic renal nerves challenging. Sham controls were not included and changes in antihypertensive medications are known to occur in a substantial number of patients who received RDN. Also, improvements of glucose regulation after RDN (an inconsistent finding) could be related to ablation of afferent or efferent nerve fibers. Experimental studies have shown that RDN reduces kidney gluconeogenesis and may, in some instances, increase renal glucose excretion by decreasing glucose transporter 2 (GLUT-2) in renal proximal tubules.164 Thus, it is still uncertain in clinical studies whether RDN reduces SNA in other organs besides the kidneys and the importance of renal afferent sensory nerves compared to the efferent sympathetic nerves in mediating BP effects of RDN is unclear.
Studies in experimental models of HT have provided mixed results on the role of renal afferent nerves in chronic BP regulation.104 We assessed the role of renal sensory nerves in obese hypertensive dogs, a model that recapitulates many of the characteristics of human obesity hypertension, with surgical removal of afferent traffic by dorsal root ganglionectomy from T10 to L2. Complete excision of renal afferents did not attenuate obesity HT in dogs fed a high fat diet suggesting that BP effects of RDN are due to removal of renal sympathetic efferent fibers rather than afferents.165
Arguing against a major role for widespread sympathoinhibition due to removal of renal sensory afferents by RDN is the finding that reductions in BP develop slowly, requiring several days or weeks to occur. Presumably, if renal afferents had a major effect on SNA in skeletal muscles or other organs, sufficient to cause peripheral vasoconstriction and increased BP, RDN should rapidly reduce BP. The slowly developing fall in BP after RDN is more consistent with removal of renal sympathetic efferent nerves that impair renal excretory function.
Hemodynamic Changes After RDN.
In subjects with HT, RDN causes hemodynamic changes that are predictable from its effects to reduce BP, including reductions in cardiac work load and various indicators of vascular stiffness such as aortic pulse pressure, carotid to femoral pulse wave velocity, and aortic augmentation index.166 RDN generally has little or no effect on cardiac output (CO) but total peripheral vascular resistance (TPR) is reduced in parallel with BP.167 Although the effect of RDN on blood flows in various tissues has not been widely studied, the fact that CO is not significantly altered implies that blood flows in most organs and tissues are also unchanged since CO represents the sum of all tissue blood flows.
Why then is TPR reduced by RDN? Is this due to a signal from the kidneys (e.g. via renal sensory afferent nerve fibers) that in some way causes vasodilation in various tissues? Thus far, no credible physiological evidence has been generated for such an effect. A more likely explanation is that RDN has important renal effects (e.g. reductions in renal NaCl reabsorption and/or renin release) that ultimately lead to decreased BP which, in turn, causes a secondary decrease in TPR due to autoregulatory reductions in vascular resistance in various organs and tissues. Multiple studies in many different organs and tissues have demonstrated that when BP is reduced, acutely or chronically, there are compensatory pressure-dependent myogenic and flow-dependent local mechanisms that maintain blood flow relatively constant as long as BP is within the autoregulatory range (typically 70–150 mmHg mean arterial pressure for many tissues).168–171
RDN in experimental animals or in clinical studies has been reported to cause minimal changes in GFR, RBF or blood flow in other tissues in normotensive or hypertensive subjects, or in patients with mild to moderate chronic kidney disease (CKD).75, 79, 142, 167, 172 These observations suggest that in most physiological conditions and in many patients with HT and/or CKD the level of RSNA is not sufficient to directly cause vasoconstriction but may be sufficient to raise BP via multiple renal actions. However, BP reductions associated with RDN may, over the long-term, have favorable effects to protect against progression of injury in target organs, including the kidneys.
Summary: Renal Denervation for Hypertension Therapy
Clinical trials that have included sham control subjects suggest that CB-RDN, using radiofrequency and ultrasound endovascular devices, lowers BP by ~5 to 8 mmHg in the presence or absence of antihypertensive medications in many hypertensive patients. Based on the few studies in which the extent of RDN has been assessed, we speculate that these reductions in BP were achieved with <50% ablation of the renal nerves. Experimental studies suggest that as much as 80% ablation of the renal nerves may be possible with improved catheter based methods that target the distal renal artery and its branches. These improved methods should produce greater reductions in BP in selected patients but there is currently lack of reliable markers of immediate procedural success confirming effective RDN. There is also a need to identify phenotypes of patients who are most likely to respond to RDN with a clinically relevant decrease in BP.
Conclusions and Perspectives
Proponents of BA and RDN for HT have suggested that these device-based therapies offer a means of reducing BP in patients who may be intolerant to antihypertensive medications, who do not adhere to prescribed medications, and who have true drug RHT.131, 173 If significant reductions in BP can be achieved and sustained, in the absence of adverse events caused by these procedures, device-based approaches for modulating sympathetic activity and HT therapy would be expected to reduce cardiovascular risk. However, further long-term studies are needed to assess safety and durability of these procedures. Several trials are now underway and will ultimately inform patients and physicians on the feasibility, safety, and efficacy of BA and RDN as treatments for RHT. In future trials, greater attention should be given to eliminating variability in concomitant antihypertensive drug therapy and patient adherence to medications, which have been major confounders in understanding the underlying physiology responsible for the decrease in BP with device-based therapy.
An important unanswered question regarding device-based therapy for HT is whether clinical trials should not only demonstrate antihypertensive efficacy and safety of a procedure, but also its durability and as well as favorable long-term clinical outcomes. Because BP has a nearly linear and strong relationship to adverse cardiovascular outcomes, such as stroke, myocardial infarction, and mortality, the U.S. Federal Drug Administration accepts BP as a surrogate endpoint for approval of devices to treat HT if they have an acceptable safety profile. However, it is conceivable that adverse or beneficial effects on long-term clinical outcomes, beyond BP control, could emerge with long-term usage of devices for BA or RDN. Time and further, carefully controlled studies of longer duration should provide the answers to these questions.
ACKNOWLEDGMENTS
We thank Kendall Ratliff and Stephanie Lucas for their expert assistance in preparation of this manuscript.
SOURCES OF FUNDING
The authors’ research was supported by grants from the National Heart, Lung, and Blood Institute (P01 HL51971) and the National Institute of General Medical Sciences (P20 GM104357 and U54 GM115428).
Non-standard Abbreviations and Acronyms
- ACE
angiotensin converting enzyme inhibitor
- AHA
American Heart Association
- ANG II
angiotensin II
- ANP
atrial natriuretic peptide
- ARB
angiotensin receptor blocker
- BA
baroreceptor activation
- BP
blood pressure
- CB-RDN
catheter-based renal denervation
- CKD
chronic kidney disease
- CO
cardiac output
- DBP
diastolic blood pressure
- GFR
glomerular filtration rate
- HR
heart rate
- HRV
heart rate variability
- HT
hypertension
- IPG
implanted pulse generator
- MSNA
muscle sympathetic nerve activity
- NE
norepinephrine
- PRA
plasma renin activity
- RAAS
renin-angiotensin-aldosterone system
- RHT
resistant hypertension
- SAD
sinoaortic denervation
- SBP
systolic blood pressure
- SSAHT
standardized stepped-care antihypertensive treatment
- RBF
renal blood flow
- RDN
renal denervation
- RSNA
renal sympathetic nerve activity
- TGF
tubuloglomerular feedback
- TPR
total peripheral resistance
- VAT
visceral adipose tissue
Footnotes
DISCLOSURES
Dr. Lohmeier received consultant fees from CVRx, Inc.
Dr. Hall was the Principal Investigator of a research grant from St. Jude Medical, Inc. to the University of Mississippi Medical Center.
REFERENCES
- 1.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B and Carey RM. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403–1419. [DOI] [PubMed] [Google Scholar]
- 2.Carey RM, Calhoun DA, Bakris GL, Brook RD, Daugherty SL, Dennison-Himmelfarb CR, Egan BM, Flack JM, Gidding SS, Judd E, Lackland DT, Laffer CL, Newton-Cheh C, Smith SM, Taler SJ, Textor SC, Turan TN, White WB, American Heart Association Professional/Public E, Publications Committee of the Council on H, Council on C, Stroke N, Council on Clinical C, Council on G, Precision M, Council on Peripheral Vascular D, Council on Quality of C, Outcomes R and Stroke C. Resistant Hypertension: Detection, Evaluation, and Management: A Scientific Statement From the American Heart Association. Hypertension. 2018;72:e53–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomaszewski M, White C, Patel P, Masca N, Damani R, Hepworth J, Samani NJ, Gupta P, Madira W, Stanley A and Williams B. High rates of non-adherence to antihypertensive treatment revealed by high-performance liquid chromatography-tandem mass spectrometry (HP LC-MS/MS) urine analysis. Heart. 2014;100:855–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung O, Gechter JL, Wunder C, Paulke A, Bartel C, Geiger H and Toennes SW. Resistant hypertension? Assessment of adherence by toxicological urine analysis. J Hypertens. 2013;31:766–74. [DOI] [PubMed] [Google Scholar]
- 5.de Jager RL, de Beus E, Beeftink MM, Sanders MF, Vonken EJ, Voskuil M, van Maarseveen EM, Bots ML, Blankestijn PJ and Investigators S. Impact of Medication Adherence on the Effect of Renal Denervation: The SYMPATHY Trial. Hypertension. 2017;69:678–684. [DOI] [PubMed] [Google Scholar]
- 6.Gupta P, Patel P, Strauch B, Lai FY, Akbarov A, Gulsin GS, Beech A, Maresova V, Topham PS, Stanley A, Thurston H, Smith PR, Horne R, Widimsky J, Keavney B, Heagerty A, Samani NJ, Williams B and Tomaszewski M. Biochemical Screening for Nonadherence Is Associated With Blood Pressure Reduction and Improvement in Adherence. Hypertension. 2017;70:1042–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esler M The sympathetic nervous system in hypertension: back to the future? Curr Hypertens Rep. 2015;17:11. [DOI] [PubMed] [Google Scholar]
- 8.Grassi G, Mark A and Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res. 2015;116:976–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lohmeier TE, Barrett AM and Irwin ED. Prolonged activation of the baroreflex: a viable approach for the treatment of hypertension? Curr Hypertens Rep. 2005;7:193–198. [DOI] [PubMed] [Google Scholar]
- 10.Lohmeier TE and Iliescu R. Chronic Lowering of Blood Pressure by Carotid Baroreflex Activation: Mechanisms and Potential for Hypertension Therapy. Hypertension. 2011;57:880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Victor RG. Carotid baroreflex activation therapy for resistant hypertension. Nat Rev Cardiol. 2015;12:451–463. [DOI] [PubMed] [Google Scholar]
- 12.Alnima T, Kroon AA and de Leeuw PW. Baroreflex activation therapy for patients with drug-resistant hypertension. Expert Rev Cardiovasc Ther. 2014;12:955–962. [DOI] [PubMed] [Google Scholar]
- 13.Gassler JP and Bisognano JD. Baroreflex activation therapy in hypertension. J Hum Hypertens. 2014;28:469–474. [DOI] [PubMed] [Google Scholar]
- 14.Iliescu R, Tudorancea I and Lohmeier TE. Baroreflex activation: from mechanisms to therapy for cardiovascular disease. Curr Hypertens Rep. 2014;16:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chobanyan-Jurgens K and Jordan J. Electrical carotid sinus stimulation: chances and challenges in the management of treatment resistant arterial hypertension. Curr Hypertens Rep. 2015;17:587. [DOI] [PubMed] [Google Scholar]
- 16.Alnima T, Goedhart EJ, Seelen R, van der Grinten CP, de Leeuw PW and Kroon AA. Baroreflex activation therapy lowers arterial pressure without apparent stimulation of the carotid bodies. Hypertension. 2015;65:1217–1222. [DOI] [PubMed] [Google Scholar]
- 17.Lohmeier TE, Iliescu R, Tudorancea I, Cazan R, Cates AW, Georgakopoulos D and Irwin ED. Chronic Interactions Between Carotid Baroreceptors and Chemoreceptors in Obesity Hypertension. Hypertension. 2016;68:227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lohmeier TE and Iliescu R. The baroreflex as a long-term controller of arterial pressure. Physiology (Bethesda). 2015;30:148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lohmeier TE, Irwin ED, Rossing MA, Serdar DJ and Kieval RS. Prolonged activation of the baroreflex produces sustained hypotension. Hypertension. 2004;43:306–311. [DOI] [PubMed] [Google Scholar]
- 20.Lohmeier TE, Iliescu R, Dwyer TM, Irwin ED, Cates AW and Rossing MA. Sustained suppression of sympathetic activity and arterial pressure during chronic activation of the carotid baroreflex. Am J Physiol Heart Circ Physiol. 2010;299:H402–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall JE, do Carmo JM, da Silva AA, Wang Z and Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116:991–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lohmeier TE, Hildebrandt DA, Warren S, May PJ and Cunningham JT. Recent insights into the interactions between the baroreflex and the kidneys in hypertension. Am J Physiol Regul Integr Comp Physiol. 2005;288:R828–R836. [DOI] [PubMed] [Google Scholar]
- 23.Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G and Lambert E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension. 2006;48:787–796. [DOI] [PubMed] [Google Scholar]
- 24.Grassi G, Facchini A, Trevano FQ, Dell’Oro R, Arenare F, Tana F, Bolla G, Monzani A, Robuschi M and Mancia G. Obstructive sleep apnea-dependent and -independent adrenergic activation in obesity. Hypertension. 2005;46:321–325. [DOI] [PubMed] [Google Scholar]
- 25.Lohmeier TE, Dwyer TM, Irwin ED, Rossing MA and Kieval RS. Prolonged activation of the baroreflex abolishes obesity-induced hypertension. Hypertension. 2007;49:1307–1314. [DOI] [PubMed] [Google Scholar]
- 26.Finke R, Gross R, Hackenthal E, Huber J and Kirchheim HR. Threshold pressure for the pressure-dependent renin release in the autoregulating kidney of conscious dogs. Pflugers Arch. 1983;399:102–10. [DOI] [PubMed] [Google Scholar]
- 27.Lohmeier TE, Dwyer TM, Hildebrandt DA, Irwin ED, Rossing MA, Serdar DJ and Kieval RS. Influence of prolonged baroreflex activation on arterial pressure in angiotensin hypertension. Hypertension. 2005;46:1194–1200. [DOI] [PubMed] [Google Scholar]
- 28.Scheffers IJ, Kroon AA, Schmidli J, Jordan J, Tordoir JJ, Mohaupt MG, Luft FC, Haller H, Menne J, Engeli S, Ceral J, Eckert S, Erglis A, Narkiewicz K, Philipp T and de Leeuw PW. Novel baroreflex activation therapy in resistant hypertension: results of a European multi-center feasibility study. J Am Coll Cardiol. 2010;56:1254–1258. [DOI] [PubMed] [Google Scholar]
- 29.Wustmann K, Kucera JP, Scheffers I, Mohaupt M, Kroon AA, de Leeuw PW, Schmidli J, Allemann Y and Delacretaz E. Effects of chronic baroreceptor stimulation on the autonomic cardiovascular regulation in patients with drug-resistant arterial hypertension. Hypertension. 2009;54:530–536. [DOI] [PubMed] [Google Scholar]
- 30.Bisognano JD, Kaufman CL, Bach DS, Lovett EG, de Leeuw P, HT DE and Rheos Feasibility Trial I. Improved cardiac structure and function with chronic treatment using an implantable device in resistant hypertension: results from European and United States trials of the Rheos system. J Am Coll Cardiol. 2011;57:1787–1788. [DOI] [PubMed] [Google Scholar]
- 31.Heusser K, Tank J, Engeli S, Diedrich A, Menne J, Eckert S, Peters T, Sweep FC, Haller H, Pichlmaier AM, Luft FC and Jordan J. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension. 2010;55:619–626. [DOI] [PubMed] [Google Scholar]
- 32.Bisognano JD, Bakris G, Nadim MK, Sanchez L, Kroon AA, Schafer J, de Leeuw PW and Sica DA. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the double-blind, randomized, placebo-controlled rheos pivotal trial. J Am Coll Cardiol. 2011;58:765–773. [DOI] [PubMed] [Google Scholar]
- 33.Bakris GL, Nadim MK, Haller H, Lovett EG, Schafer JE and Bisognano JD. Baroreflex activation therapy provides durable benefit in patients with resistant hypertension: results of long-term follow-up in the Rheos Pivotal Trial. J Am Soc Hypertens. 2012;6:152–158. [DOI] [PubMed] [Google Scholar]
- 34.de Leeuw PW, Bisognano JD, Bakris GL, Nadim MK, Haller H, Kroon AA, HT DE and Rheos Trial I. Sustained Reduction of Blood Pressure With Baroreceptor Activation Therapy: Results of the 6-Year Open Follow-Up. Hypertension. 2017;69:836–843. [DOI] [PubMed] [Google Scholar]
- 35.de Leeuw PW, Alnima T, Lovett E, Sica D, Bisognano J, Haller H and Kroon AA. Bilateral or unilateral stimulation for baroreflex activation therapy. Hypertension. 2015;65:187–192. [DOI] [PubMed] [Google Scholar]
- 36.Hoppe UC, Brandt MC, Wachter R, Beige J, Rump LC, Kroon AA, Cates AW, Lovett EG and Haller H. Minimally invasive system for baroreflex activation therapy chronically lowers blood pressure with pacemaker-like safety profile: results from the Barostim neo trial. J Am Soc Hypertens. 2012;6:270–276. [DOI] [PubMed] [Google Scholar]
- 37.Lohmeier TE, Hildebrandt DA, Dwyer TM, Barrett AM, Irwin ED, Rossing MA and Kieval RS. Renal denervation does not abolish sustained baroreflex-mediated reductions in arterial pressure. Hypertension. 2007;49:373–9. [DOI] [PubMed] [Google Scholar]
- 38.Clemmer JS, Pruett WA, Hester RL, Iliescu R and Lohmeier TE. Role of the Heart in Blood Pressure Lowering During Chronic Baroreflex Activation: Insight from an in Silico Analysis. Am J Physiol Heart Circ Physiol. 2018;315:H1368–H1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lohmeier TE, Hildebrandt DA, Dwyer TM, Iliescu R, Irwin ED, Cates AW and Rossing MA. Prolonged activation of the baroreflex decreases arterial pressure even during chronic adrenergic blockade. Hypertension. 2009;53:833–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iliescu R, Irwin ED, Georgakopoulos D and Lohmeier TE. Renal responses to chronic suppression of central sympathetic outflow. Hypertension. 2012;60:749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT and Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. [DOI] [PubMed] [Google Scholar]
- 42.Lohmeier TE, Iliescu R, Liu B, Henegar JR, Maric-Bilkan C and Irwin ED. Systemic and renal-specific sympathoinhibition in obesity hypertension. Hypertension. 2012;59:331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lohmeier TE and Iliescu R. The sympathetic nervous system in obesity hypertension. Curr Hypertens Rep. 2013;15:409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alnima T, de Leeuw PW, Tan FE, Kroon AA and Rheos Pivotal Trial I. Renal responses to long-term carotid baroreflex activation therapy in patients with drug-resistant hypertension. Hypertension. 2013;61:1334–1339. [DOI] [PubMed] [Google Scholar]
- 45.Iliescu R, Tudorancea I, Irwin ED and Lohmeier TE. Chronic baroreflex activation restores spontaneous baroreflex control and variability of heart rate in obesity-induced hypertension. Am J Physiol Heart Circ Physiol. 2013;305:H1080–H1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beske SD, Alvarez GE, Ballard TP and Davy KP. Reduced cardiovagal baroreflex gain in visceral obesity: implications for the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2002;282:H630–H635. [DOI] [PubMed] [Google Scholar]
- 47.Grassi G, Seravalle G, Dell’Oro R, Turri C, Bolla GB and Mancia G. Adrenergic and reflex abnormalities in obesity-related hypertension. Hypertension. 2000;36:538–542. [DOI] [PubMed] [Google Scholar]
- 48.Skrapari I, Tentolouris N, Perrea D, Bakoyiannis C, Papazafiropoulou A and Katsilambros N. Baroreflex sensitivity in obesity: relationship with cardiac autonomic nervous system activity. Obesity (Silver Spring). 2007;15:1685–1693. [DOI] [PubMed] [Google Scholar]
- 49.Thayer JF, Yamamoto SS and Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;141:122–131. [DOI] [PubMed] [Google Scholar]
- 50.Billman GE. Heart rate variability - a historical perspective. Front Physiol. 2011;2:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hildreth CM. Prognostic indicators of cardiovascular risk in renal disease. Front Physiol. 2011;2:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Vliet BN, Hall JE, Mizelle HL, Montani JP and Smith MJ Jr. Reduced parasympathetic control of heart rate in obese dogs. Am J Physiol. 1995;269:H629–H637. [DOI] [PubMed] [Google Scholar]
- 53.Aronne LJ, Mackintosh R, Rosenbaum M, Leibel RL and Hirsch J. Autonomic nervous system activity in weight gain and weight loss. Am J Physiol. 1995;269:R222–R225. [DOI] [PubMed] [Google Scholar]
- 54.Gordin D, Fadl Elmula FEM, Andersson B, Gottsater A, Elf J, Kahan T, Christensen KL, Vikatmaa P, Vikatmaa L, Bastholm Olesen T, Groop PH, Olsen MH, Tikkanen I and Nordic BATSG. The effects of baroreflex activation therapy on blood pressure and sympathetic function in patients with refractory hypertension: the rationale and design of the Nordic BAT study. Blood Press. 2017;26:294–302. [DOI] [PubMed] [Google Scholar]
- 55.Halbach M, Hickethier T, Madershahian N, Reuter H, Brandt MC, Hoppe UC and Muller-Ehmsen J. Acute on/off effects and chronic blood pressure reduction after long-term baroreflex activation therapy in resistant hypertension. J Hypertens. 2015;33:1697–703. [DOI] [PubMed] [Google Scholar]
- 56.Wallbach M, Halbach M, Reuter H, Passauer J, Luders S, Bohning E, Zenker D, Muller GA, Wachter R and Koziolek MJ. Baroreflex activation therapy in patients with prior renal denervation. J Hypertens. 2016;34:1630–1638. [DOI] [PubMed] [Google Scholar]
- 57.Heusser K, Tank J, Brinkmann J, Menne J, Kaufeld J, Linnenweber-Held S, Beige J, Wilhelmi M, Diedrich A, Haller H and Jordan J. Acute Response to Unilateral Unipolar Electrical Carotid Sinus Stimulation in Patients With Resistant Arterial Hypertension. Hypertension. 2016;67:585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wallbach M, Lehnig LY, Schroer C, Luders S, Bohning E, Muller GA, Wachter R and Koziolek MJ. Effects of Baroreflex Activation Therapy on Ambulatory Blood Pressure in Patients With Resistant Hypertension. Hypertension. 2016;67:701–709. [DOI] [PubMed] [Google Scholar]
- 59.Beige J, Jentzsch T, Wendt R, Hennig G, Koziolek M and Wallbach M. Blood pressure after blinded, randomized withdrawal, and resumption of baroreceptor-activating therapy. J Hypertens. 2017;35:1496–1501. [DOI] [PubMed] [Google Scholar]
- 60.Wallbach M, Bohning E, Lehnig LY, Schroer C, Muller GA, Wachter R, Luders S, Zenker D and Koziolek MJ. Safety profile of baroreflex activation therapy (NEO) in patients with resistant hypertension. J Hypertens. 2018;36:1762–1769. [DOI] [PubMed] [Google Scholar]
- 61.Hildebrandt DA, Irwin ED and Lohmeier TE. Prolonged Baroreflex Activation Abolishes Salt-Induced Hypertension After Reductions in Kidney Mass. Hypertension. 2016;68:1400–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lohmeier TE, Liu B, Hildebrandt DA, Cates AW, Georgakopoulos D and Irwin ED. Global- and renal-specific sympathoinhibition in aldosterone hypertension. Hypertension. 2015;65:1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kandzari DE, Bhatt DL, Brar S, Devireddy CM, Esler M, Fahy M, Flack JM, Katzen BT, Lea J, Lee DP, Leon MB, Ma A, Massaro J, Mauri L, Oparil S, O’Neill WW, Patel MR, Rocha-Singh K, Sobotka PA, Svetkey L, Townsend RR and Bakris GL. Predictors of blood pressure response in the SYMPLICITY HTN-3 trial. Eur Heart J. 2015;36:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spiering W, Williams B, Van der Heyden J, van Kleef M, Lo R, Versmissen J, Moelker A, Kroon A, Reuter H, Ansel G, Stone GW, Bates M and investigators C-FE. Endovascular baroreflex amplification for resistant hypertension: a safety and proof-of-principle clinical study. Lancet. 2017;390:2655–2661. [DOI] [PubMed] [Google Scholar]
- 65.Zucker IH, Hackley JF, Cornish KG, Hiser BA, Anderson NR, Kieval R, Irwin ED, Serdar DJ, Peuler JD and Rossing MA. Chronic baroreceptor activation enhances survival in dogs with pacing-induced heart failure. Hypertension. 2007;50:904–10. [DOI] [PubMed] [Google Scholar]
- 66.Sabbah HN, Gupta RC, Imai M, Irwin ED, Rastogi S, Rossing MA and Kieval RS. Chronic electrical stimulation of the carotid sinus baroreflex improves left ventricular function and promotes reversal of ventricular remodeling in dogs with advanced heart failure. Circ Heart Fail. 2011;4:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gronda E, Seravalle G, Brambilla G, Costantino G, Casini A, Alsheraei A, Lovett EG, Mancia G and Grassi G. Chronic baroreflex activation effects on sympathetic nerve traffic, baroreflex function, and cardiac haemodynamics in heart failure: a proof-of-concept study. Eur J Heart Fail. 2014;16:977–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abraham WT, Zile MR, Weaver FA, Butter C, Ducharme A, Halbach M, Klug D, Lovett EG, Muller-Ehmsen J, Schafer JE, Senni M, Swarup V, Wachter R and Little WC. Baroreflex Activation Therapy for the Treatment of Heart Failure With a Reduced Ejection Fraction. JACC Heart Fail. 2015;3:487–496. [DOI] [PubMed] [Google Scholar]
- 69.Zile MR, Abraham WT, Lindenfeld J, Weaver FA, Zannad F, Graves T, Rogers T and Galle EG. First granted example of novel FDA trial design under Expedited Access Pathway for premarket approval: BeAT-HF. Am Heart J. 2018;204:139–150. [DOI] [PubMed] [Google Scholar]
- 70.DiBona GF and Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. [DOI] [PubMed] [Google Scholar]
- 71.Gross R, Kirchheim H and Ruffmann K. Effect of carotid occlusion and of perfusion pressure on renal function in conscious dogs. Circ Res. 1981;48:777–784. [DOI] [PubMed] [Google Scholar]
- 72.Gross R, Hackenberg HM, Hackenthal E and Kirchheim H. Interaction between perfusion pressure and sympathetic nerves in renin release by carotid baroreflex in conscious dogs. J Physiol. 1981;313:237–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gross R and Kirchheim H. Effects of bilateral carotid and auditory stimulation on renal blood flow and sympathetic nerve activity in the conscious dog. Pflugers Arch. 1980;383:233–239. [DOI] [PubMed] [Google Scholar]
- 74.Barrett CJ, Navakatikyan MA and Malpas SC. Long-term control of renal blood flow: what is the role of the renal nerves? Am J Physiol Regul Integr Comp Physiol. 2001;280:R1534–R1545. [DOI] [PubMed] [Google Scholar]
- 75.Mizelle HL, Hall JE, Woods LL, Montani JP, Dzielak DJ and Pan YJ. Role of renal nerves in compensatory adaptation to chronic reductions in sodium intake. Am J Physiol. 1987;252:F291–F298. [DOI] [PubMed] [Google Scholar]
- 76.DiBona GF and Esler M. Translational medicine: the antihypertensive effect of renal denervation. Am J Physiol Regul Integr Comp Physiol. 2010;298:R245–R253. [DOI] [PubMed] [Google Scholar]
- 77.Townsend RR and Sobotka PA. Catheter-Based Renal Denervation for Hypertension. Curr Hypertens Rep. 2018;20:93. [DOI] [PubMed] [Google Scholar]
- 78.Kassab S, Kato T, Wilkins FC, Chen R, Hall JE and Granger JP. Renal denervation attenuates the sodium retention and hypertension associated with obesity. Hypertension. 1995;25:893–897. [DOI] [PubMed] [Google Scholar]
- 79.Henegar JR, Zhang Y, Rama RD, Hata C, Hall ME and Hall JE. Catheter-based radiorefrequency renal denervation lowers blood pressure in obese hypertensive dogs. Am J Hypertens. 2014;27:1285–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Armitage JA, Burke SL, Prior LJ, Barzel B, Eikelis N, Lim K and Head GA. Rapid onset of renal sympathetic nerve activation in rabbits fed a high-fat diet. Hypertension. 2012;60:163–171. [DOI] [PubMed] [Google Scholar]
- 81.Judy WV, Watanabe AM, Henry DP, Besch HR Jr., Murphy WR and Hockel GM. Sympathetic nerve activity: role in regulation of blood pressure in the spontaenously hypertensive rat. Circ Res. 1976;38:21–29. [DOI] [PubMed] [Google Scholar]
- 82.Norman RA Jr. and Dzielak DJ. Role of renal nerves in onset and maintenance of spontaneous hypertension. Am J Physiol. 1982;243:H284–H288. [DOI] [PubMed] [Google Scholar]
- 83.Winternitz SR, Katholi RE and Oparil S. Role of the renal sympathetic nerves in the development and maintenance of hypertension in the spontaneously hypertensive rat. J Clin Invest. 1980;66:971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Foss JD, Fink GD and Osborn JW. Differential role of afferent and efferent renal nerves in the maintenance of early- and late-phase Dahl S hypertension. Am J Physiol Regul Integr Comp Physiol. 2016;310:R262–R267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Foss JD, Fiege J, Shimizu Y, Collister JP, Mayerhofer T, Wood L and Osborn JW. Role of afferent and efferent renal nerves in the development of AngII-salt hypertension in rats. Physiol Rep. 2018;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barrett CJ, Ramchandra R, Guild SJ, Lala A, Budgett DM and Malpas SC. What sets the long-term level of renal sympathetic nerve activity: a role for angiotensin II and baroreflexes? Circ Res. 2003;92:1330–6. [DOI] [PubMed] [Google Scholar]
- 87.McBryde FD, Guild SJ, Barrett CJ, Osborn JW and Malpas SC. Angiotensin II-based hypertension and the sympathetic nervous system: the role of dose and increased dietary salt in rabbits. Exp Physiol. 2007;92:831–40. [DOI] [PubMed] [Google Scholar]
- 88.Xiao L, Kirabo A, Wu J, Saleh MA, Zhu L, Wang F, Takahashi T, Loperena R, Foss JD, Mernaugh RL, Chen W, Roberts J 2nd, Osborn JW, Itani HA and Harrison DG. Renal Denervation Prevents Immune Cell Activation and Renal Inflammation in Angiotensin II-Induced Hypertension. Circ Res. 2015;117:547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tudorancea I, Lohmeier TE, Alexander BT, Pieptu D, Serban DN and Iliescu R. Reduced Renal Mass, Salt-Sensitive Hypertension Is Resistant to Renal Denervation. Front Physiol. 2018;9:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Banek CT, Knuepfer MM, Foss JD, Fiege JK, Asirvatham-Jeyaraj N, Van Helden D, Shimizu Y and Osborn JW. Resting Afferent Renal Nerve Discharge and Renal Inflammation: Elucidating the Role of Afferent and Efferent Renal Nerves in Deoxycorticosterone Acetate Salt Hypertension. Hypertension. 2016;68:1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goldblatt H, Gross J and Hanzal RF. Studies on Experimental Hypertension : Ii. The Effect of Resection of Splanchnic Nerves on Experimental Renal Hypertension. J Exp Med. 1937;65:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Norman RA Jr., Murphy WR, Dzielak DJ, Khraibi AA and Carroll RG. Role of the renal nerves in one-kidney, one clip hypertension in rats. Hypertension. 1984;6:622–626. [DOI] [PubMed] [Google Scholar]
- 93.Granger J, Novak J, Schnackenberg C, Williams S and Reinhart GA. Role of renal nerves in mediating the hypertensive effects of nitric oxide synthesis inhibition. Hypertension. 1996;27:613–618. [DOI] [PubMed] [Google Scholar]
- 94.Ramchandra R, Barrett CJ, Guild SJ, McBryde F and Malpas SC. Role of renal sympathetic nerve activity in hypertension induced by chronic nitric oxide inhibition. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1479–R1485. [DOI] [PubMed] [Google Scholar]
- 95.Matsuoka H, Nishida H, Nomura G, Van Vliet BN and Toshima H. Hypertension induced by nitric oxide synthesis inhibition is renal nerve dependent. Hypertension. 1994;23:971–975. [DOI] [PubMed] [Google Scholar]
- 96.Alvarez GE, Beske SD, Ballard TP and Davy KP. Sympathetic neural activation in visceral obesity. Circulation. 2002;106:2533–2536. [DOI] [PubMed] [Google Scholar]
- 97.Davy KP and Hall JE. Obesity and hypertension: two epidemics or one? Am J Physiol Regul Integr Comp Physiol. 2004;286:R803–R813. [DOI] [PubMed] [Google Scholar]
- 98.Alvarez GE, Ballard TP, Beske SD and Davy KP. Subcutaneous obesity is not associated with sympathetic neural activation. Am J Physiol Heart Circ Physiol. 2004;287:H414–H418. [DOI] [PubMed] [Google Scholar]
- 99.Grassi G, Quarti-Trevano F, Seravalle G, Arenare F, Volpe M, Furiani S, Dell’Oro R and Mancia G. Early sympathetic activation in the initial clinical stages of chronic renal failure. Hypertension. 2011;57:846–851. [DOI] [PubMed] [Google Scholar]
- 100.Hering D, Mahfoud F, Walton AS, Krum H, Lambert GW, Lambert EA, Sobotka PA, Bohm M, Cremers B, Esler MD and Schlaich MP. Renal denervation in moderate to severe CKD. J Am Soc Nephrol. 2012;23:1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Page IH and Heuer GJ. The Effect of Renal Denervation on Patients Suffering from Nephritis. J Clin Invest. 1935;14:443–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Asirvatham-Jeyaraj N, Fiege JK, Han R, Foss J, Banek CT, Burbach BJ, Razzoli M, Bartolomucci A, Shimizu Y, Panoskaltsis-Mortari A and Osborn JW. Renal Denervation Normalizes Arterial Pressure With No Effect on Glucose Metabolism or Renal Inflammation in Obese Hypertensive Mice. Hypertension. 2016;68:929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Iliescu R, Lohmeier TE, Tudorancea I, Laffin L and Bakris GL. Renal denervation for the treatment of resistant hypertension: review and clinical perspective. Am J Physiol Renal Physiol. 2015;309:F583–F594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Osborn JW and Foss JD. Renal Nerves and Long-Term Control of Arterial Pressure. Compr Physiol. 2017;7:263–320. [DOI] [PubMed] [Google Scholar]
- 105.Page IH and Heuer GJ. The Effect of Renal Denervation on the Level of Arterial Blood Pressure and Renal Function in Essential Hypertension. J Clin Invest. 1935;14:27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smithwick RH. Surgical treatment of hypertension. Am J Med. 1948;4:744–759. [DOI] [PubMed] [Google Scholar]
- 107.Smithwick RH and Thompson JE. Splanchnicectomy for essential hypertension; results in 1,266 cases. J Am Med Assoc. 1953;152:1501–1504. [DOI] [PubMed] [Google Scholar]
- 108.Esler M, Lambert G, Schlaich M, Dixon J, Sari CI and Lambert E. Obesity Paradox in Hypertension: Is This Because Sympathetic Activation in Obesity-Hypertension Takes a Benign Form? Hypertension. 2018;71:22–33. [DOI] [PubMed] [Google Scholar]
- 109.Grassi G, Pisano A, Bolignano D, Seravalle G, D’Arrigo G, Quarti-Trevano F, Mallamaci F, Zoccali C and Mancia G. Sympathetic Nerve Traffic Activation in Essential Hypertension and Its Correlates: Systematic Reviews and Meta-Analyses. Hypertension. 2018;72:483–491. [DOI] [PubMed] [Google Scholar]
- 110.Javaheri S, Barbe F, Campos-Rodriguez F, Dempsey JA, Khayat R, Javaheri S, Malhotra A, Martinez-Garcia MA, Mehra R, Pack AI, Polotsky VY, Redline S and Somers VK. Sleep Apnea: Types, Mechanisms, and Clinical Cardiovascular Consequences. J Am Coll Cardiol. 2017;69:841–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Grassi G, Seravalle G, Brambilla G, Trabattoni D, Cuspidi C, Corso R, Pieruzzi F, Genovesi S, Stella A, Facchetti R, Spaziani D, Bartorelli A and Mancia G. Blood pressure responses to renal denervation precede and are independent of the sympathetic and baroreflex effects. Hypertension. 2015;65:1209–1216. [DOI] [PubMed] [Google Scholar]
- 112.Hall JE, Brands MW, Dixon WN and Smith MJ Jr. Obesity-induced hypertension. Renal function and systemic hemodynamics. Hypertension. 1993;22:292–299. [DOI] [PubMed] [Google Scholar]
- 113.Garrison RJ, Kannel WB, Stokes J III and Castelli WP. Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Prev Med. 1987;16:235–251. [DOI] [PubMed] [Google Scholar]
- 114.Hall ME, do Carmo JM, da Silva AA, Juncos LA, Wang Z and Hall JE. Obesity, hypertension, and chronic kidney disease. Int J Nephrol Renovasc Dis. 2014;7:75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hall JE, Granger JP, do Carmo JM, da Silva AA, Dubinion J, George E, Hamza S, Speed J and Hall ME. Hypertension: physiology and pathophysiology. Compr Physiol. 2012;2:2393–2442. [DOI] [PubMed] [Google Scholar]
- 116.Krum H, Schlaich MP, Sobotka PA, Bohm M, Mahfoud F, Rocha-Singh K, Katholi R and Esler MD. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet. 2014;383:622–629. [DOI] [PubMed] [Google Scholar]
- 117.Symplicity H-I, Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE and Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–1909. [DOI] [PubMed] [Google Scholar]
- 118.Symplicity H-I. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension. 2011;57:911–917. [DOI] [PubMed] [Google Scholar]
- 119.Worthley SG, Tsioufis CP, Worthley MI, Sinhal A, Chew DP, Meredith IT, Malaiapan Y and Papademetriou V. Safety and efficacy of a multi-electrode renal sympathetic denervation system in resistant hypertension: the EnligHTN I trial. Eur Heart J. 2013;34:2132–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Papademetriou V, Tsioufis CP, Sinhal A, Chew DP, Meredith IT, Malaiapan Y, Worthley MI and Worthley SG. Catheter-based renal denervation for resistant hypertension: 12-month results of the EnligHTN I first-in-human study using a multielectrode ablation system. Hypertension. 2014;64:565–572. [DOI] [PubMed] [Google Scholar]
- 121.Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR and Bakris GL. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401. [DOI] [PubMed] [Google Scholar]
- 122.Bakris GL, Townsend RR, Flack JM, Brar S, Cohen SA, D’Agostino R, Kandzari DE, Katzen BT, Leon MB, Mauri L, Negoita M, O’Neill WW, Oparil S, Rocha-Singh K, Bhatt DL and Investigators SH-. 12-month blood pressure results of catheter-based renal artery denervation for resistant hypertension: the SYMPLICITY HTN-3 trial. J Am Coll Cardiol. 2015;65:1314–1321. [DOI] [PubMed] [Google Scholar]
- 123.Esler M Illusions of truths in the Symplicity HTN-3 trial: generic design strengths but neuroscience failings. J Am Soc Hypertens. 2014;8:593–598. [DOI] [PubMed] [Google Scholar]
- 124.Kandzari DE. Symplicity HTN-3 Trial: Analysis of potentially confounding factors. Paper presented at: EuroPCR 2014; 2014; Paris, France. [Google Scholar]
- 125.Azizi M, Sapoval M, Gosse P, Monge M, Bobrie G, Delsart P, Midulla M, Mounier-Vehier C, Courand PY, Lantelme P, Denolle T, Dourmap-Collas C, Trillaud H, Pereira H, Plouin PF, Chatellier G and Renal Denervation for Hypertension i. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet. 2015;385:1957–1965. [DOI] [PubMed] [Google Scholar]
- 126.Townsend RR, Mahfoud F, Kandzari DE, Kario K, Pocock S, Weber MA, Ewen S, Tsioufis K, Tousoulis D, Sharp ASP, Watkinson AF, Schmieder RE, Schmid A, Choi JW, East C, Walton A, Hopper I, Cohen DL, Wilensky R, Lee DP, Ma A, Devireddy CM, Lea JP, Lurz PC, Fengler K, Davies J, Chapman N, Cohen SA, DeBruin V, Fahy M, Jones DE, Rothman M, Bohm M and investigators* SH-OMt. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet. 2017;390:2160–2170. [DOI] [PubMed] [Google Scholar]
- 127.Kandzari DE, Bohm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, Tsioufis K, Tousoulis D, Choi JW, East C, Brar S, Cohen SA, Fahy M, Pilcher G, Kario K and Investigators SH-OMT. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391:2346–2355. [DOI] [PubMed] [Google Scholar]
- 128.Kario K, Bohm M, Mahfoud F, Townsend RR, Weber MA, Patel M, Tyson CC, Weil J, Agdirlioglu T, Cohen SA, Fahy M and Kandzari DE. Twenty-Four-Hour Ambulatory Blood Pressure Reduction Patterns After Renal Denervation in the SPYRAL HTN-OFF MED Trial. Circulation. 2018;138:1602–1604. [DOI] [PubMed] [Google Scholar]
- 129.Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Davies J, Basile J, Kirtane AJ, Wang Y, Lobo MD, Saxena M, Feyz L, Rader F, Lurz P, Sayer J, Sapoval M, Levy T, Sanghvi K, Abraham J, Sharp ASP, Fisher NDL, Bloch MJ, Reeve-Stoffer H, Coleman L, Mullin C, Mauri L and Investigators R-H. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391:2335–2345. [DOI] [PubMed] [Google Scholar]
- 130.Fengler K, Rommel K, Blazek S, Besler C, Hartung P, von Roeder M, Petzold M, Winkler S, Hollriegel R, Desch S, Thiele H and Lurz P. A Three-Arm Randomized Trial of Different Renal Denervation Devices and Techniques in Patients with Resistant Hypertension (RADIOSOUND-HTN). Circulation. 2018. [DOI] [PubMed] [Google Scholar]
- 131.Mahfoud F, Schlaich M, Bohm M, Esler M and Luscher TF. Catheter-based renal denervation: the next chapter begins. Eur Heart J. 2018. [DOI] [PubMed] [Google Scholar]
- 132.Mahfoud F, Bakris G, Bhatt DL, Esler M, Ewen S, Fahy M, Kandzari D, Kario K, Mancia G, Weber M and Bohm M. Reduced blood pressure-lowering effect of catheter-based renal denervation in patients with isolated systolic hypertension: data from SYMPLICITY HTN-3 and the Global SYMPLICITY Registry. Eur Heart J. 2017;38:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ott C, Schmid A, Toennes SW, Ditting T, Veelken R, Uder M and Schmieder RE. Central pulse pressure predicts BP reduction after renal denervation in patients with treatment-resistant hypertension. EuroIntervention. 2015;11:110–6. [DOI] [PubMed] [Google Scholar]
- 134.Okon T, Rohnert K, Stiermaier T, Rommel KP, Muller U, Fengler K, Schuler G, Desch S and Lurz P. Invasive aortic pulse wave velocity as a marker for arterial stiffness predicts outcome of renal sympathetic denervation. EuroIntervention. 2016;12:e684–92. [DOI] [PubMed] [Google Scholar]
- 135.Hering D Predictive Role of Nighttime Blood Pressure in Response to Renal Denervation: Evidence From the DENER-HTN Study (Renal Denervation for Hypertension). Hypertension. 2017;69:398–400. [DOI] [PubMed] [Google Scholar]
- 136.Gosse P, Cremer A, Pereira H, Bobrie G, Chatellier G, Chamontin B, Courand PY, Delsart P, Denolle T, Dourmap C, Ferrari E, Girerd X, Michel Halimi J, Herpin D, Lantelme P, Monge M, Mounier-Vehier C, Mourad JJ, Ormezzano O, Ribstein J, Rossignol P, Sapoval M, Vaisse B, Zannad F and Azizi M. Twenty-Four-Hour Blood Pressure Monitoring to Predict and Assess Impact of Renal Denervation: The DENERHTN Study (Renal Denervation for Hypertension). Hypertension. 2017;69:494–500. [DOI] [PubMed] [Google Scholar]
- 137.Lohmeier TE and Hildebrandt DA. Renal nerves promote sodium excretion in angiotensin-induced hypertension. Hypertension. 1998;31:429–434. [DOI] [PubMed] [Google Scholar]
- 138.do Carmo JM, da Silva AA, Wang Z, Fang T, Aberdein N, de Lara Rodriguez CE and Hall JE. Obesity-Induced Hypertension: Brain Signaling Pathways. Curr Hypertens Rep. 2016;18:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G and Stec DE. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem. 2010;285:17271–17276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cai A and Calhoun DA. Resistant Hypertension: An Update of Experimental and Clinical Findings. Hypertension. 2017;70:5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mahfoud F, Moon LB, Pipenhagen CA, Jensen JA, Pathak A, Papademetriou V, Ewen S, Linz D and Bohm M. Catheter-based radio-frequency renal nerve denervation lowers blood pressure in obese hypertensive swine model. J Hypertens. 2016;34:1854–62. [DOI] [PubMed] [Google Scholar]
- 142.Mizelle HL, Hall JE and Woods LL. Interactions between angiotensin II and renal nerves during chronic sodium deprivation. Am J Physiol. 1988;255:F823–F827. [DOI] [PubMed] [Google Scholar]
- 143.Tzafriri AR, Keating JH, Markham PM, Spognardi AM, Stanley JR, Wong G, Zani BG, Highsmith D, O’Fallon P, Fuimaono K, Mahfoud F and Edelman ER. Arterial microanatomy determines the success of energy-based renal denervation in controlling hypertension. Sci Transl Med. 2015;7:285ra65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tzafriri AR, Mahfoud F, Keating JH, Markham PM, Spognardi A, Wong G, Fuimaono K, Bohm M and Edelman ER. Innervation patterns may limit response to endovascular renal denervation. J Am Coll Cardiol. 2014;64:1079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Henegar JR, Zhang Y, Hata C, Narciso I, Hall ME and Hall JE. Catheter-Based Radiofrequency Renal Denervation: Location Effects on Renal Norepinephrine. Am J Hypertens. 2015;28:909–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mahfoud F, Tunev S, Ewen S, Cremers B, Ruwart J, Schulz-Jander D, Linz D, Davies J, Kandzari DE, Whitbourn R, Bohm M and Melder RJ. Impact of Lesion Placement on Efficacy and Safety of Catheter-Based Radiofrequency Renal Denervation. J Am Coll Cardiol. 2015;66:1766–1775. [DOI] [PubMed] [Google Scholar]
- 147.Sakakura K, Ladich E, Cheng Q, Otsuka F, Yahagi K, Fowler DR, Kolodgie FD, Virmani R and Joner M. Anatomic assessment of sympathetic peri-arterial renal nerves in man. J Am Coll Cardiol. 2014;64:635–643. [DOI] [PubMed] [Google Scholar]
- 148.Singh RR and Denton KM. Renal Denervation. Hypertension. 2018;72:528–536. [DOI] [PubMed] [Google Scholar]
- 149.Dorr O, Liebetrau C, Mollmann H, Gaede L, Troidl C, Haidner V, Wiebe J, Voss S, Bauer T, Hamm C and Nef H. Brain-derived neurotrophic factor as a marker for immediate assessment of the success of renal sympathetic denervation. J Am Coll Cardiol. 2015;65:1151–3. [DOI] [PubMed] [Google Scholar]
- 150.Hoogerwaard AF, de Jong MR and Elvan A. Renal Nerve Stimulation as Procedural End Point for Renal Sympathetic Denervation. Curr Hypertens Rep. 2018;20:24. [DOI] [PubMed] [Google Scholar]
- 151.de Jong MR, Adiyaman A, Gal P, Smit JJ, Delnoy PP, Heeg JE, van Hasselt BA, Lau EO, Persu A, Staessen JA, Ramdat Misier AR, Steinberg JS and Elvan A. Renal Nerve Stimulation-Induced Blood Pressure Changes Predict Ambulatory Blood Pressure Response After Renal Denervation. Hypertension. 2016;68:707–14. [DOI] [PubMed] [Google Scholar]
- 152.Id D, Kaltenbach B, Bertog SC, Hornung M, Hofmann I, Vaskelyte L and Sievert H. Does the presence of accessory renal arteries affect the efficacy of renal denervation? JACC Cardiovasc Interv. 2013;6:1085–1091. [DOI] [PubMed] [Google Scholar]
- 153.Kline RL and Mercer PF. Functional reinnervation and development of supersensitivity to NE after renal denervation in rats. Am J Physiol. 1980;238:R353–R358. [DOI] [PubMed] [Google Scholar]
- 154.Mulder J, Hokfelt T, Knuepfer MM and Kopp UC. Renal sensory and sympathetic nerves reinnervate the kidney in a similar time-dependent fashion after renal denervation in rats. Am J Physiol Regul Integr Comp Physiol. 2013;304:R675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Booth LC, Nishi EE, Yao ST, Ramchandra R, Lambert GW, Schlaich MP and May CN. Reinnervation of renal afferent and efferent nerves at 5.5 and 11 months after catheter-based radiofrequency renal denervation in sheep. Hypertension. 2015;65:393–400. [DOI] [PubMed] [Google Scholar]
- 156.Nomura G, Kurosaki M, Takabatake T, Kibe Y and Takeuchi J. Reinnervation and renin release after unilateral renal denervation in the dog. J Appl Physiol. 1972;33:649–655. [DOI] [PubMed] [Google Scholar]
- 157.Mauriello A, Rovella V, Borri F, Anemona L, Giannini E, Giacobbi E, Saggini A, Palmieri G, Anselmo A, Bove P, Melino G, Valentina G, Tesauro M, Gabriele D and Di Daniele N. Hypertension in kidney transplantation is associated with an early renal nerve sprouting. Nephrol Dial Transplant. 2017;32:1053–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.DiBona GF. Sympathetic nervous system and hypertension. Hypertension. 2013;61:556–560. [DOI] [PubMed] [Google Scholar]
- 159.Schlaich MP, Sobotka PA, Krum H, Lambert E and Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med. 2009;361:932–934. [DOI] [PubMed] [Google Scholar]
- 160.Hering D, Lambert EA, Marusic P, Walton AS, Krum H, Lambert GW, Esler MD and Schlaich MP. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension. 2013;61:457–464. [DOI] [PubMed] [Google Scholar]
- 161.Witkowski A, Prejbisz A, Florczak E, Kadziela J, Sliwinski P, Bielen P, Michalowska I, Kabat M, Warchol E, Januszewicz M, Narkiewicz K, Somers VK, Sobotka PA and Januszewicz A. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension. 2011;58:559–565. [DOI] [PubMed] [Google Scholar]
- 162.Brinkmann J, Heusser K, Schmidt BM, Menne J, Klein G, Bauersachs J, Haller H, Sweep FC, Diedrich A, Jordan J and Tank J. Catheter-based renal nerve ablation and centrally generated sympathetic activity in difficult-to-control hypertensive patients: prospective case series. Hypertension. 2012;60:1485–1490. [DOI] [PubMed] [Google Scholar]
- 163.Verloop WL, Spiering W, Vink EE, Beeftink MM, Blankestijn PJ, Doevendans PA and Voskuil M. Denervation of the renal arteries in metabolic syndrome: the DREAMS-study. Hypertension. 2015;65:751–757. [DOI] [PubMed] [Google Scholar]
- 164.Chhabra KH, Morgan DA, Tooke BP, Adams JM, Rahmouni K and Low MJ. Reduced renal sympathetic nerve activity contributes to elevated glycosuria and improved glucose tolerance in hypothalamus-specific Pomc knockout mice. Mol Metab. 2017;6:1274–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zappe DH, Capel WT, Keen HL, Shek EW, Brands MW and Hall JE. Role of renal afferent nerves in obesity-induced hypertension. Am J Hypertens. 1996;9:20A. [Google Scholar]
- 166.Brandt MC, Reda S, Mahfoud F, Lenski M, Bohm M and Hoppe UC. Effects of renal sympathetic denervation on arterial stiffness and central hemodynamics in patients with resistant hypertension. J Am Coll Cardiol. 2012;60:1956–1965. [DOI] [PubMed] [Google Scholar]
- 167.Ewen S, Cremers B, Meyer MR, Donazzan L, Kindermann I, Ukena C, Helfer AG, Maurer HH, Laufs U, Grassi G, Bohm M and Mahfoud F. Blood pressure changes after catheter-based renal denervation are related to reductions in total peripheral resistance. J Hypertens. 2015;33:2519–2525. [DOI] [PubMed] [Google Scholar]
- 168.Coleman TG, Samar RE and Murphy WR. Autoregulation versus other vasoconstrictors in hypertension. A critical review. Hypertension. 1979;1:324–330. [DOI] [PubMed] [Google Scholar]
- 169.Cowley AW Jr. Long-term control of arterial blood pressure. Physiol Rev. 1992;72:231–300. [DOI] [PubMed] [Google Scholar]
- 170.Hall JE. Renal Dysfunction, Rather Than Nonrenal Vascular Dysfunction, Mediates Salt-Induced Hypertension. Circulation. 2016;133:894–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Carlstrom M, Wilcox CS and Arendshorst WJ. Renal autoregulation in health and disease. Physiol Rev. 2015;95:405–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ott C, Janka R, Schmid A, Titze S, Ditting T, Sobotka PA, Veelken R, Uder M and Schmieder RE. Vascular and renal hemodynamic changes after renal denervation. Clin J Am Soc Nephrol. 2013;8:1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Schlaich MP, Kiuchi MG and Esler MD. Renal Denervation-Ready for Prime Time!? The Steep SPYRAL Stairs to RADIANCE in Hypertension Treatment. Hypertension. 2018;72:287–290. [DOI] [PubMed] [Google Scholar]