Abstract
Neuropathy is a major diabetic complication. While the mechanism of this neuropathy is not well-understood, it is believed to result in part from deficient nerve regeneration. Work from our laboratory established that gp130 family of cytokines are induced in animals after axonal injury and are involved in the induction of regeneration-associated genes (RAGs) and in the conditioning lesion response. Here, we examine whether a reduction of cytokine signaling occurs in diabetes. Streptozotocin (STZ) was used to destroy pancreatic β cells, leading to chronic hyperglycemia. Mice were injected with either low doses of STZ (5× 60 mg/kg) or a single high dose (1× 200 mg/kg) and examined after three or one month, respectively. Both low and high dose STZ treatment resulted in sustained hyperglycemia and functional deficits associated with the presence of both sensory and autonomic neuropathy. Diabetic mice displayed significantly reduced intraepidermal nerve fiber density and sudomotor function. Furthermore, low and high dose diabetic mice showed significantly reduced tactile touch sensation measured with Von Frey monofilaments. To look at the regenerative and injury-induced responses in diabetic mice, neurons in both superior cervical ganglia (SCG) and the 4th and 5th lumbar dorsal root ganglia (DRG) were unilaterally axotomized. Both high and low dose diabetic mice displayed significantly less axonal regeneration in the sciatic nerve, when measure in vivo, 48 h after crush injury. Significantly reduced induction of two gp130 cytokines, leukemia inhibitory factor and interleukin-6, occurred in diabetic animals in both ganglia 6 h after injury. These effects were accompanied by reduced phosphorylation of signal transducer and activator of transcription 3 (STAT3), a downstream effector of the gp130 signaling pathway. We also found decreased induction of several gp130-dependent RAGs, including galanin and vasoactive intestinal peptide. Together, these data suggest a novel mechanism for the decreased response of diabetic sympathetic and sensory neurons to injury.
Keywords: Diabetes, Neuropathy, Axon Regeneration, Axotomy, Peripheral Nervous System
Introduction
Neuropathy is the most significant clinical complication associated with diabetes. Dysfunction of sensorimotor or autonomic nerves plays a significant role in the morbidity and mortality of patients with type I and type II diabetes (Pasnoor et al., 2013; Said, 2007). Sensory and autonomic neuropathy can often coexist and lead to symptoms such as cardiovascular irregularities, loss of sensation, pain, and in extreme cases limb amputation (Boulton et al., 2005; Tesfaye et al., 2010; Verrotti et al., 2014). While the development and persistence of diabetic polyneuropathies have been investigated, the cause has yet to be determined.
Neurons in the peripheral nervous system (PNS) have the unique ability of regenerating their axons following injury (DeFrancesco-Lisowitz et al., 2014; Fawcett and Keynes, 1990; Zochodne, 2008). However, regeneration of PNS axons following injury is significantly impaired in diabetic patients and rodent models of diabetes (Bradley et al., 1995; Christie and Zochodne, 2013; Duran-Jimenez et al., 2009; Kennedy and Zochodne, 2000, 2005a; Longo et al., 1986; Zochodne et al., 2007). Much research has focused on deficits in trophic support as a cause for this regenerative failure; yet, the mechanisms responsible for the decreased regenerative capabilities found in diabetes are unknown (Chattopadhyay et al., 2005; Wu et al., 2011). The regenerative deficit found in diabetes has been posited to underlie the development of neuropathy (Ebenezer et al., 2011; Eckersley et al., 2001; Simmons and Feldman, 2002; Vinik et al., 2003).
Peripheral nerve regeneration is supported by both intrinsic and extrinsic mechanisms, which include broad neuronal gene expression changes, macrophage accumulation at the site of injury and near injured neuronal cell bodies, and the de-differentiation of Schwann cells to support the regrowth of axons (Barrette et al., 2008; Cattin and Lloyd, 2016; Niemi et al., 2013; Tedeschi and Bradke, 2017). Injury to peripheral nerves induces a complex and coordinated change in gene expression, a response that is not seen following injury to the central nervous system (CNS) and thus is thought to be one of the more important aspects of PNS axon regeneration (Boeshore et al., 2004; Chandran et al., 2016; Costigan et al., 2002; Ma and Willis, 2015). One such group of genes that has been identified to play a significant role in nerve regeneration after injury are the gp130 cytokines (Zigmond, 2011). The gp130 family of cytokines, also known as neuropoetic cytokines, includes, but is not limited to, leukemia inhibitory factor (LIF), interleukin (IL)-6, and ciliary neurotrophic factor (CNTF) (Cheon et al., 2011; Fischer and Hilfiker-Kleiner, 2008; Gadient and Patterson, 1999; Hirano et al., 1997). The gp130 cytokines, Lif and Il6, are increased in peripheral ganglia following nerve injury and play a critical role in the conditioning lesion response of both sensory and sympathetic neurons (Banner and Patterson, 1994; Cafferty et al., 2004; Gardiner et al., 2002; Habecker et al., 2009; Hyatt Sachs et al., 2010; Sun et al., 1994; Sun and Zigmond, 1996b).
Here, we show evidence that the injury induced gene and protein expression of IL-6 and LIF are significantly impaired in sensory and sympathetic ganglia in two different mouse models of type-I diabetes. Furthermore, downstream effectors of gp130 cytokine signaling, STAT3 and expression of various neuropeptides are also significantly reduced following peripheral nerve injury in diabetic mice compared to wild type controls. This defective gp130 signaling cascade could underlie the regenerative deficit and onset of autonomic and sensory neuropathy in diabetes.
Materials and Methods
Administration of Streptozotocin
All procedures were approved by the Institutional Animal Care and Use Committee at Case Western Reserve University. Eight- to 12 week-old male C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME, USA) were housed under a 12 h light/dark cycle with ad libitum access to food and water. One week after arrival, mice were fasted for 6 h. Mice were rendered diabetic by either a low dose, 60 mg/kg, i.p. STZ (MP Biomedicals Inc., Solon, OH, USA) injection given on 5 consecutive days or a single high dose, 200 mg/kg, i.p. injection. STZ was dissolved in sodium citrate buffer (pH 4.5) right before injections. Control mice were injected i.p. with sodium citrate buffer. Body weights and fasted blood glucose levels were monitored weekly from the tail vein. Mice with blood glucose levels >275 mg/dl within 1 week for high dose and 3 weeks for low dose following injection were considered diabetic.
Animal Surgeries
Mice underwent both unilateral SCG axotomy and unilateral sciatic nerve transection either 5 weeks (high dose groups) or 15 weeks (low dose groups) after diabetes onset (Fig. 1a-b). For SCG axotomy, the right SCG was exposed and the internal and external carotid nerves were cut where they exit the ganglion. The sciatic nerve was transected at mid-thigh level and a 1–2 mm piece of the distal nerve segment was removed. For in vivo regeneration, the right sciatic nerve was crushed with ultra-fine hemostats (Fine Science Tools, Foster City, CA, USA), effectively axotomizing a population of neurons of the L4 and L5 DRG (Rigaud et al., 2008). The contralateral SCG and sciatic nerves were exposed but not injured. The contralateral SCG and L4 and L5 DRG, and sciatic nerve were used as internal controls. At 6, 24, or 48 h after injury, the mice were sacrificed by CO2 inhalation and both SCG, L4 and L5 DRG, sciatic nerves, and hind footpads were removed.
Figure 1.
Low (60 mg/kg) and high (200 mg/kg) dose administration of streptozotocin (STZ) results in decreased weight and increased fasted blood glucose and hemoglobin A1c levels in mice. Low dose mice received 5 injections of STZ at 60mg/kg on consecutive days and were tested 3 months later (a). High dose mice received a single injection of STZ at 200mg/kg and were tested 1 month post-injection (b). Both low (c) and high (d) dose STZ injections resulted in significant weight loss compared to age-matched controls (n=15–30/group) with asterisks representing a significant weight difference between groups at the final time point. Sustained hyperglycemia was observed by testing the fasted blood glucose levels (mg/dl) on a weekly basis for low (e) and high (f) dose diabetic mice (n=15–30/group). Hemoglobin A1c was significantly elevated in low dose (g) and high dose (h) mice compared to their age matched controls (n=15/group). * = p < 0.05. ** = p < 0.001.
Measurement of Hemoglobin A1c Levels
Mouse glycohemoglobin A1c was measured using the Bio-Rad Total Glycated Hemoglobin Assay (Bio-Rad, Hercules, CA, USA). The measurement required that 10 μl of blood be harvested from the tail vein before the mouse was sacrificed. The assay was carried out following the manufacturer’s guidelines. Glycohemoglobin A1c in whole blood specimens was measured in a spectrophotometer at 531nm. The data were then represented as a percentage of total hemoglobin that was glycated.
Body Temperature Regulation Assay
To monitor the ability of the diabetic mice to regulate internal body temperature, mice were placed in a cold room (4°C) with each mouse in an individual plastic housing unit. Using a rectal thermometer, the mouse’s body temperature was recorded. Temperatures were taken every 30 min for a total of 3 h. These measurements were taken one week prior to sacrificing the animals.
Sweat Assay
To measure sweating, a mold was made of the plantar surface of the hind paw with a silicone impression system using Silasoft N (Detax, Ettlingen, Germany; Vilches and Navarro, 2002). Diabetic and control mice were anesthetized with isoflurane and injected with 5 mg/kg pilocarpine nitrate (Sigma-Aldrich, St. Louis, MO, USA) subcutaneously to induce sweating. Approximately 0.2 ml of the silicone base and three to five drops of a liquid hardening catalyst, Silaplast (Detax), were mixed together and a thin layer was applied to the hind paw 10 min after pilocarpine injection. Since this material is immiscible with water, sweat droplets form impressions in the mold as it hardens. Each impression represents the activity of an individual sweat gland (Kennedy and Sakuta, 1984). The hardened mold was removed after 5 min. The molds were imaged and individual sweat droplets were counted under a dissecting microscope. Impressions in the mold were counted within 5 foot pads on each hind paw. Each hind paw was analyzed separately and five separate mice were used for each condition (control and diabetic).
Von Frey Test
To assess mechanical sensitivity, calibrated Von Frey monofilaments (Stoetling Co., Wood Dale, IL, USA) were used to measure the paw withdrawal threshold and frequency. Mice were allowed to acclimate for 3 min in an inverted Plexiglas chamber (10 cm × 7 cm × 8 cm) with a wire mesh grid bottom (1 cm spacing), allowing access to the plantar surface of the hind paws. When all four paws were touching the wire mesh, filaments (ranging from 0.16–8.0 g) were applied to the plantar surface of each hind paw for 1–2 s with an inter-stimulus interval of at least 10 s, being careful to avoid the toes, heel, and pads. Sudden paw withdrawal, sudden flinching, or sudden paw licking were considered a withdrawal response. The paw withdrawal threshold was calculated using the up-down method with 20 trials. Beginning in the middle of the filament series (1.4 g), ascending (if no response was observed a stronger stimulus was applied) or descending stimuli (if a response was observed). The resulting pattern of positive and negative responses was calculated using the 50% response threshold formulated from the mean of tabular values for the pattern of positive and negative responses. The frequency of withdrawal was determined using a standard monofilament (1.0 g; 4.08 mN) applied 10 times to the plantar hind paw. This data was then expressed as a response ratio to a 1.0 g stimulus. Eleven to twelve mice per condition were used to conduct this analysis.
Hot Plate Thermal Nociception Assay
The hot plate test was used to assess the response to thermal stimuli (Columbus Instruments, Columbus, OH, USA). The mouse was placed on a 53.5°C hot plate, and the latency to achieve a response in the hind paw (including licking, lifting, or shaking the paw, as well as attempts to jump off the surface) was measured. The mouse was removed immediately after a response was observed or if no response occurred within 30 s. A total of three trials were conducted per mouse with a 20 min inter-trial interval. The threshold was calculated as the mean latencies for the three trials. Eleven to twelve mice per condition were used to conduct this analysis.
In Vivo Regeneration
Two days after a unilateral sciatic nerve crush, animals were sacrificed and sciatic nerves were removed, cleaned, pinned down straight in a 35 mm dish, and fixed by immersion in 4% paraformaldehyde for 3 to 6 h. Nerves were cryoprotected in 30% sucrose, embedded in Tissue-Tek O.C.T. (Electron Microscopy Sciences, Hatfield, PA, USA), and sectioned. After blocking, 60 μm sections were incubated in SCG10 (1:4000; Novus Biologicals, Littleton, CO, USA) overnight at 4°C and then incubated in Alexa Fluor 555 secondary antibodies (1:400; Thermo Fisher, Waltham, MA, USA). Nerves were imaged on a Leica SP8 confocal microscope. The regeneration index was measured based on the method of Shin et al. (2014). Briefly, the amount of fluorescence was assessed using MetaMorph in a 100 pixel wide rectangle spanning the width of the nerve at the site of injury (identified by fluorescent microspheres, not shown) and another rectangle where the amount of fluorescence was 50% that of at the injury site. The distance between these two rectangles was measured and expressed as the regeneration index.
Real-time PCR
Six or 48 hours after axotomy, SCG and L4 and L5 DRG were removed, desheathed, and placed in RNALater (Thermo Fisher). Ganglia from two mice were pooled for each sample. The tissue was homogenized, and RNA was isolated using the RNAqueous Micro Kit (Thermo Fisher) and reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Thermo Fisher). Samples were run on an ABI Step ONE Plus using Prevalidated Taqman Gene Expression Assays (Thermo Fisher) for Lif, Il6, glyceraldehyde 3-phosphate dehydrogenase (GAPDH/Gapdh), cholecystokinin (CCK/Cck), galanin, growth associated protein 43 (GAP-43/Gap43), pituitary adenylate cyclase-activating peptide (PACAP/Adycap1), and vasoactive intestinal polypeptide (VIP/Vip). Relative expressions were determined using the comparative Ct model (ΔΔCt) and normalizing to GAPDH.
Immunohistochemistry
Generally, SCG and L5 DRG from diabetic and control mice were removed 48 h after injury, and the ganglia were desheathed and fixed by immersion in 4% paraformaldehyde for 1 h. The tissues were cryoprotected in 30% sucrose and embedded in Tissue-Tek O.C.T. compound. IHC was performed on 10 μm cryostat sections. For quantification of STAT3, a rabbit monoclonal antibody to phosphorylated STAT3 (Y705; 1:100; Cell Signaling Technology, Danvers, MA, USA) was incubated with tissue sections overnight at room temperature. After washing, the sections were incubated in Alexa Flour 555 secondary antibody (1:400; Jackson ImmunoResearch Laboratories, Inc.; West Grove, PA, USA). Adjacent sections were labeled with an antibody against HuC/HuD (Thermo Fisher) to label all neurons in the ganglia. In all experiments, sections not exposed to the primary antibody were included for each experimental group. Images were captured at 10x or 25x magnification using HCImage software (Hamamatsu Corporation; Bridgewater, NJ, USA). Cell counts were performed using FIJI is just ImageJ (FIJI) software and expressed as the percentage of neurons positively labeled with pSTAT3 in the nucleus.
Multiplex
IL-6 and LIF proteins were measured in both SCG and DRG using magnetic bead-based ProcartaPlex Mouse Myokine Panel for the Luminex platform (Affymetrix, Santa Clara, CA, USA). Control and diabetic mice were sacrificed 48 h after injury. SCG and L5 DRG were removed and flash frozen in liquid nitrogen. Tissue from two mice were pooled for each sample. Frozen samples were homogenized via sonication in T-Per buffer supplemented with Halt protease and phosphatase inhibitors (Thermo Fisher) and centrifuged at 1000 × g for 10 min. Supernatants were collected for further analyses; pellets were re-suspended with 50–100 μl of additional T-Per buffer and were centrifuged a final time (1000 × g, 10 min, 4°C) with supernatants added to final sample volume. Multiplex analyses were conducted according to manufacturer’s instructions. In brief, a solution containing antibody-coupled beads for LIF and IL-6 were pipetted into a 96-well microplate (25 μl/well). Twenty- five microliters of assay diluent was then added, followed by 50 μl of sample or appropriate standard. Plates were light-protected and incubated for 16–18 h on an orbital shaker (500–600 rpm) at 4°C. Following incubation, plates were washed 3x with manufacturer-provided detergent solution using a handheld magnet to keep beads in place. Twenty-five microliters of HRP-conjugated detection antibody was added to all wells and incubated at room temperature for 1 h (shaking at 500–600 rpm). Plates were washed 3x as described, and 25 μl of streptavidin-RPE was added to wells. After a final wash, 100 μl of Magpix drive fluid was added to wells, plates were vigorously shaken for 3 min, and then plates were read on a Magpix Luminex 200. Luminex Xponent® software was used to generate a standard curve for each analyte from which concentrations of unknown samples were calculated.
Statistical Analysis
Statistical analysis was performed using Sigma Plot(Version 14). Student’s t-test was used for comparison of two groups without injury, two-way ANOVA using Tukey post hoc correction was used for comparison of two groups with the presence of injury. All tests performed are two-tailed, with an alpha level of p > 0.05 used to determine significance. Results are reported as mean ± SEM.
Results
STZ-Injected Mice Develop Hyperglycemia and Weight Loss
Many different rodent models are used to study type-I and type-II diabetes mellitus. One of the most well studied and characterized models is the STZ-induced type-I diabetic model (Eleazu et al., 2013; Islam and Loots, 2009). However, the dosing and time of onset for neuropathy are not standardized for the model (Sullivan et al., 2007; Sullivan et al., 2008). Here we used two distinct STZ dosing paradigms to induce sustained hyperglycemia. Our low dose paradigm utilized five consecutive i.p. injections of STZ at 60 mg/kg (Fig. 1a) and has previously been shown to develop neuropathy within one to three months following injection (Sullivan and Feldman, 2005; Sullivan et al., 2007). Our high dose paradigm utilized a single i.p. injection of STZ at 200 mg/kg (Fig. 1b) and has been shown to develop neuropathy-like symptoms at 1 month after injection (Chen et al., 2010). For both paradigms, mice treated with STZ showed significantly lower body weights (Fig. 1c,d) and higher blood glucose levels (Fig. 1e,f) than their age-matched non-diabetic controls at all times examined after injection(s). The animals receiving low dose injections had a more gradual onset of hyperglycemia (low dose bars are averaged data over a three week period). The animals injected with a high dose of STZ became hyperglycemic within four days after injection. Mice with fasted blood glucose levels ≥ 275 mg/dl beginning one week after the final STZ injection were considered diabetic. Glycated hemoglobin is thought to be a better overall measure of increased glucose within the blood (Kilpatrick et al., 1998; Sullivan et al., 2008). The percentage of glycated hemoglobin was significantly increased in low dose (control: 3.68% ± 0.18%, diabetic – low: 6.78% ± 0.48%, ** p<0.001; Fig. 1g) and in high dose (control: 3.87% ± 0.36%, diabetic – high: 5.44% ± 0.69%, * p<0.05; Fig. 1h).
STZ Administration Induces Functional and Morphological Changes Associated with Diabetic Neuropathy
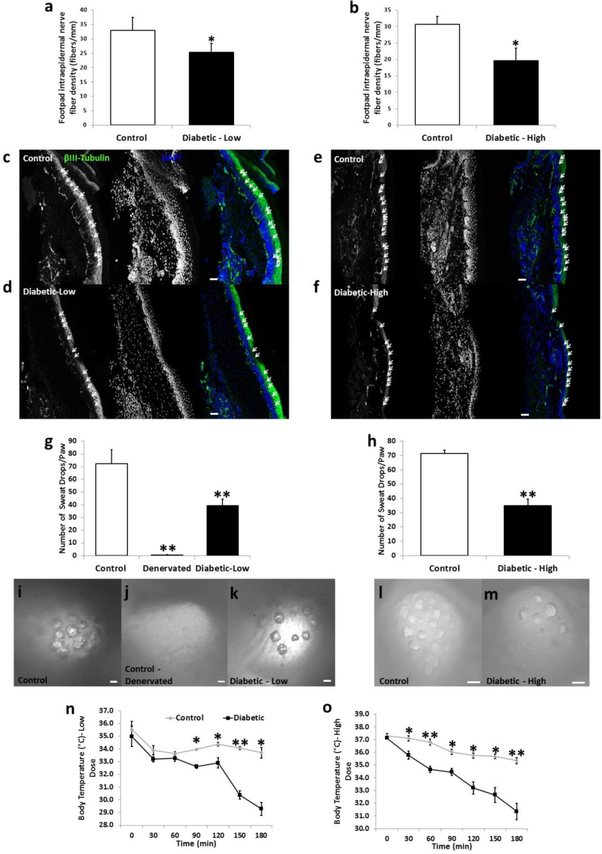
Diabetic neuropathy is a common and potentially life-threatening complication associated with all types of diabetes. Symptoms related to neuropathy are dependent upon the nerve(s) affected and can manifest in sensory or autonomic dysfunction (Said, 2007; Sharma et al., 2015; Vinik et al., 2003). To assess the possible development of diabetic neuropathy in our STZ diabetic models, we first measured intraepidermal nerve fiber density (IENFD) in the mouse hind paw. IENFD has been shown to measure accurately the presence or absence of diabetic sensory neuropathy in both rodent models and human patients with diabetes (Arimura et al., 2013; Beiswenger et al., 2008b; Navarro et al., 1995). 3 months after onset of hyperglycemia in the low dose diabetic mice and 1 month after onset of hyperglycemia in high dose diabetic mice, the IENFD was calculated by counting the number of beta-III-tubulin-positive axons projecting into the epidermal layer of the skin of the hind paw. The border between the epidermis and dermis is clearly delineated by the high density DAPI-staining seen (Fig. 2c-f). Significant reductions in IENFD were found in both low dose (Fig. 2a,c-d) and high dose (Fig. 2b, e-f) diabetic mice compared to their non-diabetic controls. This result is a direct measure of the presence of a dying-back diabetic neuropathy in these STZ mouse models.
Figure 2.
Morphological and functional changes associated with diabetic sensory and autonomic neuropathy are displayed in low and high dose diabetic mice. Intraepidermal nerve fiber density (fibers/mm) of the hind paw was reduced in low dose (a) and high dose (b) diabetic mice compared to their age-matched controls (n=5/group). Representative images displaying beta-III-tubulin-positive axons crossing into the epidermal layer are shown for control (c,e), low dose (d) and high dose (f) diabetic mice. Arrows indicate fibers counted that projected into the epidermal layer of the hind paw. Pilocarpine-induced sweating was measured using silicone molds on the hind paws of mice. The total number of sweat droplets was counted per hind paw and both low dose (g) and high dose (h) mice displayed significantly impaired sweating compared to control mice (n=10/group). Representative images of sweat droplet imprints into the silicone mold are seen for control (i,l), denervated (control mice in which the saphenous and sciatic nerves were cut; j), low dose (k) and high dose (m) mice. Internal body temperature was measured in 30 min intervals in mice placed at 4°C for low dose (n) and high dose (o) diabetic mice (n = 4–5/group). Nerve fiber density images taken at 20x. Scale bar = 50 μm. Sweat assay images taken at 9x to 20x. Scale bar = 100 μm. * = p < 0.05. ** = p < 0.001.
To measure the presence of diabetic autonomic neuropathy, we performed a silicone mold-based sweat assay (Kennedy and Navarro, 1989; Vilches and Navarro, 2002; Vilches et al., 2012). Sweat assays are used in both rodent models and humans to measure the presence of autonomic dysfunction associated with diabetes mellitus (Bharali et al., 1988; Chattopadhyay et al., 2007; Diem et al., 2003; Gibbons et al., 2009). To assess the presence of autonomic dysfunction in our diabetic mouse models, mice were injected with the muscarinic agonist pilocarpine, which induces sweating. Silicone molds are placed on the hind paws of anesthetized mice within 10 min after injection of pilocarpine. The molds harden and sweat droplets on the pads of the hind paw make indentations in the molds (ex. Fig. 2i-m). The indentations in the molds can then be counted and summed for each paw. This assay directly measures sympathetic-driven sweating which requires complete innervation of each sweat gland, as sympathetic denervation (through sciatic and saphenous nerve transection) of sweat glands completely ablates pilocarpine-induced sweating (Fig. 2 g,j). Both low dose (Fig. 2g,i-k) and high dose (Fig. 2h,l-m) diabetic mice display significantly reduced sweating compared to non-diabetic controls.
Thermoregulation during cold exposure was also assayed as an additional measure of dysautonomia in diabetic mice (Bachman et al., 2002; Campanucci et al., 2010). It was previously shown that the ability of mice to regulate their internal body temperature is reliant on proper peripheral sympathetic synaptic transmission as silencing sympathetic neurons using an nicotinic acetylcholine receptor alpha 3 subunit knockout animal reveals significantly decreased body temperature regulation (Campanucci et al., 2010). Control mice placed at 4°C only showed a 1.8°C and 2.0°C drop in body temperature for the low and high does groups respectively, over the 3 h trial (Fig. 2n-o). In contrast, both low dose (Fig. 2n) and high dose diabetic mice (Fig. 2o) had their internal body temperature drop by 6.7°C and 5.7°C over the 3 h cold exposure, respectively. These data are indicative of the presence of diabetic autonomic neuropathy in these mice.
Loss of sensation and the development of neuropathic pain are two possible consequences of diabetic neuropathy, and both have been reported to occur in STZ rodent models of diabetes (Sullivan et al., 2007; Sullivan et al., 2008). We used the Von Frey Test, to measure a possible loss of sensation, and the hotplate nociceptive pain assay, to measure a possible onset of painful neuropathy. Low dose diabetic mice displayed significantly reduced tactile sensation as measured by the increased filament weight used to yield a consistent response (Tactile Response Threshold; Fig. 3a) and the reduced paw withdrawal from a 1g monofilament over 10 trials (Response Ratio; Fig. 3c) in comparison to control mice. High dose diabetic mice also displayed a significant increase in the response threshold (Fig. 3b) and a significant decrease in the response ratio (Fig. 3d). This data indicates a loss of hind paw sensation in our STZ diabetic mouse models. However, we did not detect the presence of painful neuropathy as both the low dose (Fig. 3e) and high dose (Fig. 3e) did not show any differences in their latency to withdraw their paw from a hotplate. Taken together, the morphological and functional changes measured in the low and high dose STZ models reveals the presence of both sensory and autonomic diabetic neuropathy.
Figure 3.
Tactile sensation is significantly reduced in low and high dose diabetic mice, while thermal nociception is unaltered. The Von Frey filament assay was used to measure tactile sensation. Tactile response threshold, which is the filament weight (g) at which a withdrawal response is achieved at least 50% of the time, is significantly increased in low dose (a) and high dose (b) diabetic mice compared to controls. The response ratio to a 1 g monofilament stimulus over 10 consecutive trials was also significantly reduced in low (c) and high (d) dose diabetic mice compared to controls. The hotplate test was used to measure thermal nociception in the hind paw. The withdrawal latency (s) was calculated as the time it took for the mouse to remove their paw from a hotplate set at 53.5°C. Low (e) and high (f) dose diabetic mice displayed no difference in withdrawal latency compared to control mice. n=10–13/group for each experiment. * = p < 0.05. ** = p < 0.001.
In Vivo Regeneration of Sensory Axons is Significantly Impaired in Diabetic Mice
Diabetes has long been associated with a reduction in peripheral nerve regeneration in response to an injury (Kennedy and Zochodne, 2000, 2005b; Maxfield et al., 1995). We measured regeneration at in vivo 48 h after crushing the sciatic nerve. To measure regeneration, regenerating axons were labeled with SCG10, a label which is rapidly down regulated distal to the injury site and accumulates in regenerating fibers of the sciatic nerve (Shin et al., 2014; Shin et al., 2012). The regeneration index used to quantify in vivo regeneration identifies the distance from the lesion site to the location where levels of SCG10 are half of their levels at the crush site. This identifies the length to which approximately half of axons have regenerated (Shin et al., 2014). Low dose mice displayed significantly reduced regeneration compared to non-diabetic control mice (Fig 4a-b,e). High dose mice were trending towards a significant reduction in regeneration compared to control mice (p = 0.053; Fig. 4c-d,f).
Figure 4.
Regeneration of sensory axons is impaired in low and high dose diabetic mice. Representative images of SCG10-positive regenerating fibers in 20 μm thick sciatic nerve sections 48 h after a crush injury in control (a,c), low dose diabetic (b), and high dose diabetic (d) mice. The asterisk (*) in each image denotes the crush site. The dashed line in each image denotes the area of the nerve distal to the crush site where the percent area positively labeled for SCG10 is reduced by 50% compared to staining at the crush site. This was taken as the regeneration index with data represented as the number of μm distal to the crush site where SCG10 staining is reduced by 50% compared to the crush site. Axon regeneration, following a sciatic nerve crush, was impaired in low dose (e) and high dose (f) diabetic mice compared to non-diabetic control mice. To better illustrate the distance of the regenerating sensory axons, a regenerative ratio was calculated every 0.5 mm distal to the injury site from 0.5 mm out to 3 mm. These data are represented as a ratio of the percent area stained for SCG10 at each distance to that at the crush site compared (g,h). Using the regenerative ratio by distance, low dose diabetic mice show a significant decrease in regeneration at 1mm and 1.5 mm distal to the crush site (g) and high dose diabetic mice demonstrate a deficit at 1.5mm compared to age-matched controls (h). Images taken at 10x. Scale bar=500 μm. n=8/group. * = p < 0.05.
Injury-Induced Expression of gp130 Cytokines are Reduced in STZ Diabetic Mice
To determine a possible reason for the impaired peripheral nerve regeneration seen in diabetes, we looked at the expression of two gp130 cytokines in the SCG and DRG 6 h after axotomy. LIF and IL-6 are highly upregulated in the SCG and DRG after injury and are required for the conditioning lesion response (Banner and Patterson, 1994; Cafferty et al., 2004; Cafferty et al., 2001; Habecker et al., 2009; Hyatt Sachs et al., 2010; Sun et al., 1994). Low dose diabetic mice showed a significant reduction in the expression of IL-6 and LIF in the SCG (Fig. 5a), 6h after transection of the postganglionic fibers, and in the DRG (Fig. 5c), 6 h after a sciatic nerve injury, compared to non-diabetic control mice. High dose diabetic mice displayed a significant reduction in the injury-induced expression of IL-6 and LIF in the SCG (Fig. 5b) and of IL-6 in the DRG (Fig. 5d) compared to control mice.
Figure 5.
Injury-induced mRNA expression of IL-6 and LIF are significantly reduced in the DRG and SCG in both low and high dose diabetic mice. Expression of Lif and Il6 mRNA was determined in the SCG (a,b) and in the DRG (c,d) 6 h after axotomy for low dose (a,c) and high dose (b,d) diabetic mice. Relative expression was determined using the comparative Ct method (ΔΔCt) normalized to the housekeeping gene GAPDH. n=6–9/group. A magnetic bead multiplex array was used to quantify IL-6 and LIF protein levels in SCG and DRG 48 h after injury. IL-6 expression is displayed as picograms (pg) of IL-6 per μg of total protein for low dose (e) and high dose (f) diabetic mice. LIF expression is displayed as pg of LIF per μg of total protein for low dose (g) and high dose (h) diabetic mice. n=5/group. * = p < 0.05. ** = p < 0.001. # = p < 0.05 between injured and non-injured conditions.
To ascertain the protein expression levels of IL-6 and LIF in SCG and DRG, tissue was collected 48 h after injury and a magnetic bead multiplex array was run. Low dose diabetic mice displayed significantly attenuated protein expression of both IL-6 and LIF in the SCG (Fig. 5e,g) and of IL-6 in the DRG (Fig. 5e) compared to control mice. High dose diabetic mice displayed a reduction in IL-6 protein expression in both the SCG and DRG after injury (Fig. 5f) compared to controls while LIF protein levels were not altered compared to control mice (Fig. 5h). Given the importance of these cytokines in peripheral axon regeneration (Zigmond, 2012), the deficit in IL-6 and LIF expression could be responsible for the reduced regenerative capabilities present in diabetes.
Downstream gp130 Signaling is impaired in SCG and DRG of STZ diabetic mice
IL-6 and LIF both signal through the gp130 receptor by activating Janus kinase 2 (JAK2) and causing the phosphorylation, dimerization, and nuclear translocation of the transcription factor STAT3 (Zigmond, 2012). STAT3 activation is necessary for peripheral nerve regeneration as inhibiting its activation in peripheral neurons significantly impairs their regenerative capabilities (Bareyre et al., 2011; Heinrich et al., 1998; Hyatt Sachs et al., 2010; Niemi et al., 2016). To measure STAT3 activation, SCG and DRG from diabetic and control mice were stained for phosphorylated STAT3 (pSTAT3) and the percentage of neurons expressing pSTAT3 in their nucleus was calculated using a neuronal counterstain, HuC/HuD. Low dose diabetic mice displayed a significantly lower percentage of neurons which express pSTAT3 in both the SCG (Fig. 6a) and the DRG (Fig. 6c) 48 h after injury compared to control mice. Similarly, high dose mice displayed reduced pSTAT3 expression in the SCG (Fig. 6b) and DRG (Fig. 6d) following injury. The reduced expression of pSTAT3 is likely a direct result of the reduced IL-6 and LIF expression, as it has previously been shown that gp130 signaling accounts for a majority of the STAT3 activation after injury (Hyatt Sachs et al., 2010).
Figure 6.
Activation of STAT3 is significantly impaired in the SCG and DRG of low and high dose diabetic mice. Cell counts were performed in the SCG (a,b) and DRG (c,d) for low dose (a,c) and high dose (b,d) diabetic mice. The percentage of HuD/C-positive neurons displaying positive staining for phosphorylated STAT3 in the nucleus was calculated. n=5–7/group. * = p < 0.05 between control and diabetic groups. ** = p < 0.001 between control and diabetic groups.
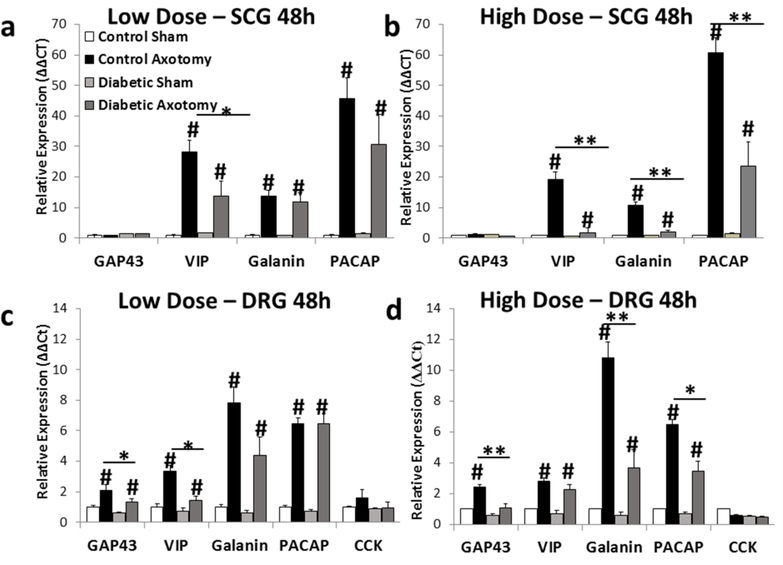
gp130 signaling is known to drive the expression of the neuropeptides Vip, galanin, Pacap/Adcyap1, and Cck (Habecker et al., 2009; Rao et al., 1993a; Sun and Zigmond, 1996a; Zigmond et al., 1998; Zigmond, 2011; Zigmond et al., 1996). Galanin and Pacap/Adcyap1 expression have been directly linked to axon regeneration, as a knockout or inhibition of these neuropeptides shows significantly impaired regeneration (Armstrong et al., 2008; Hobson et al., 2006; Holmes et al., 2000; Kerr et al., 2000). We measured the injury-induced mRNA expression of these neuropeptides, as well as the RAG GAP43, in SCG and DRG 48h after axotomy. Low dose diabetic mice displayed significant reductions in Vip in the SCG (Fig. 7a) and Gap43 and Vip in the DRG (Fig. 7c) in response to injury compared to control mice. High dose diabetic mice showed significant reductions in Vip, galanin, and Pacap/Adcyap1 in the SCG (Fig. 7b) and in GAP43, galanin, and PACAP in the DRG after injury (Fig. 7d). Taken together, deficits in the injury-induced expression, activation, and downstream signaling of gp130 in sensory and sympathetic ganglia may contribute to the regenerative deficits found in diabetes mellitus.
Figure 7.
Injury-induced mRNA expression of regeneration-associated genes downstream of gp130 is significantly reduced in the DRG and SCG in both low and high dose diabetic mice. Expression of Gap43, Vip, galanin, Pacap, and Cck mRNA was determined in the SCG (a,b) and in the DRG (c,d) 48h after axotomy for low dose (a,c) and high dose (b,d) diabetic mice. Relative expression was determined using the comparative Ct method (ΔΔCt) normalized to the housekeeping gene GAPDH. n=5–7/group. * = p < 0.05 between control and diabetic groups. ** = p < 0.001 between control and diabetic groups. # = p < 0.05 between injured and non-injured conditions.
Discussion
The results of our study indicate that the injury-induced expression and downstream signaling of the gp130 cytokine IL-6 and LIF are significantly diminished in both low and high dose diabetic STZ mice. Deficits in gp130 signaling could underlie the regenerative deficit found in diabetes. For this paper, we chose to use two different doses of STZ, 60 mg/kg given on 5 consecutive days and a single injection of 200 mg/kg, to induce hyperglycemia in our mice. The onset of hyperglycemia and the duration of time it took to observe measureable neuropathy related deficits varied greatly between the two doses. An overview of the diabetic neuropathy literature reveals a wide range of STZ doses and length of hyperglycemia prior to onset of neuropathy have been observed in rodent models (Fox et al., 1999; Islam, 2013; Islam and Loots, 2009). While many of the functional deficits associated with neuropathy remain consistent in different STZ models, the tactile sensation and thermal nociception have yielded variable results (Fox et al., 1999). Using the Von Frey monofilament assay, STZ-induced diabetic rats more readily demonstrate hyperalgesia, while in STZ mouse models it is common to observe hypoalgesia, as we did here (Christianson et al., 2003a; Christianson et al., 2003b; Greene et al., 1992; Greene et al., 1999). Reports of changes in thermal nociception are far more variable, where hyperalgesia, hypoalgesia, or no change in withdrawal latency in diabetic rodent models (Beiswenger et al., 2008a; Fox et al., 1999; Kamei et al., 1991; Raz et al., 1988; Sharma et al., 2006). Thus, the lack of a thermal nociceptive phenotype in either low or high dose STZ diabetic mice is not uncommon.
In previous studies, changes in various components of the gp130 cytokine signaling in the intact sciatic nerve have been correlated with diabetic neuropathy. LIF receptor subunit and gp130 receptor subunit expression is significantly increased in the sciatic nerve and gastrocnemius muscles up to 4 weeks after onset of hyperglycemia in a streptozotocin-induced mouse model of diabetes (Toledo-Corral and Banner, 2011). Interestingly, systemic administration of IL-6 was found to significantly attenuate the development of diabetic neuropathy in an streptozotocin rat model (Andriambeloson et al., 2006). Daily injections of IL-6 resulted in significantly improved compound muscle action potentials and sciatic nerve conduction velocity at 40 days after streptozotocin injection compared to vehicle treated diabetic rats (Andriambeloson et al., 2006).
The regenerative deficit of peripheral nerves in experimental diabetes is a well-documented aspect of neuropathy. Deficits in regeneration have been shown to significantly increase with the duration of diabetes (Ekström and Tomlinson, 1990). Alterations in the regenerative capabilities in diabetic rodent models affect both the initiation and elongation phases of axon regrowth after injury (Bisby, 1980; Ekstrom and Tomlinson, 1989). While the cause of the deficit in regeneration in diabetes remains unknown, many have hypothesized that the lack of regeneration might play a primary role in the development and onset of diabetic neuropathy. Eckersley et al. (2001) stated that “a reduced ability to regenerate peripheral axons may be partly responsible for diabetic neuropathy”, a statement echoed by others in the field (Ebenezer et al., 2011; Pittenger and Vinik, 2003; Simmons and Feldman, 2002). Interestingly, impaired nerve regeneration is a very well documented nervous system defect in diabetes in humans as well (Bengel et al., 2006; Bradley et al., 1995; Ebenezer et al., 2011; Polydefkis et al., 2004) and data indicates that diabetic patients are more susceptible to compression injuries of the nerve (Kennedy and Zochodne, 2000; Ma and Willis, 2015).
A major component of the axonal injury-induced regenerative response is the large scale gene expression changes that occur in peripheral neurons (Boeshore et al., 2004; Chandran et al., 2016; Ma and Willis, 2015; Smith and Skene, 1997). However, very few studies have measured RAG expression after injury in diabetic rodent models. Interestingly, our data showed that numerous neuropeptides, highly expressed in both SCG and DRG after axotomy in wild-type mice (Habecker et al., 2009; Hokfelt et al., 1987; Hokfelt et al., 1994; Hyatt-Sachs et al., 1996; Hyatt-Sachs et al., 1993; Mohney et al., 1994; Rao et al., 1993b; Sachs et al., 2007; Schreiber et al., 1994; Shadiack et al., 1995; Shadiack and Zigmond, 1998; Sun and Zigmond, 1996a; Zigmond et al., 1998; Zigmond, 1994), are expressed at significantly lower quantities in both a low and high dose diabetic mice. While it is clear that neuropeptide expression is significantly altered in our diabetic models after injury, the neuropeptides which displayed reduced expression were different between SCG and DRG and also between low dose and high dose STZ treatment. Il6 and Lif expression were only reduced and not completely abolished in our diabetic models which might explain why only some but not all of the neuropeptides displayed reduced mRNA expression (Habecker et al., 2009). We also showed that the quintessential RAG, Gap43, was expressed at significantly lower than controls in the DRG. Previous studies have also displayed impaired Gap43 expression in sensory neurons of diabetic rodents (Maeda et al., 1996; Pekiner et al., 1996; Scott et al., 1999). However, we were unable to detect Gap43 expression in control or diabetic mouse SCG. Although, GAP43 protein expression has been measured in sympathetic neurons of the SCG after decentralization and in dissociated cell culture (Hou and Dahlstrom, 1995; Meiri et al., 1988), a microarray study did not detect expression of Gap43 in the SCG after axotomy (Boeshore et al., 2004).
Here, we showed that two different dosing paradigms of STZ generate mouse models of diabetes with clear hyperglycemia and functional deficits which confirm the presence of both sensory and autonomic neuropathy. Both STZ mouse models also exhibit a reduction in the regeneration of axons following a sciatic nerve crush. The injury-induced upregulation of the gp130 cytokines, Il6 and Lif, normally seen at high levels in injured DRG and SCG, was significantly attenuated in both diabetic mouse models. Alterations in injury-induced gp130 downstream signaling was also observed as decreased pSTAT3 and neuropeptide expression were present in the SCG and DRG of both high dose and low dose diabetic mice. This data indicates that defective gp130 signaling in peripheral ganglia could underlie the regenerative failure seen in diabetes mellitus and identifies a possible new therapeutic target for the treatment of diabetic neuropathy.
Acknowledgements
The authors would like to thank Dr. Timothy Kern and Chieh Allen Lee in the Department of Pharmacology at Case Western Reserve for their assistance and guidance throughout this project. This work was supported by a Pilot Grant from the Juvenile Diabetes Research Fund and a National Institutes of Health grant DK097223 to R.E.Z. J.P.N was supported by training grants NS017512 and NS077888. J.A.L. was supported by F31NS093694. S.D.C. was supported by EY022358. Behavioral testing was performed by the Case Western Reserve University School of Medicine Rodent Behavioral Core. We thank Hiroyuki Arakawa for advice and guidance with the behavior tests. We would also like to acknowledge use of the Leica SP-8 Confocal Microscope in the Light Microscopy Imaging Facility at Case Western Reserve University made available through the Office of Research Infrastructure (NIH-ORIP) Shared Instrumentation Grant (S10OD016164).
Abbreviations
- CCK
cholecystokinin
- CNTF
ciliary neurotrophic factor
- DRG
dorsal root ganglia
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GAP43
growth associated protein 43
- gp130
glycoprotein 130
- IENFD
intraepidermal nerve fiber density
- IL-6
interleukin-6
- JAK2
Janus kinase 2
- LIF
leukemia inhibitory factor
- PACAP/Adcyap1
pituitary adenylate cyclase-activating peptide
- PNS
peripheral nervous system
- RAG
regeneration associated gene
- SCG
superior cervical ganglia
- STAT3
signal transducer and activator of transcription 3
- STZ
streptozotocin
- VIP
vasoactive intestinal peptide
References
- Andriambeloson E, Baillet C, Vitte PA, Garotta G, Dreano M, Callizot N, 2006. Interleukin-6 attenuates the development of experimental diabetes-related neuropathy. Neuropathology 26, 32–42. [DOI] [PubMed] [Google Scholar]
- Arimura A, Deguchi T, Sugimoto K, Uto T, Nakamura T, Arimura Y, Arimura K, Yagihashi S, Nishio Y, Takashima H, 2013. Intraepidermal nerve fiber density and nerve conduction study parameters correlate with clinical staging of diabetic polyneuropathy. Diabetes Res. Clin. Pract 99, 24–29. [DOI] [PubMed] [Google Scholar]
- Armstrong BD, Abad C, Chhith S, Cheung-Lau G, Hajji OE, Nobuta H, Waschek JA, 2008. Impaired nerve regeneration and enhanced neuroinflammatory response in mice lacking pituitary adenylyl cyclase activating peptide. Neuroscience 151, 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK, Lowell BB, 2002. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science 297, 843–845. [DOI] [PubMed] [Google Scholar]
- Banner LR, Patterson PH, 1994. Major changes in the expression of the mRNAs for cholinergic differentiation factor/leukemia inhibitory factor and its receptor after injury to adult peripheral nerves and ganglia. Proc. Natl. Acad. Sci. U.S.A 91, 7109–7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareyre FM, Garzorz N, Lang C, Misgeld T, Buning H, Kerschensteiner M, 2011. In vivo imaging reveals a phase-specific role of STAT3 during central and peripheral nervous system axon regeneration. Proc. Natl. Acd. Sci. U.S.A 108, 6282–6287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrette B, Hebert MA, Filali M, Lafortune K, Vallieres N, Gowing G, Julien JP, Lacroix S, 2008. Requirement of myeloid cells for axon regeneration. J. Neurosci 28, 9363–9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiswenger KK, Calcutt NA, Mizisin AP, 2008a. Dissociation of thermal hypoalgesia and epidermal denervation in streptozotocin-diabetic mice. Neurosci. Lett 442, 267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiswenger KK, Calcutt NA, Mizisin AP, 2008b. Epidermal nerve fiber quantification in the assessment of diabetic neuropathy. Acta Histochem. 110, 351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengel FM, Ueberfuhr P, Schafer D, Nekolla SG, Reichart B, Schwaiger M, 2006. Effect of diabetes mellitus on sympathetic neuronal regeneration studied in the model of transplant reinnervation. J. Nucl. Med 47, 1413–1419. [PubMed] [Google Scholar]
- Bharali LAM, Burgess SA, Lisney SJW, Pearson D, 1988. Reinnervation of sweat glands in the rat hind paw following peripheral-nerve injury. J. Auton. Nerv. Syst 23, 125–129. [DOI] [PubMed] [Google Scholar]
- Bisby MA, 1980. Axonal transport of labeled protein and regeneration rate in nerves of streptozocin-diabetic rats. Exp. Neurol 69, 74–84. [DOI] [PubMed] [Google Scholar]
- Boeshore KL, Schreiber RC, Vaccariello SA, Sachs HH, Salazar R, Lee J, Ratan RR, Leahy P, Zigmond RE, 2004. Novel changes in gene expression following axotomy of a sympathetic ganglion: a microarray analysis. J. Neurobiol 59, 216–235. [DOI] [PubMed] [Google Scholar]
- Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D, 2005. Diabetic neuropathies. Diabetes Care 28, 956–962. [DOI] [PubMed] [Google Scholar]
- Bradley JL, Thomas PK, King RH, Muddle JR, Ward JD, Tesfaye S, Boulton AJ, Tsigos C, Young RJ, 1995. Myelinated nerve fibre regeneration in diabetic sensory polyneuropathy: correlation with type of diabetes. Acta Neuropathol. 90, 403–410. [DOI] [PubMed] [Google Scholar]
- Cafferty WB, Gardiner NJ, Das P, Qiu J, McMahon SB, Thompson SW, 2004. Conditioning injury-induced spinal axon regeneration fails in interleukin-6 knock-out mice. J. Neurosci 24, 4432–4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferty WB, Gardiner NJ, Gavazzi I, Powell J, McMahon SB, Heath JK, Munson J, Cohen J, Thompson SW, 2001. Leukemia inhibitory factor determines the growth status of injured adult sensory neurons. J. Neurosci 21, 7161–7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanucci V, Krishnaswamy A, Cooper E, 2010. Diabetes depresses synaptic transmission in sympathetic ganglia by inactivating nAChRs through a conserved intracellular cysteine residue. Neuron 66, 827–834. [DOI] [PubMed] [Google Scholar]
- Cattin A-L, Lloyd AC, 2016. The multicellular complexity of peripheral nerve regeneration. Curr. Opin. Neurobiol 39, 38–46. [DOI] [PubMed] [Google Scholar]
- Chandran V, Coppola G, Nawabi H, Omura T, Versano R, Huebner EA, Zhang A, Costigan M, Yekkirala A, Barrett L, 2016. A Systems-Level Analysis of the Peripheral Nerve Intrinsic Axonal Growth Program. Neuron 89, 956–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay M, Krisky D, Wolfe D, Glorioso JC, Mata M, Fink DJ, 2005. HSV-mediated gene transfer of vascular endothelial growth factor to dorsal root ganglia prevents diabetic neuropathy. Gene Ther. 12, 1377–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay M, Mata M, Goss J, Wolfe D, Huang S, Glorioso JC, Fink DJ, 2007. Prolonged preservation of nerve function in diabetic neuropathy in mice by herpes simplex virus-mediated gene transfer. Diabetologia 50, 1550–1558. [DOI] [PubMed] [Google Scholar]
- Chen YS, Chung SS, Chung SK, 2010. Aldose reductase deficiency improves Wallerian degeneration and nerve regeneration in diabetic thy1-YFP mice. J. Neuropathol. Exp. Neurol 69, 294–305. [DOI] [PubMed] [Google Scholar]
- Cheon H, Yang J, Stark GR, 2011. The functions of signal transducers and activators of transcriptions 1 and 3 as cytokine-inducible proteins. J. Interferon Cytokine Res 31, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JA, Riekhof JT, Wright DE, 2003a. Restorative effects of neurotrophin treatment on diabetes-induced cutaneous axon loss in mice. Exp. Neurol 179, 188–199. [DOI] [PubMed] [Google Scholar]
- Christianson JA, Ryals JM, McCarson KE, Wright DE, 2003b. Beneficial actions of neurotrophin treatment on diabetes-induced hypoalgesia in mice. J. Pain 4, 493–504. [DOI] [PubMed] [Google Scholar]
- Christie KJ, Zochodne D, 2013. Peripheral axon regrowth: New molecular approaches. Neuroscience 240, 310–324. [DOI] [PubMed] [Google Scholar]
- Costigan M, Befort K, Karchewski L, Griffin RS, D’Urso D, Allchorne A, Sitarski J, Mannion JW, Pratt RE, Woolf CJ, 2002. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 3, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFrancesco-Lisowitz A, Lindborg J, Niemi J, Zigmond R, 2014. The neuroimmunology of degeneration and regeneration in the peripheral nervous system. Neuroscience Sept. 19, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diem P, Laederach-Hofmann K, Navarro X, Mueller B, Kennedy WR, Robertson RP, 2003. Diagnosis of diabetic autonomic neuropathy: a multivariate approach. Eur. J. Clin. Invest 33, 693–697. [DOI] [PubMed] [Google Scholar]
- Duran-Jimenez B, Dobler D, Moffatt S, Rabbani N, Streuli CH, Thornalley PJ, Tomlinson DR, Gardiner NJ, 2009. Advanced glycation end products in extracellular matrix proteins contribute to the failure of sensory nerve regeneration in diabetes. Diabetes 58, 2893–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebenezer GJ, O’Donnell R, Hauer P, Cimino NP, McArthur JC, Polydefkis M, 2011. Impaired neurovascular repair in subjects with diabetes following experimental intracutaneous axotomy. Brain 134, 1853–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckersley L, Ansselin AD, Tomlinson DR, 2001. Effects of experimental diabetes on axonal and Schwann cell changes in sciatic nerve isografts. Brain Res. Mol. Brain Res 92, 128–137. [DOI] [PubMed] [Google Scholar]
- Ekstrom AR, Tomlinson DR, 1989. Impaired nerve regeneration in streptozotocin-diabetic rats. Effects of treatment with an aldose reductase inhibitor. J. Neurol. Sci 93, 231–237. [DOI] [PubMed] [Google Scholar]
- Ekström PA, Tomlinson DR, 1990. Impaired nerve regeneration in streptozotocin-diabetic rats is improved by treatment with gangliosides. Exp. Neurol 109, 200–203. [DOI] [PubMed] [Google Scholar]
- Eleazu CO, Eleazu KC, Chukwuma S, Essien UN, 2013. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. Journal of Diabetes & Metabolic Disorders 12, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett JW, Keynes RJ, 1990. Peripheral nerve regeneration. Annu. Rev. Neurosci 13, 43–60. [DOI] [PubMed] [Google Scholar]
- Fischer P, Hilfiker-Kleiner D, 2008. Role of gp130-mediated signalling pathways in the heart and its impact on potential therapeutic aspects. Br. J. Pharmacol 153 Suppl 1, S414–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A, Eastwood C, Gentry C, Manning D, Urban L, 1999. Critical evaluation of the streptozotocin model of painful diabetic neuropathy in the rat. Pain 81, 307–316. [DOI] [PubMed] [Google Scholar]
- Gadient RA, Patterson PH, 1999. Leukemia inhibitory factor, Interleukin 6, and other cytokines using the GP130 transducing receptor: roles in inflammation and injury. Stem Cells 17, 127–137. [DOI] [PubMed] [Google Scholar]
- Gardiner NJ, Cafferty WB, Slack SE, Thompson SW, 2002. Expression of gp130 and leukaemia inhibitory factor receptor subunits in adult rat sensory neurones: regulation by nerve injury. J. Neurochem 83, 100–109. [DOI] [PubMed] [Google Scholar]
- Gibbons CH, Illigens BM, Wang N, Freeman R, 2009. Quantification of sweat gland innervation: a clinical-pathologic correlation. Neurology 72, 1479–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene DA, Sima AA, Stevens MJ, Feldman EL, Lattimer SA, 1992. Complications: neuropathy, pathogenetic considerations. Diabetes Care 15, 1902–1925. [DOI] [PubMed] [Google Scholar]
- Greene DA, Stevens MJ, Feldman EL, 1999. Diabetic neuropathy: scope of the syndrome. The American journal of medicine 107, 2–8. [DOI] [PubMed] [Google Scholar]
- Habecker BA, Sachs HH, Rohrer H, Zigmond RE, 2009. The dependence on gp130 cytokines of axotomy induced neuropeptide expression in adult sympathetic neurons. Dev. Neurobiol 69, 392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L, 1998. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. J. Biochem 334 ( Pt 2), 297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T, Nakajima K, Hibi M, 1997. Signaling mechanisms through gp130: a model of the cytokine system. Cytokine Growth Factor Rev. 8, 241–252. [DOI] [PubMed] [Google Scholar]
- Hobson SA, Holmes FE, Kerr NC, Pope RJ, Wynick D, 2006. Mice deficient for galanin receptor 2 have decreased neurite outgrowth from adult sensory neurons and impaired pain-like behaviour. J. Neurochem 99, 1000–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokfelt T, Wiesenfeld-Hallin Z, Villar M, Melander T, 1987. Increase of galanin-like immunoreactivity in rat dorsal root ganglion cells after peripheral axotomy. Neurosci. Lett 83, 217–220. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Zhang X, Wiesenfeld-Hallin Z, 1994. Messenger plasticity in primary sensory neurons following axotomy and its functional implications. Trends Neurosci. 17, 22–30. [DOI] [PubMed] [Google Scholar]
- Holmes FE, Mahoney S, King VR, Bacon A, Kerr NC, Pachnis V, Curtis R, Priestley JV, Wynick D, 2000. Targeted disruption of the galanin gene reduces the number of sensory neurons and their regenerative capacity. Proc. Natl. Acad. Sci. U. S. A 97, 11563–11568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou XE, Dahlstrom A, 1995. Effects of decentralization on the levels of GAP-43 and P38 (synaptophysin) in sympathetic adrenergic-neurons – A semiquantitative study using immunofluorescence and confocal laser-scanning microscopy. Brain Res. 679, 49–63. [DOI] [PubMed] [Google Scholar]
- Hyatt-Sachs H, Bachoo M, Schreiber R, Vaccariello SA, Zigmond RE, 1996. Chemical sympathectomy and postganglionic nerve transection produce similar increases in galanin and VIP mRNA but differ in their effects on peptide content. J. Neurobiol 30, 543–555. [DOI] [PubMed] [Google Scholar]
- Hyatt-Sachs H, Schreiber RC, Bennett TA, Zigmond RE, 1993. Phenotypic plasticity in adult sympathetic ganglia in vivo: effects of deafferentation and axotomy on the expression of vasoactive intestinal peptide. J. Neurosci 13, 1642–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt Sachs H, Rohrer H, Zigmond RE, 2010. The conditioning lesion effect on sympathetic neurite outgrowth is dependent on gp130 cytokines. Exp. Neurol 223, 516–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M, 2013. Animal models of diabetic neuropathy: progress since 1960s. Journal of diabetes research 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MS, Loots d.T., 2009. Experimental rodent models of type 2 diabetes: a review. Methods Find. Exp. Clin. Pharmacol 31, 249–261. [DOI] [PubMed] [Google Scholar]
- Kamei J, Ohhashi Y, Aoki T, Kasuya Y, 1991. Streptozotocin-induced diabetes in mice reduces the nociceptive threshold, as recognized after application of noxious mechanical stimuli but not of thermal stimuli. Pharmacol. Biochem. Behav 39, 541–544. [DOI] [PubMed] [Google Scholar]
- Kennedy JM, Zochodne DW, 2000. The regenerative deficit of peripheral nerves in experimental diabetes: its extent, timing and possible mechanisms. Brain 123 ( Pt 10), 2118–2129. [DOI] [PubMed] [Google Scholar]
- Kennedy JM, Zochodne DW, 2005a. Experimental diabetic neuropathy with spontaneous recovery: is there irreparable damage? Diabetes 54, 830–837. [DOI] [PubMed] [Google Scholar]
- Kennedy JM, Zochodne DW, 2005b. Impaired peripheral nerve regeneration in diabetes mellitus. J. Peripher. Nerv. Syst 10, 144–157. [DOI] [PubMed] [Google Scholar]
- Kennedy WR, Navarro X, 1989. Sympathetic sudomotor function in diabetic neuropathy. Arch. Neurol 46, 1182–1186. [DOI] [PubMed] [Google Scholar]
- Kennedy WR, Sakuta M, 1984. Collateral reinnervation of sweat glands. Ann. Neurol 15, 73–78. [DOI] [PubMed] [Google Scholar]
- Kerr BJ, Cafferty WB, Gupta YK, Bacon A, Wynick D, McMahon SB, Thompson SW, 2000. Galanin knockout mice reveal nociceptive deficits following peripheral nerve injury. Eur. J. Neurosci 12, 793–802. [DOI] [PubMed] [Google Scholar]
- Kilpatrick ES, Maylor PW, Keevil BG, 1998. Biological variation of glycated hemoglobin: implications for diabetes screening and monitoring. Diabetes Care 21, 261–264. [DOI] [PubMed] [Google Scholar]
- Longo FM, Powell HC, Lebeau J, Gerrero MR, Heckman H, Myers RR, 1986. Delayed nerve regeneration in streptozotocin diabetic rats. Muscle Nerve 9, 385–393. [DOI] [PubMed] [Google Scholar]
- Ma TC, Willis DE, 2015. What makes a RAG regeneration associated? Front. Mol. Neurosci 8, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Fernyhough P, Tomlinson DR, 1996. Regenerating sensory neurones of diabetic rats express reduced levels of mRNA for GAP-43, gamma-preprotachykinin and the nerve growth factor receptors, trkA and p75NGFR. Brain Res. Mol. Brain Res 37, 166–174. [DOI] [PubMed] [Google Scholar]
- Maxfield EK, Love A, Cotter MA, Cameron NE, 1995. Nerve function and regeneration in diabetic rats: effects of ZD-7155, an AT1 receptor antagonist. Am. J. Physiol 269, E530–537. [DOI] [PubMed] [Google Scholar]
- Meiri K, Willard M, Johnson M, 1988. Distribution and phosphorylation of the growth-associated protein GAP-43 in regenerating sympathetic neurons in culture. J. Neurosci 8, 2571–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohney RP, Siegel RE, Zigmond RE, 1994. Galanin and vasoactive intestinal peptide messenger RNAs increase following axotomy of adult sympathetic neurons. J. Neurobiol 25, 108–118. [DOI] [PubMed] [Google Scholar]
- Navarro X, Verdu E, Wendelscafer-Crabb G, Kennedy WR, 1995. Innervation of cutaneous structures in the mouse hind paw: a confocal microscopy immunohistochemical study. J. Neurosci. Res 41, 111–120. [DOI] [PubMed] [Google Scholar]
- Niemi JP, DeFrancesco-Lisowitz A, Cregg JM, Howarth M, Zigmond RE, 2016. Overexpression of the monocyte chemokine CCL2 in dorsal root ganglion neurons causes a conditioning-like increase in neurite outgrowth and does so via a STAT3 dependent mechanism. Exp. Neurol 275, 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemi JP, DeFrancesco-Lisowitz A, Roldan-Hernandez L, Lindborg JA, Mandell D, Zigmond RE, 2013. A critical role for macrophages near axotomized neuronal cell bodies in stimulating nerve regeneration. J. Neurosci 33, 16236–16248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasnoor M, Dimachkie MM, Kluding P, Barohn RJ, 2013. Diabetic neuropathy part 1: overview and symmetric phenotypes. Neurol. Clin 31, 425–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekiner C, Dent EW, Roberts RE, Meiri KF, McLean WG, 1996. Altered GAP-43 immunoreactivity in regenerating sciatic nerve of diabetic rats. Diabetes 45, 199–204. [DOI] [PubMed] [Google Scholar]
- Pittenger G, Vinik A, 2003. Nerve growth factor and diabetic neuropathy. Exp Diabesity Res 4, 271–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polydefkis M, Hauer P, Sheth S, Sirdofsky M, Griffin JW, McArthur JC, 2004. The time course of epidermal nerve fibre regeneration: studies in normal controls and in people with diabetes, with and without neuropathy. Brain 127, 1606–1615. [DOI] [PubMed] [Google Scholar]
- Rao MS, Sun Y, Escary JL, Perreau J, Tresser S, Patterson PH, Zigmond RE, Brulet P, Landis SC, 1993a. Leukemia inhibitory factor mediates an injury response but not a target-directed developmental transmitter switch in sympathetic neurons. Neuron 11, 1175–1185. [DOI] [PubMed] [Google Scholar]
- Rao MS, Sun Y, Vaidyanathan U, Landis SC, Zigmond RE, 1993b. Regulation of substance P is similar to that of vasoactive intestinal peptide after axotomy or explantation of the rat superior cervical ganglion. J. Neurobiol 24, 571–580. [DOI] [PubMed] [Google Scholar]
- Raz I, Hasdai D, Seltzer Z, Melmed RN, 1988. Effect of hyperglycemia on pain perception and on efficacy of morphine analgesia in rats. Diabetes 37, 1253–1259. [DOI] [PubMed] [Google Scholar]
- Rigaud M, Gemes G, Barabas ME, Chernoff DI, Abram SE, Stucky CL, Hogan QH, 2008. Species and strain differences in rodent sciatic nerve anatomy: implications for studies of neuropathic pain. Pain 136, 188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs HH, Wynick D, Zigmond RE, 2007. Galanin plays a role in the conditioning lesion effect in sensory neurons. Neuroreport 18, 1729–1733. [DOI] [PubMed] [Google Scholar]
- Said G, 2007. Diabetic neuropathy—a review. Nature clinical practice Neurology 3, 331–340. [DOI] [PubMed] [Google Scholar]
- Schreiber RC, Hyatt-Sachs H, Bennett TA, Zigmond RE, 1994. Galanin expression increases in adult rat sympathetic neurons after axotomy. Neuroscience 60, 17–27. [DOI] [PubMed] [Google Scholar]
- Scott JN, Clark AW, Zochodne DW, 1999. Neurofilament and tubulin gene expression in progressive experimental diabetes: failure of synthesis and export by sensory neurons. Brain 122 ( Pt 11), 2109–2118. [DOI] [PubMed] [Google Scholar]
- Shadiack AM, Vaccariello SA, Zigmond RE, 1995. Galanin expression in sympathetic ganglia after partial axotomy is highly localized to those neurons that are axotomized. Neuroscience 65, 1119–1127. [DOI] [PubMed] [Google Scholar]
- Shadiack AM, Zigmond RE, 1998. Galanin induced in sympathetic neurons after axotomy is anterogradely transported toward regenerating nerve endings. Neuropeptides 32, 257–264. [DOI] [PubMed] [Google Scholar]
- Sharma R, Weldekidan E, Berhane Y, Nathiya D, 2015. Diabetic Neuropathy: A Review. International Journal of Research in Pharmacy & Science. [Google Scholar]
- Sharma S, Kulkarni SK, Agrewala JN, Chopra K, 2006. Curcumin attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. Eur. J. Pharmacol 536, 256–261. [DOI] [PubMed] [Google Scholar]
- Shin JE, Geisler S, DiAntonio A, 2014. Dynamic regulation of SCG10 in regenerating axons after injury. Exp. Neurol 252, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JE, Miller BR, Babetto E, Cho Y, Sasaki Y, Qayum S, Russler EV, Cavalli V, Milbrandt J, DiAntonio A, 2012. SCG10 is a JNK target in the axonal degeneration pathway. Proceedings of the National Academy of Sciences 109, E3696–E3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons Z, Feldman EL, 2002. Update on diabetic neuropathy. Curr. Opin. Neurol 15, 595–603. [DOI] [PubMed] [Google Scholar]
- Smith DS, Skene JH, 1997. A transcription-dependent switch controls competence of adult neurons for distinct modes of axon growth. J. Neurosci 17, 646–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KA, Feldman EL, 2005. New developments in diabetic neuropathy. Curr. Opin. Neurol 18, 586–590. [DOI] [PubMed] [Google Scholar]
- Sullivan KA, Hayes JM, Wiggin TD, Backus C, Su Oh S, Lentz SI, Brosius F 3rd, Feldman EL, 2007. Mouse models of diabetic neuropathy. Neurobiol. Dis 28, 276–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KA, Lentz SI, Roberts JL Jr., Feldman EL, 2008. Criteria for creating and assessing mouse models of diabetic neuropathy. Curr. Drug Targets 9, 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Rao MS, Zigmond RE, Landis SC, 1994. Regulation of vasoactive intestinal peptide expression in sympathetic neurons in culture and after axotomy: the role of cholinergic differentiation factor/leukemia inhibitory factor. J. Neurobiol 25, 415–430. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zigmond RE, 1996a. Involvement of leukemia inhibitory factor in the increases in galanin and vasoactive intestinal peptide mRNA and the decreases in neuropeptide Y and tyrosine hydroxylase mRNA in sympathetic neurons after axotomy. J. Neurochem 67, 1751–1760. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zigmond RE, 1996b. Leukaemia inhibitory factor induced in the sciatic nerve after axotomy is involved in the induction of galanin in sensory neurons. Eur. J. Neurosci 8, 2213–2220. [DOI] [PubMed] [Google Scholar]
- Tedeschi A, Bradke F, 2017. Spatial and temporal arrangement of neuronal intrinsic and extrinsic mechanisms controlling axon regeneration. Curr. Opin. Neurobiol 42, 118–127. [DOI] [PubMed] [Google Scholar]
- Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, 2010. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 33, 2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Corral CM, Banner LR, 2011. Early changes of LIFR and gp130 in sciatic nerve and muscle of diabetic mice. Acta Histochem. 114, 159–165. [DOI] [PubMed] [Google Scholar]
- Verrotti A, Prezioso G, Scattoni R, Chiarelli F, 2014. Autonomic neuropathy in diabetes mellitus. Front. Endocrinol. (Lausanne) 5, 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilches JJ, Navarro X, 2002. New silicones for the evaluation of sudomotor function with the impression mold technique. Clin. Auton. Res 12, 20–23. [DOI] [PubMed] [Google Scholar]
- Vilches JJ, Wynick D, Kofler B, Lang R, Navarro X, 2012. Sudomotor function and sweat gland innervation in galanin knockout mice. Neuropeptides 46, 151–155. [DOI] [PubMed] [Google Scholar]
- Vinik AI, Freeman R, Erbas T, 2003. Diabetic autonomic neuropathy. Semin. Neurol 23, 365–372. [DOI] [PubMed] [Google Scholar]
- Wu Z, Mata M, Fink DJ, 2011. Prevention of diabetic neuropathy by regulatable expression of HSV-mediated erythropoietin. Mol. Ther 19, 310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond R, Mohney R, Schreiber R, Shadiack A, Sun Y, Vaccariello YS, Zhou Y, 1998. Changes in gene expression in adult sympathetic neurons after axonal injury. Adv. Pharmacol 42, 899–903. [DOI] [PubMed] [Google Scholar]
- Zigmond RE, 1994. Axotomy changes peptide expression. Trends Neurosci. 17, 297–299. [DOI] [PubMed] [Google Scholar]
- Zigmond RE, 2011. gp130 cytokines are positive signals triggering changes in gene expression and axon outgrowth in peripheral neurons following injury. Front. Mol. Neurosci 4, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond RE, 2012. gp130 cytokines are positive signals triggering changes in gene expression and axon outgrowth in peripheral neurons following injury. Front. Mol. Neurosci 4, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond RE, Hyatt-Sachs H, Mohney RP, Schreiber RC, Shadiack AM, Sun Y, Vaccariello SA, 1996. Changes in neuropeptide phenotype after axotomy of adult peripheral neurons and the role of leukemia inhibitory factor. Perspect. Dev. Neurobiol 4, 75–90. [PubMed] [Google Scholar]
- Zochodne DW, 2008. Neurobiology of Peripheral Nerve Regeneration. Cambridge University Press, Cambridge. [Google Scholar]
- Zochodne DW, Guo GF, Magnowski B, Bangash M, 2007. Regenerative failure of diabetic nerves bridging transection injuries. Diabetes Metab. Res. Rev 23, 490–496. [DOI] [PubMed] [Google Scholar]